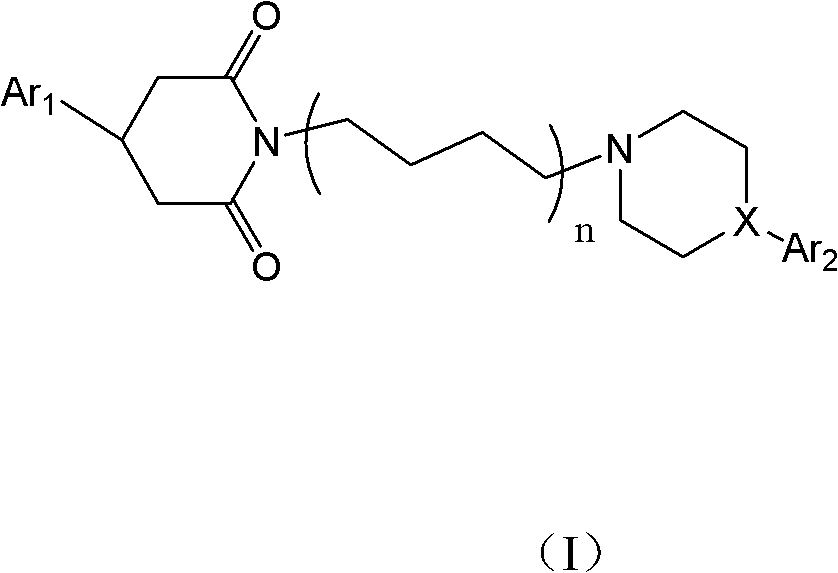
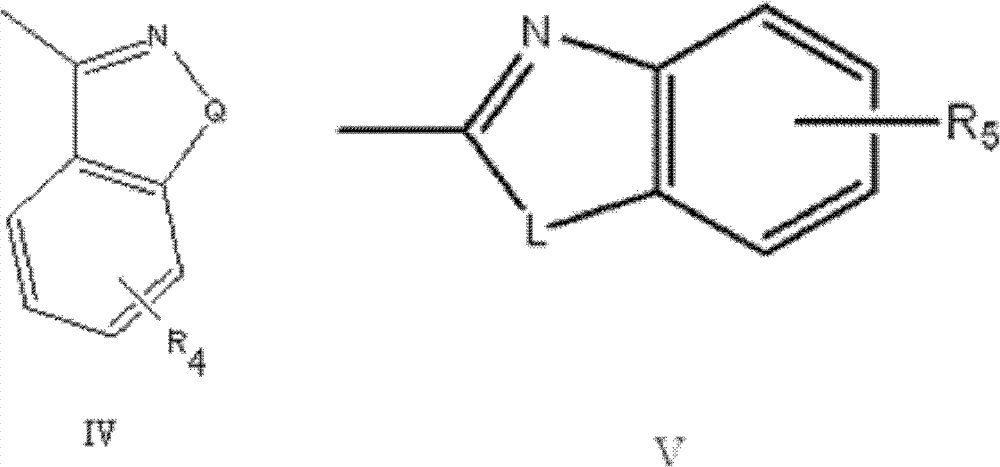
2,6-diketone-piperazine (piperidine) type derivative and application thereof
A derivative, piperidine technology, applied in the field of medicinal chemistry, can solve problems such as QT gap prolongation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1-(4-(4-(3-(6-fluoro-benzoisoxazole))-1-piperidinyl)butyl)-4-(4-chlorophenyl)-piperidine-2,6 - Diketones (No. 1)
[0067] (1) Add 52 g (0.4 mol) of ethyl acetoacetate and 28 g (0.2 mol) of p-chlorobenzaldehyde in a 500 ml three-necked flask equipped with a reflux condenser, magnetic stirring and dropping funnel. Under stirring, a mixed solution of ethanol (20ml) and piperidine (2ml) with a volume concentration of 95% was added dropwise, the solid gradually dissolved, and the stirring was continued until the reaction solution began to solidify again, about 1 hour. When the reactants are completely solidified, heat them in an electric constant temperature water bath, and place them in the dark at 40°C for 5 hours to complete the reaction. The solidified product was recrystallized with ethanol with a volume concentration of 95%, and 52.2 g of white needle crystals were obtained, with a yield of 68.2%. Melting point: 152-155°C.
[0068] (2) Add 100g of potassium hydroxid...
Embodiment 2
[0075] 1-(4-(4-(3-(6-fluoro-benzoisoxazole))-1-piperidinyl)butyl)-4-(4-fluorophenyl)-piperidine-2,6 - diketones (number 2)
[0076] Using p-fluorobenzaldehyde as the starting material, the target compound was obtained according to the method in Example 1. Melting point: 137-139°C
[0077] 1 H NMR (CDCl 3 )δ1.52-1.61 (m, 4H), 2.06-2.12 (m, 6H), 2.42 (t, 2H, J=13.6Hz), 2.75-2.83 (m, 2H), 2.96-3.07 (m, 5H) , 3.34-3.37(m, 1H), 3.83(t, 2H, J=14Hz), 7.03-7.07(m, 3H), 7.18-7.24(m, 3H), 7.71-7.74(m, 1H)
Embodiment 3
[0079] 1-(4-(4-(3-(6-fluoro-benzoisoxazole))-1-piperidinyl)butyl)-4-(phenyl)-piperidine-2,6-dione (number 3)
[0080] Using benzaldehyde as the starting material, the target compound was obtained according to the method in Example 1. Melting point: 114-116°C
[0081] 1 H NMR (CDCl 3 )δ1.55-1.59 (m, 4H), 2.05-2.13 (m, 6H), 2.42 (t, 2H, J=14.4Hz), 2.77-2.84 (m, 2H), 2.98-3.08 (m, 5H) , 3.34-3.38(m, 1H), 3.84(t, 2H, J=14Hz), 7.03-7.08(m, 1H), 7.20-7.40(m, 6H), 7.68-7.71(m, 1H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com