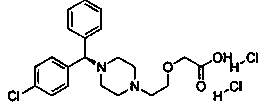
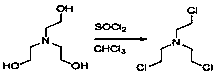
Preparation method of levocetirizine
A technology of levocetirizine and compounds, which is applied in the field of preparation of levocetirizine, can solve problems such as long synthetic routes, and achieve the effects of short synthetic routes and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
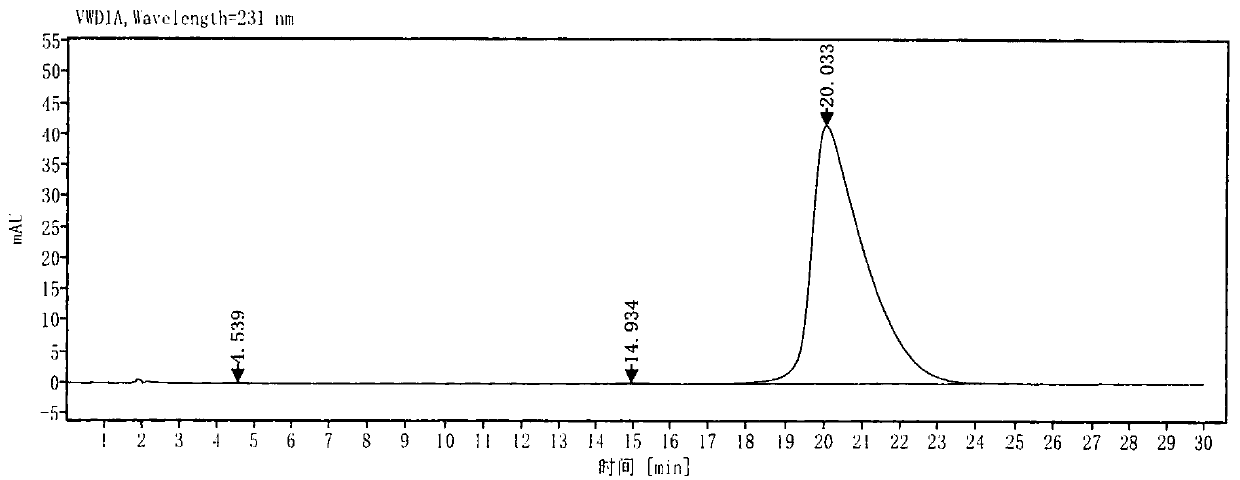
[0038] Step 1. Under nitrogen protection, 34.41g of 4-chlorobenzophenone and 48.17g of formamide were kept at 170-175°C until the raw material was less than 3% as detected by TLC / GC. The reaction system was added to 200g of water, and suction filtered. Wash the filter cake with water, dry the filter cake at 55°C, add 59.89g of 36% hydrochloric acid, 21.33g of absolute ethanol and 81.2g of water to the crude product and heat it to 75~80°C to reflux, check that the hydrolysis is complete, cool down to 25°C and suction filter, the filter cake Add water to form a paste, add sodium hydroxide and water to adjust the pH value to 10~11, add DCM to extract, wash the organic phase with saturated brine, dry over sodium sulfate and concentrate to obtain the compound of formula (III), which can be calculated by GC detection as shown in the figure , yield 80%, purity 98%,;
[0039] Formula (III);
[0040] Step 2. Add 23.00g of L-(+)-tartaric acid into a reaction flask containing 312.28g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com