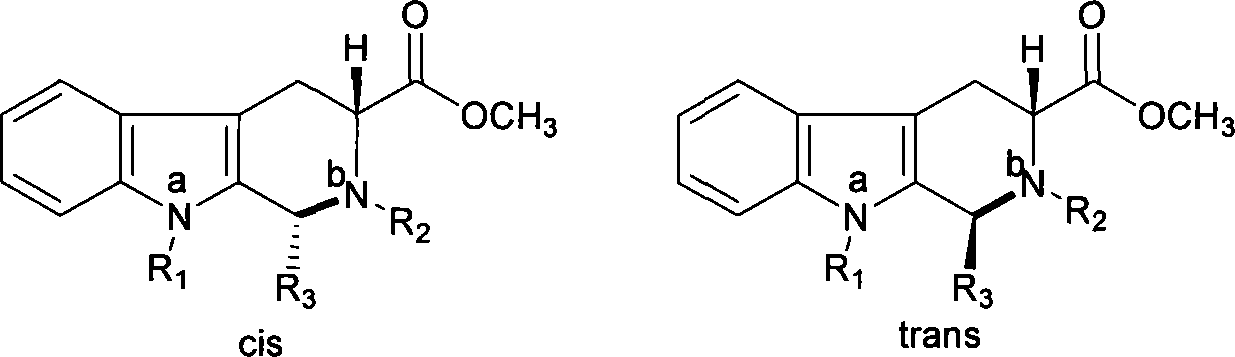
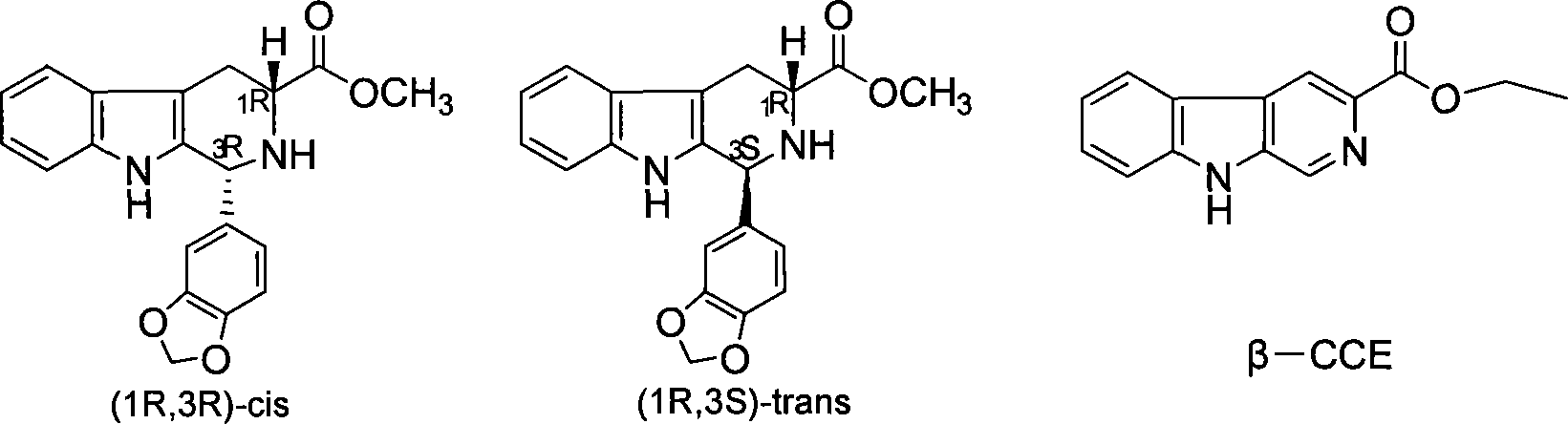
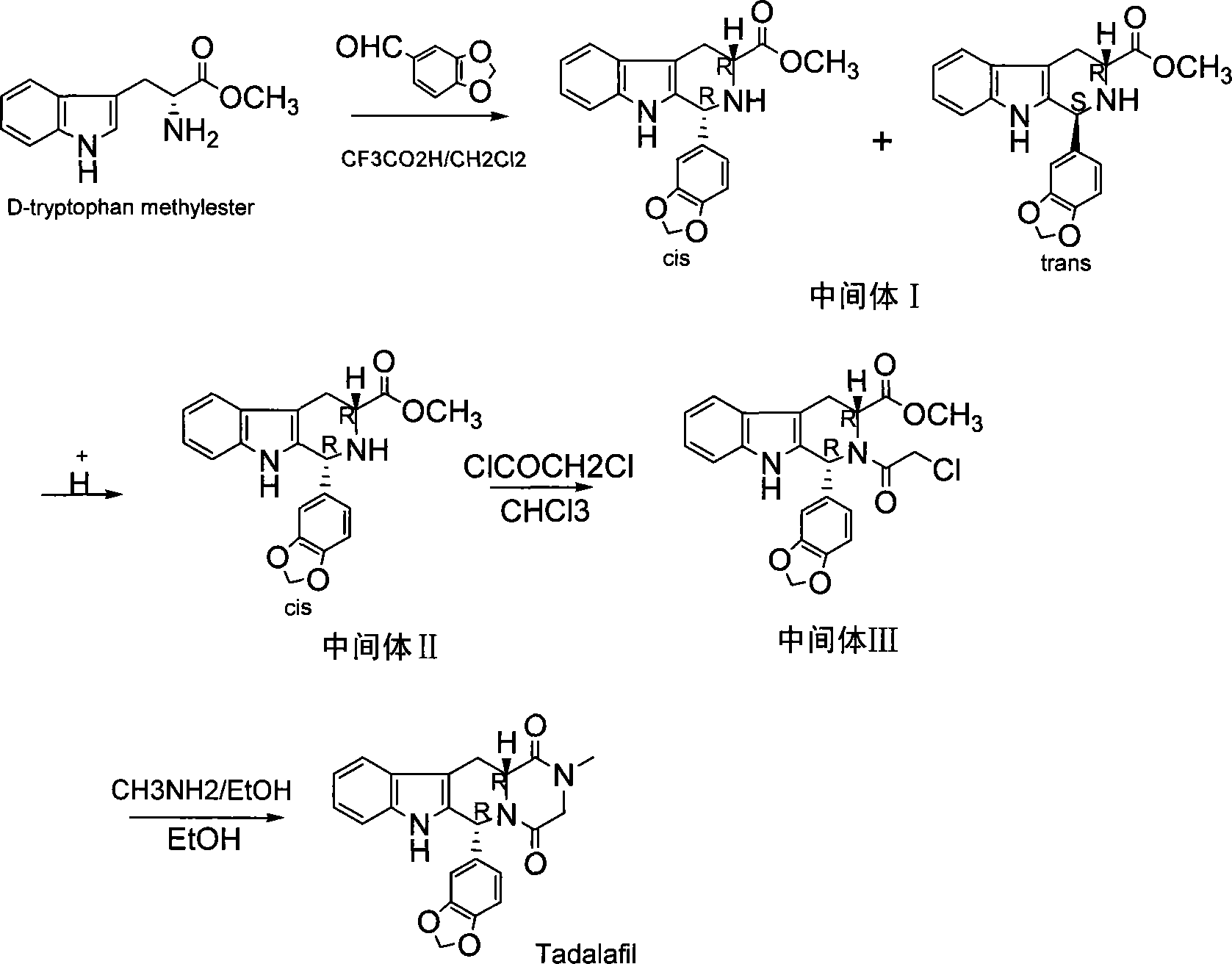
Method for synthesizing phosphodiesterase 5 inhibitor tadanafil
A synthetic method and diketone technology, applied in the field of medical engineering, can solve problems such as time-consuming and laborious, unfavorable synthetic process, and no specific conditions are specified, and achieve the effects of increasing yield, optimizing synthetic process, and simplifying separation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Intermediate I (cis (1R,3R)-1-(3,4-dimethoxy-phenyl)-2,3,4,9-tetrahydro-1 hydrogen-β-carboline-3- Methyl formate and trans (1S,3R)-1-(3,4-dimethoxy-phenyl)-2,3,4,9-tetrahydro-1hydro-β-carboline-3- Synthesis of methyl formate)
[0028] Dissolve 0.04mol, 8.72g of D-tryptophan methyl ester, 0.042mol, 6.3g (1.05eq) of piperonal in 150ml of dichloromethane, cool down to 0°C with an ice-salt bath, and slowly add 5.7ml of trifluoroacetic acid dropwise . And stirred for 1h, rose to room temperature to continue the reaction for 24h. After the reaction is complete, add 30ml of saturated sodium bicarbonate and extract the layers with dichloromethane (50ml×3). The dichloromethane layers are combined, washed with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and filtered. The filtered dichloromethane layer was concentrated to 1 / 2 volume, placed in a refrigerator to cool, and white crystals were precipitated, filtered under reduced pressure to ...
Embodiment 2
[0030]Intermediate II cis(1R,3R)-1-(3,4-dimethoxy-phenyl)-2,3,4,9-tetrahydro-1hydro-β-carboline-3-carboxylic acid Synthesis of methyl esters
[0031] Dissolve 9.7g of the intermediate I obtained in (1) in 150ml of mixed solvent of water and methanol, add hydrochloric acid to catalyze, stir to form a transparent solution, heat in a water bath to about 40-60°C and continue to react for 2-4 days, after the reaction is completed , was quenched with 30ml of saturated sodium bicarbonate, extracted with dichloromethane (50ml×3), the combined dichloromethane layers were washed with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and filtered. The filtered dichloromethane layer was evaporated to dryness under reduced pressure to obtain a white solid with a yield of 98%, mp 152-154°C, [α] D 25 =+26.76° (c=0.1, CHCl 3 ); literature value: 24.4° (c=1.03, CHCl 3 ) This is intermediate II.
[0032] Through HPLC (high performance liquid phase) (Varion ...
Embodiment 3
[0037] Intermediate III (1R,3R)-1-(3,4-dimethoxy-phenyl)-2-chloroacetyl-2,3,4,9-tetrahydro-1hydro-β-carboline -Synthesis of methyl 3-carboxylate
[0038] Dissolve 3mmol, 1.05g of the intermediate II obtained in (2), 0.41g (1.2eq) of sodium bicarbonate in 50ml of chloroform at room temperature, cool down to 0°C in an ice bath, and slowly add 3.3mmol of chloroacetyl chloride dropwise under a nitrogen atmosphere , 0.264ml (1.1eq) (diluted in 10ml chloroform for use). After the dropwise addition was completed, the temperature was raised to 25° C. to continue the reaction for 1.5 h. After the reaction was complete, 50ml of water was added, and the aqueous layer was extracted with dichloromethane (20ml×3). The combined dichloromethane layers were washed with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and filtered. Concentrate the dichloromethane filtrate to 1 / 2, add 30ml of petroleum ether and oscillate gradually to see the precipitation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com