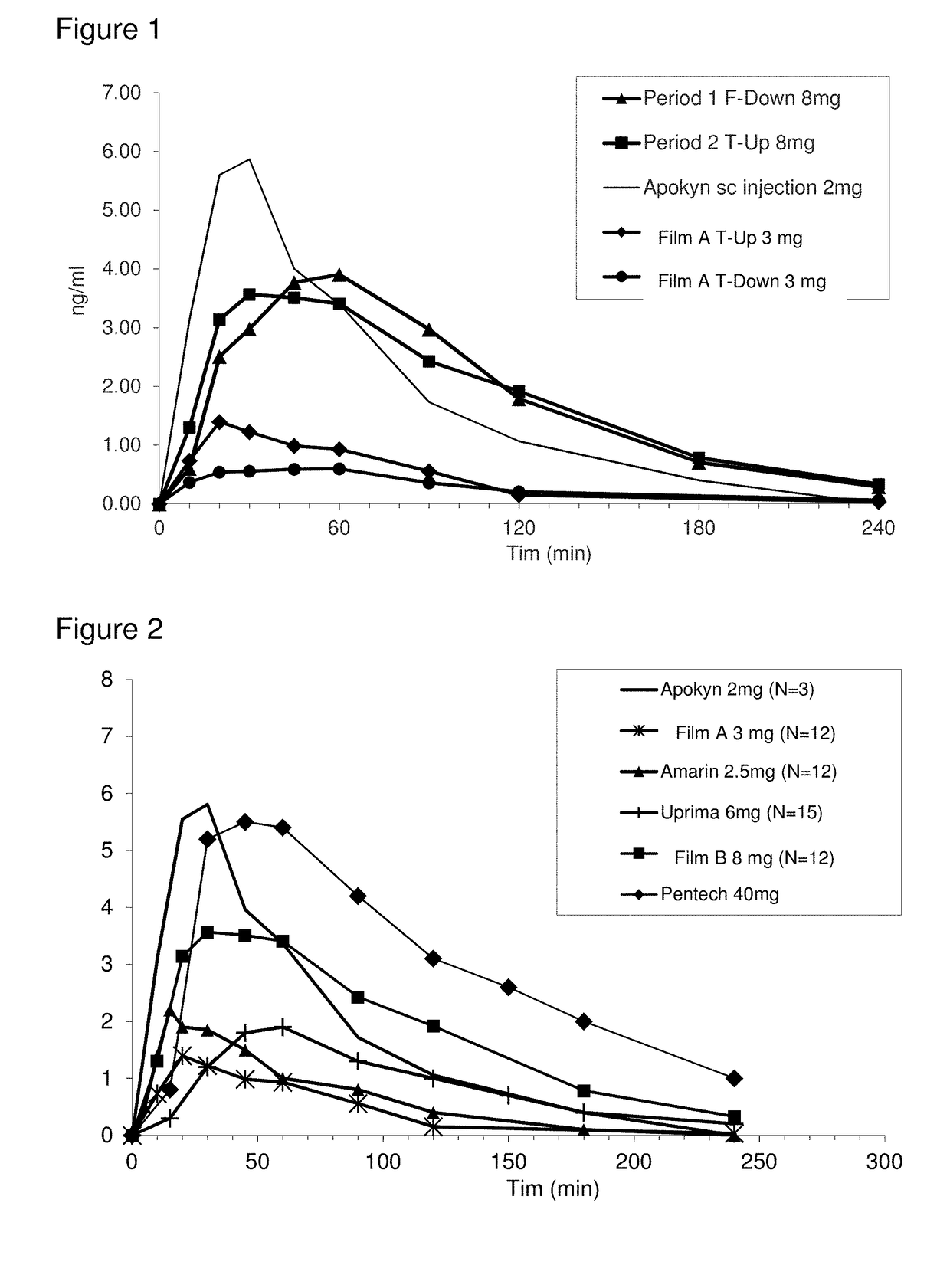
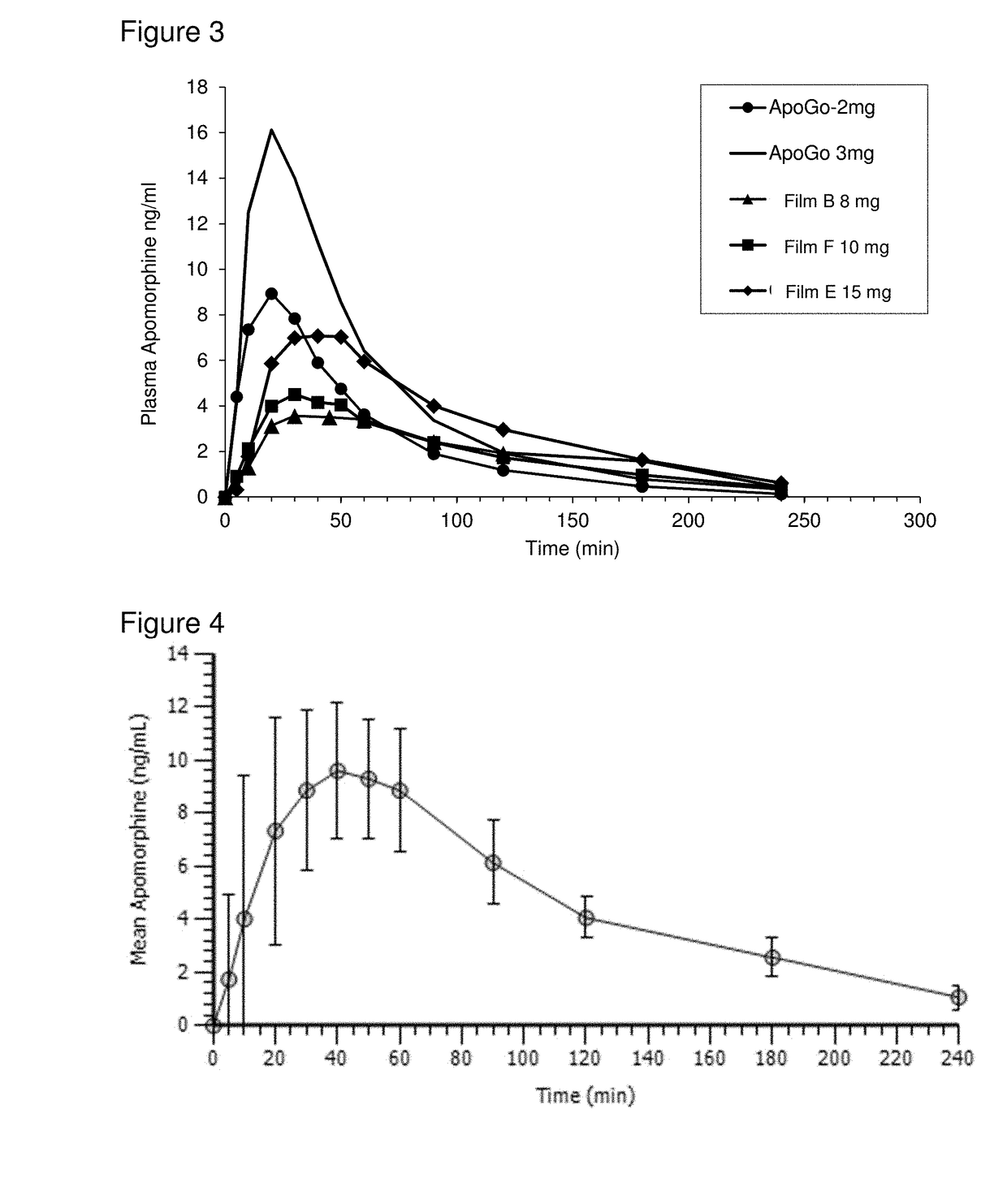
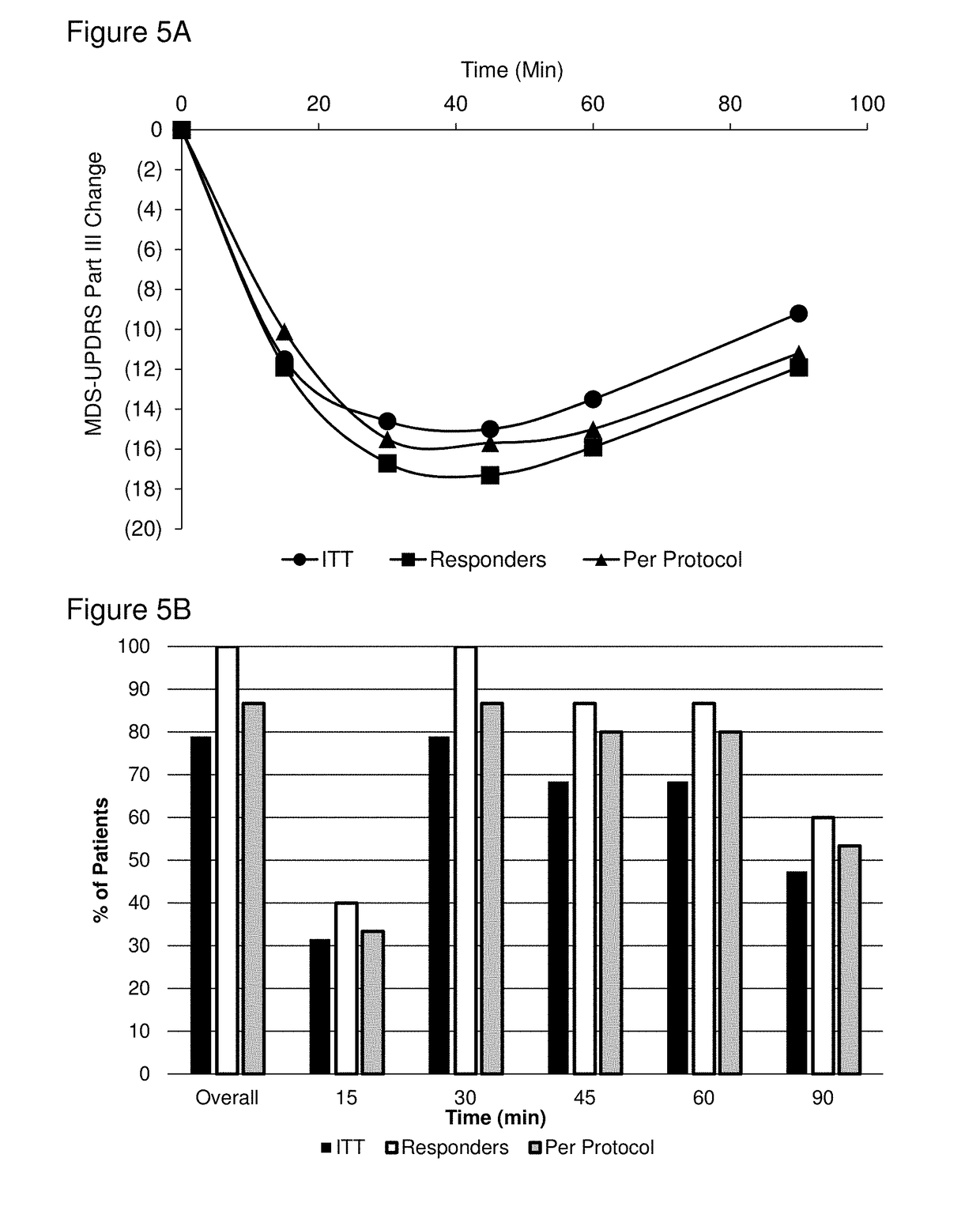
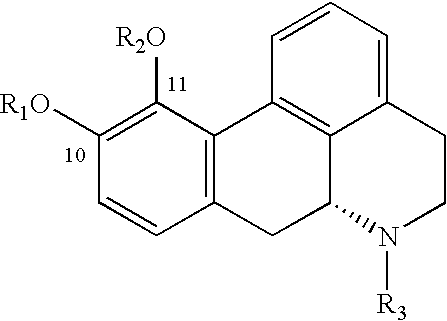
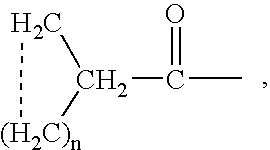
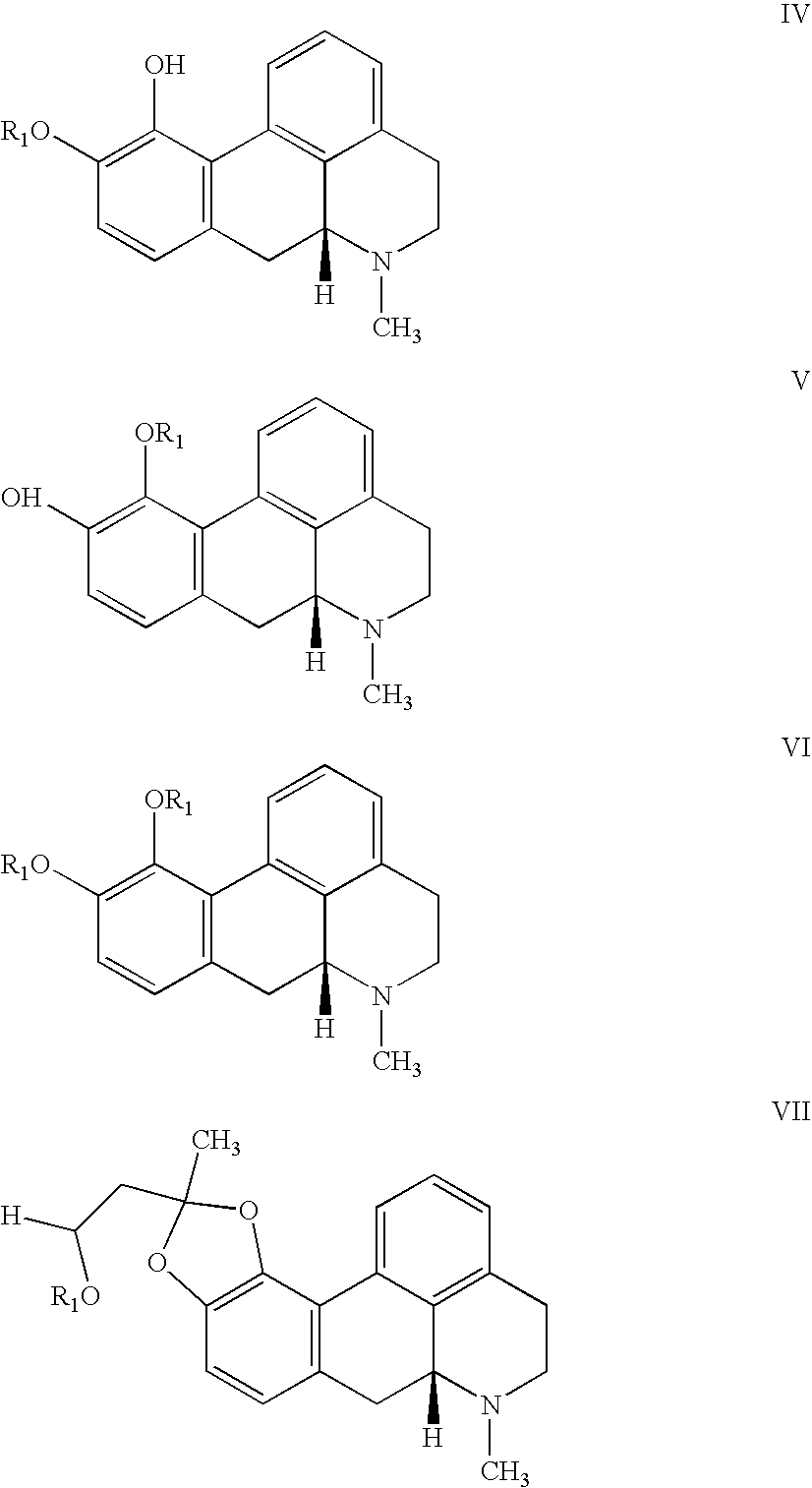
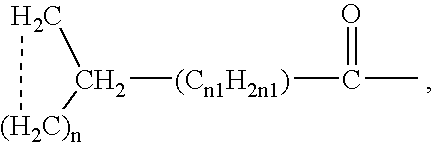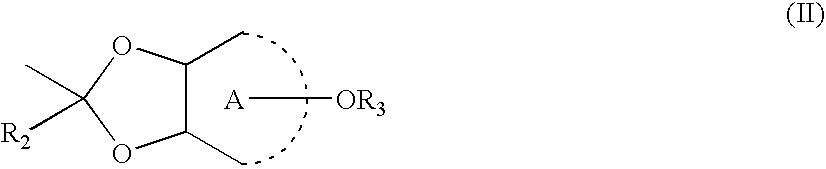Patents
Literature
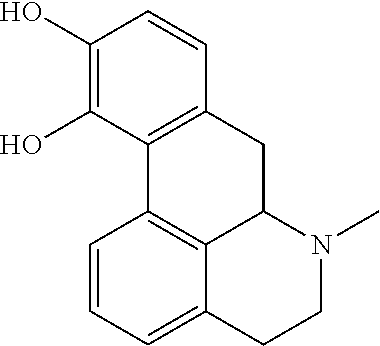
63 results about "Apomorphine" patented technology
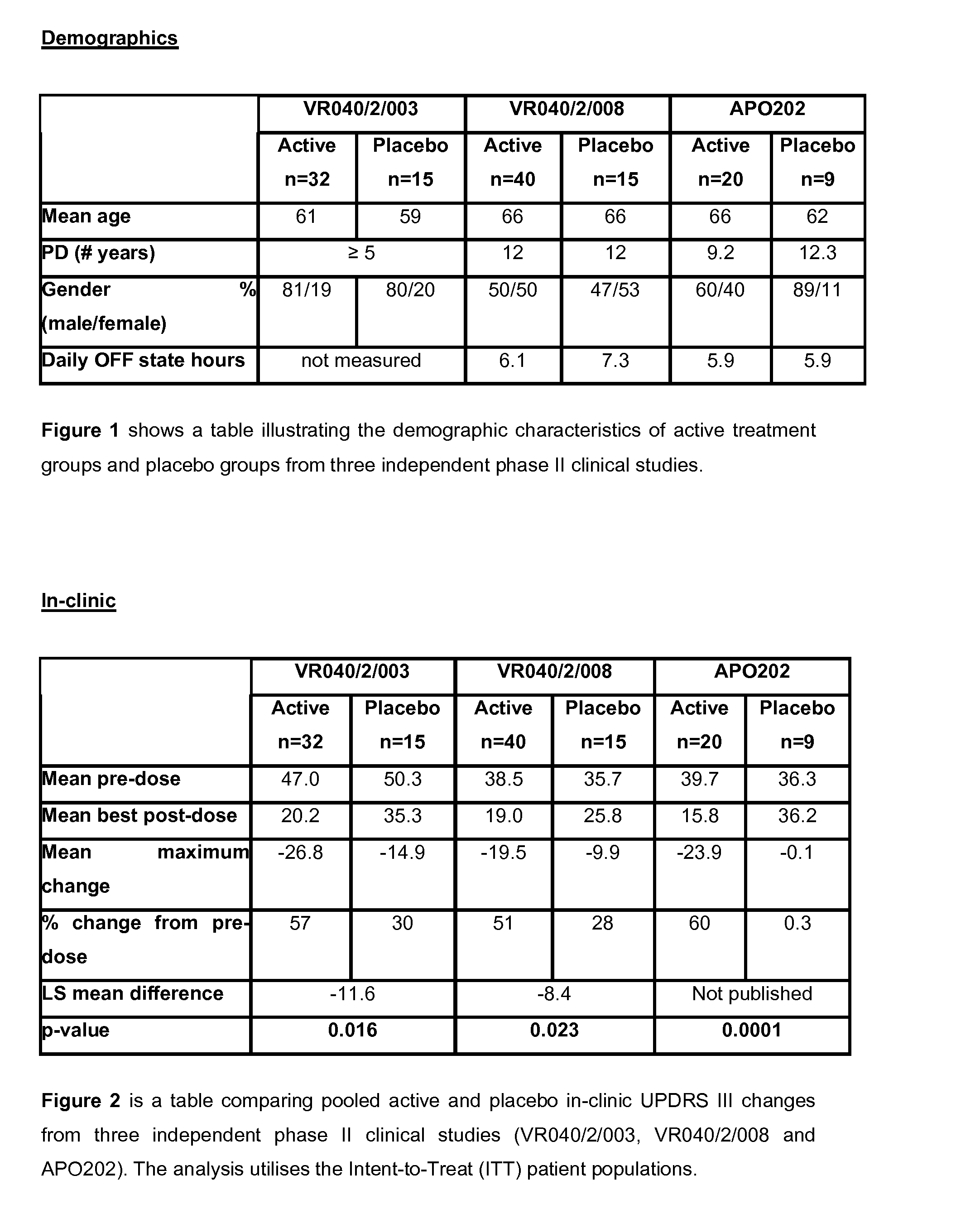
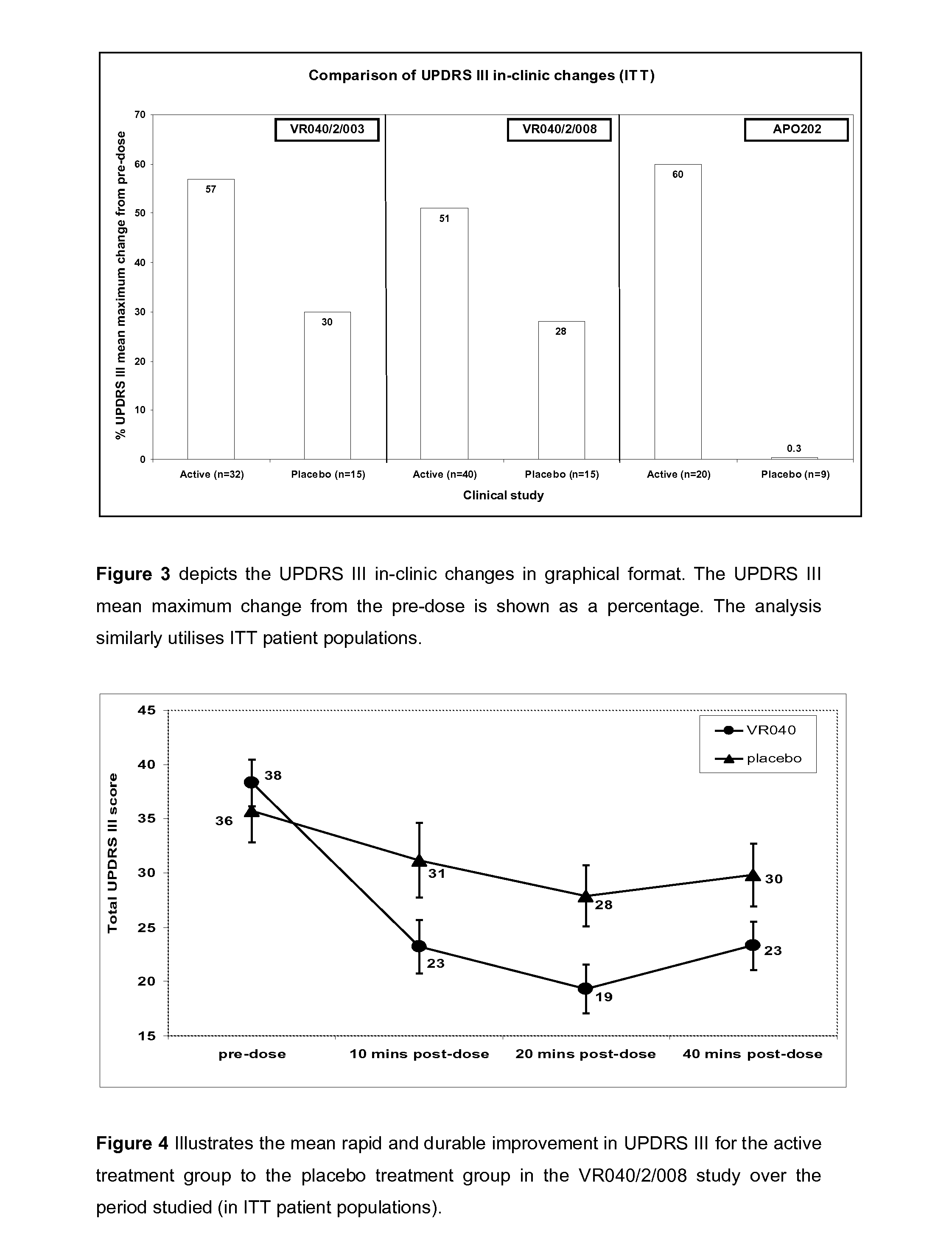
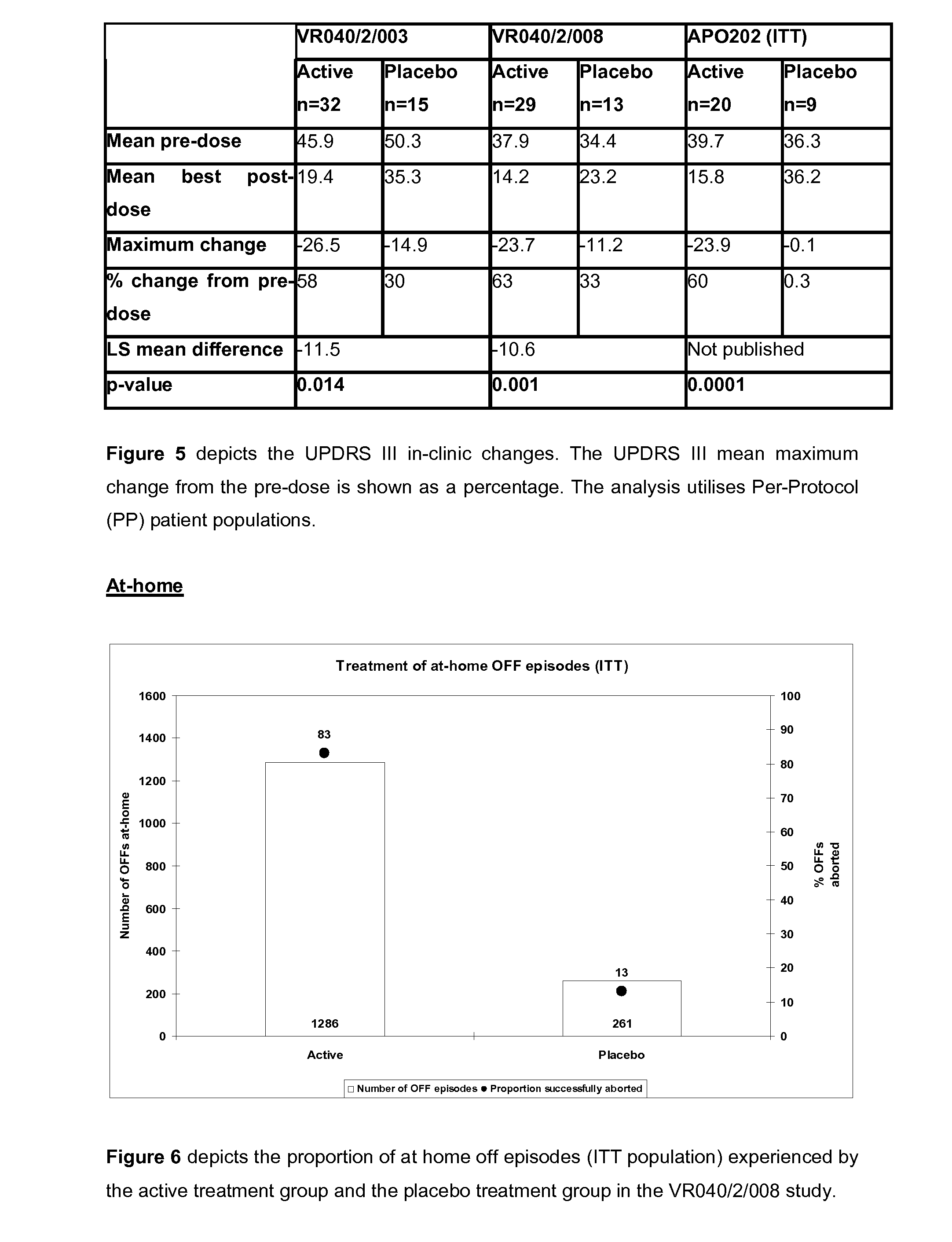
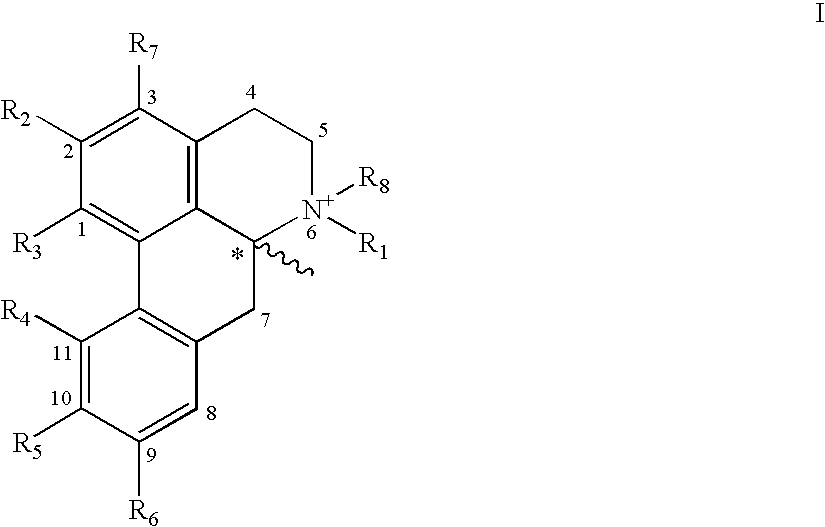
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
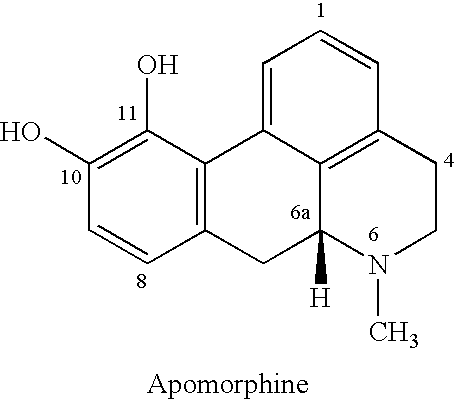
This medication is used to treat the symptoms of Parkinson's disease.
Compositions for treating parkinson's disease
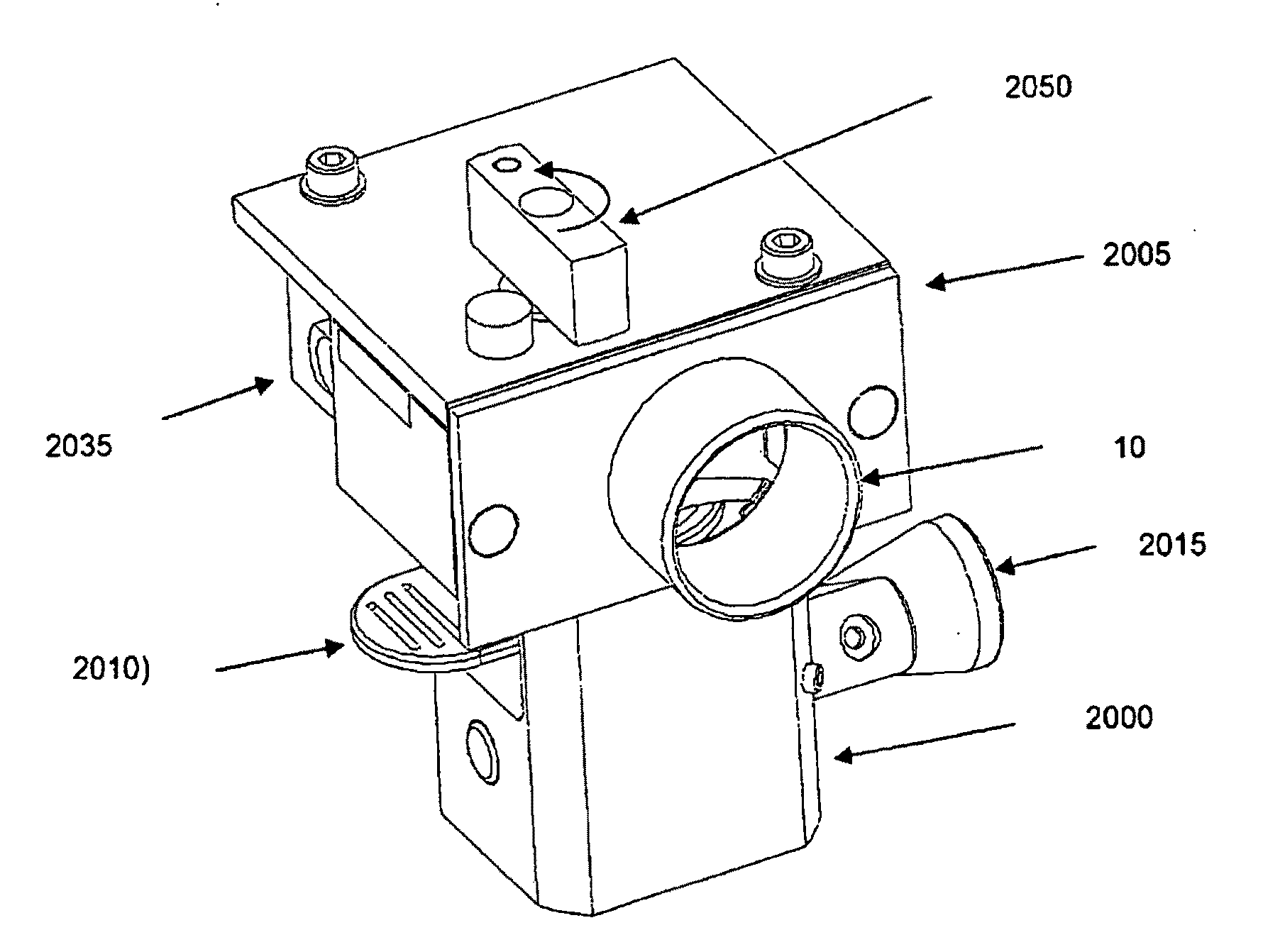
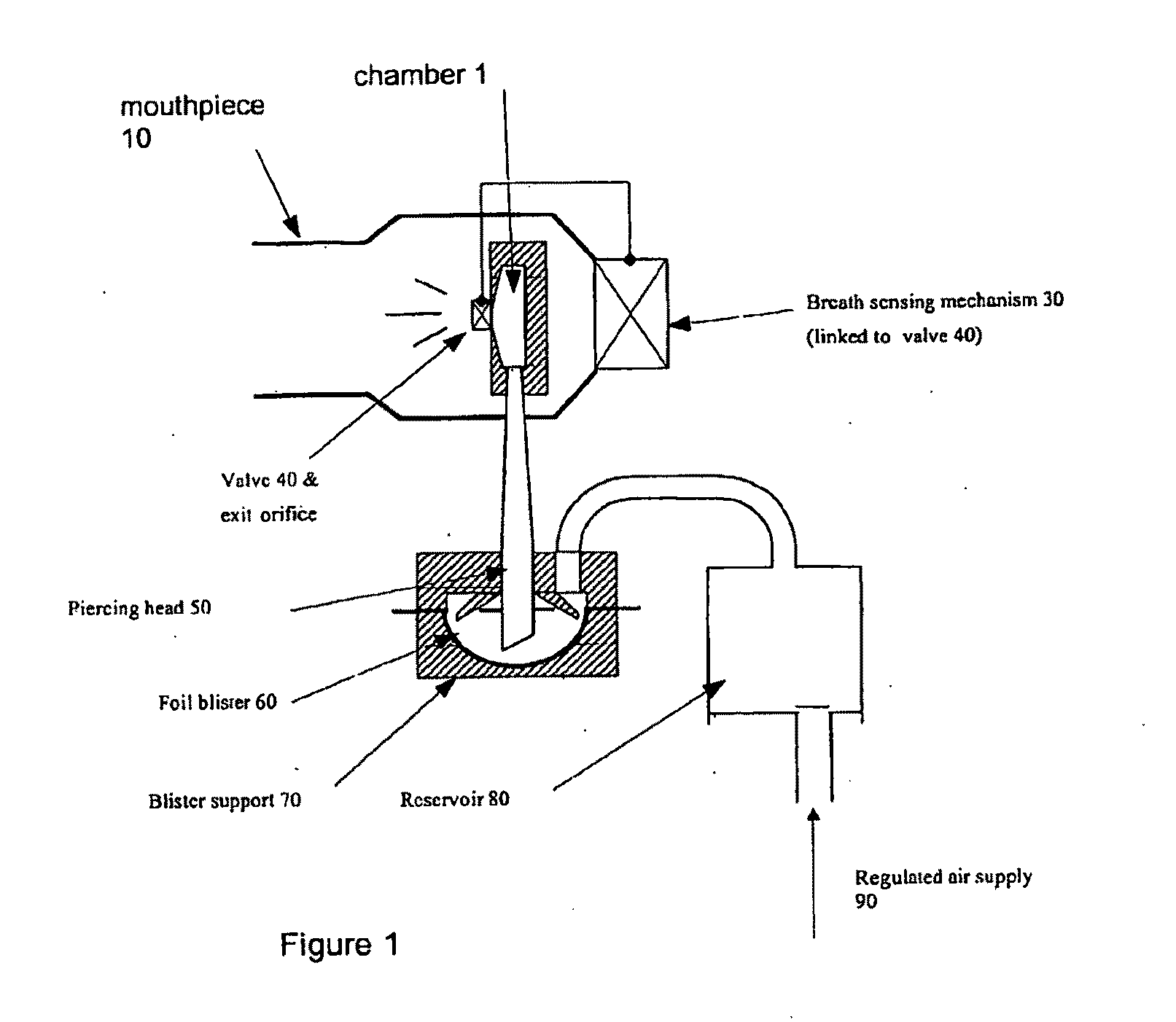
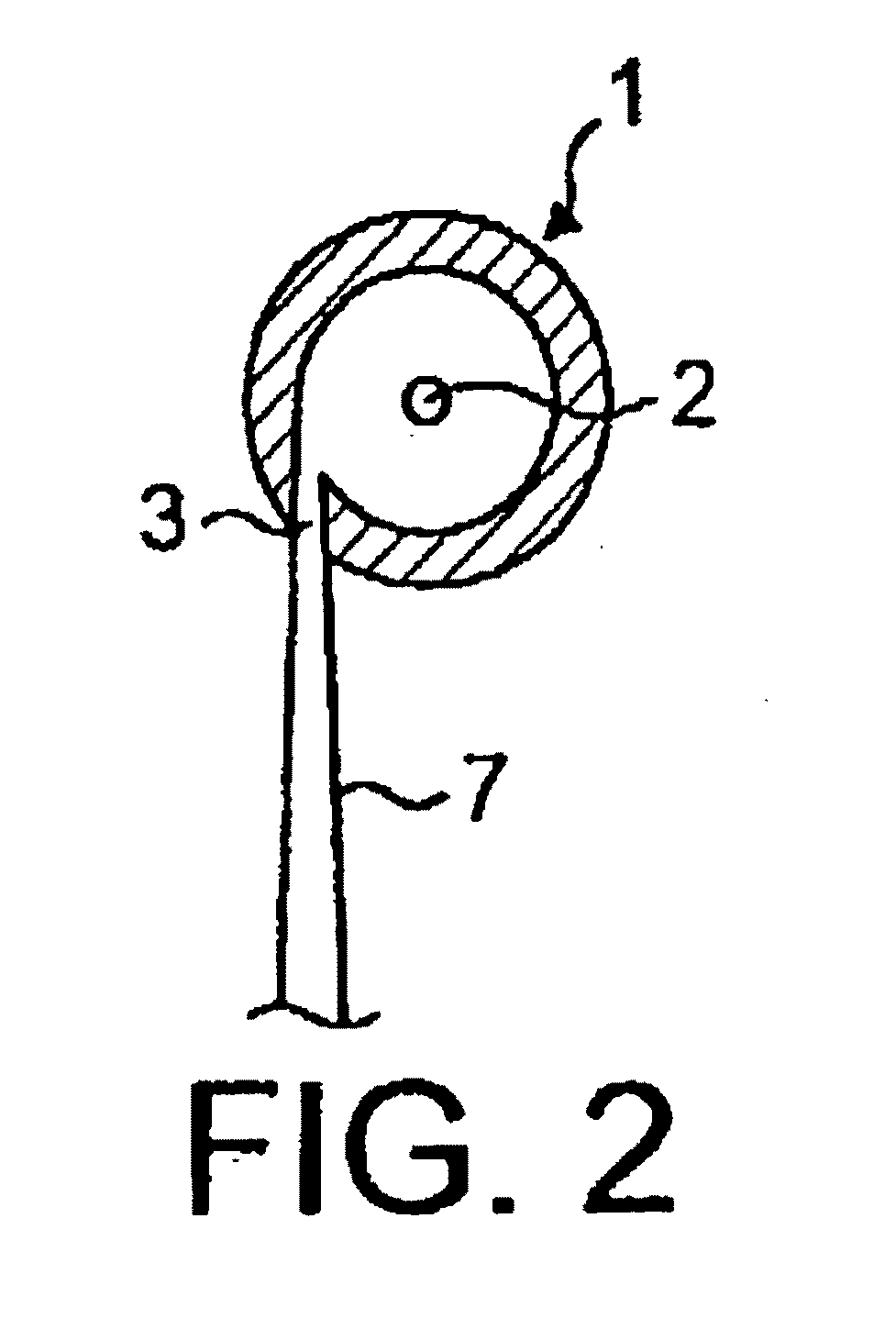
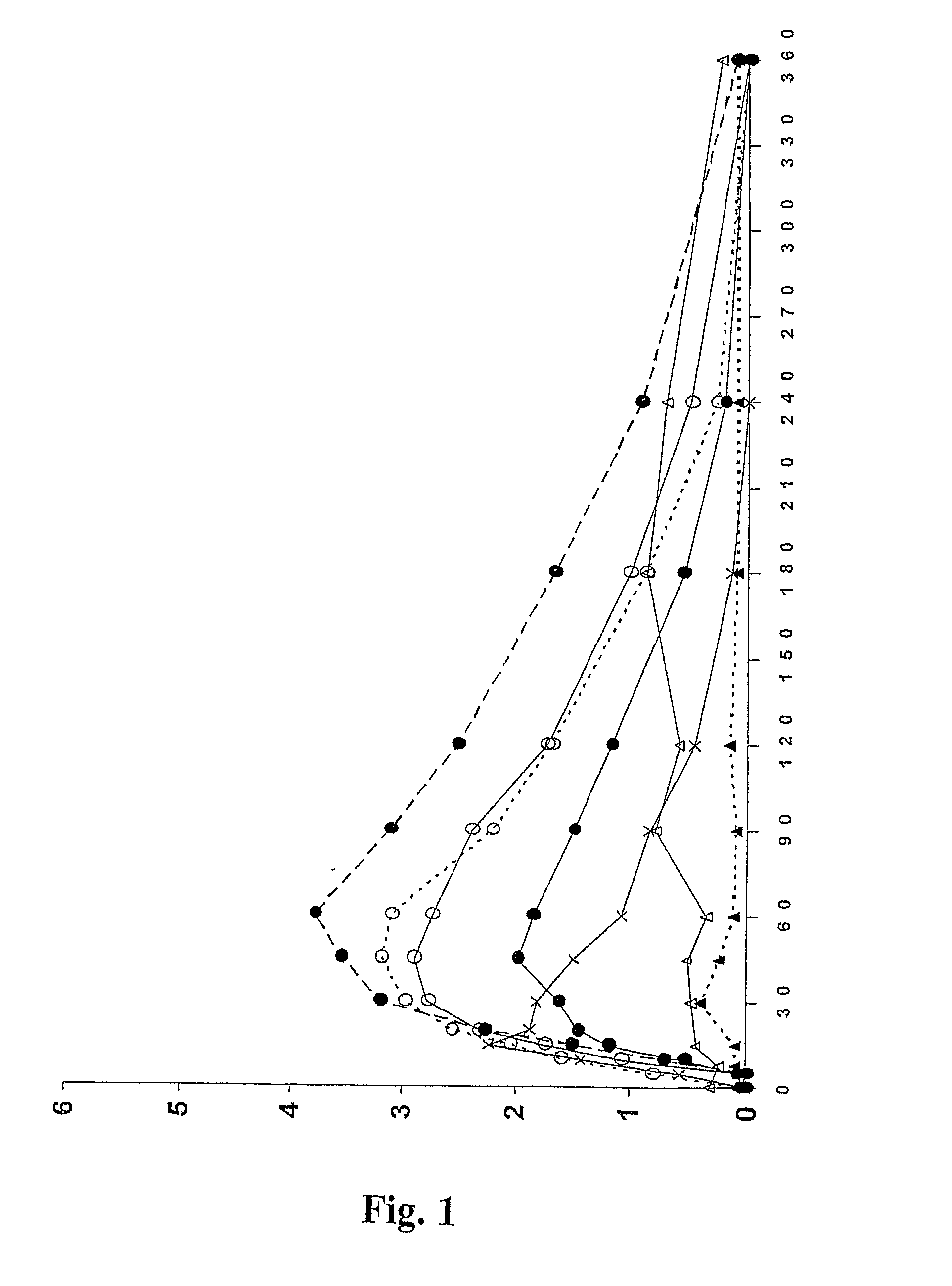
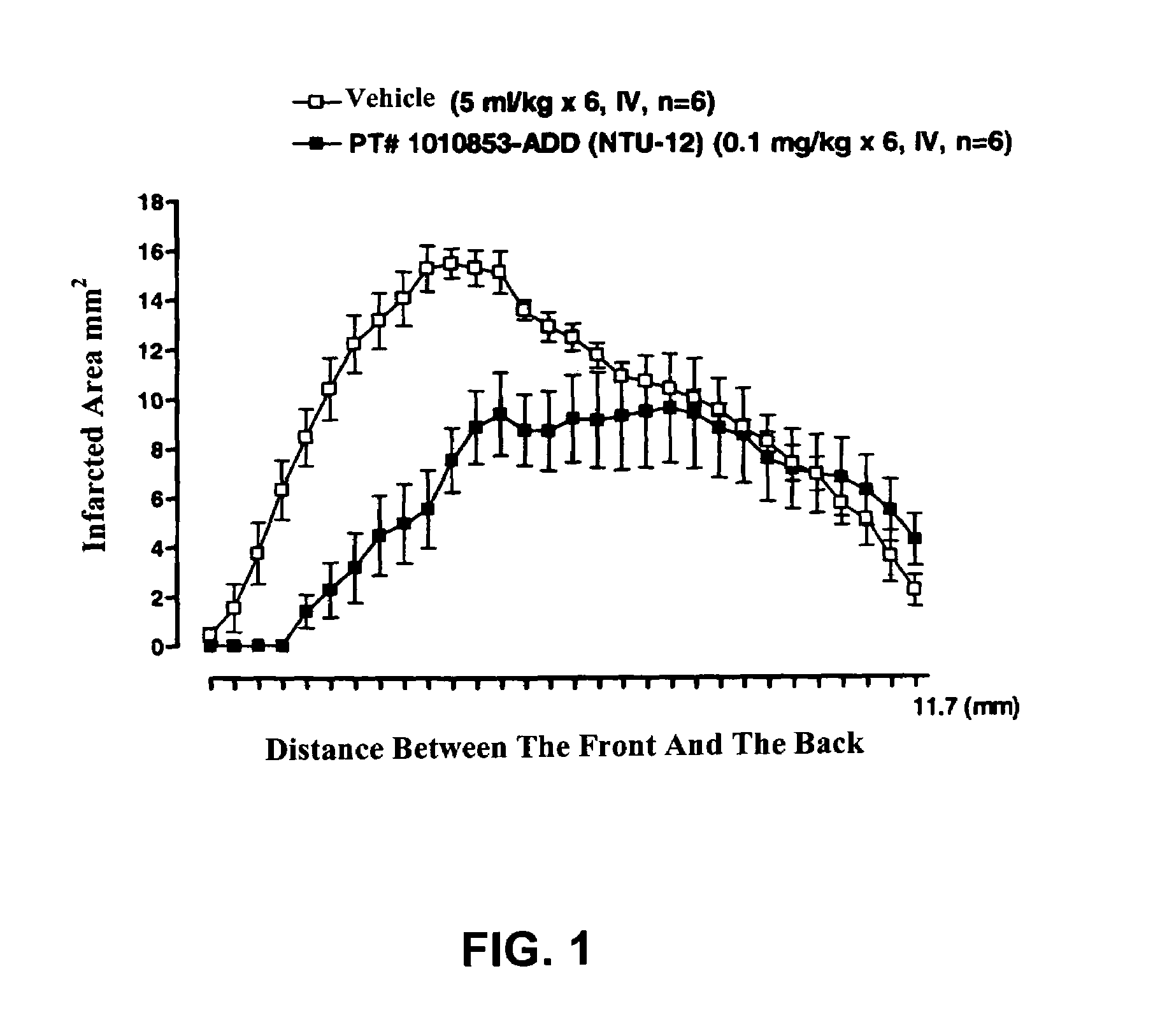
The present invention relates to improved treatment of diseases and disorders of the central nervous system by administration of apomorphine. In particular, the administration is via pulmonary inhalation. The invention provides the means for improving the treatment of a number of conditions, including Parkinson's Disease.
Owner:VECTURA LTD
Pharmaceutical formulation of apomorphine for buccal administration
InactiveUS20090023766A1Improve sexual functionIncrease libidoBiocideNervous disorderSexual functionDisease
The present invention provides a kit comprising, in separate compartments of a container, the following components (a) and (b): (a) a combination of apomorphine or a pharmaceutically acceptable acid addition salt thereof and a pharmaceutically acceptable excipient or carrier; and (b) a solution which comprises a diluent and a pH modifying agent; the components being presented such that they can be combined at the point of use into a formulation which is adjusted to a pH ranging from mildly acidic to alkaline and which is suitable for buccal administration. The formulation is useful in treating Parkinson's disease and in promoting sexual function.
Owner:AMARIN PHARMA IRELAND
Sublingual apomorphine
ActiveUS20110111011A1Alleviating dyskinesiaEffectively alleviatedPowder deliveryBiocideSexual functioningMorphine
The invention features sublingual formulations of apomorphine and apomorphine prodrugs, and methods of treating Parkinson's disease, sexual dysfunction, and depressive disorders therewith.
Owner:SUNOVION PHARMA INC
Compositions and uses
According to the invention there is provided a method of treating and / or preventing the symptoms of Parkinson's disease comprising delivering apomorphine, optionally in combination with levodopa and / or a dopamine agonist that is not apomorphine, wherein apomorphine is administered by inhalation.
Owner:VECTURA LTD
Apomorphine inhibitors of amyloid-beta (Abeta) fibril formation and their use in amyloidosis based disease
Described is a new class of small molecule inhibitors of amyloid beta protein (Abeta) aggregation, based on apomorphine. These molecules target the nucleation phase of Abeta self-assembly and interfere effectively with aggregation of Abeta 1-40 into amyloid fibrils in vitro as determined by transmission electron microscopy, Thioflavin T (ThT) fluorescence, and velocity sedimentation. Structure-activity studies using apomorphine analogues demonstrate that 10,11-dihydroxy substitutions of the D ring are preferred for the inhibitory effectiveness of these aporphines, and that methylation of these hydroxyl groups reduces their inhibitory potency. The ability of these small molecules to inhibit Abeta amyloid fibril formation appears to be linked to their ability to undergo auto-oxidation in solution, implicating an auto-oxidation product as the active Abeta inhibitor. Sedimentation velocity and electron microscopy studies demonstrate that apomorphine and analogues facilitate oligomerization of Abeta into short nonfibrillar soluble assemblies, but inhibit Abeta fibrillization.
Owner:CYTOKINE PHARMASCI
Method for the normalization of sexual response and amelioration of long term genital tissue degradation
InactiveUS20060281752A1Increase vasodilationNormalize the timing of sexual responseBiocideAnimal repellantsMammalHeifer calf
The present invention provides, in one embodiment, a method of normalizing the timing of sexual response in a mammal comprising the administration of an amount of a central nervous system sexual response initiator in an amount sufficient to produce genital vasodilation but less than the amount required to produce effective vasocongestive arousal. The method is applicable not only to adjusting or normalizing the timing of sexual response in humans, but in the breeding of valuable commercial animals such as horses, cattle, sheep, swine and the like and domesticated pets such as dogs and cats. In an alternative embodiment, the present invention provides a method for the prophylactic treatment of long-term tissue degradation in the genital organs comprising the administration to a mammal of a central nervous system sexual response initiator in an amount sufficient to produce genital vasodilation but less than the amount required to produce effective vasocongestive arousal. The preferred central nervous system sexual response initiator is apomorphine or a pharmaceutically acceptable acid addition salt thereof.
Owner:HEATON JEREMY P W +1
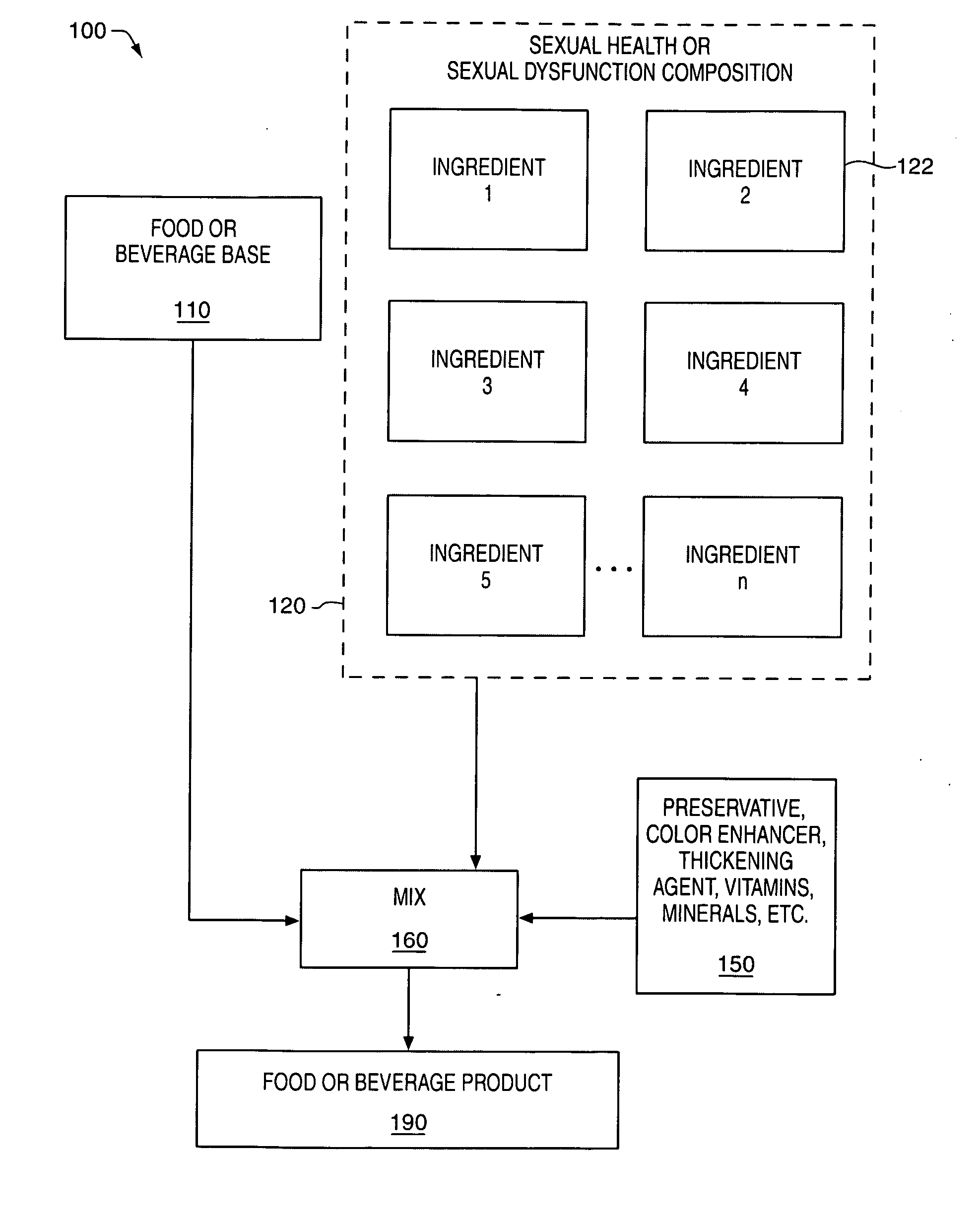
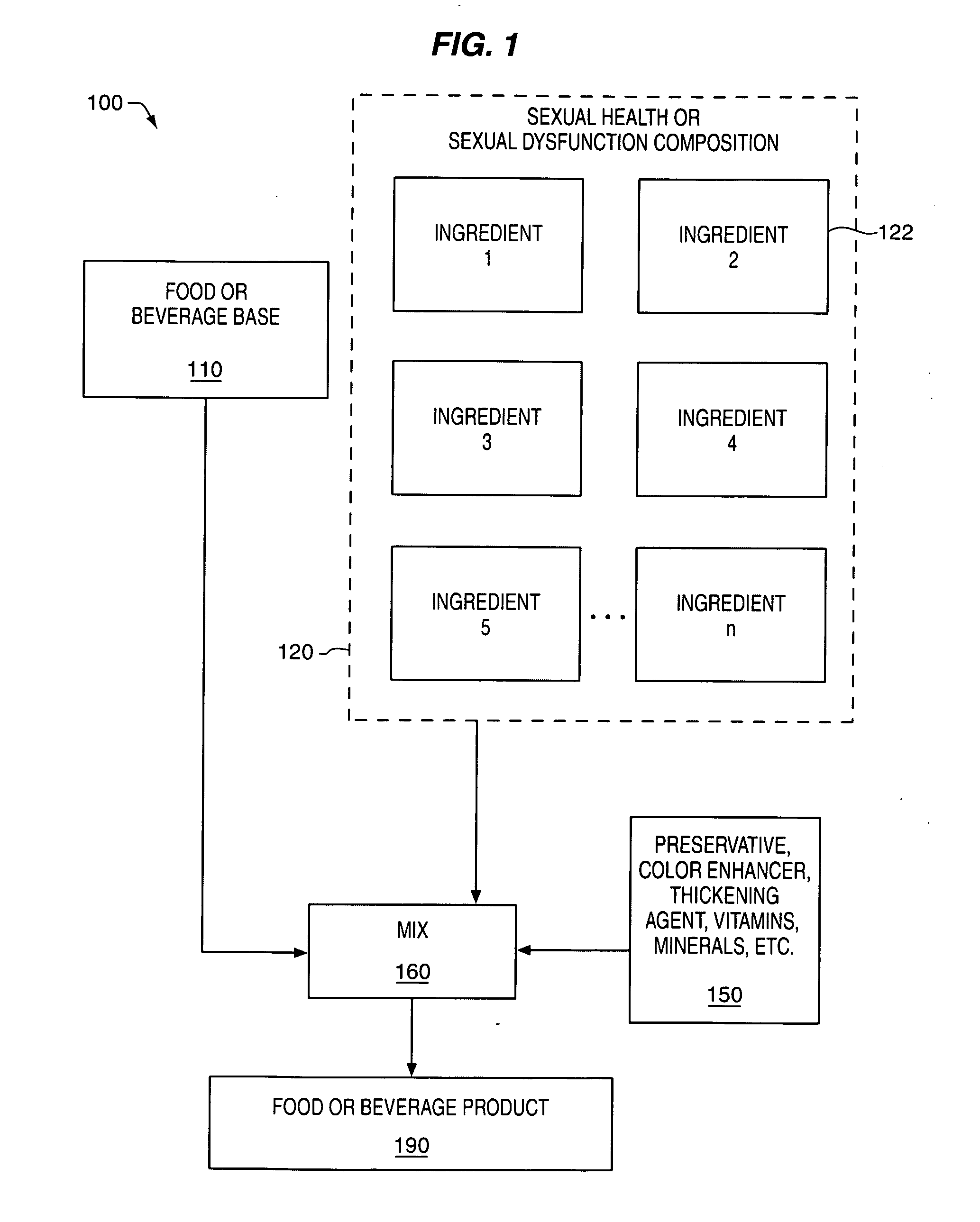
Delivery system and method for supporting and promoting healthy sexual function and prevention and treatment of sexual dysfunction
InactiveUS20060110478A1Increase in cGMPGood curative effectFood ingredient as antioxidantBiocideSexual functionVardenafil
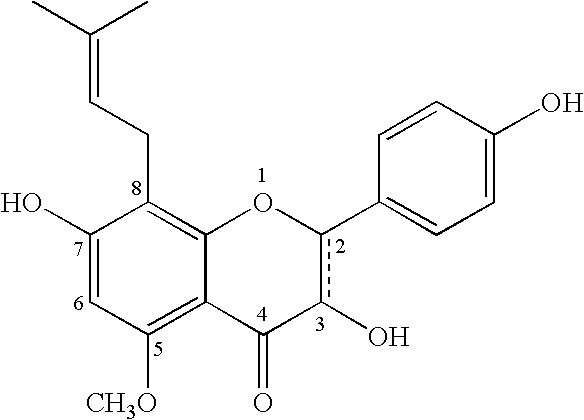
Improved delivery systems and delivery methods for supporting and promoting healthy sexual function, for preventing sexual dysfunction, or for treatment of sexual dysfunction. A compositions including one or more cGMP-specific PDE5 inhibitors and / or dopaminergic agonists is administered in the form of a breath-care strip, mint or lozenge, or a food or beverage product. The cGMP-specific PDE5 inhibitor comprises an ingredient selected from the group consisting of sophoflavescenol, vardenafil, tadalafil, and sildenafil. The dopaminergic agonist comprises apomorphine. Vitex agnus-castus extract, and one or more of lipoic acid, L-Arginine, folic acid, trimethylglycine, policosanol, carnitine, biotin, and acetyl L-Carnitine may also be included in the delivery vehicle.
Owner:MCCLEARY EDWARD LARRY +2
Sublingual apomorphine
ActiveUS9044475B2Alleviating dyskinesiaEffectively alleviatedBiocidePowder deliveryMedicineSexual dysfunction
The invention features sublingual formulations of apomorphine that is a mucoadhesive polymer film or a strip having a first portion including an acid addition salt of apomorphine and a second portion including a pH neutralizing agent, and methods of treating Parkinson's disease, sexual dysfunction, and depressive disorders by administering sublingually the film or strip.
Owner:SUNOVION PHARMA INC
Pharmaceutically acceprable salts of aporphine compounds of carboxyl group-containing agents and methods for preparing the same
The present invention discloses novel pharmaceutically acceptable salts of aporphine compounds and carboxyl-group containing agents. Also, the present invention discloses methods for preparing the pharmaceutically acceptable salts. These pharmaceutically acceptable salts are suitable for use in treating and / or preventing hyperglycemic disease and / or several oxidative stress related diseases.
Owner:STANDARD CHEM & PHARMA
Aporphine and oxoaporphine compounds and pharmaceutical use thereof
InactiveUS7057044B2Prevention and treatment of local ischemiaAvoid ischemic injuryBiocideOrganic chemistryMorphineApomorphine
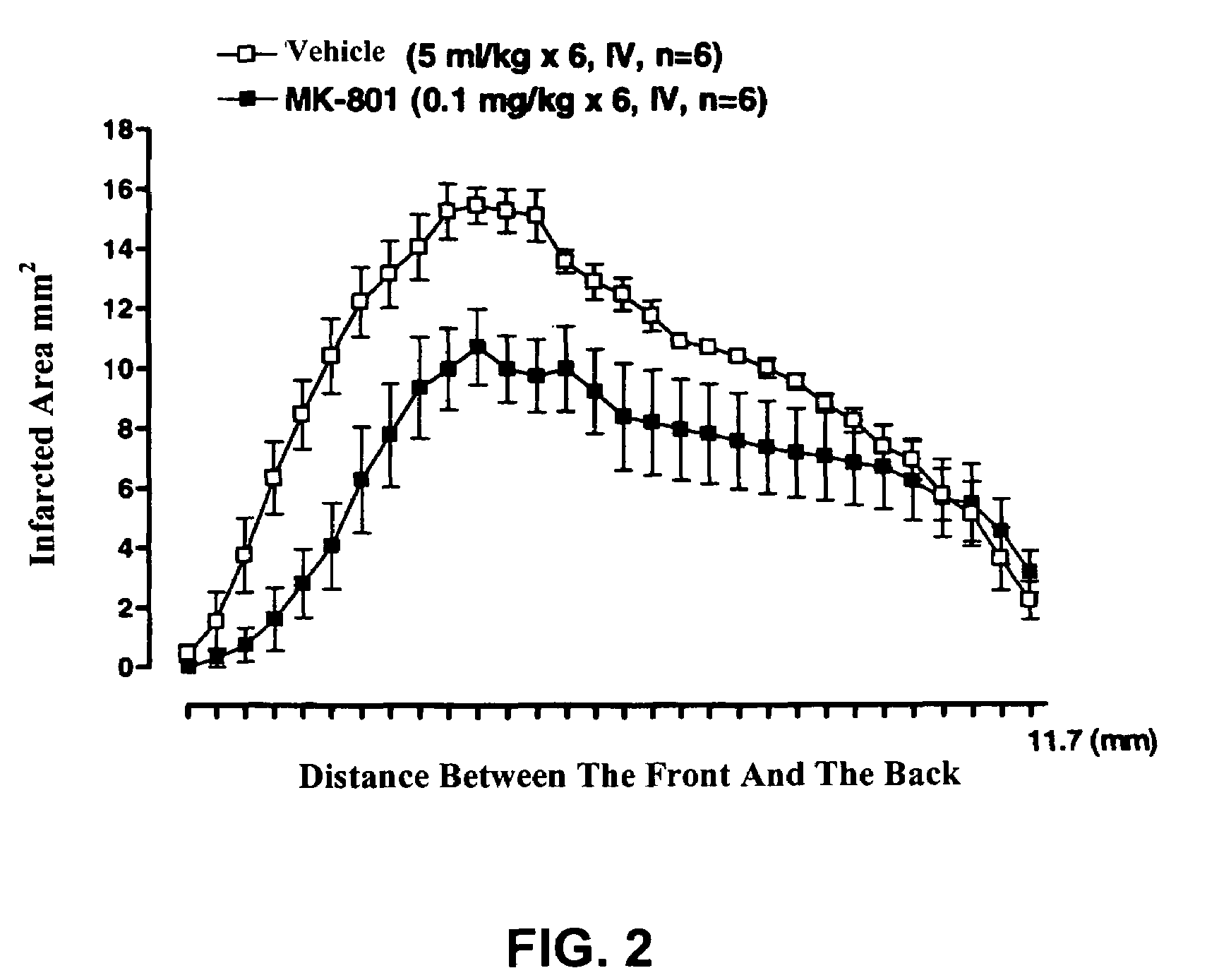
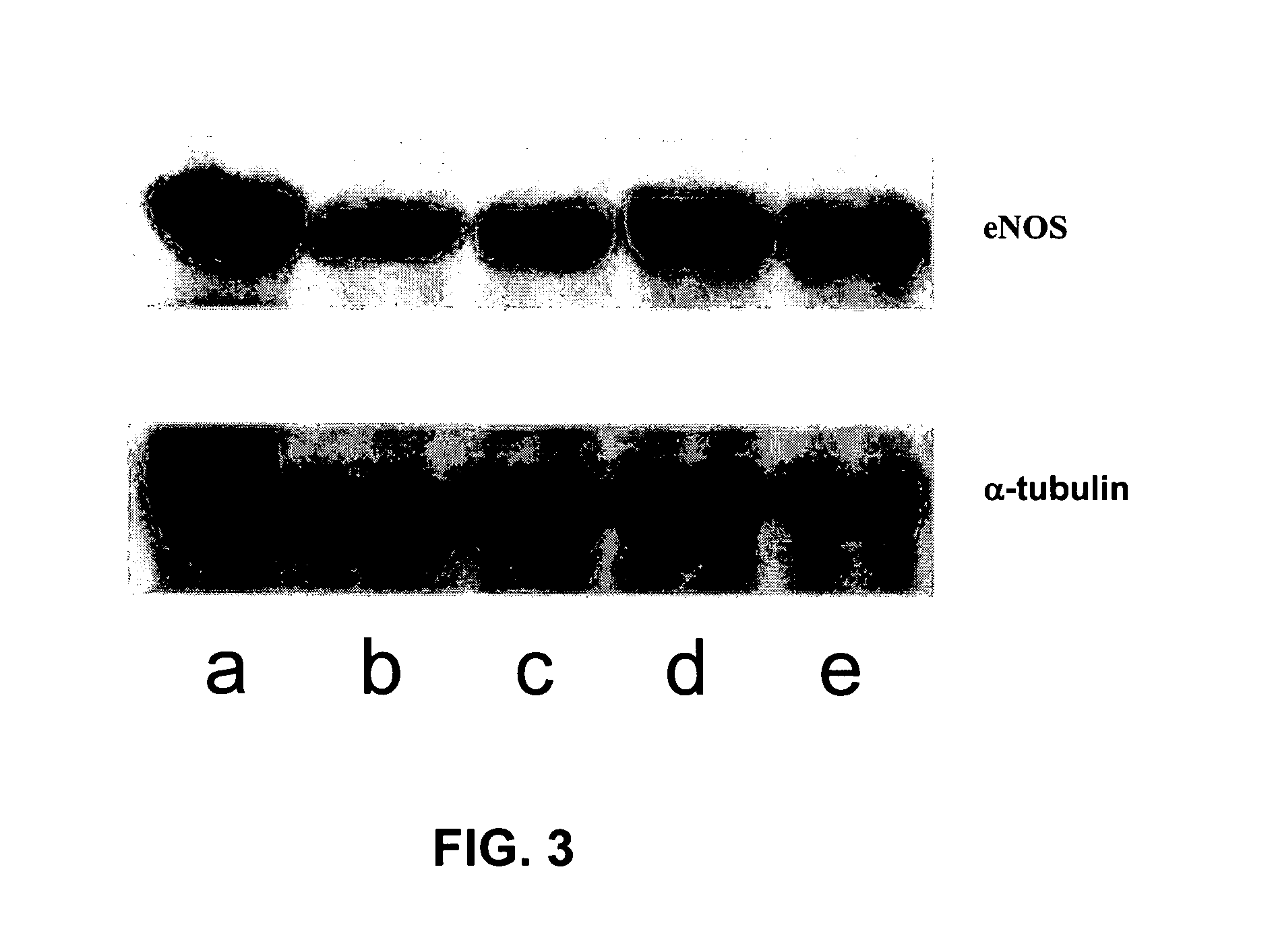
The invention provides aporphine and oxoaporphine compounds that have endothelial nitric oxide synthase (eNOS) maintaining or enhancing activities and may be used to manufacture a medicaments for preventing or treating ischemic diseases in human and mammal, and the ischemic diseases may include ischemic cerebral apoplexy, ischemic cerebral thrombosis, ischemic cerebral embolism, hypoxic ischemic encephlopathy, ischemic cardiac disease or ischemic enteropathy etc.
Owner:LOTUS PHARMA CO LTD
Pharmaceutical compositions comprising apomorphine for pulmonary inhalation
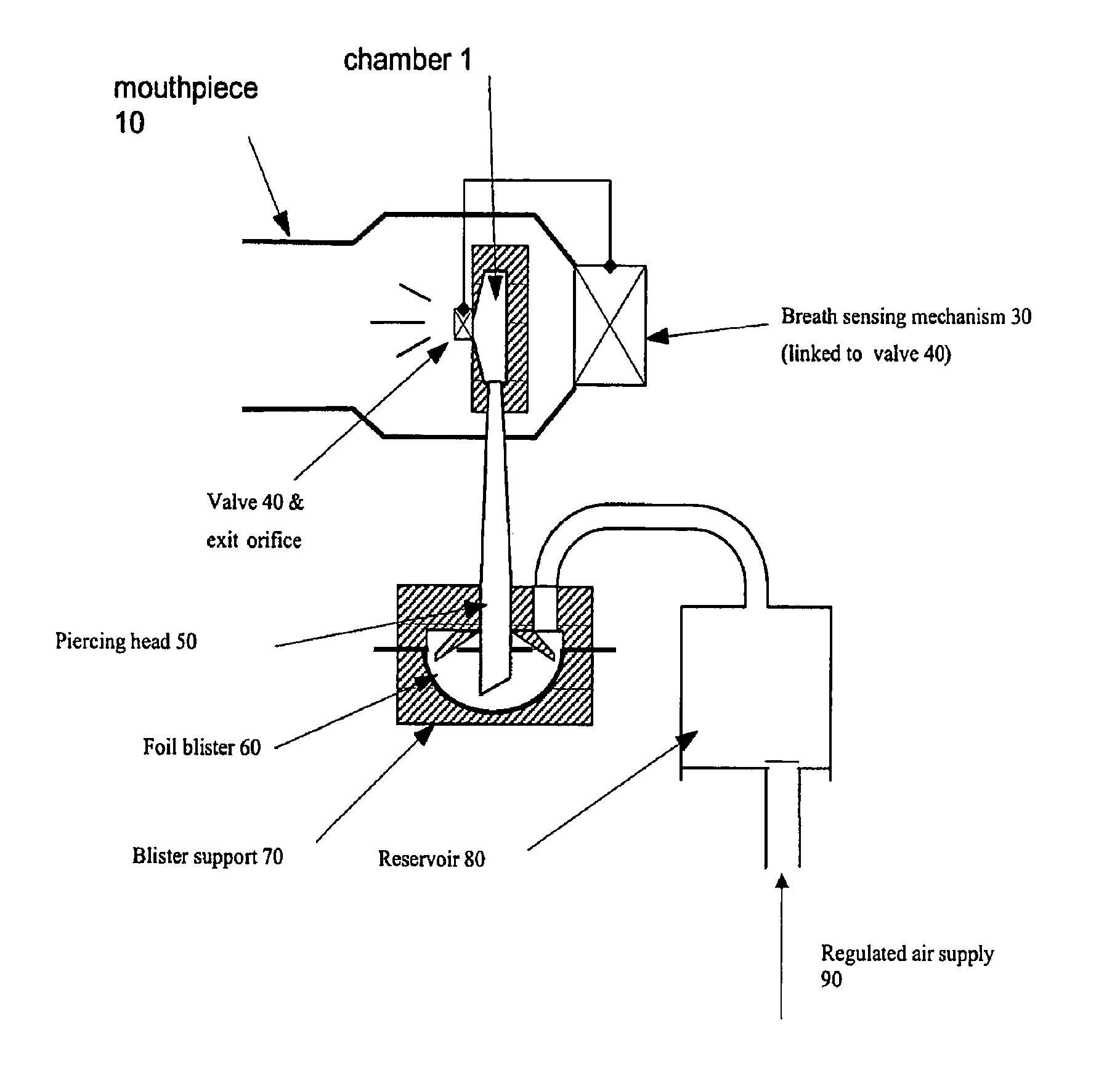
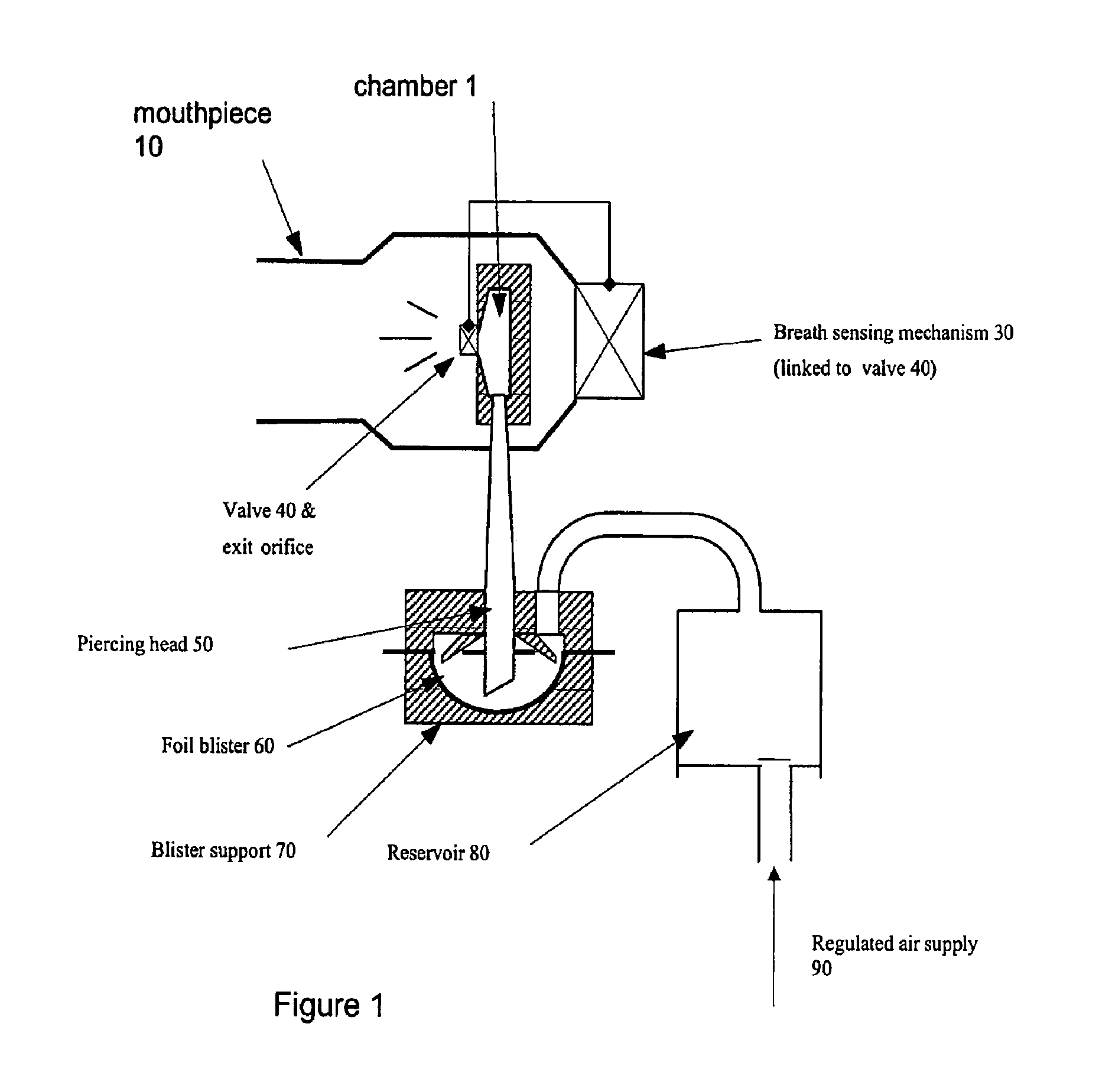
InactiveUS20060178394A1Improve performanceRapid blood levelBiocidePowder deliveryPulmonary inhalationSexual dysfunction
The present invention relates to inhalable formulations of apomorphine or its pharmaceutically acceptable salts or esters for use in treating sexual dysfunction. The present invention also relates to methods for preparing the apomorphine formulations as well as to methods for treatment of sexual dysfunction using said formulations and inhalers including said formulations. The present invention further relates to the use of apomorphine in the manufacture of a medicament for treating sexual dysfunction.
Owner:VECTURA LTD
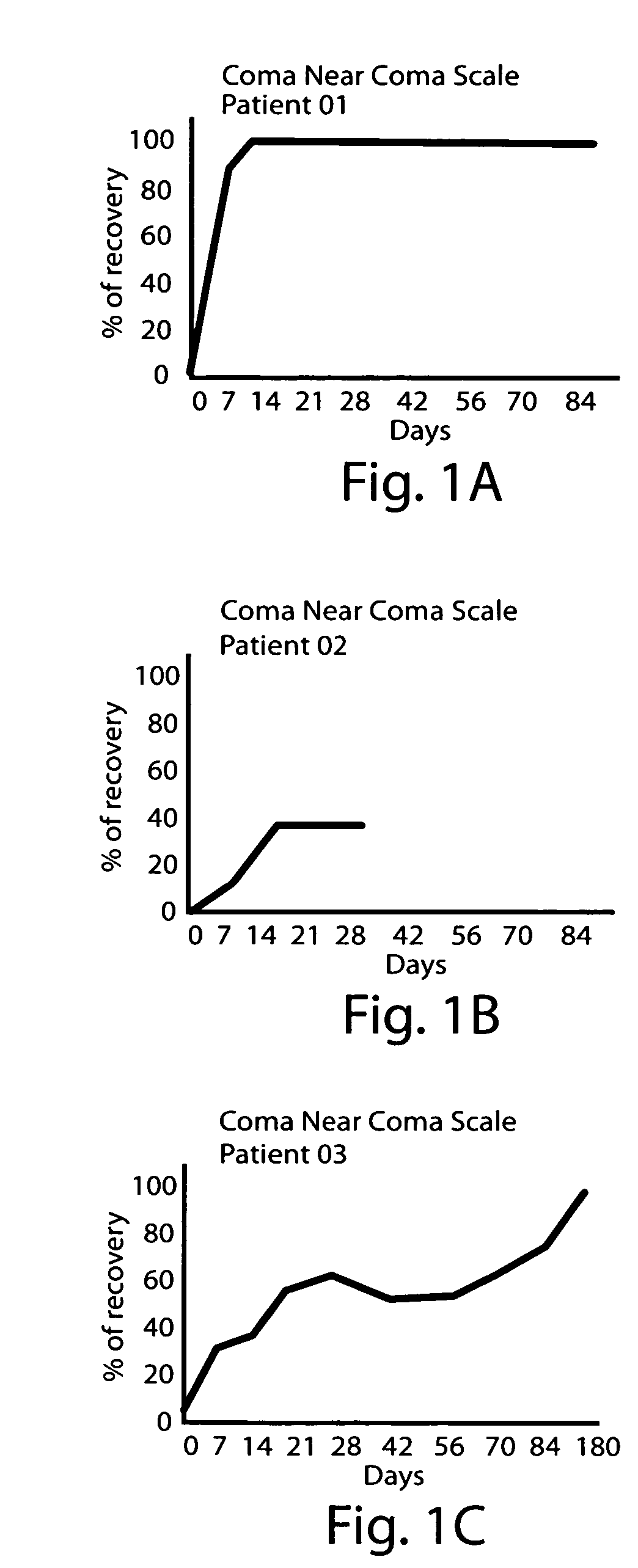
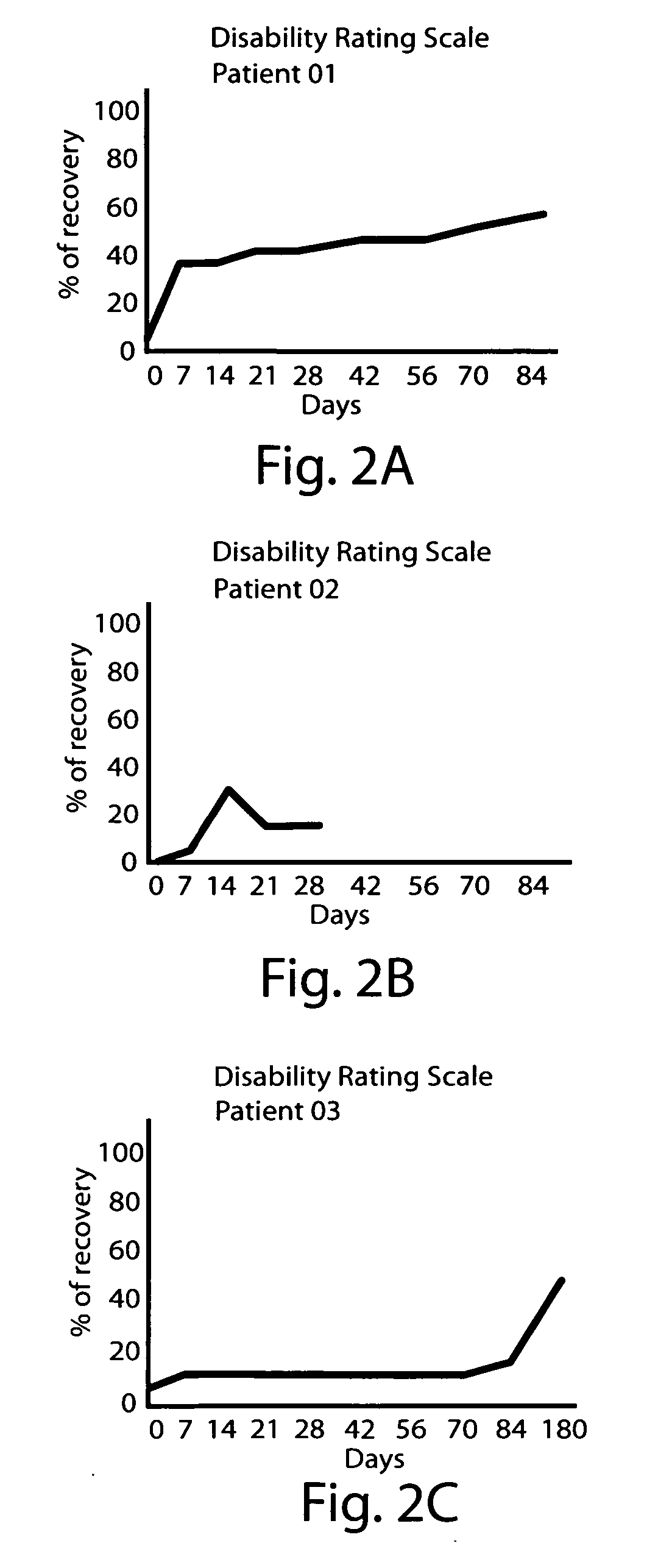
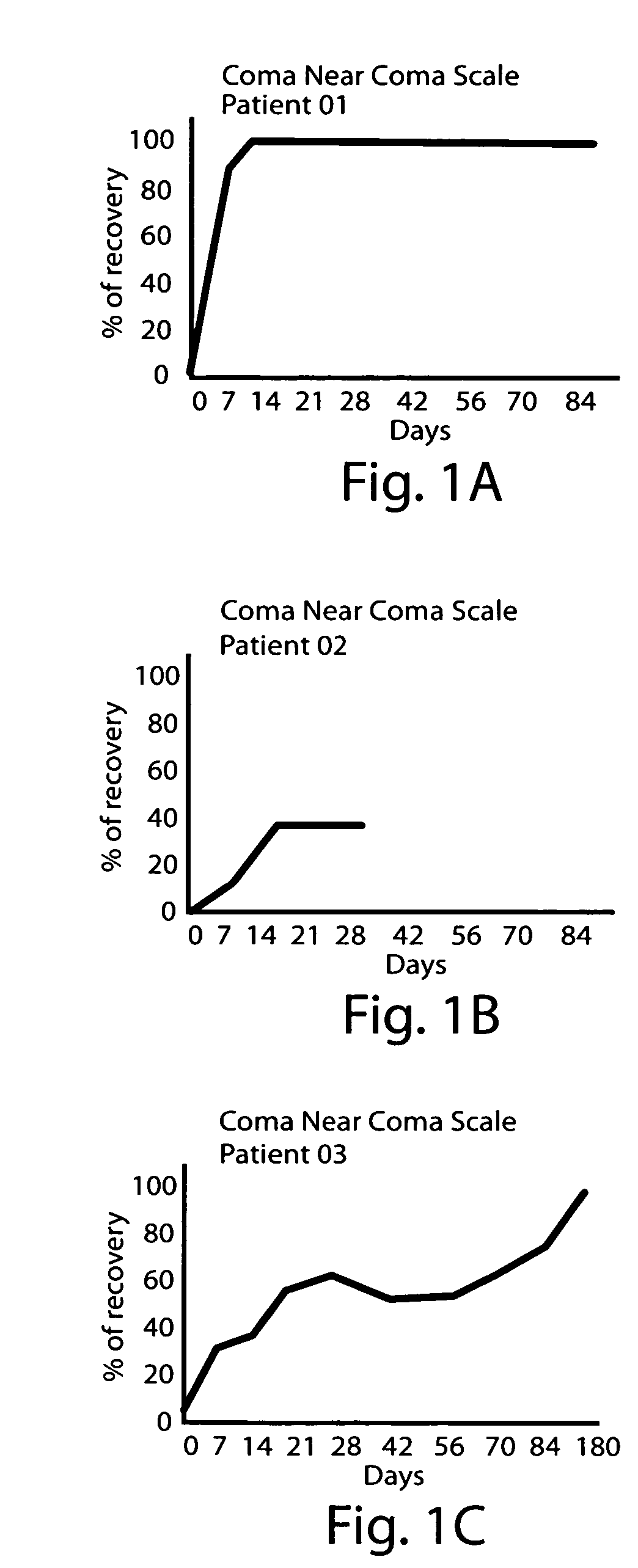
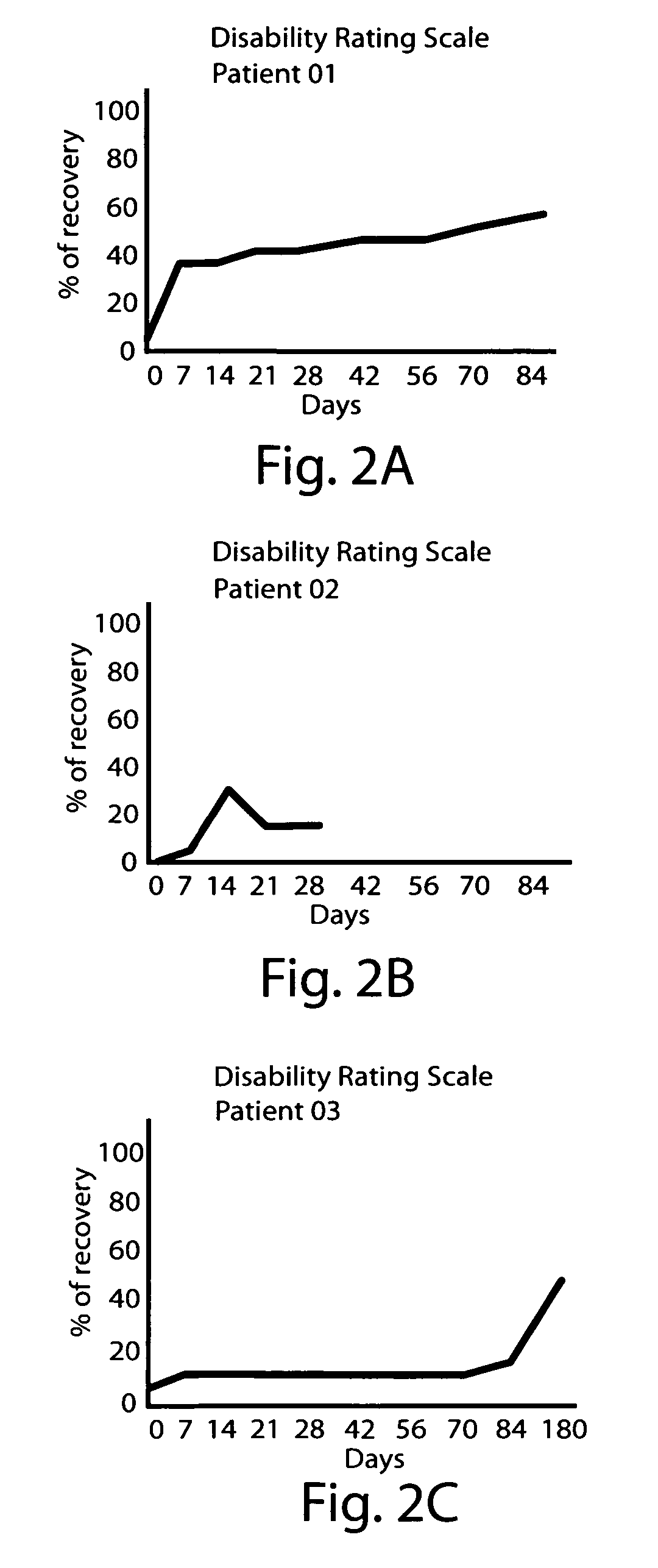
High potency dopaminergic treatment of neurological impairment associated with brain injury
Methods and compositions are described for treating impaired neurological function, including altered state of consciousness disorders, in an individual who has sustained a brain injury comprising administering to the individual apomorphine. Methods and compositions are described for treating impaired neurological function, including altered state of consciousness disorders, in an individual who has sustained a brain injury comprising administering to the individual at least 1000 mg or more of L-dopa (levodopa) per day. The use of potent dopaminergic agents to stimulate emergence from an altered consciousness state, such as a coma, is disclosed.
Owner:NEUROHEALING PHARMA INC
Erectile dysfuction treatments comprising momentary vacuum therapy alone or with medications
InactiveUS7037257B1Prevents the breakdown of these chemical messagesHeart workload is reducedElcosanoid active ingredientsDiagnosticsVascular dilatationMorphine
The present invention relates to treatments for erectile dysfunction comprising momentary vacuum therapy alone or with medications. The treatments may be effective when other treatments (PDE-5 inhibitors (e.g., VIAGRA®); vasodilators (e.g., ALPROSTADIL®); combination pharmacotherapies (e.g., VIAGRA® and MUSE); central nervous system-acting agents (e.g., Apomorphine) fail. All treatments use a finding of the present invention that momentary vacuum therapy increases the elasticity of arteries to overcome, to a significant degree, the arterial inelasticity of atherosclerosis, a primary cause of erectile dysfunction. When momentary vacuum therapy is used with VIAGRA®, e.g., the atherosclerosis problem and the chemical message problem are treated simultaneously. Momentary vacuum therapy may be useful in the treatment of peripheral (e.g., arms and legs) artery disease and congestive heart failure.
Owner:KOENIG J FR +1
Intranasal compositions
A liquid aqueous formulation for the intranasal administration of apomorphine includes:(a) at least about 15 mg / ml of apomorphine; and(b) a solubilising agent selected from(i) at least one polyoxyethylene-polyoxypropylene copolymer (poloxamer);(ii) at least one cyclodextrin; and(iii) at least one cyclodextrin together with chitosan.The formulations of the invention can be used in the treatment or management of Parkinson's disease and erectile dysfunction.
Owner:ARCHIMEDES DEVMENT
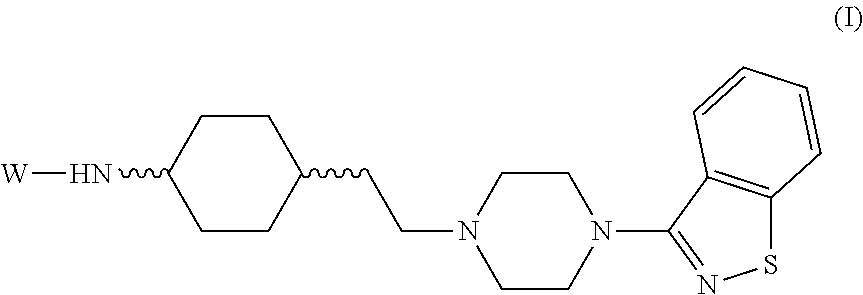
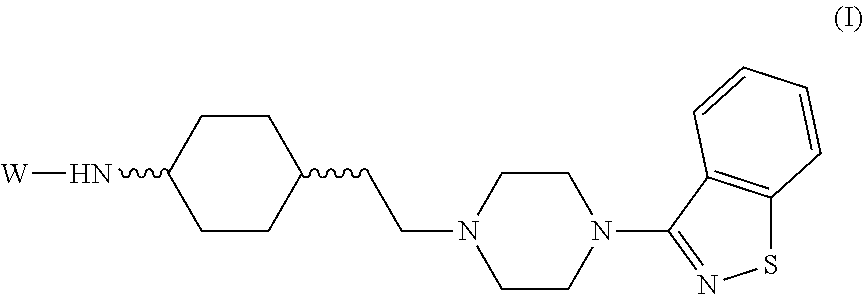
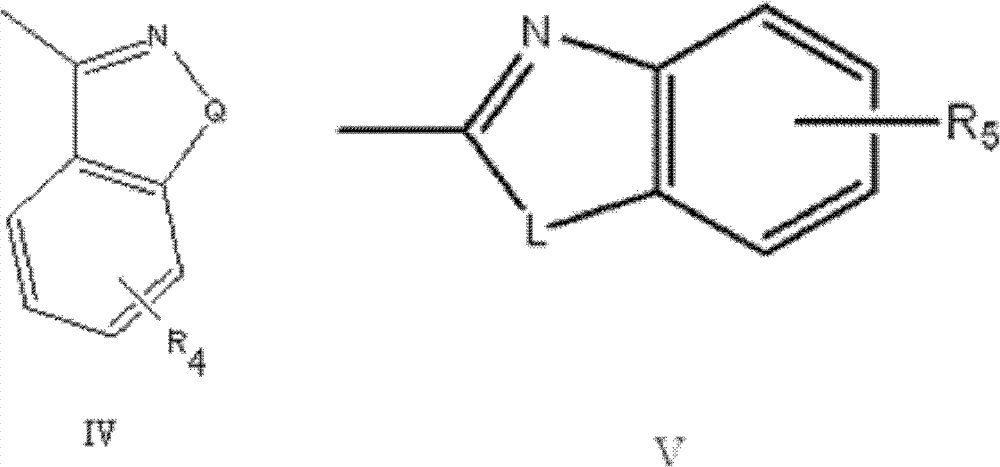
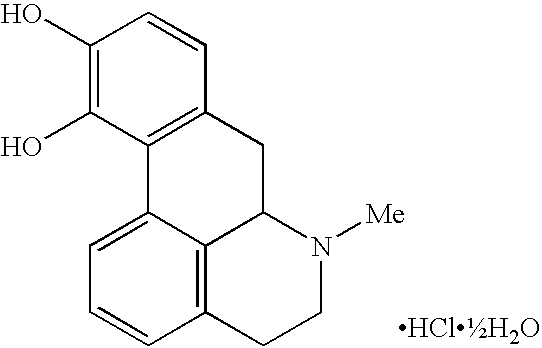
Novel substituted-1, 1-dioxo-benzo[1,2,4]thiadiazin-3ones, preparation method thereof, and pharmaceutical composition containing the same
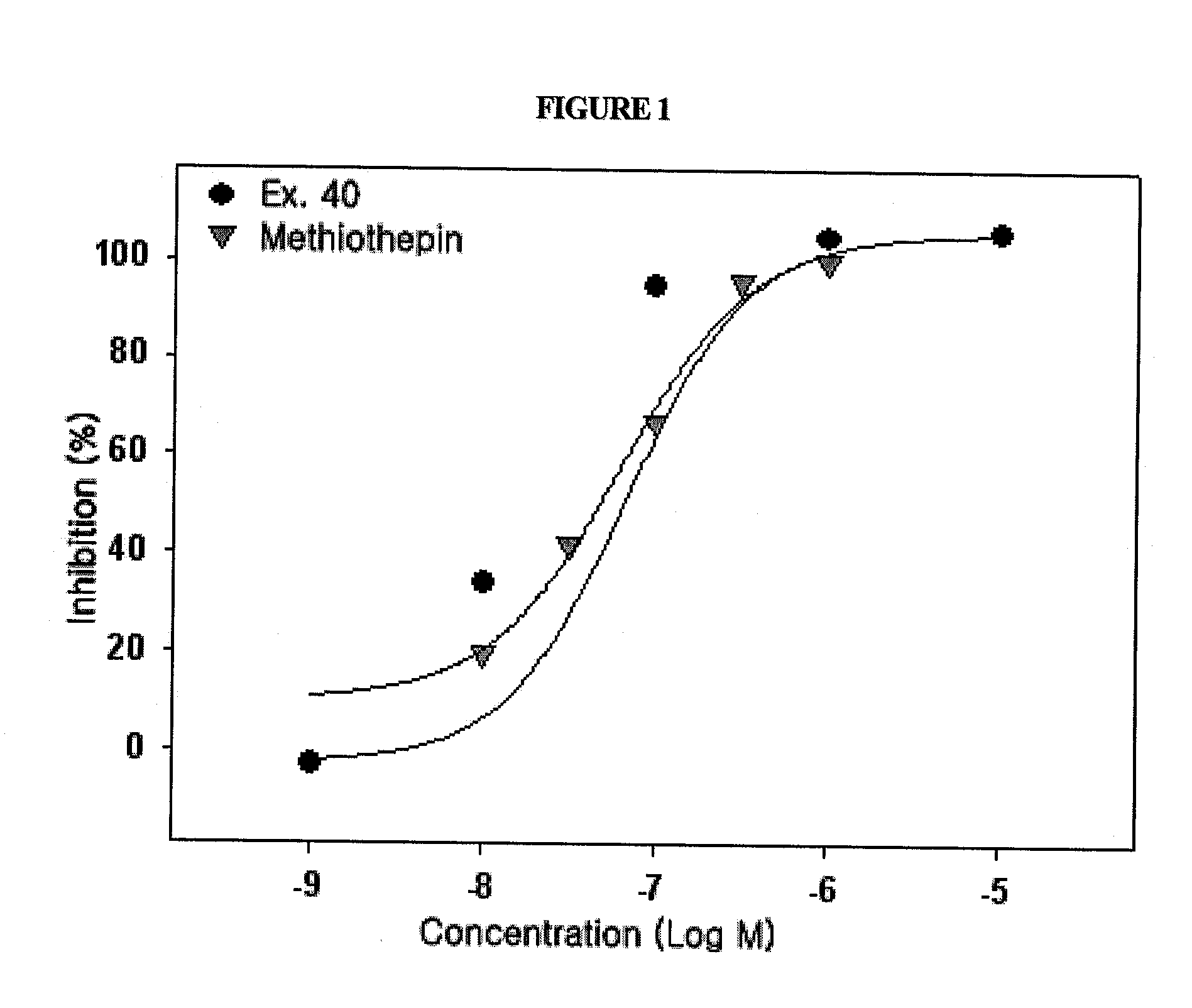
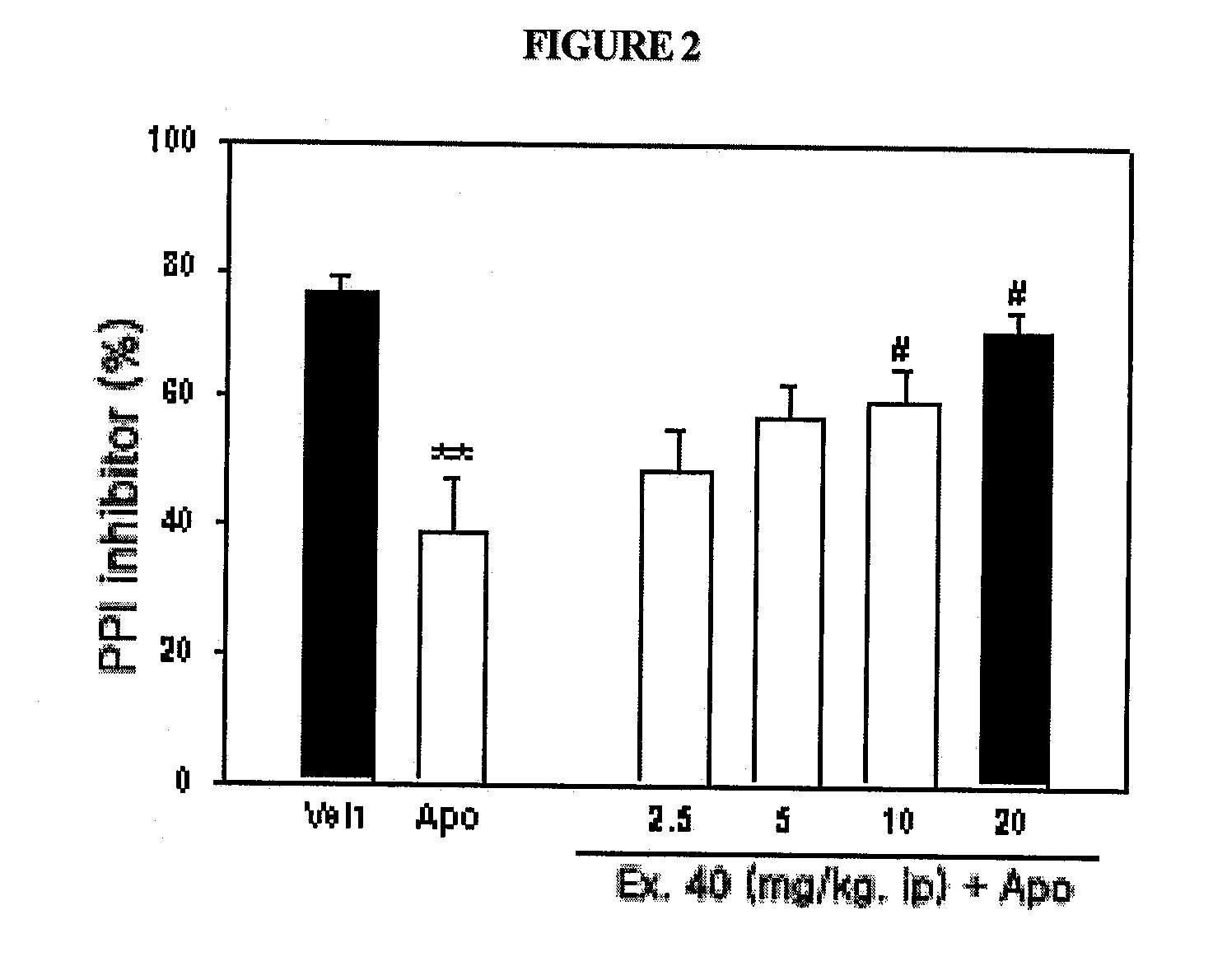
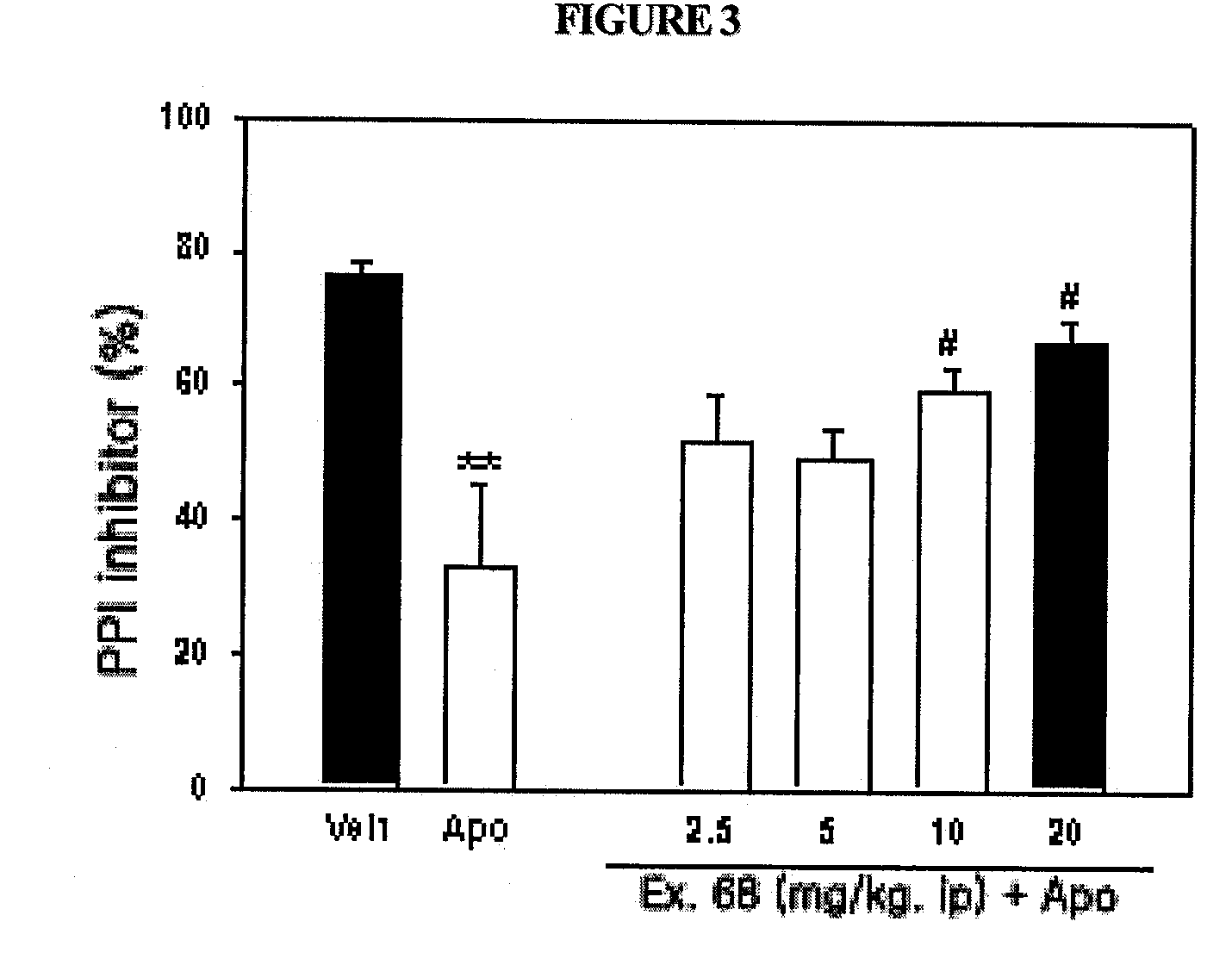
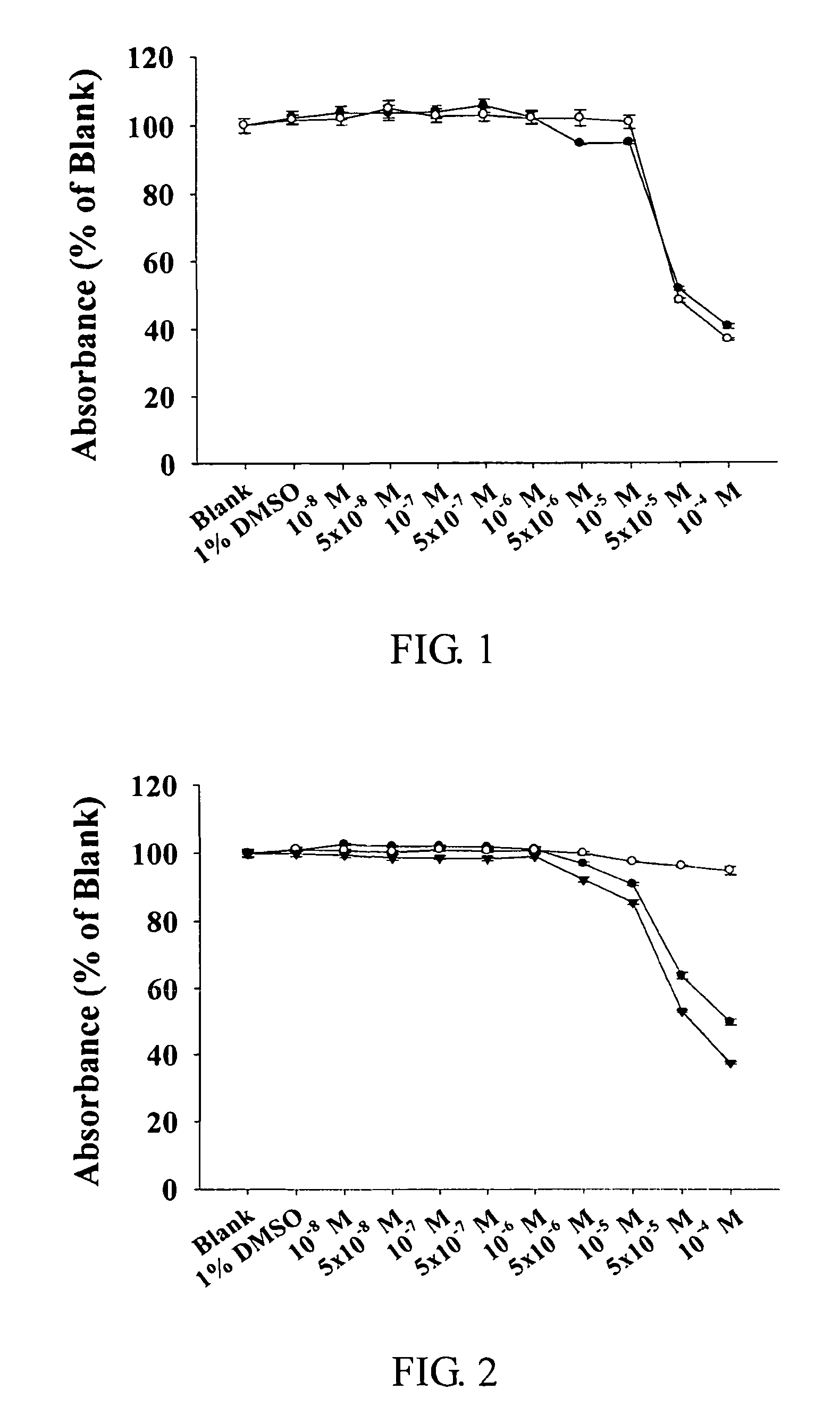
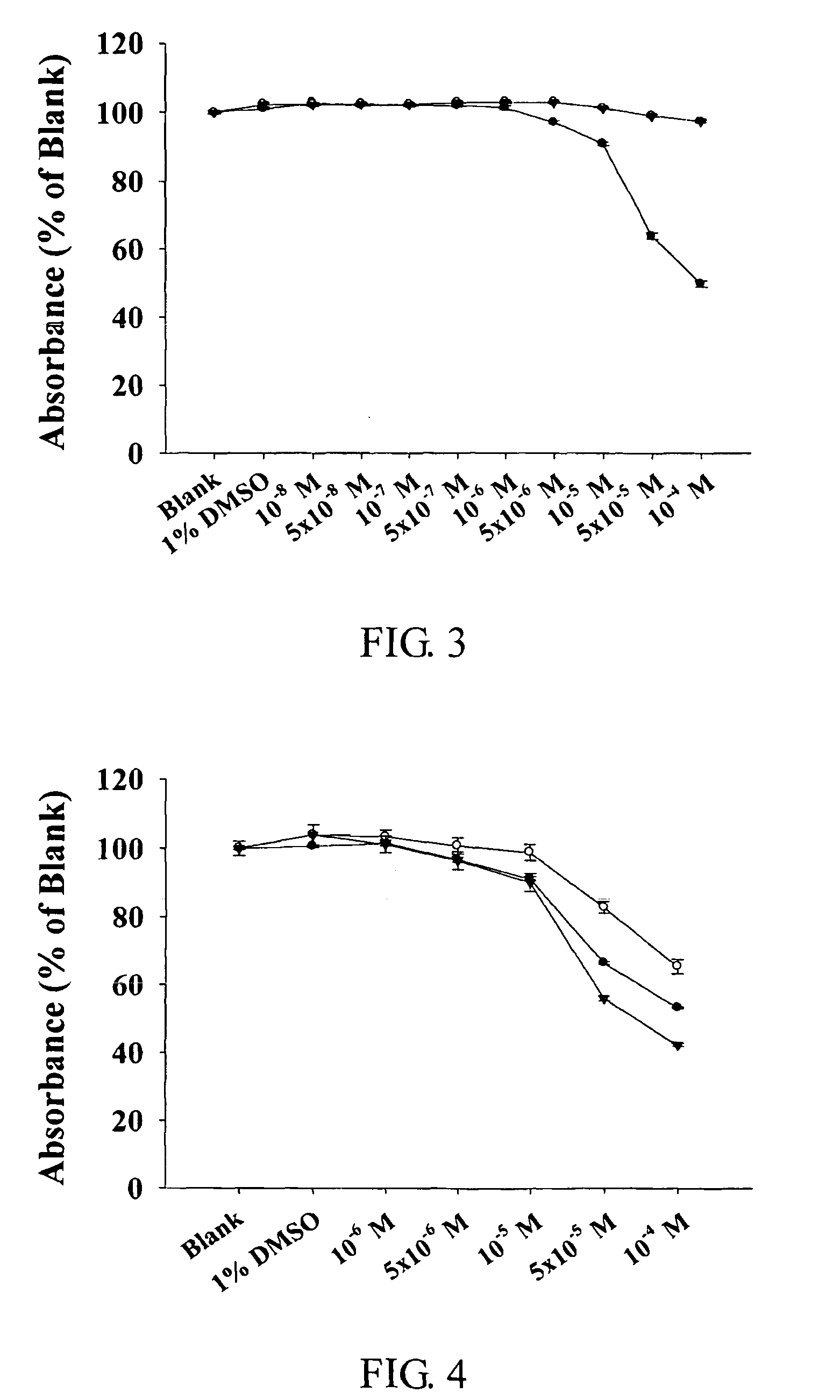
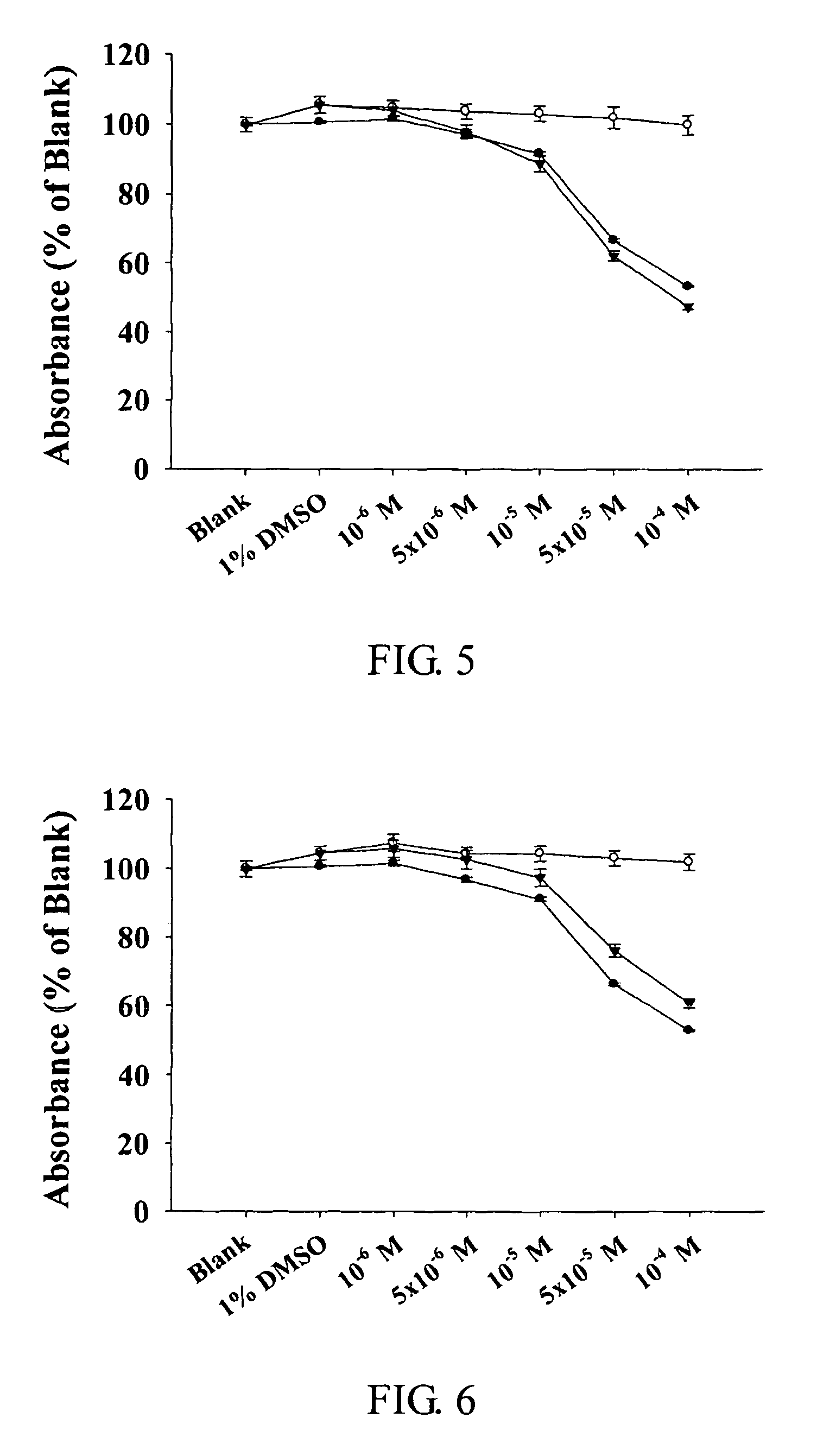
The present invention relates to compounds of substituted-1,1-dioxo-benzo[1,2,4]thiadiazin-3-ones acting as a 5HT6 receptor antagonist, a preparation method thereof, and a pharmaceutical composition containing the same for treatment of the central nervous system disorders. The compounds of substituted-1,1-dioxo-benzo[1,2,4]thiadiazin-3-ones according to the present invention have excellent binding affinity for the 5HT6 receptor and excellent selectivity for the 5HT6 receptor over other receptors. Also, the compounds reverse a disruption of PPI by apomorphine and don't show rotatod deficit in mice. Therefore the compounds according to the present invention may be valuably used for treatment of a 5HT6 receptor relating disorders.
Owner:KOREA RES INST OF CHEM TECH
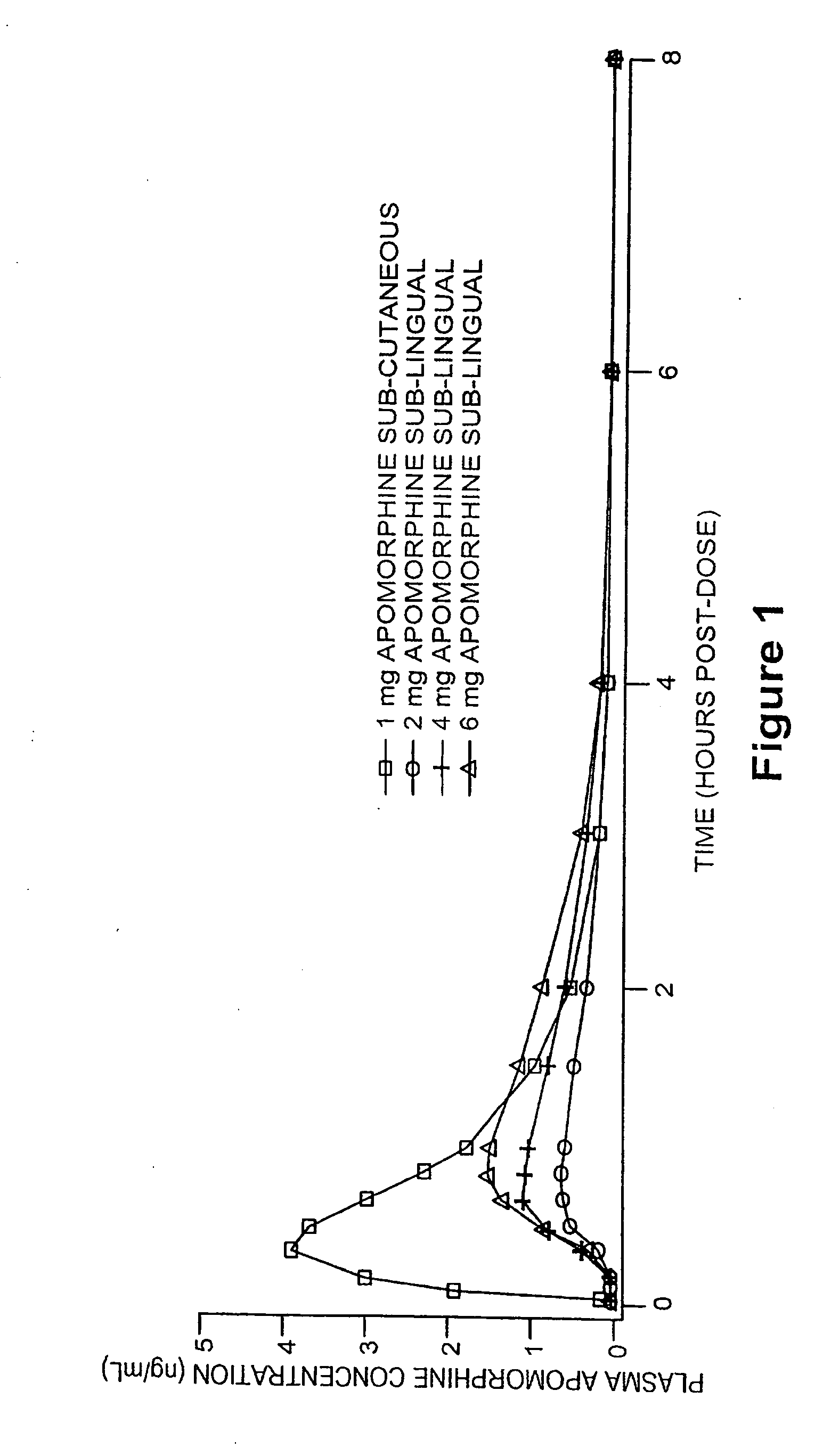
Methods of treating parkinson's disease by administration of apomorphine to an oral mucosa
ActiveUS20180133146A1Sufficient amountHigh CmaxOrganic active ingredientsNervous disorderMorphineApomorphine
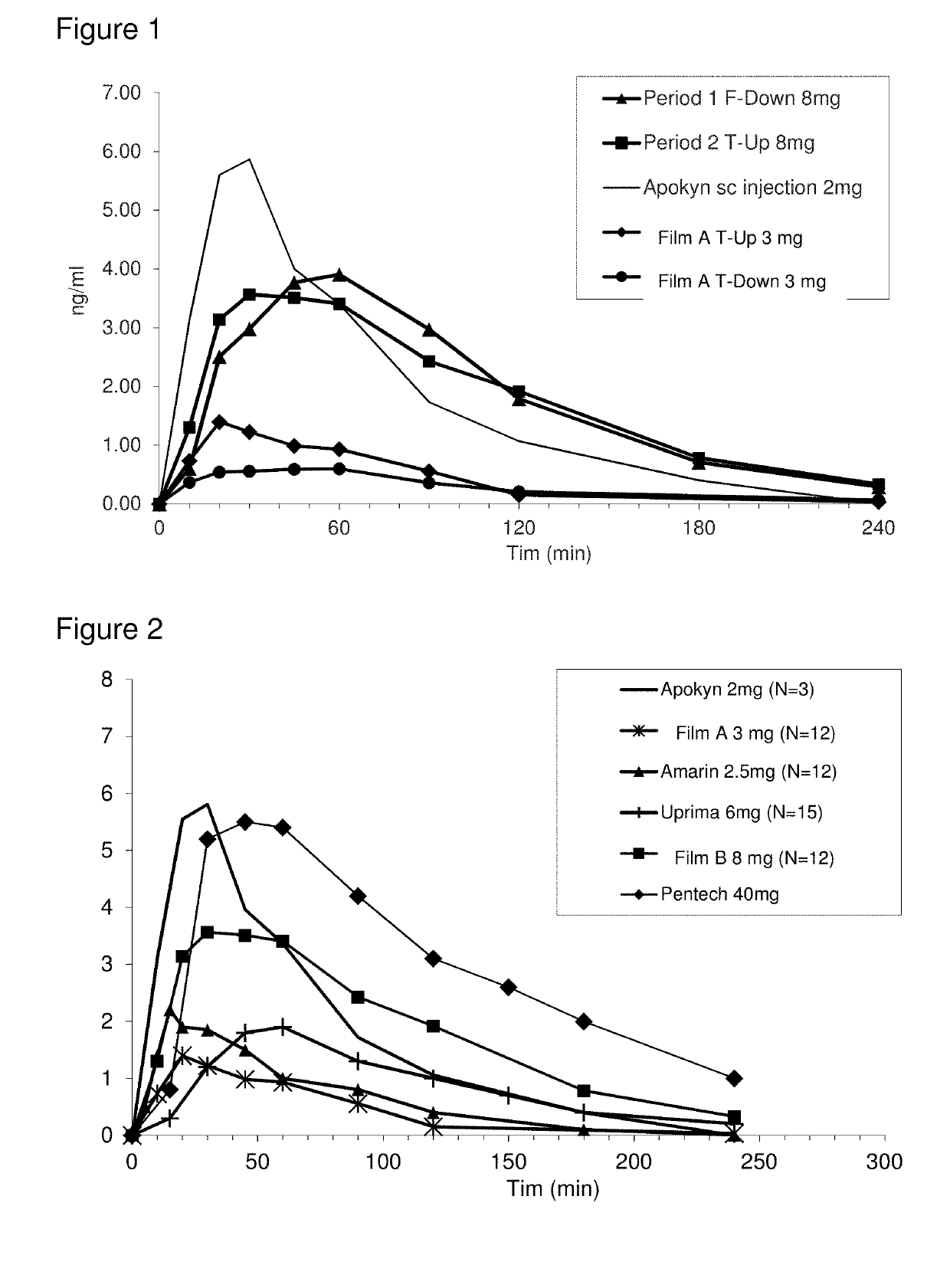
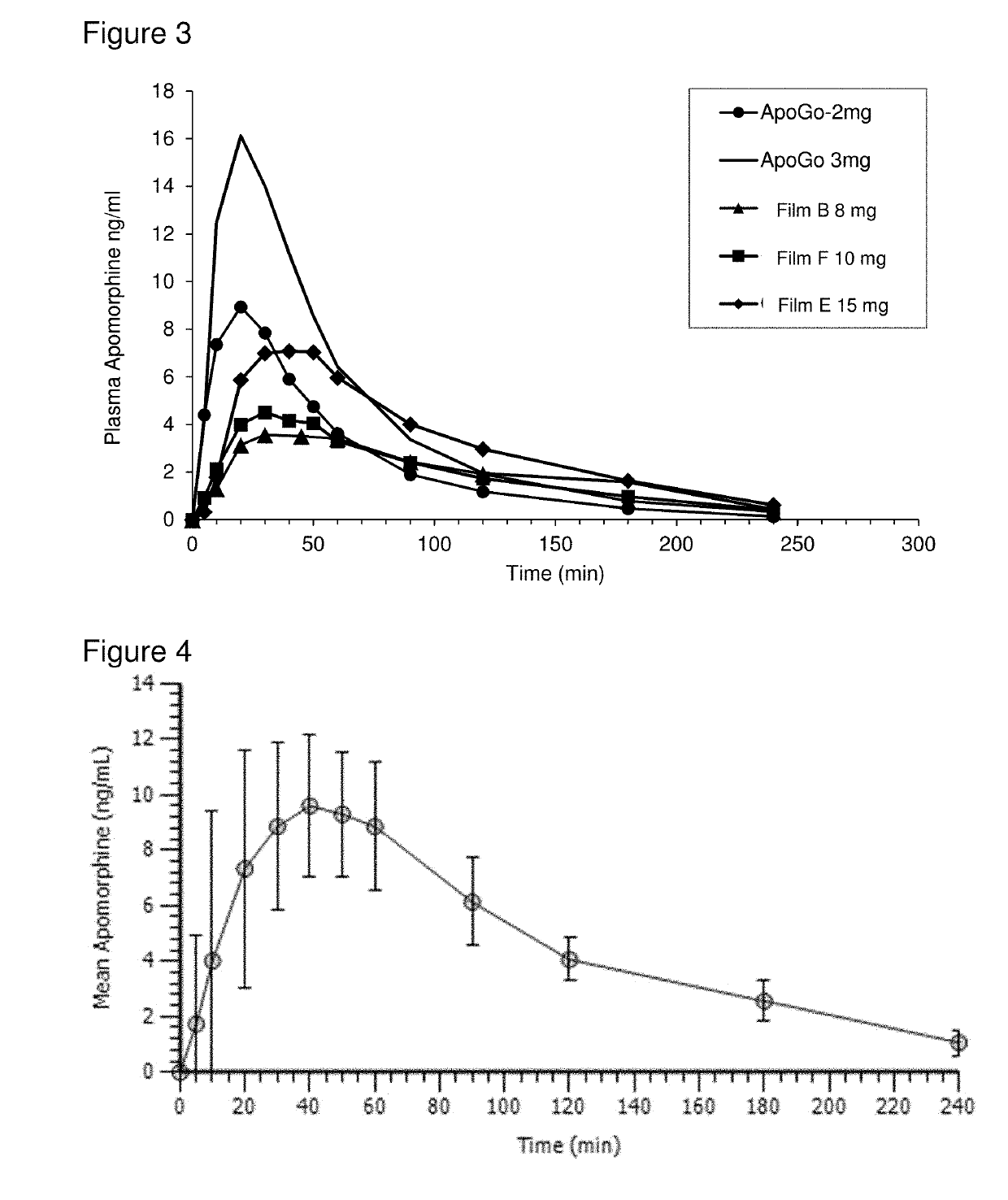
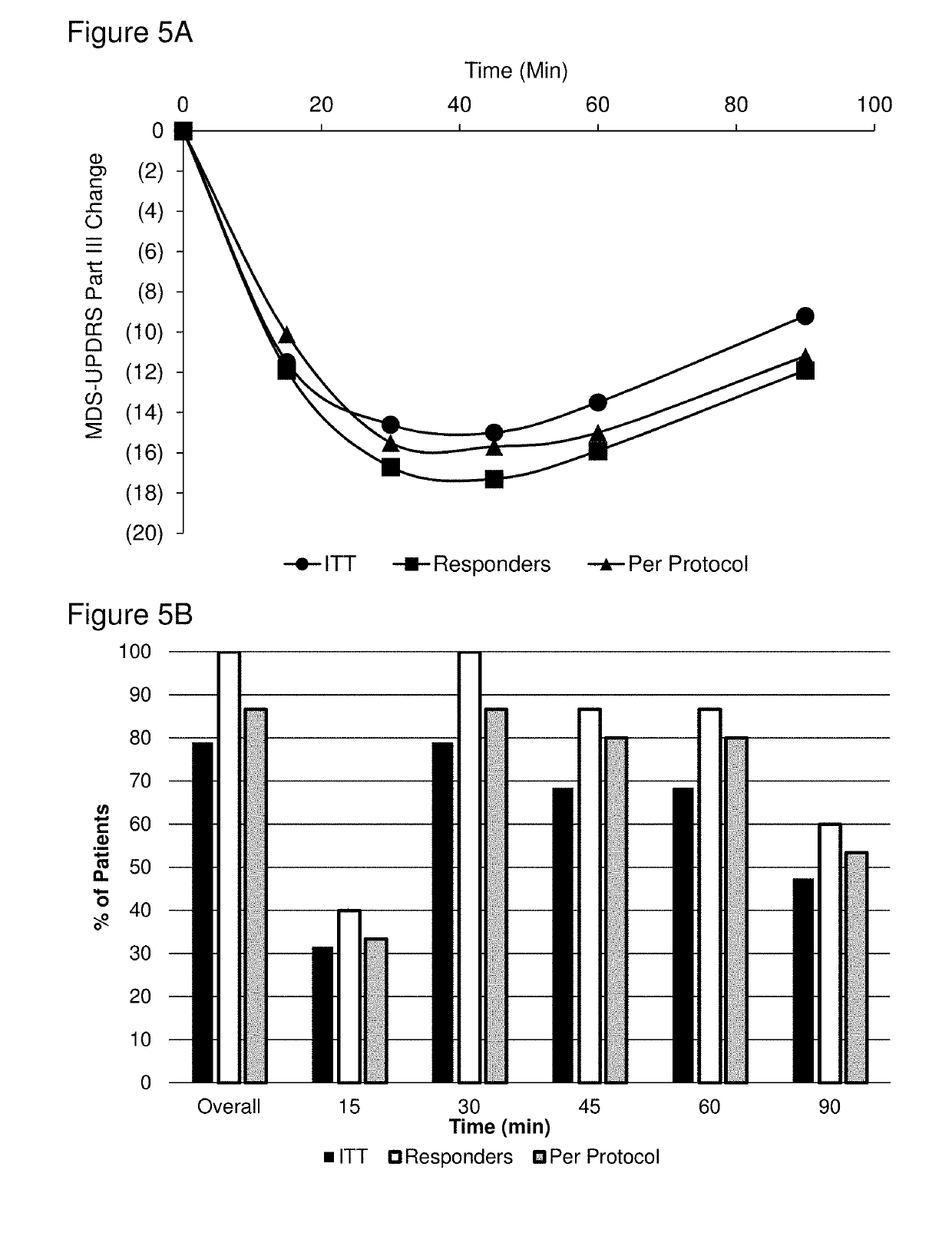
Methods and pharmaceutical unit dosage forms for treating Parkinson's disease in a subject (e.g., an “off” episode in a subject having Parkinson's disease) are described. The pharmaceutical unit dosage forms are films having a first portion including particles containing an acid addition salt of apomorphine and a second portion containing a pH neutralizing agent. The pharmaceutical unit dosage forms can be flexible and have toughness greater than 100 g×mm. The methods can involve administering to a subject having Parkinson's disease a therapeutic dose sufficient to produce an apomorphine plasma concentrate of at least 2.64 ng / mL within 45 minutes after the administration. The subject may be identified as having low uptake, medium uptake, or high uptake of apomorphine administered via oral mucosa.
Owner:SUNOVION PHARMA INC
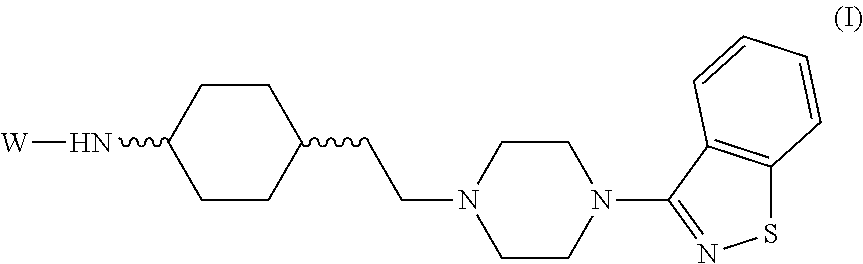
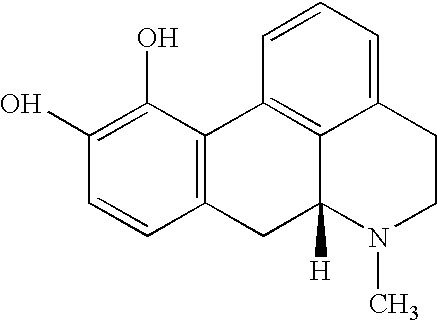
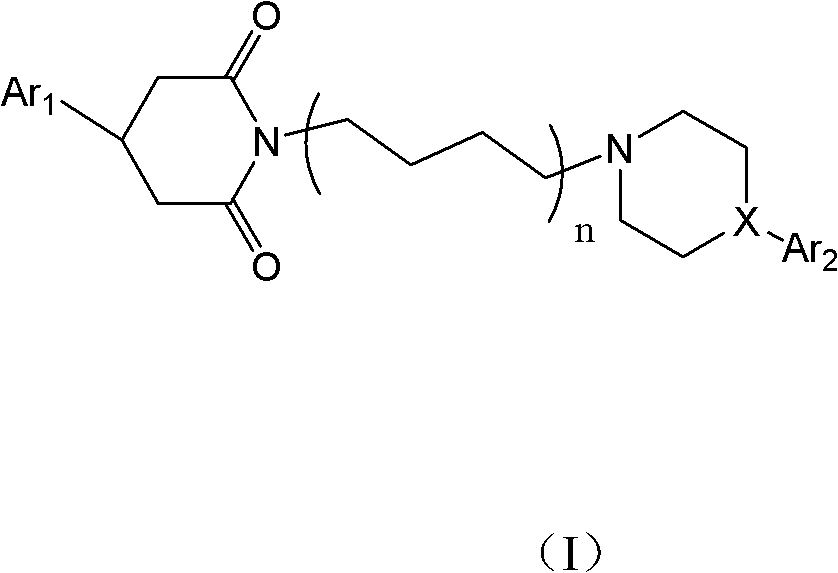
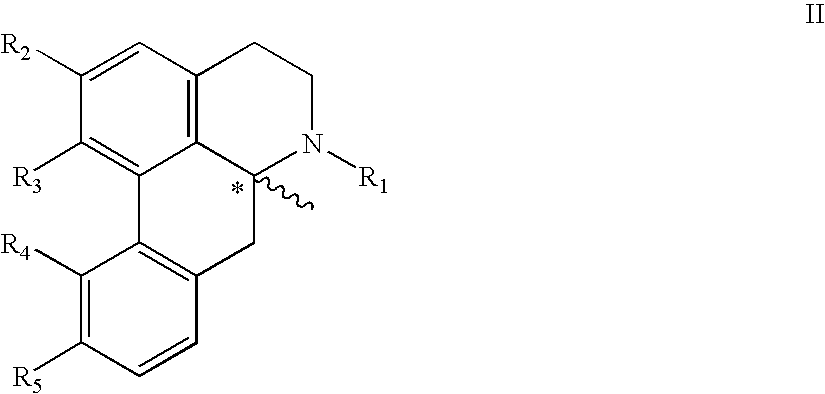
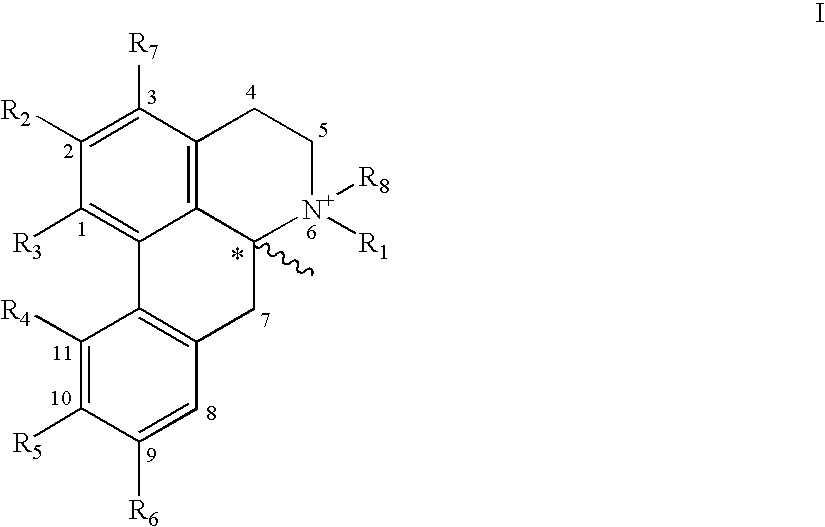
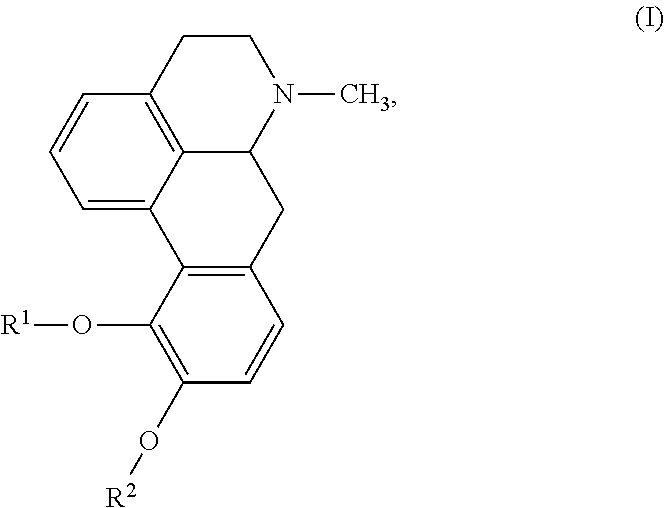
Aporphine esters and their use in therapy
InactiveUS7238705B2Improve bioavailabilityLong duration of actionBiocideNervous disorderDiseaseSexual dysfunction
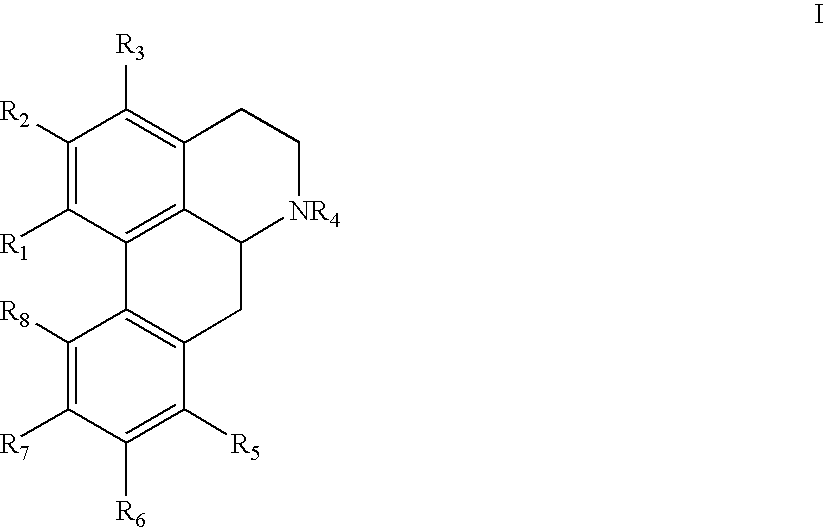
New aporphine derivatives are disclosed which have formula (I) and the physiologically acceptable salts thereof. Said derivatives may be used for the treatment of Parkinson's disease, hemicrania, restless legs syndrome (RLS), sexual dysfunction in men and women, hyperprolactemia and psychotic disorders, and / or evaluation of Parkinson's disease. Processes for the preparation of such derivatives are also disclosed.
Owner:AXON BIOCHEMICALS BV
Mixed snuff prepn. for reducing side effect of Apomorphinum
InactiveCN1375292AExtended stayEliminate or reduce nauseaOrganic active ingredientsNervous disorderNasal cavitySide effect
An apomorphine preparation compound of nasal cavity feeding used for curing Parkinson syndrom, male erection disturbance and female sexual disturbance can eliminate or decrease the side effects of bad feeling, dizziness, vomiting and abnormal taste sense occured in the treatment of apomarphine through using preparation compound adding with the vomiting stop medicine. The aromatic substance is added in order to let the nasal cavity have feelings of refreshing and comfort after the medicine is fed and the natural or synthetic high polymer compound is added to liquid preparation in order to delay the retaining period of medicine in nasal cavity. The preparation has the advantage of transmitting the medicine towards the brain and furthermore can be used with convenience, safety, effectivenessand good compliance.
Owner:ZHEJIANG ACAD OF MEDICAL SCI +1
Glycoside and orthoester glycoside derivatives of apomorphine, analogs, and uses thereof
InactiveUS20060004190A1Improve bioavailabilityLess emetic actionBiocideNervous disorderDiseaseSexual impotence
Disclosed are glycoside and orthoester glycoside derivatives of apomorphine and analogs thereof to treat conditions and diseasessuch as erectile dysfunction.
Owner:BRAIN N BEYOND BIOTECH
Composition, device, and method for treating sexual dysfunction via inhalation
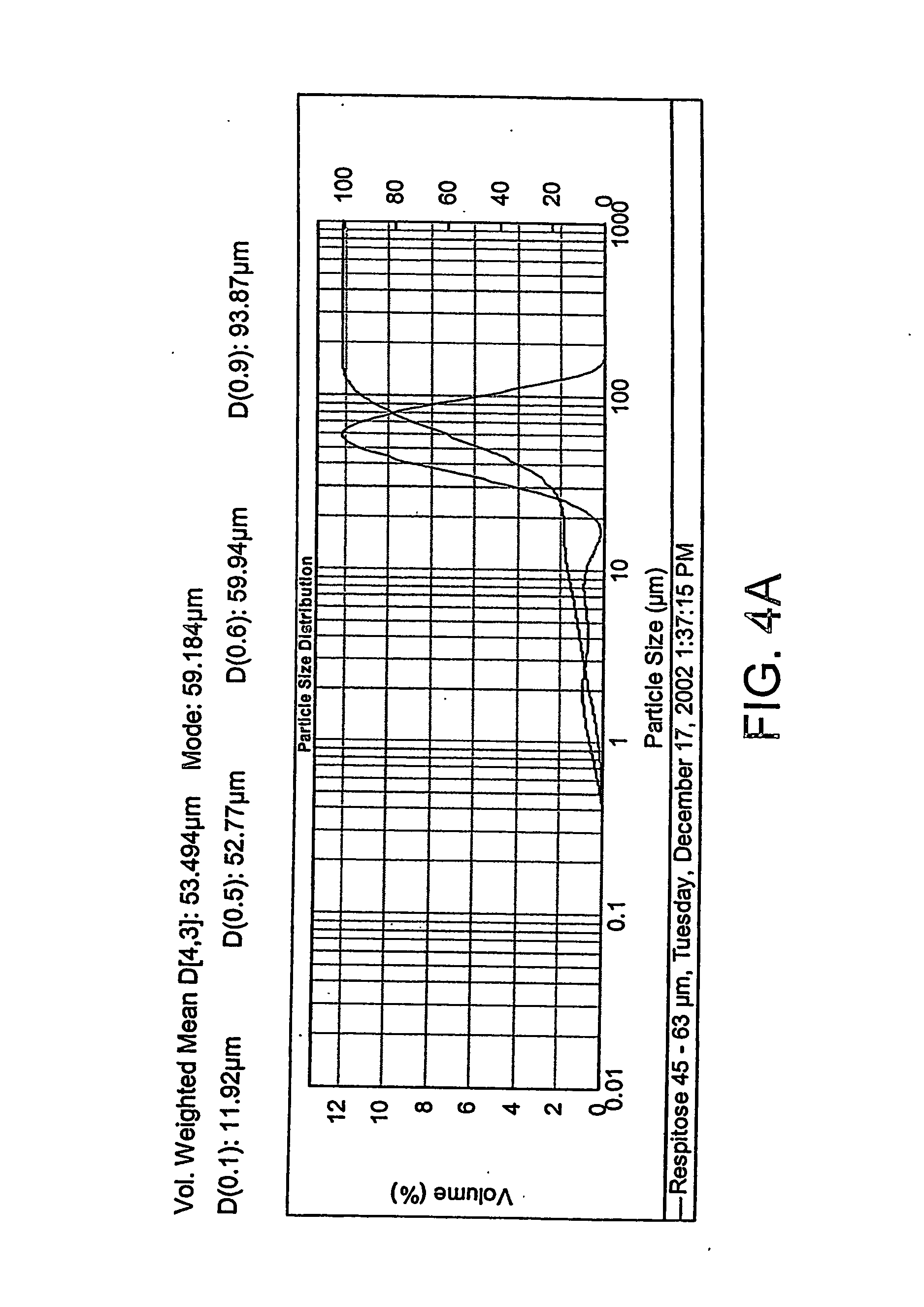
A composition, device, and method for treating sexual dysfunction via inhalation is provided which comprises inhaling a dose of a powder composition, the powder composition comprising apomorphine or pharmaceutically acceptable salts thereof. Preferably, the powder composition further includes a carrier material, the carrier material has an average particle size of from about 40 to about 70 microns, and at least 90 percent of said apomorphine has a particle size of 5 microns or less.
Owner:STANIFORTH JOHN NICOLAS +5
Benzoisothiazole compounds and use in preparation of antipsychotic drugs
ActiveUS20160096811A1Poor efficacyMeet demandOrganic active ingredientsNervous disorderDiseaseGeometric isomer
Disclosed are benzoisothiazole compounds and a use in the preparation of anti-schizophrenia drugs. The benzoisothiazole compounds of the present invention not only have strong affinity for dopamine D3 receptor, 5-HT1A receptor and 5-HT2A receptor, but also can observably improve the symptoms of schizophrenia relevant to apomorphine model and MK-801 model mice, with oral absorption being good, safety being high and side-effect being less, and having developmental value as new anti-neurotic disease drugs. The present invention is the compounds having a structure of general formula (I), or geometric isomers, free alkalies, salts, hydrates or solvates thereof.
Owner:SHANGHAI INST OF PHARMA IND
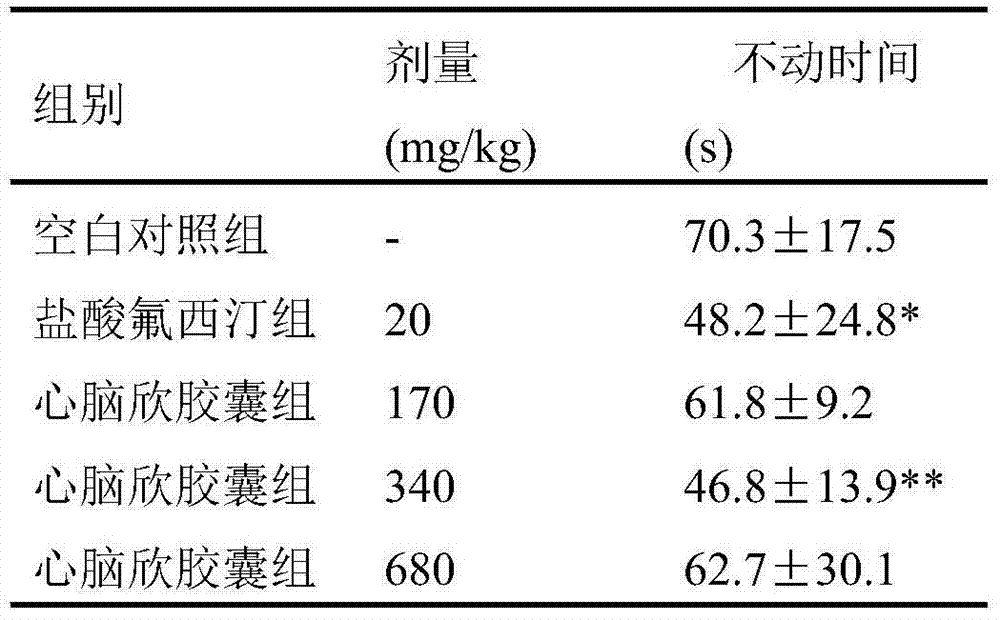
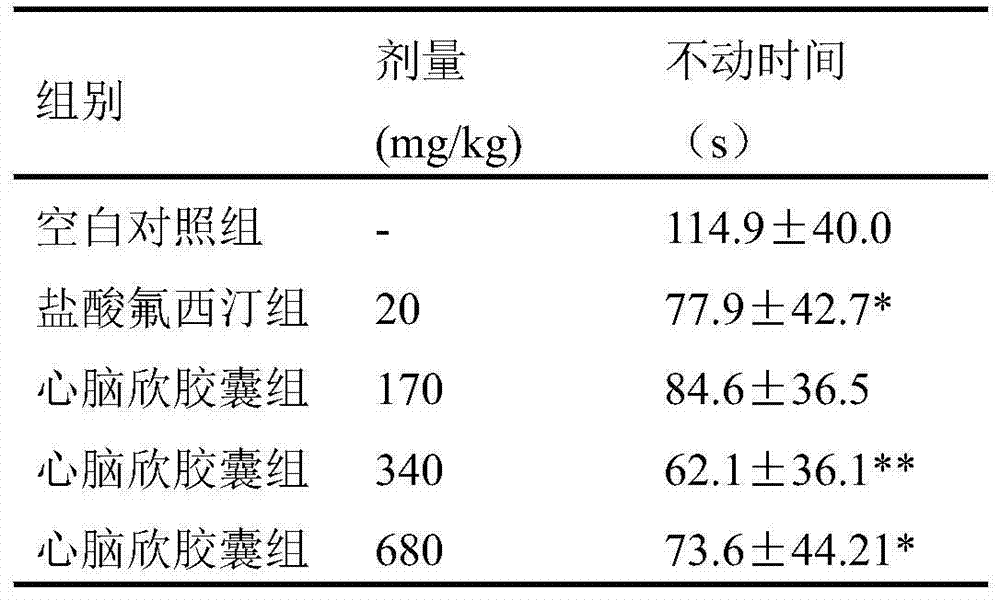
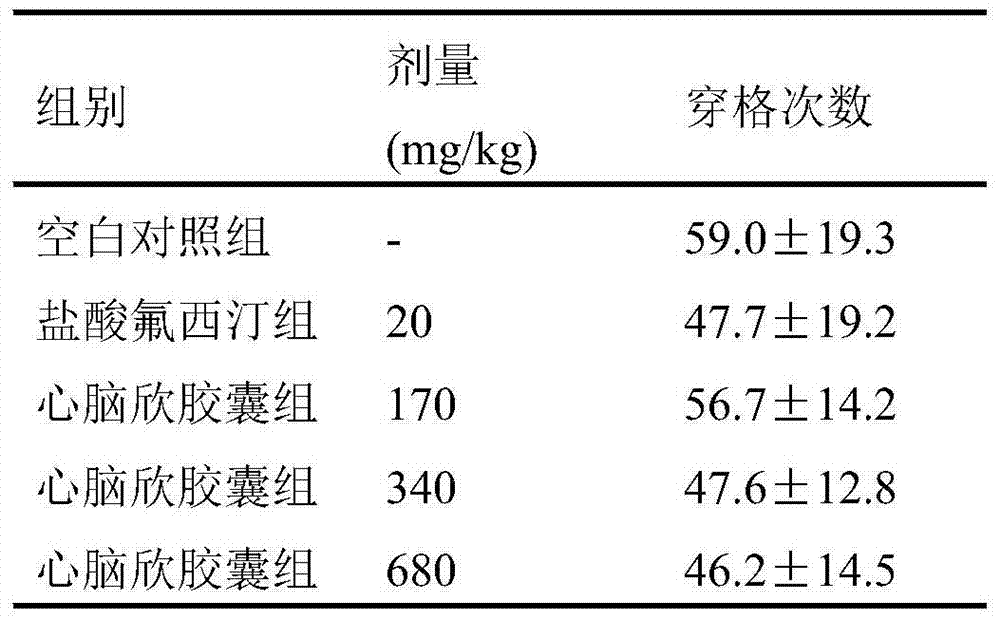
Application of Xinnaoxin capsule in preparation of antidepressant
ActiveCN103494946AAntidepressantDoes not interfere with spontaneous activityNervous disorderCapsule deliverySynaptic cleftMedicine
The invention discloses application of a Xinnaoxin capsule in preparation of an antidepressant. According to experimental studies, the Xinnaoxin capsule can substantially reduce dead time during tail suspension and forced swimming of behavioral despair model mice; according to open field experiments, spontaneous activities of the mice are not influenced while dead time is substantially decreased, which prompts that the Xinnaoxin capsule has a certain anti-depression effect. Meanwhile, the Xinnaoxin capsule can substantially inhibit activity of monoamine oxidase and increase the content of a monoamine neurotransmitter in a synaptic cleft, which proves that the Xinnaoxin capsule has the anti-depression effect. Moreover, the Xinnaoxin capsule can substantially antagonize decrease of body temperature of the mice caused by reserpine and apomorphine and increase exogenous toxicity given to NE, which reveals that the capsule has the anti-depression effect. The anti-depression effect of the capsule is realized through improvement of the level of the monoamine neurotransmitter in the synaptic cleft.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
High potency dopaminergic treatment of neurological impairment associated with brain injury
InactiveUS20060089373A1Short durationReduce doseBiocidePeptide/protein ingredientsNeurological impairmentInjury brain
Methods and compositions are described for treating impaired neurological function, including altered state of consciousness disorders, in an individual who has sustained a brain injury comprising administering to the individual apomorphine. Methods and compositions are described for treating impaired neurological function, including altered state of consciousness disorders, in an individual who has sustained a brain injury comprising administering to the individual at least 1000 mg or more of L-dopa (levodopa) per day. The use of potent dopaminergic agents to stimulate emergence from an altered consciousness state, such as a coma, is disclosed.
Owner:NEUROHEALING PHARMA INC
Composition, device, and method for treating sexual dysfunction via inhalation
A composition, device, and method for treating sexual dysfunction via inhalation is provided which comprises inhaling a dose of apomorphine or pharmaceutically acceptable salt(s) or ester(s) thereof.
Owner:VECTURA DRUG DELIVERY
2,6-diketone-piperazine (piperidine) type derivative and application thereof
The invention discloses a 2,6-diketone-piperazine (piperidine) type derivative and an application thereof. The derivative can be applied to preparation of medicines for preventing or treating central nervous system diseases. Animal experiment results show that the derivative has smaller ED50 and stronger action in MK-801-induced high activity and apomorphine-induced climbing animal models as well as has larger ED50 and larger therapeutic indexes in an animal catalepsy model. The derivative is a compound or salt thereof with a general formula (I).
Owner:NHWA PHARMA CORPORATION
Pharmaceutical formulation of apomorphine for buccal administration
The present invention provides a kit comprising, in separate compartments of a container, the following components (a) and (b): (a) a combination of apomorphine or a pharmaceutically acceptable acid addition salt thereof and a pharmaceutically acceptable excipient or carrier; and (b) a solution which comprises a diluent and a pH modifying agent; the components being presented such that they can be combined at the point of use into a formulation which is adjusted to a pH ranging from mildly acidic to alkaline and which is suitable for buccal administration. The formulation is useful in treating Parkinson's disease and in promoting sexual function.
Owner:AMARIN PHARMA IRELAND
Aporphine and oxoaporphine compounds and pharmaceutical use thereof
InactiveUS20040198759A1Not cause memory loss or hypothermic side effectsBetter indexBiocideOrganic chemistryMorphineIschaemic encephalopathy
The invention provides aporphine and oxoaporphine compounds that have endothelial nitric oxide synthase (eNOS) maintaining or enhancing activities and may be used to manufacture a medicaments for preventing or treating ischemic diseases in human and mammal, and the ischemic diseases may include ischemic cerebral apoplexy, ischemic cerebral thrombosis, ischemic cerebral embolism, hypoxic ischemic encephlopathy, ischemic cardiac disease or ischemic enteropathy etc.
Owner:LOTUS PHARMA CO LTD
New therapeutical composition containing apomorphine as active ingredient
ActiveUS20140128422A1Increase local toleranceGood chemical stabilityBiocideNervous disorderHas active ingredientAntioxidant
A pharmaceutical composition contains apomorphine as the active pharmaceutical ingredient, a water-miscible co-solvent, an antioxidant, and water. The solution has a pH greater than 4. The pharmaceutical composition is suitable for parenteral administration for the treatment of Parkinson's disease. The process for the manufacture of the pharmaceutical composition includes weighing the apomorphine and introducing it into a container with the co-solvent and the antioxidant under agitation until complete dissolution takes place.
Owner:BRITANNIA PHARMA LTD
Methods of treating parkinson's disease by administration of apomorphine to an oral mucosa
ActiveUS10449146B2High CmaxSufficient amountOrganic active ingredientsNervous disorderMorphinePharmaceutical drug
Methods and pharmaceutical unit dosage forms for treating Parkinson's disease in a subject (e.g., an “off” episode in a subject having Parkinson's disease) are described. The pharmaceutical unit dosage forms are films having a first portion including particles containing an acid addition salt of apomorphine and a second portion containing a pH neutralizing agent. The pharmaceutical unit dosage forms can be flexible and have toughness greater than 100 g×mm. The methods can involve administering to a subject having Parkinson's disease a therapeutic dose sufficient to produce an apomorphine plasma concentrate of at least 2.64 ng / mL within 45 minutes after the administration. The subject may be identified as having low uptake, medium uptake, or high uptake of apomorphine administered via oral mucosa.
Owner:SUNOVION PHARMA INC
Novel substituted-1-h-quinazoline-2,4-dione derivatives, preparation method thereof and pharmaceutical composition containing the same
InactiveUS20090203708A1High affinityHigh selectivityOrganic active ingredientsNervous disorderSerotoninMorphine
Disclosed herein are novel substituted-1H-quinazoline-2,4-dione derivatives, a preparation method thereof, and a pharmaceutical composition containing the same. The novel substituted-1H-quinazoline-2,4-dione derivatives are excellent in binding affinity and selectivity for 5-HT6 receptors over other receptors, inhibit serotonin(5-HT)-stimulated cAMP accumulation, and disrupt apomorphine(2 mg / kg, i.p.)-induced hyperactivity in rats. Thanks to these effects, the derivatives are useful in the treatment of 5-HT6 receptor-related central nervous system diseases.
Owner:KOREA RES INST OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
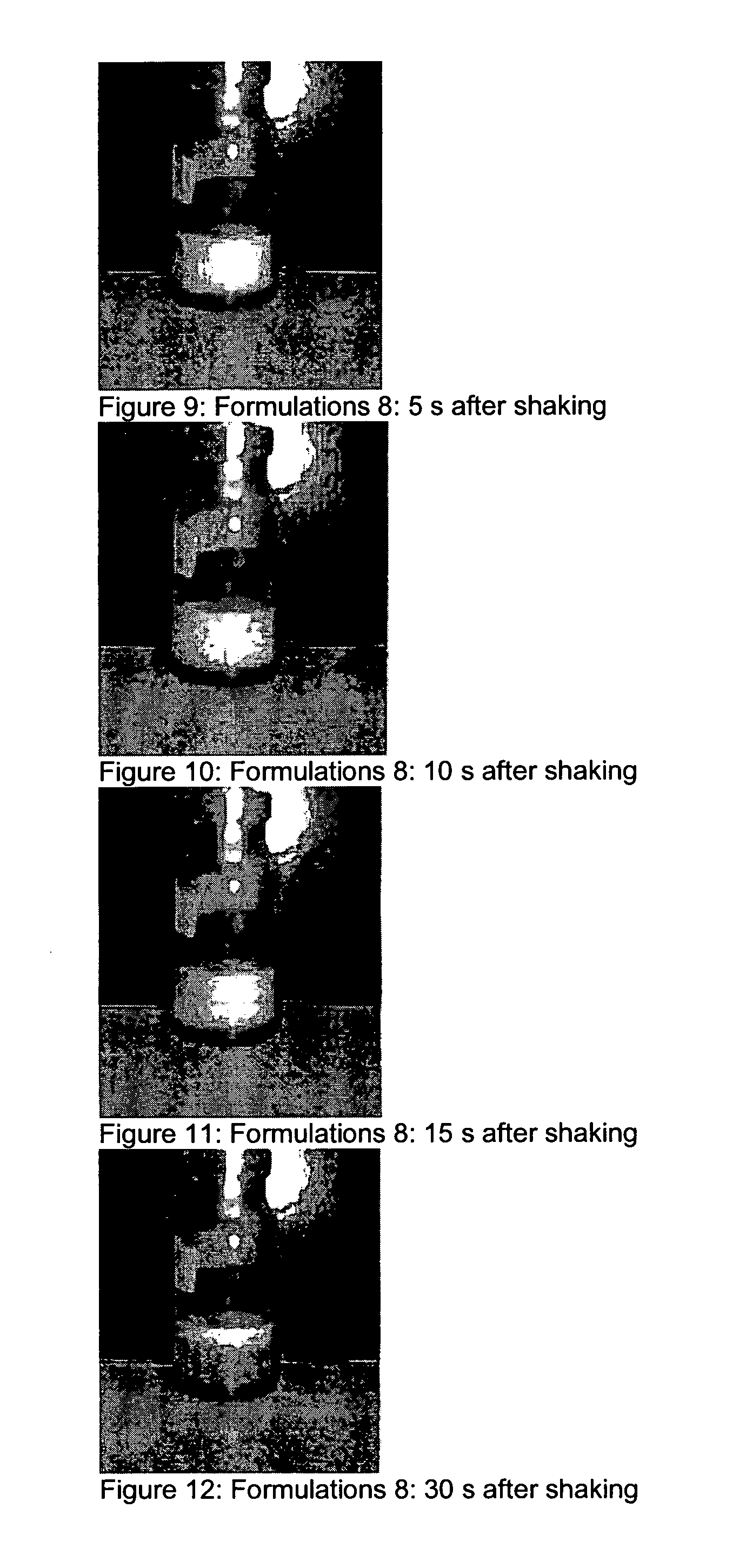
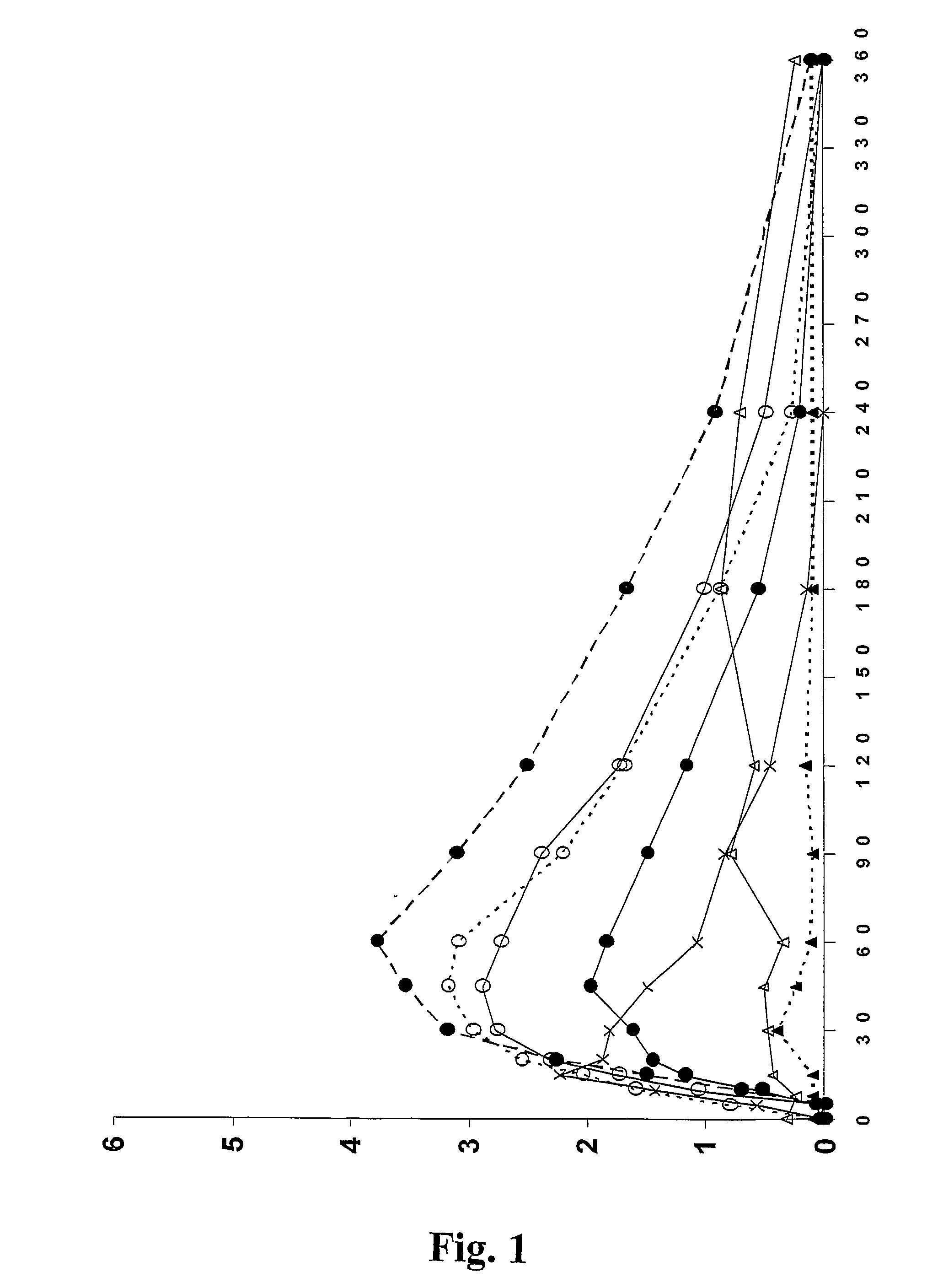
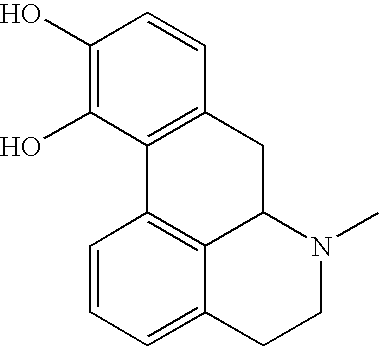
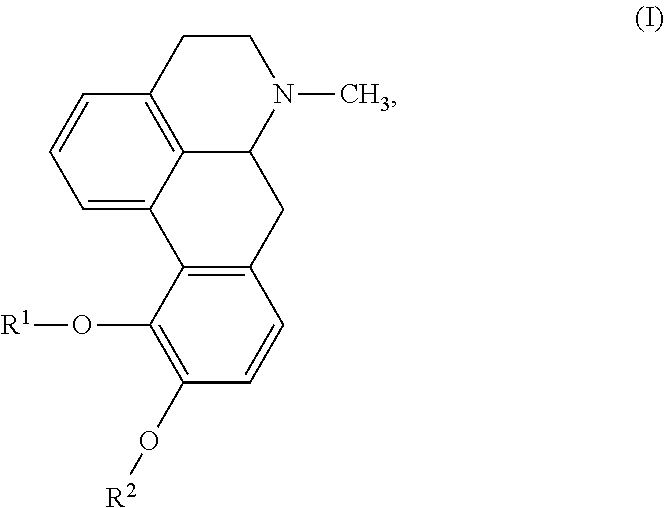
![Novel substituted-1, 1-dioxo-benzo[1,2,4]thiadiazin-3ones, preparation method thereof, and pharmaceutical composition containing the same Novel substituted-1, 1-dioxo-benzo[1,2,4]thiadiazin-3ones, preparation method thereof, and pharmaceutical composition containing the same](https://images-eureka.patsnap.com/patent_img/13d94ce4-873f-4f98-8523-7dc5cfd1a9b5/US20100035866A1-20100211-D00000.png)
![Novel substituted-1, 1-dioxo-benzo[1,2,4]thiadiazin-3ones, preparation method thereof, and pharmaceutical composition containing the same Novel substituted-1, 1-dioxo-benzo[1,2,4]thiadiazin-3ones, preparation method thereof, and pharmaceutical composition containing the same](https://images-eureka.patsnap.com/patent_img/13d94ce4-873f-4f98-8523-7dc5cfd1a9b5/US20100035866A1-20100211-D00001.png)
![Novel substituted-1, 1-dioxo-benzo[1,2,4]thiadiazin-3ones, preparation method thereof, and pharmaceutical composition containing the same Novel substituted-1, 1-dioxo-benzo[1,2,4]thiadiazin-3ones, preparation method thereof, and pharmaceutical composition containing the same](https://images-eureka.patsnap.com/patent_img/13d94ce4-873f-4f98-8523-7dc5cfd1a9b5/US20100035866A1-20100211-C00001.png)