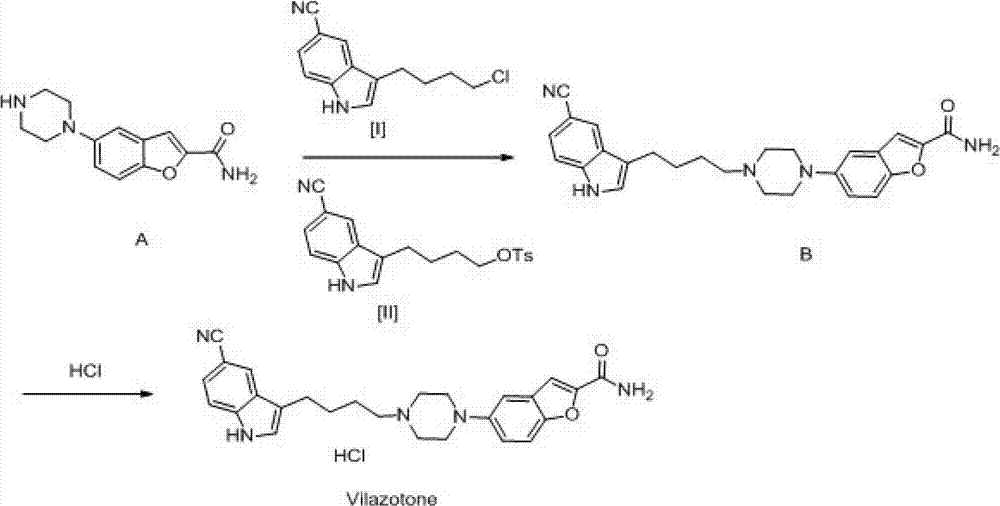
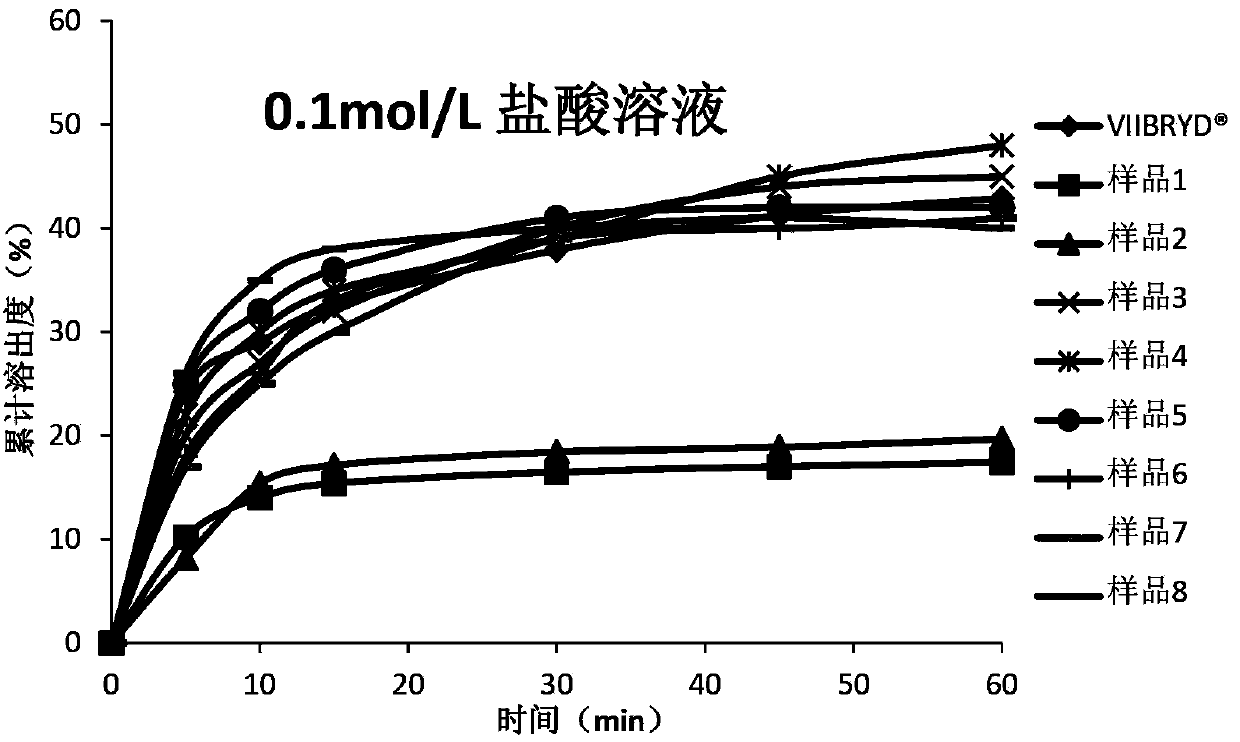
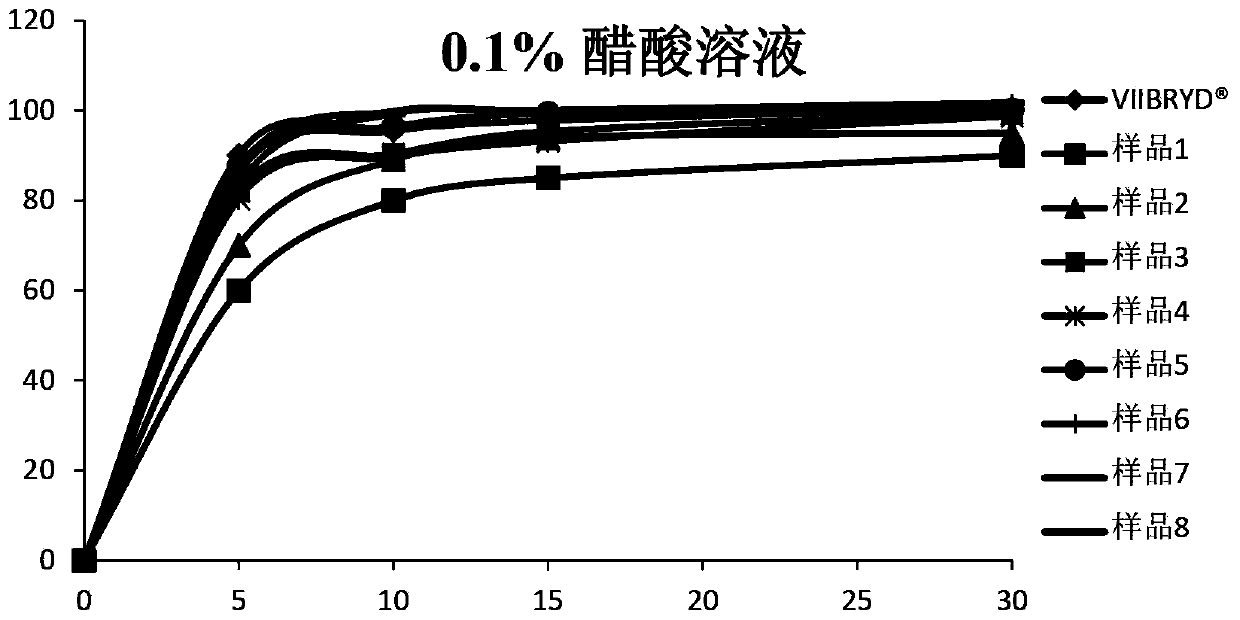
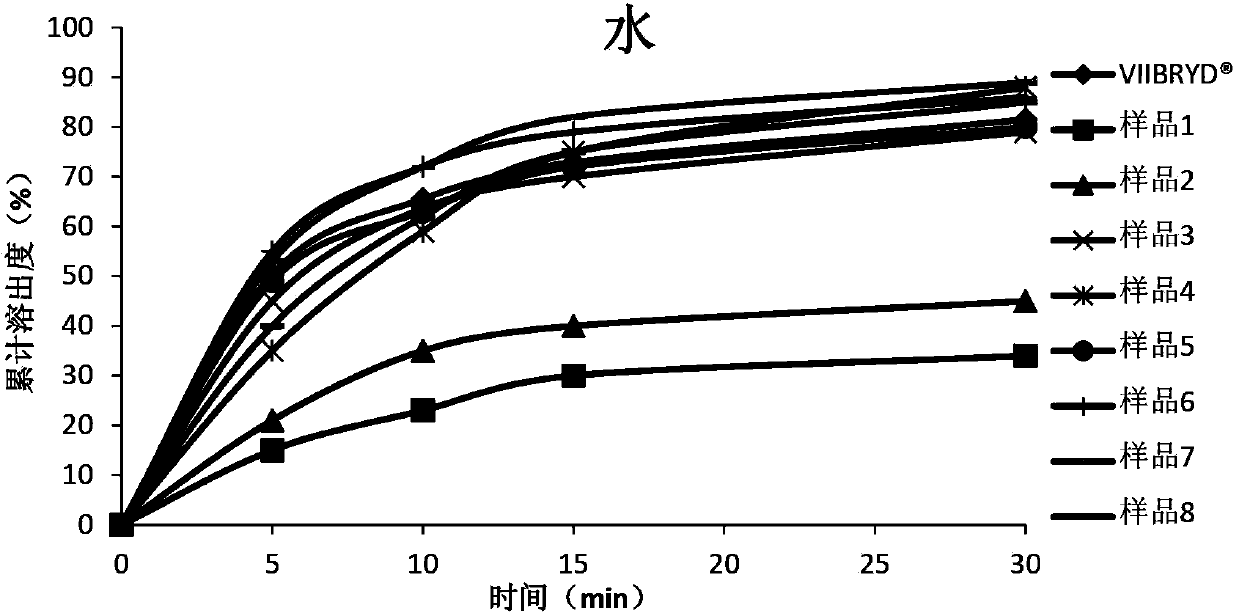
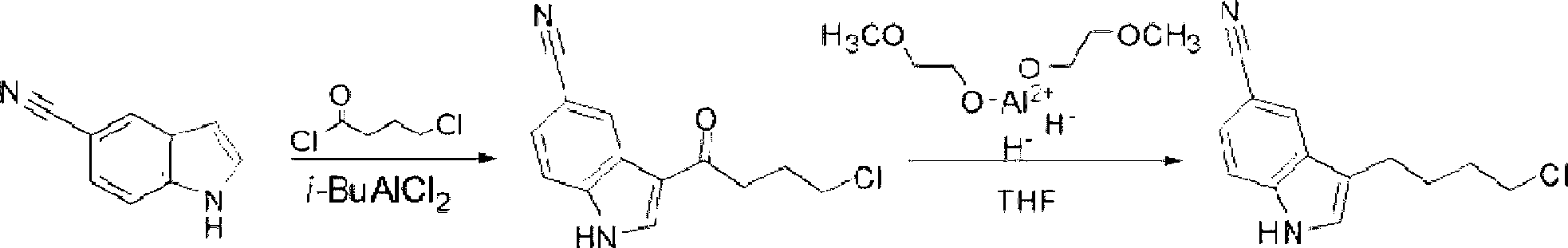
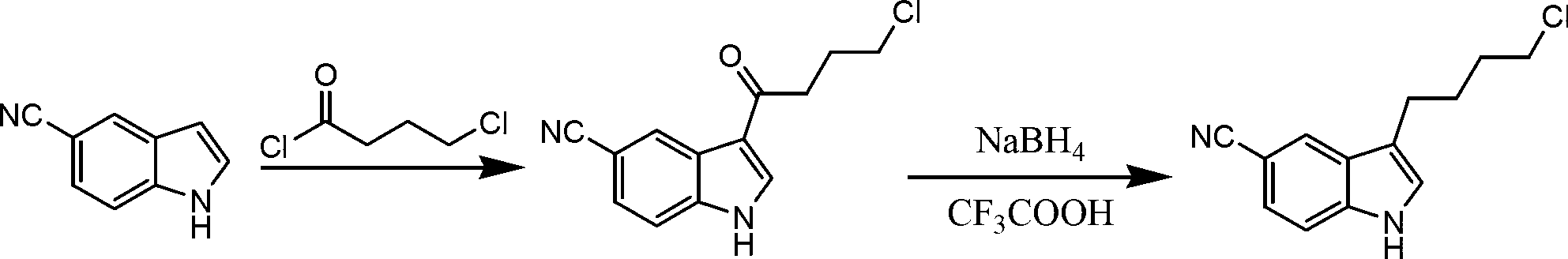
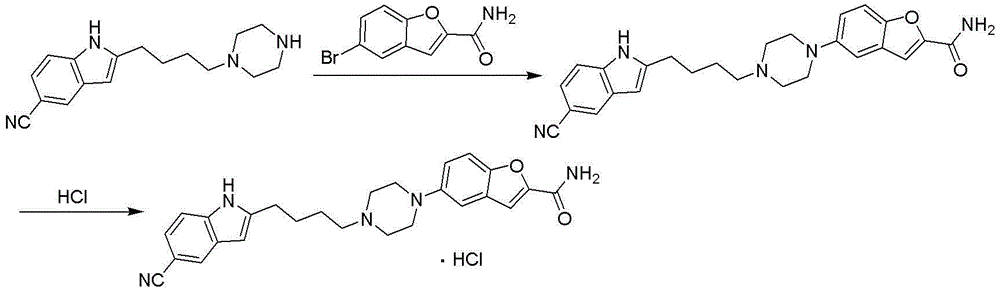
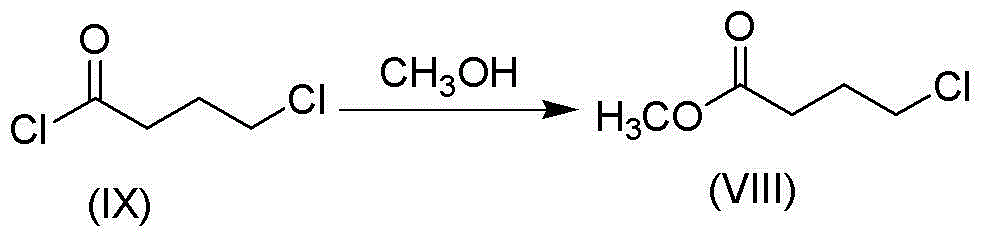
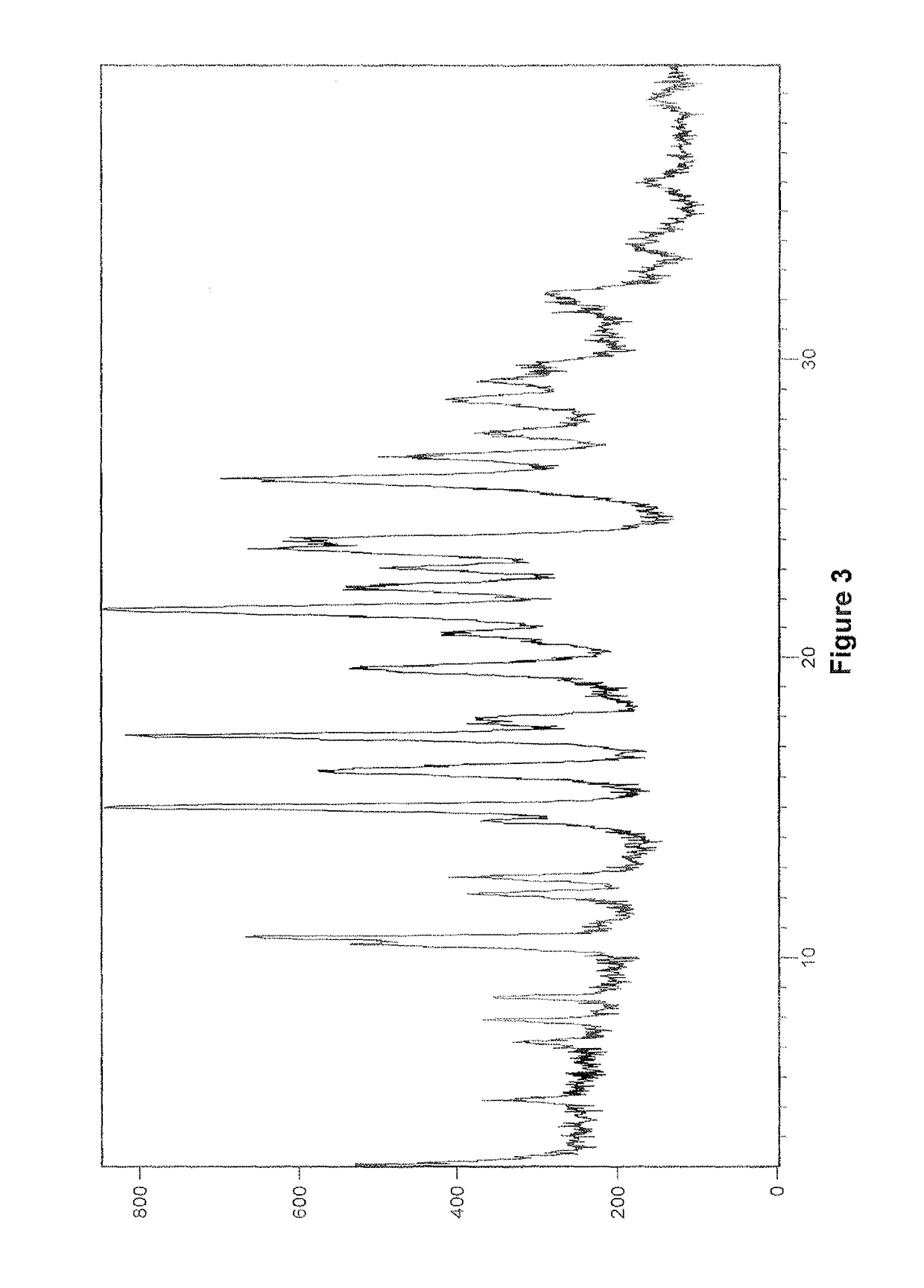
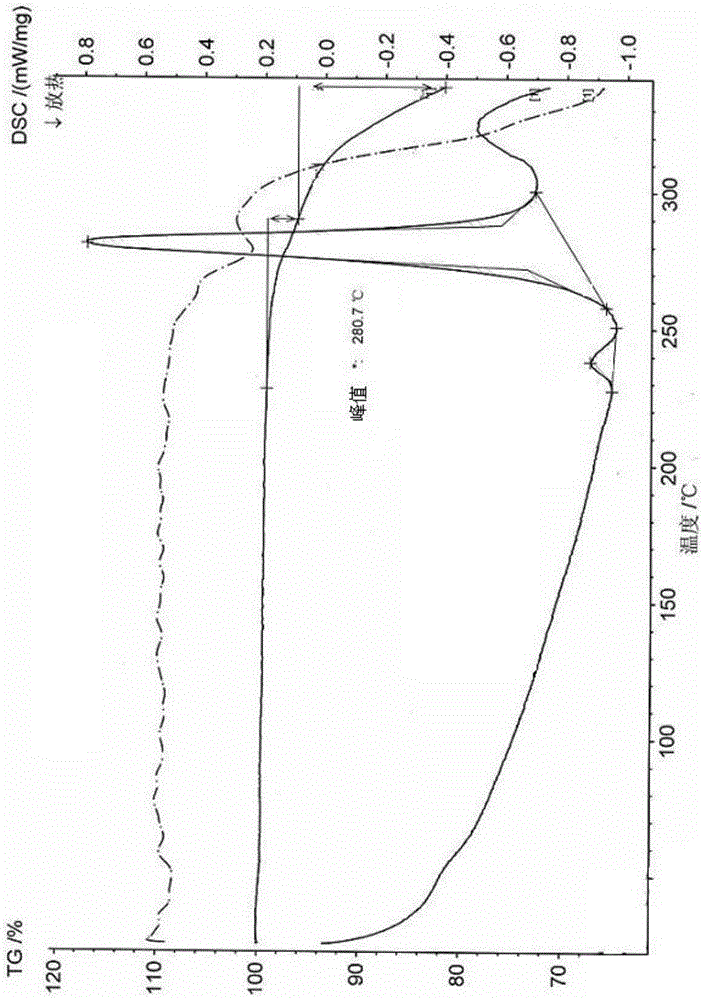
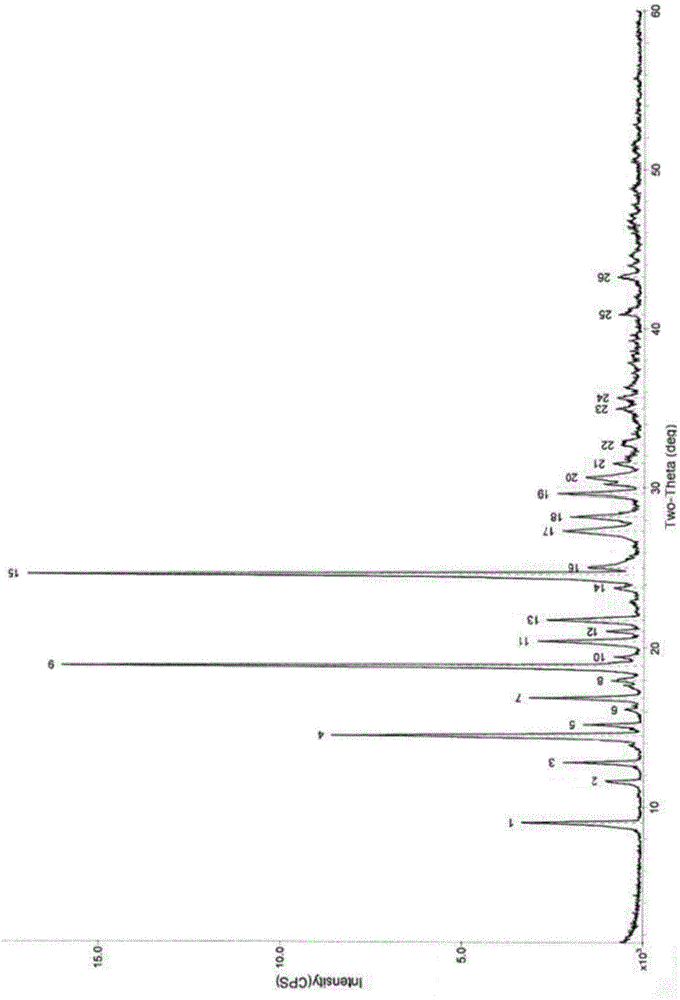
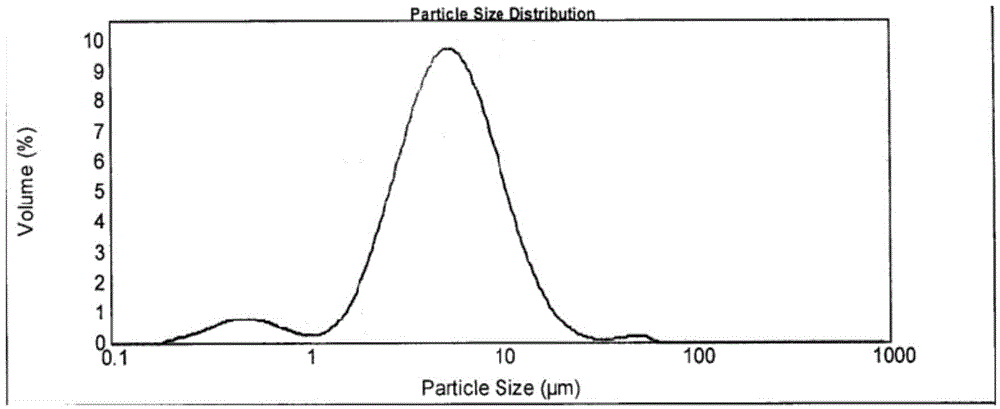
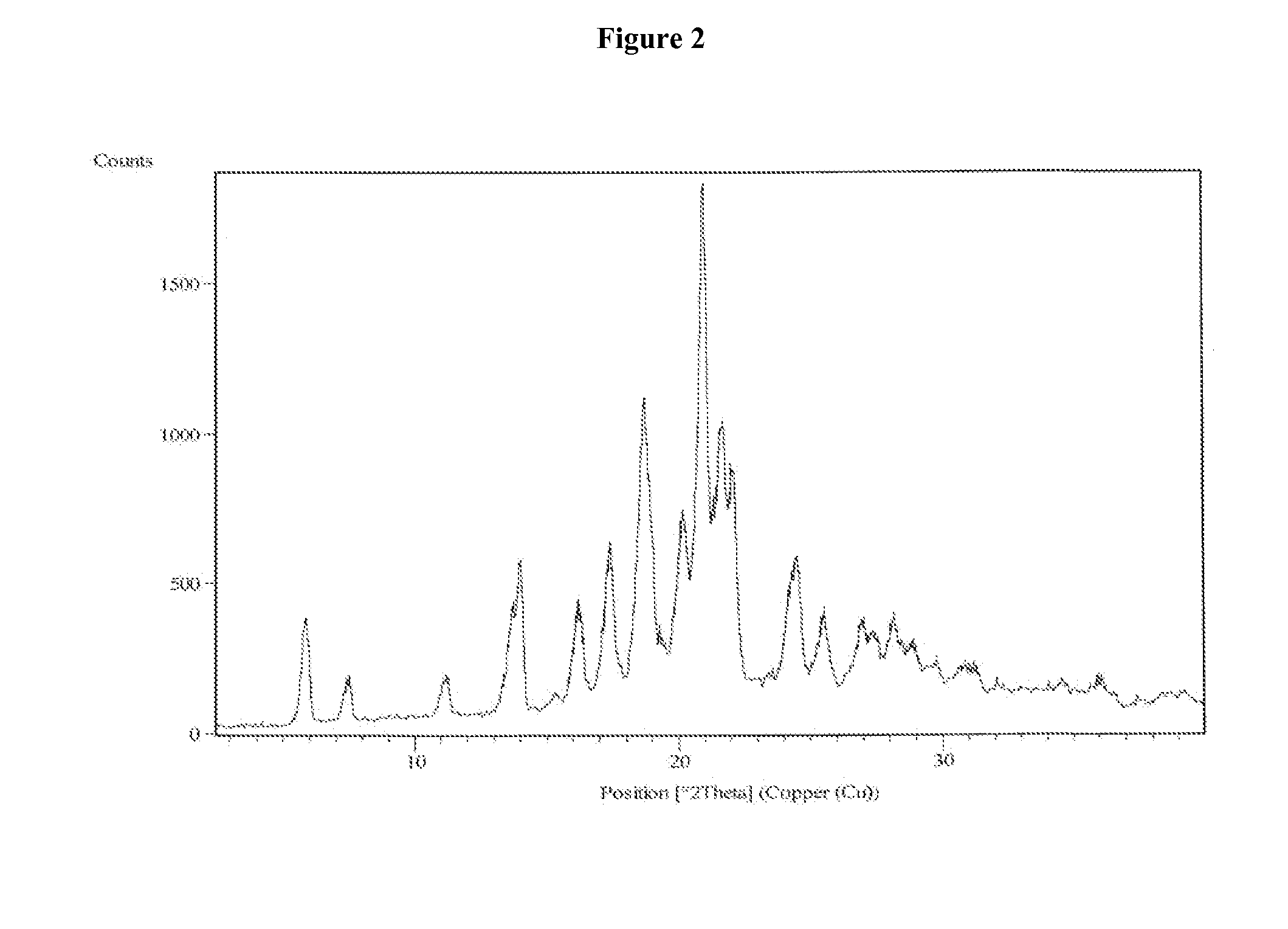
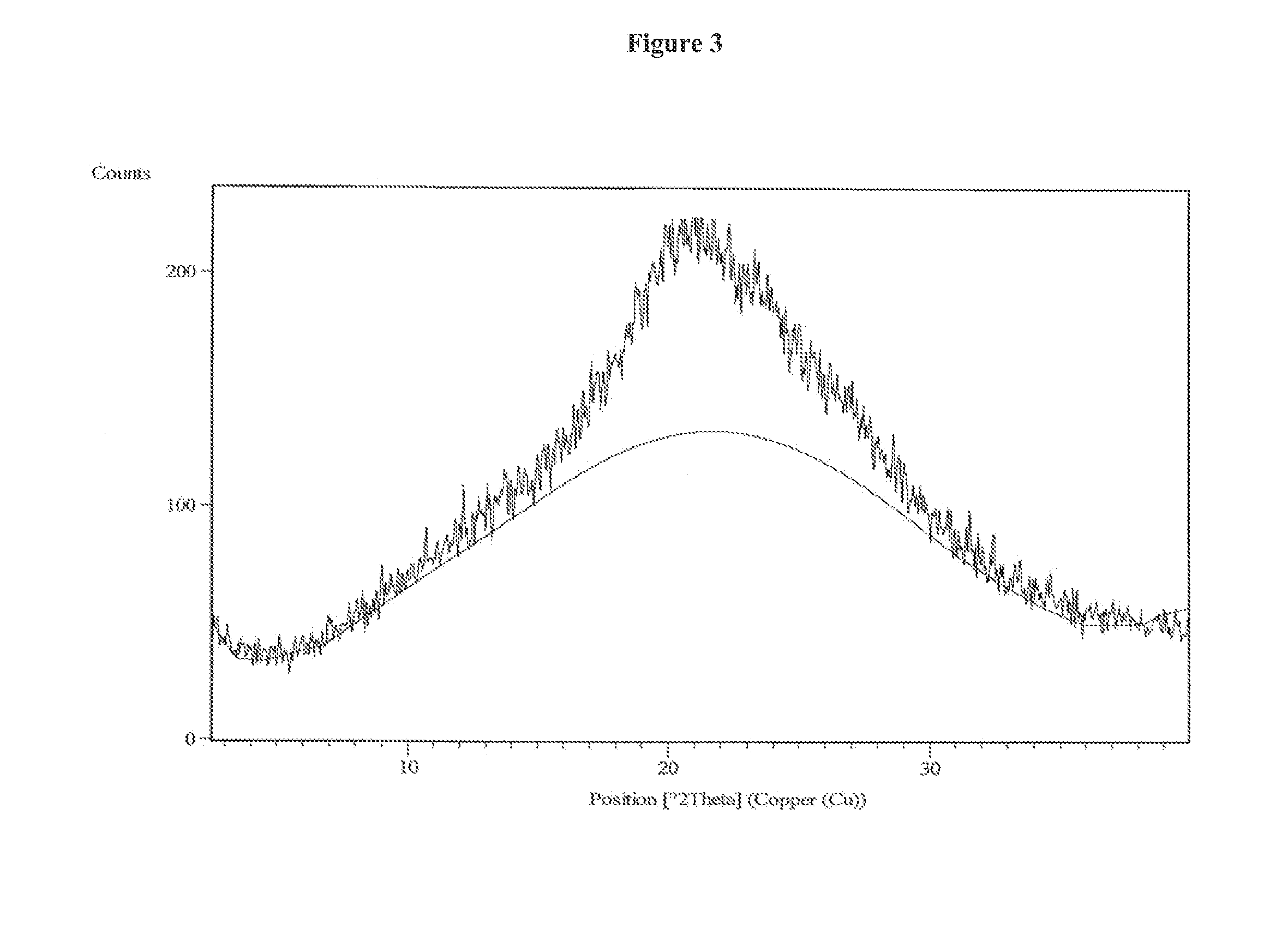
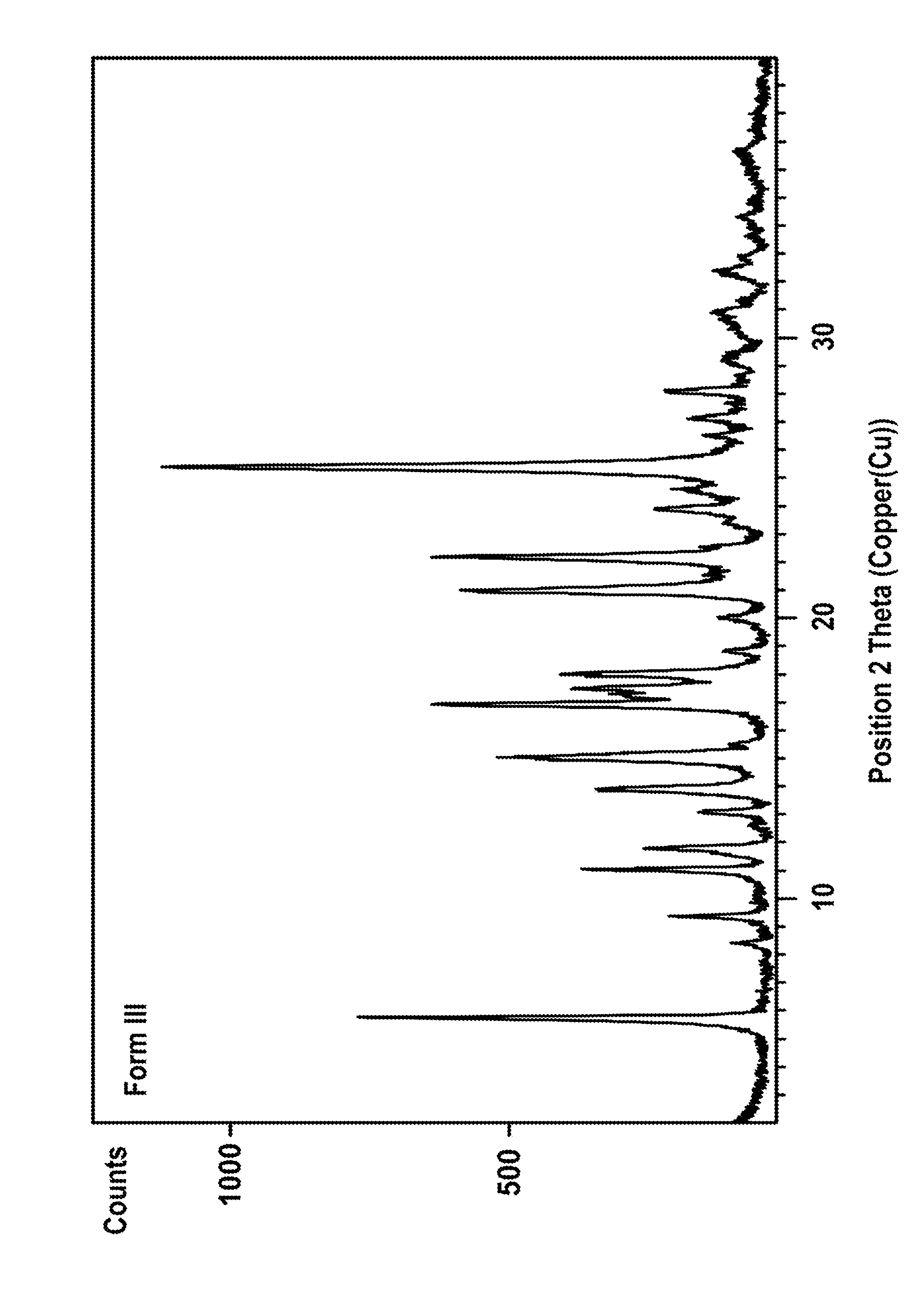
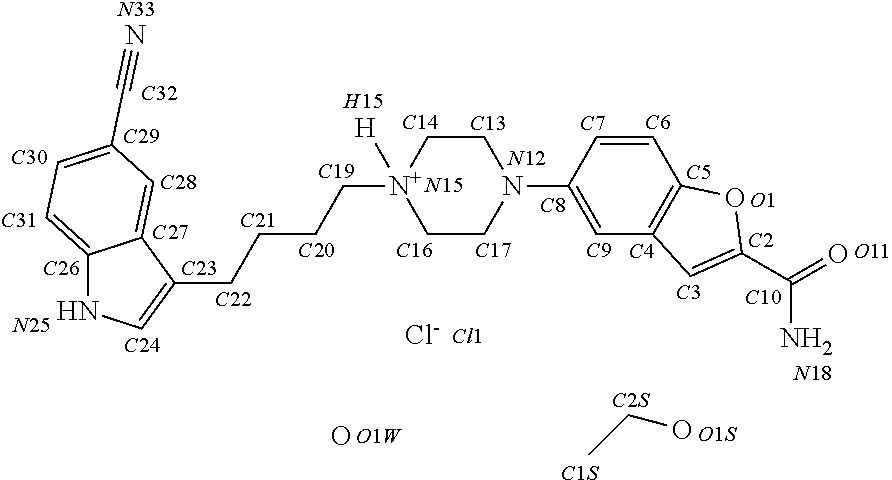
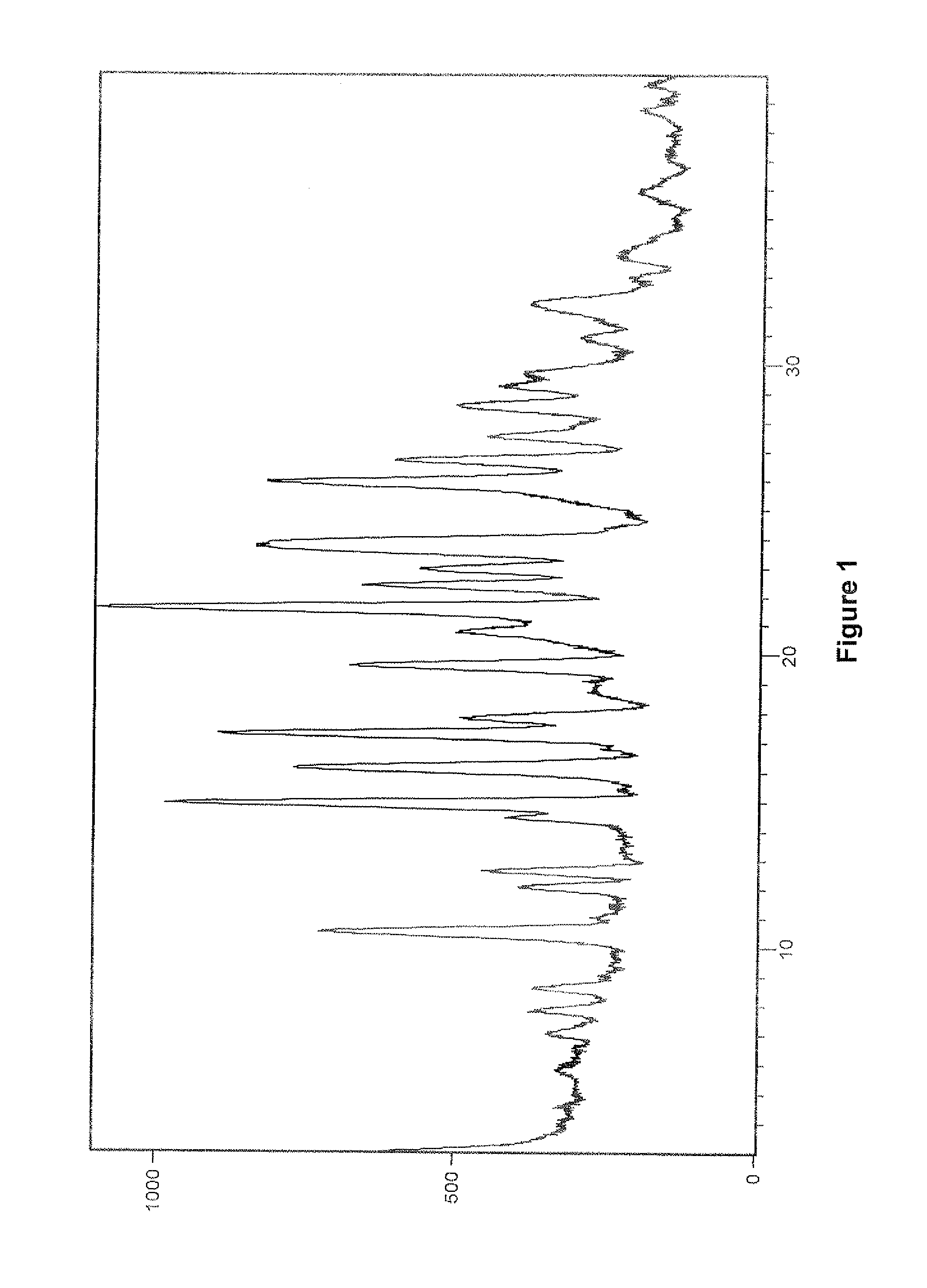
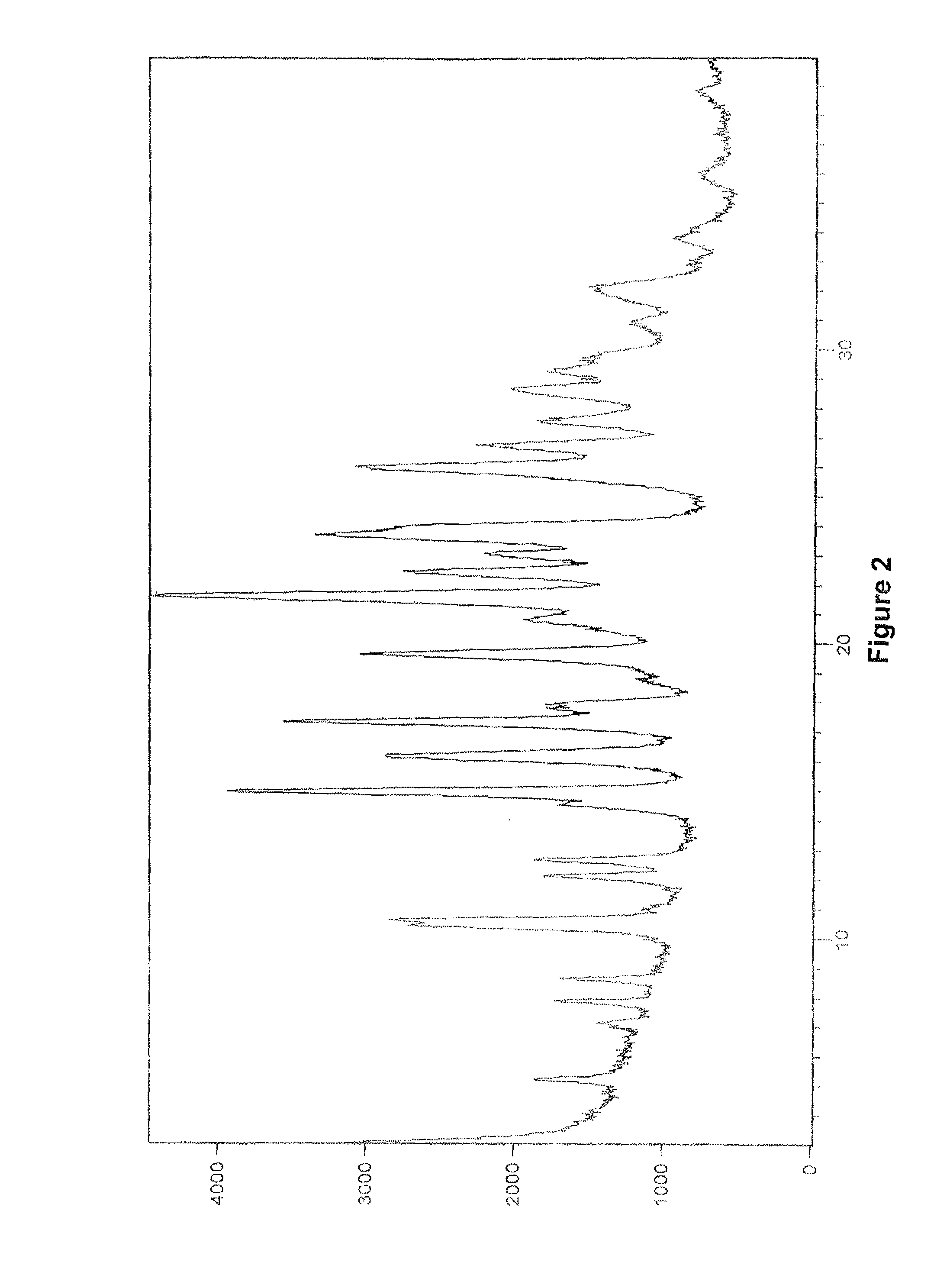
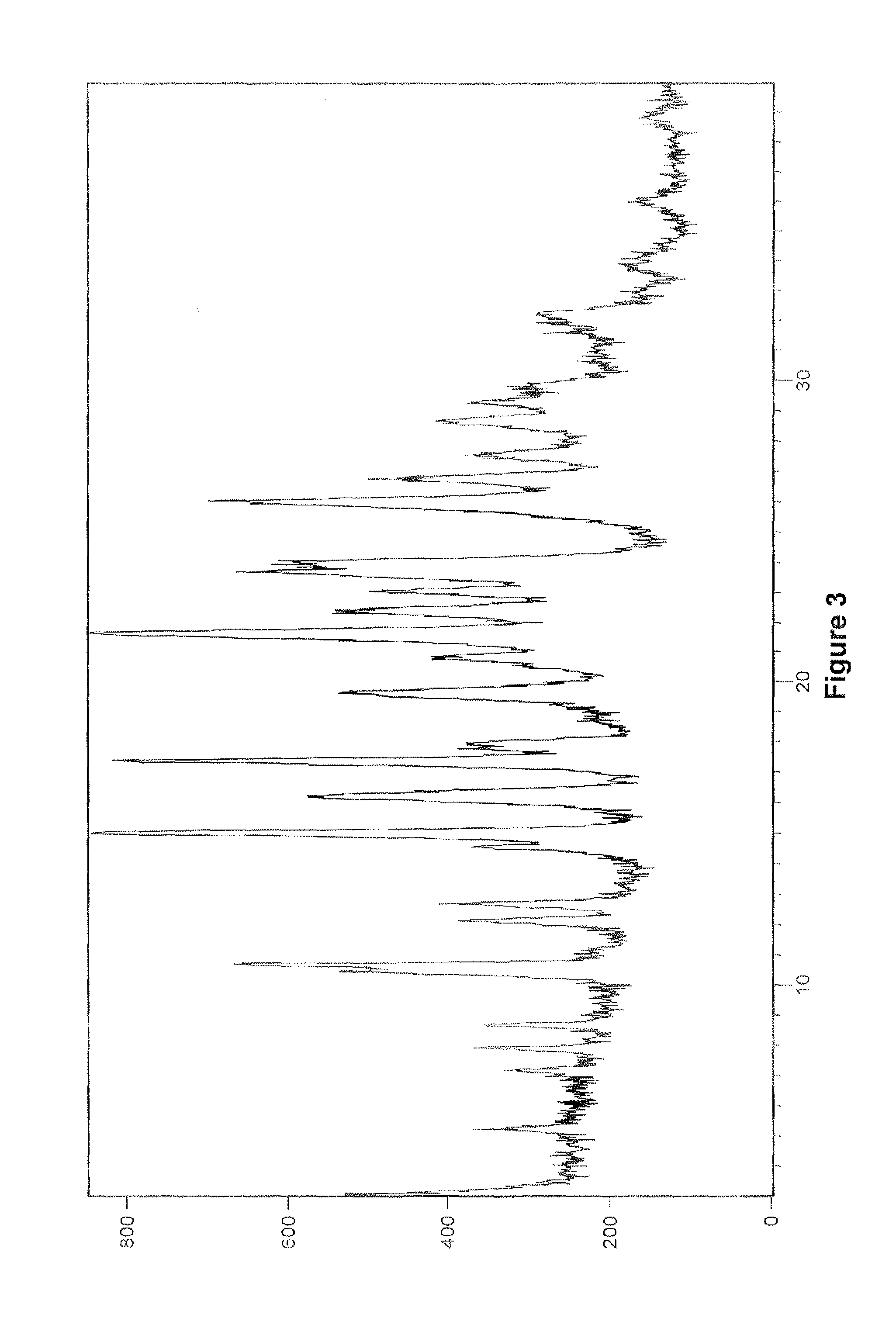
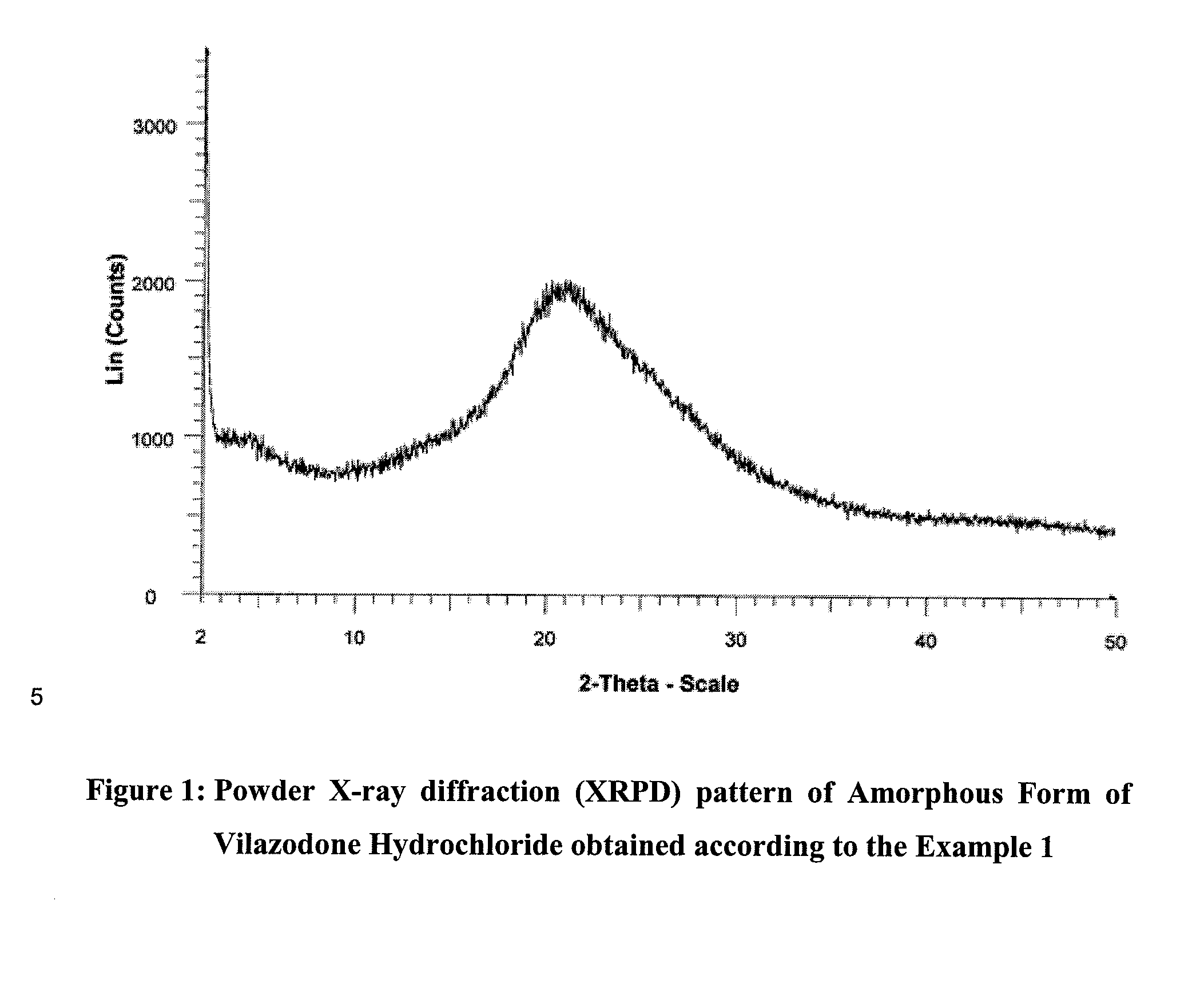
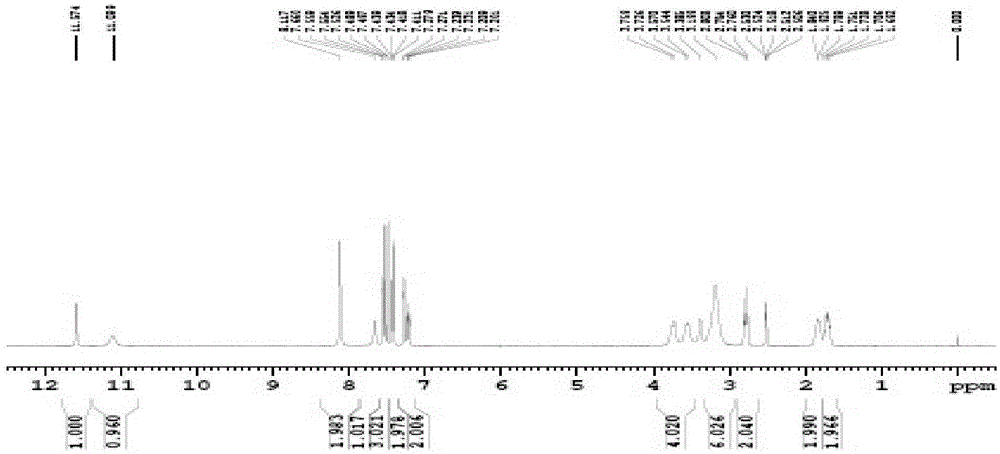
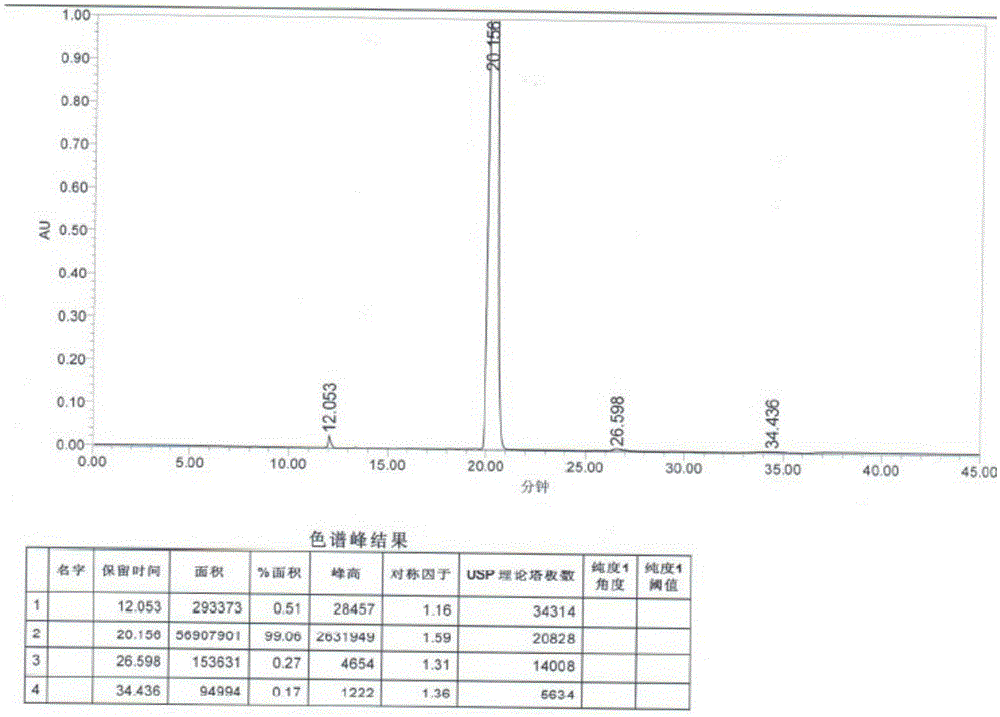
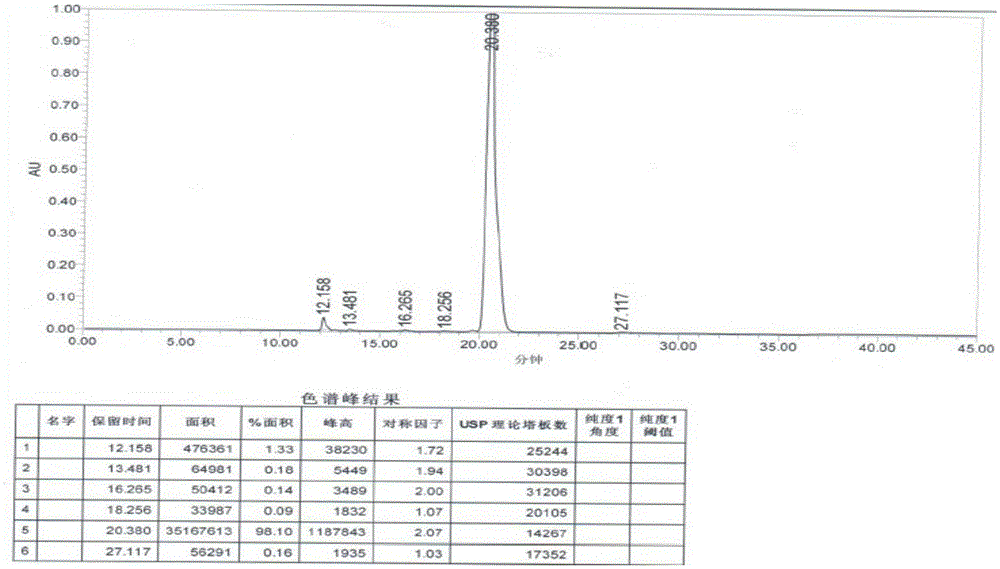
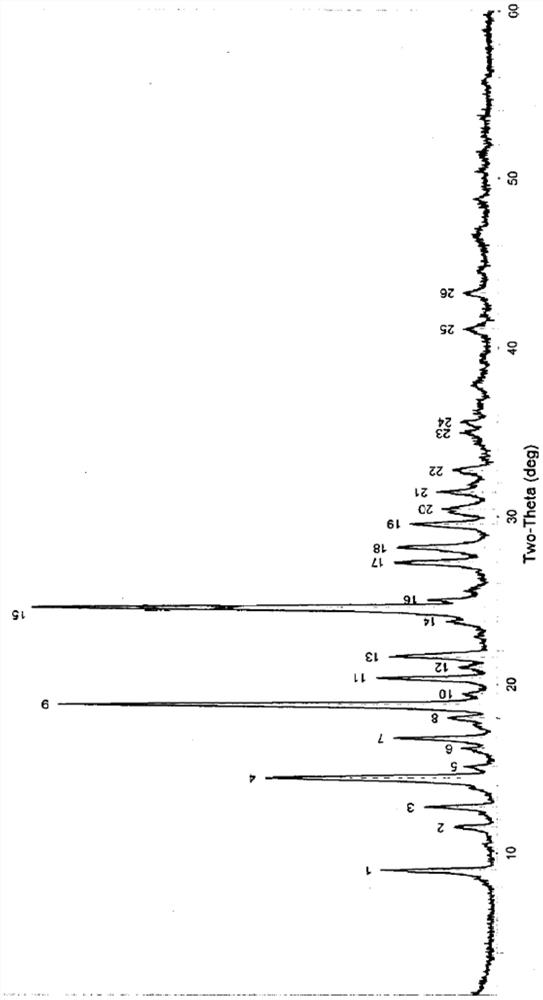
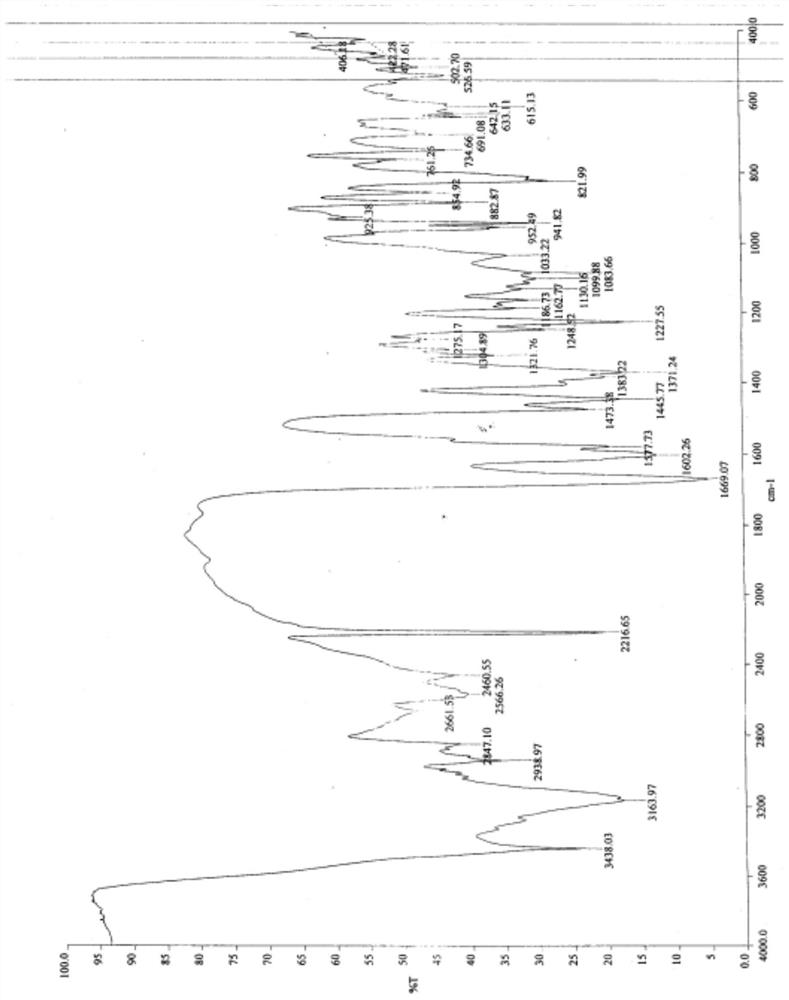
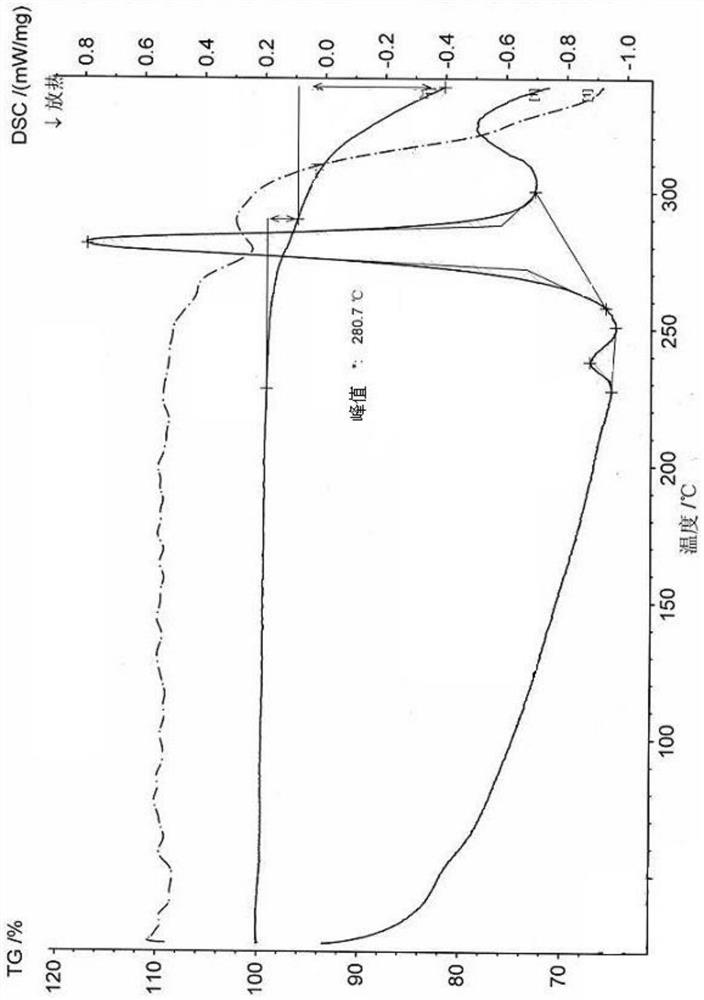
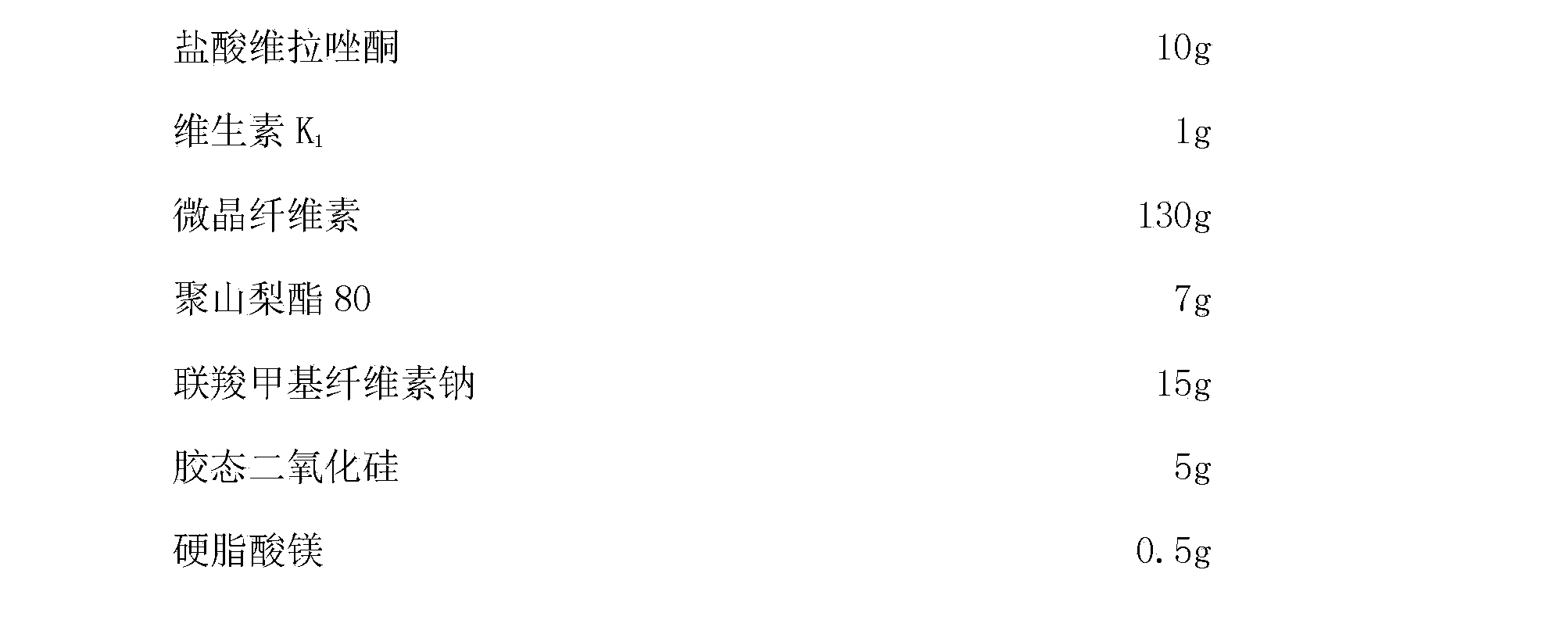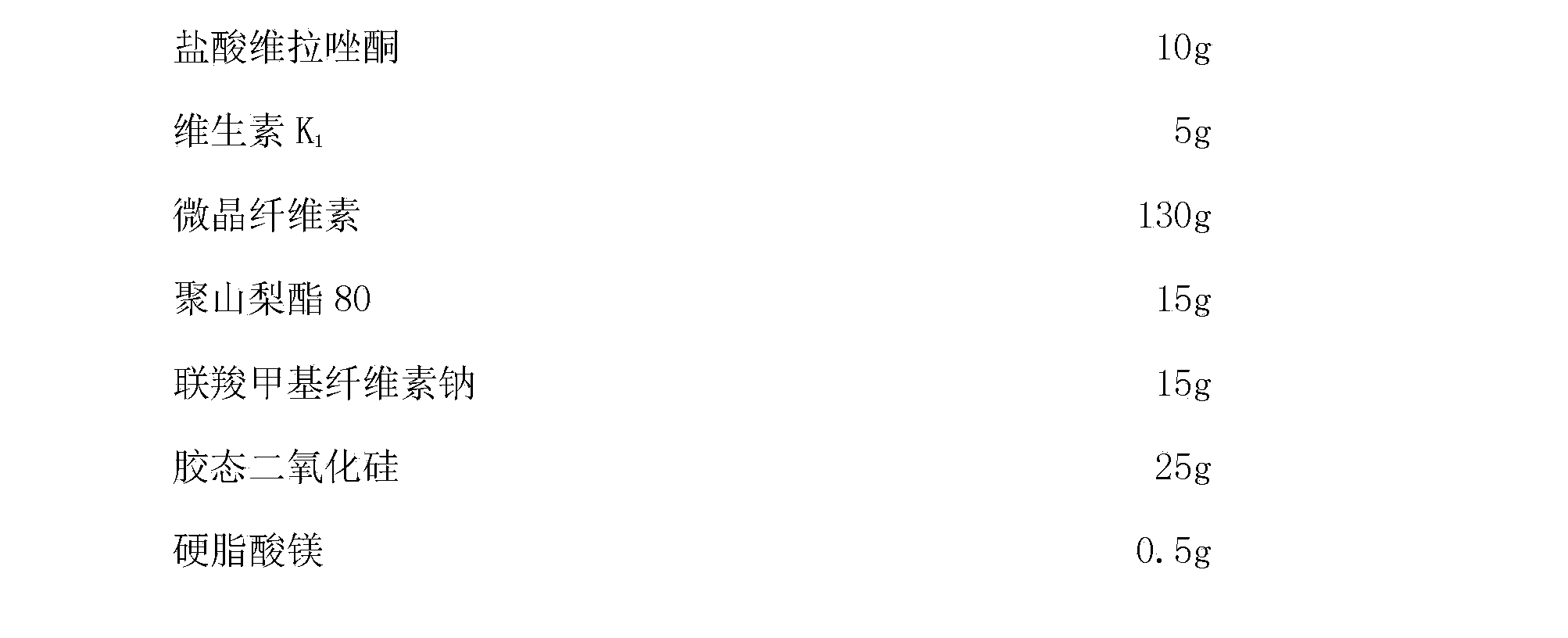Patents
Literature
49 results about "Vilazodone Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
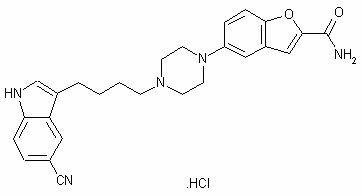
A benzofuran, indole, and piperazine derivative that functions as a SEROTONIN UPTAKE INHIBITOR and partial SEROTONIN 5-HT1 RECEPTOR AGONIST. It is used as an ANTIDEPRESSIVE AGENT.
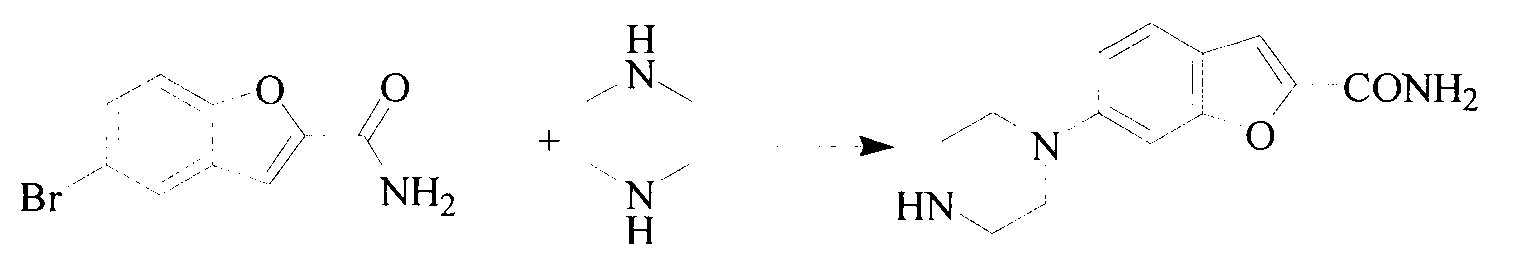
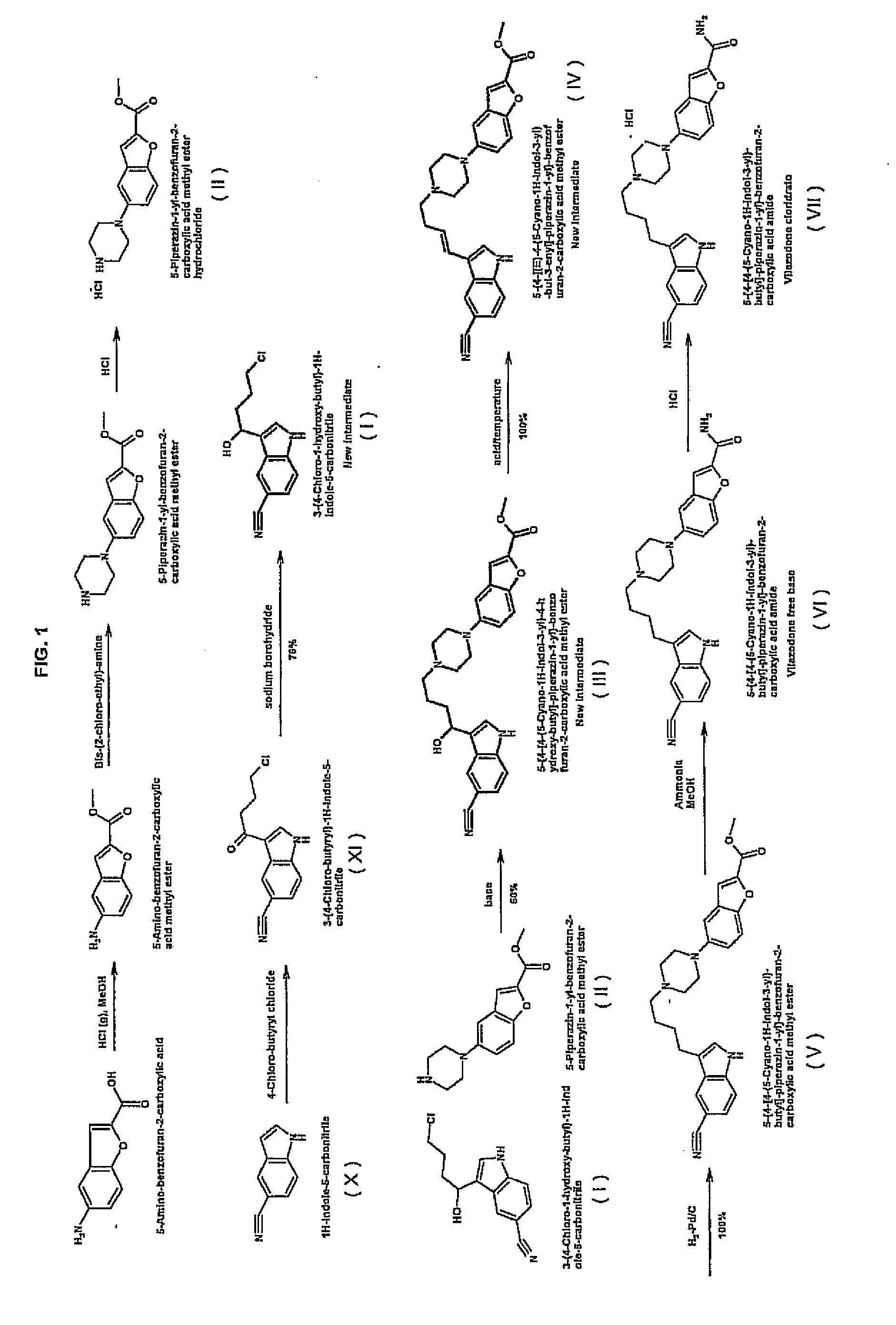
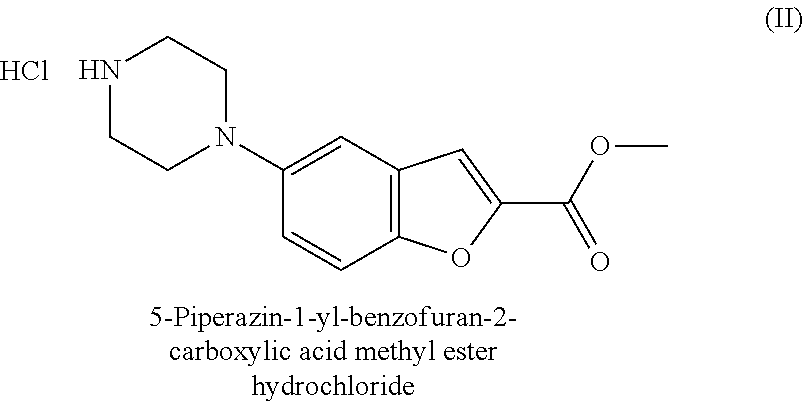
Synthesis method of 5-(piperazino-1-yl)benzofuryl-2-formamide
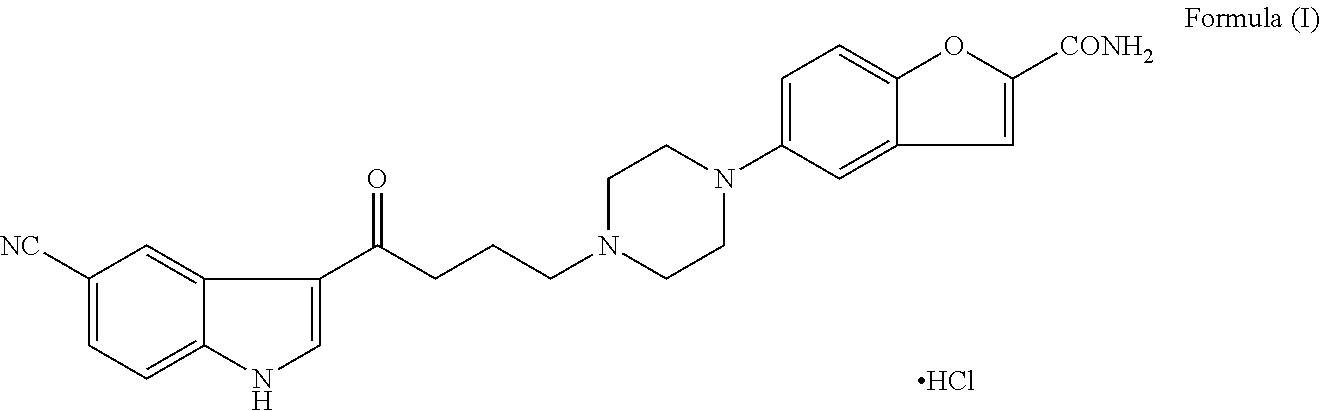
ActiveCN102964323ASolve environmental problemsLow costOrganic chemistryBulk chemical productionSynthesis methodsStructural formula
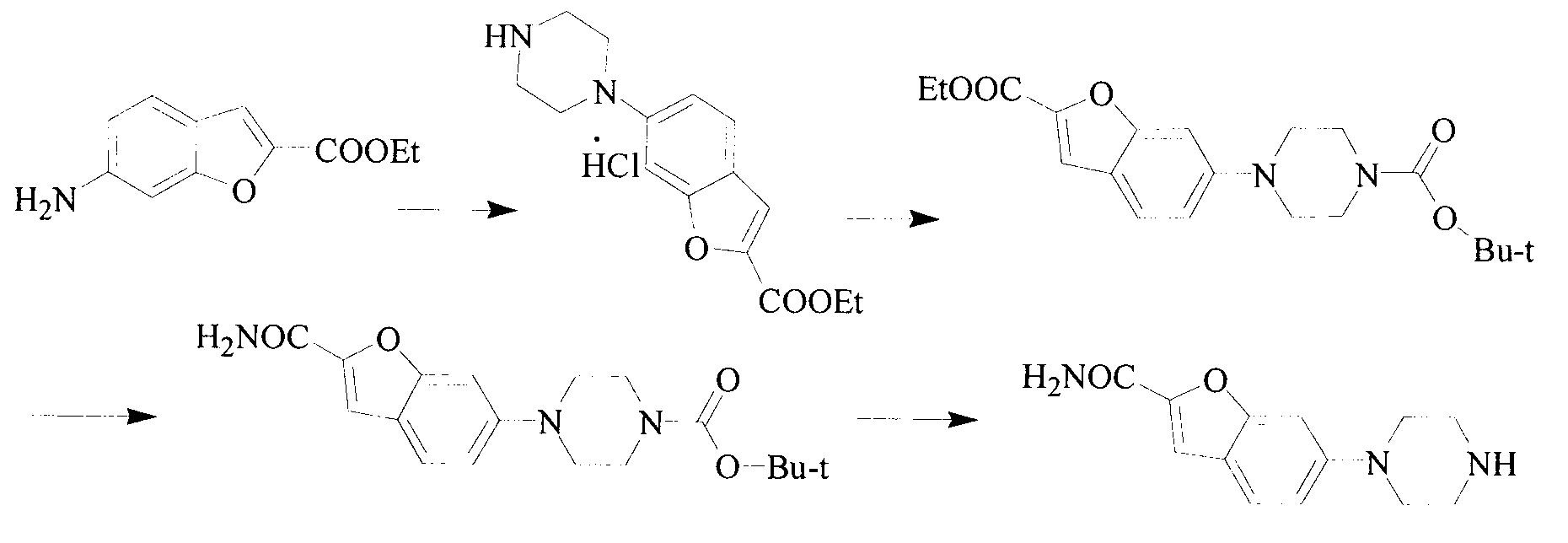
The invention relates to a synthesis method of a vilazodone hydrochloride intermediate 5-(piperazino-1-yl)benzofuryl-2-formamide, belonging to the technical field of medicine synthesis. The synthesis method comprises the following steps: reacting a compound disclosed as Formula (I) with polyformaldehyde under reflux for 3-10 hours, carrying out cyclization reaction with ethyl chloroacetate at 80-120 DEG C for 3-24 hours, dissolving in alcohol, introducing ammonia gas, and reacting at 10-80 DEG C under the pressure of 0.1-2.0 Mpa for 3-24 hours to obtain a compound disclosed as Formula (IV), wherein the structural formulae of the Formula (I) and Formula (IV) are disclosed in the specification: when R is H, the compound disclosed as Formula (IV) is the product 5-(piperazino-1-yl)benzofuryl-2-formamide, and when R is a nitrogen protecting group, the protecting group can be removed to obtain the target product 5-(piperazino-1-yl)benzofuryl-2-formamide. The synthesis method has the advantages of low cost, short production procedure, high product yield and purity, and good product quality.
Owner:SUZHOU UUGENE BIOPHARMA
Vilazodone hydrochloride composition and preparation method thereof
ActiveCN104116741ASolving In Vitro Dissolution ProblemsImprove bioavailabilityOrganic active ingredientsNervous disorderPharmaceutical medicineMicrocarrier
The invention relates to a vilazodone hydrochloride composition and a preparation method thereof. The composition includes vilazodone hydrochloride, a microcarrier and pharmaceutically acceptable excipients for an oral solid preparation. The preparation method is as below: mixing and crushing vilazodone hydrochloride and the microcarrier, and evenly mixing the mixture with pharmaceutically acceptable excipients for oral solid preparation. The obtained composition preparation has greatly improved dissolution in vitro, and in vivo bioavailability reaching bioequivalence to VIIBRYD.
Owner:JIANGSU SIMCERE PHARMA
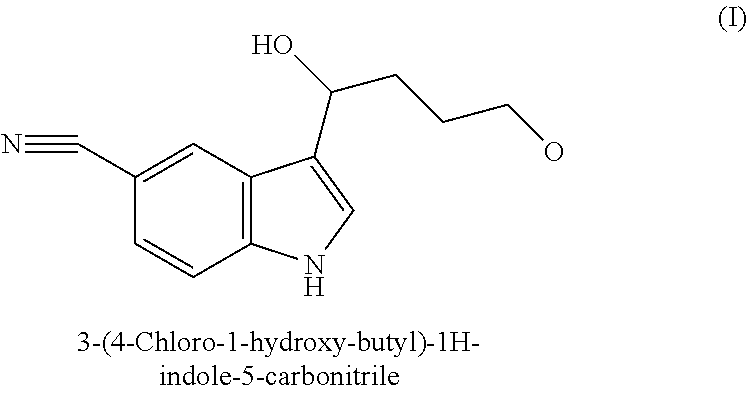
Preparation method and application of 3-(4-chlorobutyl)-5-cyano-1H-indole
The invention relates to a preparation method and application of 3-(4-chlorobutyl)-5-cyano-1H-indole. The preparation method comprises the following steps: after dissolving 3-(4-chlorobutyryl)-5-cyano-1H-indole in a solvent, adding trifluoroacetic acid, adding sodium borohydride in batches, and treating the reaction liquid to obtain the 3-(4-chlorobutyl)-5-cyano-1H-indole. The method overcomes the defects in the existing preparation method of an important intermediate 3-(4-chlorobutyl)-5-cyano-1H-indole of an antidepressant vilazodone hydrochloride, and has obvious creativity and practical application value. The reaction formula is disclosed in the specification.
Owner:HANGZHOU HEZE PHARMA TECH
Vilazodone hydrochloride and composition thereof
The invention relates to a crystal form of vilazodone dihydrochloride and a preparation method thereof, and also relates a medicinal composition containing vilazodone in the crystal form and application of the crystal form to the preparation of medicaments for treating melancholia.
Owner:天津汉一医药科技有限公司
Preparation method of 3-(4-chlorobutyl)indole-5-formonitrile
InactiveCN103058912AHigh yieldHigh product purityOrganic chemistryTrifluoroacetic acidEconomic benefits
The invention relates to a preparation method of 3-(4-chlorobutyl)indole-5-formonitrile. 3-(4-chlorobutyl)indole-5-formonitrile is an important intermediate for synthesis of vilazodone hydrochloride. 5-cyanoindole and 4-chlorobutyryl chloride as raw materials undergo a Friedel-Crafts acylation reaction and then the product is reduced by sodium borohydride / trifluoroacetic acid into 3-(4-chlorobutyl)indole-5-formonitrile. The preparation method utilizes the cheap and easily acquired reagents, has a high yield and high product purity, is simple and reliable, and has a low production cost, a large implement value and social and economic benefits.
Owner:SHANDONG ZOUPING DAZHAN NEW MATERIALS
Method for preparing vilazodone or hydrochloride thereof
The invention relates to a method for preparing 5-(4-[4-(5-cyan-3-indolyl)-butyl]-1-piperazinyl) benzofuran-2-formamide (vilazodone) or hydrochloride thereof. The method comprises the following steps of: performing reaction between a formula-I compound and a formula-II compound 5-amino-2-formamido-benzofuran in a solvent under the action of alkaline matter, and separating and purifying products to obtain a formula-1 compound, i.e. the vilazodone; and salifying the vilazodone and hydrochloric acid in the solvent to prepare vilazodone hydrochloride. The invention also relates to a method for preparing the formula-I compound.
Owner:BEIJING TRADE STAR MEDICAL TECH
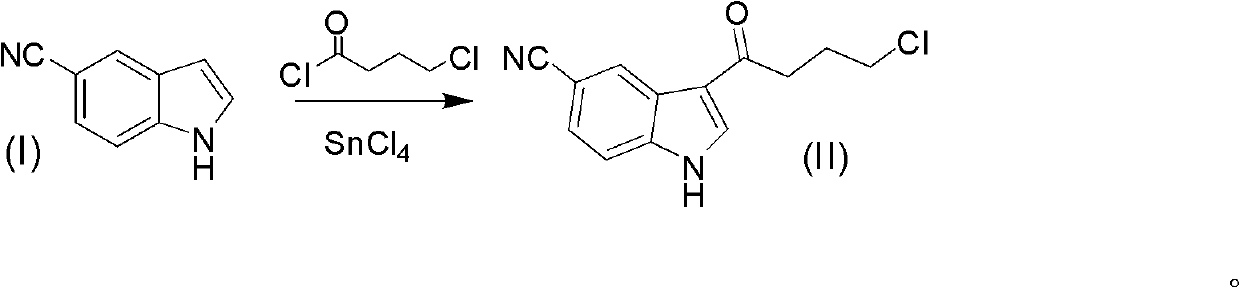
Method for preparing 3-(4-chlorobutyryl)-1H-indole-5-methylcyanogen
The invention provides a method for preparing 3-(4-chlorobutyryl)-1H-indole-5-methylcyanogen. The method provided by the invention comprises the following steps of: dissolving a compound 1H-indole-5-methylcyanogen shown as a formula (I) into a solvent and a cosolvent to react with tetrachlorobutyryl chloride in the presence of a catalyst tin tetrachloride; then collecting the compound 3-(4-chlorobutyryl)-1H-indole-5-methylcyanogen shown as a formula (II) from the reaction product. The method provided by the invention is beneficial to overcoming of defects of the traditional method for preparing antidepressant vilazodone hydrochloride intermediate, namely 3-(4-chlorobutyryl)-1H-indole-5-methylcyanogen, is more suitable for the industrial production in a large scale, and has obvious creativeness and a practical application value. The reaction formula is shown as in the specification.
Owner:上海开义医药化工有限公司
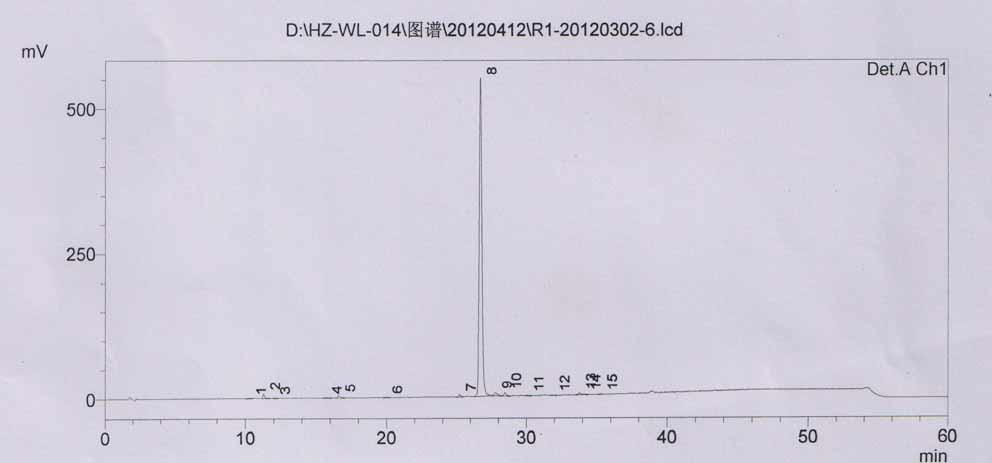
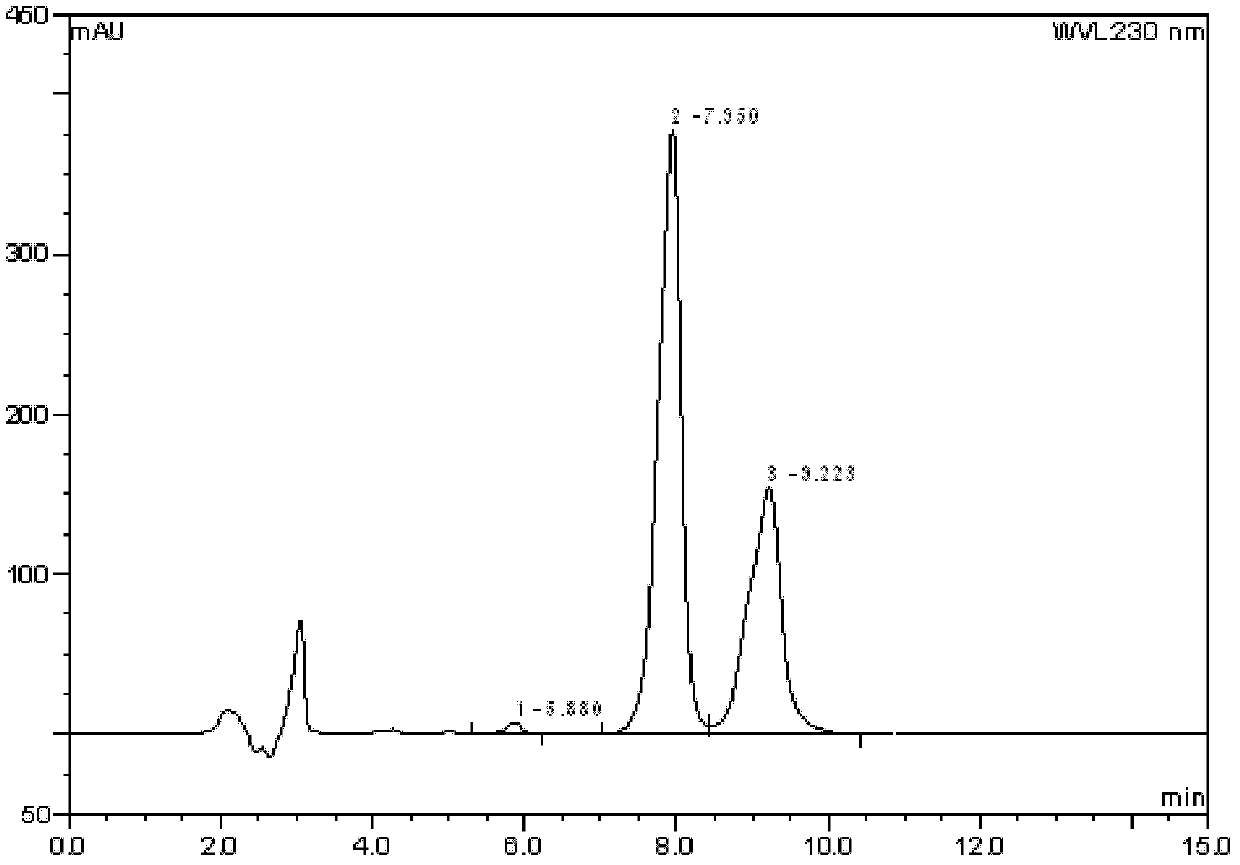
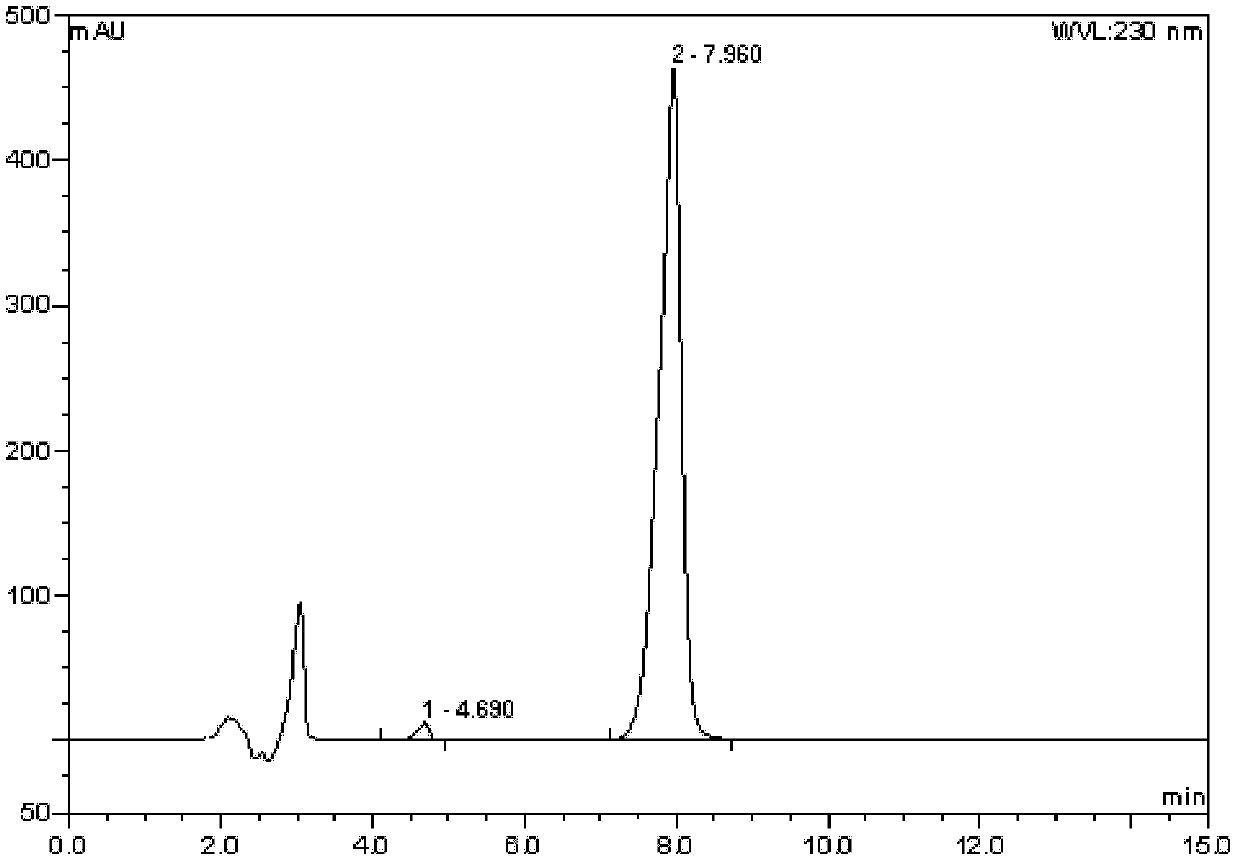
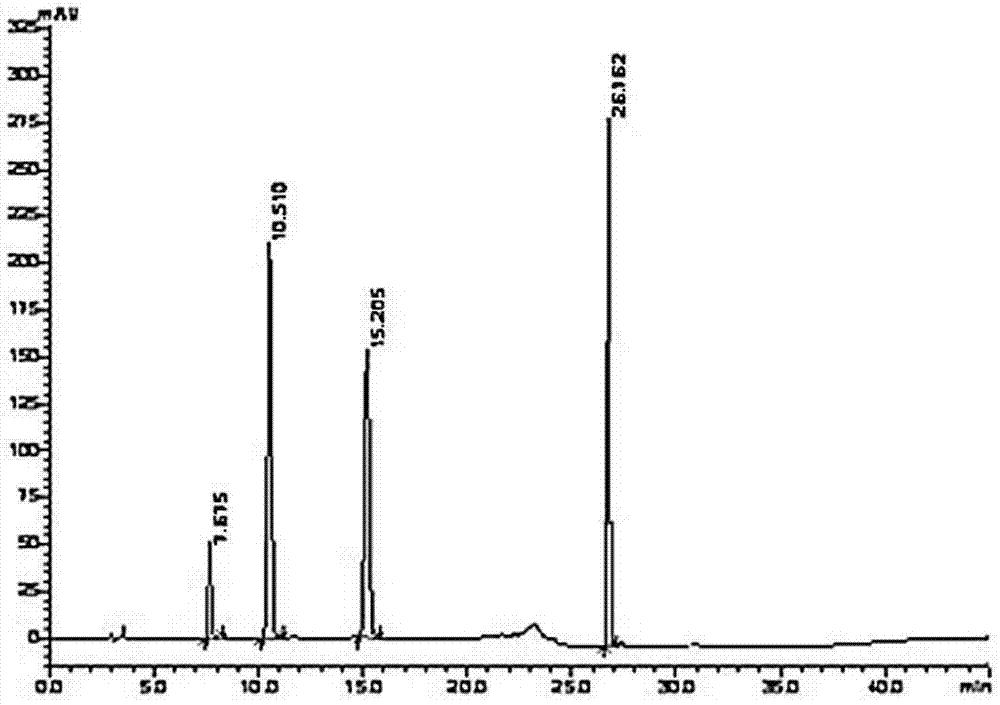
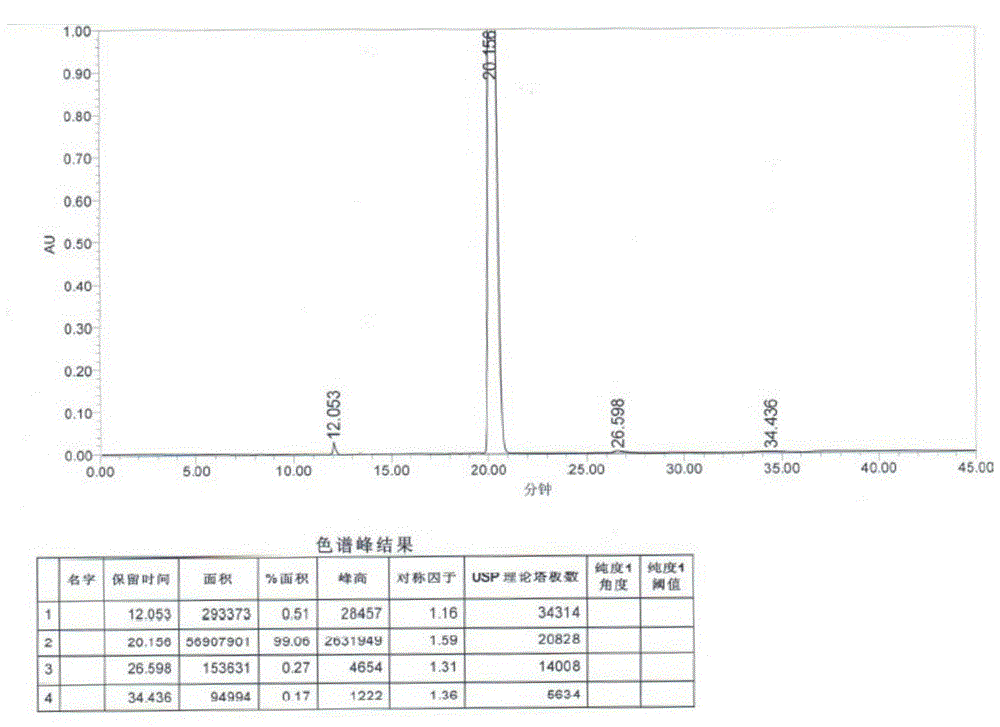
Method for measuring content of 2-chloro-1-methylpyridinium iodide in Vilazodone hydrochloride by separating with liquid chromatography
ActiveCN106018617AEffective separation assayQuality is easy to controlComponent separationIodideSilanes
The invention belongs to the field of analytical chemistry and discloses a method for detecting 2-chloro-1-methylpyridinium iodide in Vilazodone hydrochloride by separating with a liquid chromatography. According to the method disclosed by the invention, the content of the 2-chloro-1-methylpyridinium iodide is calculated with an external standard method by adopting a chromatographic column with octadecyl silane-bonded silica gel as filler and taking a certain proportion of buffer salt solution-organic phase as a flowing phase. The method disclosed by the invention is strong in specificity, high in accuracy, favorable in reproducibility and simple and convenient to operate, can be used for qualitatively or quantitatively detecting the 2-chloro-1-methylpyridinium iodide in the Vilazodone hydrochloride, and ensures the quality of the Vilazodone hydrochloride, thereby improving the safety of clinical medication.
Owner:BEIJING VENTUREPHARM BIOTECH
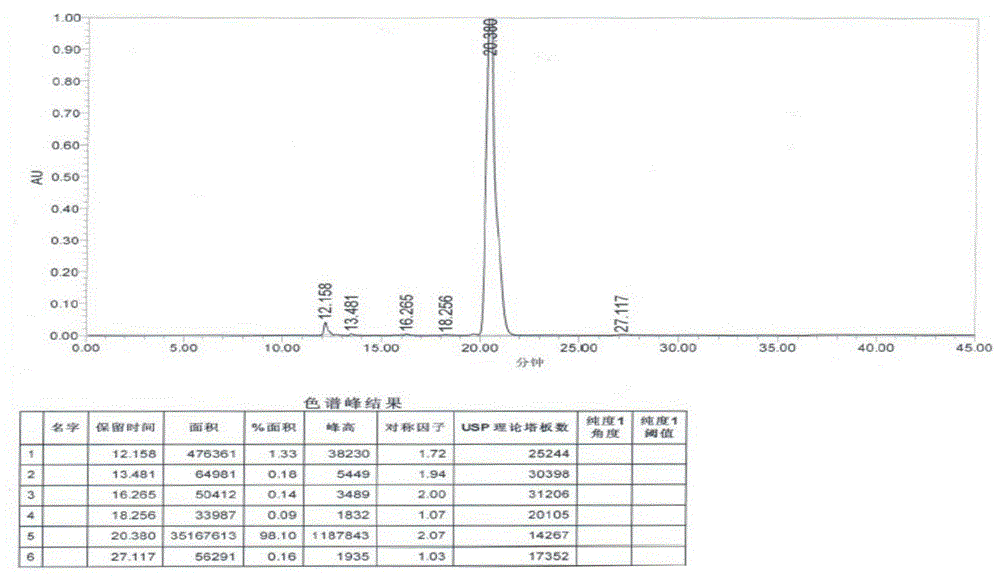
Method for separating and determining vilazodone hydrochloride raw materials and preparations thereof by liquid chromatography
ActiveCN103487532AEfficient separationSolving Separation Assay ProblemsComponent separationFluid phaseSilica gel
The invention belongs to the field of analysis chemistry, and specifically relates to a method for separating and determining vilazodone hydrochloride raw materials and the preparation thereof by liquid chromatography. According to the method, a chromatographic column with ctadecylsilane bonded silica gel as the filling material is employed, the mobile phase is a mixture of an acid aqueous solution and an organic phase with a certain ratio, and thus the contents of vilazodone hydrochloride and other related substances, so that the quality of vilazodone hydrochloride and preparations containing vilazodone hydrochloride are effectively controlled. The method is strong in specificity, high in accuracy and simple to operate.
Owner:BEIJING VENTUREPHARM BIOTECH
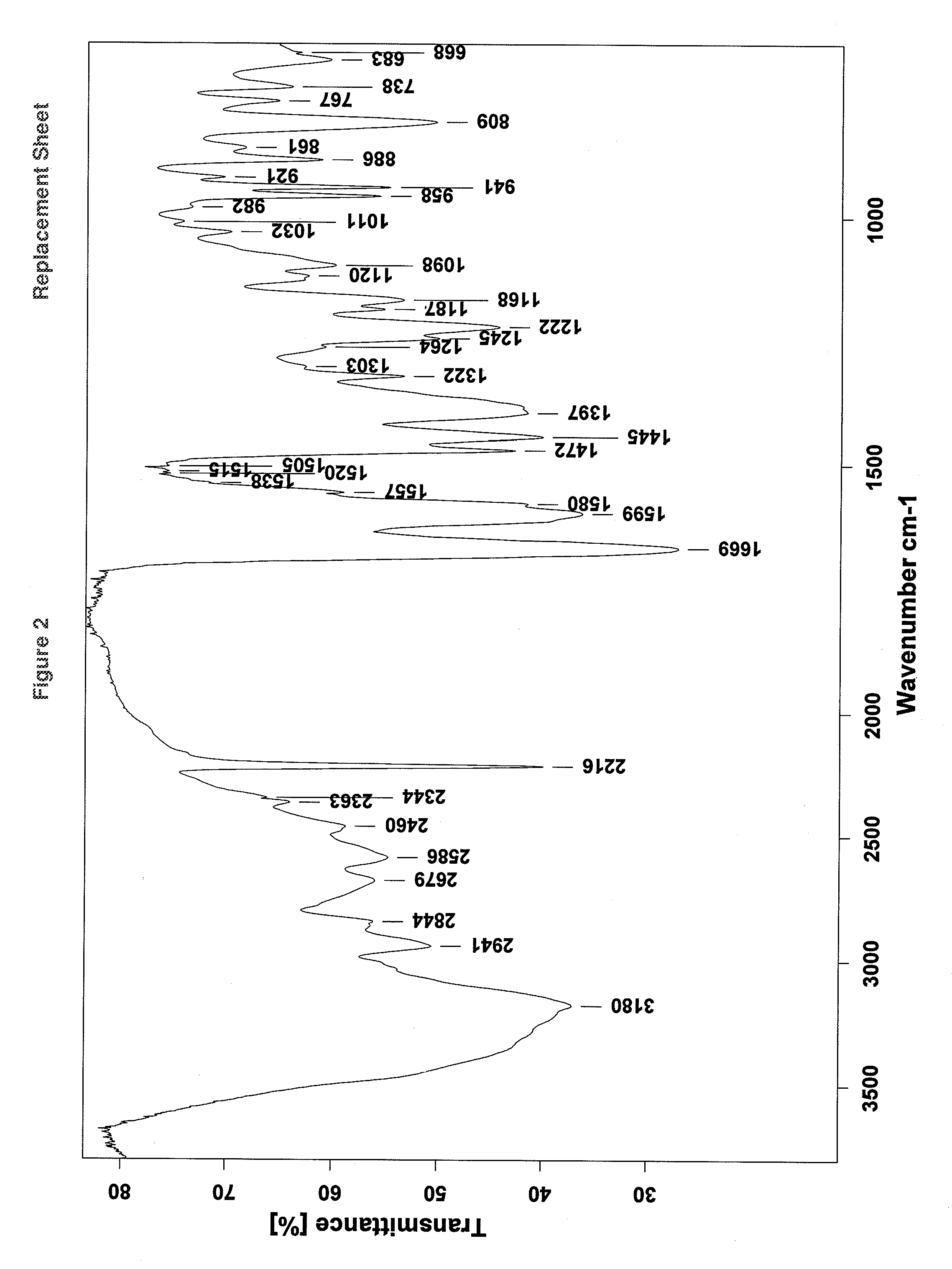
Process for the preparation of vilazodone hydrochloride and its amorphous form
InactiveUS20150087835A1High purityImprove stabilityOrganic chemistryOrganic chemistryVilazodone Hydrochloride
The present invention relates to an improved process for the preparation of vilazodone Hydrochloride and a process for preparation of novel pure amorphous form of vilazodone hydrochloride.
Owner:ALEMBIC PHARMA
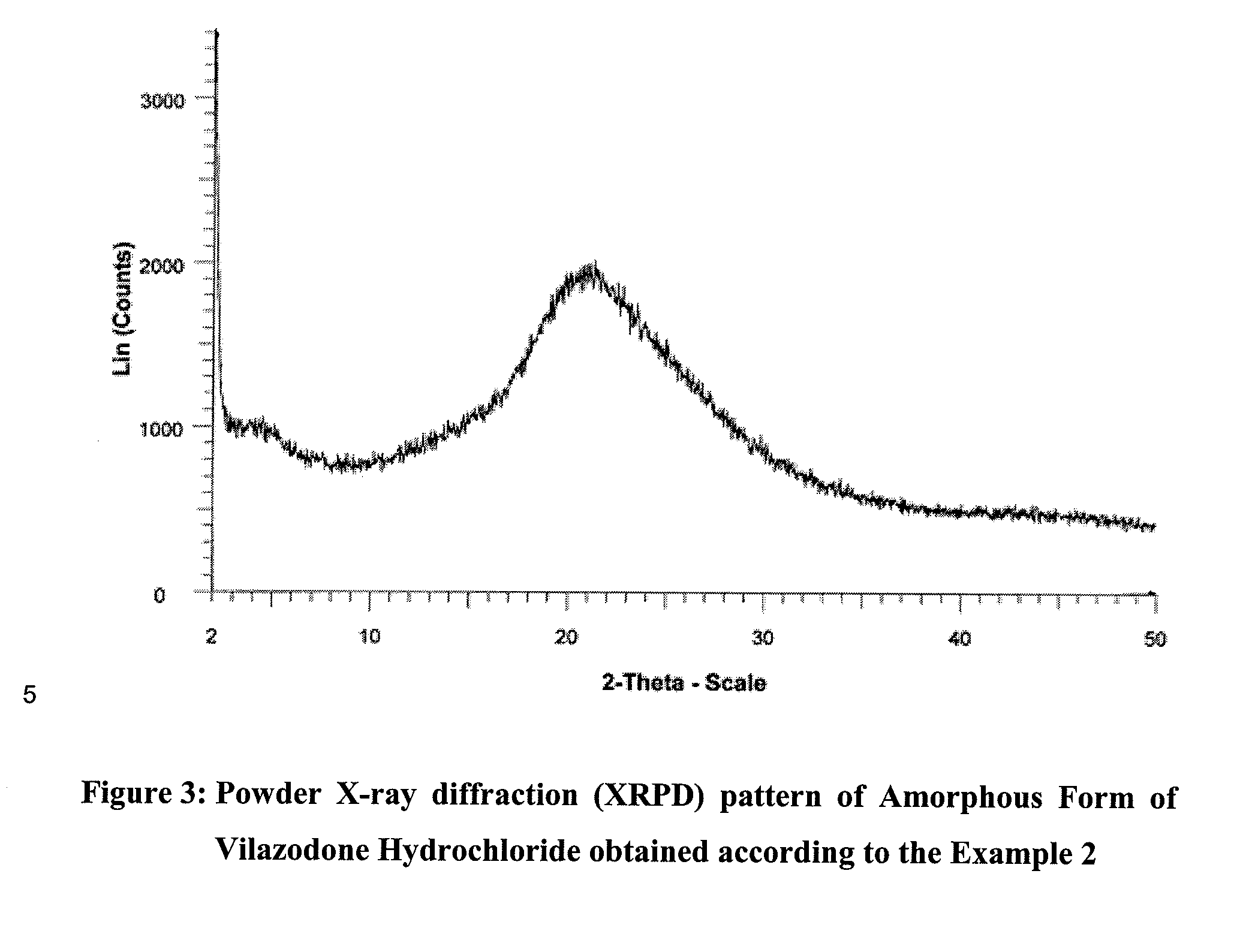
Amorphous form of vilazodone hydrochloride and process for its preparation
InactiveUS20140057925A1High purityProcess stabilityOrganic active ingredientsNervous disorderRecurrent major depressive episodesPharmacology
The present invention relates to an amorphous form of vilazodone hydrochloride and process for the preparation of amorphous form of vilazodone hydrochloride. The invention also relates to pharmaceutical compositions that include a therapeutically effective amount of the amorphous form of vilazodone hydrochloride and use of said compositions for the treatment of major depressive disorder (MDD).
Owner:CADILA HEALTHCARE LTD
Preparation method and application of vilazodone hydrochloride IV crystal
ActiveCN103772368AEliminate the trouble of preparing hydrochloric acidSimple processOrganic chemistryRefluxOrganic solvent
The invention discloses a preparation method and application of a 5-(4-(4-(5-cyano-3-indolyl)butyl)-1-piperazinyl)benzofuran-2-formamide hydrochloride IV crystal. The method comprises the steps of: 1) under certain temperature, dissolving 5-(4-(4-(5-cyano-3-indolyl)butyl)-1-piperazinyl)benzofuran-2-formamide in an organic solvent to obtain a solution; 2) heating the solution obtained in step 1) to a reflux temperature of 40DEG C; 3) slowly adding concentrated hydrochloric acid dropwise; 4) subjecting the system obtained in step 3) to heat preservation reaction for 0.5-12 hours; 5) conducting filtering to obtain an IV crystal form wet product; and 6) conducting vacuum drying to the wet product obtained in step 5) at 80-140DEG C to a constant weight, thus obtaining the IV crystal. By adopting the technical scheme, the process is simplified, a complex preparation process of the existing IV crystal is overcome, and a mixed crystal can be avoided. The method provided by the invention can achieve high crystal purity, stable volume production and high yield, and is easy to realize industrialized production.
Owner:HANGZHOU HEZE PHARMA TECH
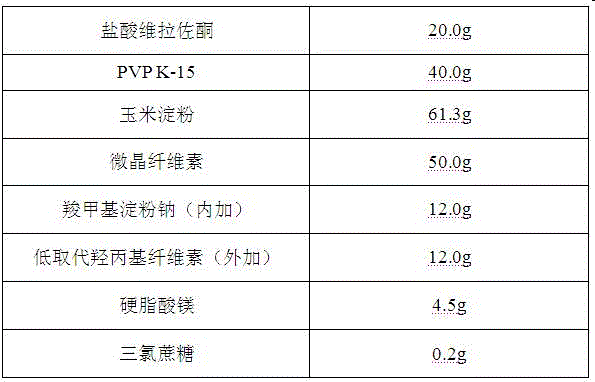
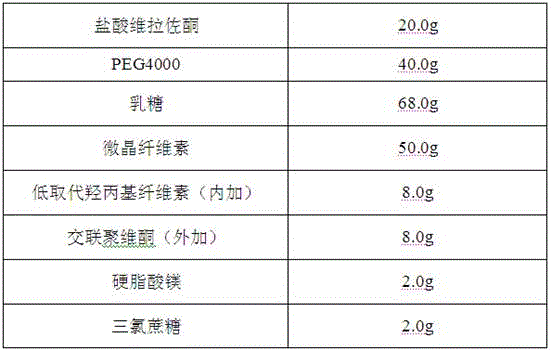
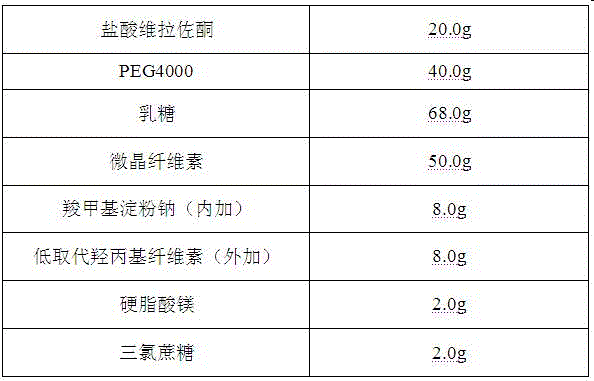
Orally disintegrating tablet containing vilazodone hydrochloride solid dispersions and preparation method thereof
InactiveCN106580895AImprove solubilityHigh dissolution rateOrganic active ingredientsNervous disorderSolubilityOrally disintegrating tablet
The invention belongs to the field of a medicine preparation, and particularly relates to an orally disintegrating tablet containing vilazodone hydrochloride solid dispersions and a preparation method thereof. By aiming at the problems of poor water solubility and low preparation dissolubility of the vilazodone hydrochloride, the vilazodone hydrochloride and carriers are prepared into the solid dispersions for improving the solubility. The solid dispersions are used for preparing the orally disintegrating tablet; the orally disintegrating tablet can fast disintegrate in 30s; and the main medicine dissolubility is obviously improved.
Owner:万全万特制药(厦门)有限公司
Vilazodone hydrochloride softgel and preparation method thereof
InactiveCN106580914AOrganic active ingredientsNervous disorderTherapeutic effectVilazodone Hydrochloride
The invention relates to a preparation method of vilazodone hydrochloride softgel. The vilazodone hydrochloride softgel prepared in the invention has the advantages of good stability and stable properties. The above depression treatment medicine combination has a reasonable ratio, rapidly releases a medicine, and has a very good treatment effect on diseases.
Owner:佛山市弘泰药物研发有限公司
Method for preparing vilazodone hydrochloride midbody
The invention provides a method for preparing vilazodone hydrochloride midbody. The method comprises the steps of: based on 5-amino coumarone ethyl formate as an initial raw material, reacting with bi(2-chloroethy) benzylamine hydrochloride under the effect of tetrabutylammonium bromide to generate compound II, and reacting with ammonia to prepare compound I 5-(1-piperazinyl)-benzofuran-2-formamide. The used method is easy in raw material obtaining, low in cost, high in reaction yield, simple to operate and suitable for industrial production.
Owner:NANJING HUAWE MEDICINE TECH DEV
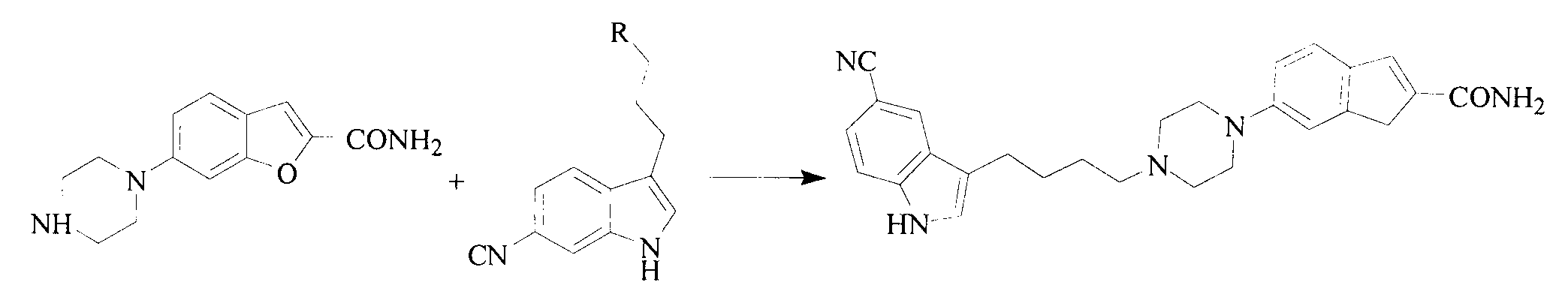
Preparation method of vilazodone hydrochloride
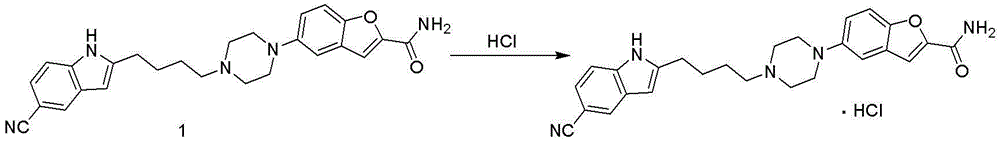
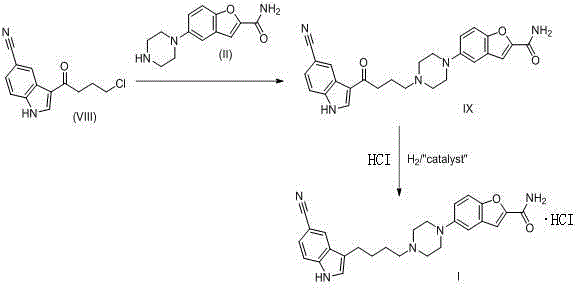
The invention discloses a preparation method of vilazodone hydrochloride and especially relates to a method for preparing vilazodone hydrochloride from 3-(4-chlorobutyryl)-1H-indole-5-carbonitrile and 5-(piperazin-1-yl)benzofuran-2-carboxamide as initial raw materials. The preparation method comprises that through one-step two-intermediate coupling reaction, the final product is prepared from the initial raw materials and through catalytic hydrogenation and salt formation, vilazodone hydrochloride is prepared, wherein two intermediates are prepared through two parallel synthesis routes. The vilazodone hydrochloride has stable quality, high purity, total impurity content less than 0.3%, single impurity content less than 0.1% and a yield of 85-90%. The preparation method has less reaction processes, can be operated simply, has mild reaction conditions and is convenient for industrial production.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Process for preparing vilazodone hydrochloride
Owner:ERREGIERRE
Preparation method of vilazodone hydrochloride intermediate 3-(4-chlorobutyl)-1H-5-cyanindole
ActiveCN104592087AMild reaction conditionsEasy post-processingOrganic chemistrySodium borohydrideSolvent
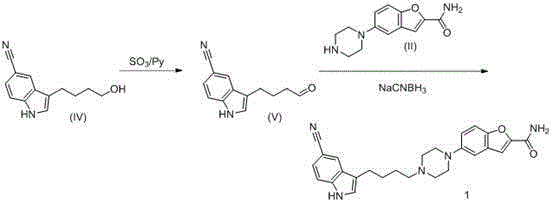
The invention relates to a preparation method of 3-(4-chlorobutyl)-1H-5-cyanindole. The above compound is an important intermediate for preparing an antidepressant vilazodone hydrochloride. The method comprises the following steps: dispersing sodium borohydride in a solvent, adding a trichloroacetic acid organic solution, and adding 3-(4-chlorobutylacyl)-1H-5-cyanindole in batches. The preparation method of the vilazodone hydrochloride intermediate 3-(4-chlorobutyl)-1H-5-cyanindole has the advantages of simple operation, low device requirements, high yield, good purity, suitableness for industrial production, and obvious creativity and practical application values. The reaction equation of the method is shown in the specification.
Owner:BEIJING SINICA TECH
Crystalline forms of vilazodone hydrochloride and vilazodone free base
InactiveUS20170217939A1Influence acceptanceInfluence lifeNervous disorderOrganic chemistry methodsVilazodone HydrochlorideCrystallization
The present application relates to crystalline and amorphous Vilazodone hydrochloride. The present application further relates to amorphous solid dispersions of vilazodone hydrochloride with pharmaceutically acceptable carriers. The present application also relates to a process for the preparation of form I of vilazodone free base.
Owner:DR REDDYS LAB LTD
Pharmaceutical composition for treating insomnia
InactiveCN103893180AHigh dissolution rateImprove sleep and treat insomniaOrganic active ingredientsNervous disorderSide effectOrally disintegrating tablet
The invention discloses use of a pharmaceutical composition in a medicament for treating insomnia. The study finds that a novel medicament vilazodone hydrochloride for treating a major depressive disorder generates sedative-hypnotic action and treats insomnia of animals at the dosage lower than the clinical recommended dose. The invention also discloses preparation forms of the pharmaceutical composition for treating insomnia, preferably orally disintegrating tablets, components of the disintegrating tablets, and a preparation method thereof. The clinical research shows that the medicament disclosed by the invention is free of obvious toxic and side effects, significant in effect on treatment of insomnia, quick to take effect, convenient to use, and easy to accept by a sufferer. A new choice is provided for treatment of insomnia, and the pharmaceutical composition has a broad market prospect.
Owner:BEIJING KEYUAN CHUANGXIN TECH
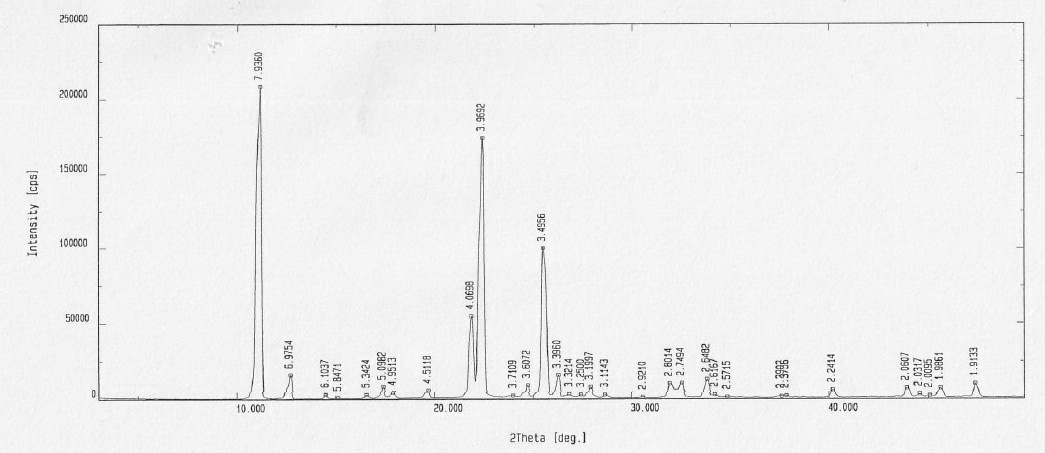
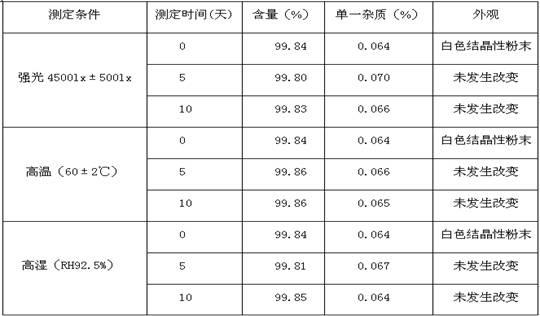
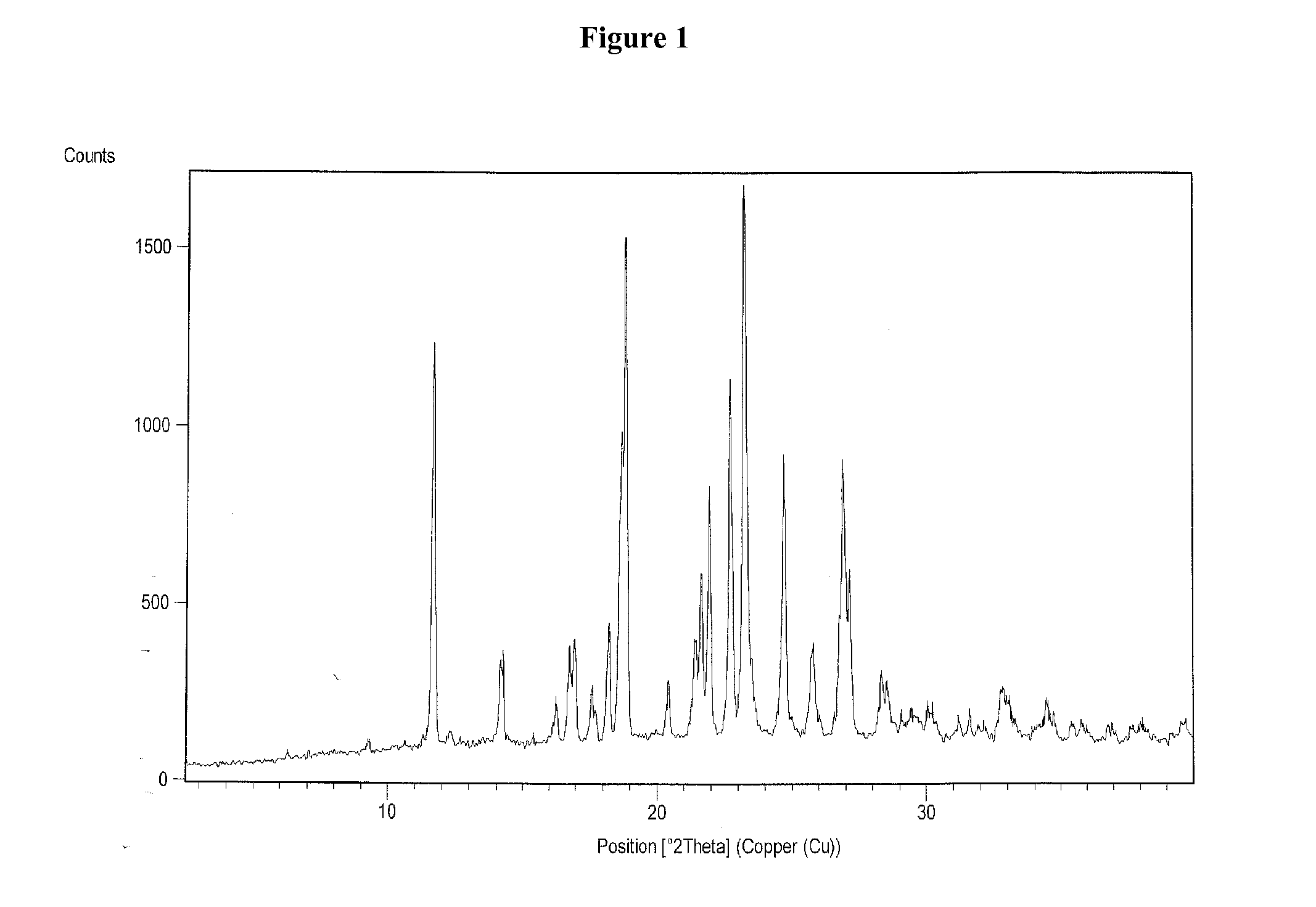
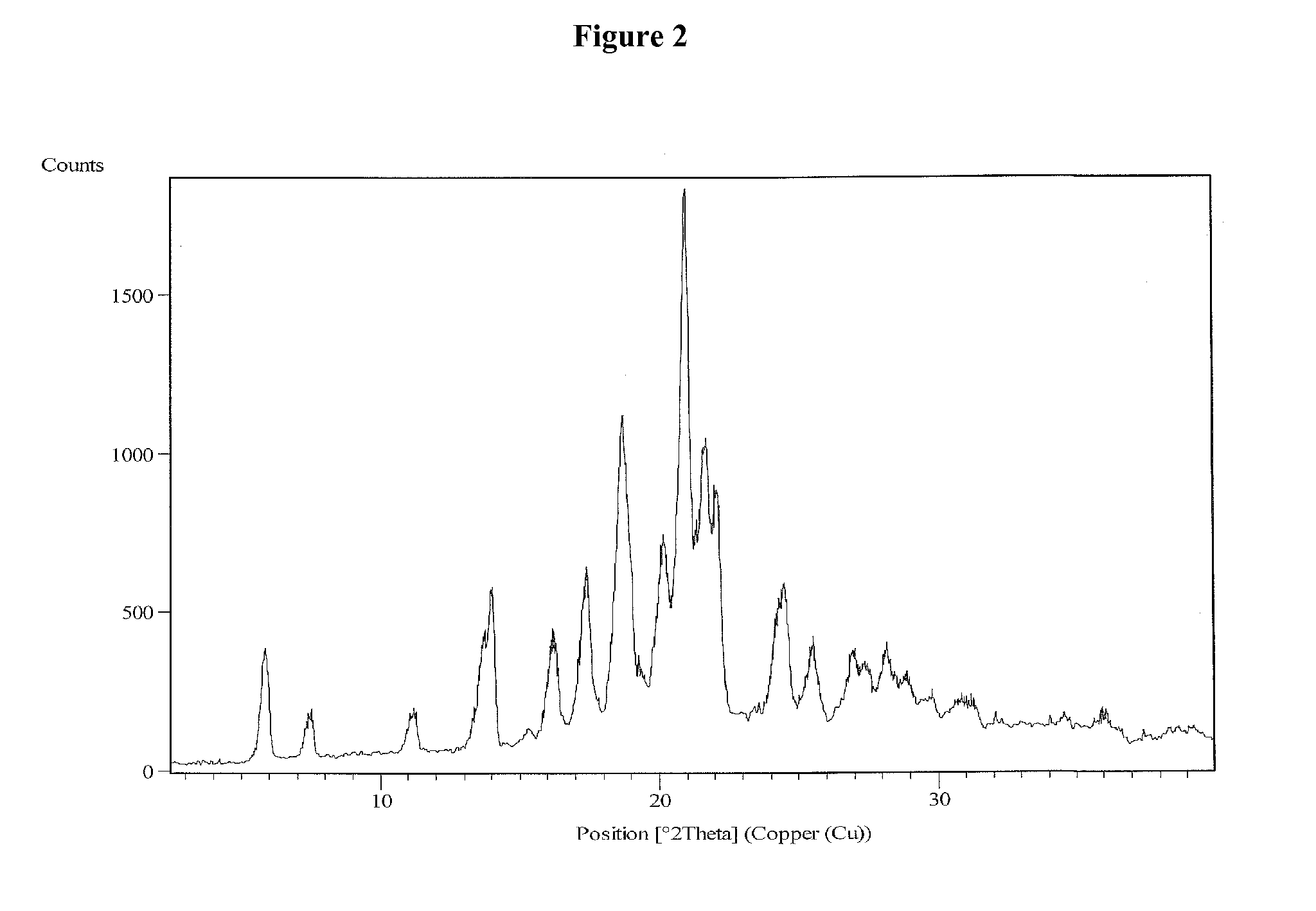
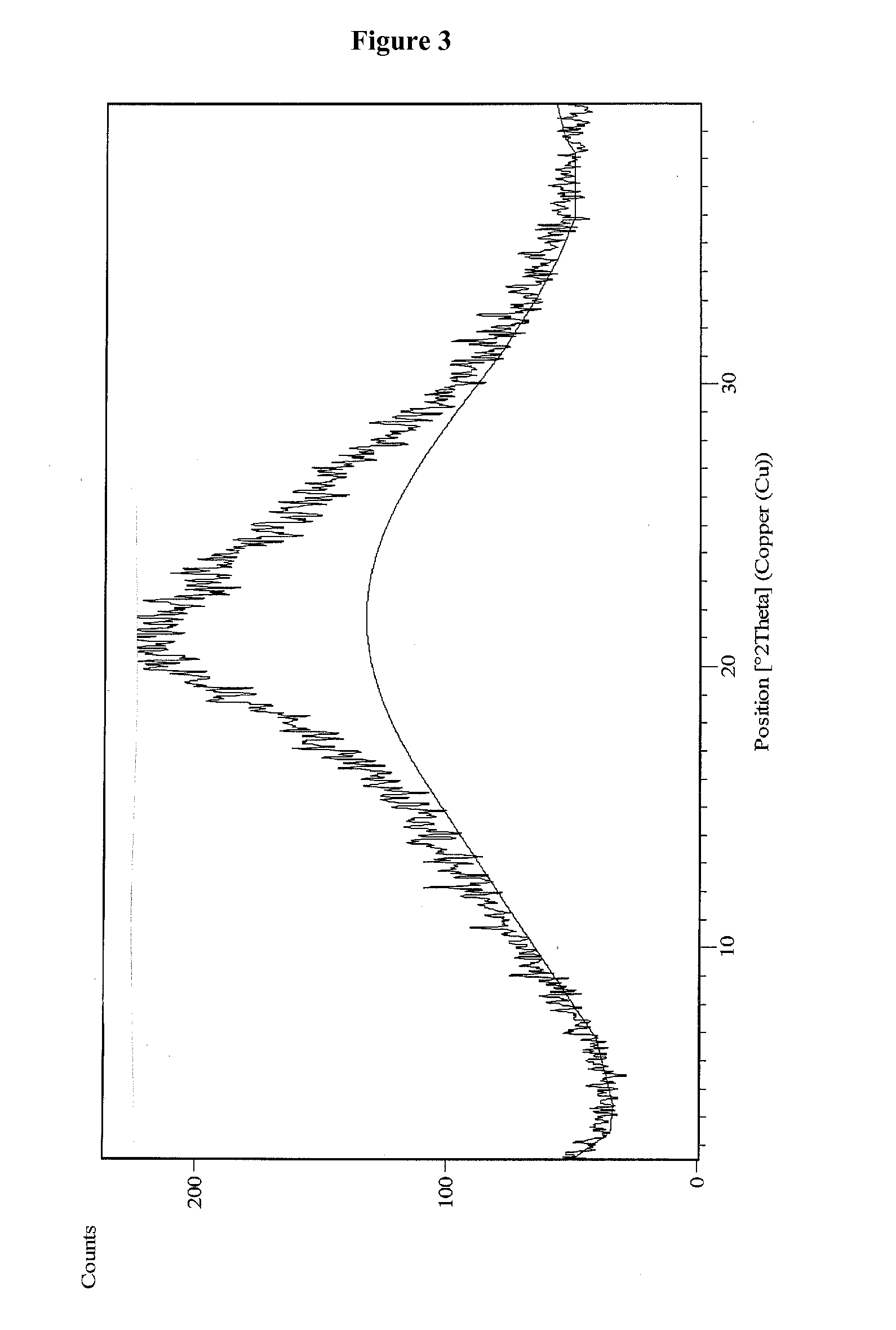
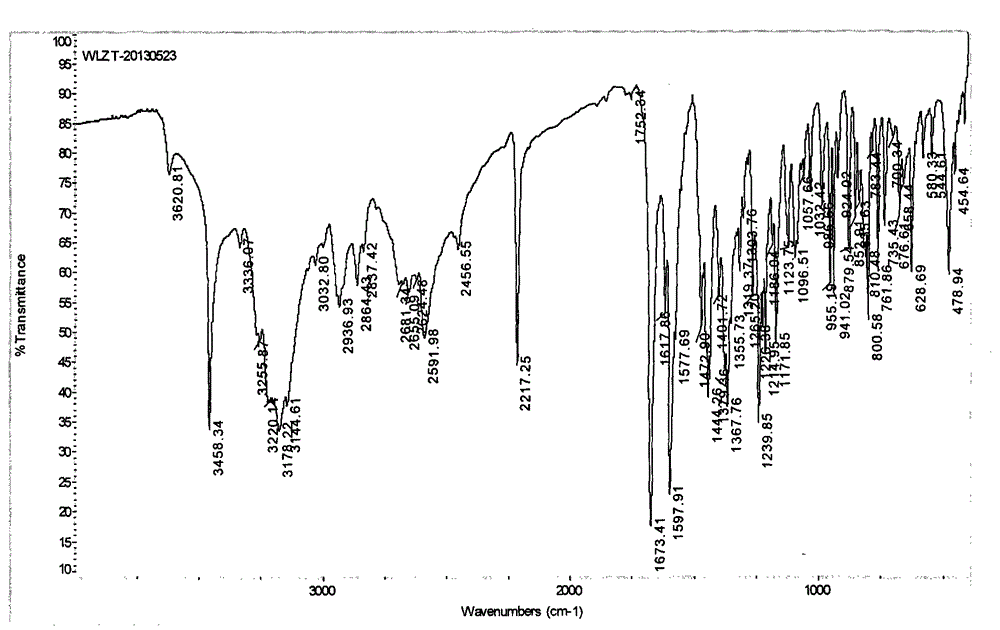
Vilazodone hydrochloride crystal form and preparation method thereof
ActiveCN105820157AImprove stabilityEasy to prepareOrganic active ingredientsNervous disorderPowder diffractionVilazodone Hydrochloride
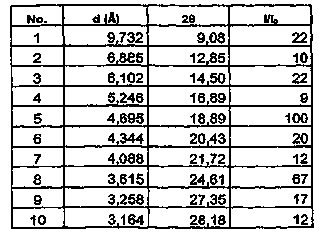
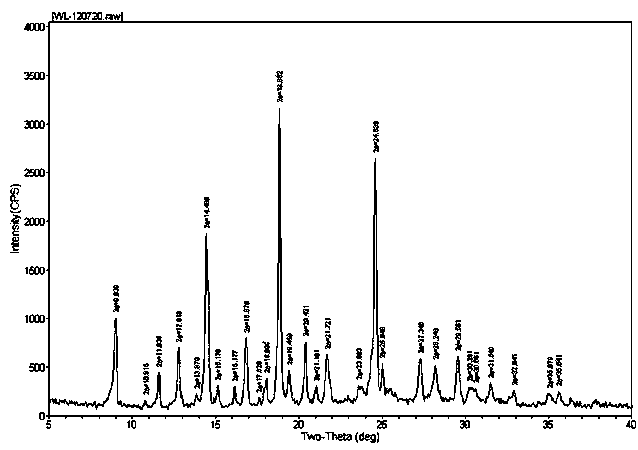
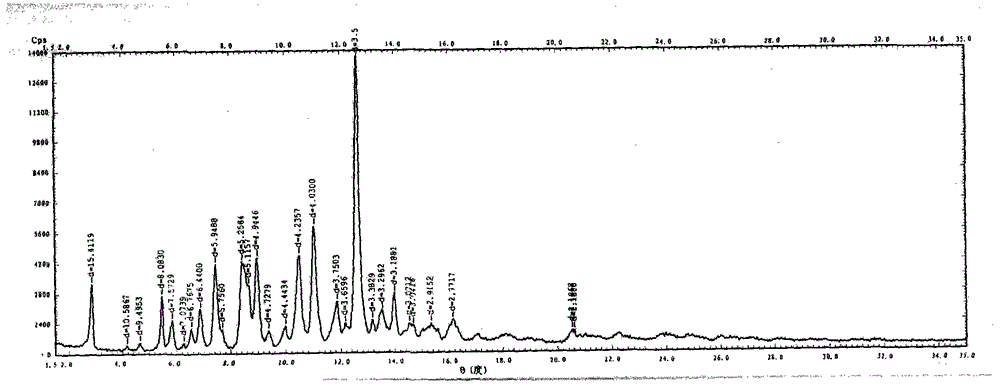
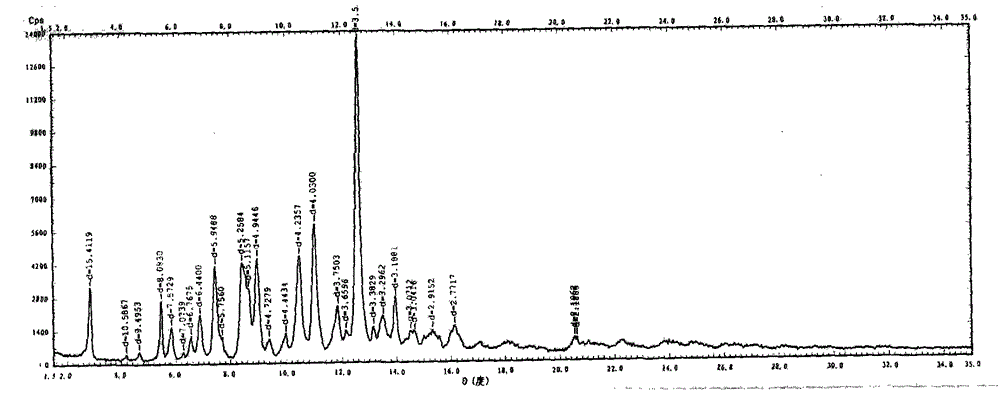
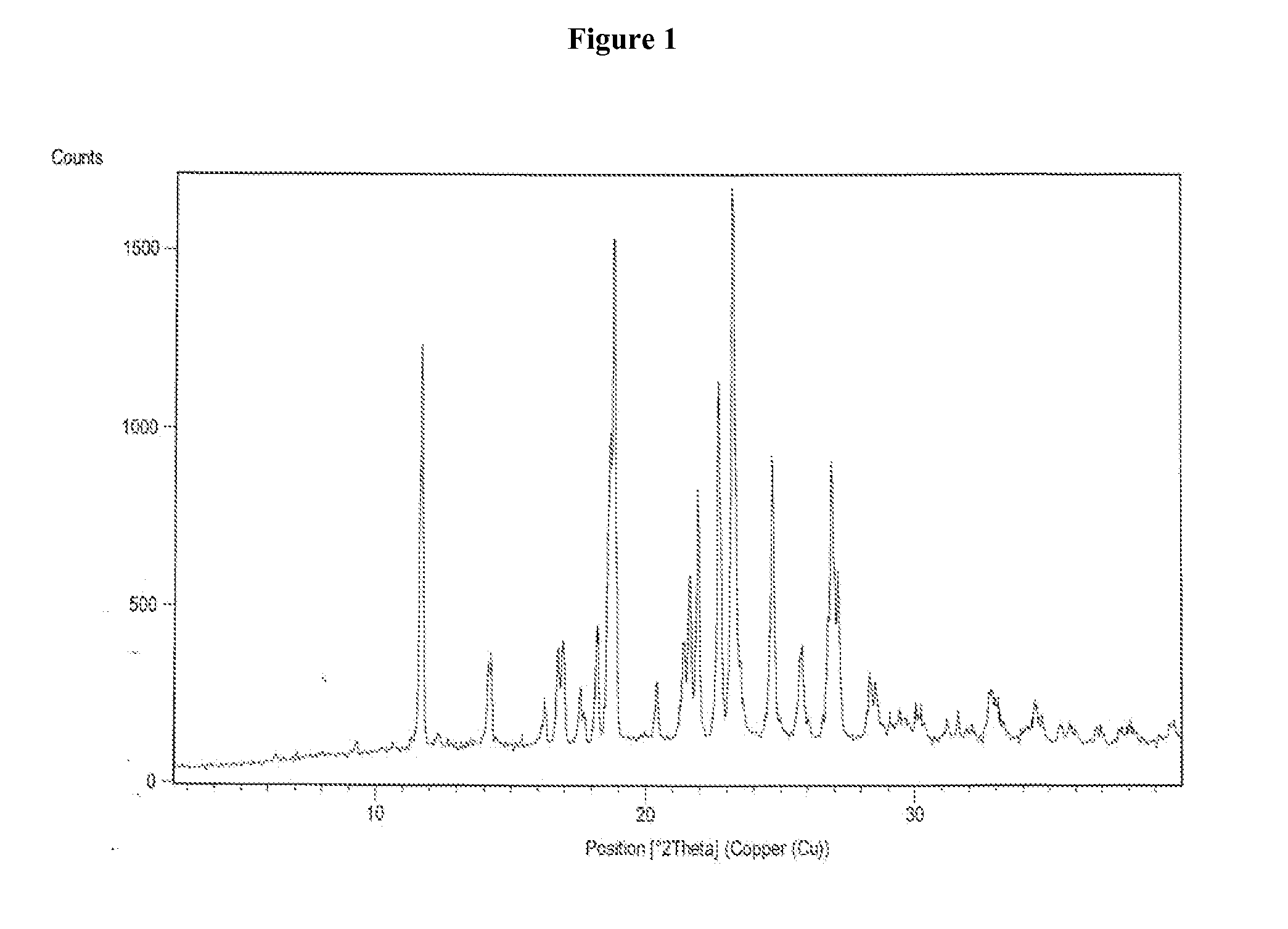
The invention relates to a vilazodone hydrochloride crystal form. The powder X-ray powder diffraction pattern of the crystal form has characteristic peaks when a diffraction angle 2theta is 9.019+ / -0.2 DEG, 14.481+ / -0.2 DEG, 18.839+ / -0.2 DEG, and 24.580+ / -0.2 DEG, 27.271+ / -0.2 DEG, the relative intensity of the diffraction angle 2theta at 24.580+ / -0.2 DEG is 100%, the relative intensity of the diffraction angle 2theta at 18.839+ / -0.2 DEG is no less than 70%, the relative intensity of the diffraction angle 2theta at 14.481+ / -0.2 DEG is no less than 30%, the relative intensity of the diffraction angle 2theta at 9.019+ / -0.2 DEG is no less than 20%, and the relative intensity of the diffraction angle 2theta at 27.271+ / -0.2 DEG is no less than 18%. The invention also relates to a preparation method of the vilazodone hydrochloride crystal form, a pharmaceutical composition and the vilazodone hydrochloride crystal form in preparation of medicine for treating depression.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Novel preparation method of III crystal-form vilazodone hydrochloride
InactiveCN104610238AReduce processing timeLow costOrganic chemistry methodsVacuum dryingPiperazine hydrochloride
The invention belongs to the field of pharmaceutical chemistry, and more specifically to a novel preparation of III crystal-form 1-[4-(5-cyanoindole-3-butyl)]-4-(2-carbamoyl-coumarone-5-yl)-piperazine hydrochloride. The preparation method comprises reacting a solvate of hydrochloric acid, vilazodone and tetrahydrofuran at a temperature range of 120-140 DEG C and performing vacuum drying for 12-36 hours. The III crystal-form vilazodone hydrochloride is stable and is convenient to obtain.
Owner:BEIJING SINICA TECH
Process for the preparation of vilazodone hydrochloride and its amorphous form
ActiveUS20150239871A1Good yield and pharmaceutical qualityLow costOrganic chemistryOrganic chemistryVilazodone Hydrochloride
The present invention relates to an improved process for the preparation of vilazodone hydrochloride and a process for preparation of novel pure amorphous form of vilazodone hydrochloride.
Owner:ALEMBIC PHARMA
Process for the preparation of form III of Vilazodone hydrochloride
The invention relates to a new solvate, Vilazodone hydrochloride monoethanol monohydrate solvate, and to a process for the preparation of polymorphic form III of Vilazodone hydrochloride via the Vilazodone hydrochloride monoethanol monohydrate solvate.
Owner:SANDOZ GMBH +1
Crystalline forms of vilazodone hydrochloride and vilazodone free base
InactiveUS20150126525A1Influence acceptanceInfluence lifeOrganic active ingredientsNervous disorderMedicinal chemistryFree base
Provided are crystalline and amorphous vilazodone hydrochloride. Further provided are amorphous solid dispersions of vilazodone hydrochloride with pharmaceutically acceptable carries. Also provided is a process for the preparation of form I of vilazodone free base.
Owner:DR REDDYS LAB LTD
Amorphous form of vilazodone hydrochloride substantially free of crystalline forms
InactiveUS8835635B2Improve stabilityImprove solubilityOrganic active ingredientsOrganic chemistryExcipientPharmaceutical preservatives
Disclosed herein is a stable amorphous form of vilazodone hydrochloride substantially free of crystalline forms, a process for the preparation, pharmaceutical compositions, and methods of treating thereof. Disclosed also herein are stable amorphous co-precipitates of vilazodone hydrochloride and a pharmaceutically acceptable excipient, methods for the preparation, pharmaceutical compositions, and method of treating thereof.
Owner:SYMED LABS
Compound composition for treatment of depression
The invention discloses a compound composition for treatment of depression and a preparation method thereof. The compound composition takes a novel antidepressant vilazodone hydrochloride and vitamin K as active components. Vitamin K participates in vilazodone hydrochloride to treat severe depression, on the one hand, the clinical dosage of vilazodone hydrochloride can be reduced, a very good antidepressant effect can be generated while maintaining a low dosage, more serious side effects caused by dosage fluctuation similar to gradual dosage increase and gradual dosage decrease during drug withdrawal, and on the other hand, the adverse reaction of abnormal bleeding generated after use of vilazodone hydrochloride can be reduced. Combined use of the two drugs can show stronger antidepressant activity. The compound composition has good application prospects.
Owner:BEIJING KEYUAN CHUANGXIN TECH
A kind of preparation method of vilazodone hydrochloride intermediate 3-(4-chlorobutyl)-1h-5-cyanindole
ActiveCN104592087BMild reaction conditionsEasy post-processingOrganic chemistrySolventTrichloroacetic acid
The invention relates to a preparation method of 3-(4-chlorobutyl)-1H-5-cyanindole. The above compound is an important intermediate for preparing an antidepressant vilazodone hydrochloride. The method comprises the following steps: dispersing sodium borohydride in a solvent, adding a trichloroacetic acid organic solution, and adding 3-(4-chlorobutylacyl)-1H-5-cyanindole in batches. The preparation method of the vilazodone hydrochloride intermediate 3-(4-chlorobutyl)-1H-5-cyanindole has the advantages of simple operation, low device requirements, high yield, good purity, suitableness for industrial production, and obvious creativity and practical application values. The reaction equation of the method is shown in the specification.
Owner:BEIJING SINICA TECH
A kind of vilazodone hydrochloride crystal form and preparation method thereof
ActiveCN105820157BImprove stabilityEasy to prepareOrganic active ingredientsNervous disorderMedicinePhysical chemistry
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Preparation method for vilazodone hydrochloride crystal form XI
InactiveCN109503560AMild reaction conditionsRealize industrial productionOrganic chemistry methodsFibromyalgiaDisease injury
The invention provides a preparation route for a vilazodone hydrochloride crystal form XI: vilazodone hydrochloride with the crystal form XI is obtained by a series of operations such as dilution, pHregulation, crystallization and drying, and the problem that the crystal form XI cannot be obtained by the route of the prior patent. The drug is used for treating or preventing depression, anxiety, bipolar disorder, mania, dementia, mental disorders associated with psychoactive substances, sexual dysfunction, eating disorder, obesity, fibromyalgia, sleep disorder, psychosis-like mental disorders,cerebral infarction, tension, side effects in hypertension treatment, brain diseases, chronic pain, acromegaly, hypogonadism, secondary amenorrhea and premenstrual syndrome.
Owner:BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com