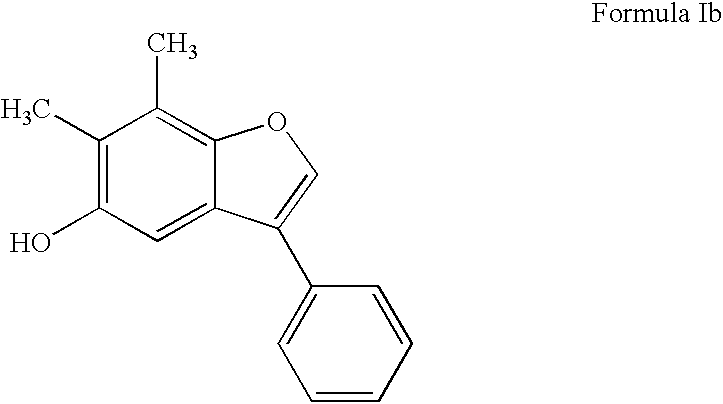
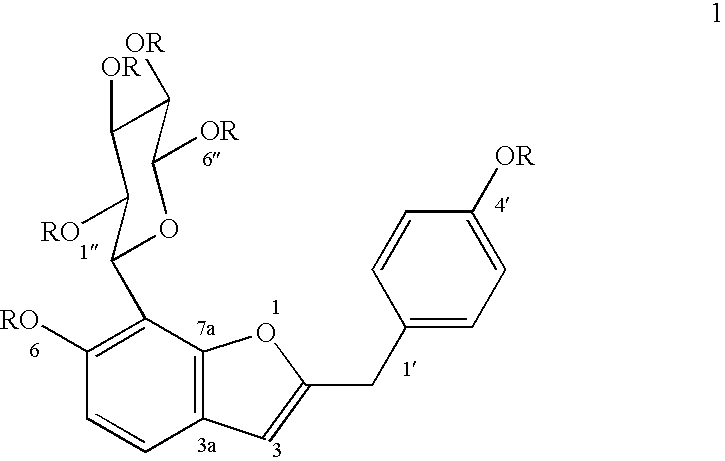
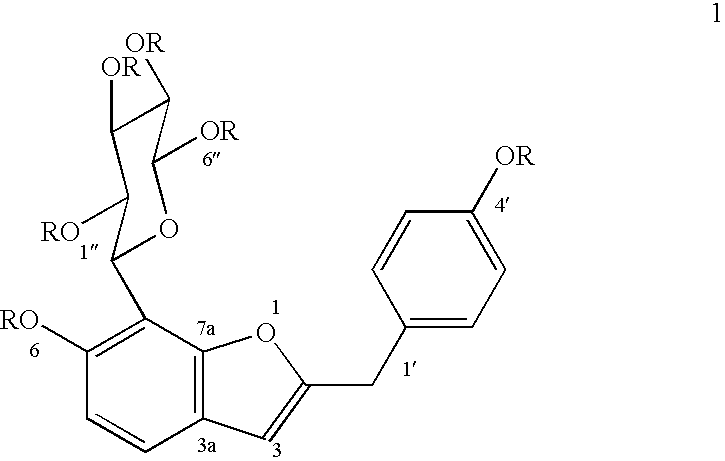
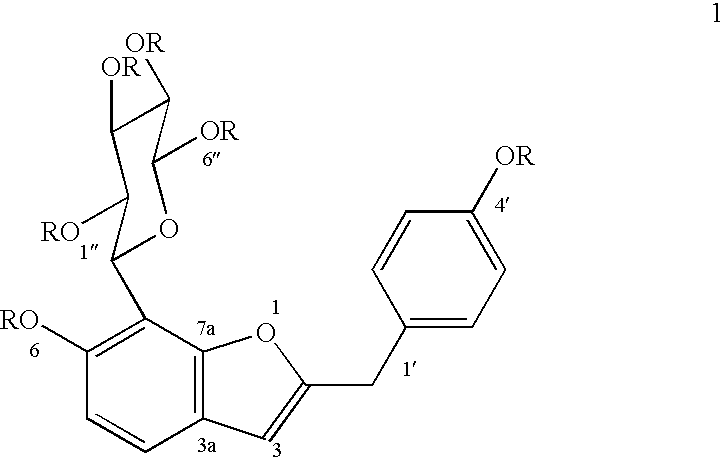
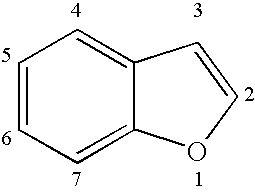
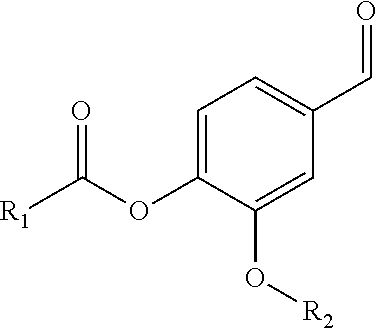
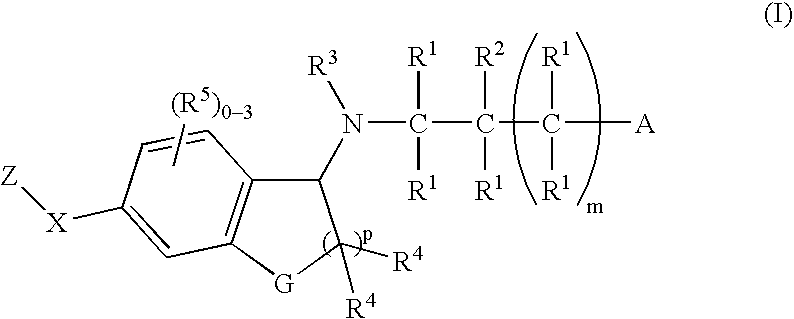
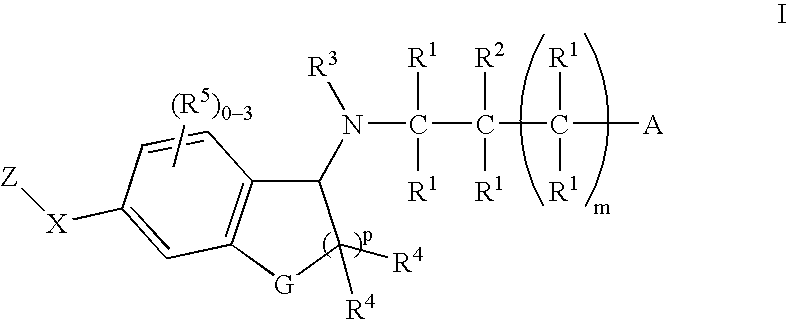
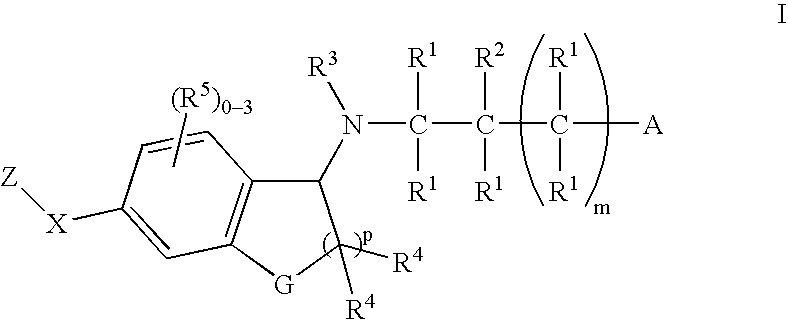
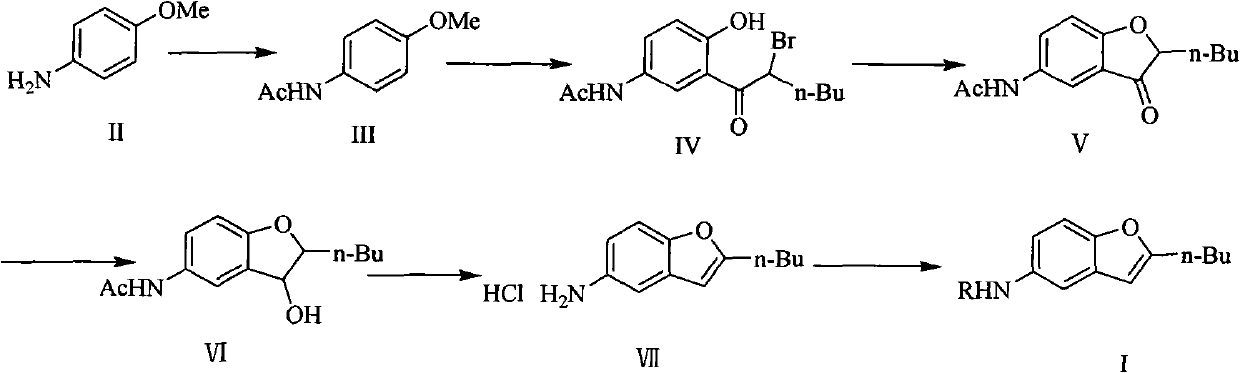
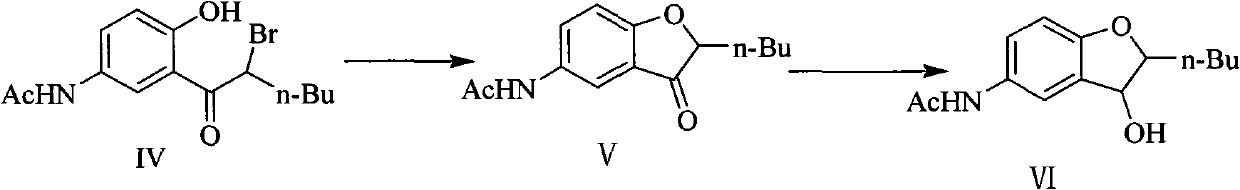
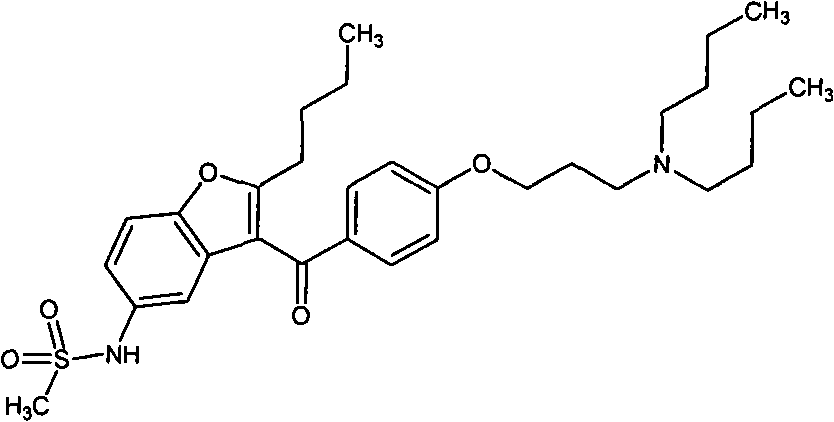
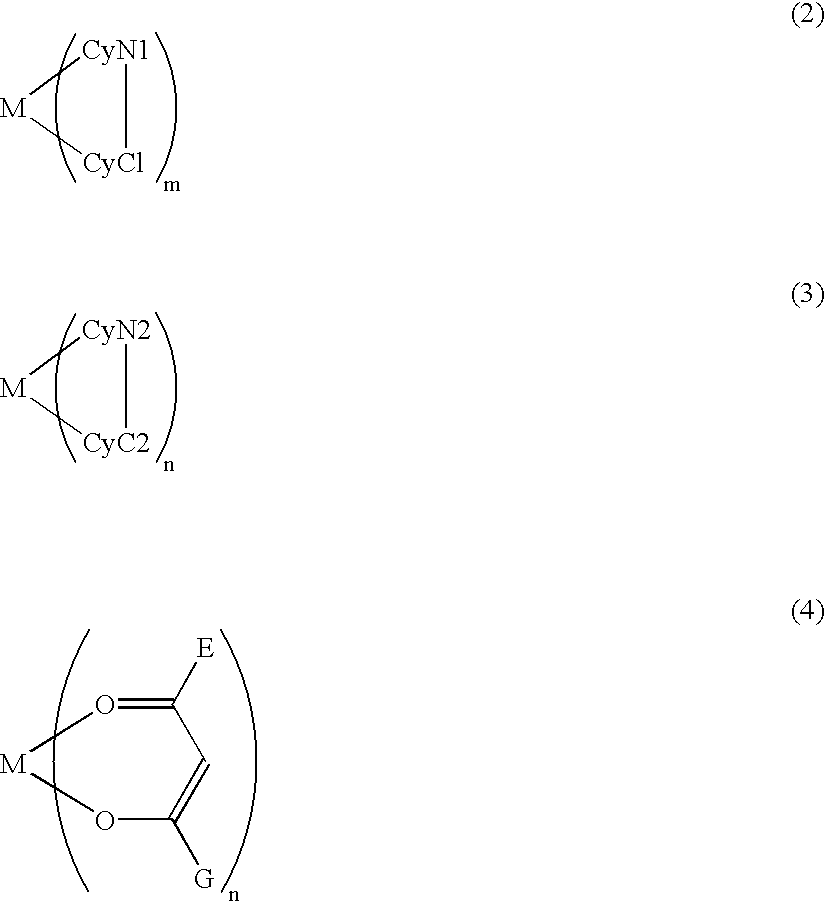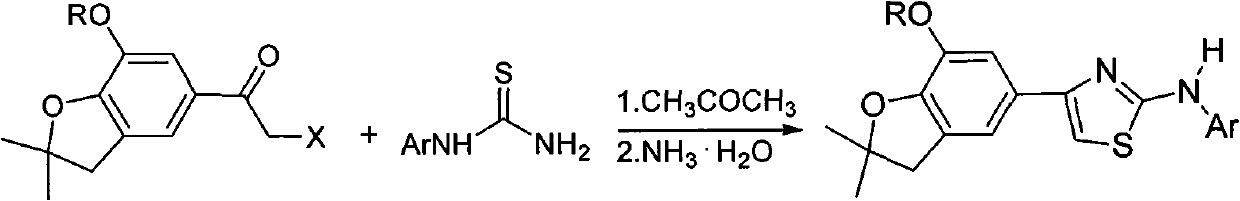Patents
Literature
1551 results about "Benzofuran" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
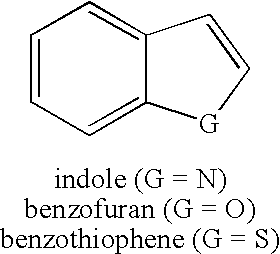
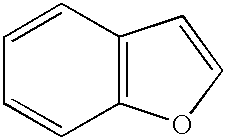
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the "parent" of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
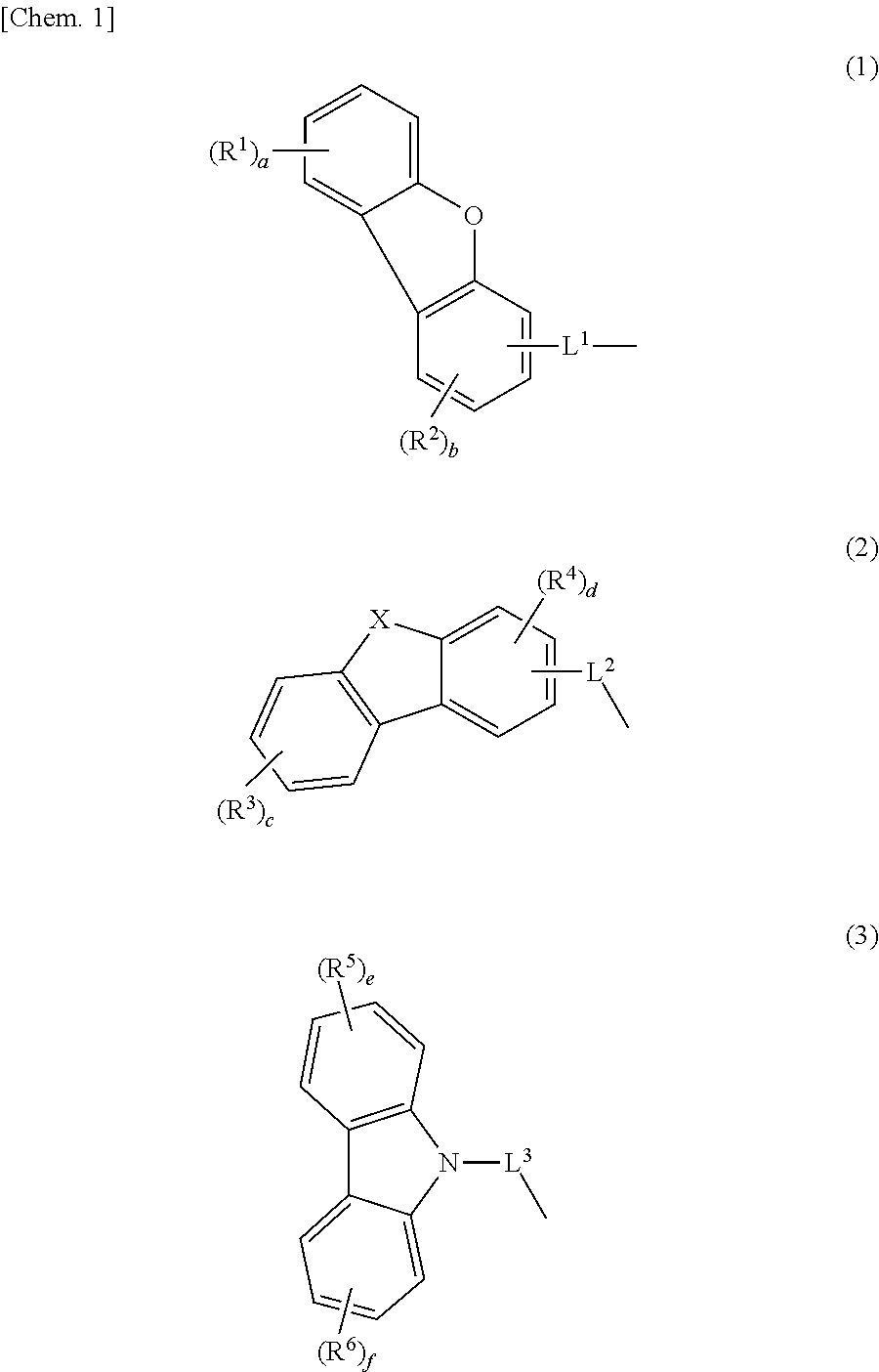
Aromatic amine derivative, and organic electroluminescent element
ActiveUS20110278551A1Improve efficiencyLong life-timeOrganic chemistryElectroluminescent light sourcesOrganic filmHole transport layer
Provided are an organic electroluminescence device that not only provides high efficiency but also has a long lifetime, and an aromatic amine derivative that realizes the device. The organic electroluminescence device includes an aromatic amine derivative, including at least one substituent A having dibenzofuran and at least one substituent B selected from groups each having dibenzofuran or carbazole, in a molecule thereof, in which the substituent A and the substituent B include groups different from each other, and the substituent A and the substituent B are bonded to the same nitrogen atom, or different nitrogen atoms, in the molecule. The molecules of the aromatic amine derivative hardly crystallize, which improves a yield in the production of the organic electroluminescence device. In the organic electroluminescence device, including an organic thin film layer formed of one or more layers including at least a light emitting layer, the organic thin film layer being interposed between a cathode and an anode, the aromatic amine derivative is contained in at least one layer, particularly a hole transport layer, in the organic thin film layer.
Owner:IDEMITSU KOSAN CO LTD
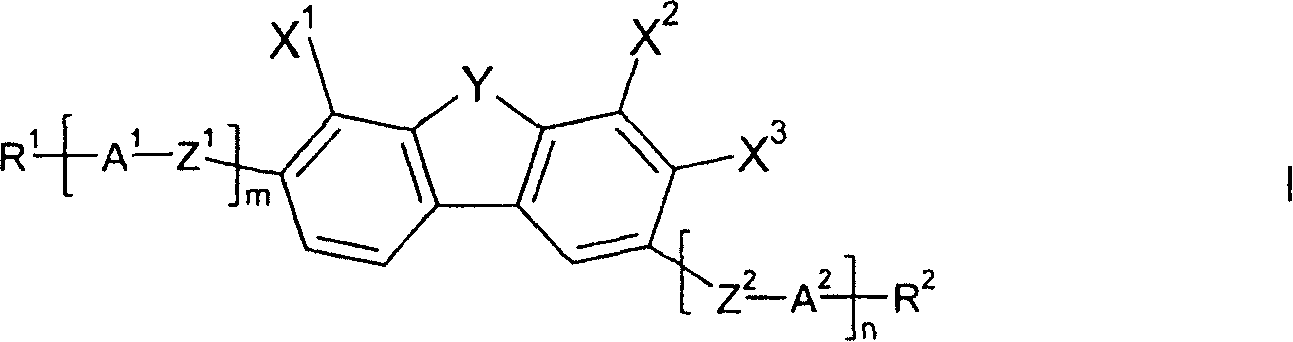
Dibenzofuran-, dibenzothiophene- and fluorene derivatives
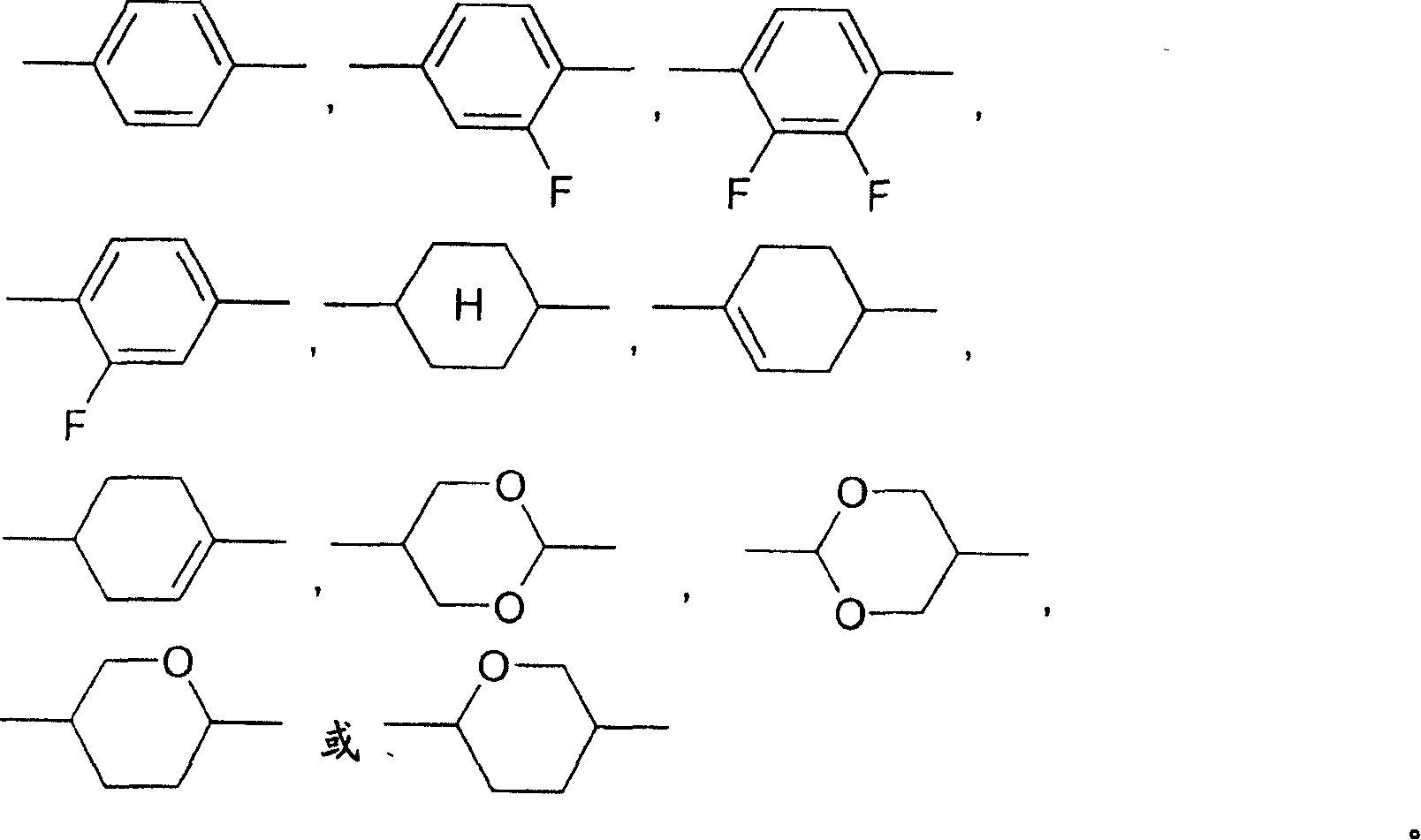
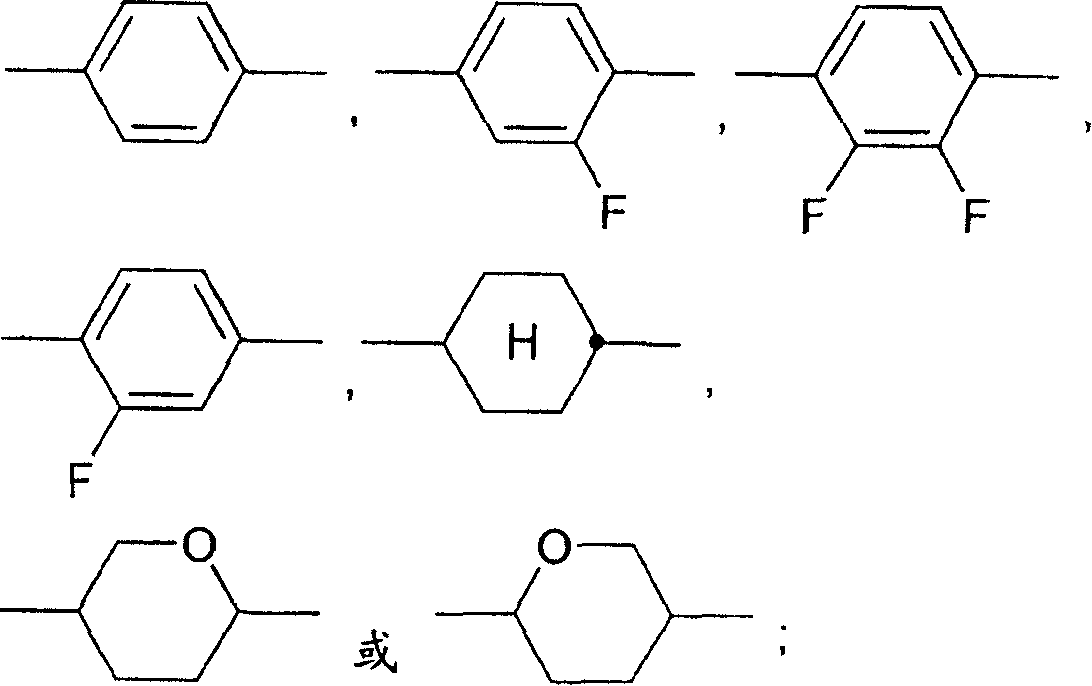
The present invention relates to compounds of formula I, wherein Y, X1, X2, X3, R1, R2, A1, A2, Z1, Z2, m and n are as defined in claim 1 as components in liquid crystal media and to electro-optical display elements comprising a liquid-crystalline medium according to the invention.
Owner:MERCK PATENT GMBH
Cytoprotective benzofuran derivatives
Cytoprotective compounds, many of which are benzofuran derivatives are useful in the treatment of certain ischemic or inflammatory conditions, including but not limited to stroke, myocardial infarction, congestive heart failure, and skin disorders characterized by inflammation or oxidative damage. They are also useful in the manufacture of pharmaceutical and cosmetic formulations for the treatment of such conditions.
Owner:EDISON PHARMA
Glucopyranoside, process for isolation thereof, pharmaceutical composition containing same and use thereof
Owner:COUNCIL OF SCI & IND RES
Method for synthesis of AZA-annelated pyrroles, thiophenes, and furans
Methods of synthesis of intermediates that are useful as bioisosteres of the indole, benzofuran and benzothiophene scaffold are disclosed.
Owner:ADESIS
Nicotinamide acids, amides, and their mimetics active as inhibitors of PDE4 isozymes
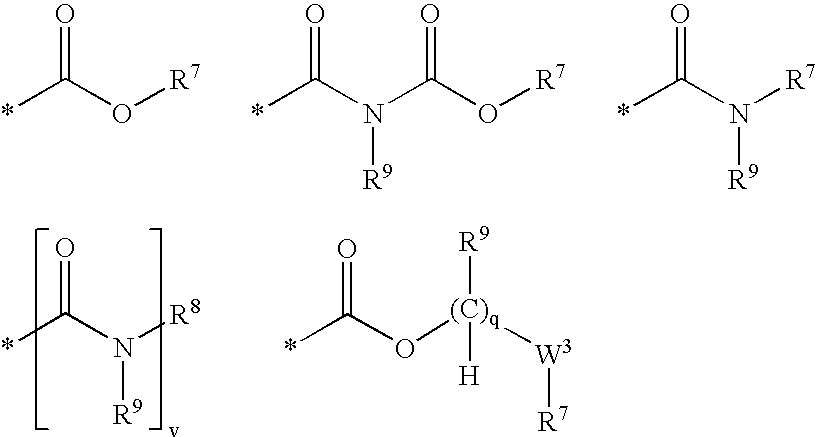
Compounds useful as inhibitors of PDE4 in the treatment of diseases regulated by the activation and degranulation of eosinophils, especially asthma, chronic bronchitis, and chronic obstructuive pulmonary disease, of the formula: wherein j is 0 or 1, k is 0 or 1, m is 0, 1, or 2; n is 1 or 2; A is selected from the partial Formulas: where q is 1, 2, or 3, W3 is -O-; -N(R9)-; or -OC(=O)-; R7 is selected from -H; -(C1-C6) alkyl, -(C2-C6) alkenyl, or -(C2-C6) alkynyl substituted by 0 to 3 substituents R10; -(CH2)u-(C3-C7) cycloalkyl where u is 0, 1 or 2, substituted by 0 to 3 R10; and phenyl or benzyl substituted by 0 to 3 R14; R8 is tetrazol-5-yl; 1,2,4-triazol-3-yl; 1,2,4-triazol-3-on-5-yl; 1,2,3-triazol-5-yl; imidazol-2-yl; imidazol-4-yl; imidazolidin-2-on-4-yl; 1,3,4-oxadiazolyl; 1,3,4-oxadiazol-2-on-5-yl; 1,2,4-oxadiazol-3-yl; 1,2,4-oxadiazol-5-on-3-yl; 1,2,4-oxadiazol-5-yl; 1,2,4-oxadiazol-3-on-5-yl; 1,2,5-thiadiazolyl; 1,3,4-thiadiazolyl; morpholinyl; parathiazinyl; oxazolyl; isoxazolyl; thiazolyl; isothiazolyl; pyrrolyl; pyrazolyl; succinimidyl; glutarimidyl; pyrrolidonyl; 2-piperidonyl; 2-pyridonyl; 4-pyridonyl; pyridazin-3-onyl; pyridyl; pyrimidinyl; pyrazinyl; pyridazinyl; indolyl; indolinyl; isoindolinyl; benzo[b]furanyl; 2,3-dihydrobenzofuranyl; 1,3-dihydroisobenzofuranyl; 2H-1-benzopyranyl; 2-H-chromenyl; chromanyl; benzothienyl; 1H-indazolyl; benzimidazolyl; benzoxazolyl; benzisoxazolyl; benzothiazolyl; benzotriazolyl; benzotriazinyl; phthalazinyl; 1,8-naphthyridinyl; quinolinyl; isoquinolinyl; quinazolinyl; quinoxalinyl; pyrazolo[3,4-d]pyrimidinyl; pyrimido[4,5-d]pyrimidinyl; imidazo[1,2-a]pyridinyl; pyridopyridinyl; pteridinyl; or 1H-purinyl; or A is selected from phosphorous and sulfur acid groups; W is -O-; -S(=O)t-, where t is 0, 1, or 2; or -N(R3)-; Y is =C(R1a)-, or -[N<custom-character file="US20020111495A1-20020815-P00900.TIF" wi="20" he="20" id="custom-character-00001" / >(O)k] where k is 0 or 1; R4, R5 and R6 are (1) -H; provided that R5 and R6 are not both -H at the same time, -F; -Cl; -(C2-C4) alkynyl; -R16; -OR16; -S(=O)pR16; -C(=O)R16, -C(=O)OR16, -C(=O)OR<highlight><sup
Owner:PFIZER INC
Aromatic amine derivative and organic electroluminescence device
InactiveUS20100001636A1Improves yield upon productionExtended service lifeOrganic chemistryDischarge tube luminescnet screensOrganic filmDibenzofuran
An aromatic amine derivative having a specific structure. An organic electroluminescence device which is composed of one or more organic thin film layers sandwiched between a cathode and an anode, wherein at least one of the organic thin film layers, especially a hole transporting layer, contains the aromatic amine derivative. The aromatic amine derivative has at least one substituted or unsubstituted dibenzofuran skeleton and at least one substituted or unsubstituted terphenylene skeleton. Because the molecules in the aromatic amine derivative hardly crystallize, organic electroluminescence devices improving their production yield and having prolonged lifetime are provided.
Owner:IDEMITSU KOSAN CO LTD
Glucopyranoside and process of isolation thereof from pterocarpus marsupium pharmaceutical composition containing the same and use thereof
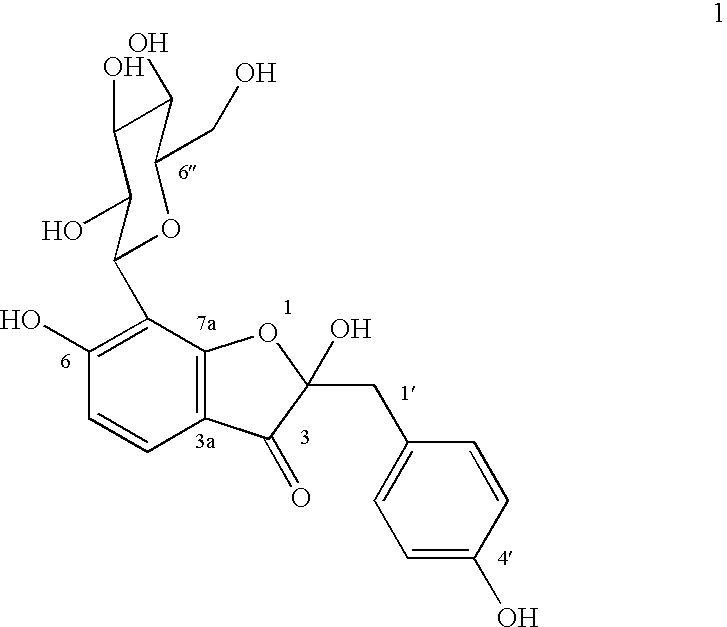
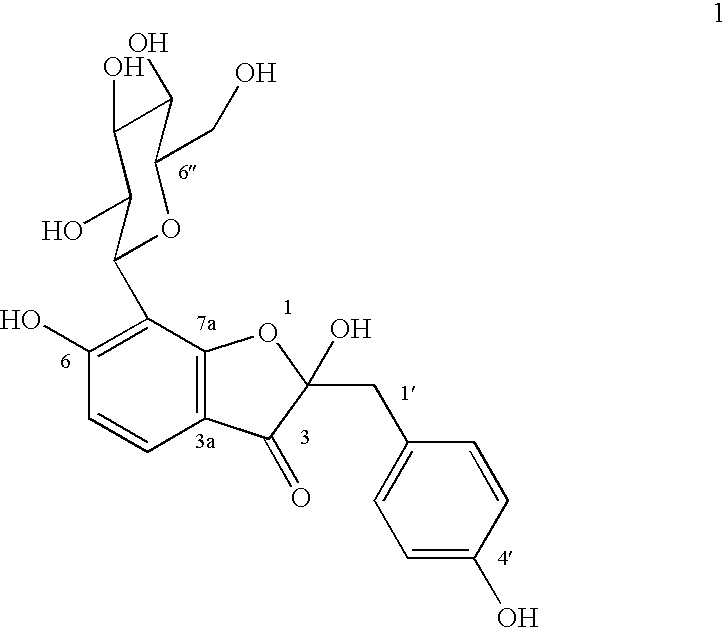
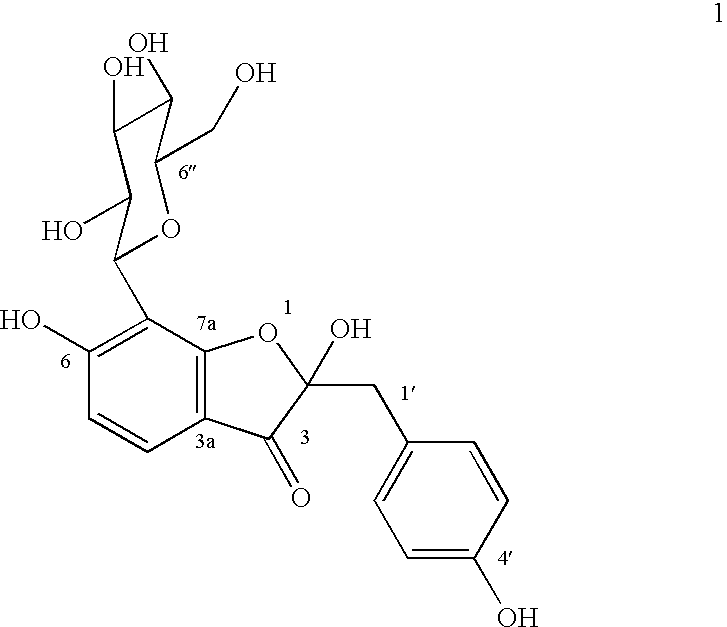
A novel glucopyranoside, 2,6-dihydroxy-2-(P-hydroxybenzyl)-3(2H) benzofuran-7-C-beta-D-glucopyranoside of the formula 1isolated from Pterocarpus marsupium and to a process for the isolation thereof is disclosed. The invention also relates to a pharmaceutical composition containing 2,6-dihydroxy-2-(P-hydroxybenzyl)-3(2H)benzofuran-7-C-beta-D-glucopyranoside and to method for the treatment of diabetes using said compound.
Owner:COUNCIL OF SCI & IND RES
Anthracene derivative and organic electroluminescence device using the same
ActiveUS20080315754A1Solve low luminous efficiencyLong life-timeOrganic chemistryDischarge tube luminescnet screensAnthraceneBenzothiophene
Provided are a novel anthracene derivative of a specific structure in which benzofuran or benzothiophene is bonded to anthracene through an arylene group, a material for an organic electroluminescence device and a light emitting material for an organic electroluminescence device each containing the anthracene derivative, and an organic electroluminescence device including an organic thin film layer formed of one or plural layers including at least a light emitting layer, the organic thin film layer being interposed between a cathode and an anode, in which at least one layer of the organic thin film layer contains the anthracene derivative alone or as a component of a mixture. The organic electroluminescence device has high luminous efficiency and is capable of emitting light with a long lifetime, and the device can be realized by the anthracene derivative.
Owner:JOLED INC +1
Benzofuran compounds
Owner:XTL BIOPHARMLS
Heterocyclic compound
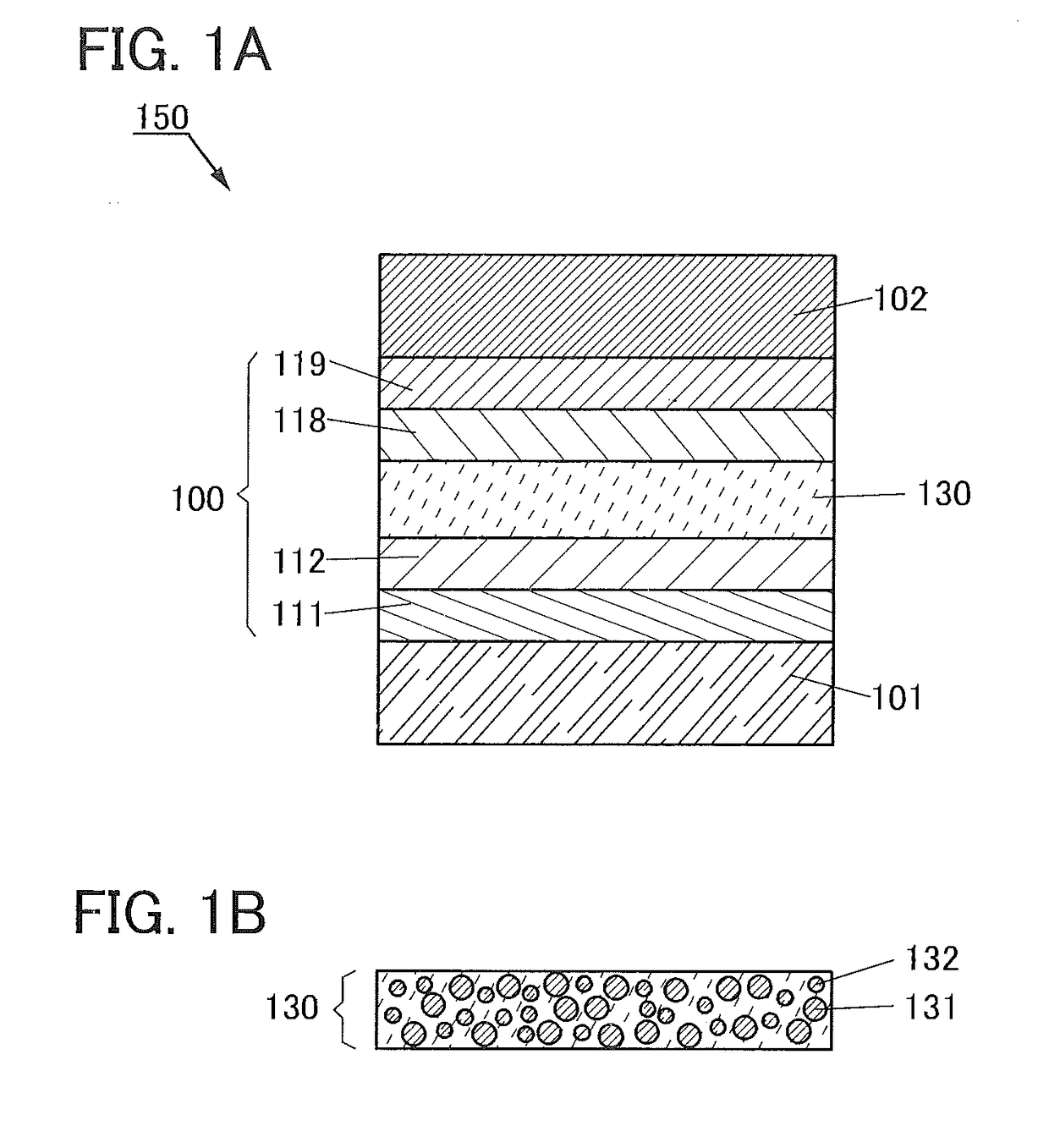
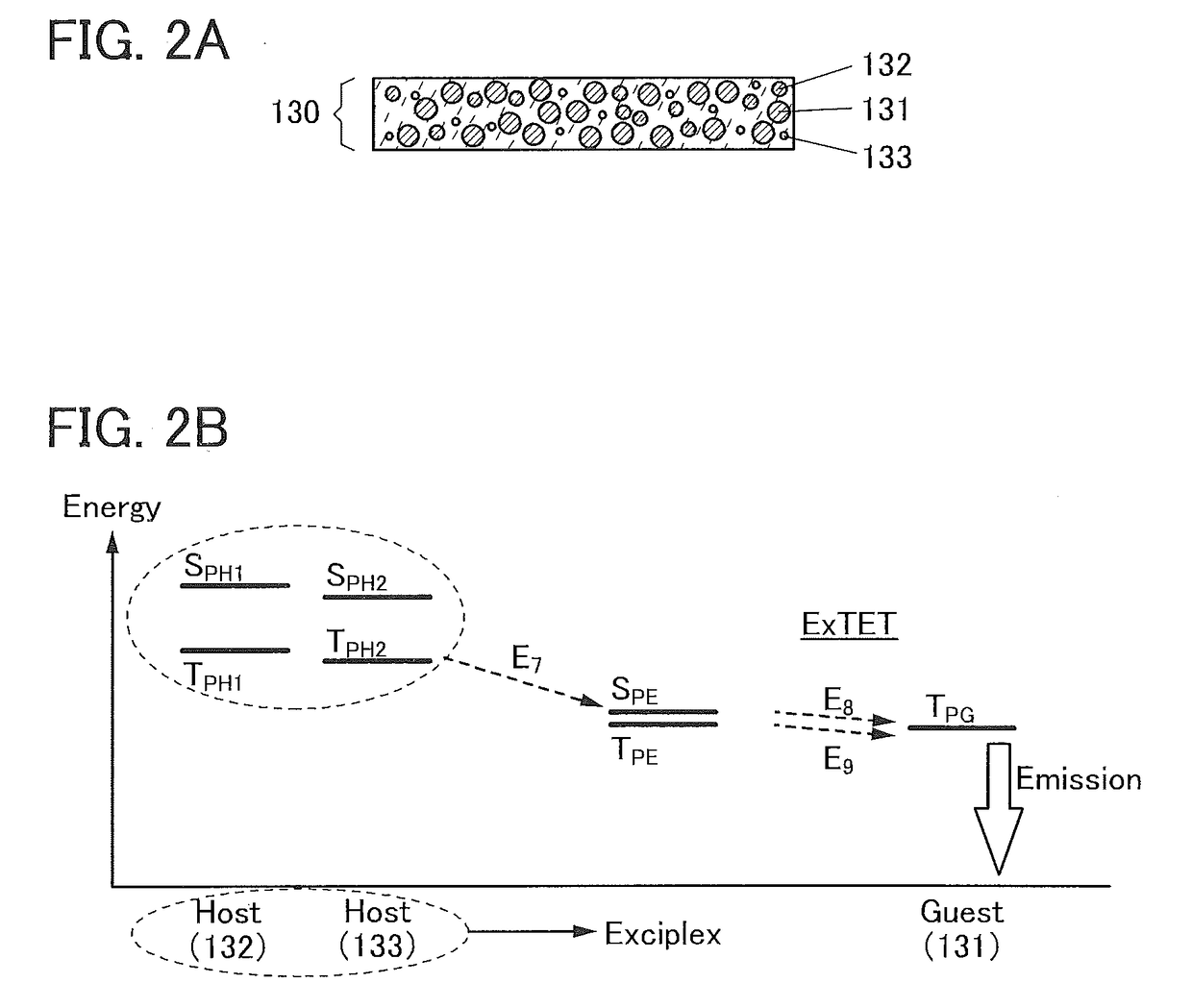
ActiveUS20120197020A1Reduce the driving voltageImprove current efficiencyOrganic chemistrySolid-state devicesCarbazoleDibenzofuran
Provided is a novel heterocyclic compound which can be used for a light-emitting element, as a host material of a light-emitting layer in which a light-emitting substance is dispersed. A heterocyclic compound represented by a general formula (G1) is provided. In the formula, A represents any of a substituted or unsubstituted dibenzothiophenyl group, a substituted or unsubstituted dibenzofuranyl group, and a substituted or unsubstituted carbazolyl group, R11 to R19 separately represent any of hydrogen, an alkyl group having 1 to 4 carbon atoms, and a substituted or unsubstituted aryl group having 6 to 14 carbon atoms, and Ar represents a substituted or unsubstituted arylene group having 6 to 13 carbon atoms.
Owner:SEMICON ENERGY LAB CO LTD
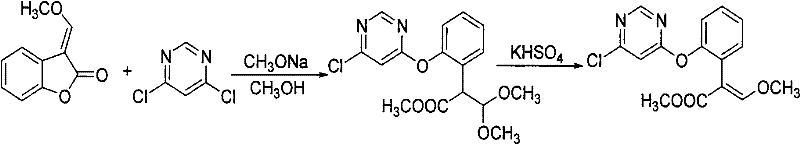
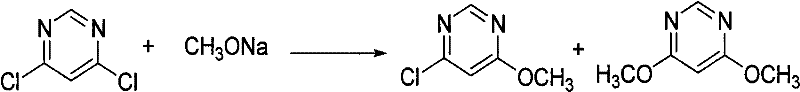
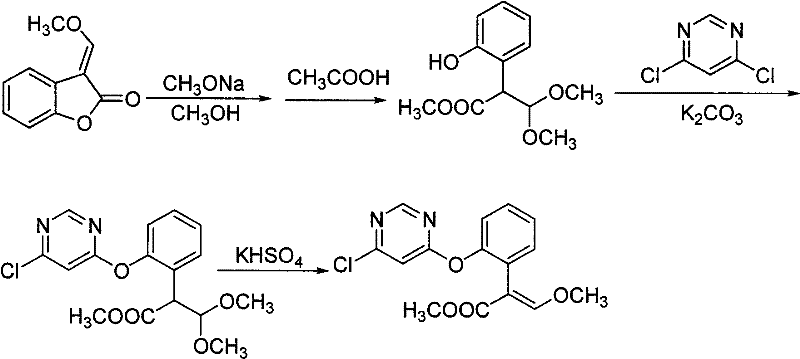
Synthetic method of azoxystrobin and special intermediate for synthesis
The invention relates to a synthetic process of a chemical substance, and particularly relates to a synthetic method for synthesizing (E)-2-[2-(6-chloro pyrimidine-4-yloxy)phenyl]-3-methoxy methacrylate and azoxystrobin; the method comprises the following steps: mixing a raw material of 3-(alpha-methoxy)methylene benzofuran-2-(3H)-ketone and potassium carbonate in a toluene solvent, cooling to 0-10 DEG C, adding sodium methoxide, reacting for 0.4-0.6 hours; adding 4,6-dichloropyrimidine and a catalyst of DABCO, reacting for 1-2 hours, filtering to remove inorganic salts, washing the filtrate with water, performing distillation to recover toluene; adding a catalyst of potassium bisulfate into the distillation residues of the above reaction, heating to 132-145 DEG C in a reduced-pressure condition for reaction; directly adding salicylonitrile to synthesize azoxystrobin or performing toluene dissolution, water washing, solvent recovery, recrystallization and filtration to obtain an intermediate. The production and synthesis of (E)-2-[2-(6-chloro pyrimidine-4-yloxy)phenyl]-3-methoxy methacrylate by the production process of the invention has high yield, and simple operations, and the used raw materials and processes are routine reagents and methods.
Owner:CHONGQING UNISPLENDOUR CHEM
Aryl- and heteroaryl-substituted tetrahydroisoquinolines and use thereof to block reuptake of norepinephrine, dopamine, and serotonin
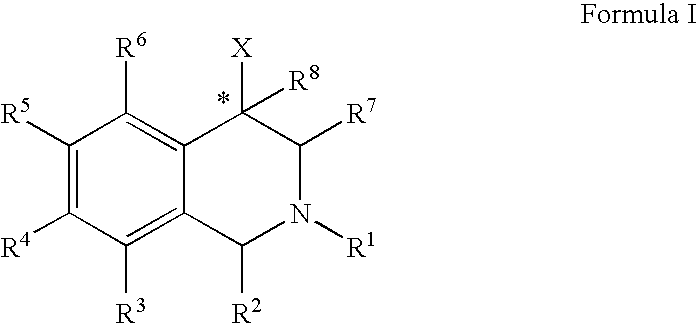
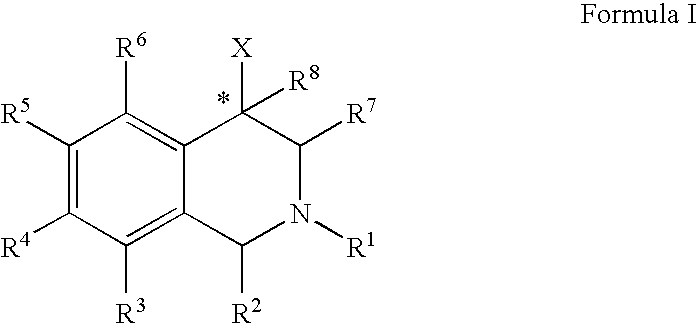
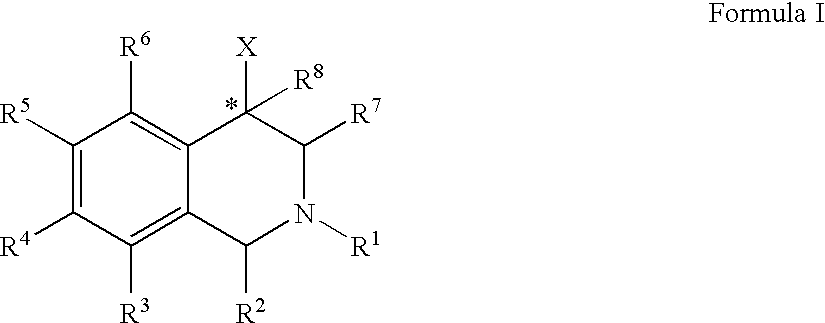
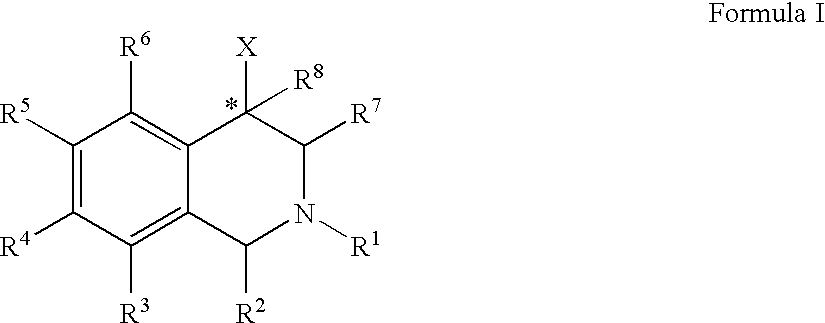
ActiveUS20060052378A1Good curative effectQuick effectBiocideNervous disorderBenzoxazoleChemical structure
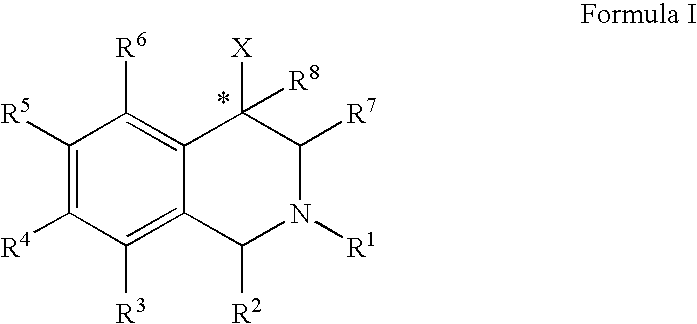
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Oral Care Compositions With Improved Flavor
Oral care compositions having improved taste, said compositions comprising: a carrier material; from about 0.001 to about 10%, by weight of the composition, of an oral care component selected from metal salts, antimicrobial agents, bad breath reduction agents, bleaching agents, surfactants, or a combination thereof; and from about 0.0001 to about 1%, by weight of the composition, of a TRPA1 agonist selected from vanillin esters; benzoate esters; hydroxybenzoate derivatives; methoxy benzoate derivatives; hydroxybutanedioate derivatives; benzamidobenzoate derivatives; methylpropanoate derivatives; phenyl acetate derivatives; hex-3-enoate derivatives; 2-(furan-2-ylmethylsulfanyl)-3-methylpyrazine; phenylmethoxymethylbenzene; (2R)-2-azaniumyl-3-[(2R)-2-azaniumyl-3-oxido-3-oxopropyl]disulfanylpropanoate; (3E)-2-hydroxy-4,8-dimethylnona-3,7-dienal; (2R)-2-azaniumyl-3-[(2S)-2-azaniumyl-3-oxido-3-oxopropyl]disulfanylpropanoate; (3Z)-3-butylidene-2-benzofuran-1-one; 3-methyl-N-(3-methylbutyl)butan-1-imine; 2-(furan-2-ylmethyldisulfanylmethyl)furan; and combinations thereof. Uses thereof and methods of improving the taste of an oral care composition.
Owner:THE PROCTER & GAMBLE COMPANY
1-(Amino)indanes and (1,2-dihydro-3-amino)-benzofurans, benzothiophenes and indoles as edg receptor agonists
The present invention encompasses compounds of Formula (I): as well as the pharmaceutically acceptable salts and hydrates thereof. The compounds are useful for treating immune mediated diseases and conditions, such as bone marrow, organ and tissue transplant rejection. Pharmaceutical compositions and methods of use are included.
Owner:MERCK SHARP & DOHME CORP
Preparation method of vilazodone or its hydrochloride
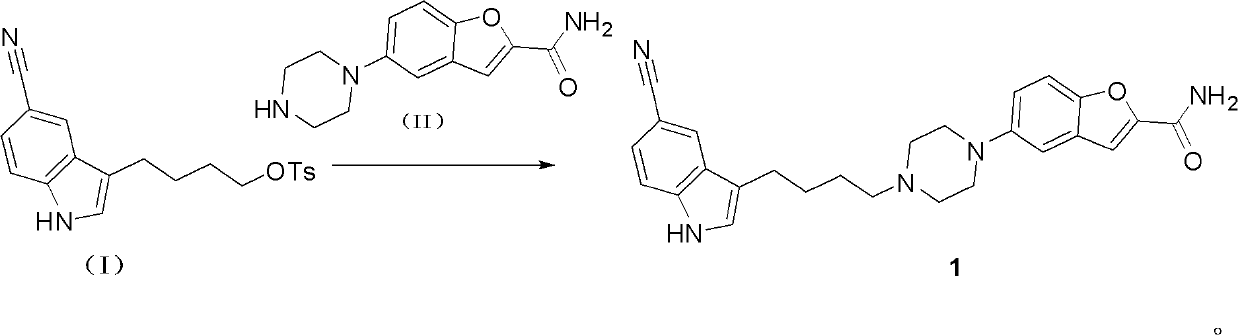
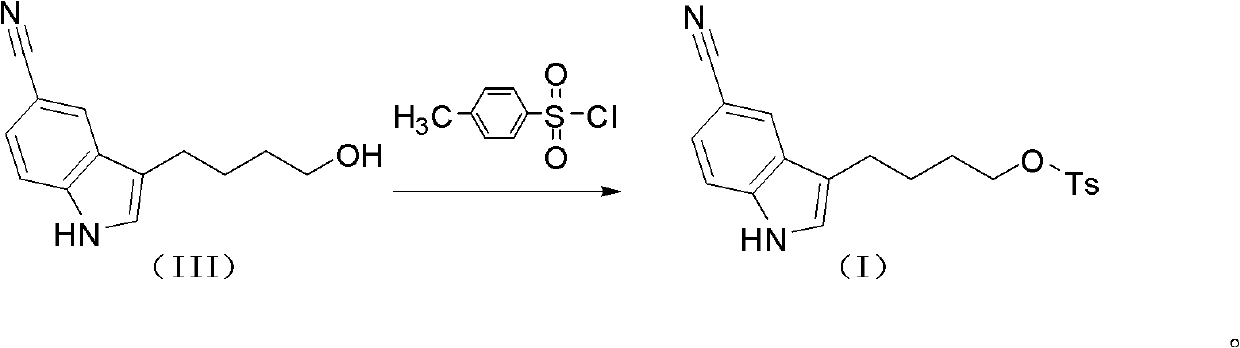
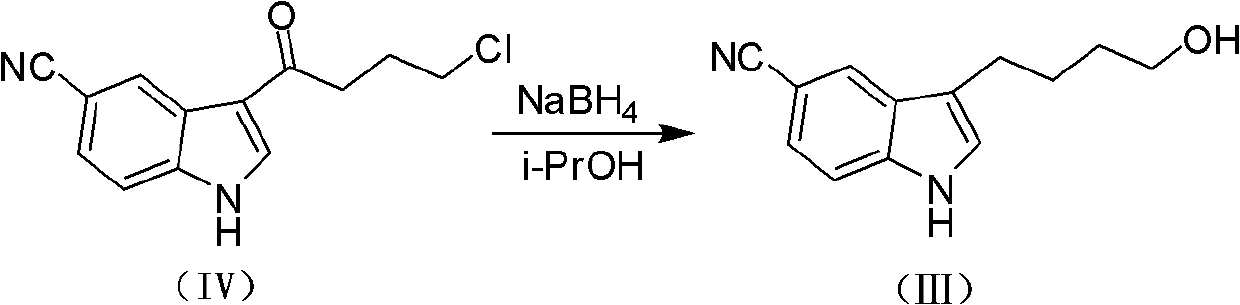
The invention discloses a preparation method of vilazodone or its hydrochloride, which comprises the following steps: the compound of formula (I) is mixed with the compound of formula (II) 5-(1 -piperazinyl)-benzofuran-2-formamide reaction, then collect vilazodone shown in formula 1 from the reaction product; Gained vilazodone 1 is in a solvent, and hydrochloric acid is salified to prepare formula (A ) shown vilazodone hydrochloride. The present invention overcomes the defects and deficiencies in the existing preparation methods of vilazodone and its intermediates, is more suitable for the large-scale industrial preparation of vilazodone hydrochloride, has obvious creativity, and has a relatively large positive progress effect and practical application value. The reaction formula is as follows:
Owner:SHANGHAI INST OF PHARMA IND
Metal coordination compound, luminescence device and display apparatus
InactiveUS20030085646A1Indium organic compoundsCathode ray tubes/electron beam tubesLuminescenceHigh luminance
An electroluminescence device having a layer containing a specific metal coordination compound is provided. The metal coordination compound is represented by formula (1) below:MLmL'n (1),wherein M is a metal atom of Ir, Pt, Rh or Pd; L and L' are mutually different bidentate ligands; m is 1, 2 or 3 and n is 0, 1 or 2 with the proviso that m+n is 2 or 3; a partial structure MLm is represented by formula (2) shown below and a partial structure ML'n is represented by formula (3) or (4) shown below: at least one of the optional substituent(s) of the cyclic groups, and the cyclic groups CyC1 and CyC2 includes a benzofuran structure capable of having a substituent represented by the following formula (5): The metal coordination compound having the benzofuran structure is effective in providing high-efficiency luminescence and long-term high luminance.
Owner:CANON KK
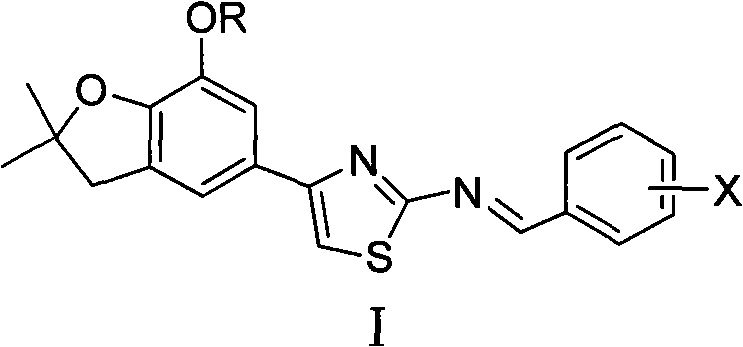
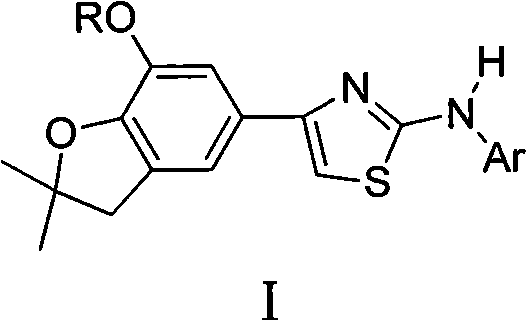
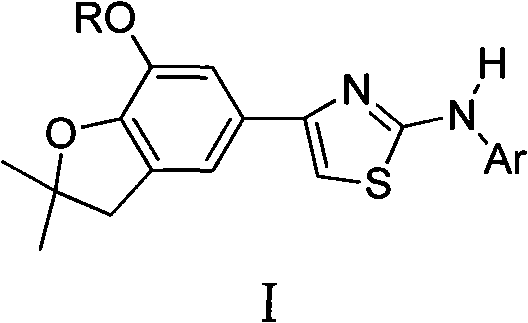
4-(benzofuran-5-yl)-2-benzal aminothiazole and application of 4-(benzofuran-5-base)-2-benzal aminothiazole as antineoplastic agent
The invention discloses 4-(benzofuran-5-yl)-2-benzal aminothiazole as shown in a chemical structural formula I. The preparation method of the 4-(benzofuran-5-yl)-2-benzal aminothiazole is as follows: 1-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl) butanone is subject to bromination and reacts with thiourea to obtain 4-(7-hydroxy / alkoxy-2,2-dimethyl-2,3-dihydro-benzofuran-5-yl)-2-aminothiazole which reacts with aromatic aldehyde to prepare the 4-(benzofuran-5-yl)-2-benzal aminothiazole. The 4-(benzofuran-5-yl)-2-benzal aminothiazole has good activity inhabiting activity on Hela cells, human liver cancer cells (Bel 7402 cells) and lung carcinoma cells (A549 cells) and can be used for preparing the antineoplastic agent.
Owner:HUNAN UNIV
Bioavailable formulations of heterocyclic compounds
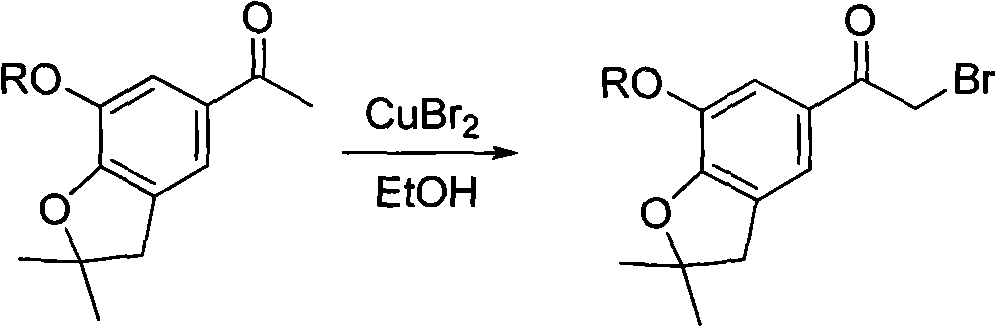
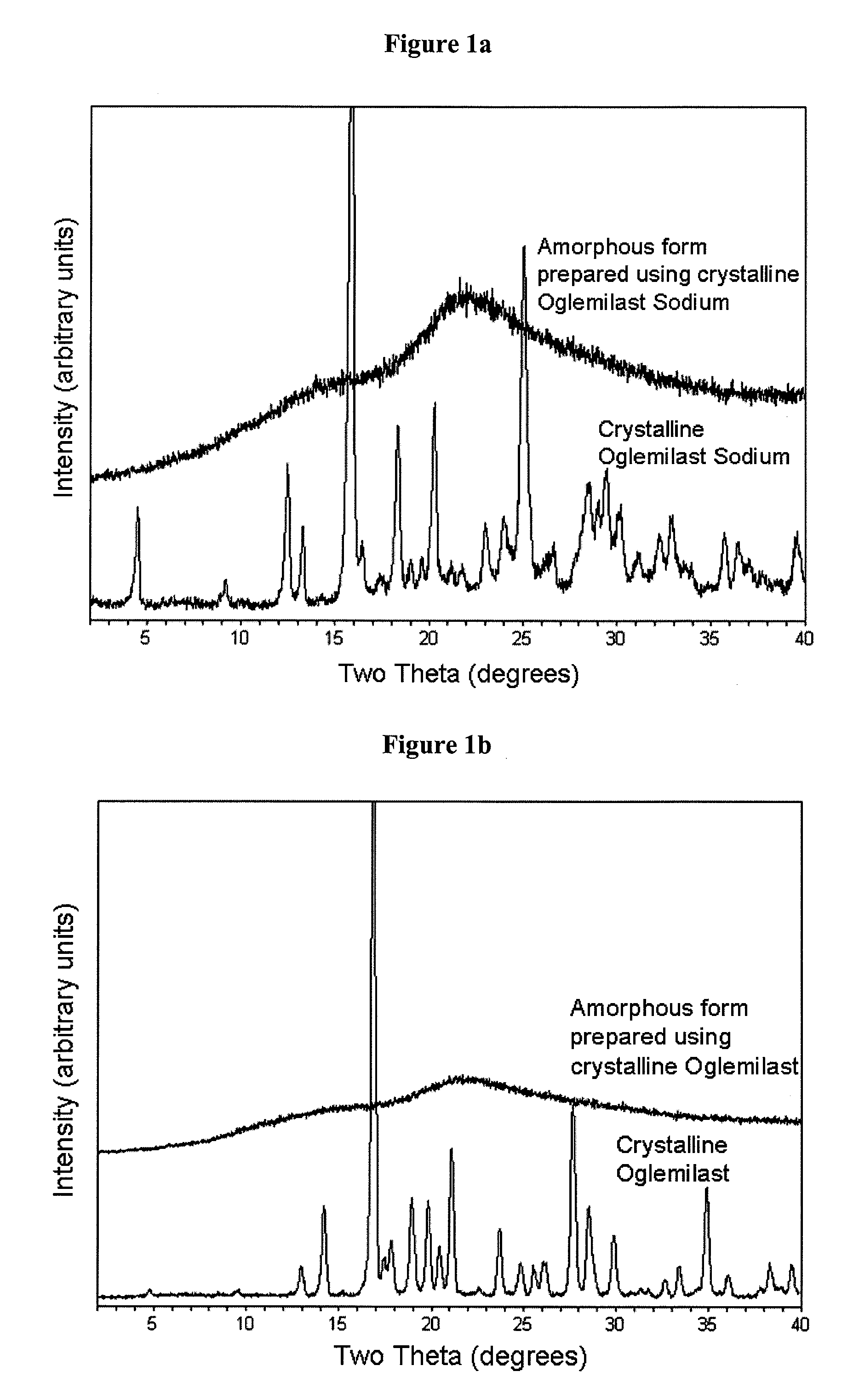
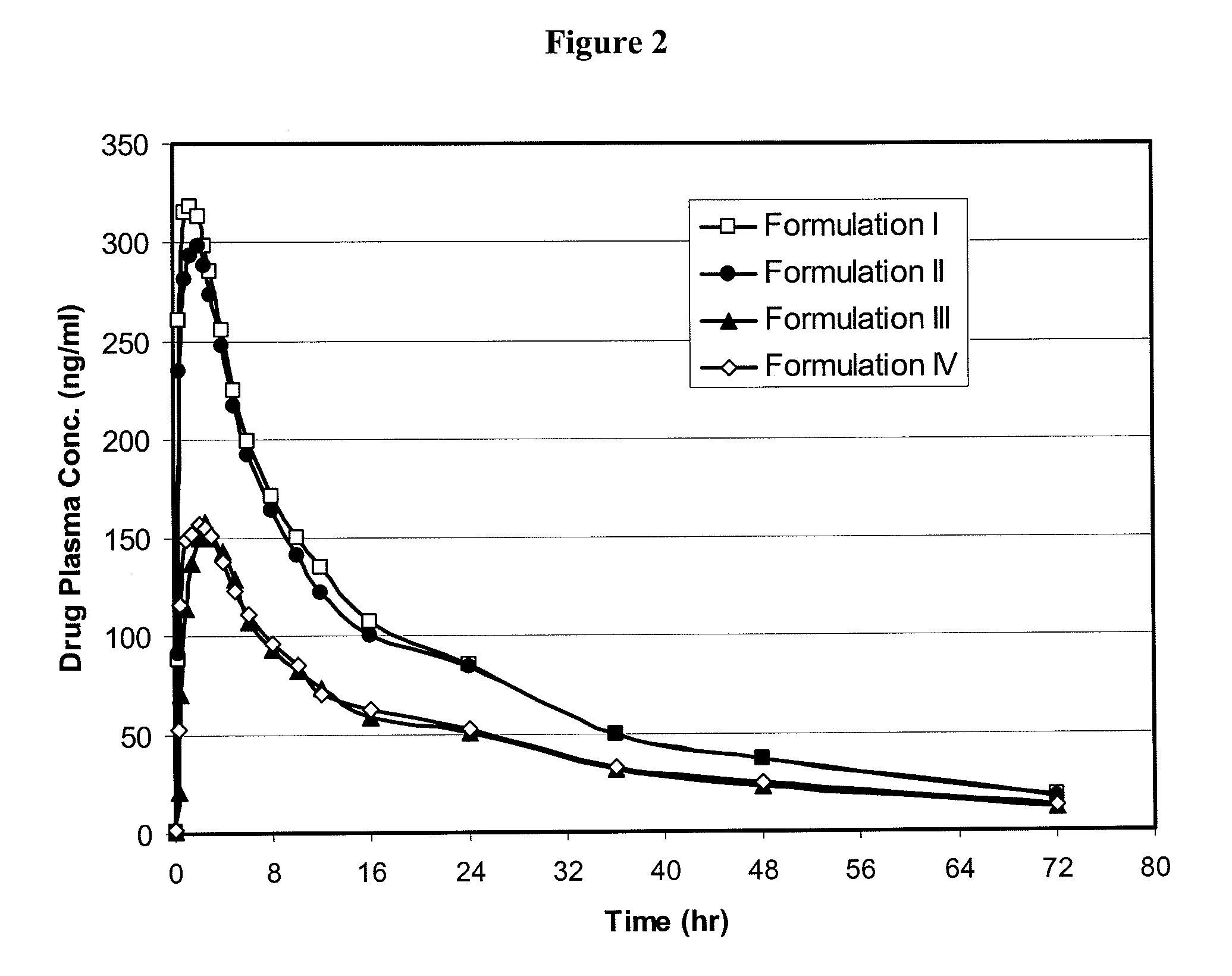
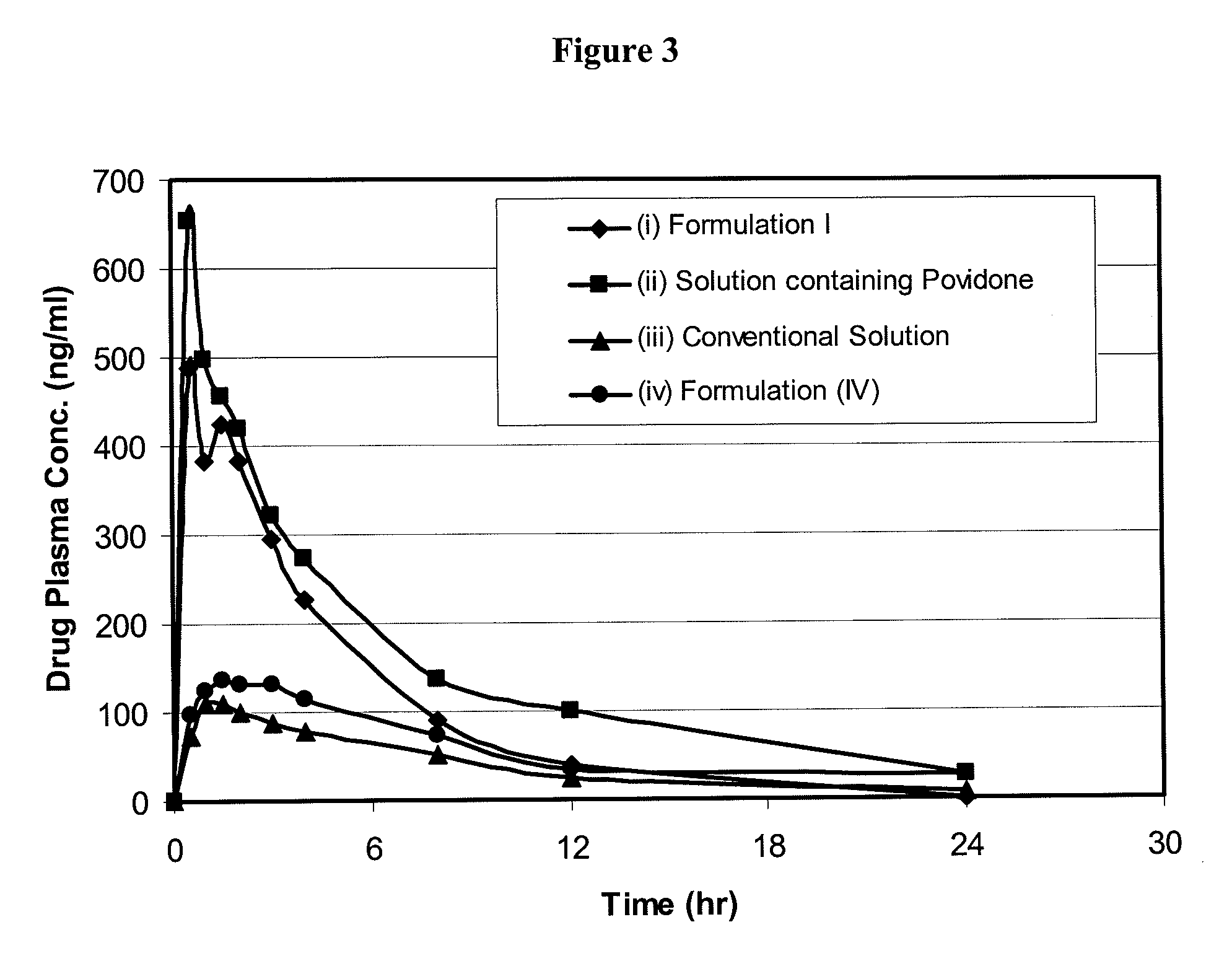
The present invention relates to bioavailable pharmaceutical formulations of heterocyclic compounds, such as such as N-(3,5-dichloropyrid-4-yl)-4-difluoromethoxy-8-methanesulfonamido-dibenzo[b,d]furan-1-carboxamide (oglemilast) and pharmaceutically acceptable salts thereof, to processes for their preparation and to methods of treatment using the same. The present invention also relates to substantially pure amorphous forms of heterocyclic compounds, such as oglemilast. The invention is particularly directed to bioavailable pharmaceutical oral dosage forms containing amorphous oglemilast.
Owner:FOREST LAB HLDG LTD
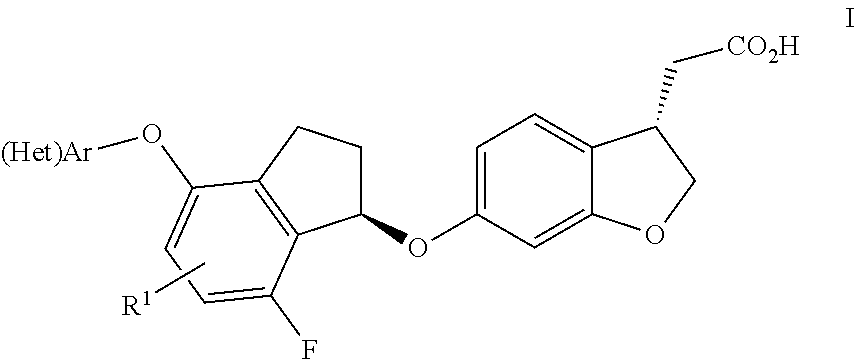
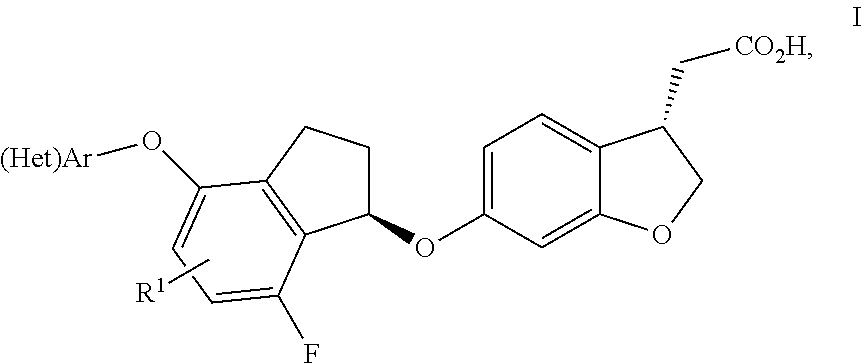
Indanyloxydihydrobenzofuranylacetic acids
InactiveUS20140163025A1Improve effectivenessImprove stabilityBiocideOrganic chemistryDiabetes mellitusDisease
The present invention relates to compounds of general formula I,wherein the groups (Het)Ar and R1 are defined as in claim 1, which have valuable pharmacological properties, in particular bind to the GPR40 receptor and modulate its activity. The compounds are suitable for treatment and prevention of diseases which can be influenced by this receptor, such as metabolic diseases, in particular diabetes type 2.
Owner:BOEHRINGER INGELHEIM INT GMBH
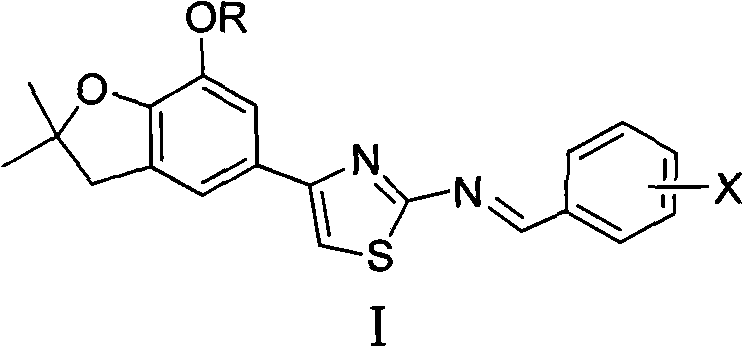
4-(benzofuran-5-yl)-2-aromatic aminothiazole and preparation method and application thereof
The invention discloses 4-(benzofuran-5-yl)-2-aromatic aminothiazole shown as a chemical structural formula I. The preparation method of the 4-(benzofuran-5-yl)-2-aromatic aminothiazole comprises the following steps of: heating and stirring 2-halogen-1-(7-hydroxyl / alkoxyl-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl) butanone and arylthiourea in acetone for reaction to obtain 4-(benzofuran-5-yl)-2-aromatic aminothiazole salt; and neutralizing the 4-(benzofuran-5-yl)-2-aromatic aminothiazole salt with stronger ammonia water to obtain the 4-(benzofuran-5-yl)-2-aromatic aminothiazole. The 4-(benzofuran-5-yl)-2-aromatic aminothiazole has high insecticidal activity and is applied to the preparation of pesticides.
Owner:HUNAN UNIV
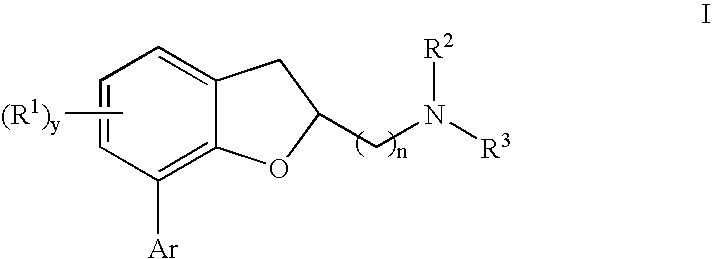
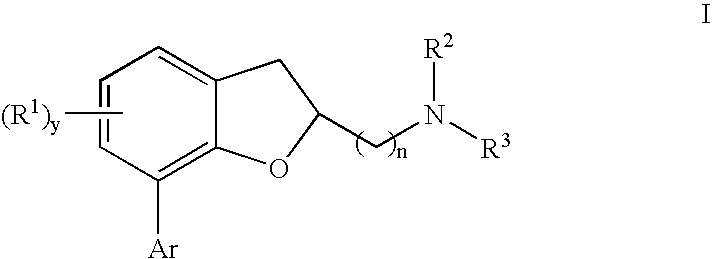
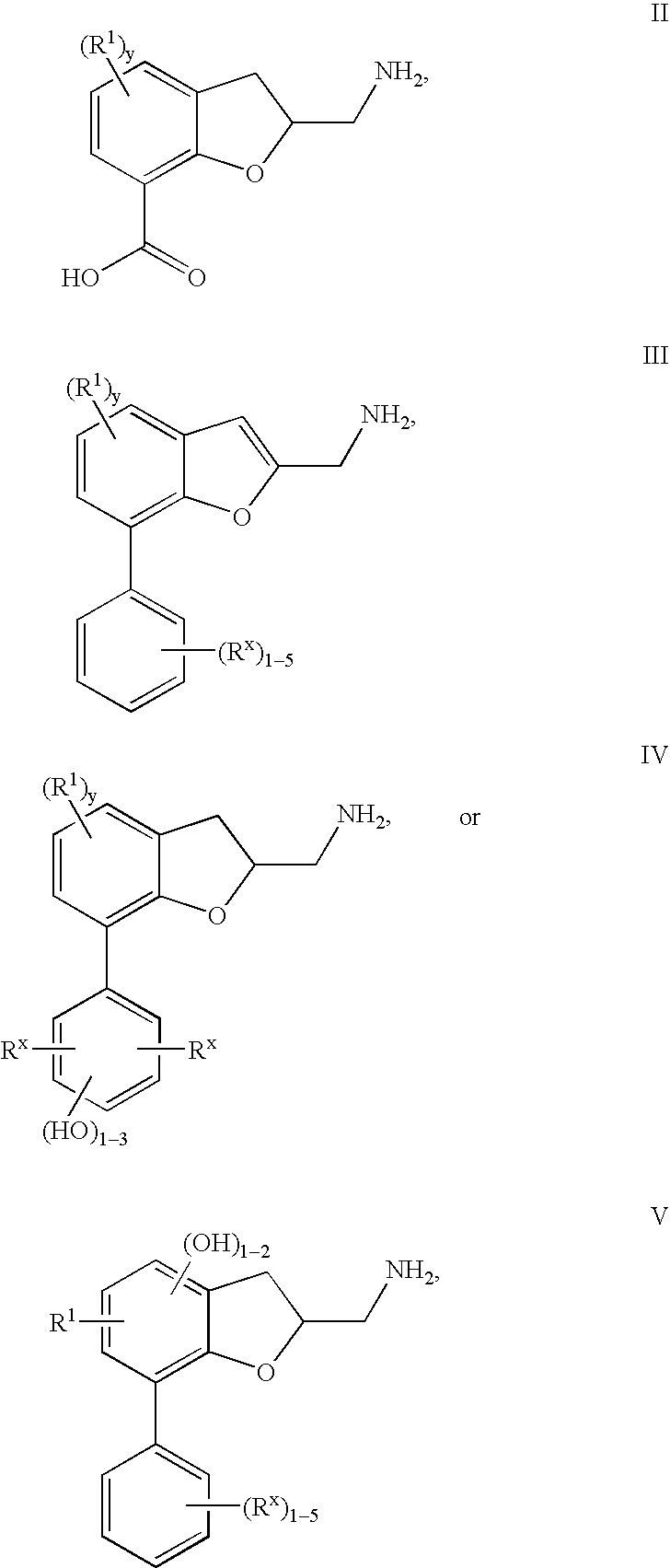
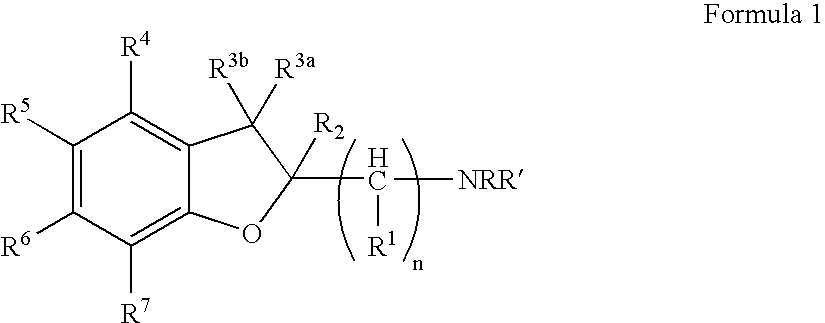
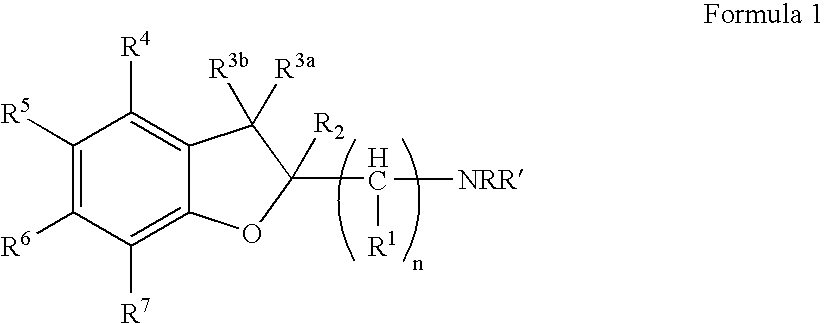
Dihydrobenzofuran derivatives and uses therof
The present invention provides a composition comprising a compound of formula I: or a pharmaceutically acceptable salt thereof, wherein each of R1, R2, R3, y, n, and Ar are as defined, and described in classes and subclasses herein, which are agonists or partial agonists of the 2C subtype of brain serotonin receptors. Such compounds, and compositions thereof, are useful for treating a variety of central nervous system disorders such as schizophrenia.
Owner:WYETH LLC
Benzofuranyl alkanamine derivatives and uses thereof
InactiveUS20060247276A1Increase body weightUseful in treatmentBiocideNervous disorderSerotoninMedicine
Compounds of formula I or pharmaceutically acceptable salts thereof are provided: wherein each of R1, R1′, R2, R3, R4, n, and Ar are as defined, and described in classes and subclasses herein, which are agonists or partial agonists of the 2C subtype of brain serotonin receptors. The compounds, and compositions containing the compounds, can be used to treat a variety of central nervous system disorders such as schizophrenia.
Owner:WYETH LLC
Dihydrobenzofuranyl alkanamine derivatives and methods for using same
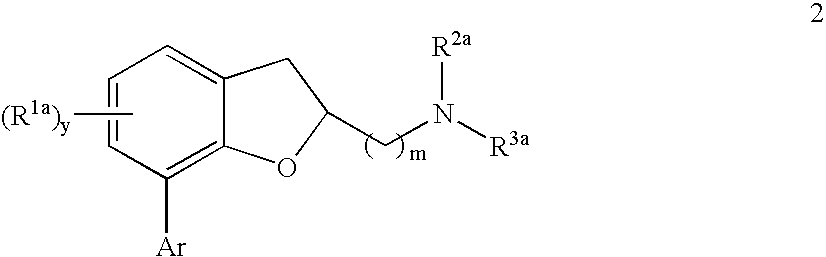
Compounds of formula 2 or pharmaceutically acceptable salts thereof are provided: wherein each of R1a, R2a, R3a, Ar, y, and m are as defined herein, which are agonists or partial agonists of the 2C subtype of brain serotonin receptors. The compounds, and compositions containing the compounds, are used to treat a variety of central nervous system disorders such as schizophrenia.
Owner:WYETH LLC
Benzofuranone derivatives and application of the same
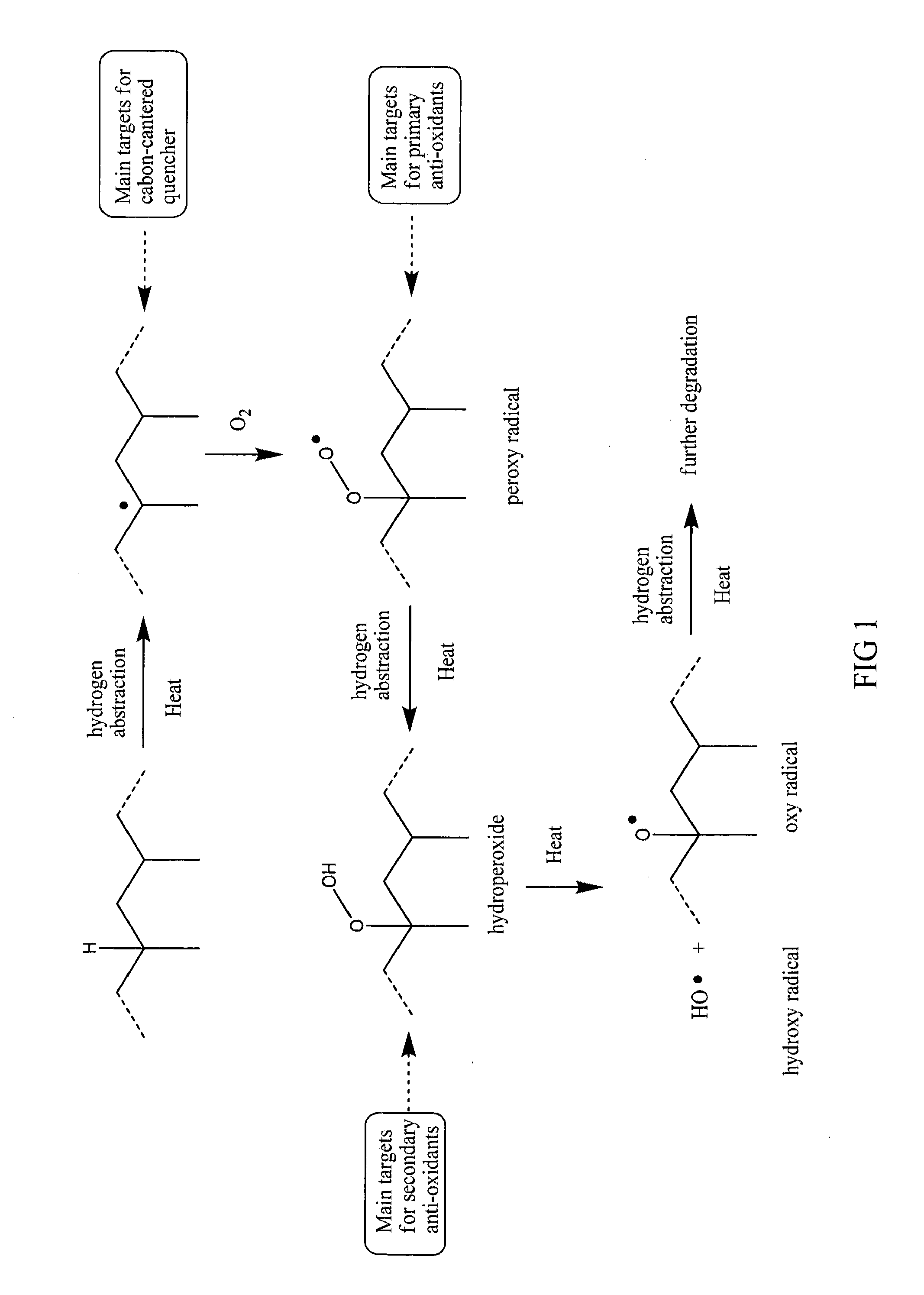
ActiveUS20120238677A1Improve heat resistanceLess discolorationOrganic chemistryChemical inhibitorsBenzoic acidCarbon centered radicals
The present invention relates to the antioxidant compounds which are synthesized or derived from benzofuranone compound and benzoic acid compound. The antioxidant compound with remarkable heat resistance possesses carbon-centered radical quencher and primary antioxidant synergism. It can be use as additive for polymer to enhance its stability of melting flow and color.
Owner:CHITEC TECH +2
Aromatic chalcogen compounds and their use
This invention relates to dibenzothiophene, dibenzofuran, dibenzopyran, and dibenzothiapyran compounds. This invention also relates to layers and devices including at least one of the above compounds.
Owner:EI DU PONT DE NEMOURS & CO
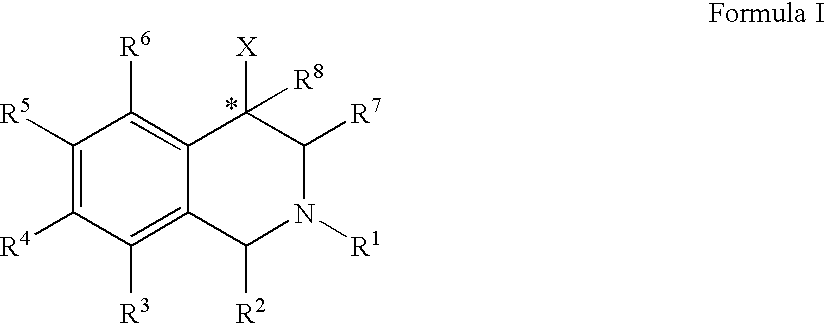
Use of aryl- and heteroaryl-substituted tetrahydroisoquinolines to block reuptake of norepinephrine, dopamine, and serotonin
InactiveUS20060063766A1Little and no activityMinimal potential for substance abuseBiocideNervous disorderBenzoxazoleBenzene
The compounds of the present invention are represented by the chemical structure found in Formula (I): wherein: the carbon atom designated * is in the R or S configuration; and X is a fused bicyclic carbocycle or heterocycle selected from the group consisting of benzofuranyl, benzo[b]thiophenyl, benzoisothiazolyl, benzoisoxazolyl, indazolyl, indolyl, isoindolyl, indolizinyl, benzoimidazolyl, benzooxazolyl, benzothiazolyl, benzotriazolyl, imidazo[1,2-a]pyridinyl, pyrazolo[1,5-a]pyridinyl, [1,2,4]triazolo[4,3-a]pyridinyl, thieno[2,3-b]pyridinyl, thieno[3,2-b]pyridinyl, 1H-pyrrolo[2,3-b]pyridinyl, indenyl, indanyl, dihydrobenzocycloheptenyl, tetrahydrobenzocycloheptenyl, dihydrobenzothiophenyl, dihydrobenzofuranyl, indolinyl, naphthyl, tetrahydronaphthyl, quinolinyl, isoquinolinyl, 4H-quinolizinyl, 9aH-quinolizinyl, quinazolinyl, cinnolinyl, phthalazinyl, quinoxalinyl, benzo[1,2,3]triazinyl, benzo[1,2,4]triazinyl, 2H-chromenyl, 4H-chromenyl, and a fused bicyclic carbocycle or fused bicyclic heterocycle optionally substituted with substituents (1 to 4 in number) as defined in R14; with R1, R2, R3, R4, R5, R6, R7, R8, and R14 defined herein.
Owner:ALBANY MOLECULAR RESEARCH INC
2- normal-butyl-5-substituted amino benzofuran and preparation method thereof
The invention discloses an important intermediate of dronedarone, which relates to a dronedarone intermediate shown by a formula I, wherein R is phenylmethoxycarbonyl, or tert-butoxycarbonyl or 9-fluorenylmethoxycarbonyl.
Owner:AVENTIS PHARMA HAINAN
Compound, Light-Emitting Element, Display Device, Electronic Device, and Lighting Device
ActiveUS20170186971A1High triplet excitation energy levelImprove reliabilityOrganic chemistrySolid-state devicesDisplay devicePyrrole
A compound includes a benzofuropyrimidine skeleton or a benzothienopyrimidine skeleton, a first substituent, and a second substituent. Each of the first substituent and the second substituent includes a furan skeleton, a thiophene skeleton, or a pyrrole skeleton. The first substituent is bonded to a pyrimidine ring included in the benzofuropyrimidine skeleton or a pyrimidine ring included in the benzothienopyrimidine skeleton. The second substituent is bonded to a benzene ring included in the benzofuropyrimidine skeleton or a benzene ring included in the benzothienopyrimidine skeleton. The light-emitting element includes the compound.
Owner:SEMICON ENERGY LAB CO LTD +1
Benzofuran-3-yl(indol-3-yl) maleimides as potent GSK3 inhibitors
Compounds of formula:and pharmaceutically acceptable salts, esters and solvates thereof, where variables are defined in the specification, useful generally as inhibitors of protein kinases and particularly useful for inhibition of GSK-3.Pharmaceutically compositions and medicaments containing a compound of the invention are provided. The invention provides methods of treatment of protein kinase-related disease, disorders or conditions. The invention provides methods of treatment of GSK-3-related diseases, disorders or conditions. More specifically, methods of treatment of bipolar disorder, including mania, schizophrenia, stroke, epilepsy, motor neuron disease, cranial or spinal trauma, neurodegenerative disorders, including multiple sclerosis (MS), Alzheimer's disease, Fragile X syndrome, autism, Huntington's disease, Parkinson's disease, amylotrophic lateral sclerosis (ALS), AIDS-associated dementia, diabetes, particularly type II diabetes, cardiomycete hypertrophy, reperfusion / ischemia, cancer, particularly colorectal cancer, pancreatic cancer, allergies and / or asthma and hair loss or baldness.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com