Method for separating and determining vilazodone hydrochloride raw materials and preparations thereof by liquid chromatography
A technology for vilazodone hydrochloride and its determination method, which is applied in the field of separation and determination of vilazodone hydrochloride and its related substances by liquid chromatography, can solve the problems of not knowing the effect of the net result, and achieve the effect of ensuring quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Instruments and Conditions
[0036] High performance liquid chromatography: Shimadzu: LC-10ATvp, SPD-M10Avp;
[0037] Column: C 18 (Agela, 250 × 4.6 mm, 5 μm)
[0038] Mobile phase: A phase: 0.1% trifluoroacetic acid aqueous solution (pH3.0), B phase: acetonitrile, using gradient elution;
[0039] T(min) 0 15 17 30 35 45 B% 30 30 60 65 30 30
[0040] Flow rate: 1.0mL / min
[0041] Detection wavelength: 240nm
[0042] Column temperature: 25°C
[0043] Injection volume: 10μL
[0044] Experimental procedure
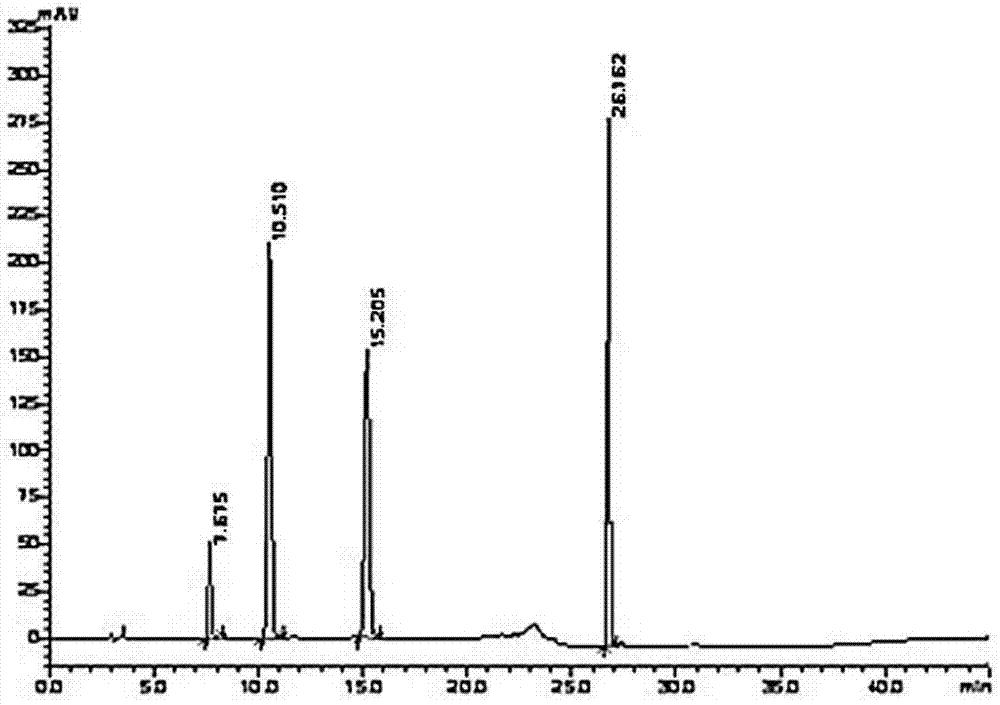
[0045] Take about 25mg of vilazodone hydrochloride and its intermediates respectively, put them in a 50mL measuring bottle, add methanol to dissolve and dilute to the mark, shake well, and take an appropriate amount of each to prepare a mixed sample solution.
[0046] Take reagent blank solution and mixed sample solution respectively, carry out high performance liquid chromatography analysis according to the above conditions, recor...
Embodiment 2
[0049] Instruments and Conditions
[0050] High performance liquid chromatography: Shimadzu: LC-10ATvp, SPD-M10Avp;
[0051] Column: C 18 (Agela, 250 × 4.6 mm, 5 μm)
[0052] Mobile phase: A phase: 0.1% trifluoroacetic acid aqueous solution (pH3.0), B phase: acetonitrile, using gradient elution;
[0053] T(min) 0 15 17 30 35 45 B% 30 30 60 65 30 30
[0054] Flow rate: 1.0mL / min
[0055] Detection wavelength: 240nm
[0056] Column temperature: 20°C
[0057] Injection volume: 10μL
[0058] Experimental procedure
[0059] Take about 25 mg of vilazodone hydrochloride raw material, put it in a 50 mL measuring bottle, add methanol to dissolve and dilute to the mark, shake well, and use it as the test solution.
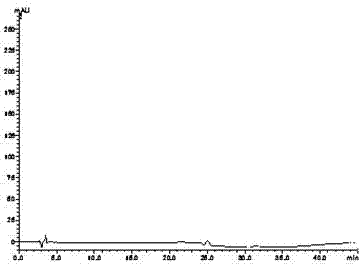
[0060] Get need testing solution, carry out high performance liquid chromatography analysis by above-mentioned condition, record chromatogram, the result sees image 3 .
[0061] image 3 The chromatographic peak whose retention time ...
Embodiment 3
[0063] Instruments and Conditions
[0064] Column: C 18 (Agela, 250 × 4.6 mm, 5 μm)
[0065]Mobile phase: A phase: 0.1% trifluoroacetic acid aqueous solution (pH3.0), B phase: acetonitrile, using gradient elution;
[0066] T(min) 0 15 35 55 60 70 B% 30 30 60 65 30 30
[0067] Flow rate: 1.0mL / min
[0068] Detection wavelength: 240nm
[0069] Column temperature: 25°C
[0070] Injection volume: 10μL
[0071] Experimental procedure
[0072] Take an appropriate amount of vilazodone hydrochloride tablets, approximately equivalent to 12.5mg of vilazodone hydrochloride, put it in a 25mL measuring bottle, add mobile phase to ultrasonic treatment to dissolve, and dilute to the mark with methanol, shake well, filter, and take the subsequent filtrate as the supply Test solution.
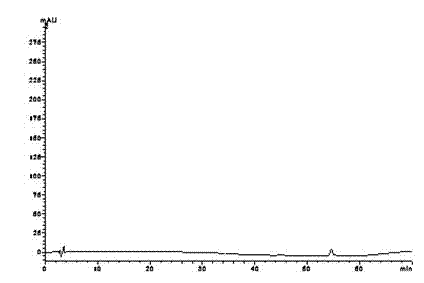
[0073] Get the test solution, carry out high performance liquid chromatography analysis according to the above conditions, record the chromatogram, and carry out the blank ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com