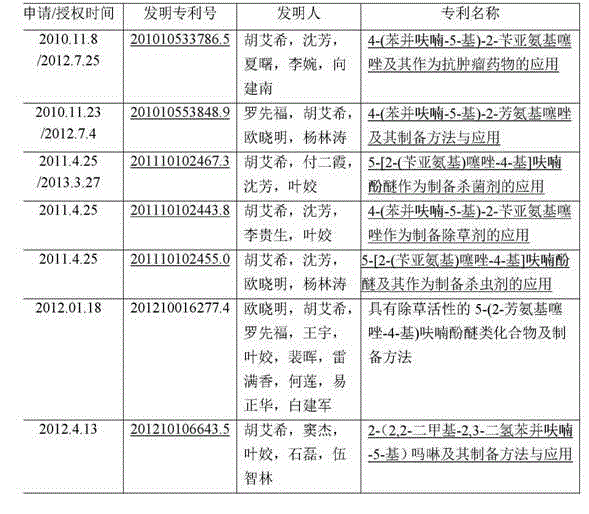
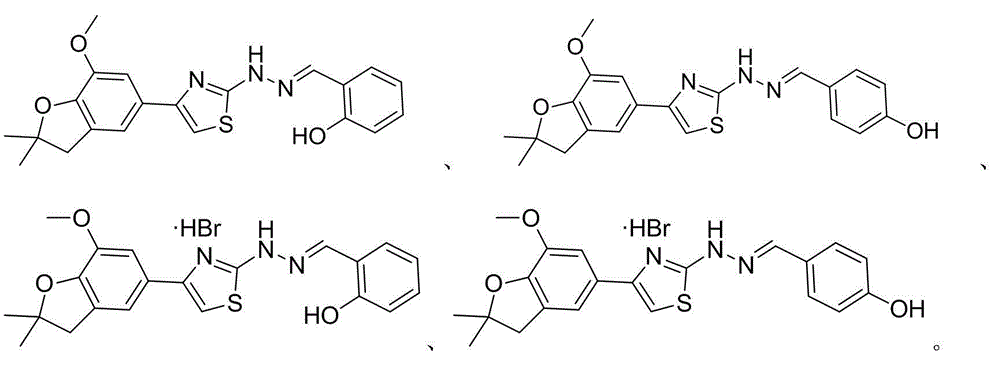
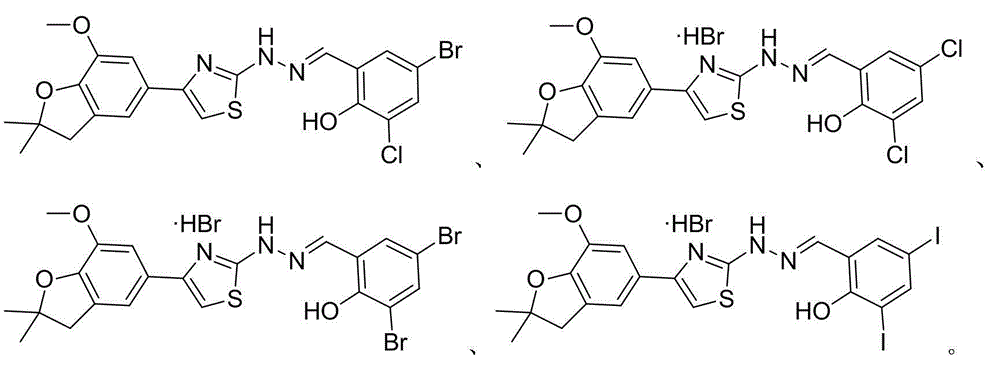
2-(2-benzyl hydrazono)-4-(benzofuran-5-yl) thiazole and preparation method and application thereof
A technology of dichlorobenzylhydrazine and nitrobenzylhydrazine, which is applied in the field of new compounds and their preparation, and can solve problems such as high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of 2-bromo-1-(7-ethoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone (C1)
[0038]
[0039] C1
[0040] 4.66 g of 1-(7-ethoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone, 80 mL of ethanol, stirred and refluxed, added 8.96 g of copper bromide, After reacting for 2 h, the reaction solution was filtered while it was hot, and the solvent was recovered by rotary evaporation, dissolved in ethyl acetate, washed with dilute acid, filtered, washed with water, separated, dried, and distilled under reduced pressure to obtain a crude product, which was recrystallized from ethanol to obtain a white solid C1; yield 72.0 %, m.p. 98~100 ℃.
Embodiment 2
[0042] Preparation of 2-bromo-1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone (C2)
[0043]
[0044] C2
[0045] 4.38 g of 1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)ethanone, 80 mL of ethanol, stirred and refluxed, added 8.96 g of copper bromide, After reacting for 2 h, the reaction solution was filtered while it was hot, and the solvent was recovered by rotary evaporation, dissolved in ethyl acetate, washed with dilute acid, filtered, washed with water, separated, dried, and distilled under reduced pressure to obtain a crude product, which was recrystallized from ethanol to obtain a white solid C2; yield 50.0 %, m.p. 90~91 ℃.
Embodiment 3
[0047] Preparation of 2-bromo-1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)acetone (C3)
[0048]
[0049] C3
[0050] 9.37 g of 1-(7-methoxy-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)acetone, 120 mL of ethanol were stirred and refluxed, and 18.02 g (0.08 mol) of bromine were added in batches Copper chloride, reacted for 2 h, filtered the reaction solution while it was hot, evaporated the solvent, dissolved in ethyl acetate, washed with dilute acid, filtered, washed the filtrate with water, separated, and distilled under reduced pressure to obtain a crude product, which was recrystallized from ethanol to obtain a white solid C3; Rate 97.8%, m.p. 85~87 ℃.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com