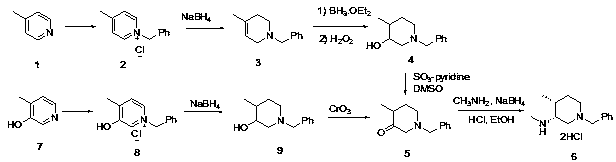
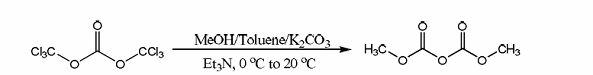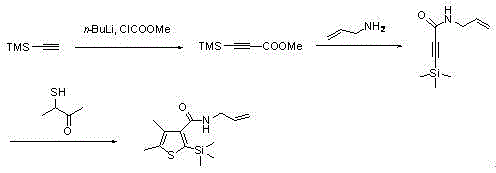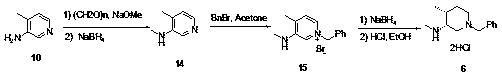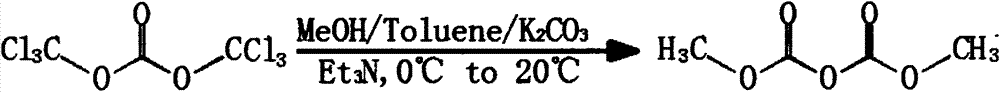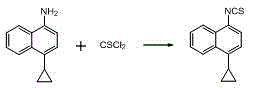Patents
Literature
101 results about "Methyl chloroformate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methyl chloroformate is the methyl ester of chloroformic acid and is an oily liquid with a color that is anywhere from yellow to colorless. It is also known for its pungent odor.
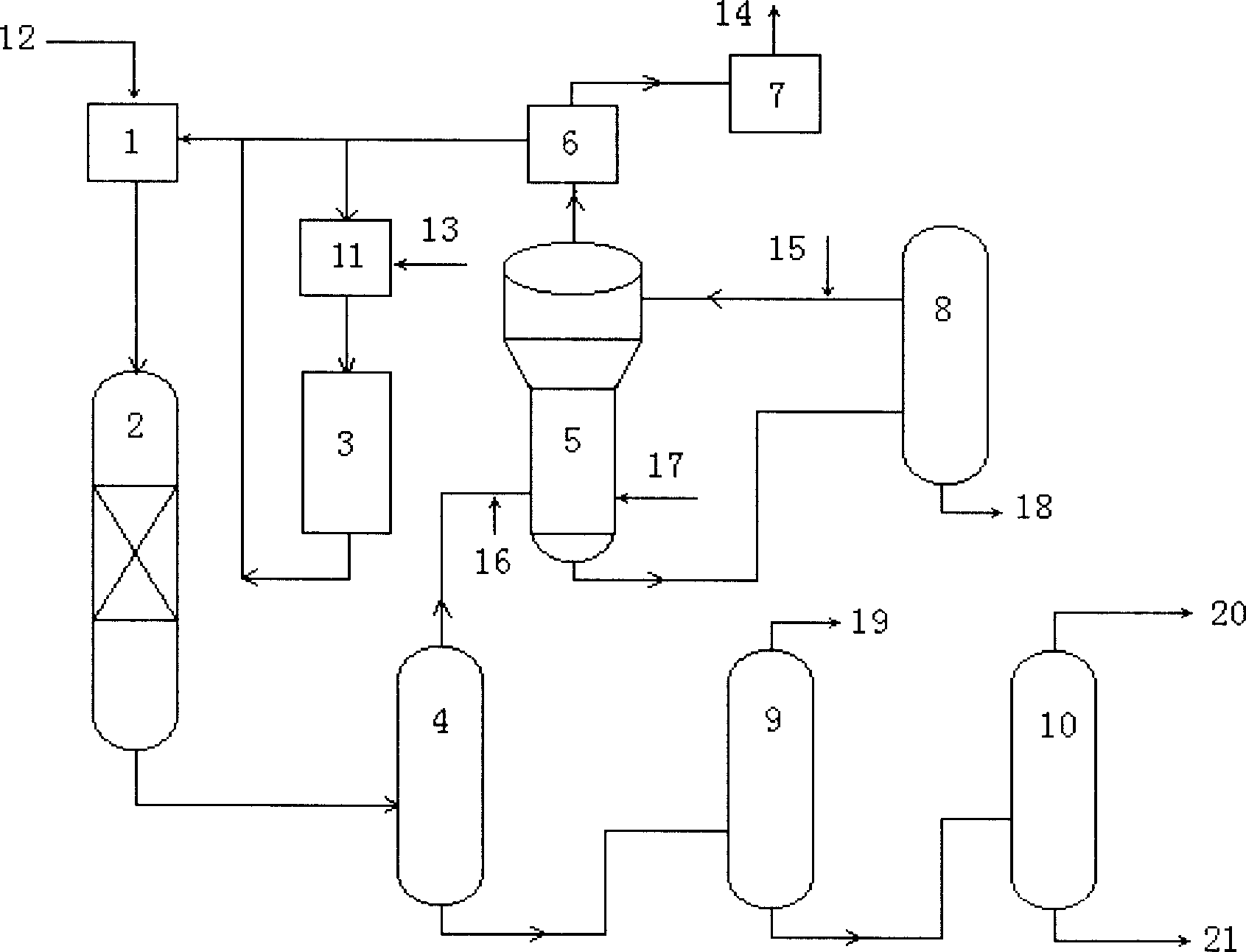
Method of producing dimethyl carbonate
InactiveCN1724506AReduce volumeSolution to short lifeOrganic compound preparationCarbonic/haloformic acid esters preparationNitrogen monooxideMethyl carbonate
The invention discloses a process for producing dimethyl carbonate which comprises, mixing HCl with the gaseous mixture of carbon monoxide and methyl nitrite, then entering auxiliary reaction area, mixing the product yield of the subsidiary reaction zone with the gaseous mixture of carbon monoxide raw material gas and methyl nitrite, entering main reaction zone, condensing and absorbing the product yield of the main reaction zone, separating and rectifcating the condensate liquid, thus obtaining the dimethyl carbonate product, while the non-condensed gas, oxygen, nitrogen monoxide reacting with methanol and obtaining and methyl nitrite for use by the subsidiary reaction zone and the main reaction zone. The process provided by the invention can sustain catalytic activity, extend period of catalyst use, and avoid the use of hypertoxic methyl chloroformate.
Owner:CHINA PETROCHEMICAL CORP +1
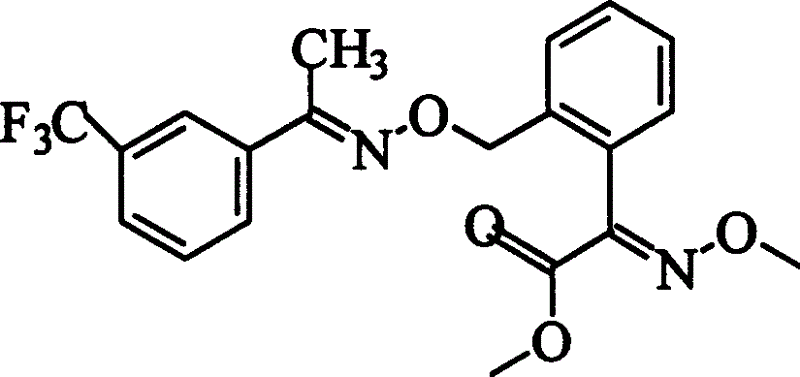
Preparation process of oxime strain ester
The invention discloses a trifloxystrobin preparing method, including the steps as follows: (a) using ortho-methyl hypnone as raw material and using potassium permanganate to oxidize so as to obtain 2-(2'-methyl-phenyl)-2-carbonyl acetic acid; (b) making the product in step (a) react with methanol to obtain 2-(2'-methyl-phenyl)-2-carbonyl methyl acetate; (c) bromizing the product in step (b) to obtain 2-(2'-bromomethyl-phenyl)-2-carbonyl methyl acetate; (d) making the product in step (c) react with methoxy amine to obtain (E)-2-(2'-bromomethyl-phenyl)-2-carbonyl methyl acetate-O-methyl ketone oxime; (e) making the product in step (d) react with meta-trifluoromethyl hypnone oxime to obtain the product trifloxystrobin. It reduces the discharge of large amount of waste water in course of oxidization reaction, avoids esterification by adopting methyl- chloroformate, most operating conditions are moderate.
Owner:TONGJI UNIV
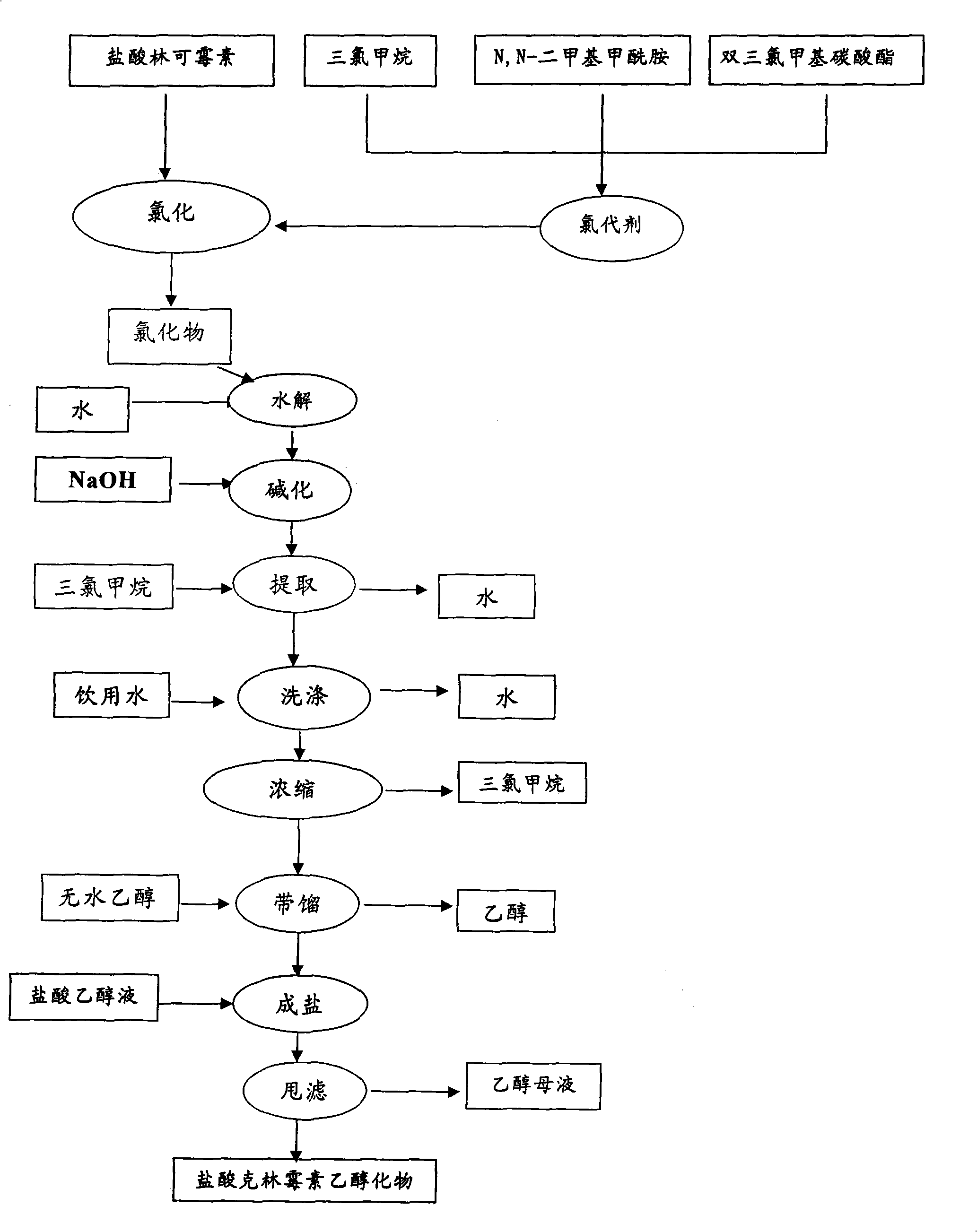
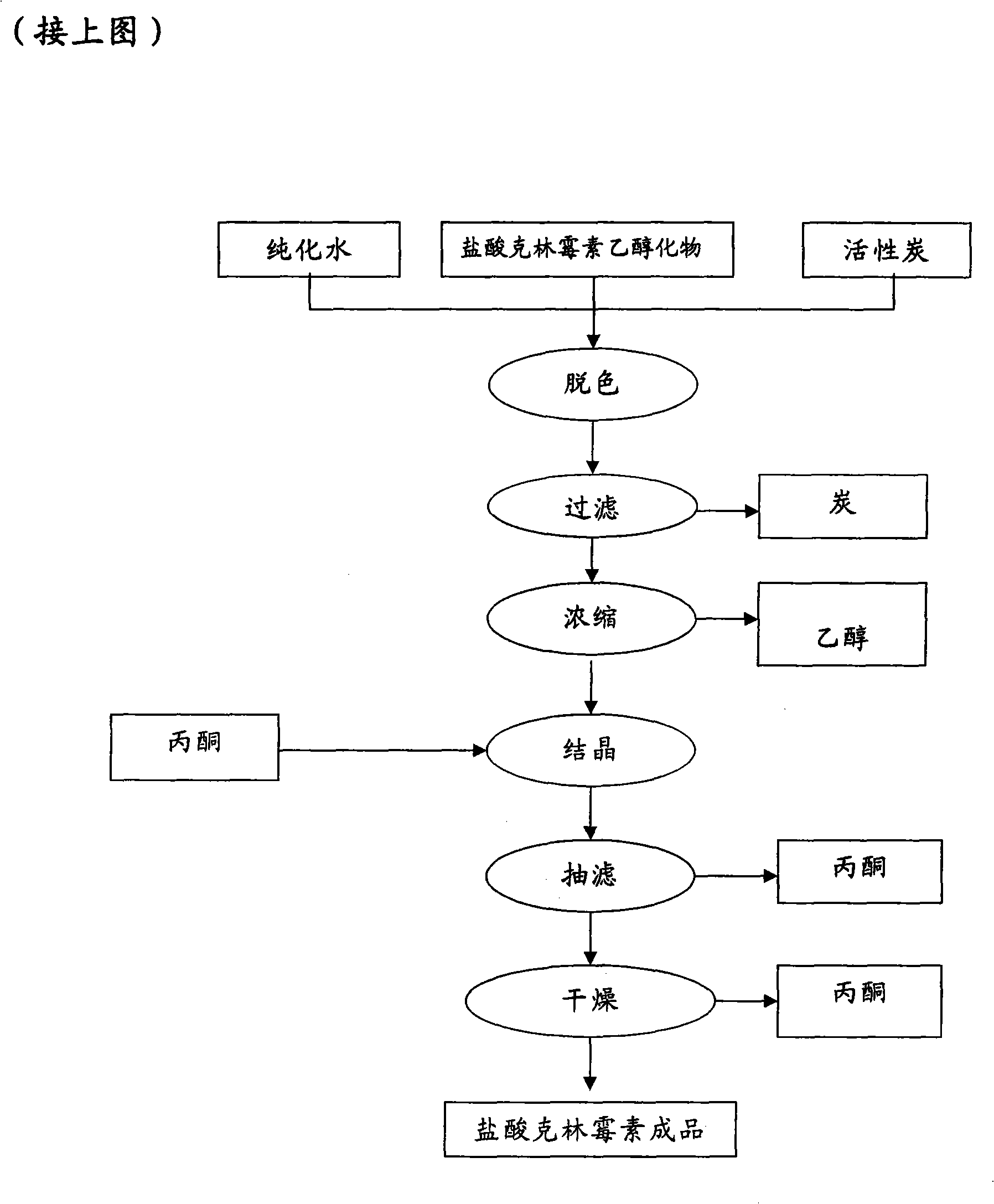
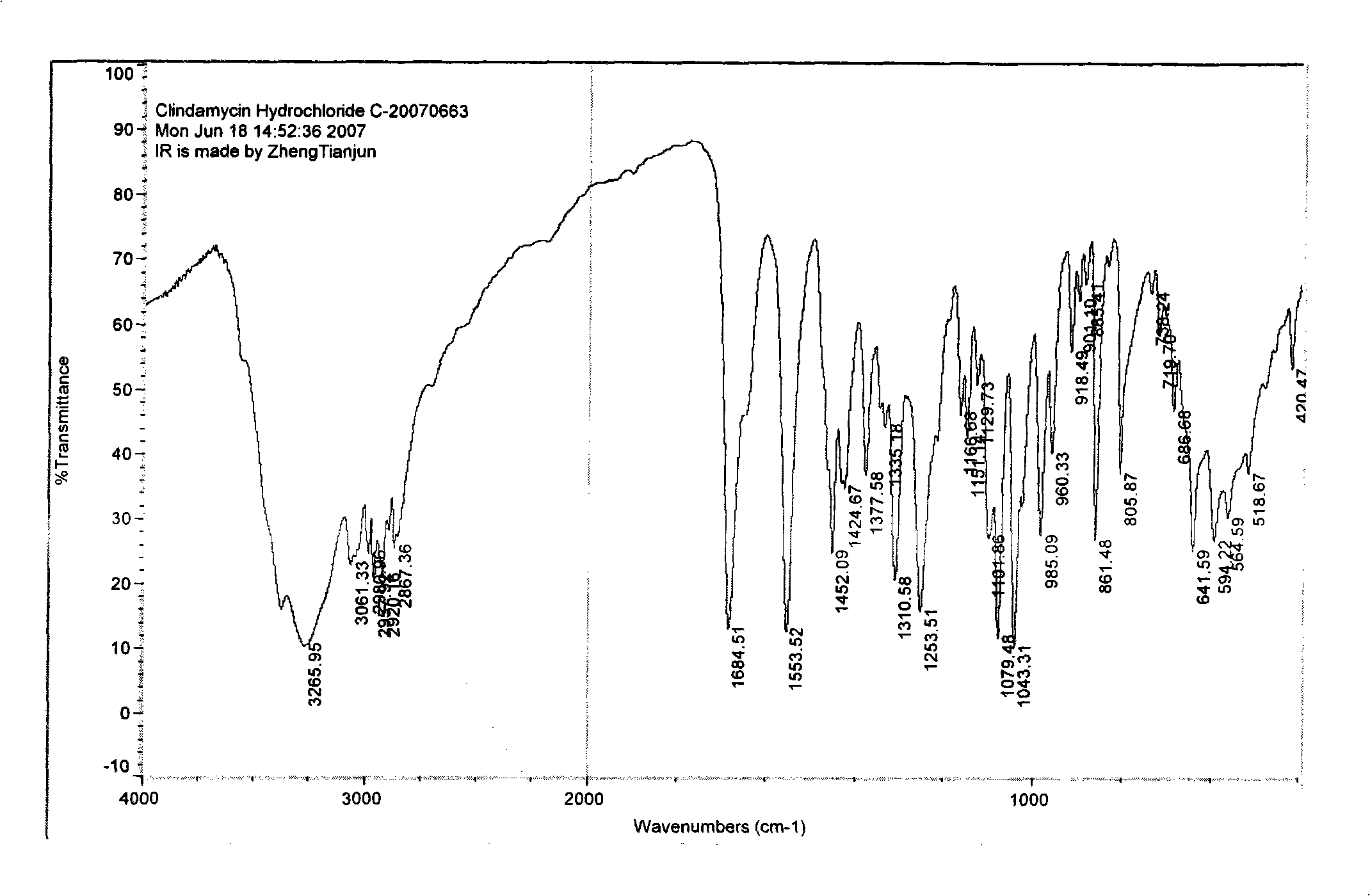
Industrial production method for clindamycin or salts thereof
The invention relates to an industrial production method for producing clindamycin or the salts of clindamycin. The method uses dual-trichloromethyl carbonate or trichloromethyl methyl chloroformate and amide to prepare the intermediate chlorophthalic agent. The method of the invention has high product purity, high yield, easier post-processing and other technical advantages, compared with the prior art.
Owner:重庆凯林制药有限公司 +1
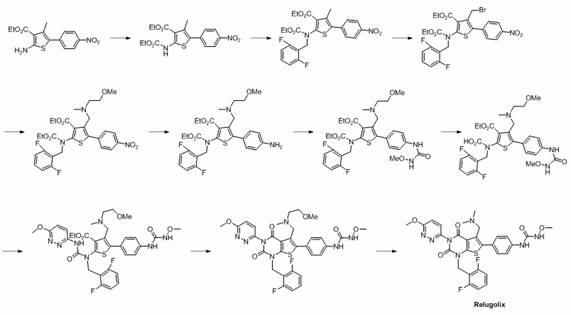
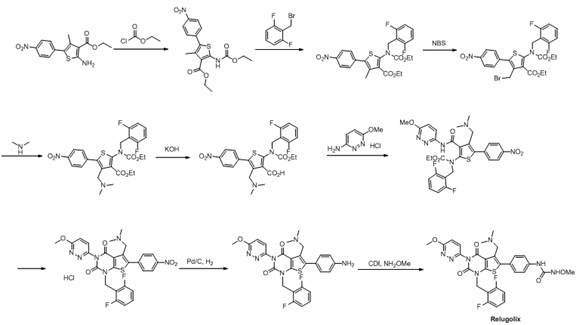
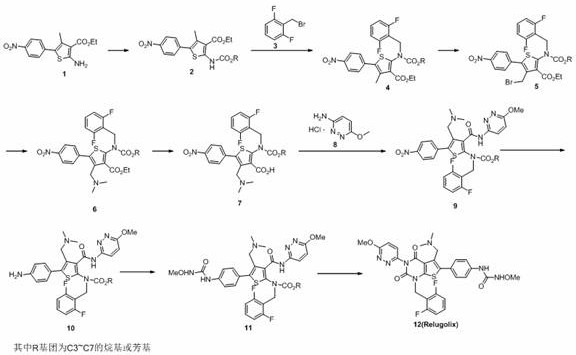
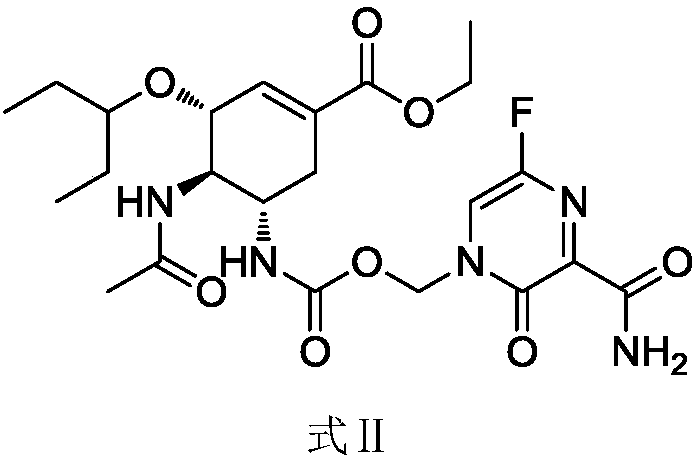
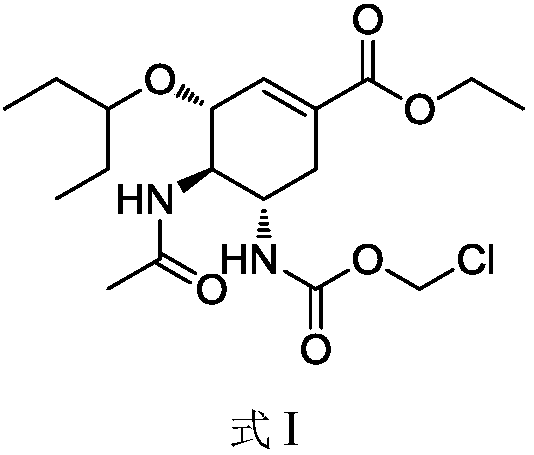
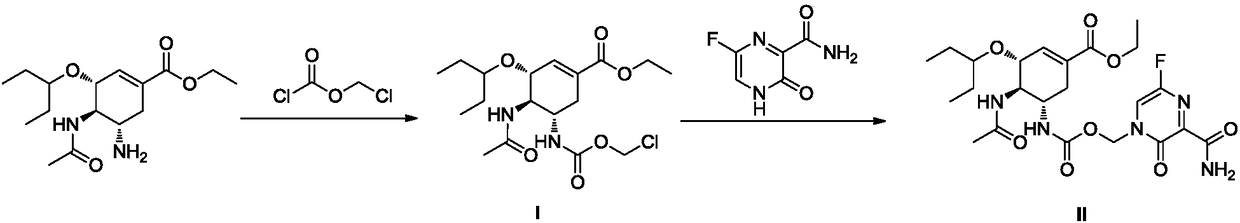
Preparation method of relugolix drug intermediate
The invention provides a relugolix intermediate and a synthesis scheme of relugolix, the synthesis scheme adopted by the invention effectively avoids the use of a high-toxicity substance methyl chloroformate or ethyl chloroformate, and other types of chloroformate which is low in toxicity and convenient to use are adopted, so that the use risk in the production process of crude drugs can be reduced, and the production cost is reduced. Operation is easy, the process is safer, and industrial production is facilitated.
Owner:SUZHOU PENGXU PHARM TECH CO LTD
Synthetic method of methyl-4-(trifluoromethoxy) phenyl carbamate
InactiveCN102219712AImprove economyLow toxicityCarbamic acid derivatives preparationOrganic compound preparationCarbamateSide effect
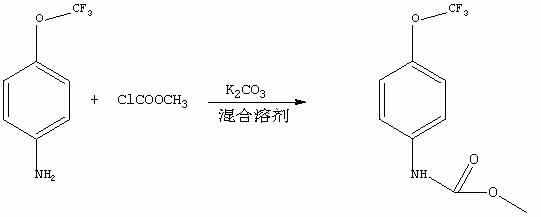
A synthetic method of methyl-4-(trifluoromethoxy) phenyl carbamate. Raw materials of 4-trifluoromethoxy-aniline, methyl chloroformate and alkalis and a mixed solvent of water and organic solvents are mixed and undergo directly a reaction to produce a product of methyl-4-(trifluoromethoxy) phenyl carbamate. The synthetic method comprises the following steps of 1, adding 4-trifluoromethoxy-aniline, sodium hydroxide, tetrahydrofuran and water into a container, mixing well and make the mixture undergo a reaction at a temperature of 0 to 5 DEG C for 2 hours, 2, heating the reaction products to room temperature and stirring for 30 minutes, adding slowly methyl chloroformate into the heated reaction products and stirring at room temperature for 6 to 7 hours, 3, cooling the reaction products obtained from the step 2 to 0 DEG C and stirring for 30 minutes, and 4, carrying out a suction filtration process for the reaction products obtained from the step 3 to collect solids, and washing and drying the solids to obtain desired products. The synthetic method has the advantages that the synthetic method is simple and practical, has a high efficiency and can realize an end product yield more than 95%; reagents adopted in synthesizing processes have the advantages of low toxicity, high economic efficiency, less side effects, less corrosion on equipment and less pollution on environment; and prices of raw materials are cheap thus a production cost is low and the synthetic method has a high economic efficiency and good application prospects.
Owner:NANKAI UNIV
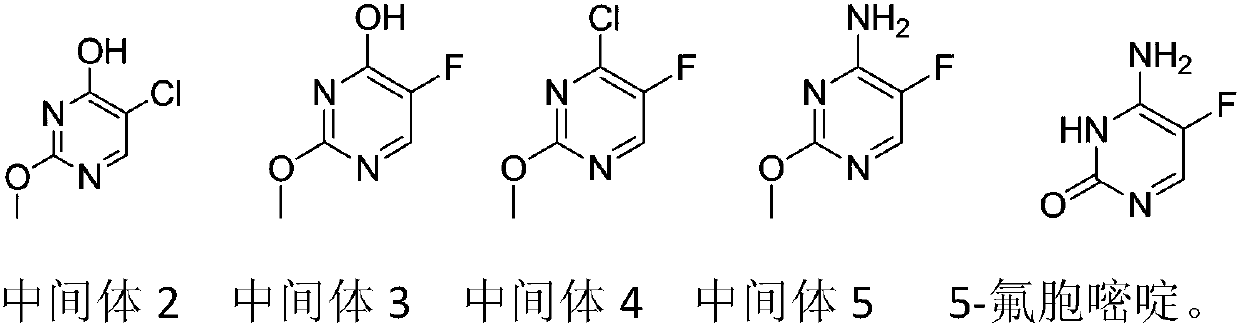
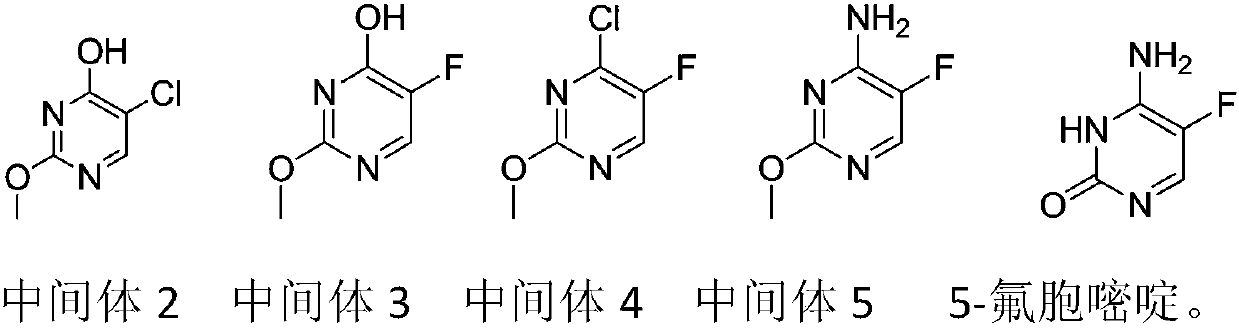
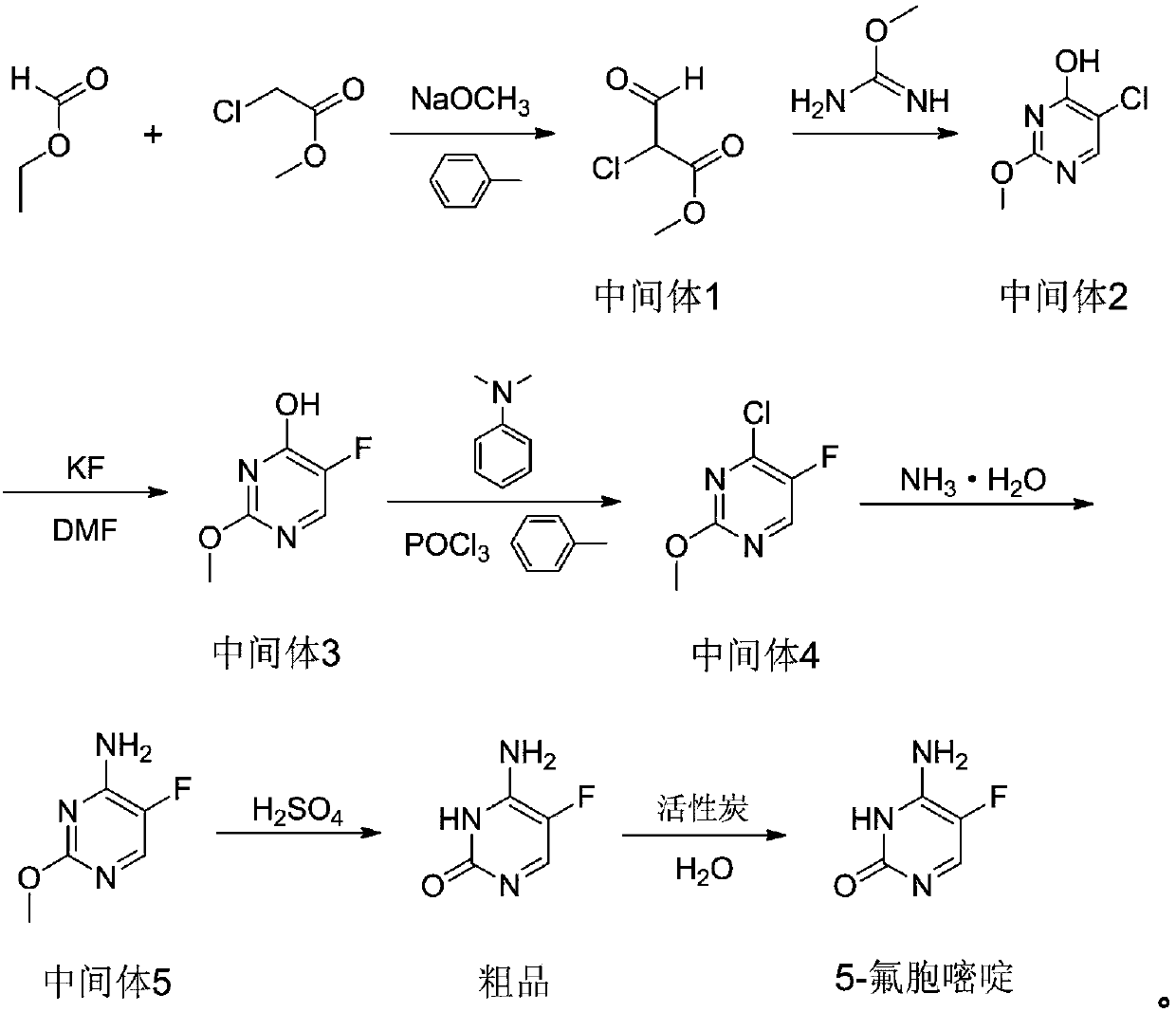
Preparation method of 5-flucytosine
ActiveCN108033917AReduce usageThe process is environmentally friendlyOrganic chemistryChemical synthesisPotassium fluoride
The invention belongs to the technical field of chemical synthesis of medicines and relates to a preparation method of 5-flucytosine. The preparation method comprises the following steps: utilizing ethyl formate and methyl chloroformate to synthesize 2-chloro-3-oxo methyl propionate, then utilizing oxymethylisourea to close rings to obtain pyrimidine rings, utilizing potassium fluoride to substitute chlorine on the pyrimidine rings, utilizing phosphorus oxytrichloride to substitute hydroxyl groups on the pyrimidine rings, then adding ammonia water to lead chloride to be substituted with aminogroups, and hydrolyzing under an acid condition to obtain a product, namely the 5-flucytosine. The preparation method has the beneficial effects that the methyl chloroacetate is adopted for substituting methyl fluoroacetate to be used as a synthetic raw material of the 5-flucytosine, so that the use of highly-toxic chemicals such as the methyl fluoroacetate is avoided; simultaneously, since the price of the methyl chloroacetate is much lower than the price of the methyl fluoroacetate, the production cost can be saved; by utilization of the synthetic route provided by the invention, the higher-purity 5-flucytosine can be prepared without need of complex aftertreatment steps; simultaneously, the preparation method has higher overall yield and obvious industrial value and is worthy of being promoted and used on a large scale.
Owner:ZHEJIANG XIANFENG TECH
Preparation method of indoxacarb technical concentrate
InactiveCN107235926ANo pollution in the processHigh response rateOrganic chemistrySodium methoxideEthylenediamine
The invention discloses a preparation method of an indoxacarb mother drug, comprising the following steps: using 5-chloroindanone as a raw material to synthesize: (S)-5-chloro-2,3-dihydro-2-hydroxyl-1- Methyl oxo-1H-indene-2-carboxylate; from (S)-5-chloro-2,3-dihydro-2-hydroxy-1-oxo-1H-indene-2-methyl carboxylate and Benzyl carbazate is first condensed and then synthesized with diethoxymethane: 2‑benzyloxycarboxy‑7‑chloroindeno[1,2‑P][1,3,4]oxadiazine‑2, 4a-methyl carboxylate; use p-trifluoromethoxyaniline as raw material and methyl chloroformate, 1,2-diphenylethylenediamine as acid-binding agent to generate 4-(trifluoromethoxy)phenyl Methyl carbamate is then photoacylated with sodium methoxide to generate chloroformyl (4-trifluoromethoxyphenyl) methyl carbamate; by 2-benzyloxycarboxy-7-chloroindeno[1,2 ‑P][1,3,4]Oxadiazine‑2,4a‑carboxylate methyl ester is deprotected by hydrogenation, and then synthesized with methyl chloroformyl (4‑trifluoromethoxyphenyl) carbamate prestige. The invention has the advantages of high reaction yield, greatly improved production efficiency, high product purity and no pollution to the environment.
Owner:NANTONG SHI ZHUANG CHEM
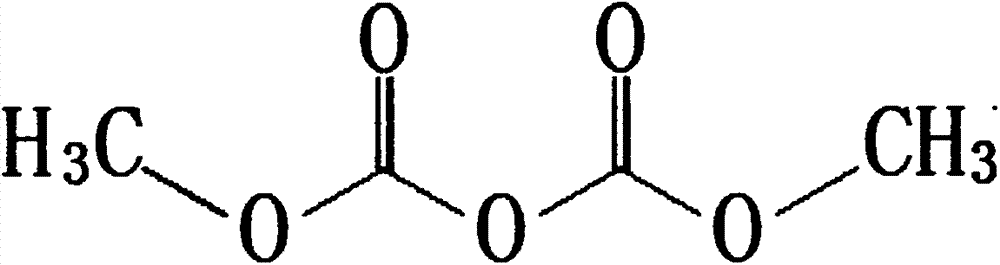
Preparation method for dimethyl dicarbonate
ActiveCN102219690APromote completeEasy to removeOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsFood additiveOrganic solvent
The invention discloses a preparation method for dimethyl dicarbonate, and belongs to the technical field of fine chemical industry. The method is characterized in that: in a organic solvent which is insoluble in water, quaternary ammonium salt is adopted as a catalyst, methyl chloroformate and a alkaline solutionare are adopted as raw materials to be subjected to a reaction for 0.5-3 hours at a temperature of 0-25 DEG C; after completing the reaction, the resulting solution is stood for demixing; the resulting organic layer is processed, and is subjected to reduced pressure distillation to collect fractions under pressure of 200 Pa at the temperature of 30-35 DEG C to obtain the product of the dimethyl dicarbonate. With the present invention, the quaternary ammonium salt is adopted as the catalyst, such that complete performance of the reaction is prompted, and the quaternary ammonium salt is easily removed after completing the reaction and is not remained in the product so as to do not influence on purity of the product; tertiary amine used in the prior art is avoided, because the tertiary amine can prompt the reaction, and can also inhibit the reaction when reaction conditions can not be controlled well, and the tertiary amine is difficult to be removed completely and can be remained in the product so as to enable the product to have disadvantages of decomposition and deterioration; reaction yield of the dimethyl dicarbonate is higher than 81%, purity of the dimethyl dicarbonate is high, GC of the dimethyl dicarbonate is more than equal to 99.8%, a solidification point of the dimethyl dicarbonate is 17 DEG C, such that each index of the dimethyl dicarbonate meets requirements of food additives, and the dimethyl dicarbonate is applicable for industrial production.
Owner:杭州元素添加剂科技有限公司
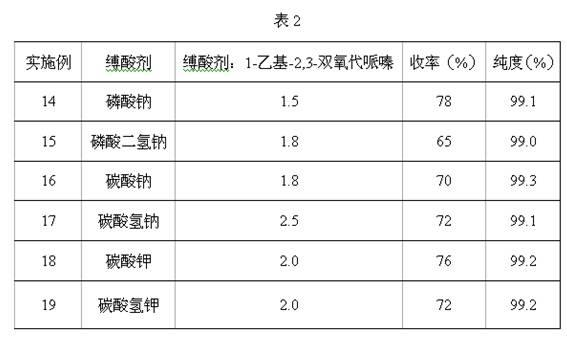
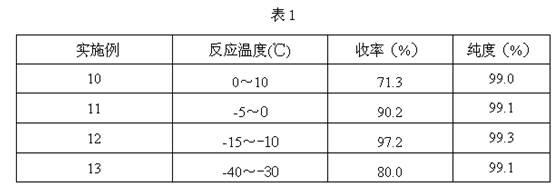
Method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate
The invention discloses a method for preparing 4-ethyl-2,3-dioxypiperazine-1-formate. In the method, 1-ethyl-2,3-dioxypiperazine and chloro-formate are used as raw materials and reacted in an organic solvent system in the presence of an acid binding agent to form 4-ethyl-2,3-dioxypiperazine-1-formate, wherein the molar ratio of the 1-ethyl-2,3-dioxypiperazine to the acid binding agent to the chloro-formate is 1:1.0-3.0:1.0-2.0; the chloro-formate is methyl chloroformate or ethyl chloroformate; and the 4-ethyl-2,3-dioxypiperazine-1-formate is 4-ethyl-2,3-dioxypiperazine-1-methyl formate or 4-ethyl-2,3-dioxypiperazine-1-ethyl formate. The method greatly reduces cost, simplifies process, reduces byproducts, improves product purity and reduces solvent separation processes; and the prepared product can be used as an intermediate for piperacillin and cefoperazone and is suitable for industrial production.
Owner:山东艾孚特科技有限公司
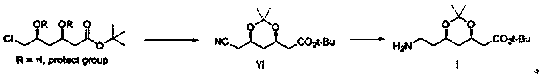
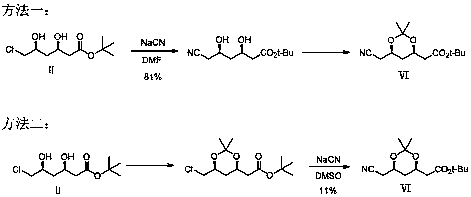
Preparation method of atorvastatin calcium intermediate
ActiveCN108586427ASuitable for industrial productionPreparation method suitable for industrialized productionOrganic chemistryHydrobromideLithium
The invention belongs to the field of medicines and chemical industry, and particularly relates to the field of pharmacy, in particular to a preparation method of an atorvastatin calcium intermediate.The preparation method comprises the following steps: firstly preparing a compound II into a lithium reagent, then reacting with methyl chloroformate, introducing an ester group, aminolyzing the ester group into amine, dehydrating the amide, and finally reducing a cyano group to obtain a target compound. By the brand-new preparation method of the atorvastatin calcium intermediate, a compound VI can be synthesized under the premise that highly toxic substances such as hydrocyanic acid, hydrobromide and the like are not used, and the compound VI is used for preparing a compound I; reagents usedin the whole preparation process are safe and environment-friendly, and the preparation method is more suitable for industrial production.
Owner:JIANGSU ALPHA PHARM CO LTD
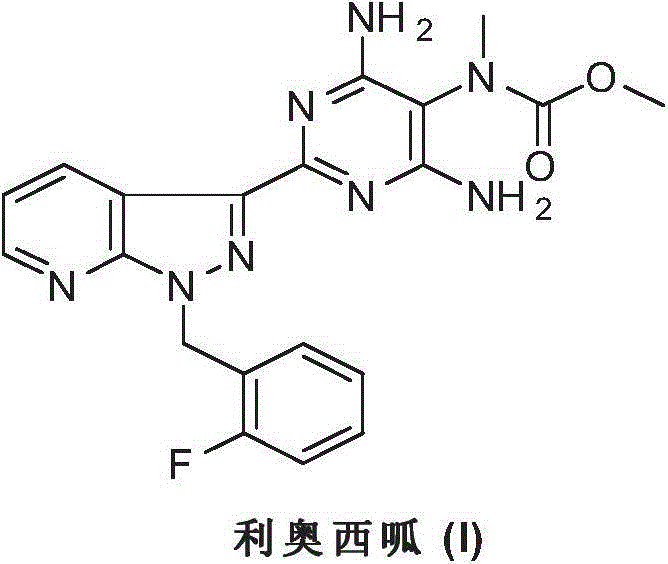
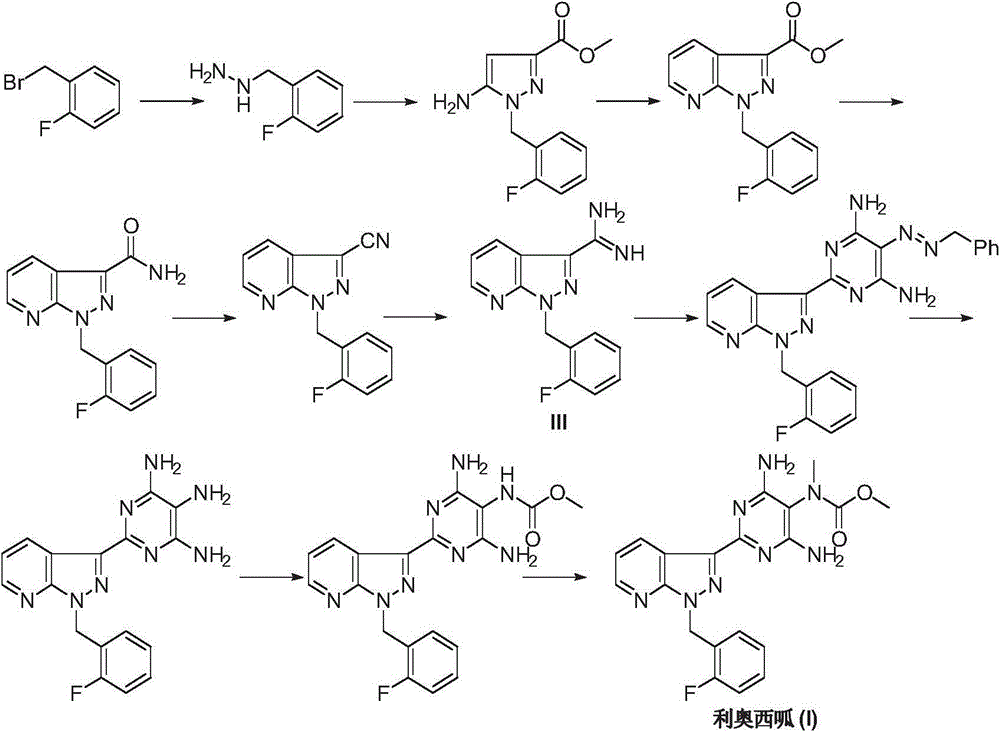
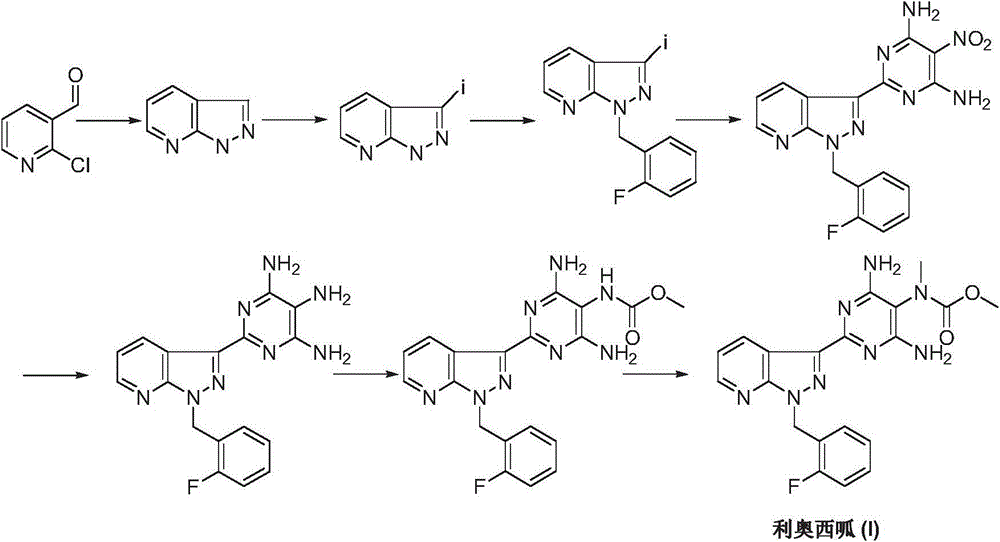
Riociguat intermediate and preparation method thereof
InactiveCN104892459AEase of industrial productionRaw materials are easy to getCarbamic acid derivatives preparationOrganic compound preparationAminomalononitrileN methylation
The invention discloses a Riociguat (I) intermediate, namely, N-methyl-N-methyl formate-2-amino malononitrile (II), and a preparation method thereof. The preparation method comprises preparation steps as follows: 2-amino malononitrile has an N-methylation reaction to prepare N-methyl-2-amino malononitrile (IV); N-methyl-2-amino malononitrile (IV) and methyl chloroformate or methyl bromoformate have an amidation reaction to prepare N-methyl-N-methyl formate-2-amino malononitrile (II). The intermediate II and 1-(2-fluorobenzyl)-1H-pyrazolo [3,4-b] pyridine-3-formamidine (III) have a cyclization reaction to prepare Riociguat (I). According to the preparation method, the raw materials are easy to obtain, the process is concise, and the preparation method is economic and environment-friendly and suitable for industrial production.
Owner:SUZHOU MIRACPHARMA TECH
Multi-stage formation method of lithium ion battery
ActiveCN112201870AImprove film formationGood synergySecondary cells charging/dischargingElectrolytic agentPhysical chemistry
The invention provides a multi-stage formation method of a lithium ion battery. The method comprises the following steps that a first electrolyte is injected into the battery,vinylene carbonate is used as an additive in the first electrolyte,charging is carried out to a first predetermined voltage, and constant-voltage charging is carried out at the first predetermined voltage; a second electrolyte is injected, an additive in the second electrolyte is methyl chloroformate, and constant-voltage charging is carried out under a second preset voltage after charging is carried out to the second preset voltage; a third electrolyte is injected, dimethyl sulfoxide serves as an additive of the third electrolyte, and constant-voltage charging is carried out under third preset voltage after chargingis carried out to reach the third preset voltage. The method is characterized in that the first, second and third predetermined voltages are respectively related to the content of additives in the first, second and third electrolyte solutions; according to the multi-stage formation method, the proper formation voltage can be accurately positioned, and the lithium ion battery with stable performance is obtained.
Owner:苏州极闪控电信息技术有限公司
N-pyridylurea chitosan quaternary ammonium salt and preparation method and application thereof
ActiveCN107383239AImprove biological activityGood water solubilityChemical inhibitorsChemical industryPyridine
The invention discloses N-pyridylurea chitosan quaternary ammonium salt and a preparation method and an application thereof. The N-pyridylurea chitosan quaternary ammonium salt takes chitosan, methyl chloroformate, aminopyridine and iodomethane as raw materials, the preparation method comprises the following steps: preparing N-methoxycarbonyl chitosan, reacting N-methoxycarbonyl chitosan and aminopyridine, reacting the obtained N-pyridylurea chitosan and iodomethane, and purifying the product to obtain the N-pyridylurea chitosan quaternary ammonium salt. The prepared N-pyridylurea chitosan quaternary ammonium salt can be used for preparing an anti-oxidant, and can be widely used in the fields of biology, medicine, food and chemical industry, the biological activity such as antioxidant activity is increased, the preparation raw materials are easily available, the preparation is simple, the condition is mild, and the preparation method establishes a base for application and exploitation for high-value chitosan.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Synthetic method for silthiopham
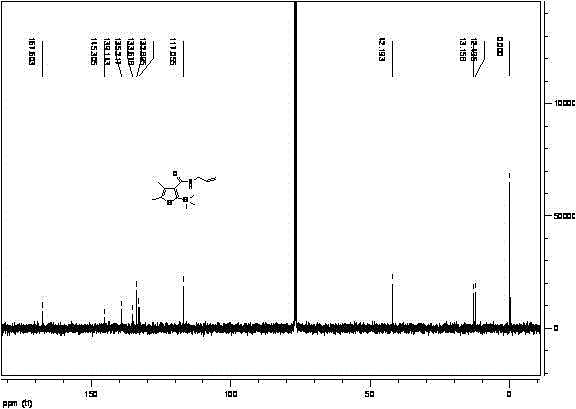
ActiveCN105111229AAvoid pollutionRaw materials are cheap and easy to getGroup 4/14 element organic compoundsOrganic baseOrganic synthesis
The invention provides a synthetic method for silthiopham, which belongs to the technical field of organic synthesis. The objective of the invention is to overcome the problems of complicated synthesis process, a great number of byproducts and severe environmental pollution of conventional synthetic methods for silthiopham. The synthetic method provided by the invention comprises the following steps: (1) with trimethylsilylacetylene as a raw material, under the protection of inert gas, reacting trimethylsilylacetylene with methyl chloroformate under the action of organic base so as to obtain methyl (trimethylsilyl)propiolate; (2) reacting methyl (trimethylsilyl)propiolate with allyl amine in a solvent under the action of a catalyst so as to obtain N-allyl-3-(trimethylsilyl)propiolamide; and (3) subjecting N-allyl-3-(trimethylsilyl)propiolamide with 3-mercapto-2-butanone to a heating reflux reaction under the action of an alkali catalyst and carrying out dehydration so as to obtain the final product silthiopham. The method has the advantages of usage of cheap and easily available raw materials, simple route, high yield and no usage of reagents severely polluting the environment, e.g., t-butyl nitrous acid and thionyl chloride.
Owner:HANGZHOU NORMAL UNIVERSITY
Method for detecting urine metabolite based on deriving method
InactiveCN101206203AEasy to operateRapid responseComponent separationPreparing sample for investigationMetaboliteGas phase
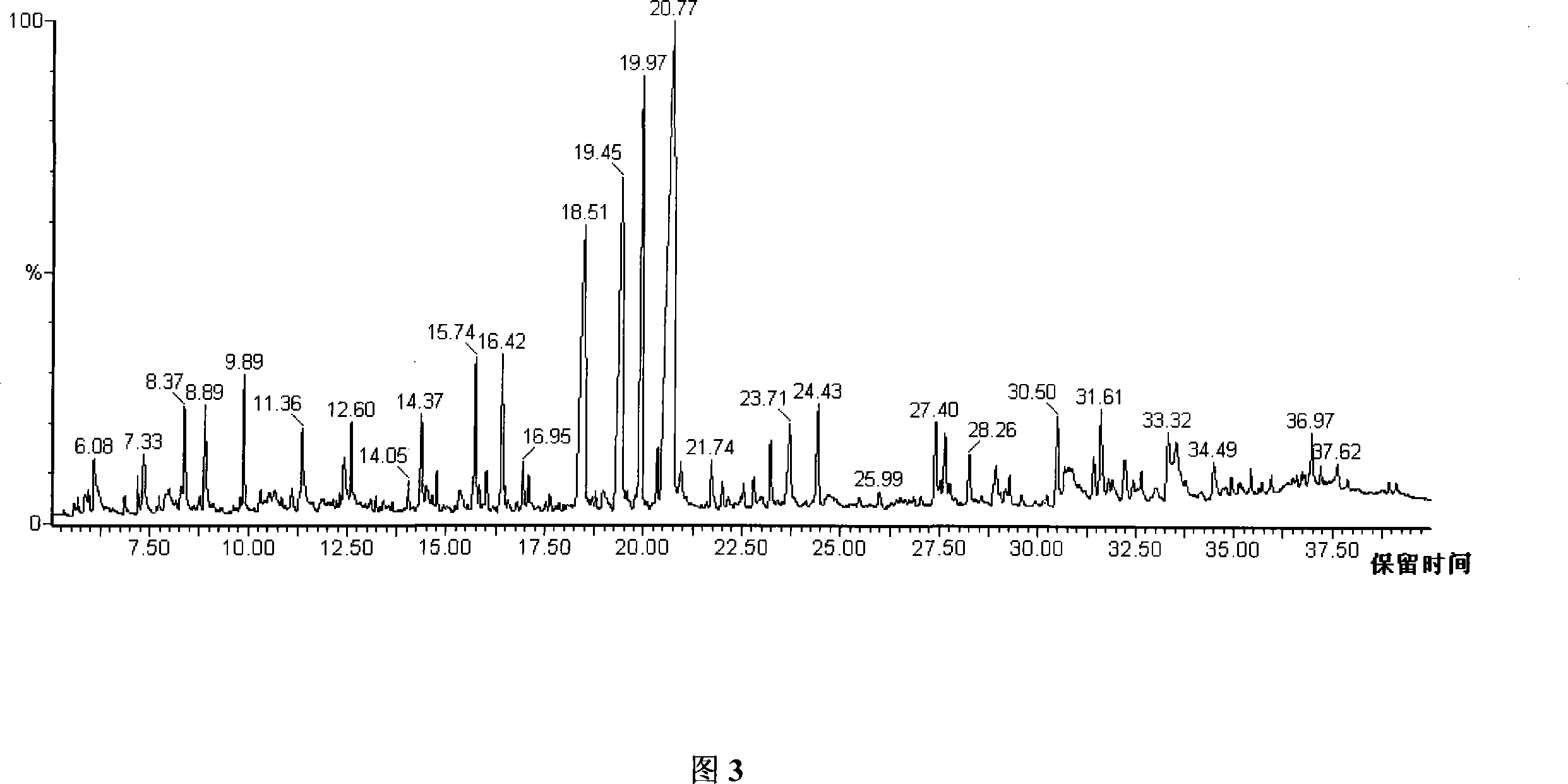
The invention relates to a measuring method of urine metabolic characteristic substance based on derivation method in the bioengineering technical field, including the following steps that: collected urine is stored after centrifugation; methanol, pyridine and methyl chloroformate are added in the urine and then derivation reaction of the mixture is carried out under the ultrasonic action of an ultrasonic instrument; chloroform is adopted to complete extraction of the derivation mixed solution and then sodium hydroxide solution is added in the mixed solution; methyl chloroformate is added in the obtained solution to be mixed with the solution once again; moreover, secondary derivation reaction is carried out under the ultrasonic action of the ultrasonic instrument; after the reaction, rotating recentrifuging of the reaction solution is carried out to eliminate the aqueous layer, and the remaining chloroform layer is dried by a little anhydrous sodium bisulphate to be used for gas phase chromatogram mass spectrum combination measuring; finally, the chloroform layer is used for gas phase chromatogram mass spectrum combination measuring. The invention has the characteristics of simple operation (capable of realizing reaction in water phase), rapid reaction (with ultrasonic reaction completed within 2 minutes), outstanding stability and high reproducibility, etc.
Owner:SHANGHAI JIAO TONG UNIV
New synthesis method for improving quality of thiophanate methyl
InactiveCN108516946AReduce generationImprove securityOrganic chemistrySynthesis methodsSodium thiocyanate
The invention relates to a new synthesis method for improving the quality of thiophanate methyl. The synthesis method specifically comprises the following steps that ethyl acetate is added into a reaction kettle as a solvent, then sodium thiocyanate is added, the reaction temperature is controlled to be about 59 DEG C, stirring is started, and methyl chloroformate is dropwise added at a medium orhigh speed; methyl chloroformate and sodium thiocyanate react in the reaction kettle and subjected to reduced pressure distillation after being centrifuged, and methyl isothiocyanate is obtained; theobtained methyl isothiocyanate is put into an enamel reaction kettle, stirring is started, o-phenylenediamine is added in the ratio of 2:1 for addition reaction, the reaction temperature is controlledto be 40-70 DEG C, and centrifugation is carried out after the reaction stops; the temperature of solid is increased to 110-160 DEG C, and thiophanate methyl is obtained after liquid-solid separation. The purity of the thiophanate methyl synthesized through the method reaches 98% or above, the reaction conversion rate reaches 95% or above, the safety is good, the temperature is easy to control, generation of pollutants is reduced, and generation of by-products is reduced.
Owner:ANHUI GUANGXIN AGROCHEM
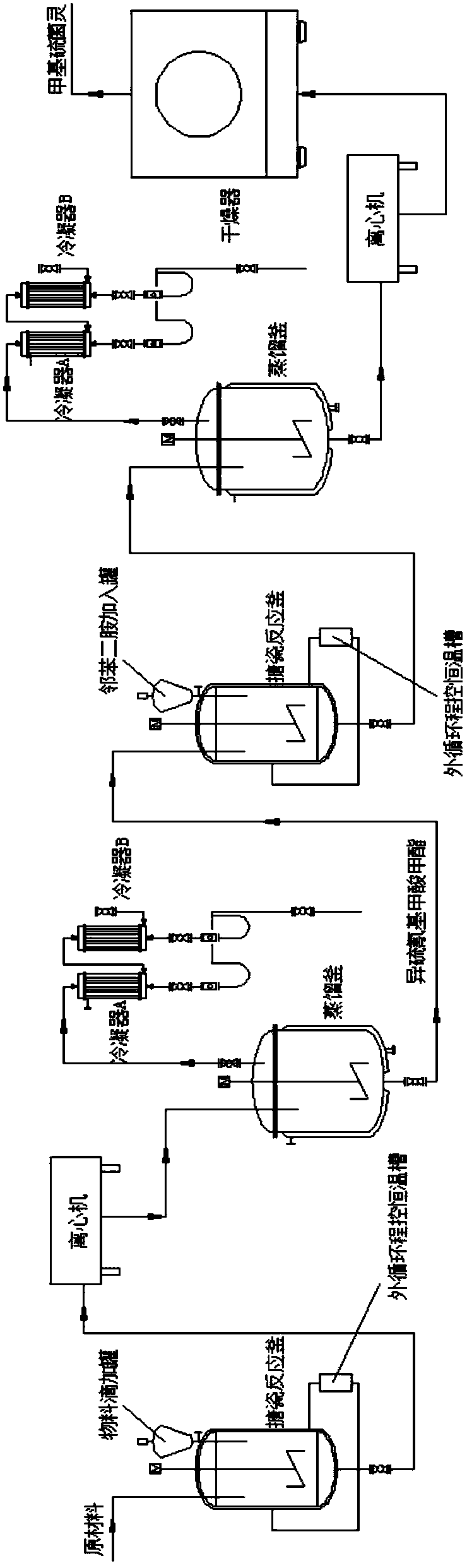
Methyl chloroformate exhaust gas treatment process
The present invention provides a methyl chloroformate exhaust gas treatment process. The process is as below: synthesis of methyl chloroformate: first adding methanol into a reaction tower, then introducing the methanol from one side of an esterification column bottom and introducing phosgene from the other side into the esterification column, keeping the molar ratio of phosgene to methanol at (1.05-1.1):1, and expelling the exhaust gas by nitrogen; a first trap: sending the exhaust gas into a trap tower through a pipe from the one side of the bottom of the trap tower, and discharging the exhaust gas from the top of the trap tower; and a second trap: sending the exhaust gas after the first trap into a trap autoclave from one side of the trap autoclave and sending into an exhaust gas damage system. A trapping system installed at the exhaust outlet of the methyl chloroformate tower comprise a trap column and a trap autoclave, so that the methyl chloroformate carried by the exhaust gas is fully captured down. According to the production data, the load of tail broken system is greatly reduced, while the yield of methyl chloroformate is increased by 5%.
Owner:ANHUI GUANGXIN AGROCHEM
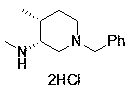
Novel method for synthesizing cis-1-benzyl-3-methylamino-4-methyl-piperidine
ActiveCN108610279AThere are no harsh conditions for feedingShorten the production cycleOrganic chemistryTwo stepMethyl chloroformate
The invention provides a novel method for synthesizing cis-1-benzyl-3-methylamino-4-methyl-piperidine. The method has the advantages that aminopyridine is used as the raw material, the original two-step arylamine methylation becomes one-step reaction, unfriendly lithium aluminum hydrogen reduction is avoided, toxic controlled product methyl chloroformate is avoided, the used raw materials are simple and easy to obtain, the method is suitable for industrial production, the generation of a large amount of aluminum-containing wastewater is reduced, production cycle is shortened evidently, and productivity is increased.
Owner:SULI PHARMA TECH JIANGYIN
Pharmaceutical composition with Meclofenoxate hydrochloride capable of preventing hydrolysis
ActiveCN1951501AAvoid hydrolysisOrganic active ingredientsNervous disorderActive componentCombinatorial chemistry
The invention relates to an alcaine methyl chloroformate compound which can avoid hydrolysis. Wherein, said compound comprises active component alcaine methyl chloroformate, findings, lubricant and stabilizer, while the stabilizer can keep the compound at weak acid. And the invention uses full powder compress method to avoid hydrolysis problem.
Owner:GUANGDONG XIANQIANG PHARMA +2
Continuous preparing technology for dimethyl dicarbonate
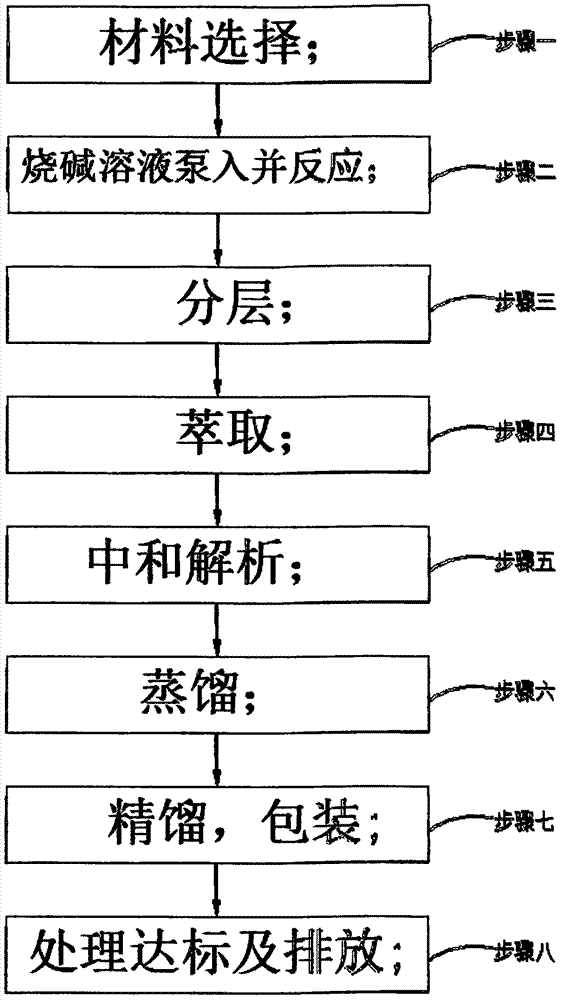
InactiveCN107188805AShort reaction timeLow costOrganic compound preparationCarbonic/haloformic acid esters purification/separationOrganic solventDistillation
The invention discloses a continuous preparing technology for dimethyl dicarbonate. The continuous preparing technology comprises the first step of material selection, the second step of caustic soda solution pumping and reacting, the third step of layering, the fourth step of extraction, the fifth step of neutralizing dissolving, the sixth step of distillation, the seventh step of rectifying and packaging and the eighth step of treatment standard reaching and discharging. In the first step, selected materials are organic solvent methylbenzene, a phase transfer catalyst and methylclhlorofonmate, and the charging molar ratio catalyst: methylbenzene: methylclhlorofonmate of the organic solvent methylbenzene to the phase transfer catalyst to the methylclhlorofonmate is 1:(50-150):(100-260). In the second step, the continuously-pumped 14%-20% caustic soda solution is subjected to the reaction at the constant temperature ranging from 0 DEG C to 20 DEG C under the situation of outer cooling. The continuous preparing technology is short in reaction time, low in cost, safe, environmentally friendly and suitable for continuous industrial production.
Owner:重庆长风生物科技有限公司
Method for the Preparation of (S)-N-Methyl-3-(1-Naphthyloxy)-3-(2-Thienyl)Propylamine Hydrochloride (Duloxetine)
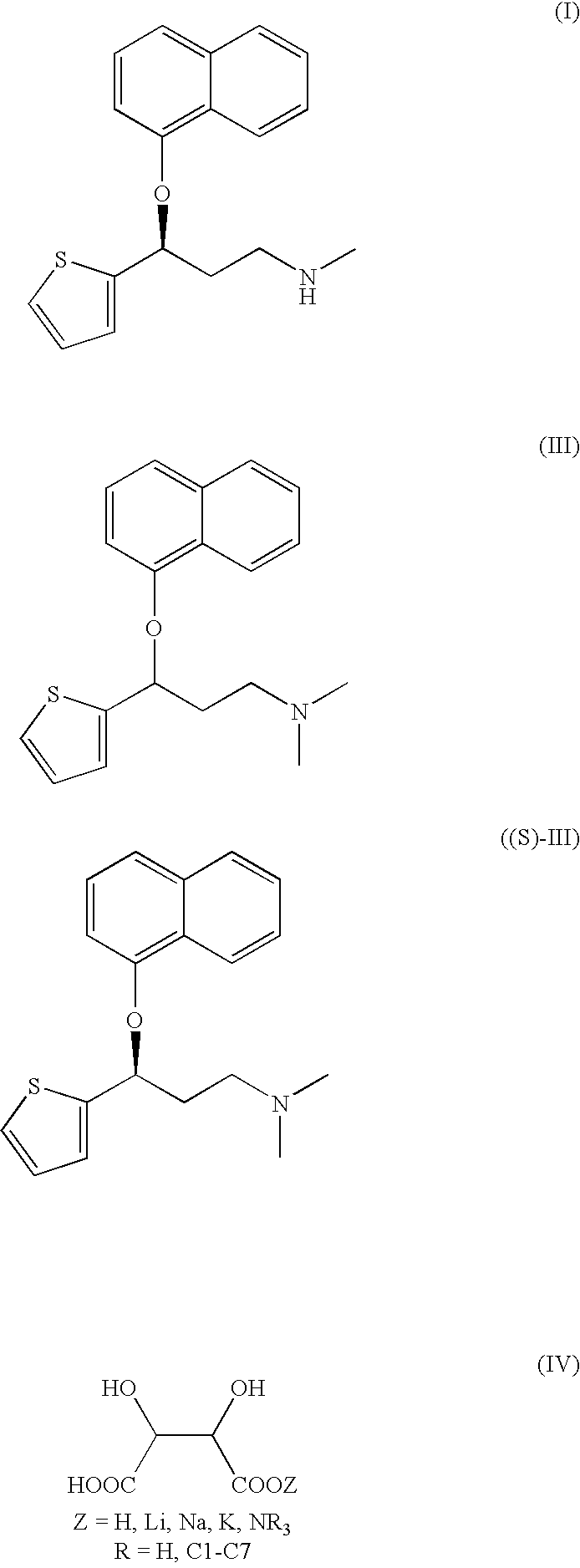
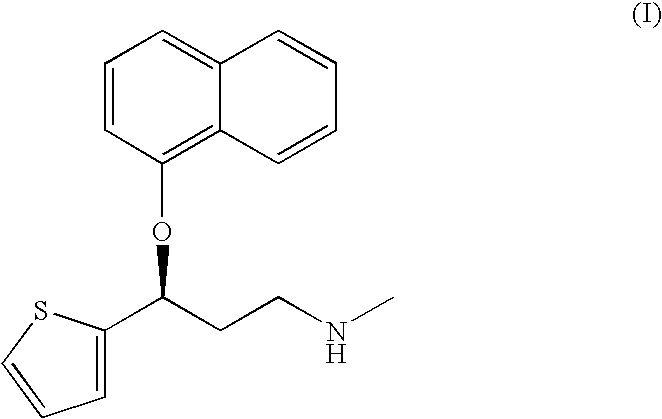
A method of preparation of (S)—N-methyl-3-(1-naphthyloxy)-3-(2-mienyl)propylamine of Formula (I) and its pharmaceutically acceptable salts, comprising a) reaction of (RS)—N,N-dimethyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine with optically active D-tartaric acid or an acid salt derived from D-tartaric acid forming a mixture of diastereoisomeric salts of N,N-dimethyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine and D-tartaric acid (2:1), b) isolation of the salt (S)—N,N-dimethyl-3-(naphthyloxy)-3-(2-thienyl)propylamine / D-tartrate (2:1) from the mixture of diastereoisomeric salts in an organic solvent, water or a mixture thereof and release of (S)—N,N-dimethyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine by action of an inorganic or organic base, c) demethylation of (S)—N,N-dimethyl-3-(1-naphthyloxy)-3-(2-thienyl)propylamine by action of an alkylchloroformate of formula ClCOOR (R=C1-C5 alkyl, or C6-C12 aryl or alkylraryl), especially phenyl, ethyl or methyl chloroformate, and d) hydrolytic release of the duloxetine base of formula I and optionally conversion of the base to a salt with the respective acid, or salt of a weak base.
Owner:ZENTIVA AS
Refining method of methyl chloroformate
InactiveCN107459458AIncrease speedSpeed up the responseCarbonic/haloformic acid esters purification/separationPreparation from phosgene or haloformatesIce waterMethyl chloroformate
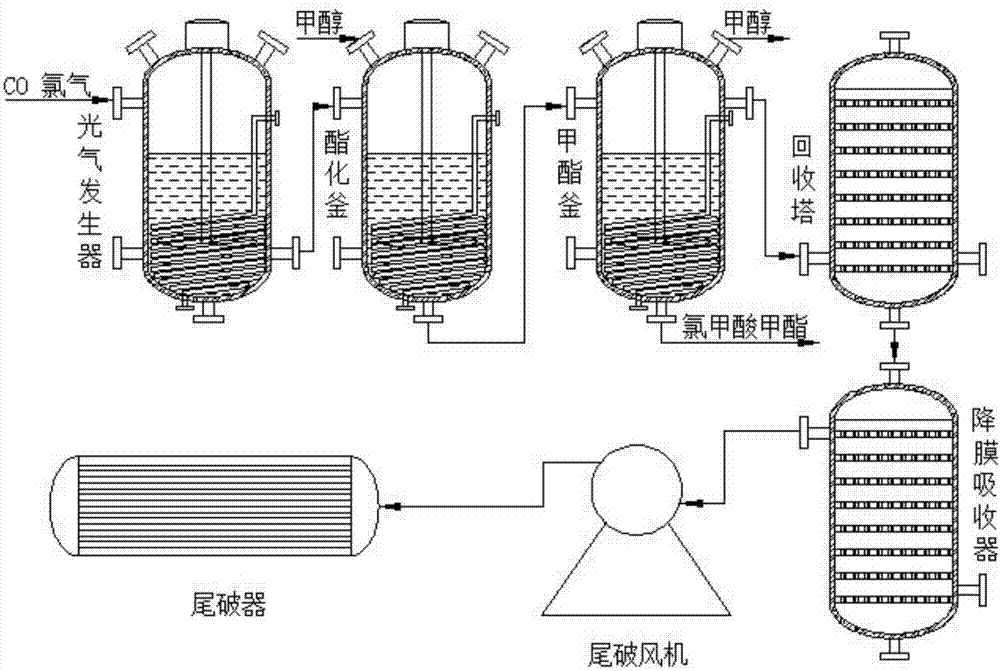
The invention discloses a refining method of methyl chloroformate. The refining method specifically comprises the following steps: introducing CO and chlorine into a phosgene generator according to the molar ratio of 1 to 1.05 and generating synthetic reaction through the phosgene generator under the catalytic action of coconut shell activated carbon to generate phosgene; introducing the phosgene and methanol into an esterification tower for esterification reaction, acyl chlorohydrolysis and gas-liquid bubbling reaction according to the molar ratio of 1 to 1.1 to synthesize the methyl chloroformate; after the completion of esterification, overflowing and transferring a methanol and methyl chloroformate reaction liquid phase from the top of a reactor, into a methyl ester kettle, performing gas expulsion after collection and refining; moving out heat released by the reaction from a shell-side refrigerant to ensure that the reaction is performed at a temperature of below 35 DEG C; pumping tail gas at the top of the reactor to a tail-breaking damage area for continuous treatment of light-containing tail gas after effective components cooled and recovered by two-stage ice water enter ice cold and low temperature methanol falling film absorption in a centralized manner. The refining method disclosed by the invention has the benefits that after the reaction of the methyl chloroformate, toxic gas containing hydrogen chloride can be treated, useful substances are effectively recovered, the synthetic cost is reduced, and the economic benefits are improved.
Owner:ANHUI DONGZHI GUANGXIN AGROCHEMICAL CO LTD
Preparation method of metharcylic acid, 2-isocyanatoethyl ester
InactiveCN103113261AReduce generationNo pollution in the processPreparation from carbamatesPtru catalystOrganosolv
The invention discloses a preparation method of a binder, namely, metharcylic acid, 2-isocyanatoethyl ester. The preparation method comprises the following steps of: (1) adding ethanol amine and an organic solvent into a reactor with a stirrer, a thermometer and a condensing tube, slowly dropping methylclhlorofonmate at 50-60 DEG C, and after dropping, continuously reacting for 2-4 hours; (2) adding a catalyst into the reactor, slowly dropping methylacryloyl at 80-100 DEG C, and after dropping, continuously reacting for 3-8 hours; (3) adding phosphorus pentachloride into the reactor to react at 50-100 DEG C and reacting for 48-72 hours; and (4) carrying out solid-liquid separation on the reactant obtained in step (3), depressurizing and distilling the filtrate, and collecting the fraction so as to obtain the metharcylic acid, 2-isocyanatoethyl ester. By utilizing the method, the generation of toxic phosgene in the production process is greatly reduced, so that potential safety hazard is reduced; the total yield is over 85%; and the whole process is generally free of environmental pollution.
Owner:ZHANGJIAGANG HICOMER CHEM CO LTD
Preparation process of amino acid N-carboxylic acid anhydride
InactiveCN112898219AQuality assuranceReduce pollutionOrganic chemistryReaction temperatureCombinatorial chemistry
The invention provides a preparation process of amino acid N-carboxylic acid anhydride, which comprises the following operations: amino acid and methyl chloroformate are used as starting raw materials to react under an alkaline condition to generate N-methoxycarbonyl-amino acid, and the N-methoxycarbonyl-amino acid is separated and purified under an acidic condition; dissolving the purified and dried N-methoxycarbonyl-amino acid in a solvent, mixing with a ring closing reagent thionyl chloride, and reacting at a certain reaction temperature to generate an amino acid N-carboxylic acid anhydride crude product; and adding a good solvent into the obtained amino acid N-carboxylic acid anhydride crude product, filtering to remove impurities, adding a poor solvent into the filtrate, recrystallizing under freezing, and drying to obtain the amino acid N-carboxylic acid anhydride (NCA). The preparation process is simple and convenient in method, easy to purify, stable in process, controllable in quality, low in anhydrous requirement on a reaction system, greatly improved in product yield, greatly reduced in reaction risk and suitable for industrial mass production.
Owner:JINGCHU UNIV OF TECH
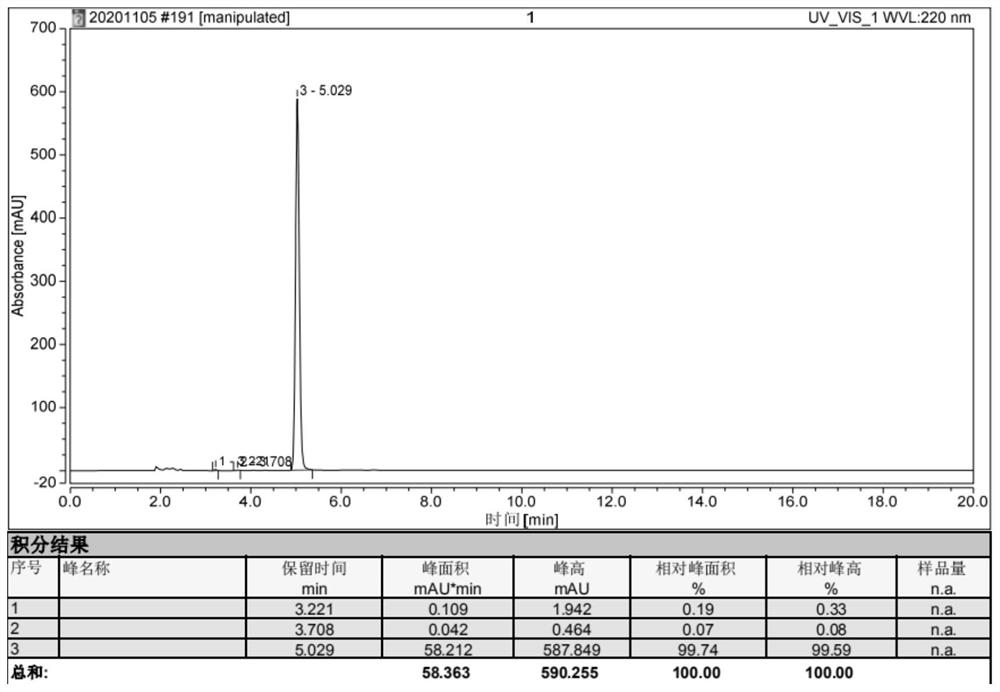
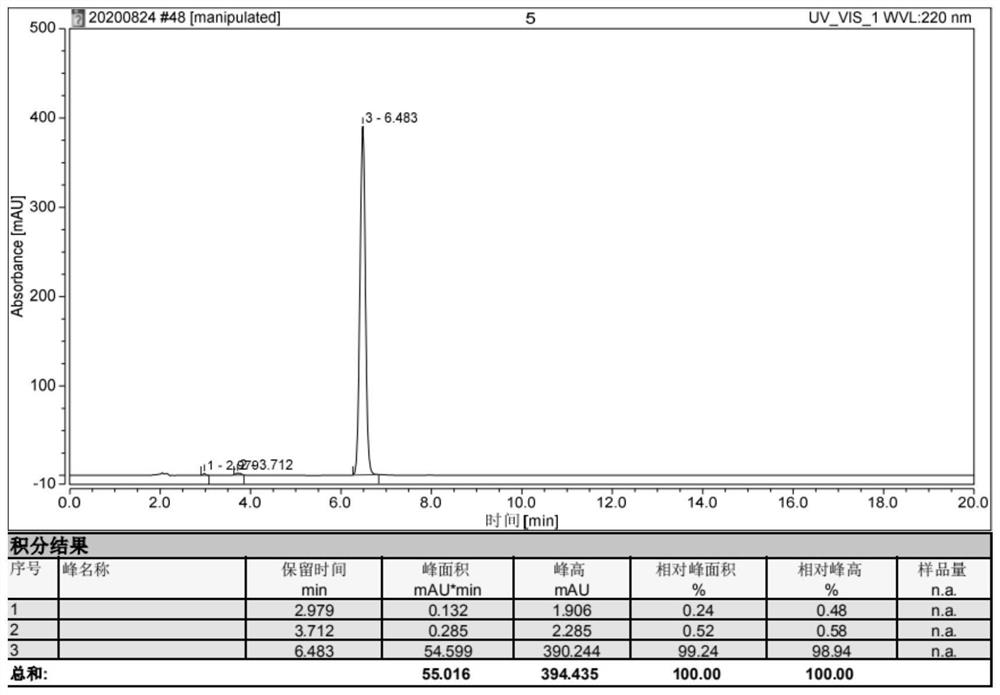
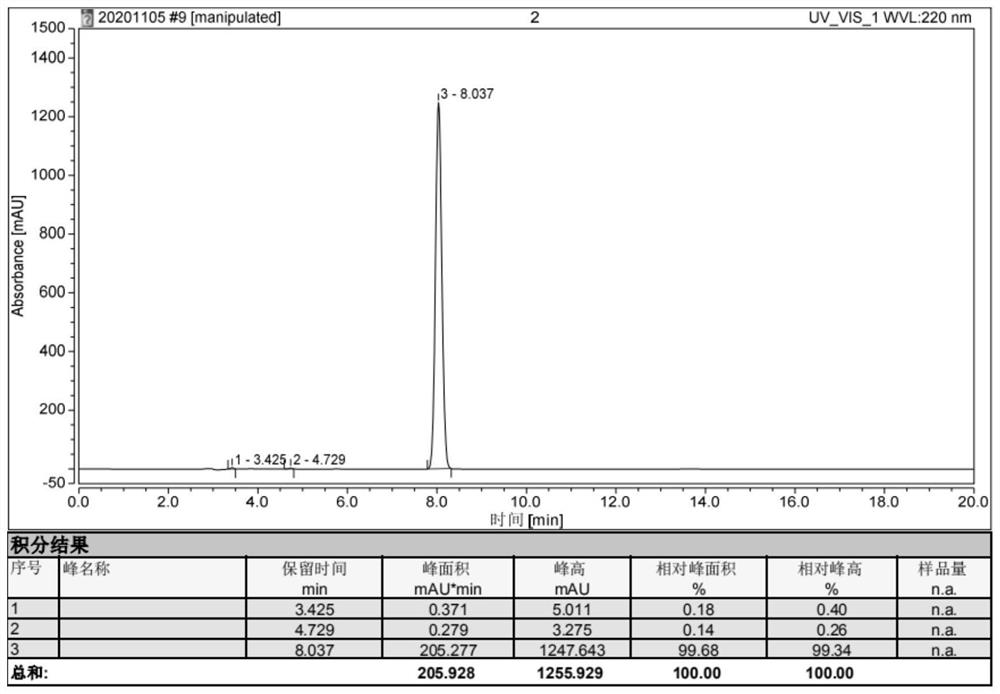
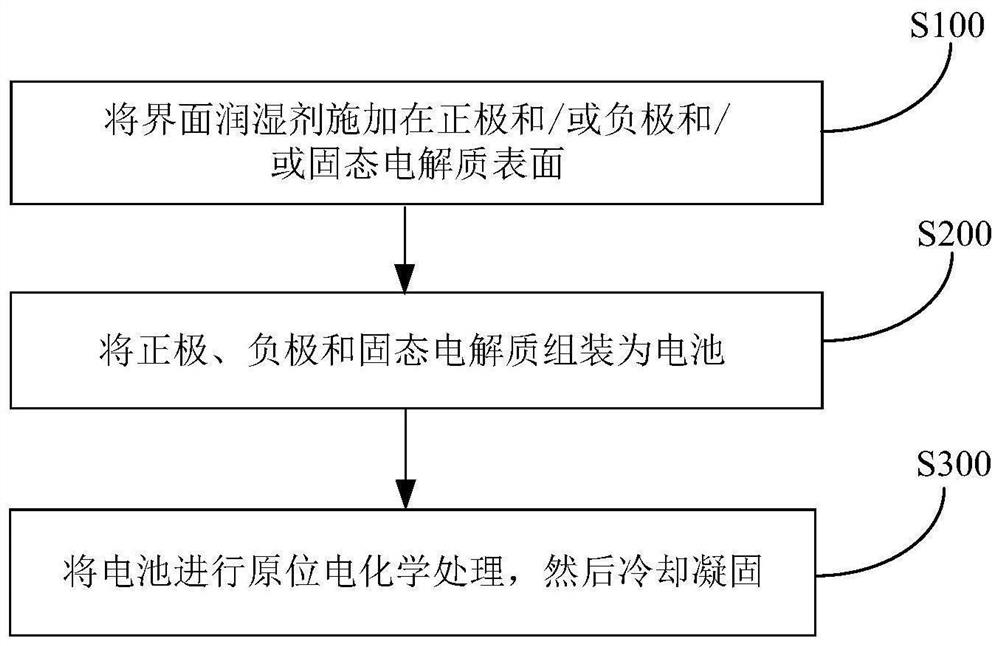
Interface wetting agent and preparation method and application thereof
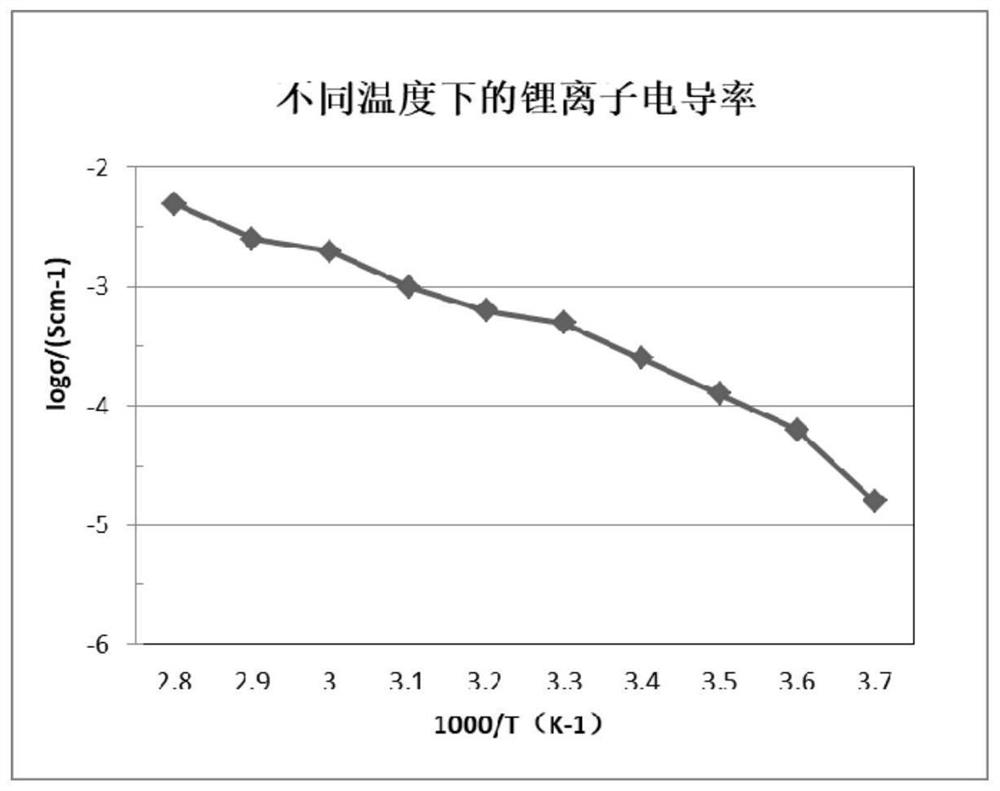
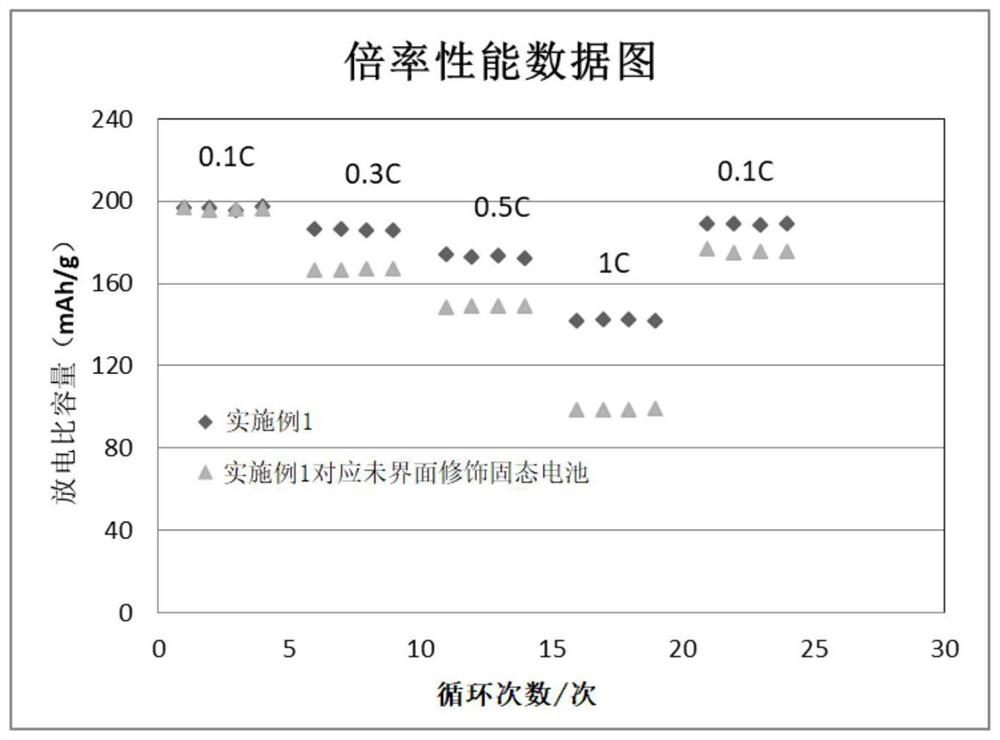
InactiveCN112002951AHigh lithium ion conductivityGuaranteed uniformityFinal product manufacturePropulsion by batteries/cellsPhosphorous acidMethyl carbonate
The invention discloses an interface wetting agent and a preparation method and application thereof. The interface wetting agent comprises a plastic crystal compound, inorganic lithium salt, a first additive and a second additive, wherein the first additive comprises at least one of ethyl carbonate / propylene ester, dimethyl carbonate / ethyl carbonate, ethyl methyl carbonate, trifluoropropenyl esterand lithium difluorophosphate; the second additive comprises at least one from lithium carbonate, trifluoromethyl phosphorous acid, methyl chloroformate, bromo-butyrolactone, fluoroacetate ethane, fluoroethylene carbonate, vinylene carbonate, vinylethylene carbonate, vinyl acetate, styrene carbonate, ethyl aminocarbonate, methyl aminocarbonate, 1, 3-dioxolane, 1, 4-dioxane, 1, 2-bis (cyanoethoxy)ethane, ethylene sulfate and dimethyl sulfite. The interface wetting agent has the advantages of high lithium ion conductivity and better electrochemical stability, the interface resistance of the solid-state battery can be effectively reduced, and the multiplying power and the cycle performance of the solid-state battery are improved.
Owner:KUNSHAN BAOTRON NEW ENERGY TECH CO LTD
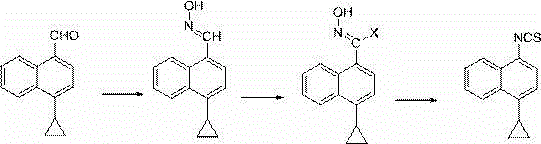
A method for synthesizing 4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione
The present invention discloses a method for synthesizing 4-(4-cyclopropylnaphthalen-1-yl)-1H-1,2,4-triazole-5(4H)-thione. The method includes the following steps: using 4-cyclopropyl-1-naphthylamine (formula A) as a starting reactant, reacting the formula A with carbon disulfide to generate 4-cyclopropyl-1-naphthylamino dithiocarbamate (formula B) under a organic alkaline condition, reacting the formula B with bis (trichloromethyl) carbonate (BTC) or acylating reagents like ethyl chloroformate and methyl chloroformate, etc. to generate 4-cyclopropyl-1-naphthylamino dithiocarbamate chloro-carbonic acid anhydride, conducting decomposition reaction to the resulting product without separation and purification to produce 4-cyclopropyl-1-naphthyl isothiocyanate. The method uses carbon disulfide instead of thiophosgene with greater toxicity, and provides simple process and stable reaction. Furthermore, raw materials are readily available, and industrialization is easy to be realized with a total recovery of more than 65%.
Owner:ANHUI WANBANG MEDICAL TECH
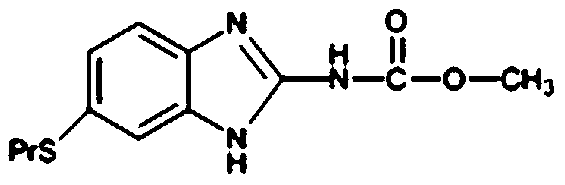
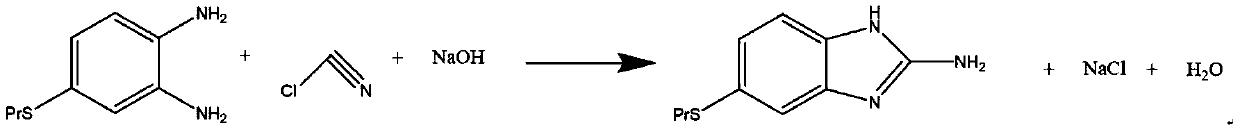
Preparation method of albendazole
The invention discloses a preparation method of albendazole, which comprises the following steps of: dissolving 4-propylthio-o-phenylenediamine in methanol, cooling, keeping the temperature at 0-5 DEGC, slowly dropwise adding cyanogen chloride for reaction under the condition of uniform stirring, and regulating and keeping the pH value at 4-5 in the process; heating to 35-45 DEG C after dropwiseadding is finished, carrying out heat preservation for 1-2 hours, then carrying out atmospheric distillation to recover methanol to obtain a solid-liquid mixed aqueous solution, and carrying out cooling, filtering and drying to obtain 6-propylthio-2-amino-1-hydrogen-benzo [d] imidazole; adding the obtained product into methanol, stirring, cooling, maintaining the temperature at 5-10 DEG C, slowlydropwise adding methyl chloroformate, and adjusting and maintaining the pH value at 6-7; after dropwise adding is finished, heating to 35-45 DEG C, keeping the temperature for 1-2 hours, distilling atnormal pressure to recover methanol, cooling to 15-20 DEG C, and performing filtering and drying to obtain an albendazole product. The method has the advantages of mild process conditions, low cost of used raw materials, water saving, strong reaction activity, good selectivity, high product yield and good product quality.
Owner:SHANDONG GUOBANG PHARMA +1
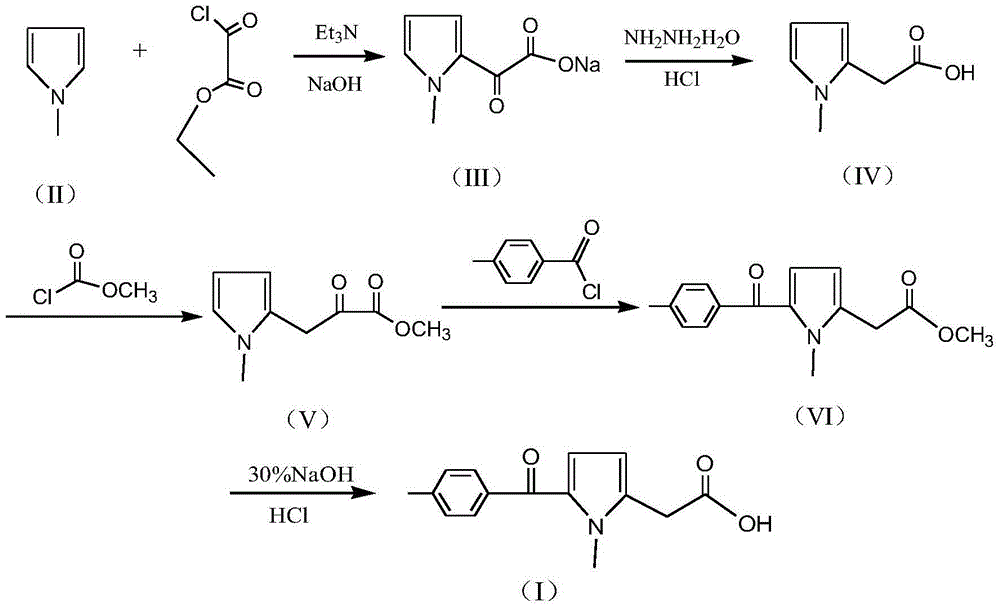
A kind of preparation method of non-steroidal anti-inflammatory drug tolmetine
InactiveCN103435527BAvoid pollutionAvoid environmental problemsOrganic chemistrySodium acetateTolmetin
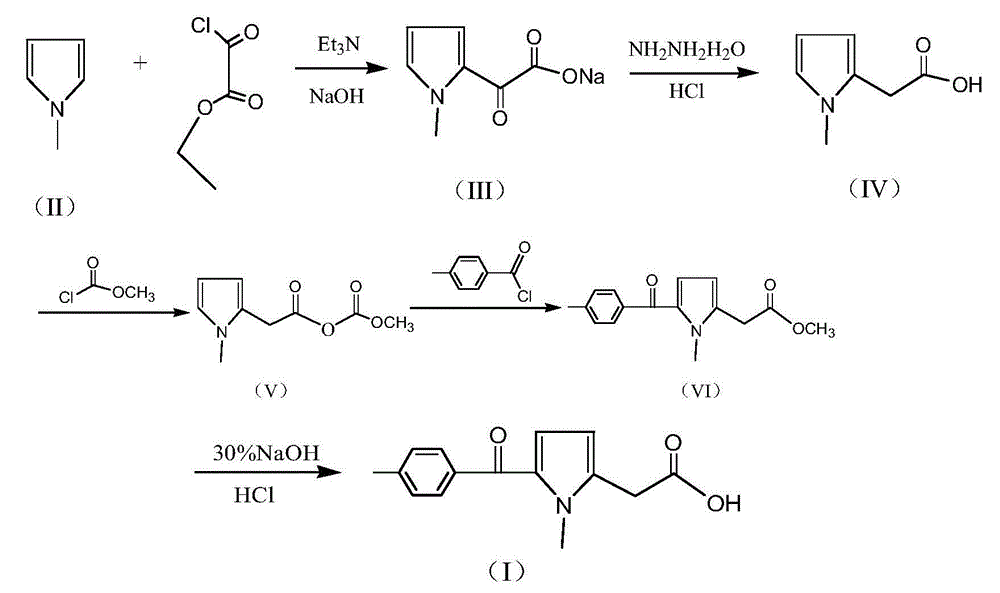
The invention discloses a preparation method of non-steroid anti-inflammatory drug tolmetin. The preparation method comprises the steps of: reacting N-methylpyrrole (II) with oxalyl chloride mono-ethyl ester in the presence of solvents, namely methyl benzene and acid binding agent, to obtain (1-methyl-1H-pyrrole-2-radical)-oxo-sodium acetate (III); by reduction with hydrazine hydrate, acidizing hydrochloric acid to form N-methylpyrrole-2-acetic acid (IV); condensing with methyl chloroformate at low temperature to produce 3-(1-methyl-1H-pyrrole-2-radical)-2-oxo-methyl propionate (V); condensing with p-toluoyl chloride to produce (1-methyl-5-(4-methyl-benzoyl)-1H-pyrrole-2-radical)-methyl acetate; and reacting with a sodium hydroxide solution to obtain a tolmetin product. Rare precious and dangerous raw materials are replaced by common safe raw materials, serious pollution problems and production operation problems are avoided, and meanwhile the production cost is reduced remarkably; the preparation method is suitable to large-scale industrial production.
Owner:ZHANGJIAGANG XINYI CHEM
Anti-influenza virus compound as well as preparation method and application thereof
ActiveCN108299316AOrganic active ingredientsOrganic chemistry methodsEthyl esterMethyl chloroformate
The invention relates to an anti-influenza virus compound as well as a preparation method and an application thereof, and belongs to the technical field of pharmaceutical synthesis. The compound contains oseltamivir and favipiravir structures and has the structure shown in the formula II in the description. The method comprises the following steps: oseltamivir and methyl chloroformate are subjected to condensation reaction, a compound (I) (3R,4R,5S)-4-acetamido-5-[chloromethoxycarbonyl]amino-3-(1-propoxyethyl)- -1-cyclohexane-1-carboxylic ethyl ester is obtained; the compound (I) and favipiravir are linked by nucleophilic substitution reaction, and a target compound (II) is obtained. The compound (II) has different degrees of inhibiting effects on H5N2, H5N6 and H5N8, and has better antiviral activity on H5N2 in vitro. Therefore, the invention also provides the application of the compound shown in the formula II in preparation of anti-influenza virus medicines.
Owner:SHANDONG UNIV
Mildew proof and insect prevention sterilizing agent for bamboo wood vine willow herb
InactiveCN109760162AImprove efficiencyEasy to useBiocideSilicon halogen compoundsMethyl chloroformateChlorothalonil
The invention discloses a mildew proof and insect prevention sterilizing agent for a bamboo wood vine willow herb. The mildew proof and insect prevention sterilizing agent for the bamboo wood vine willow herb is prepared from the components in percentage by weight: 15%-25% of methylene dithiocyanate, 25%-30% of carbendazim, 20%-30% of chlorothalonil and 20%-30% of sodium fluosilicate, the mildew proof and insect prevention sterilizing agent for the bamboo wood vine willow herb is prepared from the components in percentage by mass: 20% of methylene dithiocyanate, 30% of carbendazim, 25% of chlorothalonil and 25% of sodium fluosilicate, the carbendazim is prepared from the raw materials: a single cyanamide water solution, methyl chloroformate, sodium hydroxide, o-phenylenediamine and concentrated hydrochloric acid, the carbendazim with a high specification is prepared, and a preparation method of the chlorothalonil comprises the step that metric isophthalonitrile is put into a vaporizerfor vaporization. The sterilizing agent is prepared by adopting the raw materials of high-purity methylene dithiocyanate, carbendazim, chlorothalonil and sodium fluosilicate, the mildew proof and insect prevention high efficiency can be better ensured, the long-time mildew proof can be ensured, pests are killed thoroughly, so that the using effect of the mildew proof and insect prevention sterilizing agent for the bamboo wood vine willow herb is better ensured, and the efficiency of the mildew proof and insect prevention sterilizing agent for the bamboo wood vine willow herb is better ensured.
Owner:深圳市至霸化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com