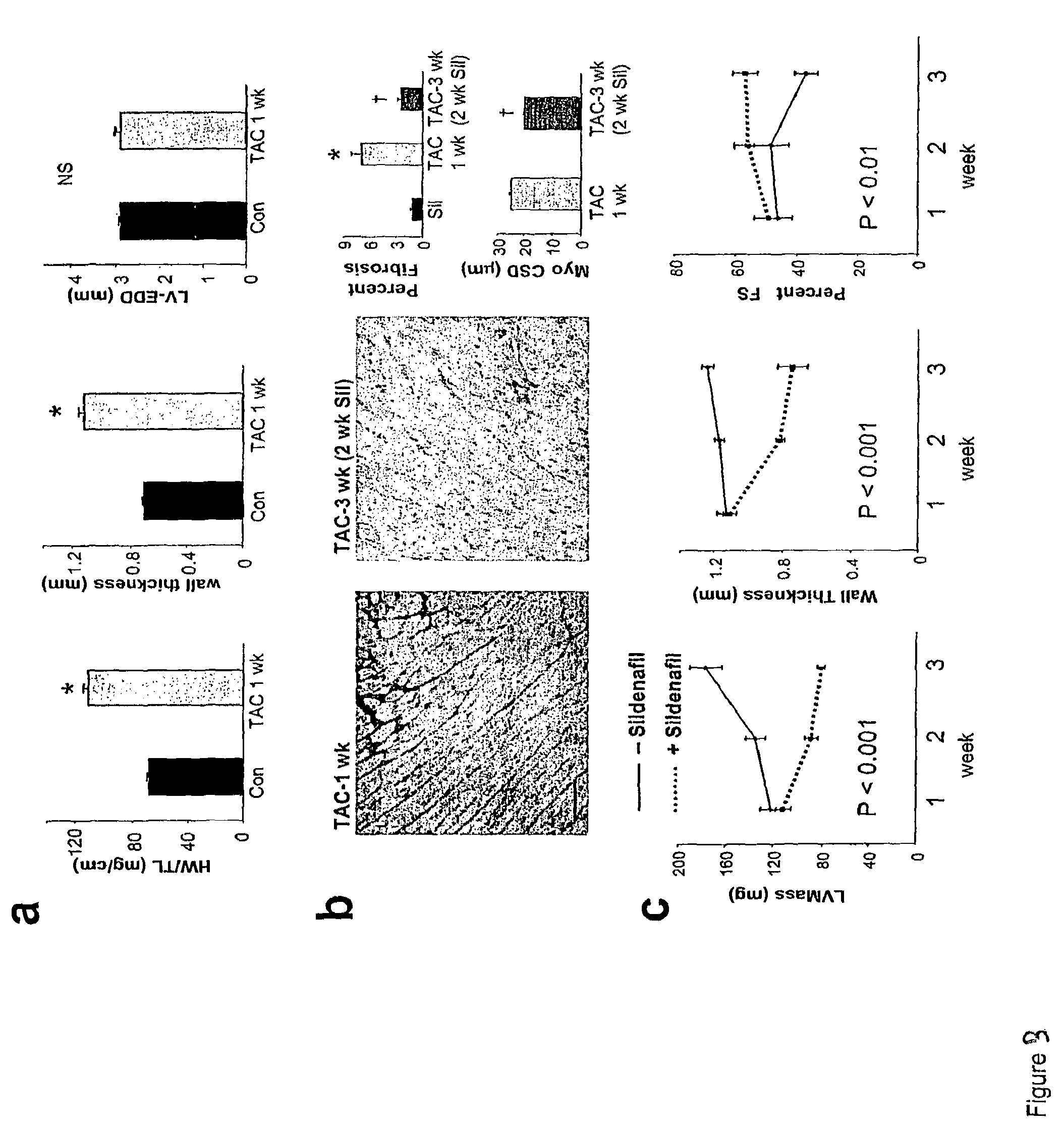
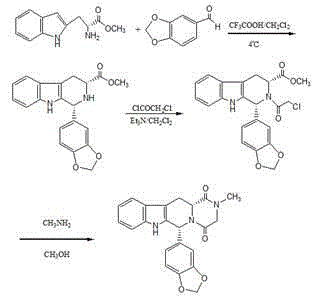
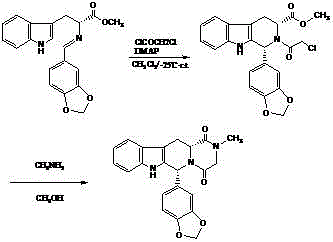
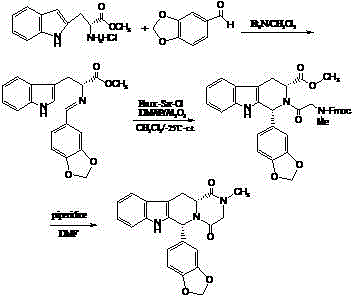
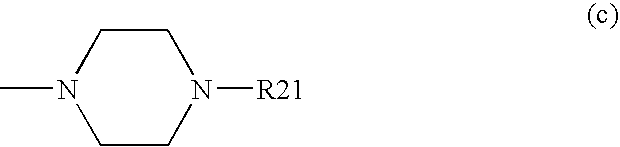Patents
Literature
103 results about "Phosphodiesterase 5 inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
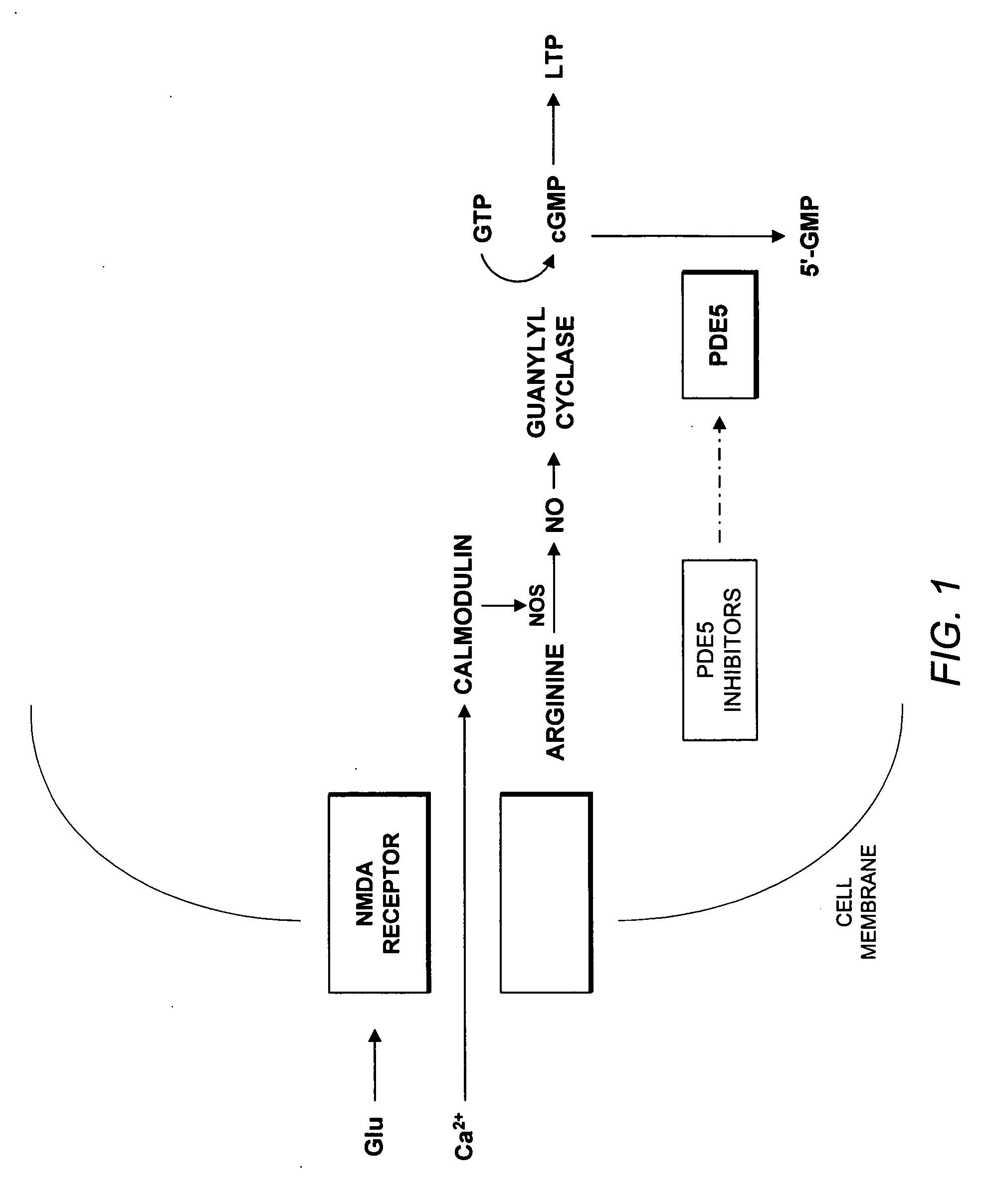
A phosphodiesterase type 5 inhibitor (PDE5 inhibitor) is a drug used to block the degradative action of cGMP-specific phosphodiesterase type 5 (PDE5) on cyclic GMP in the smooth muscle cells lining the blood vessels supplying various tissues. These drugs dilate the corpora cavernosa of the penis, facilitating erection with sexual stimulation, and are used in the treatment of erectile dysfunction (ED). Sildenafil was the first effective oral treatment available for ED. Because PDE5 is also present in the smooth muscle of the walls of the arterioles within the lungs, sildenafil and tadalafil dilates those vessels, and are FDA-approved for the treatment of pulmonary hypertension.
Pulmonary delivery of inhibitors of phosphodiesterase type 5
InactiveUS20060099269A1BiocideOrganic active ingredientsPhosphodiesterase 5 inhibitorSexual dysfunction
Owner:MANNKIND CORP
Treatment of sexual dysfunction
Bombesin receptor antagonists have been found to be useful in the treatment of sexual dysfunction in both males and females. They may be selective BB1 antagonists or mixed BB1 / BB2 antagonists. Combinations are disclosed of bombesin receptor antagonists with a range of other active compounds, for example PDE5 inhibitors, NEP inhibitors and lasofoxifene.
Owner:WARNER-LAMBERT CO
Beta-carboline pharmaceutical compositions
InactiveUS6841167B1Organic active ingredientsOrganic chemistryPhosphodiesterase 5 inhibitorSexual dysfunction
A soft capsule containing a solution or suspension of a PDE5 inhibitor, and use of the capsule in treating sexual dysfunction.
Owner:ICOS CORP
Use of phosphodiesterase 5 (PDE5) inhibitors in the treatment of schizophrenia
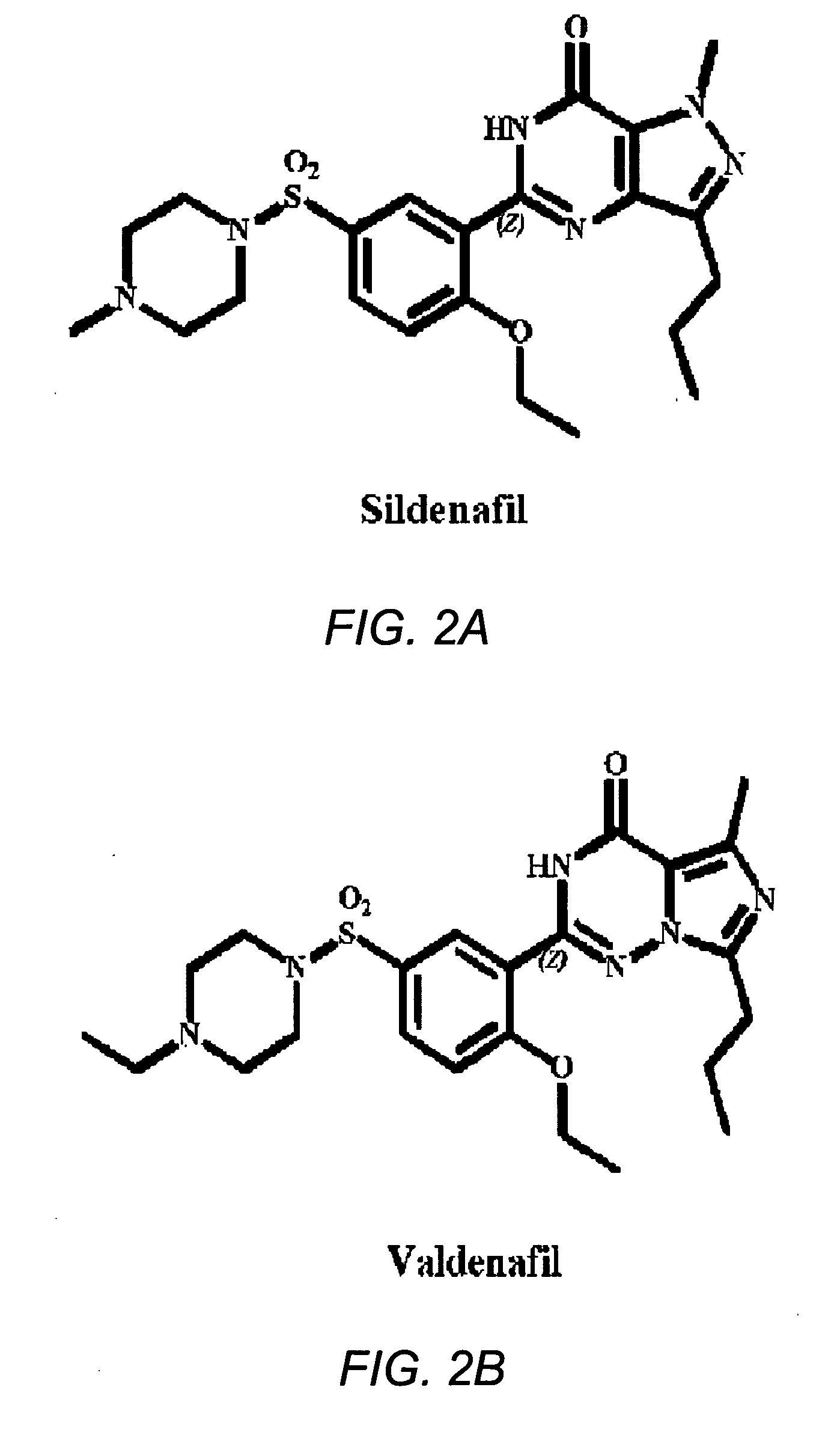
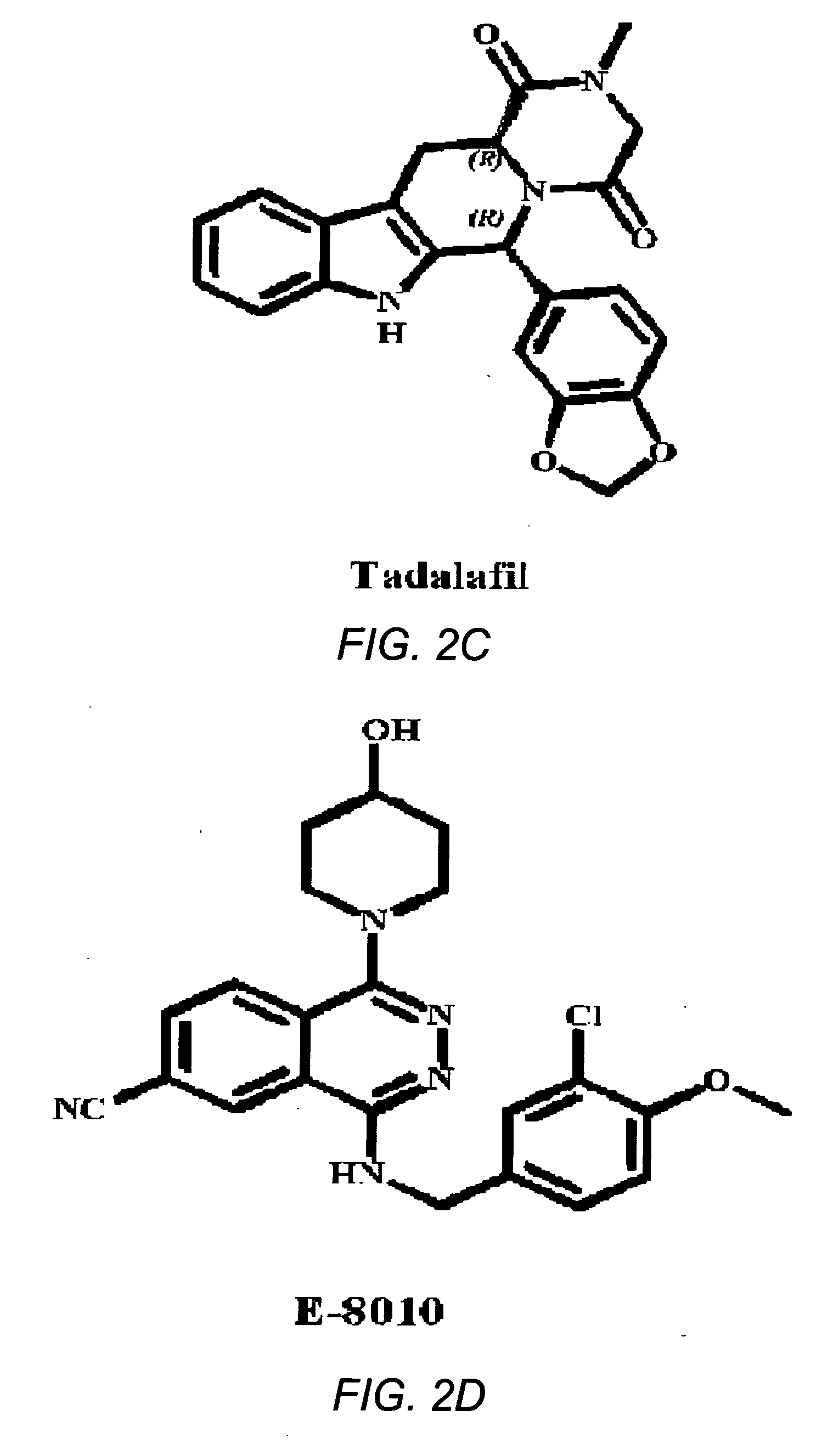
The use of phosphodiesterase 5 (PDE5) inhibitors for treatment of schizophrenia is described. Suitable PDE5 inhibitors for use for treatment of schizophrenia include sildenafil, vardenafil, tadalafil, E-8010, zaprinast, and E-4021. In one embodiment, for example, a method is described for treating schizophrenia in a patient which comprises treating the patient with an effective amount of a PDE5 inhibitor, or a pharmaceutically acceptable salt, solvate, or composition thereof. The PDE5 inhibitor may be administered orally. The PDE5 inhibitor may also be administered together with one or more conventional antipsychotic medications such as risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, clozapine, haloperidol, and fluphenazine.
Owner:SHARY CIRCLE
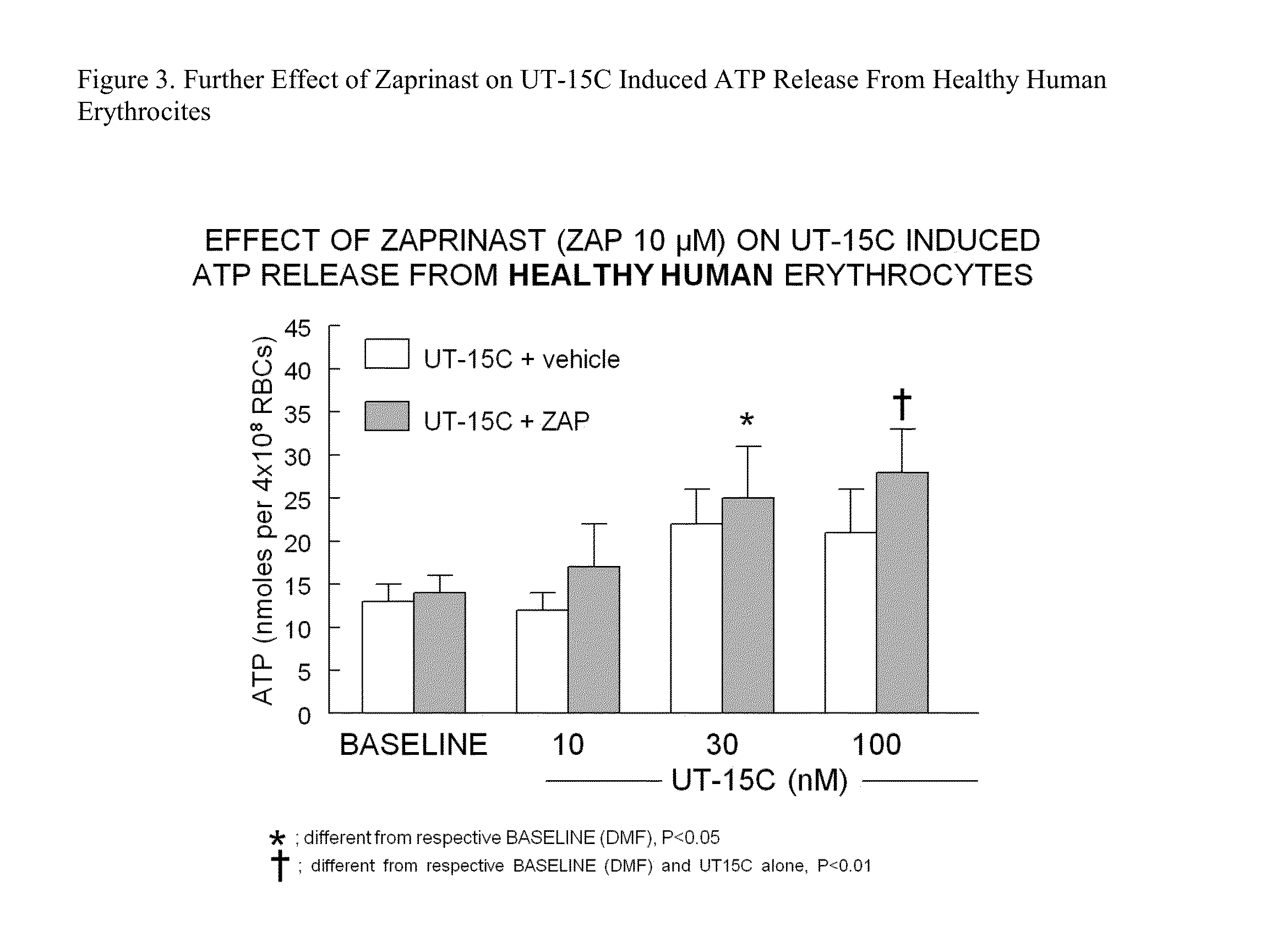
Method of identifying therapies for pulmonary hypertension
ActiveUS20130184295A1High activityBiocideOrganic active ingredientsPhosphodiesterase 5 inhibitorPde5 inhibition
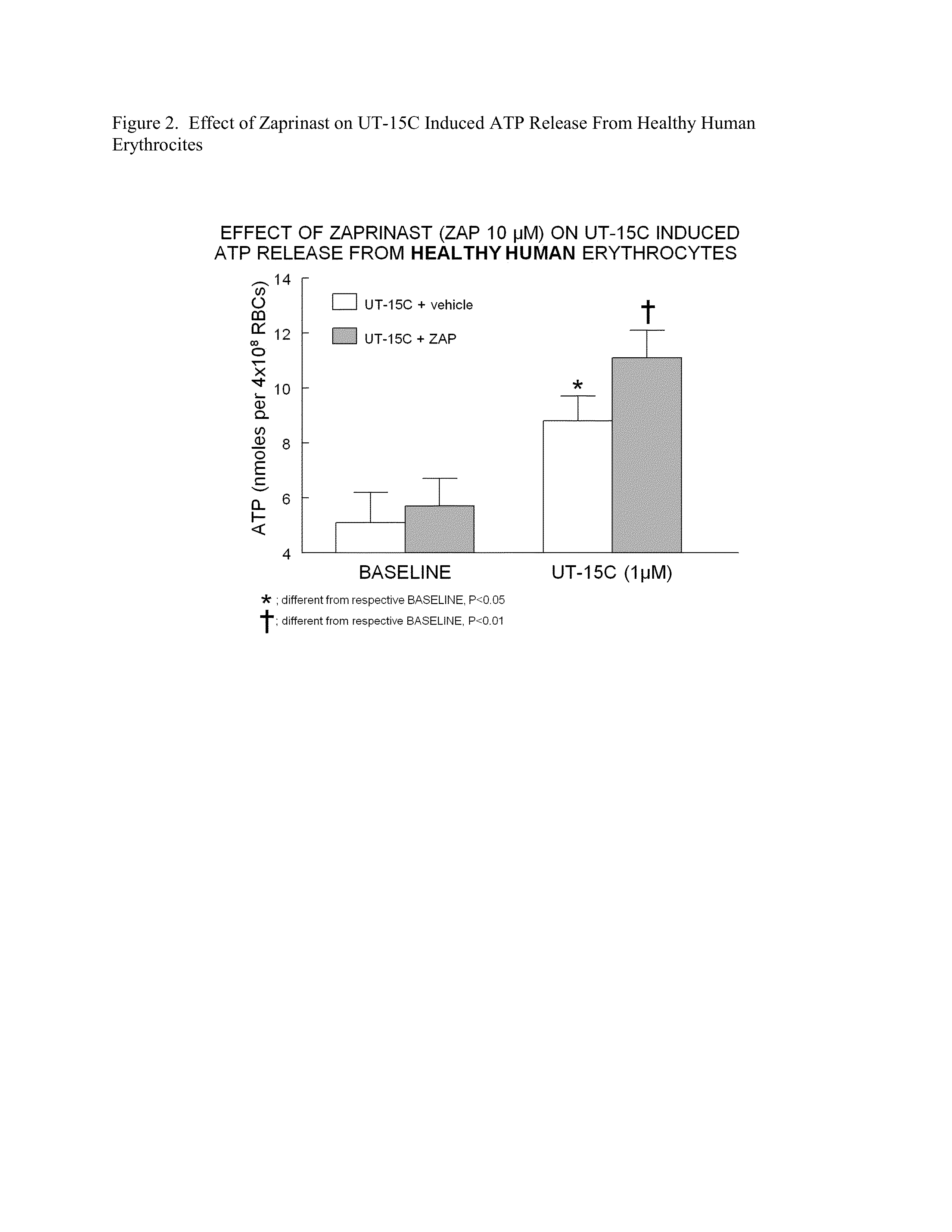
The present invention is directed to a method of screening for a therapeutic agent useful for treating pulmonary hypertension comprising: contacting an erythrocyte with a candidate therapeutic agent; and detecting a presence or absence of erythrocyte-derived adenosine triphosphate, wherein a greater erythrocyte-derived adenosine triphosphate level indicates the candidate therapeutic agent has greater activity in treating pulmonary hypertension. Additionally, the present invention is directed to methods of treating pulmonary arterial hypertension by stimulating ATP release from erythrocytes through co-administration to a subject in need thereof an amount of a PDE5 inhibitor compound, and an amount of a prostacyclin compound.
Owner:UNITED THERAPEUTICS CORP
Beta-carboline pharmaceutical compositions
Formulations containing a PDE5 inhibitor, a water-soluble diluent, a lubricant, a hydrophilic binder, a disintegrant, and optional microcrystalline cellulose and / or a wetting agent, and their use in treating sexual dysfunction, are disclosed.
Owner:ICOS CORP
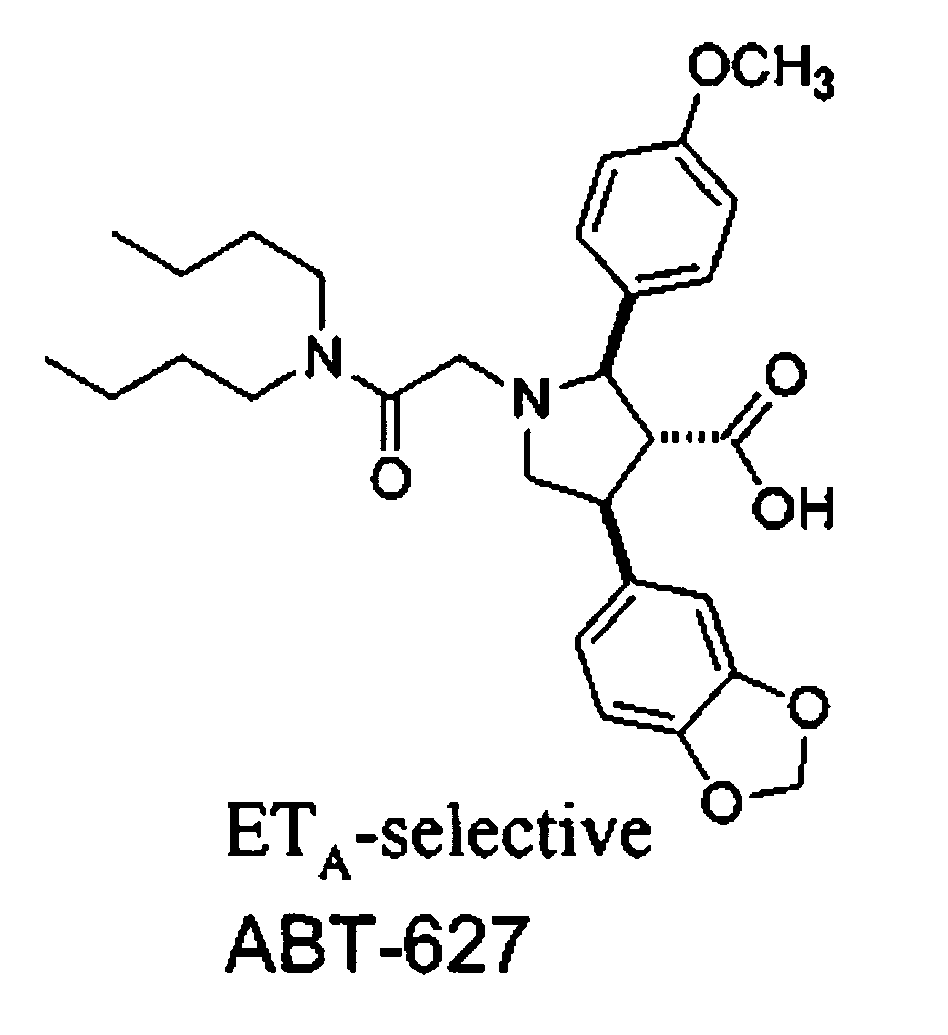
Endothelin a receptor antagonists in combination with phosphodiesterase 5 inhibitors and uses thereof
InactiveUS20060205733A1Eliminate side effectsLow toxicityBiocideNervous disorderDiseasePhosphodiesterase 5 inhibitor
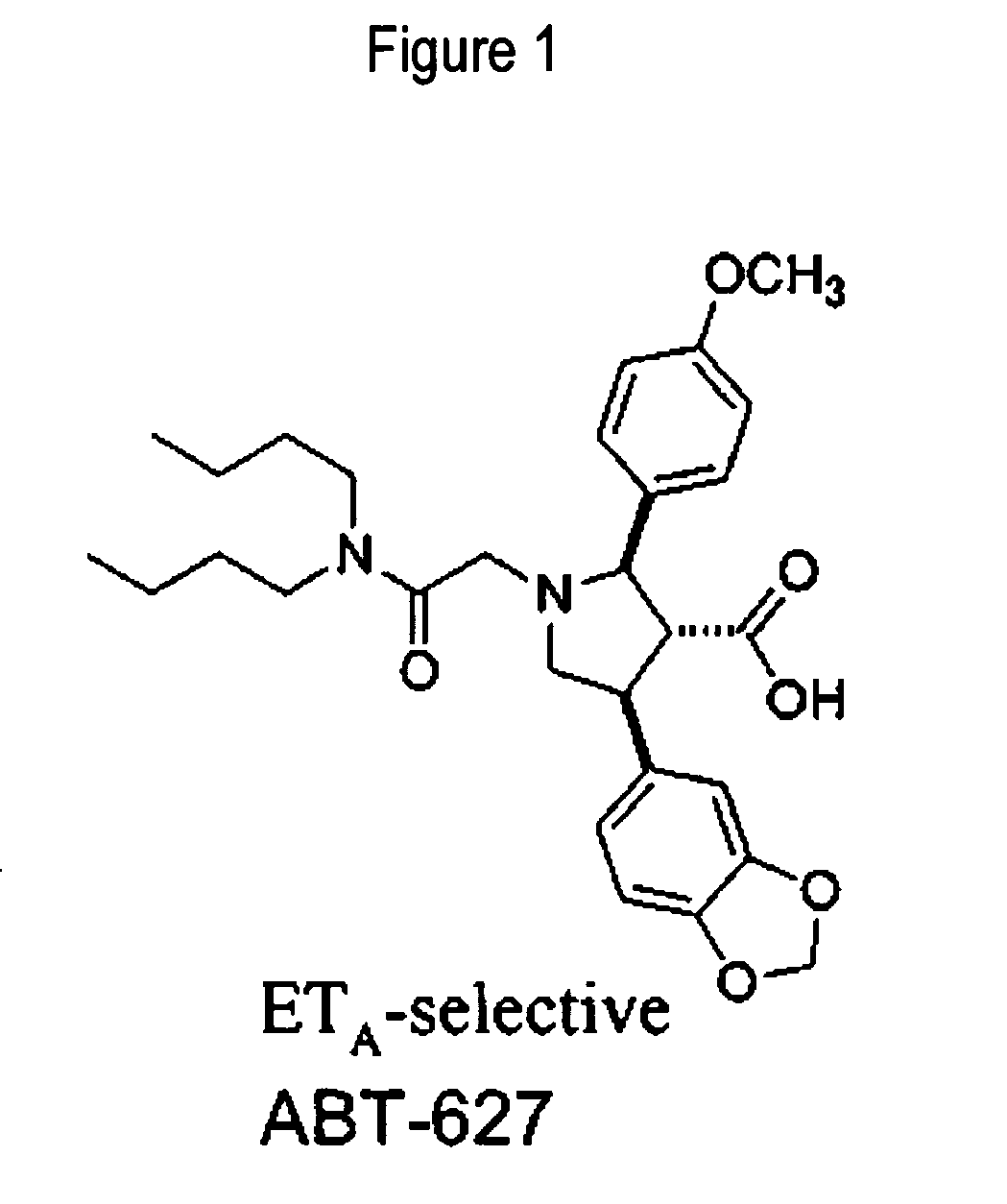
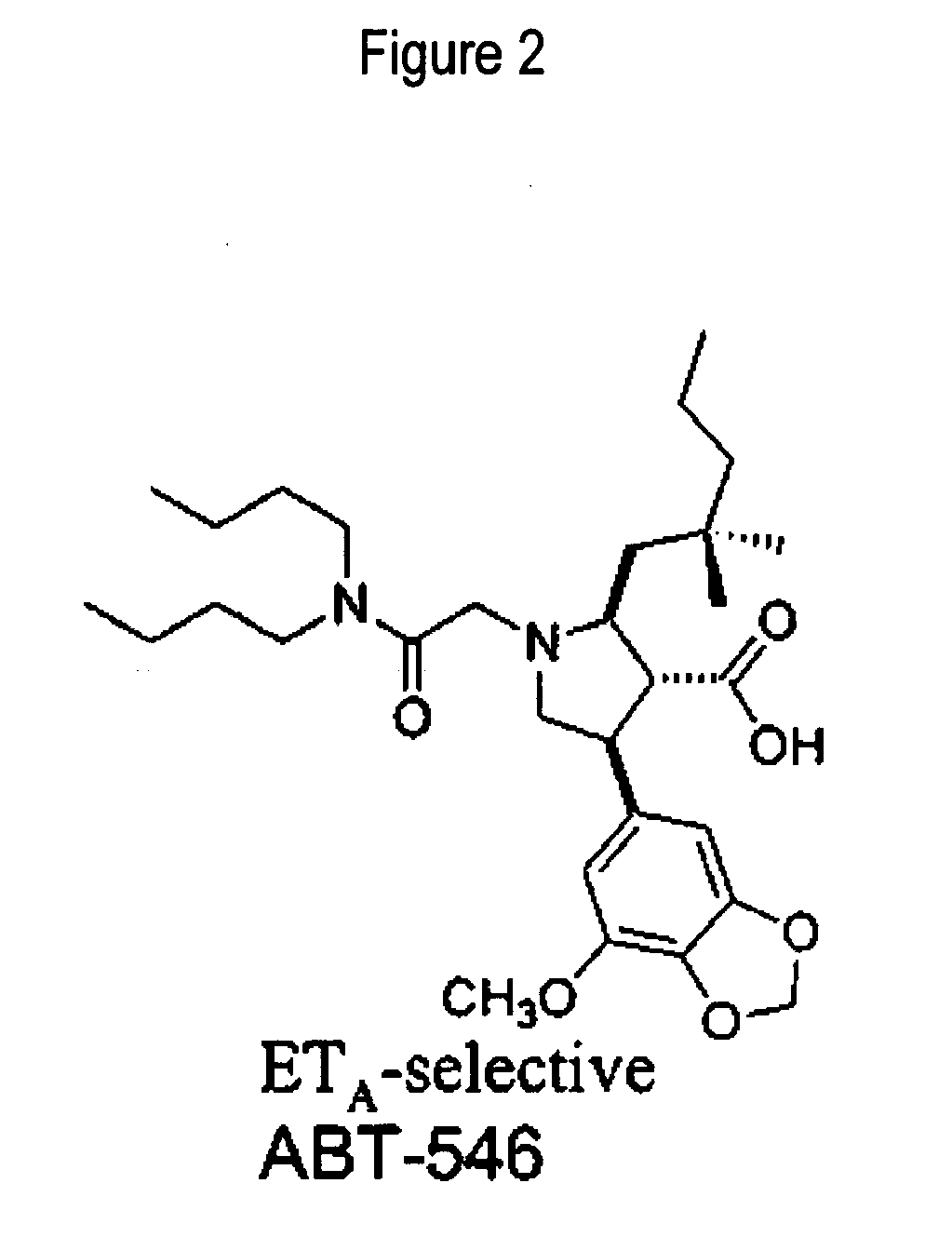
The invention relates generally to combination therapies comprising an endothelin A receptor (ETA) antagonist and a phosphodiesterase 5 (PDE5) inhibitor, pharmaceutical compositions comprising ETA antagonist and PDE5 inhibitor and methods of treating various disorders comprising administering an ETA antagonist and a PDE5 inhibitor. In particular, the combination therapies and pharmaceutical compositions are useful for the treatment and / or prevention of cardiac disorders such as pulmonary arterial hypertension (PAH).
Owner:ENCYSIVE PHARMA INC
Rapidly absorbing oral formulations of PDE 5 inhibitors
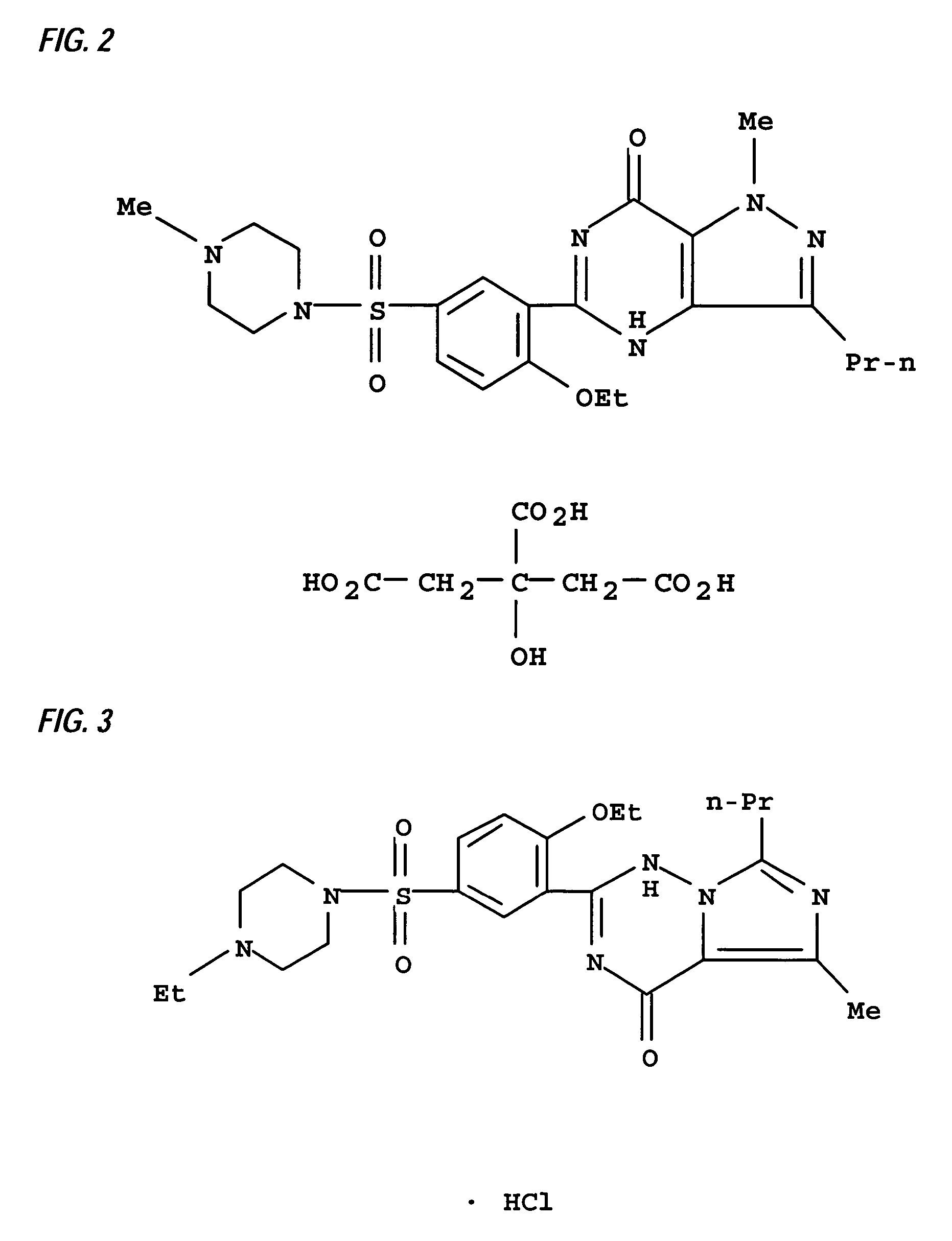
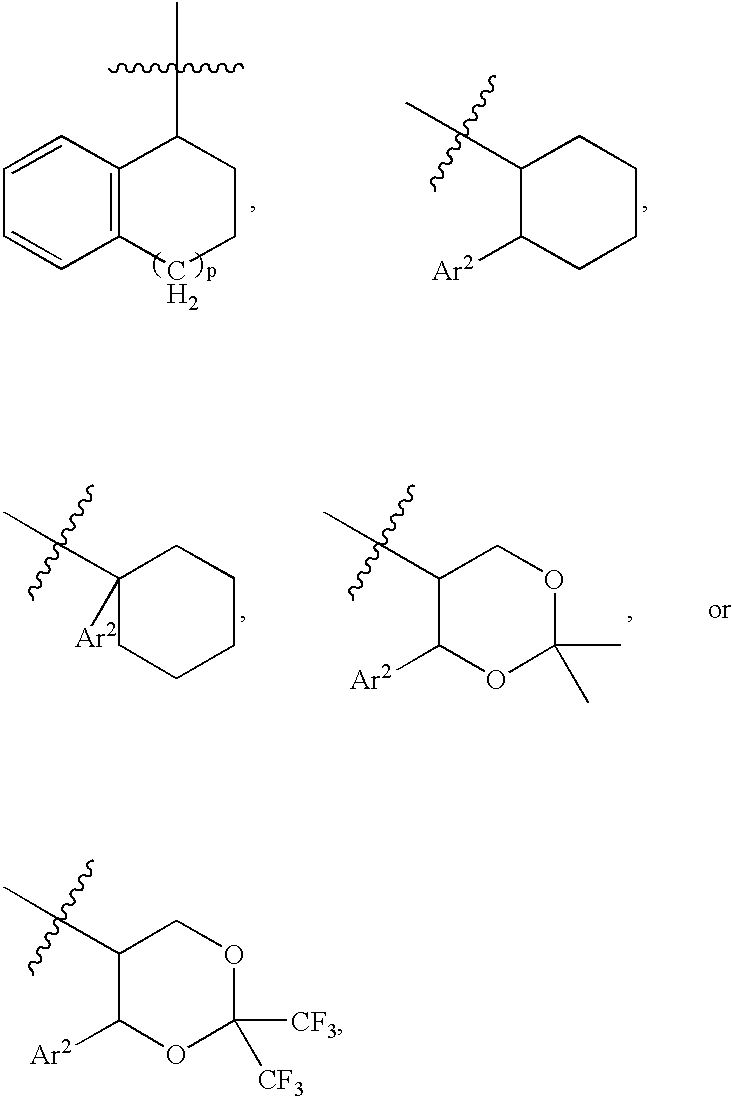
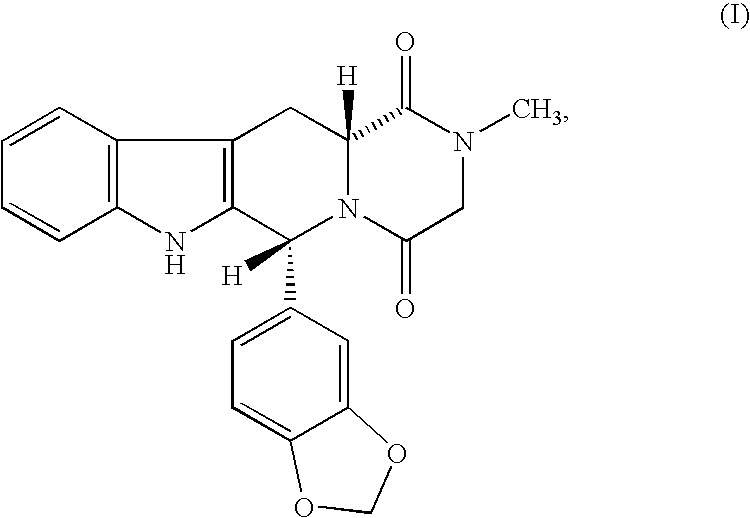
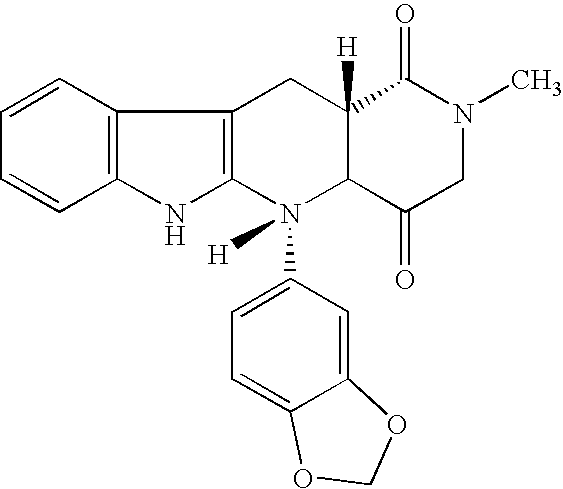
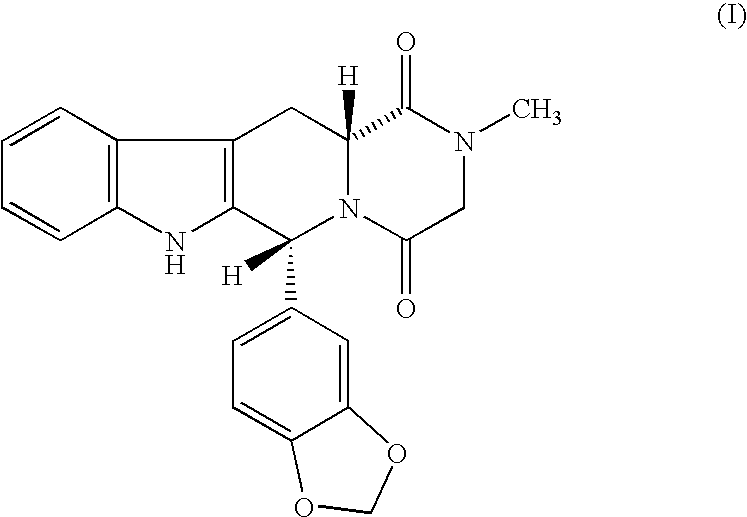
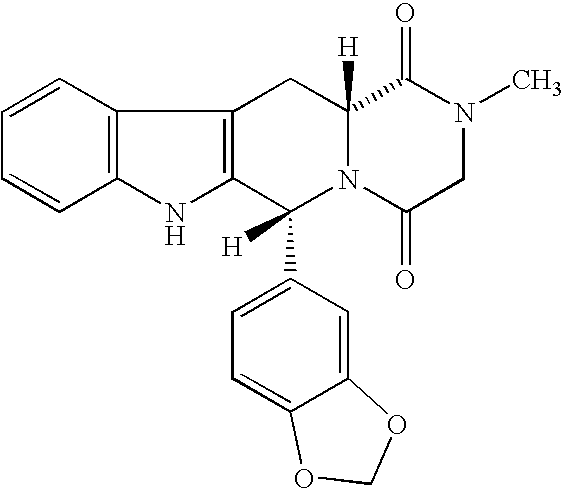
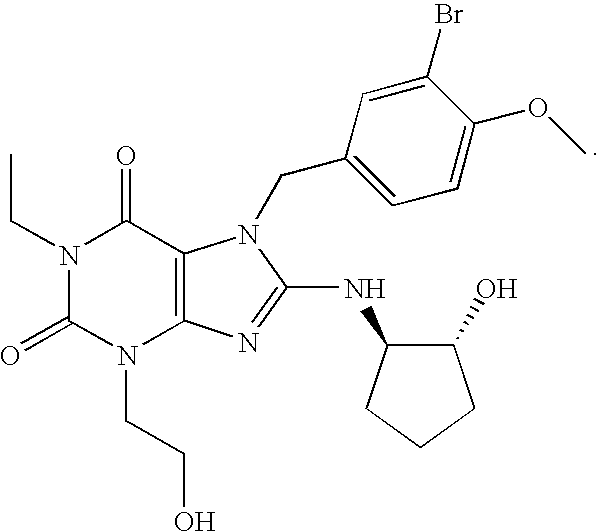
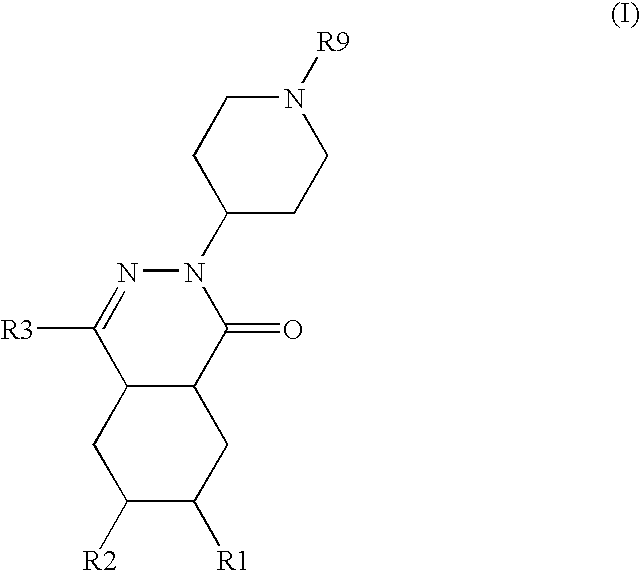
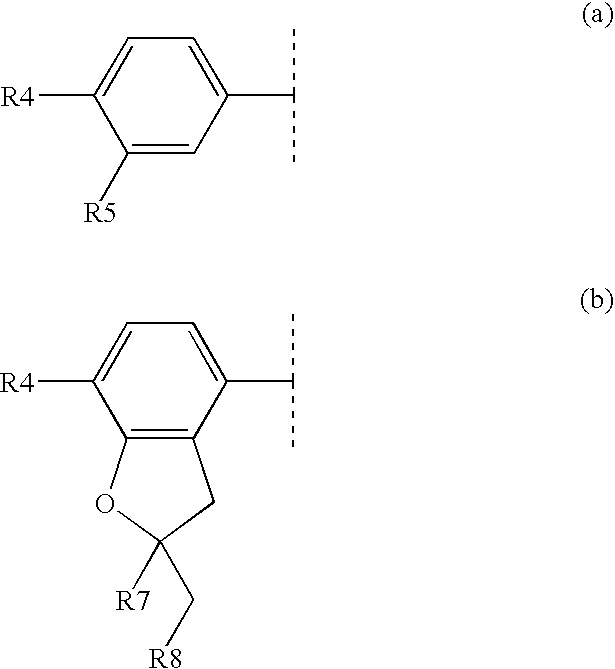
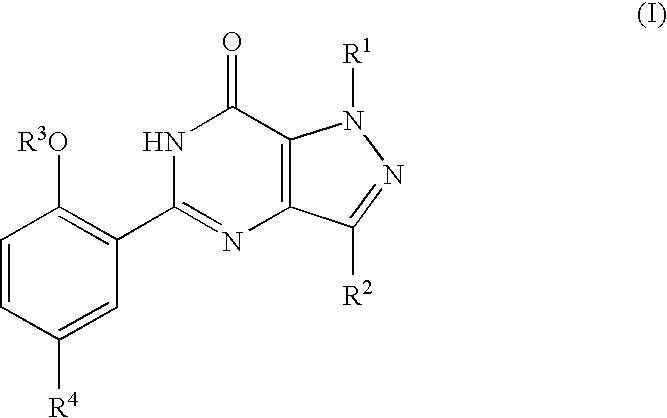
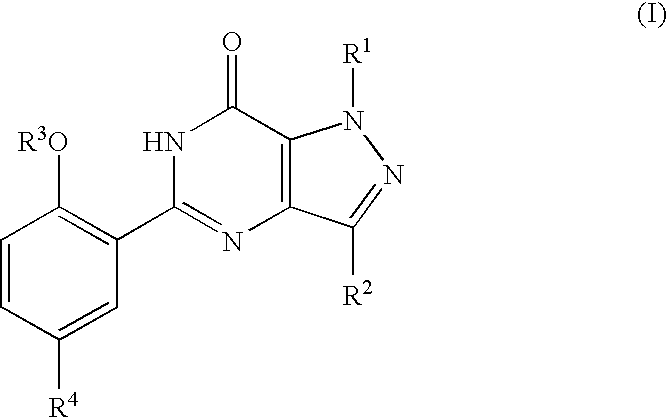
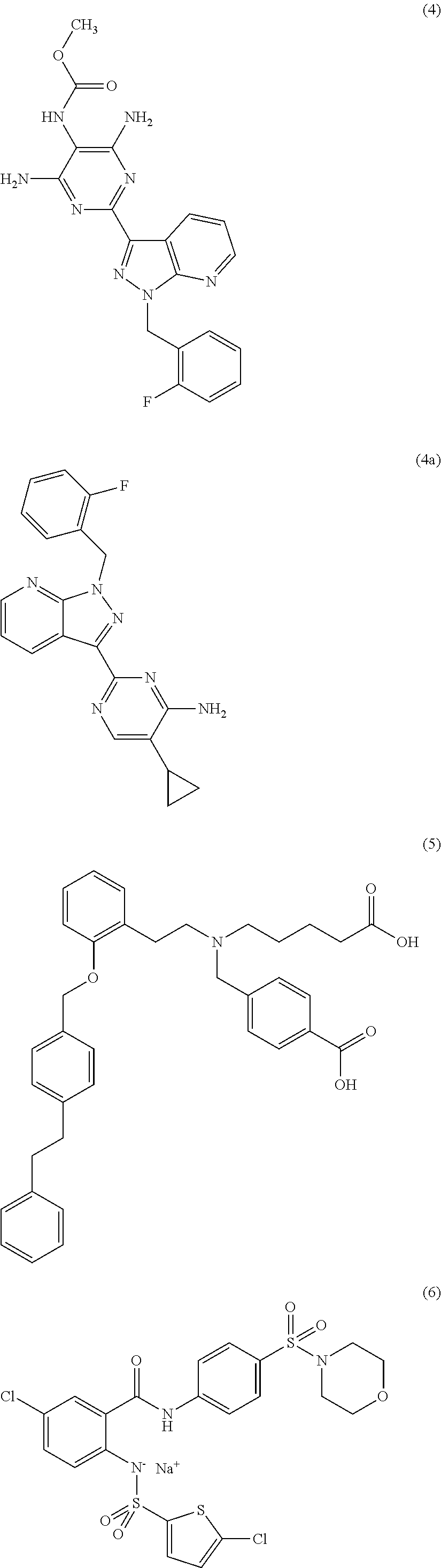
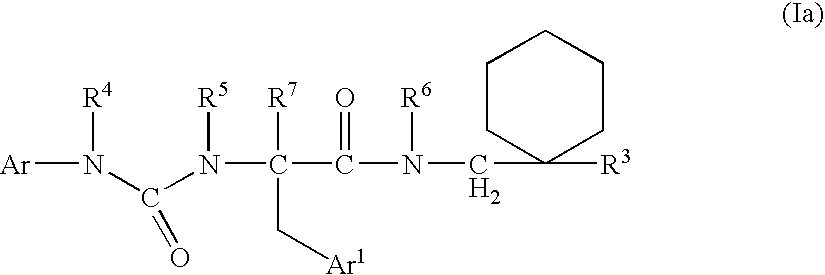
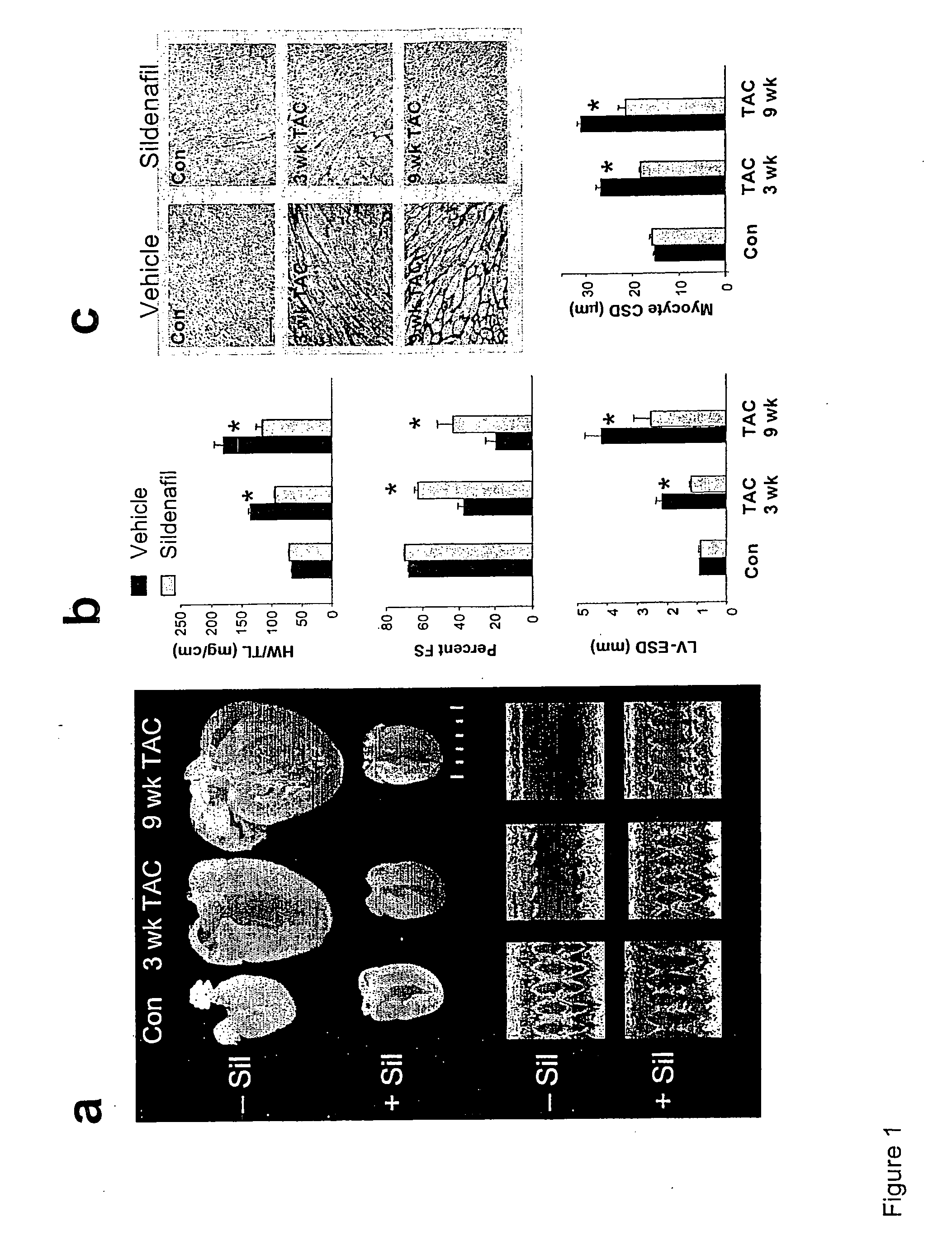
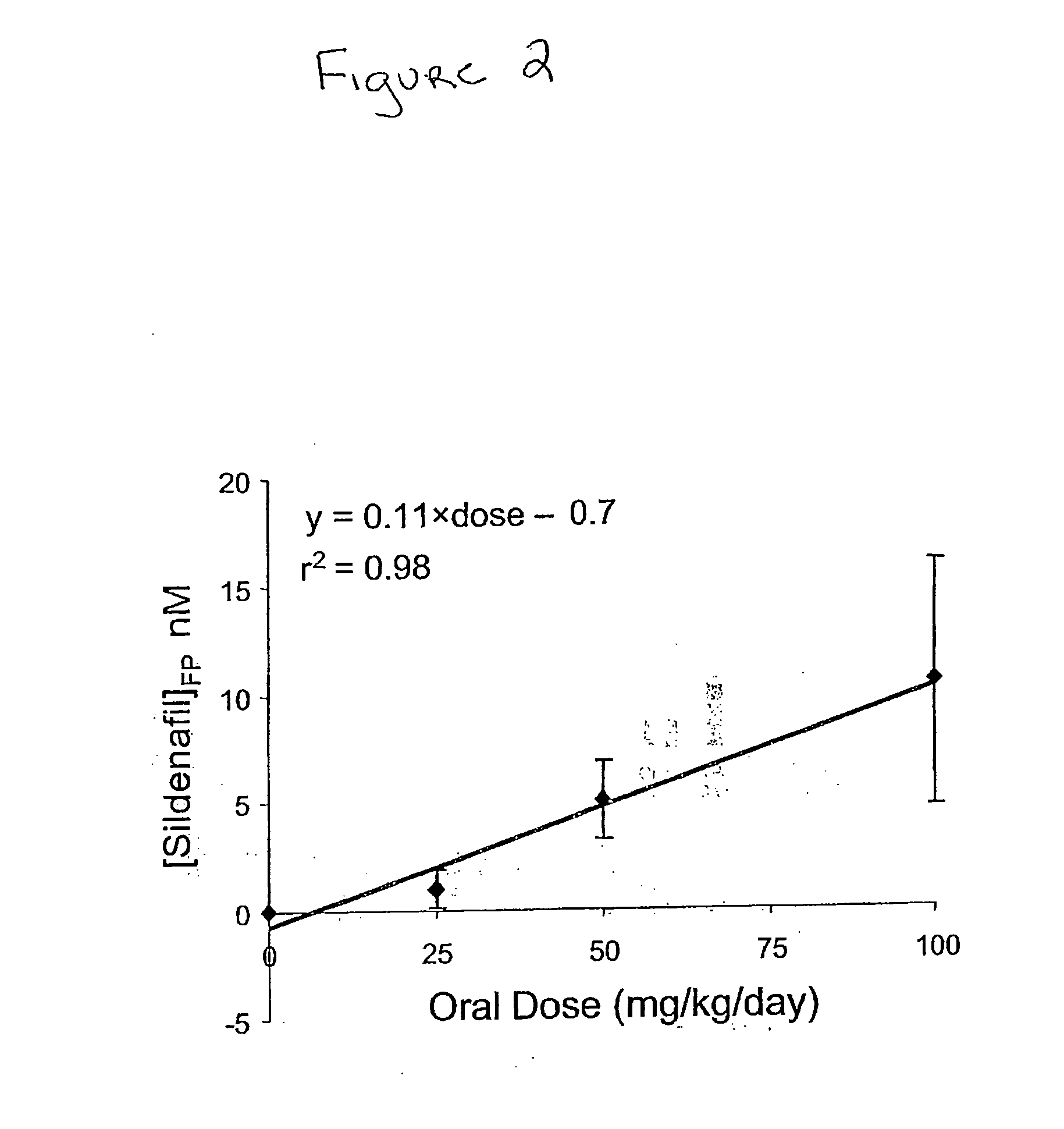
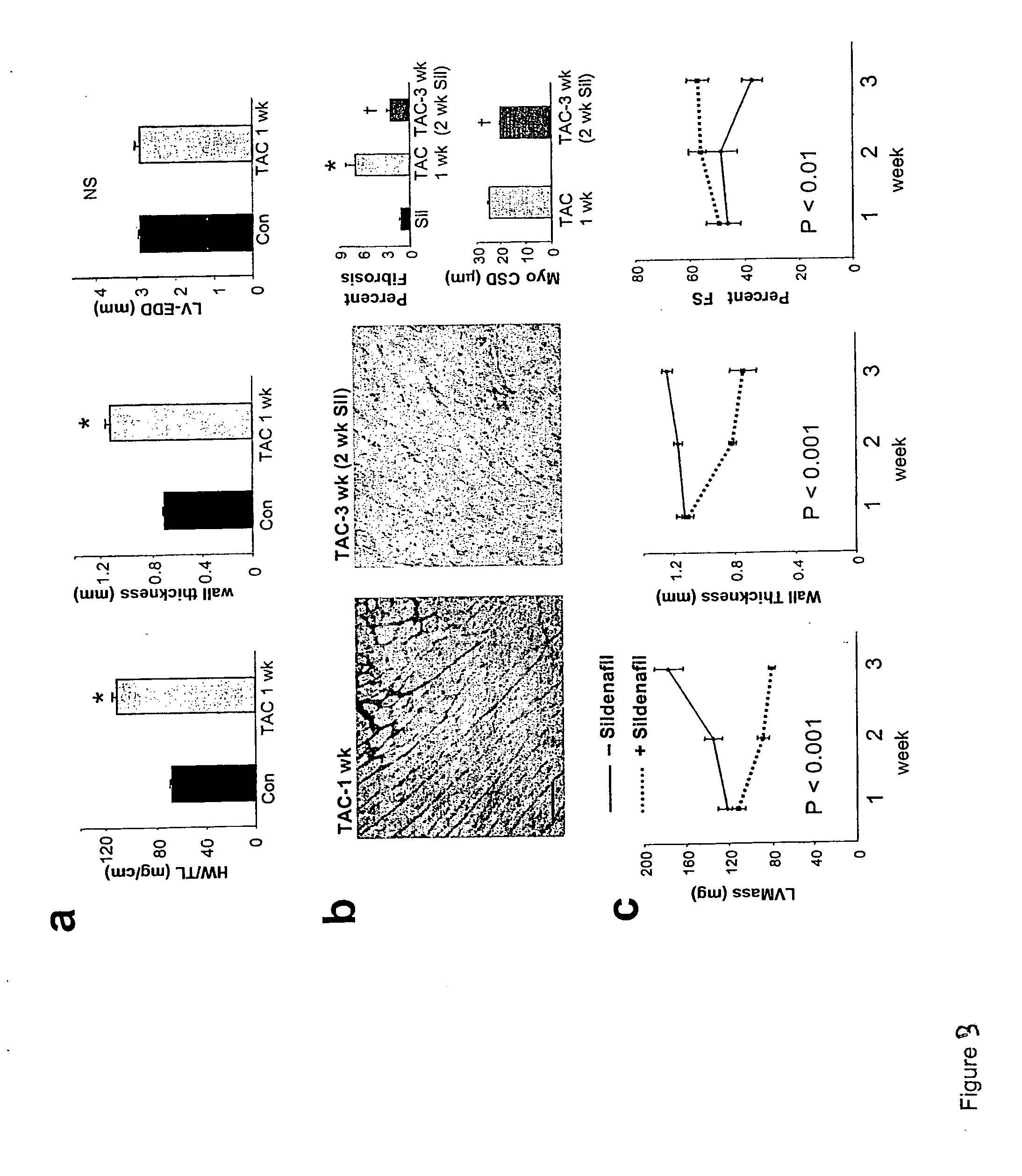
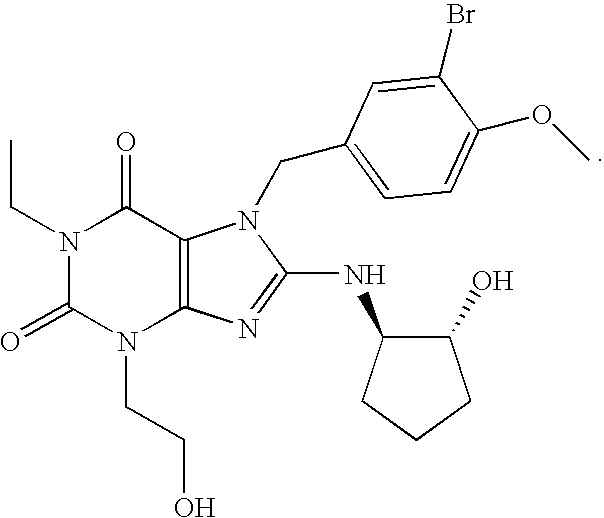
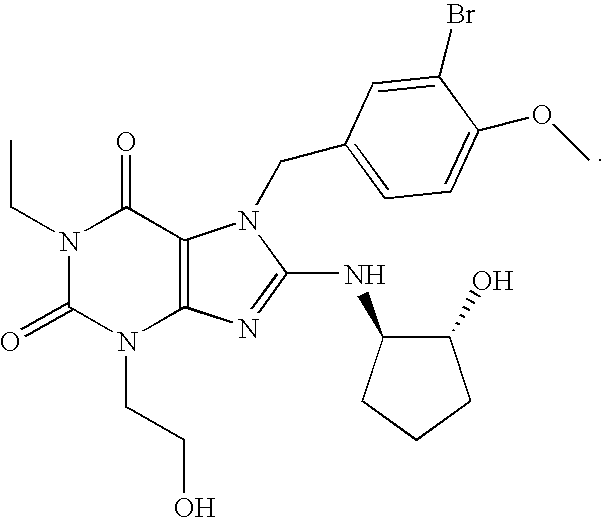
The present invention encompasses oral formulations of a PDE5 inhibitor which provide rapid disintegration after introduction to the oral cavity, followed by buccal and / or sublingual absorption. The orally disintegrating formulations can be in a variety of dosage forms including lingual strip, sublingual strip, oral mist, rapidly disintegrating tablet, lyophilized wafer, granulated particles and gum. The formulations can include an extended release component that allows the PDE5 inhibitor to be swallowed for gastrointestinal absorption. Combination therapies with a second pharmaceutical agent known to cause a PDE5-treatable condition as a side effect, such as erectile dysfunction, are also described. The PDE5 inhibitor of the following chemical structure is particularly favored for these formulations:
Owner:SCHERING CORP
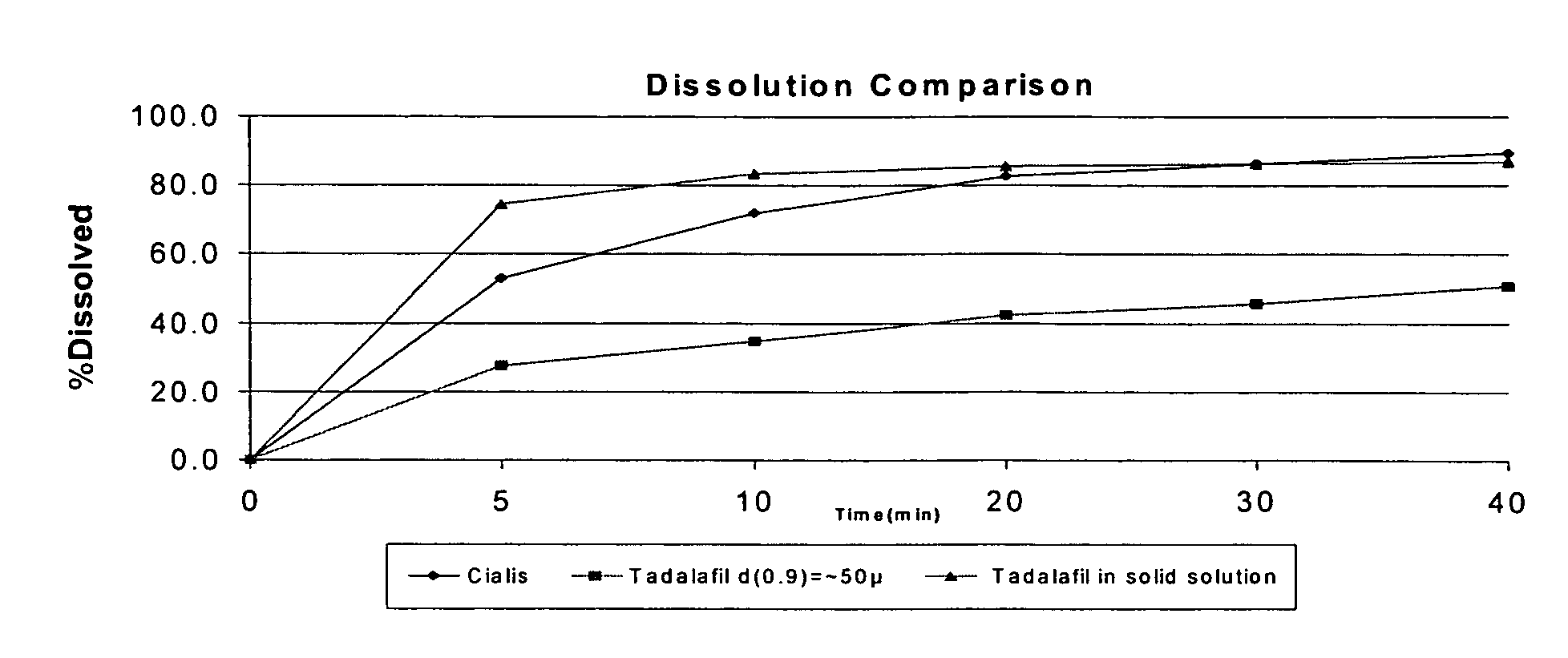
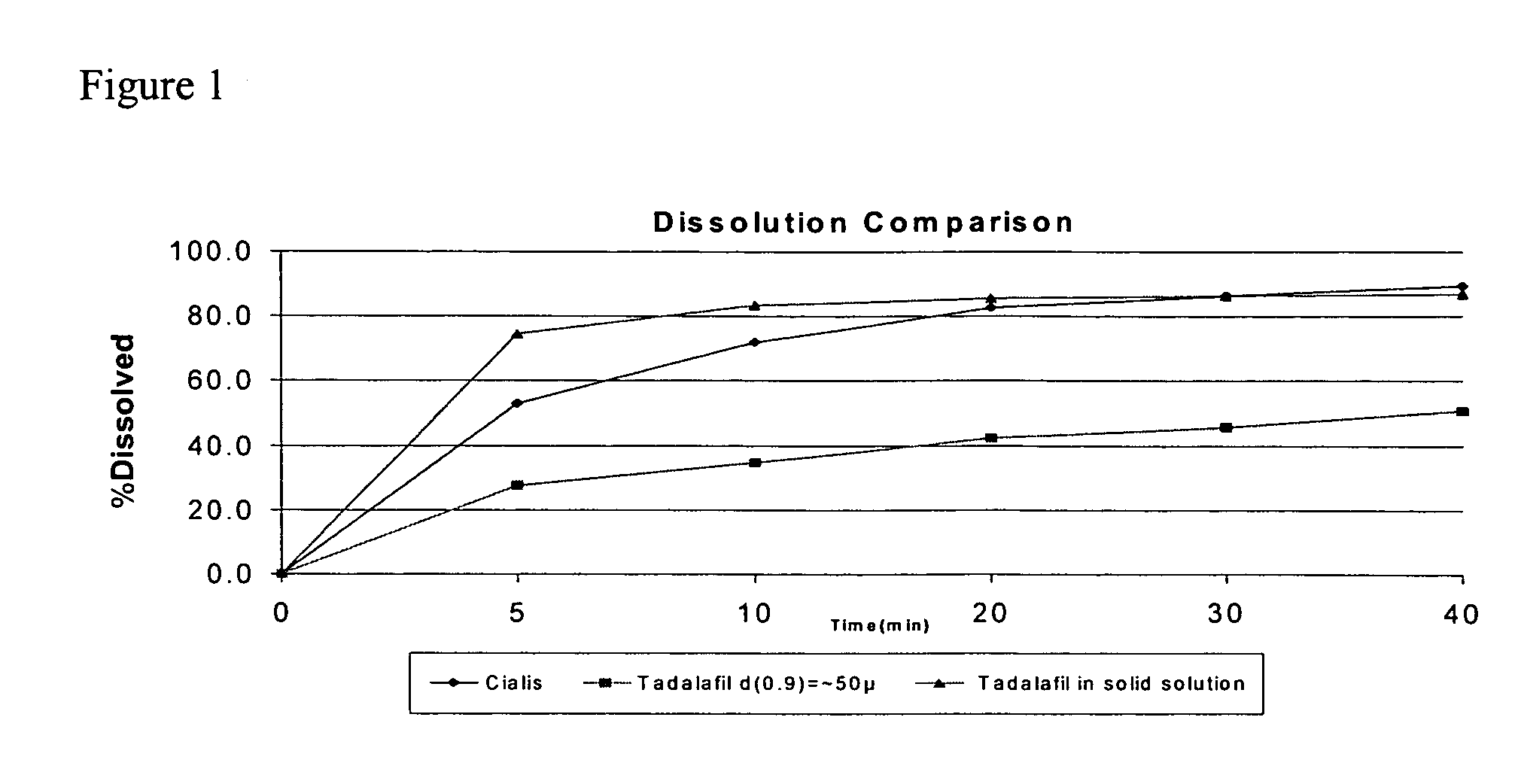
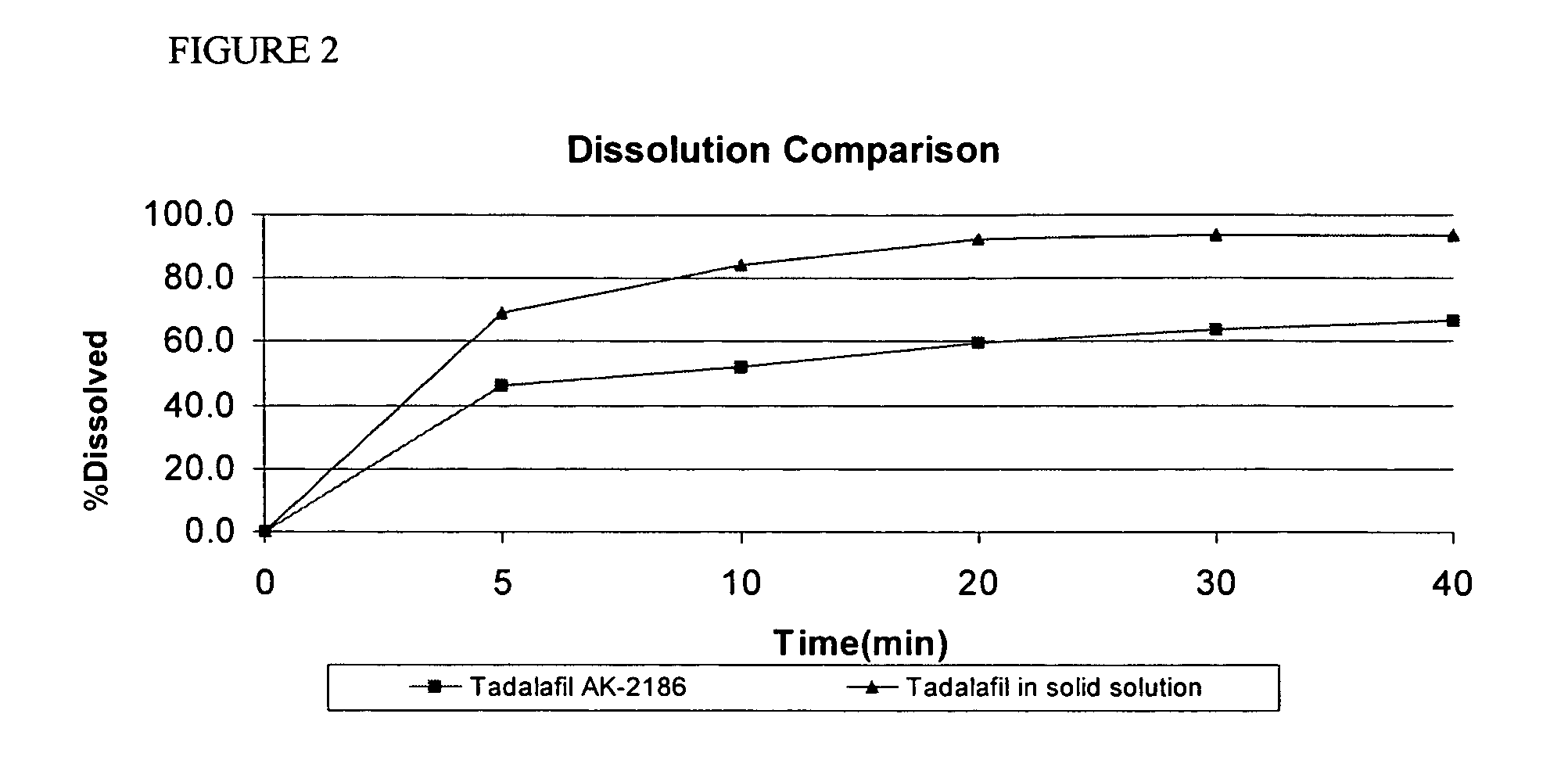
Tadalafil solid composites
This invention relates to oral pharmaceutical compositions suitable for making pharmaceutical formulations for oral administration that provide for the rapid dissolution of the phosphodiesterase 5 inhibitor tadalafil. In particular, the pharmaceutical compositions comprise solid composites of tadalafil exhibiting high solubility and rate of dissolution. The invention further relates to methods of preparing these pharmaceutical formulations and the use of such pharmaceutical formulations for treating diseases associated with PDE5 inhibitors.
Owner:TEVA PHARM USA INC
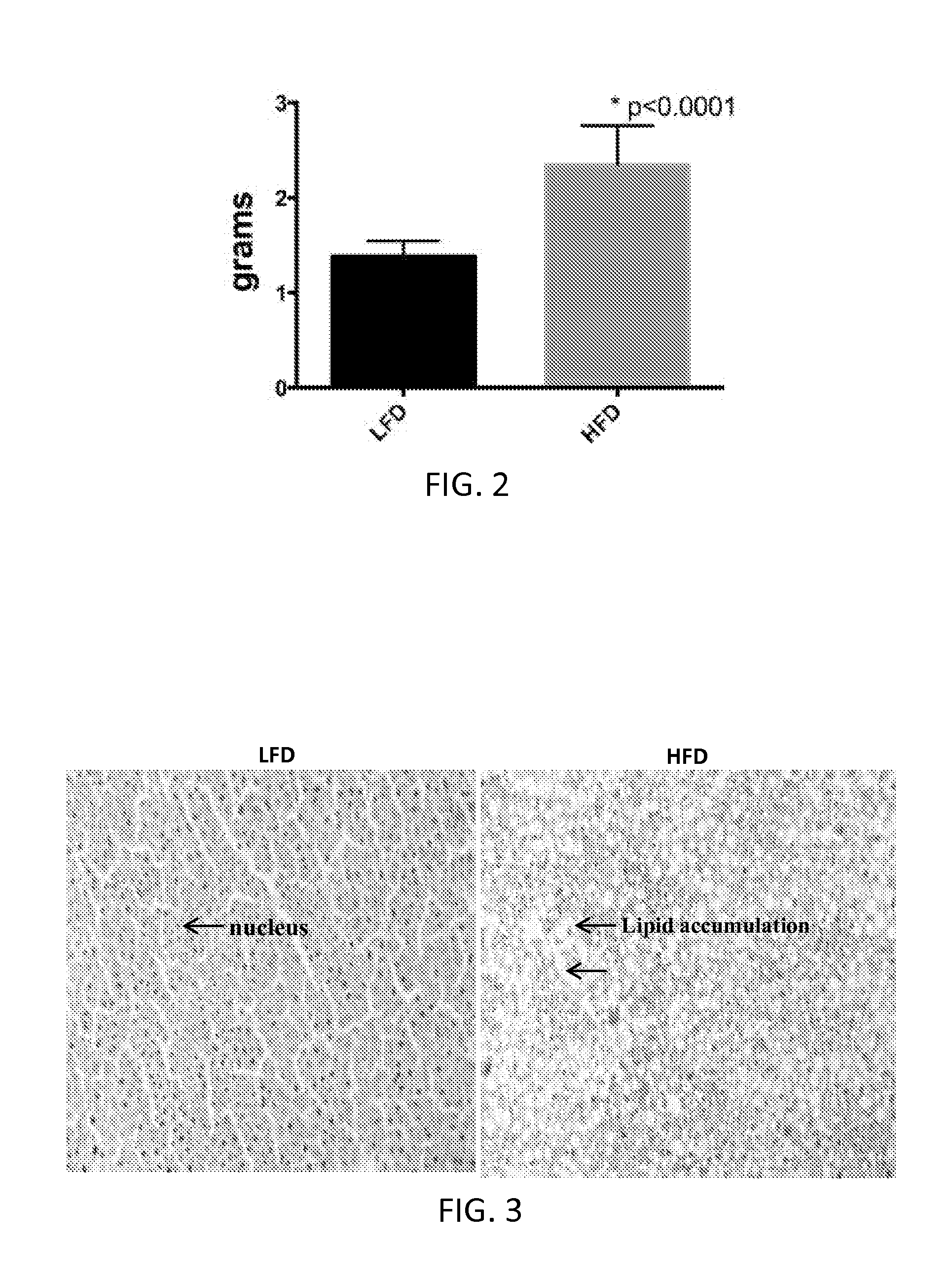
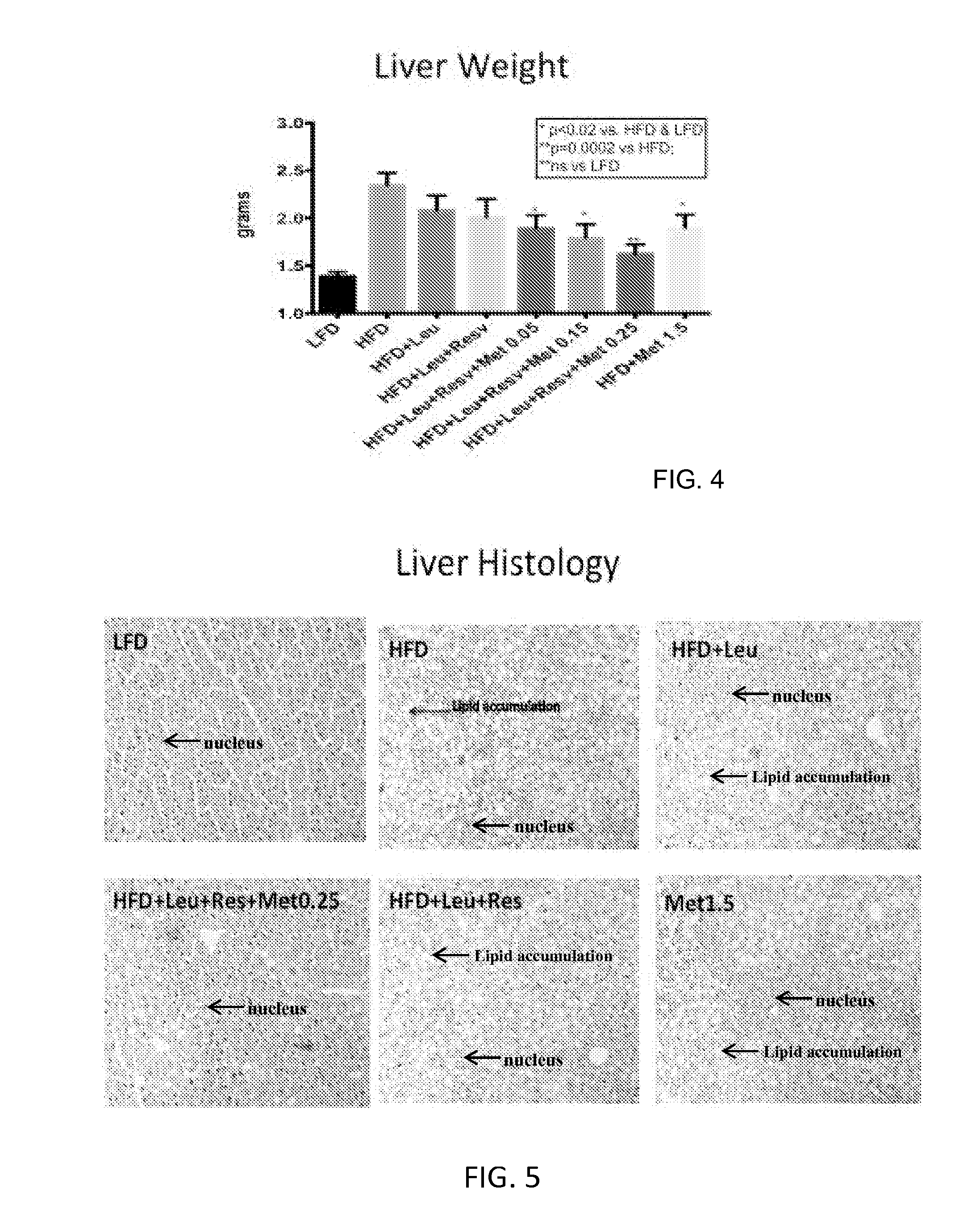
Compositions and methods for the reduction or prevention of hepatic steatosis
ActiveUS20160067201A1Lower Level RequirementsReduction of non-alcoholic steatohepatitis (NASH)BiocidePeptide/protein ingredientsSteatosisMetabolite
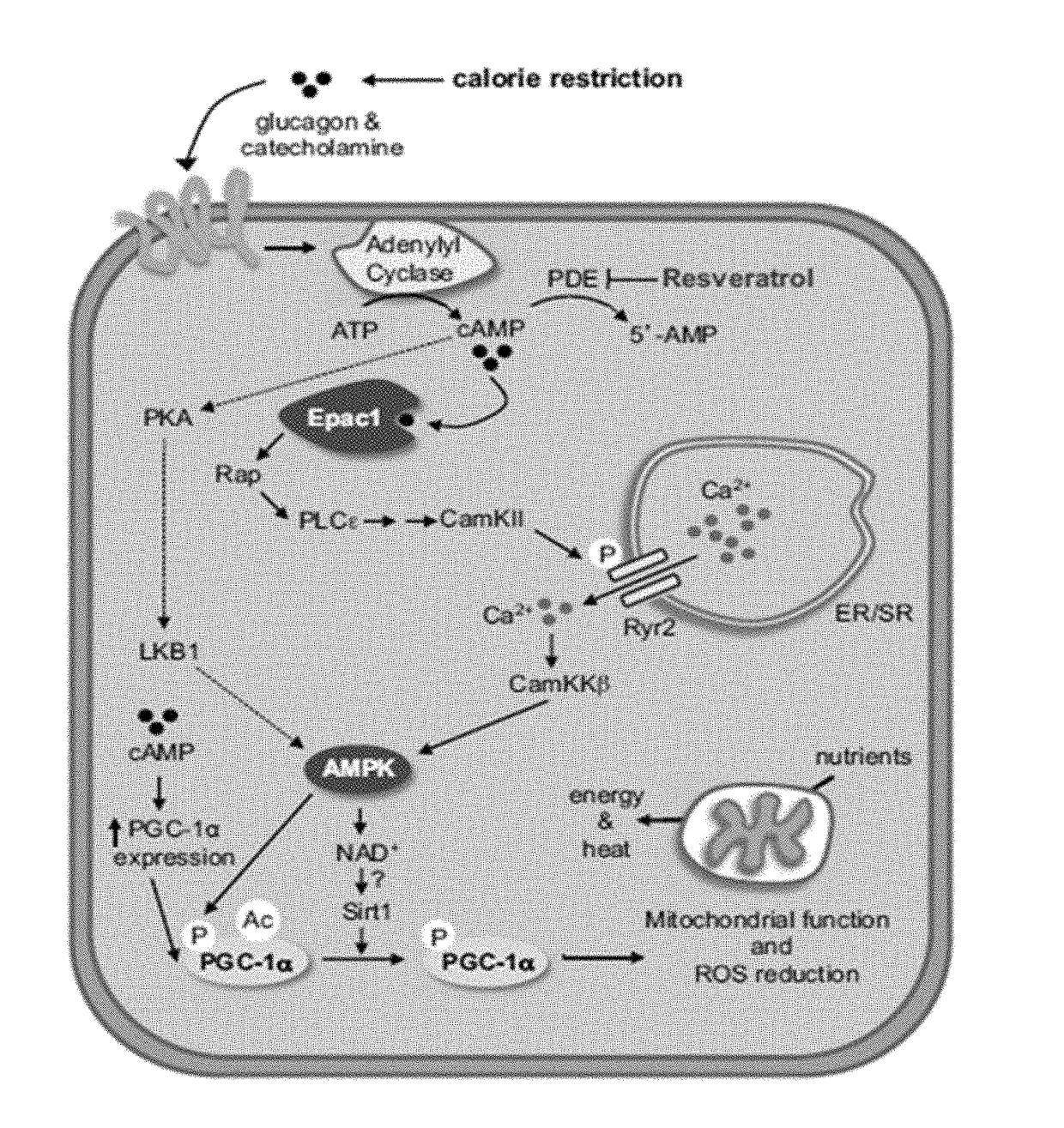
Methods useful for reducing or preventing non-alcoholic steatohepatitis or hepatic steatosis are provided herein. Such methods may comprise administering to a subject in need thereof a sirtuin pathway activator and / or PDE5 inhibitor alone or in combination with an amount of a branched amino acid in free amino acid form, or a metabolite thereof. Also provided herein are compositions and kits for practicing any of the methods described herein.
Owner:NUSIRT SCI
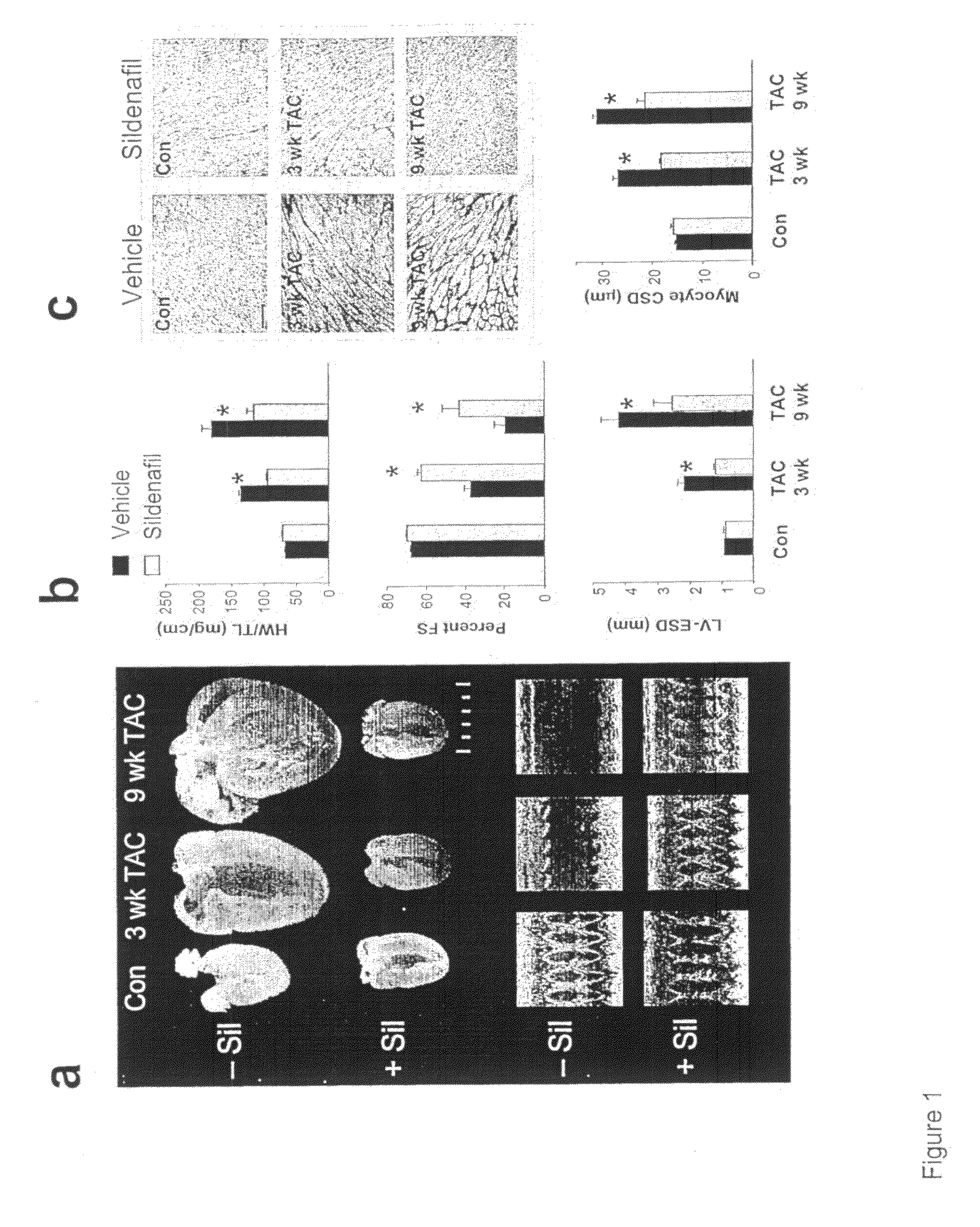
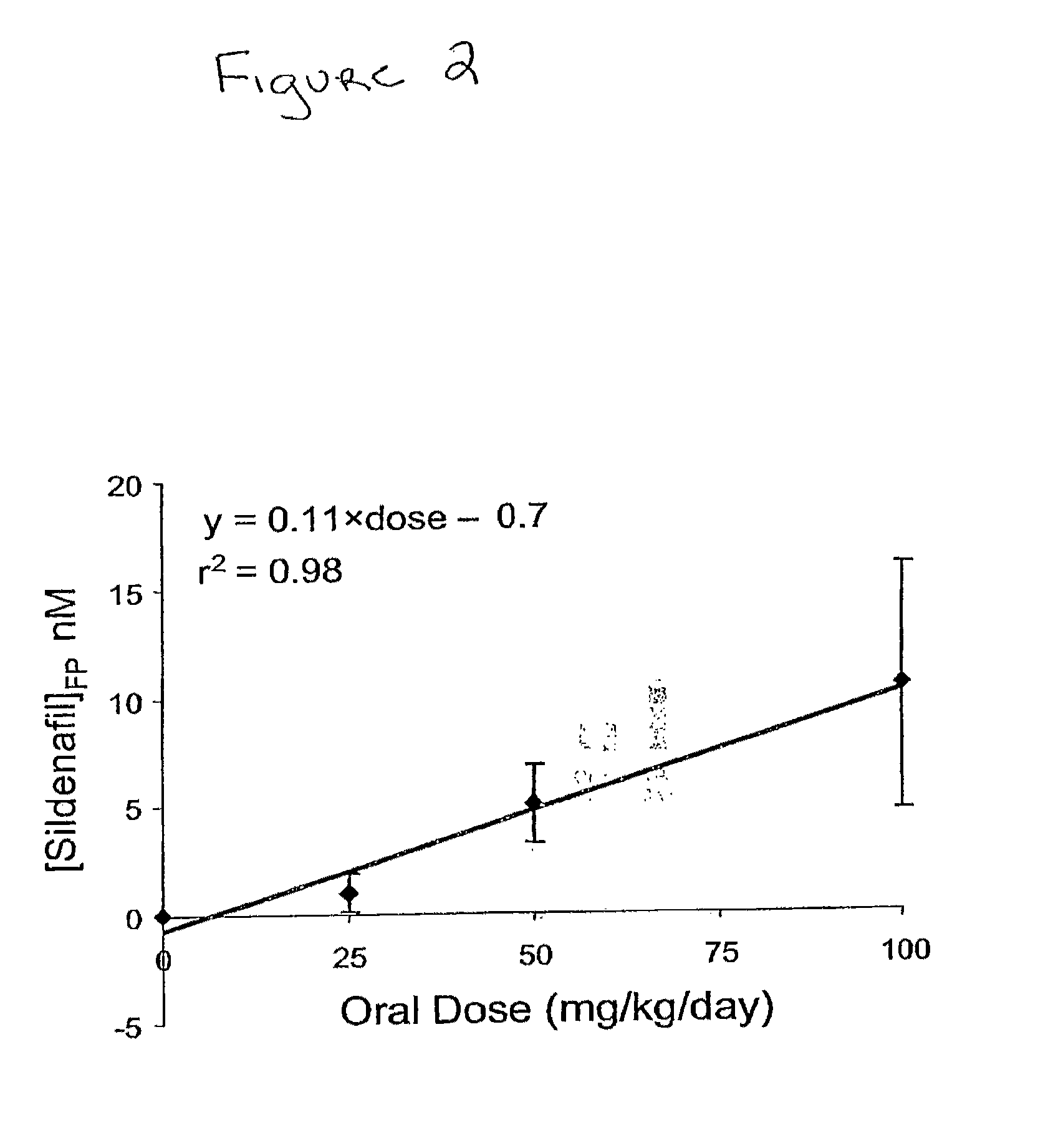
PDE5 inhibitor compositions and methods for treating cardiac indications
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Preparing method of phosphodiesterase 5 inhibitor tadalafil
ActiveCN103980275AGood removal effectHigh chiral purityOrganic chemistryPhosphodiesterase 5 inhibitorTadalafil
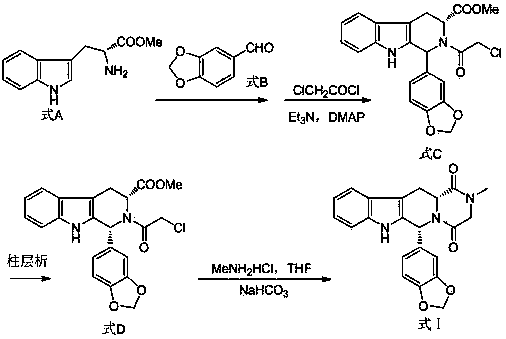
The invention relates to a preparing method of a phosphodiesterase 5 inhibitor tadalafil. D-methyl tryptophanate hydrochloride is adopted as an initial raw material, and is subjected to cyclization with heliotropin, N-acylation, aminolysis-cyclization, and other reactions to obtain a tadalafil crude product. The tadalafil crude product is recrystallized to obtain a tadalafil finished product. The method has characteristics of mild reaction conditions, short reaction time, high yield, good product stability and convenience for industrial production.
Owner:湖北省医药工业研究院有限公司
Composition comprising a pde4 inhibitor and a pde5 inhibitor
InactiveUS20060094723A1Preventing and reducing onsetReduce the severity of the diseaseBiocideAntipyreticPhosphodiesterase 5 inhibitorPDE4 Inhibitors
The invention relates to the combined administration of a PDE4 inhibitor and a PDE5 inhibitor for the treatment of a disease in which phosphodiesterase 4 (PDE4) and / or phosphodiesterase 5 (PDE5) is detrimental.
Owner:NYCOMED GMBH
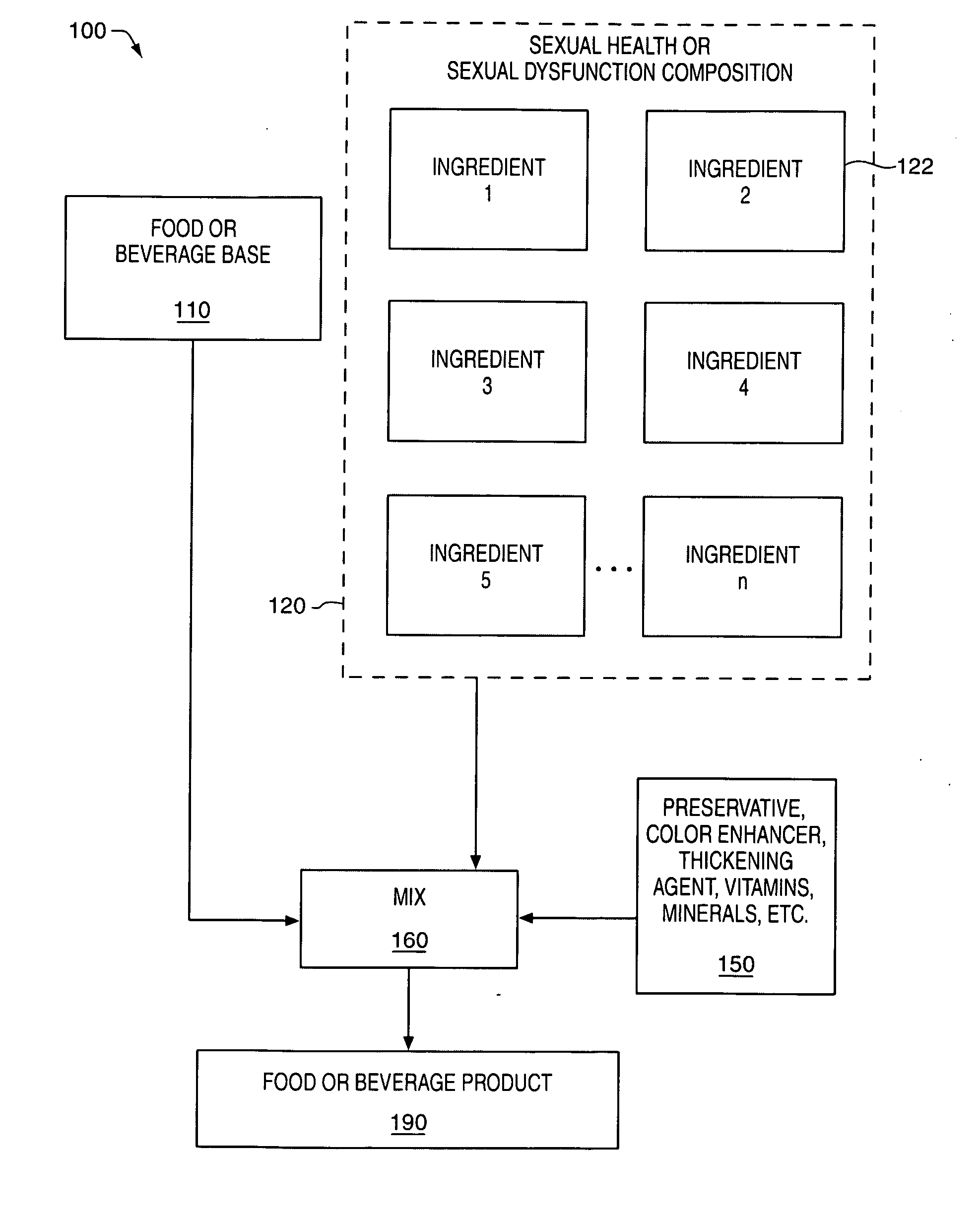
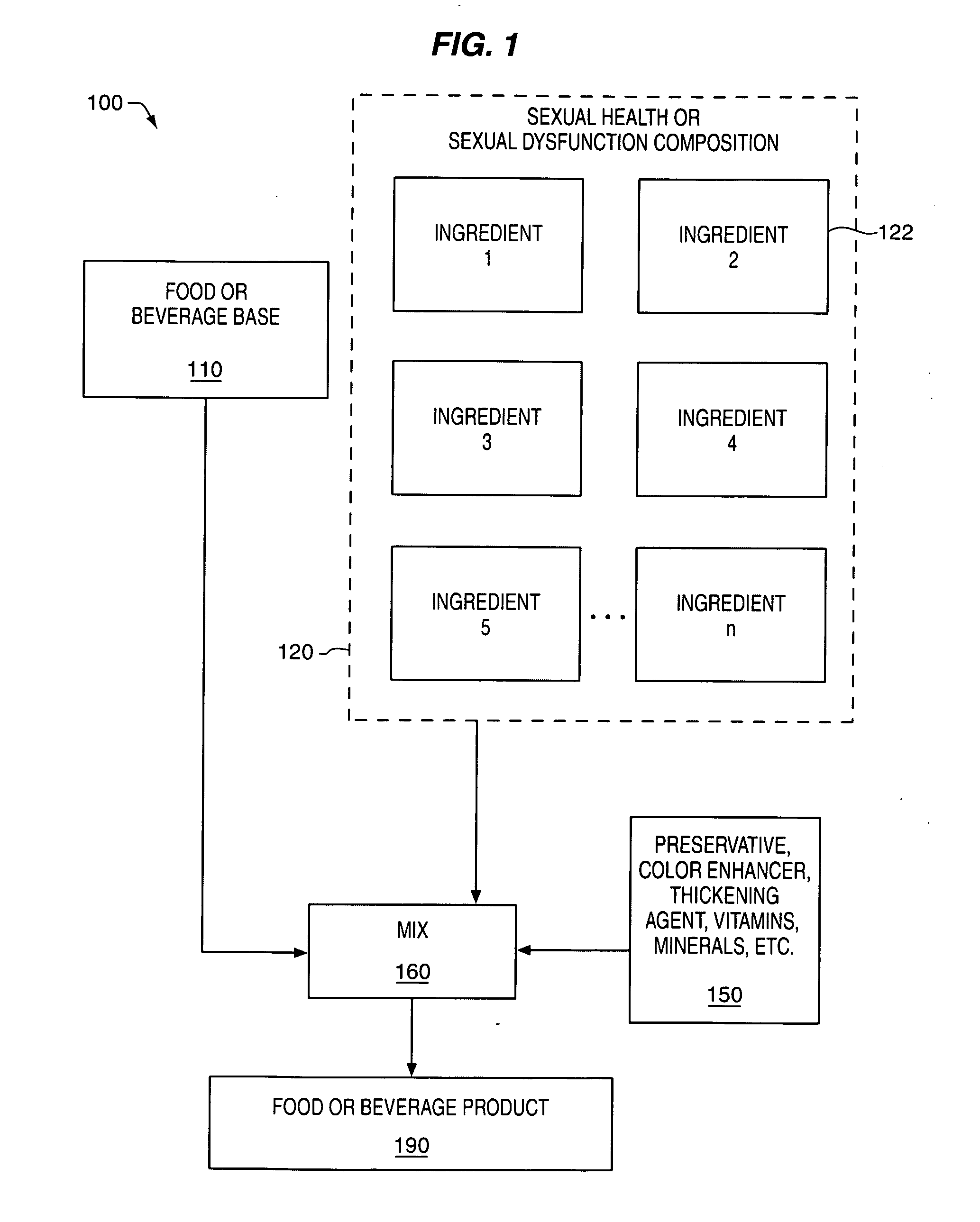
Delivery system and method for supporting and promoting healthy sexual function and prevention and treatment of sexual dysfunction
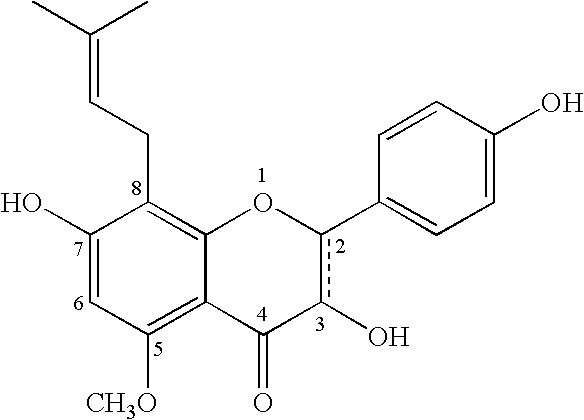
InactiveUS20060110478A1Increase in cGMPGood curative effectFood ingredient as antioxidantBiocideSexual functionVardenafil
Improved delivery systems and delivery methods for supporting and promoting healthy sexual function, for preventing sexual dysfunction, or for treatment of sexual dysfunction. A compositions including one or more cGMP-specific PDE5 inhibitors and / or dopaminergic agonists is administered in the form of a breath-care strip, mint or lozenge, or a food or beverage product. The cGMP-specific PDE5 inhibitor comprises an ingredient selected from the group consisting of sophoflavescenol, vardenafil, tadalafil, and sildenafil. The dopaminergic agonist comprises apomorphine. Vitex agnus-castus extract, and one or more of lipoic acid, L-Arginine, folic acid, trimethylglycine, policosanol, carnitine, biotin, and acetyl L-Carnitine may also be included in the delivery vehicle.
Owner:MCCLEARY EDWARD LARRY +2
Novel compositions comprising a phosphodiesterase-5 inhibitor and their use in methods of treatment
InactiveUS20130296324A1Enhance health and appearanceFacilitating accelerating healingBiocideNervous disorderDiseaseSynaptic cleft
The invention relates generally to novel pharmaceutical methods for the treatment of various conditions. Compositions comprising: at least one phosphodiesterase-5-inhibitor in combination with one or more of the following medications: a selective serotonin reuptake inhibitor; a serotonin-norepinephrine reuptake inhibitor; a cholinesterase inhibitor; a dopamine agonist; or a medication suitable to increase the chemical concentrations of the neurotransmitters, selected from amino acids, monoamines, neuropeptides and other agents capable of primary neurotransmission in the synaptic clefts, and their use for treating a neurodegenerative disease in a subject. The invention also relates to: Compositions comprising: at least one phosphodiesterase-5-inhibitor in combination with one or more of the following medications: a selective serotonin reuptake inhibitor; or a cholinesterase inhibitor, and their use for treating damaged skin in a subject.
Owner:HELD JERRY M
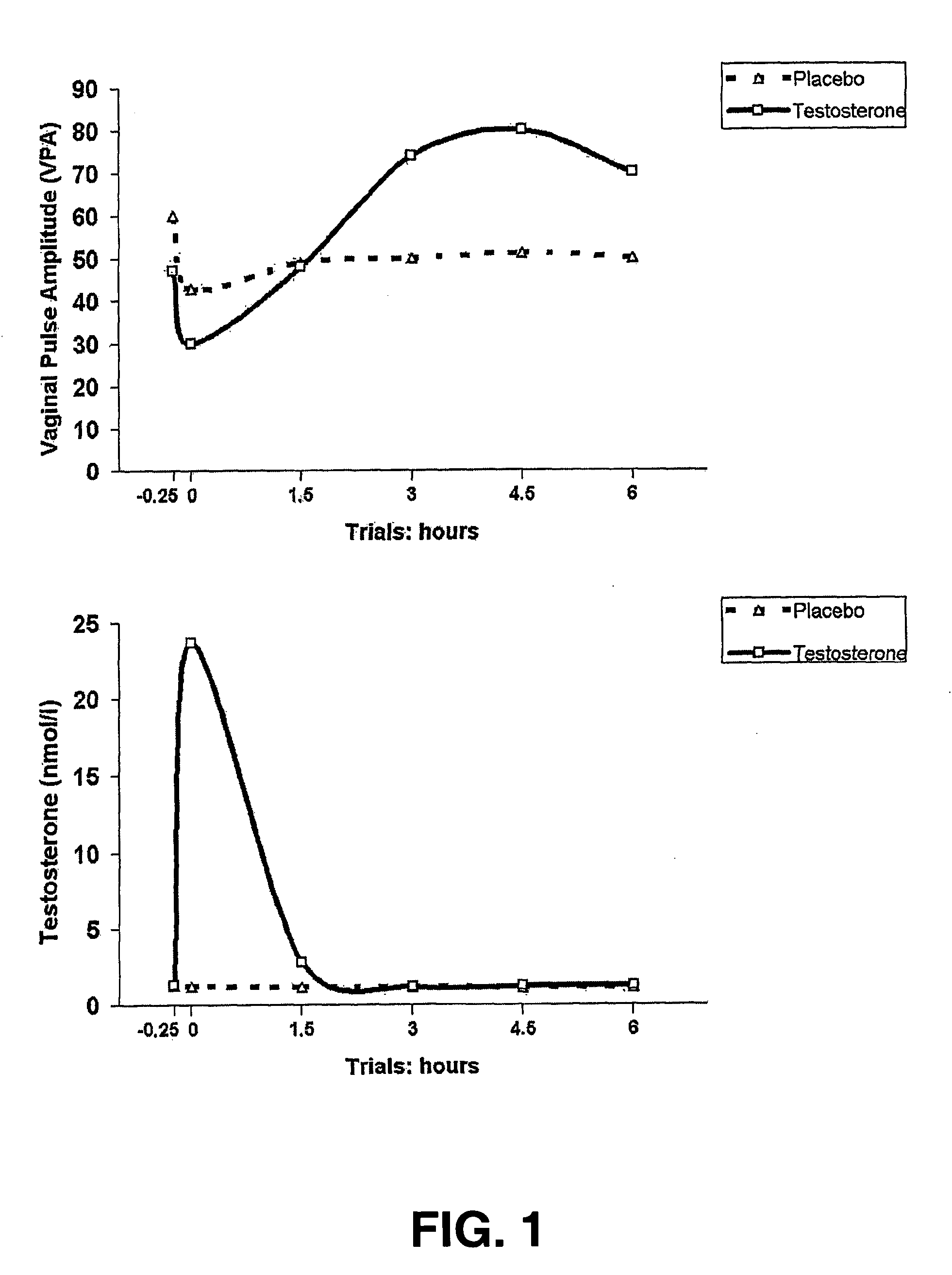
Pharmaceutical formulations and uses thereof in the treatment of female sexual dysfunction
ActiveUS20070093450A1Good effectIncrease heightBiocideData processing applicationsPhosphodiesterase 5 inhibitorPde5 inhibition
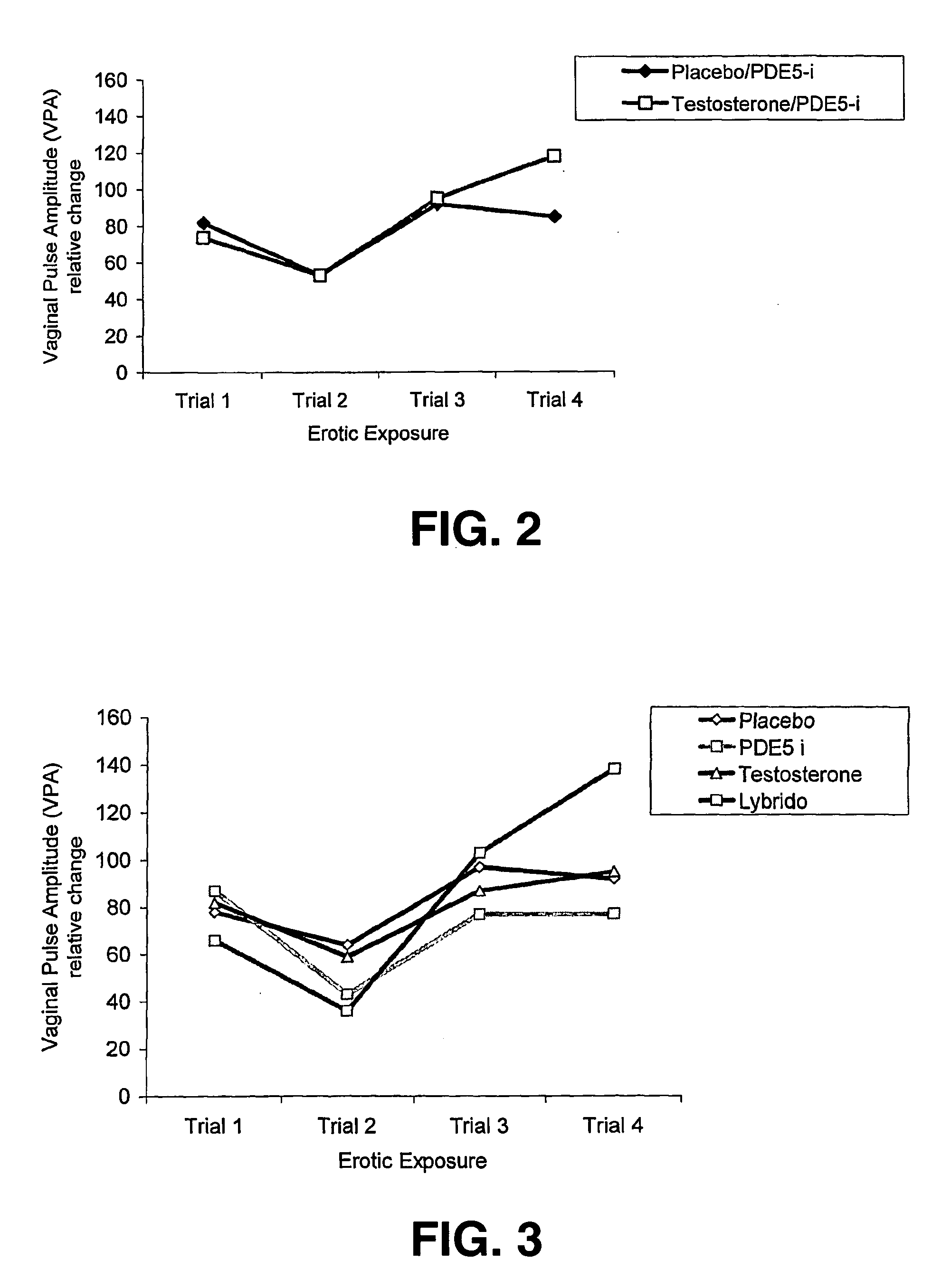
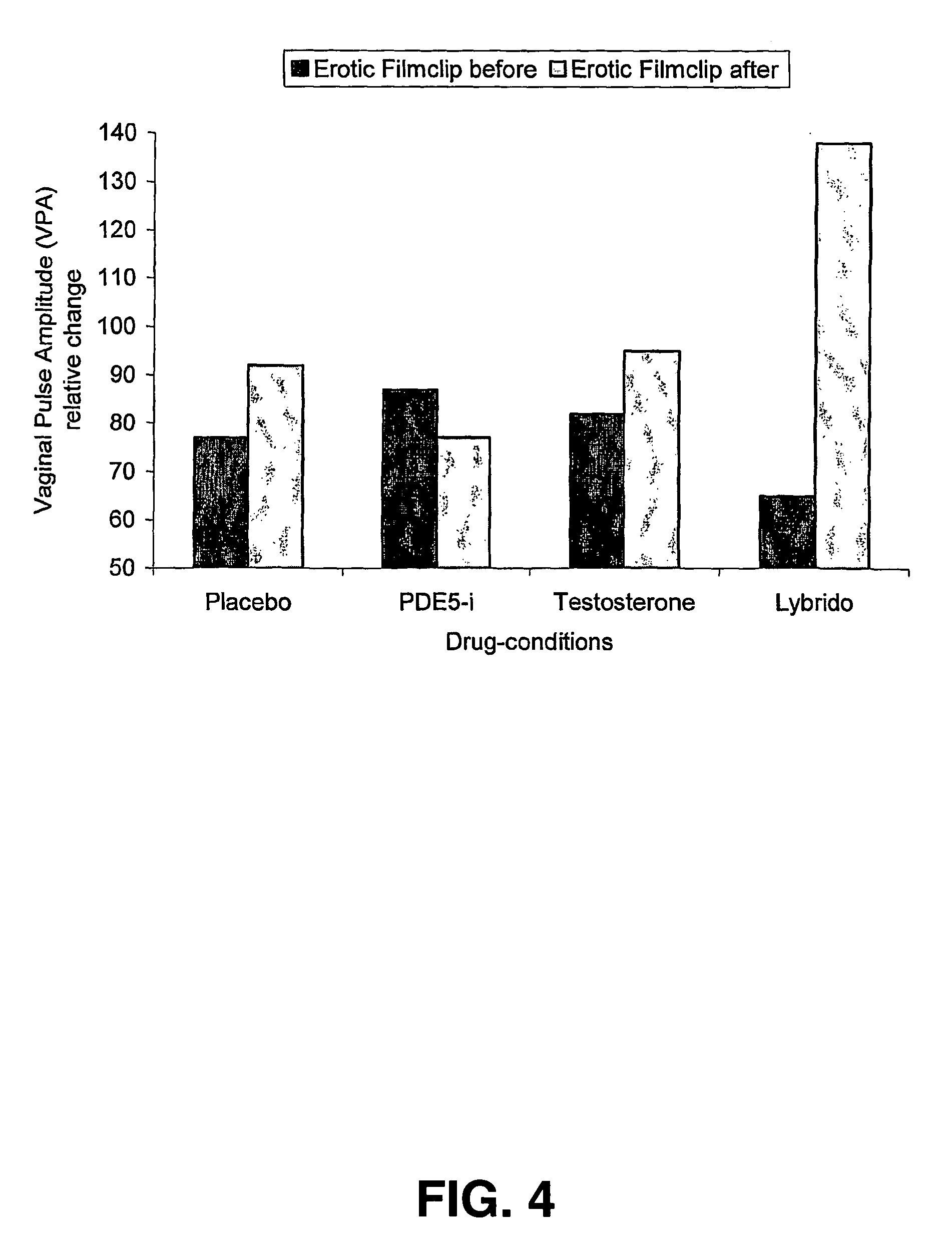
The present invention relates to the use of a combination of a PDE5-inhibitor and testosterone for the preparation of a medicament for the treatment of Female Sexual Dysfunction.
Owner:EB IP LYBRIDO
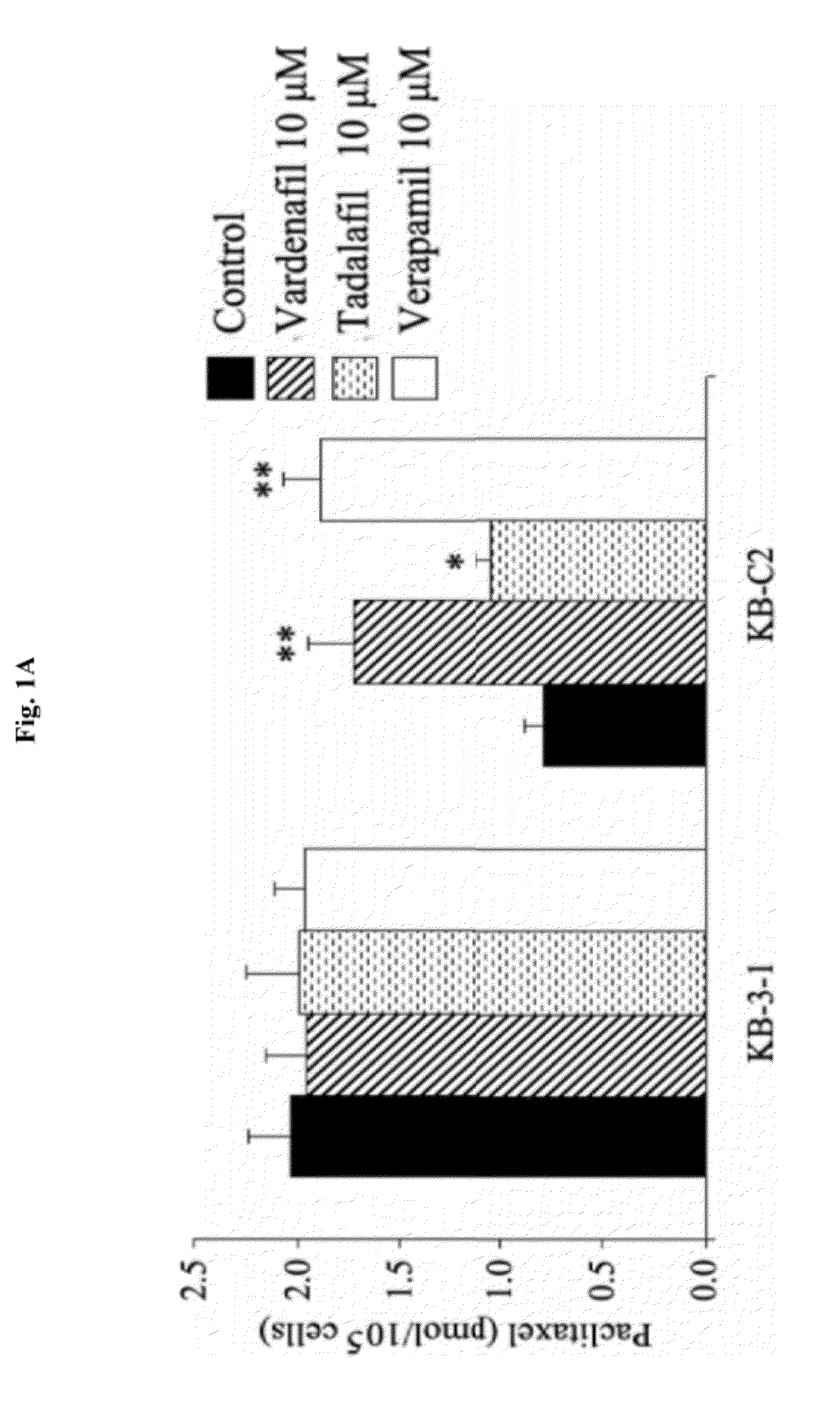
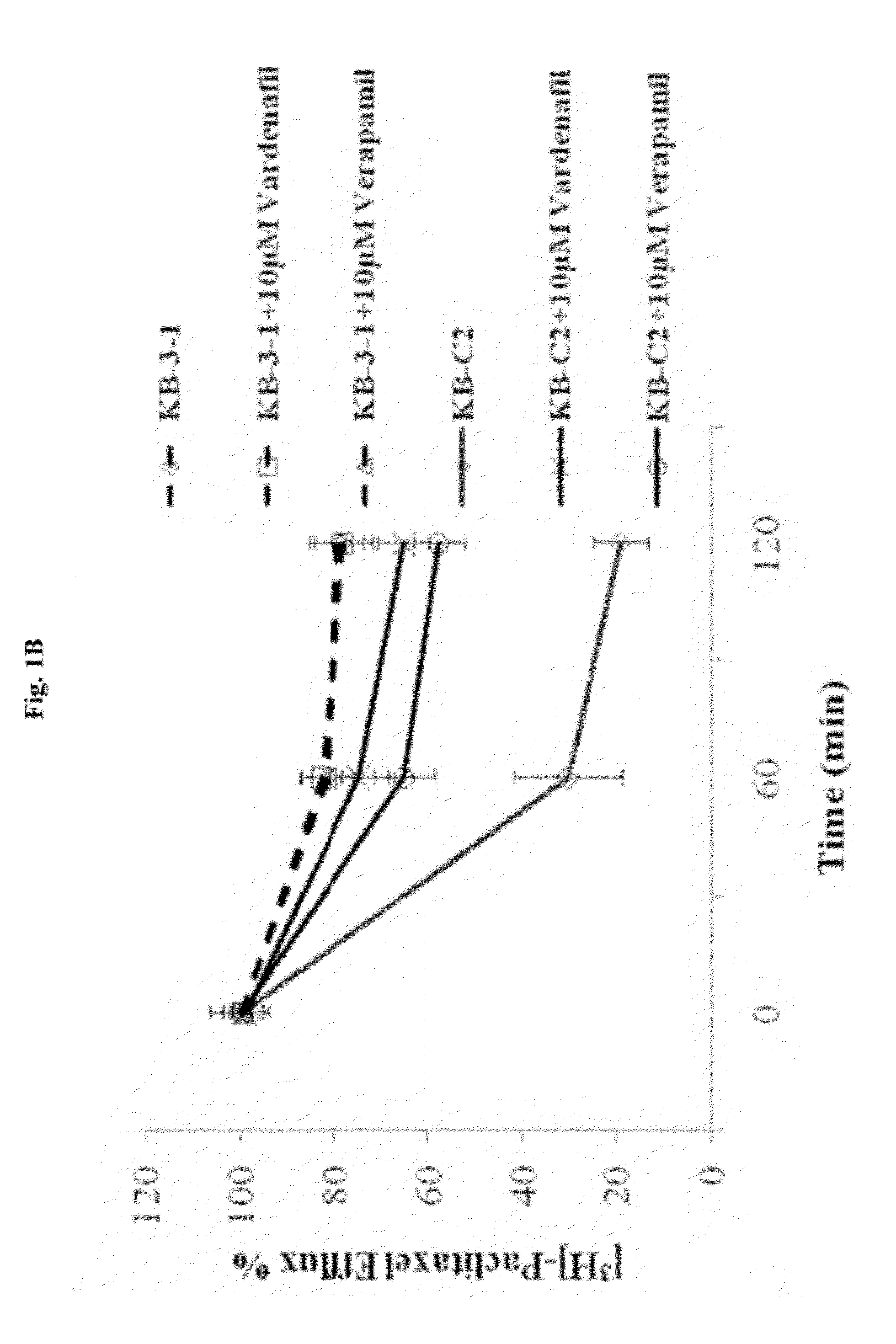
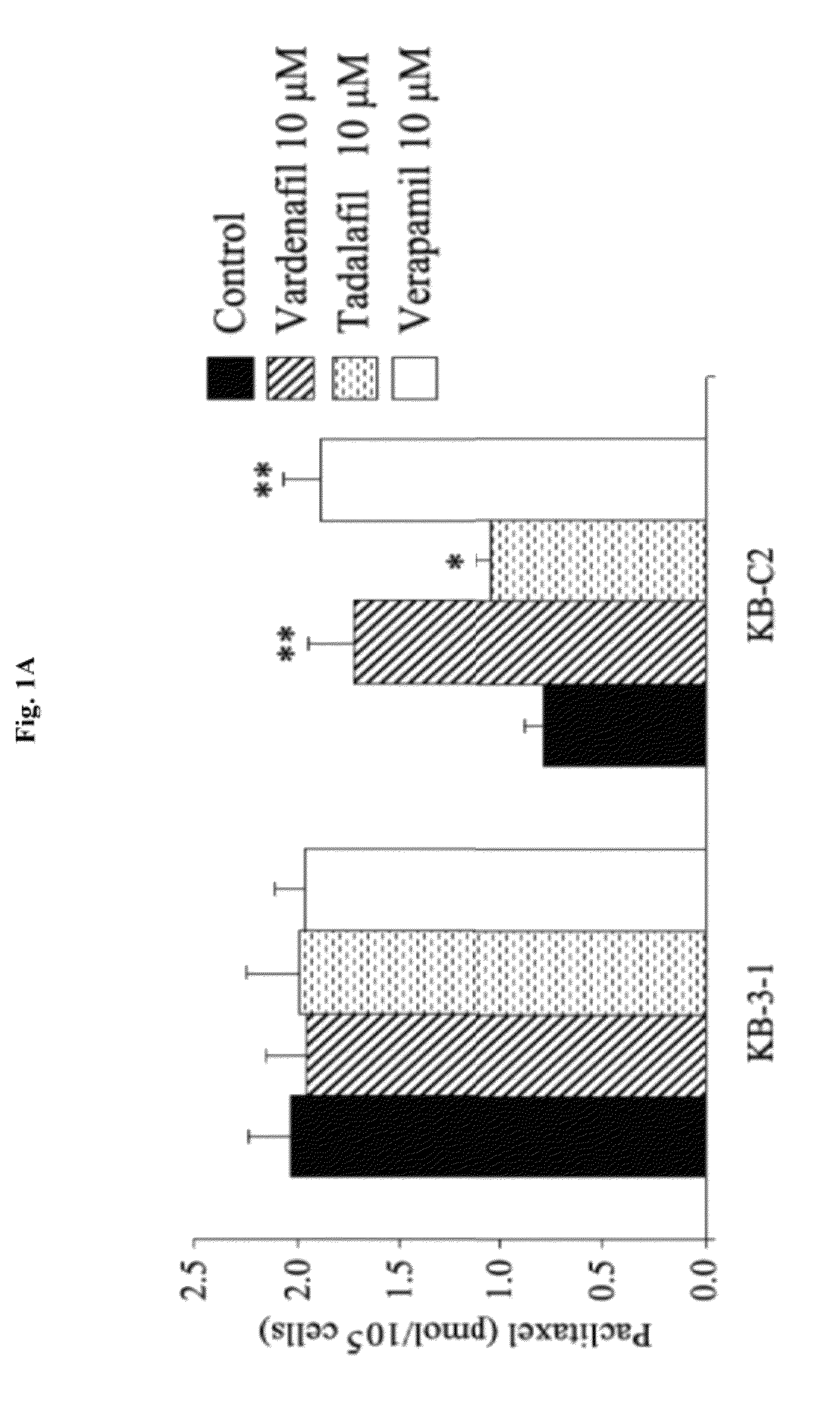
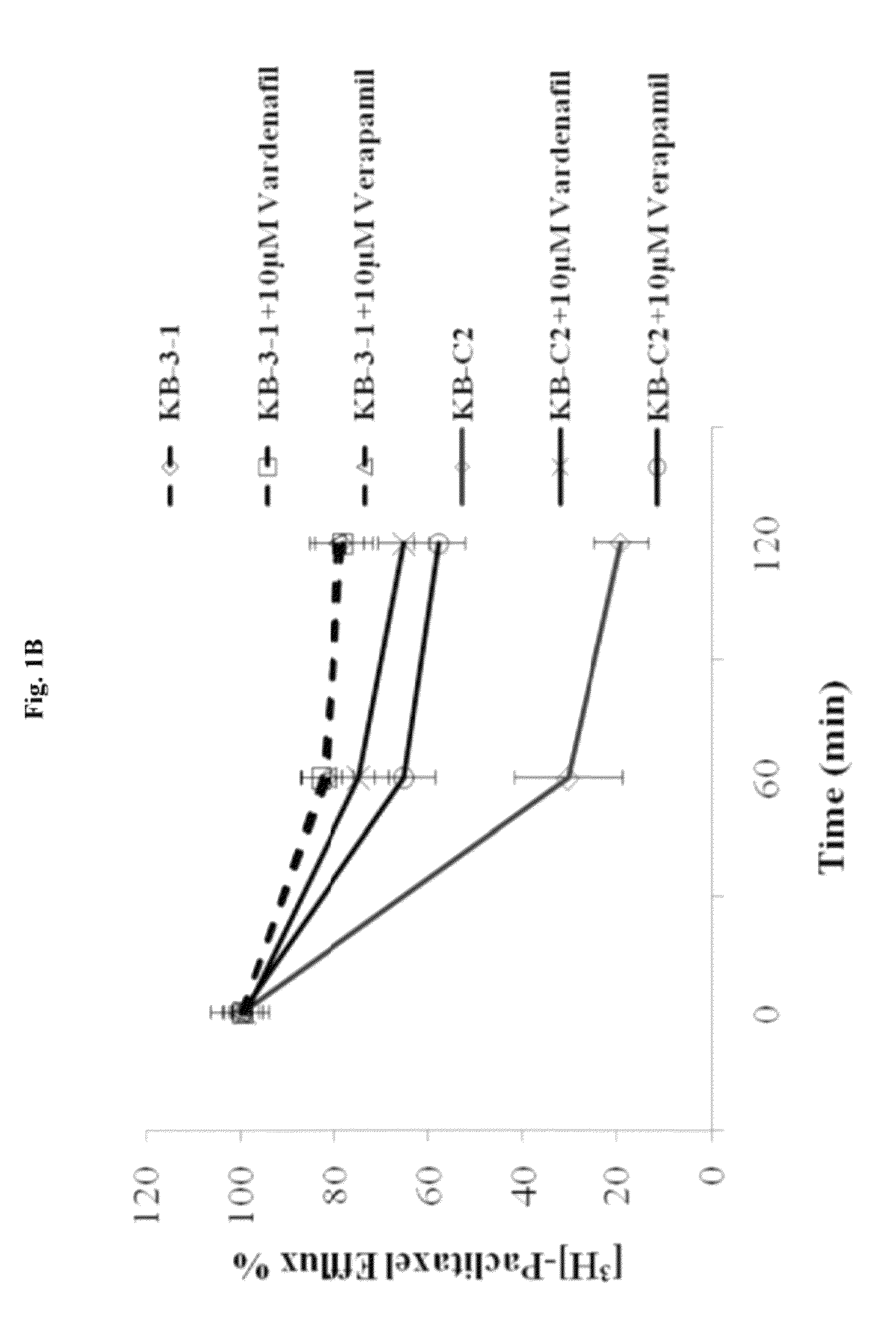
Use of phosphodiesterase inhibitors for treating multidrug resistance
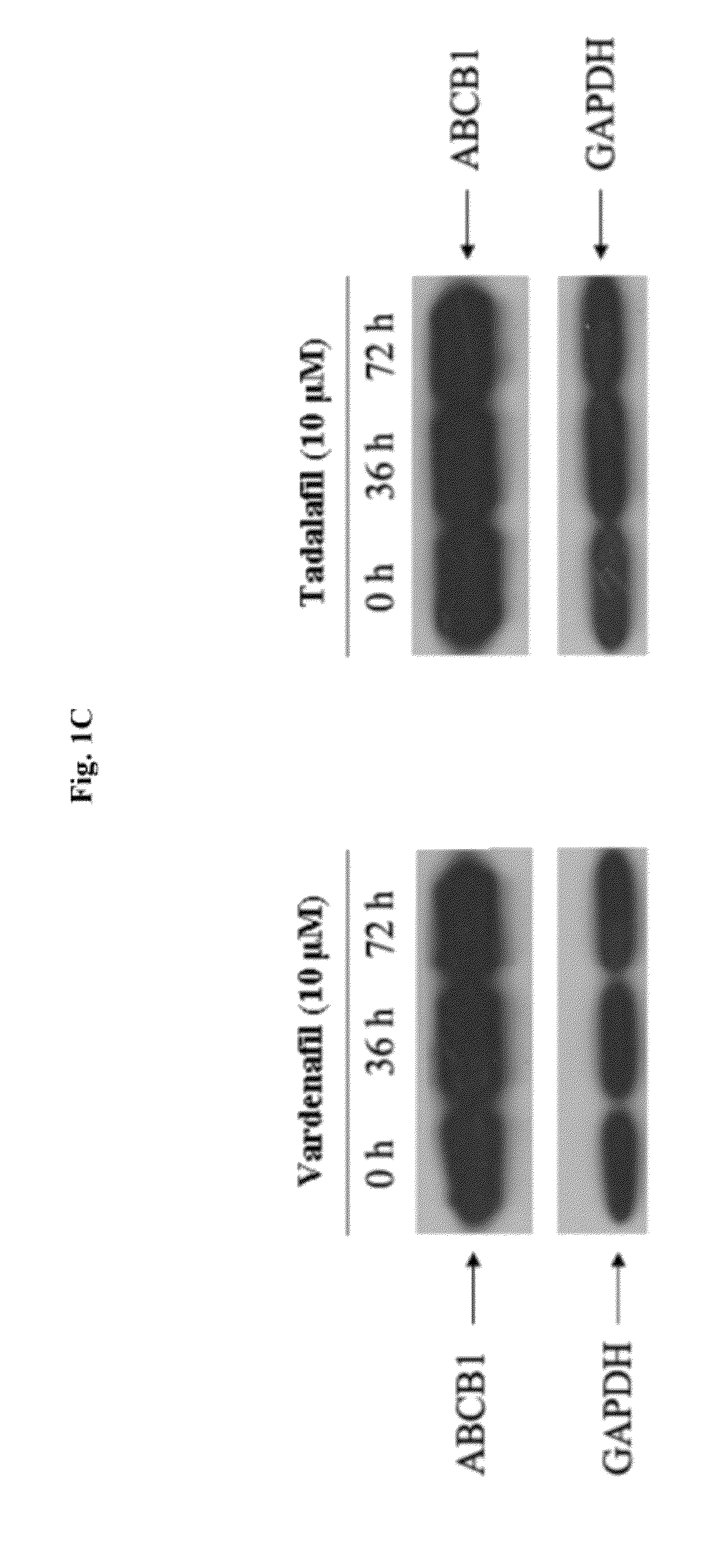
InactiveUS20120252816A1Suppression problemInhibiting ABCG transporter activityBiocideAnimal repellantsVardenafilPde5 inhibition
The present invention relates to methods of treating multidrug resistance in cancerous cells with phosphodiesterase (PDE) inhibitors, e.g., PDE5 inhibitors. More specifically, the invention relates to methods of treating multidrug resistance that arises, e.g., during administration of chemotherapeutic / antineoplastic (anticancer) agents for treatment of cancer, with a PDE5 inhibitor (e.g., sildenafil, vardenafil, and tadalafil). The invention also relates to methods of treating cancer, e.g., multidrug resistant cancer, using a PDE5 inhibitor in combination with an antineoplastic therapeutic agent. Further, the invention relates to pharmaceutical compositions for treating multidrug resistant cancers comprising a PDE5 inhibitor, or a combination of a PDE5 inhibitor and an antineoplastic agent.
Owner:ST JOHNS UNIV
Composition comprising a pulmonary surfactant and a pde5 inhibitor for the treatment of lung diseases
InactiveUS20060148693A1Preventing and reducing onsetReduce severityBiocidePeptide/protein ingredientsPhosphodiesterase 5 inhibitorPde5 inhibition
The invention relates to the combined administration of a pulmonary surfactant and a PDE5 inhibitor for the treatment of a disease in which pulmonary surfactant malfunction and / or phosphodiesterase 5 (PDE5) activity is detrimental.
Owner:TAKEDA GMBH
Improved tadalafil preparation method
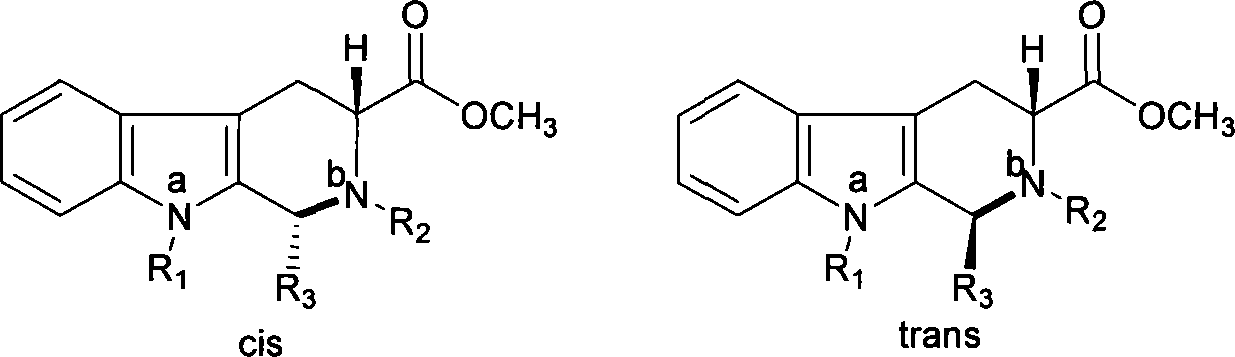
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
Method for synthesizing phosphodiesterase 5 inhibitor tadanafil
InactiveCN101205228AIncreased Stereospecific YieldThe separation method is simpleOrganic chemistryDiketoneBenzene
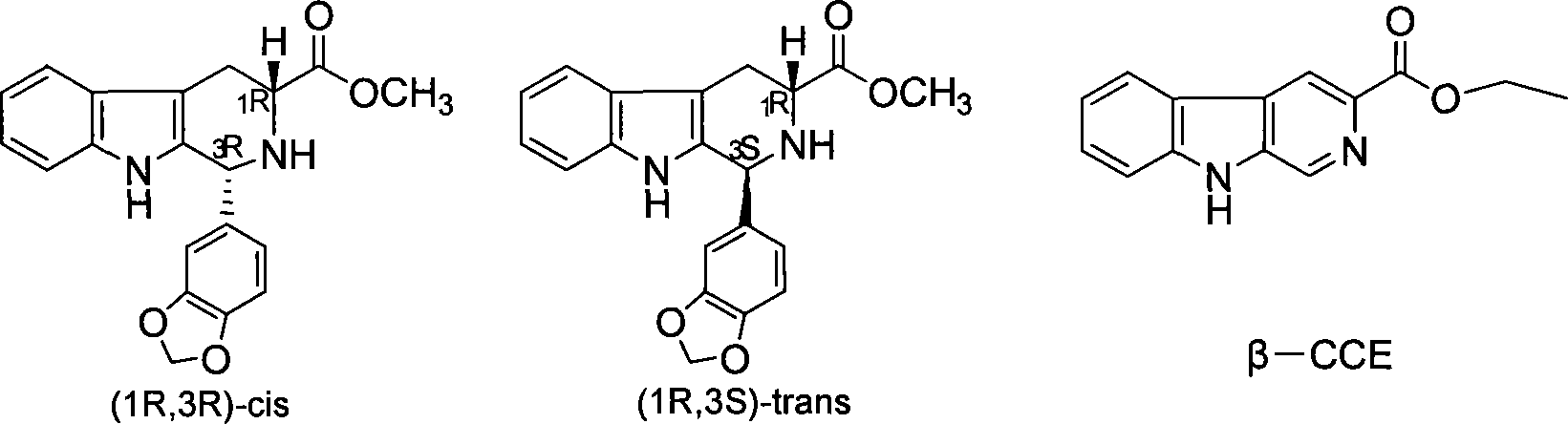
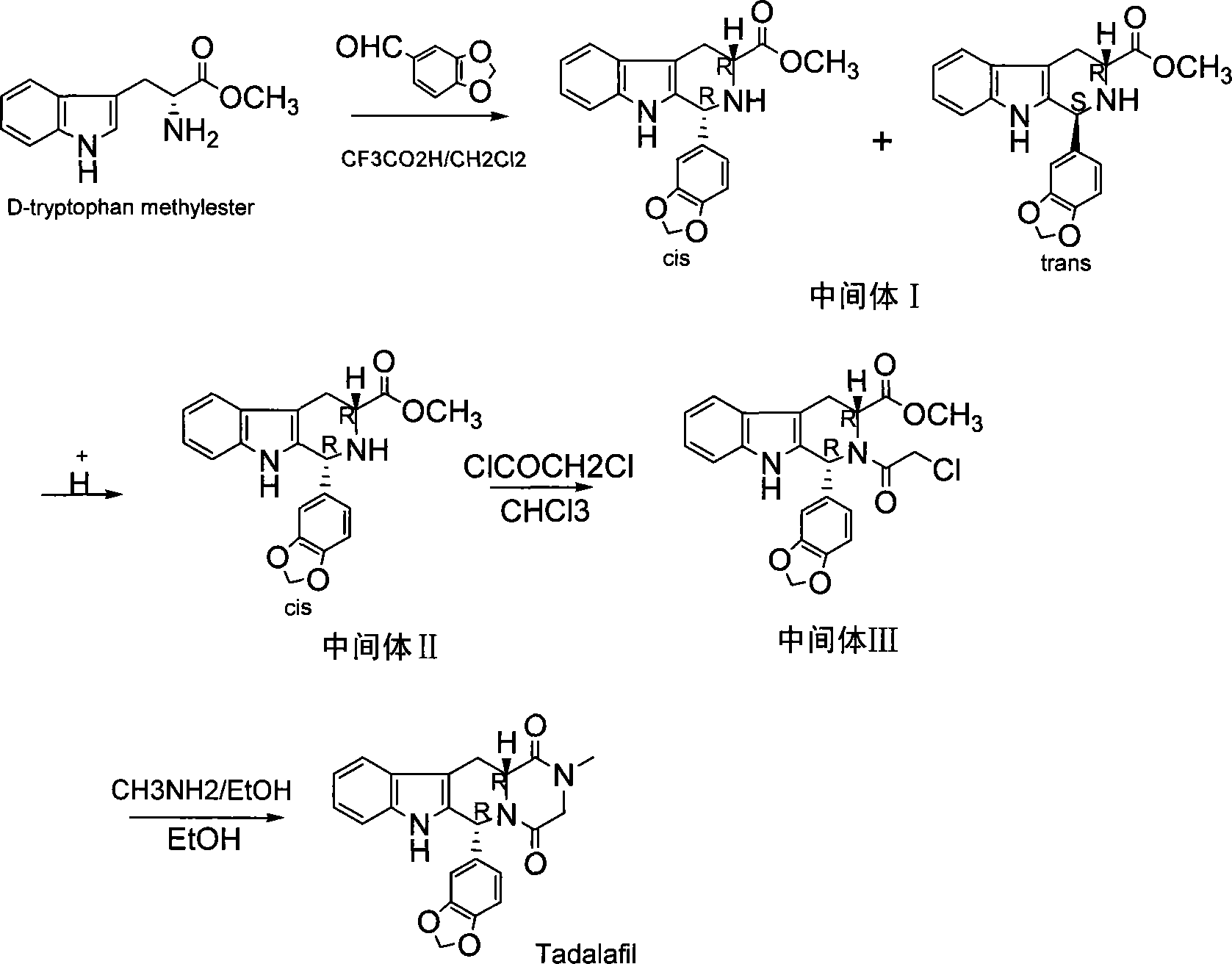
The invention relates to a synthesis method for (6R, 12aR)-6-(1, 3-benzodioxane heterocyclas-5-base)-2-methyl-2, 3, 6, 7, 12, -12a-hexahydro pyrazinyl [1', 2':1, 6] pyridyl [3, 4-b] indole-1, 4-diketone in the technical field of the pharmaceutical engineering. With the D-methyl tryptophan and piperonal as raw materials, the cis-1, 3-disubstituted carboline intermediates are prepared and generated through the Pictet-Spengler cyclization and then the products called tadalafil are obtained through the acylation ammoniation of the 3-secondary amino group and the final MTX substitution and condensation close-loop. By adopting the acidic condition protic solvents in the invention, the trans-1, 3-disubstituted carboline intermediates generated through the Pictet-Spengler cyclization, which is a key step in the existing process, are converted into those with a cis-configuration, thus greatly enhancing the stereospecific configuration yield of the carboline intermediates so as to enhace the overal yield of the tadalafil with reaching the goal of optimizing and improving the synthesis process.
Owner:SHANGHAI JIAO TONG UNIV
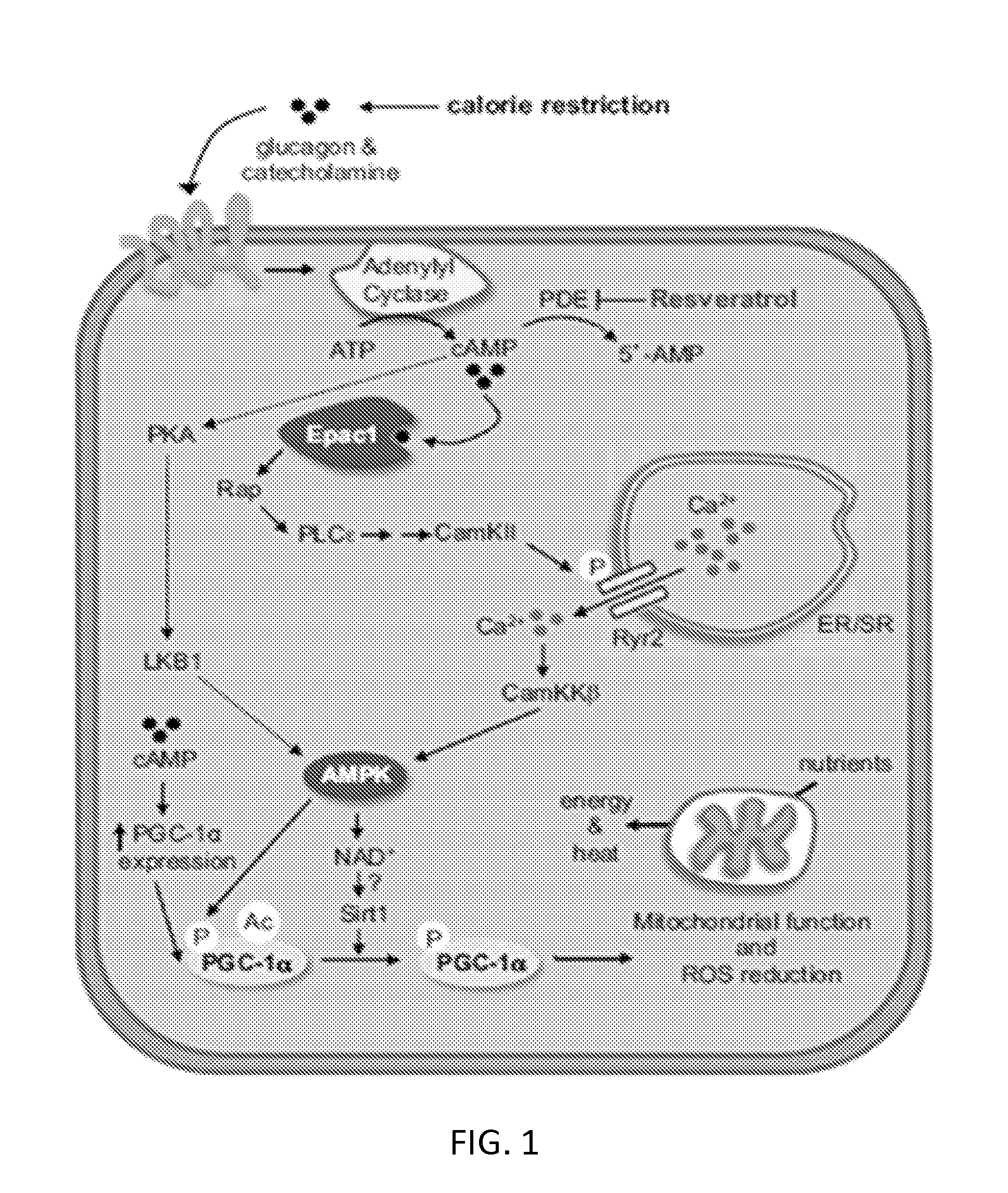
Use of sGC stimulators, sGC activators, alone and combinations with PDE5 inhibitors for the treatment of systemic sclerosis (SSc).
The use of sGC stimulators, sGC activators alone, or in combination with PDE5 inhibitors for the prevention and treatment of fibrotic diseases, such as systemic sclerosis, scleroderma, and the concomitant fibrosis of internal organs.
Owner:ADVERIO PHARMA
Pharmaceutical composition for alleviating pain or spasticity in a patient suffering from spinal cord injury
To provide a novel pharmaceutical composition for alleviating pain or spasticity in a patient suffering from spinal cord injury. The present invention relates to a method for alleviating pain or spasticity in a patient suffering from spinal cord injury comprising administering to the patient an effective amount of cGMP PDE5 inhibitor, to a pharmaceutical composition comprising such an effective amount of the inhibitor, and to a use of the inhibitor in the manufacture of such a pharmaceutical composition.
Owner:PFIZER INC
Use of phosphodiesterase inhibitors for treating multidrug resistance
The present invention relates to methods of treating multidrug resistance in cancerous cells with phosphodiesterase (PDE) inhibitors, e.g., PDE5 inhibitors. More specifically, the invention relates to methods of treating multidrug resistance that arises, e.g., during administration of chemotherapeutic / antineoplastic (anticancer) agents for treatment of cancer, with a PDE5 inhibitor (e.g., sildenafil, vardenafil, and tadalafil). The invention also relates to methods of treating cancer, e.g., multidrug resistant cancer, using a PDE5 inhibitor in combination with an antineoplastic therapeutic agent. Further, the invention relates to pharmaceutical compositions for treating multidrug resistant cancers comprising a PDE5 inhibitor, or a combination of a PDE5 inhibitor and an antineoplastic agent.
Owner:ST JOHNS UNIV
Methods for treating erectile dysfunction in patients with insulin-dependent diabetes
InactiveUS20110190192A1Enhances erection durationEasy maintenanceOrganic active ingredientsPeptide/protein ingredientsInsulin dependent diabetesRegimen
The present invention relates to the development of improved methods for treating erectile dysfunction associated with diabetes. Significantly, such dosing regimens can be combined with established methods for treating sexual dysfunction, including PDE5 inhibitors such as those sold under the trademark VIAGRA® to provide for significantly improved efficacy compared to the PDE5 inhibitor alone.
Owner:CEBIX
Use for PDE5 inhibitors
Owner:TAKEDA GMBH
sGC STIMULATORS OR sGC ACTIVATORS ALONE AND IN COMBINATION WITH PDE5 INHBITORS FOR THE TREATMENT OF CYSTIC FIBROSIS
The present invention relates to soluble guanylate cyclase (sGC) and to phosphodiesterases (PDEs) and the pharmacology of sGC stimulators, sGC activators and PDE inhibitors. More particularly, the invention relates to the use of sGC stimulators and sGC activators in combination with PDE5 inhibitors for preparation of medicaments for the treatment of Cystic Fibrosis (CF).
Owner:ADVERIO PHARMA
Treatment of sexual dysfunction
Bombesin receptor antagonists have been found to be useful in the treatment of sexual dysfunction in both males and females. They may be selective BB1 antagonists or mixed BB1 / BB2 antagonists. Combinations are disclosed of bombesin receptor antagonists with a range of other active compounds, for example PDE5 inhibitors, NEP inhibitors and lasofoxifene.
Owner:WARNER-LAMBERT CO
Compositions and methods for the reduction or prevention of non-alcoholic steatohepatitis (NASH)
InactiveUS20170239253A1Lower Level RequirementsReduction of non-alcoholic steatohepatitis (NASH)Disease diagnosisPill deliveryMetabolitePhosphodiesterase 5 inhibitor
Methods useful for sustaining a reduction of non-alcoholic steatohepatitis are provided herein. Such methods may comprise administering to a subject in need thereof a sirtuin pathway activator and / or PDE5 inhibitor alone or in combination with an amount of a branched amino acid in free amino acid form, or a metabolite thereof. Also provided herein are compositions and kits for practicing any of the methods described herein.
Owner:NUSIRT SCI
Pde5 inhibitor compositions and methods for treating cardiac indications
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Rapidly absorbing oral formulations of PDE 5 inhibitors
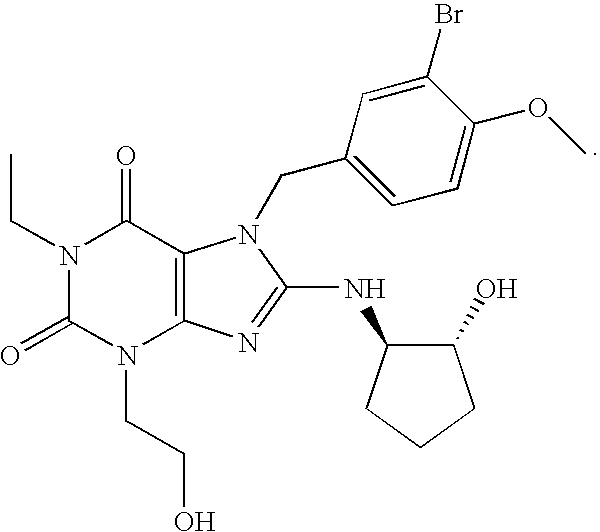
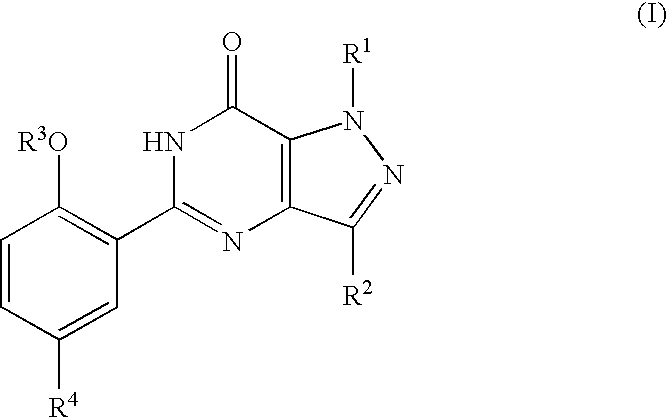
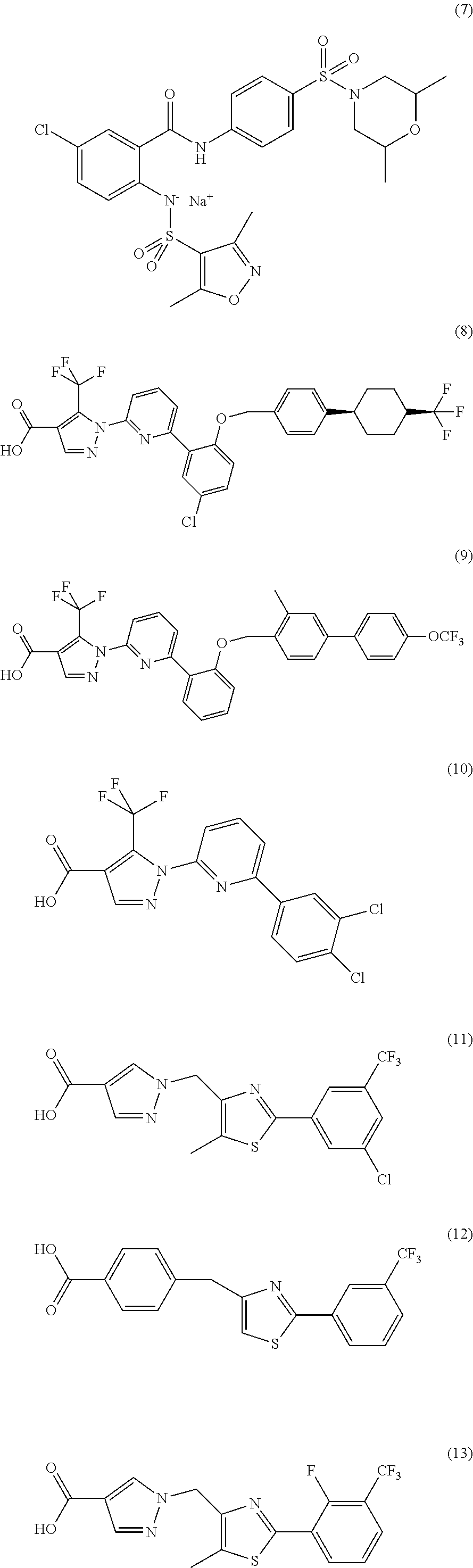
The present invention encompasses oral formulations of a PDE5 inhibitor which provide rapid disintegration after introduction to the oral cavity, followed by buccal and / or sublingual absorption. The orally disintegrating formulations can be in a variety of dosage forms including lingual strip, sublingual strip, oral mist, rapidly disintegrating tablet, lyophilized wafer, granulated particles and gum. The formulations can include an extended release component that allows the PDE5 inhibitor to be swallowed for gastrointestinal absorption. Combination therapies with a second pharmaceutical agent known to cause a PDE5-treatable condition as a side effect, such as erectile dysfunction, are also described. The PDE5 inhibitor of the following chemical structure is particularly favored for these formulations:
Owner:SCHERING CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com