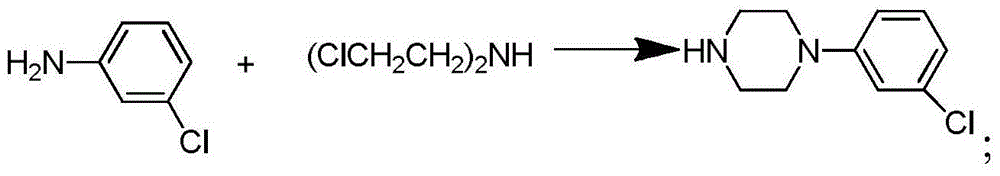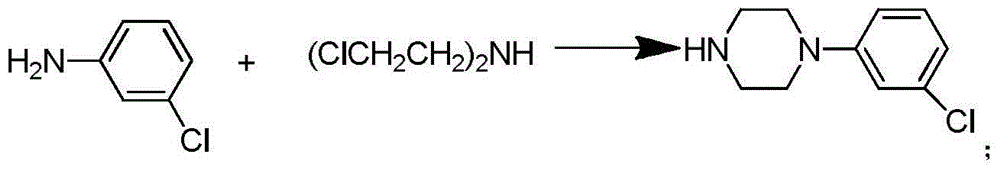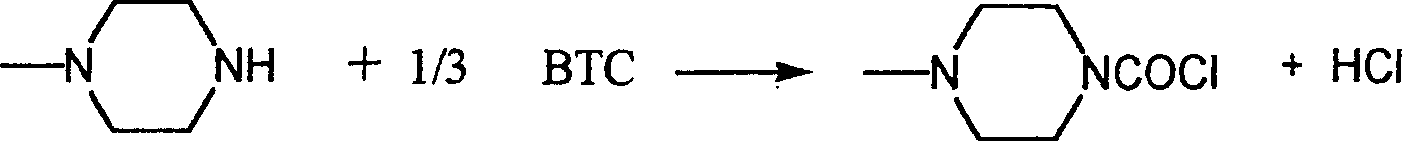Patents
Literature
50 results about "Piperazine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
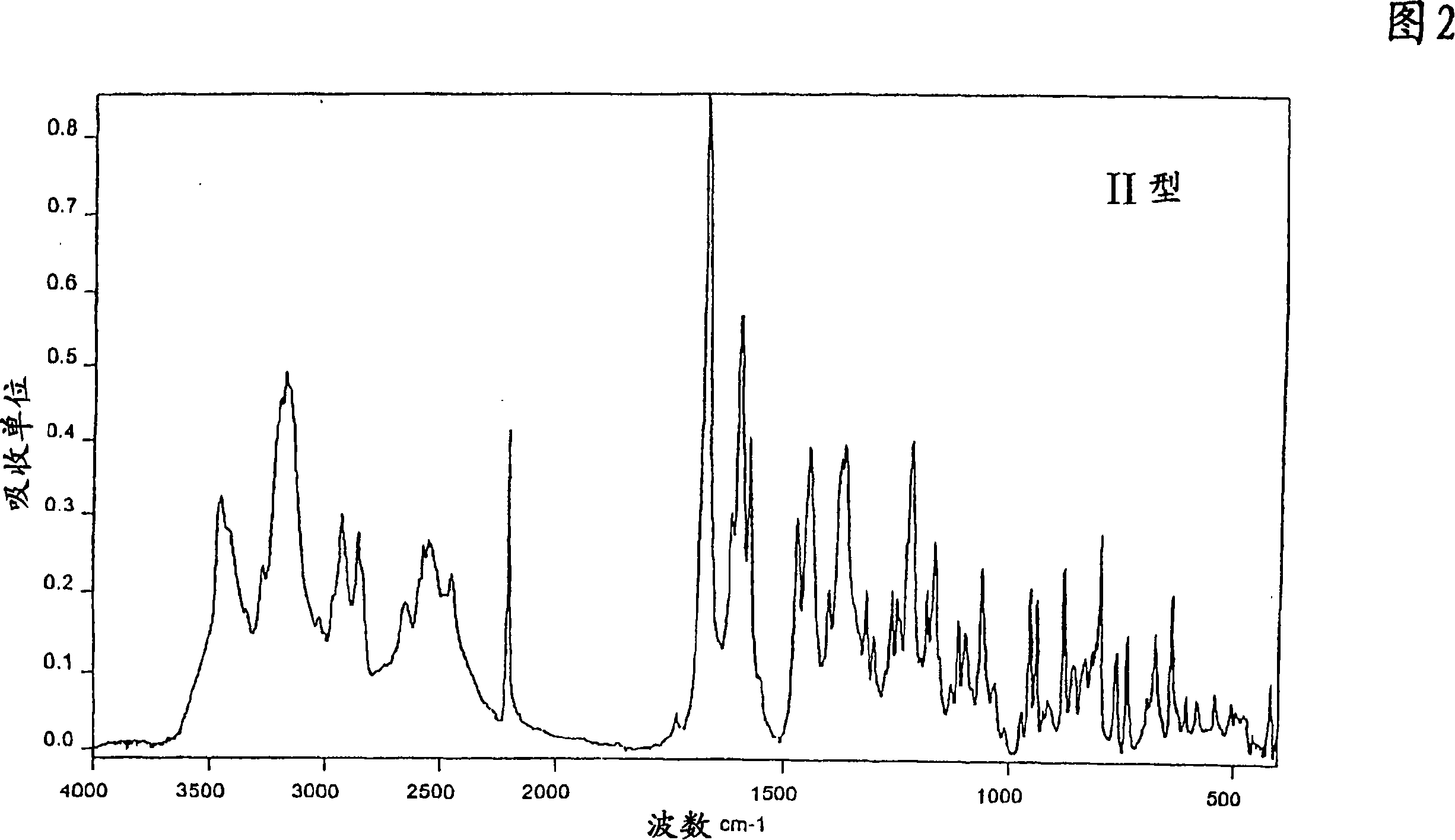
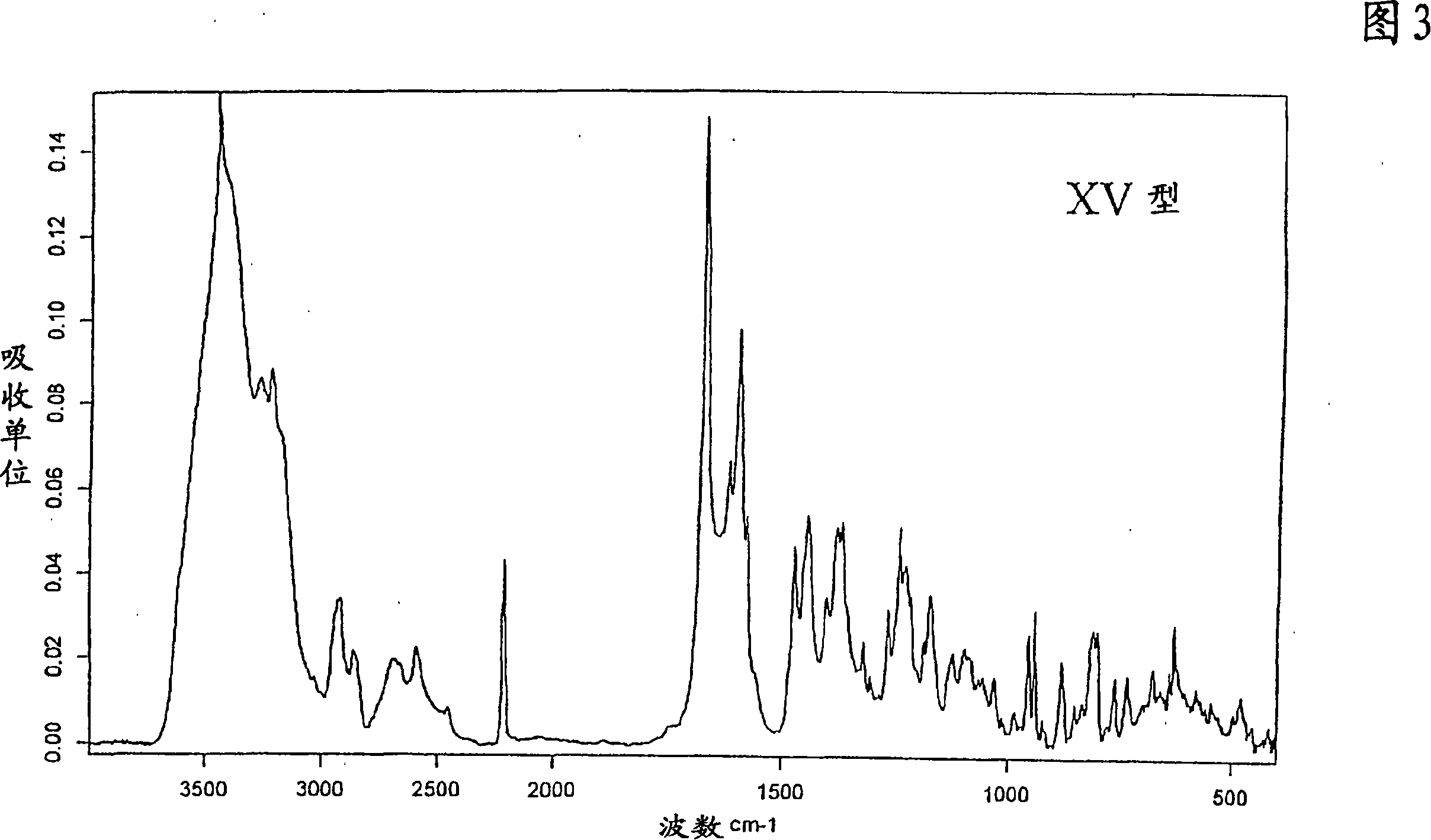
Polymorphic forms of 1-4-(5-cyanoindol-3-yl) butyl-4-(2-carbamoylbenzofuran-5-yl) piperazine hydrochloride
InactiveCN1516699ALow hygroscopicityEasy to compressOrganic active ingredientsNervous disorderSide effectCerebral infarction
The invention relates to new crystalline modifications of the hydrochloride of 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine, crystalline modification of the dihydrochloride of 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine and amorphous 1-[4-(5-cyanoindol-3-yl)butyl]-4-(2-carbamoyl-benzofuran-5-yl)-piperazine hydrochloride which are suitable in particular for the preparation of solid medicaments for the treatment or prevention of depressive disorders, anxiety disorders, bipolar disorders, mania, dementia, substance-related disorders, sexual dysfunctions, eating disorders, obesity, fibromyalgia, sleeping disorders, psychiatric disorders, cerebral infarct, tension, for the therapy of side-effects in the treatment of hypertension, cerebral disorders, chronic pain, acromegaly, hypogonadism, secondary amenorrhea, premenstrual syndrome and undesired puerperal lactation.
Owner:MERCK PATENT GMBH
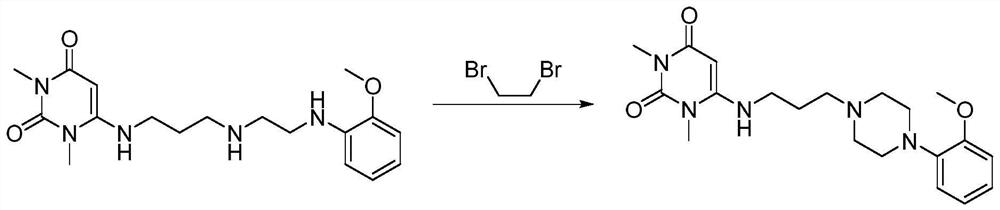
Inverse phase transfer catalysis preparation method for urapidil
InactiveCN102295607BShort reaction timeHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsOrganic synthesisUracil
The invention discloses a preparation method for urapidil, and relates to an inverse phase transfer catalysis method for preparing urapidil, and belongs to the technical field of organic synthesis. The method comprises the following steps that: water is adopted as a solvent; an inorganic alkaline substance is adopted as an acid binding agent; 6-(3-chloropropyl)-1,3-dimethyluracil (I) reacts with 1-(2-methoxyphenyl)piperazine hydrochloride (II) in the presence of an inverse phase transfer catalyst to generate 6-[[3-[4-(2-methoxyphenyl)-1-piperazinyl]-propyl]-amino]-1,3-dimethyl-uracil (III, urapidil). With the method, the high-selectivity and high-yield preparation of the urapidil can be realized; the prepared urapidil has high purity, wherein the purity of the urapidil is more than 99.5%;the conversion rate is high; the yield can reach 80%; the reaction conditions are mild; the selectivity is good; the post-treatment is simple; the method is environmental-friendly, and is suitable for the industrial production.
Owner:ZHENGZHOU UNIV +1
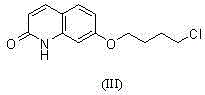
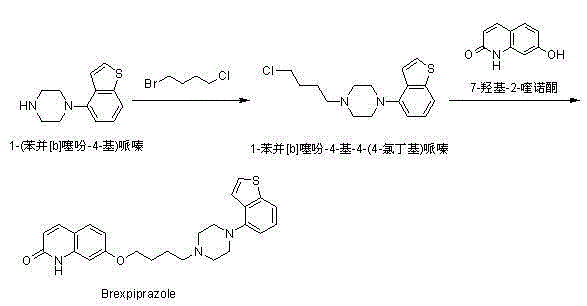
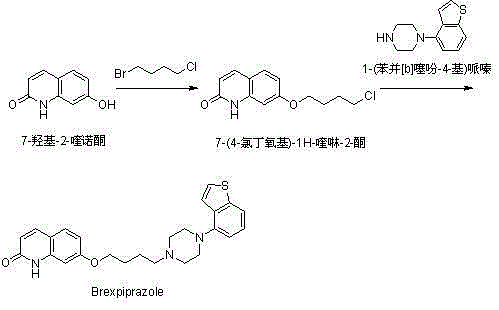
Method for preparing Brexpiprazole with one-pot process
ActiveCN105061414ASolve the problem of insufficient reaction and difficult purificationEasy to operateOrganic chemistryBenzeneAlcohol
The invention relates to a method for preparing Brexpiprazole with a one-pot process. 7-hydroxyl-2-quinolone reacts with added 1-bromine-4-chlorobutane in the presence of alcohol and alkali, 1-(benzo[B]thiophen-4-yl) piperazine hydrochloride and water are added for a further reaction, finally, filtration, separation and drying are performed, and Brexpiprazole is obtained. Compared with the prior art, the method has the benefits as follows: 1, the problems of insufficient reaction and difficulty in purification in the prior art are solved; 2, the operation process is simplified, and the production efficiency is greatly improved; 3, used solvents are safe, and less environment pollution is caused.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
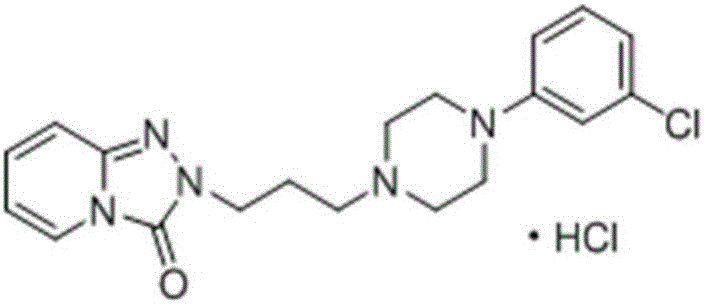
Preparation method of trazodone hydrochloride
The invention is applicable to the field of chemistry and chemical engineering and provides a preparation method of trazodone hydrochloride. The preparation method comprises the following steps: mixing N-(3-chloro-phenyl)-N'-(3-chloropropyl)-piperazine hydrochloride and 1,2,4-triazolo[4,3-a]pyridin-3(2H)-one in a solvent; adding an alkali, and heating and reflowing; carrying out heat filtering into a reaction system, adding alkali liquid and heating and reflowing again in sequence; crystallizing to obtain trazodone; taking the trazodone to react with hydrochloric acid to obtain the trazodone hydrochloride. According to the preparation method of the trazodone hydrochloride, provided by the invention, the total yield of the product is improved, and the content of an impurity N-(3-chloro-phenyl)-N'-(3-chloropropyl)-piperazine is remarkably reduced, so that the industry limitation requirements can be directly met. The preparation method has simple flow steps so that the production cost is reduced; the process is stable and industrial production can be carried out.
Owner:SHENZHEN FONCOO PHARMACEUTICAL CO LTD
Preparation method of 1-(2,3-dichlorophenyl) piperazine hydrochloride
The invention discloses a preparation method of 1-(2,3-dichlorophenyl) piperazine hydrochloride, which is characterized by comprising the following steps: carrying out cyclization reaction 2,3-dichloroaniline as a raw material with bis(2-chloroethyl) amine hydrochloride, wherein the charging temperature is 90-120 DEG C, and the reaction temperature is 120-220 DEG C; and treating reaction liquid after the reaction by using an after-treatment solvent, so as to obtain a coarse product, and carrying out refining on the coarse product so as to obtain a refining after-treatment solvent so as to obtain a product with purity meeting the requirement. The mass ratio of the 2,3-dichloroaniline to the bis(2-chloroethyl) amine hydrochloride is 1:(0.8-2.0), and the after-treatment solvent and the refining after-treatment solvent are protonic solvents. According to the method, the yield is high, waste liquid is relatively little, the cost is low, the purity (HPLC (High Performance Liquid Chromatography)) is up to more than 99.5%, and the yield is up to more than 59.5%; and the method is suitable for industrial production of the 1-(2,3-dichlorophenyl) piperazine hydrochloride.
Owner:江西华龙化工有限公司
New synthesis method of aripiprazole
InactiveCN103787965AControl generationSimple equipment requirementsOrganic chemistrySynthesis methodsZinc
A preparation method of aripiprazole comprises the following steps: reacting a raw material tetrahydrofuran with paratoluensulfonyl chloride under the catalysis of zinc chloride to obtain 4-chlorobutyl paratoluenesulfonate in a rapid mild high-output manner; reacting 4-chlorobutyl paratoluenesulfonate with 7-hydroxyquinolinone under the action of a solvent and an alkali to generate 4-chlorobutoxyquinolinone; and reacting chlorobutoxyquinolinone with piperazine hydrochloride in the certain solvent and the alkali to generate aripiprazole. Each of the intermediate 4-chlorobutoxyquinolinone and the finally obtained product aripiprazole contains a low content of dimer, so the tedious low-efficiency time-consuming removal process of the dimer can be avoided. The method has the advantages of simple process, high output, safety and low cost.
Owner:ZHANGJIAGANG JIUMU TECH
New preparation technique of urapidil hydrochloride
The invention relates to a new preparation technique of urapidil hydrochloride, belonging to the technical field of drug synthesis. According to the new preparation technique, a Pd / NHC catalytic system is directly utilized to catalyze 6-(3-chloropropyl)-1,3-dimethyluracil and 1-(2-methoxyphenyl)piperazino hydrochloride to prepare urapidil, and the urapidil is further acidified to prepare the urapidil hydrochloride. The new preparation technique of urapidil hydrochloride has the advantages of simple structure of raw materials, no need of abundant phase-transfer catalysts, fewer side reactions, easy after-treatment, favorable product selectivity and higher yield, and is suitable for industrial production; and the related substance content is less than 0.0047%, and the total yield is greater than 58%.
Owner:HEBEI YIPIN PHARMA
Preparation method of urapidil by reverse phase transfer catalysis
InactiveCN102295607AShort reaction timeHigh yieldOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsUracilOrganic synthesis
The invention discloses a preparation method for urapidil, and relates to an inverse phase transfer catalysis method for preparing urapidil, and belongs to the technical field of organic synthesis. The method comprises the following steps that: water is adopted as a solvent; an inorganic alkaline substance is adopted as an acid binding agent; 6-(3-chloropropyl)-1,3-dimethyluracil (I) reacts with 1-(2-methoxyphenyl)piperazine hydrochloride (II) in the presence of an inverse phase transfer catalyst to generate 6-[[3-[4-(2-methoxyphenyl)-1-piperazinyl]-propyl]-amino]-1,3-dimethyl-uracil (III, urapidil). With the method, the high-selectivity and high-yield preparation of the urapidil can be realized; the prepared urapidil has high purity, wherein the purity of the urapidil is more than 99.5%;the conversion rate is high; the yield can reach 80%; the reaction conditions are mild; the selectivity is good; the post-treatment is simple; the method is environmental-friendly, and is suitable for the industrial production.
Owner:ZHENGZHOU UNIV +1
Process for preparing aripiprazole
The invention encompasses the synthesis of aripiprazole from BBQ and DCP, and comprises mixing 7-(4-bromobutoxy)-3,4-dihydrocarbostyril (BBQ) and 1-(2,3-dichlorophenyl)piperazine hydrochloride (DCP) in the presence of at least one base and at least one organic solvent to form a reaction mixture; heating the reaction mixture for a sufficient amount of time to effect the reaction; and isolating aripiprazole. The invention also encompasses the use of phase transfer catalysts in synthesizing aripiprazole from BBQ and DCP.
Owner:TEVA PHARM USA INC
Aryl piperazine modified benzo [b] thiophene compound, and its preparing method and use
The invention discloses aryl piperazine attributive benzo[b] thiophene compound with antidepressant activity structure and its preparing method and the application. Its preparing method includes the following steps: adding 1mol 3-propionyl benzo[b] thiophene, 1-5mol paraformaldehyde, and 1-5mol aryl piperazine hydrochloride into the alcohol with 1-50 times weight ratio; reacting for 1-80h at back flow to generate 1-(benzo[b]thiophene-3-group)-2-methyl-3-(4-aryl piperazine-1-group)-1-acetone; adding 1mol the above generated compound and 0.2-5mol sodium borohydride into the alcohol with 1-50 times weight ratio; reacting for 1-20h at 0-100 degree centigrade to generate 1-(benzo[b] thiophene -3-group)-2-methyl-3-(4-aryl piperazine-1-group) -1-propanol. This compound can be used in antidepressant preparing by itself free alkali or salt form. The invention has the advantages of simple preparing process and easy operation.
Owner:TIANJIN UNIV
Method for preparing 4-trifluoromethylphenyl piperazine
InactiveCN102060712AHigh reaction yieldIncrease contentOrganic compound preparationAmino compound preparationTrifluoromethylphenylpiperazineN-Butyl Alcohol
The invention relates to a method for preparing 4-trifluoromethylphenyl piperazine, which comprises the following steps of: firstly, reacting diethanol amine with thionyl chloride to obtain bis(2-chloroethyl)amine, reacting 4-trifluoromethylaniline with bis(2-chloroethyl)amine in normal butanol or diethylene glycol monomethyl ether under the heating condition to obtain 4-trifluoromethylphenyl piperazine hydrochloride, and neutralizing with an alkaline to obtain the 4-trifluoromethylphenyl piperazine. The method for preparing the 4-trifluoromethylphenyl piperazine has the advantages of high reaction yield, less production of three wastes, easy reaction control, small production danger, simple product purification, high content and the like.
Owner:天津均凯农业科技有限公司
Method for preparing sitagliptin phosphate side chain
InactiveCN101973997AShort routeRaw materials are cheap and easy to getOrganic chemistryPhosphorus pentasulfideThioketone
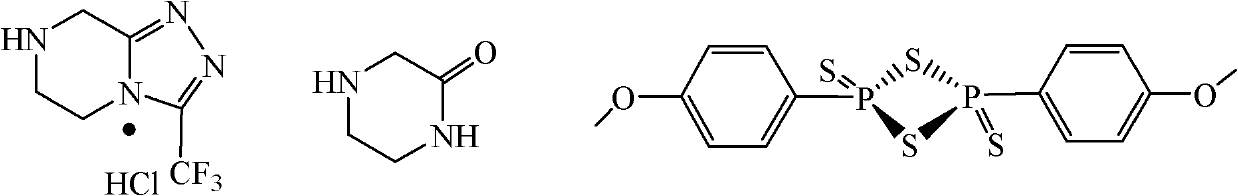
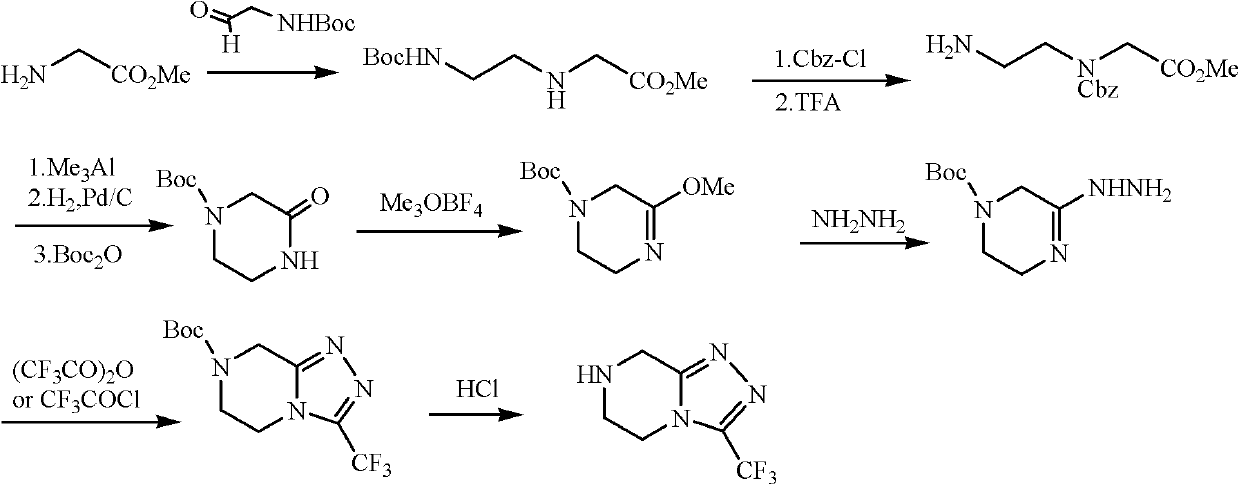
The invention discloses a method for preparing a sitagliptin phosphate side chain which has a structure shown by a formula 1. The method comprises the following steps of: a) preparing 2-piperazine thioketone with structure shown by a formula 4 by vulcanizing 2-piperazine ketone with structure shown by a formula 2 with a vulcanizing reagent, wherein the vulcanizing reagent is phosphorus pentasulfide or a Lawesson reagent with structure shown by a formula 3; and b) performing cyclization on trifluoro ethyl hydrazine and the 2-piperazine thioketone through Pellizzari reaction and adding hydrochloric acid to form salt so as to obtain 3-trifluoromethyl-[1,2,4] triazole [4,3-a] piperazine hydrochloride serving as a target product. The method of the invention has short route, cheap and readily available raw material, high yield of each step and relatively low cost and is simple to operate.
Owner:ZHEJIANG UNIV
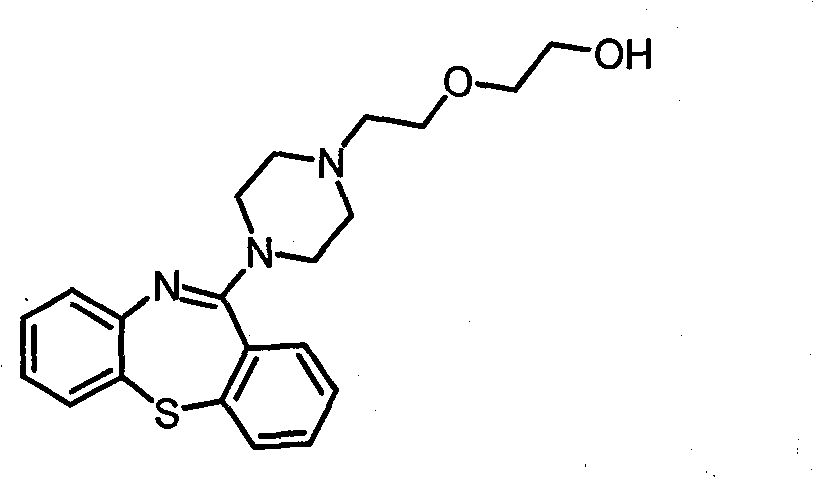
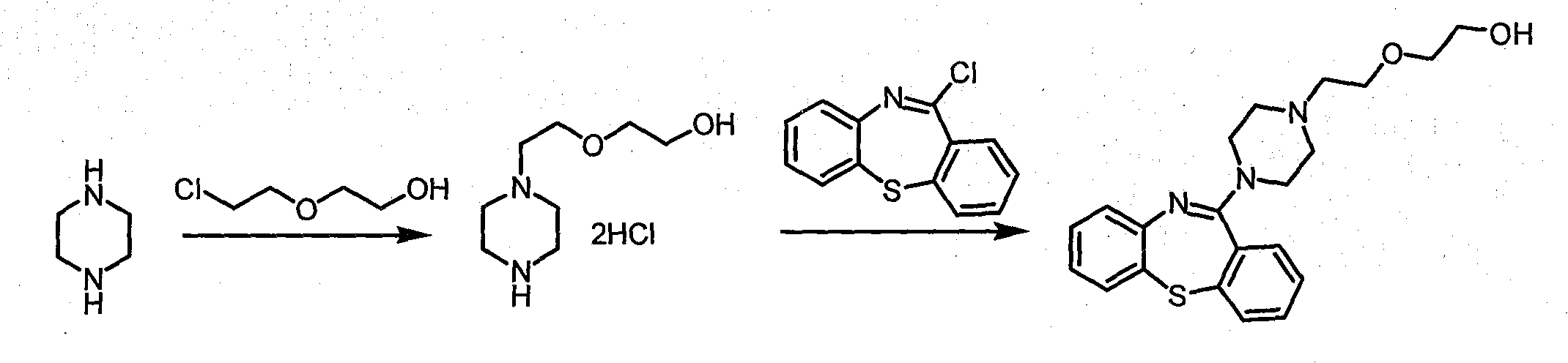
Novel preparation method of quetiapine
InactiveCN103724294AHigh yieldReduce consumptionCarboxylic acid salt preparationThiazepineQuetiapine
The invention discloses a high-efficient and simple method used for preparing high purity 1-[2-(2-hydroxyethoxy)ethyl]piperazine hydrochloride. The preparation method is used for replacing a method used for preparing quetiapine by reacting a free alkali derivative with 11-chloro-dibenzo[b, f](1, 4)thiazepine. According to the preparation method, low-temperature recrystallization is adopted for purifying 1-[2-(2-hydroxyethoxy)ethyl]piperazine hydrochloride so as to avoid residue of unknown piperazidine impurities in high-temperature purification processes, and high purity quetiapine can be obtained in subsequent reactions.
Owner:WUXI QIANHAO BIOPHARMA
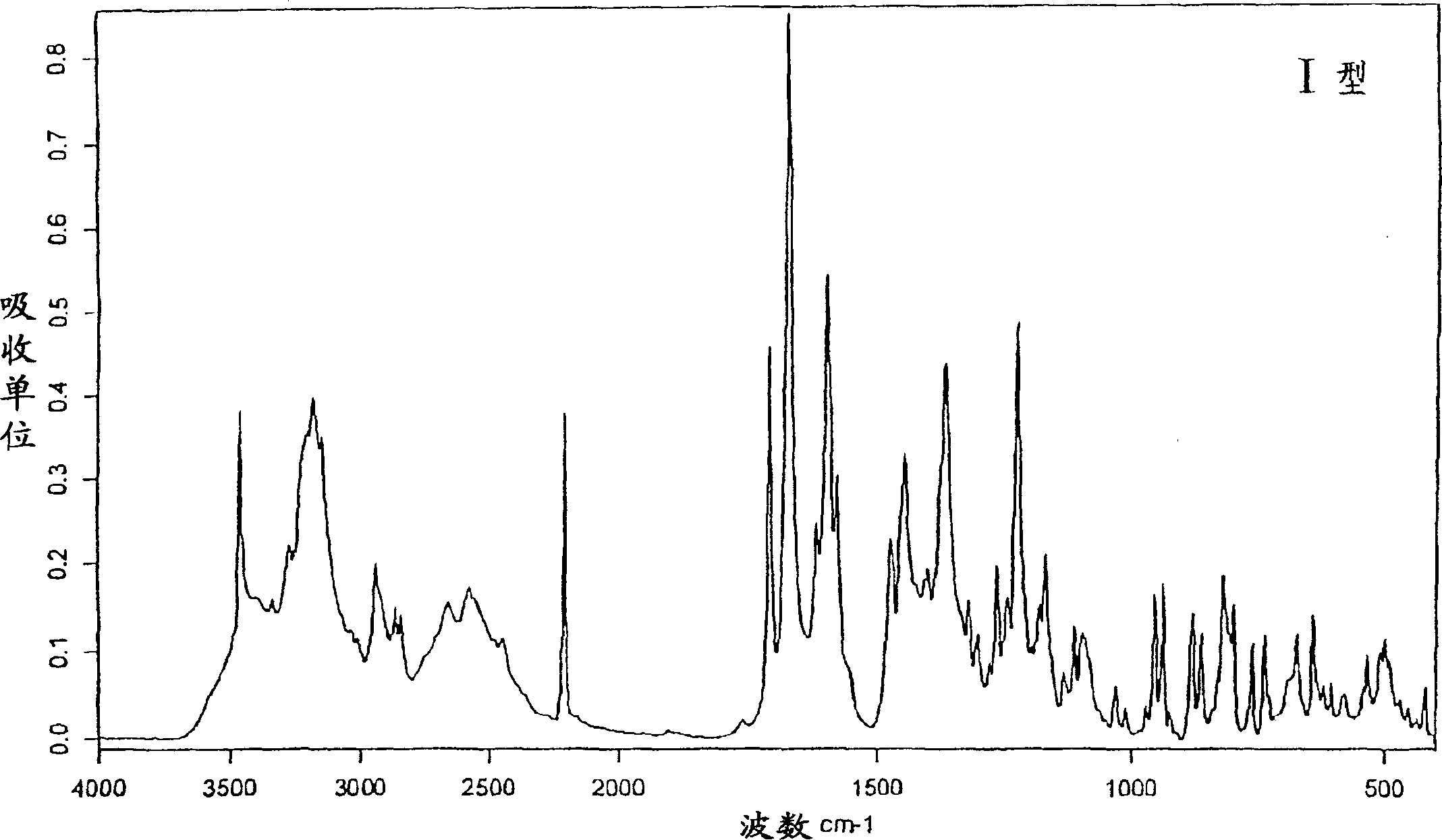
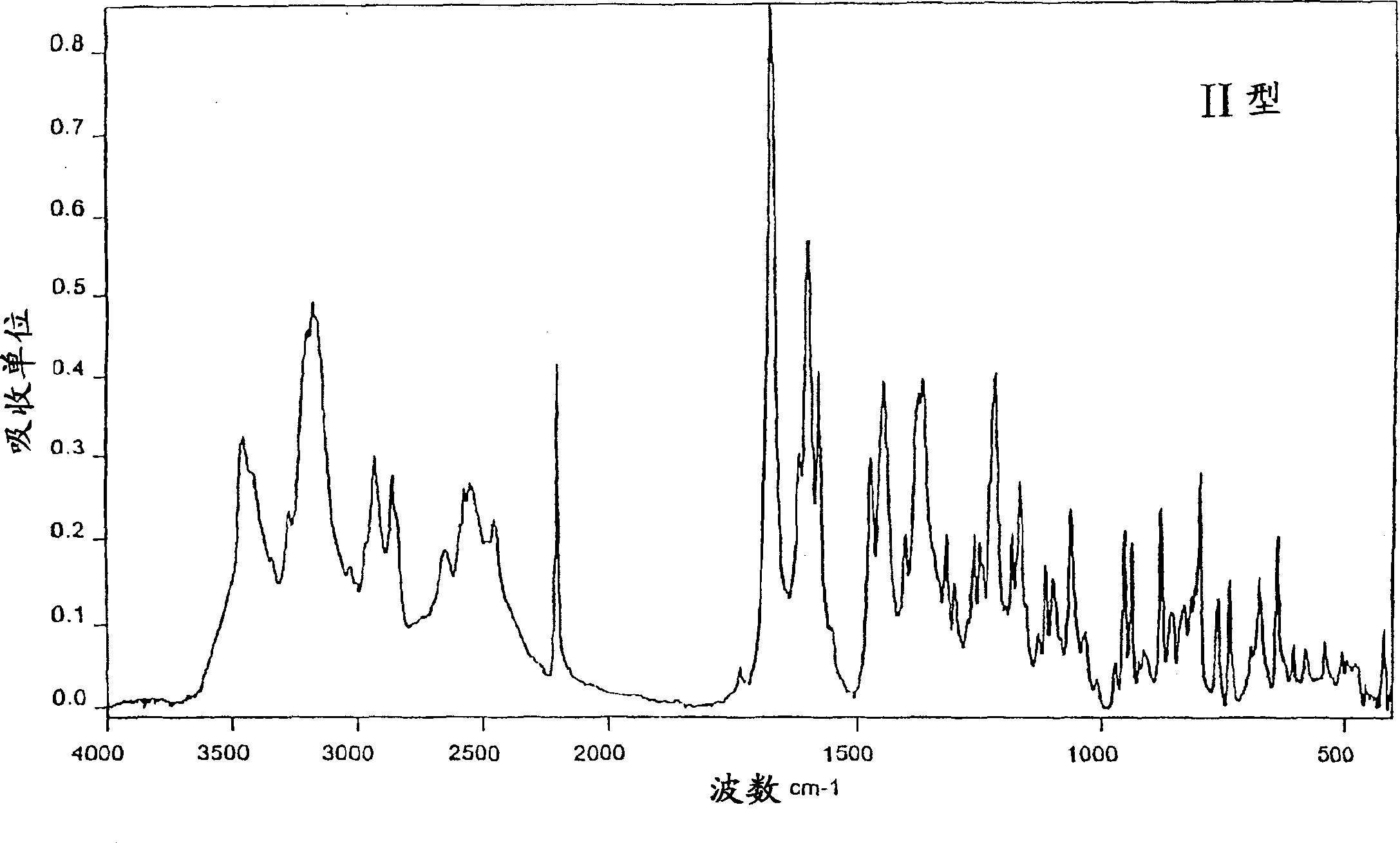
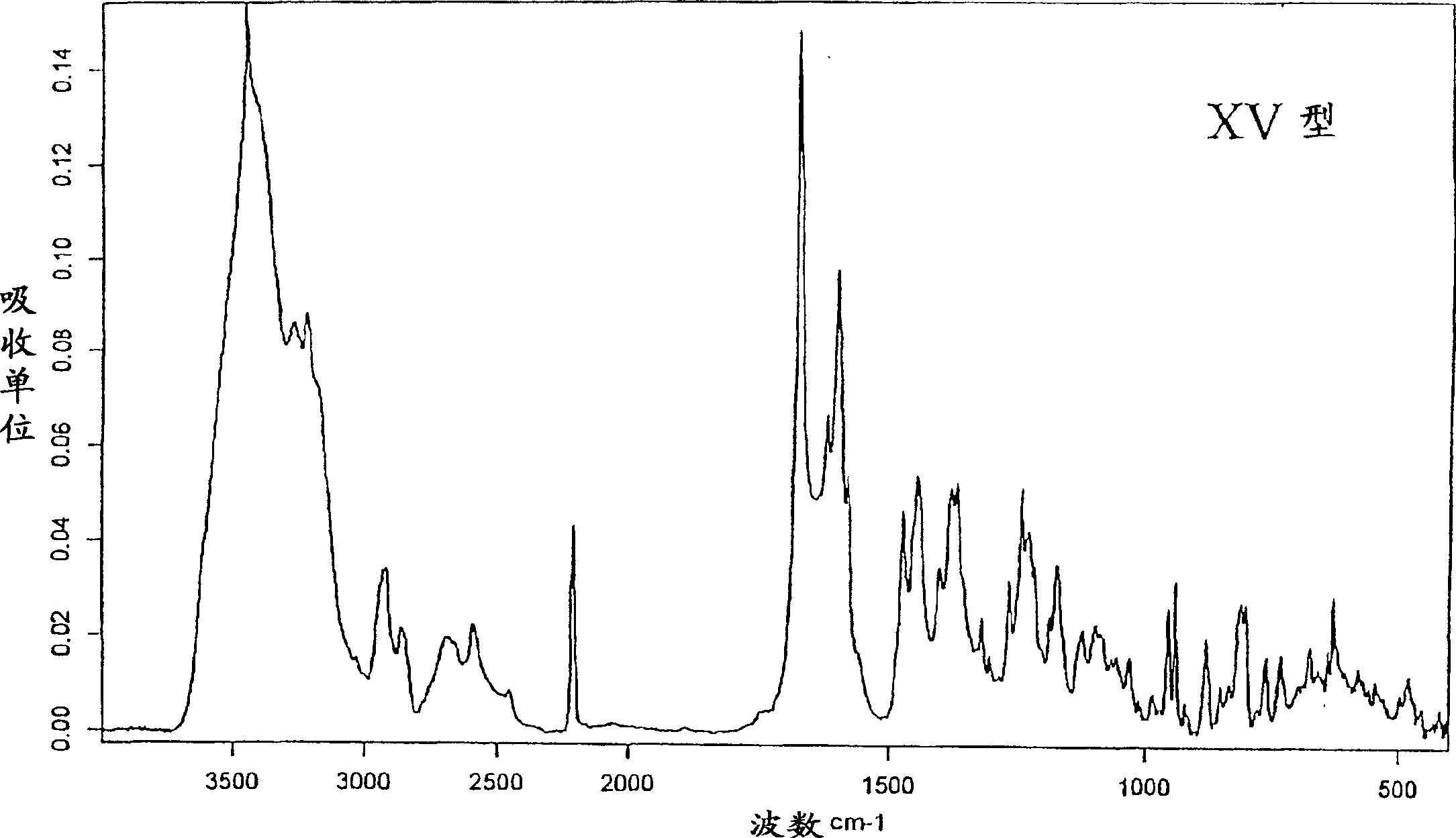
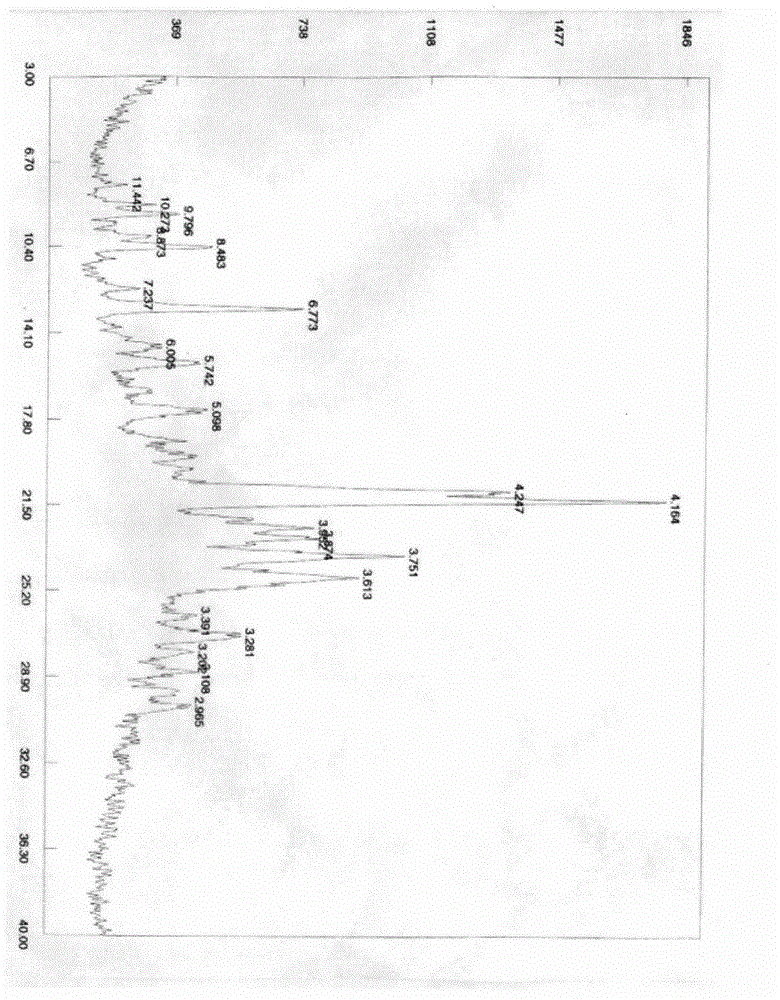
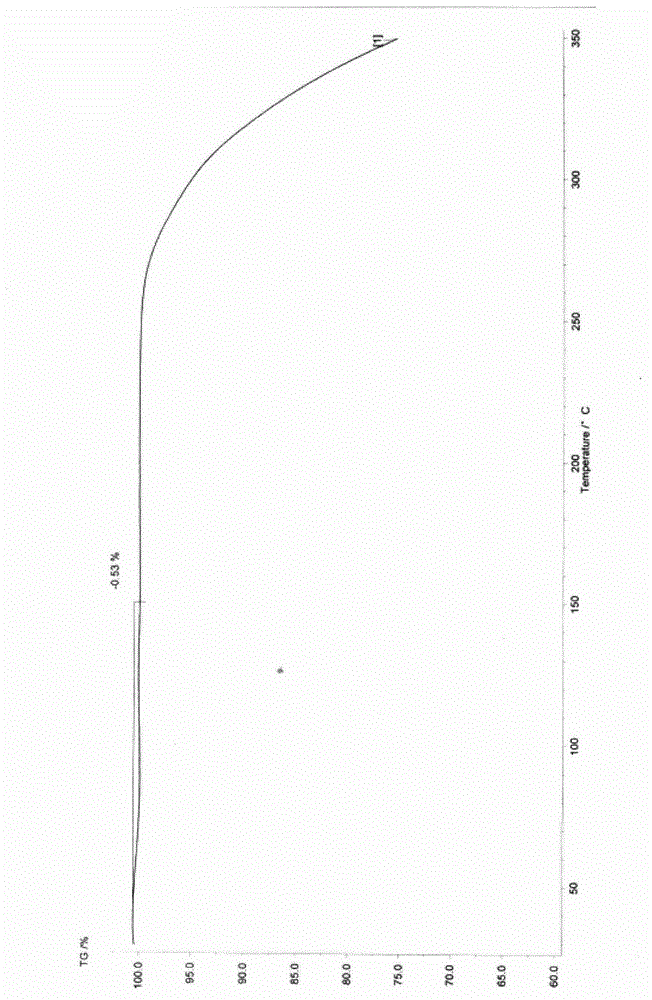
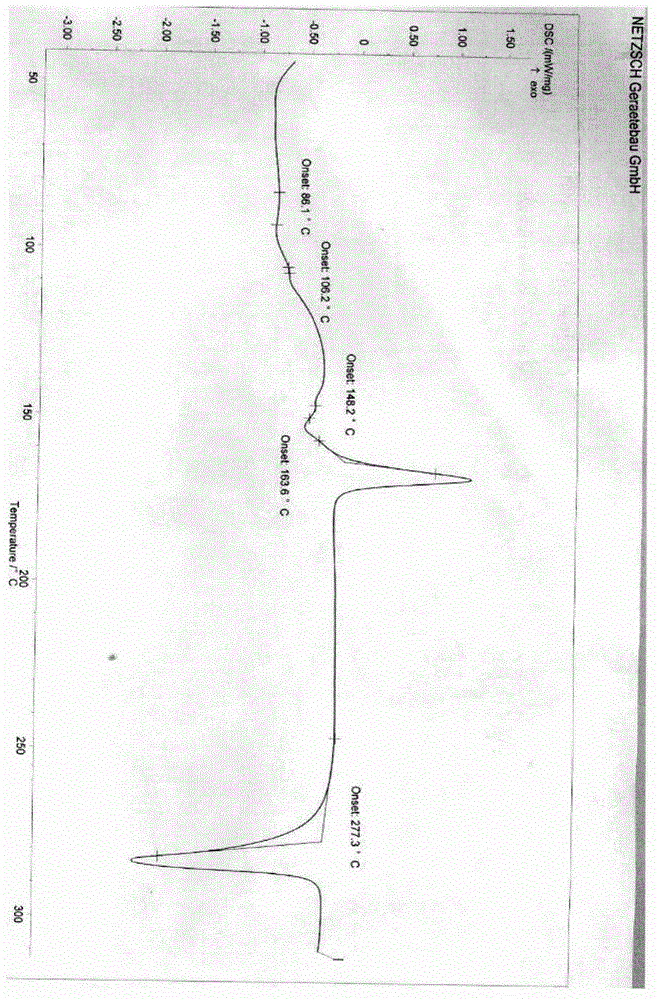
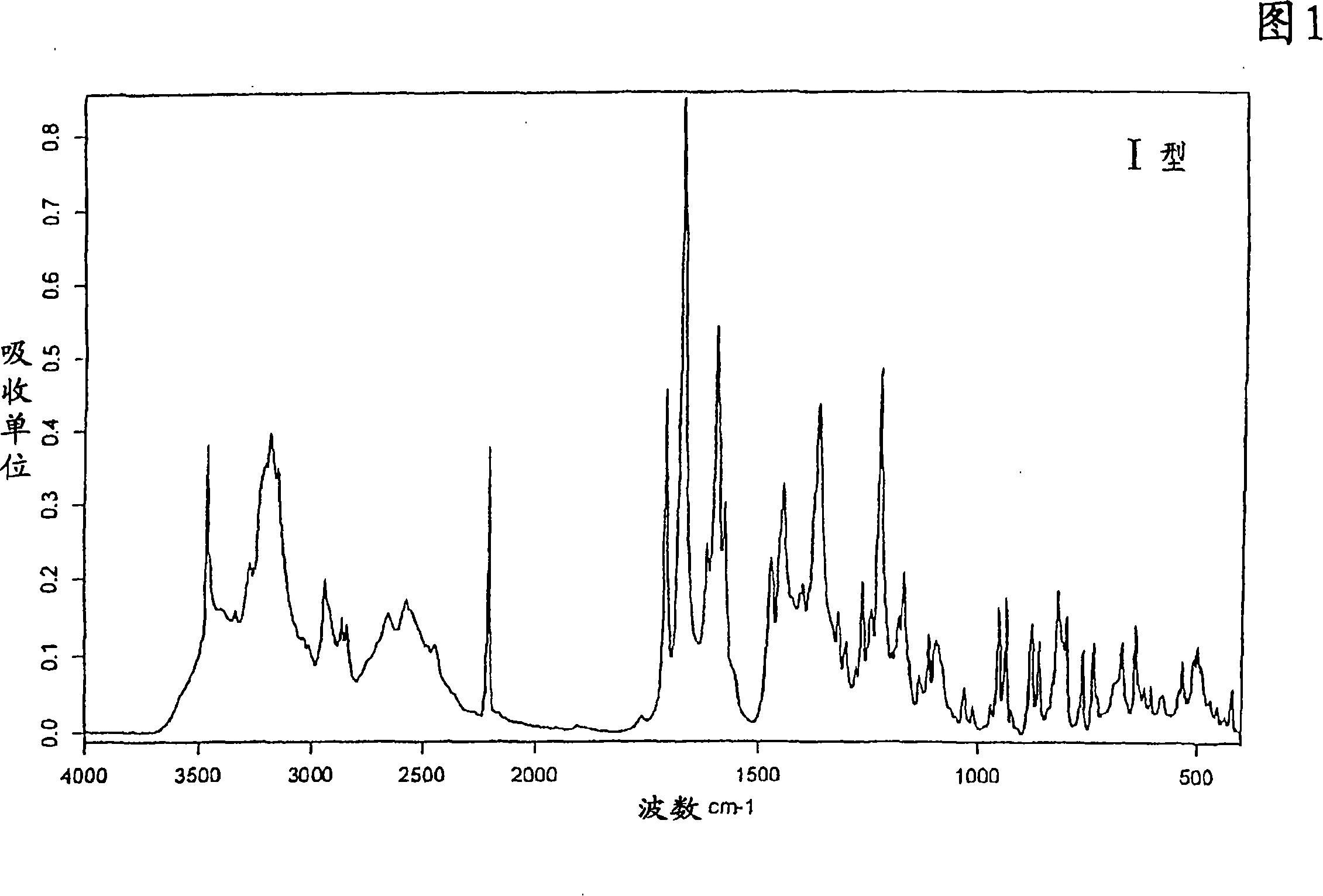
New crystal form XVII of 1-(4-(5-cyanoindole-3-yl) butyl)-4-(2-carbamyl-benzofuran-5-yl)-piperazine hydrochloride and preparation method of new crystal form XVII
The invention relates to a new crystal form XVII of 1-(4-(5-cyanoindole-3-yl) butyl)-4-(2-carbamyl-benzofuran-5-yl)-piperazine hydrochloride and a preparation method of the new crystal form XVII. Peaks of an X-ray diffraction pattern of powder of the new crystal form XVII are located at 9.02 degrees, 9.96 degrees, 13.06 degrees, 20.90 degrees, 21.32 degrees, 22.94 degrees, 23.70 degrees and 24.62 degrees+ / -0.2 degrees and 2 theta, initial melting is shown at about 277 DEG C in a differential scanning calorimetry spectrum, and thermogravimetric analysis shows that the product has no water of crystallization.
Owner:NANJING HEALTHNICE MEDICAL TECH
Polymorphic forms of 1-'4-(5-cyanoindol-3-yl)butyl-4-(2-carbamoylbenzofuran-5-yl)piperazine hydrochloride
InactiveCN101139345ALow hygroscopicityEasy to compressOrganic active ingredientsNervous disorderFibromyalgiaPsychotic illness
Owner:MERCK PATENT GMBH
Lomerizine Hydrochloride isomeride and preparation method therefor
InactiveCN105175359AEfficient productionEfficient Separation ProcessOrganic chemistryHalogenBenzaldehyde
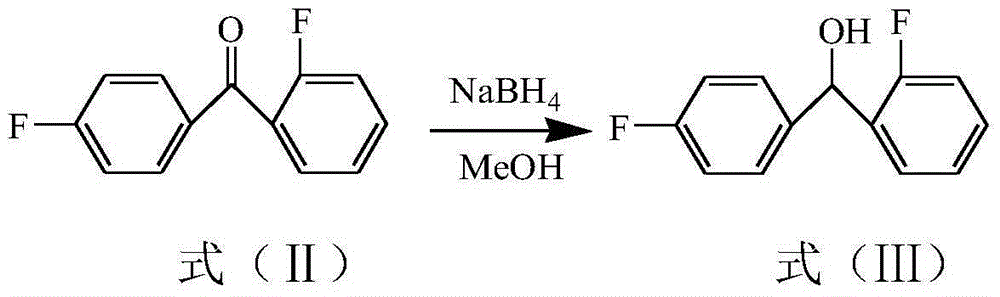
The invention relates to a lomerizine Hydrochloride isomeride and a preparation method therefor. The structural formula of the lomerizine Hydrochloride isomeride is shown in the specification. The chemical name is 1-(2,4'-difluoro benzhydryl)-4-[(2,3,4- trimethoxyphenyl)methyl]piperazine hydrochloride. 2,4'-difluoro benzhydryl ketone is subjected to reduction and halogenation, halogen substitution is carried out by utilization of piperazine, the obtained compound and 2,3,4- trimethoxy benzaldehyde are subjected to a condensation reaction, finally a salt forming reaction with concentrated hydrochloric acid is carried out, and the lomerizine Hydrochloride isomeride is prepared.
Owner:四川省百草生物药业有限公司
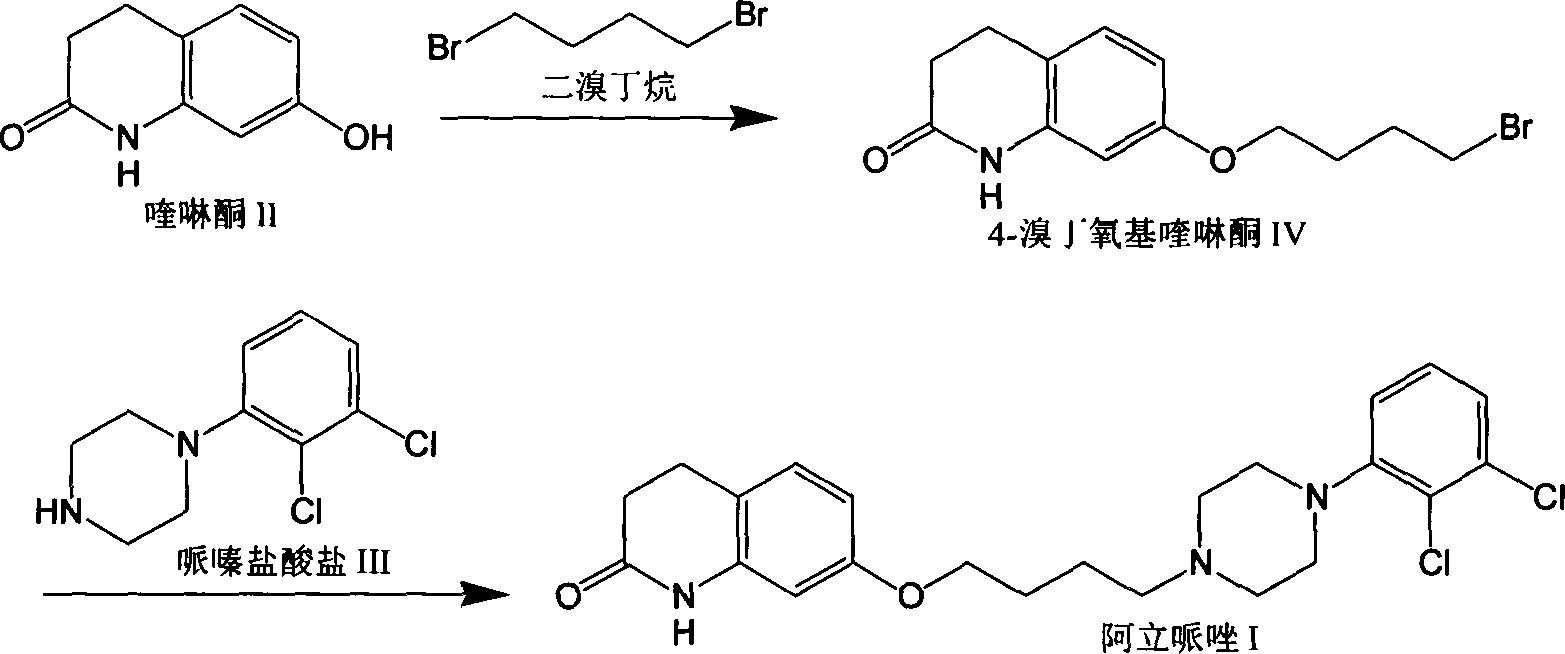
Preparation method of aripiprazole intermediate 1-(2,3-dichlorophenyl)piperazine hydrochloride
The invention discloses a preparation method of an aripiprazole intermediate 1-(2,3-dichlorophenyl)piperazine hydrochloride. The preparation comprises the step of reacting by taking 2,3-dichloronitrobenzene and bis(2-chloroethyl)amine hydrochloride as starting materials and utilizing a one-pot method. The method is simple in operation, and acidic hydrogen chloride generated in the reaction process is dissolved into an ammonium chloride solution to participate in reduction of nitryl, so that the cost is saved, and energy conservation and environment protection are achieved; and furthermore, the method is simple in operation, high in cost, relatively low in cost and suitable for industrial production.
Owner:山东安信制药有限公司
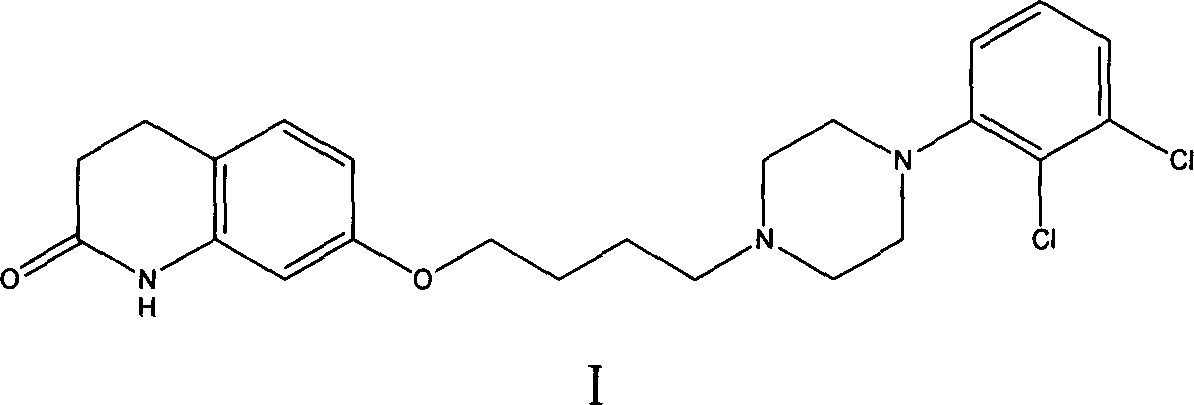
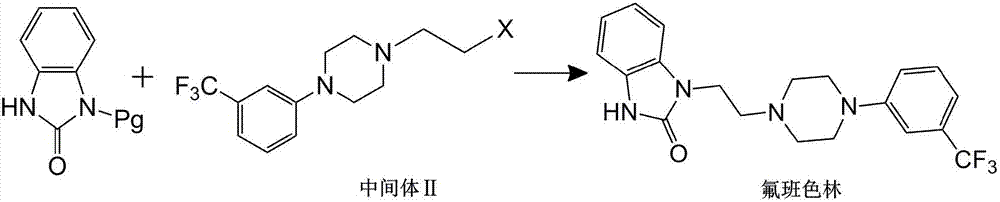
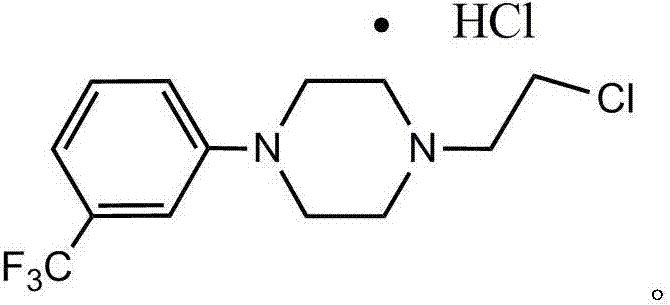
Preparation method of flibaserin intermediate
The invention discloses a method belonging to the field of heterocyclic compounds, and concretely relates to a preparation method of a flibaserin intermediate. The preparation method comprises the following steps of (1) dissolving 1-(3-trifluoromethylphenyl) piperazine hydrochloride in a solvent a, and reacting with 2-halogenated ethanol or ethylene oxide in the presence of alkali to obtain 2-(4-(3-trifluoromethylphenyl) piperazine-1-ethanol; (2) reacting the 2-(4-(3-trifluoromethylphenyl) piperazine-1-ethanol with a chloride agent compound to obtain a compound as shown in a formula I. According to the preparation method, the reaction selectivity is improved, the generation of impurities is reduced, the product purity is improved, and the preparation method is simple and convenient to operate, environmentally-friendly, and beneficial for industrialized mass production.
Owner:广州隽沐生物科技股份有限公司
Process for preparing aripiprazole
The invention includes the synthesis of aripiprazole from BBQ and DCP, and comprises mixing 7-(4-bromobutoxy)-3,4-dihydrocarbostyril (BBQ) and 1-(2,3-dichlorophenyl)piperazine hydrochloride (DCP) in the presence of at least one base and at least one organic solvent to form a reaction mixture; heating the reaction mixture for a sufficient amount of time to effect the reaction; and isolating aripiprazole. The invention also includes the use of phase transfer catalysts in synthesizing aripiprazole from BBQ and DCP.
Owner:TEVA PHARMA IND LTD
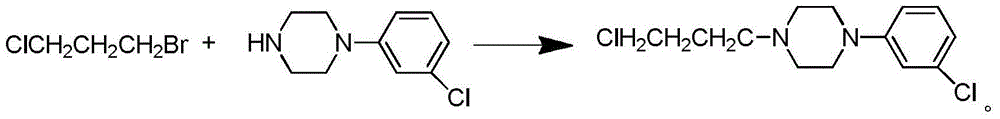
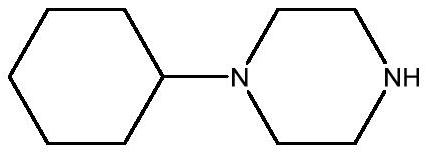
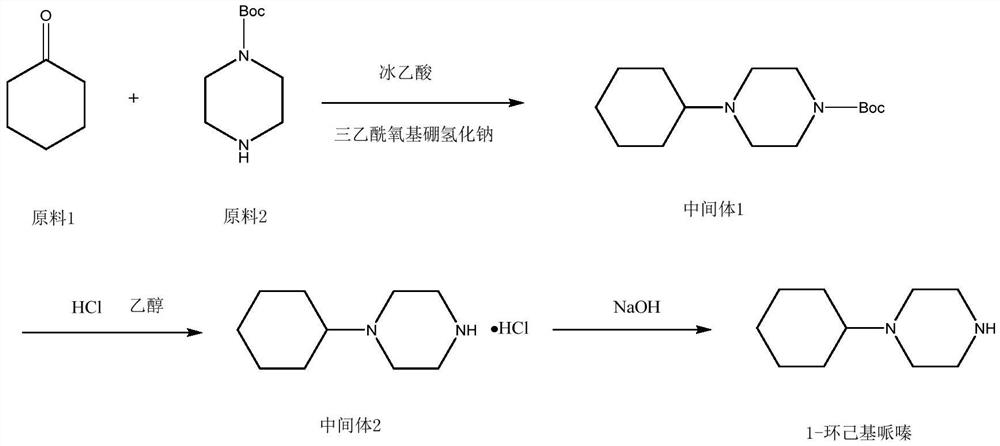
Preparation method of 1-cyclohexylpiperazine
InactiveCN112645901AReduce usageReduce manufacturing costOrganic chemistrySodium triacetoxyborohydrideOrganic solvent
The invention discloses a preparation method of 1-cyclohexylpiperazine. The method comprises the steps of carrying out reflux reaction on cyclohexyl halide, 1-Boc-piperazine and inorganic base in an organic solvent, filtering after the reaction is finished, and concentrating to obtain an intermediate 1; removing Boc from the intermediate 1 under an acidic condition, drying by distillation after the reaction is finished, pulping with isopropanol, and filtering to obtain solid 1-cyclohexyl piperazine hydrochloride; dissolving with water, adding an inorganic base to adjust the pH value to 12 to 14, extracting with an extraction solvent, drying the solvent by distillation to obtain a crude product of 1-cyclohexylpiperazine, and carrying out reduced pressure distillation on the crude product through an oil pump to obtain a pure product of 1-cyclohexylpiperazine. In the synthesis process of the intermediate 1, use of sodium triacetoxyborohydride and sodium hydroxide is avoided, the production cost is greatly saved, meanwhile, only filtering is needed for aftertreatment, the method is very simple, and time and labor are saved.
Owner:SHANDONG BOYUAN PHARM CO LTD
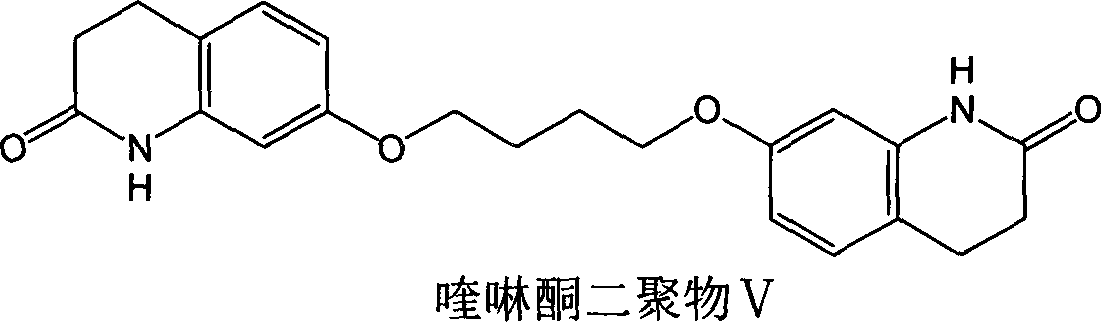
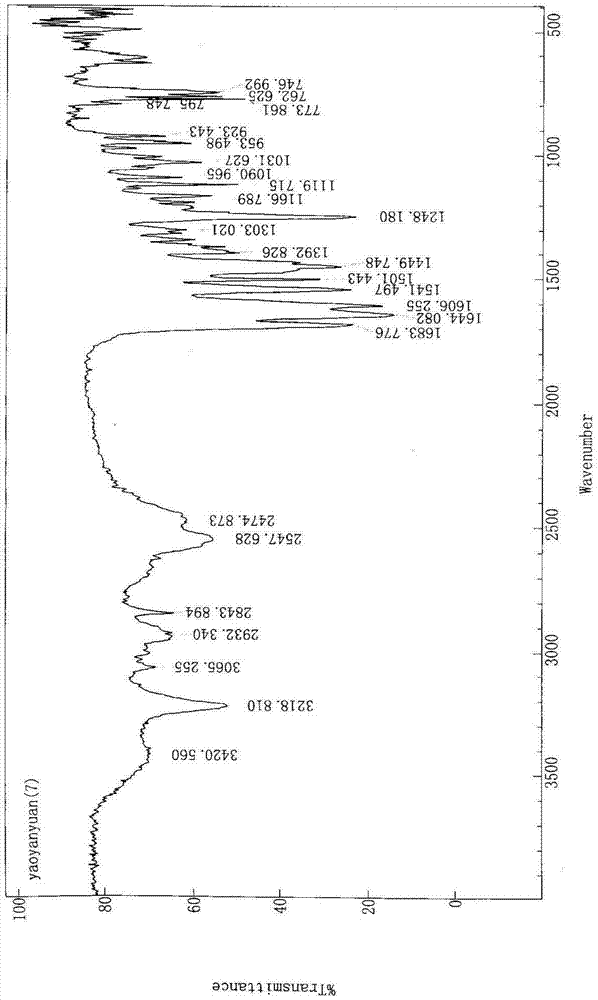
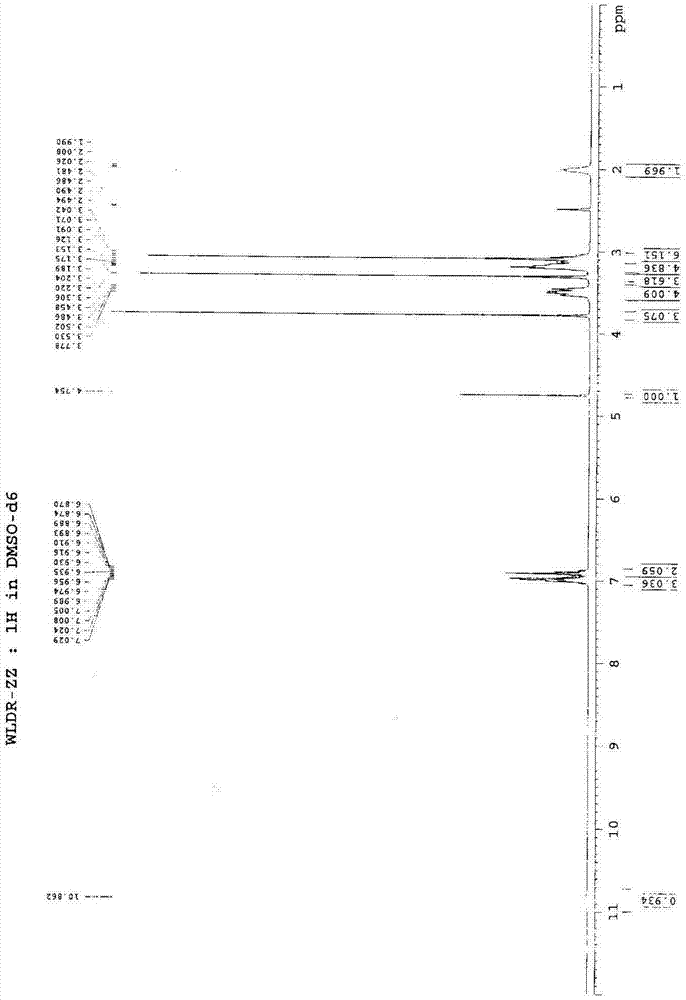
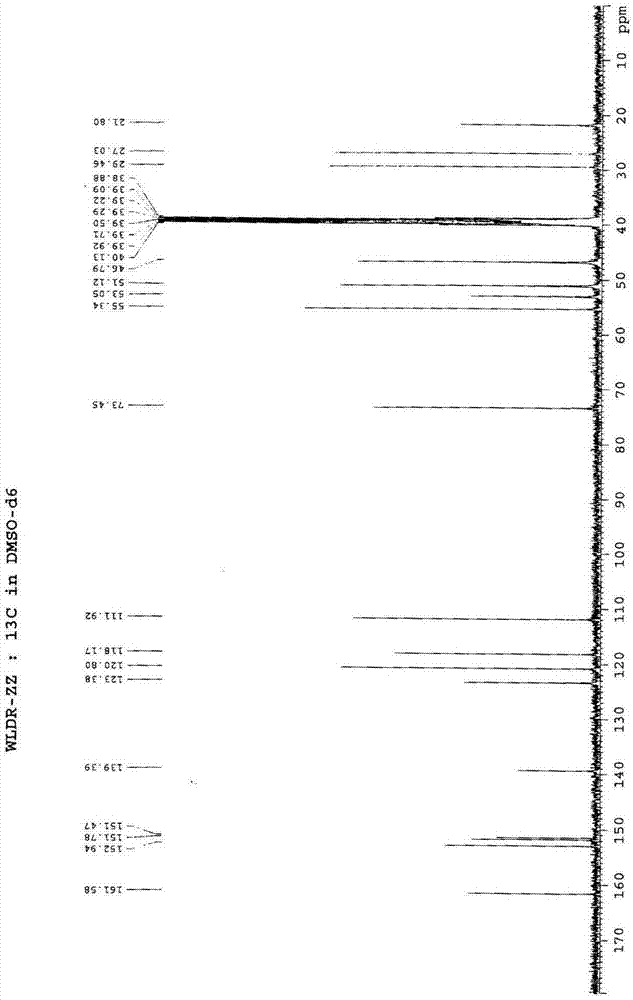
Novel preparation method of III crystal-form vilazodone hydrochloride
InactiveCN104610238AReduce processing timeLow costOrganic chemistry methodsVacuum dryingPiperazine hydrochloride
The invention belongs to the field of pharmaceutical chemistry, and more specifically to a novel preparation of III crystal-form 1-[4-(5-cyanoindole-3-butyl)]-4-(2-carbamoyl-coumarone-5-yl)-piperazine hydrochloride. The preparation method comprises reacting a solvate of hydrochloric acid, vilazodone and tetrahydrofuran at a temperature range of 120-140 DEG C and performing vacuum drying for 12-36 hours. The III crystal-form vilazodone hydrochloride is stable and is convenient to obtain.
Owner:BEIJING SINICA TECH
Preparation method of urapidil
The invention provides a preparation method of urapidil. The preparation method comprises the following steps: mixing 1, 3-dimethyl-6-aminouracil with 3-amino-1-propanol, and carrying out a reaction so as to prepare 6-(3-hydroxypropylamino)-1, 3-dimethyluracil; mixing 6-(3-hydroxypropylamino)-1, 3-dimethyluracil and thionyl chloride to react so as to prepare 6-(3-chloropropylamino)-1, 3-dimethyluracil; and enabling 6-(3-chloropropyl amino)-1, 3-dimethyluracil to react with 1-(2-methoxyphenyl) piperazine hydrochloride so as to obtain the urapidil. The preparation method has the advantages of simple operation, cheap and easily available reagents, few side reactions, high yield, good purity of the obtained product, and facilitation of industrial production.
Owner:苏州中科新药篮生物医药科技有限公司
Novel preparation method of 1-[2-(2-aminoethyoxyl)ethyl]piperazine hydrochloride
The invention discloses a practical synthesis method for preparing an important medicine raw material 1-[2-(2-aminoethyoxyl)ethyl]piperazine hydrochloride. In the presence of a proper solvent or in the absence of a solvent, piperazine hydrochloride and 2-(2-aminoethyoxyl)ethylamine hydrochloride are stirred to react at 100-150 DEG C; 1-[2-(2-aminoethyoxyl)ethyl]piperazine hydrochloride is directlyobtained with high yield without protection of one amino of piperazine.
Owner:WUXI QIANHAO BIOPHARMA
Novel preparation method of 1-[((2-hydroxyethyoxyl)ethyoxyl)ethyl]piperazine hydrochloride
The invention discloses a practical synthesis method for preparing an important medicine raw material 1-[((2-hydroxyethyoxyl)ethyoxyl)ethyl]piperazine hydrochloride. In the presence of a proper solvent or in the absence of a solvent, piperazine and 1-[((2-chloroethyoxyl)ethyoxyl)ethanol are stirred to react at 100-150 DEG C; 1-[((2-hydroxyethyoxyl)ethyoxyl)ethyl]piperazine hydrochloride is directly obtained with high yield without protection of one amino of piperazine.
Owner:WUXI QIANHAO BIOPHARMA
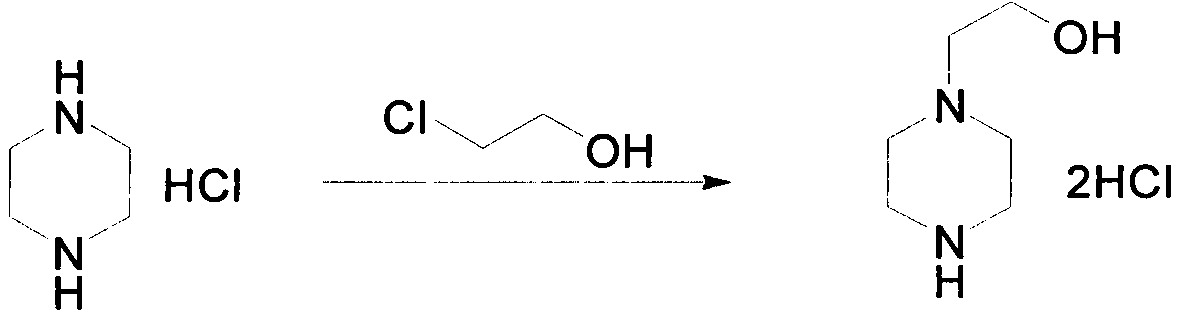
Novel preparation method of 1-(2-hydroxyethyl)piperazine hydrochloride
The invention discloses a practical synthesis method for preparing an important medicine raw material 1-(2-hydroxyethyl)piperazine hydrochloride. In the presence of a proper solvent or in the absenceof a solvent, piperazine hydrochloride and 2-chloroethanol are stirred to react at 100-150 DEG C; 1-(2-hydroxyethyl)piperazine hydrochloride is directly obtained with high yield without protection ofone amino of piperazine.
Owner:WUXI QIANHAO BIOPHARMA
1-[2-(2,4-dimethylphenylsulfydryl)phenyl]piperazine hydrochloride and preparation method thereof
InactiveCN107915694AReduce usageThe synthesis process is simpleOrganic chemistryImpurityPiperazine hydrochloride
The invention discloses 1-[2-(2,4-dimethylphenylsulfydryl)phenyl]piperazine hydrochloride and a preparation method thereof. By means of the specific preparation method, the HPLC purity of vortioxetinehydrochloride is higher than 99.5%, and the content of single impurities is smaller than 0.1%. The invention aims to provide a synthesis process of a compound vortioxetine and vortioxetine hydrochloride, which is high in yield, low in cost and suitable for large-scale industrial production.
Owner:FUKANGREN BIO PHARMA
Synthetic method of piperazidines drug intermediate
The invention discloses a synthetic method of a piperazidines drug intermediate. The synthetic method comprises the following steps: step one, reacting diethanol amine with thionyl chloride to prepare di(2-chloroethyl) methylamine hydrochloride; step two, reacting 3-chloroaniline with di(2-chloroethyl) methylamine hydrochloride to prepare 1-(3-chlorphenyl) piperazine hydrochlorid; step three, reacting 1-(3-chlorphenyl) piperazine hydrochloride with 1-bromine-3-chloropropane to prepare 1-(3-chlorphenyl)-4-(3-chloropropyl) piperazine hydrochloride. The line is simple, convenient and easy to perform, the aftertreatment operation is also greatly simplified, the reaction conditions are mild, and the product purity is high.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Chemical synthesis of 1-chloroformyl-4-methylpiperazine hydrochloride
InactiveCN1227242CEasy to operateOperational securityOrganic chemistryChemical synthesisOrganic solvent
1-chloroformyl-4-methylpiperazine hydrochloride is an important organic synthesis intermediate, and the present invention uses bis(trichloromethyl)carbonate and N-methylpiperazine as raw materials to react in an organic solvent have to. The chemical synthesis method eliminates safety hazards and sources of three wastes from the source of the process, and is a 1-chloroformyl-4-methylpiperazine with easy-to-obtain raw materials, safe and reliable production, high reaction yield, low production cost, and basically no three wastes Synthesis method of hydrochloride.
Owner:ZHEJIANG UNIV OF TECH
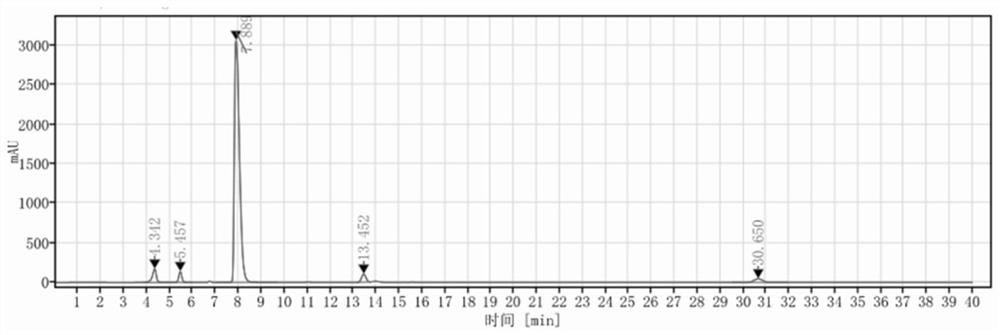
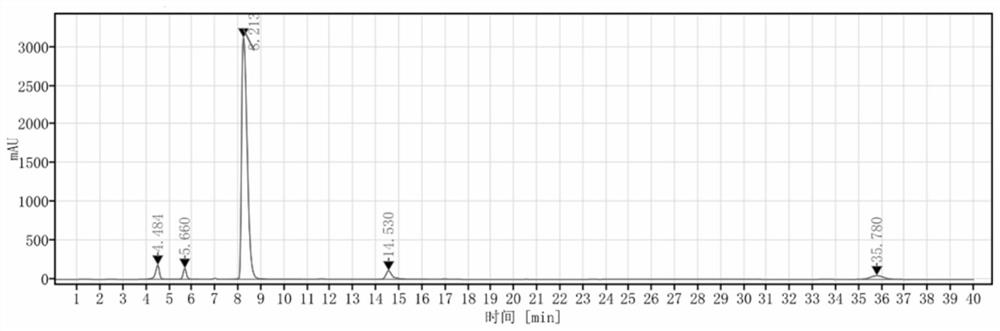
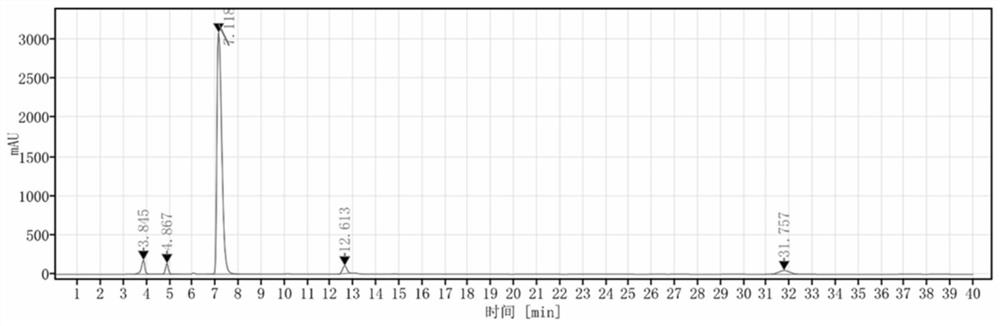
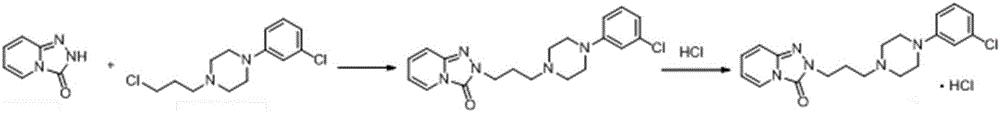
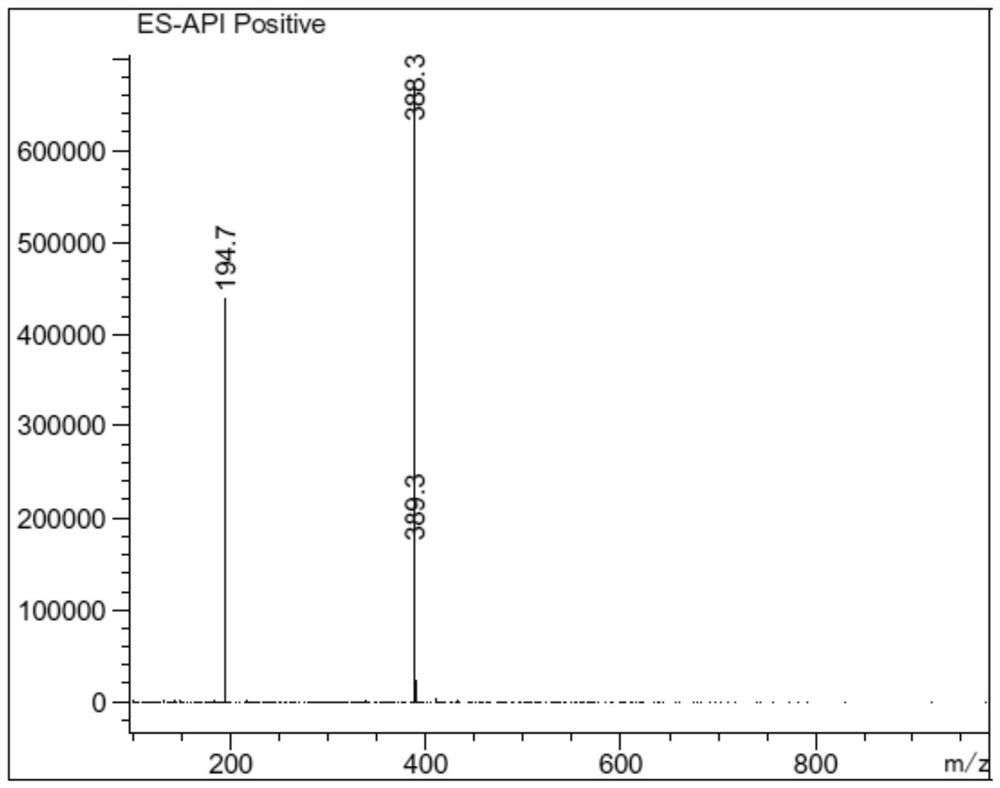
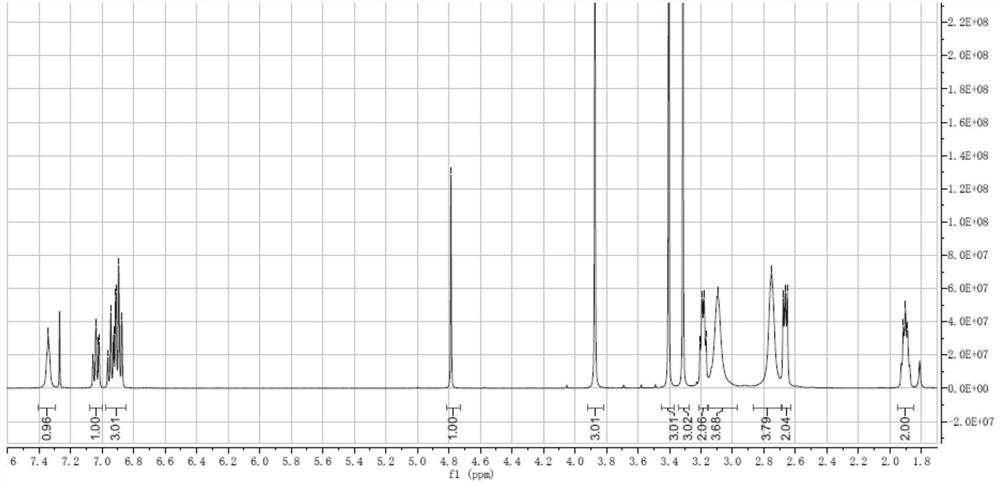
Detection method and application of brexpiprazole related substances
The invention discloses a detection method and application of brexpiprazole related substances. According to the detection method of the brexpiprazole related substances, the brexpiprazole related substances such as an intermediate 7-hydroxy-1H-quinoline-2-ketone (intermediate 1), 7-(4-chlorobutoxy)-1H-quinoline-2-ketone (intermediate 2) and 1-(benzo [b] thiophene-4-yl) piperazine hydrochloride (intermediate 3) and an impurity 1-bromo-4-chlorobutane can be detected at one time; the separation degree is good, the detection time is short, and the elution procedure is simple. The method is applied to preparation of brexpiprazole, the quality of each intermediate can be controlled, and the product quality can be more effectively controlled.
Owner:LIVZON NEW NORTH RIVER PHARMA
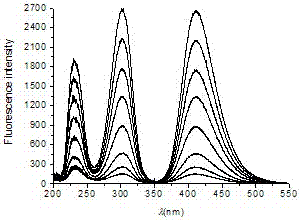
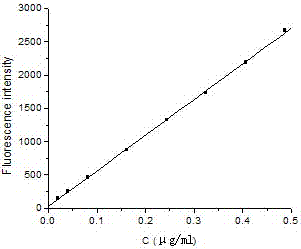
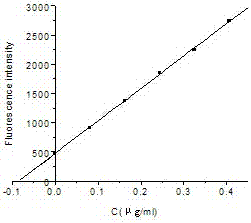
A kind of fluorescence assay method of flavoxate hydrochloride
ActiveCN104777142BSolve the problem of not being able to make it produce sensitized fluorescenceHigh sensitivityFluorescence/phosphorescenceWater bathsFluorescence spectrometry
Owner:HEBEI COLLEGE OF IND & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
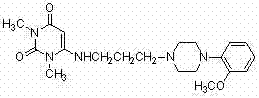
![Aryl piperazine modified benzo [b] thiophene compound, and its preparing method and use Aryl piperazine modified benzo [b] thiophene compound, and its preparing method and use](https://images-eureka.patsnap.com/patent_img/31a181f7-894f-4a48-bcba-9030b38c1af2/A2006101532760002C1.PNG)
![Aryl piperazine modified benzo [b] thiophene compound, and its preparing method and use Aryl piperazine modified benzo [b] thiophene compound, and its preparing method and use](https://images-eureka.patsnap.com/patent_img/31a181f7-894f-4a48-bcba-9030b38c1af2/A2006101532760002C2.PNG)
![Aryl piperazine modified benzo [b] thiophene compound, and its preparing method and use Aryl piperazine modified benzo [b] thiophene compound, and its preparing method and use](https://images-eureka.patsnap.com/patent_img/31a181f7-894f-4a48-bcba-9030b38c1af2/A2006101532760002C3.PNG)
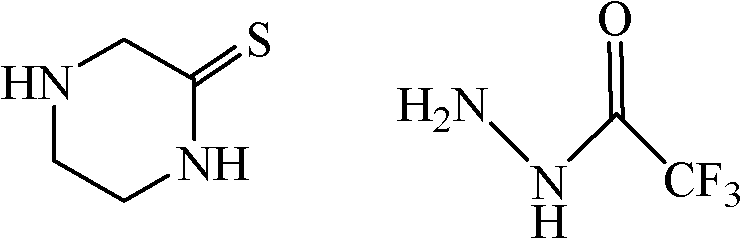
![Novel preparation method of 1-[2-(2-aminoethyoxyl)ethyl]piperazine hydrochloride Novel preparation method of 1-[2-(2-aminoethyoxyl)ethyl]piperazine hydrochloride](https://images-eureka.patsnap.com/patent_img/7fbc1bed-863d-4554-9f42-81ddd6249b94/BSA0000149579390000011.png)
![Novel preparation method of 1-[((2-hydroxyethyoxyl)ethyoxyl)ethyl]piperazine hydrochloride Novel preparation method of 1-[((2-hydroxyethyoxyl)ethyoxyl)ethyl]piperazine hydrochloride](https://images-eureka.patsnap.com/patent_img/8746ac85-5d5e-43b3-a2b7-3b57301f6dca/BSA0000149579510000011.png)
![1-[2-(2,4-dimethylphenylsulfydryl)phenyl]piperazine hydrochloride and preparation method thereof 1-[2-(2,4-dimethylphenylsulfydryl)phenyl]piperazine hydrochloride and preparation method thereof](https://images-eureka.patsnap.com/patent_img/a8295fae-7d7e-4362-a4ce-842e7c4b6243/FDA0001126951440000011.png)
![1-[2-(2,4-dimethylphenylsulfydryl)phenyl]piperazine hydrochloride and preparation method thereof 1-[2-(2,4-dimethylphenylsulfydryl)phenyl]piperazine hydrochloride and preparation method thereof](https://images-eureka.patsnap.com/patent_img/a8295fae-7d7e-4362-a4ce-842e7c4b6243/BDA0001126951450000011.png)
![1-[2-(2,4-dimethylphenylsulfydryl)phenyl]piperazine hydrochloride and preparation method thereof 1-[2-(2,4-dimethylphenylsulfydryl)phenyl]piperazine hydrochloride and preparation method thereof](https://images-eureka.patsnap.com/patent_img/a8295fae-7d7e-4362-a4ce-842e7c4b6243/BDA0001126951450000021.png)