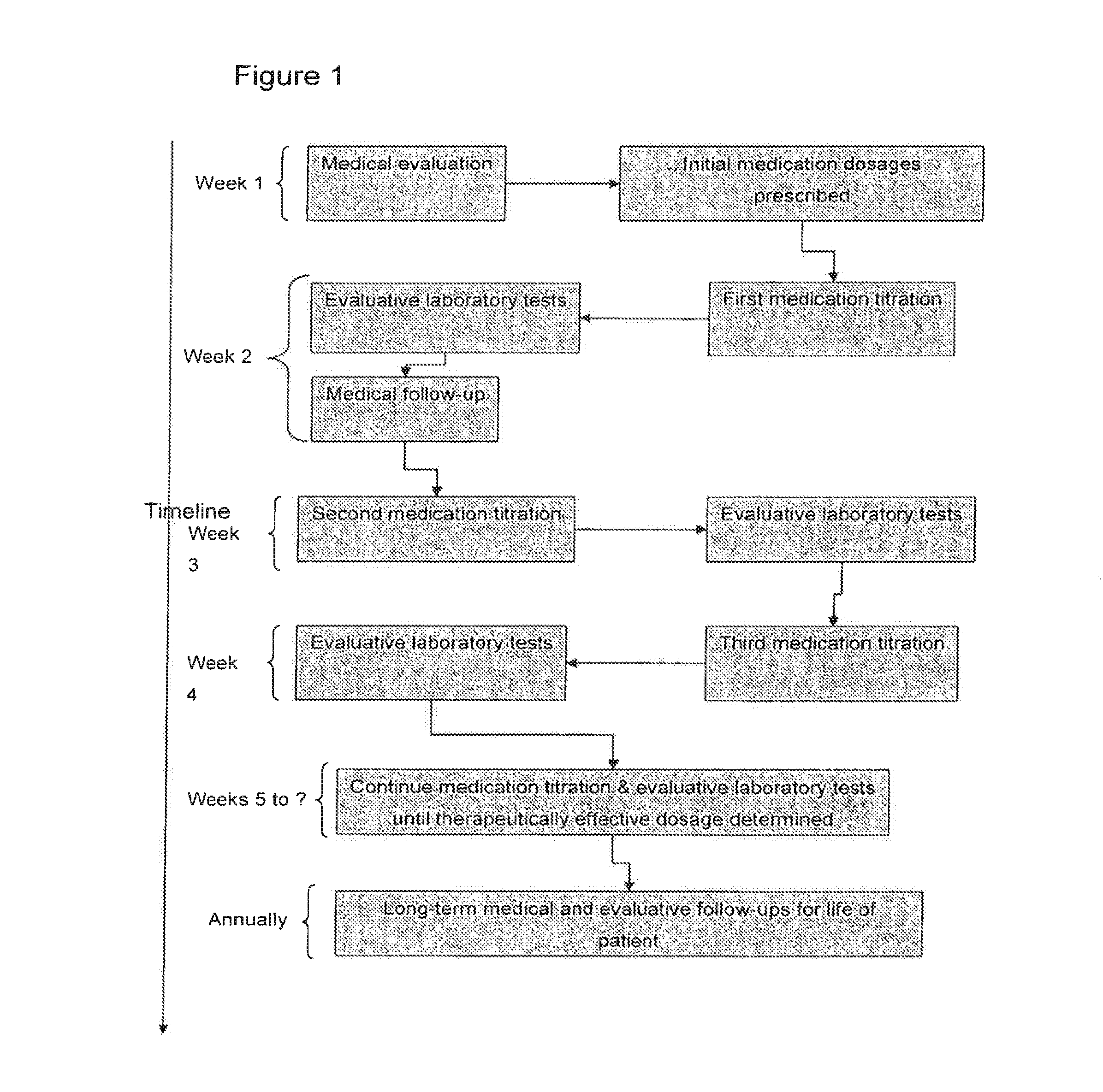
Novel compositions comprising a phosphodiesterase-5 inhibitor and their use in methods of treatment
a technology of phosphodiesterase and composition, which is applied in the direction of drug composition, biocide, peptide/protein ingredients, etc., can solve the problems of affecting the effect of lipid metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0309]A 58-year old Caucasian male patient with moderate NDD secondary to Parkinson's Disease was administered a combination of PI-5, SSRI, and Cl. Patient has been suffering from Parkinson's for an approximate 12-year duration with no other significant co-morbidities. Patient weighed 210 lbs with a body mass index (bmi) of 0.20. Patient has had a recent, seven-month progression of somatic symptoms with moderate progression of apraxia, bradykinesia and loss of short-term and intermediate visual and verbal memory, combined with decreased analytic ability, mathematic ability, creativity and organizational skills. Initial tests were run for kidney function and liver function. The kidney function tests showed a creatinine level of 1.0, and a liver function test (LFT), both of which were within normal limits. Workup as standard for dementia, including CBC, SMAC, UA, EKG, CXR, MRI brain (no gadolinium), syphilis titres, AIDS test, pulse oximitry, sedimentation rate, thyroid function tests...
example 2
[0312]A second subject was diagnosed with nonspecific but rapidly progressive multi-system neurodegenerative disease at age 57. Clinical progression and further diagnostic findings suggest probable frontotemproral dementia with cortico-bulbar disease. Initial presentation was with abrupt onset dementia with rapid progression over 3-4 months. Severe, generalized myoclonus of high amplitude with ataxia, dyspahagia, central and peripheral respirator), distress (requiring CPAP), dizziness, and hypokinetic rigidity followed some one month later and have continued to progress as well as dementia.
[0313]Patient also suffered for approximately 10 years from mild facial acne which has been responsive to topical and oral antibiotics, digital vitiligo, and one scar of 4×4 cm atrophic hypopigmentation on left upper thigh secondary to a chemical burn thirty years ago. Mild intermittent gingival infections requiring minimal surgical intervention with retraction from tooth line for overten years.
[0...
example 3
[0326]A third subject was diagnosed with atypical Parkinson's Disease with an initial presentation fifteen years earlier with cogwheel type diffuse tremor of low amplitude, diffuse muscular rigidity, mild bradykinesia, gait abnormalities. Patient also suffered from severe seborrheic dermatitis and scarring facial acne rosacea, for over 25 years and noted only a minimal response to topical and oral antibiotics. All symptoms slowly progressed to the present state of advanced severity in all symptoms and signs. Significant work-up has included the following:[0327]1 / MRI brain (without contrast)×2 / 3 years post onset, 15 years later)—wnl[0328]2 / PET brain (unclear isotopes / 10 years into disease)—symmetrical abnormalities in cortex (further details unavailable at this time).[0329]3 / paradoxical response to IV Apomorphine with decreased blood pressure and worsening of rigidity and tremor.
[0330]Patient is a non-smoker and social drinker with minimal alcohol consumption and no history of drug a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com