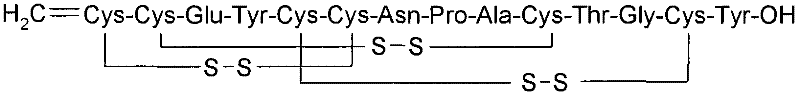Stable solid formulation of a GC-C receptor agonist polypeptide suitable for oral administration
A drug and dosage form technology, applied in the field of stable solid preparations of guanylate cyclase-C receptor agonist polypeptides suitable for oral administration, can solve the problems of inconvenient freezing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0087] Example 1-15: Preparation of Linaclotide Preparations
[0088] The linaclotide formulations of Examples 1-15 were produced essentially as described in Formulation Protocol A, wherein Table 1 provides the amounts of cation, sterically hindered primary amine, binder, linaclotide, and beads, while Table 1 2 provides the conditions for coated beads:
[0089] Table 1
[0090]
[0091]
[0092] * "Cation" means a divalent cation contained in the salt used in the examples, "amine" means a sterically hindered primary amine, and [ ] means a molar ratio of cation and / or amine to linaclotide.
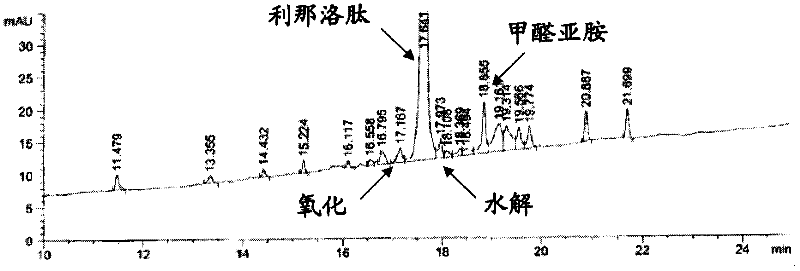
[0093] ** The concentration of linaclotide in this and all following examples was determined based on the peptide content and chromatographic purity listed on the certificate of analysis provided for each manufacturing batch of linaclotide active pharmaceutical ingredient (API). quantity.
[0094] Table 2
[0095]
Embodiment 16
[0096] Example 16: Preparation of Linaclotide Preparations
[0097] The linaclotide formulation of Example 16 was produced essentially as described in formulation protocol B, wherein Table 3 provides the amounts of cation, sterically hindered primary amine, binder, linaclotide, and beads, while Table 4 provides Conditions for coated beads:
[0098] table 3
[0099]
[0100] Table 4
[0101]
Embodiment 17
[0102] Example 17: Preparation of Linaclotide Preparations
[0103] The linaclotide formulation of Example 17 was produced essentially as described in formulation protocol A, except that the formulation contained 22.96 mg of butylated hydroxyanisole (BHA), where Table 5 provides the cation, sterically hindered primary amine, binder , linaclotide and the amount of beads, while Table 6 provides the conditions for coating the beads.
[0104] table 5
[0105]
[0106] Table 6
[0107]
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com