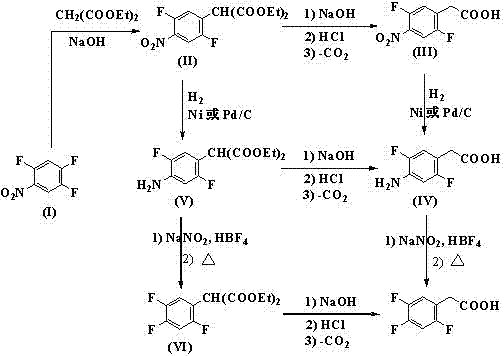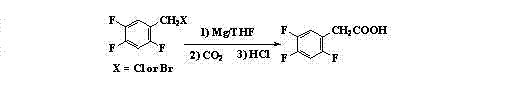Preparation method 2,4,5-trifluorophenylacetic acid
A technology of trifluorophenylacetic acid and trifluoronitrobenzene, applied in the field of antidiabetic drugs, can solve the problems of high cost, large pollution, complicated process and the like, and achieve the effects of few synthesis steps, low cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
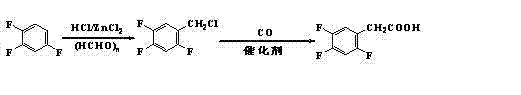
Image
Examples
Embodiment 1
[0044]Add sodium hydroxide (7.8g, 0.195mol), 2,4,5-trifluoronitrobenzene (35.4g, 0.2mol), diethyl malonate (30.4g, 0.19mol) into the four-necked flask and N,N-dimethylformamide (200mL), stirred and reacted at 40°C for 6h, added saturated brine, extracted with dichloromethane (3x100mL), washed the organic phase with saturated brine, dried over anhydrous magnesium sulfate, concentrated to obtain shallow The brown oil was directly used in the next reaction without purification.
[0045] The product 2,5-difluoro-4-nitrophenylmalonate diethyl ester obtained above, water (180mL), acetic acid (220mL) and concentrated sulfuric acid (65mL) were mixed, stirred and heated to reflux for 24h, after cooling Extract with dichloromethane (3x100mL), wash the organic phase with saturated brine, then adjust the pH to 10 with potassium carbonate solution, acidify the separated aqueous phase to pH 2 with 3mol / L hydrochloric acid, then extract with dichloromethane, and use the organic phase with W...
Embodiment 2
[0050] Add potassium hydroxide (85% content, 12.8g, 0.195mol), 2,4,5-trifluoronitrobenzene (35.4 g, 0.2 mol), diethyl malonate (30.4 g, 0 .,19 mol) and N,N-dimethylacetamide (200mL), stirred at 40°C for 6 h, added saturated brine, extracted with dichloromethane (3x100mL), washed the organic phase with saturated brine, anhydrous sulfuric acid It was dried over sodium and concentrated to obtain a light brown oil, which was directly used in the next reaction without further purification.
[0051] After the product 2,5-difluoro-4-nitrophenylmalonate diethyl ester, water (180mL), acetic acid (220mL) and concentrated sulfuric acid (65mL) were mixed, stirred and heated to reflux for 24h, after cooling Extract with dichloromethane (3x100mL), wash the organic phase with saturated brine, then adjust the pH to 10 with sodium carbonate solution, acidify the separated aqueous phase to pH 2 with 3mol / L hydrochloric acid, then extract with dichloromethane, and use the organic phase with Was...
Embodiment 3
[0056] Add sodium methylate (10.5g, 0.195mol), 2,4,5-trifluoronitrobenzene (35.4g, 0.2mol), diethyl malonate (30.4g, 0.19mol) and N- Methylpyrrolidone (200mL), stirred at 40°C for 6h, added saturated brine, extracted with dichloromethane (3x100mL), washed the organic phase with saturated brine, dried over anhydrous magnesium sulfate, concentrated to give a light brown oil without purification used directly in the next reaction.
[0057] The product 2,5-difluoro-4-nitrophenylmalonate diethyl ester obtained above, water (180mL), acetic acid (220mL) and concentrated sulfuric acid (65mL) were mixed, stirred and heated to reflux for 24h, after cooling Extract with dichloroethane (3x100mL), wash the organic phase with saturated brine, then adjust to pH10 with sodium carbonate solution, acidify the separated aqueous phase with 2mol / L hydrochloric acid to pH2, then extract with dichloroethane, organic The phase was washed with saturated brine, dried over anhydrous magnesium sulfate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com