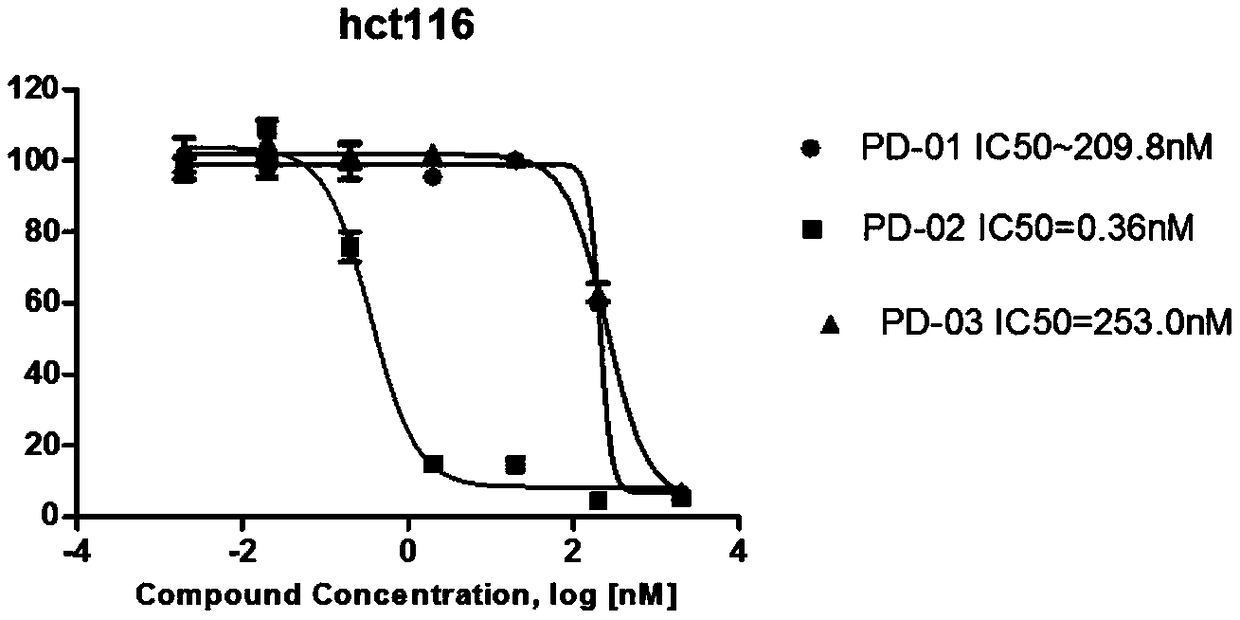
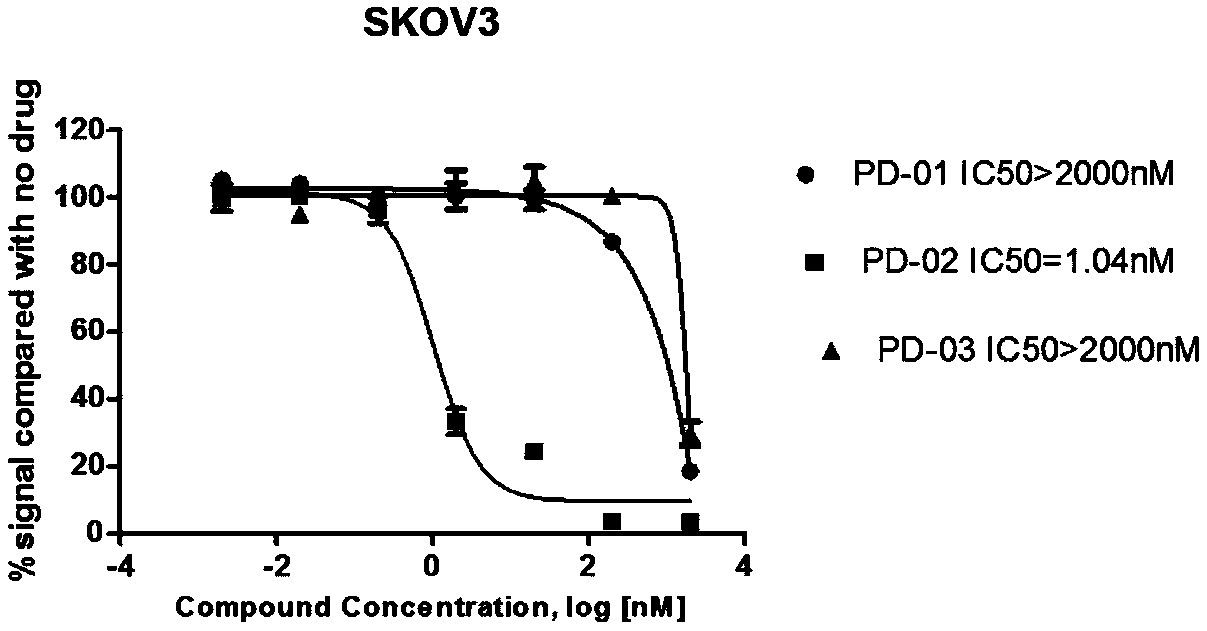
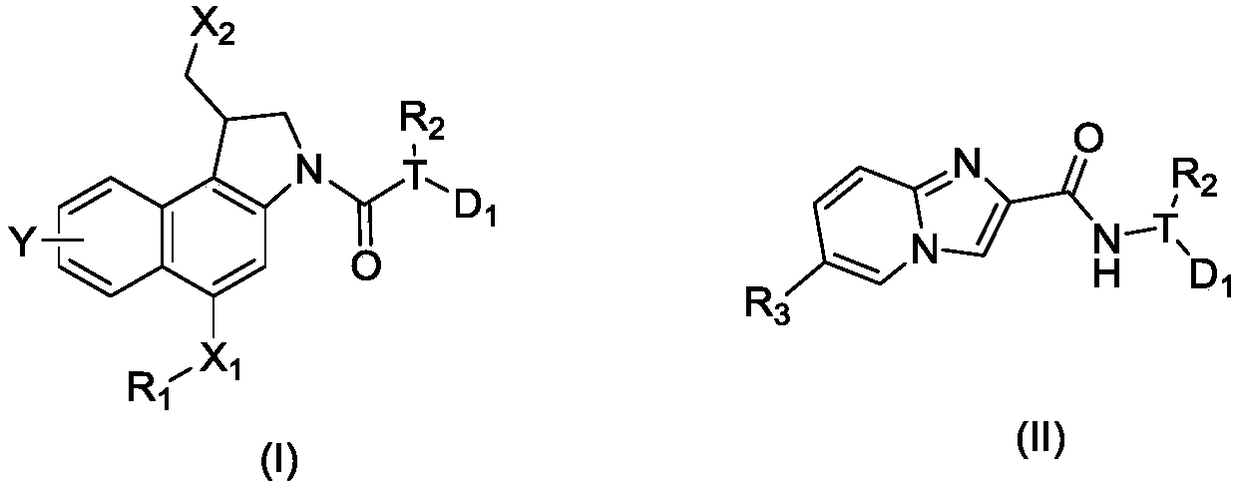
DNA toxic dimer compound
A hydrate and solvate technology, applied in the field of new cytotoxic compounds, can solve the problems of toxicity and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 Preparation of 3-(methoxyformyl)bicyclo[1.1.1]pentane-1-carboxylic acid
[0076]
[0077] Add 1.48g methyl bicyclo[1.1.1]pentane-1,3-dicarboxylate into a 50ml three-necked flask, dissolve it with 20ml methanol, slowly add NaOH (324mg) in methanol (5ml) solution dropwise at room temperature, add After completion, the temperature was raised to 80°C for 2 hours. After the reaction is completed, concentrate to dryness, add 40ml of water, extract with dichloromethane (50ml*3), adjust the pH of the aqueous layer to acidic with dilute hydrochloric acid, add dichloromethane for extraction (40ml*4), combine the organic layers, dry and concentrate 925mg of white solid, yield 68%. 1 H NMR (400MHz, CDCl3) δ3.71(s, 3H), 2.38(s, 6H); MS m / z 171(M+1).
Embodiment 2
[0078] Example 2 Preparation of 3-(hydroxymethyl)bicyclo[1.1.1]pentane-1-methyl carboxylate
[0079]
[0080] Add 6.34g of 3-(methoxyformyl)bicyclo[1.1.1]pentane-1-carboxylic acid, 30ml of tetrahydrofuran, and 4.89g of triethylamine to a 500ml three-necked flask successively, cool the reaction system to -10°C, and slowly add After adding 3.87g of methyl chloroformate, react at 0°C for 1 hour, add 2.54g of NaBH4 in batches, and continue to react at 0°C until the raw material is complete. The reaction was quenched by adding 400ml of water, extracted with ethyl acetate (600ml*3), the organic layers were combined, dried and concentrated, and the resulting residue was purified by column chromatography (DCM:MeOH=20:1) to obtain 3.83g of light yellow oil , yield 66%. 1 H NMR (400MHz, CDCl3) δ3.71(s,3H),2.38(s,6H); 3.48(s,2H); MS=157(M+1)
Embodiment 3
[0081] Example 3 Methyl 3-((p-toluenesulfonyloxy)methyl)bicyclo[1.1.1]pentane-1-carboxylate
[0082]
[0083] Add 700mg of 3-(hydroxymethyl)bicyclo[1.1.1]pentane-1-methyl carboxylate to a 100ml flask, dissolve it with 10ml of dichloromethane, add 1.2ml of triethylamine and 0.55g of DMAP in sequence, Add a dichloromethane solution of p-toluenesulfonyl chloride dropwise, and then react at room temperature until the raw materials are completely reacted. The organic layer was washed successively with dilute hydrochloric acid and saturated brine, dried over anhydrous Na2SO4, and concentrated to obtain 1.4 g of a yellow solid, which was directly used in the next reaction without further purification.
[0084]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com