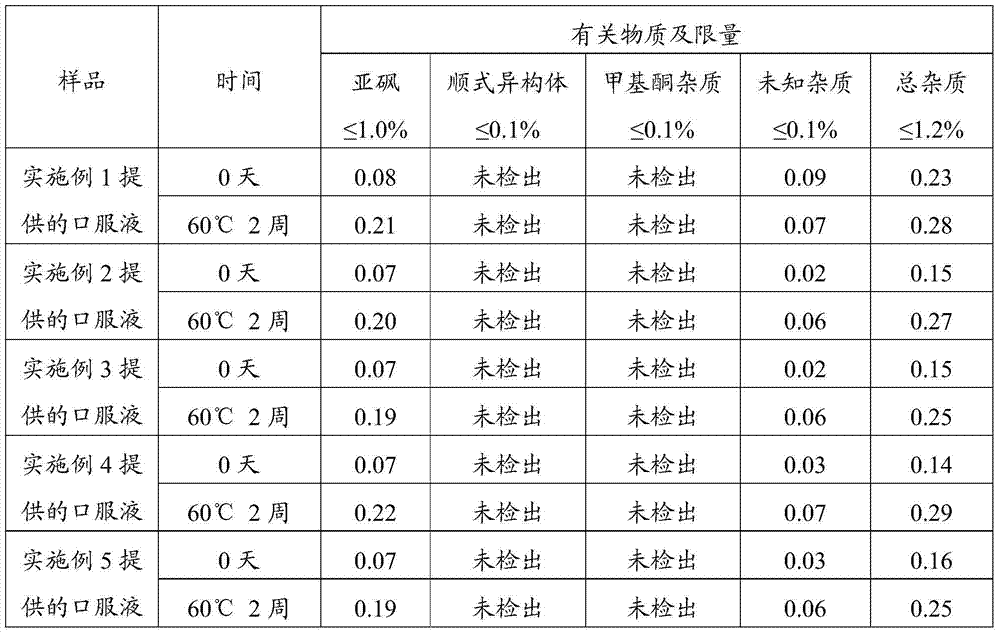
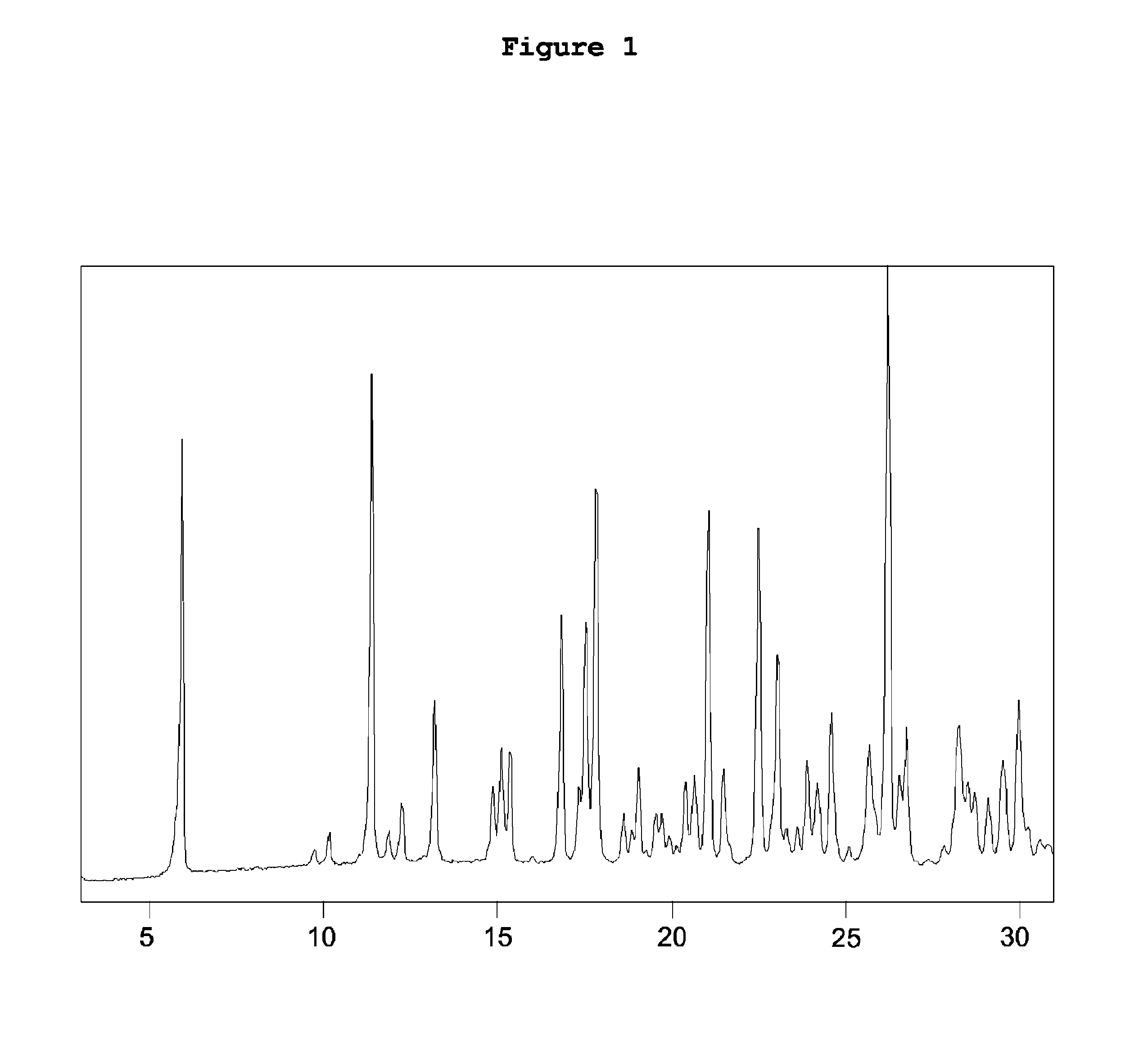
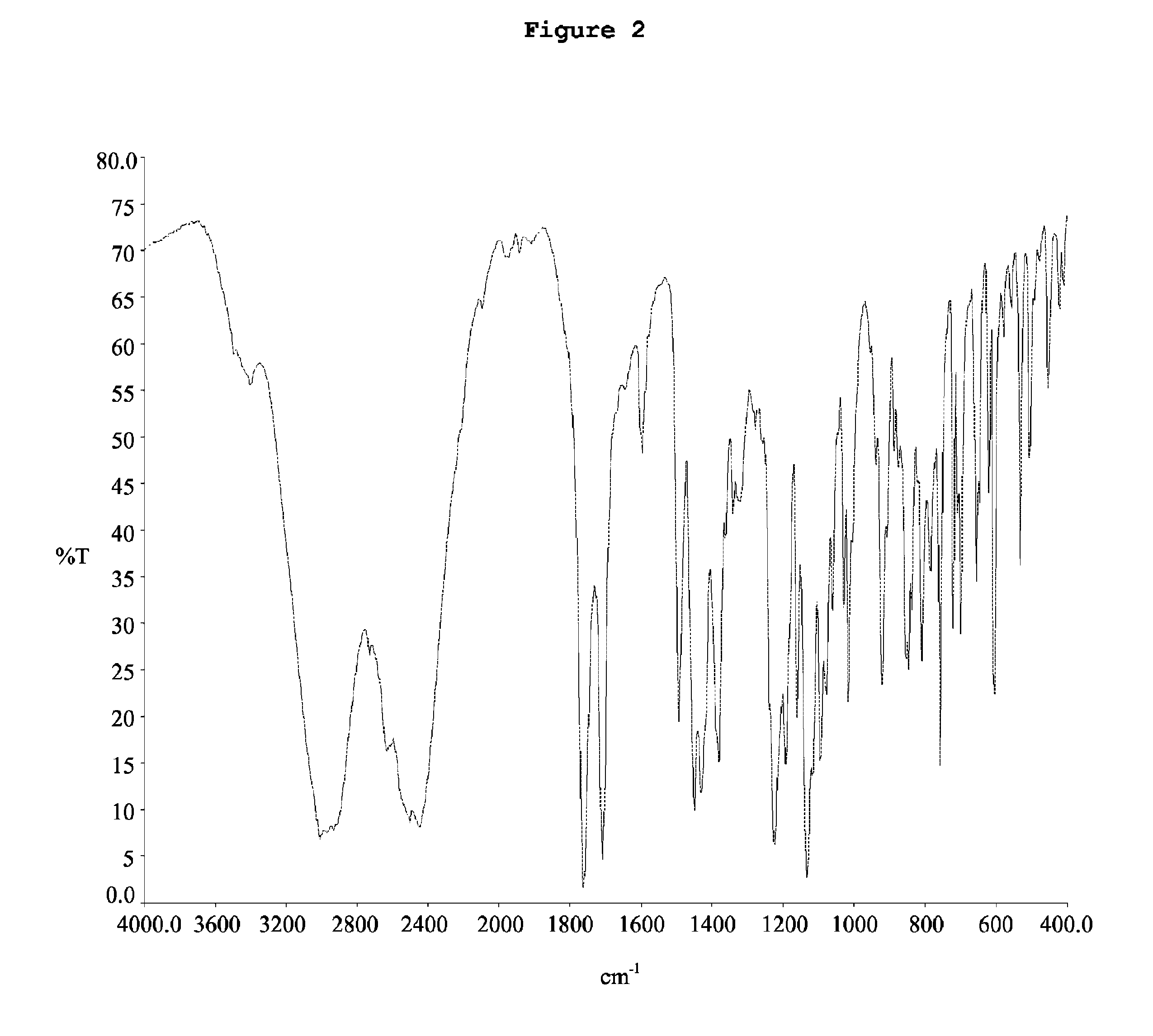
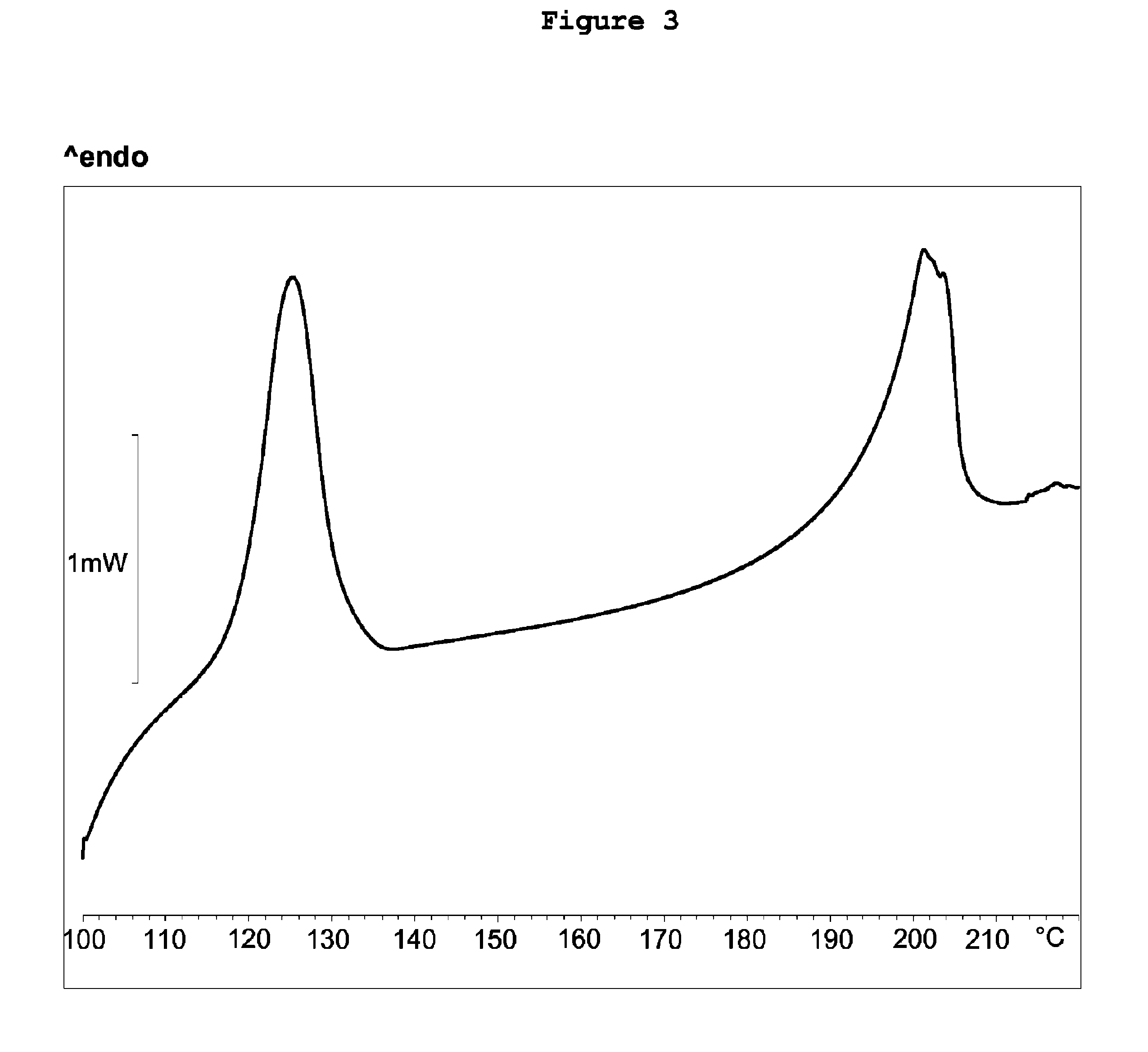
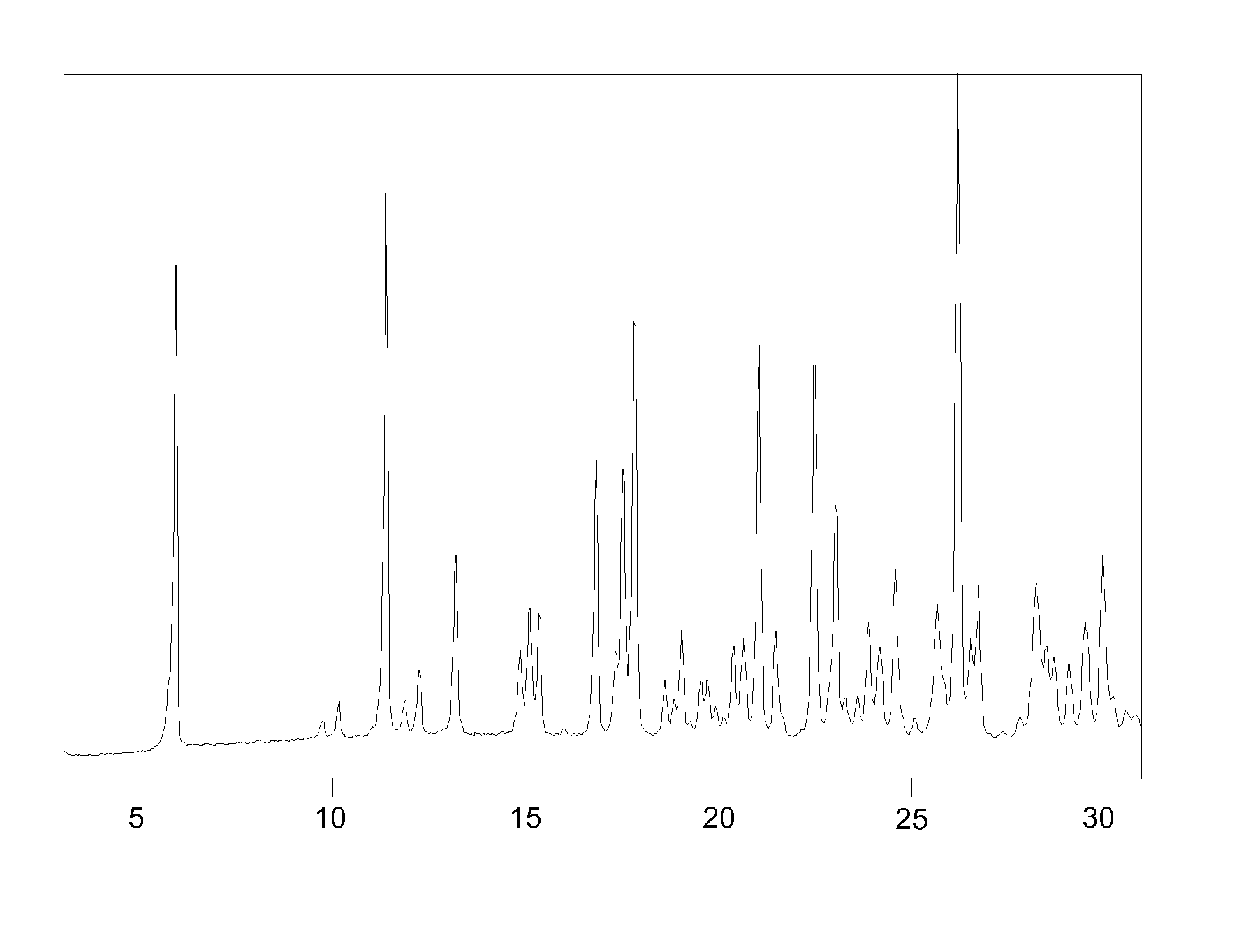
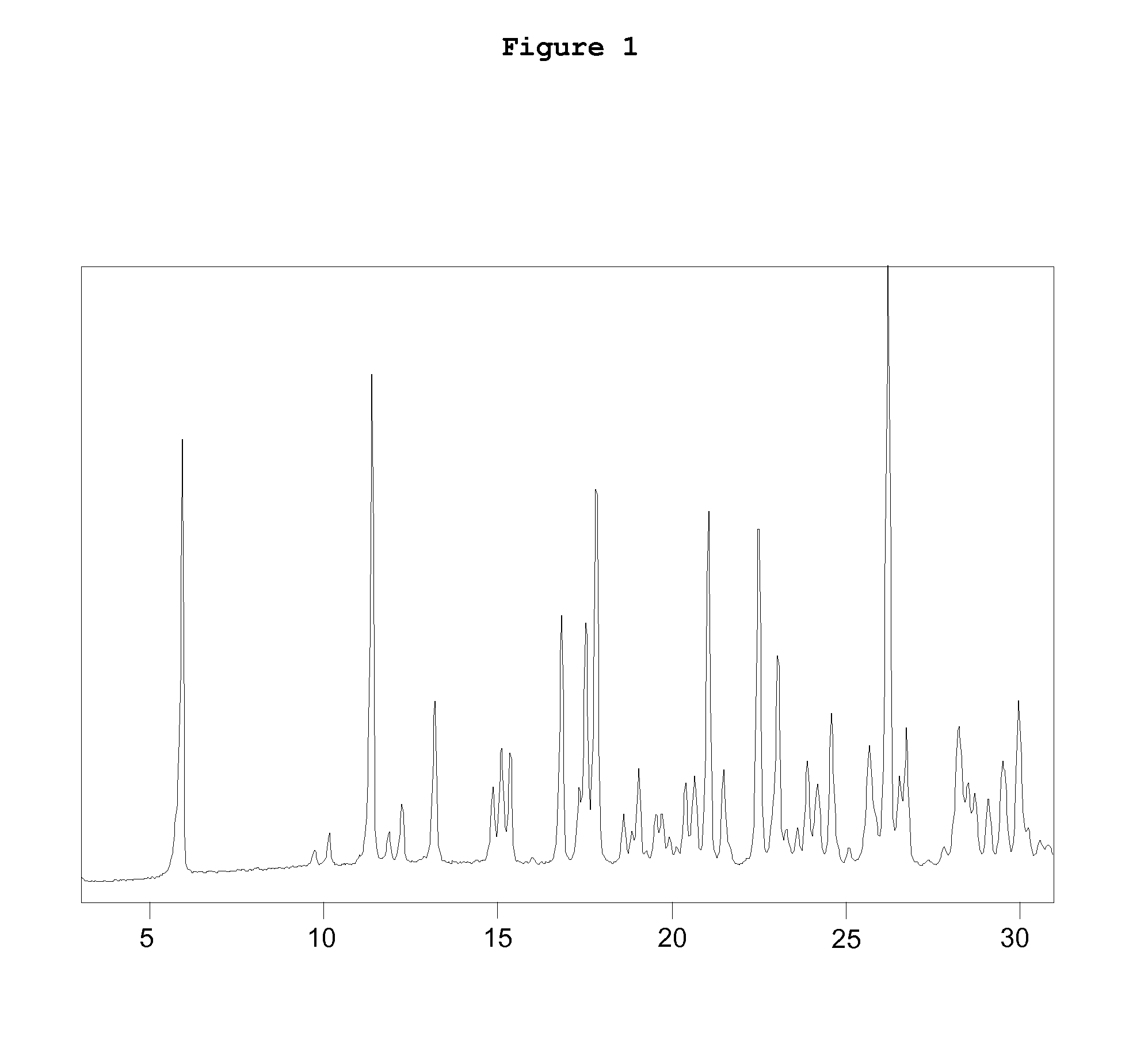
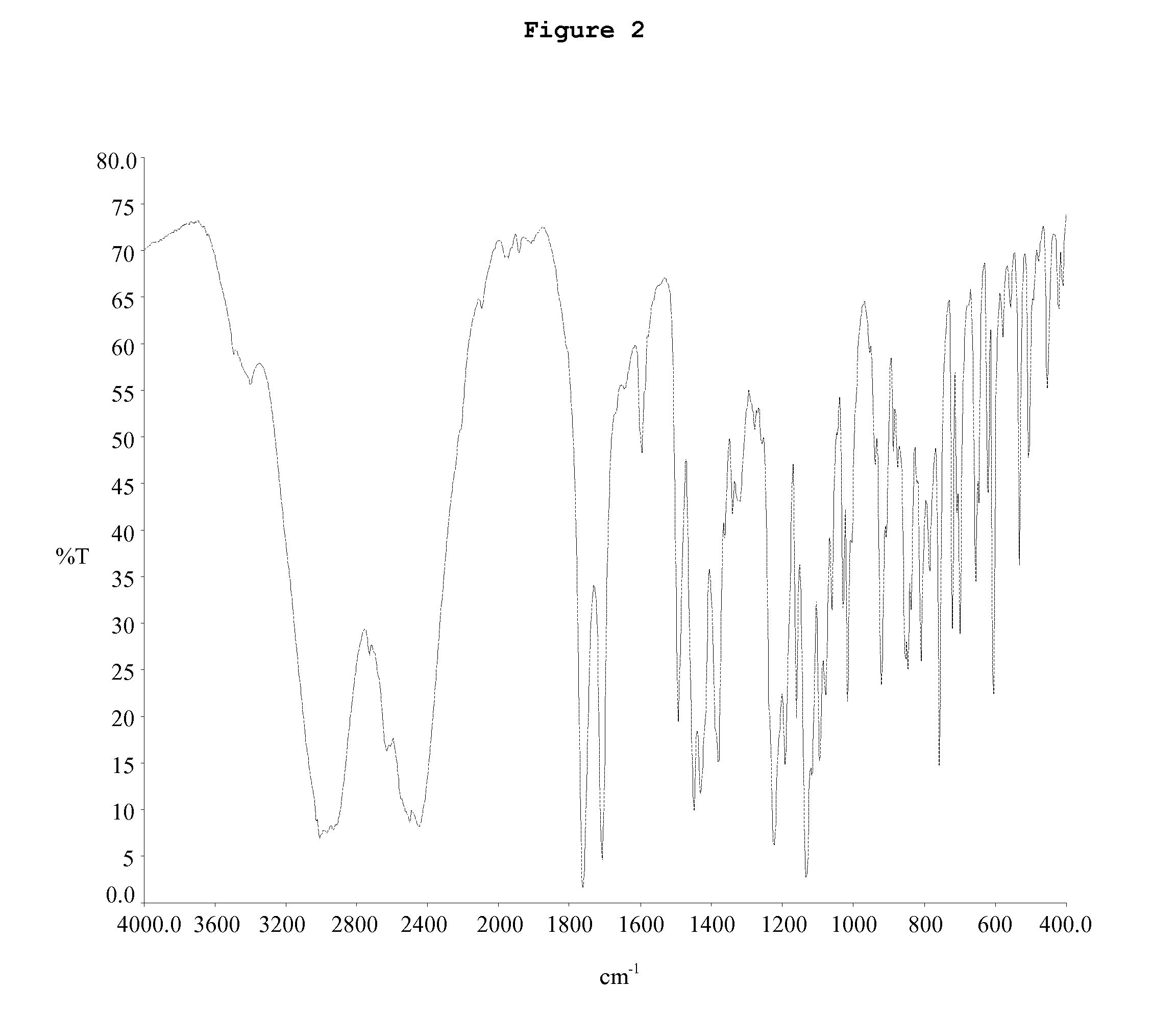
Patents
Literature
45 results about "Levocetirizine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
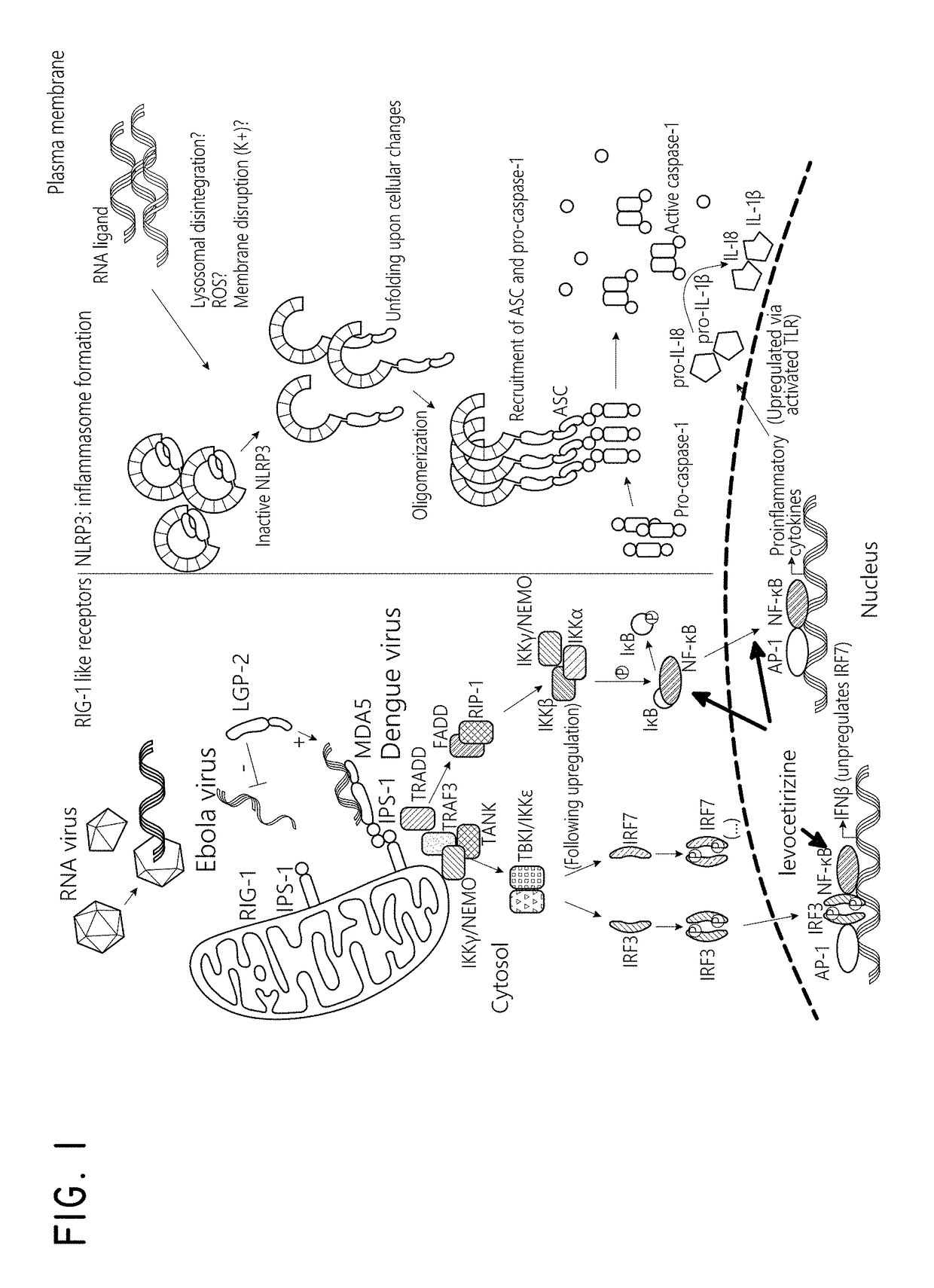
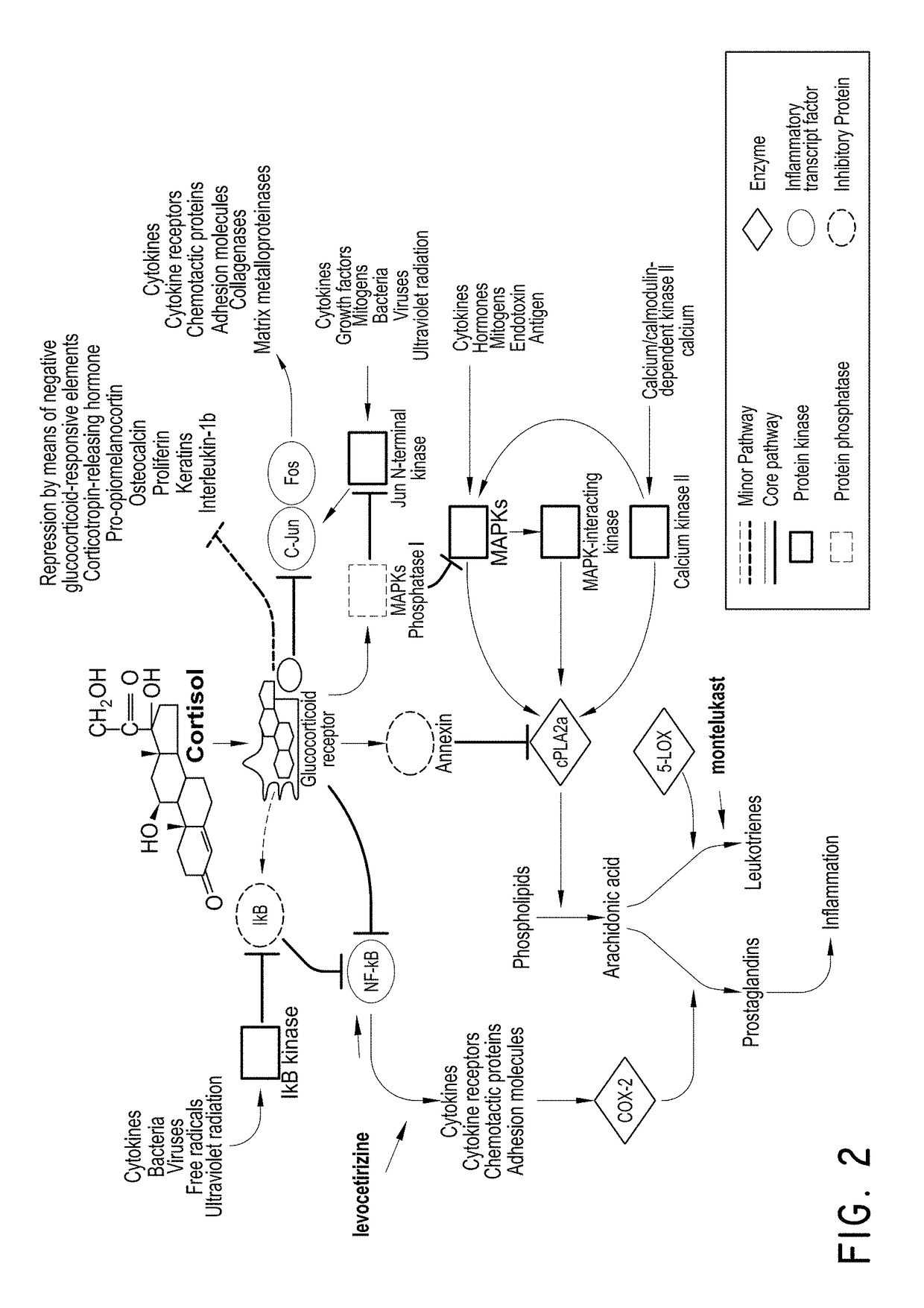
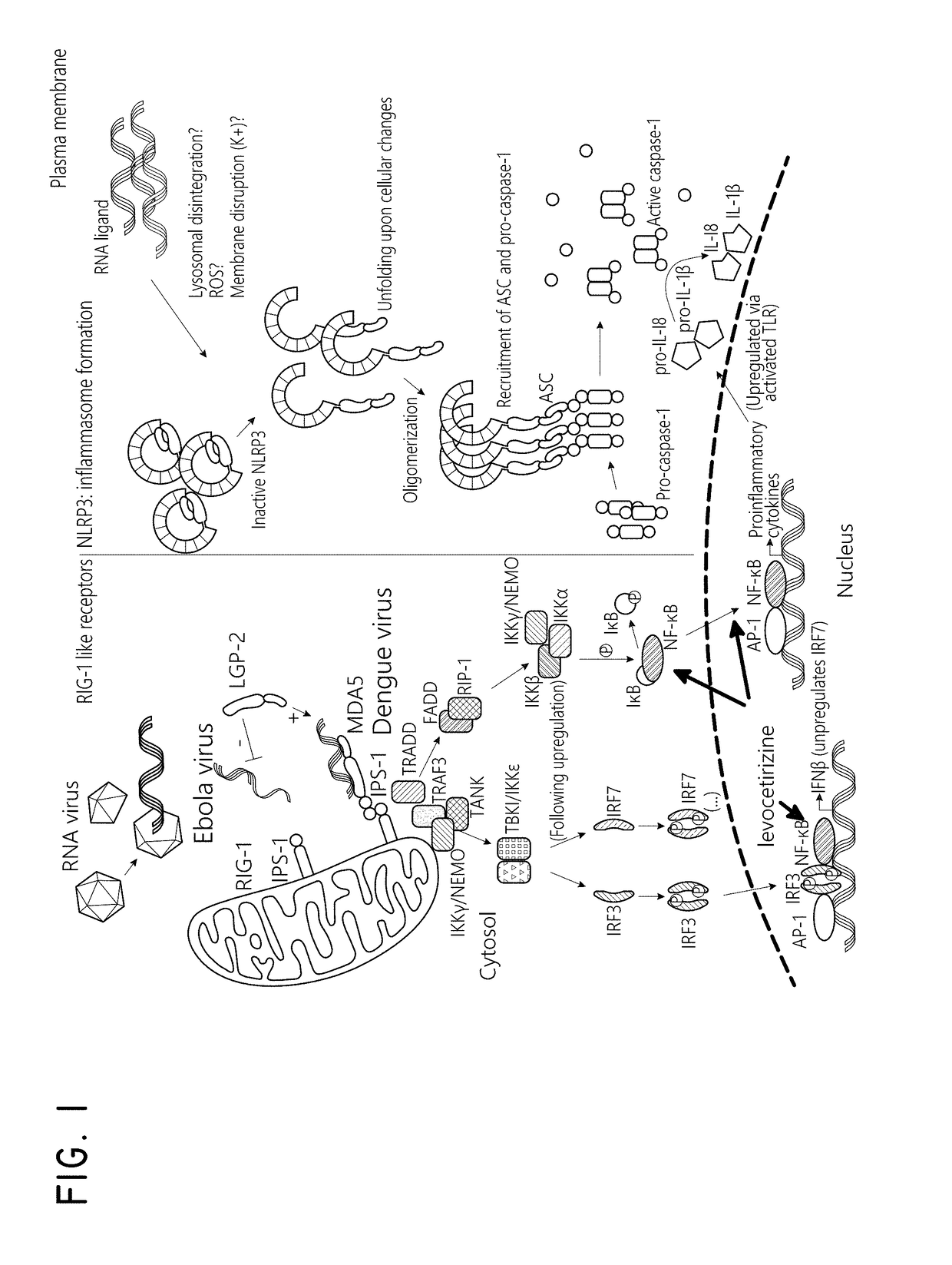
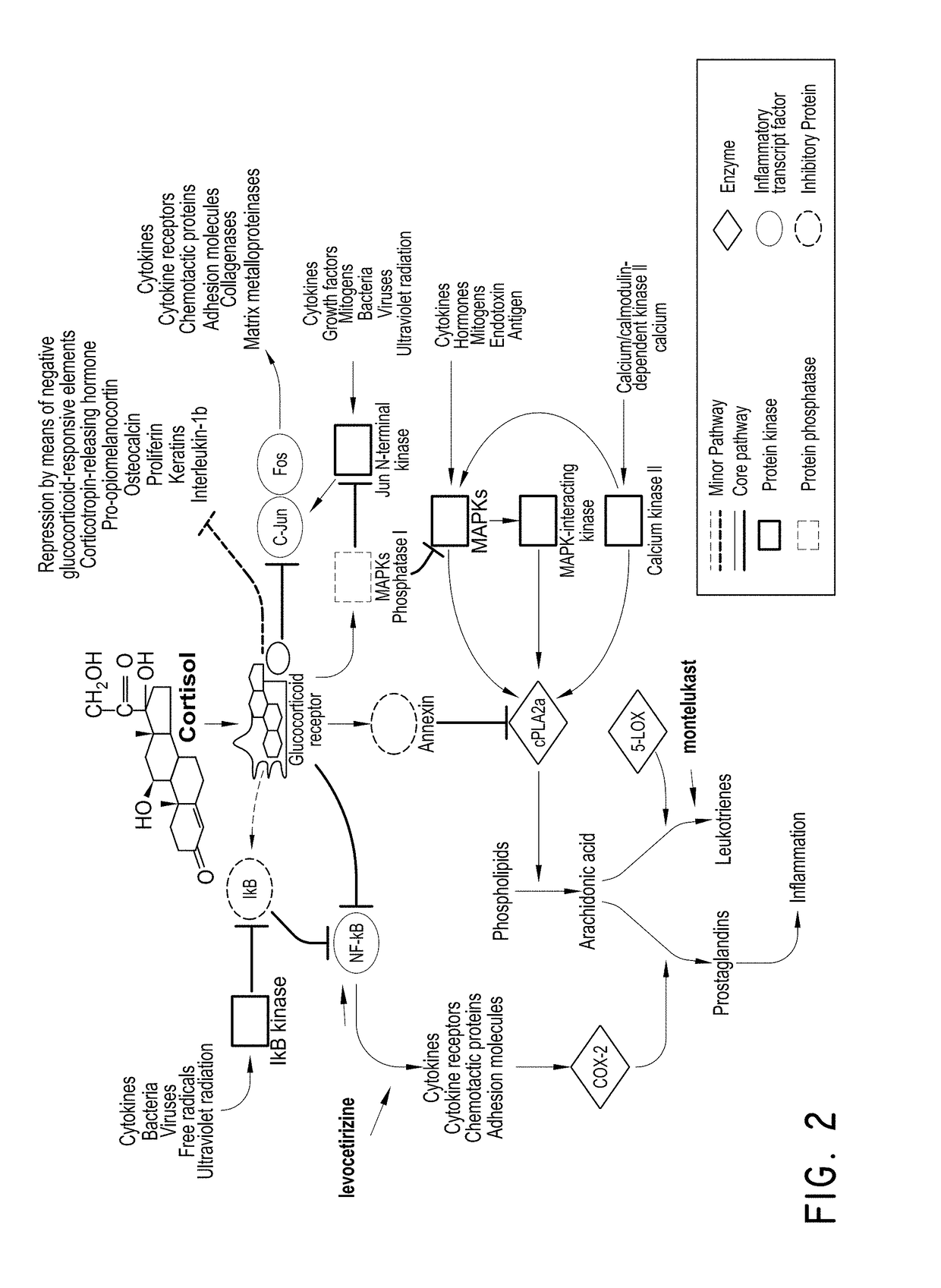
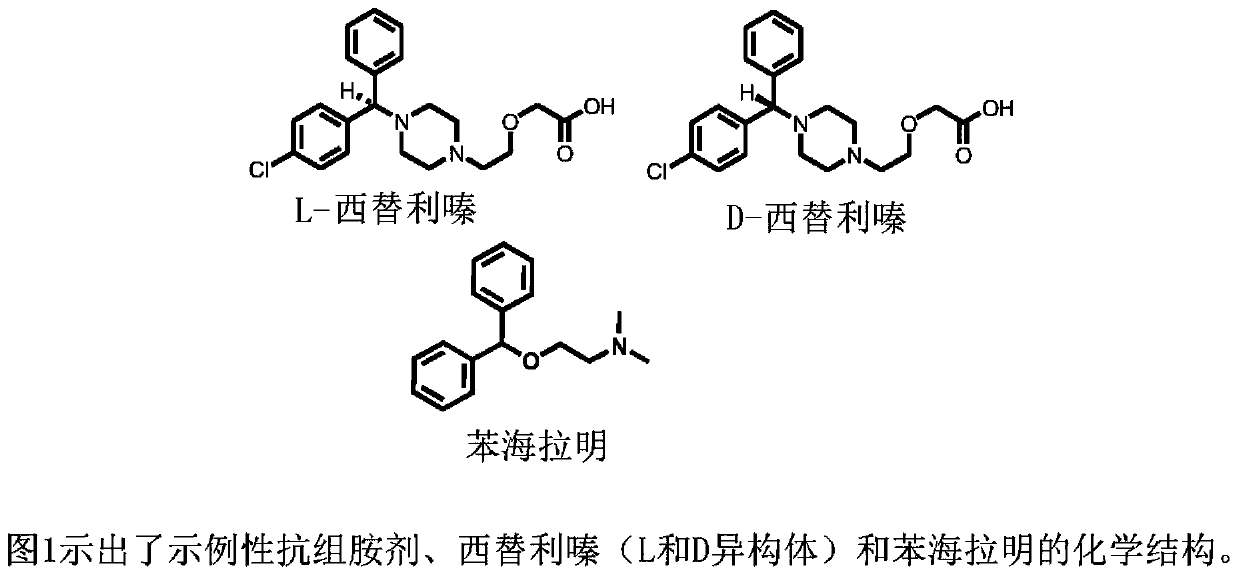
Levocetirizine is an antihistamine used to relieve allergy symptoms such as watery eyes, runny nose, itching eyes/nose, and sneezing. It is also used to relieve itching and hives.
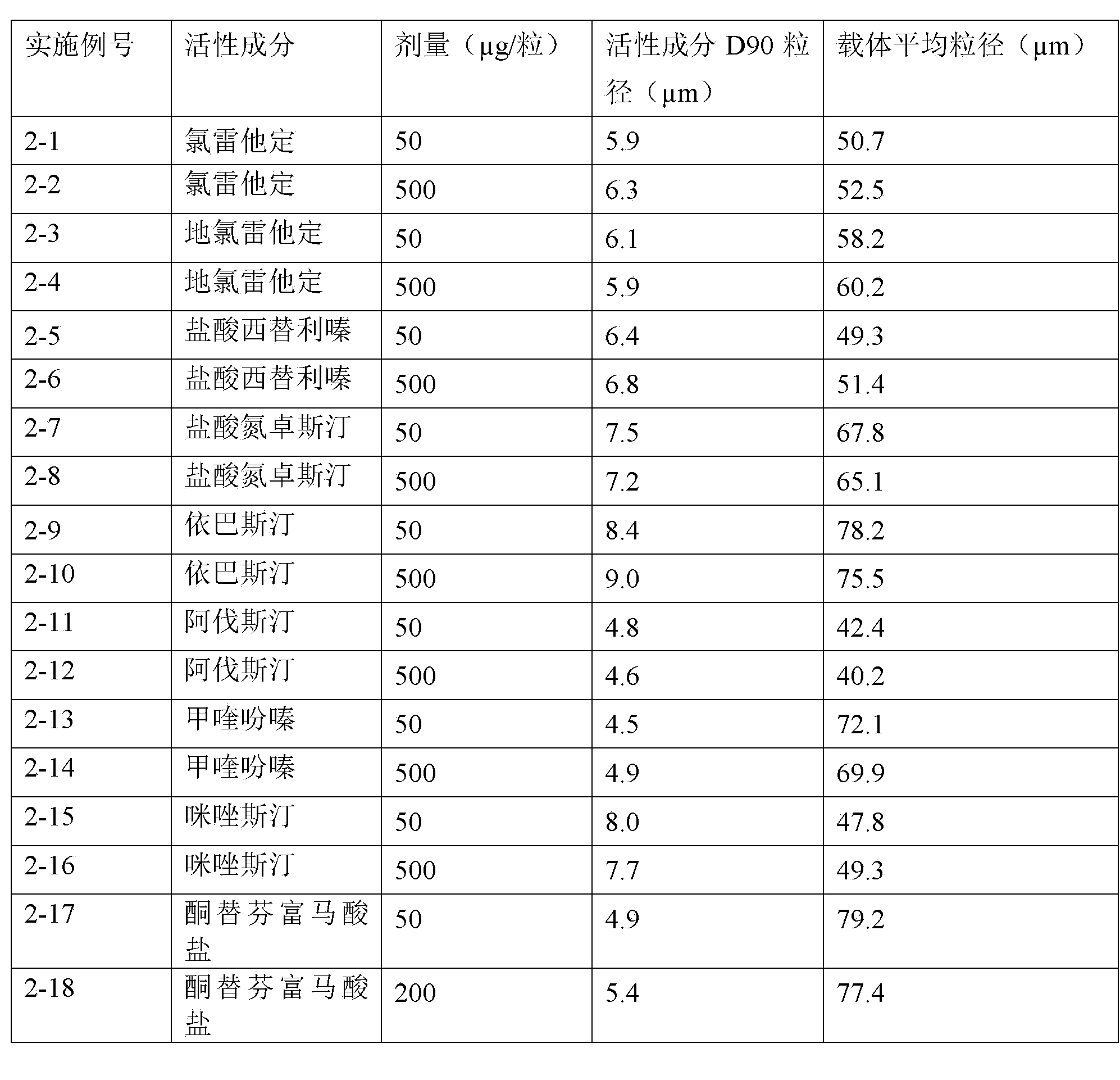
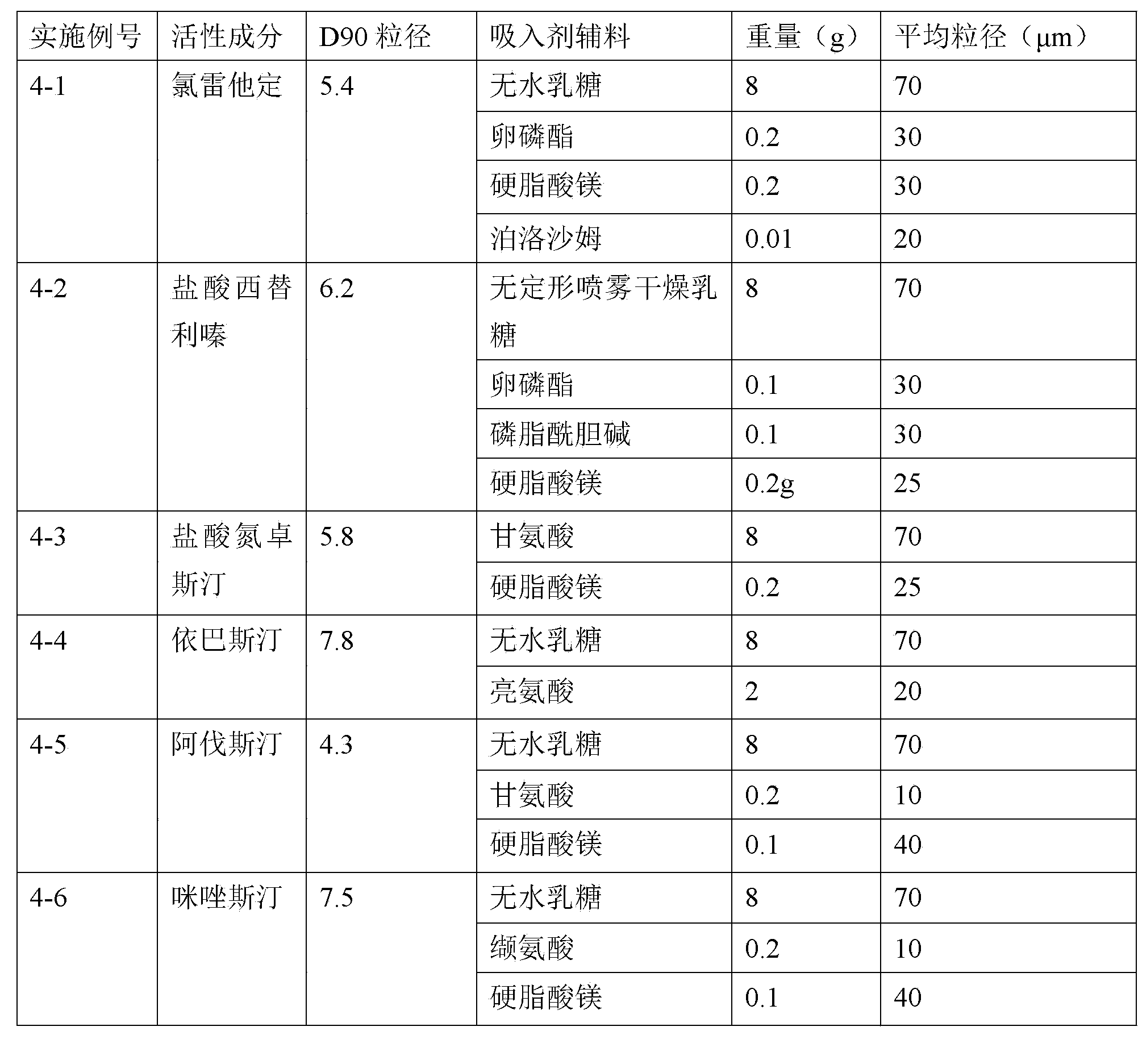
Taste masking pharmaceutical composition containing levocetirizine
InactiveUS20060083786A1Increase surface areaOrganic active ingredientsPill deliveryAdditive ingredientLevocetirizine hydrochloride
A solid oral dosage composition is provided comprising a prophilactically or therapeutically effective amount of an active pharmaceutical ingredient comprising levocetirizine or a pharmaceutically acceptable salt thereof, the solid oral dosage composition having a coating thereon capable of providing taste masking of the levocetirizine or pharmaceutically acceptable salt thereof.
Owner:GLENMARK PHARMACEUTICALS LIMITED
Levocetirizine and montelukast in the treatment of inflammation mediated conditions
The embodiments described herein include methods and formulations for treating viruses and diseases that are exacerbated by inflammatory responses in the body. The methods and formulations include, but are not limited to, methods and formulations for delivering effective concentrations of levocetirizine and montelukast to a patient in need. The methods and formulations can comprise conventional and / or modified-release elements, providing for drug delivery to the patient.
Owner:IRR INC
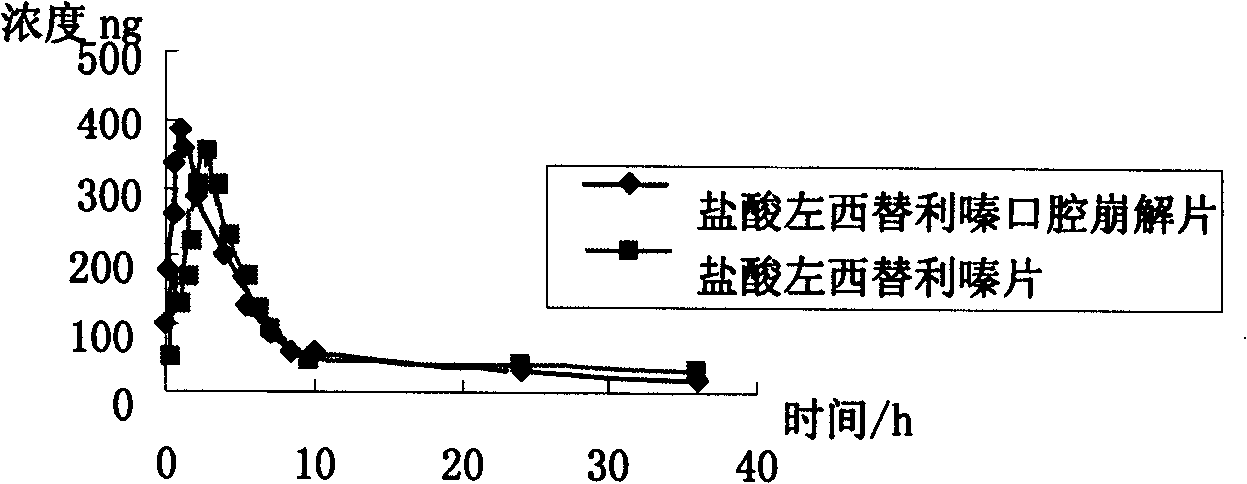
Levo-cetirizine hydrochloride orally disintegrating tablets and preparation method thereof
InactiveCN101310711AFast disintegrationFast absorptionOrganic active ingredientsPill deliveryCetirizine HydrochlorideTreatment effect
The invention relates to a hydrochloric acid levocetirizine orally disintegrating tablet and a preparation method thereof. The hydrochloric acid levocetirizine orally disintegrating tablet is obtained by directly pressing principal medicine and accessories. The tablet of the invention can be dissolved fast in a mouth cavity and has no grit feeling and high biological availability, which takes effect fast and can have curative effect faster.
Owner:海南高升医药科技开发股份有限公司
Resolution method of levocetirizine chiral intermediates
ActiveCN102351810AEasy to operateImprove efficiencyOptically-active compound separationBulk chemical productionSpecific rotationSolvent
A resolution method of a levocetirizine chiral intermediates belongs to the technical field of fine organic chemical engineering. The method comprises the following specific steps: adding racemic 1-[(4-chlorophenyl)benzyl]piperazine and chiral ionic liquid in a reaction container to react at 10-100 DEG C for 1-10 hours, extracting with toluene for layering, adjusting the pH value of the aqueous phase with 10wt% of sodium hydroxide solution to 11-12, extracting with toluene again, and concentrating to crystallize after the post-treatment, thus obtaining the product. The chiral ionic liquid is used as the solvent and resolving agent, the technology is easy to operate, the efficiency is high, the yield of three wastes is low, the yield of the product is more than 34%, the melting range of the product is narrow, the melting point is 94-96 DEG C, the specific rotation [alpha] D is not more than -21.5 degrees (C1, toluene), the optical purity is not less than 99.0%, and the content is not less than 99.0%. The post-treatment is convenient, the ionic liquid can be used repeatedly and the resolution method is an economical, practical, green and environmentally friendly technology.
Owner:浙江永合新材料科技有限公司
Levocetirizine and montelukast in the treatment of inflammation mediated conditions
The embodiments described herein include methods and formulations for treating viruses and diseases that are exacerbated by inflammatory responses in the body. The methods and formulations include, but are not limited to, methods and formulations for delivering effective concentrations of levocetirizine and montelukast to a patient in need. The methods and formulations can comprise conventional and / or modified-release elements, providing for drug delivery to the patient.
Owner:IRR INC
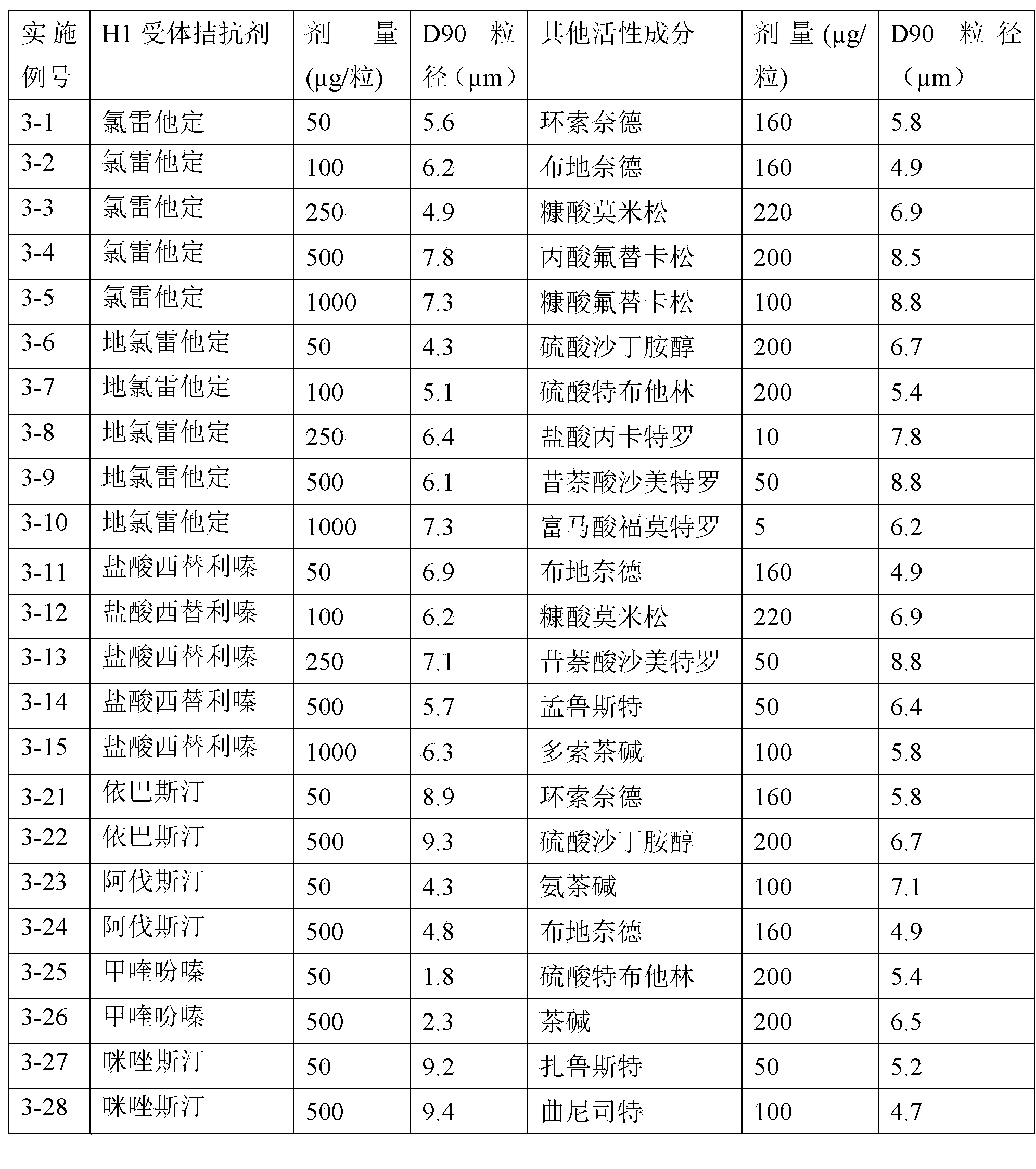
H1-receptor-antagonist-containing inhalation preparation
The invention relates to an H1-receptor-antagonist-containing inhalation preparation which contains an H1 receptor antagonist and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
Oral anti-allergy compound pharmaceutical composition
The invention relates to an oral anti-allergy compound pharmaceutical composition, which contains levocetirizine and mast cell membrane stabilizer. The dosage forms of the composition comprise tablet, capsule, suspending agent, dry suspending agent, oral solution, granule, and the like. The oral medicament is applied to the treatment of allergic diseases such as urticaria, eczema, dermatitis, allergic rhinitis, skin pruritus, and the like.
Owner:CHONGQING HUAPONT PHARMA
External-use formulation of levocetirizine hydrochloride
InactiveCN1813743AEffectively antagonizes sensitizationIdeal for delayed hypersensitivity reactionsOrganic active ingredientsAerosol deliveryDrugLevocetirizine
The present invention relates to a medicine levocetirizine hydrochloride preparation for external application. Said preparation is made up by using levocetirizine hydrochloride as active effective component and medicinal auxiliary material, and can be used for effectively curing various dermal allergic diseases.
Owner:LUNAN PHARMA GROUP CORPORATION
New procedure for preparation of levocetirizine and its intermediates
The present invention describes a novel process for the preparation of levocetirizine and pharmaceutically acceptable acid addition salts thereof using diglycolic acid or derivatives thereof and new intermediates used in that process.
Owner:克卡制药新梅斯托股份公司
Processes for the synthesis of levocetirizine and intermediates for use therein
InactiveCN102046612AOxygen-containing compound preparationOrganic compound preparationPiperazinePhotochemistry
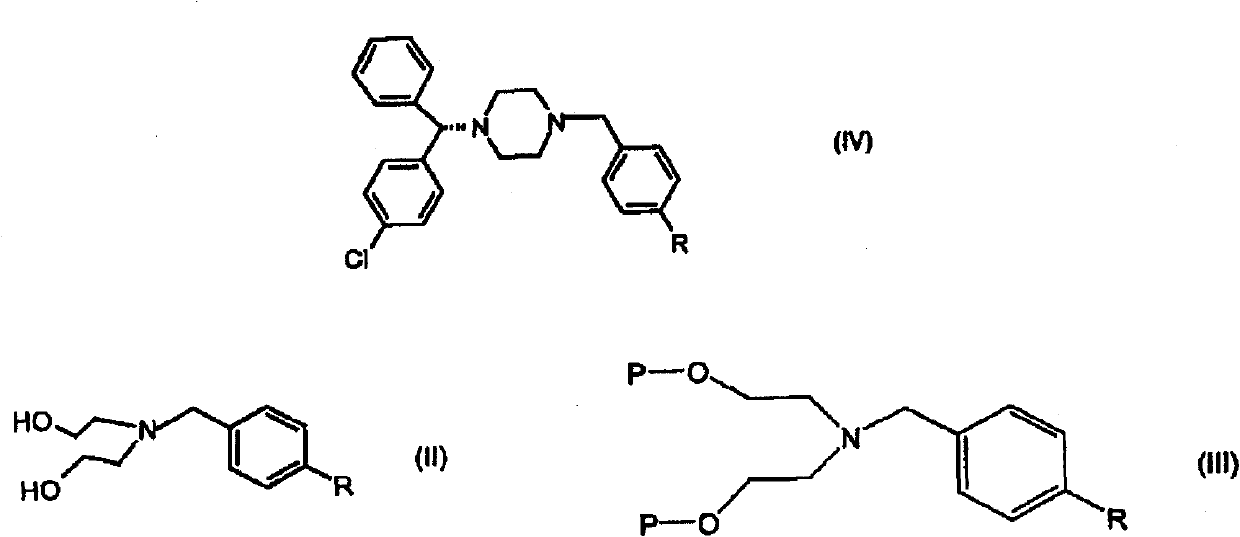
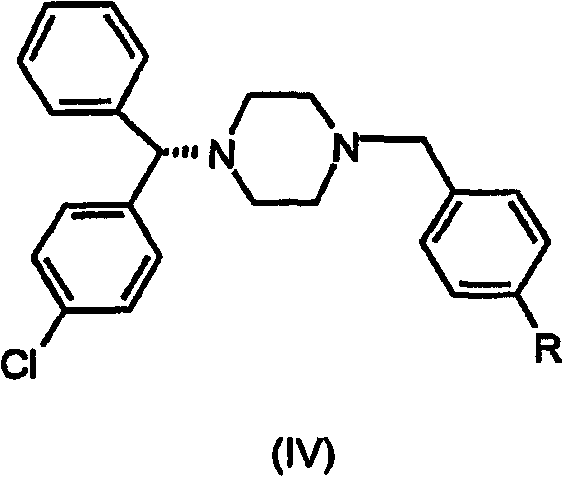
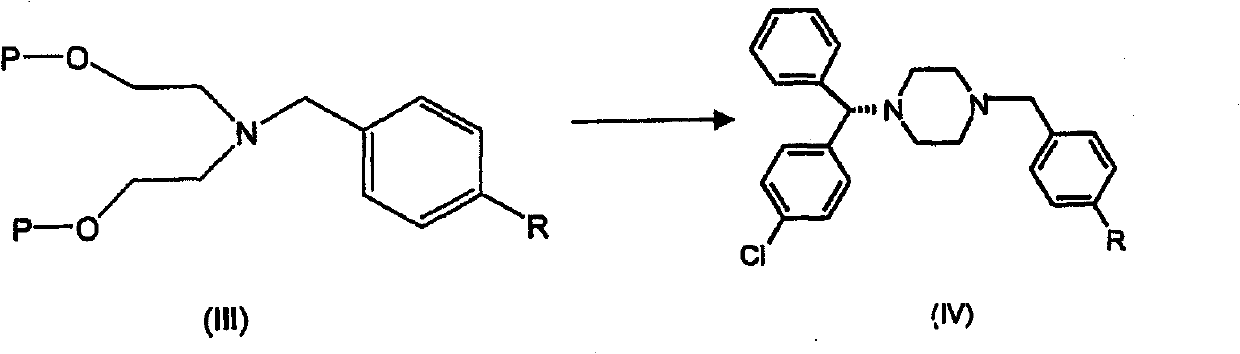
The present invention provides a compound of formula (IV) wherein R is Cl, Br, NO2, OH or OR', and R' is alkyl, and its use in the synthesis of levocetirizine, including its use in the synthesis of (-)-1-[(4-chlorophenyl)-phenyl methyl] piperazine, an intermediate useful in the synthesis of levocetirizine. The present invention also provides compounds (II) and (III) which are useful in the synthesis of compound (IV).
Owner:CIPLA LTD
External preparation of Levocetirizine hydrochloric acid
ActiveCN1957942AAntagonism of sensitizationPromote transdermal absorptionOrganic active ingredientsAerosol deliverySkin sensitizationWhole body
An exterior-applied levocetirizine hydrochloride for preventing and treating the anaphylaxia and inflammation without untoward effect is disclosed.
Owner:LUNAN PHARMA GROUP CORPORATION
Levocetirizine preparation method and levocetirizine dihydrochloride preparation method
ActiveCN103351361AImprove conversion rateHigh selectivityOrganic chemistryHydroxyzineCatalytic oxidation
The invention relates to a levocetirizine preparation method and a levocetirizine dihydrochloride preparation method. Levocetirizine is prepared through a synthesis route shown in the specification, and levocetirizine dihydrochloride is prepared through hydrochlorinating the prepared levocetirizine to form a salt, and recrystallizing the salt. The preparation method which adopts cheap and easily available Pd-M / C to realize the catalytic oxidation of L-hydroxyzine as a catalyst in order to prepare levocetirizine realizes the high conversion rate of the substrate L-hydroxyzine and the high selectivity and high optical purity of the target product levocetirizine, and is an environmentally-friendly technology.
Owner:HAISO TECH
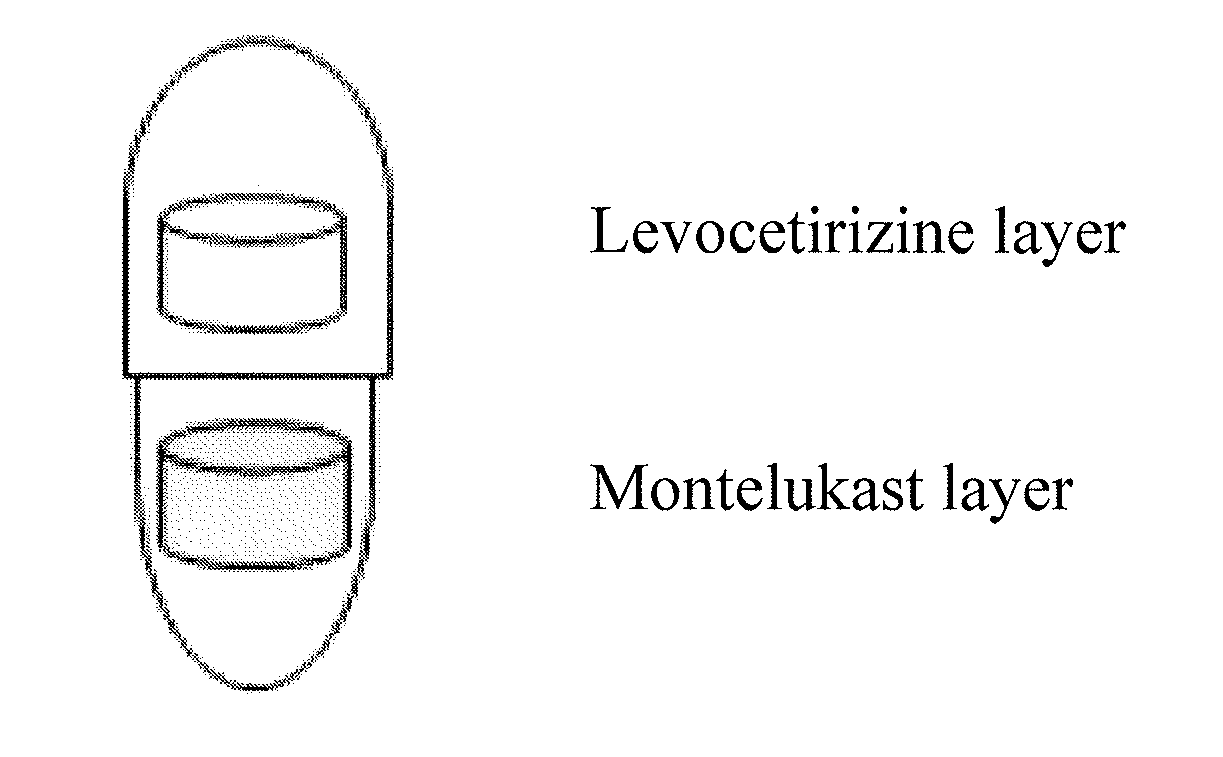
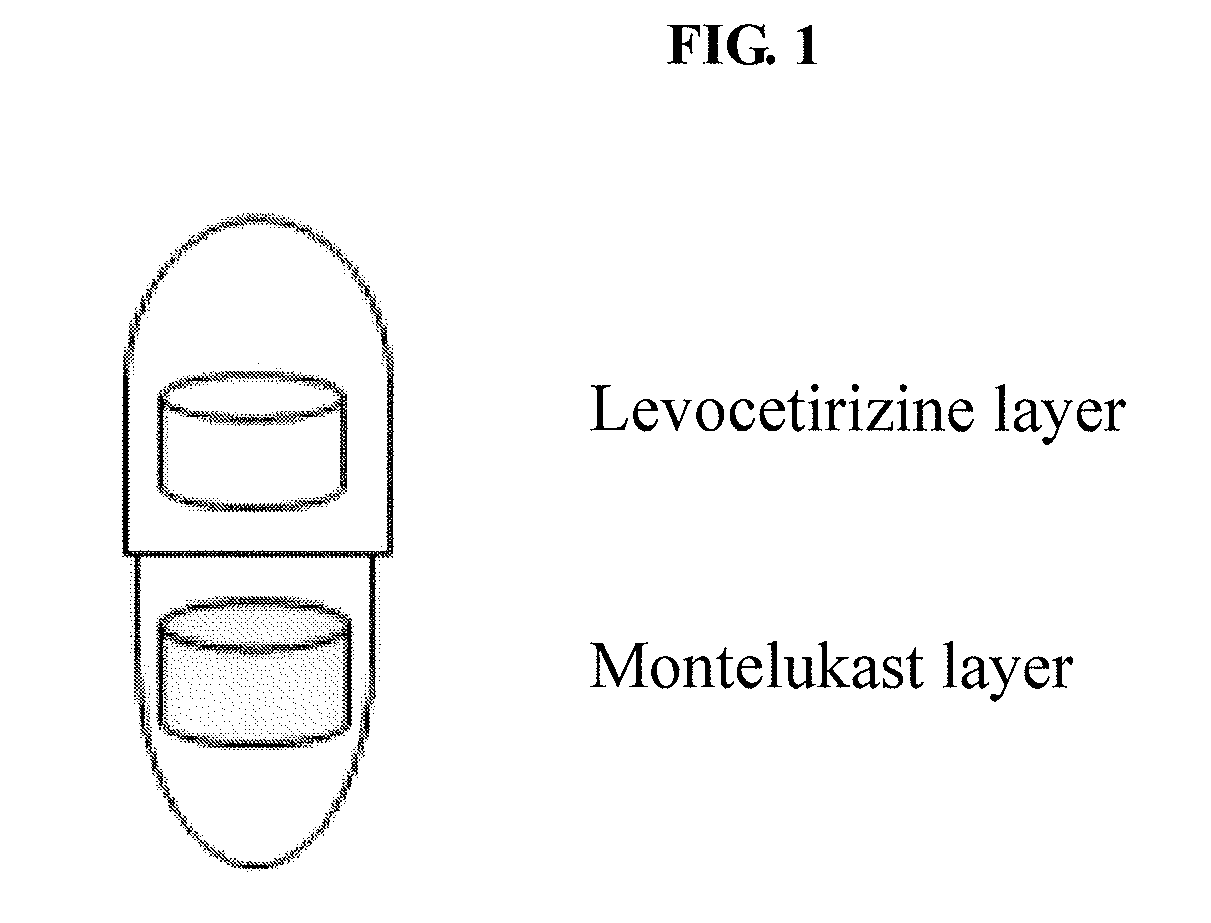
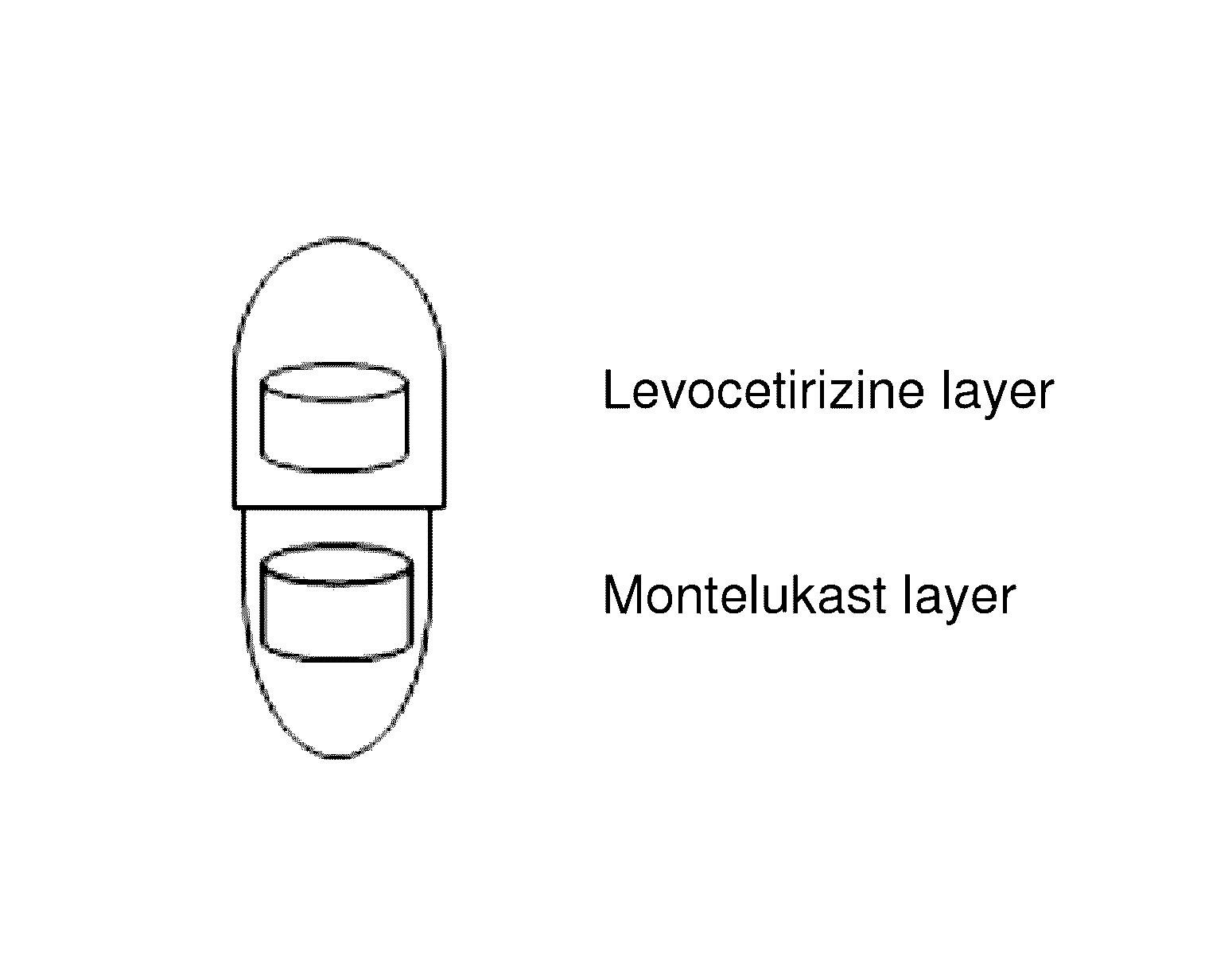
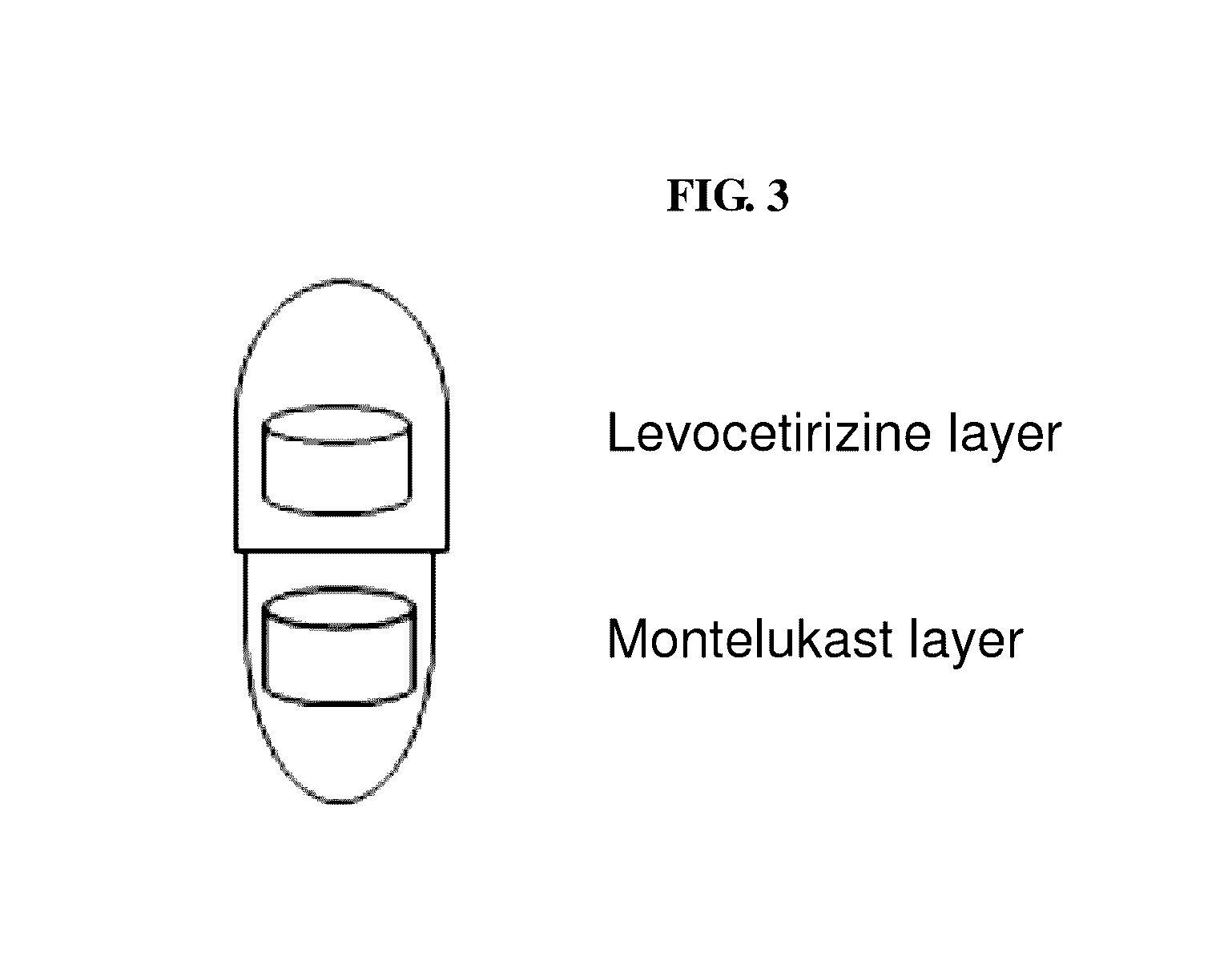
Capsule formulation comprising montelukast and levocetirizine
Disclosed is a capsule formulation for preventing or treating allergic rhinitis and asthma, which comprises two separate layers of: (1) a Montelukast layer comprising montelukast or a pharmaceutically acceptable salt thereof; and (2) a Levocetirizine layer comprising levocetirizine or a pharmaceutically acceptable salt thereof; and a method for the preparation thereof. The capsule formulation according to the present invention can completely separate two active ingredients, thereby minimizing the reactivity between them and improving product stability against aging effects, and thus, can optimize the therapeutic effects.
Owner:HANMI PHARMA
Oral compound levocetirizine pseudoephedrine formulation and its preparation
The present invention belongs to the field of pharmaceutical technology, and discloses one kind of orally taken compound levo-cetirizine pseudoephedrine preparation. The preparation has activity similar to that of compound cetirizine pseudoephedrine preparation and is single optical isomer with half reduced dosage and thus long effect and reduced side effect. The preparation may be taken by adult and children. The preparation process is also provided.
Owner:北京曙光药业有限责任公司
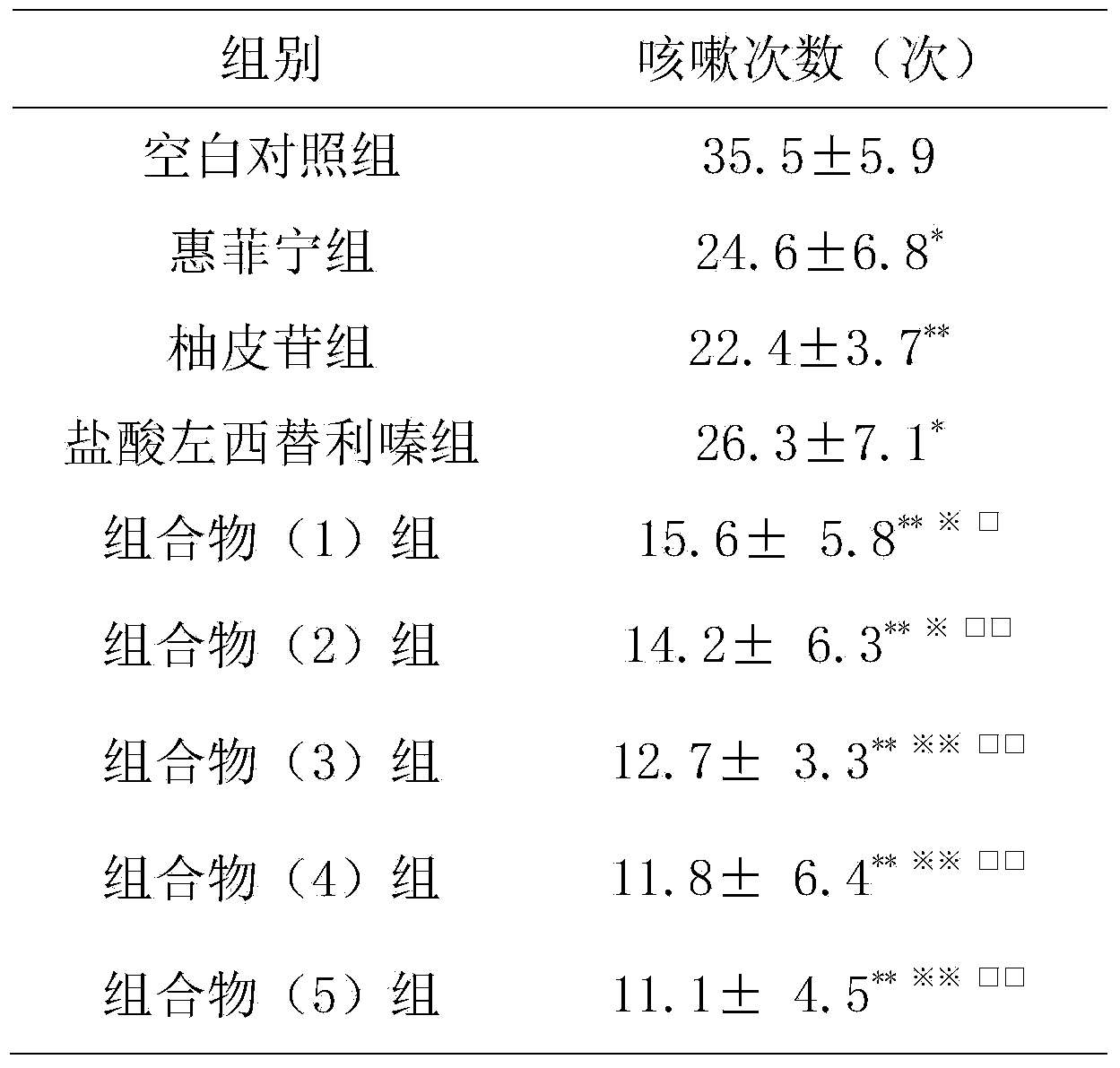
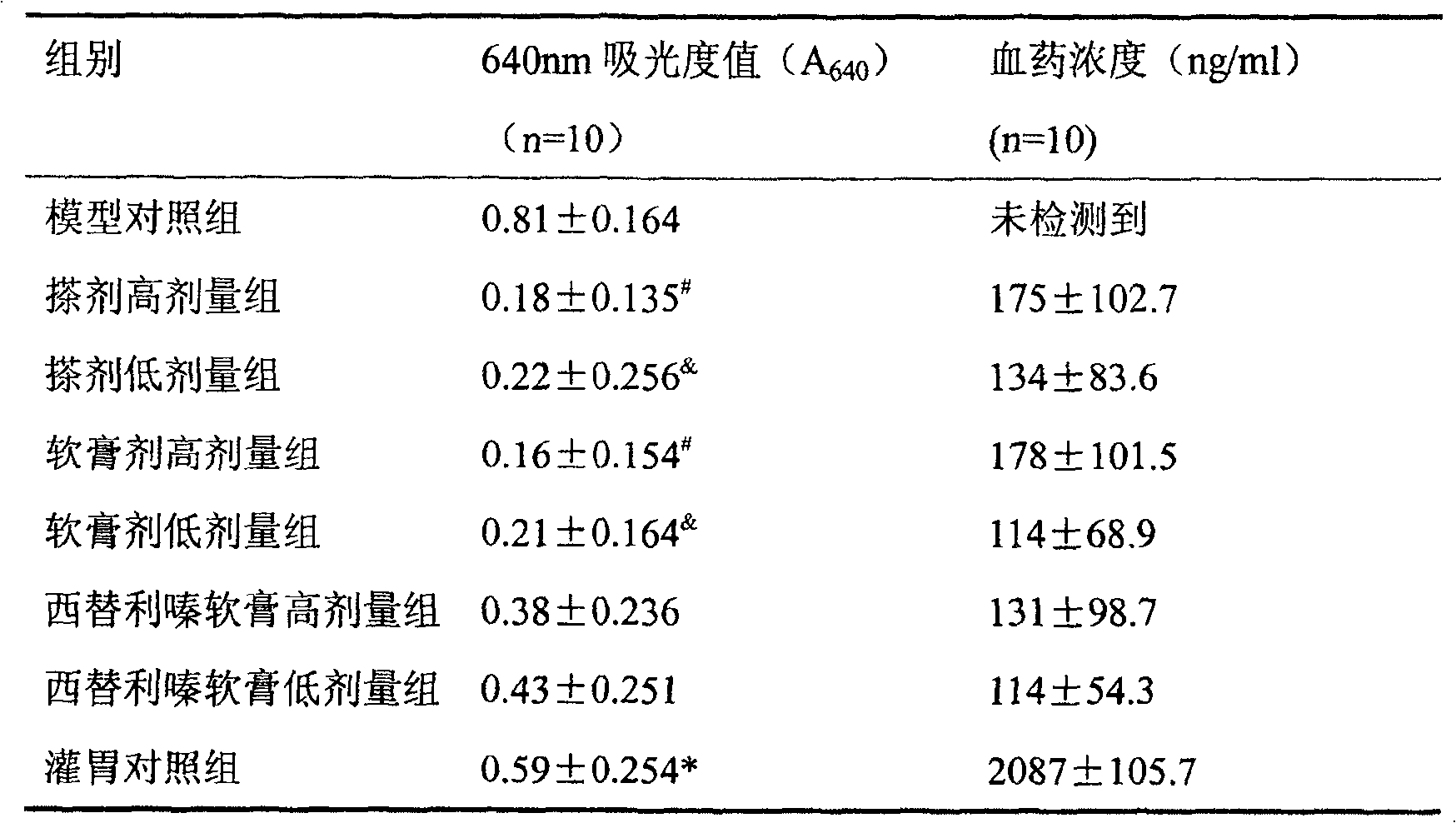
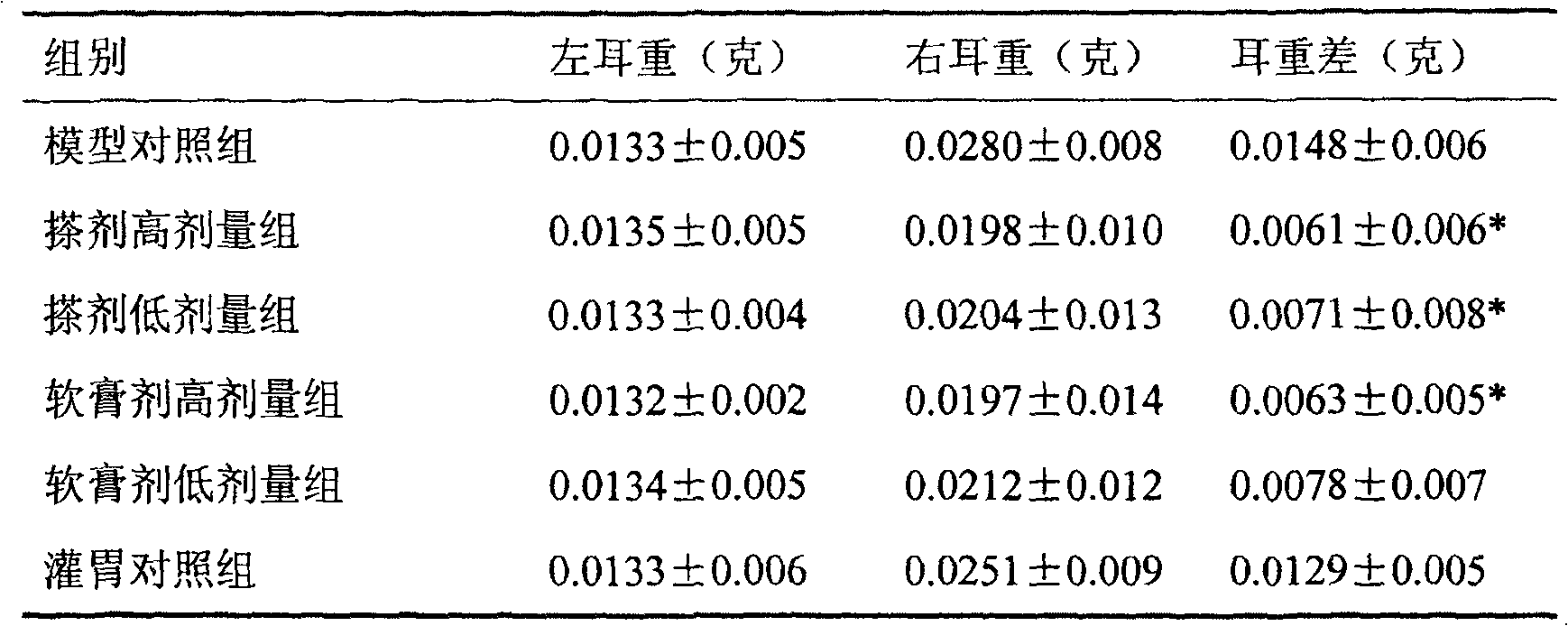
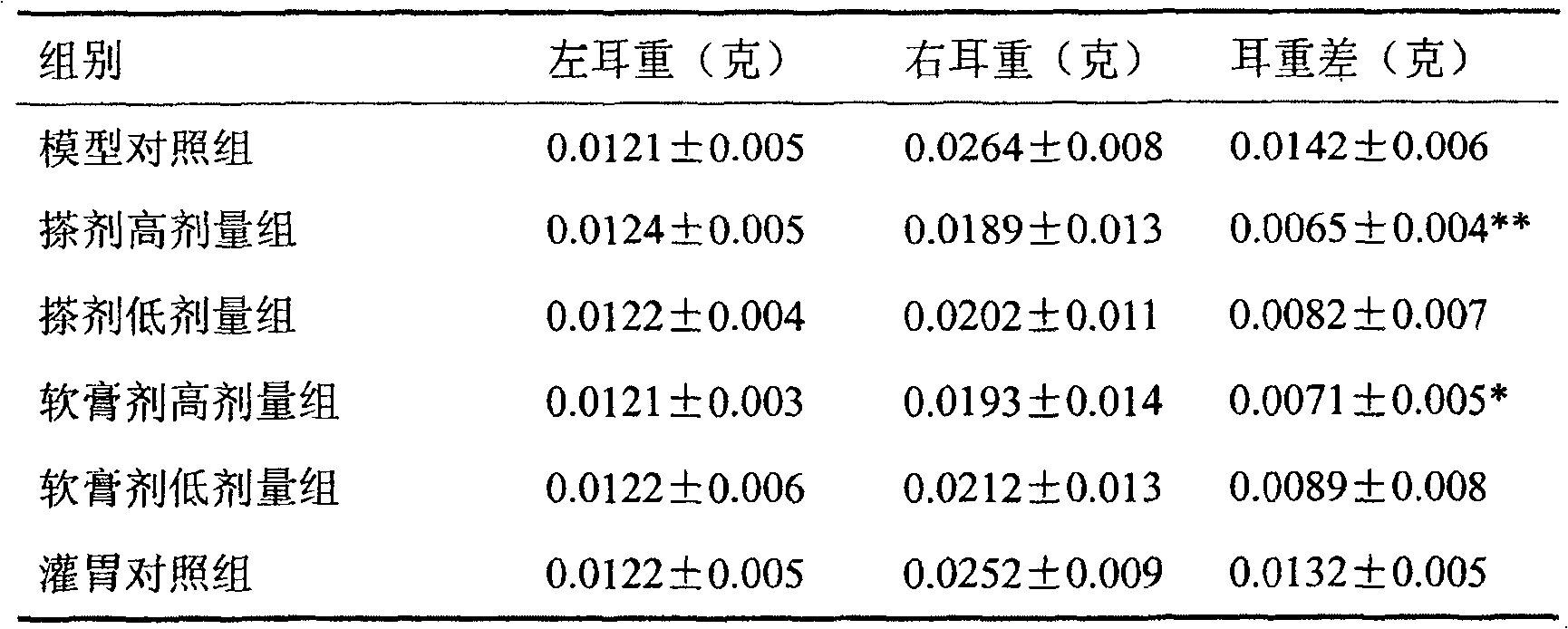
Naringin and levocetirizine hydrochloride pharmaceutical composition and preparation thereof
The invention relates to naringin composition and a preparation thereof. The pharmaceutical composition is characterized by comprising naringin and levocetirizine hydrochloride, wherein preferably, the naringin is in a range of 27.5-275 mg, and the levocetirizine hydrochloride is in a range of 1.25-12.5 mg; the preferable mass ratio of the naringin to the levocetirizine hydrochloride is 20:1. Tests prove that the pharmaceutical composition has good curative effects on coughs and excessive phlegm caused by various reasons and the cough variant asthma, and the efficacy of the composition is obviously superior to the efficacy of the independently-used naringin or the independently-used levocetirizine hydrochloride.
Owner:SUN YAT SEN UNIV
New composition for treating seasonal and perennial allergic rhinitis
The present invention relates to one new kind of medicine composition containing levocetirizine (or its pharmaceutically acceptable salt) and pseudoepherine (or its pharmaceutically acceptable salt). The medicine composition is pressed into double layer including the first layer containing levocetirizine (or its pharmaceutically acceptable salt) in the amount for resisting allergy effectively and the second layer containing pseudoepherine (or its pharmaceutically acceptable salt) in the amount as effective decongestant for nose. The medicine composition is used in treating seasonal and perennial allergic rhinitis.
Owner:HANGZHOU MINSHENG PHARM CO LTD
External preparation of Levocetirizine hydrochloric acid
ActiveCN100589805CAntagonism of sensitizationPromote transdermal absorptionOrganic active ingredientsAerosol deliverySkin sensitizationWhole body
An exterior-applied levocetirizine hydrochloride for preventing and treating the anaphylaxia and inflammation without untoward effect is disclosed.
Owner:LUNAN PHARMA GROUP CORPORATION
Oral complex composition comprising pseudoephedrine and levocetirizine
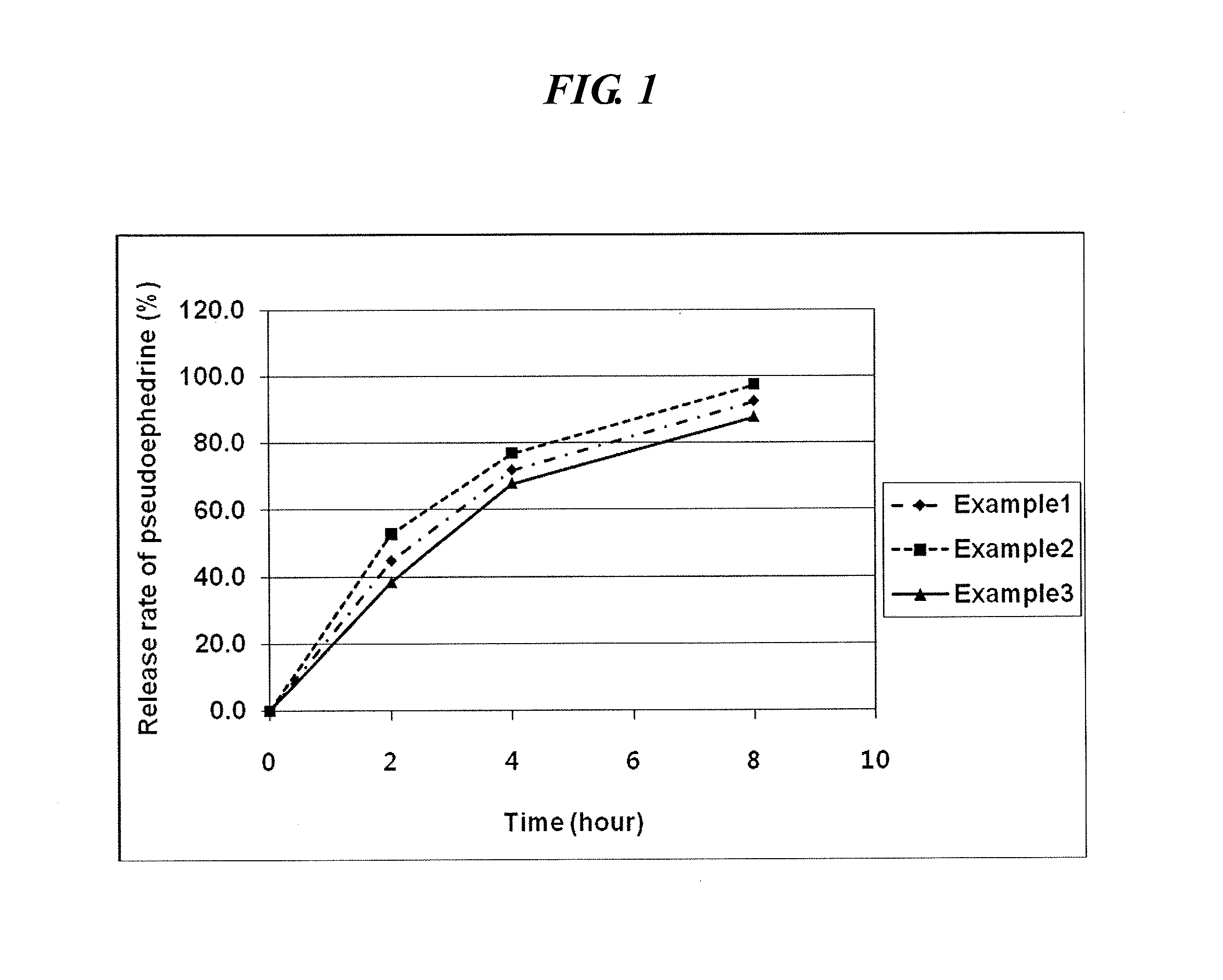
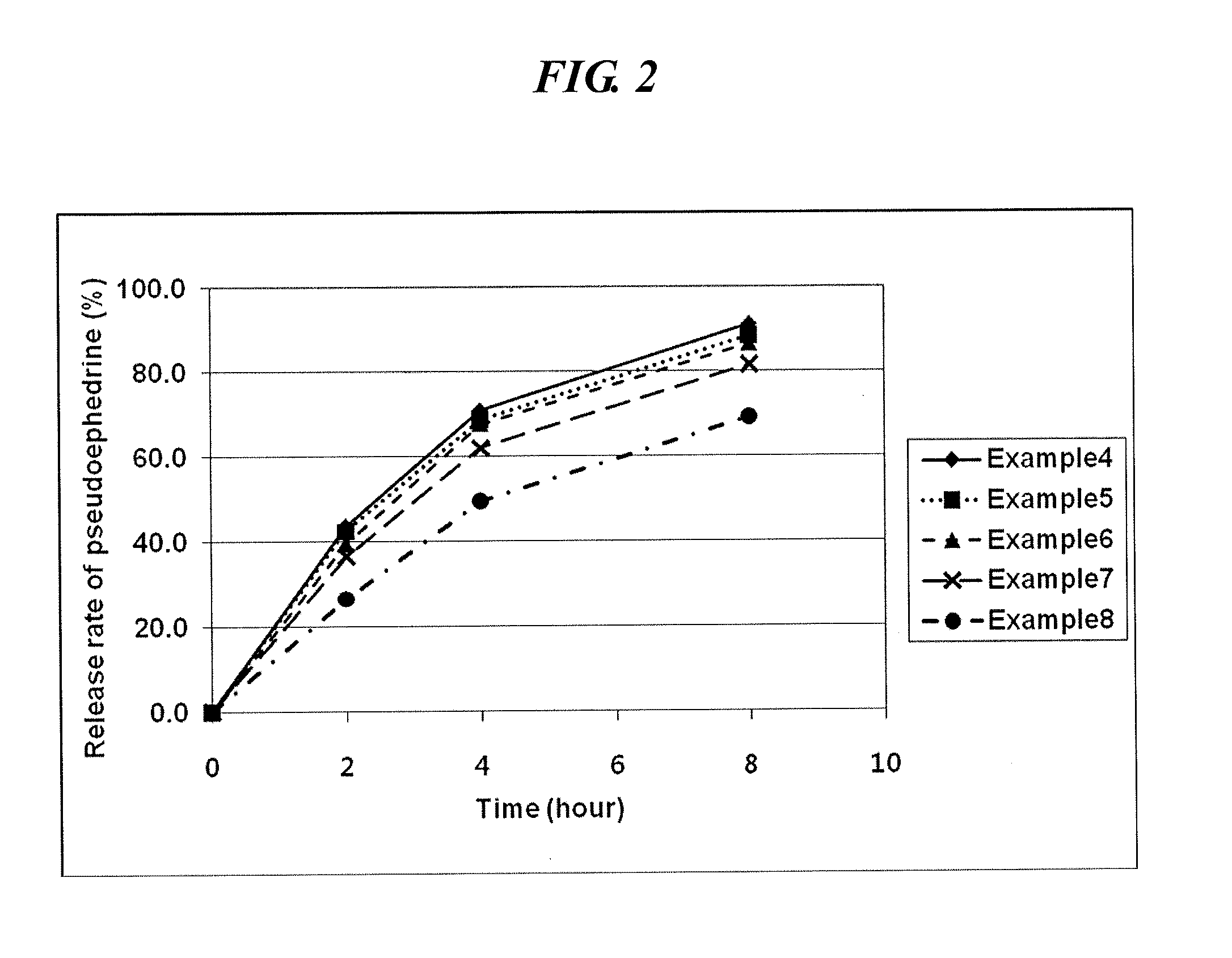
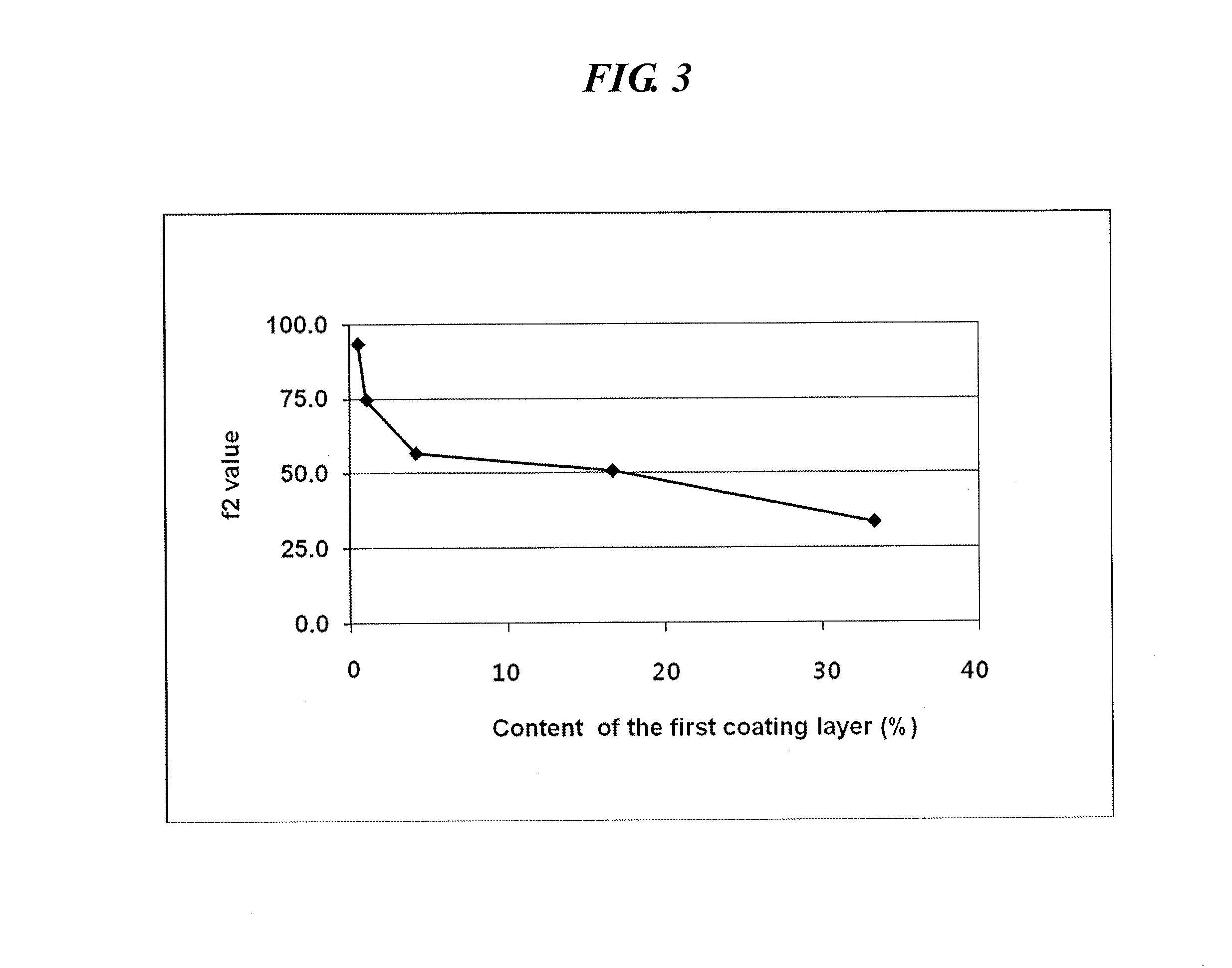
InactiveUS20120301548A1Increase release rateOrganic active ingredientsPill deliveryPseudoephedrinePolyethylene glycol
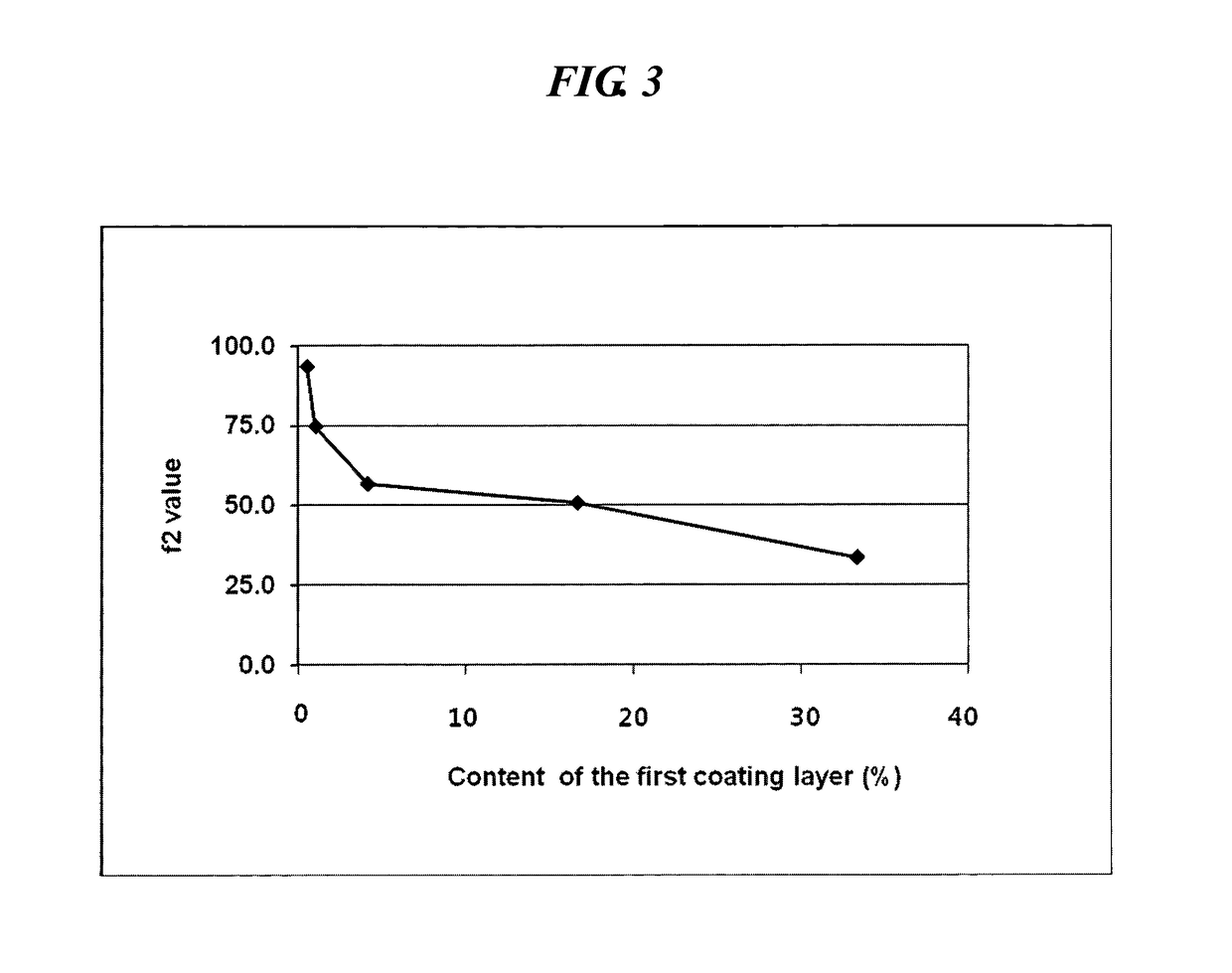
An oral complex composition which comprises (i) a core comprising a swellable hydrogel-forming agent and pseudoephedrine, or a pharmaceutically acceptable salt thereof; (ii) a first coating layer encasing the core which comprises a water-soluble substance; and (iii) a second coating layer deposited on the first coating layer which comprises levocetirizine or a pharmaceutically acceptable salt thereof together with polyvinylalcohol, povidone, polyvinylalcohol-polyethyleneglycol graft copolymer or a mixture thereof, has an improved levocetirizine releasing rate and does not show a delayed release behavior even after a long storage period. Accordingly, the inventive oral complex composition is useful for treating perennial or seasonal allergic diseases including nasal obstruction, sneezing, and rhinorrhea.
Owner:HANMI SCI CO LTD
Semi-solid Anti-histamine compositions and methods of making and using thereof
PendingCN111587106AHeavy metal active ingredientsHydroxy compound active ingredientsDesloratadineLevocetirizine
The disclosure provides a semi-solid composition, comprising a gelling component in a sufficient amount to provide a cohesive gelled product, an anti-histamine composition, comprising an antihistamine, and a complexing agent, wherein the complexing agent is capable of interacting with the antihistamine and forming an antihistamine complex. In one embodiment, the antihistamine comprises diphenhydramine, cetirizine, levocetirizine, loratadine, desloratadine, fexofenadine, azelastine, bilastine, rupatadine, or a derivative, salt or a combination thereof.
Owner:SEATTLE GUMMY CO
Oral complex composition comprising pseudoephedrine and levocetirizine
InactiveUS9694007B2Increase release rateOrganic active ingredientsGranular deliveryDiseasePseudoephedrine
An oral complex composition which comprises (i) a core comprising a swellable hydrogel-forming agent and pseudoephedrine, or a pharmaceutically acceptable salt thereof; (ii) a first coating layer encasing the core which comprises a water-soluble substance; and (iii) a second coating layer deposited on the first coating layer which comprises levocetirizine or a pharmaceutically acceptable salt thereof together with polyvinylalcohol, povidone, polyvinylalcohol-polyethyleneglycol graft copolymer or a mixture thereof, has an improved levocetirizine releasing rate and does not show a delayed release behavior even after a long storage period. Accordingly, the inventive oral complex composition is useful for treating perennial or seasonal allergic diseases including nasal obstruction, sneezing, and rhinorrhea.
Owner:HANMI SCI CO LTD
Resolution method of levocetirizine chiral intermediates
ActiveCN102351810BEasy to operateImprove efficiencyOptically-active compound separationBulk chemical productionSpecific rotationSolvent
A resolution method of a levocetirizine chiral intermediates belongs to the technical field of fine organic chemical engineering. The method comprises the following specific steps: adding racemic 1-[(4-chlorophenyl)benzyl]piperazine and chiral ionic liquid in a reaction container to react at 10-100 DEG C for 1-10 hours, extracting with toluene for layering, adjusting the pH value of the aqueous phase with 10wt% of sodium hydroxide solution to 11-12, extracting with toluene again, and concentrating to crystallize after the post-treatment, thus obtaining the product. The chiral ionic liquid is used as the solvent and resolving agent, the technology is easy to operate, the efficiency is high, the yield of three wastes is low, the yield of the product is more than 34%, the melting range of the product is narrow, the melting point is 94-96 DEG C, the specific rotation [alpha] D is not more than -21.5 degrees (C1, toluene), the optical purity is not less than 99.0%, and the content is not less than 99.0%. The post-treatment is convenient, the ionic liquid can be used repeatedly and the resolution method is an economical, practical, green and environmentally friendly technology.
Owner:浙江永合新材料科技有限公司
Fluticasone and fluticasone ester/H1 receptor antagonist inhalant
InactiveCN103830729AOrganic active ingredientsPharmaceutical delivery mechanismFexofenadineLoratadine
The invention relates to a fluticasone and fluticasone ester / H1 receptor antagonist inhalant which contains fluticasone and fluticasone ester and one or more H1 receptor antagonists used as active components, and one or more pharmaceutical auxiliary materials suitable for inhalation administration. The H1 receptor antagonist is one or more of loratadine, desloratadine, cetirizine, levocetirizine, astemizole, ketotifen, ebastine, fexofenadine, avastin, mequitazine, mizolastine and salts thereof, and preferably one or more of loratadine, desloratadine, cetirizine, levocetirizine, ebastine, mizolastine, avastin, mequitazine, ketotifen and hydrochlorides or fumarates thereof.
Owner:TIANJIN JINYAO GRP
Stable pharmaceutical formulation for oral administration comprising levocetirizine or a pharmaceutically acceptable salt thereof, and montelukast or a pharmaceutically acceptable salt thereof
ActiveUS9486528B2Excellent long-term storage stabilityPrevention or treatment of allergic rhinitisPowder deliverySenses disorderPharmaceutical formulationBuccal administration
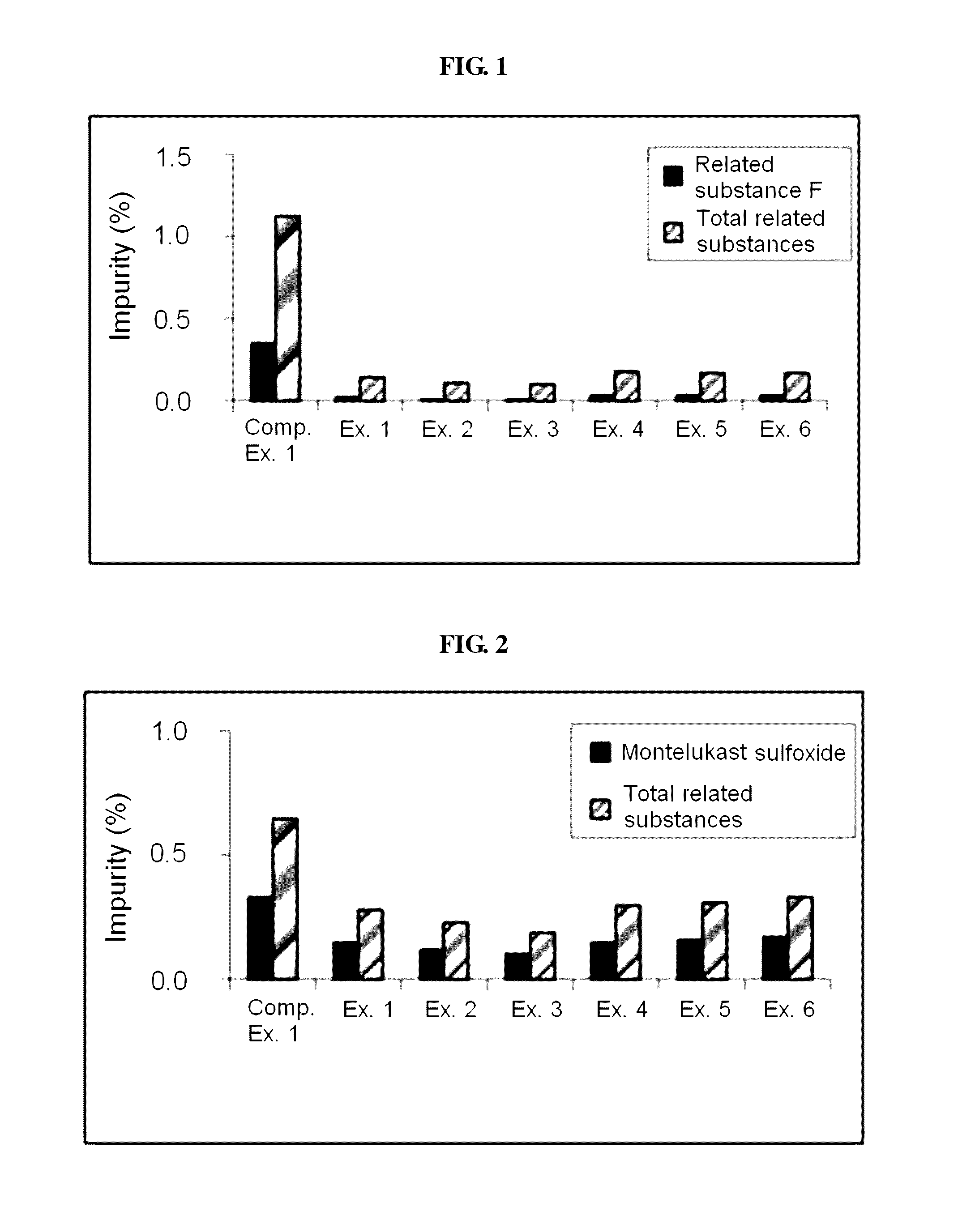
The present invention relates to a pharmaceutical formulation for oral administration for preventing or treating allergic rhinitis or asthma, which comprises: (a) a first particle part comprising levocetirizine or a pharmaceutically acceptable salt thereof and an organic acid; and (b) a second particle part comprising montelukast or a pharmaceutically acceptable salt thereof. The pharmaceutical formulation according to the present invention comprises an organic acid as a stabilizing agent, which can effectively inhibit the production of levocetirizine and montelukast related substances, and thus, show good stability.
Owner:HANMI PHARMA
Preparation method of levocetirizine
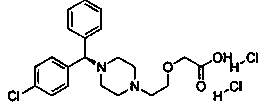
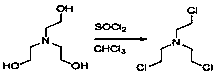
The invention provides a preparation method of levocetirizine. The method comprises the following steps of: the step 1, carrying out a cyclization reaction on (R)-4-chlorodiphenyl methylamine and tris(2-chloroethyl)amine to obtain a compound represented by a formula (I); 2, performing condensation reaction of the compound shown in the formula (I) and 2-ethyl glycolate to obtain a compound shown ina formula (II); and the step 3, converting the compound shown in the formula (II) into levocetirizine. According to the preparation method, (R)-4-chlorodiphenyl methylamine and tris(2-chloroethyl)amine are taken as the initial raw materials, and cyclization reaction, condensation reaction and hydrolysis reaction are carried out so as to obtain levocetirizine. The synthetic route provided by the invention is short, the yield is high, and experimental results show that the yield of the levocetirizine prepared by the method provided by the invention can reach 47%, and the purity can reach 99.7%.
Owner:湖南九典宏阳制药有限公司 +1
Composition and its preparation method, oral liquid and its preparation method
ActiveCN104666302BImprove stabilityPromote growthInorganic non-active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideOrganic solvent
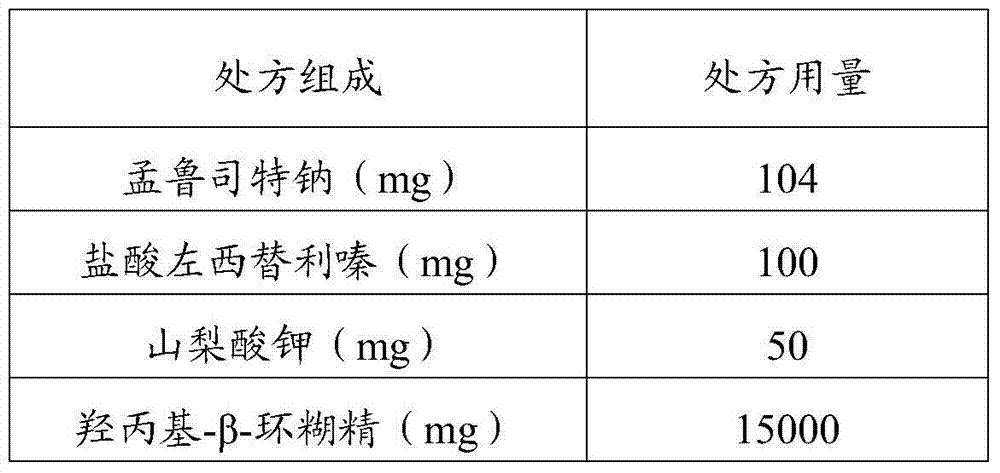
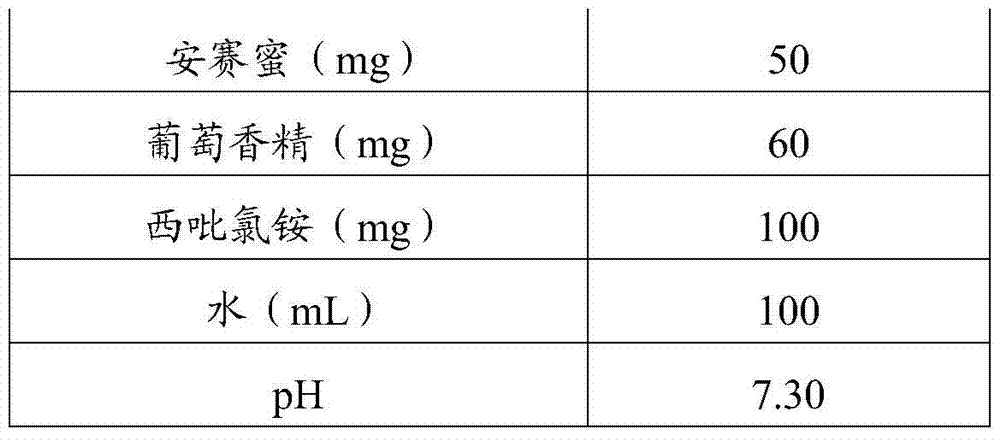
The invention relates to the field of pharmaceutical preparations, in particular to a composition and a preparation method thereof, an oral liquid and a preparation method thereof. The preparation method of the composition is as follows: mixing montelukast sodium with the first water to obtain a montelukast sodium solution; mixing levocetirizine hydrochloride with the second water to adjust the pH value to 6.5-9 to obtain Levocetirizine hydrochloride solution; get montelukast sodium solution and levocetirizine hydrochloride solution to mix. The montelukast sodium in the oral liquid provided by the invention has high stability, does not contain organic solvents and surfactants, is convenient for children to take medicine, and has a simple preparation process.
Owner:BEIJING HANMI PHARMA CO LTD
New procedure for preparation of levocetirizine and its intermediates
The present invention describes a novel process for the preparation of levocetirizine and pharmaceutically acceptable acid addition salts thereof using diglycolic acid or derivatives thereof and new intermediates used in that process.
Owner:克卡制药新梅斯托股份公司
Solution agent of antiallergi medicine contg. levocetirizine
InactiveCN1166362CDelicious fruity aromaComfortable tasteOrganic active ingredientsImmunological disordersPEG 400Levocetirizine hydrochloride
The present invention provides an antianaphylactic medicine solution preparation containing Levocetirizine. In 1000 ml of medicine composite solution 0.5-20g of zocetirizine or its pharmacal acceptable salt, 20-100 g of polyvinyl pyrrolidone, 5-50g of poloxamer and 10-250g of polyethylene glycol 400 are contained.
Owner:CHONGQING HUAPONT PHARMA
Process for the preparation of levocetirizine and intermediates thereof
The present invention describes a novel process for the preparation of levocetirizine and pharmaceutically acceptable acid addition salts thereof using diglycolic acid or derivatives thereof and new intermediates used in that process.
Owner:KRKA TOVARNA ZDRAVIL D D
Process For The Preparation Of Levocetirizine And Intermediates Thereof
The present invention describes a novel process for the preparation of levocetirizine and pharmaceutically acceptable acid addition salts thereof using diglycolic acid or derivatives thereof and new intermediates used in that process.
Owner:KRKA TOVARNA ZDRAVIL D D
New composition for treating seasonal and perennial allergic rhinitis
InactiveCN1706385BOrganic active ingredientsPharmaceutical non-active ingredientsPseudoephedrineDecongestant
The present invention relates to one new kind of medicine composition containing levocetirizine (or its pharmaceutically acceptable salt) and pseudoepherine (or its pharmaceutically acceptable salt). The medicine composition is pressed into double layer including the first layer containing levocetirizine (or its pharmaceutically acceptable salt) in the amount for resisting allergy effectively andthe second layer containing pseudoepherine (or its pharmaceutically acceptable salt) in the amount as effective decongestant for nose. The medicine composition is used in treating seasonal and perennial allergic rhinitis.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com