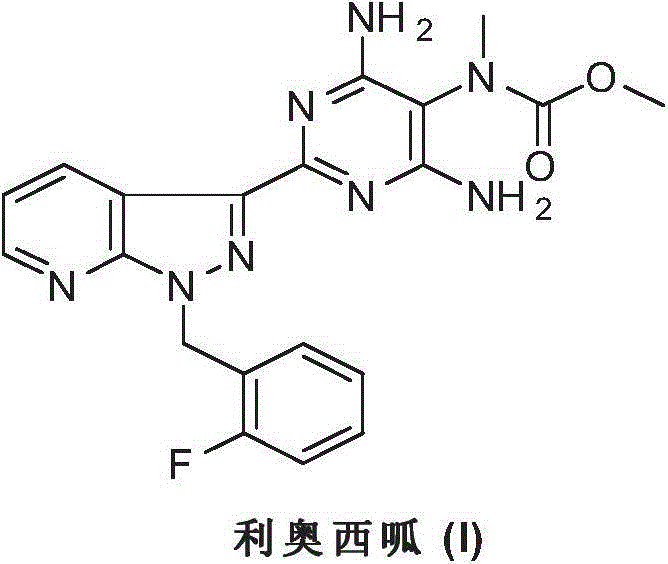
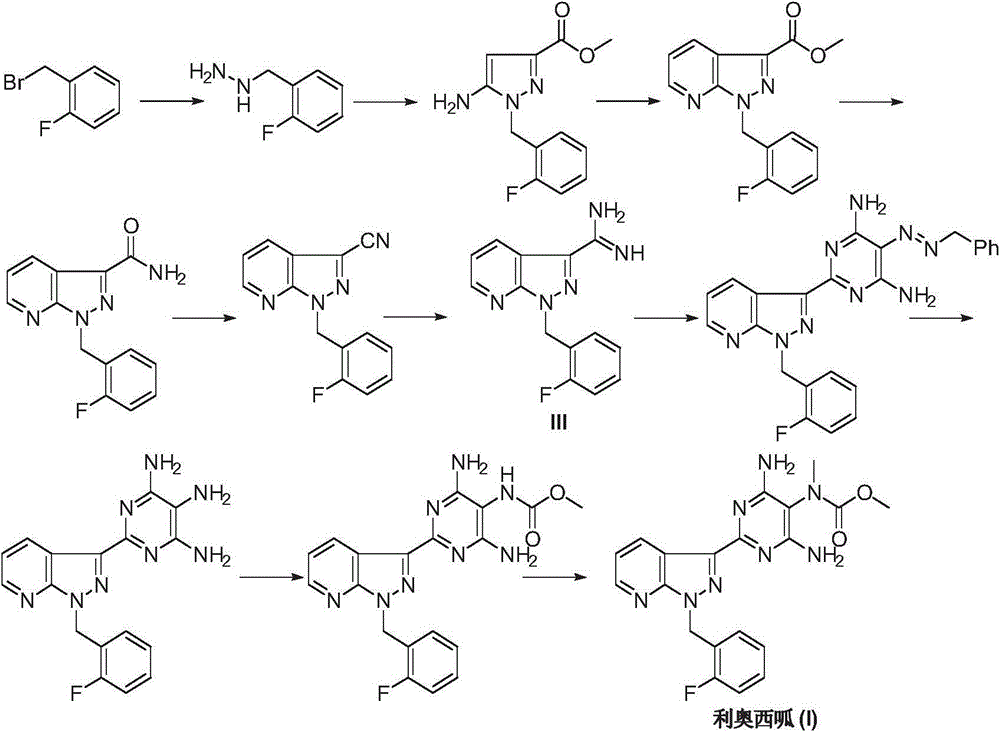
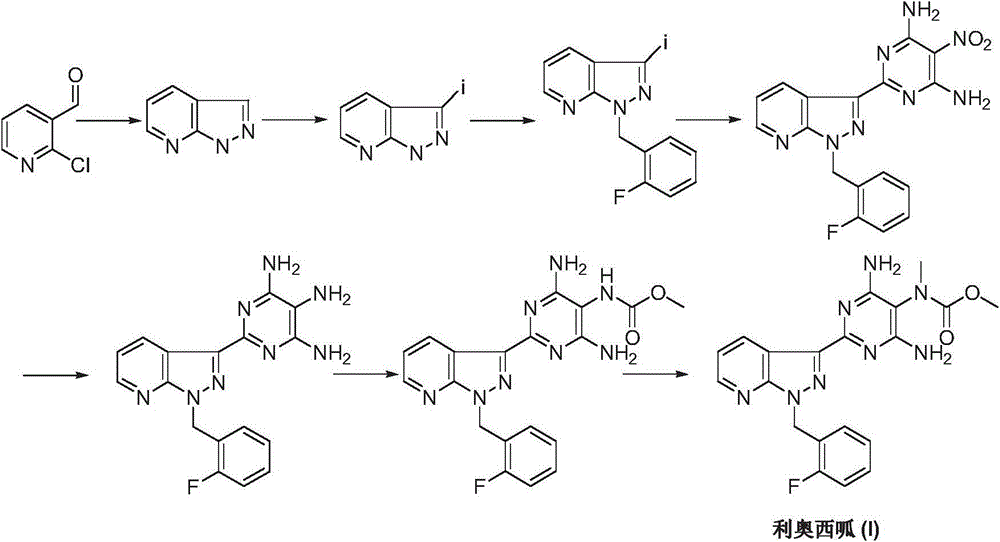
Riociguat intermediate and preparation method thereof
A technology of methyl and aminomalononitrile, which is applied in the design of organic synthesis routes and the preparation of raw materials and intermediates, can solve the problems of rare raw materials, many steps, and inconsistencies, and achieve easy-to-obtain raw materials, simple processes, and promote The effect of development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add 2-aminomalononitrile (IV) (8.1 g, 0.1 mol), dimethyl sulfate (25.2 g, 0.20 mol) and 150 mL of toluene into the reaction flask, raise the temperature to 35-45 ° C, and stir until the system is uniformly dissolved. Potassium carbonate (27.6 g, 0.2 mol) was added in batches, and the reaction was continued to stir at the temperature for 3 hours, and the reaction was detected by TLC. The solvent was recovered under reduced pressure, the residue was recrystallized with hydrochloric acid / methanol, and dried in vacuo to obtain the hydrochloride salt of N-methyl-2-aminomalononitrile as a pale yellow solid. Add the solid to ethyl acetate, adjust to alkalinity with 10% by weight sodium bicarbonate, separate the organic phase, dry over anhydrous sodium sulfate, and concentrate under reduced pressure to obtain light brown liquid N-methyl-2-amino Malononitrile (V) 8.1g, yield 85.3%; EI-MS m / z: 96[M+H] + .
Embodiment 2
[0038] Add 2-aminomalononitrile (IV) (8.1g, 0.1mol), anhydrous potassium carbonate (41.3g, 0.3mol) and N,N-dimethylformamide (150mL) in a dry reaction flask, and Methane iodide (28.4 g, 0.2 mol) was slowly added dropwise, and the reaction was continued for 3-5 hours after the addition was complete, and the reaction was detected by TLC. Concentrate under reduced pressure, and the residue is post-treated as in Example 1 to obtain 8.2 g of N-methyl-2-aminomalononitrile (V), with a yield of 87.4%.
Embodiment 3
[0040] Add 2-aminomalononitrile (IV) (8.1g, 0.1mol) and 50mL of formic acid into the reaction flask, raise the temperature to 45-55°C, stir the reaction for 5-6 hours, recover the excess formic acid under reduced pressure, and wash the residue with tetrahydrofuran Dissolve, cool to 0-5°C, add sodium borohydride (7.6g, 0.2mol) three times, heat up to reflux, react for 4-6 hours, TLC detects that the reaction is complete. Cool to room temperature, quench the reaction with methanol, concentrate under reduced pressure, and carry out the post-treatment of the residue as in Example 1 to obtain 7.7 g of N-methyl-2-aminomalononitrile (V), with a yield of 81.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com