Preparation method of vilazodone or its hydrochloride
A technology of vilazodone hydrochloride and vilazodone, which is applied in the field of preparing vilazodone or its hydrochloride, can solve the problems of being unsuitable for mass industrialized preparation, high preparation cost, expensive and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
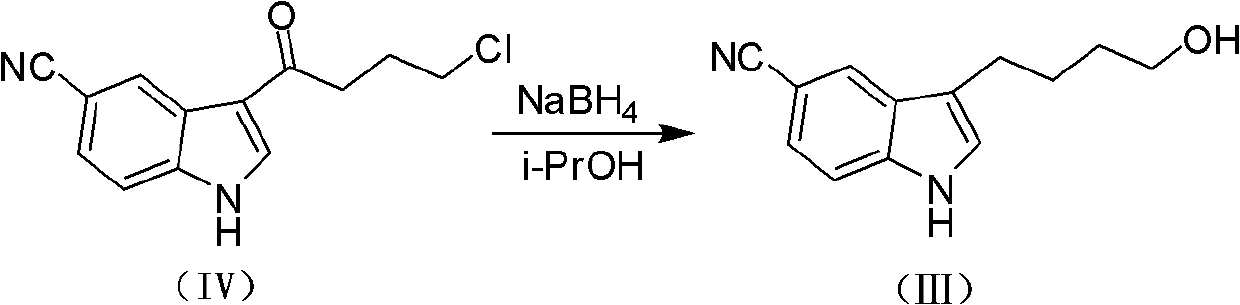
[0066] Preparation of 3-(4-Hydroxybutyl)indole-5-cyanocyanide(III)
[0067] At room temperature, dissolve 3-(4-chlorobutyryl)indole-5-cyanide (0.4mol, 99g) in 2000ml of isopropanol, stir for 5min, add sodium borohydride (1mol, 38g), and heat up to reflux React for 6 hours, slowly pour the reaction solution into 500ml of 1N ice-diluted hydrochloric acid, stir for 30min, and rotate the reaction solution under reduced pressure until there are basically only water and solids, dissolve the solids with 1000ml ethyl acetate, separate the liquid, and The phases were extracted with 250ml of ethyl acetate, the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and evaporated to dryness to obtain 86g of a yellow oily crude product, which was washed with 1L of petroleum ether to obtain 83g, which was directly used in the next reaction .
[0068] ESI-MS[M+H] + : 215.12
[0069] 1 H-NMR (CDCl 3 ): δ1.63-1.70(m, 2H), 1.74-1.83(m,...
Embodiment 2
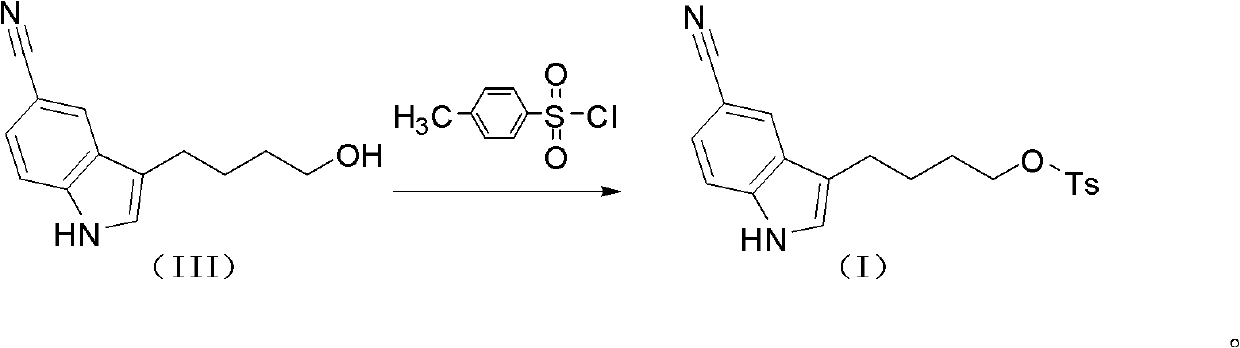
[0071] Preparation of 4-(5-cyanindol-3-yl)butyl p-toluenesulfonate (I)
[0072] The crude 3-(4-hydroxybutyl)indole-5-cyanide (83g) prepared in the previous step reaction was dissolved in 1200ml of methylene chloride, the temperature was controlled in an ice-water bath at 0-5°C, stirred for 5min, and pyridine (68g ), and p-toluenesulfonyl chloride (0.436mol, 83g) was added after stirring for 10min. Raise the temperature to 15-20°C and react for 2 hours, pour the reaction solution into 500ml of water, stir for 0.5 hours, separate the liquids, extract the water phase with 30ml of dichloromethane, combine the organic phases, wash with saturated saline, evaporate to dryness, and place the obtained solid in 700ml of ethanol Stir at 40° C. for 2 h, cool to room temperature, filter and dry to obtain 88 g of white powder, the two-step yield is 60%.
[0073] ESI-MS[M+Na] + : 391.09
[0074] 1 H-NMR (DMSO-d6): δ1.60-1.64 (m, 4H), 2.40 (s, 3H), 2.66 (t, 2H, J=7.8Hz), 4.08 (t, 2H, J=5....
Embodiment 3
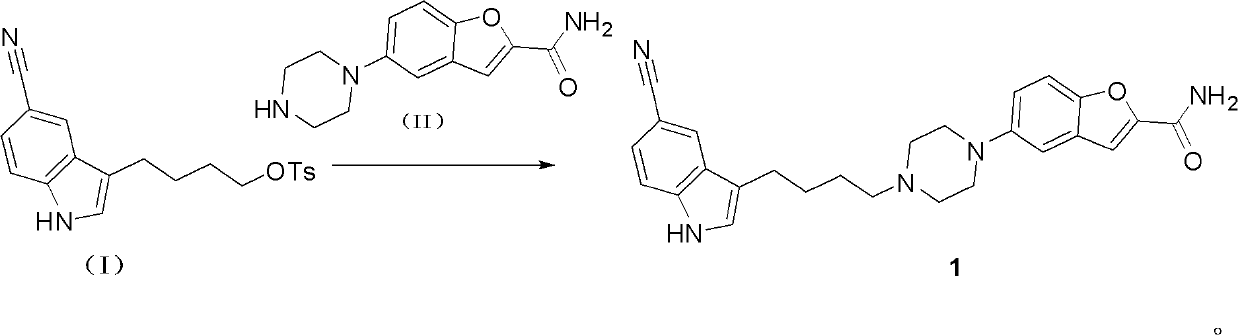
[0076] Preparation of 5-(4-(4-(5-cyano-3-indolyl)butyl)-1-piperazinyl)benzofuran-2-carboxamide (compound 1, vilazodone)
[0077] Dissolve 4-(5-cyanindol-3-yl)butyl p-toluenesulfonate (0.12mol, 44.2g) in 800ml acetonitrile, add 5-(1-piperazinyl)-benzofuran -2-Carboxamide (0.1mol, 24.5g), diisopropylethylamine (0.3mol, 38g) was then heated to reflux for 3 hours, the solvent was evaporated under reduced pressure, and the residue was added with 200ml of water and 400ml of ethyl acetate, After stirring for 1 h, the insoluble matter was collected by filtration to obtain 42 g of a light yellow solid crude product, which was recrystallized in 150 ml of methanol to obtain 35 g of a white powder with a yield of 79%.
[0078] ESI-MS[M+H] + : 442.22.
[0079] 1 H-NMR (DMSO-d6): δ1.49-1.51 (m, 2H), 1.62-1.67 (m, 2H), 2.30-2.34 (m, 2H), 2.47-2.50 (m, 4H), 2.72 (t , 2H, J=7.2Hz), 3.04-3.09(m, 4H), 7.09-7.12(m, 2H), 7.32(s, 1H), 7.38-7.51(m, 4H), 7.60(br.s, 1H ,D 2 O heavy water exchang...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com