Application of Xinnaoxin capsule in preparation of antidepressant
A technology of antidepressants and capsules, applied in the field of medicine, to achieve the effects of reducing immobility time, antagonizing body temperature drop, and increasing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
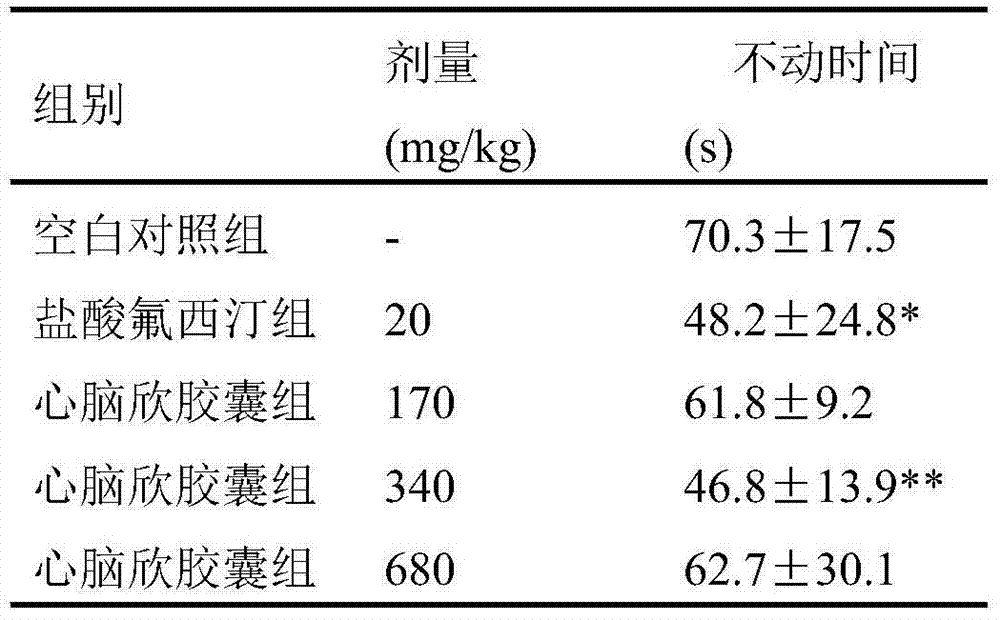
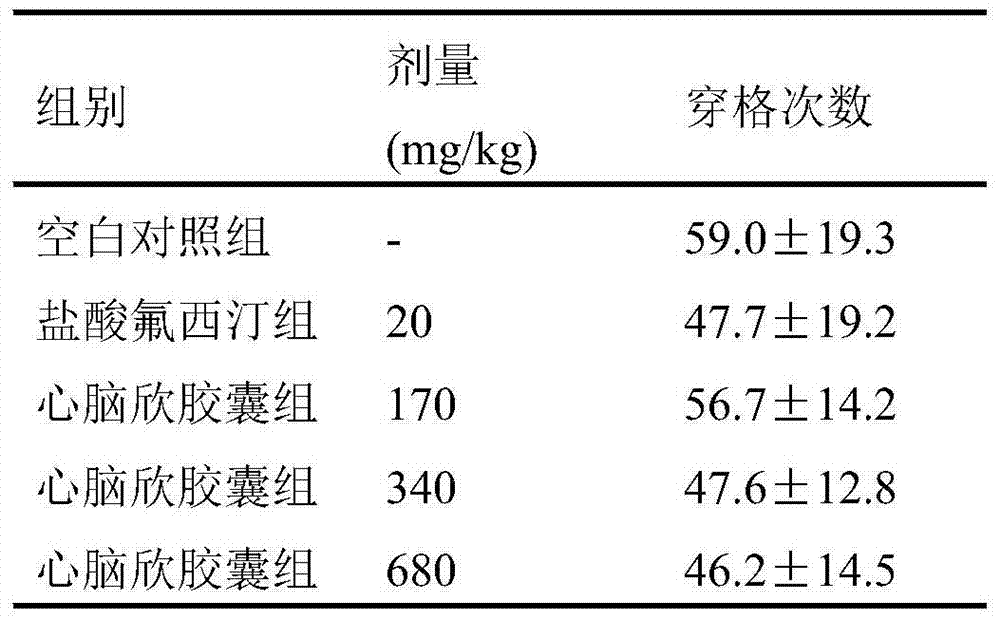
[0010] Effects of Xinnaoxin Capsules on Depressive Behavior in Behavioral Hopelessness Model Mice
[0011] 1 Materials and methods
[0012] 1.1 Materials
[0013] 1.1.1 Main Drugs and Reagents
[0014] Xinnaoxin Capsules, the content is tan powder, 0.5g / capsule, batch number: 120803 Sanpu Pharmaceutical Co., Ltd.;
[0015] Fluoxetine hydrochloride tablets (Changzhou Siyao Pharmaceutical Co., Ltd., 20120308).
[0016] 1.1.2 Experimental animals
[0017] Clean grade ICR mice, male, weight (20±2) g, Nantong University Experimental Animal Center, certificate number: SCXK (Su) 2008-0010. The animals were housed in separate cages, maintained a 12-h circadian rhythm, at a room temperature of 22±1°C, and had free access to water and food.
[0018] 1.1.3 Main experimental instruments
[0019] Mine behavior tester for mice (homemade, 30cm in diameter, 20cm in height, 19 equal parts at the bottom);
[0020] Mouse forced swimming behavior tester (self-made, height 50cm, diameter 20...
Embodiment 2
[0050] Effects of Acute Administration of Xinnaoxin Capsules on MAO-B Activity in Brain Tissue of Mice
[0051] 1 Materials and methods
[0052] 1.1.1 Main Drugs and Reagents
[0053] Xinnaoxin Capsules, the content is tan powder, 0.5g / capsule, batch number: 120803 Sanpu Pharmaceutical Co., Ltd.;
[0054] Fluoxetine Hydrochloride Tablets (Changzhou Siyao Pharmaceutical Co., Ltd., 20120308);
[0055] BCA protein concentration determination kit (Beiyuntian Biotechnology Research Institute, P0012)
[0056] Monoamine oxidase (MAO) test kit (Nanjing Jiancheng Bioengineering Institute, A034)
[0057] 1.1.2 Experimental animals
[0058] Clean grade ICR mice, male, weight (20±2) g, Nantong University Experimental Animal Center, certificate number: SCXK (Su) 2008-0010. The animals were housed in separate cages, maintained a 12-h circadian rhythm, at a room temperature of 22±1°C, and had free access to water and food.
[0059] 1.1.3 Main experimental instruments
[0060] Mouse fo...
Embodiment 3
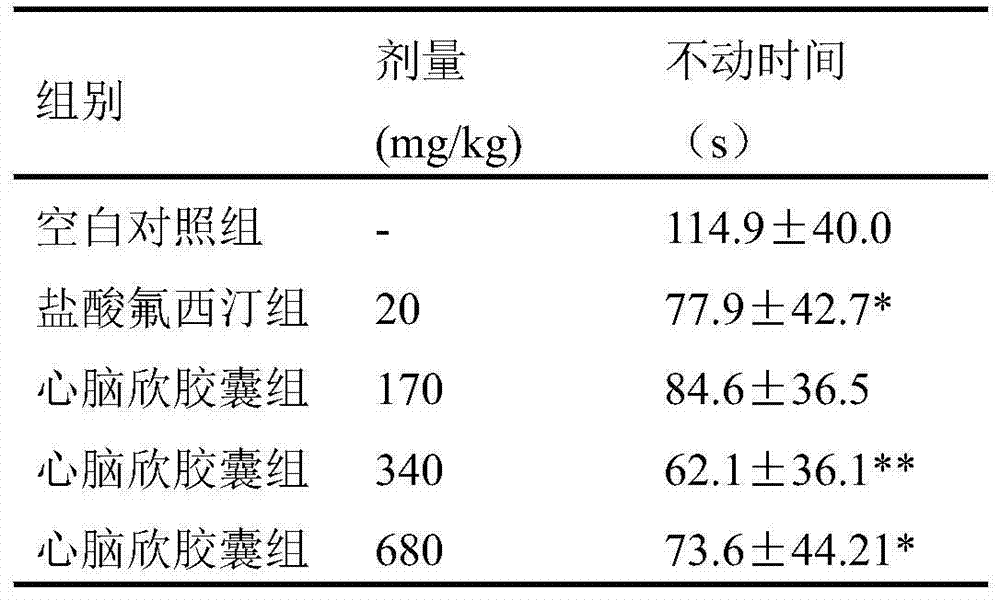
[0081] Effects of Xinnaoxin Capsules on the Behavior of Drug-Induced Depression Model Mice
[0082] 1 Materials and methods
[0083] 1.1 Materials
[0084] Main Drugs and Reagents
[0085] Xinnaoxin Capsules, the content is tan powder, 0.5g / capsule, batch number: 120803 Sanpu Pharmaceutical Co., Ltd.;
[0086] Fluoxetine Hydrochloride Tablets (Changzhou Siyao Pharmaceutical Co., Ltd., 20120308);
[0087] Normal saline (0.86%);
[0088] 5-HTP: produced by Hangzhou Zhongxiang Chemical Co., Ltd., batch number: CAC071205;
[0089] 5-Hydroxytryptophan (5-Hydroxy-DL-tryptophan, 5-HTP), Fluka Company;
[0090] Apomorphine (Apomorphine Hydrochloride Hemihydrate), Sigma Company;
[0091] Reserpine: The injection is a product of Tianjin Jinyao Co., Ltd., batch number 1107171.
[0092] Noradrenaline Bitartrate Injection (Noradrenaline Bitartrate Injection), Shanghai Hefeng Pharmaceutical Co., Ltd., batch number 110703.
[0093] 1.2 Experimental animals
[0094] Clean grade ICR m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com