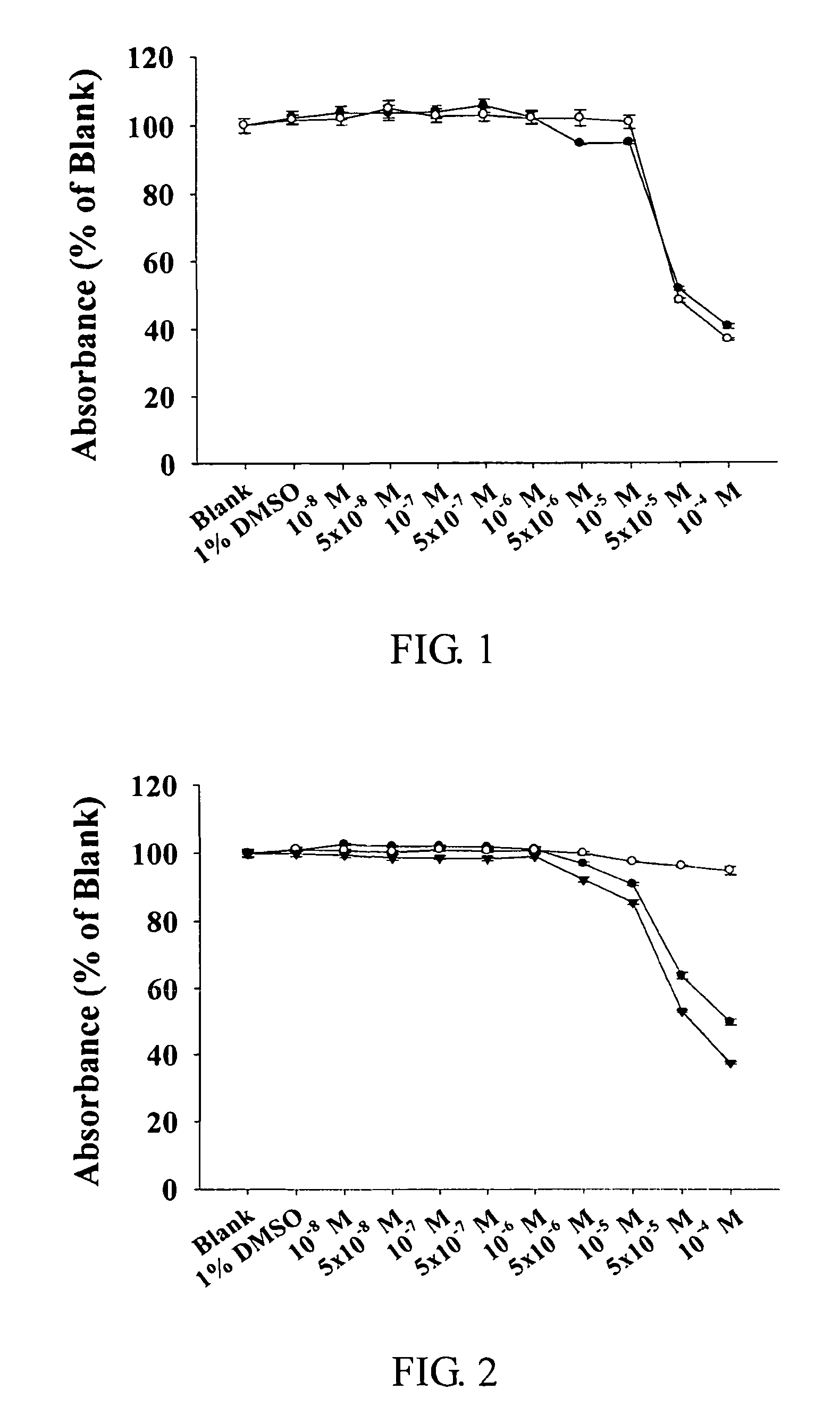
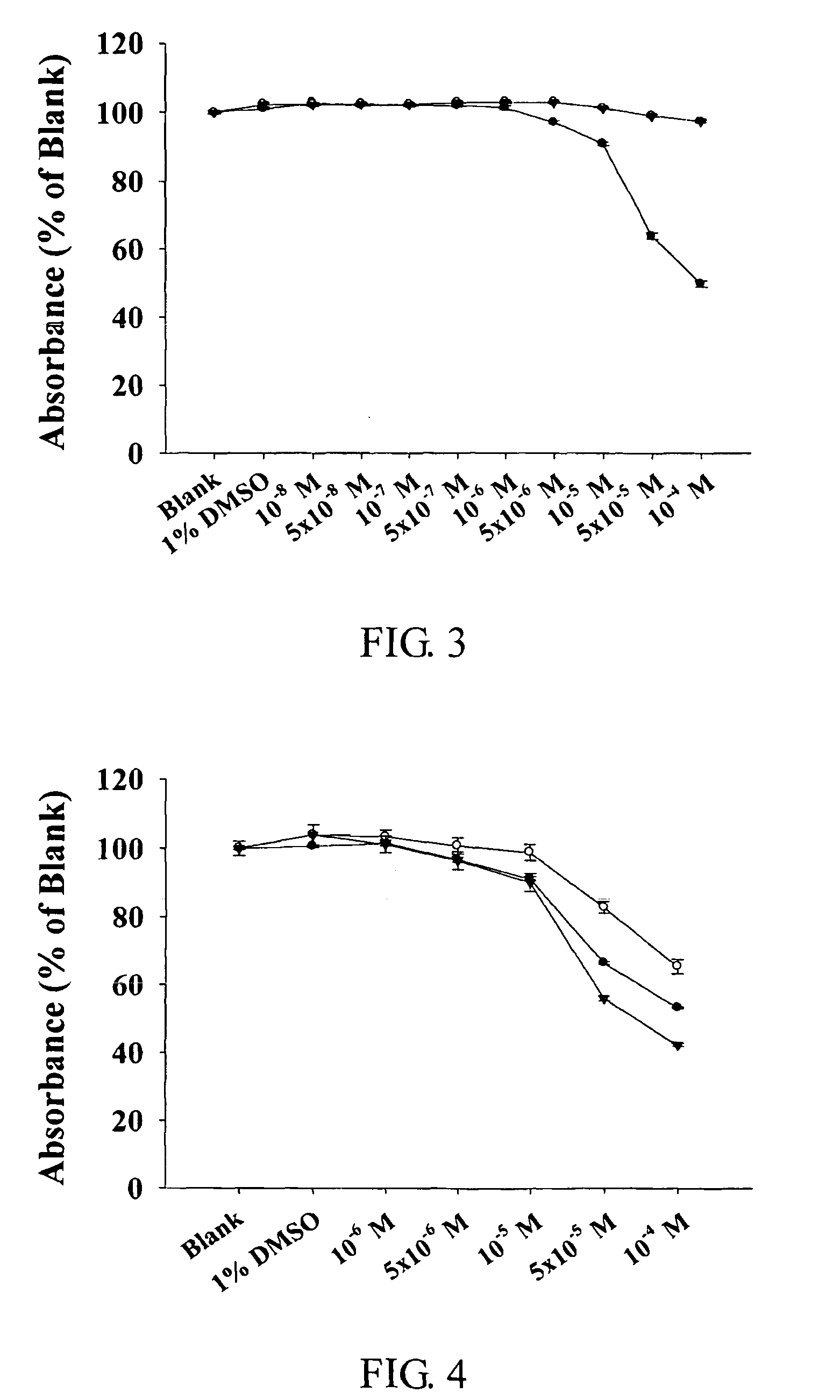
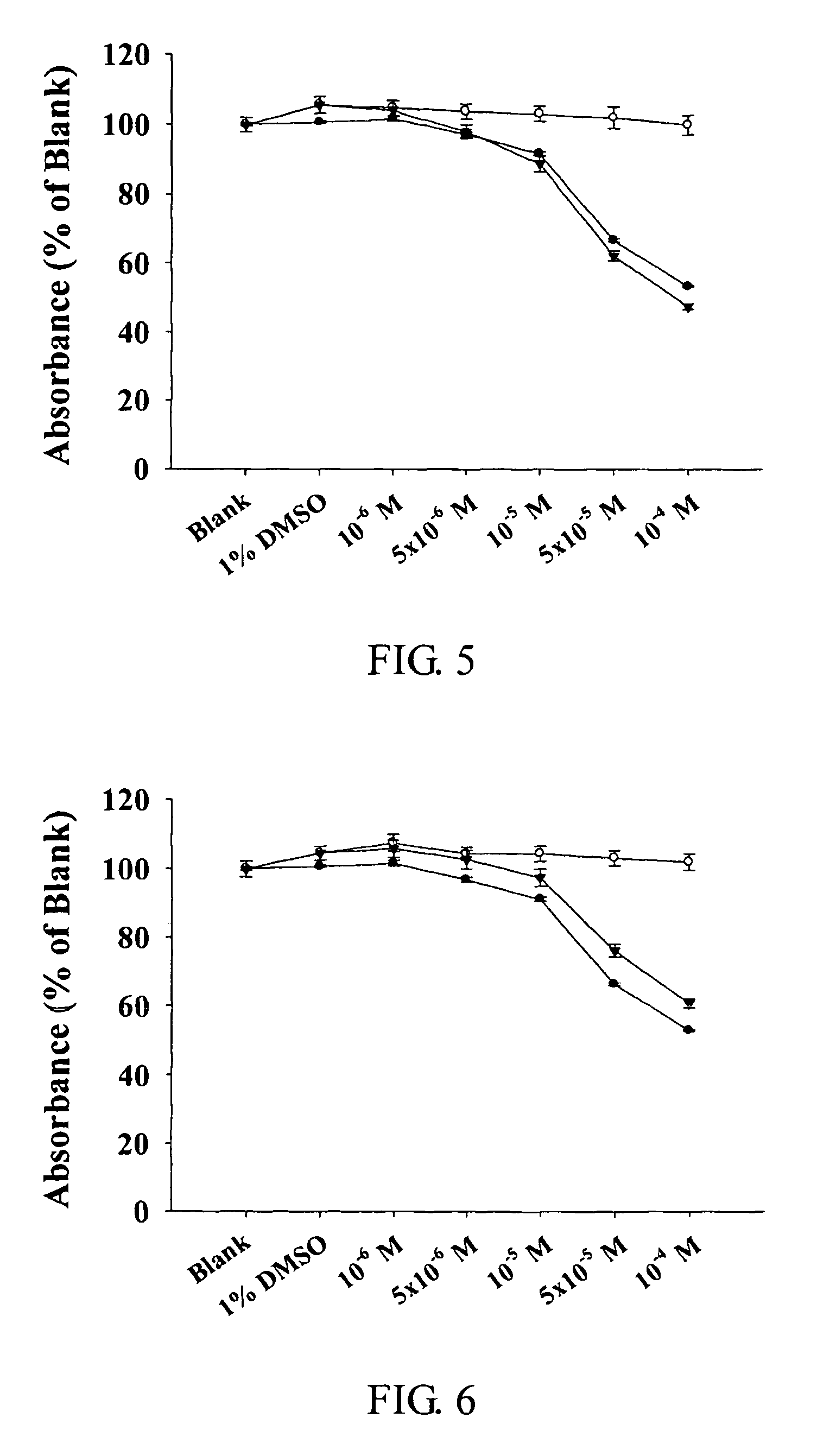
Pharmaceutically acceprable salts of aporphine compounds of carboxyl group-containing agents and methods for preparing the same
a technology of carboxyl group and aporphine, which is applied in the field of pharmaceutically acceptable salts of aporphine compounds and carboxyl group-containing agents and methods for preparing the same, can solve the problems of nitrate tolerance, hypertension, and artery gradually narrowing and can become clogged, and achieves dysregulation of physiological processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example 1
Preparation of Compound 1
[0118]
[0119]Northaliporphine (380 mg, 1.16 mmol), methanol (MeOH, 10 mL) and 2-[(2-methoxy-phenox)methyl]oxirane (167 mg, 0.92 mmol) were added into a 50 mL round bottom flask and stirred at 70° C. for 16 hours. The mixture was evaporated to dryness. The residue was purified by chromatography (silica gel: 70-230 mesh 30 g, mobile phase: 2% MeOH / CH2Cl2, v / v) to obtain Compound 1, Rf 0.15 (2% MeOH / CH2Cl2, v / v); Physical data were as follows: mp: 63-68° C. (CH2Cl2); IR(KBr)vmax: 3500, 2931, 1605, 1506, 1464, 1253, 1112 cm−1; 1H NMR (CDCl3, 500 MHz):δ 8.00 (s, 1H), 6.98-6.88 (m, 4H), 6.75 and 6.73 (s, 1H), 6.53 (s, 1H), 6.12 (brs, 1H), 4.24-4.07 (m, 3H), 3.90 (s, 3H), 3.88 (s, 3H), 3.85 (s, 3H), 3.79 (s, 3H), 3.39-2.53 (m, 9H); EIMS (70 eV): m / z (%) 507 [M]+, 339 (100).
preparation example 2
Preparation of Compound 2
[0120]
[0121]Northaliporphine (260 mg, 0.794 mmol), MeOH (10 mL) and 2-chloro-N-(2,6-dimethyl-phenyl)acetamide (187 mg, 0.946 mmol) were added into a 50 mL two-necked round bottom flask. Then triethylamine (Et3N, 0.5 mL, 3.55 mmol) was dropped into the mixture, and the reaction was allowed to proceed at 60° C. for two days. The mixture was evaporated to dryness. The residue was partitioned with water (50 mL) and dichloromethane (50 mL×3), and the organic layers were collected. The organic layer was dried with anhydrous MgSO4 and then filtered. The filtrate was evaporated to dryness. The residue was purified by chromatography (silica gel: 70-230 mesh 30 g, mobile phase: 2% MeOH / CH2Cl2, v / v) to obtain Compound 2, Rf 0.58 (2% MeOH / CH2Cl2, v / v); Physical data were as follows: mp: 205-207° C. (CH2Cl2); IR(KBr)vmax: 3312, 2945, 1663,1 604, 1511, 1477, 1258, 1087 cm−1; 1H NMR(CDCl3, 500 MHz):δ 8.99 (s, 1H), 8.02 (s, 1H), 7.10 (s, 3H), 6.76 (s, 1H), 6.56 (s, 1H), 6.1...
preparation example 3
Preparation of Compound 3
[0122]
[0123]Norglaucine (300 mg, 0.88 mmol), MgSO4 (1 g), MeOH (7 mL), 2-thiophenecarboxaldehyde (0.14 mL, 1.5 mmol) and AcOH (0.5 mL, 8.88 mmol) were added into a 100 mL two-necked round bottom flask and stirred at room temperature. Sodium cyanoborohydride (NaBH3CN, 100 mg, 1.58 mmol) was added into the mixture after 1 hour, and the reaction was allowed to proceed at 70° C. for 4 hours. The mixture was evaporated to dryness. The residue was partitioned with water (50 mL) and dichloromethane (50 mL×2), and the organic layers were collected. The organic layer was dried with anhydrous MgSO4 and then filtered. The filtrate was evaporated to dryness. The residue was purified by chromatography (silica gel: 230-400 mesh 30 g, mobile phase: EA / Hex=1 / 2, v / v) to obtain Compound 3, Rf 0.77 (EA / Hex=1 / 1, v / v); Physical data were as follows: mp: 143-148° C. (CH2Cl2); IR(KBr)vmax: 2958, 1578, 1514, 1466, 1110 cm−1; 1H NMR(CDCl3, 400 MHz):δ 8.05 (s, 1H), 7.22-7.20 (m, 1H),...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| oxidative stress | aaaaa | aaaaa |
| physico-chemical | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com