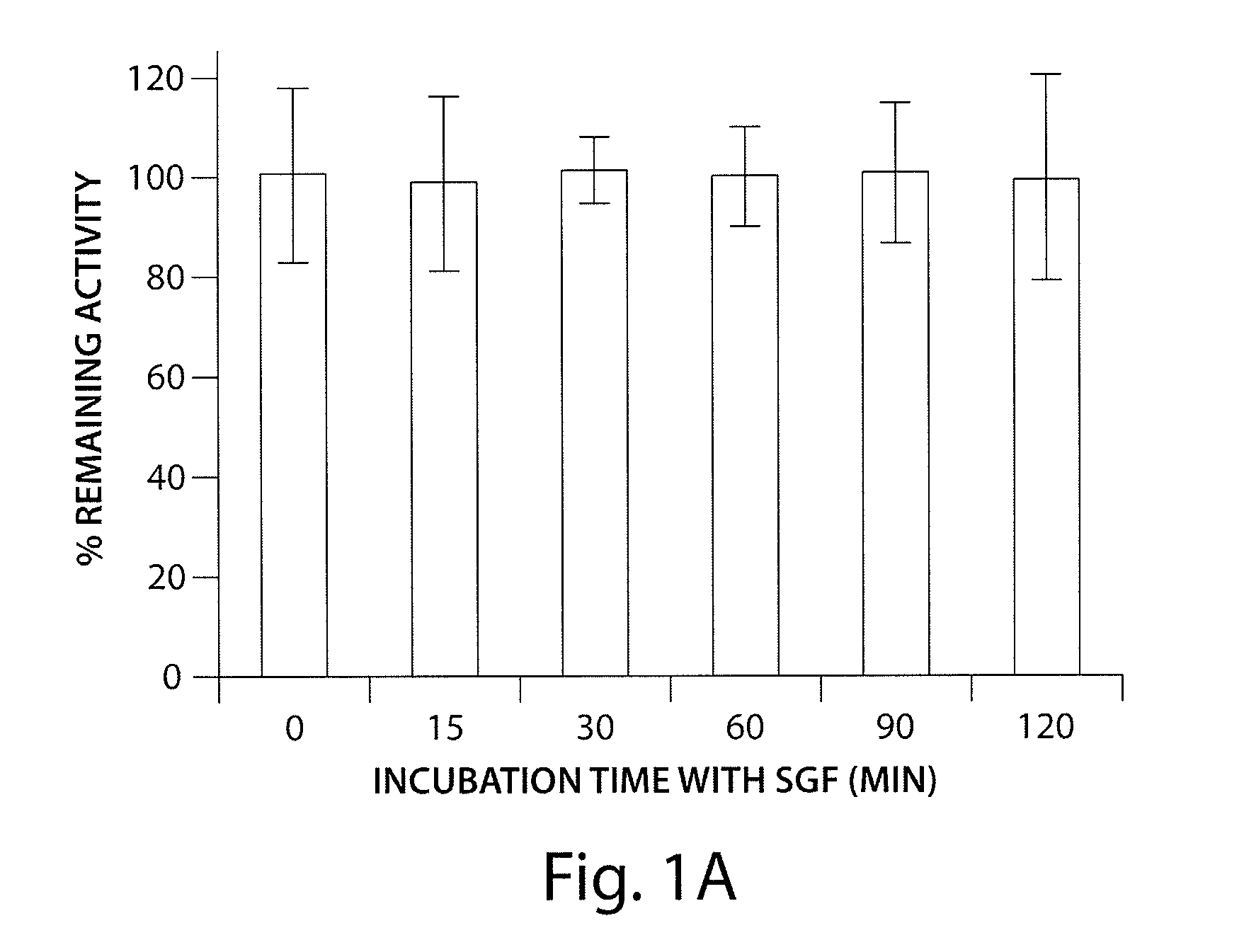
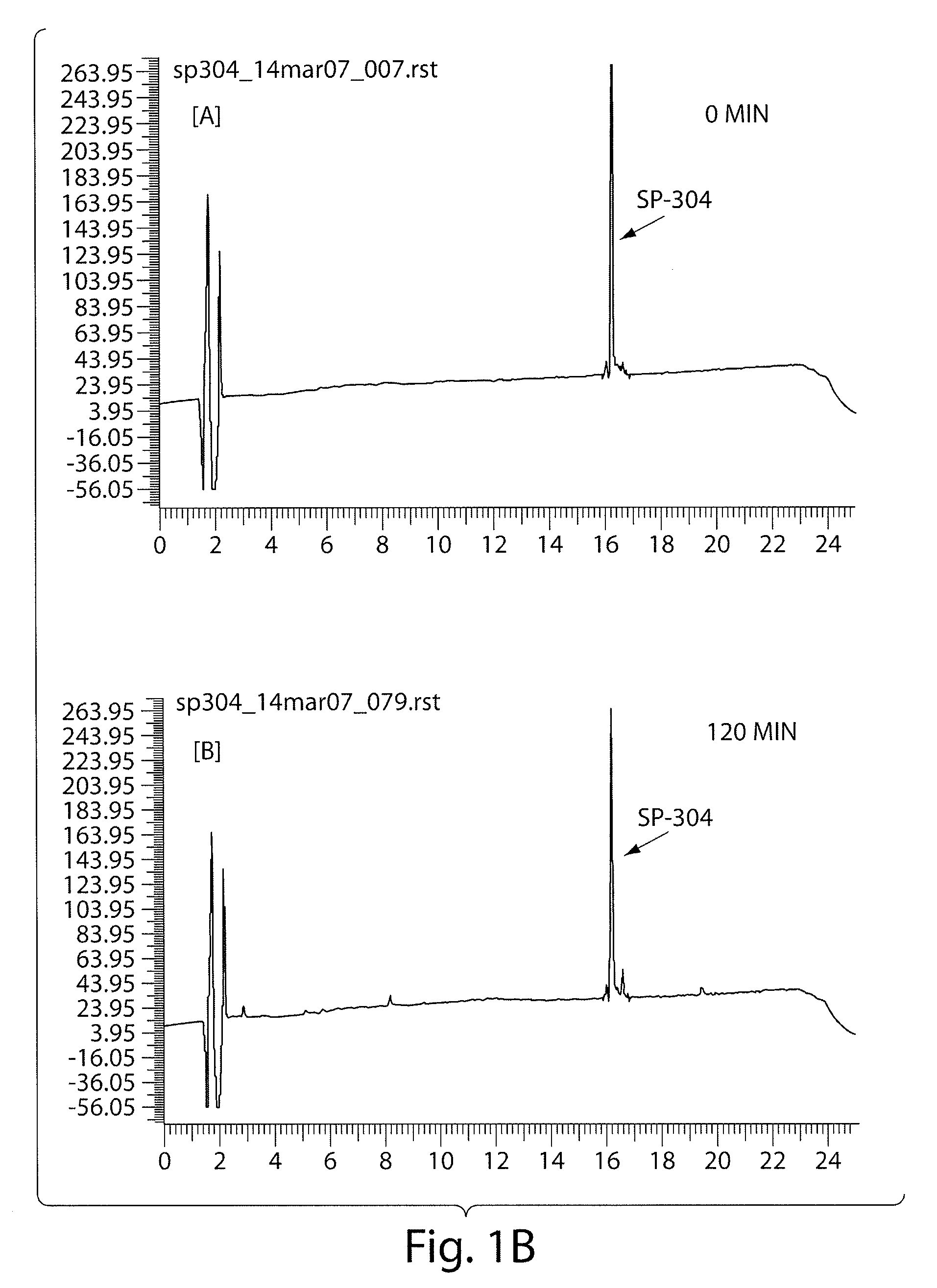
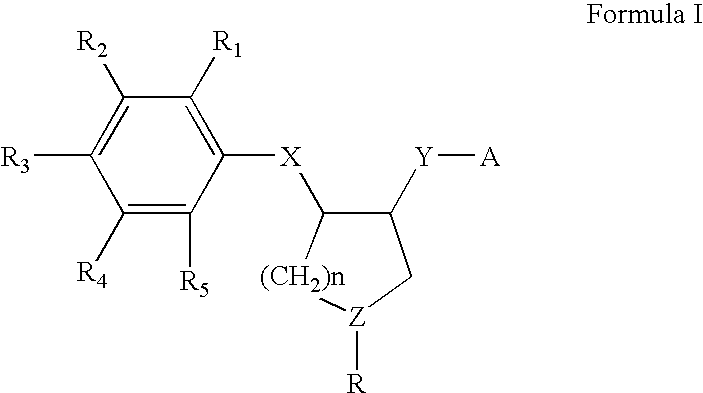
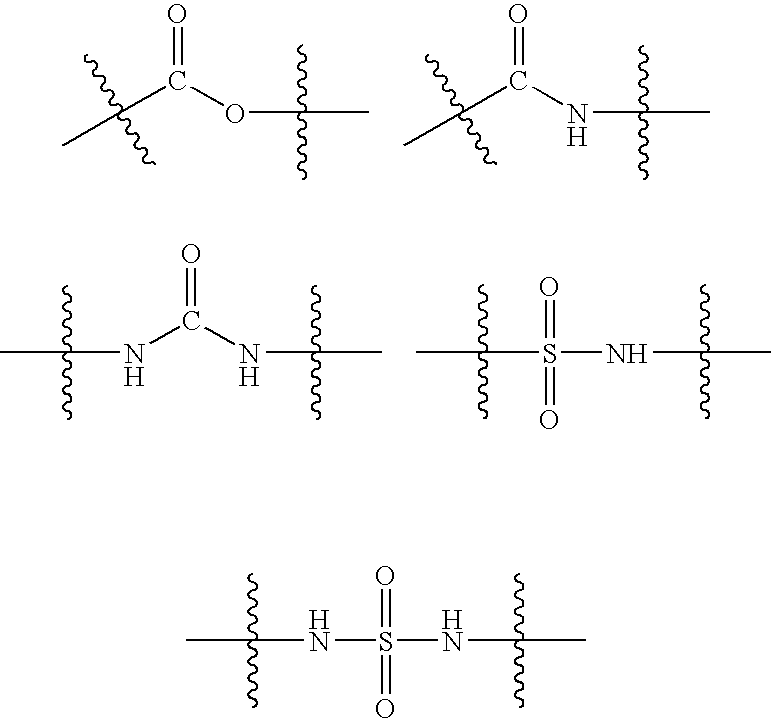
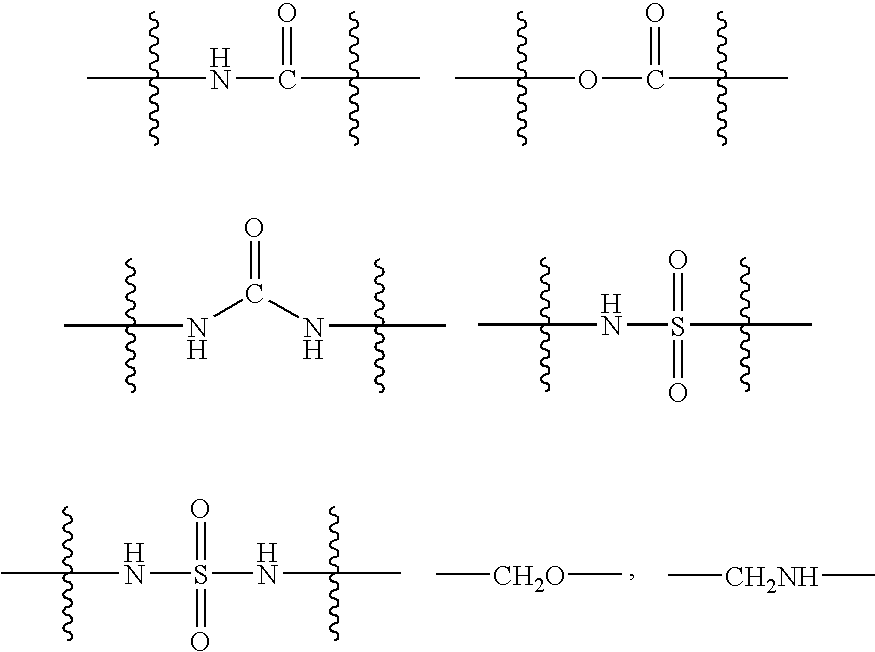
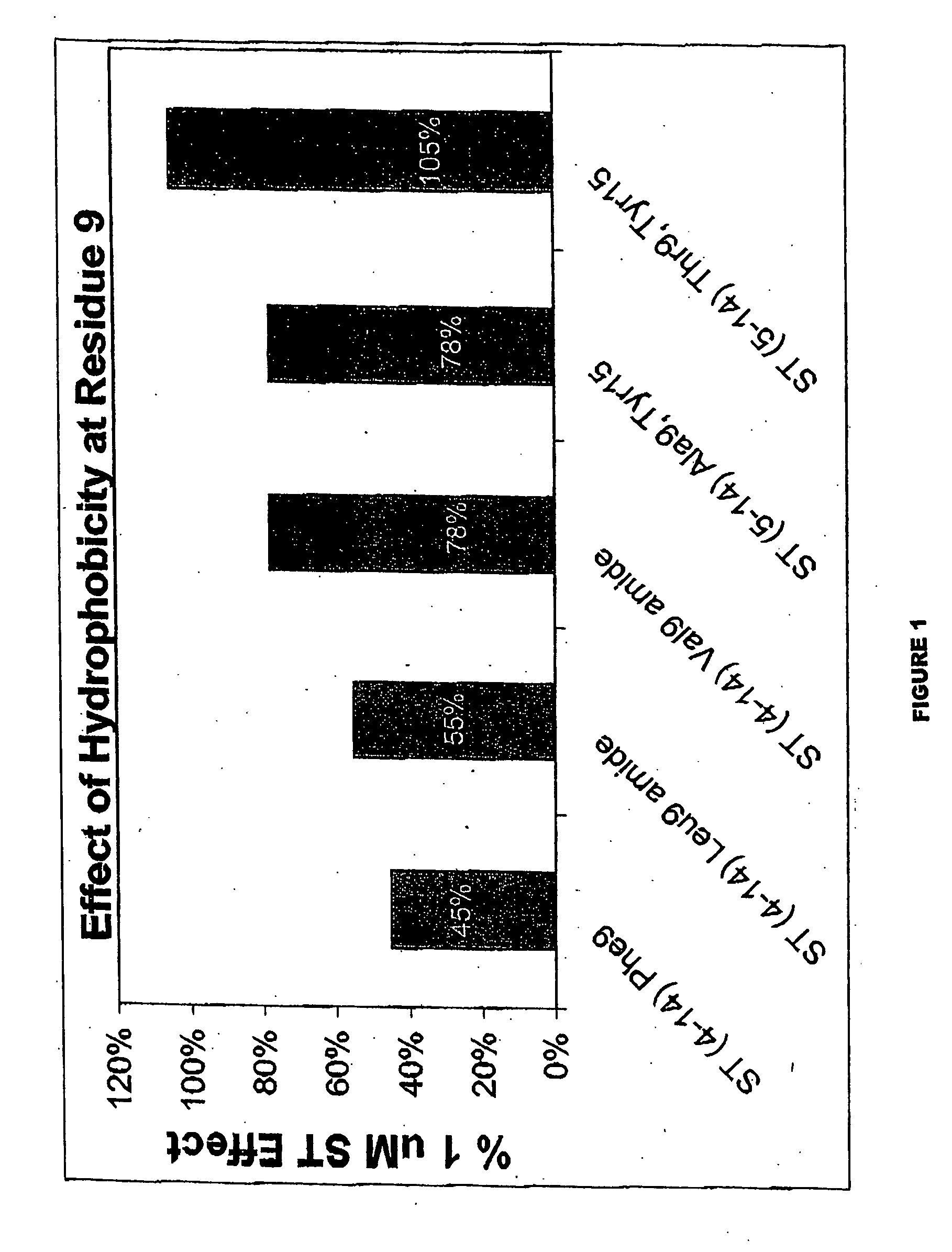
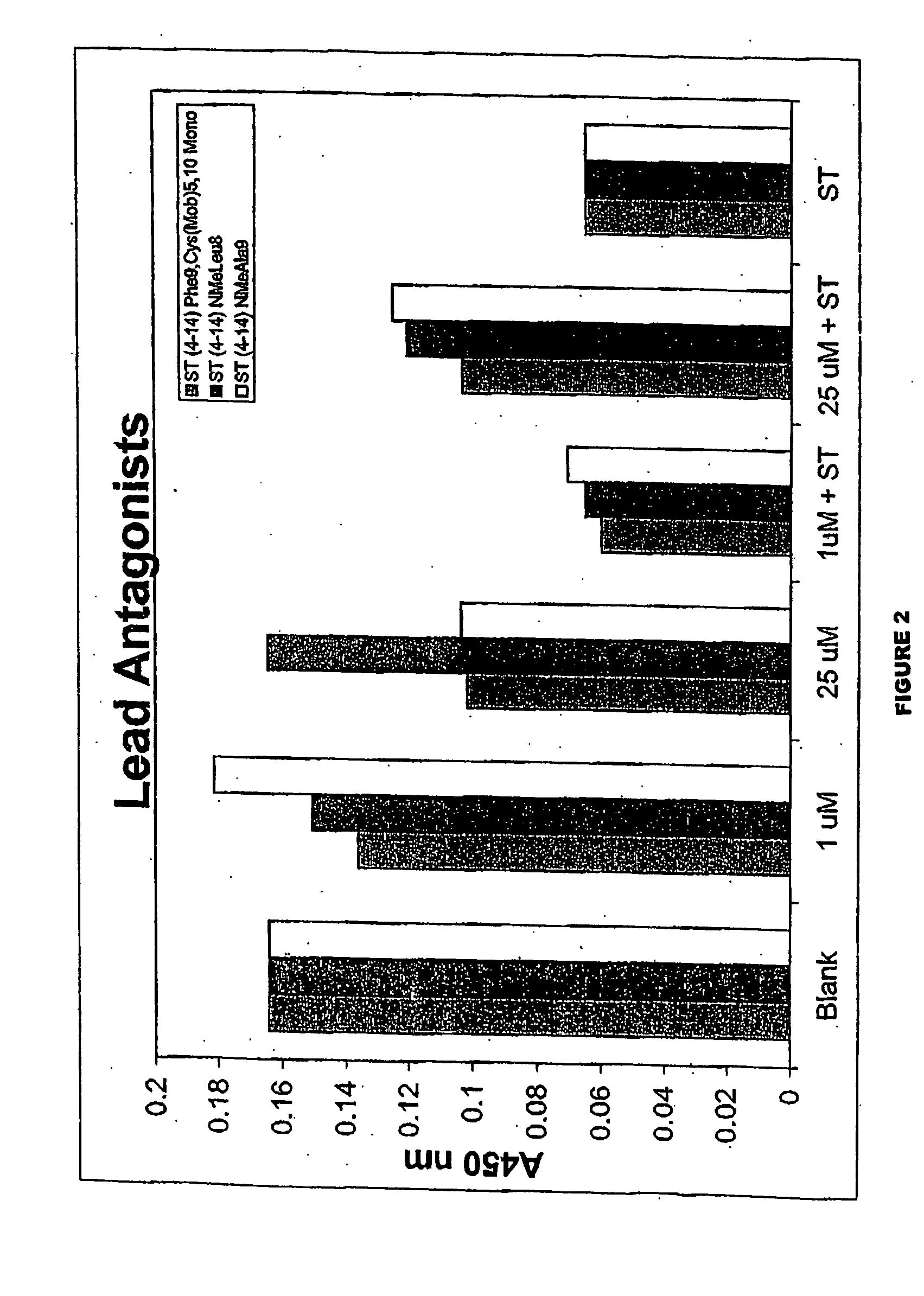
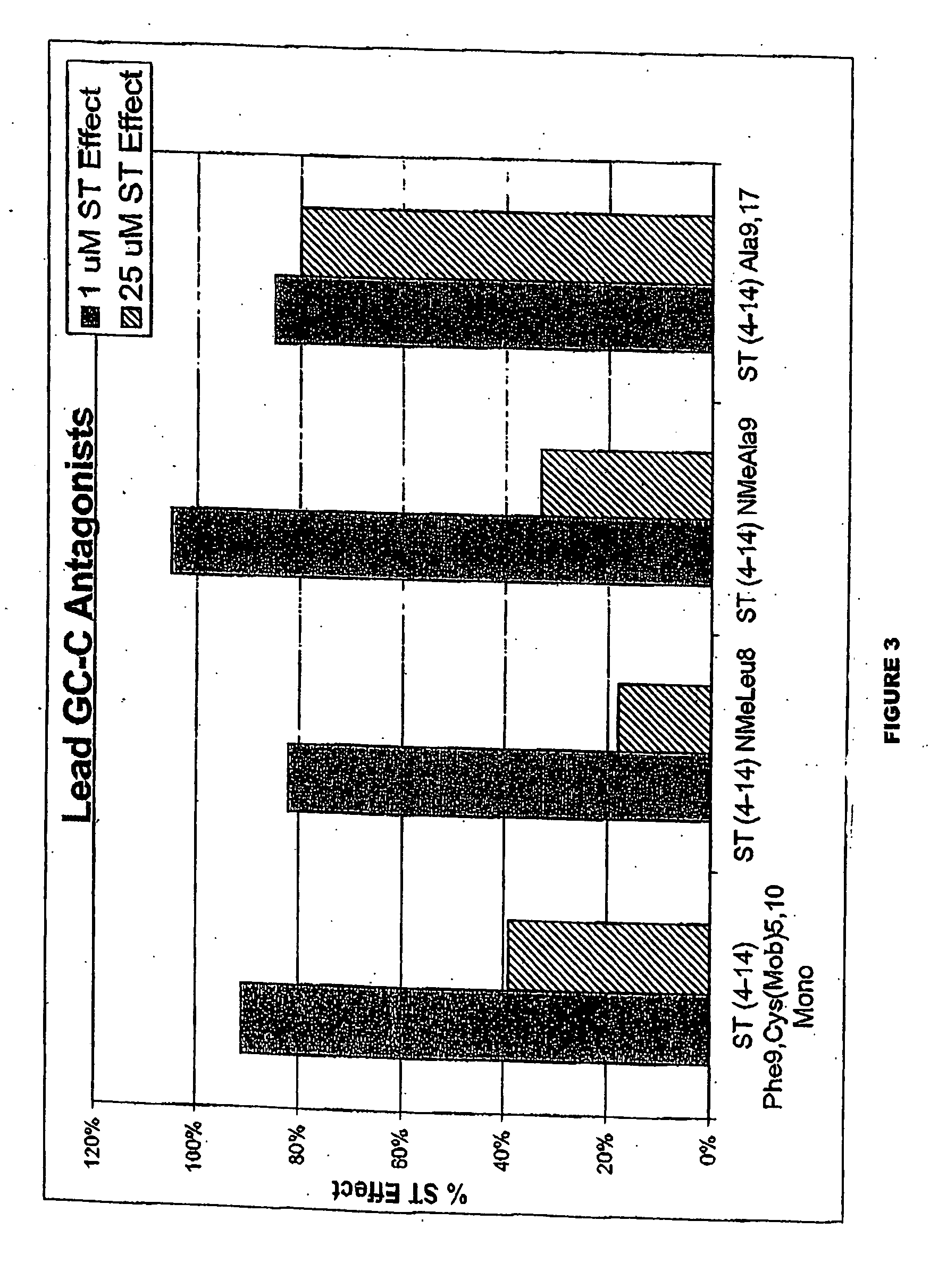
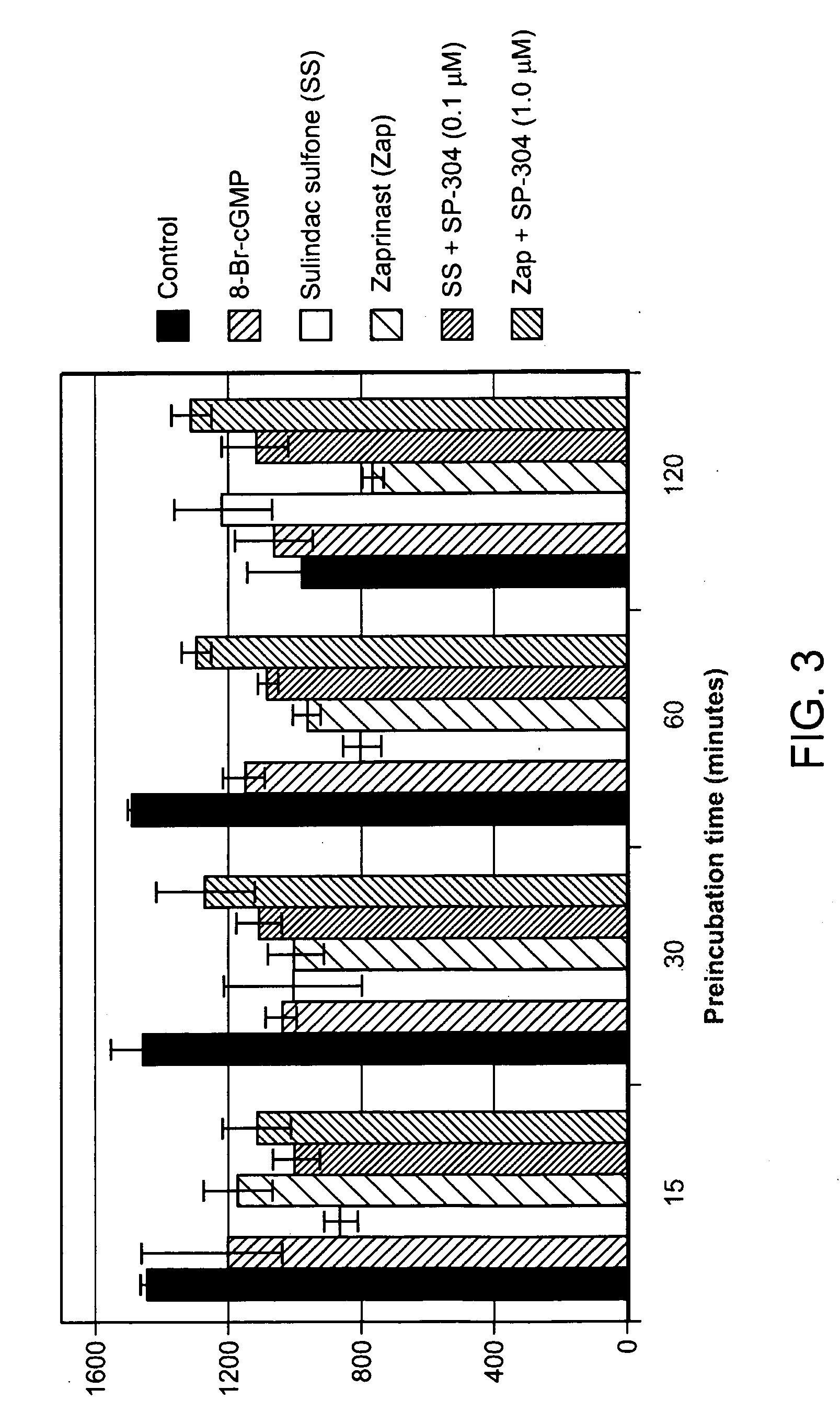
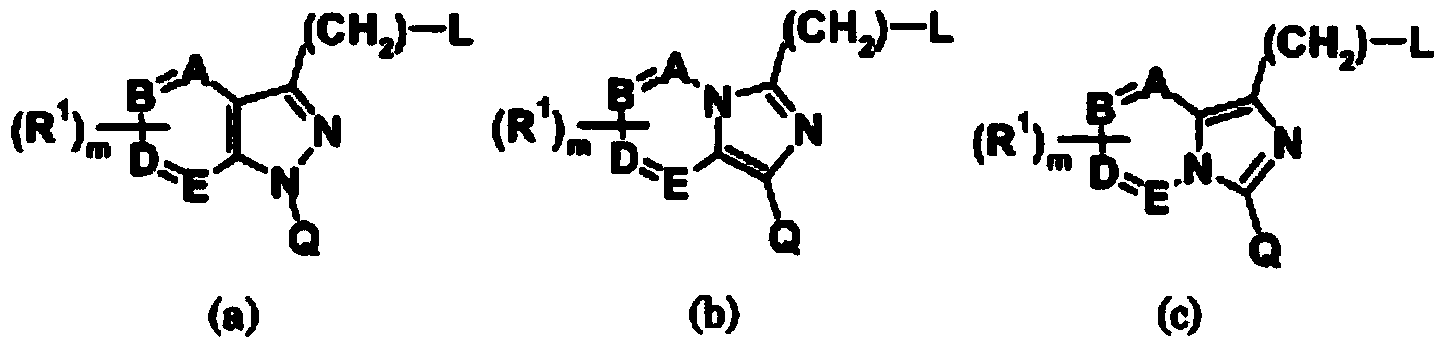
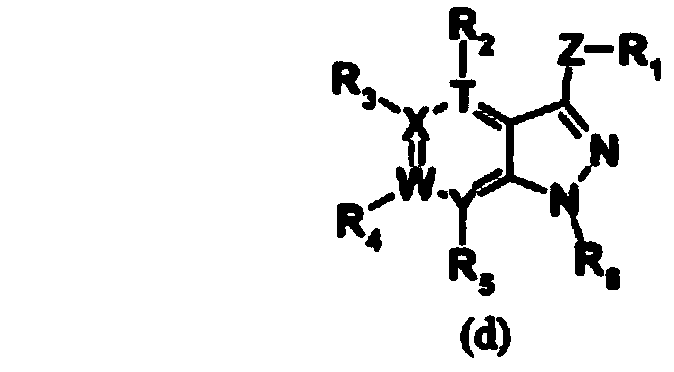
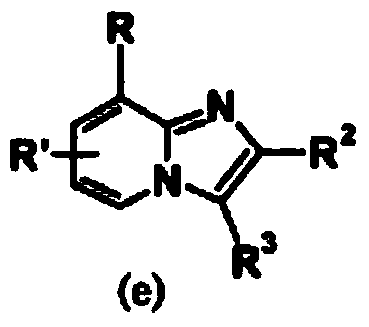
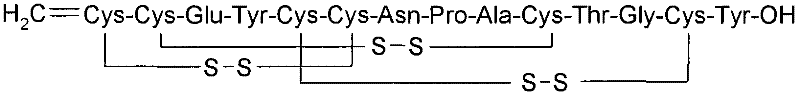Patents
Literature
91 results about "Guanylate cyclase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
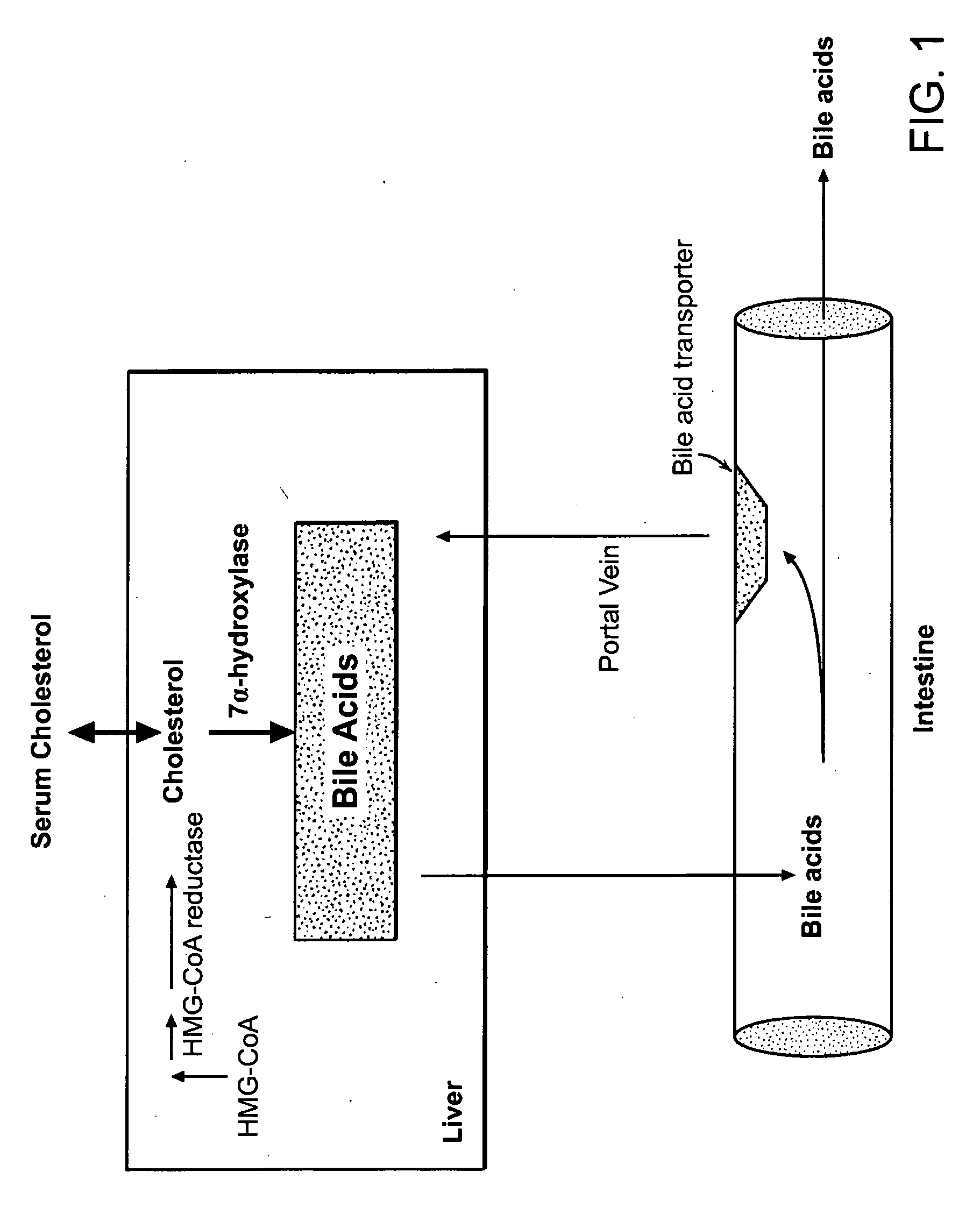
Guanylate cyclase (EC 4.6.1.2, also known as guanyl cyclase, guanylyl cyclase, or GC) is a lyase enzyme. Guanylate cyclase is often part of the G protein signaling cascade that is activated by low intracellular calcium levels and inhibited by high intracellular calcium levels. In response to calcium levels, guanylate cyclase synthesizes cGMP from GTP. cGMP keeps cGMP-gated channels open, allowing for the entry of calcium into the cell. Like cAMP, cGMP is an important second messenger that internalizes the message carried by intercellular messengers such as peptide hormones and nitric oxide, and can also function as an autocrine signal. Depending on cell type, it can drive adaptive/developmental changes requiring protein synthesis. In smooth muscle, cGMP is the signal for relaxation, and is coupled to many homeostatic mechanisms including regulation of vasodilation, vocal tone, insulin secretion, and peristalsis. Once formed, cGMP can be degraded by phosphodiesterases, which themselves are under different forms of regulation, depending on the tissue.
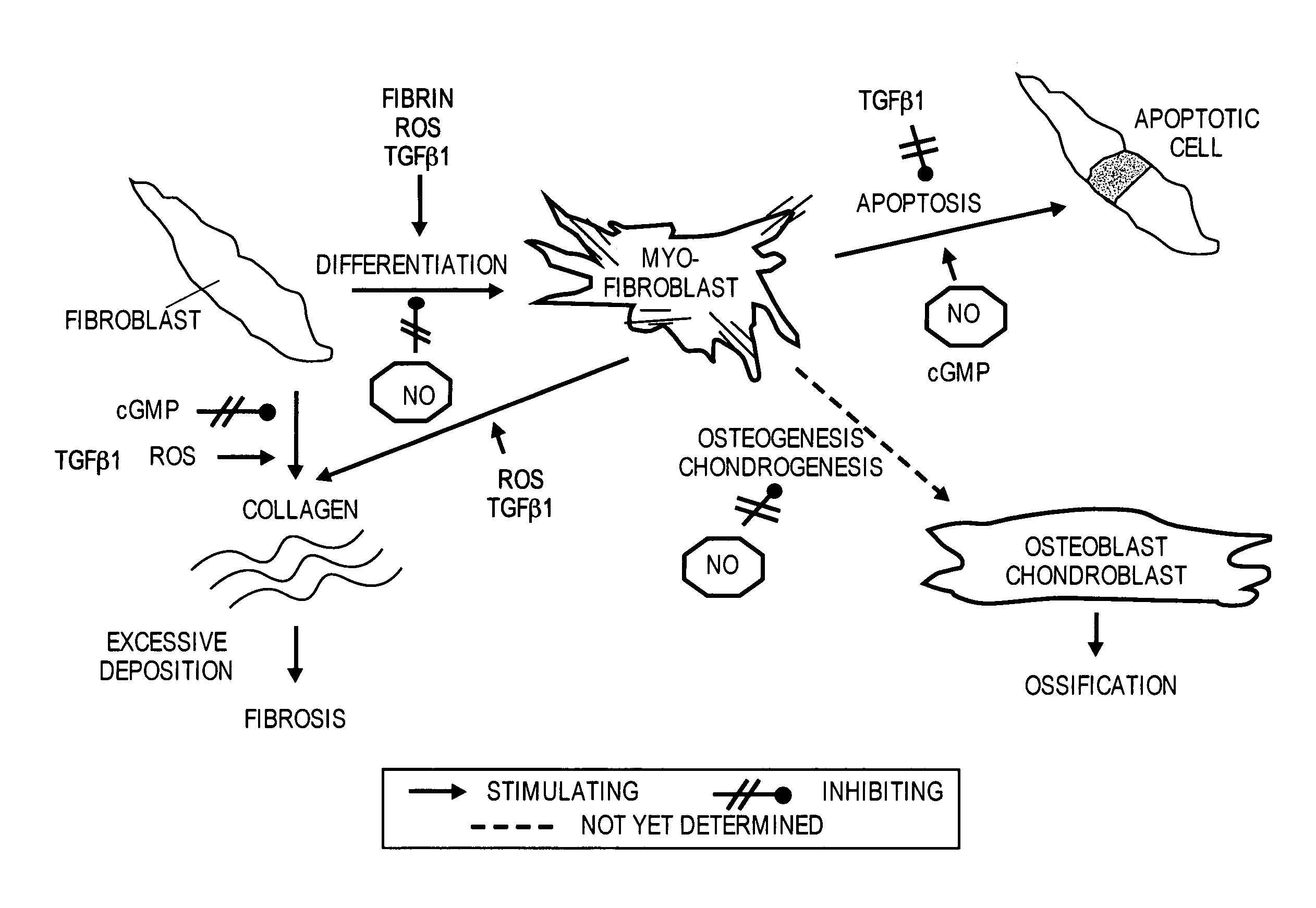
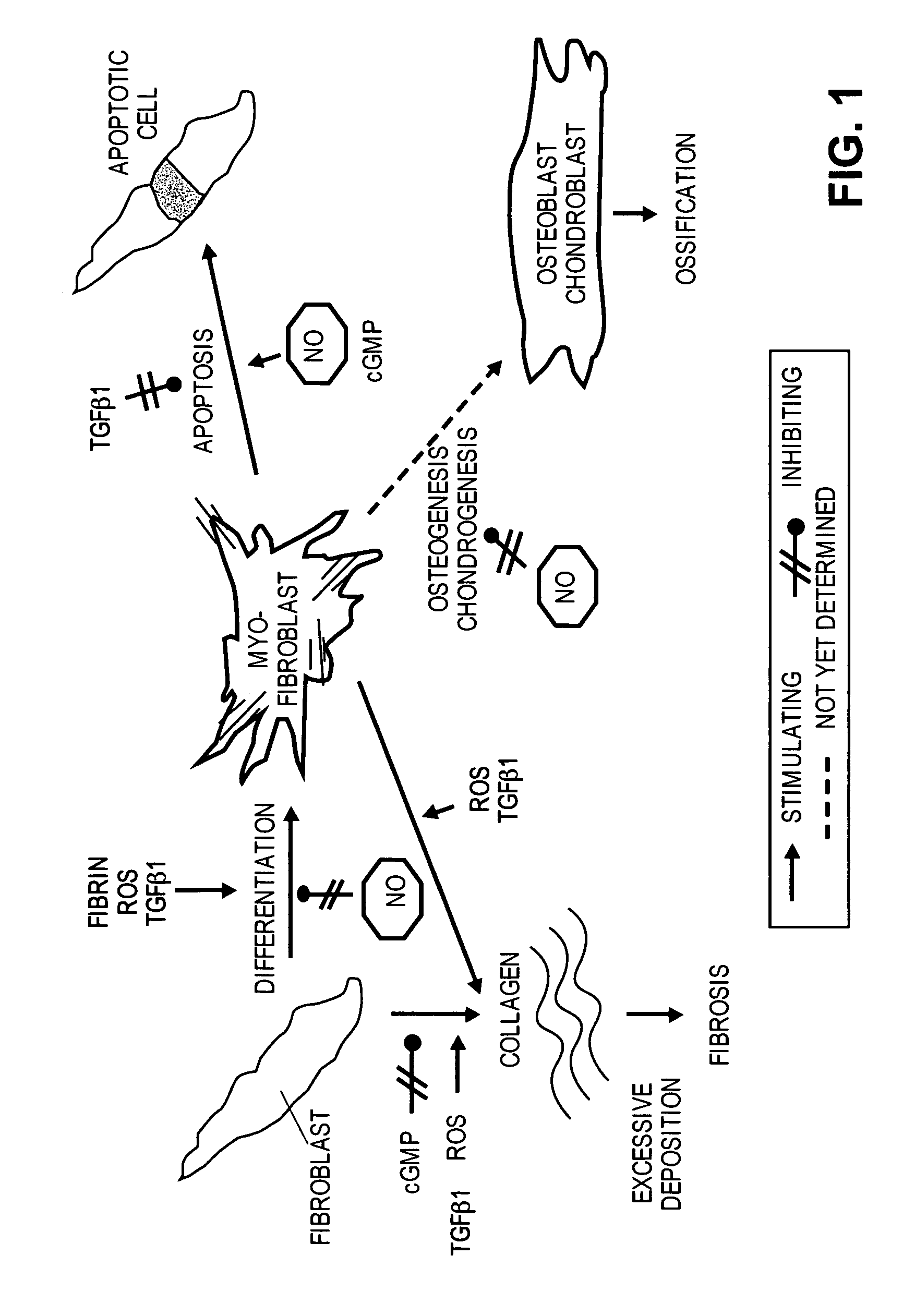
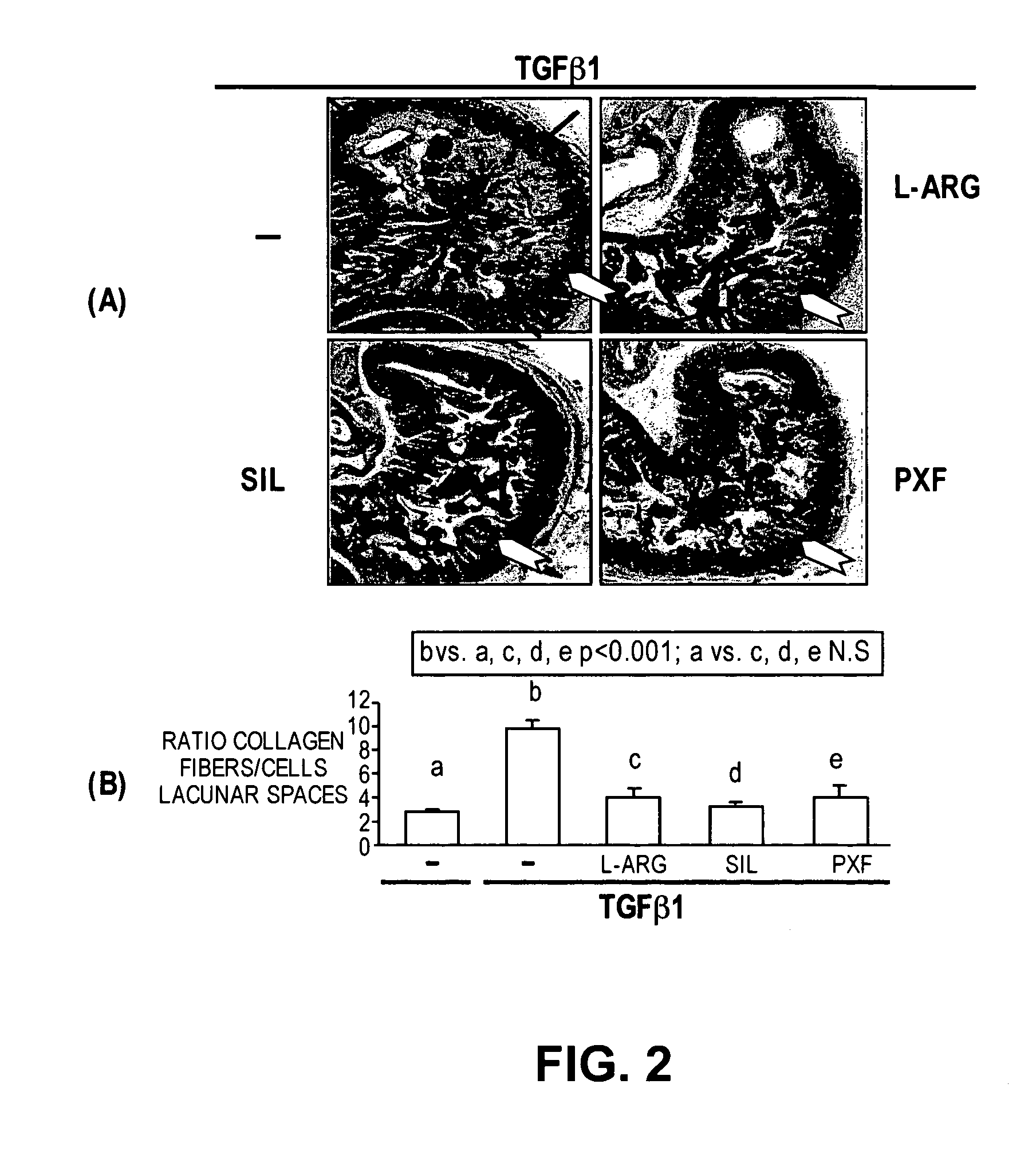
Methods of use of inhibitors of phosphodiesterases and modulators of nitric oxide, reactive oxygen species, and metalloproteinases in the treatment of peyronie's disease, arteriosclerosis and other fibrotic diseases
ActiveUS20050085486A1Increasing NO levelReduce expressionBiocidePharmaceutical delivery mechanismFemale Sexual Arousal DisorderCyclase
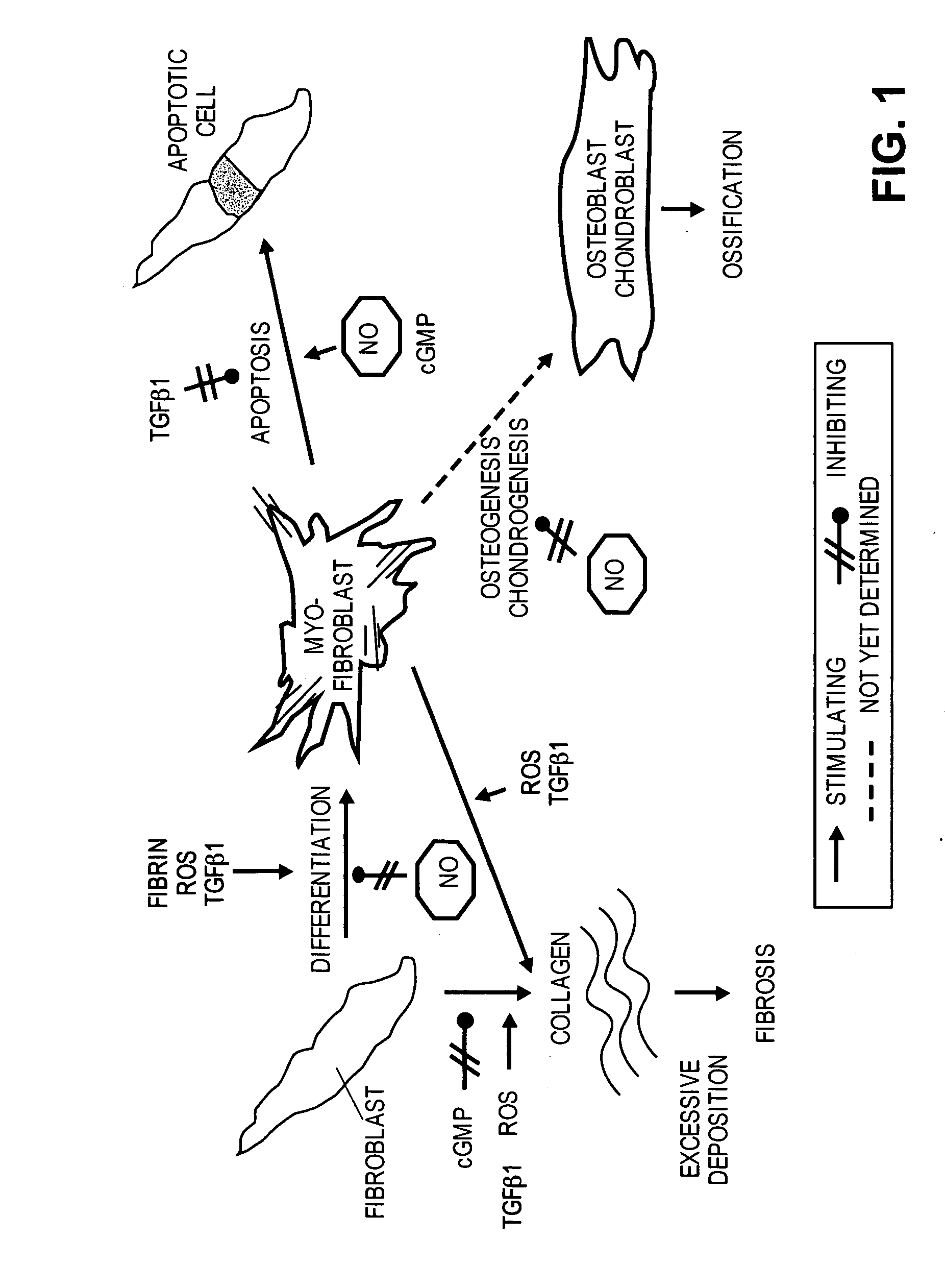
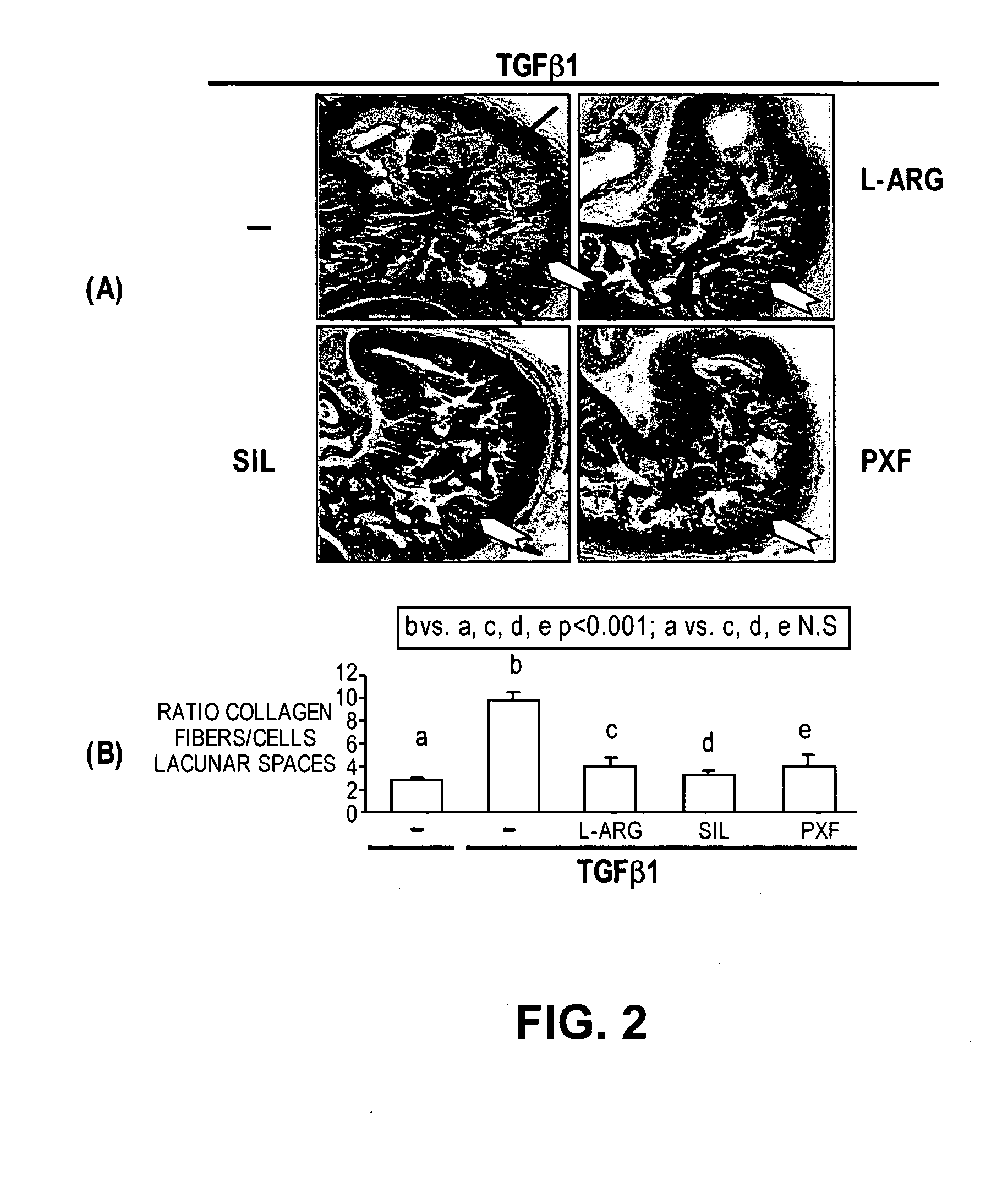
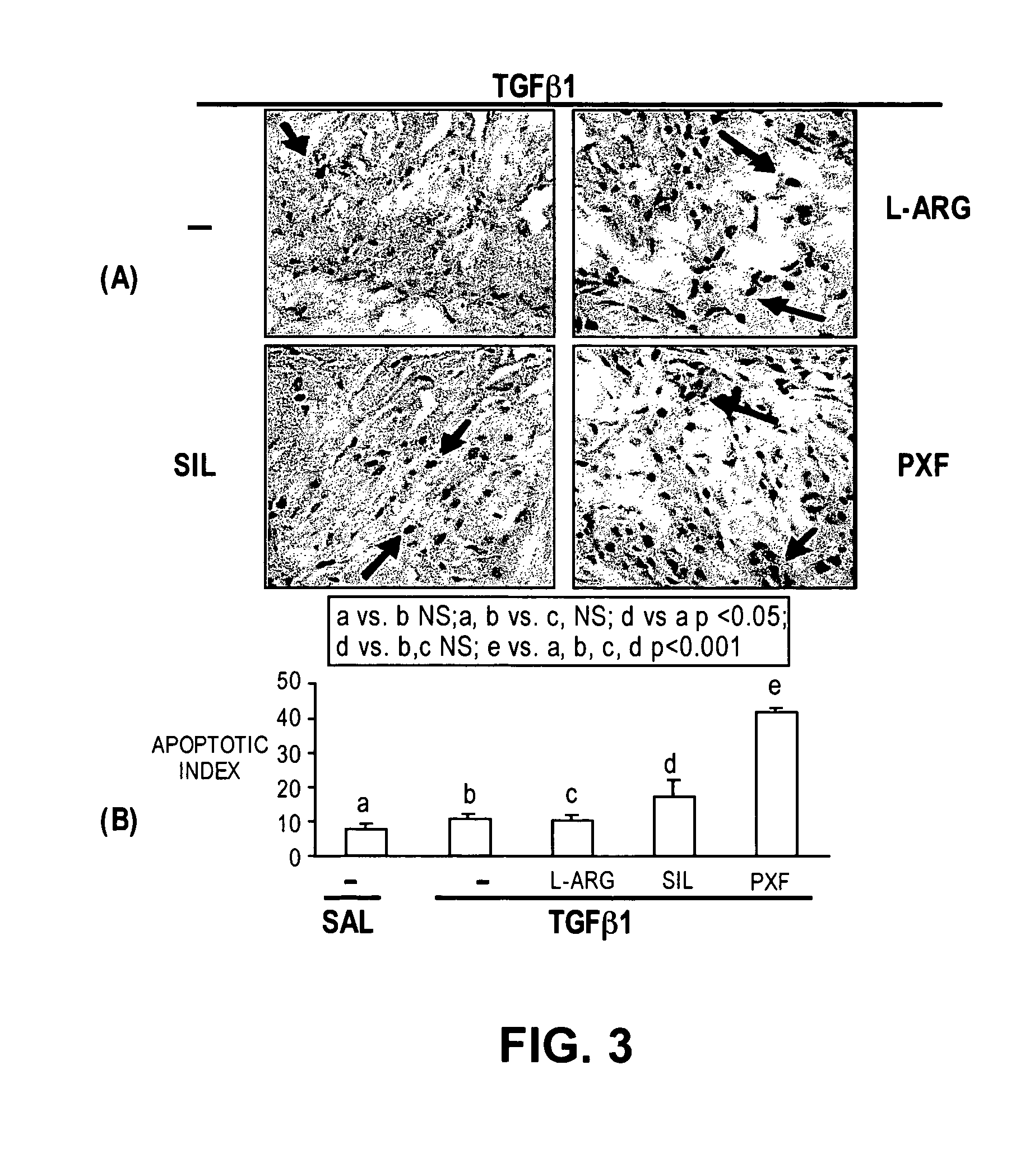
The present methods and compositions are of use for treatment of conditions involving fibrosis, such as Peyronie's disease plaque, penile corporal fibrosis, penile veno-occlusive dysfunction, Dupuytren's disease nodules, vaginal fibrosis, clitoral fibrosis, female sexual arousal disorder, abnormal wound healing, keloid formation, general fibrosis of the kidney, bladder, prostate, skin, liver, lung, heart, intestines or any other localized or generalized fibrotic condition, vascular fibrosis, arterial intima hyperplasia, atherosclerosis, arteriosclerosis, restenosis, cardiac hypertrophy, hypertension or any condition characterized by excessive fibroblast or smooth muscle cell proliferation or deposition of collagen and extracellular matrix in the blood vessels and / or heart. In certain embodiments, the compositions may comprise a PDE-4 inhibitor, a PDE-5 inhibitor, a compound that elevates cGMP and / or PKG, a stimulator of guanylyl cyclase and / or PKG, a combination of a compound that elevates cGMP, PKG or NO with an antioxidant that decreases ROS, or a compound that increases MMP activity.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Agonists of guanylate cyclase useful for the treatment of gastrointestinal disorders, inflammation, cancer and other disorders
ActiveUS20090048175A1Maintain good propertiesIncrease resistanceOrganic active ingredientsSenses disorderPhosphodiesteraseGastrointestinal cancer
The invention provides novel guanylate cyclase-C agonist peptides and their use in the treatment of human diseases including gastrointestinal disorders, inflammation or cancer (e.g., a gastrointestinal cancer). The peptides can be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The gastrointestinal disorder may be classified as either irritable bowel syndrome, constipation, or excessive acidity etc. The gastrointestinal disease may be classified as either inflammatory bowel disease or other GI condition, including Crohn's disease and ulcerative colitis, and cancer.
Owner:BAUSCH HEALTH IRELAND LTD
Neuronal pain pathway
ActiveUS20060216339A1High activityImprove the level ofNervous disorderDipeptide ingredientsCyclasePhosphorylation
The present invention relates to the discovery of a novel molecular pathway involved in long-term hyperexcitability of sensory neurons, which, in higher animals, is associated with persistent pain. It is based on the discovery that, following injury to an axon of a neuron, an increase in nitric oxide synthase activity results in increased nitric oxide production, which, in turn, activates guanylyl cyclase, thereby increasing levels of cGMP. Increased cGMP results in activation of protein kinase G (“PKG”), which then is retrogradely transported along the axon to the neuron cell body, where it phosphorylates MAPKerk.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Theurapeutic or prophyiactic agent for arthritis
ActiveUS20070197434A1Slow changeIncrease the number ofAntibacterial agentsAntimycoticsCyclaseArthritis therapy
This invention provides a new therapeutic or prophylactic agent for arthritis such as osteoarthritis. Specifically, it provides a therapeutic or prophylactic agent for arthritis such as osteoarthritis, or an agent for promoting the growth of articular chondrocyte, comprising a guanyl cyclase B (GC-B) activator as an active ingredient; or a method for inhibiting arthritis or for promoting the growth of articular chondrocyte by activating GC-B; or a method for screening an agent for promoting the growth of articular chondrocyte or an agent capable of treating arthritis using the GC-B activity as an indication.
Owner:KAZUWA NAKAO +1
Compounds of the inventions of guanylyl cyclase C
InactiveUS20050287067A1Activity can be delayedControllable conversionCompound screeningApoptosis detectionCyclaseMedicine
Owner:THOMAS JEFFERSON UNIV
Agonists of guanylate cyclase useful for the treatment of hypercholesterolemia, atherosclerosis, coronary heart disease, gallstone, obesity and other cardiovascular diseases
ActiveUS20100152118A1Widen the optionsOrganic active ingredientsMetabolism disorderCyclaseCoronary artery disease
This invention also provides a method to prevent, control, and treata lipid metabolism disorder, a billary disorder, cardiovascular disease, obesity or an endocrine disorder by administering at least one agonist of guanalyte cyclase receptor either alone or in combination with a compound typically used to treat the disorder and or with an inhibitor of cGMP-dependent phosphodieasterases.
Owner:BAUSCH HEALTH IRELAND LTD
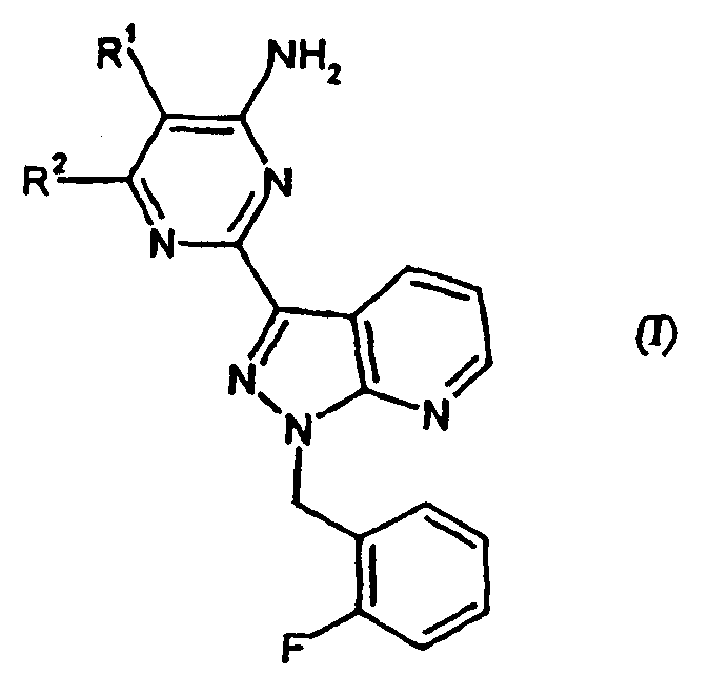
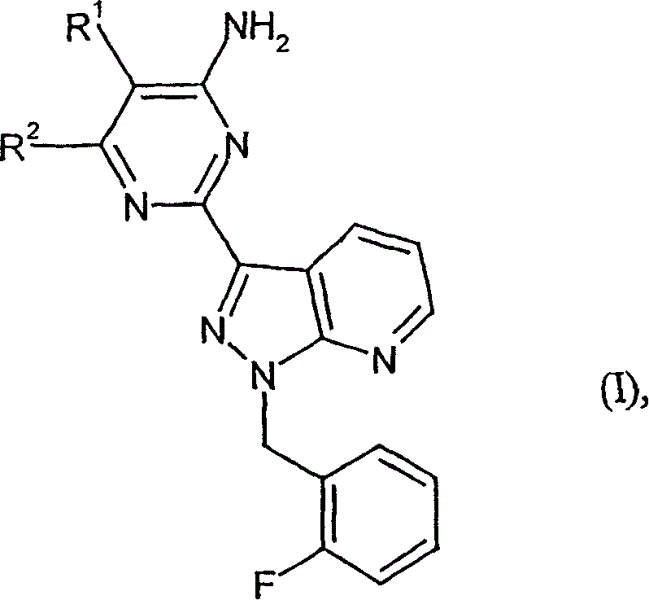
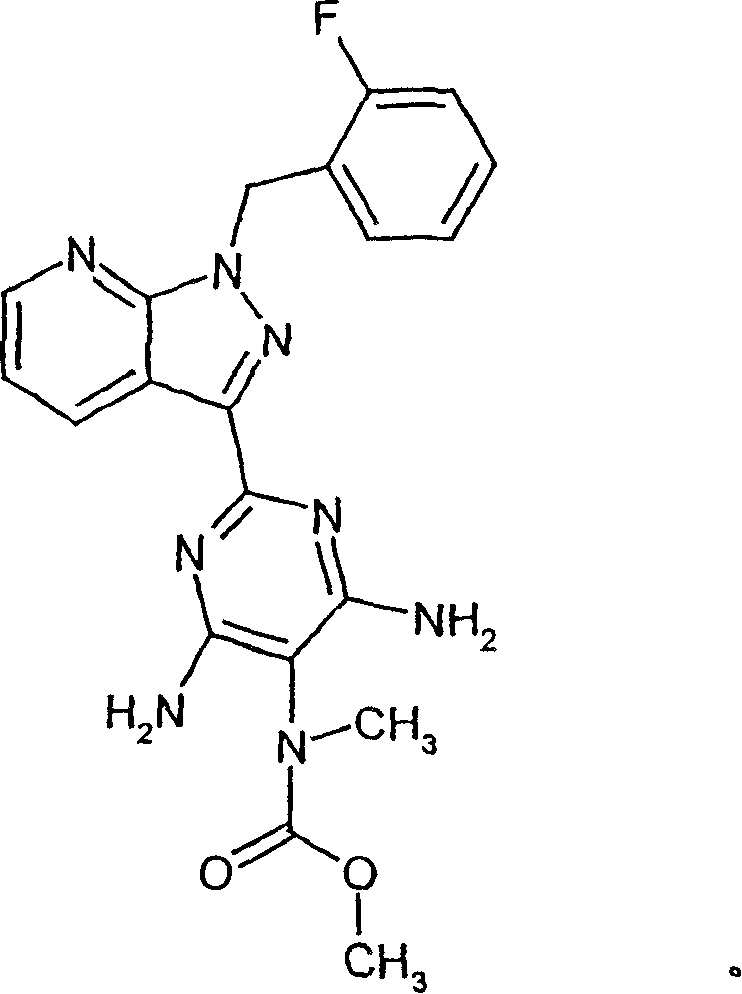
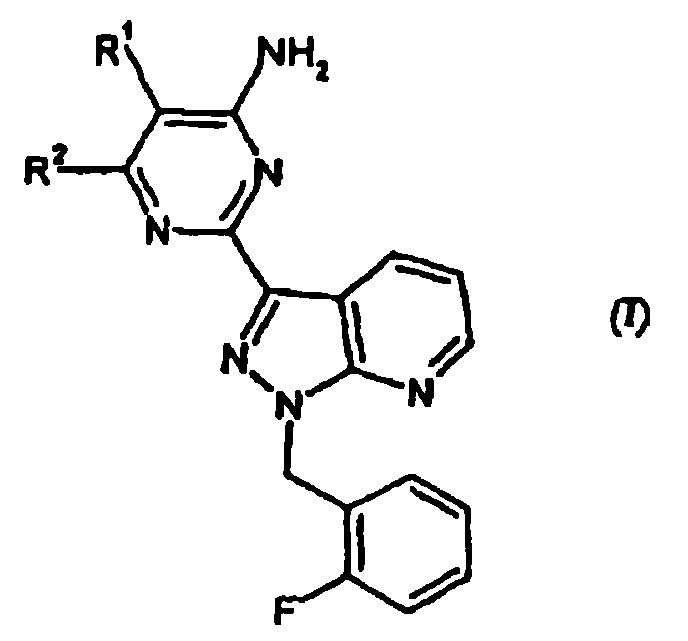
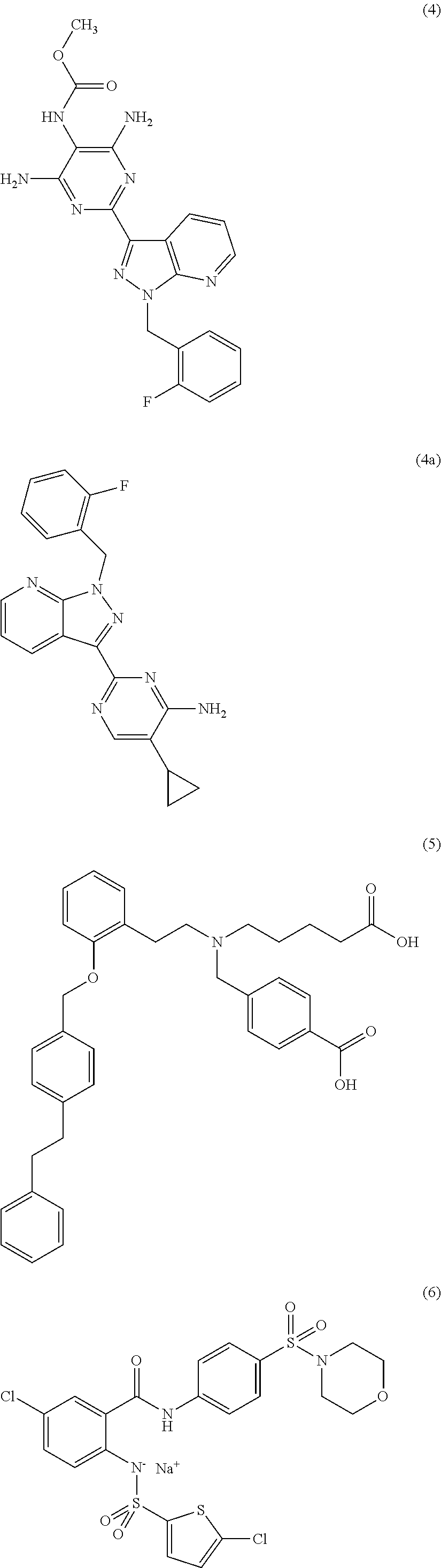
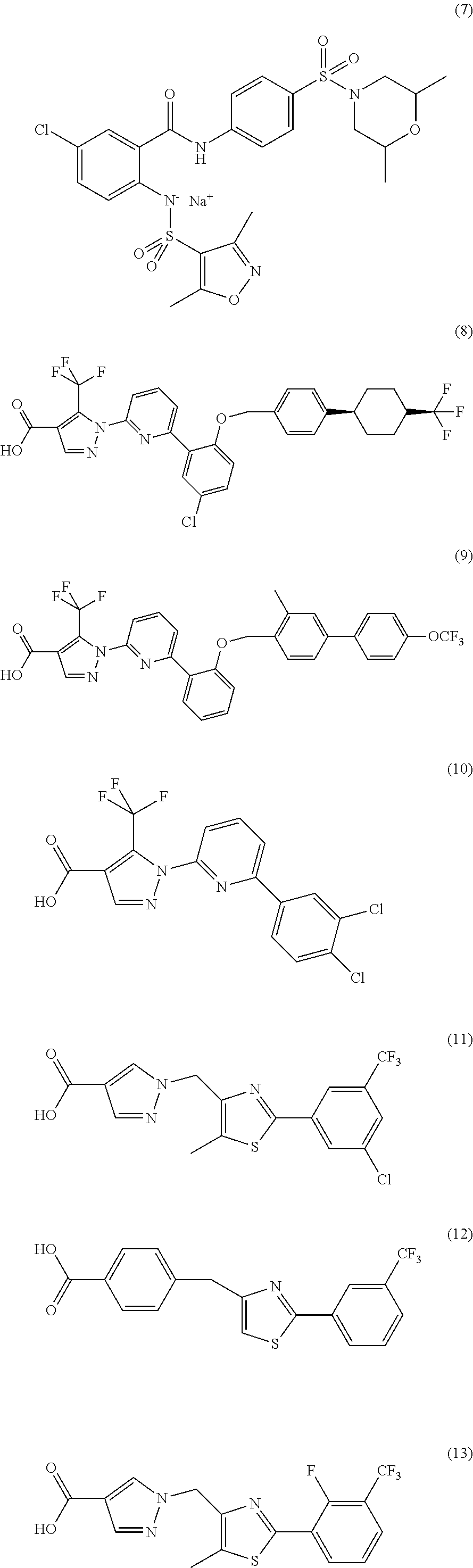
Carbamate-substituted pyrazolopyridines
The present invention relates to compounds which stimulate soluble guanylate cyclase, to the preparation thereof and to the use thereof as medicaments, in particular as medicaments for the treatment of cardiovascular disorders and / or sexual dysfunction.
Owner:ADVERIO PHARMA
Agonists of Guanylate Cyclase Useful for the Treatment of Hypercholesterolemia, Atherosclerosis, Coronary Heart Disease, Gallstone, Obesity and Other Cardiovascular Diseases
This invention also provides a method to prevent, control, and treata lipid metabolism disorder, a biliary disorder, cardiovascular disease, obesity or an endocrine disorder by administering at least one agonist of guanalyte cyclase receptor either alone or in combination with a compound typically used to treat the disorder and or with an inhibitor of cGMP-dependent phosphodieasterases.
Owner:BAUSCH HEALTH IRELAND LTD
Imidazopyridine compound
[Problem] To provide an excellent therapeutic or prophylactic agent for cardiovascular diseases on the basis of cGMP production-increasing activity attributed to soluble guanylate cyclase-activating activity. [Solution] It was discovered that an imidazopyridine compound having a carbamoyl group at the 3-position of an imidazo[1,2-a]pyridine skeleton and having a substituent attached to the 8-position of the skeleton through an oxygen atom exhibits cGMP production-increasing activity attributed to potent soluble guanylate cyclase-activating activity and can be used in an excellent therapeutic or prophylactic agent for various cardiovascular diseases associated with soluble guanylate cyclase, and the invention was completed.
Owner:ASTELLAS PHARMA INC
Agonists of guanylate cyclase useful for the treatment of hypercholesterolemia, atherosclerosis, coronary heart disease, gallstone, obesity and other cardiovascular diseases
This invention also provides a method to prevent, control, and treat a lipid metabolism disorder, a billary disorder, cardiovascular disease, obesity or an endocrine disorder by administering at least one agonist of guanalyte cyclase receptor either alone or in combination with a compound typically used to treat the disorder and or with an inhibitor of cGMP-dependent phosphodieasterases.
Owner:BAUSCH HEALTH IRELAND LTD
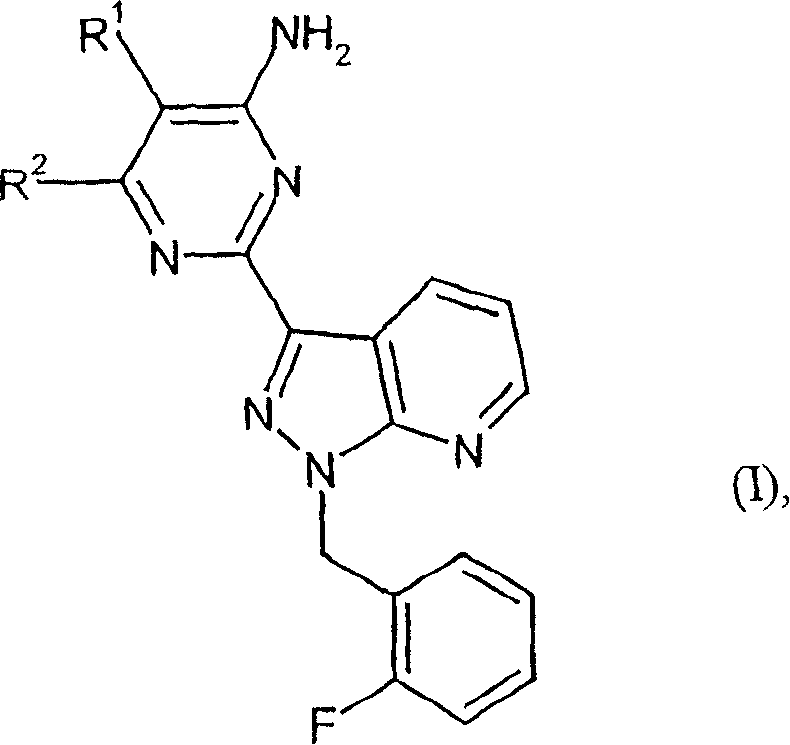
Carbamate-substituted pyrazolopyridines
The present invention relates to compounds which stimulate soluble guanylate cyclase, to the preparation thereof and to the use thereof as medicaments, in particular as medicaments for the treatment of cardiovascular disorders and / or sexual dysfunction.
Owner:ADVERIO PHARMA
Use of inhibitors of glutaminyl cyclase and glutamate cyclase for treatment and prevention of neurodegenerative diseases
Physiological substrates of mammalian glutaminyl cyclase (QC, EC 2.3.2.5), new effectors of QC, methods for screening for such effectors, and the use of such effectors and pharmaceutical compositions comprising such effectors for the treatment of conditions that can be treated by modulation of QC-activity. Preferred compositions additionally comprise inhibitors of DP IV or DP IV-like enzymes for the treatment or alleviation of conditions that can be treated by modulation of QC- and DP IV-activity.
Owner:VIVORYON THERAPEUTICS NV
Inhibitors of Cyclic Nucleotide Synthesis and Their Use for Therapy of Various Diseases
InactiveUS20100035867A1Shorter treatmentMore reversible effectOrganic chemistryHeterocyclic compound active ingredientsDiseaseCyclase
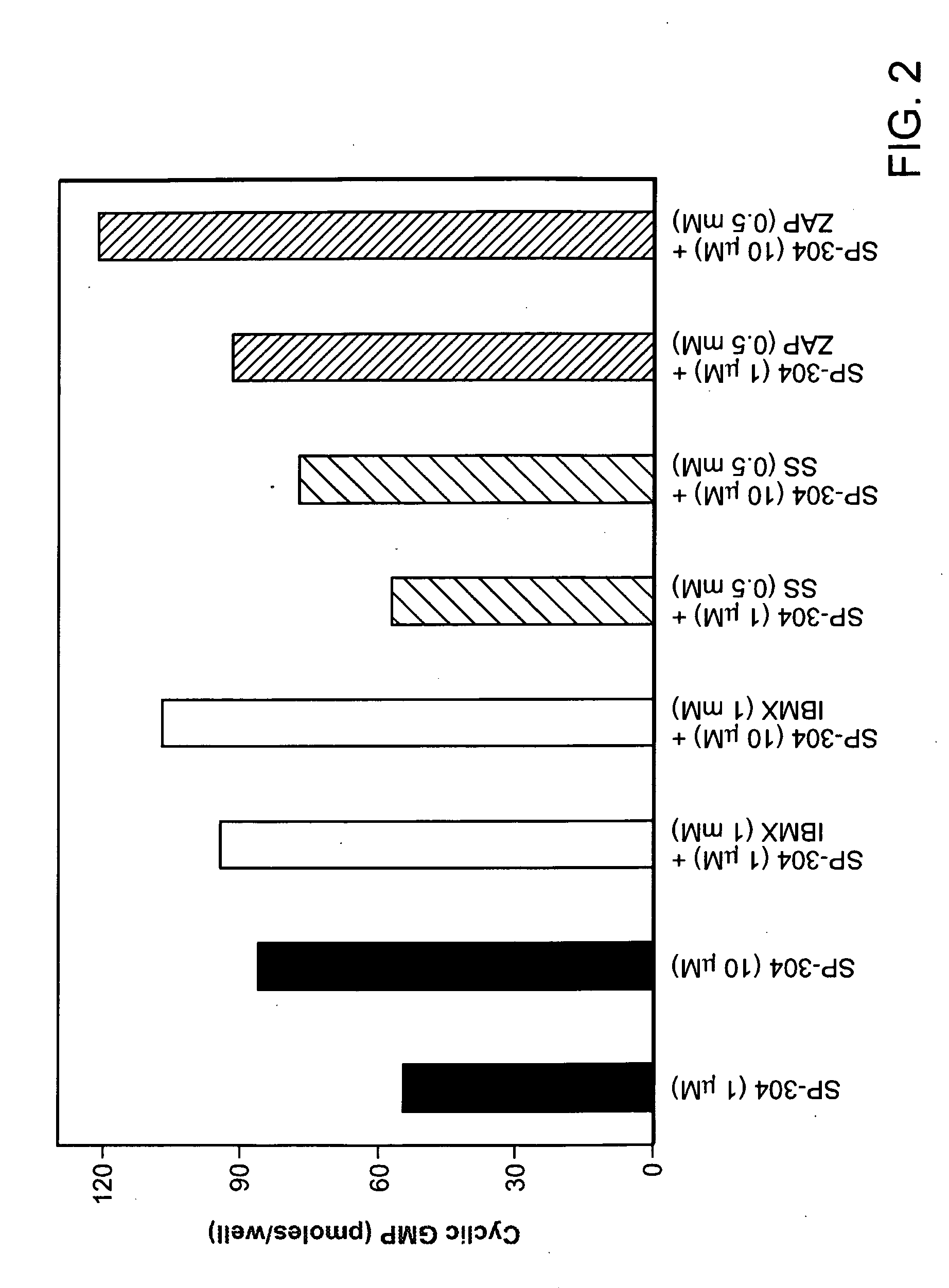
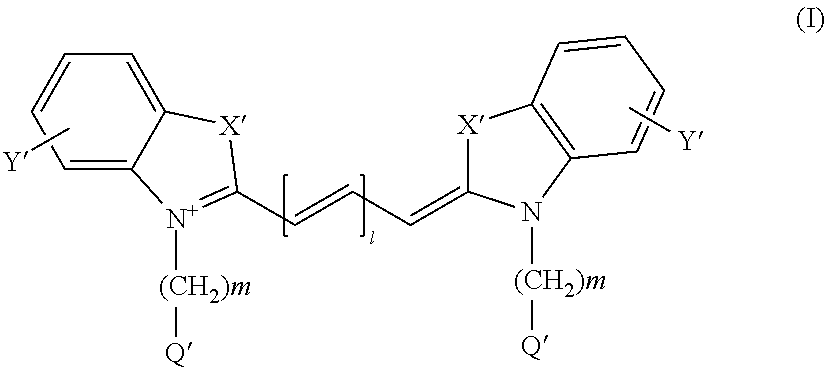
We disclose a method of inhibiting activity of adenylyl cyclase or guanylyl cyclase in a mammal by administering to the mammal an amount of a composition effective to inhibit the activity, wherein the composition contains at least one compound selected from the group consisting of structural formulae (Ia) and (Ib) and salts thereof, wherein R1 is —H or has the structure —C(═O)R8; R2 is ═O or has the structure —OC(═O)R9; and R3, R4, R5, R6, and R7 are each independently selected from the group consisting of —H, —NO2, formula (I), -halogen, —OC(═O)R9, —OR9, —OH, —R8OH, —CH3, —OC(═O)CH2Ph, formulae (II), (III), (IV), —OPh, —CF3, —R8, —C(═O)OR9, -Ph, —R8Ph, formulae (V), (VI), (VII), (VIII), (IX), (X), (XI), (XII), (XIII), (XIV), (XV), (XVI), (XVII), (XVIII), (XIX), (XX), and (XXI), wherein each R8 is independently a linear or branched hydrocarbon group having from 1 to 4 carbon atoms and each R9 is independently a hydrocarbon group having from 1 to 2 carbon atoms. Administering the composition can be used to treat a disease in a mammal mediated by activity of adenylyl cyclase or guanylyl cyclase and effected by a toxin produced by a pathogenic organism or to reduce cyclic AMP or cyclic GMP levels in a mammal in need of reduction thereof. The composition can also be administered to mammalian cells in vitro. The above methods of inhibiting activity of adenylyl cyclase or guanylyl cyclase and treating diseases via such inhibition can be effective without prolonged treatment, have reversible effects, have low or no toxicity, are highly potent, are unlikely to have side effects, do not act on purinergic receptors, or can negate pathogenic toxins independently of whether the pathogenic organism survives.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
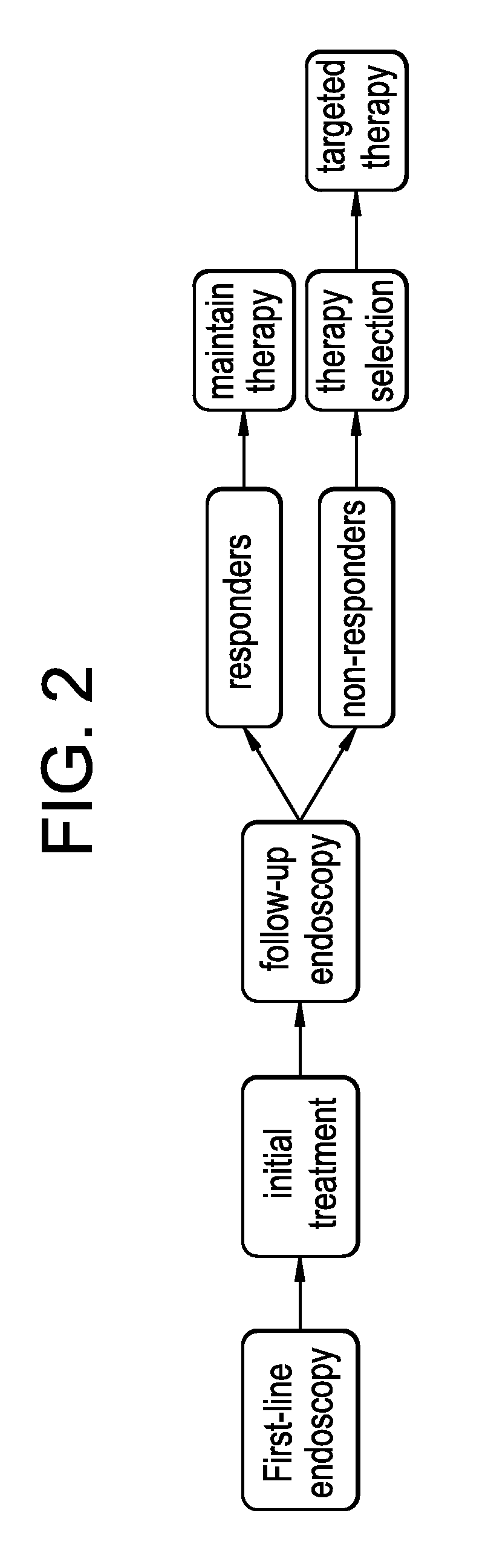
Therapy selection method
InactiveUS8568693B2Efficient use ofIn-vivo radioactive preparationsAntineoplastic agentsCyclaseRadio isotopes
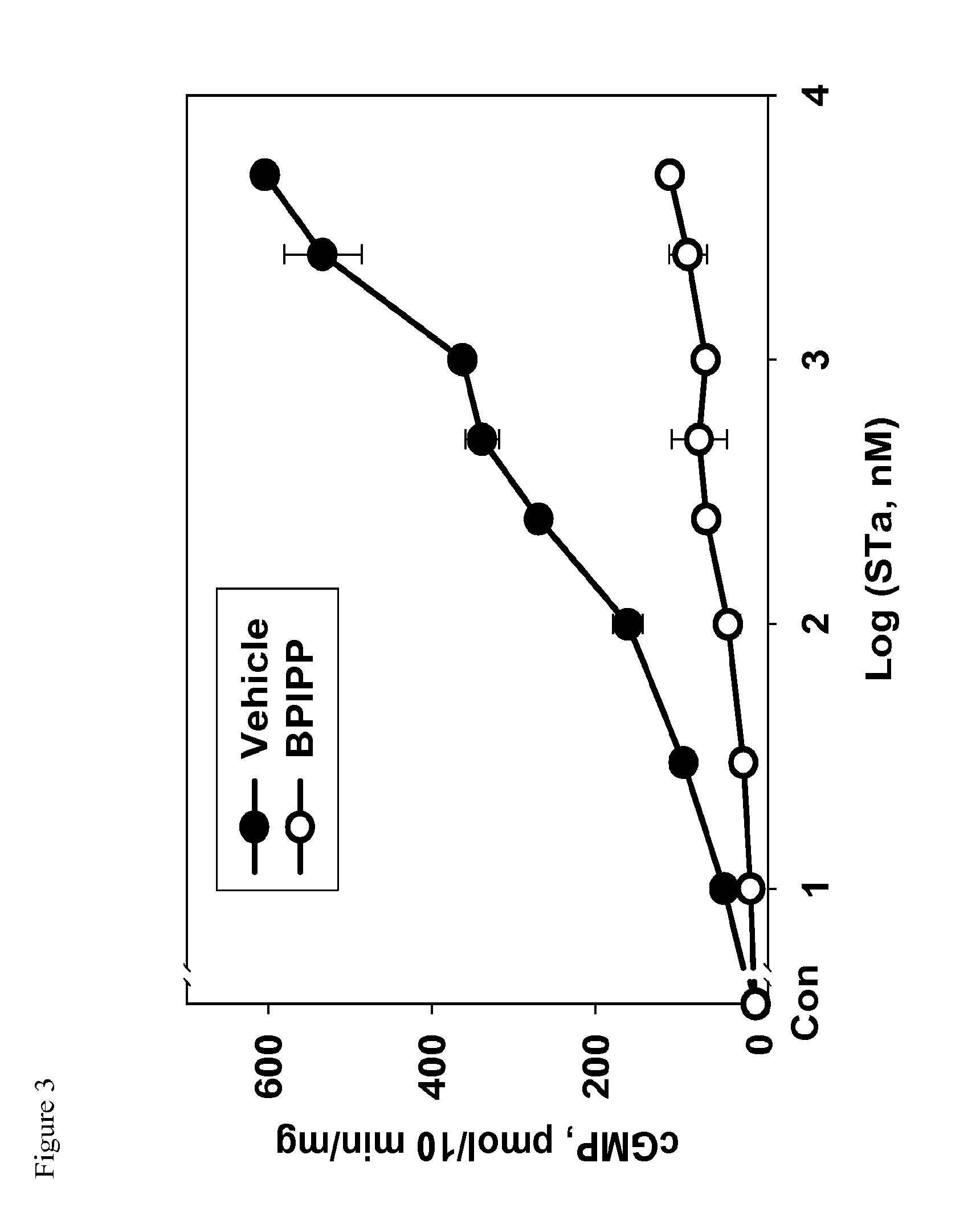
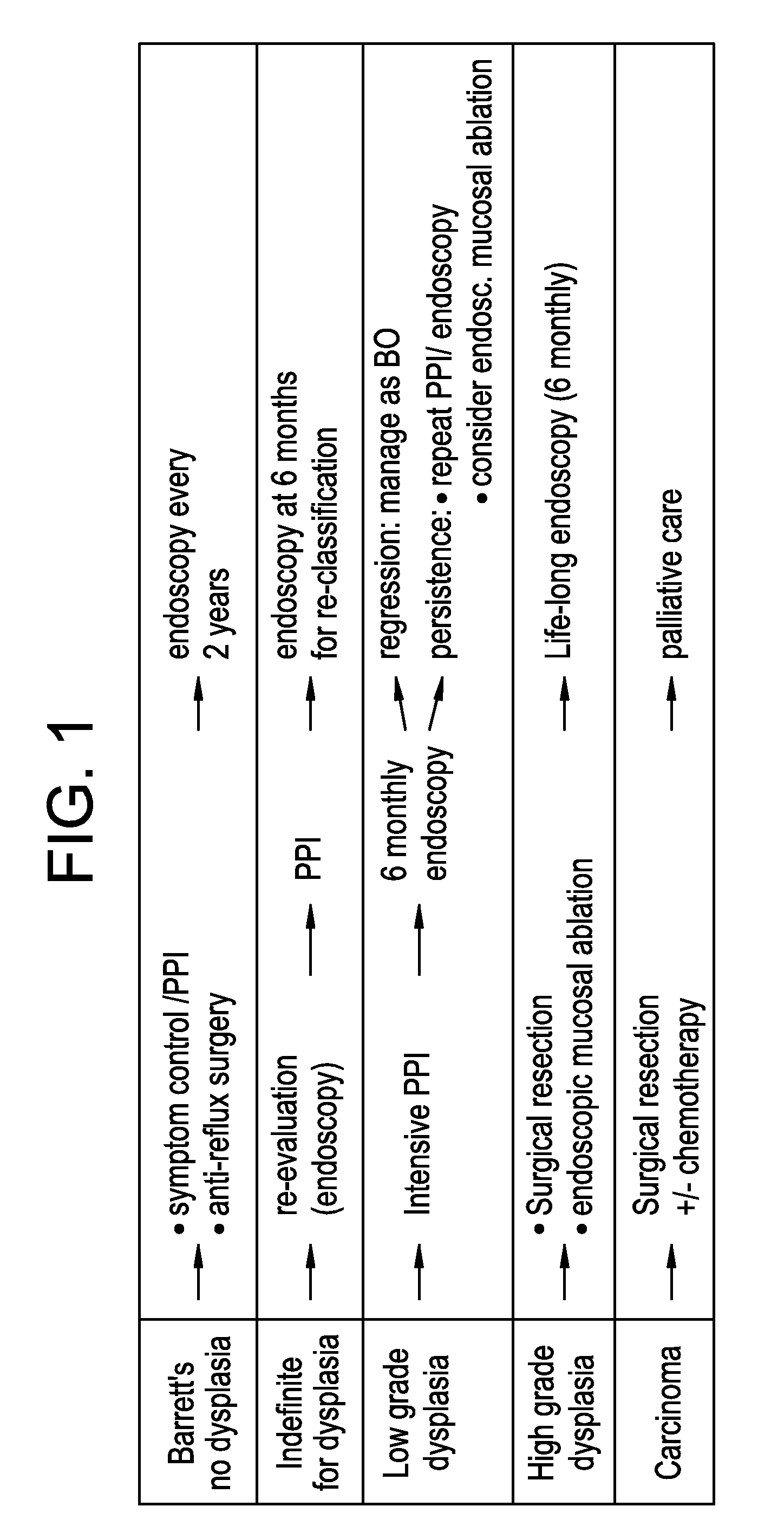
The present invention relates to a method to assist in the determination of therapy for a patient suffering from Barrett's oesophagus, especially where first-line therapy has been unsuccessful and when dysplasia has been diagnosed. The method comprises the use of an imaging agent comprising a vector which targets (a) Her2, (b) cMet, (c) guanylyl cyclase or (d) IGF1R. The imaging agent is suitable for radioisotope or optical imaging in vitro or preferably in vivo.
Owner:GE HEALTHCARE AS
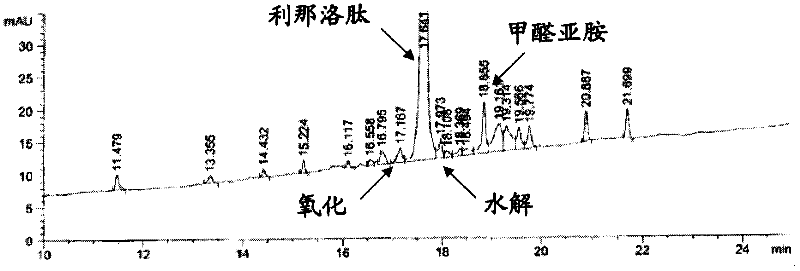
Stable solid formulation of a GC-C receptor agonist polypeptide suitable for oral administration
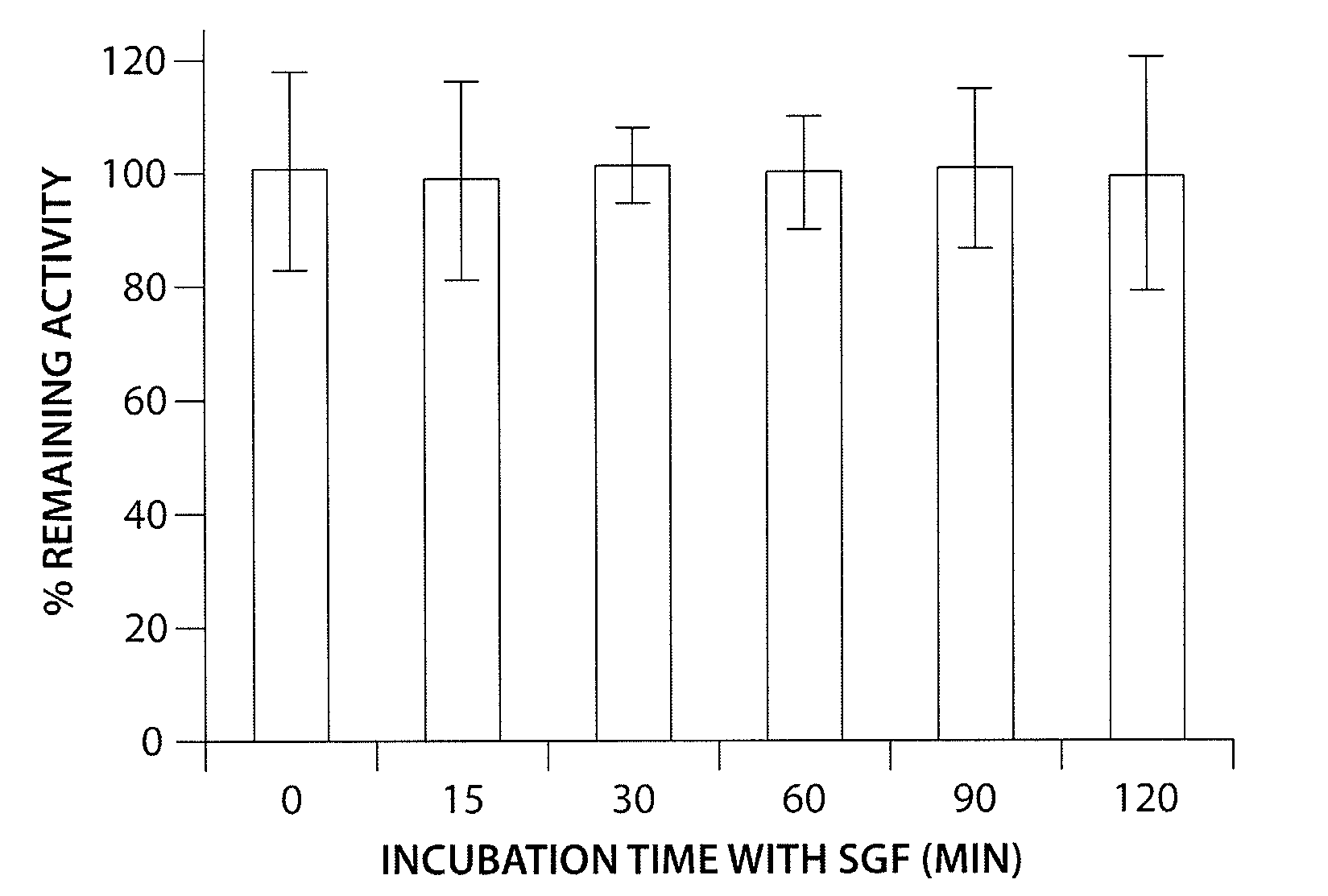
Solid, stable formulations of linaclotide suitable for oral administration are described herein as are methods for preparing such formulations. The formulations described herein contain a polypeptide consisting of the amino acid sequence Cys Cys GIu Tyr Cys Cys Asn Pro Ala Cys Thr GIy Cys Tyr ("linaclotide") or a pharmaceutically acceptable salt thereof. The linaclotide formulations described herein are stable and have a sufficient shelf life for manufacturing, storing and distributing the drug.
Owner:硬木药品公司
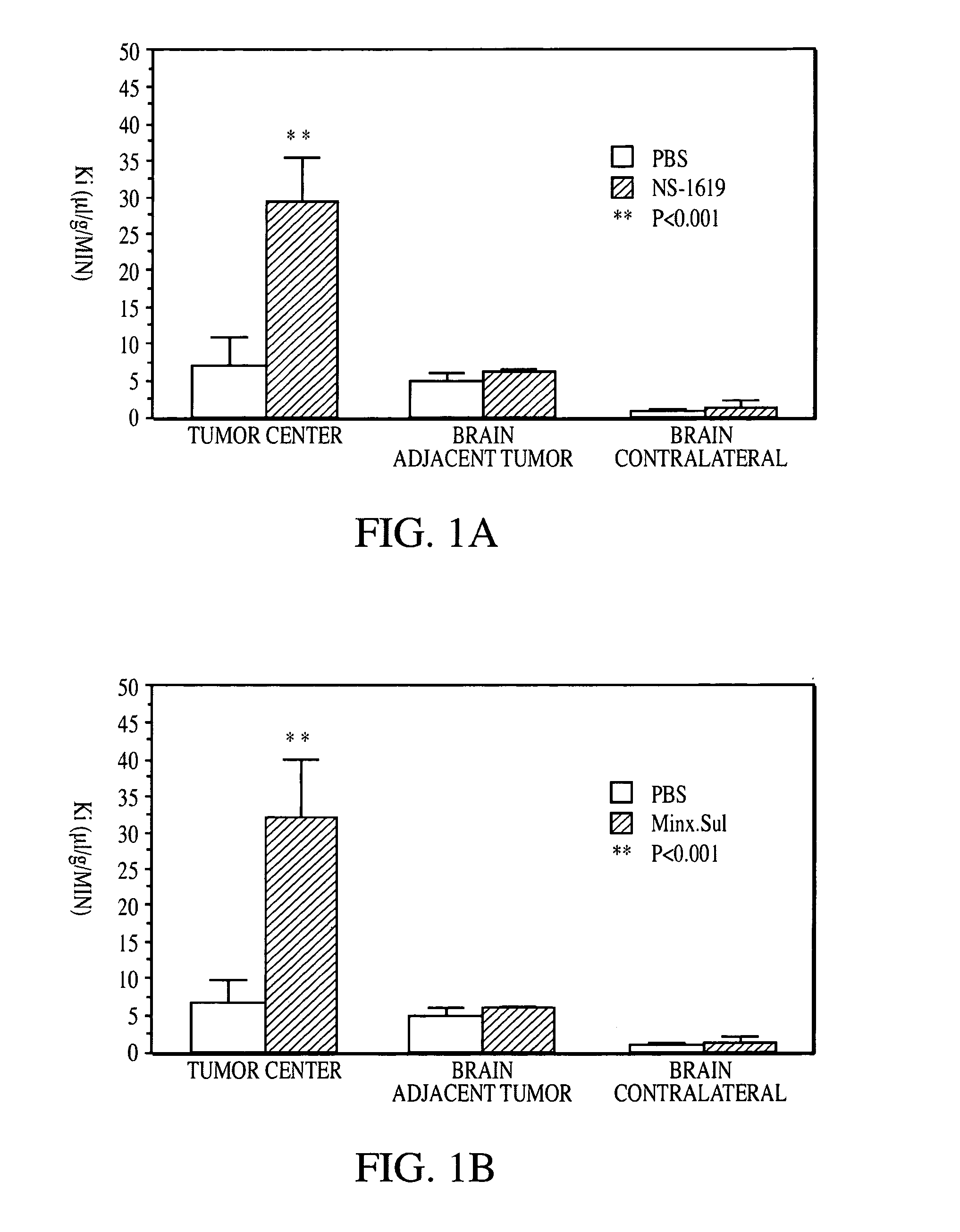
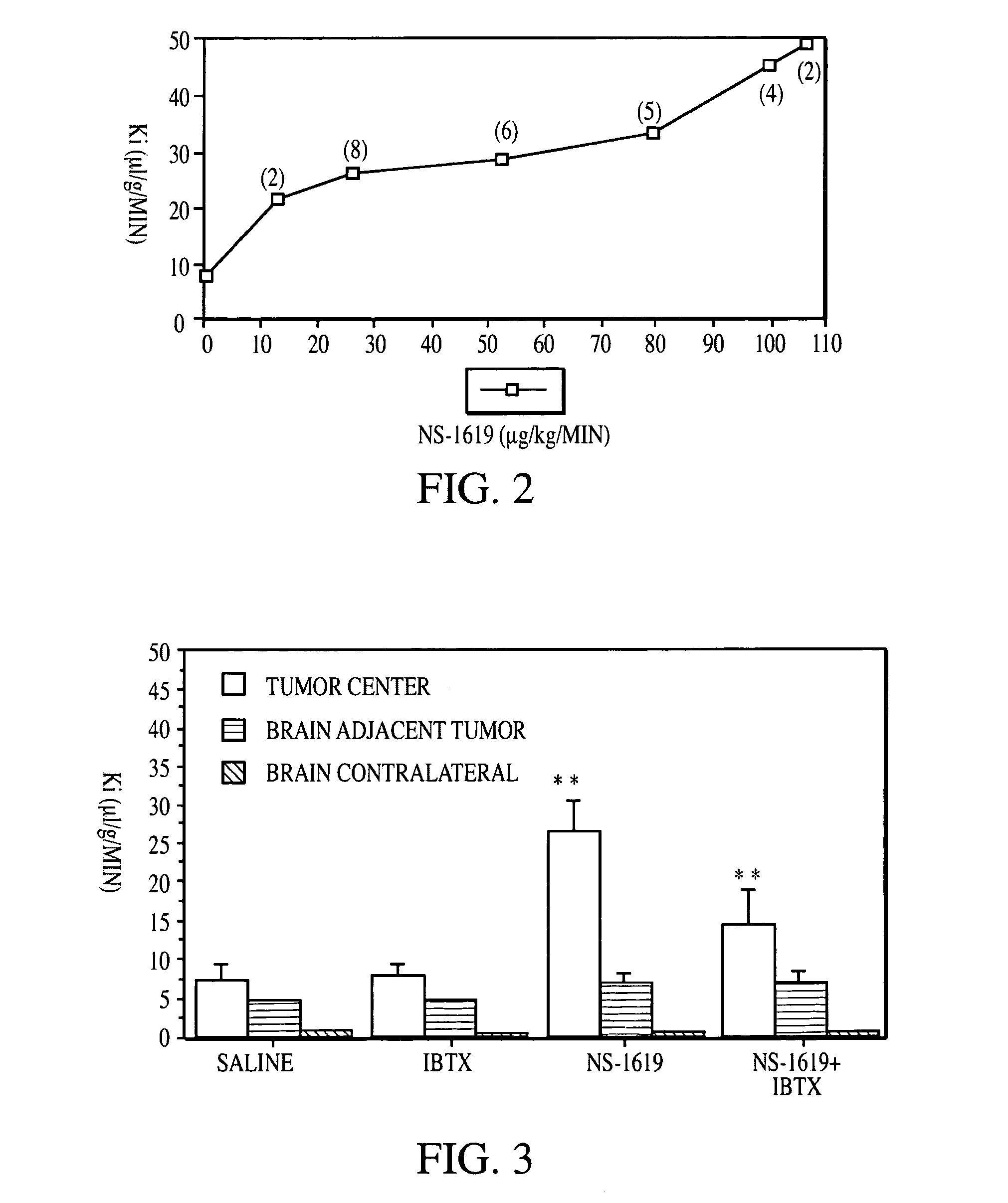
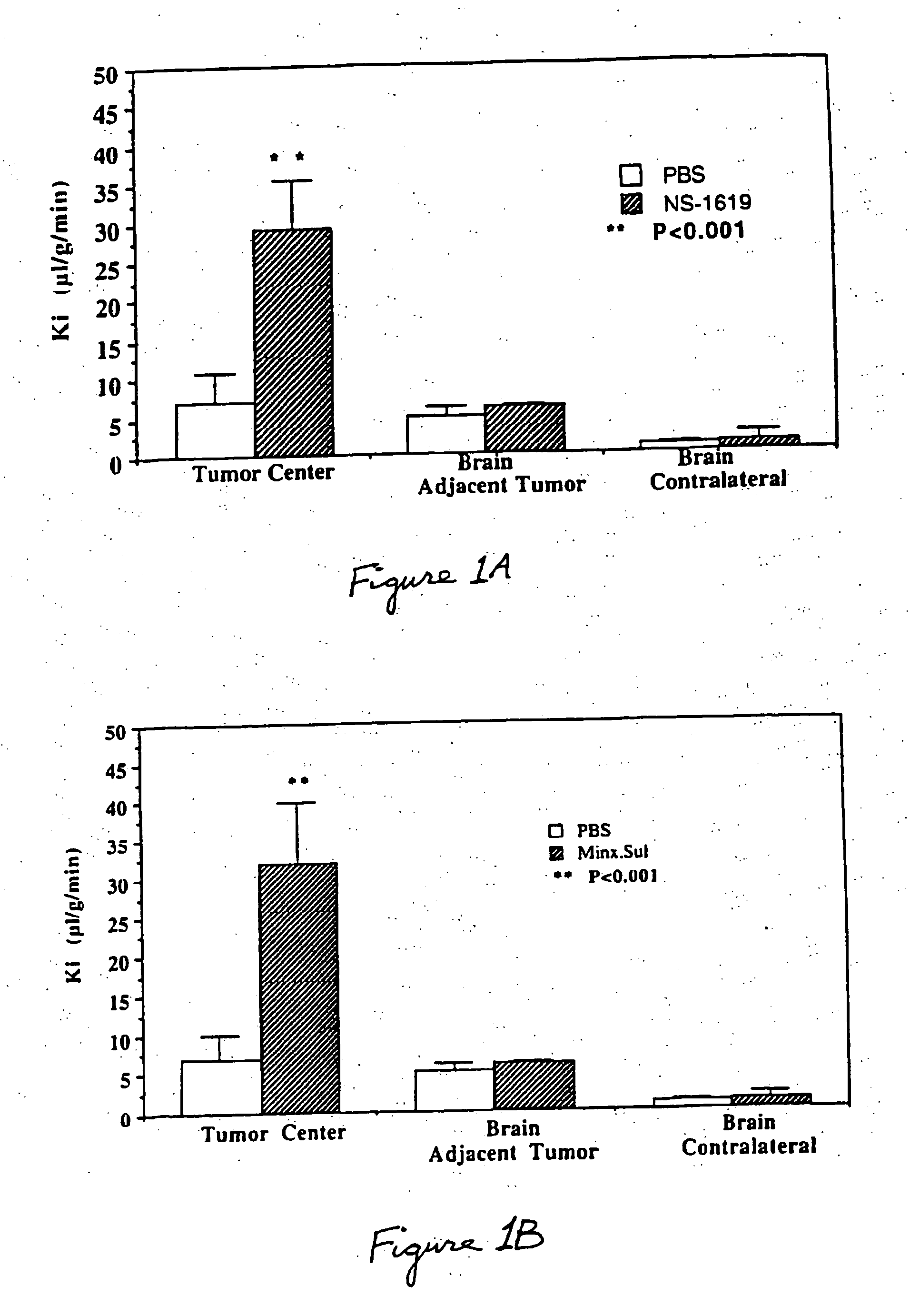
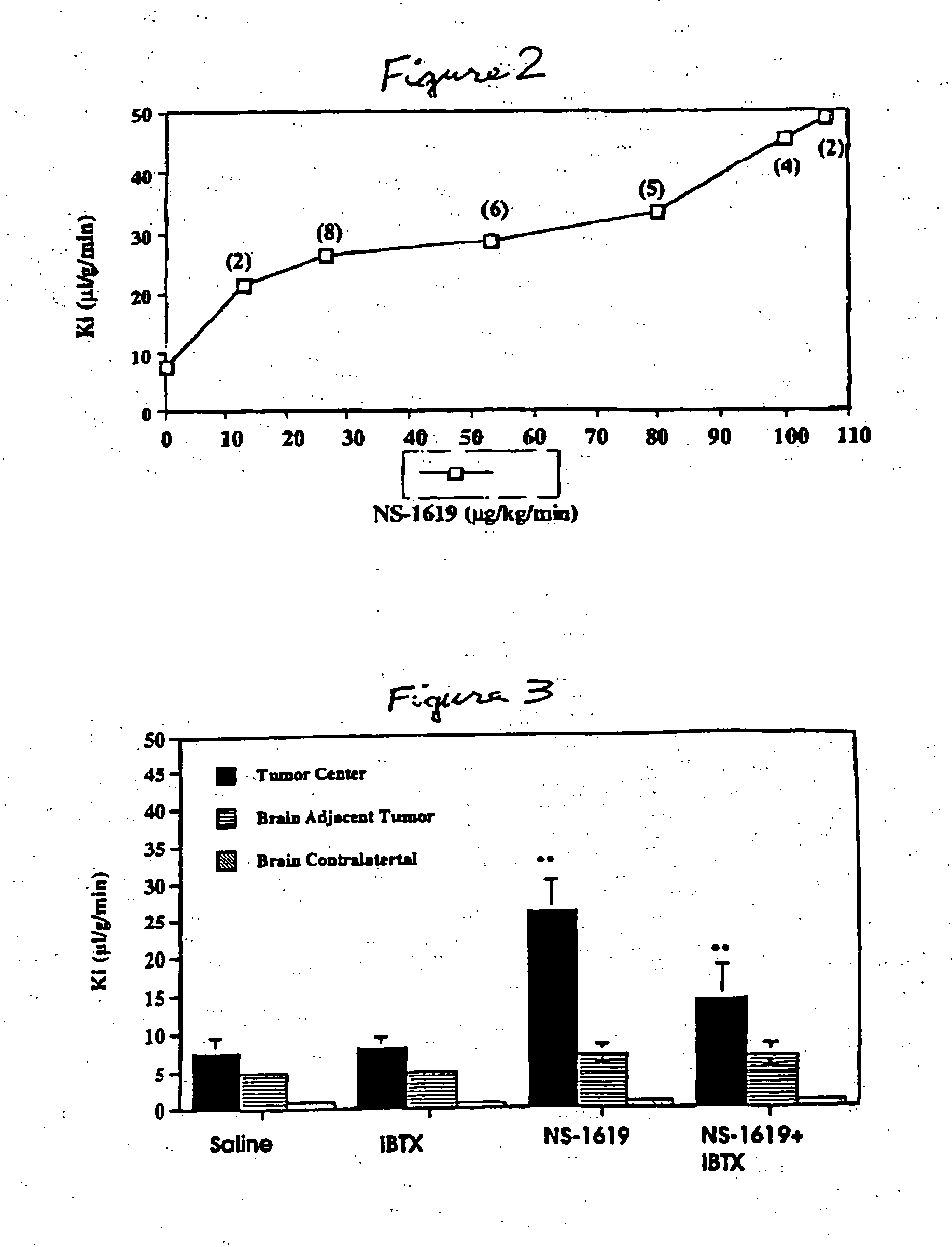
Method for using potassium channel agonists for delivering a medicant to an abnormal brain region and/or a malignant tumor
InactiveUS7018979B1Improve breathabilityHigh selectivityOrganic active ingredientsBiocideCyclasePotassium
Disclosed are methods of selectively delivering a medicant to an abnormal brain region and / or to a malignant tumor in a mammalian subject, including a human. A medicant is administered simultaneously or substantially simultaneously with a potassium channel agonist (other than bradykinin or a bradykinin analog), such as NS-1619,1-EBIO, a guanylyl cyclase activator, a guanylyl cyclase activating protein, minoxidil, pinacidil, cromakalim, or levcromakalim, whereby the medicant is delivered selectively to the cells of the abnormal brain region and / or to the tumor, compared to normal tissues. Thus, among the disclosures is a method of treating a malignant tumor in a human subject. Also disclosed are pharmaceutical compositions that combine a potassium channel agonist together with a medicant and a kit for enhancing the delivery of a medicant to an abnormal brain region and / or to a malignant tumor.
Owner:CEDARS SINAI MEDICAL CENT
Agonists of guanylate cyclase useful for downregulation of pro-inflammatory cytokines
InactiveUS20160235807A1Relieve symptomsInhibition of activationPeptide/protein ingredientsAntipyreticCyclaseDisease
This invention provides a method to prevent, control, and / or treat an inflammatory disease or disorder by administering at least one agonist of guanalyte cyclase receptor, or pharmaceutical compositions thereof, either alone or either concurrently or sequentially with another compound or an active agent used to treat the disease or disorder, and / or with an inhibitor of cGMP-dependent phosphodieasterases.
Owner:SYNERGY PHARMA
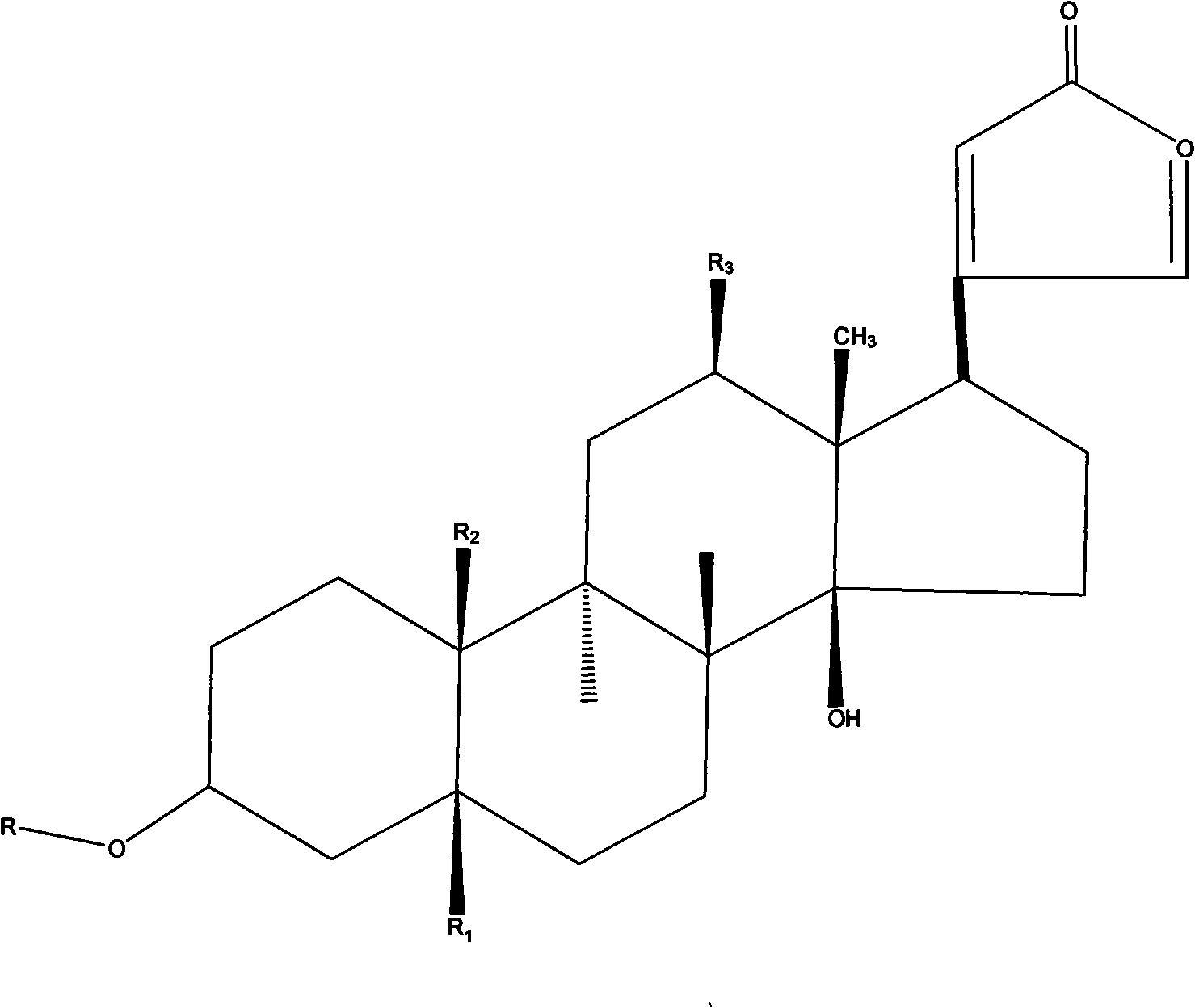
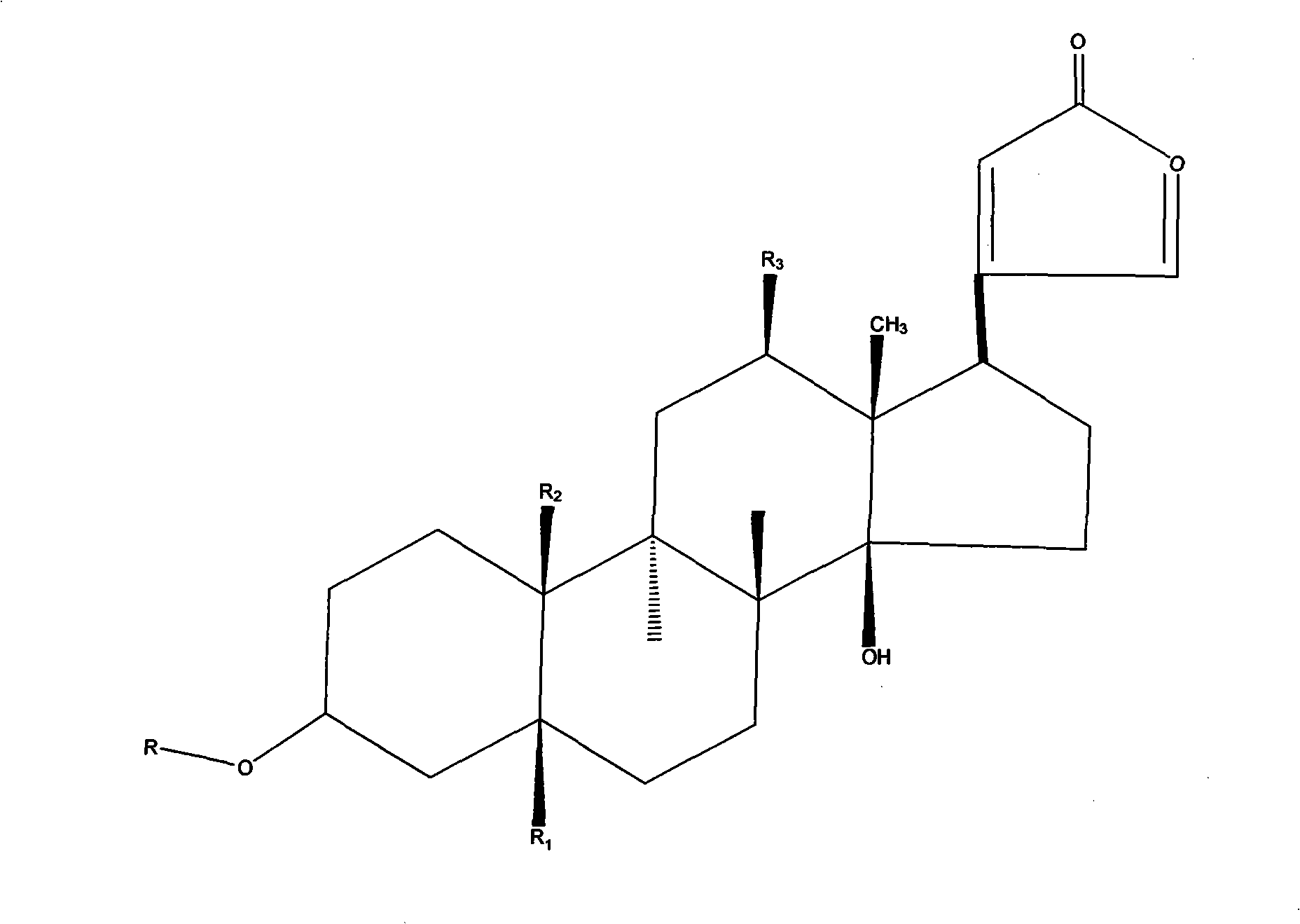
Heterocyclic carboxylic acids as activators of soluble guanylate cyclase
ActiveUS9353090B2Useful in treatmentReduce activationSenses disorderNervous disorderCarboxylic acidMedicinal chemistry
Owner:BOEHRINGER INGELHEIM INT GMBH
sGC STIMULATORS OR sGC ACTIVATORS ALONE AND IN COMBINATION WITH PDE5 INHBITORS FOR THE TREATMENT OF CYSTIC FIBROSIS
The present invention relates to soluble guanylate cyclase (sGC) and to phosphodiesterases (PDEs) and the pharmacology of sGC stimulators, sGC activators and PDE inhibitors. More particularly, the invention relates to the use of sGC stimulators and sGC activators in combination with PDE5 inhibitors for preparation of medicaments for the treatment of Cystic Fibrosis (CF).
Owner:ADVERIO PHARMA
Guanylate cyclase receptor agonists for the treatment of tissue inflammation and carcinogenesis
A method of treatment of inflamed, pre-cancerous or cancerous tissue or polyps in a mammalian subject is disclosed. The treatment involves administration of a composition of at least one peptide agonist of a guanylate cyclase receptor and / or other small molecules that enhance intracellular production of cGMP. The at least one peptide agonist of a guanylate cyclase receptor may be administered either alone or in combination with an inhibitor of cGMP-dependent phosphodiesterase. The inhibitor may be a small molecule, peptide, protein or other compound that inhibits the degradation of cGMP. Without requiring a particular mechanism of action, this treatment may restore a healthy balance between proliferation and apoptosis in the subject's population of epithelial cells, and also suppress carcinogenesis. Thus, the method may be used to treat, inter alia, inflammation, including gastrointestinal inflammatory disorders, general organ inflammation and asthma, and carcinogenesis of the lung, gastrointestinal tract, bladder, testis, prostate and pancreas, or polyps.
Owner:SYNERGY PHARMA
Natural activator of soluble guanylate cyclase
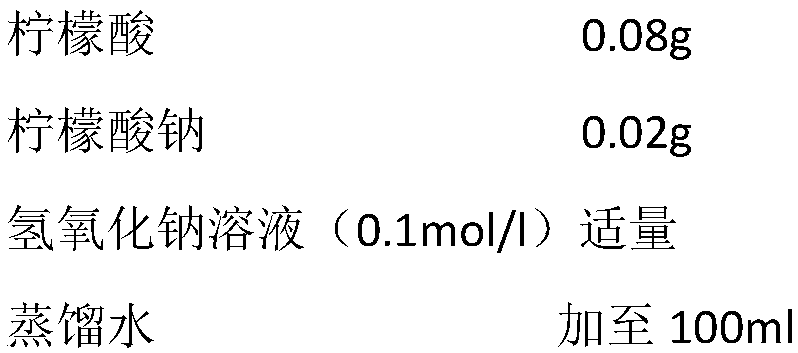
The invention relates to a natural activator of soluble guanylate cyclase, which is characterized in that the soluble guanylate cyclase in a human circulatory system is used as a selected target; <3>Hisotope is used for marking; and natural components capable of activating the soluble guanylate cyclase are selected from plant medicaments for treating cardiovascular-related diseases in Chinese, Indian and European traditional medicine. The natural components capable of activating the soluble guanylate cyclase in the human circulatory system under the condition of vitro experiments are obtainedthrough screening, and structure searches and further activation analyses are carried out on the natural components and metabolic components thereof in the human body. The mother nucleus structure ofa substance capable of activating the soluble guanylate cyclase and the side chain structure information of the mother nucleus are obtained so as to provide the pertinent information for the researchand development of medicaments related to the treatment of cardiovascular diseases relating to the soluble guanylate cyclase-cGMP signal conduction path.
Owner:JILIN UNIV
Isolated and purified human soluble guanylyl cyclase α1/β1 (hsgcα1/β1)
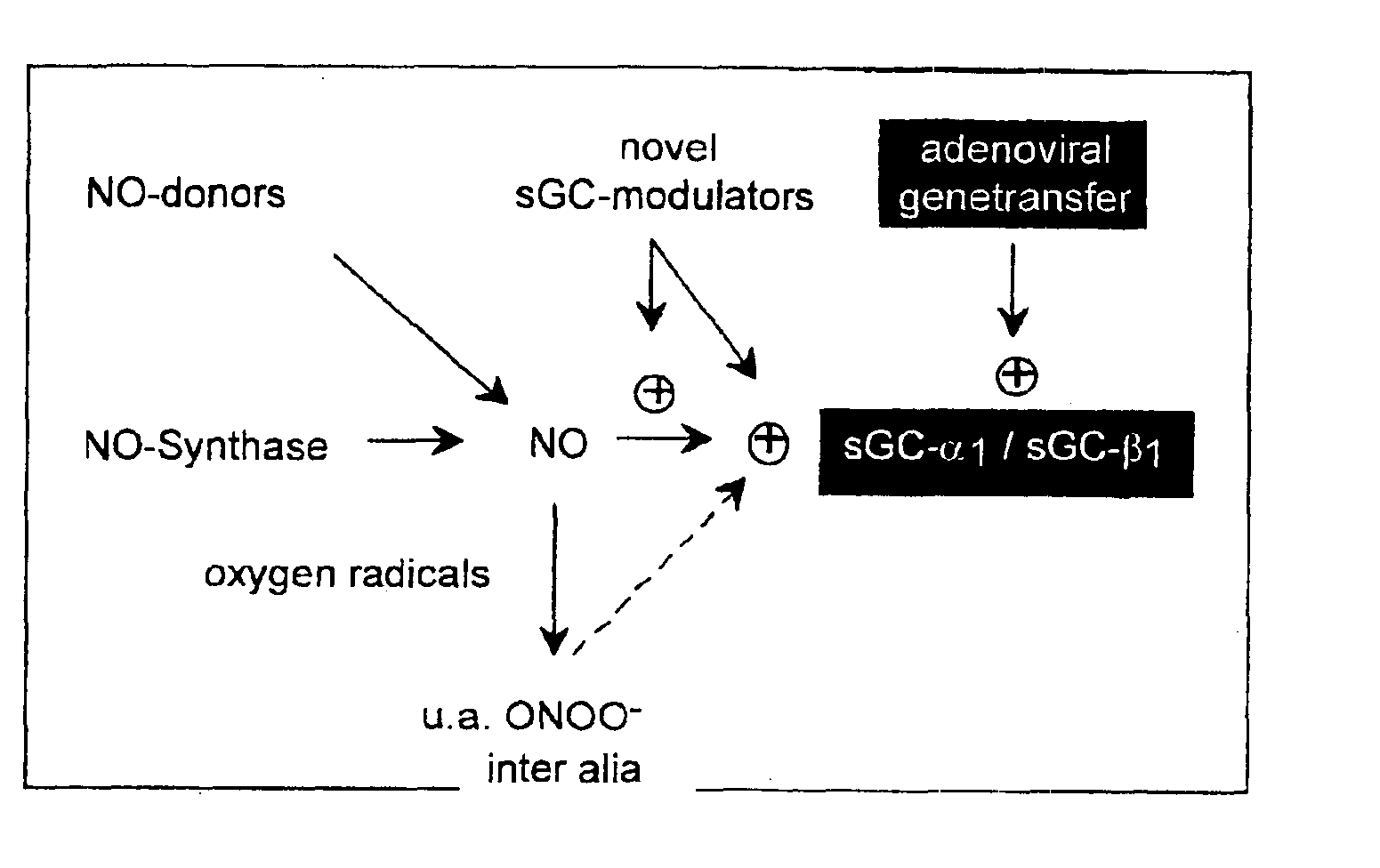
The invention relates to the expression of the cDNA clone for sub-units a1 (hsGCa1) and b1 (hsGCb1) of human soluble guanylylcyclase and the subsequent purification of the active enzyme and use thereof, the medical application of the expression of this clone by gene transfer, in addition to antibodies against peptides derived from said sequence and the use thereof.
Owner:VASOPHARM BIOTECH
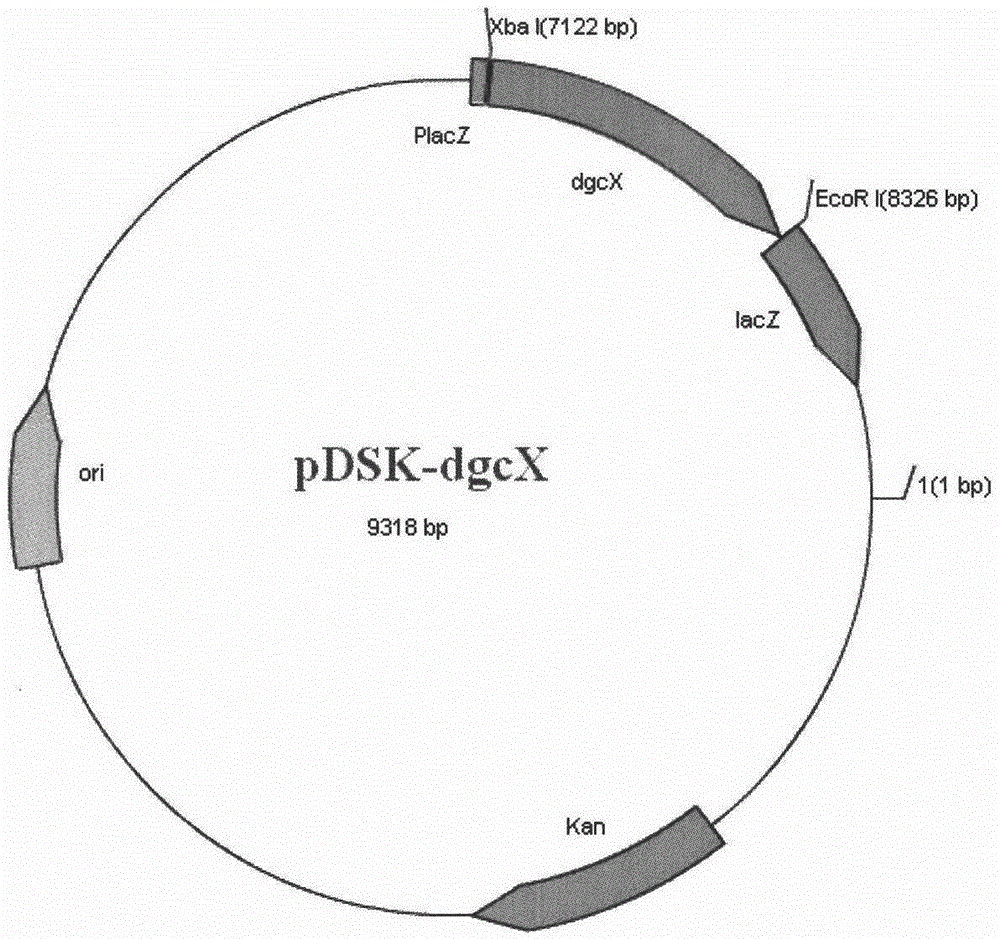
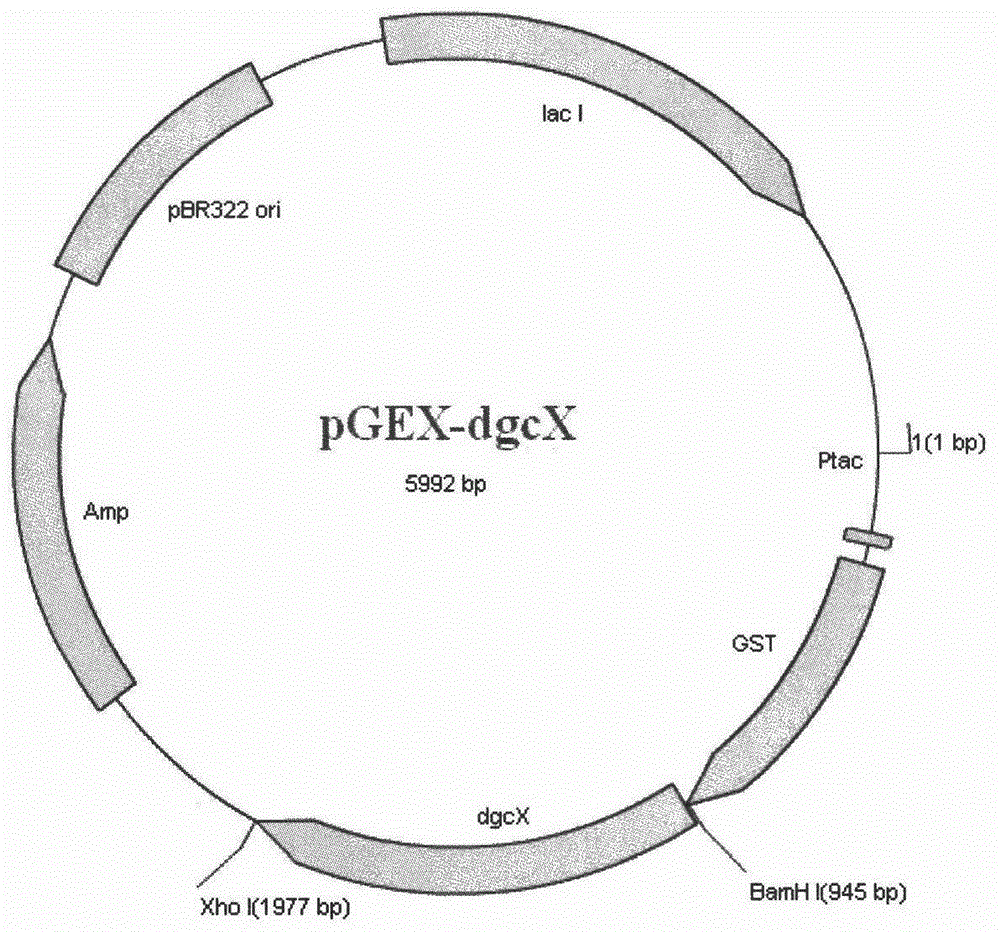
Diguanylate cyclase gene, vector, engineering bacteria and application
InactiveCN104152472ABacteriaMicroorganism based processesEscherichia coliRecombinant escherichia coli
The invention provides a diguanylate cyclase gene for coding diguanylate cyclase, a recombinant vector containing the gene, recombinant gene engineering bacteria obtained by transformation of the recombinant vector, and application thereof in the preparation of recombinant diguanylate cyclase. The diguanylate cyclase can be bonded with an expression vector to construct intracellular expression recombinant plasmid containing the genes for respectively correspondingly transforming to escherichia coli to obtain recombinant escherichia coli. The recombinant escherichia coli containing the recombinant diguanylate cyclase can be used as an enzyme source for biological catalysis and transformation. The recombinant diguanylate cyclase as a transformation enzyme can use 5'-guanosine triphosphate (GTP) trisodium as a substrate for transformation for preparation of cyclic di-guanylic monophosphate (c-di-GMP).
Owner:陶飞
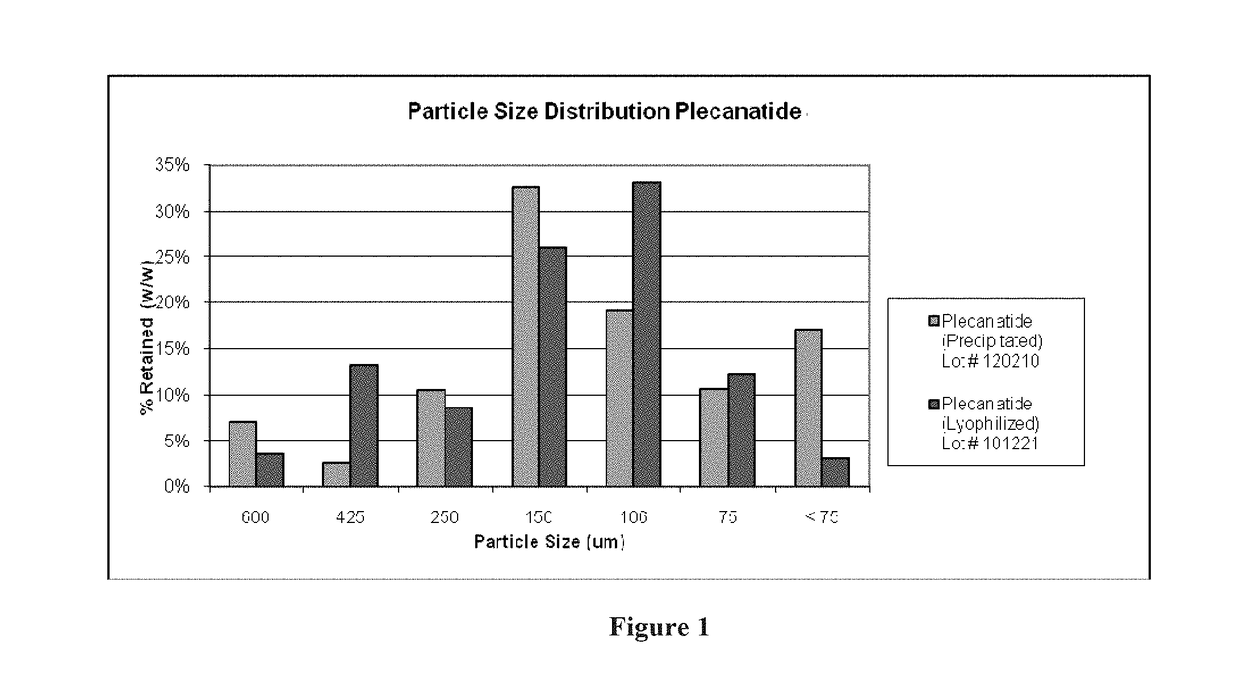
Ultra-pure agonists of guanylate cyclase C, method of making and using same
ActiveUS10011637B2Reduce the amount requiredPeptide/protein ingredientsPhosphorus-oxygen lyasesFreeze-dryingSolvent
Owner:BAUSCH HEALTH IRELAND LTD
Methods of use of inhibitors of phosphodiesterases and modulators of nitric oxide, reactive oxygen species, and metalloproteinases in the treatment of peyronie's disease, arteriosclerosis and other fibrotic diseases
ActiveUS8133903B2Small sizeReduce synthesisBiocidePharmaceutical delivery mechanismCyclaseFemale Sexual Arousal Disorder
The present methods and compositions are of use for treatment of conditions involving fibrosis, such as Peyronie's disease plaque, penile corporal fibrosis, penile veno-occlusive dysfunction, Dupuytren's disease nodules, vaginal fibrosis, clitoral fibrosis, female sexual arousal disorder, abnormal wound healing, keloid formation, general fibrosis of the kidney, bladder, prostate, skin, liver, lung, heart, intestines or any other localized or generalized fibrotic condition, vascular fibrosis, arterial intima hyperplasia, atherosclerosis, arteriosclerosis, restenosis, cardiac hypertrophy, hypertension or any condition characterized by excessive fibroblast or smooth muscle cell proliferation or deposition of collagen and extracellular matrix in the blood vessels and / or heart. In certain embodiments, the compositions may comprise a PDE-4 inhibitor, a PDE-5 inhibitor, a compound that elevates cGMP and / or PKG, a stimulator of guanylyl cyclase and / or PKG, a combination of a compound that elevates cGMP, PKG or NO with an antioxidant that decreases ROS, or a compound that increases MMP activity.
Owner:LOS ANGELES BIOMEDICAL RES INST AT HARBOR UCLA MEDICAL CENT
Agonists of guanylate cyclase and their uses
Owner:BAUSCH HEALTH IRELAND LTD
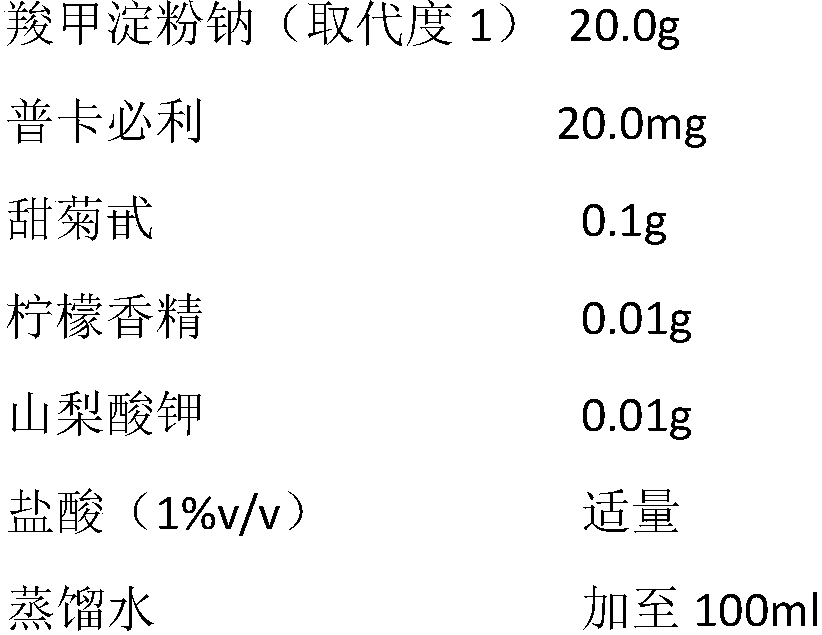
Pharmaceutical composition for treating constipation and application thereof
InactiveCN108904808AIncrease the average daily defecation volumeIncrease the number ofElcosanoid active ingredientsPeptide/protein ingredientsOral suspensionsMotility
The invention discloses the combined application of a pharmaceutical composition composed of water-soluble starch salt modified by carboxylic acid groups and gastrointestinal motility promoting drugsor secretion promoting drugs in the prevention and treatment of constipation, the pharmaceutical composition can be suitable for various functional constipation, especially has good curative effects on old age refractory constipation, chronic constipation, habitual constipation, drug-induced constipation and the like, has slight adverse reactions, and can be suitable for various crowds. The representative drug of the water-soluble starch salt modified by the carboxylic acid groups is sodium carboxymethyl starch, and the gastrointestinal motility promoting drugs or secretion promoting drugs areselected from dopamine receptor antagonist, 5-HT4 receptor agonist, motilin receptor agonist, chloridion channel activator, guanylate cyclase agonist. The two components are combined according to a suitable weight ratio and can be prepared into any oral pharmaceutical dosage form including oral solution, oral suspension, granule, powder, tablet, capsule and the like.
Owner:XIAN LIBANG PHARMA TECH
Novel genes related to glutaminyl cyclase
ActiveCN101573450AOrganic active ingredientsOrganic chemistryIsozymeGlutaminyl-Peptide Cyclotransferase
The present invention relates to novel glutaminyl-peptide cyclotransferase-like proteins (QPCTLs), which are isoenzymes of glutaminyl cyclase (QC, EC 2.3.2.5), and to isolated nucleic acids coding for these isoenzymes, all of which are useful for the discovery of new therapeutic agents, for measuring cyclase activity, and for determining the inhibitory activity of compounds against these glutaminyl cyclase isoenzymes.
Owner:VIVORYON THERAPEUTICS NV
Method for using potassium channel agonists for delivering a medicant to an abnormal brain region and/or a malignant tumor
InactiveUS20050095196A1Improve breathabilityHigh selectivityHeavy metal active ingredientsBiocideCyclasePotassium
Disclosed are methods of selectively delivering a medicant to an abnormal brain region and / or to a malignant tumor In a mammalian subject, including a human. A medicant is administered simultaneously or substantially simultaneously with a potassium channel agonist (other than bradykinin or a bradykinin analog), such as NS-1619,1-EBIO, a guanylyl cyclase activator, a guanylyl cyclase activating protein, minoxidil, pinacidil, cromakalim, or levcromakalim, whereby the medicant is delivered selectively to the cells of the abnormal brain region and / or to the tumor, compared to normal tissues. Thus, among the disclosures is a method of treating a malignant tumor in a human subject. Also disclosed are pharmaceutical compositions that combine a potassium channel agonist together with a medicant and a kit for enhancing the delivery of a medicant to an abnormal brain region and / or to a malignant tumor.
Owner:CEDARS SINAI MEDICAL CENT
Bioremediation, detoxication and plant-growth enhancing compositions and methods of making and using such compositions
InactiveUS6268206B1Enhance and facilitate plant growthImprove scalabilityBiocideSolid waste disposalMicroorganismGrowth plant
A composition containing cAMP, cGMP, forskolin, adenylate cyclase or guanylate cyclase and microorganisms is provided to facilitate bioremediation, detoxication and to enhance plant growth in media contaminated with petroleum hydrocarbons. Methods to make the composition and apply it to the contaminated media in order to facilitate bioremediation and detoxication of such contaminants are also provided.
Owner:LIPTAK DAVID
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com