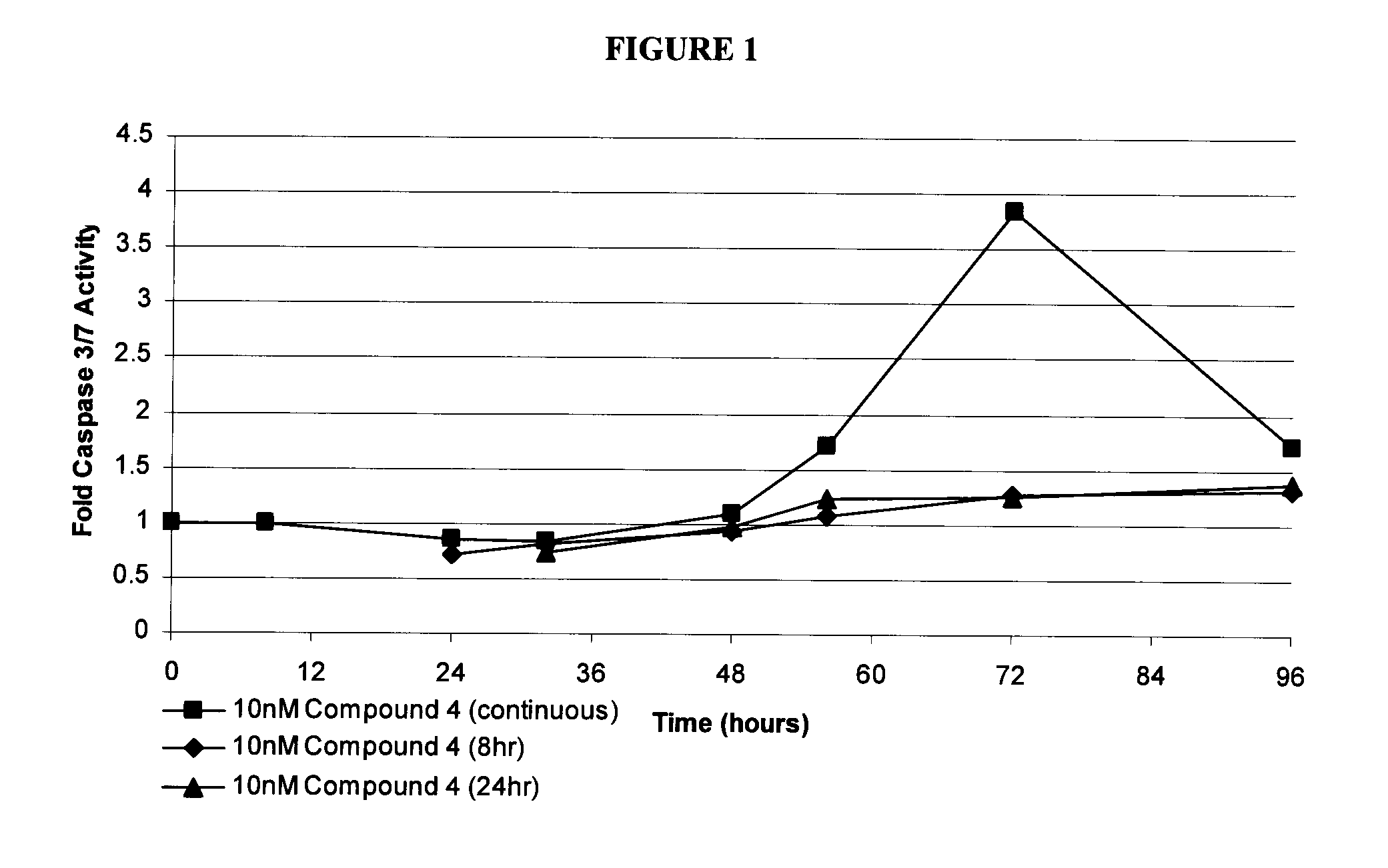
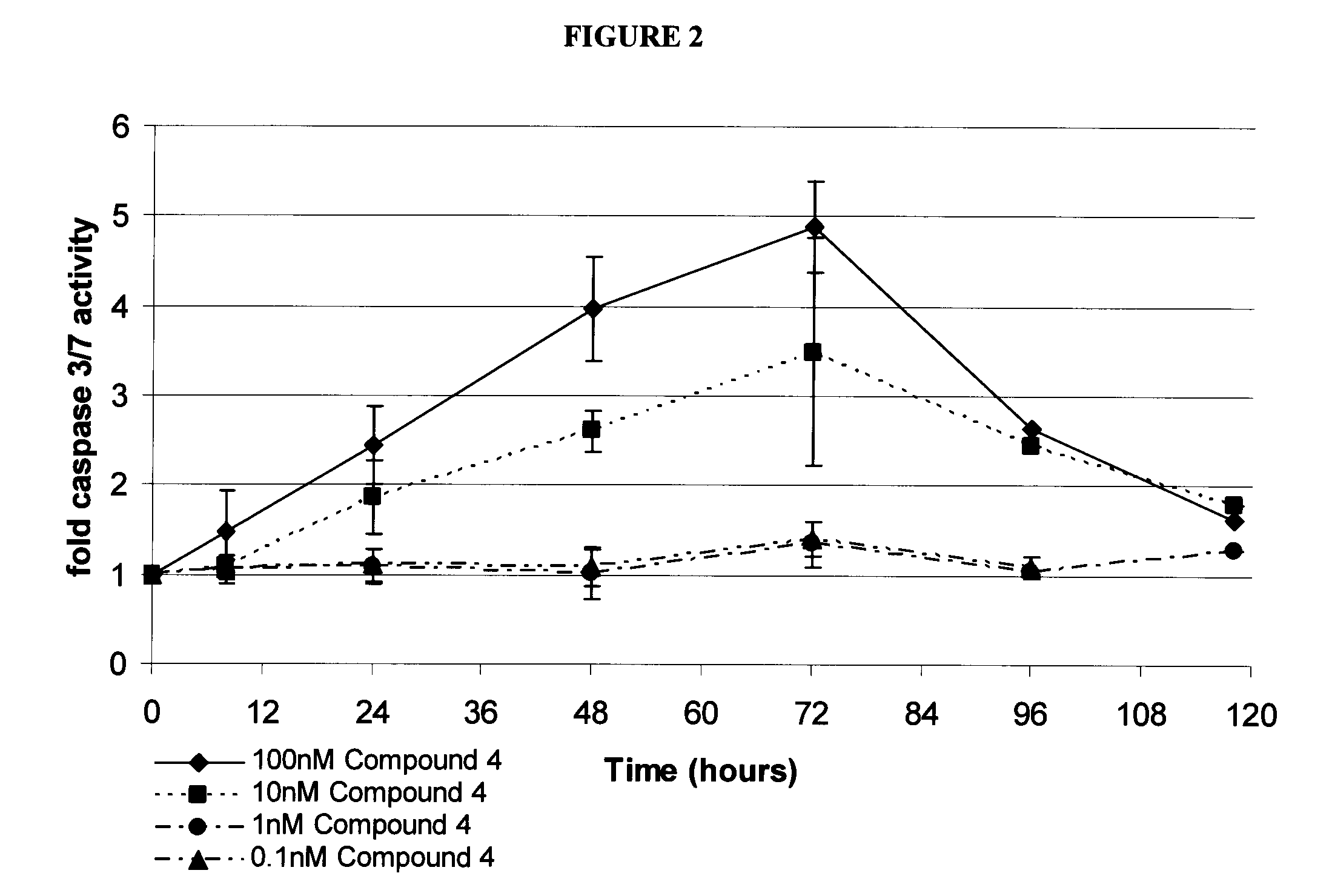
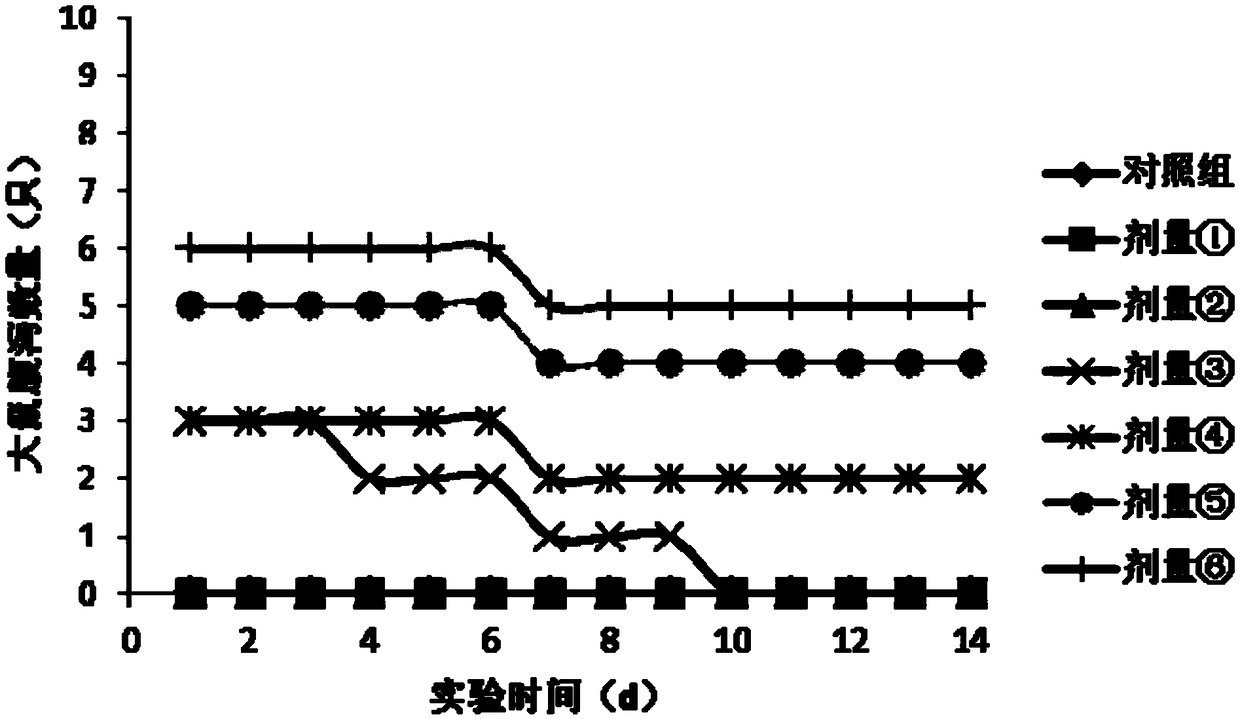
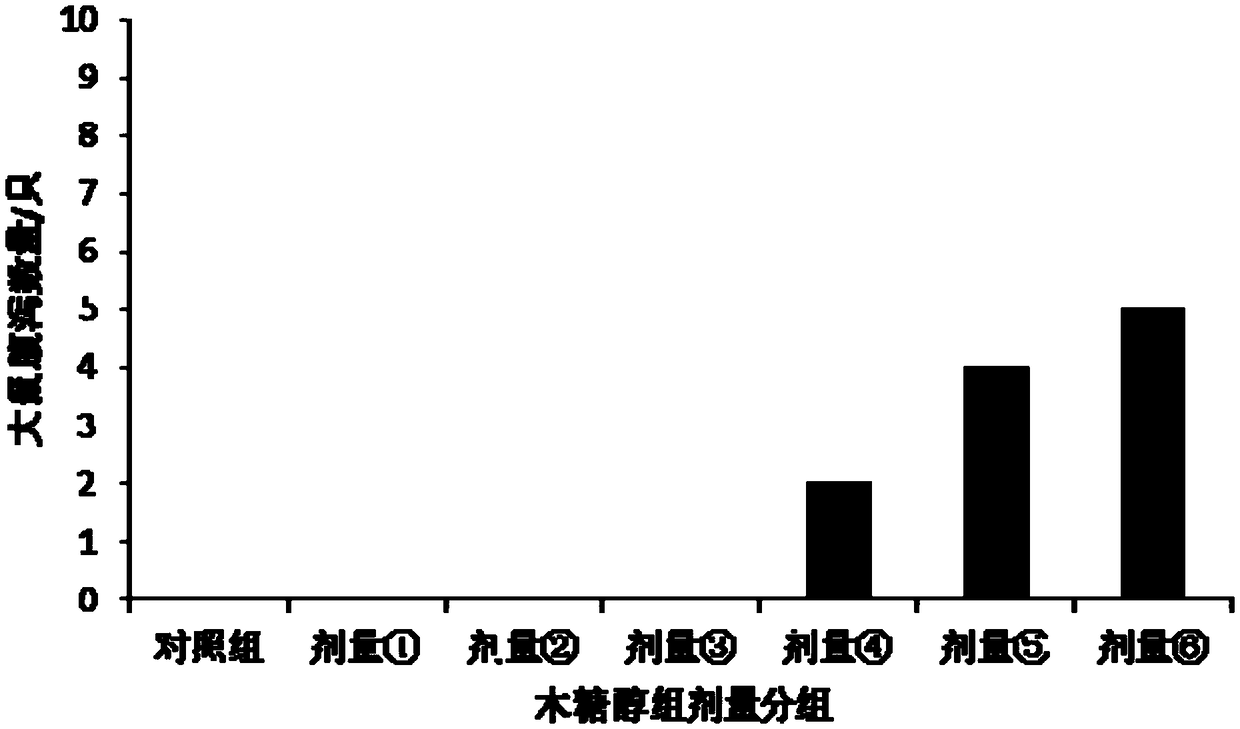
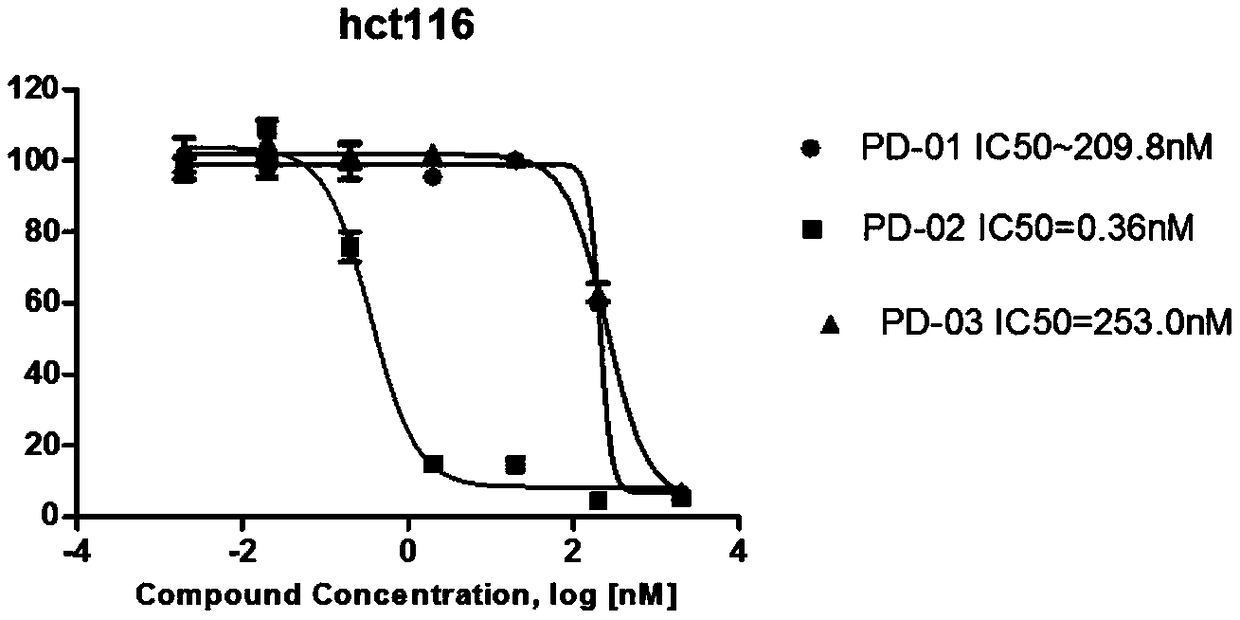
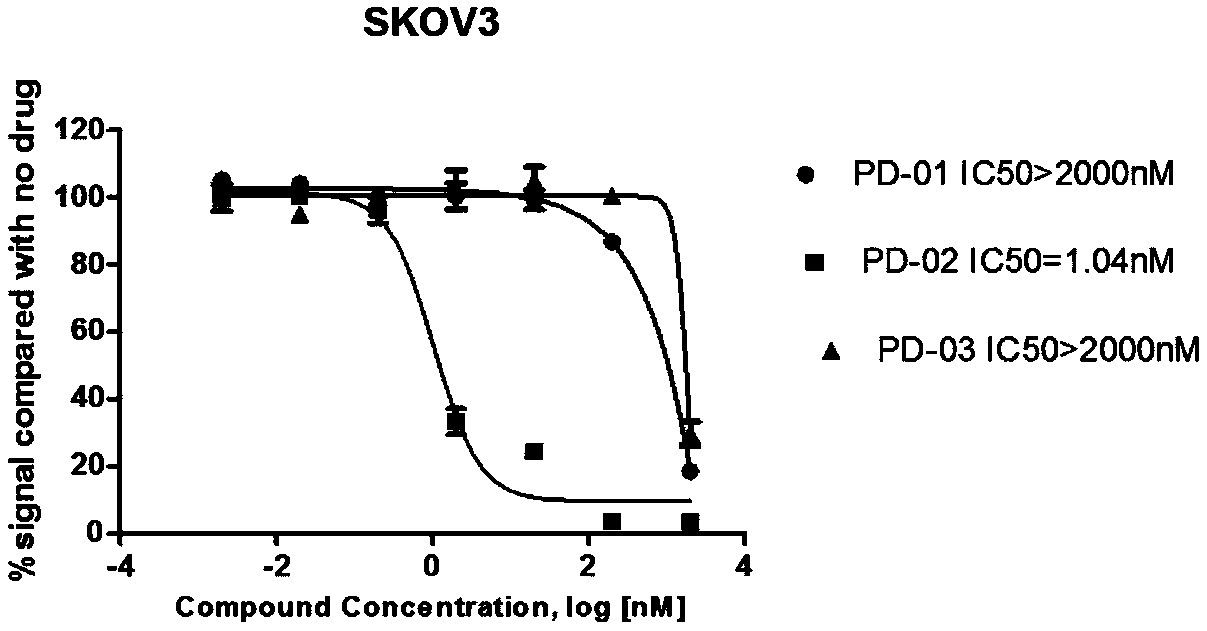
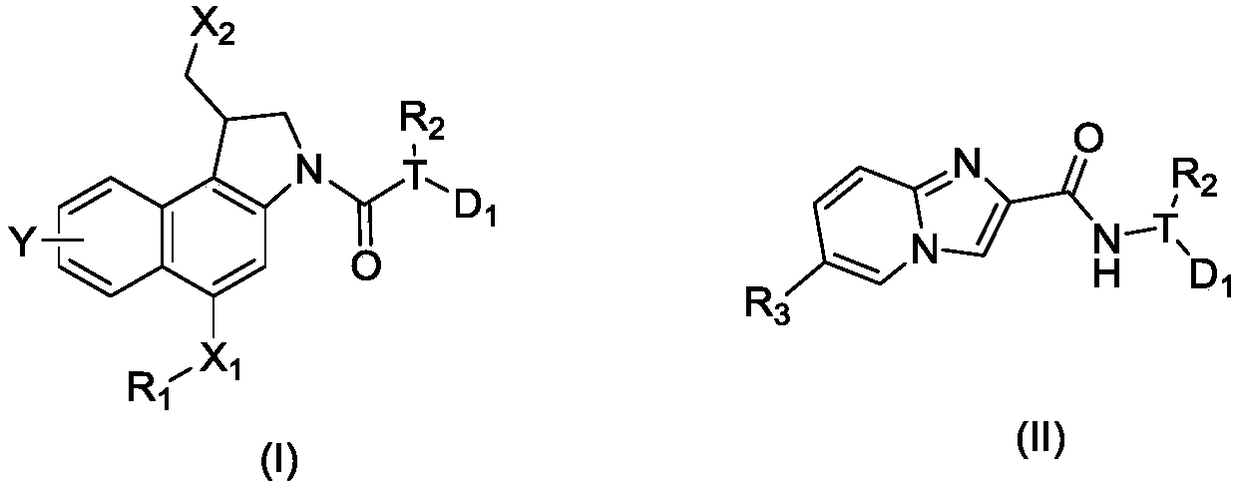
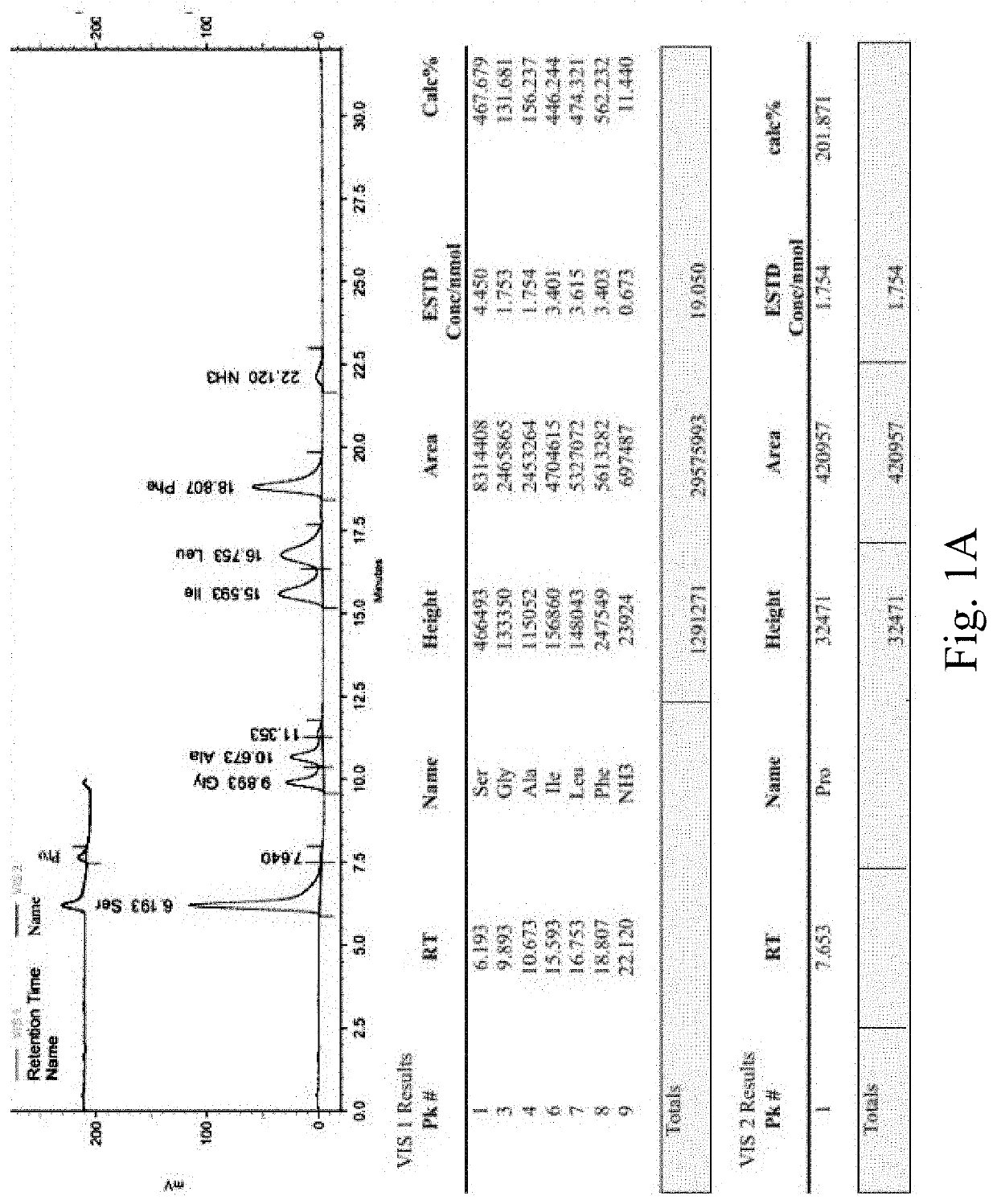
Patents
Literature
41 results about "Maximum tolerated dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
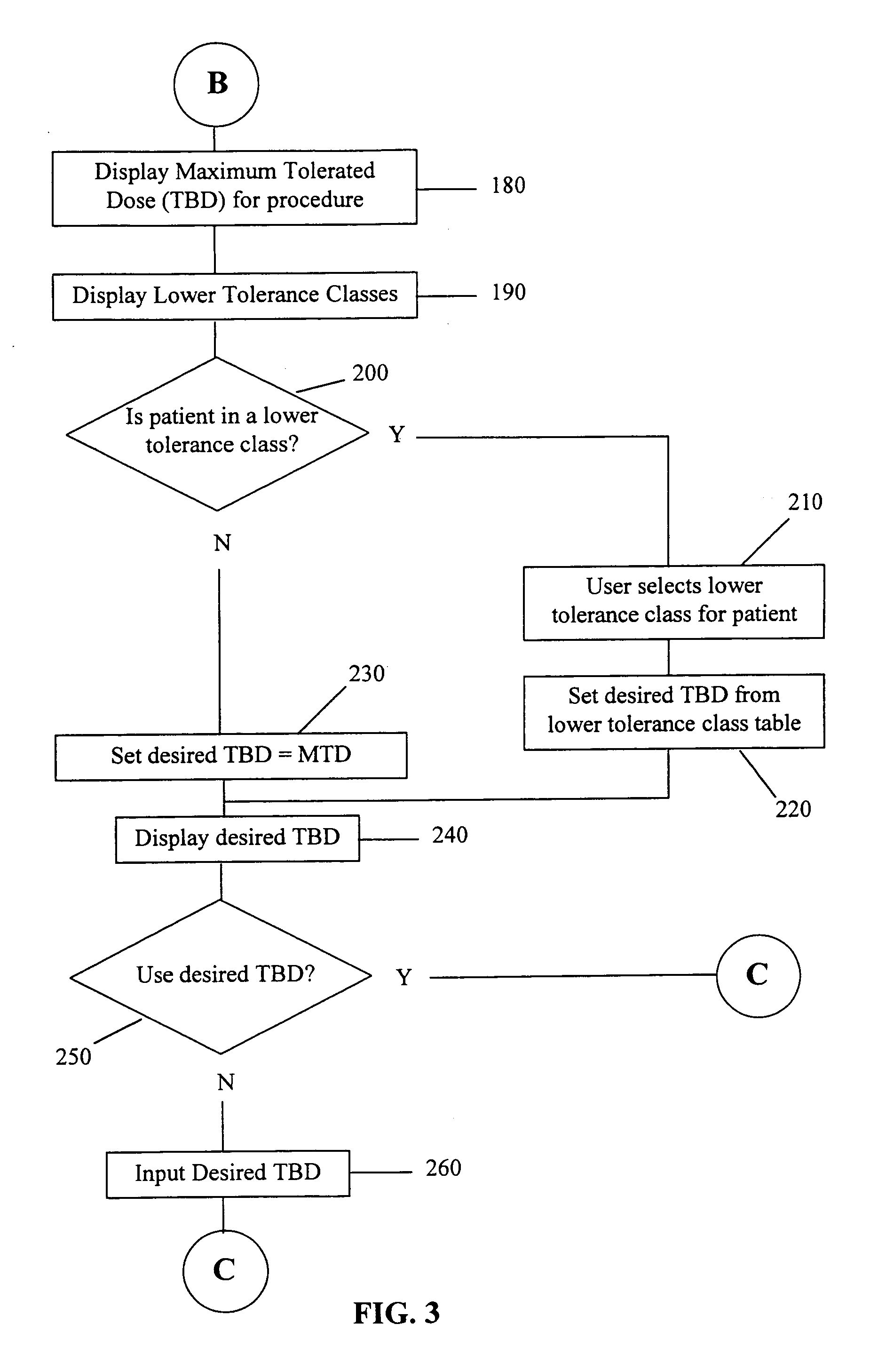
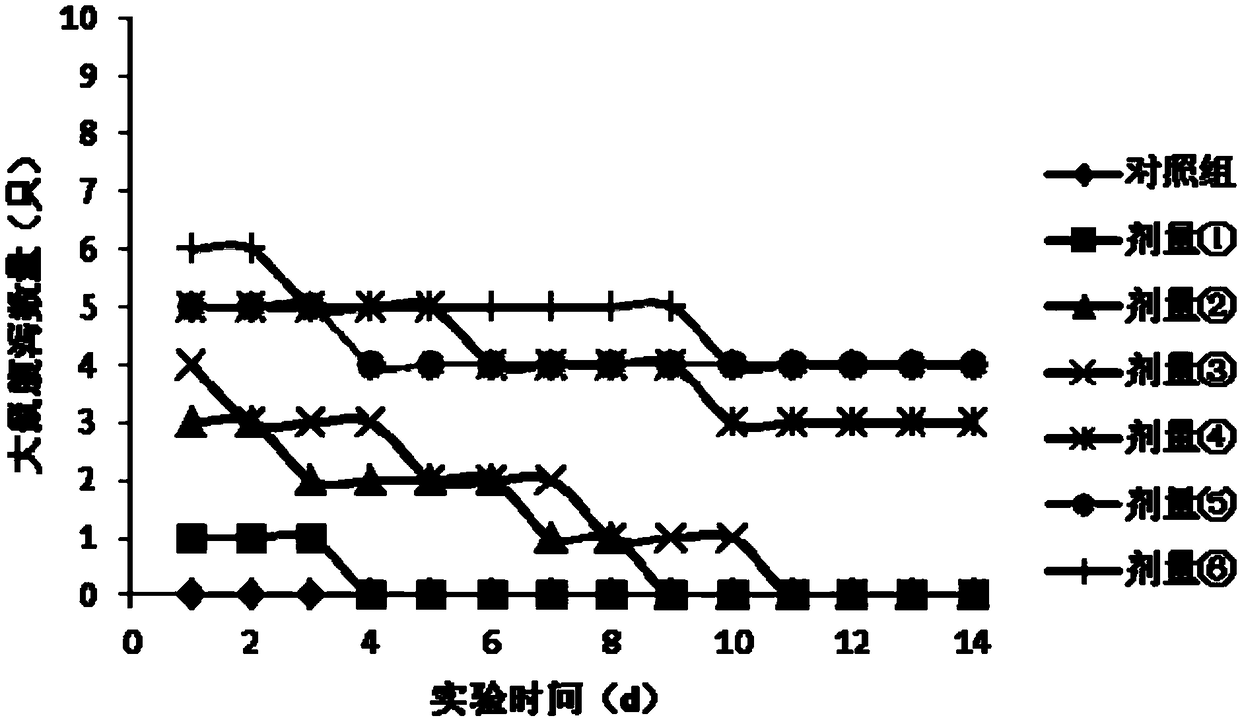
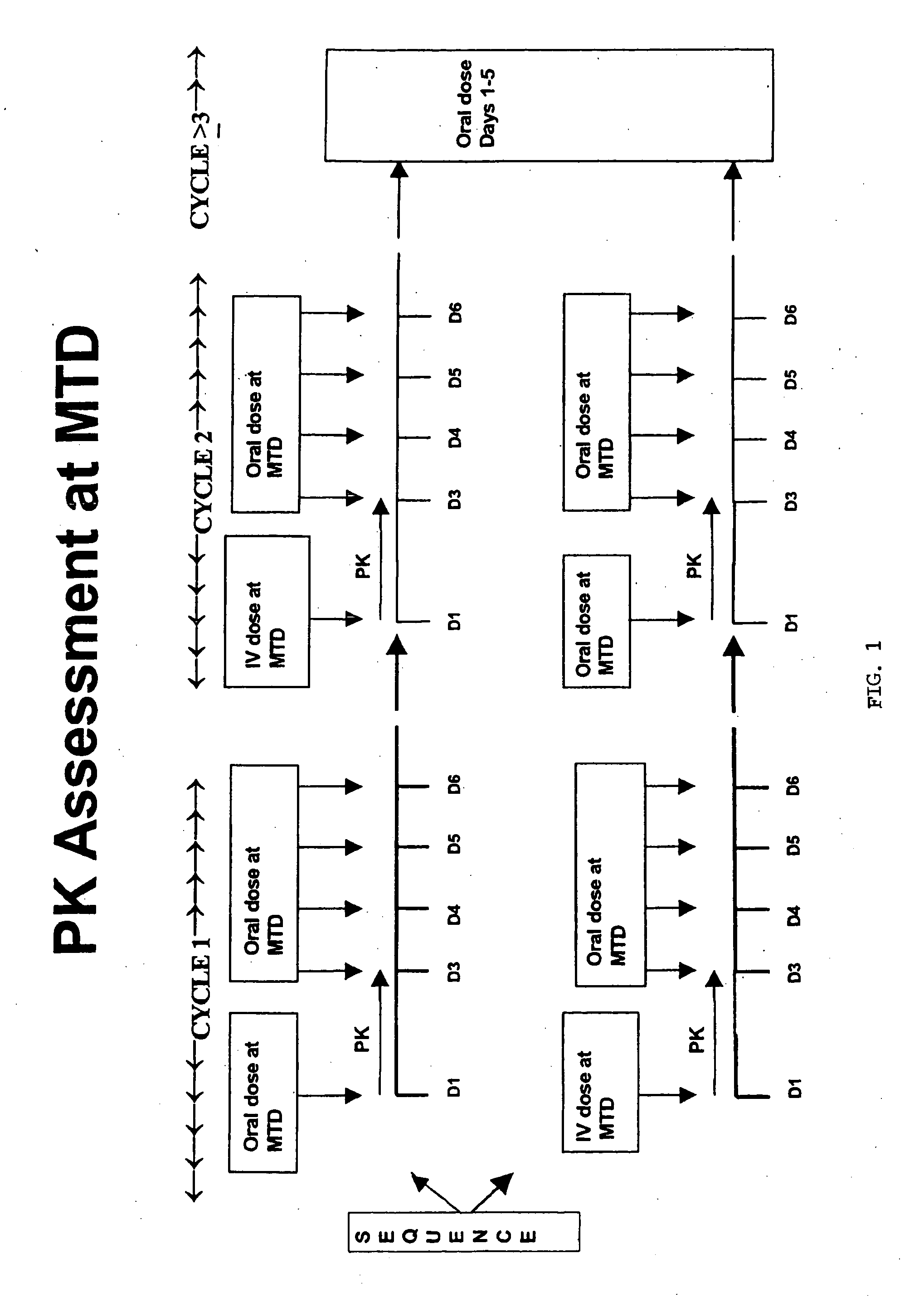
Maximum tolerated dose refers to the highest dose of a radiological or pharmacological treatment that will produce the desired effect without unacceptable toxicity. The purpose of administering MTD is to determine whether long-term exposure to a chemical might lead to unacceptable adverse health effects in a population, when the level of exposure is not sufficient to cause premature mortality due to short-term toxic effects. The maximum dose is used, rather than a lower dose, to reduce the number of test subjects, in order to detect an effect that might occur only rarely. This type of analysis is also used in establishing chemical residue tolerances in foods. Maximum tolerated dose studies are also done in clinical trials.
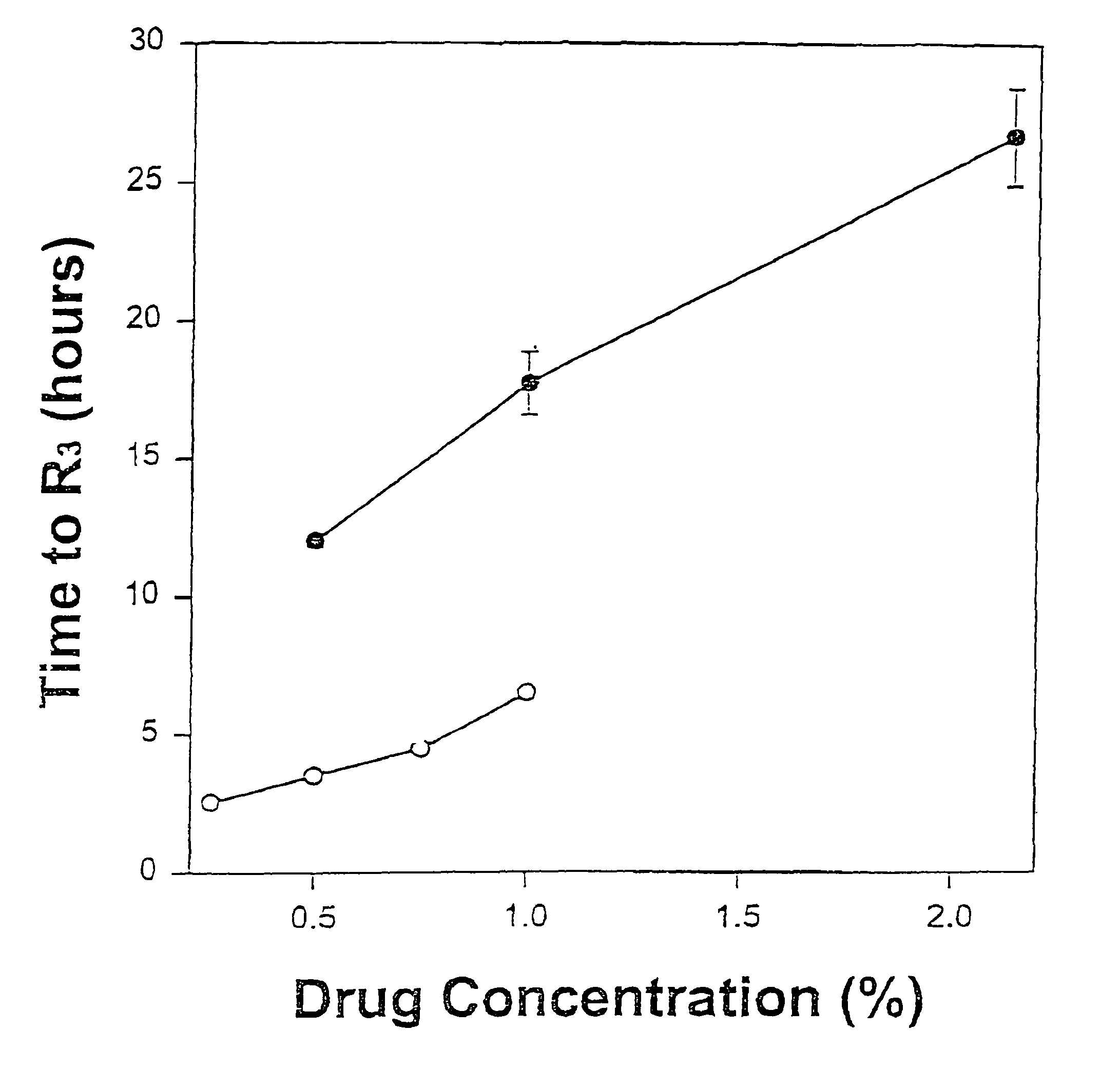
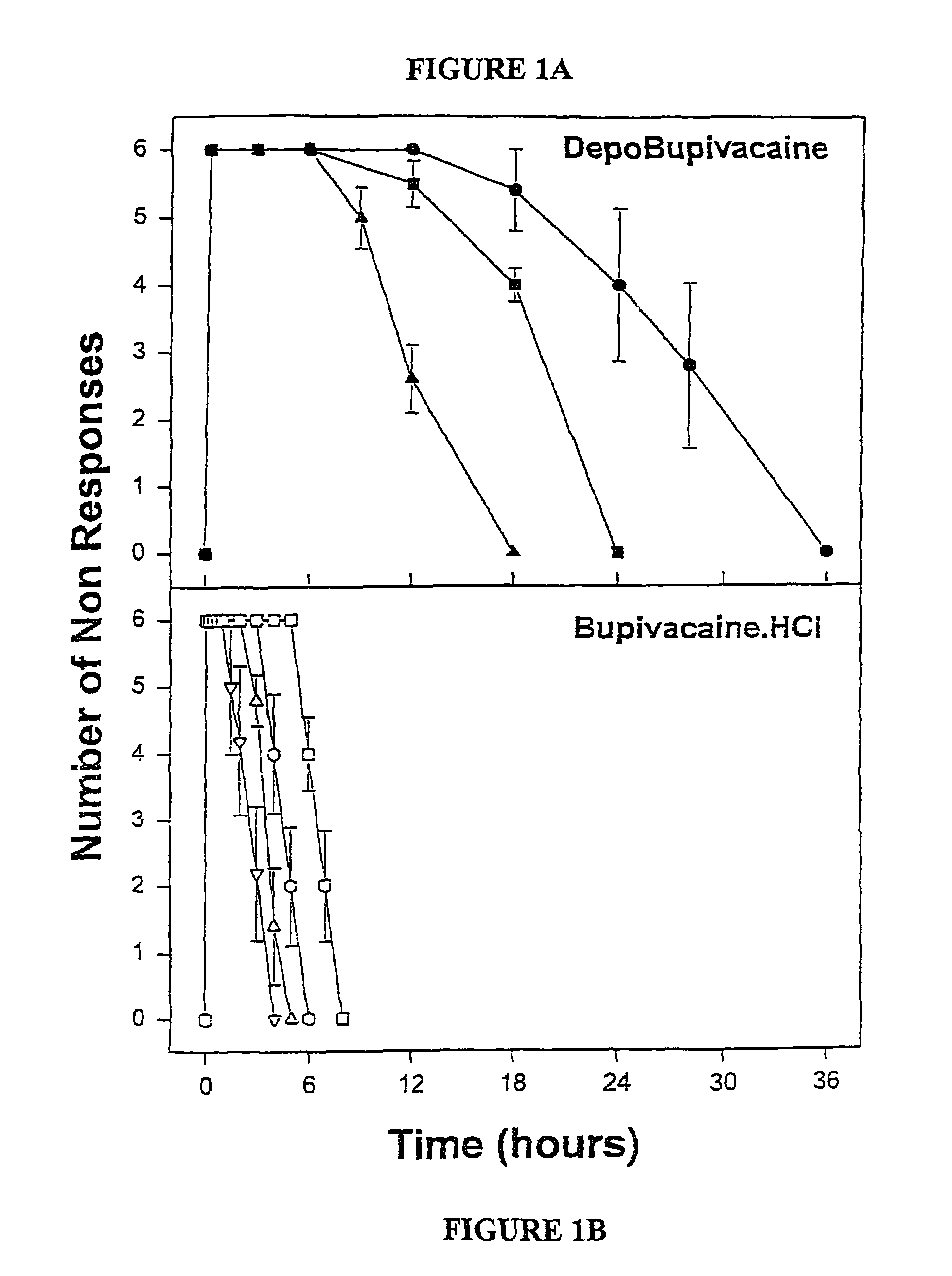
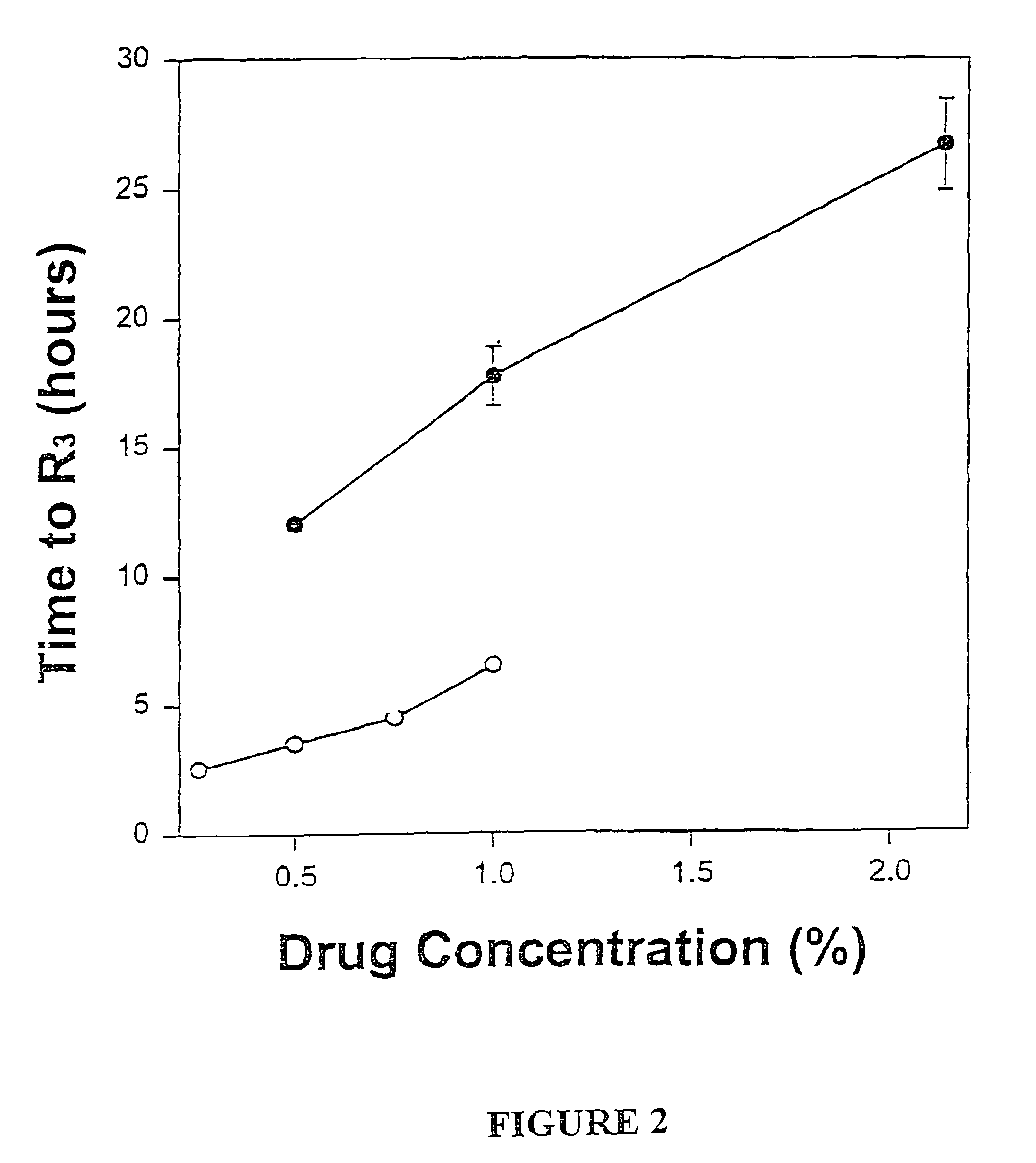
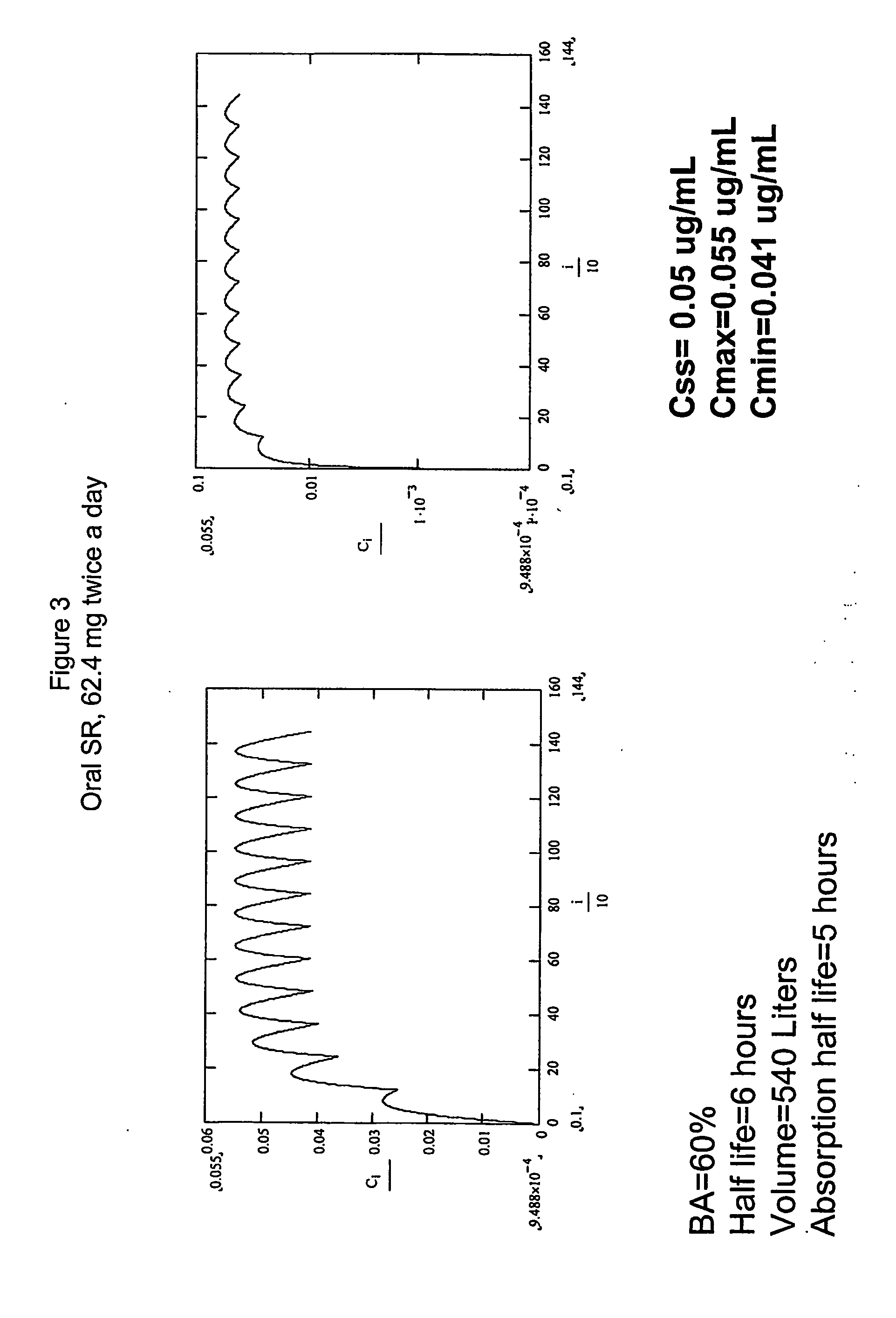
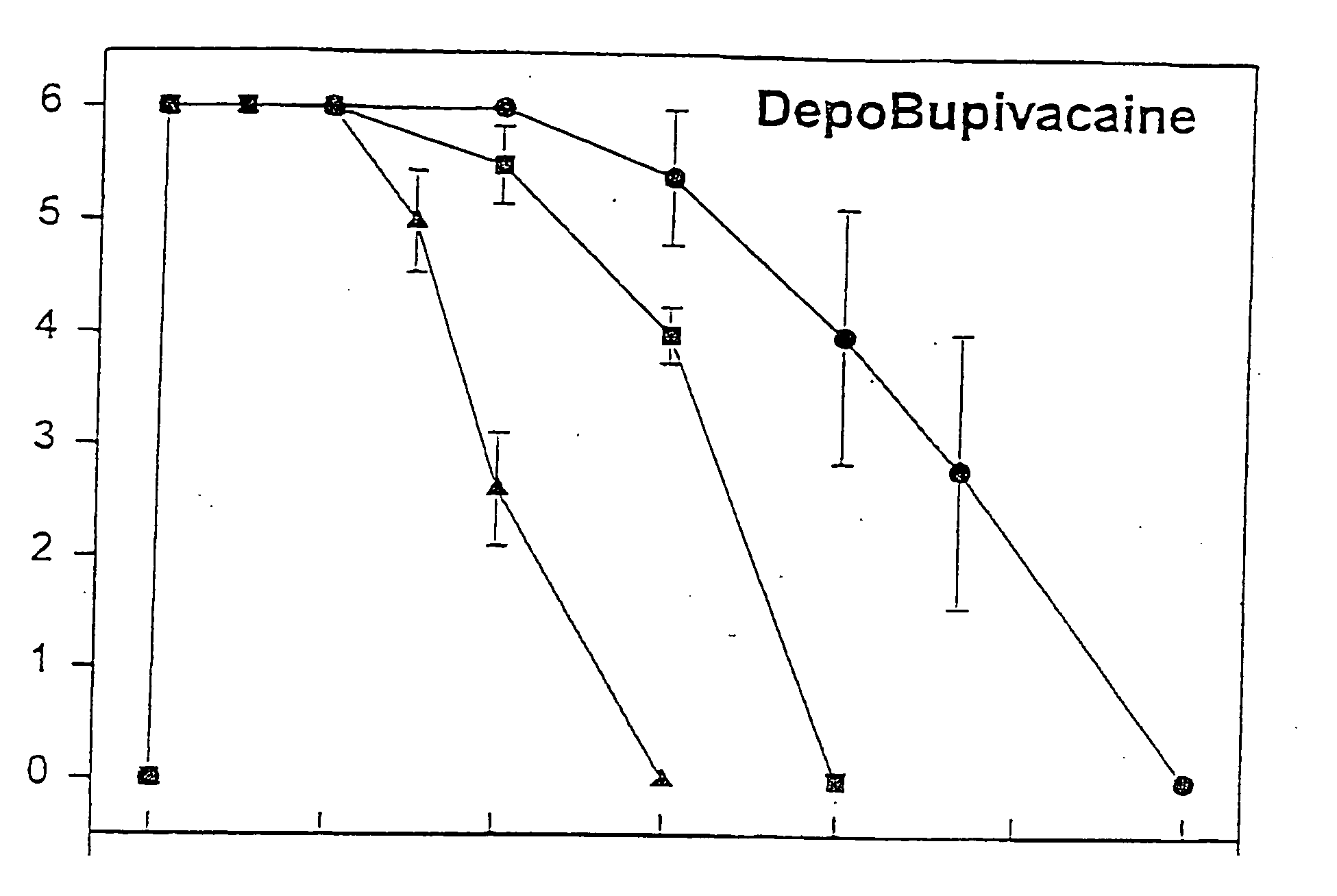
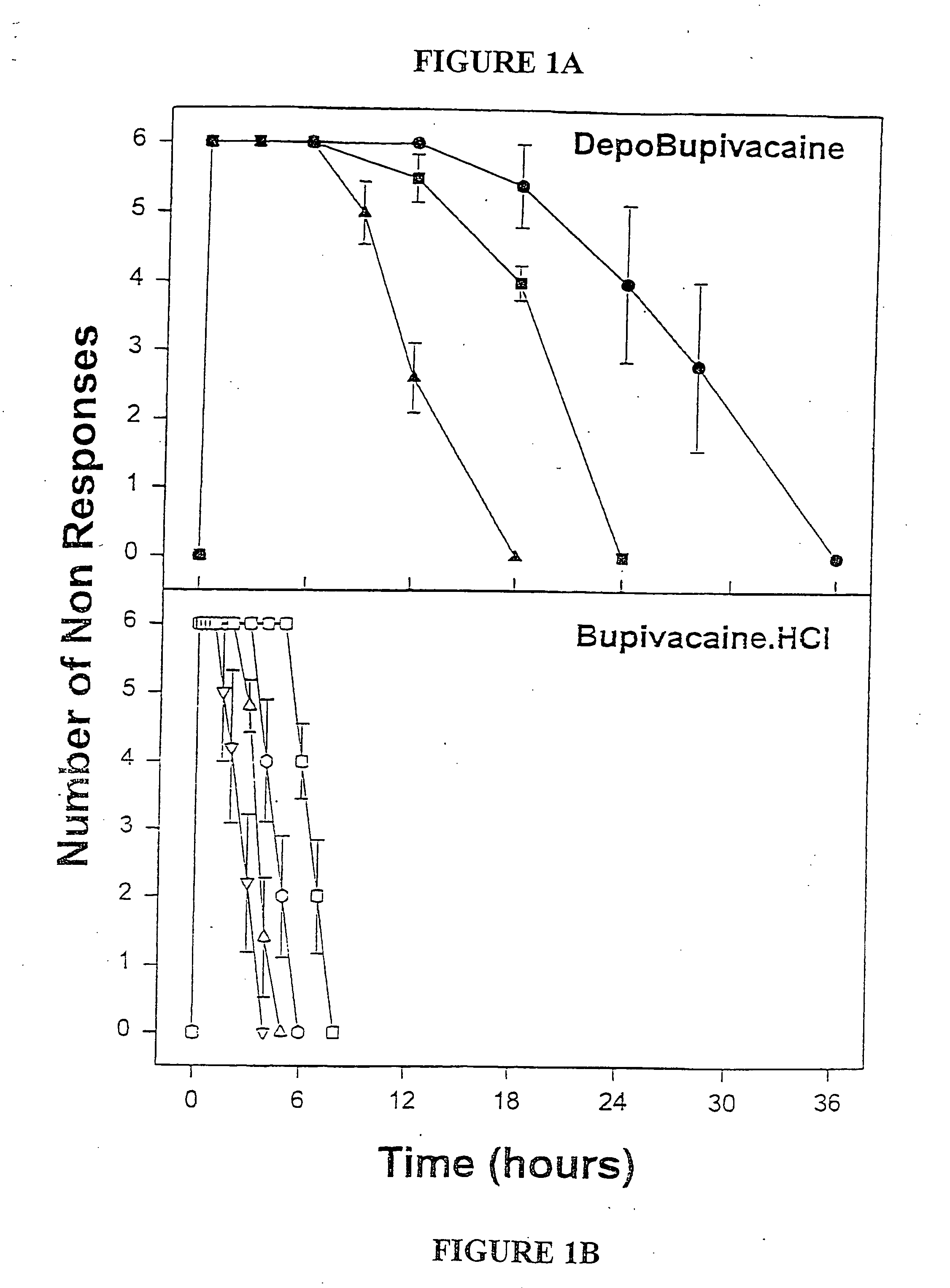
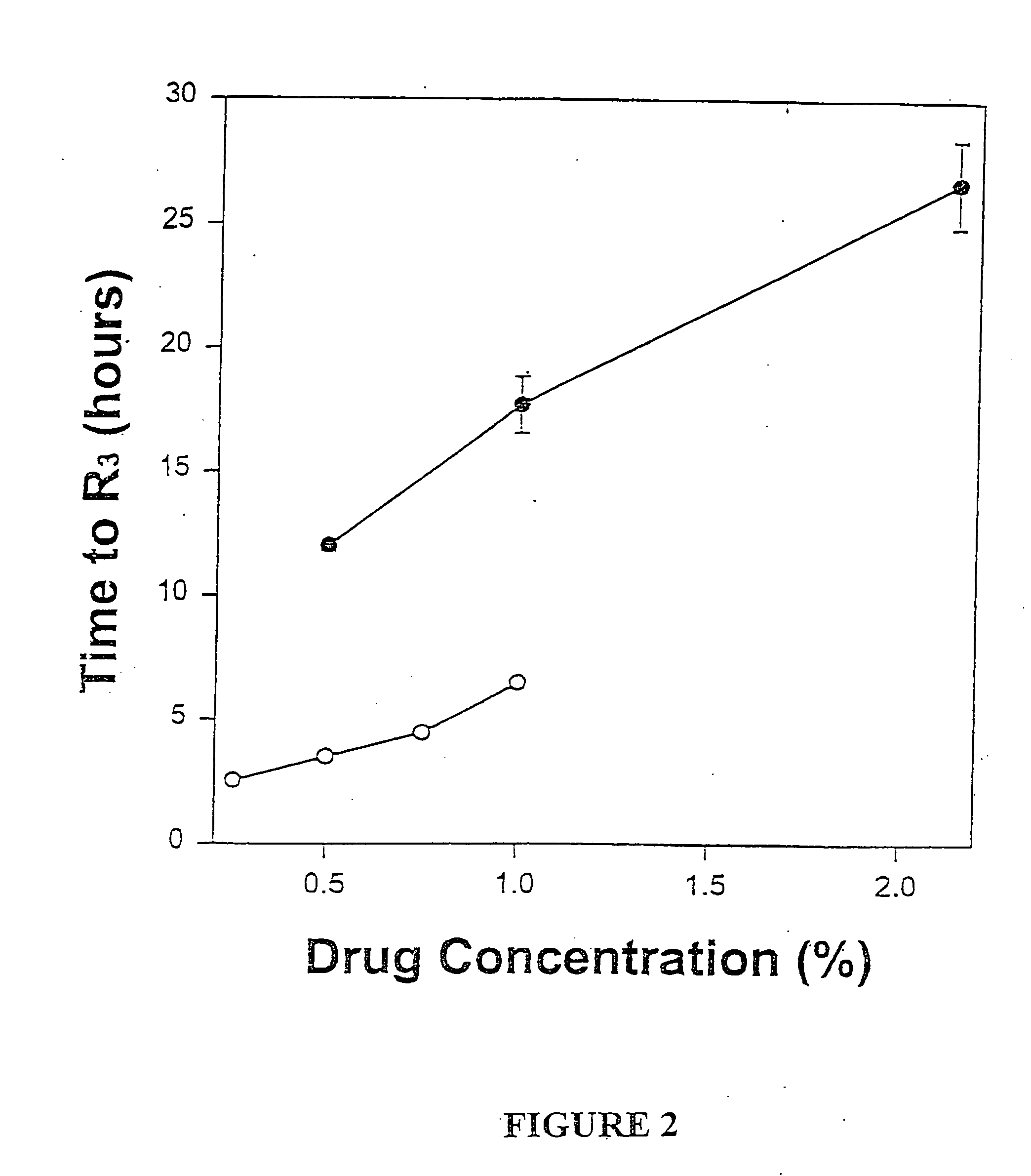
Sustained-release liposomal anesthetic compositions
InactiveUS8182835B2High acceptabilityImprove encapsulationInorganic non-active ingredientsAnaestheticsHalf-lifeMaximum tolerated dose
Owner:PACIRA PHARMA INC
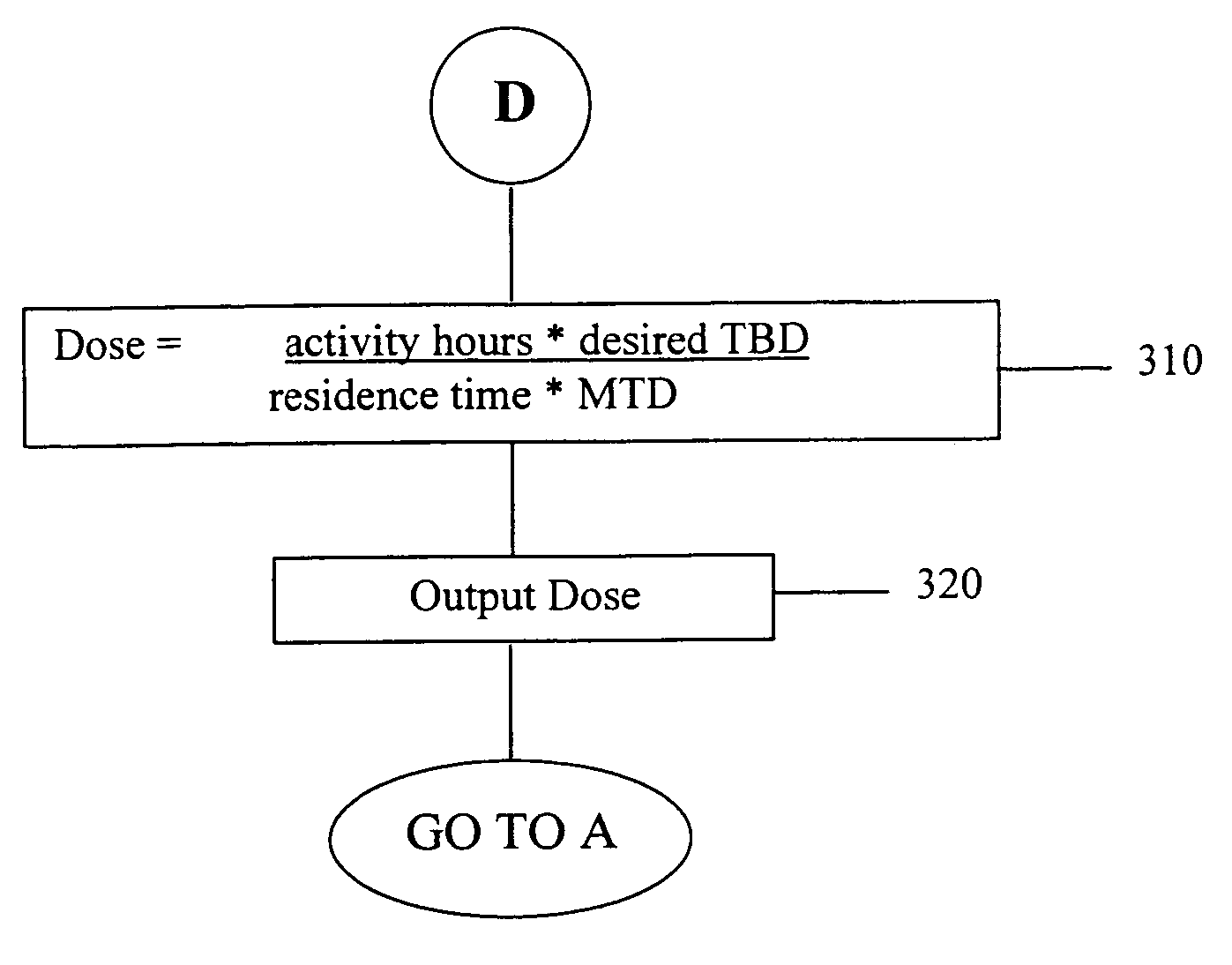
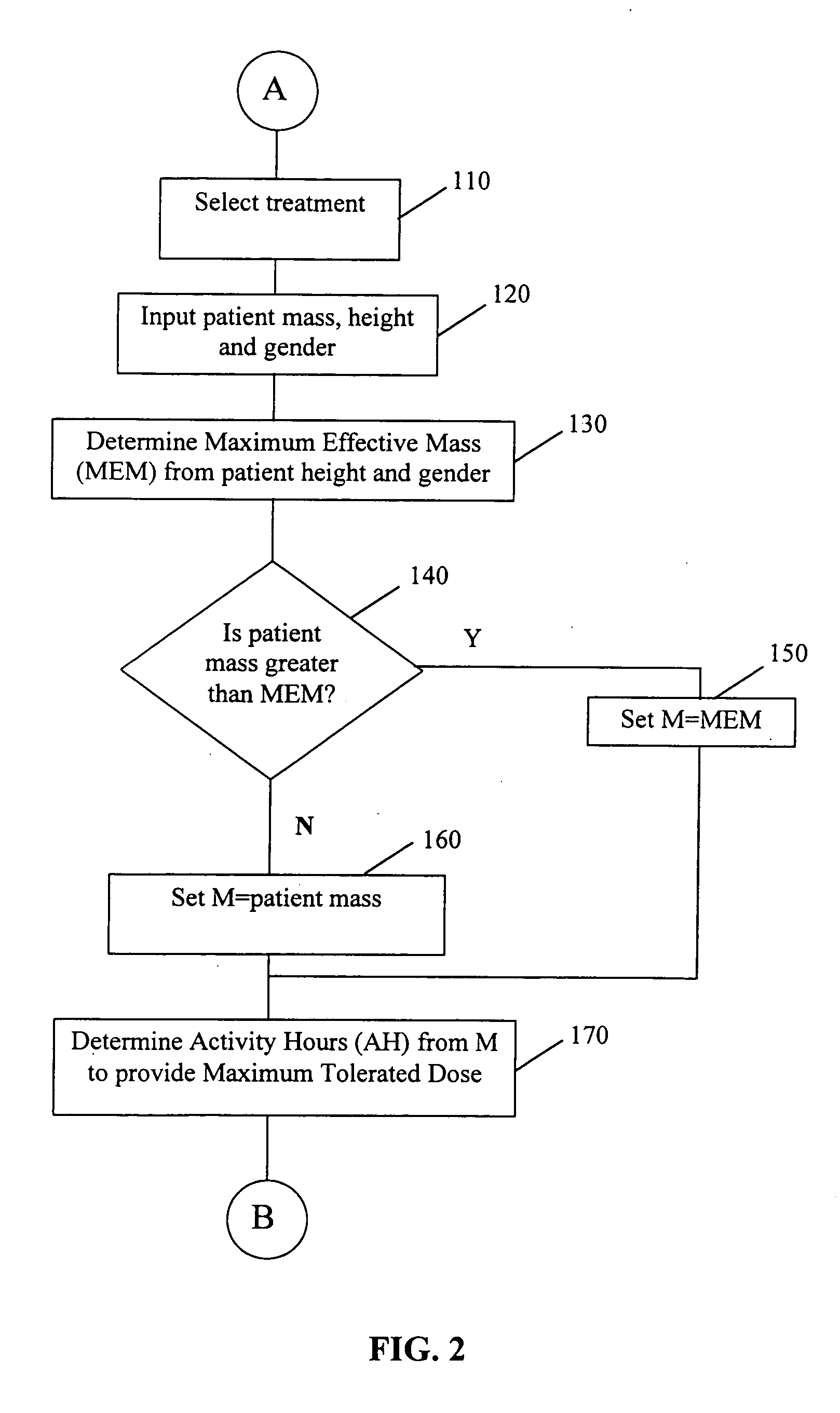
Patient-specific dosimetry
InactiveUS20050288869A1Simple methodRadioactive preparation carriersImmunoglobulinsDosimetry radiationWhole body
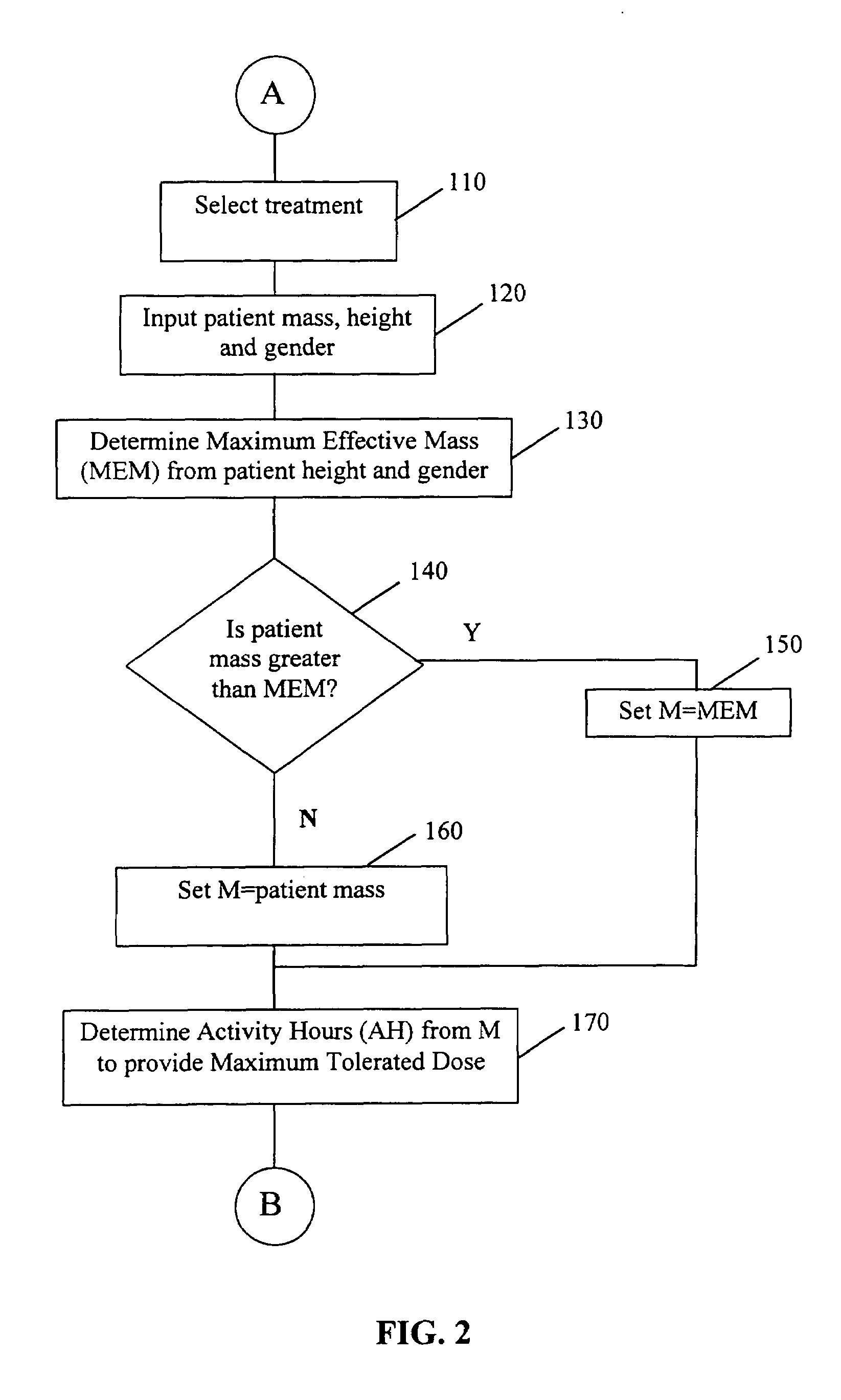
A patient-specific optimally effective radiation dose for administration of a radiopharmaceutical to a patient for treatment of a disease may be established by basing the calculation of the appropriate therapeutic dose on factors such as the desired total body dose, the maximum tolerated dose, the typical clearance profile of the radiopharmaceutical, the patient's mass or maximum effective mass, and the patient-specific residence time of the radiopharmaceutical or an analog in the whole body of the patient. The use of the method allows for treatment of a patient with an appropriate dose which is maximally effective against the disease yet minimally toxic. The determination of a patient-specific therapeutic dose may be assisted by the use of a software program set to the particular parameters of the radiopharmaceutical.
Owner:GLAXO SMITHKLINE LLC +1
Prolonged administration of NMDA antagonist and safener drug to alter neuropathic pain condition
InactiveUS20050148673A1Reduce neurotoxic side effectInherent activityBiocideOrganic active ingredientsNR1 NMDA receptorSide effect
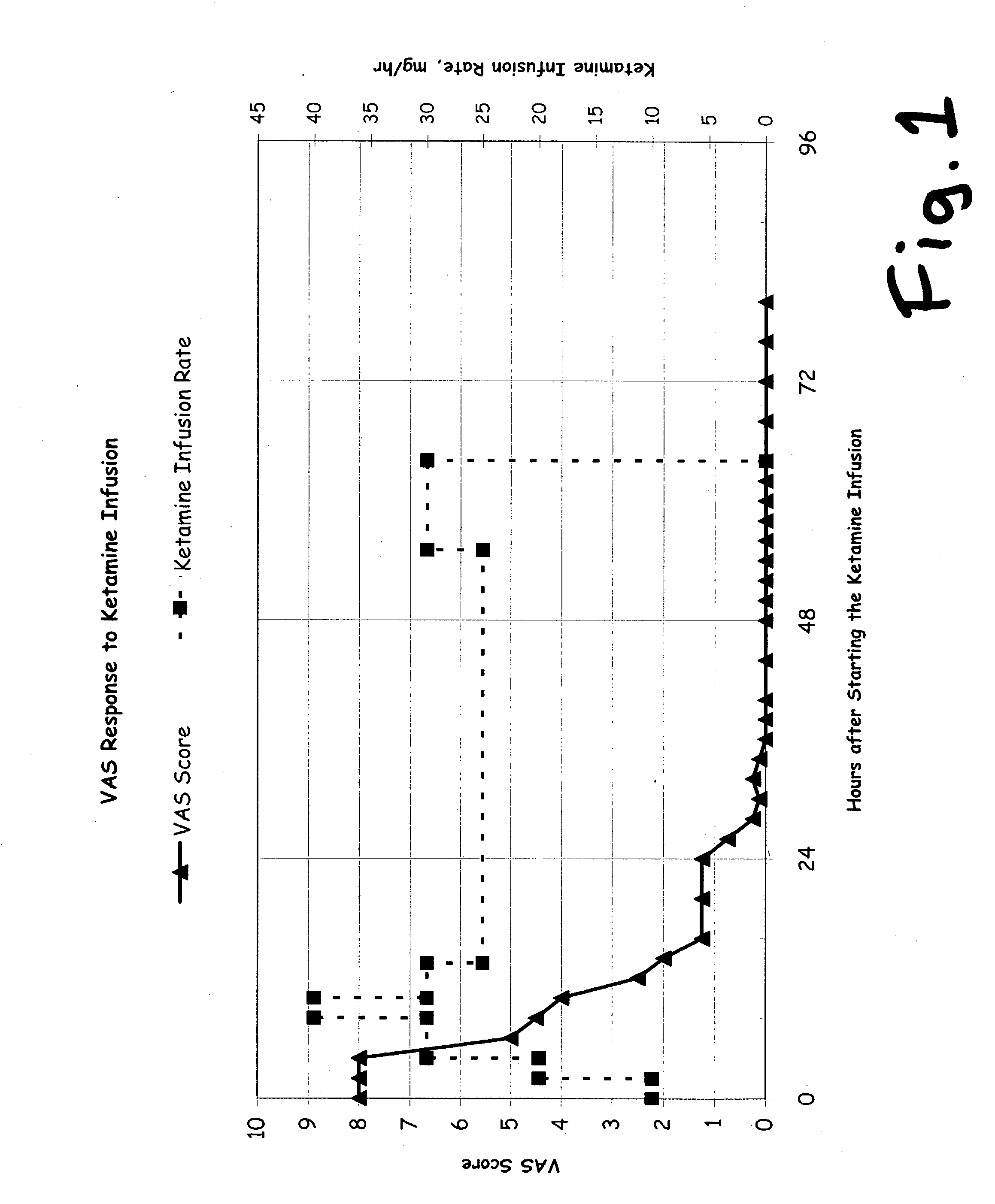
A drug that inhibits NMDA receptors (such as ketamine, a surgical anesthetic) is continuously administered to patients suffering from neuropathic pain. Unless the NMDA antagonist drug has inherent safening activity, this treatment requires a “safener” drug to prevent the neurotoxic side effects of NMDA antagonists. One class of safener drugs that increase the efficacy of the treatment include alpha-2 adrenergic agonists, such as clonidine. The treatment lasts for several days and nights, continuously. A maximum tolerated dosage is titered for each patient, such as by observing slurring of speech, and the patient does not lose consciousness except during normal sleep. Magnesium and / or drugs that inhibit ketamine-degrading enzymes can also be used. Patients who suffered for years from chronic intractable pain emerged from this treatment with apparently permanent relief, or with lasting reductions in their levels of pain.
Owner:HARBUT RONALD E +2
Use and composition for treating dementia
ActiveUS8404701B2Maximize the effectSymptoms improvedBiocideNervous disorderMaximum tolerated doseAnti cholinergic
Owner:CHASE PHARMA CORP
Dosing regimens for the treatment of cancer
The invention relates to a method of treating cancer. The method includes administering systemically a therapeutically effective amount of a small molecule hedgehog pathway inhibitor, such that the concentration of the inhibitor in the blood does not vary by more than about ±30% from the average concentration, and such that the concentration remains at or below the maximum tolerated dose of the inhibitor for a time period of at least about one day.
Owner:INFINITY PHARMA
Sustained-release liposomal anesthetic compositions
InactiveUS20060078606A1High acceptabilityImprove encapsulationInorganic non-active ingredientsRotary piston pumpsHalf-lifeMaximum tolerated dose
The invention provides a method for obtaining local anesthetics encapsulated in liposomes, such as multivesicular liposomes, with high encapsulation efficiency and slow release in vivo. When the encapsulated anesthetic is administered as a single intracutaneous dose, the duration of anesthesia and half-life of the drug at the local injection site is increased as compared to injection of unencapsulated anesthetic. The maximum tolerated dose of the encapsulated anesthetic is also markedly increased in the liposomal formulation over injection of unencapsulated anesthetic. These results show that the liposomal formulation of local anesthetic is useful for sustained local infiltration and nerve block anesthesia.
Owner:PACIRA PHARMA INC
Methods for reducing cardiovascular risk
PendingUS20190292273A1Reduce cardiovascular riskReduce riskAntibody ingredientsDisease diagnosisMaximum tolerated doseCvd risk
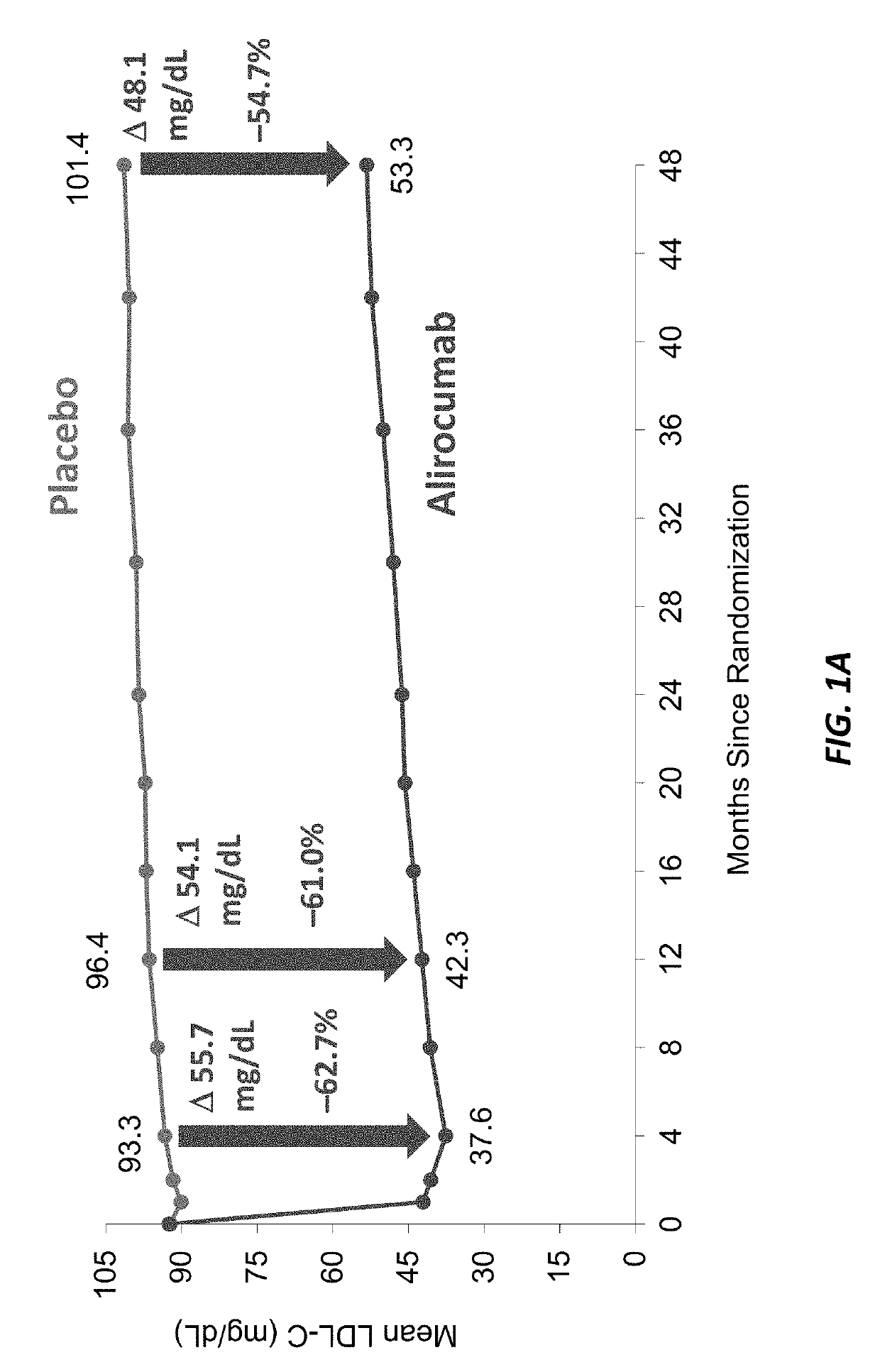
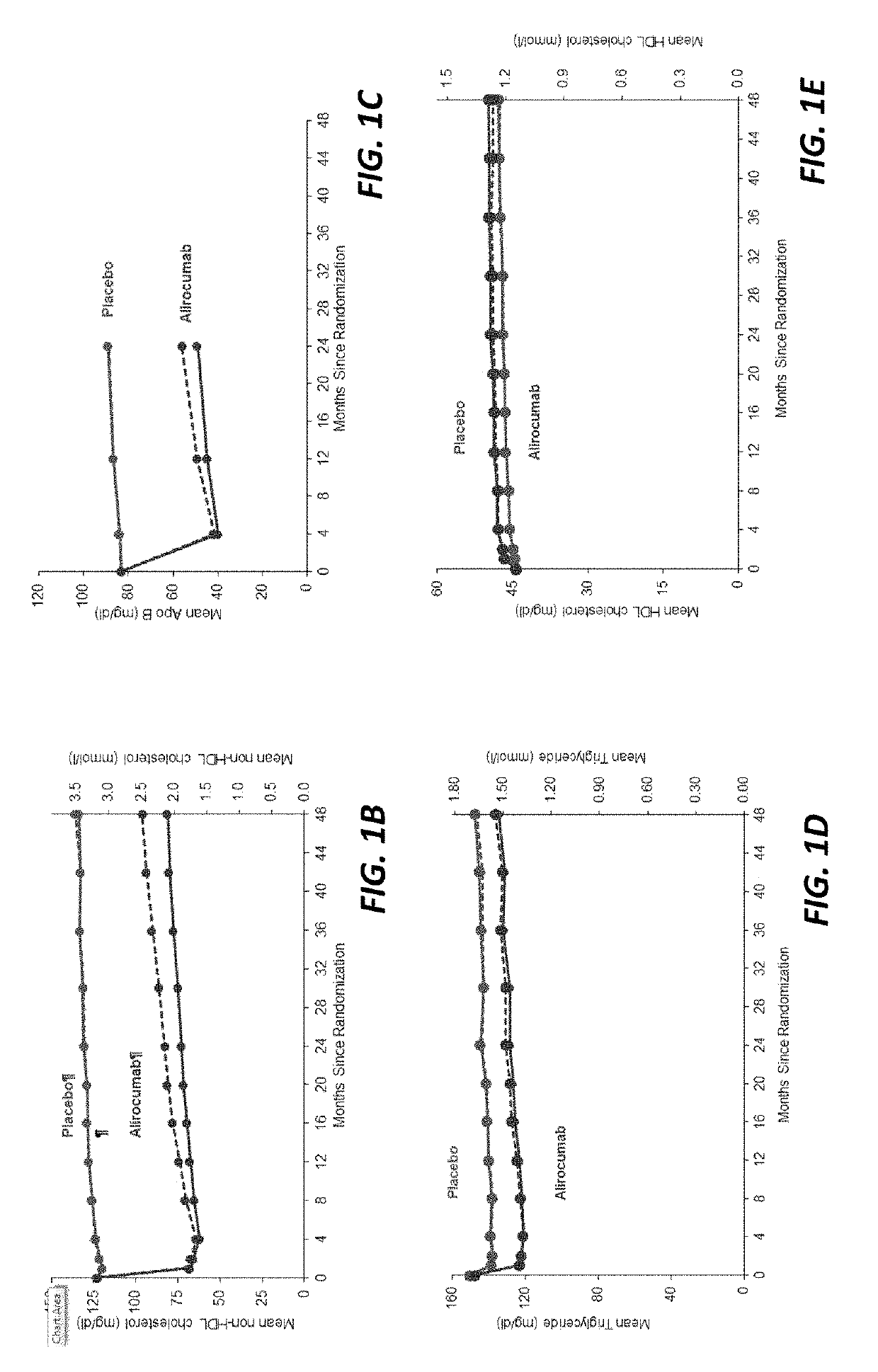
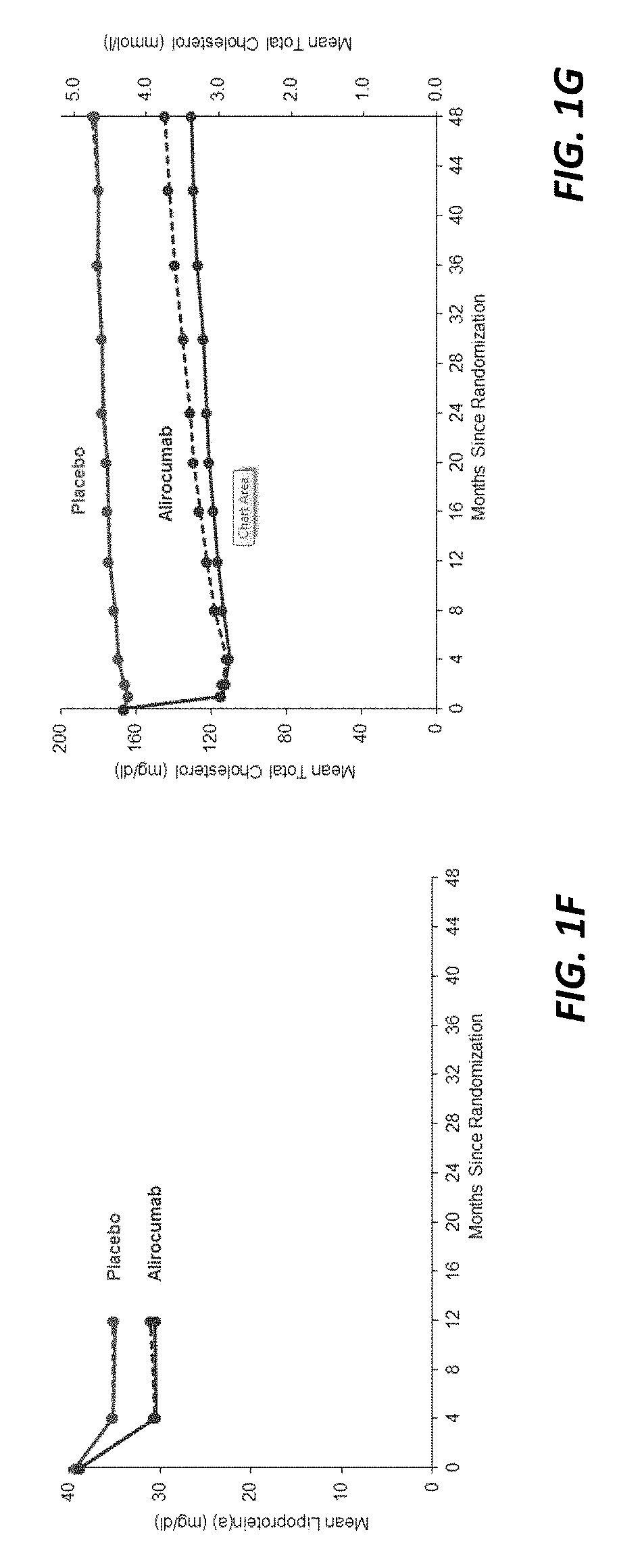
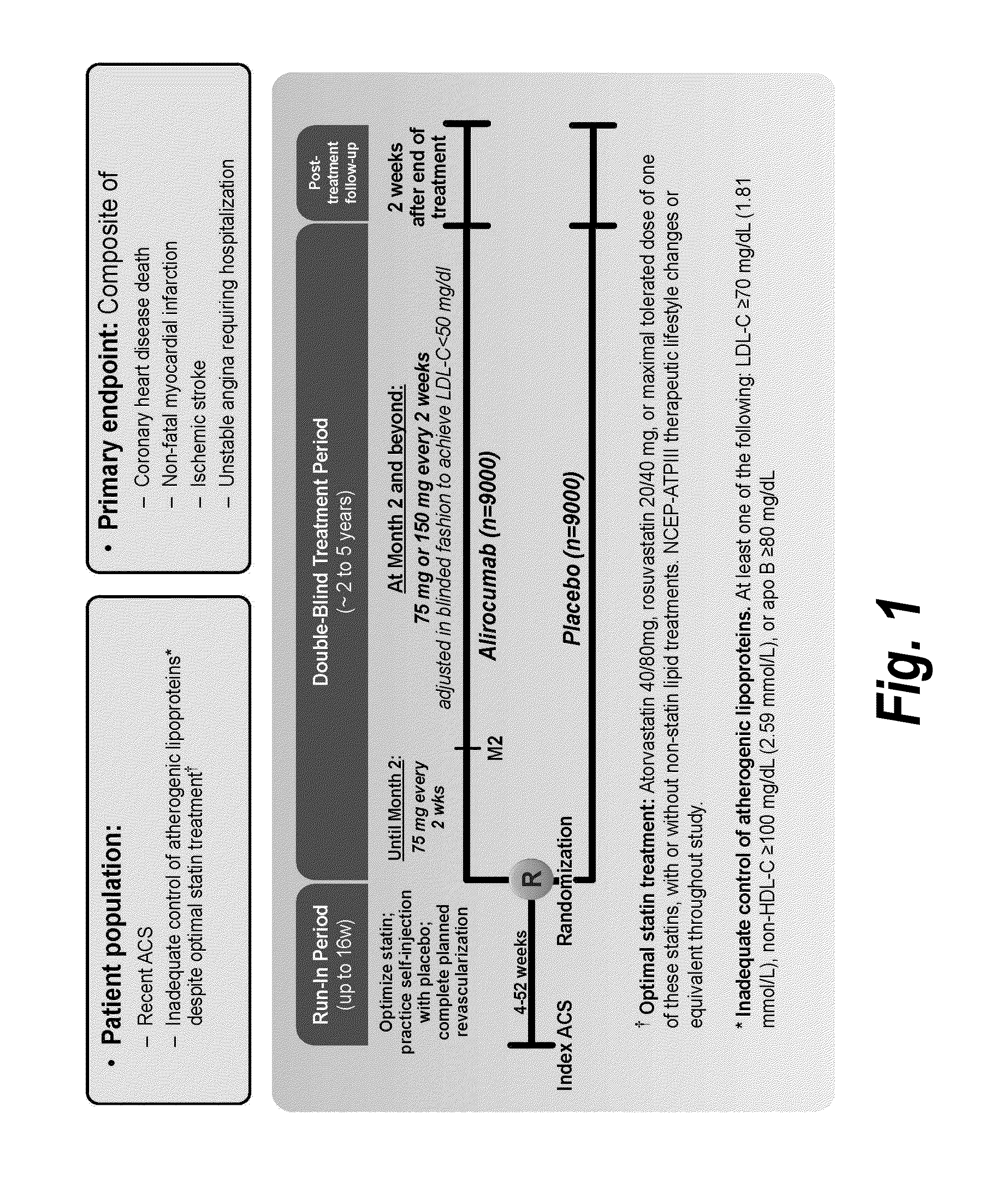
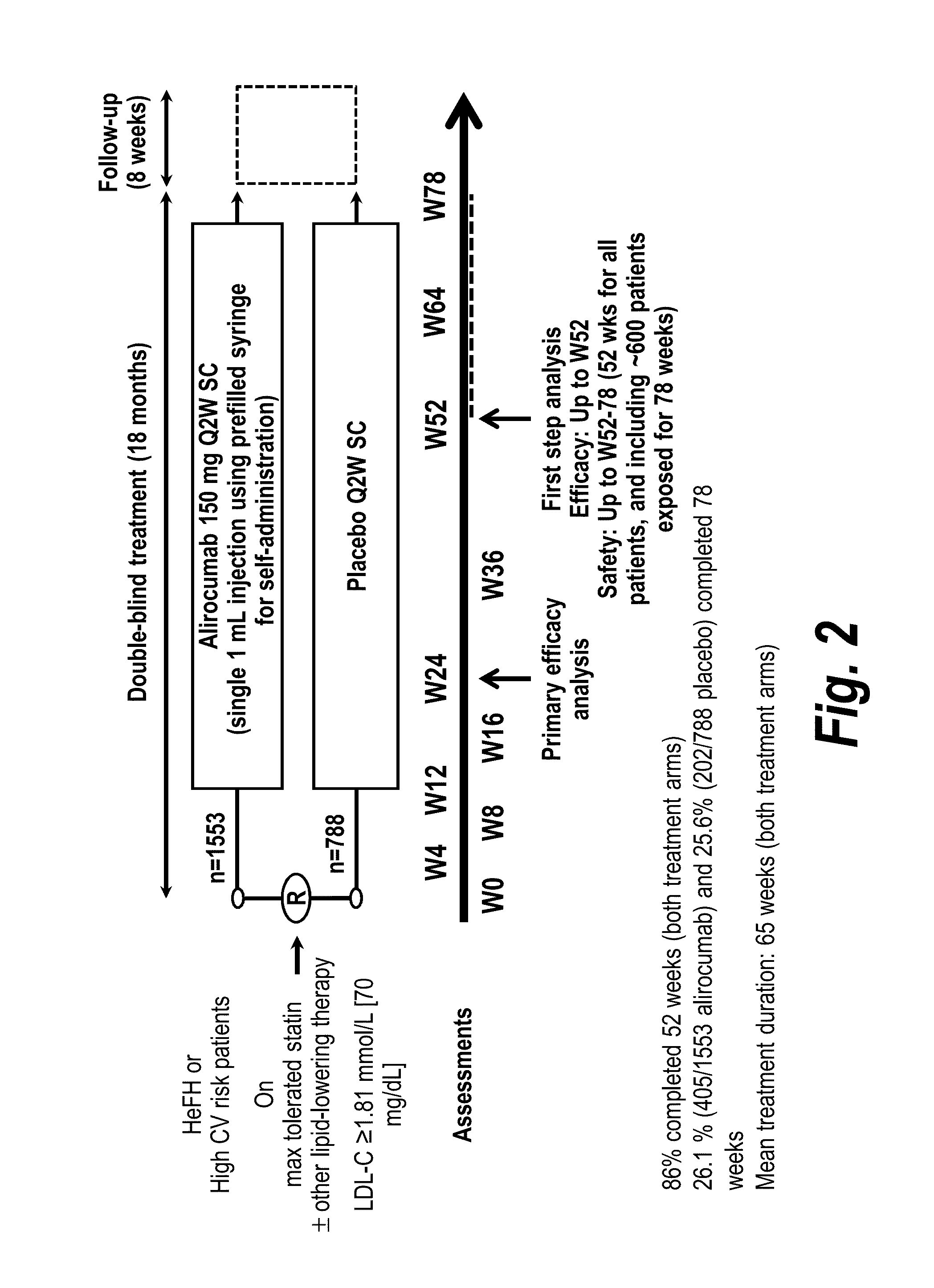
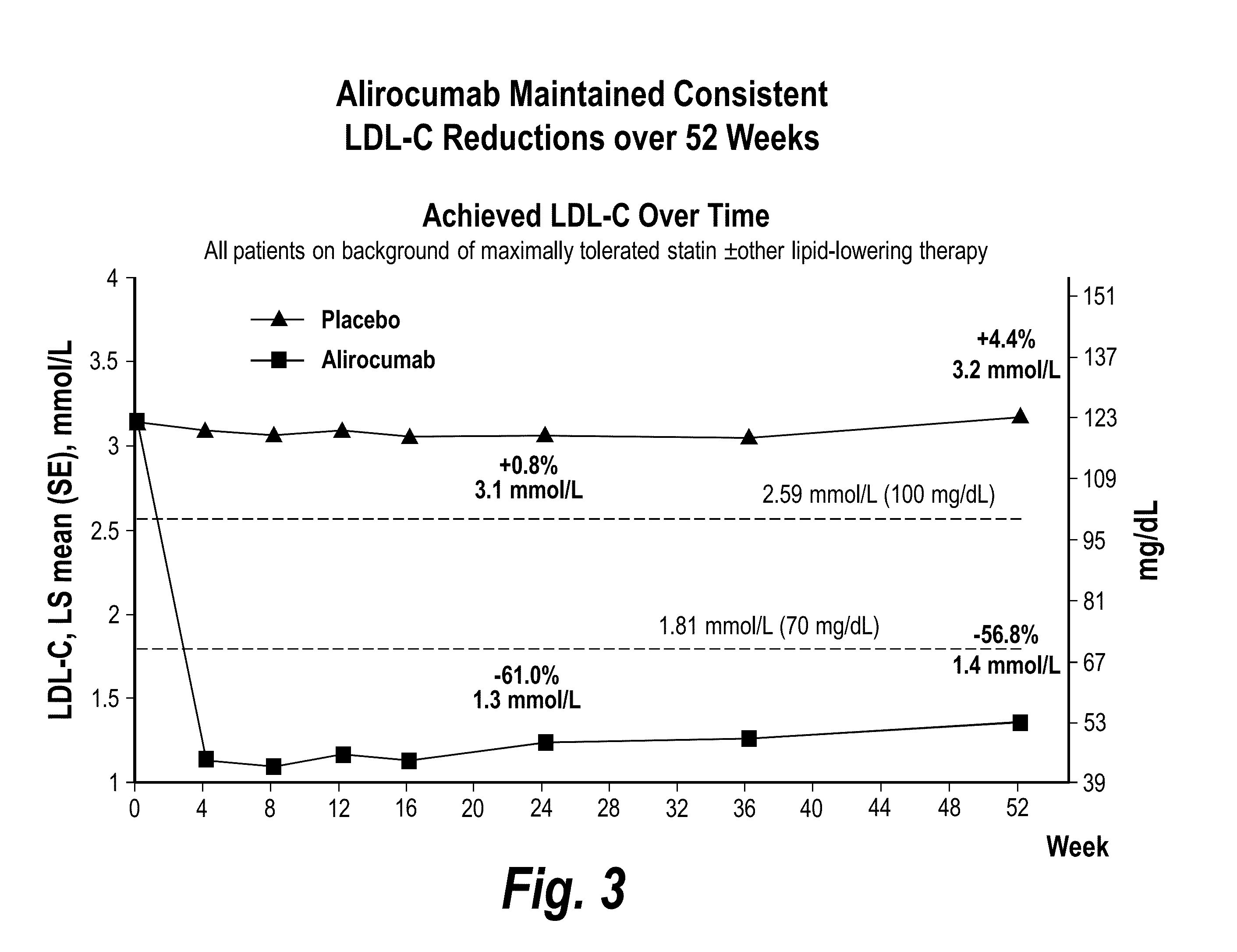
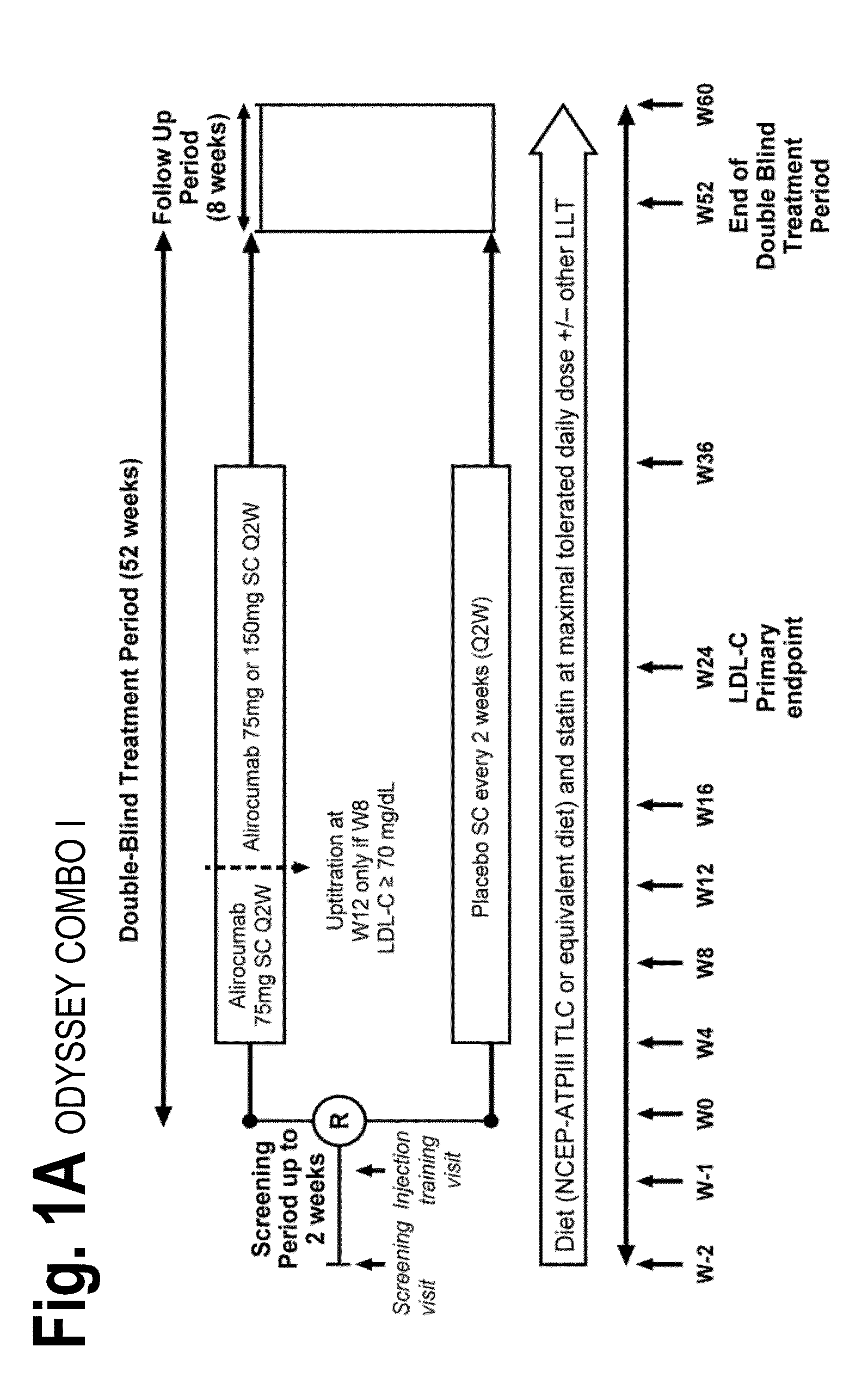
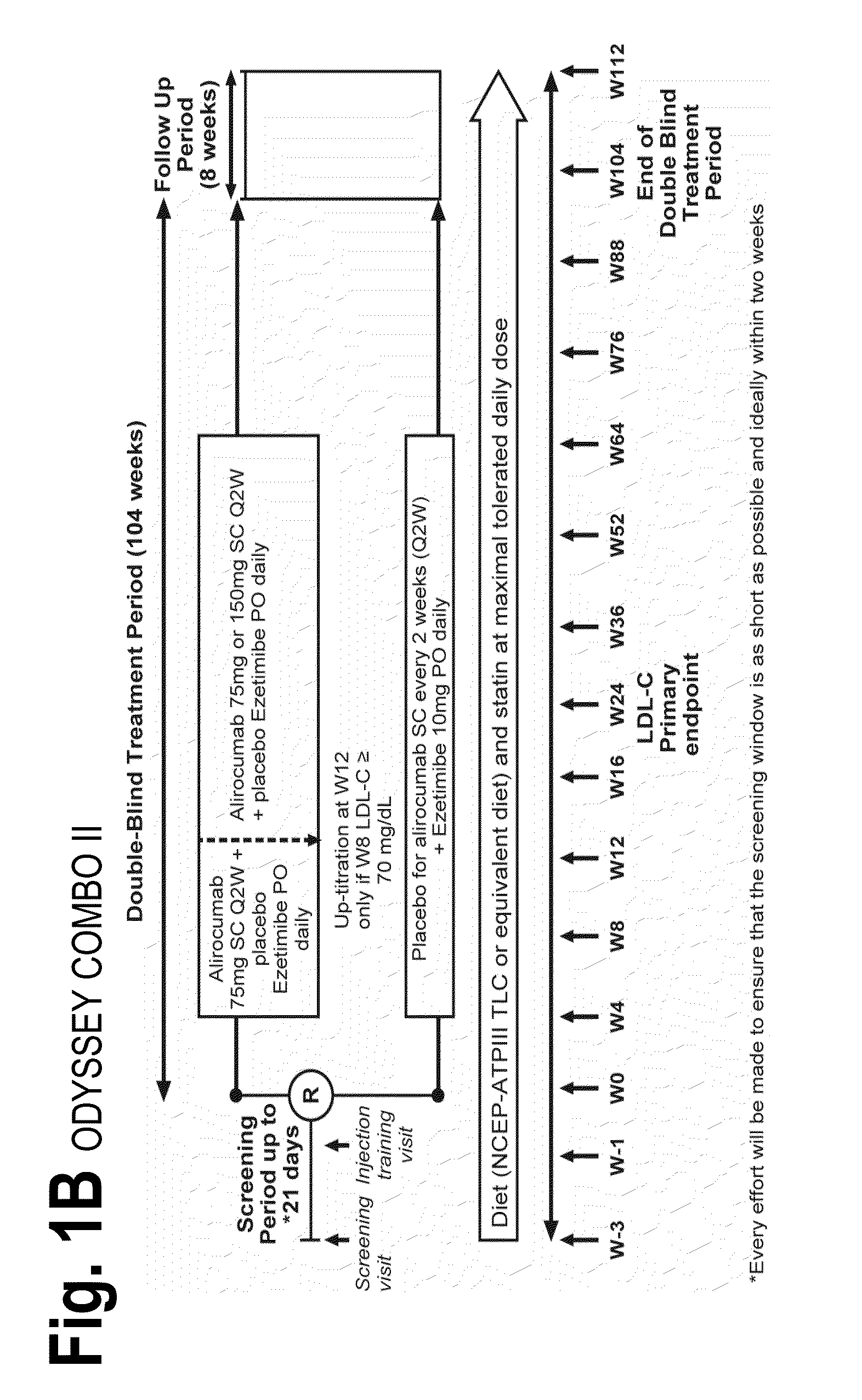
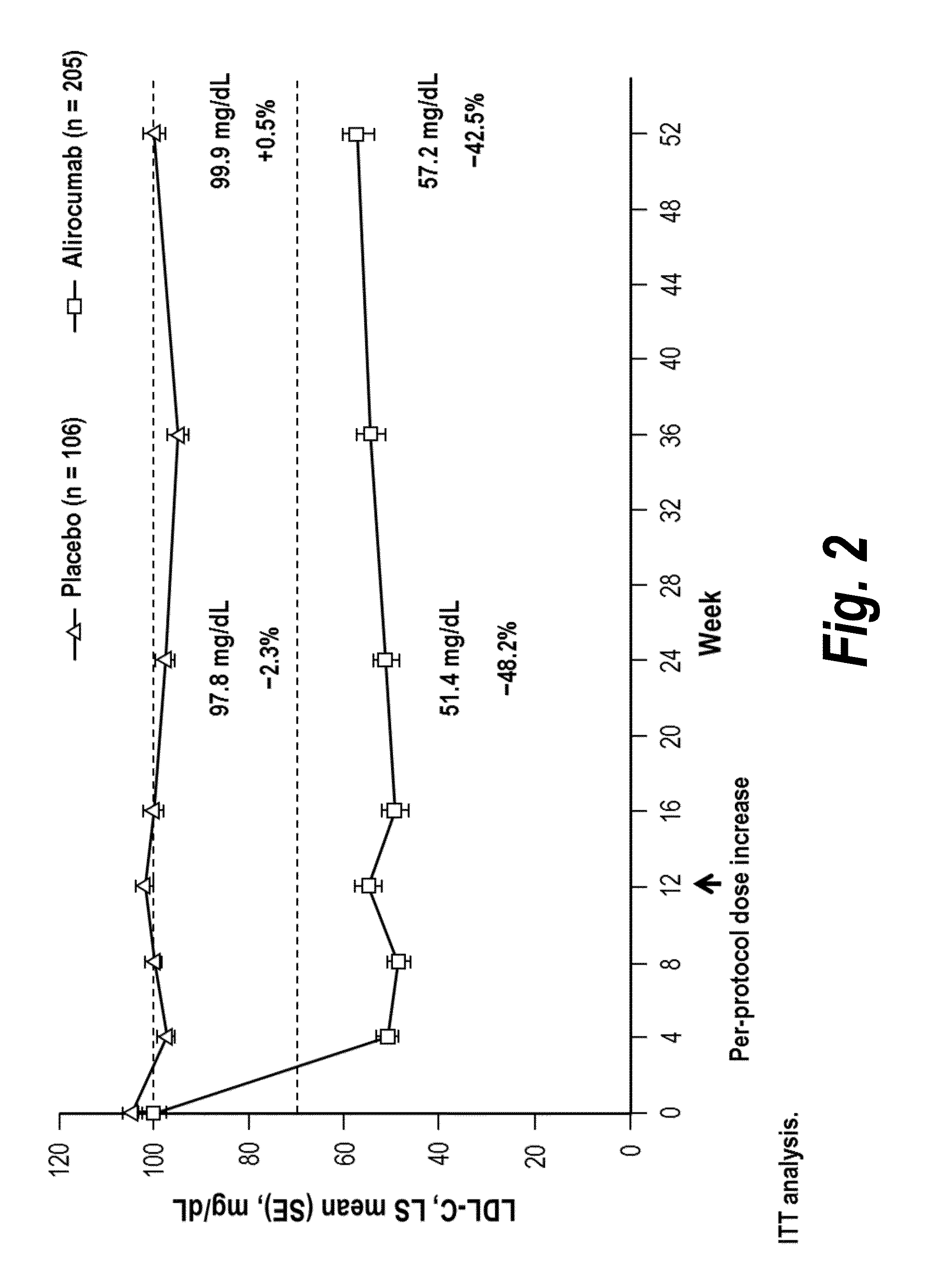
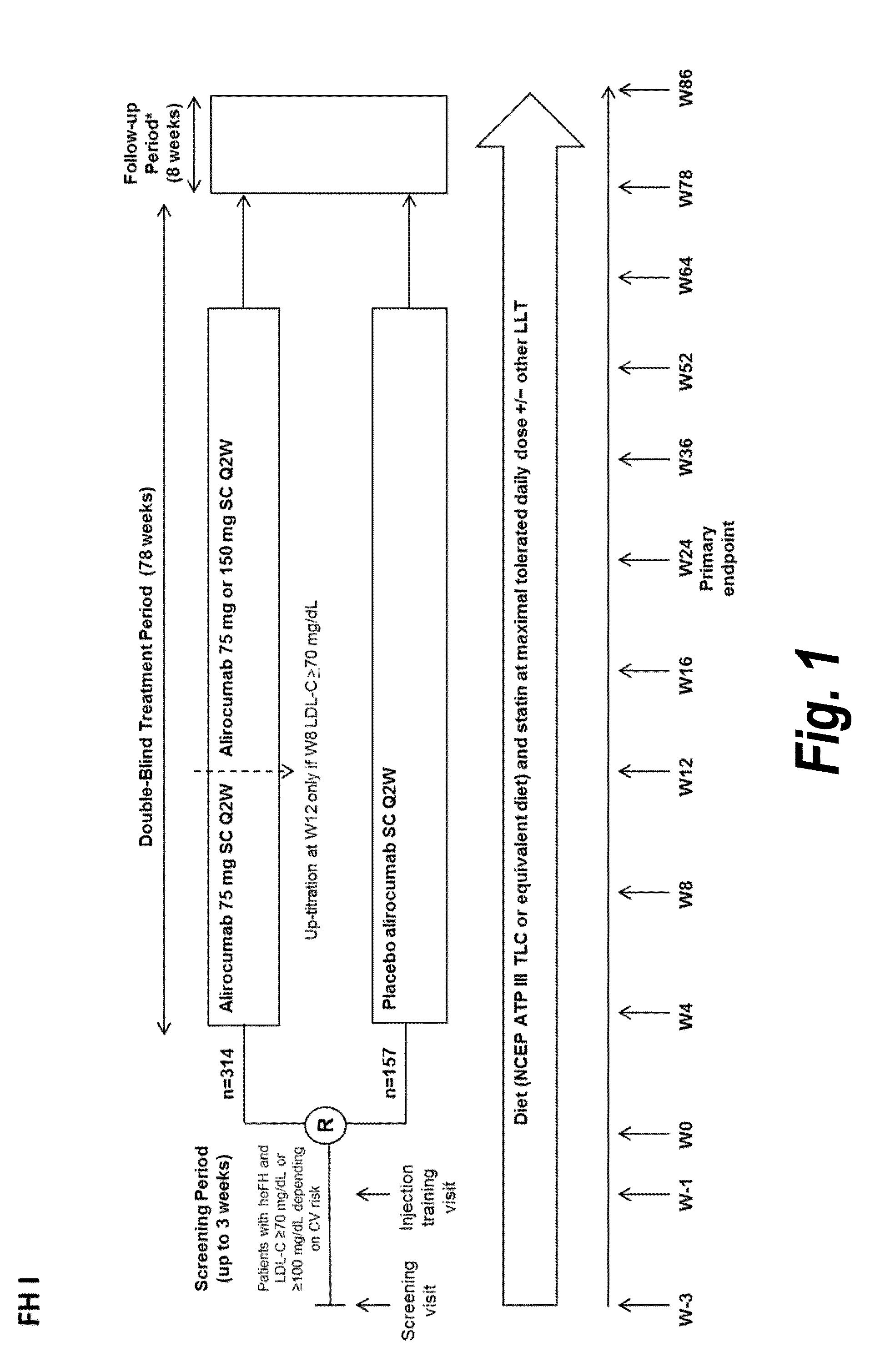
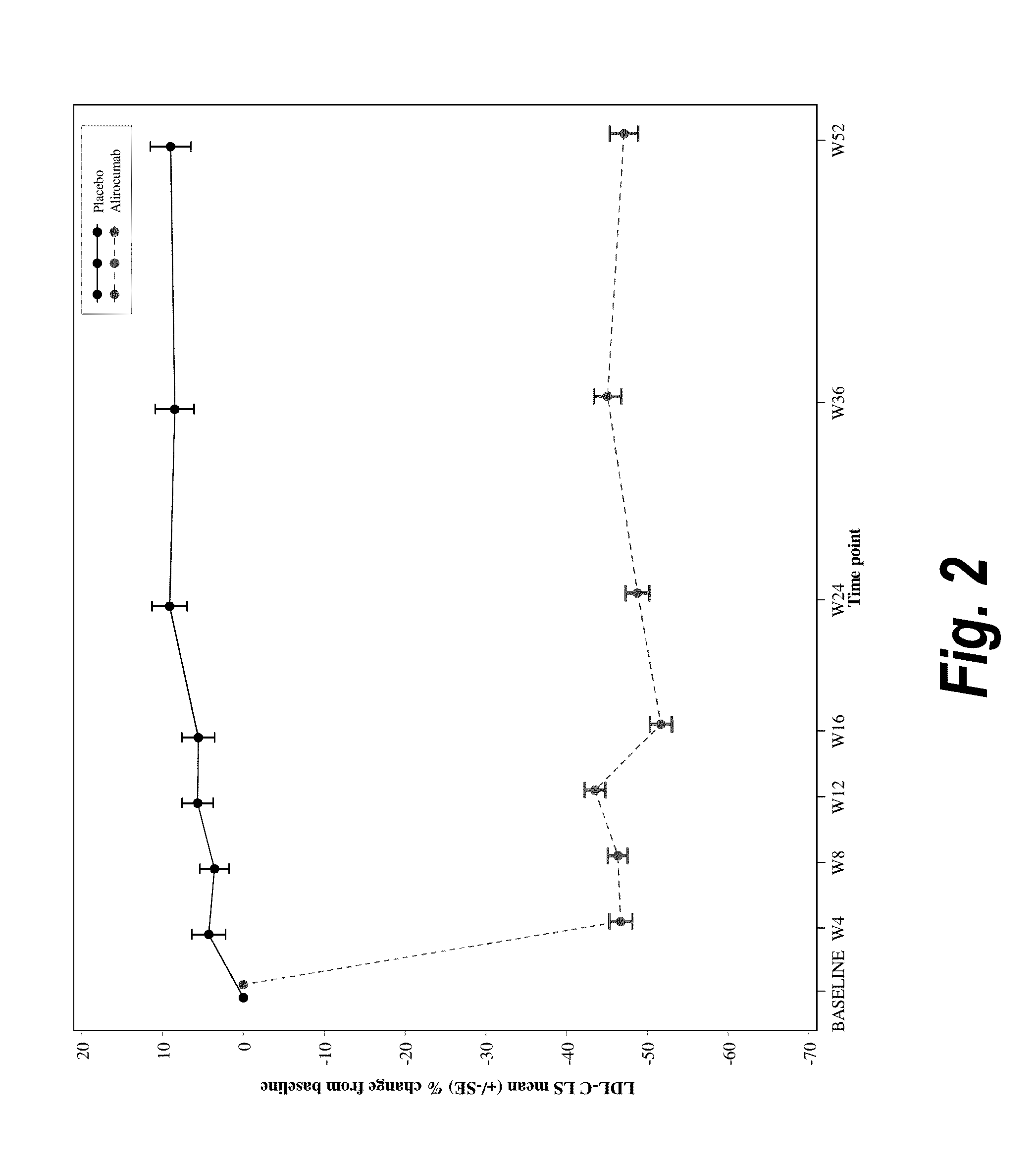
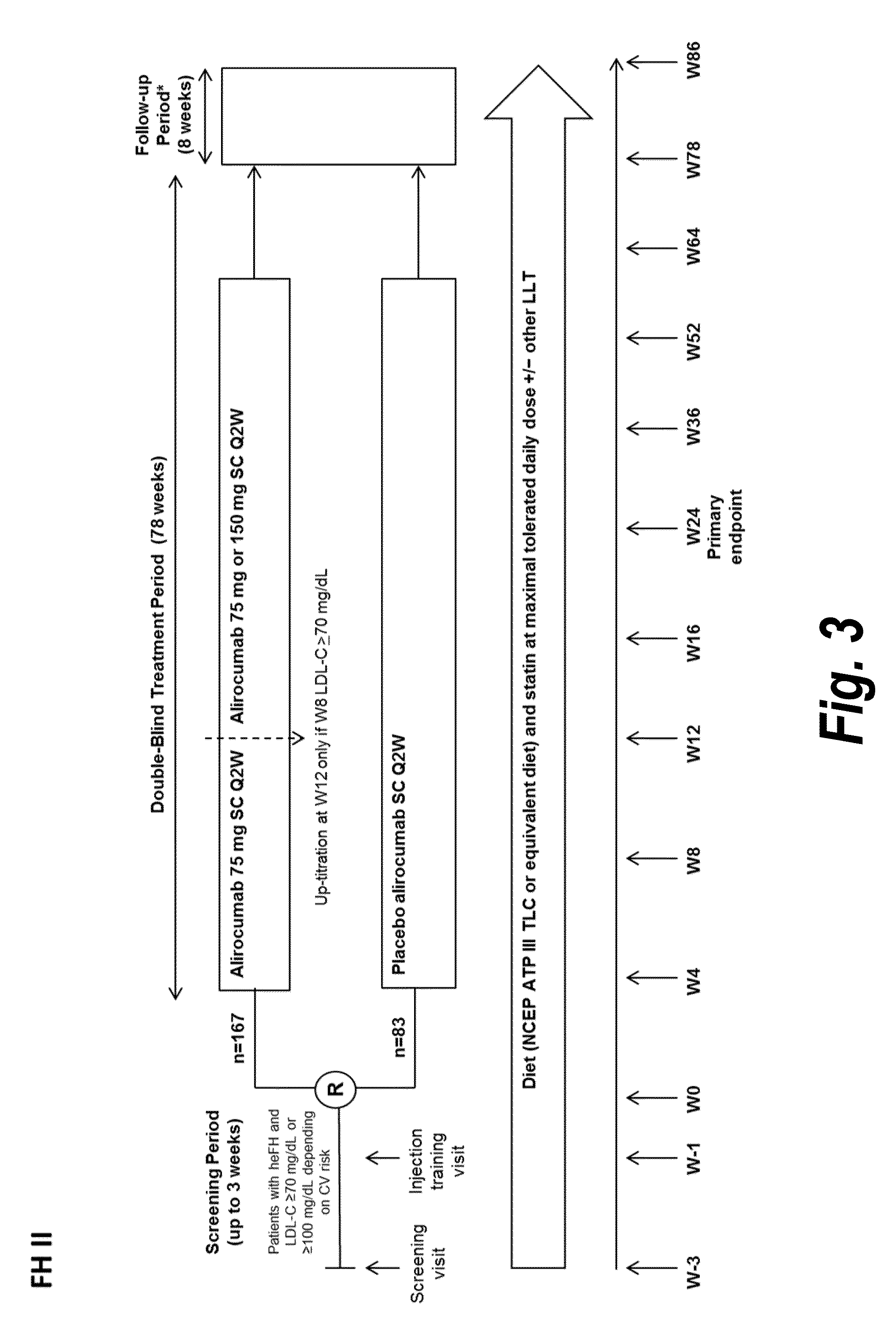
The present invention provides methods for treating diseases and disorders that are associated with elevated levels of lipids and lipoproteins. The methods of the present invention comprise administering to a high cardiovascular risk patient a pharmaceutical composition comprising a PCSK9 inhibitor. In certain embodiments, the PCSK9 inhibitor is an anti-PCSK9 antibody such as the exemplary antibody referred to herein as mAb316P or alirocumab. The methods of the present invention are useful for treating high cardiovascular risk patients with hypercholesterolemia and elevated levels of other atherogenic lipoproteins that are not adequately controlled by maximum tolerated dose statin therapy. In particular, the methods of the present invention are useful for reducing cardiovascular risk and lowering atherogenic lipoproteins in high cardiovascular risk patients within 12 months following an acute coronary syndrome event despite a maximum tolerated dose statin therapy.
Owner:REGENERON PHARM INC
Methods for reducing cardiovascular risk
InactiveUS20150284473A1Reduce cardiovascular riskReduce riskAntibody ingredientsCardiovascular disorderCholesterol bloodMaximum tolerated dose
The present invention provides methods for treating diseases and disorders that are associated with elevated levels of lipids and lipoproteins. The methods of the present invention comprise administering to a high cardiovascular risk patient a pharmaceutical composition comprising a PCSK9 inhibitor. In certain embodiments, the PCSK9 inhibitor is an anti-PCSK9 antibody such as the exemplary antibody referred to herein as mAb316P or alirocumab. The methods of the present invention are useful for treating high cardiovascular risk patients with hypercholesterolemia and elevated levels of other atherogenic lipoproteins that are not adequately controlled by maximum tolerated dose statin therapy. In particular, the methods of the present invention are useful for reducing cardiovascular risk and lowering atherogenic lipoproteins in high cardiovascular risk patients within 12 months following an acute coronary syndrome event despite a maximum tolerated dose statin therapy.
Owner:SANOFI BIOTECH +1
Patient-specific dosimetry
A patient-specific optimally effective radiation dose for administration of a radiopharmaceutical to a patient for treatment of a disease may be established by basing the calculation of the appropriate therapeutic dose on factors such as the desired total body dose, the maximum tolerated dose, the typical clearance profile of the radiopharmaceutical, the patient's mass or maximum effective mass, and the patient-specific residence time of the radiopharmaceutical or an analog in the whole body of the patient. The use of the method allows for treatment of a patient with an appropriate dose which is maximally effective against the disease yet minimally toxic. The determination of a patient-specific therapeutic dose may be assisted by the use of a software program set to the particular parameters of the radiopharmaceutical.
Owner:GLAXO SMITHKLINE LLC +1
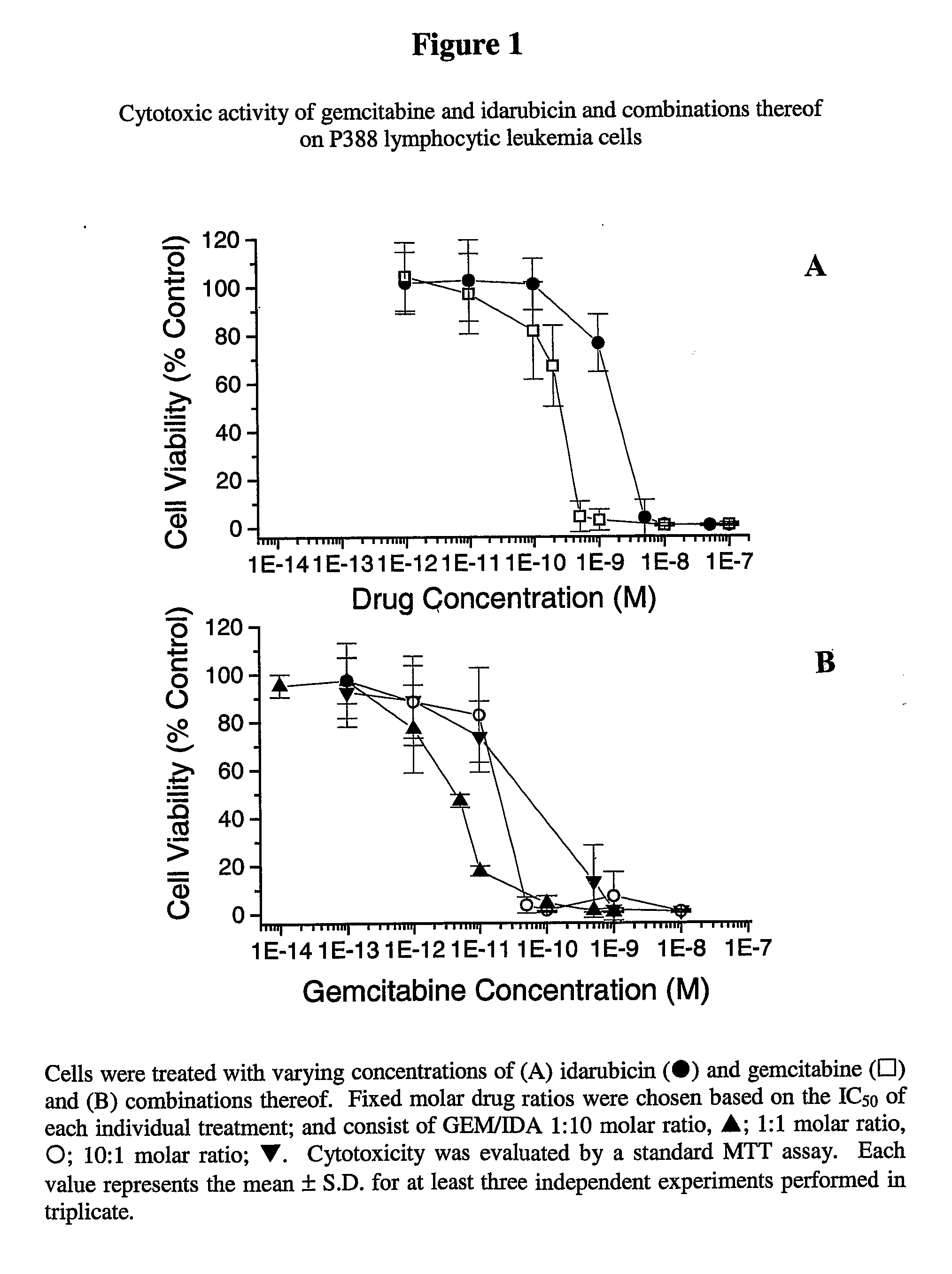
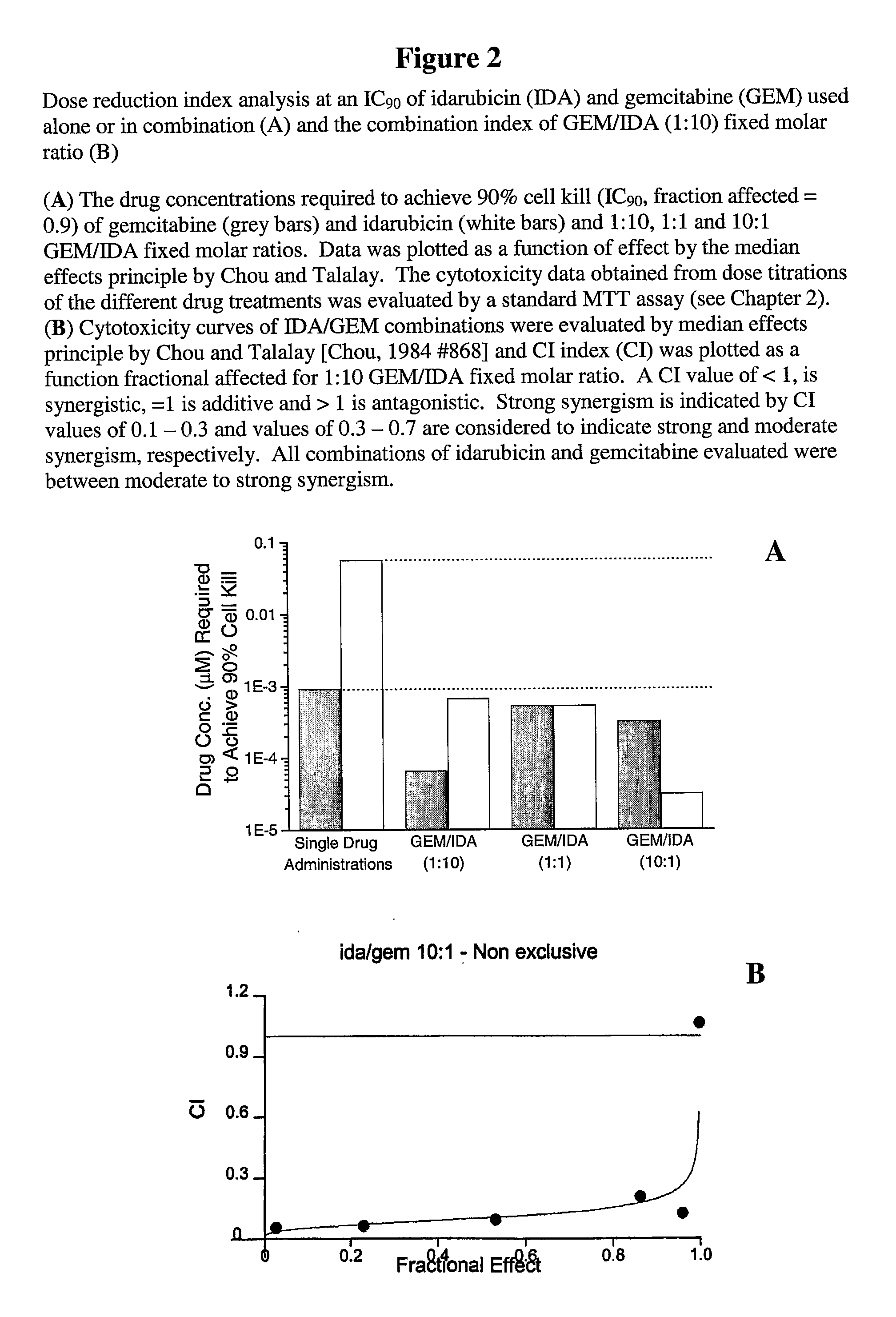
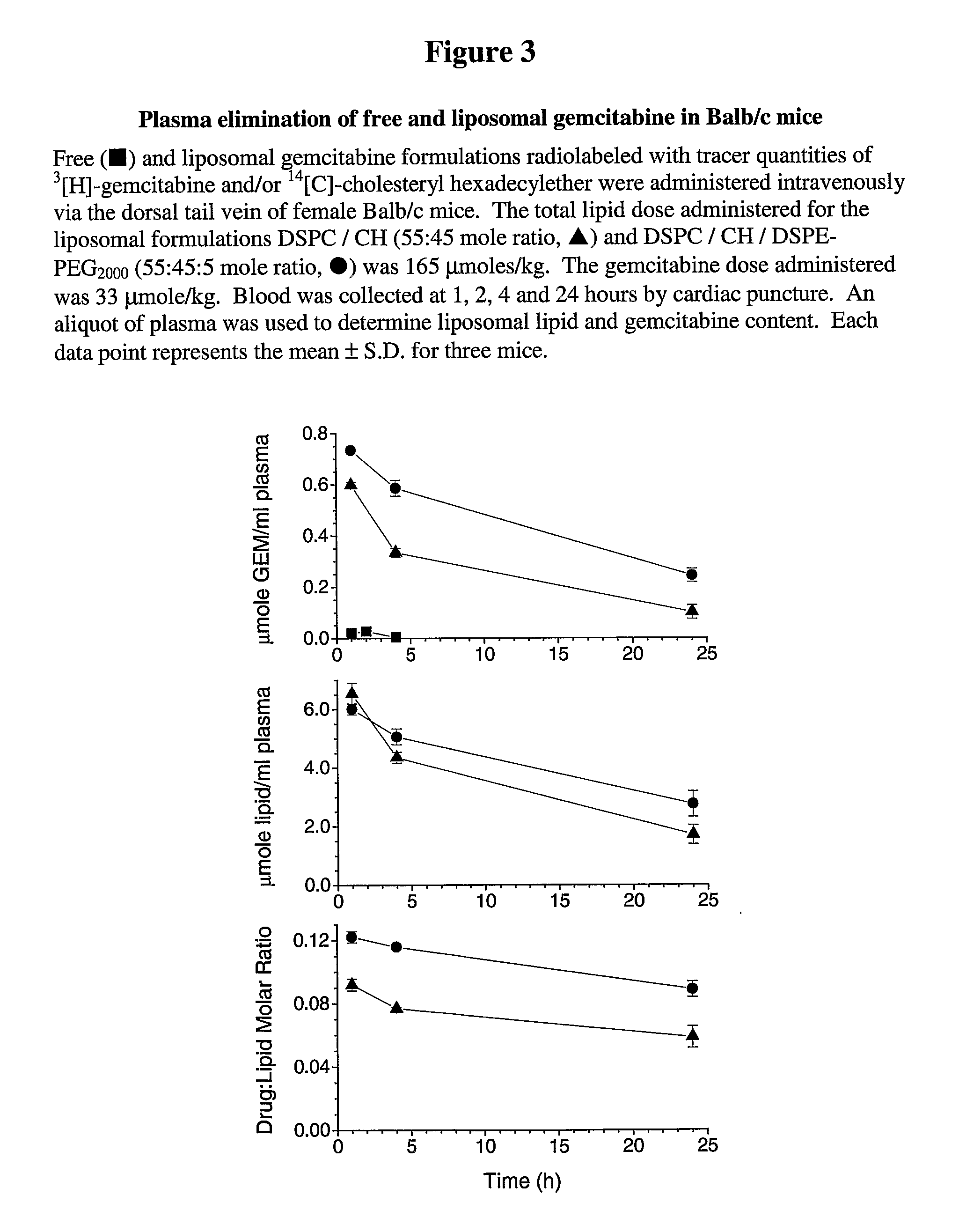
Free or Liposomal Gemcitabine Alone or in Combination with Free or Liposomal Idarubicin
InactiveUS20080213183A1Organic active ingredientsIn-vivo testing preparationsCisplatinMaximum tolerated dose
The use of the maximum tolerated dose (MTD) of individual drugs to determine appropriate administration ratios of drugs for combination therapy, wherein the ratios of drugs are fixed based on the same percentage of the MTD for each drug. Furthermore, antineoplastic compositions comprising liposomal encapsulated gemcitabine alone or in combination with free or liposomal encapsulated antineoplastic agents, such as idarubicin, irinotecan, etopside, cisplatin, cyclophosphamide, doxorubicin, or vincristine are diclosed.
Owner:BRITISH COLUMBIA CANCER AGENCY
Method and composition for treating alzheimer-type dementia
InactiveUS20110201597A1Extended durationFunction increaseBiocideNervous disorderNK1 receptor antagonistMaximum tolerated dose
There is described a method for increasing the maximal tolerated dose and thus the efficacy of an acetylcholinesterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetylcholinesterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetylcholinesterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholinesterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Cancer Treatments with Radiation and Immunocytokines
InactiveUS20100330029A1Reducing tumor and cancer cell growthPromote lowerPeptide/protein ingredientsImmunological disordersPrimary tumorCancer cell
The present invention is directed to a method for treating tumors and cancer cells by administering an immunocytokine following radiation treatment. This combination of treatments can stimulate an immune response at irradiated and non-irradiated sites, which is useful in eradicating cancer cells that have spread from the site of the primary tumor. In addition, immunocytokines can be administered at a dose that is less that the maximum tolerated dose, which reduces the side effects associated with immunocytokine therapy.
Owner:MERCK PATENT GMBH
Radar target simulation method based on correction network
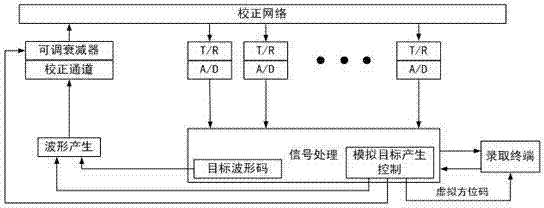
InactiveCN102778671AExtended service lifeReduce boot timeWave based measurement systemsDecompositionRadar
The invention relates to the technical field of radars, in particular to a target simulation method based on a correction network. The target simulation method simulates a target position, distance, height, speed, range and static target from a source. A simulation target generated by the method is more realistic than a simulation target generated by a terminal monitoring and can be used for receiving data base file (DBF) validation, maximum tolerated dose (MTD) validation, a small target detection capability validation and tracking capability validation. The target simulation method comprises steps of simulation target decomposition, target trace point appearing time calculation, target trace point distance simulation, target trace point height simulation, target trace point position simulation, target trace point doppler frequency simulation and target trace point range simulation. The target simulation method can reduce development cost of the radars, can be used for simulating training after being loaded along with devices, reduces staring time, prolongs service life of the radars, and reduces training cost.
Owner:WUHAN BINHU ELECTRONICS
Method of Treatment Using Inhibitors of Mitosis
ActiveUS20100099697A1Recovery and subsiding of side effectBiocideAnimal repellantsDiseaseCell division
Methods of treating diseases caused by cell division or that are treated by inhibiting mitosis by administering two doses of an inhibitor of mitosis between the biologically effective dose and the maximum tolerated dose in a dosing cycle that allows for the recovery or subsiding of side effects, wherein the second dose is administered 24 to 48 hours after the first dose.
Owner:ARRAY BIOPHARMA
Method for calculating tolerance dosage of sugar alcohol and function sugar of human body
PendingCN109493974ACompounds screening/testingHealth-index calculationDihydrogen oxideAlcohol sugars
Owner:ZHEJIANG HUAKANG PHARMA
Methods for treating high cardiovascular risk patients with hypercholesterolemia
InactiveUS20160137746A1High densityImproves at least one hypercholesterolemia-associated parameterSenses disorderMetabolism disorderCholesterol bloodMaximum tolerated dose
The present invention provides methods for treating hypercholesterolemia. The methods of the present invention comprise administering to a high cardiovascular risk patient a pharmaceutical composition comprising a PCSK9 inhibitor. In certain embodiments, the PCSK9 inhibitor is an anti-PCSK9 antibody such as the exemplary antibody referred to herein as mAb316P. The methods of the present invention are useful for treating high cardiovascular risk patients with hypercholesterolemia and established CHD or CHD risk equivalents that are not adequately controlled by maximum tolerated dose statin therapy.
Owner:SANOFI BIOTECH SAS +1
METHODS FOR TREATING PATIENTS WITH HETEROZYGOUS FAMILIAL HYPERCHOLESTEROLEMIA (heFH)
ActiveUS20160137745A1High densityImproves at least one hypercholesterolemia-associated parameterCell receptors/surface-antigens/surface-determinantsMetabolism disorderCholesterol bloodMaximum tolerated dose
The present invention provides methods for treating hypercholesterolemia. The methods of the present invention comprise administering to patients with heterozygous familial hypercholesterolemia a pharmaceutical composition comprising a PCSK9 inhibitor. In certain embodiments, the PCSK9 inhibitor is an anti-PCSK9 antibody such as the exemplary antibody referred to herein as mAb316P. The methods of the present invention are useful for treating patients with heterozygous familial hypercholesterolemia who are not adequately controlled by maximum tolerated dose statin therapy with or without other lipid lowering therapy.
Owner:REGENERON PHARM INC +1
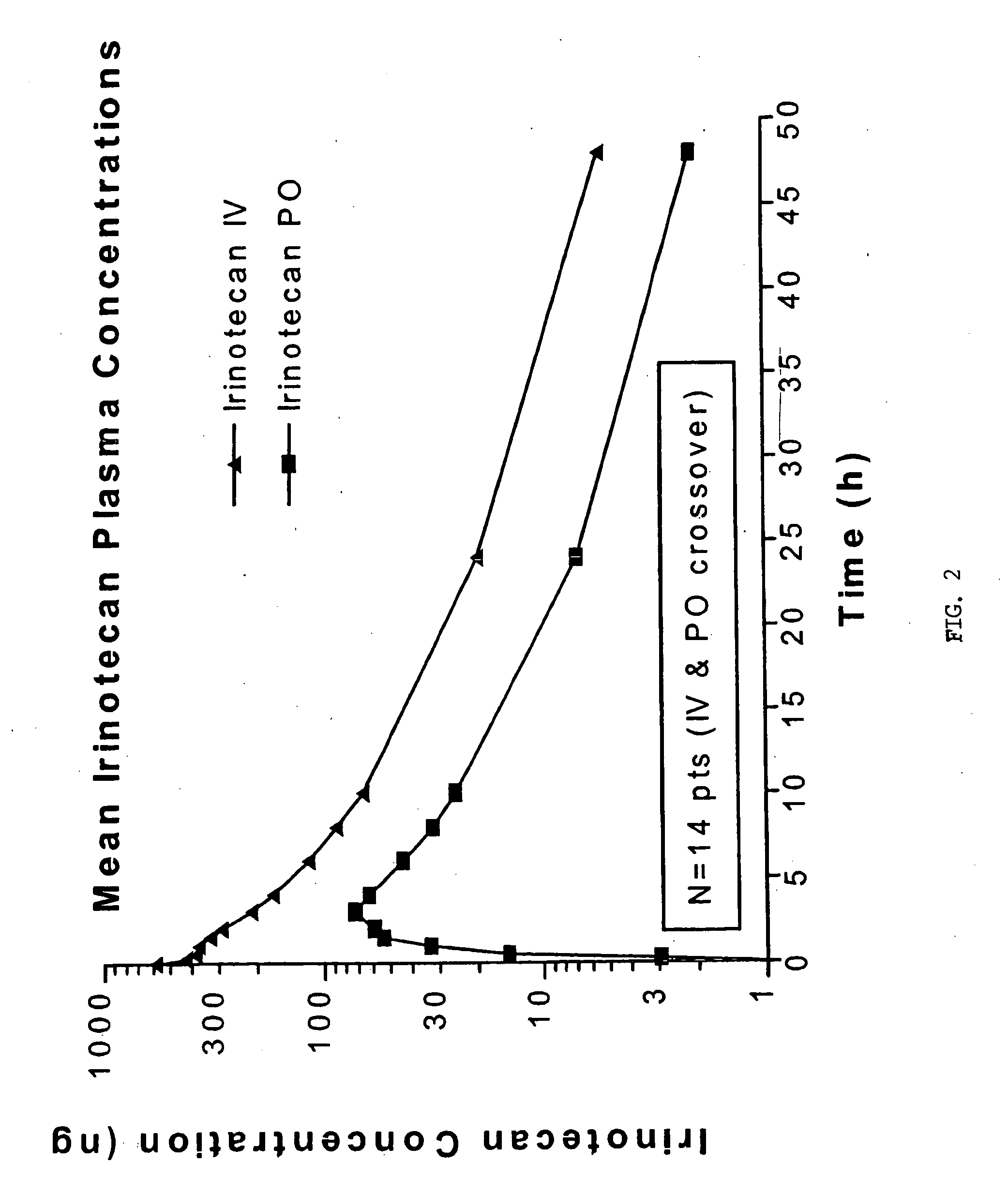
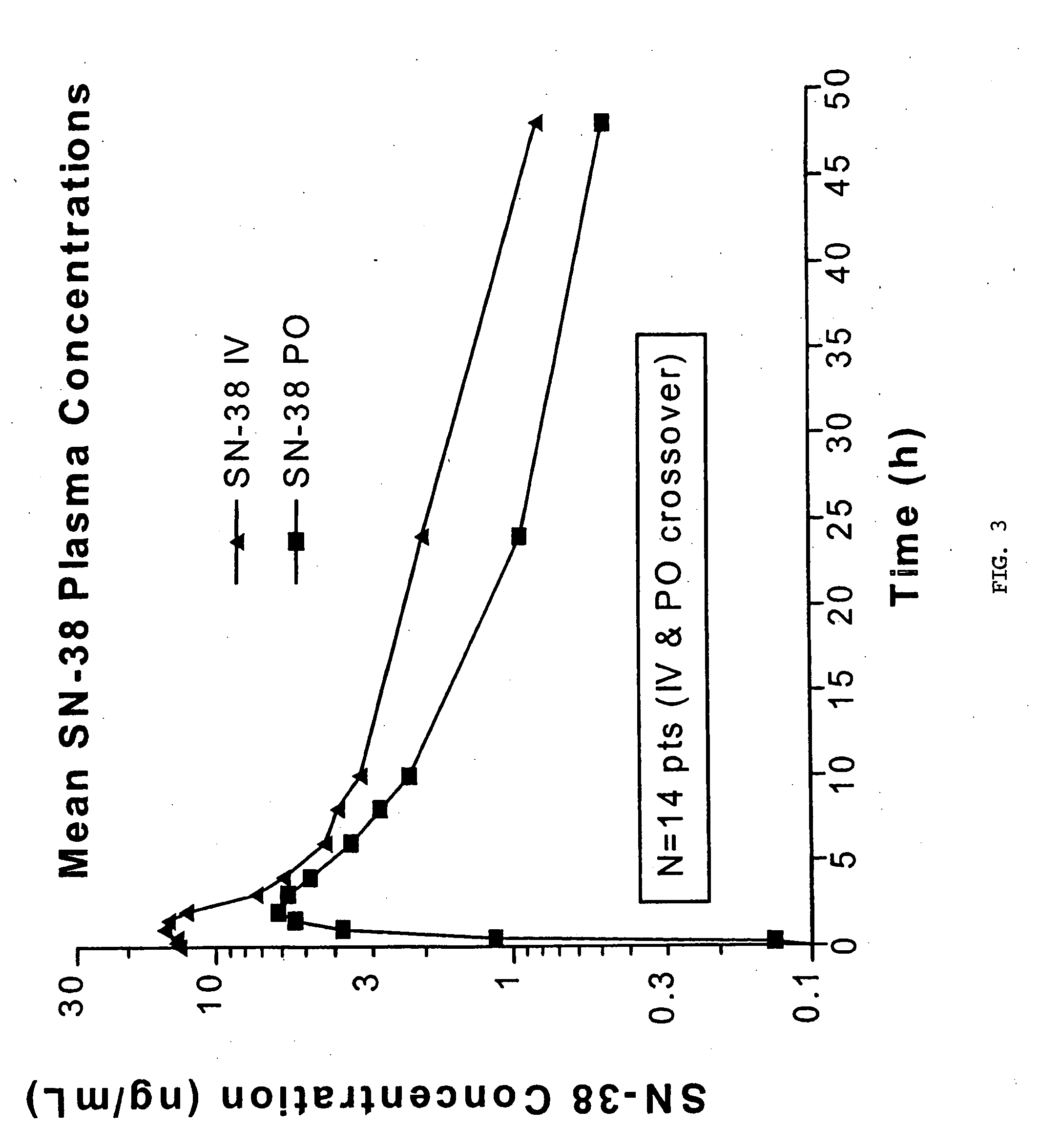
Novel dosage and administration method for oral camptosar
The present invention relates to a maximally tolerable dosage of oral irinotecan encapsulated in a semi-solid filling medium which comprises the irinotecan or a derivative thereof; a pharmaceutically acceptable carrier matrix which is a polyglycolized glyceride; and an effective thickening-reducing and stabilizing-promoting amount of one or more pharmaceutically acceptable excipients, where the maximally tolerable oral daily dose is about 60 mg / m2 when administered daily for five days every three weeks. The invention also relates to a method of oral administration of irinotecan or a derivative thereof by administering irinotecan, or a derivative thereof, in an encapsulated semi-solid matrix formulation given daily for five days every three weeks. The method is suitable for treatment of cancer in a mammal.
Owner:PFIZER INC
Cancer treatments with radiation and immunocytokines
InactiveCN102196815AEnhance systemic immune responseEnhance immune responsePeptide/protein ingredientsImmunological disordersPrimary tumorCancer cell
Owner:MERCK PATENT GMBH
Method for administration of pegylated liposomal doxorubicin
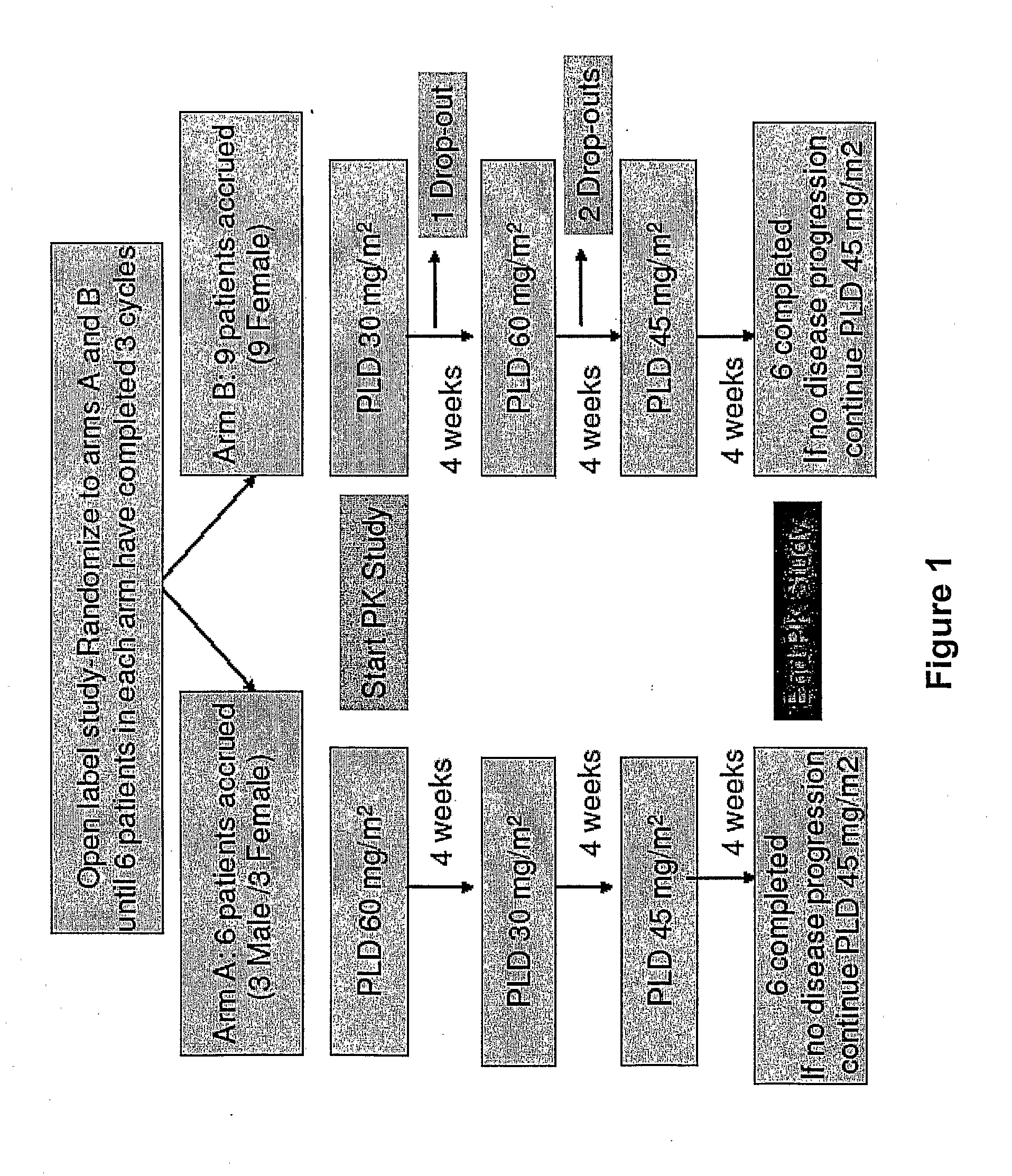
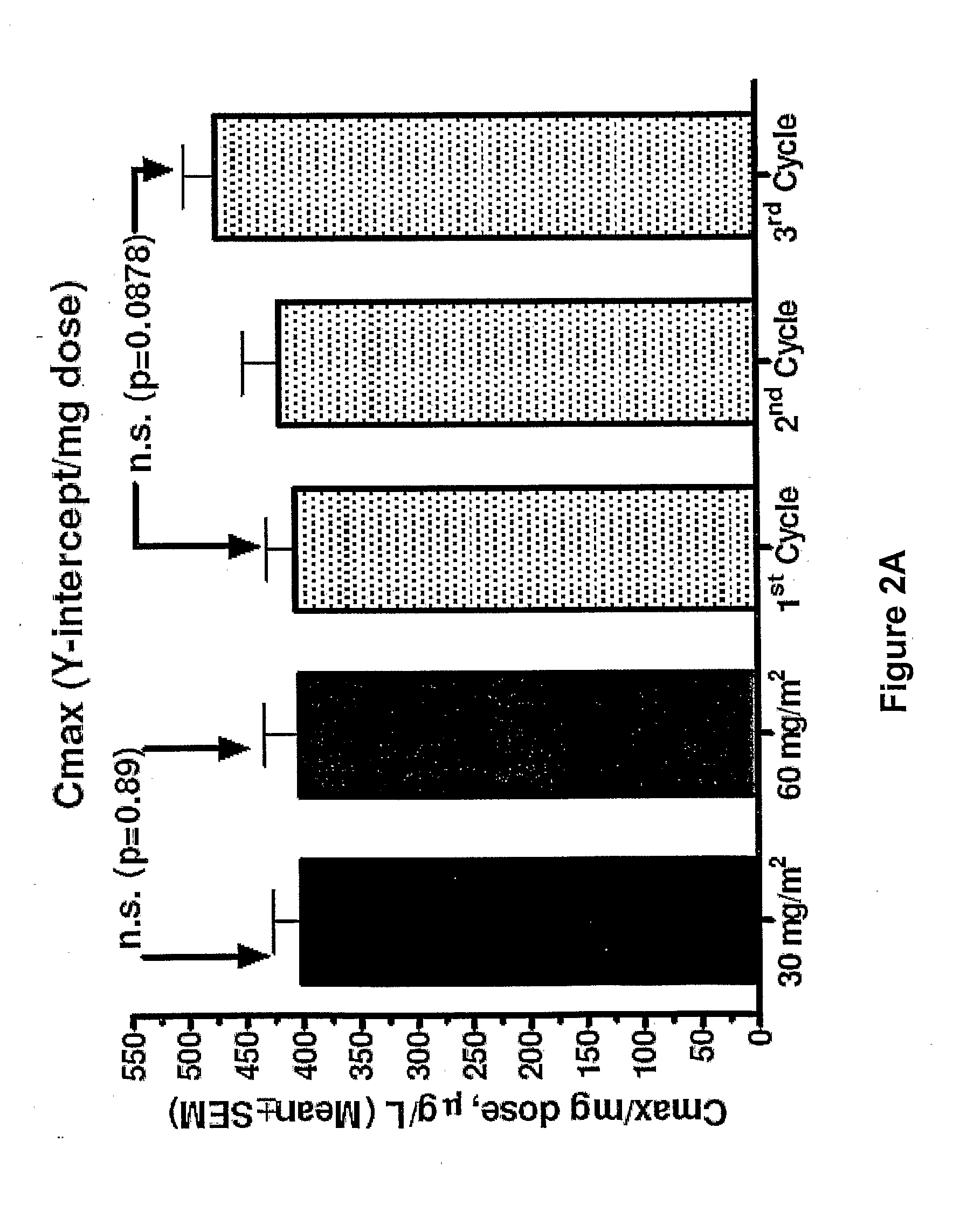
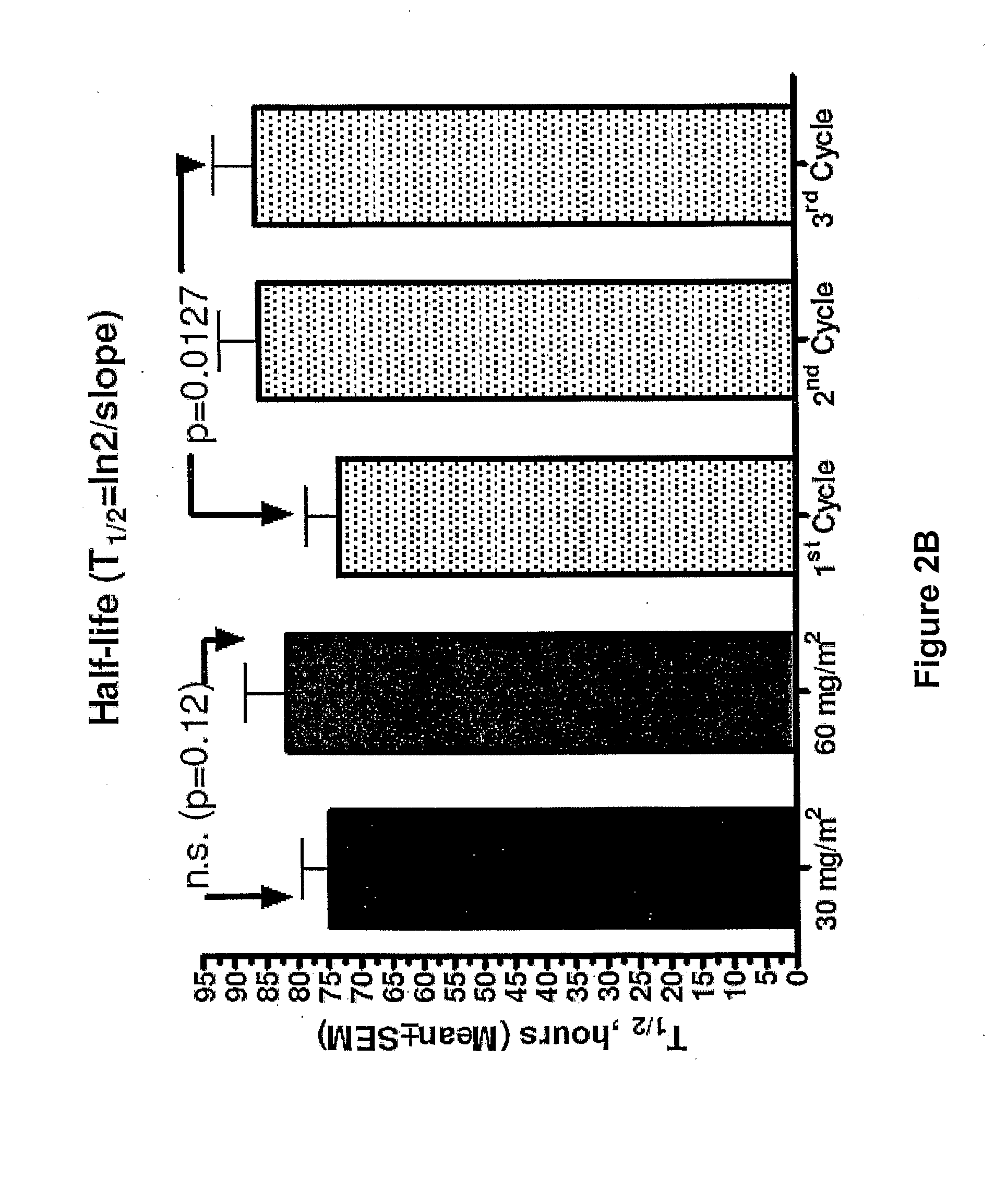
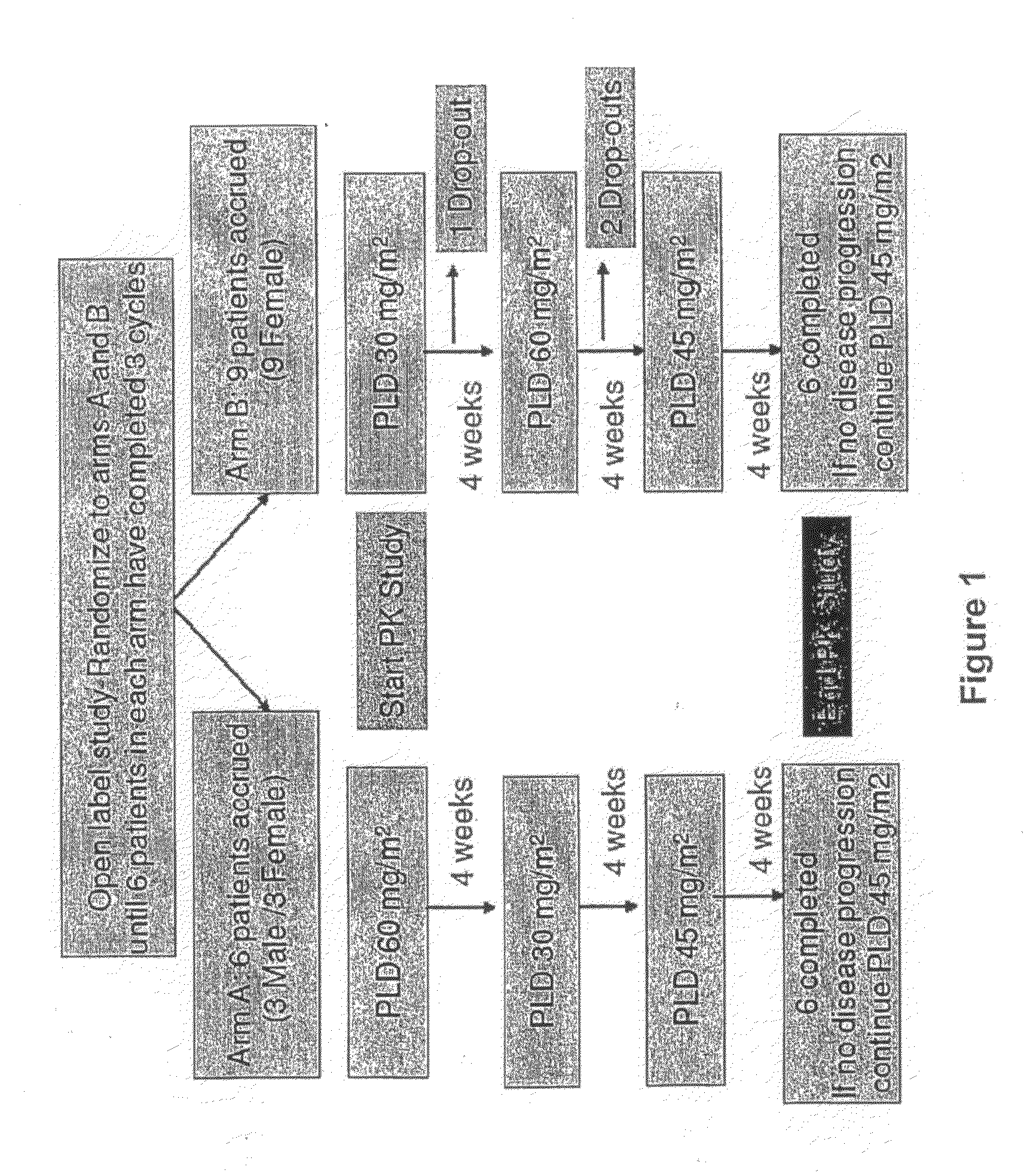
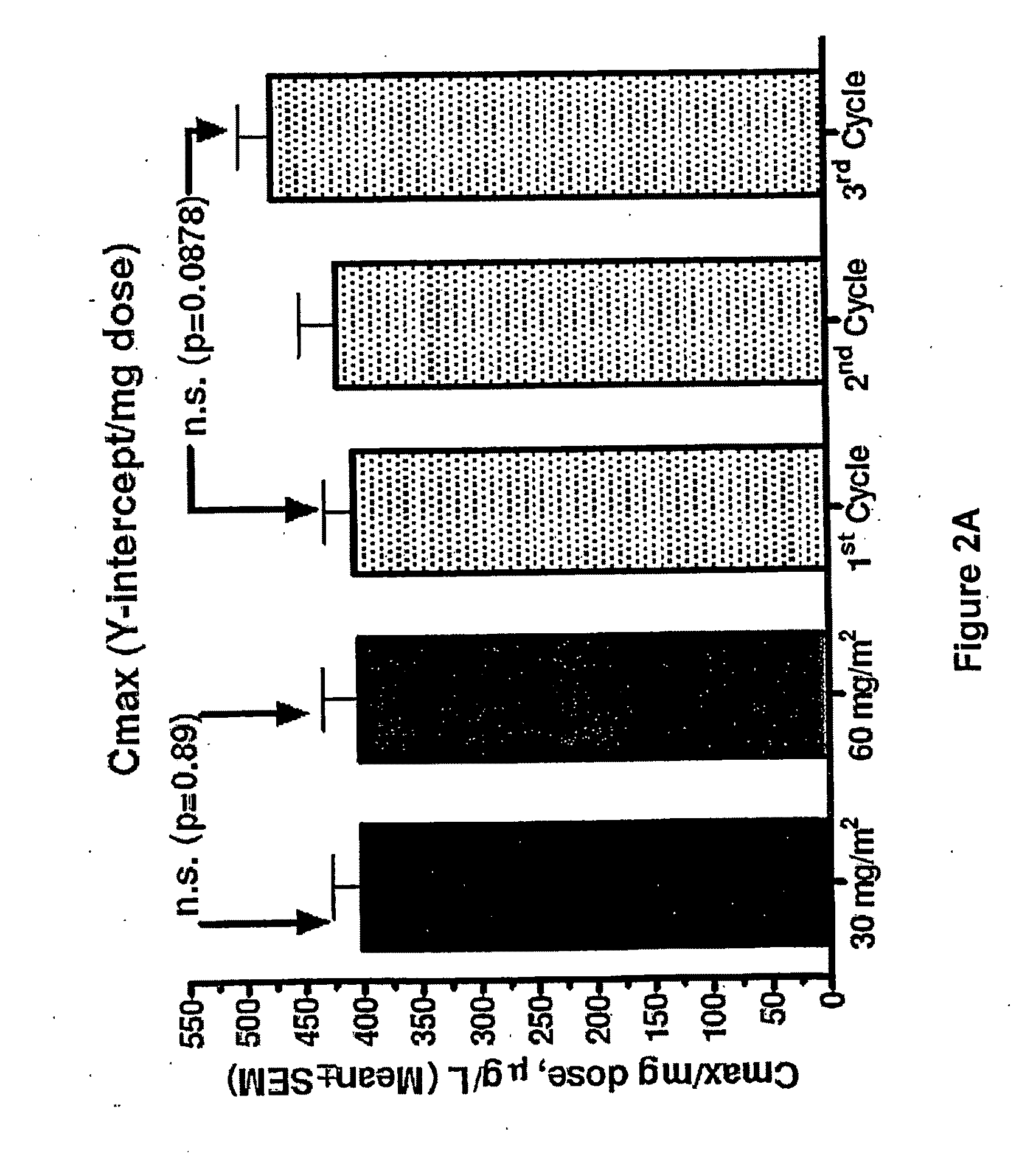
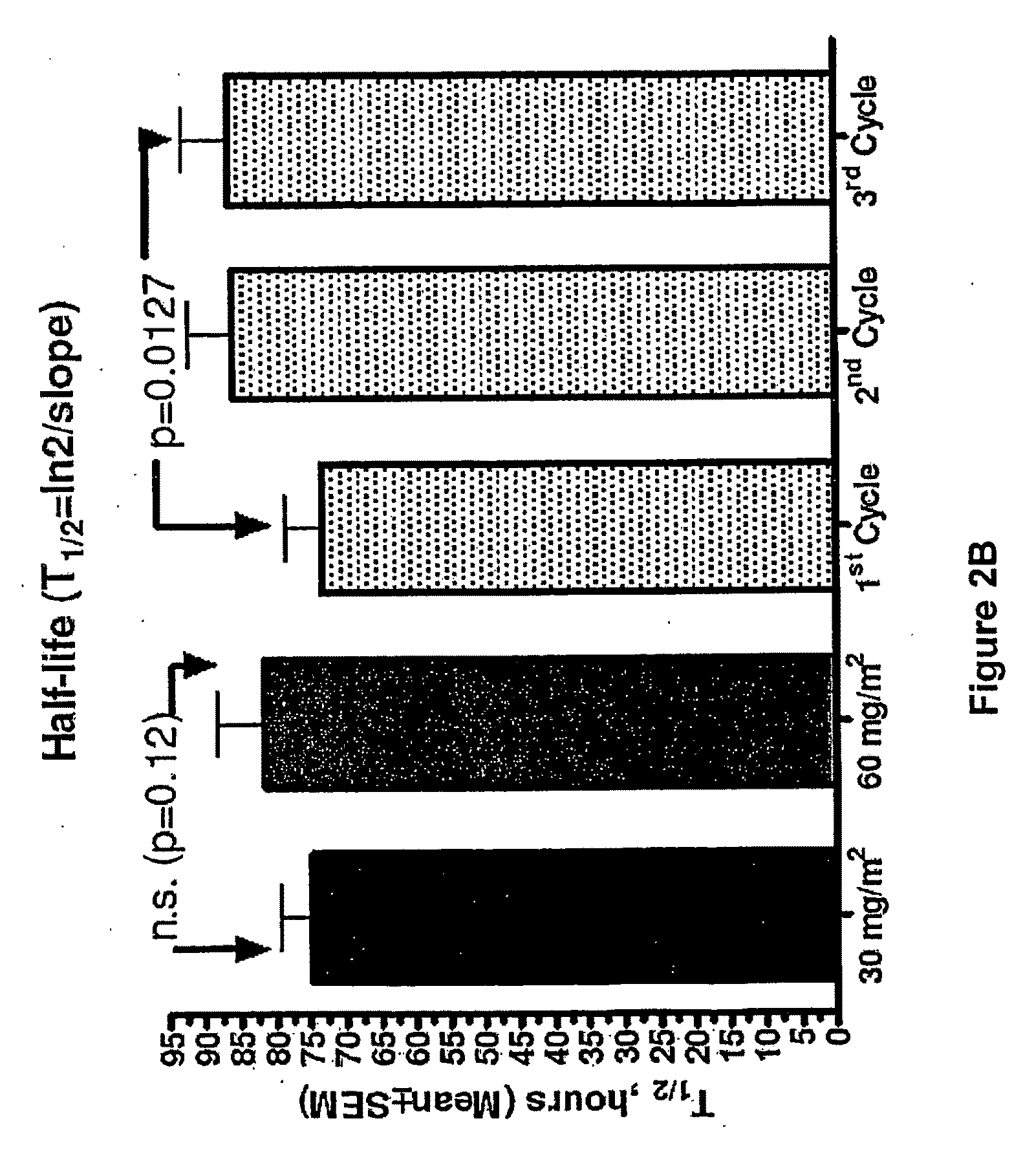
An embodiment of the present invention comprises a method of treating malignancies in a subject in need of treatment comprising administering to the subject a high loading dose of a pegylated liposomal doxorubicin (PLD) in an initial cycle, followed by a reduced dose in a second cycle, wherein the second cycle reduced dose is in the range of 20% to 50%, preferably 50%, of the initial loading dose, and thereafter one or more maintenance doses in further cycles. The interval between dose cycles is in the range of about three-to-four weeks, preferably about four weeks. The initial loading dose is in the range of between the maximum tolerated dose (MTD) and the recommended dose, preferably the MTD (for instance, in the range of about 70 mg / m2 to 50 mg / m2, preferably 60 mg / m2). The one or more maintenance doses are in the range of about 40 mg / m2 to 50 mg / m2, preferably 45 mg / m2).
Owner:GABIZON ALBERTO A
Method for administration of pegylated liposomal doxorubicin
An embodiment of the present invention comprises a method of treating malignancies in a subject in need of treatment comprising administering to the subject a high loading dose of a pegylated liposomal doxorubicin (PLD) in an initial cycle, followed by a reduced dose in a second cycle, wherein the second cycle reduced dose is in the range of 20% to 50%, preferably 50%, of the initial loading dose, and thereafter one or more maintenance doses in further cycles. The interval between dose cycles is in the range of about three-to-four weeks, preferably about four weeks. The initial loading dose is in the range of between the maximum tolerated dose (MTD) and the recommended dose, preferably the MTD (for instance, in the range of about 70 mg / m2 to 50 mg / m2, preferably 60 mg / m2). The one or more maintenance doses are in the range of about 40 mg / m2 to 50 mg / m2, preferably 45 mg / m2).
Owner:GABIZON ALBERTO A
Application of cytotoxic anti-tumor drugs to preparation of tumor-related tissues
InactiveCN110694070APromote growthPromote proliferationOrganic active ingredientsInorganic active ingredientsMaximum tolerated dosePharmaceutical drug
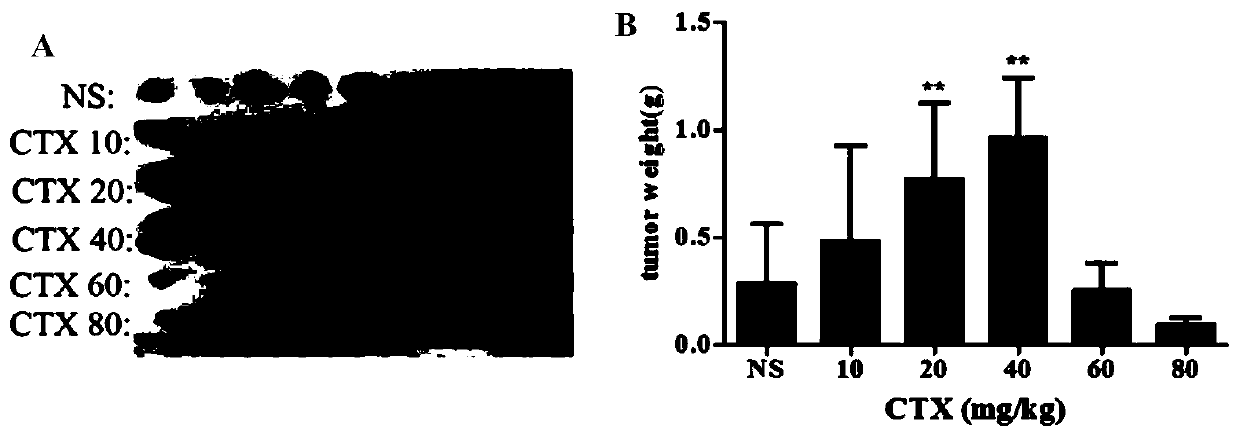
The invention provides application of cytotoxic anti-tumor drugs to preparation of tumor-related tissues. A research proves that under a drug administration condition of certain concentration or dosage being less than conventional maximum tolerance dose, a result proves that the drugs have an effect of obviously promoting tumor growth; and by utilizing the phenomenon, the drugs can be applied to building of a tumor growth model, production culturing of in vitro and in vivo tumor tissues and tumor cells, culturing of tissue cells closely related with tumor tissue growth in a tumor micro-environment, and separation and extraction of specific tissue components.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Method and composition for treating alzheimer-type dementia
ActiveUS20140288057A1Maximize the effectSymptoms improvedBiocideNervous disorderNK1 receptor antagonistMaximum tolerated dose
There is described a method for increasing the maximal tolerated dose and thus the efficacy of an acetylcholinesterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetylcholinesterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetylcholinesterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholinesterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Phototoxicity detection method based on recombinant skin model
InactiveCN110487997AExpand the scope of detectionGuaranteed accuracyBiological testingMaximum tolerated doseOrganism
The invention provides a phototoxicity detection method based on a recombinant skin model. The method comprises steps of S1, receiving and resuscitating the recombinant skin model; S2, selecting a light source; S3, determining the maximum tolerated dose of the recombinant skin model; and S4, testing the phototoxicity of a test object. The method is advantaged in that the method can detect not onlywater-soluble chemicals and cosmetic raw materials, but also can detect oily and creamy cosmetic finished products and different proportions of formulated substances, the method not only simulates the change in biological response of the human skin to different types of exogenous substances, but also expands the detection range of to-be-tested substances, is no longer limited to the water-solublechemicals, further includes finished cosmetics and formulas of various properties.
Owner:GUANGDONG BOXI BIO TECH CO LTD
Method of treatment using inhibitors of mitosis
Owner:ARRAY BIOPHARMA INC
Combination treatments of hsp90 inhibitors for enhancing tumor immunogenicity and methods of use thereof
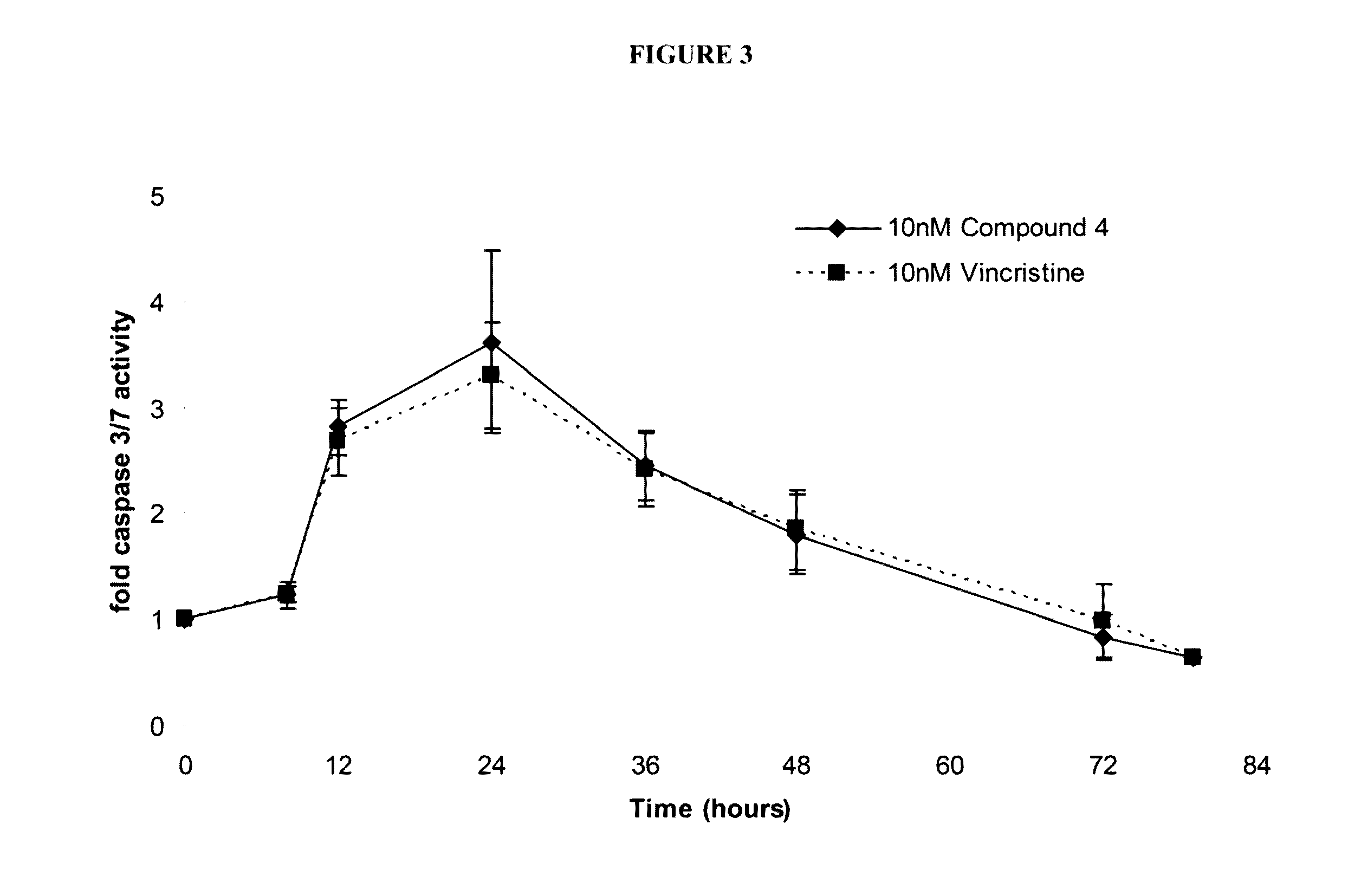
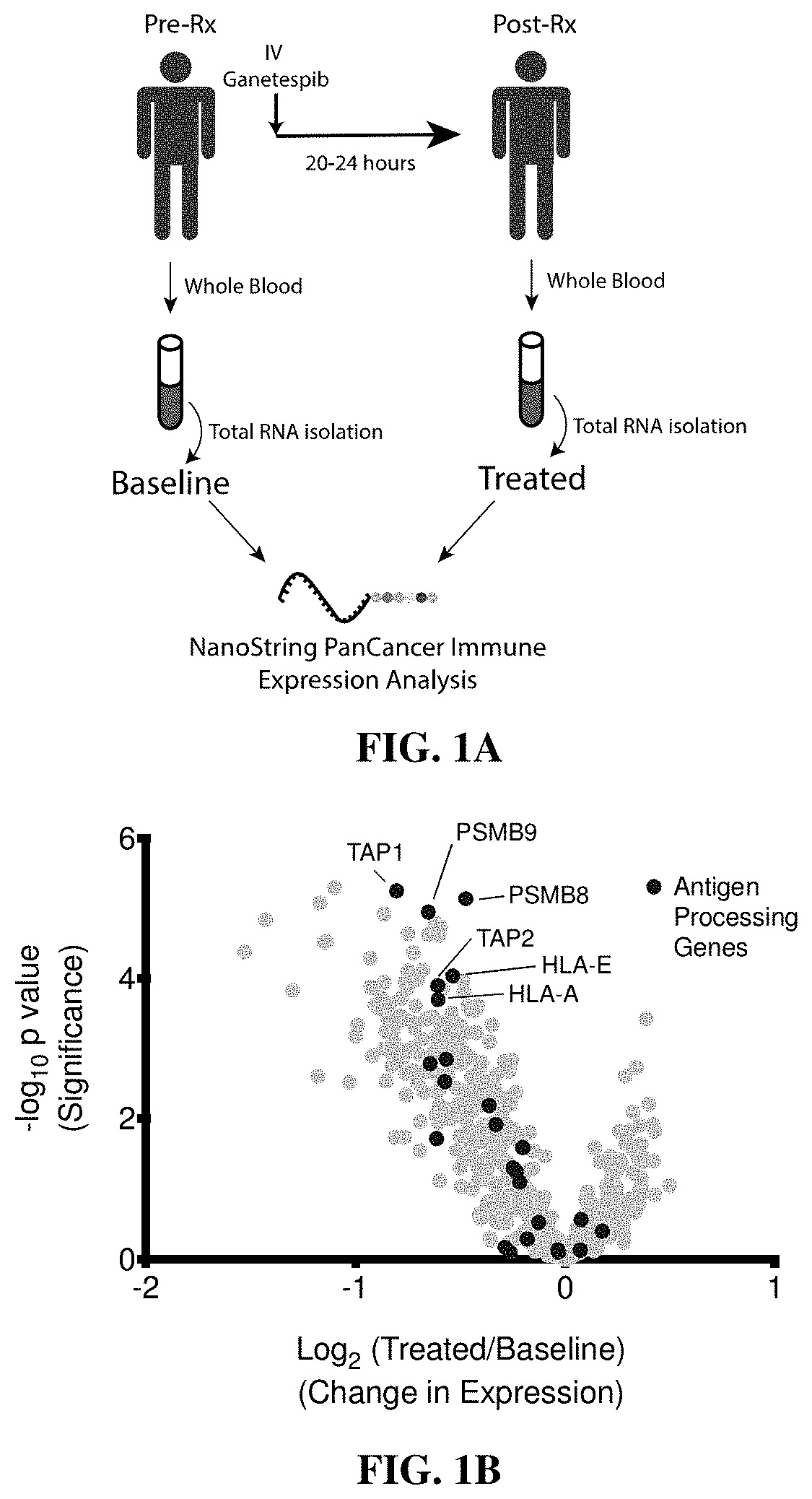
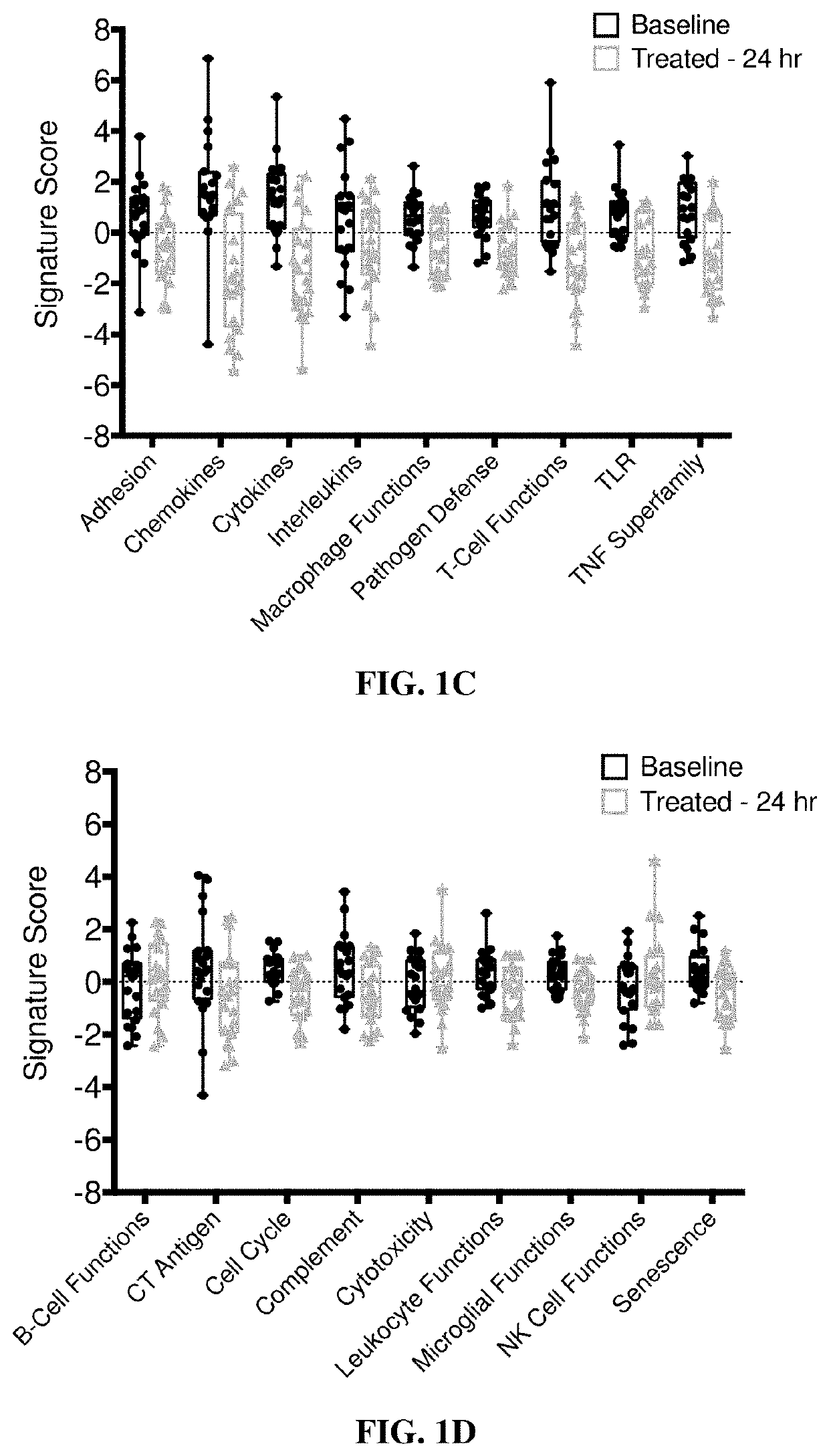
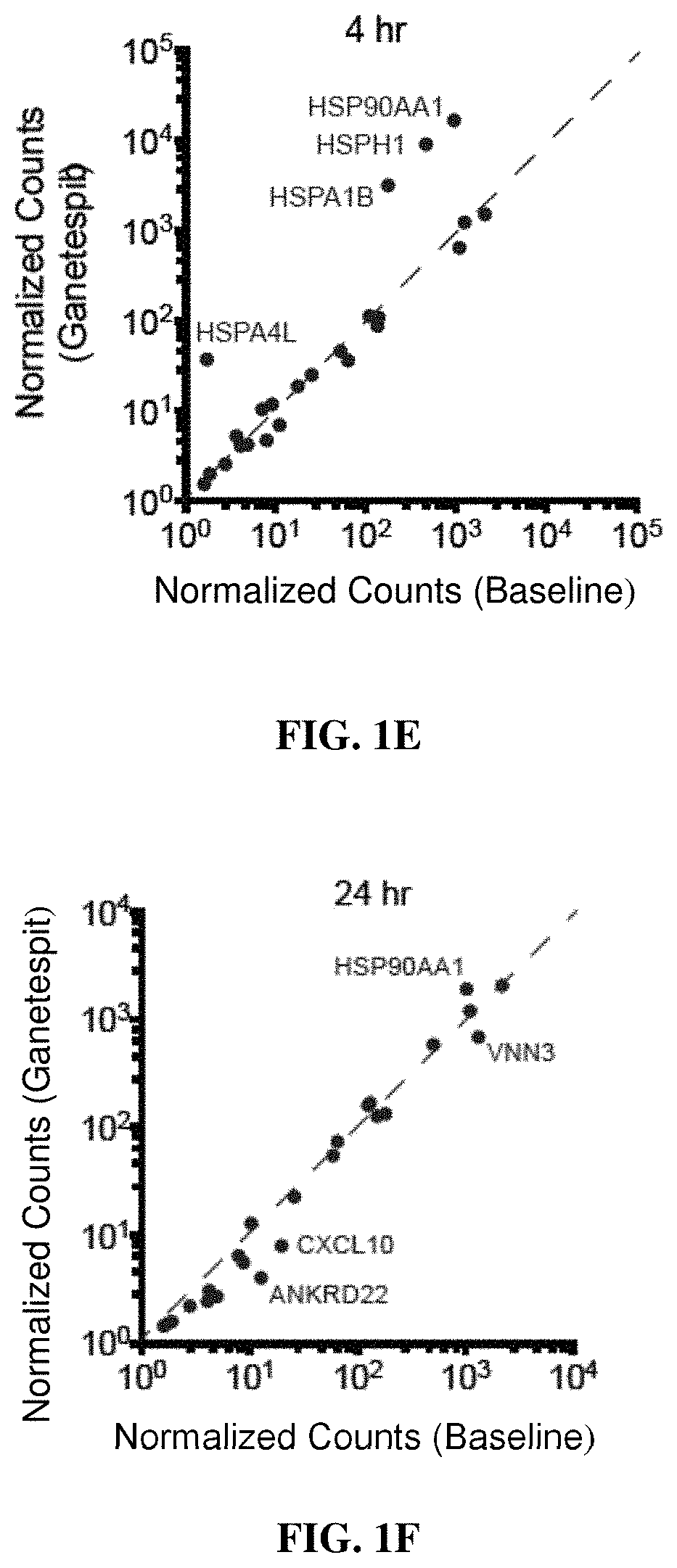
PendingUS20190365719A1Enhance anti-cancer efficacyReduce proliferationOrganic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMaximum tolerated doseCytotoxicity
It has been established that exposure to cytotoxic doses of HSP90 inhibitor is broadly immunosuppressive, whereas continuous exposure to low-dosages of the same inhibitor exerts anti-tumor activity. The anti-tumor activity is mediated by the host immune system. Compositions and methods for continuous, low-dose exposure to HSP90 inhibitors in combination with one or more immunostimulatory agents for the treatment of cancer are described. Typically, the HSP90 inhibitor is administered in an amount that is between 1% and 20% of the clinically-determined maximum tolerate dose. The immunostimulatory agent can be administered simultaneously with the HSP90 inhibitor, or at some time before or after the HSP90 inhibitor. Compositions including a sub-toxic dose of HSP90 inhibitor in combination with an immunostimulatory agent in an amount effective to treat cancer are also provided.
Owner:WHITEHEAD INST FOR BIOMEDICAL RES +2
Article comprising calcium for reducing the production of TSST-1
Articles comprising one or more calcium salts are provided. The articles can contain one or more calcium salts in an amount effective to reduce the production of TSST-1 by at least about 50% when measured by the Shake Flask Method. In certain embodiments, the one or more calcium salts can be substantially non-lethal to Staphylococcus aureus when measured by the Shake Flask Method, and / or to Lactobacillus crispatus, Lactobacillus gasseri, and / or Lactobacillus iners when measured by the Maximum Tolerated Dose Test.
Owner:THE PROCTER & GAMBLE COMPANY
DNA toxic dimer compound
The invention discloses a DNA toxic dimer compound. Compared with a benzodiazepine conjugate disclosed in the prior art, a cytotoxic compound disclosed by the invention presents much higher in vivo treatment index (ratio of maximum tolerated dose and minimum effective dose).
Owner:バイリバイオ(チェンドゥ)ファーマスーティカル シーオーエルティーディー
Preparation method for radiopolymerized slow-release high-polymer material
InactiveCN102382238ALittle side effectsReduce or eliminate adverse effectsPharmaceutical non-active ingredientsOn/in organic carrierNitrogenMaximum tolerated dose
The invention relates to a preparation method for radiopolymerized slow-release high-polymer material. The method includes the following steps: slow-release material as high-polymer material blended according to different proportions or slow-release high-polymer material uniformly mixed with drug to be slowly released is put into a plastic container without chlorine atoms, sealed, vacuumized, then saturated with high-purity nitrogen, put into a thermal insulating container under minus 18 DEG C and irradiated by 60 Co Gamma rays or 5 MeV to 10MeV of electron beam, the radiation dose is 9.9kGy to 20kGy, the lowest effective dose is 9.9kGy, the maximum tolerated dose is 20kGy, the ununiformity of the radiation dose is lower than 1.3, the high-polymer material is polymerized, and the radiopolymerized slow-release high-polymer material is then produced into the shapes of tablets, granules and needles according to different purposes, and is used for fixing chemicals, enzyme and bacteria after water swelling. The invention overcomes the defects of the prior art, and provides the method for preparing the slow-release material, which can better promote radiopolymerization.
Owner:余姚市顺诚电子加速器技术服务有限公司
Synthetic peptide sp4 and use thereof
InactiveUS20210179664A1Maintain cell divisionStrong inhibitory activityPeptide/protein ingredientsPeptidesTelomeraseEfficacy
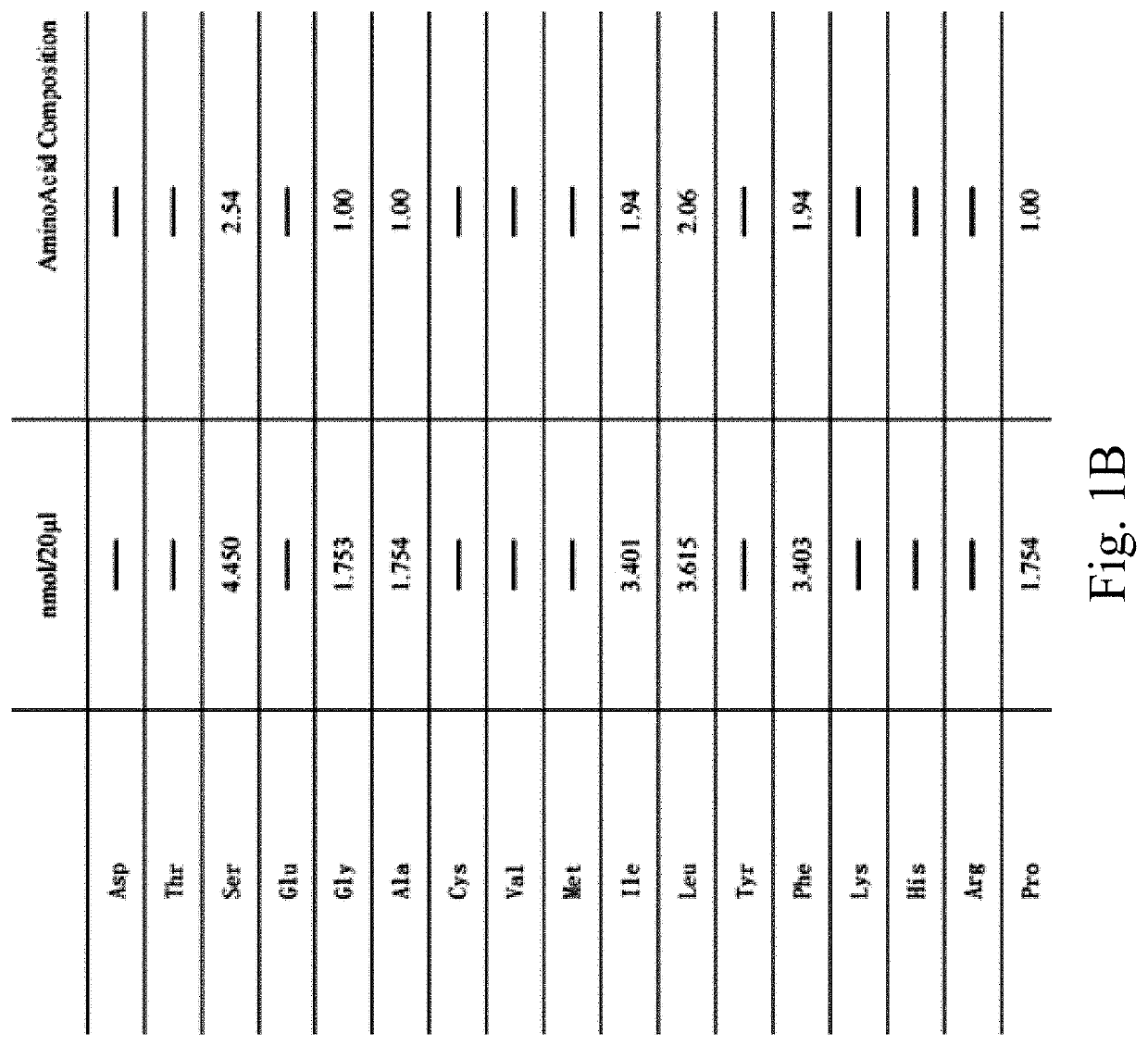
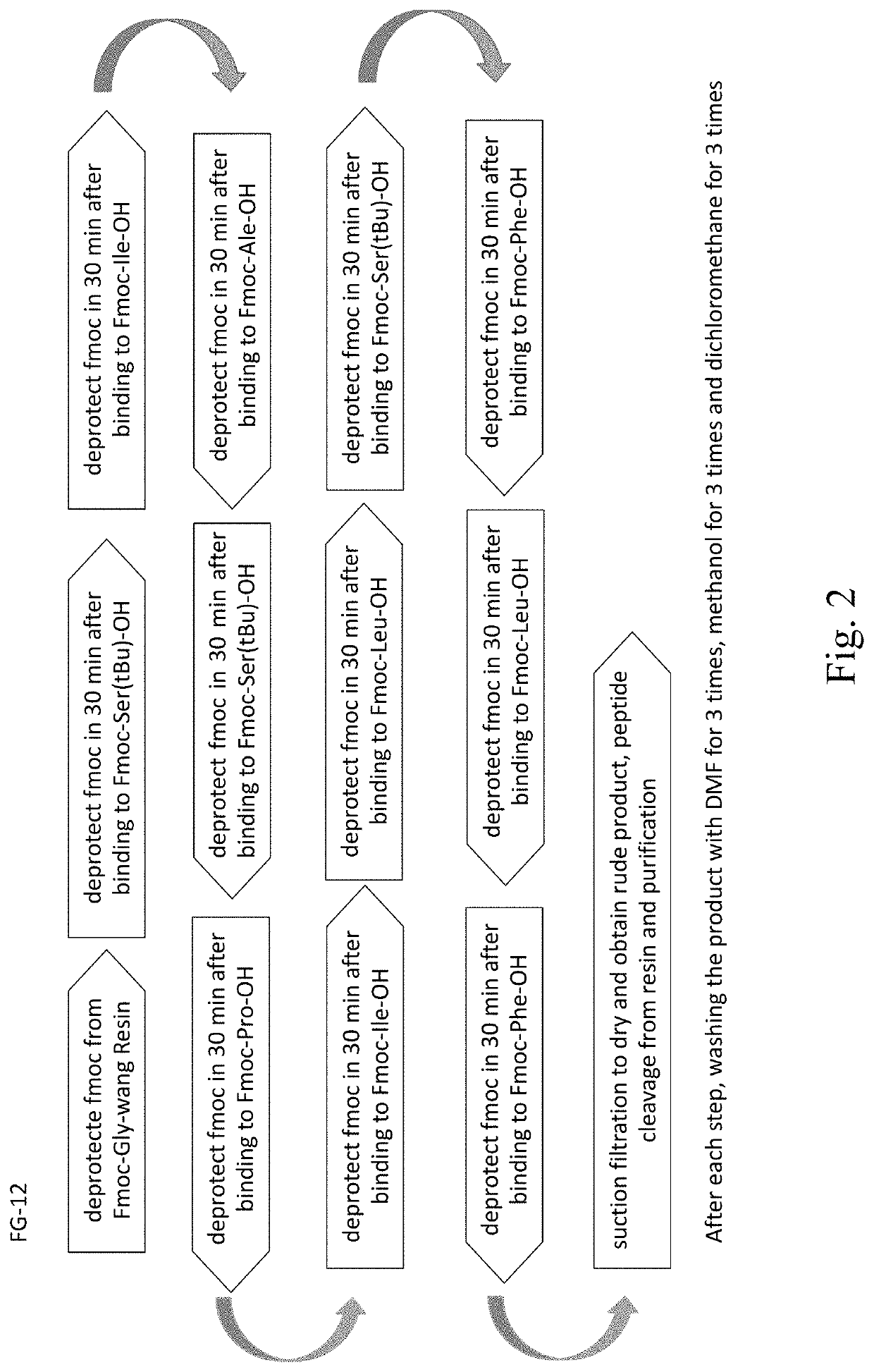
A synthetic peptide sp4 has an amino acid sequence shown in SEQ ID NO: 1. As the sole effective ingredient in the antitumor efficacy test, it has a significant inhibitory effect on both the tumor volume and tumor weight of human osteosarcoma MG-63 in nude mice and has a significant dose-effect and time-effect relationship. There is no difference between the relative tumor growth rate T / C (%) of sp4 and that of the positive control group of Paclitaxel. The safety of sp4 is that its maximal tolerated dose for intravenous administration is 700 mg / kgBW. Sp4 has clear targets of pharmacological effects, while having efficacy in vivo, it also has an inhibitory effect on tumor telomerase, has an arrest on G1 phase of the tumor cell cycle, and inhibits the high expressions of tumor PD-L1 and CD47. The present disclosure relates to multiple uses of sp4, especially in the preparation of a class of antitumor drugs.
Owner:TAIAN CITY QIHANG BIOTECH CO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com