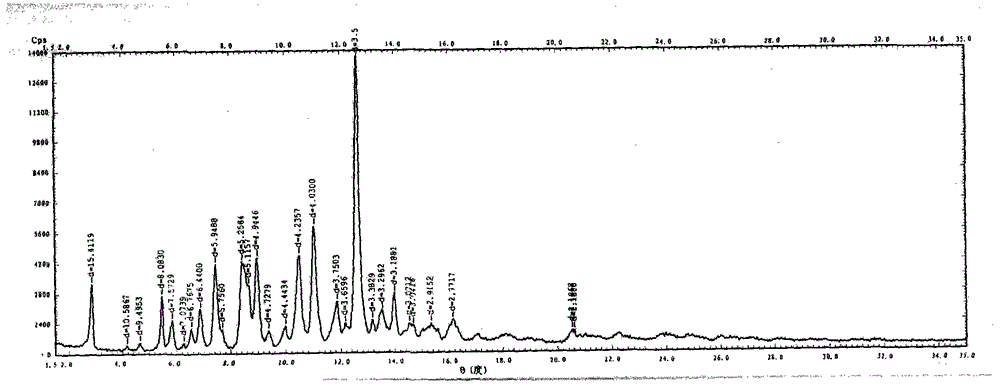
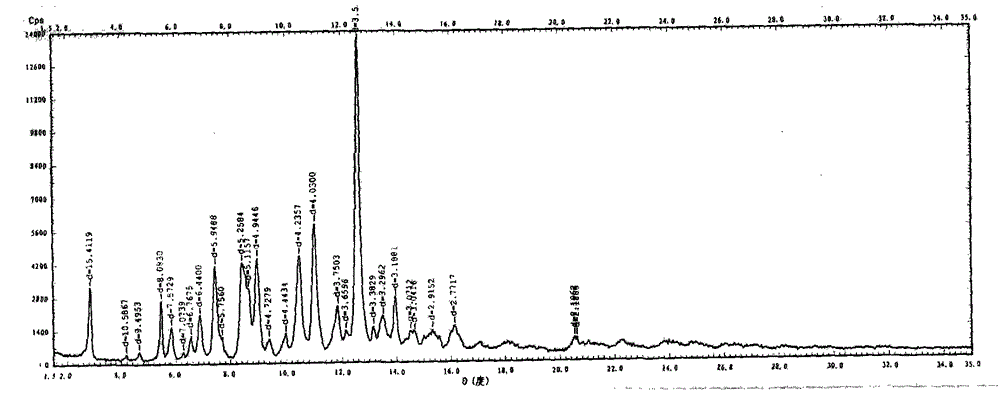
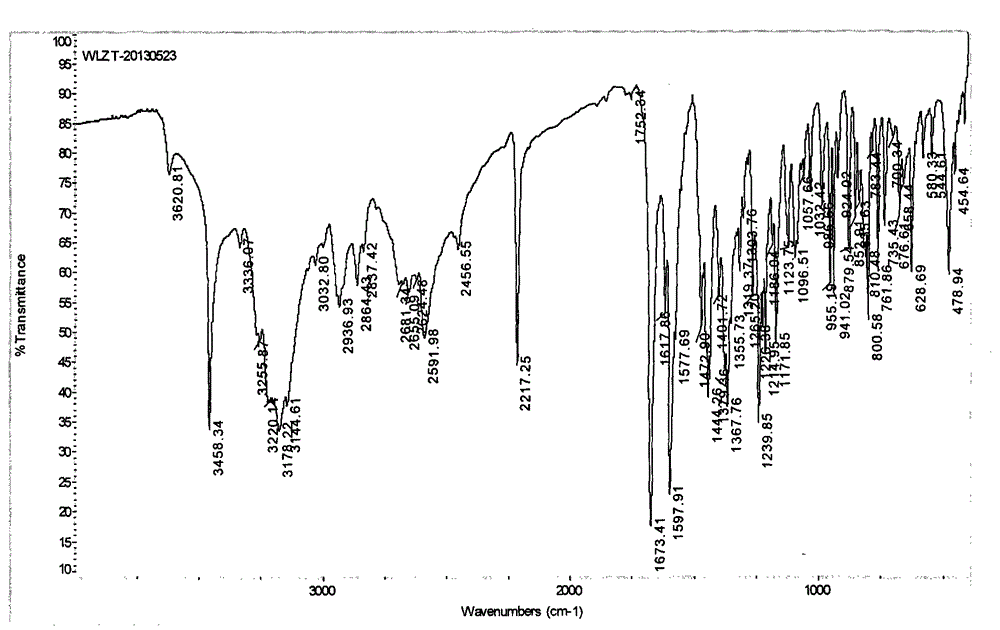
Novel preparation method of III crystal-form vilazodone hydrochloride
A technology of vilazodone and vilazodone hydrochloride, applied in the directions of organic chemistry, organic chemistry, etc., can solve problems such as inability to realize industrialized production, and achieve a shortened process time, good reproducibility, and strong operability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve vilazodone (4.41g, 10mmol) in 30mL of tetrahydrofuran, control the temperature at 25°C, slowly add 3mL of 1N HCl, stir for 30 minutes, a white solid precipitates, filter, and vacuum dry at 120°C for 72 hours to obtain hydrochloric acid Vilazodone III crystal form 4.2g, yield 88%.
Embodiment 2
[0024] Dissolve vilazodone (4.41g, 10mmol) in 30mL of tetrahydrofuran, control the temperature at 30°C, slowly add 3mL of 1N HCl, stir for 30 minutes, a white solid precipitates, filter, and vacuum dry at 130°C for 24 hours to obtain hydrochloric acid Vilazodone III crystal form 4.3g, yield 90%.
Embodiment 3
[0026] Dissolve vilazodone (4.41g, 10mmol) in 30mL of tetrahydrofuran, control the temperature at 40°C, slowly add 3mL of 1N HCl, stir for 30 minutes, a white solid precipitates, filter, and vacuum dry at 150°C for 20 hours to obtain hydrochloric acid Vilazodone III crystal form 4.1g, yield 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com