Polyhalogenated isoquinoline class derivate and synthetic method thereof
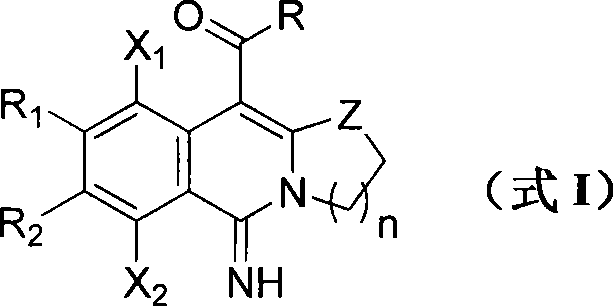
A technology for isoquinolines and a synthesis method, which is applied in the field of polyhalogenated isoquinoline derivatives and the synthesis thereof, can solve the problems of long route, low total yield, large limitation of substrate range, etc. The synthesis process is simple, the route is simple, and the effect of molecular diversity is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
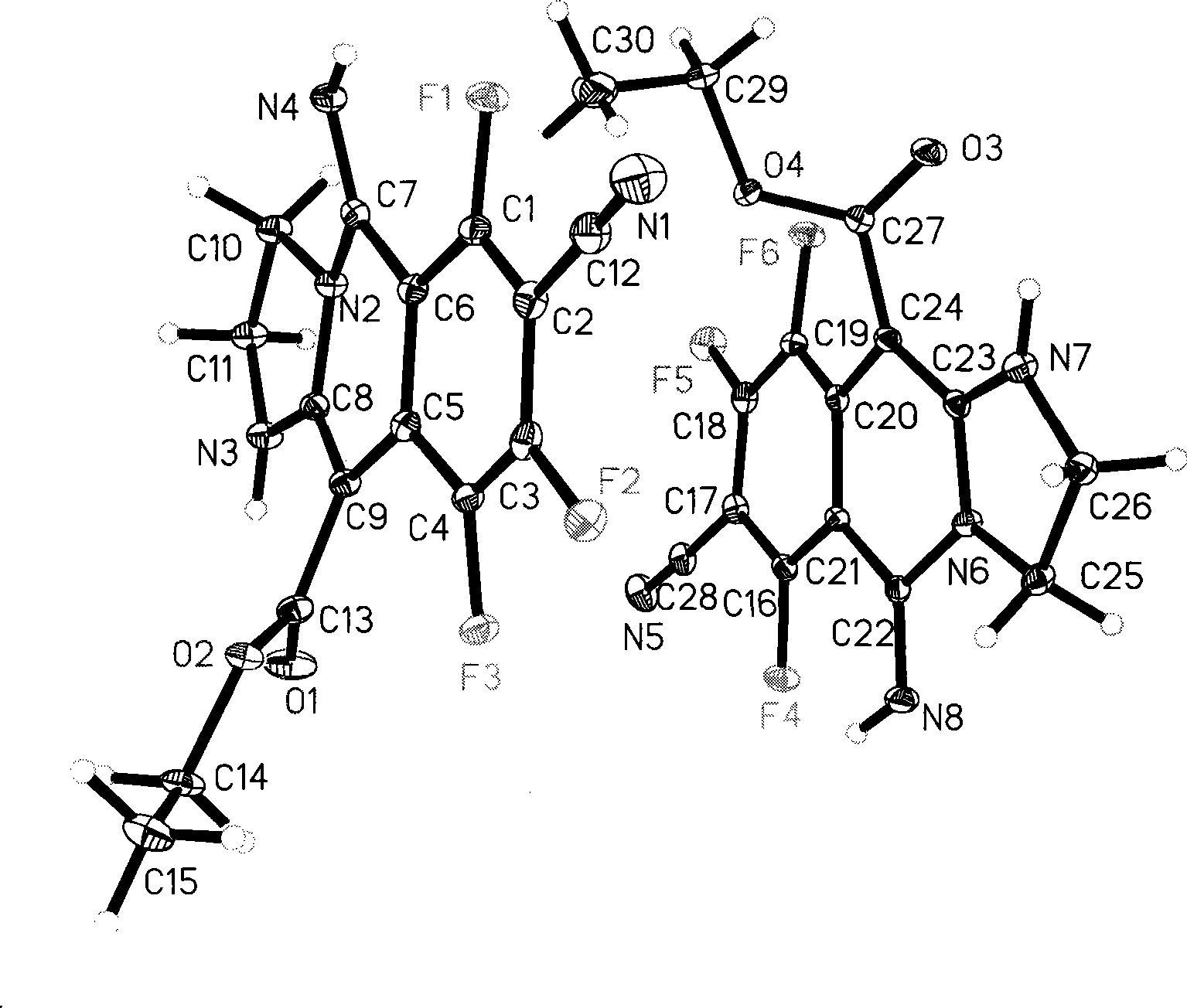
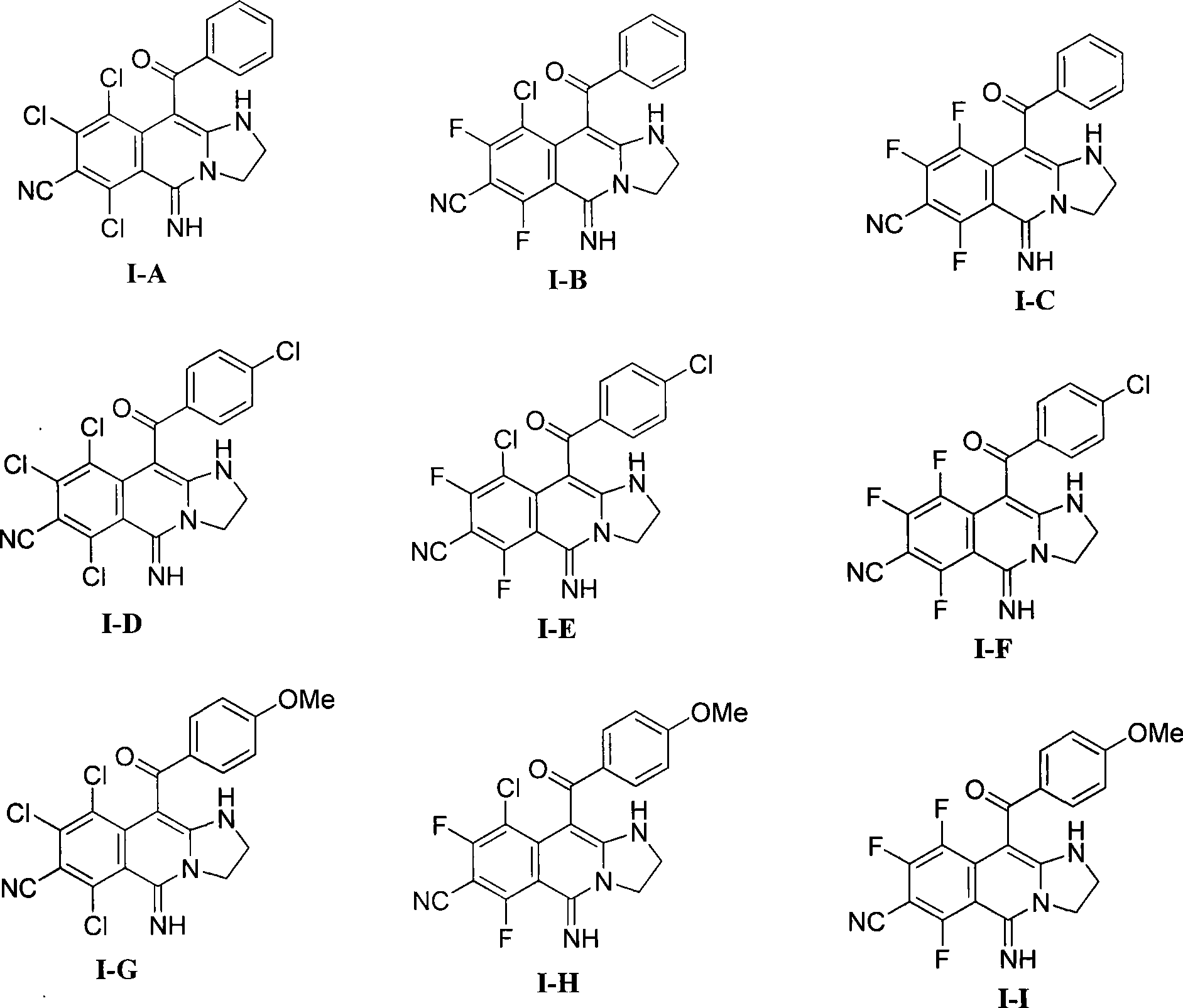
[0026] Example 1: 10-benzoyl-6,8,9-trichloro-7-cyano-5-imino-1,2,3,5-tetrahydroimidazo[1,2-b]isoquine Synthesis of phenoline (I-A): Add 0.53 g (2 mmol) 2,4,5,6-tetrachloro-1,3-phthalonitrile and 0.38 g (2 mmol) 2-benzoyl to a mortar Methyleneimidazolinidine, after being fully ground, was heated to 140°C for 30 minutes while grinding on a heating mantle, and the reaction mixture changed from colorless to dark yellow. After TLC showed that the raw materials disappeared completely, 25 ml of 1, 4-Dioxane was transferred to a 50 ml round bottom flask, and 0.22 g (2 mmol) of potassium tert-butoxide was added to react at room temperature for 30 minutes. TLC showed that the reaction of the intermediate was complete, and the reaction was stopped. Column chromatography (ethyl acetate / petroleum ether=2:1) yielded the product 10-benzoyl-6,8,9-trichloro-7-cyano-5-imino-1,2,3,5 - Tetrahydroimidazo[1,2-b]isoquinoline. Yellow solid, 80% yield. Its melting point is: 216-219°C.
[0027] P...
Embodiment 2
[0029] Example 2: 10-benzoyl-6,8-difluoro-9-chloro-7-cyano-5-imino-1,2,3,5-tetrahydroimidazo[1,2-b] Synthesis of isoquinoline (I-B): 0.43 g (2 mmol) 2,4,6-trifluoro-5-chloro-1,3-phthalonitrile and 0.38 g (2 mmol) 2 - Benzoylmethyleneimidazolinidine, after fully pulverized, heated to 120°C for 15 minutes while grinding on the heating mantle, the reaction mixture changed from colorless to dark yellow, TLC showed that the raw materials disappeared completely, and then used 25 ml of 1,4-dioxane was transferred to a 50 ml round bottom flask, and 0.22 g (2 mmol) of potassium tert-butoxide was added to react at room temperature for 30 minutes. TLC showed that the reaction of the intermediate was complete, and the reaction was stopped. Column chromatography (ethyl acetate / petroleum ether=2:1) gave a yellow solid with a yield of 87%. Its melting point is: 260-262°C.
[0030] Proton NMR spectrum (deuterated dimethyl sulfoxide as solvent, Bruker AM 500 instrument): δ=8.64 (br, 2H, NH...
Embodiment 3
[0034]Example 3: 10-Benzoyl-6,8,9-trifluoro-7-cyano-5-imino-1,2,3,5-tetrahydroimidazo[1,2-b]isoquine Synthesis of phenoline (I-C): Add 0.40 g (2 mmol) 2,4,5,6-tetrafluoro-1,3-phthalonitrile and 0.38 g (2 mmol) 2-benzoyl to a mortar Methyleneimidazolinidine, after being fully ground, was heated to 90°C for 10 minutes while grinding on a heating mantle, and the reaction mixture changed from colorless to dark yellow. After TLC showed that the raw materials disappeared completely, 25 ml of 1, 4-Dioxane was transferred to a 50 ml round bottom flask, and 0.22 g (2 mmol) of potassium tert-butoxide was added to react at room temperature for 30 minutes. TLC showed that the reaction of the intermediate was complete, and the reaction was stopped. Column chromatography (ethyl acetate / petroleum ether=2:1) gave a yellow solid with a yield of 94%. Its melting point is: 257-259°C.
[0035] Fluorine nuclear magnetic resonance spectrum (deuterated dimethyl sulfoxide as solvent, Bruker AM 50...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com