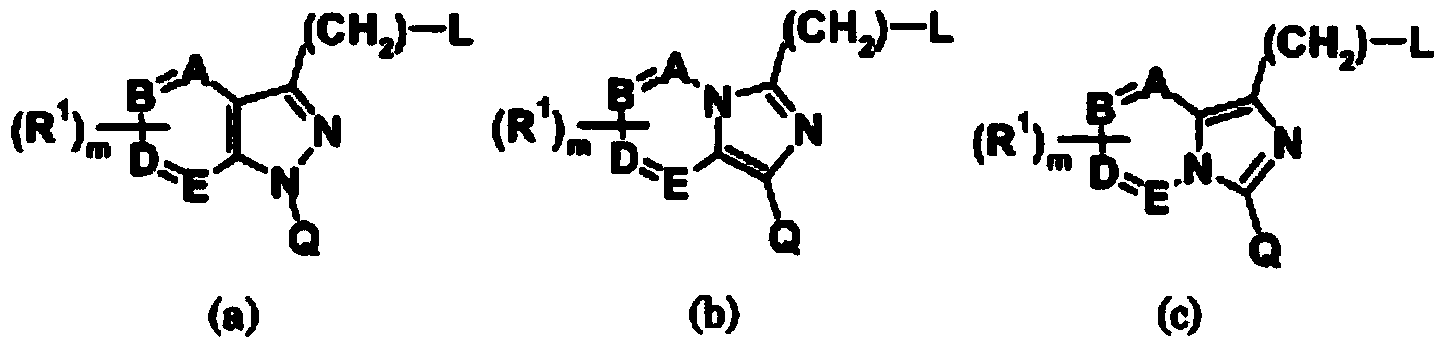
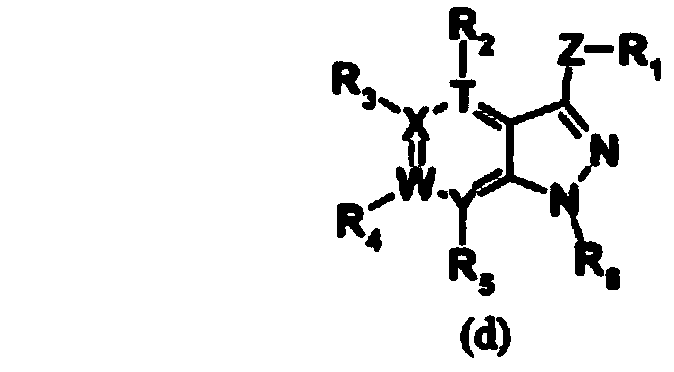
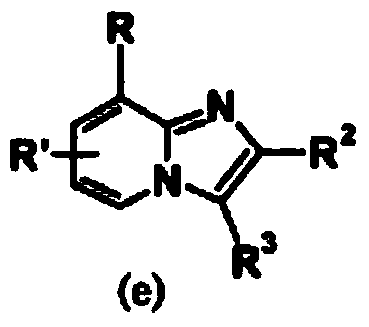
Imidazopyridine compound
A compound and group technology, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problems of compounds not being disclosed, not enlightening, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0322] Hereinafter, the production method of the compound of formula (I) is demonstrated in more detail based on an Example. It should be noted that the present invention is not limited to the compounds described in the following examples. In addition, the production method of the raw material compound is shown in the production example. The compound of formula (I) is not limited to the production methods described in the examples below, but can also be produced using a combination of these production methods or a method obvious to those skilled in the art.
[0323] In addition, the following abbreviations may be used in Examples, Production Examples, and Tables described later. PEx: Manufacturing example number, Ex: Example number, Str: Structural formula, Dat: Physical data (ESI+: ESI-MS[M+H] + or ESI-MS[M] + ;ESI-:ESI-MS[M-H] - ;FAB+: FAB-MS[M+H] + or FAB-MS[M] + ;EI+:EI[M] + ;APCI / ESI+:APCI / ESI-MS[M+H] + or APCI / ESI-MS[M] + (APCI / ESI refers to the simultaneous det...
manufacture example 1
[0329] A suspension of 1 g of 5-methyl-2-nitropyridin-3-ol, 1.35 ml of (bromomethyl)cyclohexane, and 1.79 g of potassium carbonate in 10 ml of DMF was stirred at 78° C. for 12 hours. After cooling naturally at room temperature, water and hexane / ethyl acetate were added to the reaction liquid, and a liquid separation operation was performed. The organic layer was washed with water and saturated brine, and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure to obtain 1.8 g of 3-(cyclohexylmethoxy)-5-methyl-2-nitropyridine.
manufacture example 2
[0331] To a solution of 1.8 g of 3-(cyclohexylmethoxy)-5-methyl-2-nitropyridine in 16 ml of THF was added 325 mg of 10% palladium-carbon (hydrate) and stirred under a hydrogen atmosphere for 3 hours. After filtering the reaction liquid through celite, the solvent was distilled off under reduced pressure to obtain 1.38 g of 3-(cyclohexylmethoxy)-5-methylpyridin-2-amine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com