Antiallergic nasal medicine composition with high moisture retention and preparation methods and applications thereof
A technology with high moisture retention and composition, which is applied in the field of medicine, can solve the problems of organic lesions that can only relieve symptoms, inconvenience for patients, and rapid nasal dryness, so as to overcome the problem of bioavailability, reduce gastrointestinal excretion, The effect of lowering the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
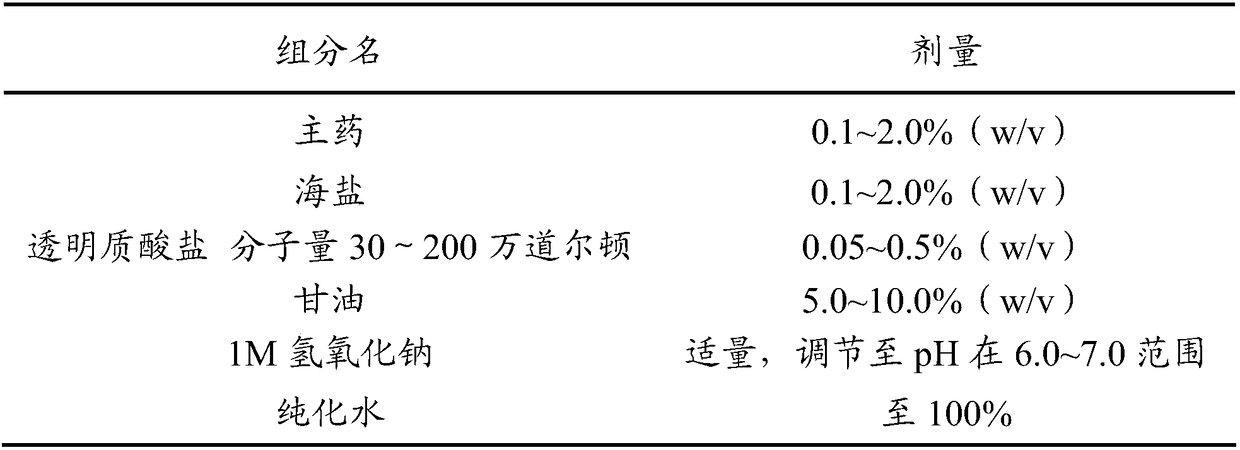
[0115] The basic composition of the sea saline solution for nasal use provided by the invention (excluding main ingredients), as shown in Table 1.
[0116] Table 1 Basic composition of nasal pharmaceutical composition (aqueous solution)
[0117]
[0118] The preparation method of the aqueous solution is as follows: taking the prescribed amount of sodium hyaluronate and dissolving it in purified water, fully stirring until completely dissolved, then adding the prescribed amount of glycerin, sea salt and main ingredients in sequence and stirring evenly to obtain the product.
preparation example 2
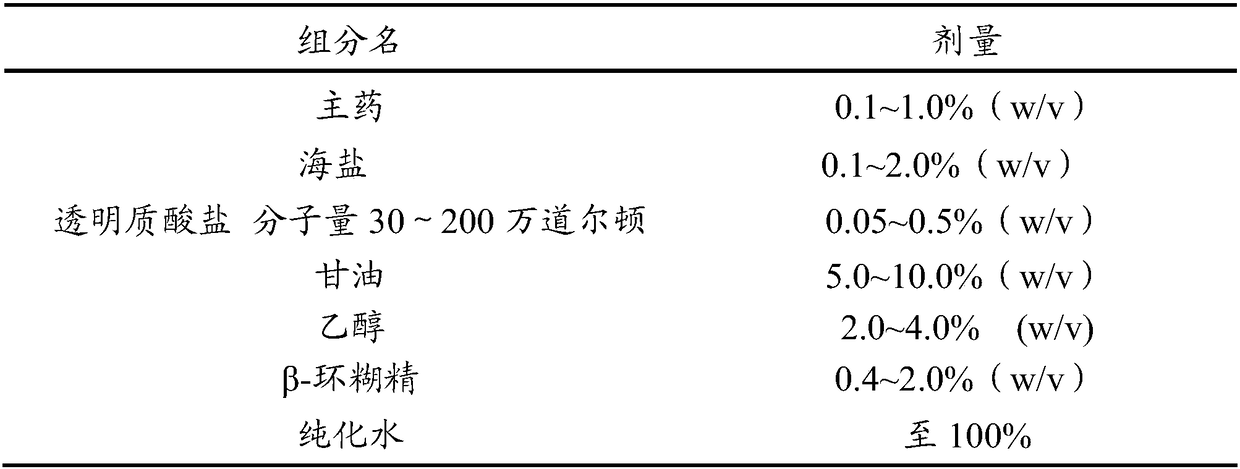
[0120] The basic composition of the nasal pharmaceutical composition (aqueous solution) provided by the present invention is shown in Table 2.
[0121] Table 2 Basic composition of nasal pharmaceutical composition (aqueous solution)
[0122]
[0123] The main drug is selected from levocetirizine hydrochloride, azastatine maleate, stastatine hydrochloride, bilastine fumarate, clemastine fumarate, epinastine hydrochloride, moxifloxacin hydrochloride one or more of.
[0124] The preparation method of the aqueous solution is as follows: take the prescribed amount of sodium hyaluronate and dissolve it in purified water, fully stir until it is completely dissolved, then add the prescribed amount of glycerin, sea salt and the main ingredient and stir evenly, and adjust the appropriate amount of pH regulator to pH 6.0 It can be obtained within the range of ~7.0.
preparation example 3
[0126] The basic composition of the nasal pharmaceutical composition (beta-cyclodextrin inclusion solution) provided by the present invention is shown in Table 3.
[0127] Table 3 Basic composition of nasal pharmaceutical composition (β-cyclodextrin inclusion solution)
[0128]
[0129] Note: The main drug is loratadine.
[0130] The preparation method of the β-cyclodextrin inclusion solution is as follows: weighing the raw materials of the prescription amount and adding an appropriate amount of ethanol to dissolve to obtain the ethanol solution. Dissolve hyaluronate in purified water and stir until it is completely dissolved. Add the prescribed amount of glycerin and sea salt to the solution, stir evenly, then add the prescribed amount of β-cyclodextrin, and heat to 40°C to obtain an aqueous solution. Slowly add the ethanol solution to the mixture, stirring while adding, the stirring speed is 50rpm. After the addition, stir for 20 minutes, let it stand overnight, and fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com