New preparation technique of urapidil hydrochloride
A technology of urapidil hydrochloride and preparation process, applied in the field of pharmaceutical synthesis, can solve problems such as water environment pollution, and achieve the effects of easy post-processing, less side reactions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 The new preparation technology of urapidil hydrochloride of the present invention
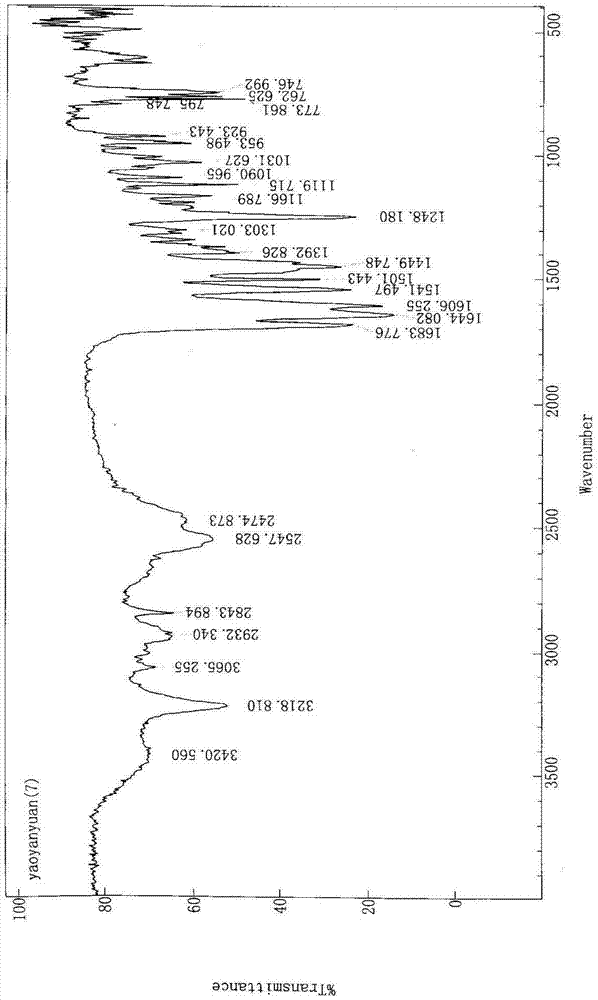
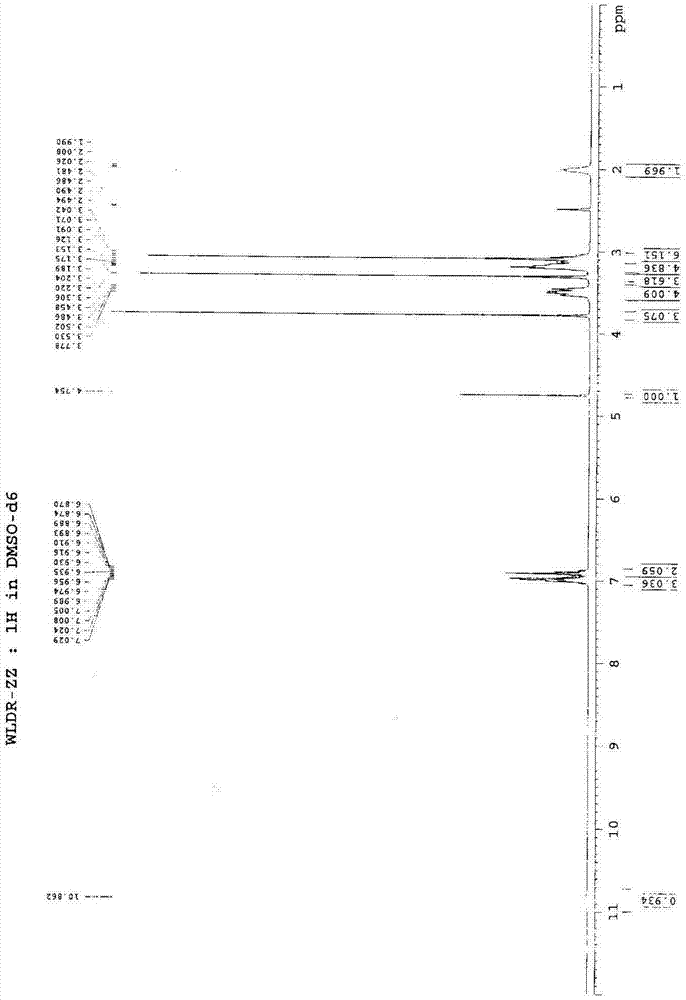
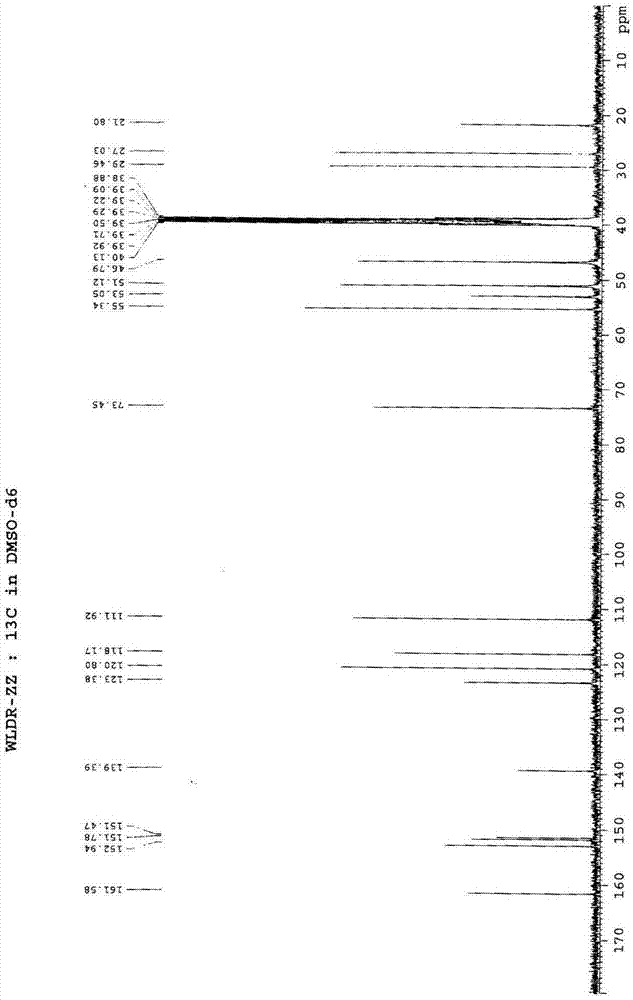
[0037] A. Under nitrogen protection, in a 50L reactor, add 1,3-bis(2,6-diisopropylphenyl)imidazolium chloride (8.50g, 0.02mol), Pd(OAc) in sequence 2 (2.25g, 0.01mol), degassing solvent dioxane (20L) and potassium carbonate (691.1g, 5.0mol) stirred at room temperature and reacted for 0.5h (stirring speed 120r / min), after adding 1-(2-formazan Oxyphenyl)piperazine hydrochloride (571.8g, 2.5mol), 6-(3-chloropropyl)-1,3-dimethyluracil (634.4g, 2.75mol), heated for 6h (stirring speed 120r / min, heating temperature 80°C), after cooling to room temperature, a solid precipitated, filtered under reduced pressure (vacuum degree -0.08MPa~0.1MPa) to obtain the crude product, the crude product was dissolved in 20L of hot ethanol (temperature 70°C) Afterwards, filter while it is hot, and the filtrate stands overnight for 12 hours to obtain white crystals. The filter cake is filtered under...
Embodiment 2
[0043] Embodiment 2 The new preparation technology of urapidil hydrochloride of the present invention
[0044] The difference between this embodiment and embodiment 1 is that the A step uses PdCl 2 (1.77g, 0.01mol) instead of Pd(OAc) 2 (2.25g, 0.01mol), degassing solvent tetrahydrofuran (20L) instead of degassing solvent dioxane (20L), to obtain urapidil 783.6g, yield 81.1%, melting range 157-158 ℃; B step with methanol / isopropanol mixed solvent (20L, V 甲醇 :V 异丙醇 =1:1) instead of methanol / ethanol mixed solvent (20L, V 甲醇 :V 乙醇 =1:1); The present embodiment obtains 304.5 g of urapidil hydrochloride, and the crystallization yield is 72.0%. The total yield is 58.4%, the content of related substances is 0.0041%, and the spectral data are the same as in Example 1.
Embodiment 3
[0045] Embodiment 3 The new preparation technology of urapidil hydrochloride of the present invention
[0046] The difference between this embodiment and embodiment 1 is that the A step uses PdCl 2 (1.77g, 0.01mol) instead of Pd(OAc) 2 (2.25g, 0.01mol), with sodium carbonate (530.0g, .5.0mol) instead of potassium carbonate (691.1g, 5.0mol), degassed tetrahydrofuran (20L) instead of degassed dioxane (20L), to uradi Mole 795.2g, yield 82.3%, melting range 157-158 ℃; B step uses methanol / acetone mixed solvent (20L, V 甲醇 :V 丙酮 =1:1) instead of methanol / ethanol mixed solvent (20L, V 甲醇 :V 乙醇 =1:1); The present embodiment obtains 313.4 g of urapidil hydrochloride, and the crystallization yield is 74.1%. The total yield is 61.0%, the content of related substances is 0.0040%, and the spectral data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com