Patents
Literature
679 results about "Nitryl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
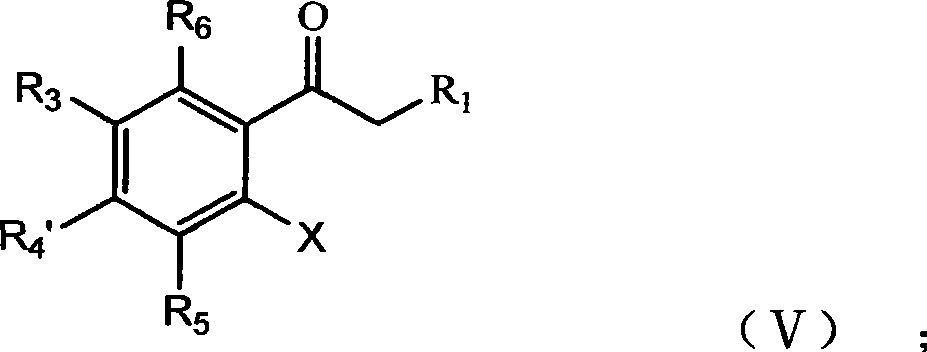
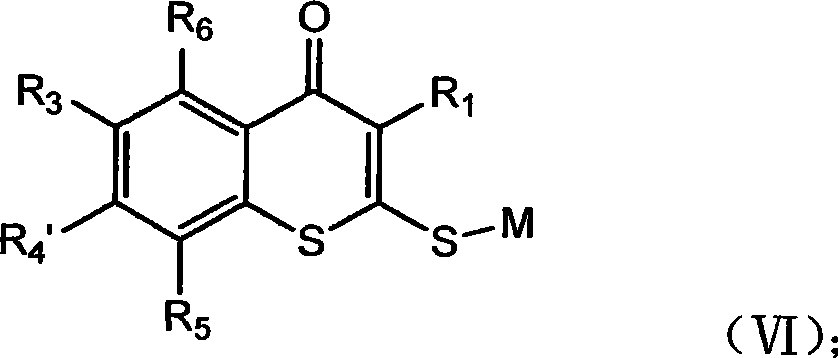
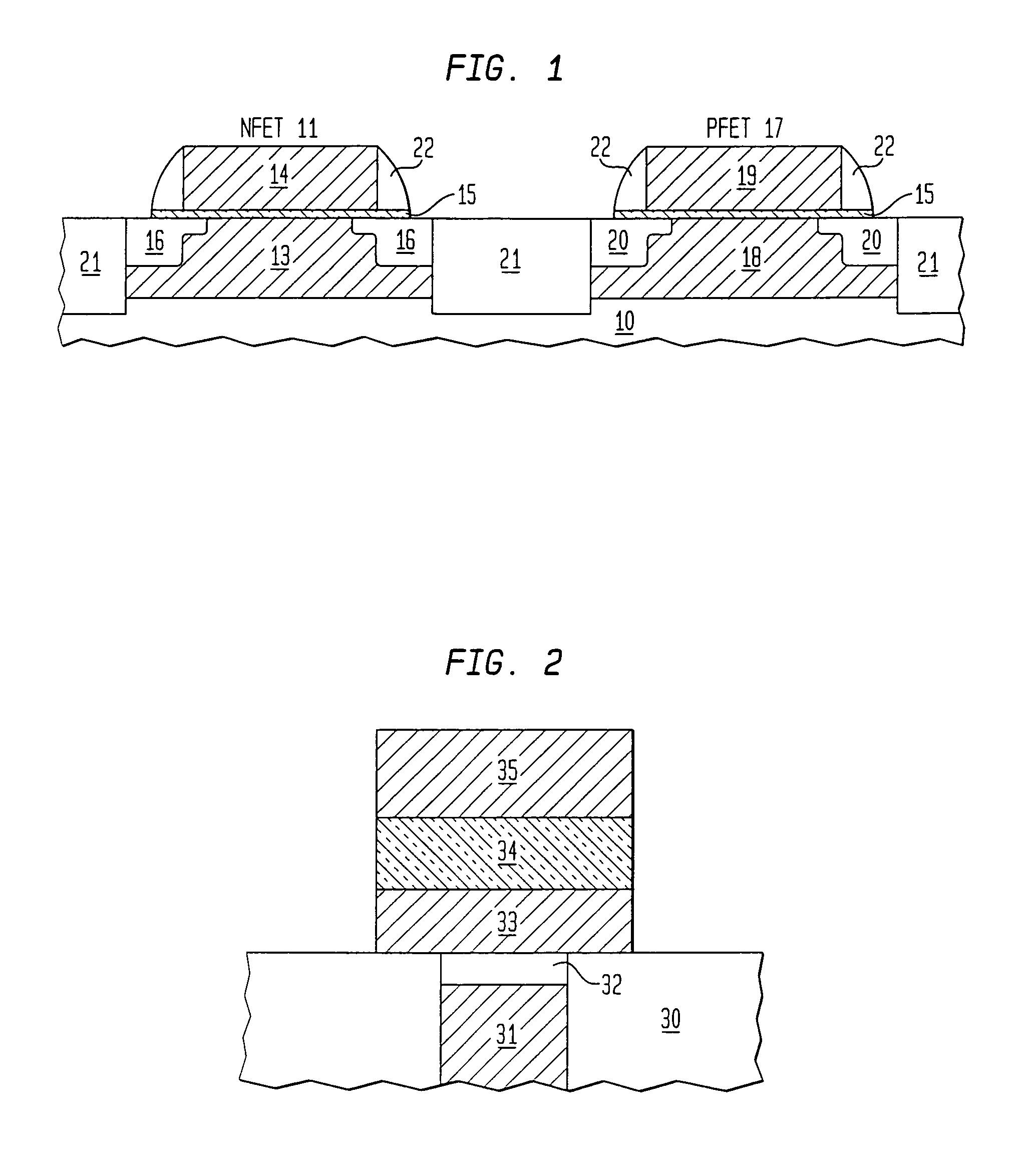
Nitryl is the nitrogen dioxide (NO₂) moiety when it occurs in a larger compound as a univalent fragment. Examples include nitryl fluoride (NO₂F) and nitryl chloride (NO₂Cl). Like nitrogen dioxide, the nitryl moiety contains a nitrogen atom with two bonds to the two oxygen atoms, and a third bond shared equally between the nitrogen and the two oxygen atoms. The nitrogen-centred radical is then free to form a bond with another univalent fragment (X) to produce an N-X bond, where X can be F, Cl, OH, etc.
Precursor source mixtures
A precursor source mixture useful for CVD or ALD of a film comprising: at least one precursor composed of an element selected from the group consisting of Li, Na, K, Rb, Cs, Fr, Be, Mg, Ti, Zr, Hf, Sc, Y, La, V, Nb, Ta, Cr, Mo, W, Mn, Re, Fe, Ru, Os, Co, Rh, Ir, Ni, Pd, Pt, Cu, Ag, Au, Zn, Cd, Hg, B, Al, Ga, In, Tl, Si, Ge, Sn, Pb, As, P, Sb and Bi, to which is bound at least one ligand selected from the group consisting of hydride, alkyl, alkenyl, cycloalkenyl, aryl, alkyne, carbonyl, amido, imido, hydrazido, phosphido, nitrosyl, nitryl, nitrate, nitrile, halide, azide, alkoxy, siloxy, silyl, and halogenated, sulfonated or silyated derivatives thereof, which is dissolved, emulsified or suspended in an inert liquid selected from the group consisting of aliphatic hydrocarbons, aromatic hydrocarbons, alcohols, ethers, aldehydes, ketones, acids, phenols, esters, amines, alkylnitrile, halogenated hydrocarbons, silyated hydrocarbons, thioethers, amines, cyanates, isocyanates, thiocyanates, silicone oils, nitroalkyl, alkylnitrate, and mixtures thereof. The precursor source mixture may be a solution, emulsion or suspension and may consist of a mixture of solid, liquid and gas phases which are distributed throughout the mixture.
Owner:GLOBALFOUNDRIES INC
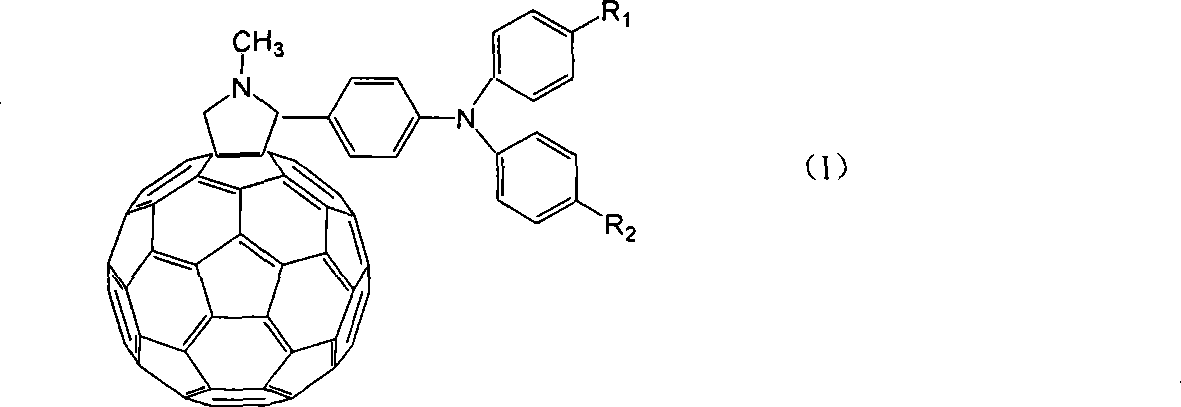
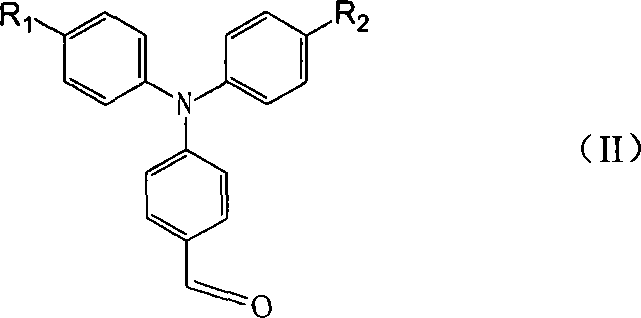
Organic optoelectronic material, preparation method thereof and organic light emitting diode containing organic material
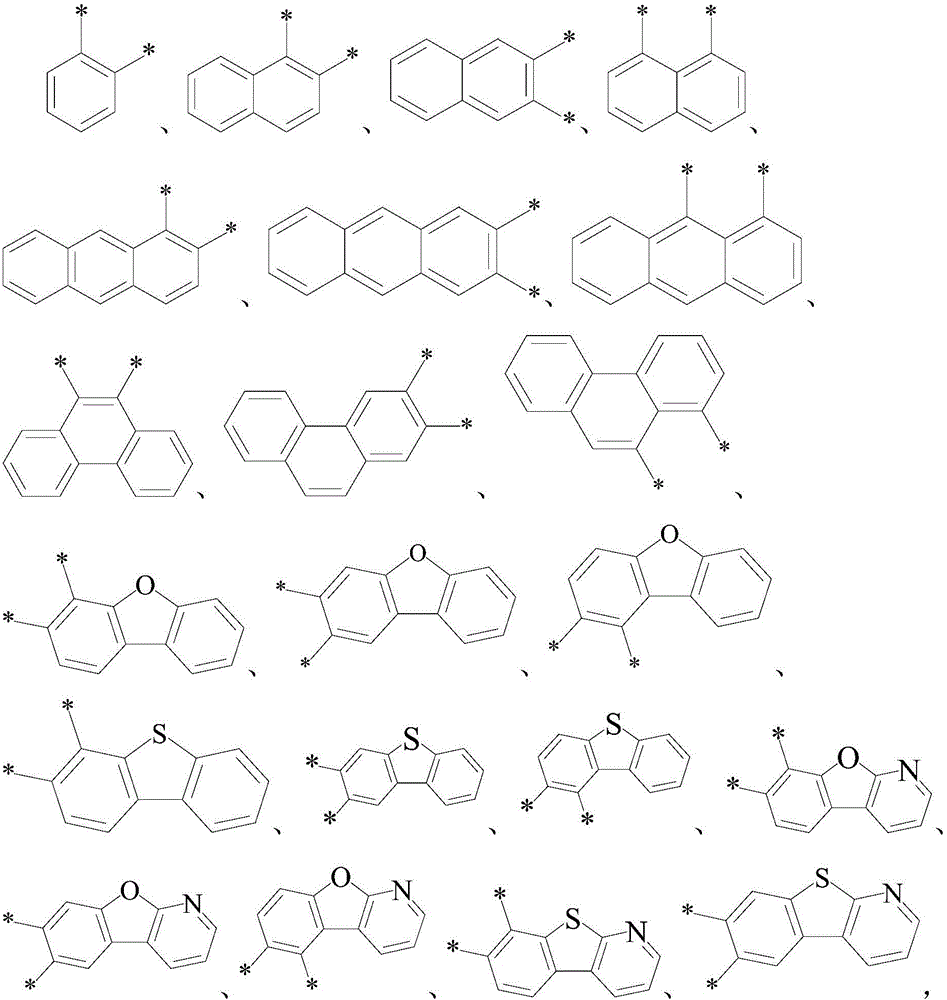
ActiveCN105778891AImprove thermal stabilityHigh glass transition temperatureOrganic chemistrySolid-state devicesBenzeneAryl
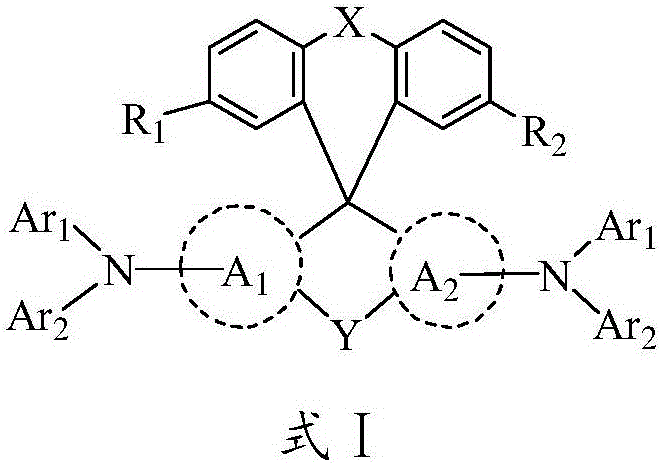
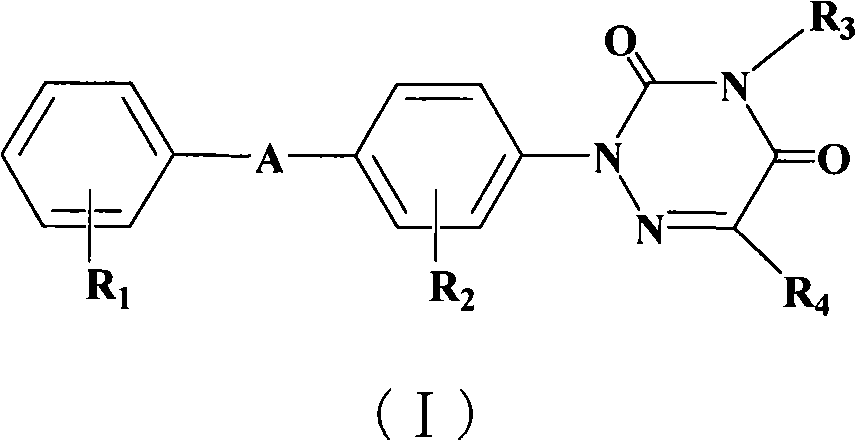
The invention relates to an organic optoelectronic material, a preparation method of the organic optoelectronic material and an organic light emitting diode containing the organic material. The material is shown in the formula I, wherein R1 and R2 are respectively and independently hydrogen, halogen, cyanogroup, nitryl, isothiocyano, sulfonyl, sulfuryl, acylamino, alkyl of which the carbon atom number is 1 to 10 or alkoxy of which the carbon atom number is 1 to 12; A1 and A2 are respectively and independently phenyl, polycyclic conjugate aryl of which the carbon atom number is 10 to 60 or aromatic heterocyclic group containing at least one of N, S and O; two phenyls having groups R1 and R2 and two para-positions relative to the groups R1 and R2 are connected by directly bonding or connected through X; A1 is connected with A2 by directly bonding or through Y; X and Y are respectively and independently sulphur, oxygen, alkylene of which the carbon atom number is 1 to 6 or alkenyl of which the carbon atom number is 2 to 6; Ar1 and Ar2 are respectively and independently phenyl, cyanophenyl, alkyl benzene, polycyclic conjugate aryl of which the carbon atom number is 10 to 60 or aromatic heterocyclic group containing at least one of N, S and O.
Owner:VALIANT CO LTD
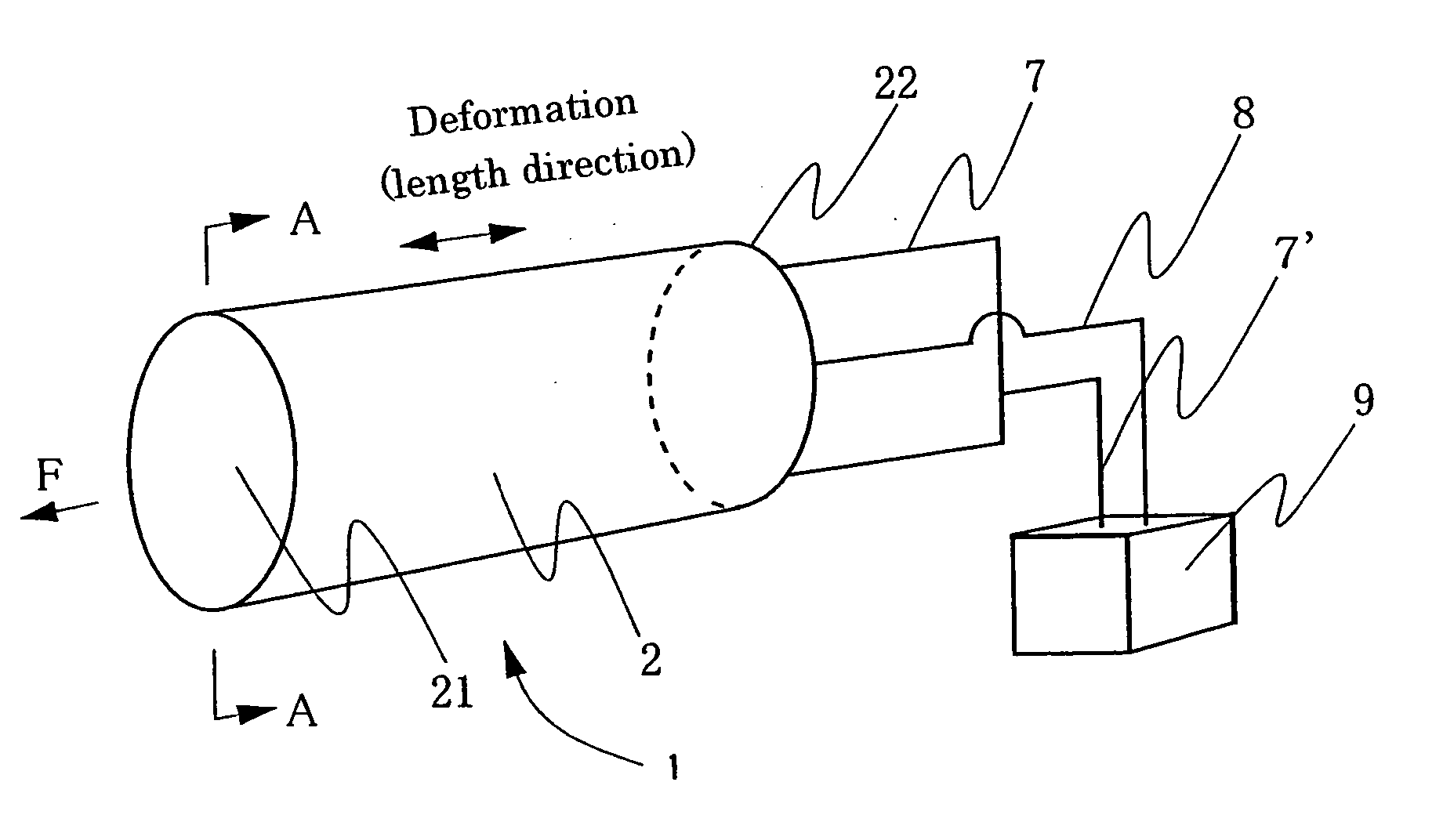
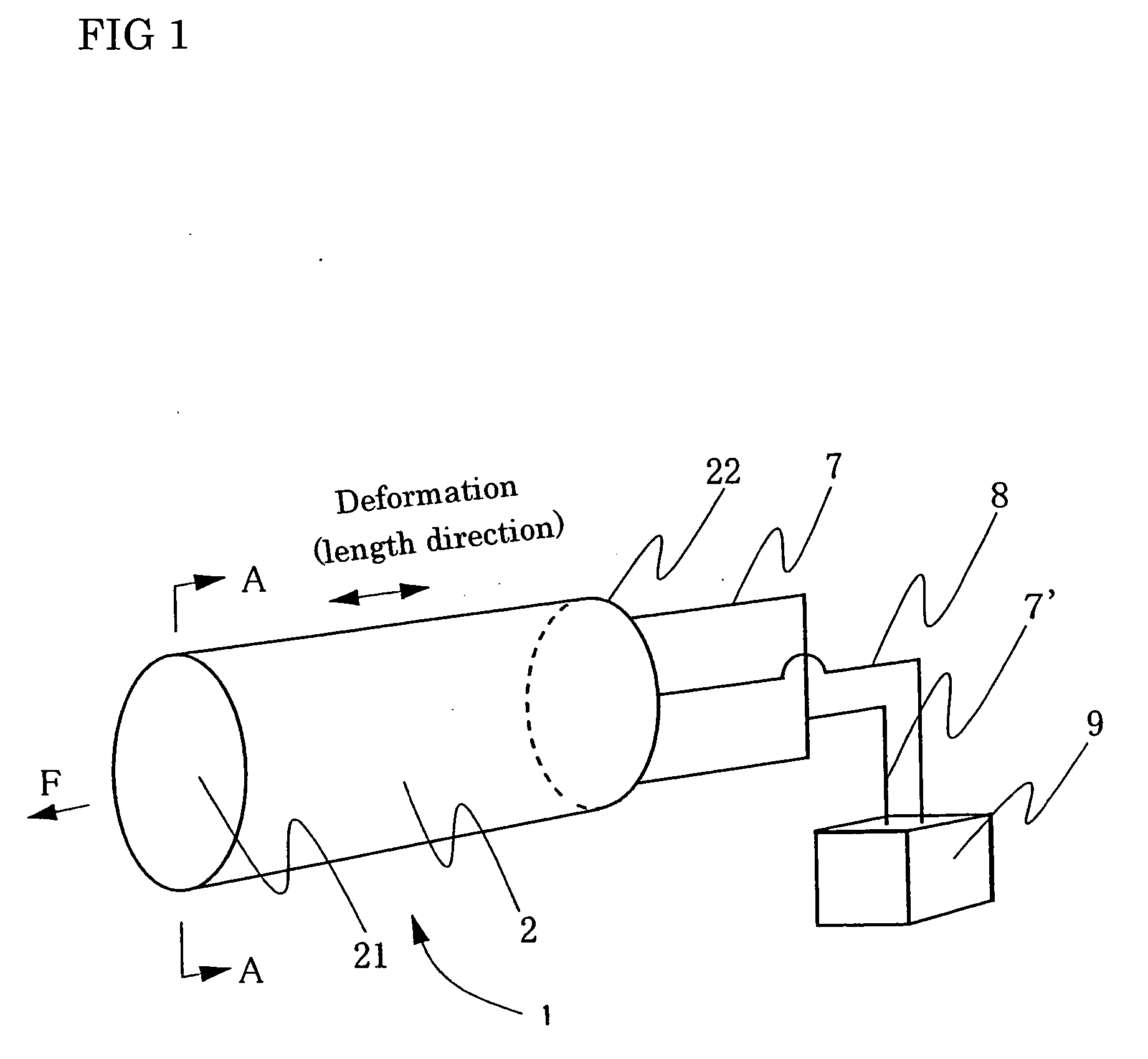
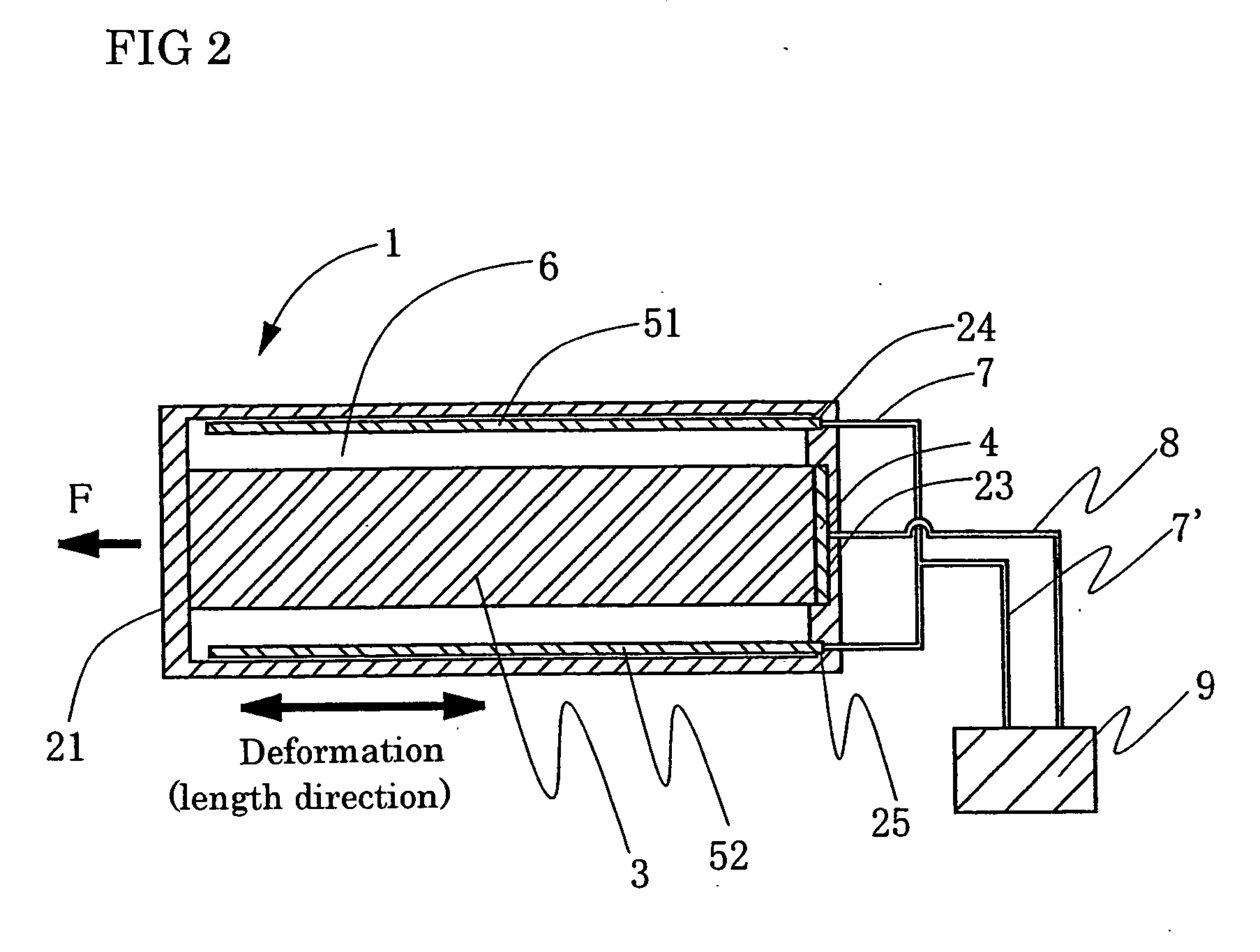
Process for producing conductive polymer
A process for producing conductive polymers with excellent electrochemical strain per redox cycle is provided. A process for producing conductive polymers by an electrochemical polymerization method, wherein said conductive polymers have deformation property by electrochemical redox, said electrochemical polymerization method is a polymerization method using electrolyte including organic compounds as solvents, and wherein said organic compounds include (1) chemical bond species selected at least one from a group composed of the chemical bond consisting of ether bond, ester bond, carbon-halogen bond, and carbonate bond and / or (2) functional groups selected at least one from a group composed of functional groups consisting of hydroxyl group, nitro group, sulfone group, and nitryl group in a molecule, and said electrolyte includes anions which include trifluoromethanesulfonate ion and / or plural of fluorine atoms which bond to central atom is used.
Owner:EAMEX
Compound for pigment
ActiveCN103626717AImprove heat resistanceOptical filtersSulfonic acid amide preparationSulfurHydroxy group
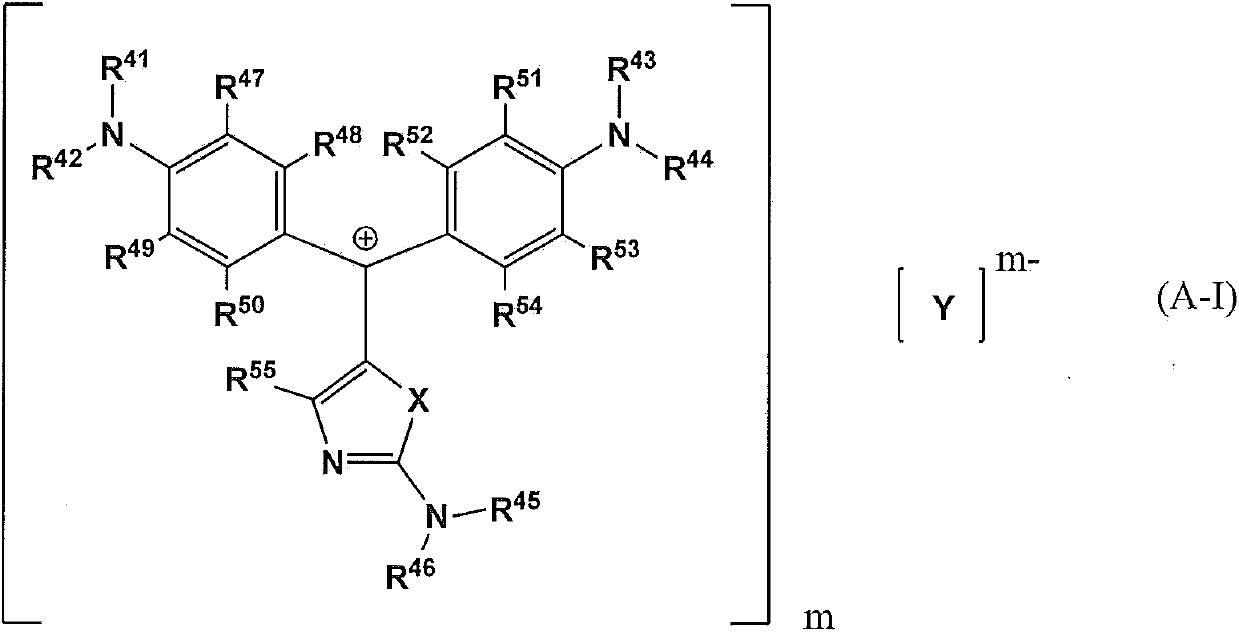
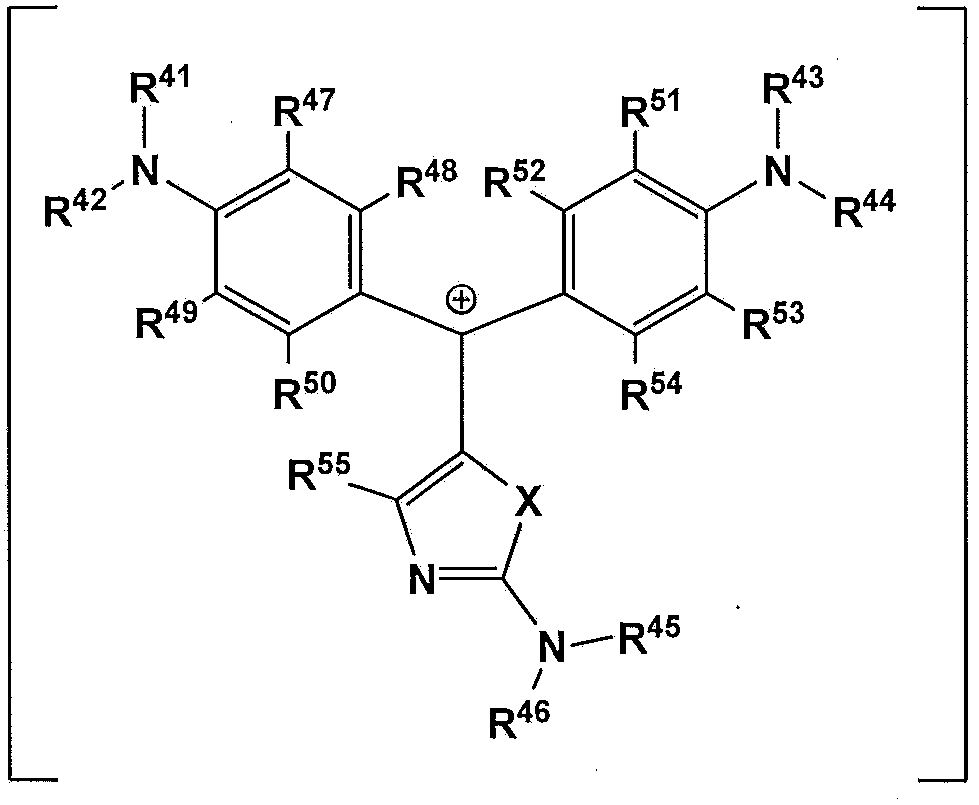
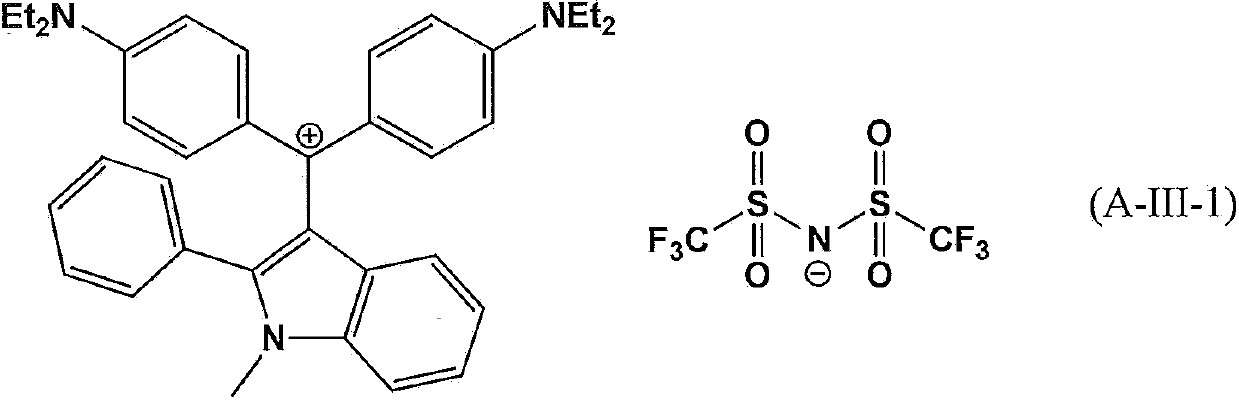
The present invention provides a compound which is represented by a formula (A-I), a coloring curable resin composition with the compound, a color filter formed by the resin composition, and a display device with the color filter. In the formula (A-I), X represents an oxygen atom, a nitrogen atom or sulfur atom; [Y]m represents a random m-valued anion; R41-R46 independently represents hydrogen atom or an alkyl which can be substituted by an amino group or halogen atom and has 1-20 carbon atoms; R47-R54 independently represents a hydrogen atom, a halogen atom, a nitryl, a hydroxyl or an alkyl with 1-8 carbon atoms; and R55 represents a hydrogen atom, an alkyl with 1-20 carbon atoms or a substitutable aryl.
Owner:SUMITOMO CHEM CO LTD +1
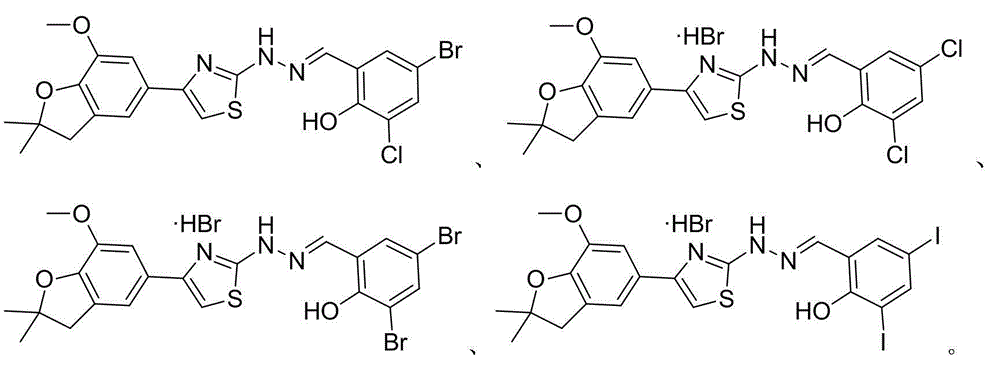
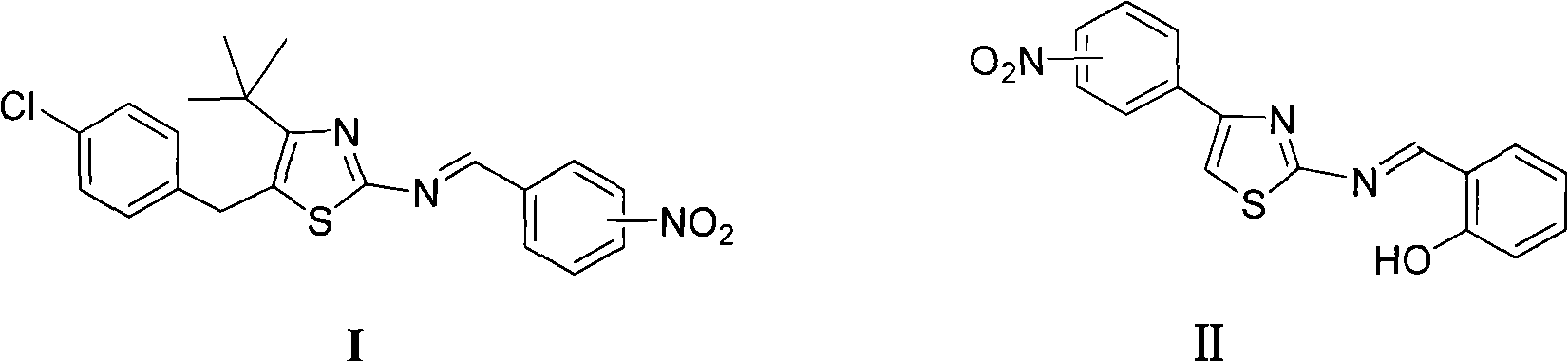
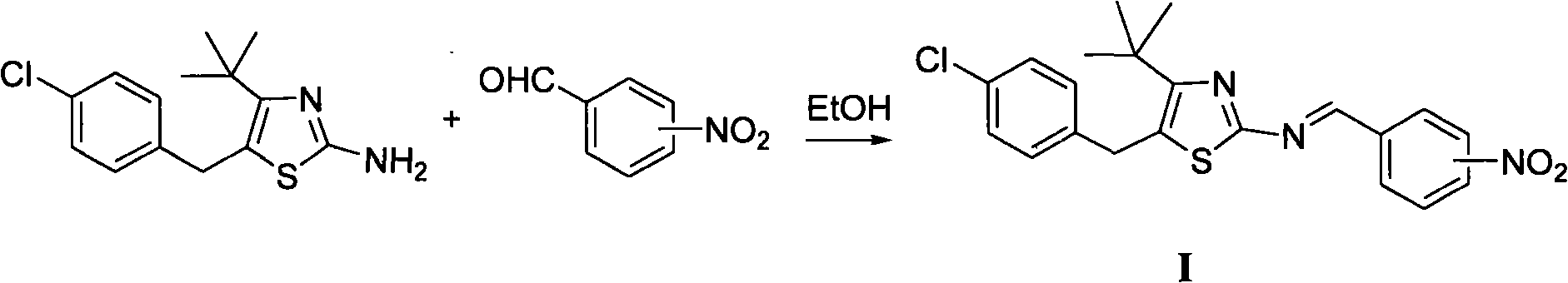
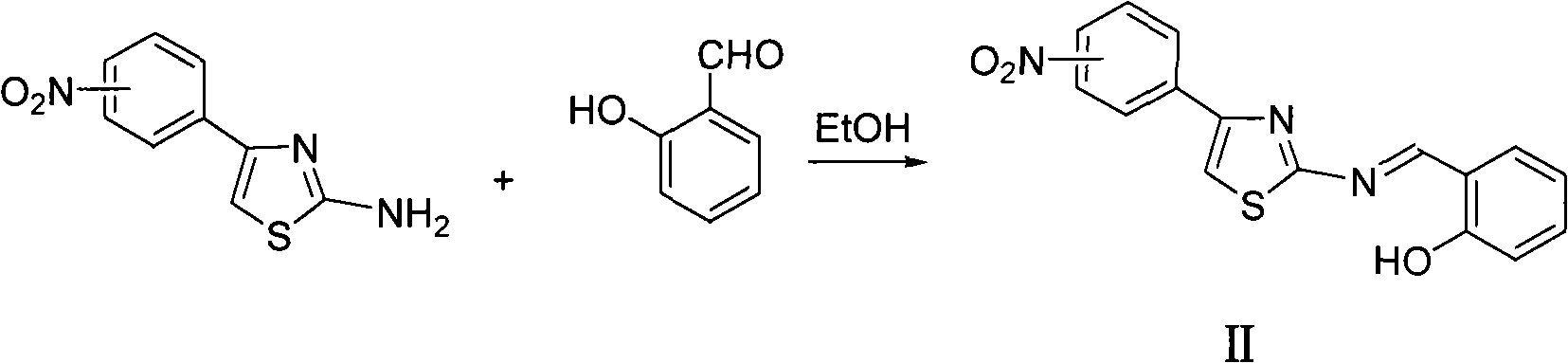
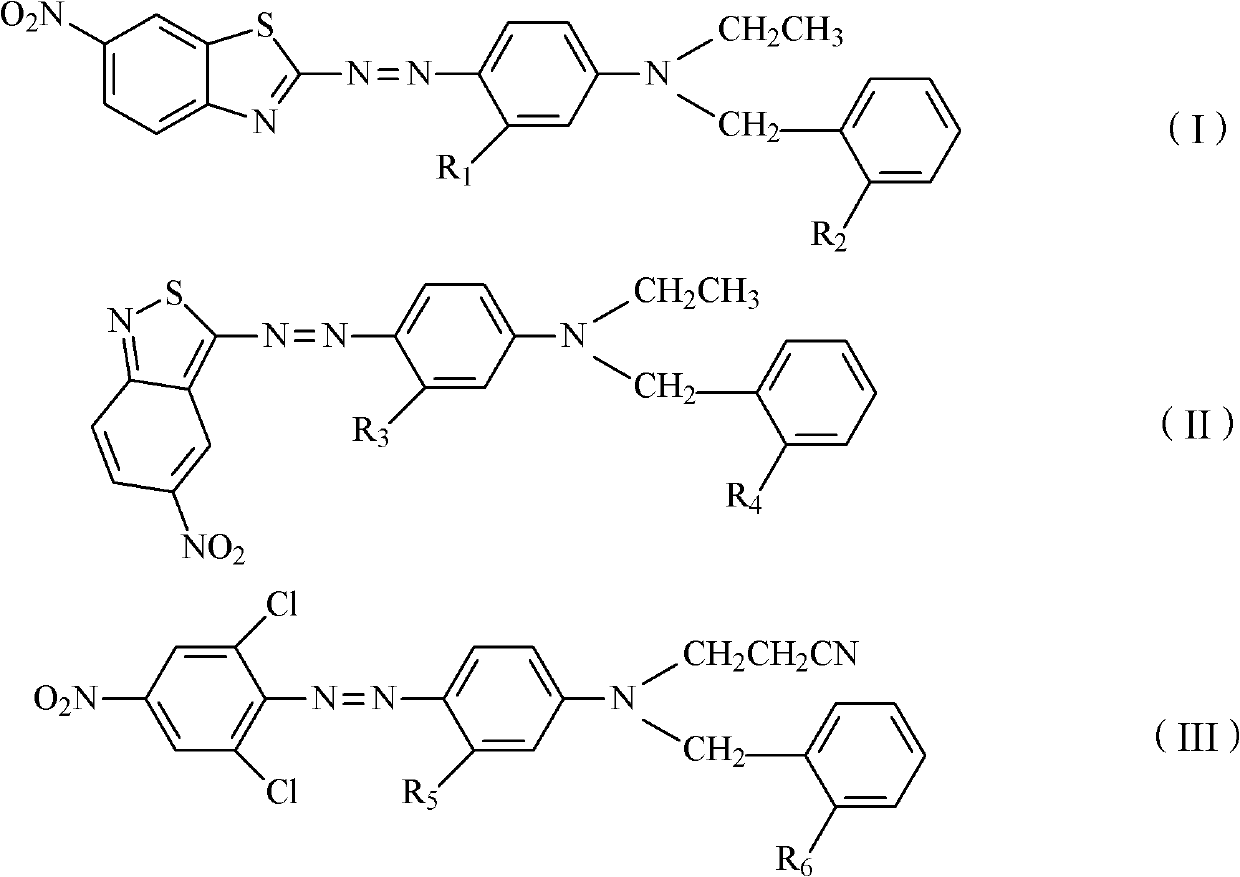
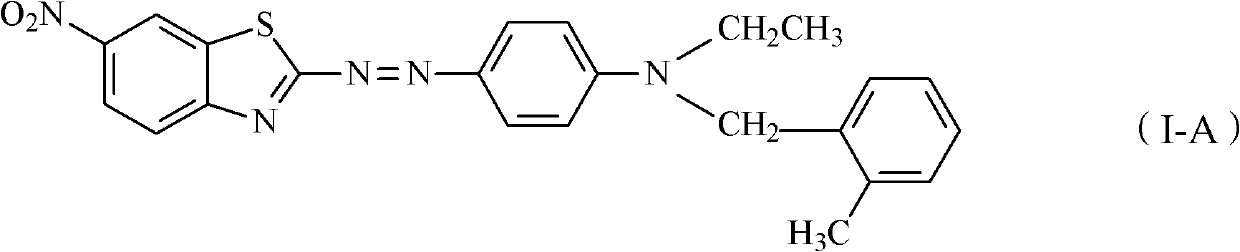
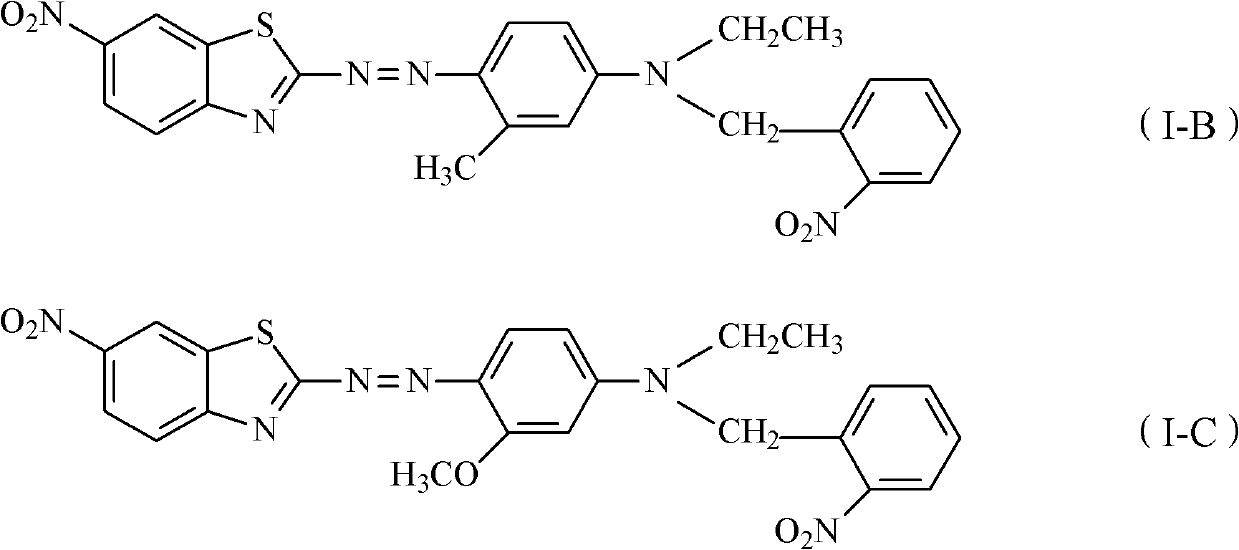
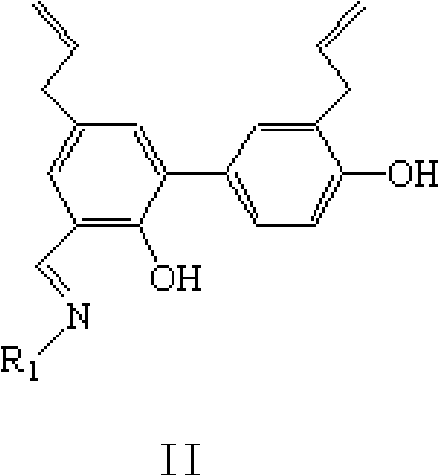
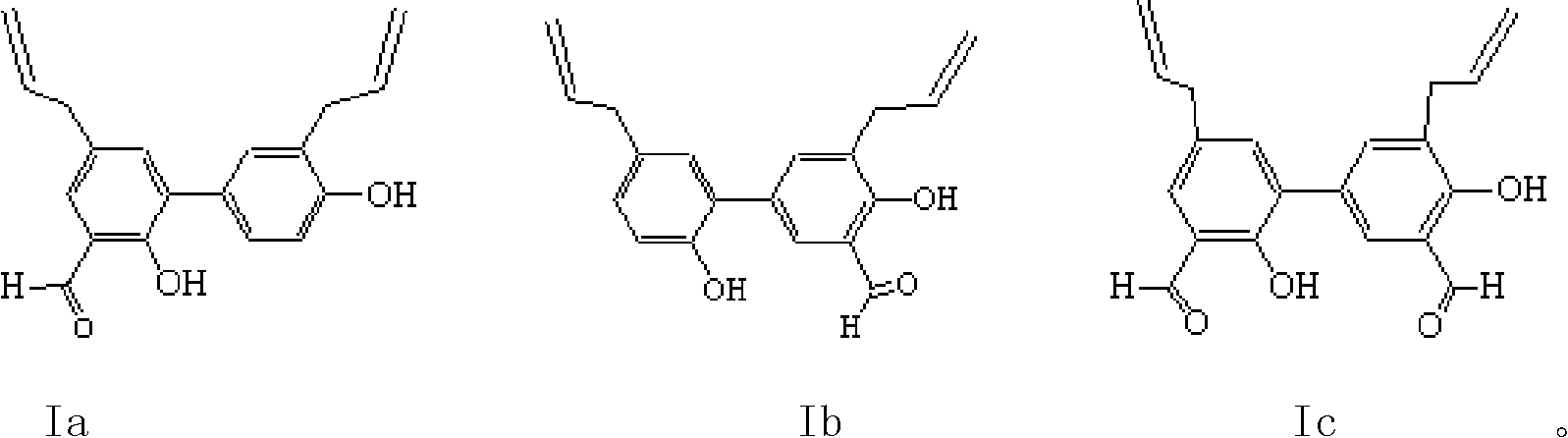
Thiazole schiff base containing nitryl, preparation and uses thereof
InactiveCN101492426AHas anti-inflammatory activityOrganic active ingredientsOrganic chemistrySalicylaldehydeStructural formula
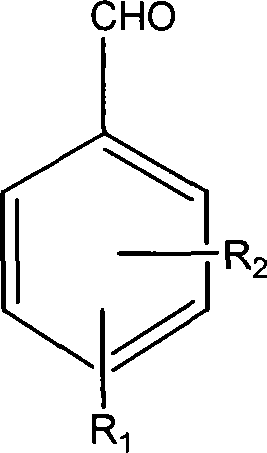
The invention discloses thiazole Schiff base (I, II) containing nitryl with a chemical structural formula on the right. A method for preparing the thiazole Schiff base containing the nitryl comprises the steps as follows: nitrobenzene formaldehyde takes reflux reaction with 5-(4-chlorobenzyl)-4-tert-butyl-2-amido thiazole in ethanol to prepare and obtain the thiazole Schiff base [I with chemical name of 5-(4-chlorobenzyl)-4-tert-butyl-2-(nitrobenzyl imino) thiazole] containing the nitryl; or salicylaldehyde takes reflux reaction with 4-(nitrophenyl)-2-amido thiazole in the ethanol to prepare and obtain the thiazole Schiff base [II with chemical name of 4-( nitrophenyl)-2-(2-hydroxy benzyl imino) thiazole] containing the nitryl. The thiazole Schiff base containing the nitryl can be used for preparing anti-inflammatory analgesics.
Owner:HUNAN UNIV
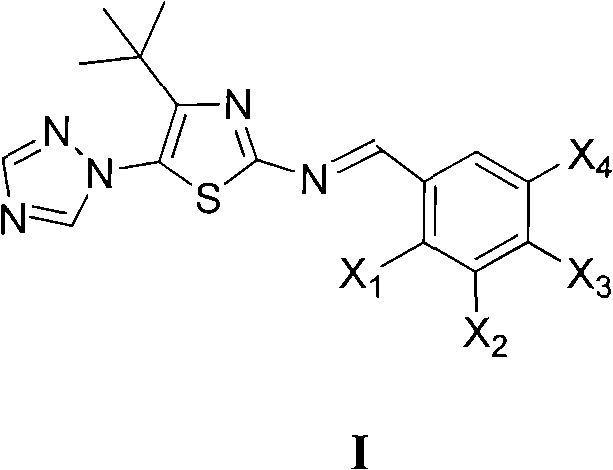
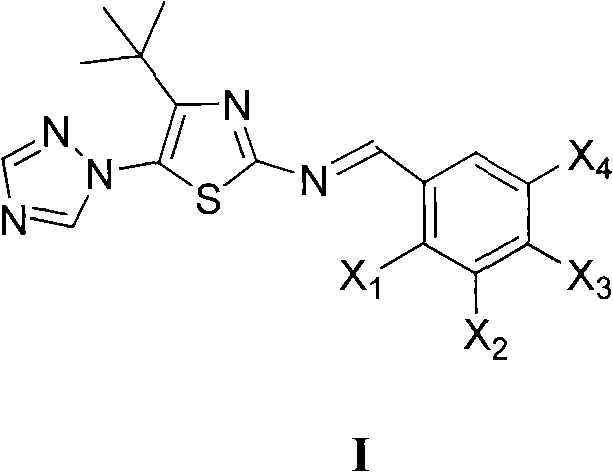
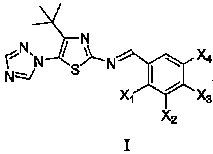
Medication application of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzyliminothiazole
InactiveCN101836979AStrong inhibitory activityOrganic active ingredientsAntineoplastic agentsHydrogenBromine
The invention discloses a medical application of 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzyliminothiazole as shown in a chemical structural formula (I), wherein the X in the formula (I) is selected from hydrogen, hydroxyl, methoxyl, nitryl, amido and chlorine; the X2 is selected from hydrogen, nitryl, amido, chlorine, bromine and iodine; the X3 is selected from hydrogen, methyl, ethyl, nitryl, methoxyl, chlorine, bromine, amido and dimethylamino; and the X4 is selected from hydrogen, nitryl, amido, chlorine, bromine and iodine. The 4-tert-butyl-5-(1,2,4-triazol-1-yl)-2-benzyliminothiazole has better inhibitory activity to human cervical carcinoma cells, human hepatoma cells, human nasopharyngeal carcinoma cells and the like and can be used for preparing antitumor drugs.
Owner:HUNAN UNIV
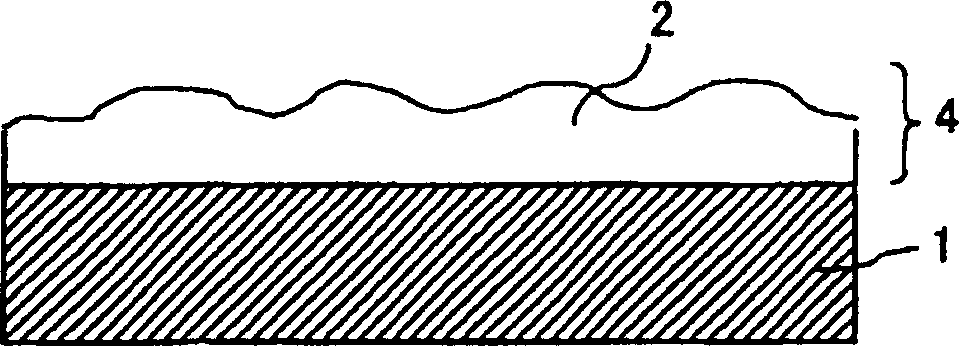
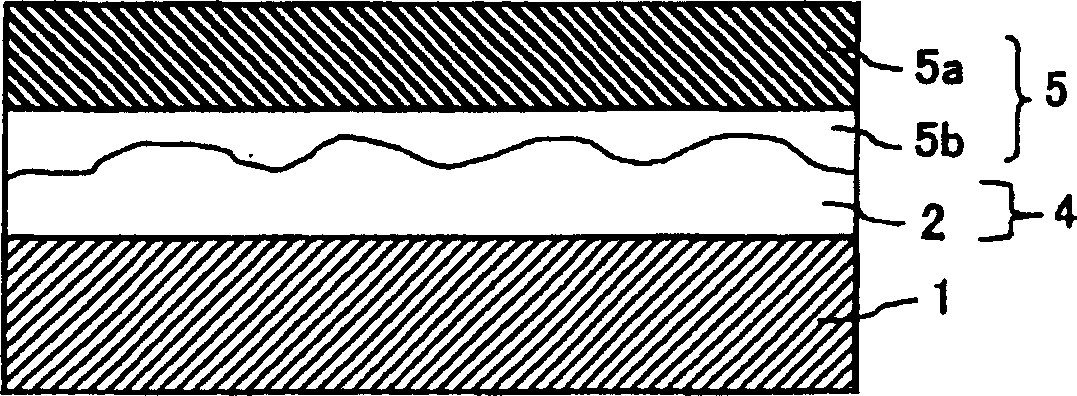
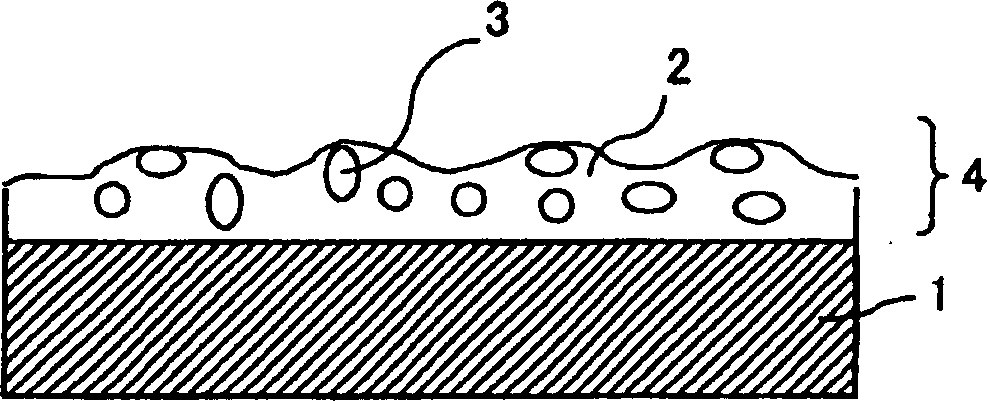
Light-diffusing sheet, optical device, and image display
A light-diffusing sheet comprises a transparent film and a light-diffusing layer composed of a resin coating layer having a rough surface and provided on at least one side of the transparent film. The transparent film contains (A) a thermoplastic resin having a substituted and / or unsubstituted imide group at a side chain and (B) a thermoplastic resin having a substituted and / or unsubstituted phenyl group and a nitryl group. The light-diffusing sheet hardly exhibits birefringence and is excellent in adhesion and durability. The ratio (internal haze / total haze) of the total haze of the light-diffusing sheet to the internal haze is 0.5 to 1.0 and the total haze is preferably 30 to 70%. When this sheet is applied to a high-definition LCD, the antiglareness is maintained, the glittering of the screen is prevented, and the whitening is hardly seen.
Owner:NITTO DENKO CORP
Light-sensitive resin composite and method for manufacturing printed circuit board using same
ActiveCN101738861AHigh sensitivityImprove resolutionPrinted circuit manufacturePhotosensitive materials for photomechanical apparatusCarbon numberChemical compound
The invention relates to a light-sensitive resin composite and a method for manufacturing a printed circuit board using the same. The resin composition comprises the following components: (A) an adhesive polymer, (B) a light polymeric compound with an ethylene unsaturated bond and (C1) a stilbazole compound represented by the following general formula (1); in the formula (1), R1, R2 and R3 respectively represent alkyl with the carbon number of 1-20, alkoxyl with the carbon number of 1-6, alkyl ester with the carbon number of 1-6, amido, and alkyl amido, carboxyl, cyanogen group, nitryl or (methyl) acryloyl group with the carbon number of 1-20 independently, m and n respectively represent the integer of 1-3 independently, and a, b and c respectively represent the integer of 0-5 independently.
Owner:株式会社力森诺科 +1
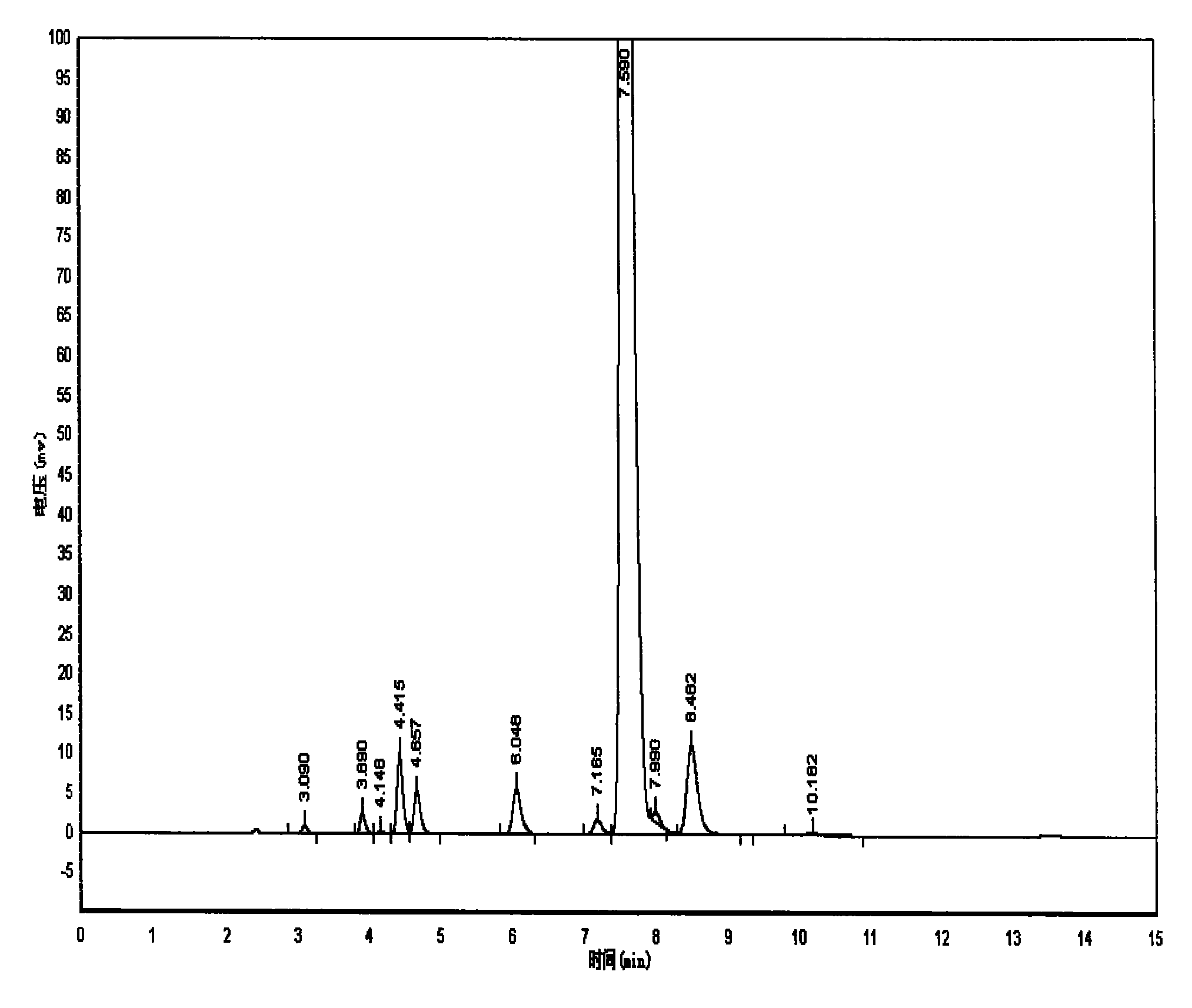
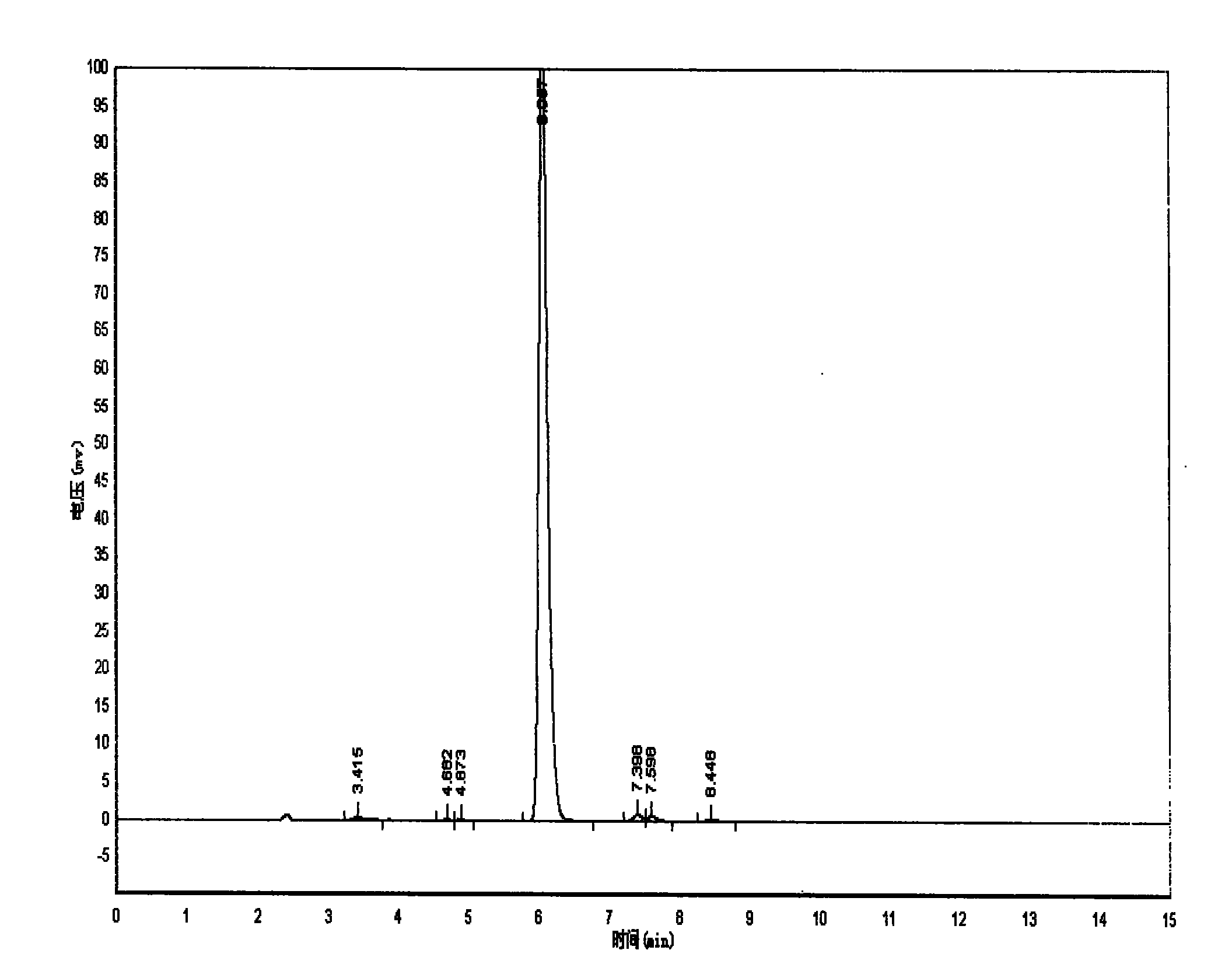
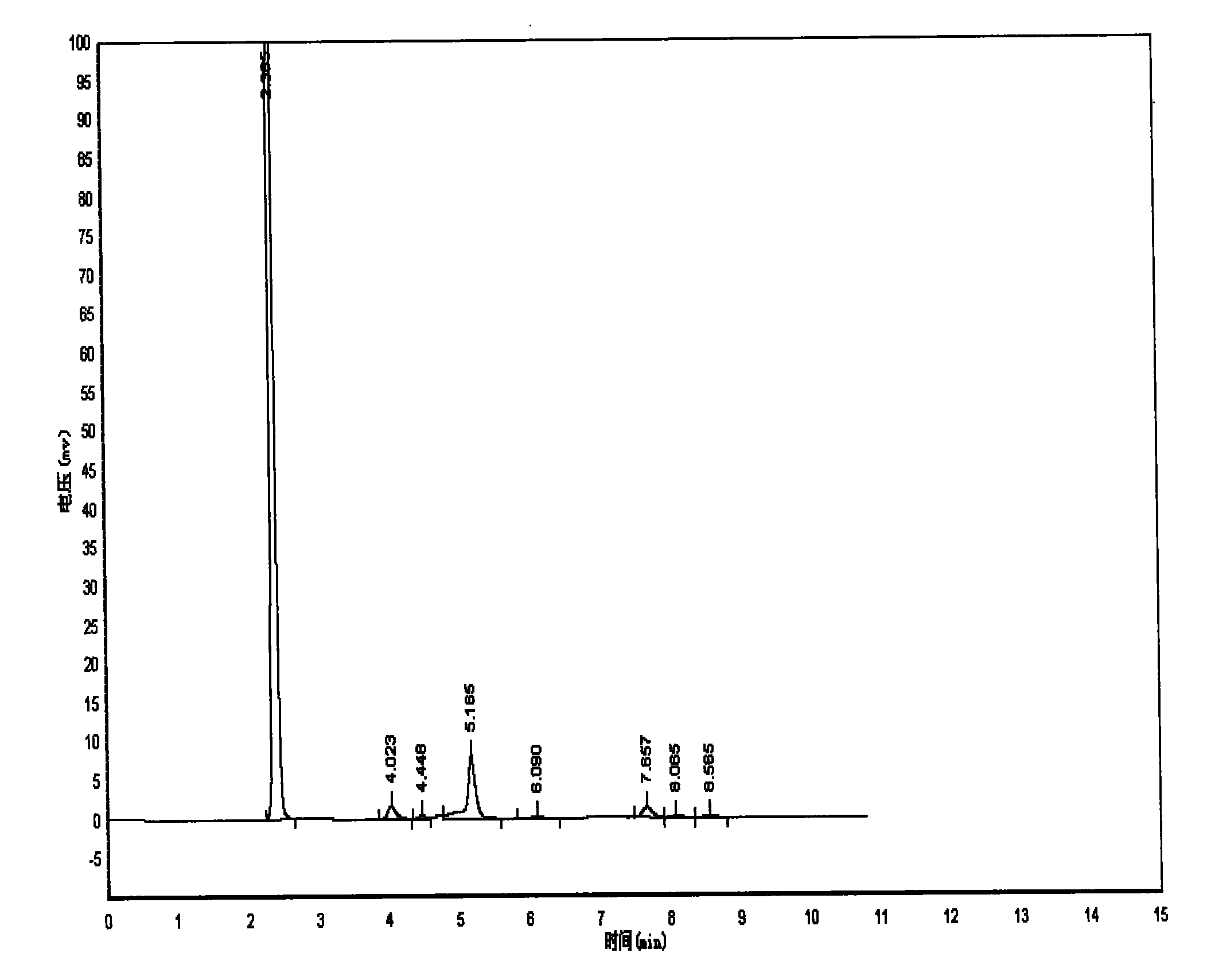
Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline
ActiveCN101570516AReduce pollutionReduce manufacturing costOrganic active ingredientsOrganic chemistry4 FluorophenylalanineNitration
The invention relates to a method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline (I). The method takes 4-methoxy-2-nitrobenzoic acid as initial material which is subjected to reduction and nitration to obtain 4-methoxy-5-nitryl-2-aminobenzoic acid, is then subjected to cyclization to obtain 7-methoxy-6-nitryl quinazoline-4(3H)-ketone, is again subjected to reduction and diazo reaction hydrolysis to obtain 7-methoxy-6-hydroxy quinazoline-4(3H)-ketone, after etherification reaction is carried out, phosphorus oxychloride is used for preparing 7-methoxy-6-[3-(4-morphjolinyl) propoxy]-4-chloroquinazoline, which is subjected to amination to obtain the compound in the formula (I). The initial material adopted by the preparation method is convenient and accessible, the cost is low, the process route is simple and reasonable, the three wastes produced in the process of preparation generates less pollution, therefore the preparation method can prepare final product with high quality and yield in mass production, and is suitable for industrialized production.
Owner:CHONGQING WORLD HAORUI PHARM CHEM
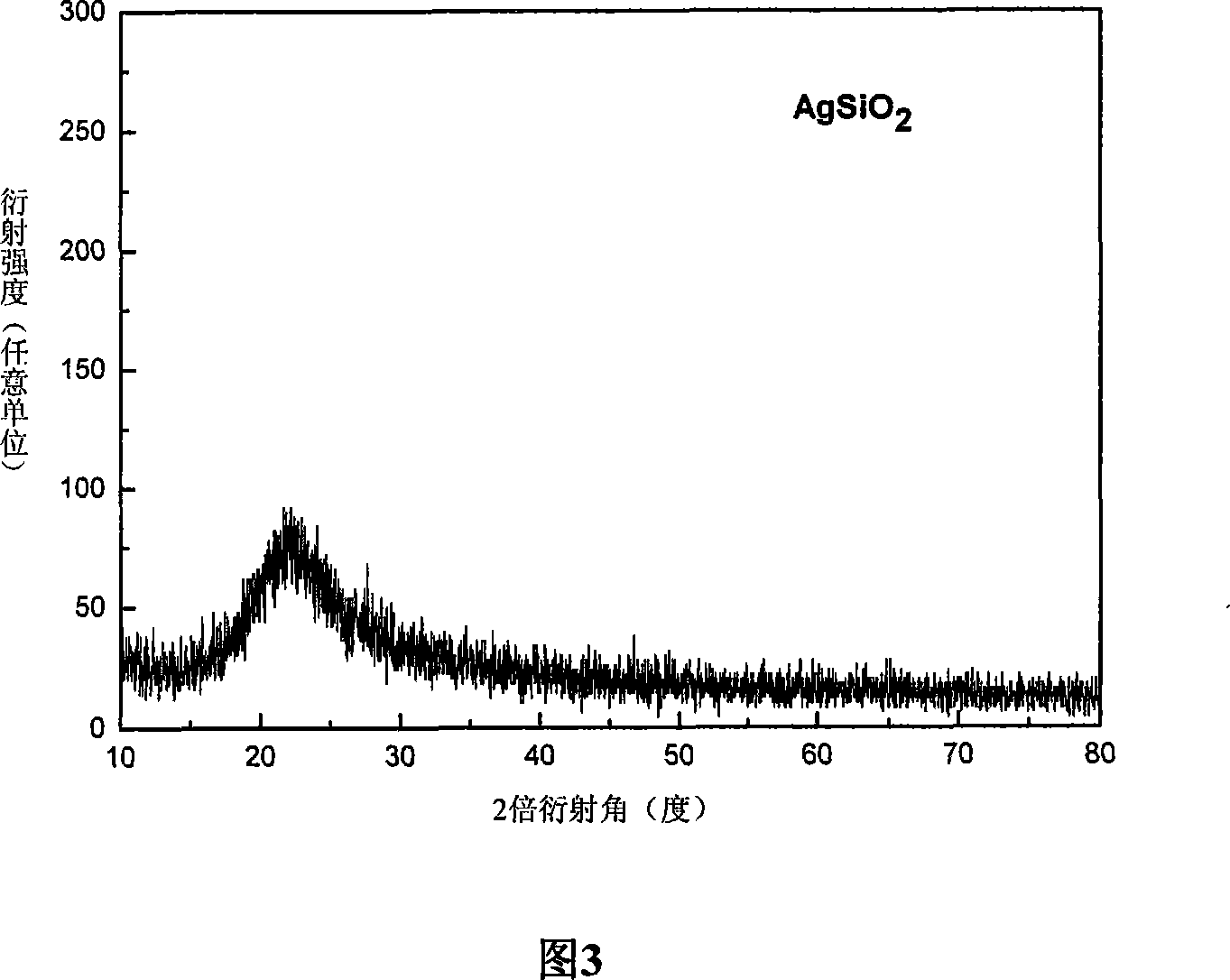
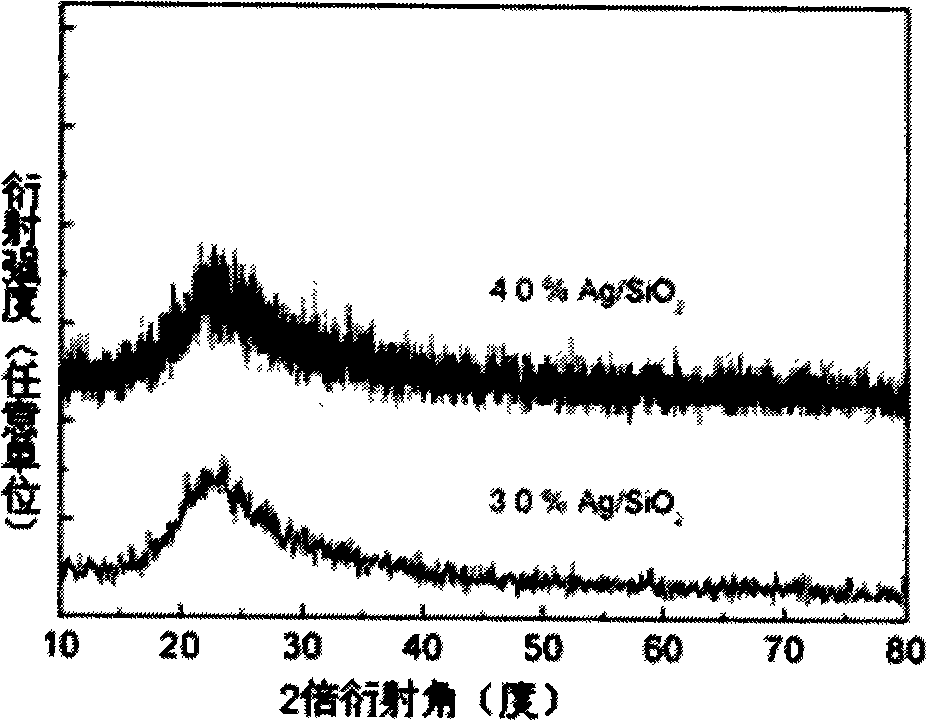
SiO2 supported nanometer silver catalyst, preparing method and applications thereof
InactiveCN101224422ALow costHigh yieldOrganic compound preparationAmino compound preparationNitro compoundPolyamide
The invention discloses a catalyst for loading silver on SiO2, a preparation method and application in a selective catalytic hydrogenation of chloro-aromatic compounds, nitryl-aromatic ene, nitryl-aromatic aldehydes, nitryl-aromatic ketone, and nitryl-aromatic polyamide. The synthetic method is that tetraethoxysilane and aminopropyl-triethoxysilane are added by weight proportion of 1: 0.1-1: 25 into an ammonia solution containing definite AgNO3, then the solution is stirred to form gelatinate under room temperature and dried under 100-200 DEG C for about 4-12 hours, thus obtaining the catalyst. The synthetic catalyst of the invention solves two problems of current catalytic hydrogenation of aromatic nitro-compounds: (1) a secondary reaction of dechlorination is generated during the hydrogenation of chloronitrobenzenes of aromatic nitro-compounds; (2) the selectivity of reaction is poor when alkene, aldehydes, ketone, nitrile and amide groups coexist.
Owner:NANJING UNIV
Method for synthesizing permanent violet
The invention discloses a method for synthesizing permanent violet. The method comprises the following steps of: 1) alkylating, namely, in a closed reaction kettle, putting carbazole into solution consisting of an inert halogenated aryl hydrocarbon solvent and an alkali metal hydroxide; and performing a reaction with bromoethane in the presence of a quaternary ammonium salt catalyst; 2) nitrifying, namely, treating an obtained N-ethyl carbazole-contianing organic phase by using dilute nitric acid; and after cooling, crystallizing and separating, obtaining a midbody 3-nitryl-N-ethyl carbazole by washing paste; 3) reducing, namely, after dissolving a 3-nitryl-N-ethyl carbazole material in inert halogenated aryl hydrocarbon, performing hydrogenated reduction by using fermium-containing Raney's nickel as a catalyst; and 4) performing a condensation reaction and a ring closing reaction. The method has the advantages that: 1) in the alkylation of the invention, the inert halogenated aryl hydrocarbon is taken as a solvent, so that pollution and cost are reduced and production devices are simplified at the same time; 2) in the method, the temperature for the nitration is improved, so that raw material consumption and the discharge of waste water are reduced; and 3) the process of industrial hydrogenated reduction is perfected, so that the problem of waste water reduction treatment is solved basically, and energy consumption is reduced obviously.
Owner:江西紫荆颜料化工有限公司
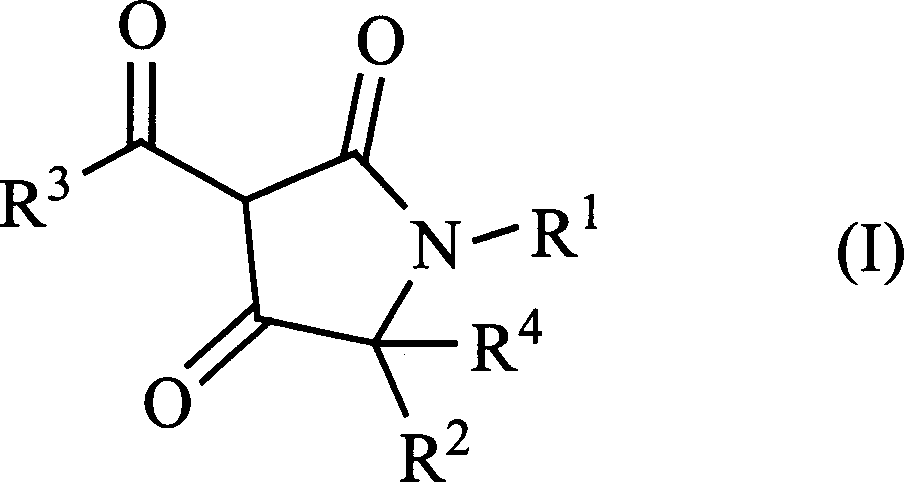
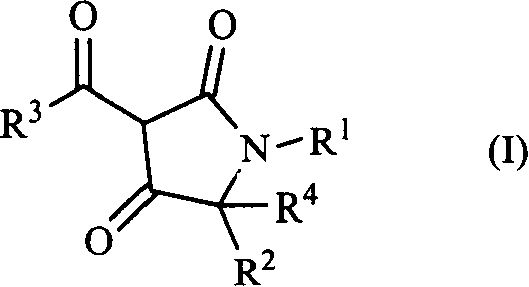
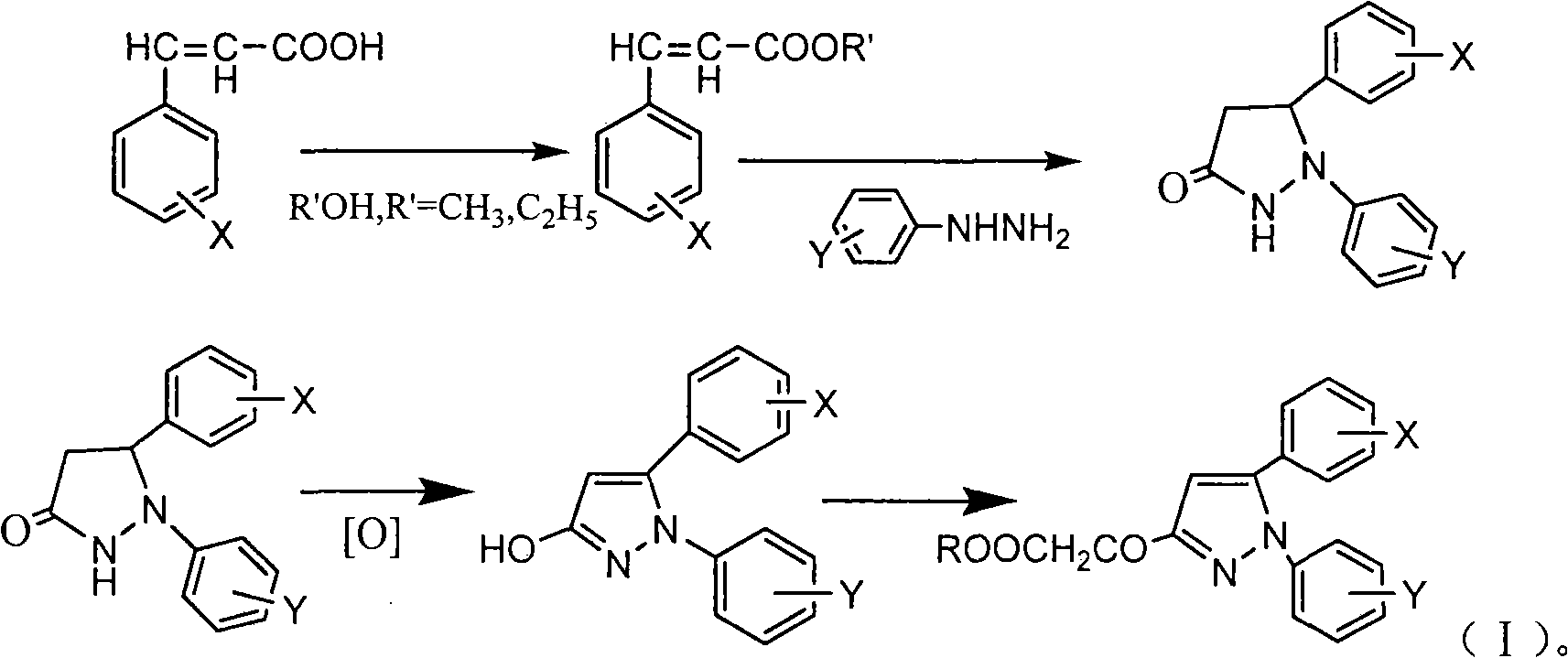
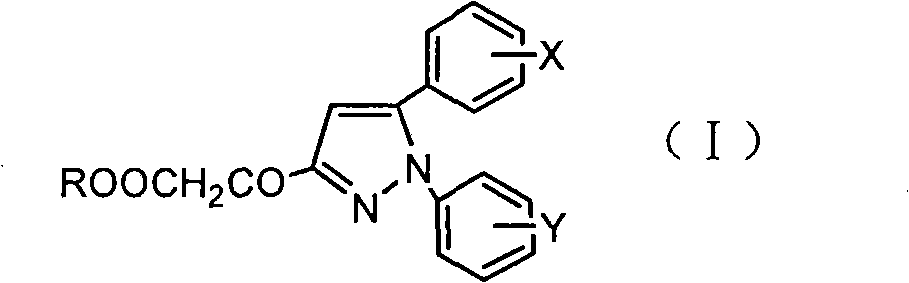
3-acyl-pyrrolidine-2,4-diketo compound and herbicidal activity
This invention relates to 3-acyl-pentazane-2,4-diketo compound. The compound (I) has high plant growing restraining and weed killing reactivity, and shows good selectivity to the plants. It can be the weed killing solvent in the broad-leave field to remove single-leave weed. There into, R1 : H, alkyl, alkoxyl alkyl, alkylogen, alkylogen alkoxyl alkyl, substituted aromatic base and etc; R2R4: H, alkyl, alkoxyl, phenyl alkoxyl, hydroxyl alkyl, alkoxyl, alkylogen, alkylogen alkoxyl, nitryl, cyan and etc, R2, R4 can be the same or different; R3: substituted phenyl, substituted naphthyl, substituted pyridyl, substituted thiazolyl, cyan and etc.
Owner:NANKAI UNIV
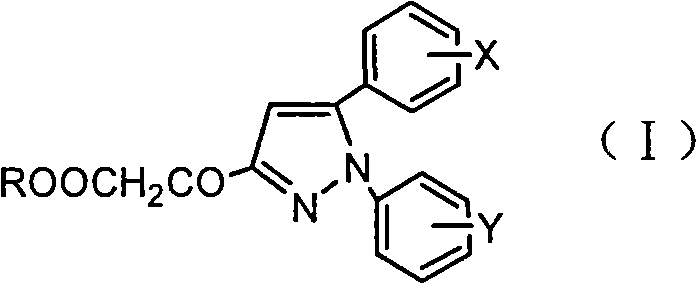
Pyrazoleoxy acetic acid compounds, preparation method and use
The invention discloses a pyrazolyloxyacetic acid compound, a preparation method and use thereof. In the compound with a structural formula (I), X or Y is respectively hydrogen, C 1-4 alkyl, C 1-4 alkoxyl, substituted or non-substituted phenoxyl, halogen, nitryl or trifluoromethyl; and R is hydrogen or C 1-4 alkyl. The invention also discloses a preparation method for the compound and application thereof in preparing bactericide.
Owner:NANJING UNIV OF TECH
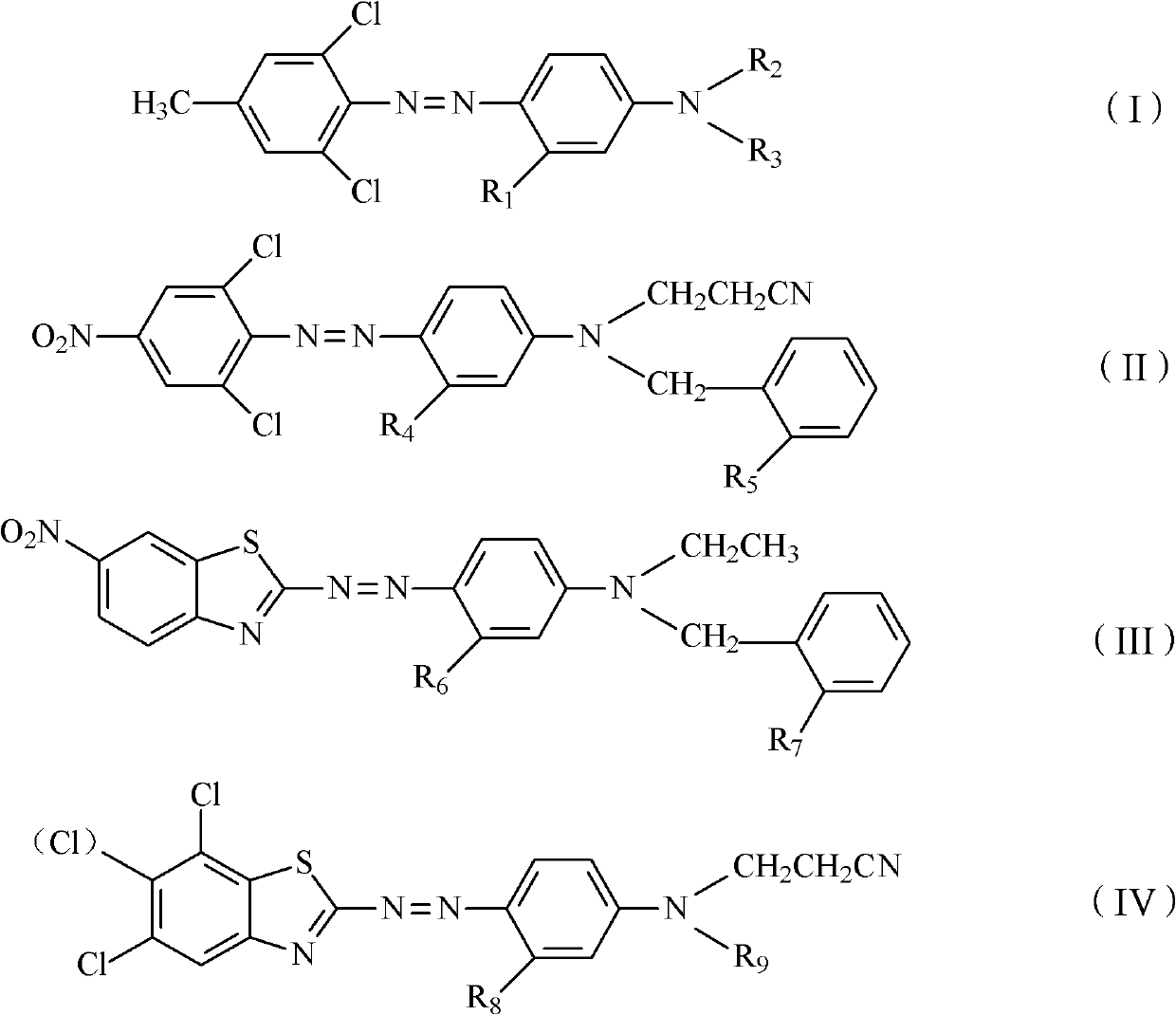
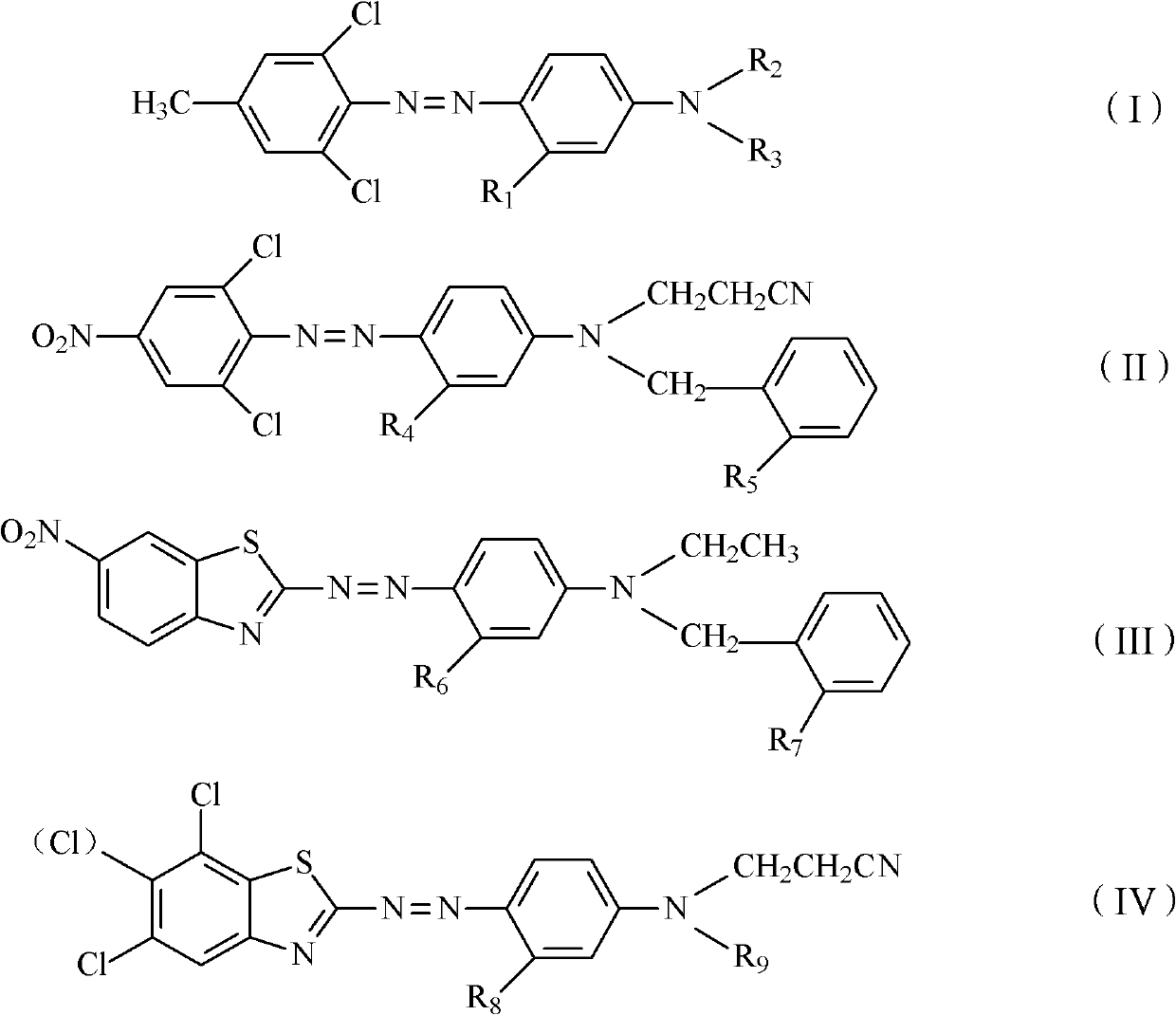
Disperse dye composition, dyeing method and preparation method of disperse dye composition
ActiveCN102618078AImprove oxidation resistanceGood alkali resistanceFibre treatmentOrganic dyesDisperse dyeHydrogen
The invention discloses a disperse dye composition, which consists of an original dye and an aid, wherein the original dye consists of any 2-4 kinds of dyes selected from 0-2 kinds of dyes shown as a general formula (I), 0-2 kinds of dyes shown as a general formula (II), 0-2 kinds of dyes shown as a general formula (III) and 0-2 kinds of dyes shown as a general formula (IV), wherein R1, R4, R6 and R8 in the formula (I), formula (II), formula (III) and formula (IV) are independently hydrogen, methyl or methoxy; when R2 is hydrogen, R3 is 2-nitrobenzophenone or 2,4-dinitrophenyl; when R2 is methyl, ethide or cyanoethyl, R3 is benzyl or 2-methyl benzyl; R5 and R7 are independently hydrogen, nitryl, methyl or ethyl; and R9 is methyl, ethyl, benzyl, 2-methyl benzyl or 2-nitrobenzophenone. The invention further discloses a dyeing method for a dacron. The disperse dye composition provided by the invention has the advantages of high dispersing property and high oxygen resistance.
Owner:ZHEJIANG WANFENG CHEM
Silicon dioxide supported nano-silver catalyst, preparation and use thereof
InactiveCN101301608ALow costHigh yieldOrganic compound preparationAmino compound preparationNitro compoundPtru catalyst
The invention discloses a silicon dioxide load nanometer silver catalyst, preparation method and application in nitro compound catalytic hydrogenation reaction thereof. The preparing method comprises adding 1.0g of silicon dioxide in 20-50ml aqueous solution with 0.016g-0.064g of silver nitrate dissolved therein; stirring for 8-12 hours at 40 DEG C; adding 10-50ml of alcohol; heating up to 110 DEG C solvent thermal reaction for 8-12 hours; filtrating pale yellow solid; drying the solid at 100-120 DEG C for 8-12 hours to obtain the silicon dioxide load nanometer silver catalyst. The catalyst prepared by the invention is used for nitryl aromatic compound selective hydrogenation to prepare amido aromatic compound, and can realize complete transformation under optimization condition.
Owner:NANJING UNIV
Method for preparing aromatic amine, alcohol and alkane by heterogeneous catalytic hydrogen transfer
InactiveCN101182274AWide variety of sourcesHigh activityOrganic reductionOrganic compound preparationChemical industryNitro compound
The invention belongs to the chemical industry technical field and concretely provides a method of preparing for aromatic amine, alcohol or alkane by multi-phase catalytic hydrogenation transfer. The invention considers aromatic nitryl compound, aldehyde ketone and alkene compound as a substrate, hydrogenous polyatomic molecule as a hydrogen donor and loading type nano-gold as catalyst for reflux and stirring for the multi-phase catalytic hydrogenation transfer reaction under 80 DEG C to 200 DEG C and 0.4 to 0.6MPa to prepare for the aromatic amine, alcohol or alkane. The reaction condition of the method of the invention is gentle; the environment is friendly; the applied catalyst has high reaction activity and good selectivity.
Owner:FUDAN UNIV
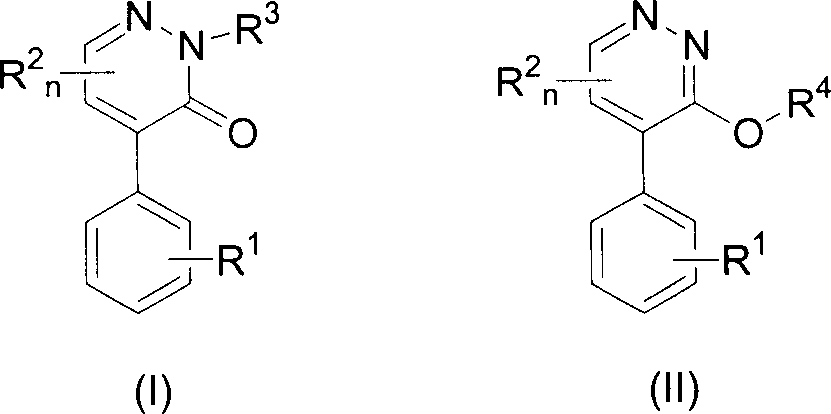
4-substituted phenyl pyridazine compound and herbicidal activity
This invention relates to 4-substitued phenyl pyridazine chemical compound and its weed killing reactivity. The chemical compounds (I)(II) have high weed killing reactivity. It can effectively kill the one-year ruderal and broad-leave weed. There after, R1: H, alkyl, alkoxyl, alkylogen, alkylogen oxide, halogen and etc; R2, H, alkyl, alkoxyl, phenyl alkoxyl, hydroxyl phenyl, alkoxyl alkyl, alkylogen, alkylogen alkoxyl, nitryl, cyan, alkyl amine, dialkyl, acetylamino and etc; R3:H, phenyl, hydroxyl phenyl, alkoxyl, alkylogen, halide, alkyl halide and etc; R4: H, benzoxazole, substituted benzoxazole base, benzothiazole and etc; R5: H, alkyl, alkylogen: R6R7: H, alkyl, R6, R7 can be the same or different, n=1 or 2.
Owner:NANKAI UNIV
Phosphorescent dye parent materials with tetrahedral structure, and application thereof in electroluminescent device
InactiveCN102775432AImprove migration abilityHigh electroluminescence efficiencyGroup 4/14 element organic compoundsGroup 5/15 element organic compoundsDisplay deviceQuinoline
The invention belongs to the technical field of organic electroluminescence material, in particular to a series of phosphorescent dye parent materials with a tetrahedral structure, and the application of the materials in an electroluminescent device. The phosphorescent dye parent materials can be used for a panel display device, a light-emitting diode (LED) and an electronic imaging device. The phosphorescent dye parent material has excellent electronic and hole transmission properties, can be independently used as a luminous layer and a current carrier transmission layer, and can be used as matrix and doped with other fluorescent or phosphorescent dyes. The compound has stronger fluorescent property in a state of solution or solid, can be used for forming an even film, and has better optical stability and thermal stability. R1, R2, R3 and R4 are respectively connected with phenyl group by a single bond, and are respectively selected from one of hydrogen, alkyl group, alkoxy, nitryl, hydroxyl, cyano-group, benzene cyano-group, amino, sulfydryl, halogen, diphenyl phosphoryl, furan, thiophene, pyrrole, pyridina, diazine, triazine, pyran, quinoline, benzpyrole, carbazole, aniline or phenothiazine, and X is C or Si.
Owner:JILIN UNIV
Disperse dye composition, dyeing method and preparation method of disperse dye composition
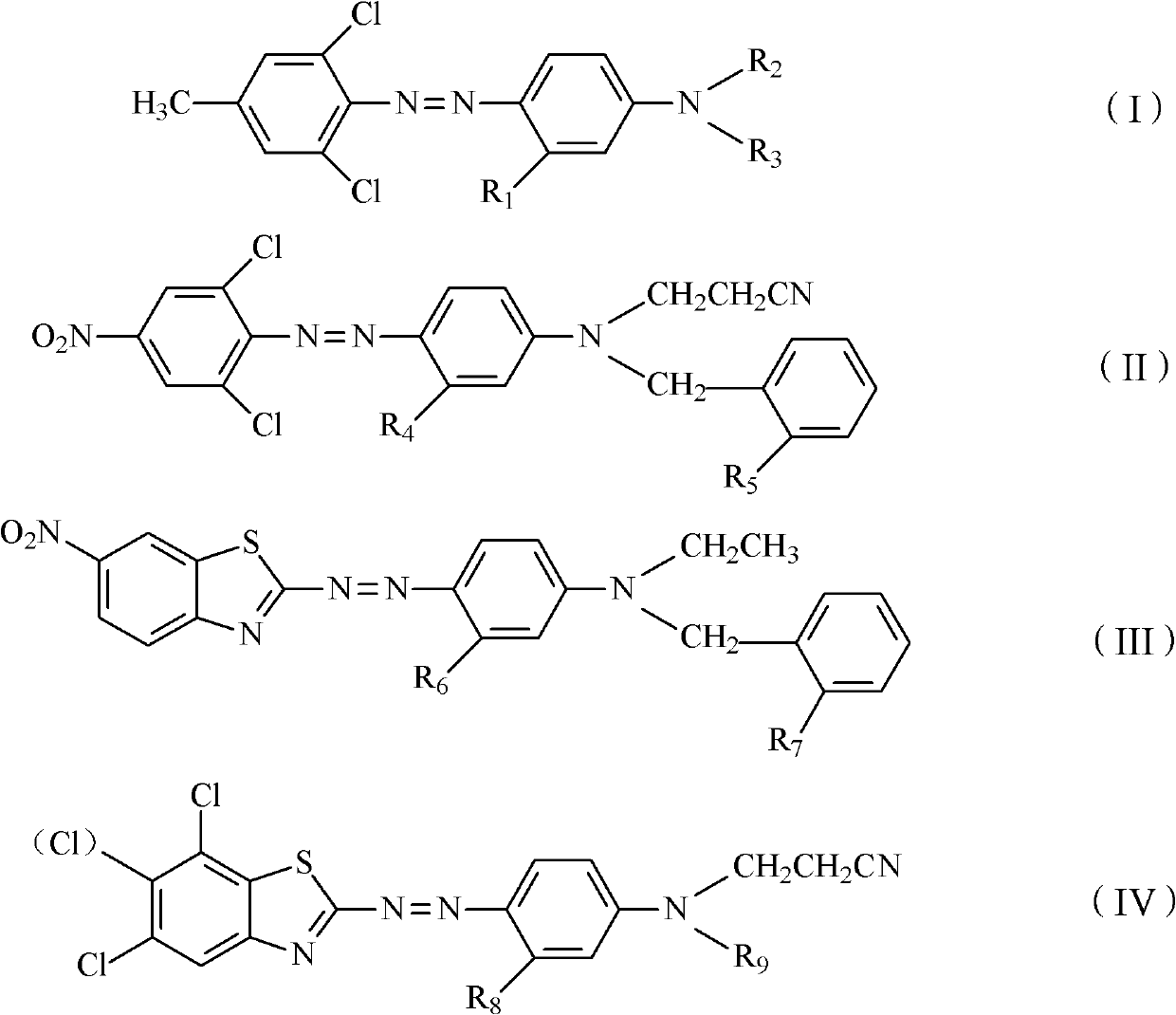
InactiveCN102618080AImprove oxidation resistanceGood alkali resistanceOrganic dyesDyeing processDisperse dyeHydrogen
The invention discloses a disperse dye composition, which consists of a dye A shown as a general formula (I), a dye B shown as a general formula (II), a dye C shown as a general formula (III) and an aid D, wherein in the formulae (I), (II) and (III), R1, R3 and R5 are independently hydrogen, methyl or methoxy; and R2, R4, R6 are independently hydrogen, nitryl, methyl or ethyl. The invention further discloses a dyeing method of a dacron and a preparation method of the disperse dye composition. The disperse dye composition provided by the invention has the advantages of high dispersing performance and oxidation resistance, and is suitable for a processing technology for pretreating and dyeing the dacron in one bath in the presence of an oxidant; and the dacron obtained by dyeing the disperse dye composition has the advantages of bright chromatic light, high soaping fastness, high dry rubbing fastness and superior wet rubbing fastness.
Owner:ZHEJIANG WANFENG CHEM
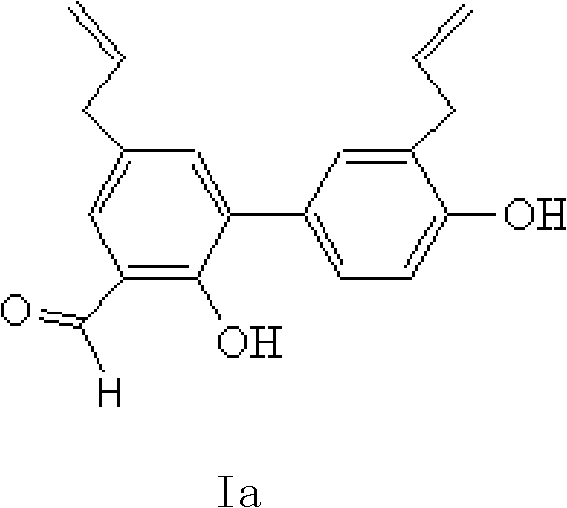
Honokiol series derivates, preparation and use thereof
ActiveCN101279901AGood antitumor activityHydroxy compound active ingredientsCarboxylic acid nitrile preparationHalohydrocarbonMagnolol
The invention relates to magnolola derivatives, the preparation method thereof, medical compounds with the magnolola derivatives as active components, as well as the application of the medical compounds in cancer treatment, pertaining to the technical field of pharmaceutical chemistry. The magnolola derivatives of the invention mainly include 3-substituted derivatives of magnolola; wherein the magnolola derivative with the formula I a is an intermediate product; the structural formula of the magnolola derivatives is II, wherein R1 is one of the following: H, halogen, hydroxyl, cyano, nitryl, amidocyanogen, alkyl, halogenated alkyl, cyanogens alkyl, hydroxide alkyl, allyl, amide, alkyloxyacyl, alkoxy, thiol group, alkyl, phenyl or heterocyclic radical. Cell poison tests and anti-tumor cell tests show that the magnolola derivatives have good anti-tumor effects.
Owner:CHENGDU JINRUI FOUND BIOTECH CO LTD
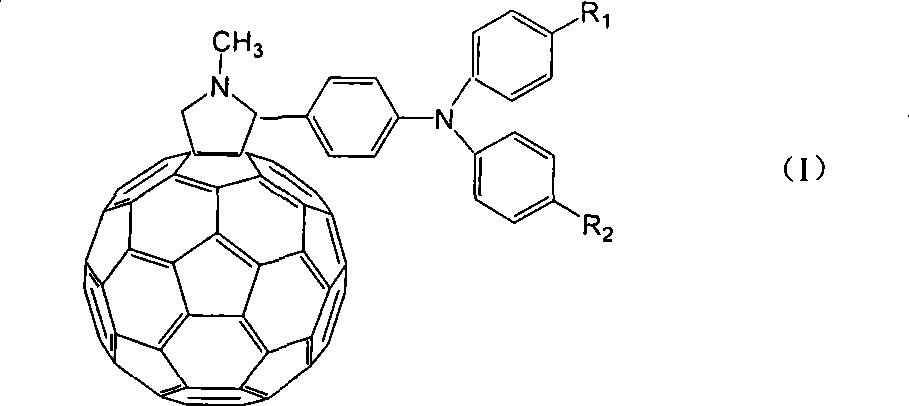
Application of C60 triarylamine derivative in solar energy battery
InactiveCN101186593AImprove solubilitySimple preparation processOrganic chemistryFinal product manufactureFuranKetone
The invention discloses a C60 triarylamine derivative of D-A type, and relative application in solar battery, wherein R1 and R2 are selected from hydrogen atom, halogen atom, nitryl, amidogen, alkyl, isoalkyl, alkoxy, alkylene, alkyne, alkyl aldehyde, alkyl ketone, pyridine or pyridine derivative, furan or furan derivative, imidazole or imidazole derivative, thiofuran or thiofuran derivative, aromatic radical or substituted aromatic radical, while R1 and R2 are not both hydrogen atoms. The inventive compound has significantly improved solubility than fullerene in organic solvent, to simplify the manufacture of large-area solar battery.
Owner:JIANGNAN UNIV
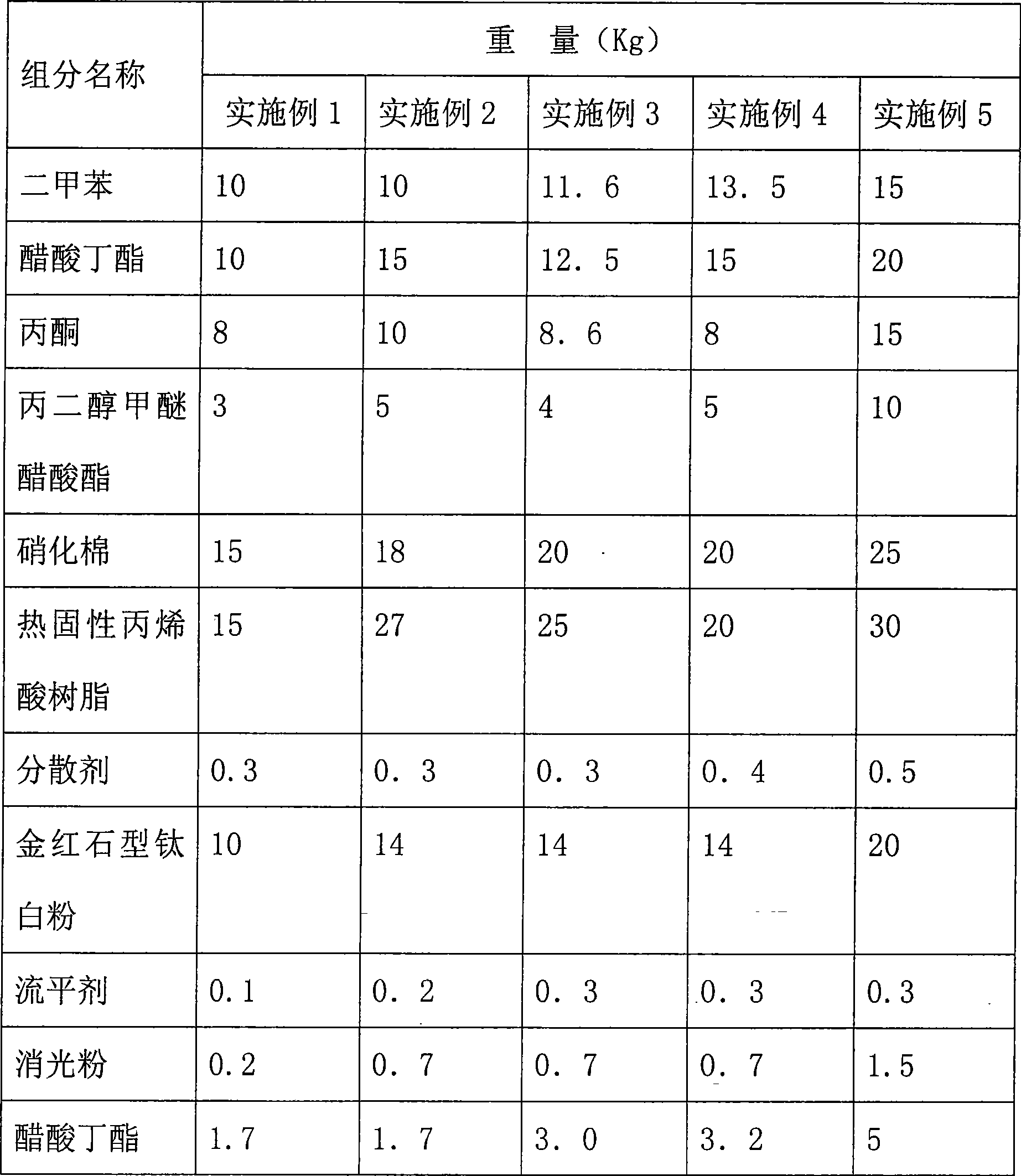
Yellowing-resistant nitro white matt finish and preparation method thereof, use method
ActiveCN101386727AGood weather resistanceHigh gloss retentionLiquid surface applicatorsCoatingsNitrocelluloseLow speed
The invention relates to anti-yellowing nitryl white matt surface lacquer which is prepared from the following components in weight percentage: 31 to 70 percent of a mixed solvent, 15 to 25 percent of nitrocellulose, 15 to 30 percent of thermosetting acrylic resin, 0.3 to 0.5 percent of a dispersant, 10 to 20 percent of rutile type titanium pigment, 0.1 to 0.3 percent of a leveling agent, 0.2 to 1.5 percent of flatting powder and 1.7 to 5 percent of butyl acetate. The preparation method comprises the following steps: the nitrocellulose is pre-dissolved; the mixed solvent and the nitrocellulose are mixed and are dispersed at intermediate speed till the nitrocellulose is fully dissolved; the thermosetting acrylic resin and the dispersant are evenly stirred at low speed and are added with the rutile type titanium pigment, the mixture is dispersed for 15 to 20 minutes at high speed till the fineness is less than or equal to 20 um; the flatting powder is added to the mixture and the mixture is dispersed for 10 to 15 minutes at high speed till the fineness is less than or equal to 25 um; the leveling agent and the butyl acetate are added to the mixture, and the mixture is dispersed for 10 minutes at intermediate speed until the mixture is even; and after the dissolved nitrocellulose is added to the mixture under the low-speed stirring, the mixture is evenly stirred for 10 to 15 minutes at low speed and is filtered to obtain the anti-yellowing nitryl white matt surface lacquer. The lacquer has good anti-yellowing property; when a lacquer film is placed in an indoor place where no sunlight irradiates, the lacquer film has no obvious yellowing within one year; and the lacquer has high weather resistance and gloss retention.
Owner:SHENZHEN GRANDLAND ENVIRONMENTAL COATING CO LTD
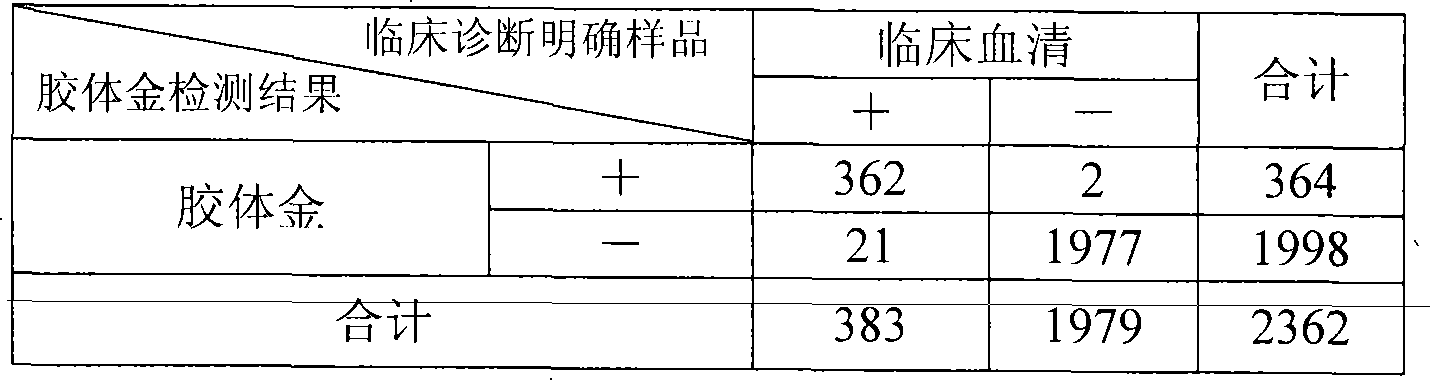
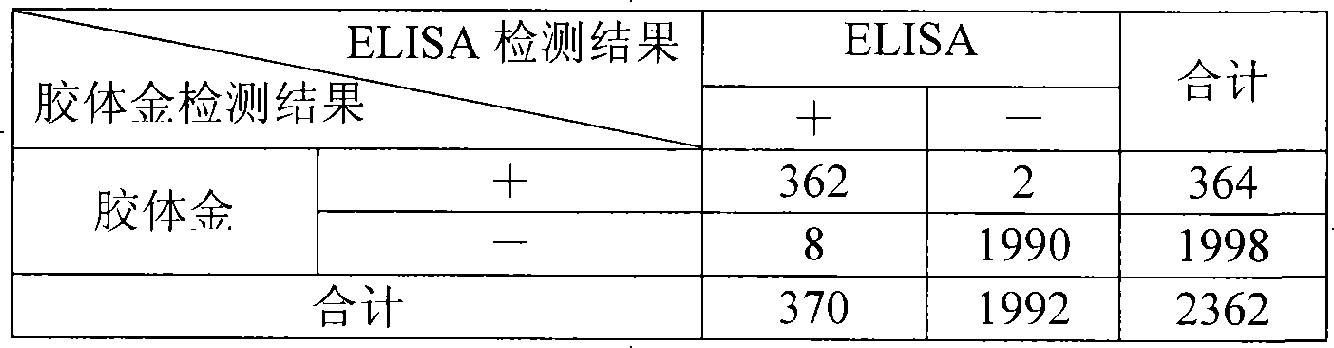
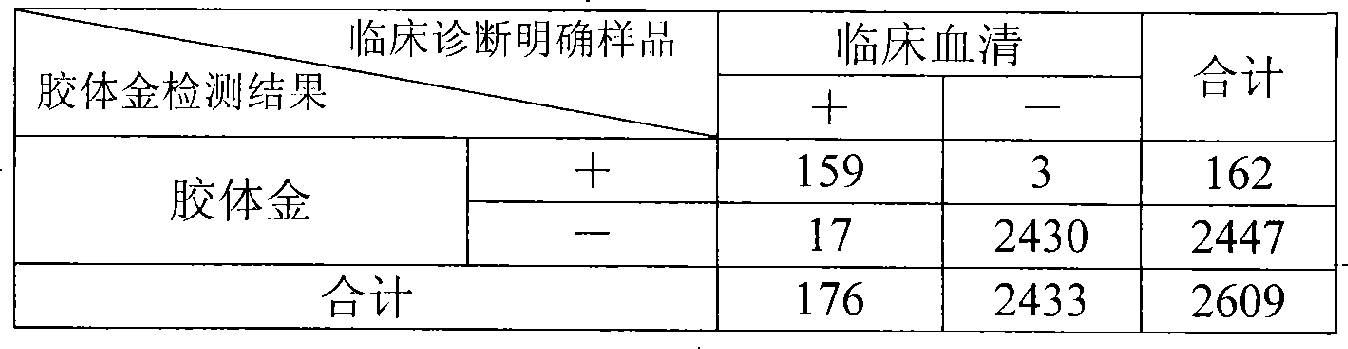
Colloidal gold chromatographic band for joint detecting specific IgM. IgG antibody and producing method thereof
The invention provides a colloidal gold chromatography band and the preparing technology to detect the special IgM,IgG. The colloidal gold is as the labeled thing of the colloidal gold. One end of the PVC back plate is pasted with the sample pad, the compound pad, the nitryl fibrous membrane, the other end is pasted with the absorbing pad; the compound pad is the glass fibrous membrane covered with the antigen-colloidal gold compound. The character is in that: the fibrous membrane includes three cover lines which cover the anti IgM antibody, the special antigen and the antibody according to the antigen separately. The invention detects the IgM antibody using the immune capture method principle and detects the IgG antibody by the double antigen sandwich method. So it can detect the special IgM, IgG antibody by one operation and the result has the high whole according ratio.
Owner:北京英诺特生物技术股份有限公司
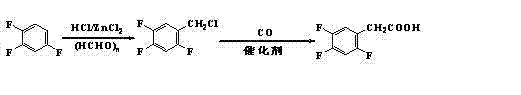
Preparation method 2,4,5-trifluorophenylacetic acid
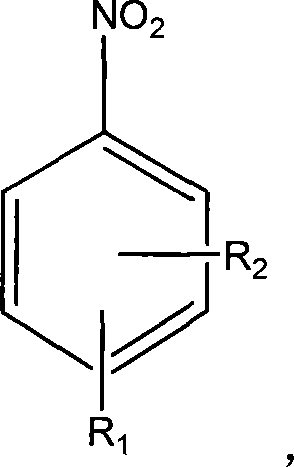
ActiveCN103012111AFew synthetic stepsMild reaction conditionsOrganic compound preparationCarboxylic preparation by ozone oxidationPhenylacetic acidNitrobenzene
The invention discloses a preparation method 2,4,5-trifluorophenylacetic acid. The method is characterized by consisting of four reaction steps of: A, reaction of 2,4,5-trifluoronitrobenzene (I) and diethyl malonate which are condensed to prepare 2,5-difloro-4-nitrobenzophenone diethyl malonate; B, hydrolysis, acidification and decarboxylic reaction of dibasic ester; C, reduction reaction of nitryl; and D, diazotization fluoridation of amino. The four reaction steps can be sequentially carried out according to A, B, C and D, or A, C, B and D, or A, C, D and B. According to the preparation method provided by the invention, condensation of 2,4,5-trifluoronitrobenzene (I) and diethyl malonate is easy to realize by means of high substituting activity of nitryl p-fluorine, and the raw material 2,4,5-trifluoronitrobenzene (I) is low in cost and easy to obtain and can be easily prepared by nitration and fluorination of 2,4-dichlor fluorbenzene. Compared with the prior art, the preparation method provided by the invention has the characteristics of low-cost and easily obtained raw materials, mild reaction condition, high total yield, low production cost and the like, and is comparatively suitable for industrialized production.
Owner:江苏中丽新材料有限公司
Triazine compounds having coccidiostat activity and preparation thereof
The invention discloses triazine compounds of formula (I) which have anti-coccidia activity and the pharmaceutically acceptable acid or alkali salts of the compounds, wherein, A stands for oxygen or sulfur, R1 and R2 stand for one or several groups of hydrogen, halogen atoms, alkyl, naphthene base, alkoxy, nitryl, trifluoromethyl, trichloromethyl, COR5, naphthene base and heterocyclic rings; R1 and R2 stand for same or different groups; R3 stands for hydrogen atoms, alkyl or naphthene base; R4 stands for hydrogen atoms, CO2R5 or CONHR6; and the compounds have good inhabitation effect for animal coccidiosis.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof
InactiveCN103755697AHas anti-influenza drug neuraminidase activityOrganic chemistryAntiviralsThiazoleHydrogen
The invention relates to 3-[[2-(benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as shown in chemical structural formulas I and II or a salt thereof. In the chemical structural formulas, R, X<1> and X<2> are selected from hydrogen, deuterium, C1-C2 alkyl, C3-C4 linear-chain alkyl or branched-chain alkyl; X<3> is selected from hydroxyl, methoxyl and ethyoxyl; is selected from hydrogen, deuterium, C1-C2 alkyl, fluorine, fluorine or bromine; X4 and X6 are selected from hydrogen, deuterium, C1-C2 alkyl, fluorine, fluorine, bromine or nitryl; X5 and X7 are selected from hydrogen, deuterium and C1-C2 alkyl. The invention also provides an application of the 3-[[2-(benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone in preparation of an influenza virus neuraminidase inhibitor.
Owner:HUNAN UNIV
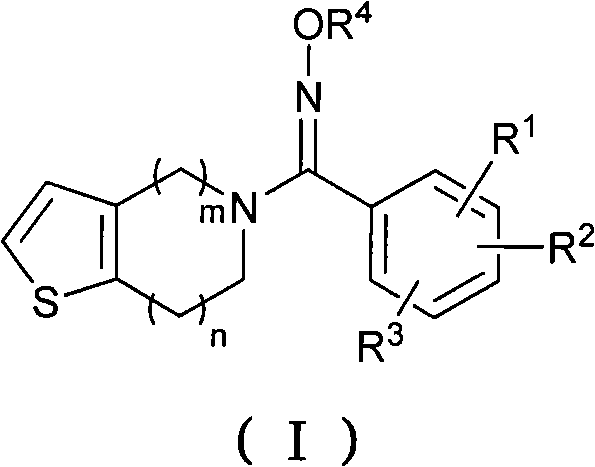
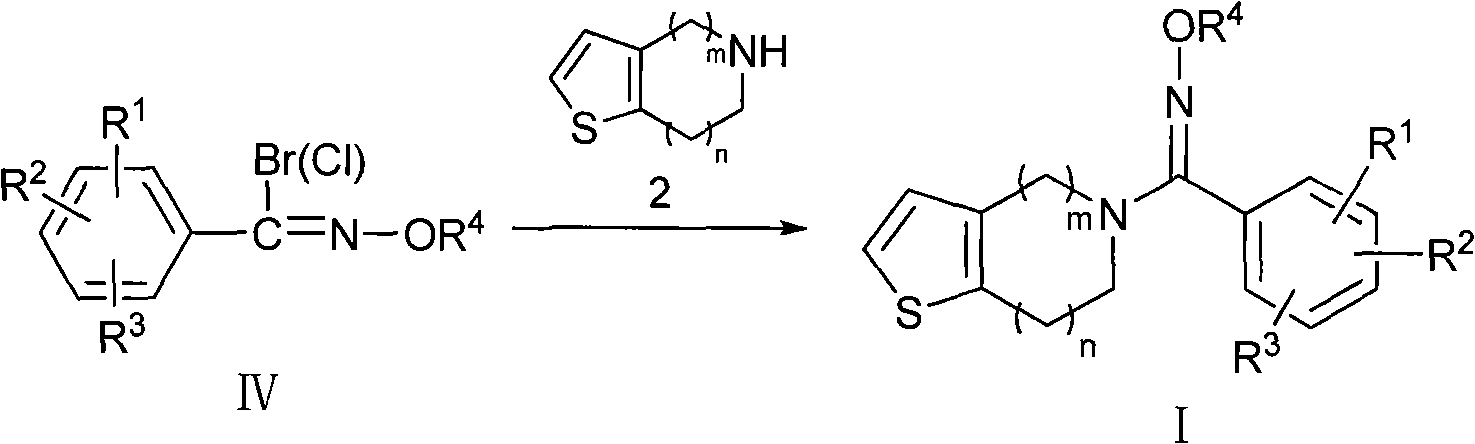
Oxime derivatives containing thienopyridine, preparation method and application thereof
The invention pertains to the technical field of medicaments for preventing platelet aggregation and provides an oxime derivative which contains thienopyridine and has a structure as shown in the general formula (I), and pharmaceutically acceptable salt thereof, wherein, m equals to 1 and 2 and n equals to 0 and 1; R <1 >, R <2> and R <3> collectively or individually refer to hydrogen, C1-C6 straight-chain or branched-chain alkyl, C3-C6 naphthenic base, halogen, hydroxyl, carboxyl, amino, amido, cyano, nitryl, C1-C4 alkoxy, substituted phenyl and substituted heterocyclic radical; and R<4> refers to hydrogen, C1-C4 straight-chain or branched-chain alkyl as defined in the Instruction in details. In addition, the invention also relates to a preparation method of the compounds and discloses medicament combinations of which the compound or the pharmaceutically acceptable salt of the compound is taken as active ingredient and the application of such medicament combinations as drugs for preventing platelet aggregation.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
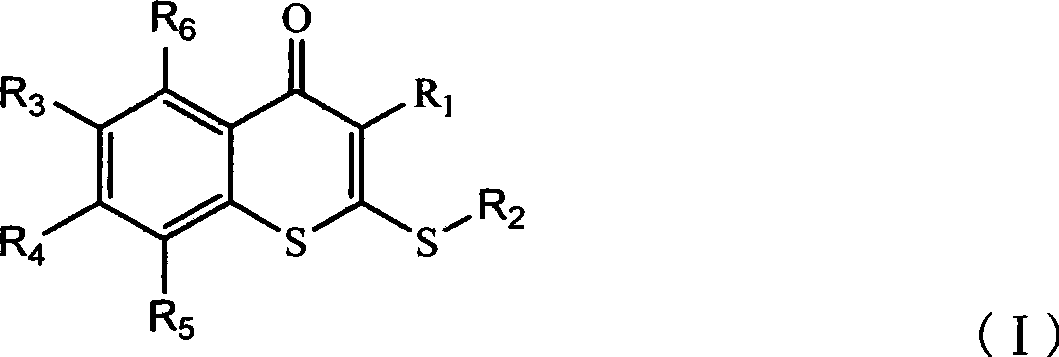
Thiochromone compound, synthetic method and application thereof in preparing antifungal medicaments
The invention relates to a thiochromone compound, which has the structure as right: wherein R1 is five-membered nitrogen-containing aromatic heterocycle substituted by hydrogen, methyl, nitryl or cyano; R2 is aliphatic alkyl of C1 to C12 or unsubstituted five-membered or six-membered aromatic ring aliphatic alkyl or five-membered or six-membered aromatic ring aliphatic alkyl selectively substituted by amino group, methyl, trifluoromethyl, trifluoromethoxy, nitryl, halogen and cyano; R3 is alkyl, hydrogen, fluorine, chlorine, bromine or iodine of C1 to C4; R4 is hydrogen, fluorine, chlorine, bromine, iodine or five-membered or six-membered heterocycle containing nitrogen; and R5 and R6 are hydrogen, fluorine, chlorine, bromine, iodine, alkyl of C1 to C4, hydroxyl, oxyl of C1 to C4, cyano, nitryl, amino group or amino group substituted by alkyl of C1 to C4. The compound has application in preparing antifungal medicaments, strong bacteriostatic activity for common pathogenic fungus and deep fungal infection, low toxicity, good stability and broad antifungal spectrum.
Owner:NANJING UNIV OF TECH
2-(2-benzyl hydrazono)-4-(benzofuran-5-yl) thiazole and preparation method and application thereof
The invention relates to 2-(2-benzyl hydrazono)-4-(benzofuran-5-yl) thiazole as shown in the chemical structural formula I and salt thereof, wherein R and R1 are selected from H, C1-C2 alkyl, C3-C4 straight chain alkyl or branch chain alkyl; X1-X5 are selected from hydrogen, C1-C2 alkyl, hydroxyl, methoxyl, ethoxyl, trifluoromethyl, fluorine, chlorine, bromine and nitryl; thiosemicarbazone is reacted with 2-holo-1-(7-methoxyl-2, 2-dimethyl-2, 3-dihydrobenzofuran-5-yl) alkyl ketone, and is neutralized to prepare 2-(2-benzyl hydrazono)-4-(2,2-dimethly-2, 3-dihydrobenzofuran-5-yl) thiazole. The compound is applied to preparing bactericide.
Owner:HUNAN UNIV
Phthalocyanine metal complex as well as preparation method and application thereof
ActiveCN104262350AHigh molar absorption coefficientGood spectral propertiesBiocideOrganic active ingredientsAntifungalPhotosens
The invention discloses a phthalocyanine metal complex as well as a preparation method and an application of the phthalocyanine metal complex. 3-[2,4,6-tri(dimethylamino methyl)-phenoxyl] phthalonitrile is prepared by using 2-phthalonitrile nitryl and 2,4,6-tri(dimethylamino methyl)-phenol as reactants, and further, the phthalocyanine metal complex substituted with amido or amido alpha bit is prepared. The phthalocyanine metal complex has the characteristics of high photosensitive activity and high water solubility; the phthalocyanine metal complex exists in water in a monomer mode, so that the photodynamic activity is facilitated in the water; the phthalocyanine metal complex is subjected to absorption spectroscopic redshift and shifted to a near-infrared area where is beneficial for penetrating through human tissues. The phthalocyanine metal complex is specially high in photodynamic antifungal activity and can be used for preparing photosensitizers or photodynamic medicines or photosensitive medicaments.
Owner:FUZHOU UNIV
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline](https://images-eureka.patsnap.com/patent_img/ab0c394b-e0e0-4d69-a4b9-9f77c6cd947e/A20091010360500051.PNG)
![Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline](https://images-eureka.patsnap.com/patent_img/ab0c394b-e0e0-4d69-a4b9-9f77c6cd947e/A20091010360500091.PNG)
![Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline Method for preparing 4-(3-chlorine-4-fluorophenylalanine)-7-methoxy-6-[3-(4-morpholinyl) propoxy] quinazoline](https://images-eureka.patsnap.com/patent_img/ab0c394b-e0e0-4d69-a4b9-9f77c6cd947e/A20091010360500101.PNG)
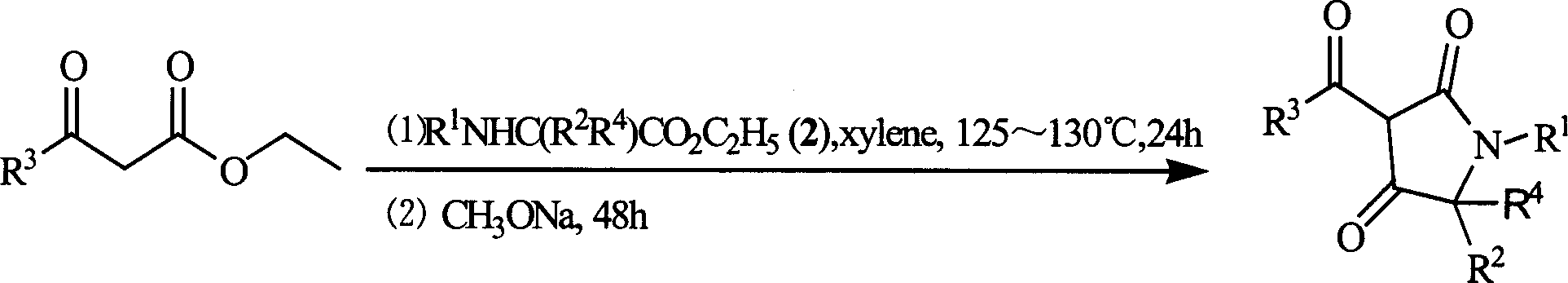

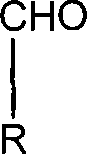
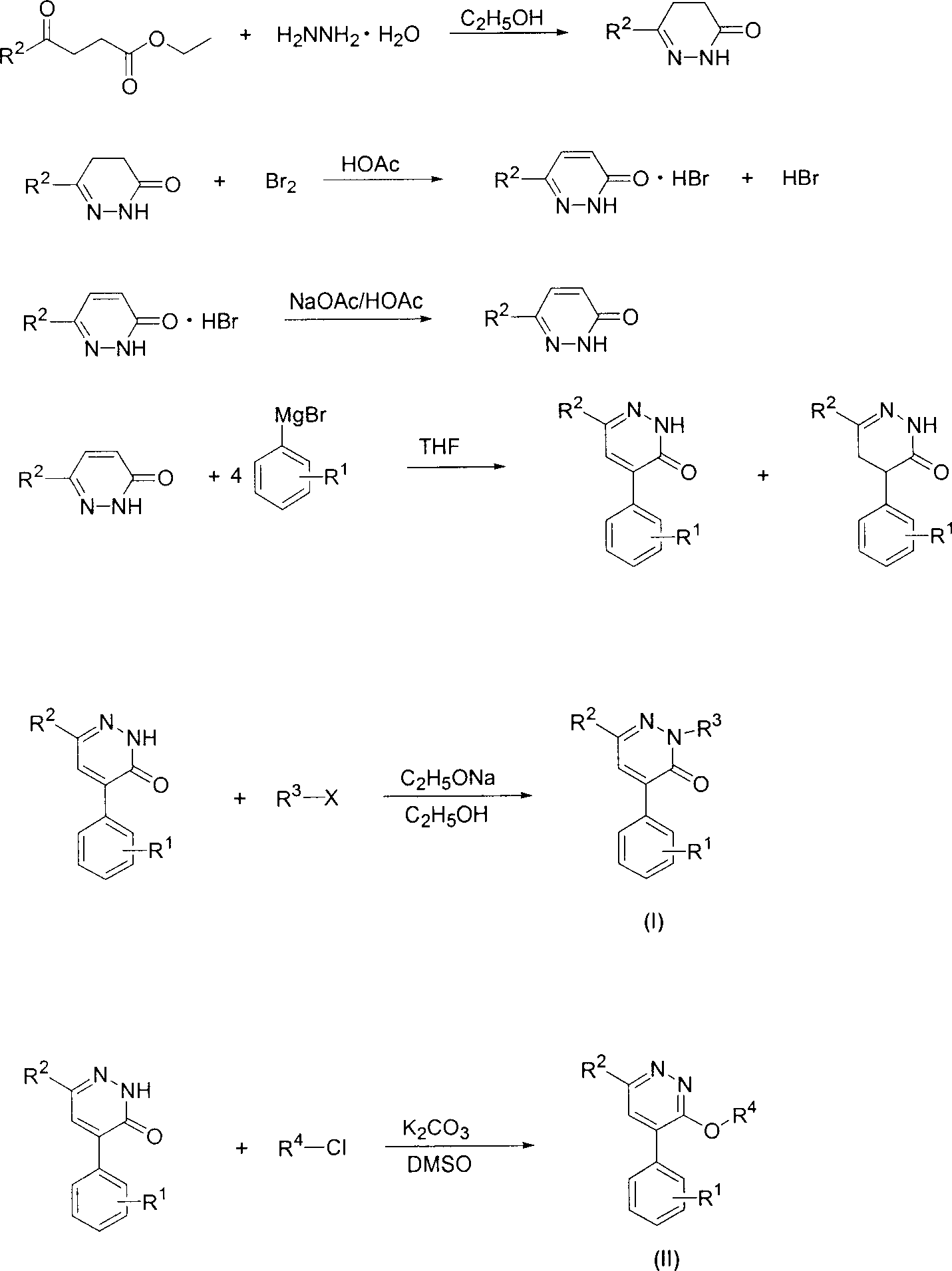
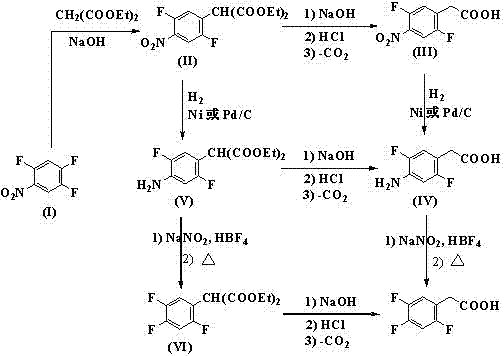
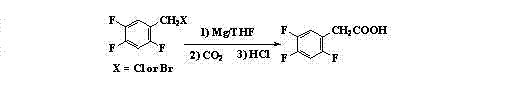

![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000021.PNG)
![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000022.PNG)
![3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof 3-[[2-(2-benzylamino) thiazole-5-yl]-methyl] quinolone-2(1H)-ketone as well as preparation and application thereof](https://images-eureka.patsnap.com/patent_img/1ec64ec5-fc1c-433f-bd19-00bb4ff43c61/BDA0000456427430000023.PNG)