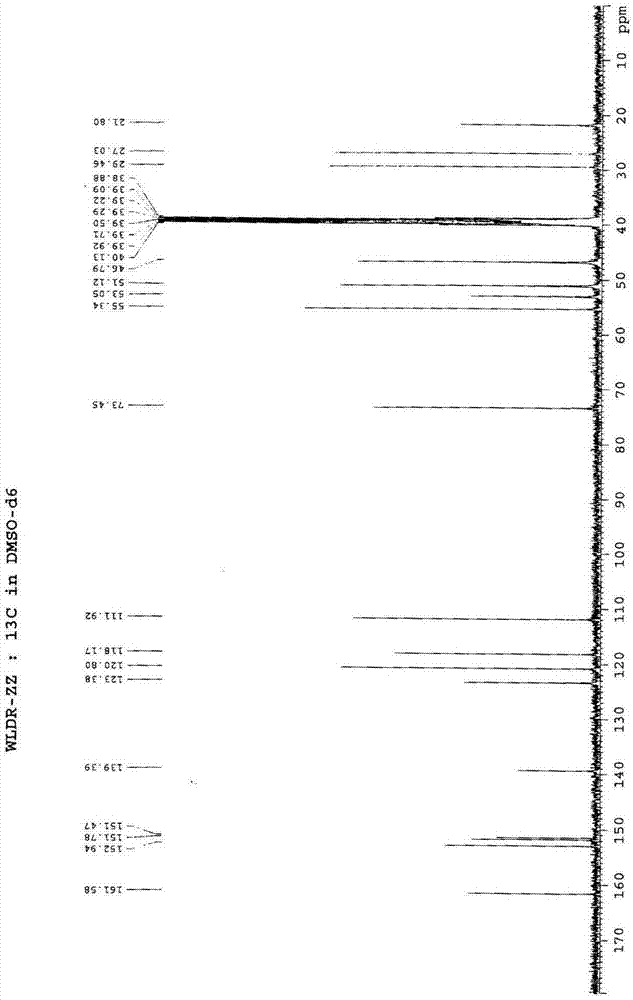
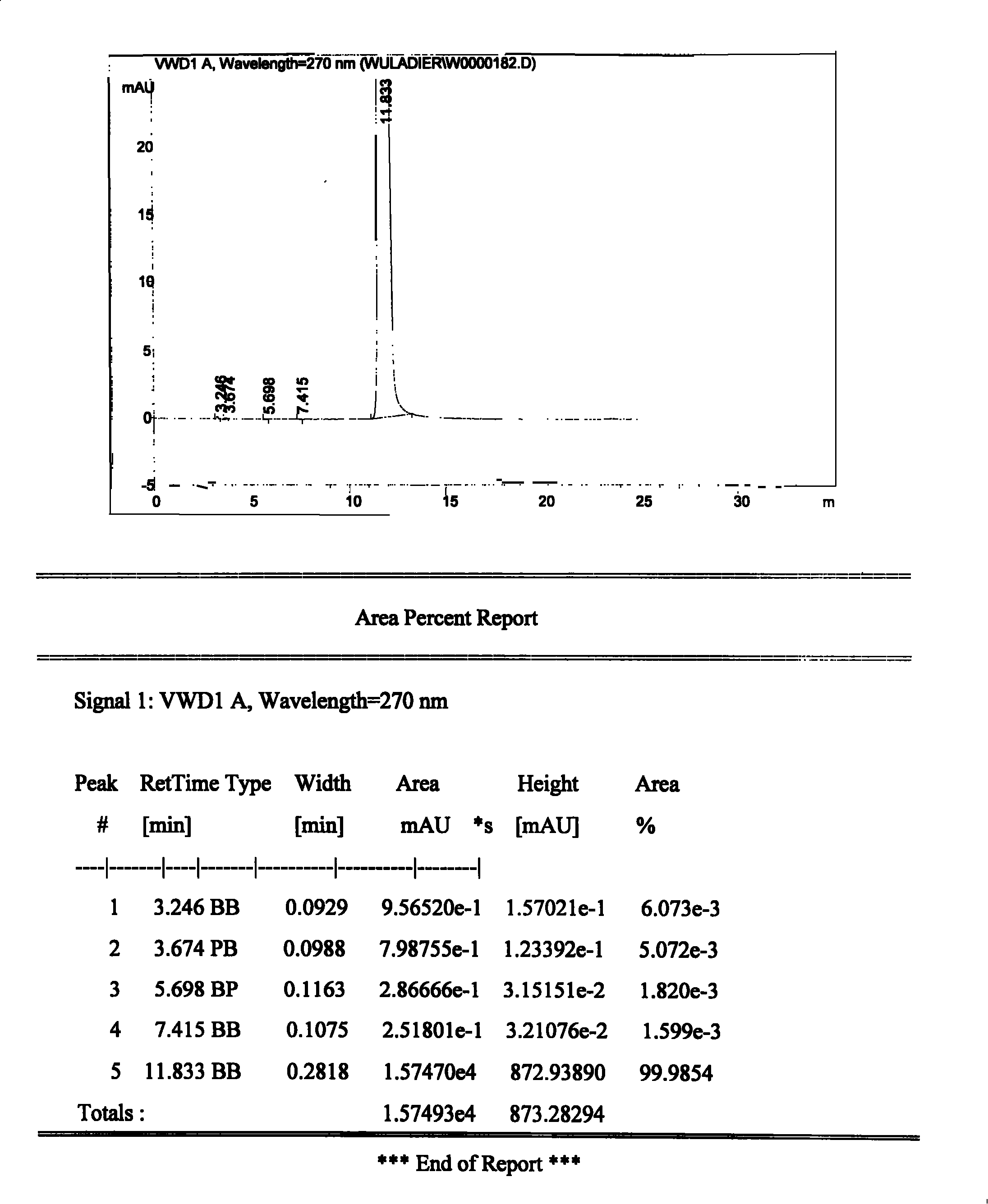
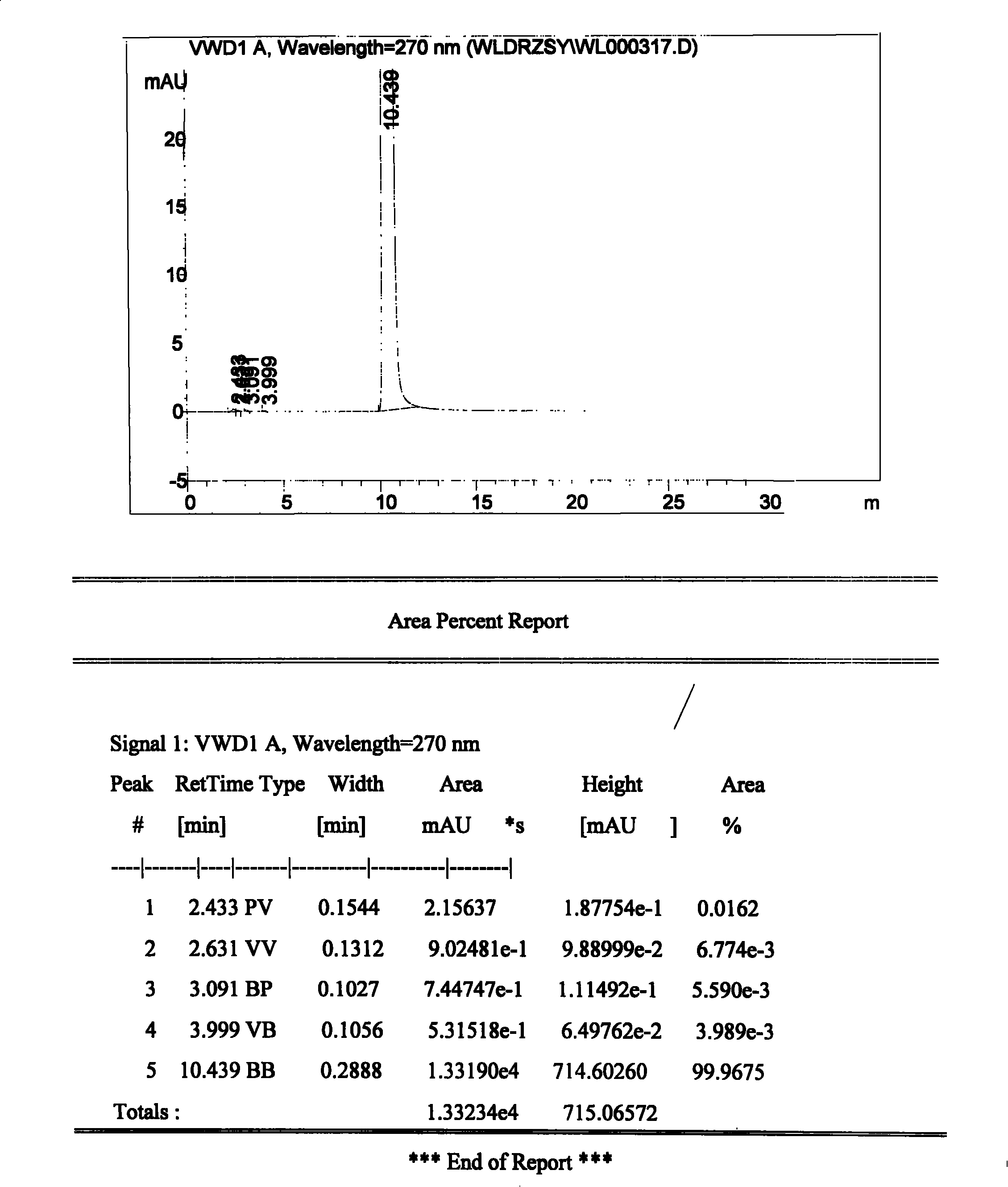
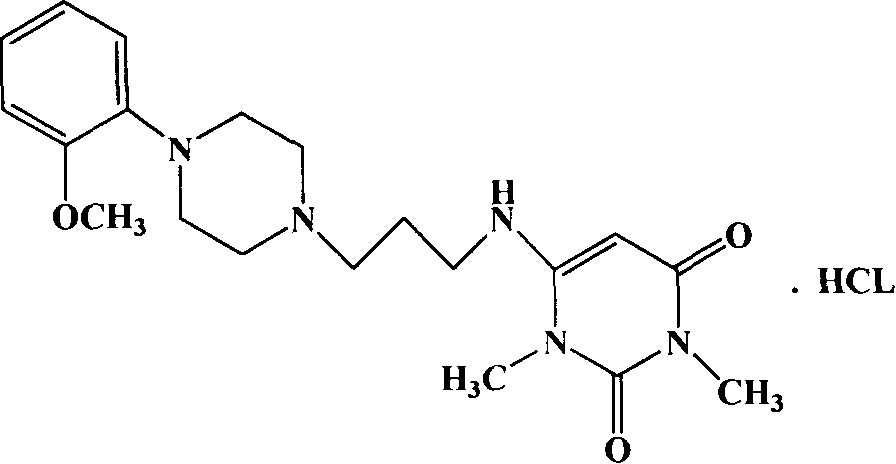
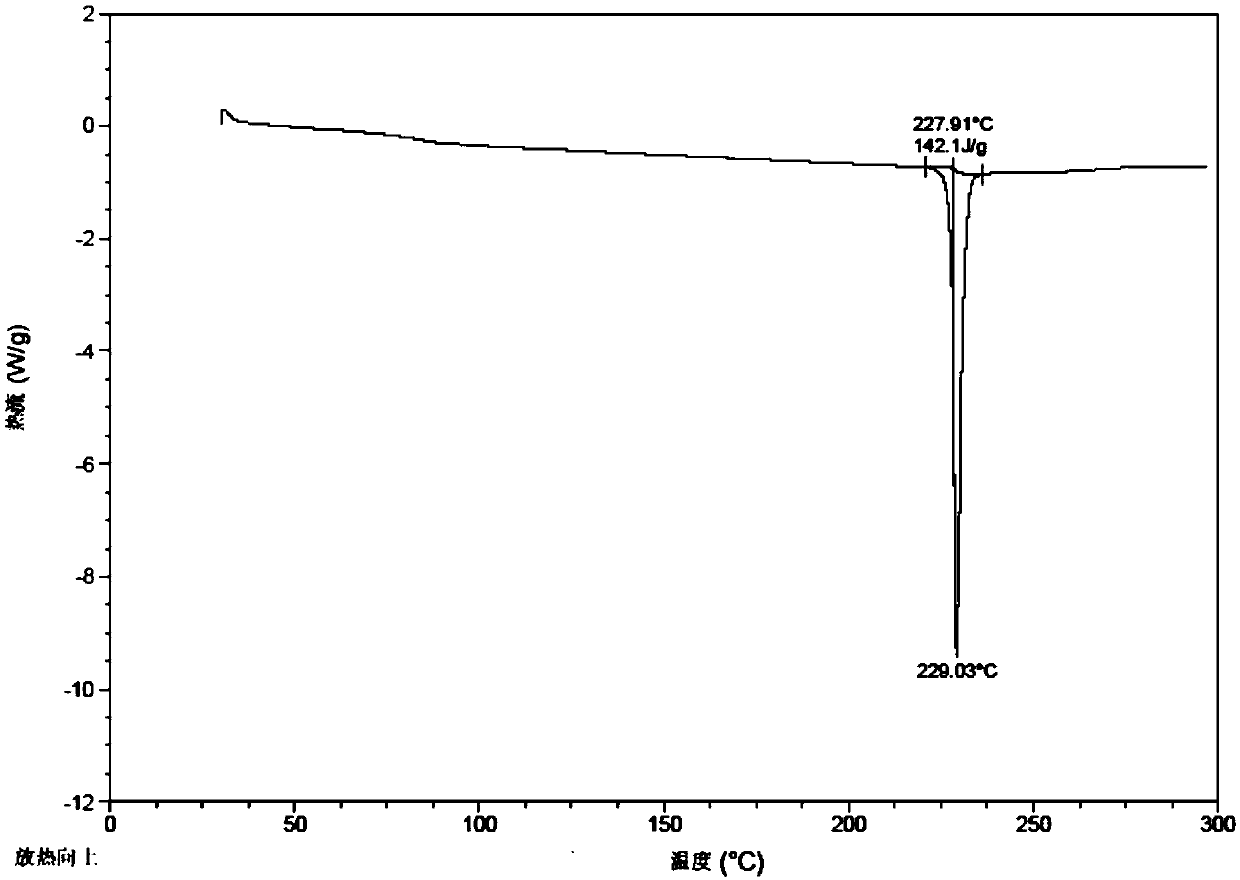
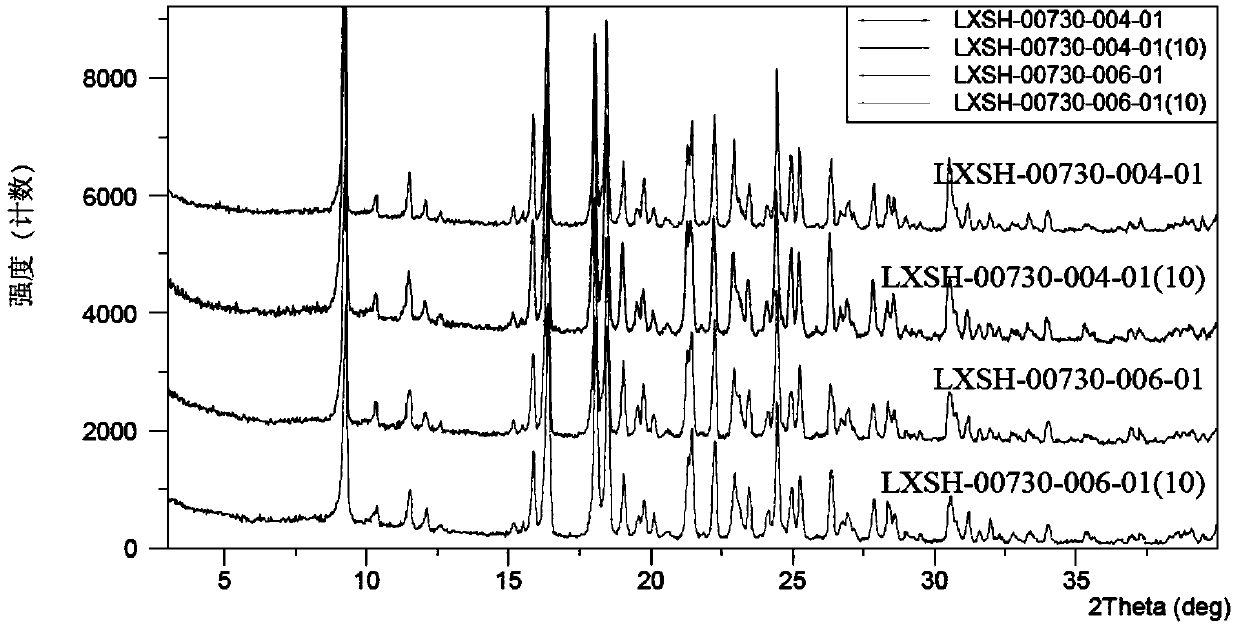
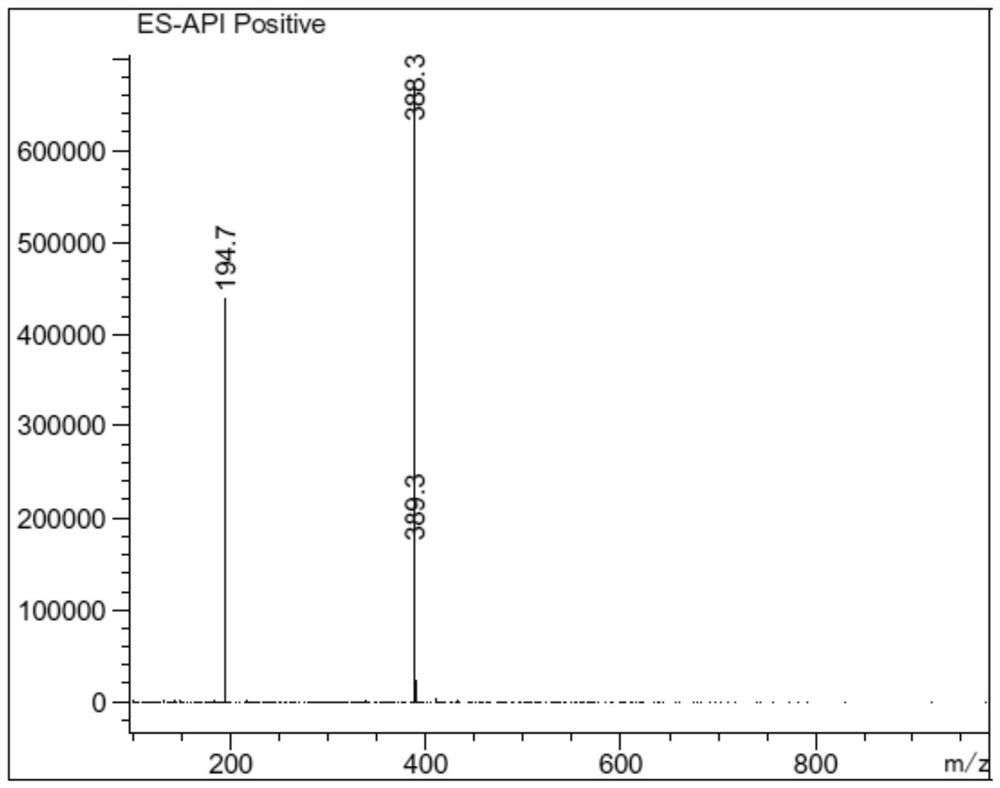
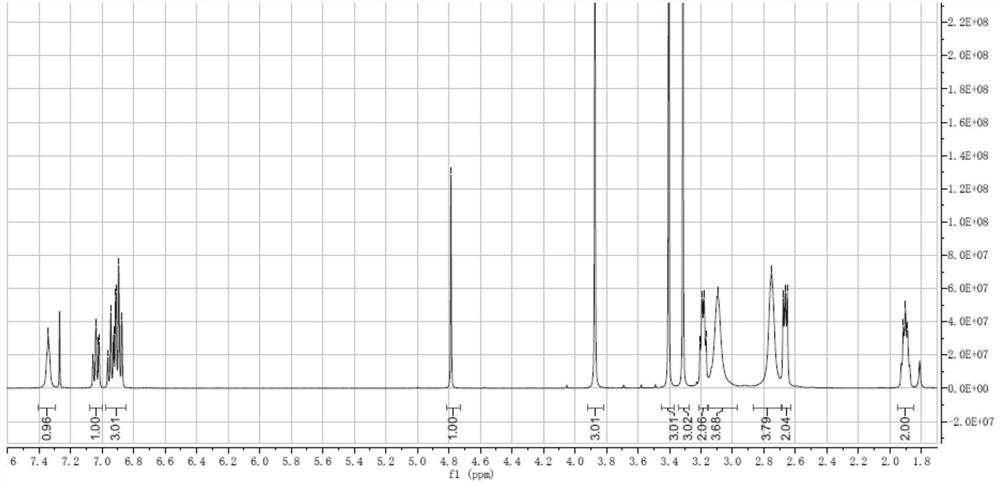
Patents
Literature
33 results about "Urapidil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
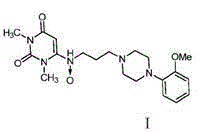
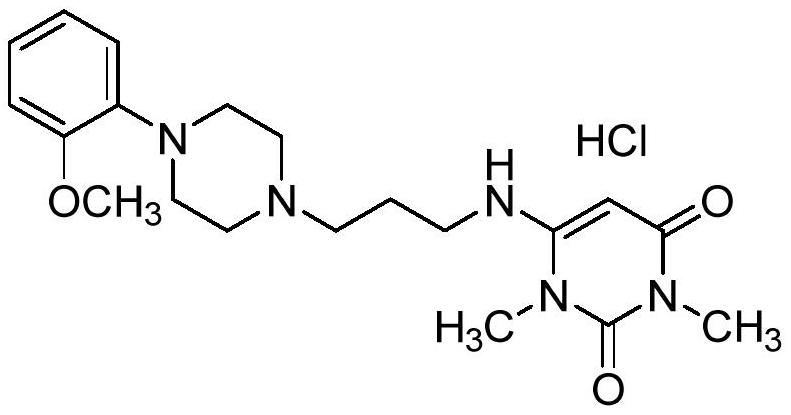
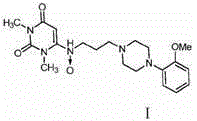
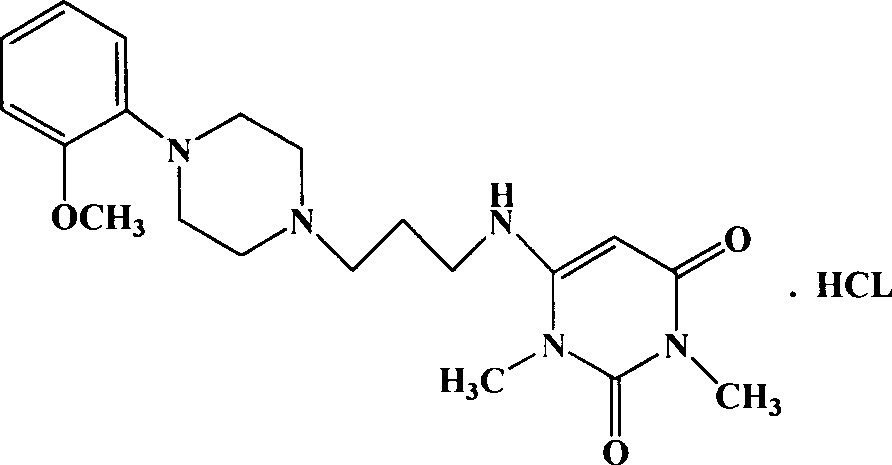
Urapidil is a sympatholytic antihypertensive drug. It acts as an α₁-adrenoceptor antagonist and as an 5-HT1A receptor agonist. Although an initial report suggested that urapidil was also an α₂-adrenoceptor agonist, this was not substantiated in later studies that demonstrated it was devoid of agonist actions in the dog saphenous vein and the guinea-pig ileum. Unlike some other α₁-adrenoceptor antagonists, urapidil does not elicit reflex tachycardia, and this may be related to its weak β₁-adrenoceptor antagonist activity, as well as its effect on cardiac vagal drive. Urapidil is currently not approved by the U.S. Food and Drug Administration, but it is available in Europe.
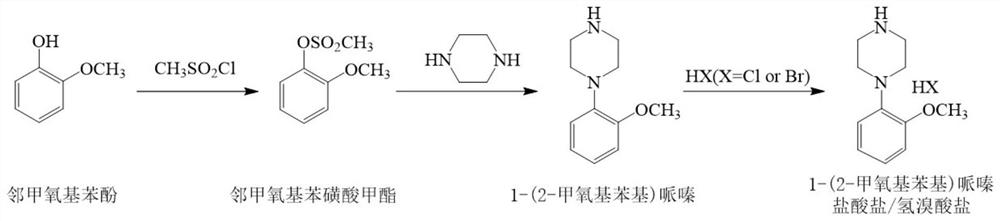
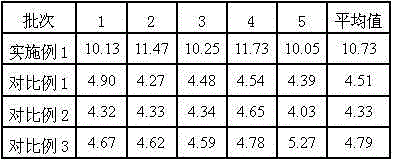
Method for purifying urapidil with counter solvent recrystallization method
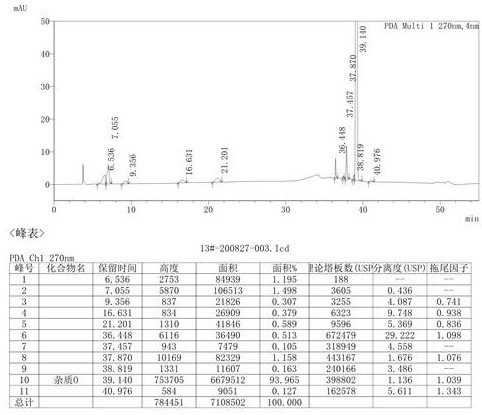
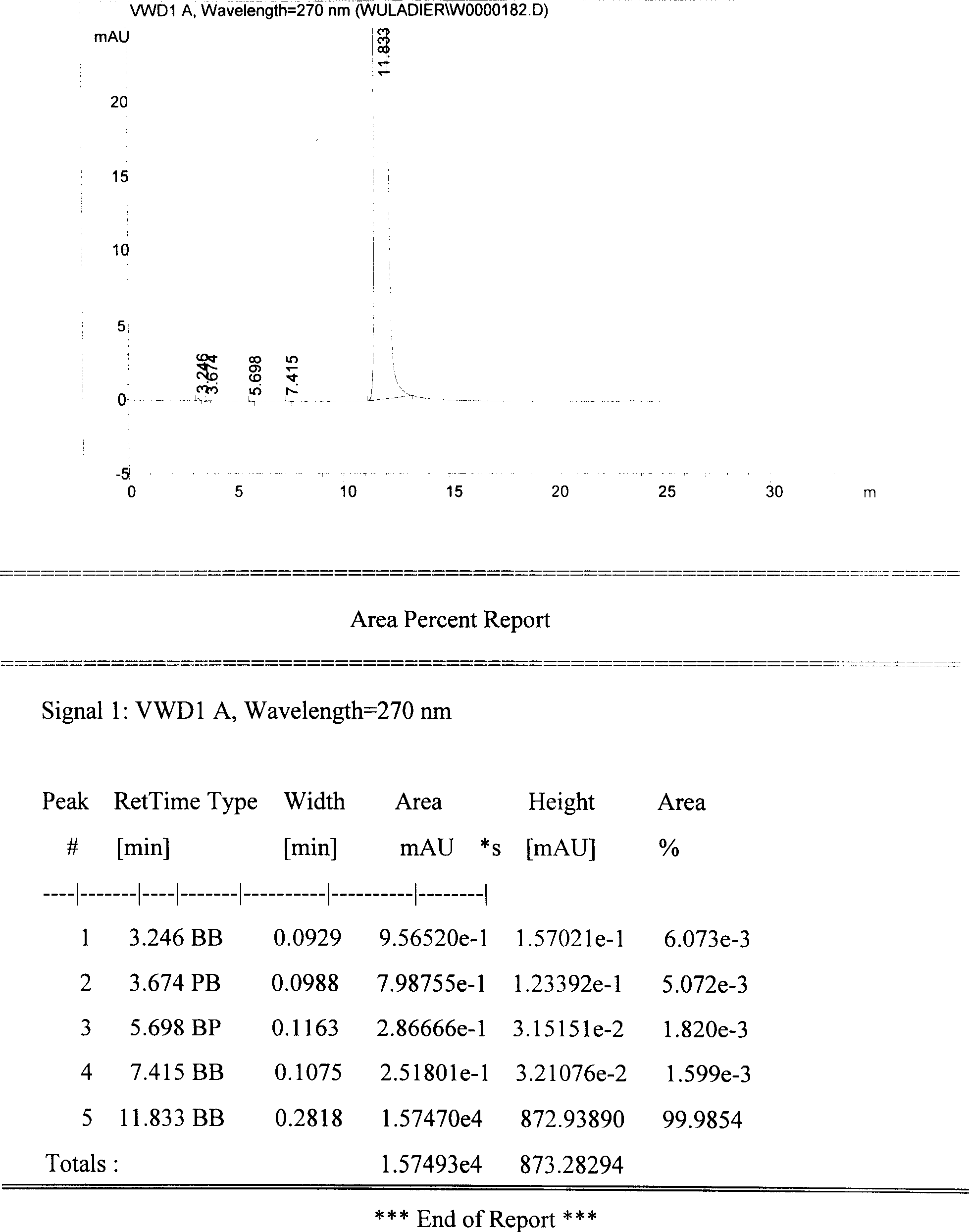
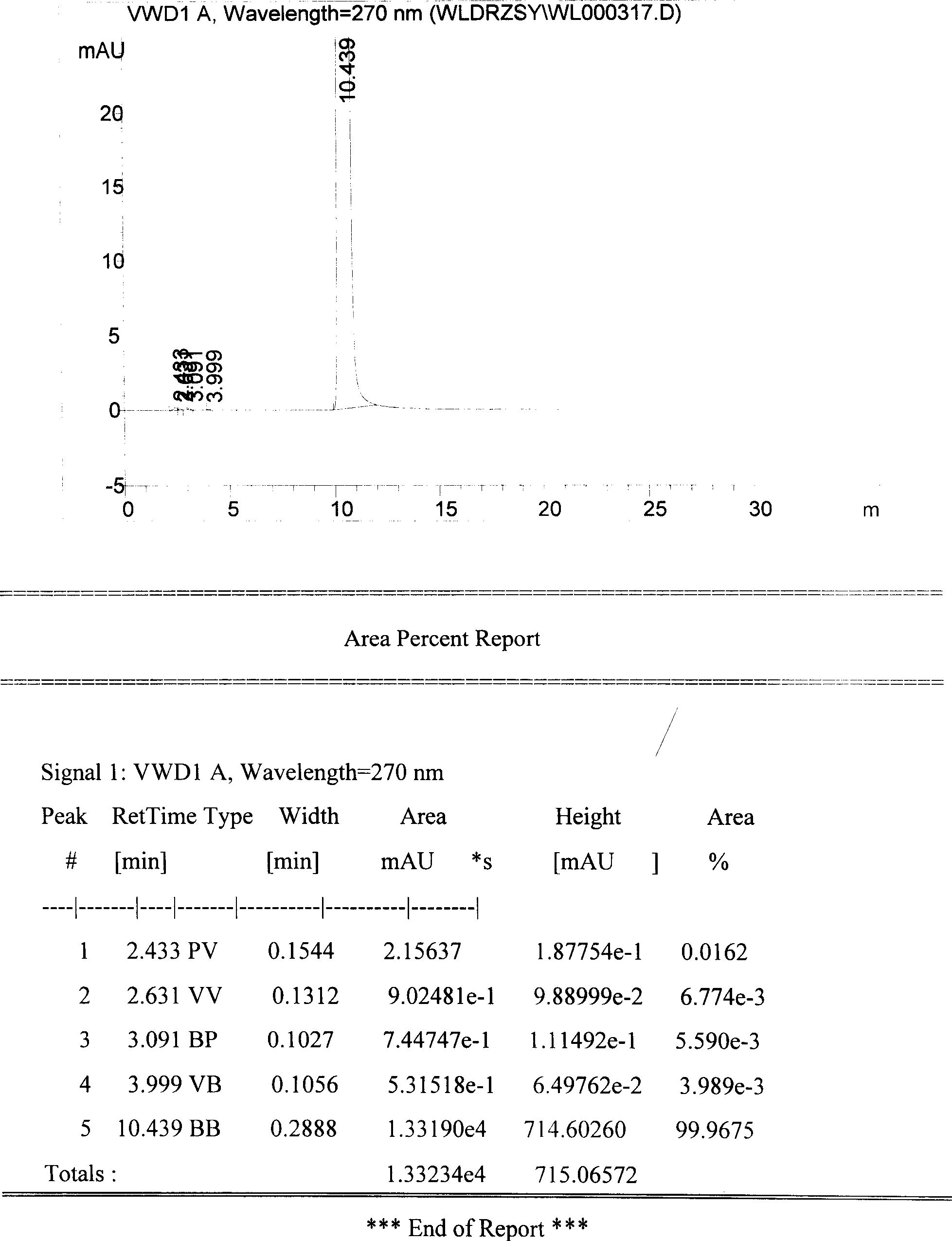
The invention discloses a method for purifying urapidil, in particular to a method for purifying urapidil with a counter solvent recrystallization method, belonging to the field of purification of compounds. The method is implemented by the following steps of: adding urapidil of which the purity is over 96 percent into an organic solvent; heating and dissolving; performing suction filtration; pouring the filtrate into a counter solvent; standing; performing suction filtration under reduced pressure; and performing vacuum drying to obtain the urapidil. The method has the advantages of simpleness in operation, short time needed for precipitating a product, higher yield of the obtained urapidil than that of the prior art, and purity of over 99.82 percent (HPLC (High Performance Liquid Chromatography)); and the method is extremely suitable for industrial production.
Owner:HENAN FUREN MEDICAL TECH DEV +1
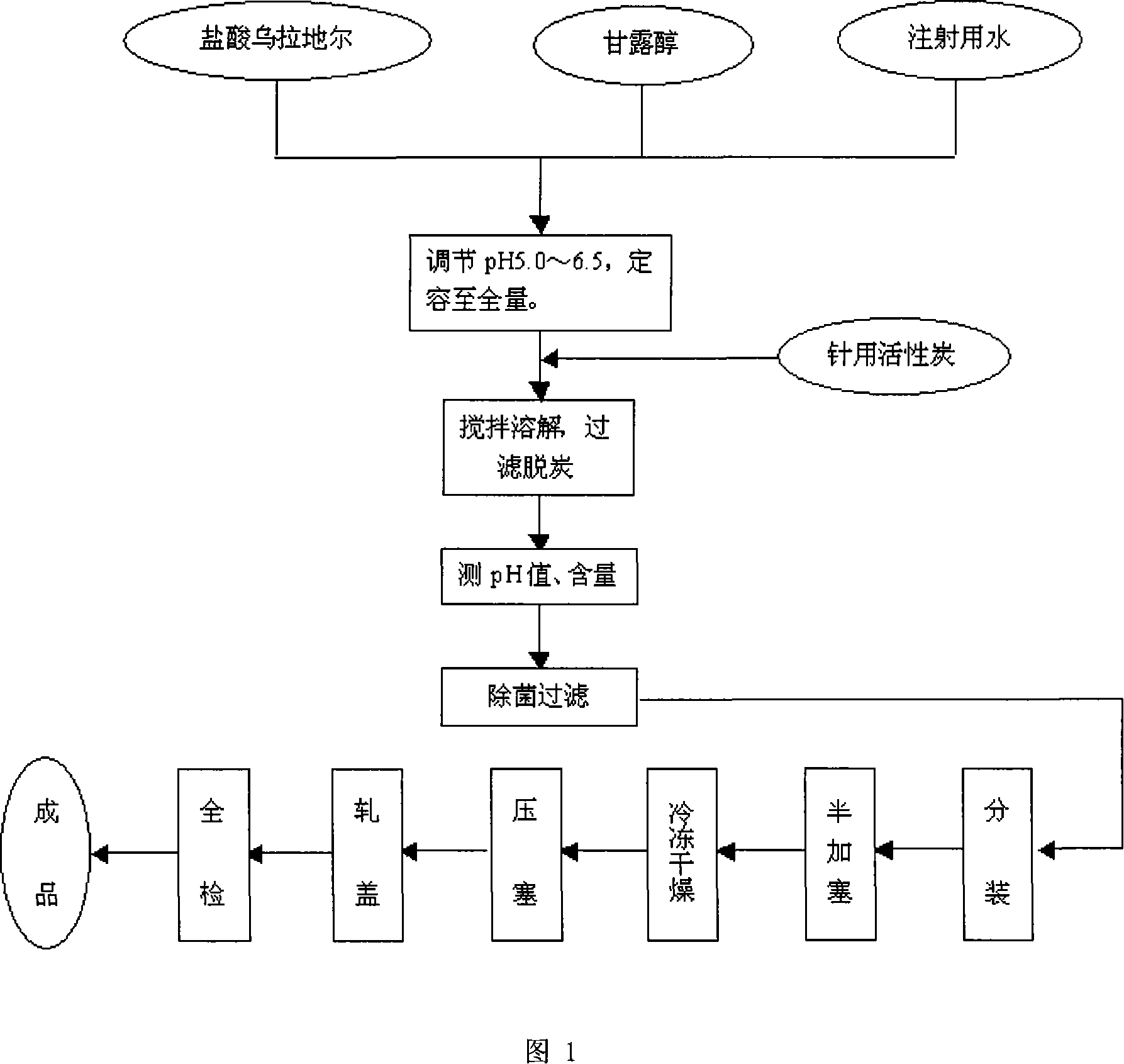
Urapidil large volume injection, its preparation method and application
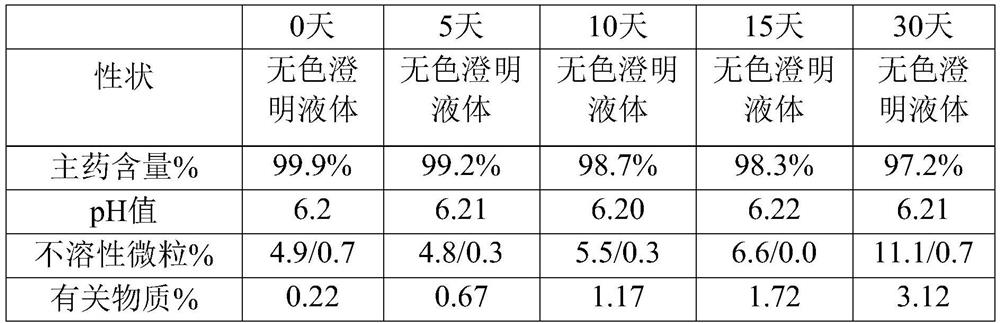
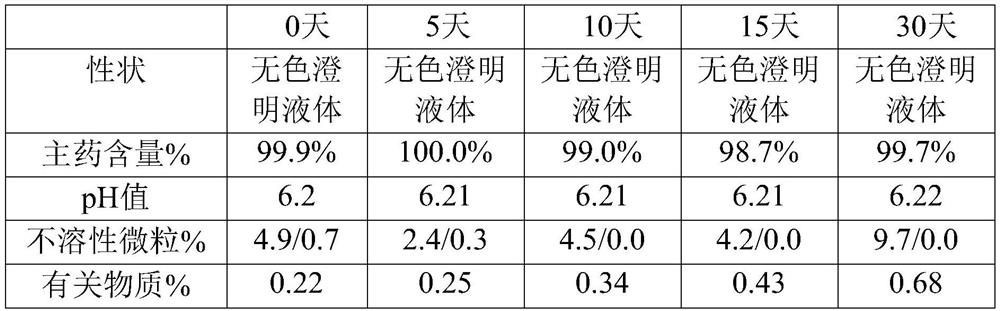
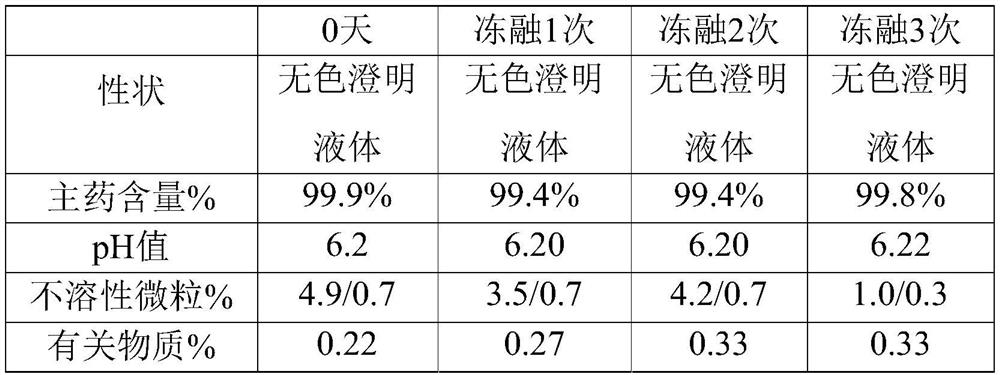
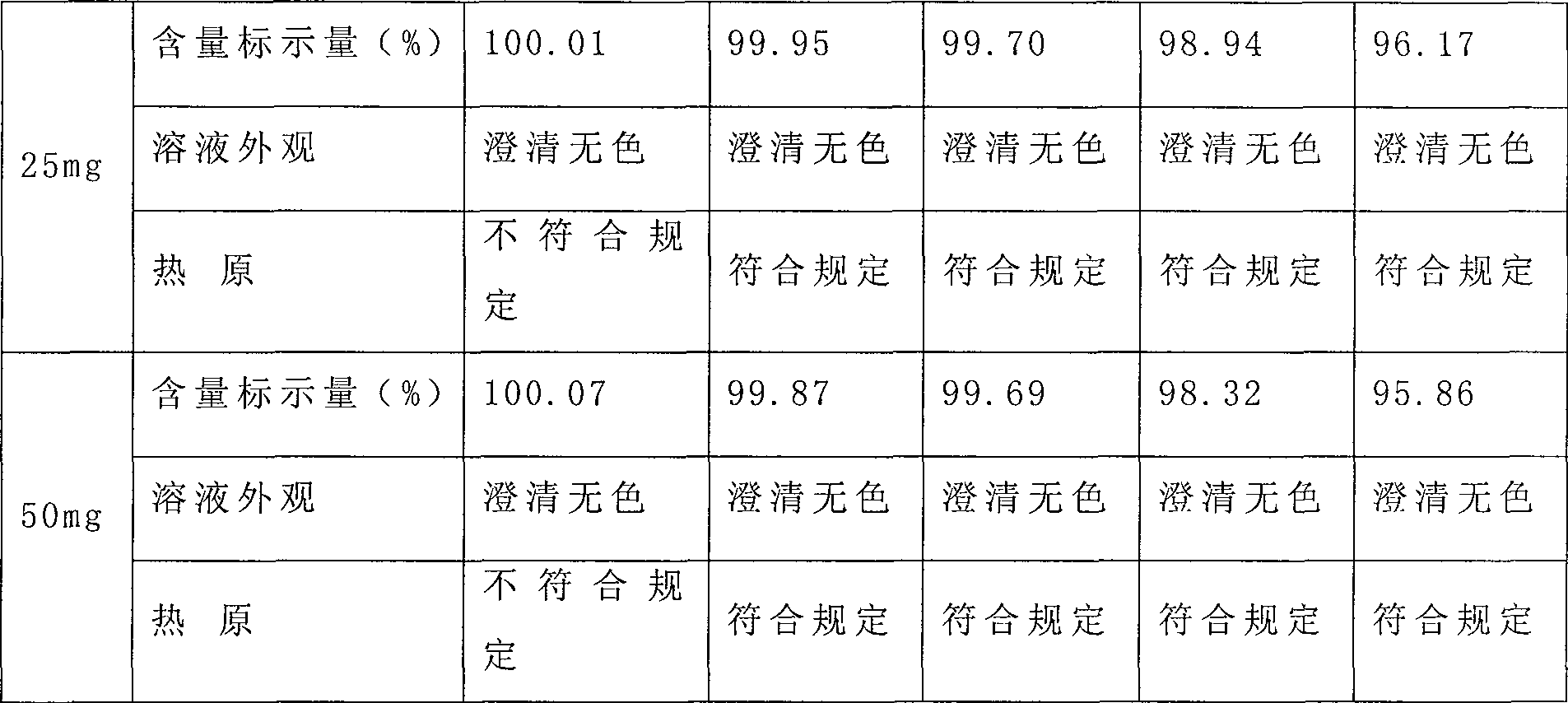
ActiveCN1593419AOrganic active ingredientsPharmaceutical delivery mechanismMANNITOL/SORBITOLCurative effect
The invention provides a Urapidil large volume injection, its preparation method and use in making medicament for treating hypertension, wherein the preparation comprises Urapidil, isotonic conditioning agent, pH regulator and water for injection, wherein the isotonic conditioning agent is selected from sodium chloride, glucose, dextran, mannitol or sorbierite, the pH regulator is selected from hydrochloric acid and sodium hydroxide.
Owner:杨立新
Urapidil powder and injection preparation and preparing method
Owner:GUANGDONG QIFANG MEDICINES CO LTD
Method for purifying urapidil by anti-solvent recrystallization method
The invention discloses a method for purifying urapidil, in particular to a method for purifying urapidil with a counter solvent recrystallization method, belonging to the field of purification of compounds. The method is implemented by the following steps of: adding urapidil of which the purity is over 96 percent into an organic solvent; heating and dissolving; performing suction filtration; pouring the filtrate into a counter solvent; standing; performing suction filtration under reduced pressure; and performing vacuum drying to obtain the urapidil. The method has the advantages of simpleness in operation, short time needed for precipitating a product, higher yield of the obtained urapidil than that of the prior art, and purity of over 99.82 percent (HPLC (High Performance Liquid Chromatography)); and the method is extremely suitable for industrial production.
Owner:HENAN FUREN MEDICAL TECH DEV +1
New preparation technique of urapidil hydrochloride
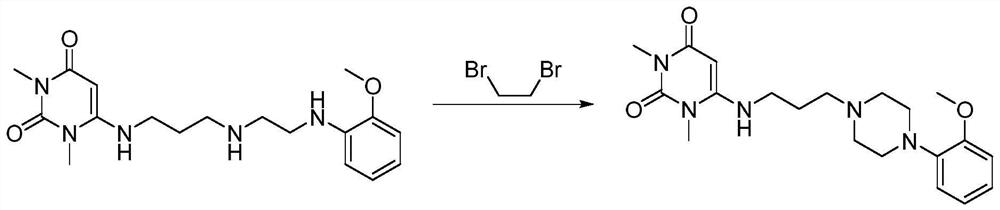
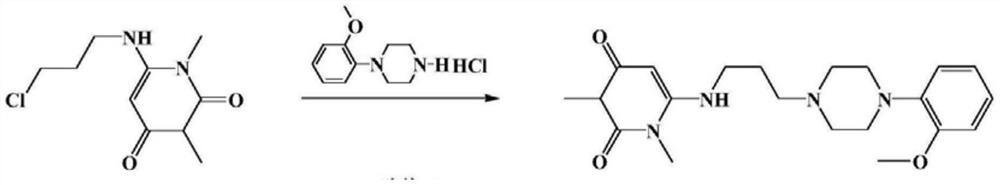
The invention relates to a new preparation technique of urapidil hydrochloride, belonging to the technical field of drug synthesis. According to the new preparation technique, a Pd / NHC catalytic system is directly utilized to catalyze 6-(3-chloropropyl)-1,3-dimethyluracil and 1-(2-methoxyphenyl)piperazino hydrochloride to prepare urapidil, and the urapidil is further acidified to prepare the urapidil hydrochloride. The new preparation technique of urapidil hydrochloride has the advantages of simple structure of raw materials, no need of abundant phase-transfer catalysts, fewer side reactions, easy after-treatment, favorable product selectivity and higher yield, and is suitable for industrial production; and the related substance content is less than 0.0047%, and the total yield is greater than 58%.
Owner:HEBEI YIPIN PHARMA
Method for preparing hydrochloride urapidil
The invention relates to a preparation method of the urapidil hydrochloride. The method comprises the following procedures, the urapidil is dissolved in the alcohols or / and the ketones solvent, the organic solution of the hydrogen chloride is slowly added after the heating dissolution for controlling pH at the range of 2.0 to 4.0, then the mixture is crystallized by decreasing the temperature into the range of 20 to 70 DEG C, and the urapidil hydrochloride is filtrated and dried by the decompression drying. The content is not lower than 99.5 percent, the clarity and the pH meet the purity requirement of the raw material used in the injection. The invention has the advantages of simple method, stable quality, easy operation and being suitable for the industrial production.
Owner:YAOPHARMA CO LTD
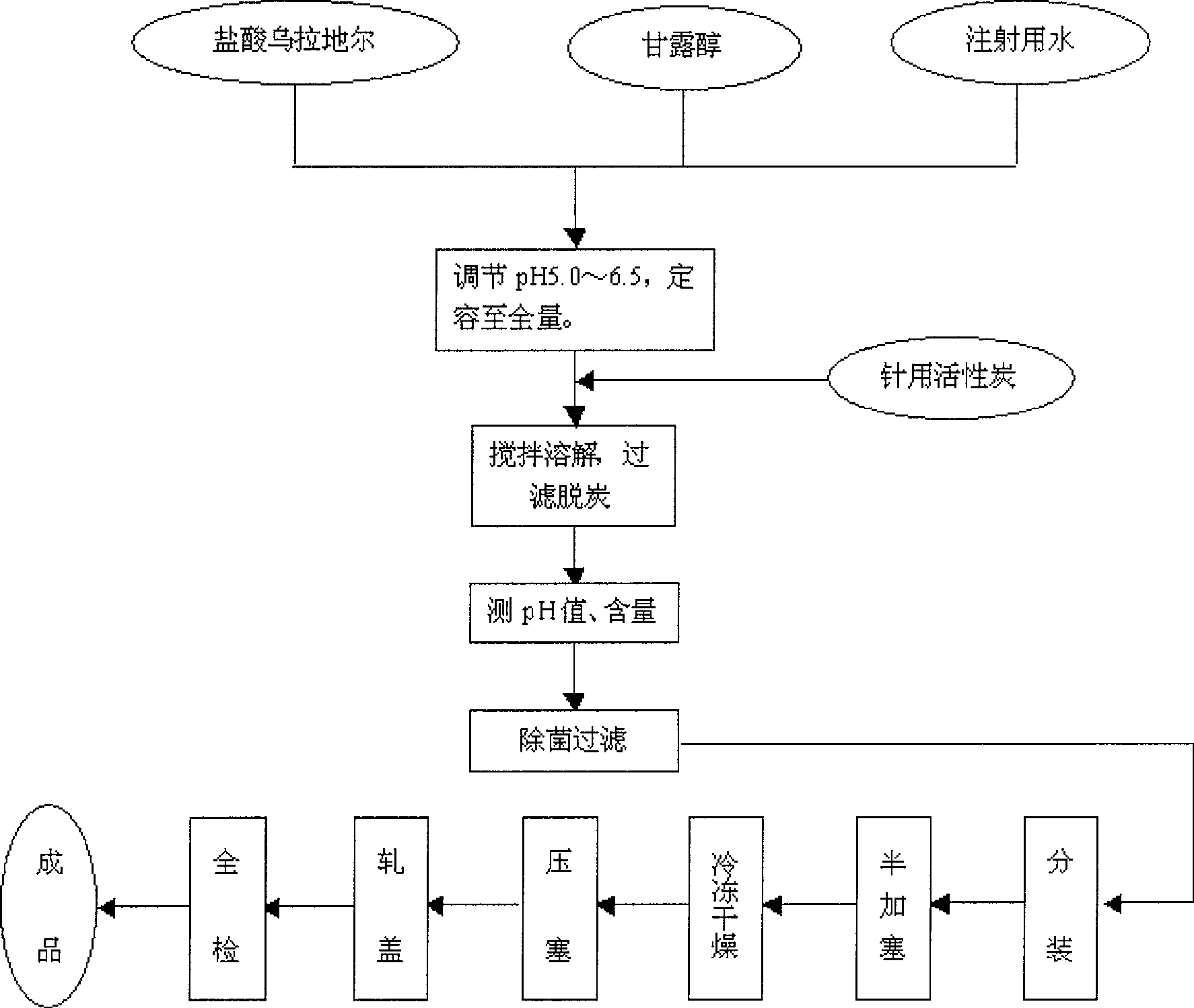
Urapidil hydrochloride freeze-dried powder for injection and preparing method thereof
ActiveCN101229134AEliminate first pass effectEasy to storePowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention relates to an urapidil hydrochloride freeze-dried powder used for injection and a preparation method. The freeze-dried powder consists of the urapidil hydrochloride and excipents in proportion. The preparation is realized according to the following steps: dissolving the main materials and excipents in water, decolorizing activated carbon, being frozen to minus 60 to minus 30 DEG C and for 3 to 8 hours, vacuum pumping, slow heating to 0 to 20 DEG C, the heating time is from 10 hours to 20 hours, keeping the heating temperature for 2 to 6 hours, rising temperature to 20 to 50 DEG C, the temperature rising time is 2 to 6 hours, heating insulation for 0.5 to 4 hours, being packaged and put in the stock room after tamponade, capping and qualified and complete test. The method utilizes gradient cooling processes and the prepared products are full in exterior, good in solubility, easy to transport and good in stability.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
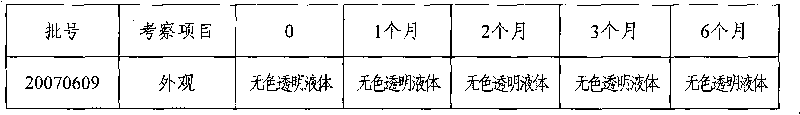
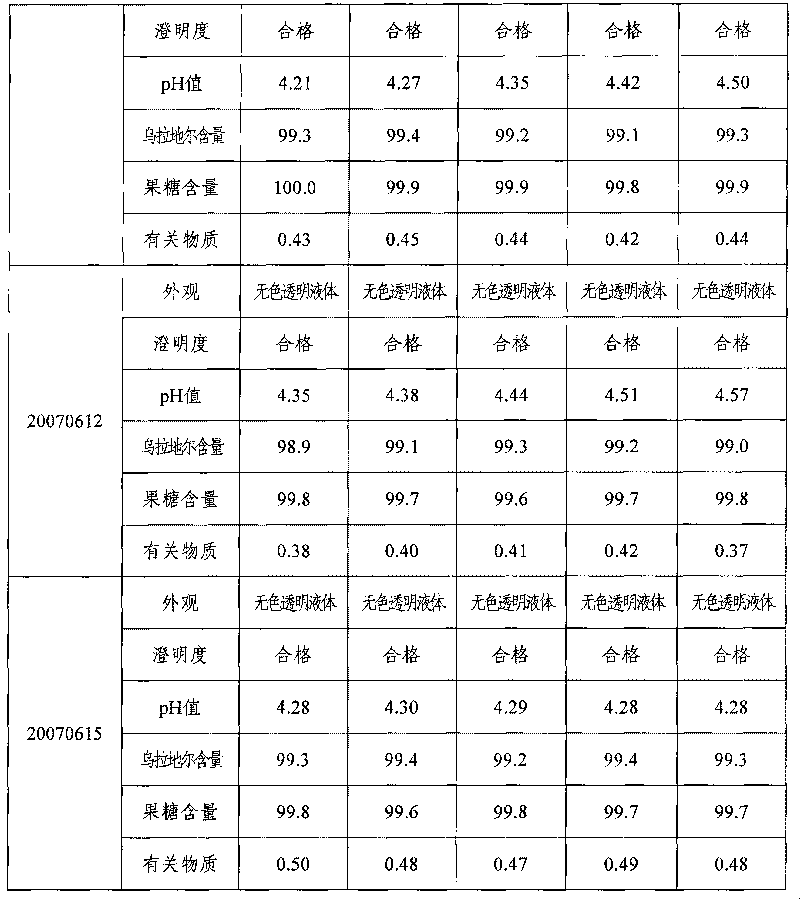
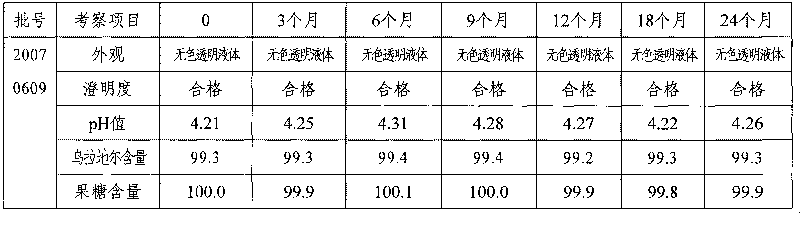
Urapidil fructose injection and preparation method thereof
The invention relates to an urapidil fructose injection and a preparation method thereof. The injection consists of urapidil, fructose, water for injection and an acidity and alkalinity regulator, wherein the mass and volume concentration of urapidil is 0.01-0.05%, the mass and volume concentration of fructose is 5-10% and the pH value is 3.1-6.1. Compared with the traditional urapidil injection, the invention has the advantages that the amount of insulin in vivo of a patient is not needed to consider when the urapidil injection is applied, wherein the urapidil injection is particularly suitable for the hypertension patient suffering from diabetes and the hypertension patient in a perioperative period; infection and pollution in the intermediate link in the use process of the traditional product can be avoided in the process of applying the urapidil injection, thereby the urapidil injection is safer, faster and more convenient. The preparation method of the urapidil fructose injection has simple operation and is suitable for industrial production in large scale.
Owner:BENGBU BBCA MEDICINE SCI DEV
Urapidil hydrochloride injection and preparation method thereof
InactiveCN113876705AImprove stabilityPromote high-volume industrial productionOrganic active ingredientsInorganic non-active ingredientsSodium acetateO-Phosphoric Acid
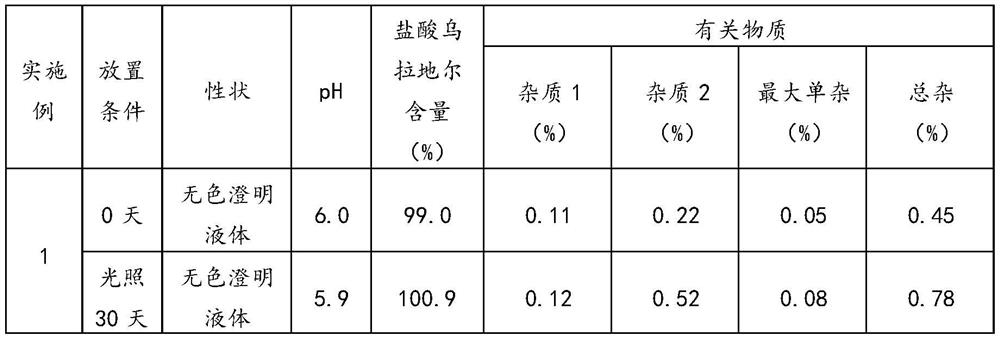
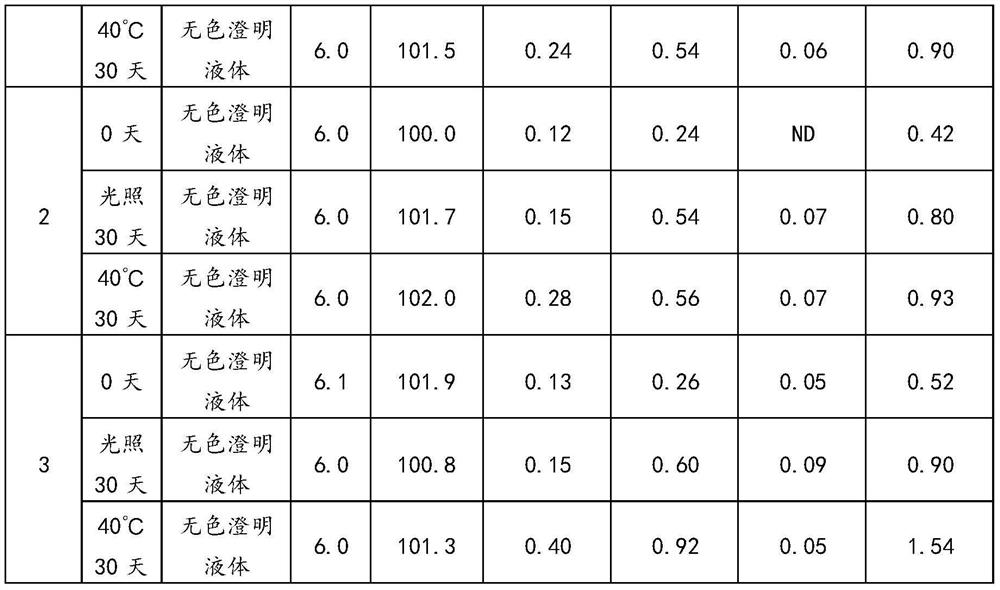
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses an urapidil hydrochloride injection and a preparation method thereof. In the urapidil hydrochloride injection, every 1000 mL of the urapidil hydrochloride injection comprises the following raw materials: 5 g of urapidil hydrochloride, 100 g of an osmotic pressure regulator and an acid-base buffer pair, and the pH value of the urapidil hydrochloride injection is 5.9-6.5. According to the invention, citric acid-sodium citrate, acetic acid-sodium acetate, citric acid-disodium hydrogen phosphate or phosphoric acid-sodium phosphate are used as acid-base buffer pairs, and the pH value of the injection is adjusted to 5.9-6.5, so that the stability of the injection is remarkably improved. In the preparation process of the injection, the adding and mixing sequence of the main drug urapidil hydrochloride and other auxiliary materials is adjusted, and the temperature in the preparation process is controlled to be lower than 60 DEG C, so that the deterioration probability of urapidil hydrochloride is effectively reduced, and the stability of the injection is remarkably improved.
Owner:SHIJIAZHUANG NO 4 PHARMA
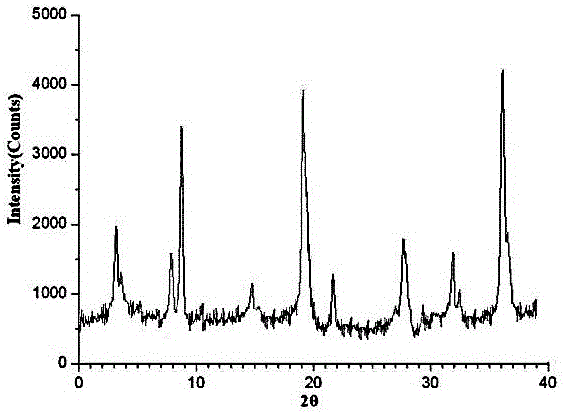
Urapidil hydrochloride crystal form and preparation method thereof
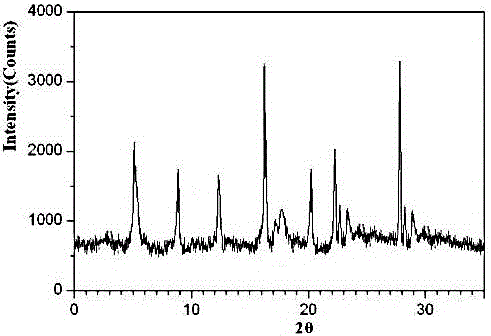
PendingCN111116491AHigh purityHigh clarityOrganic chemistry methodsCardiovascular disorderPowder diffractionHydrochloride
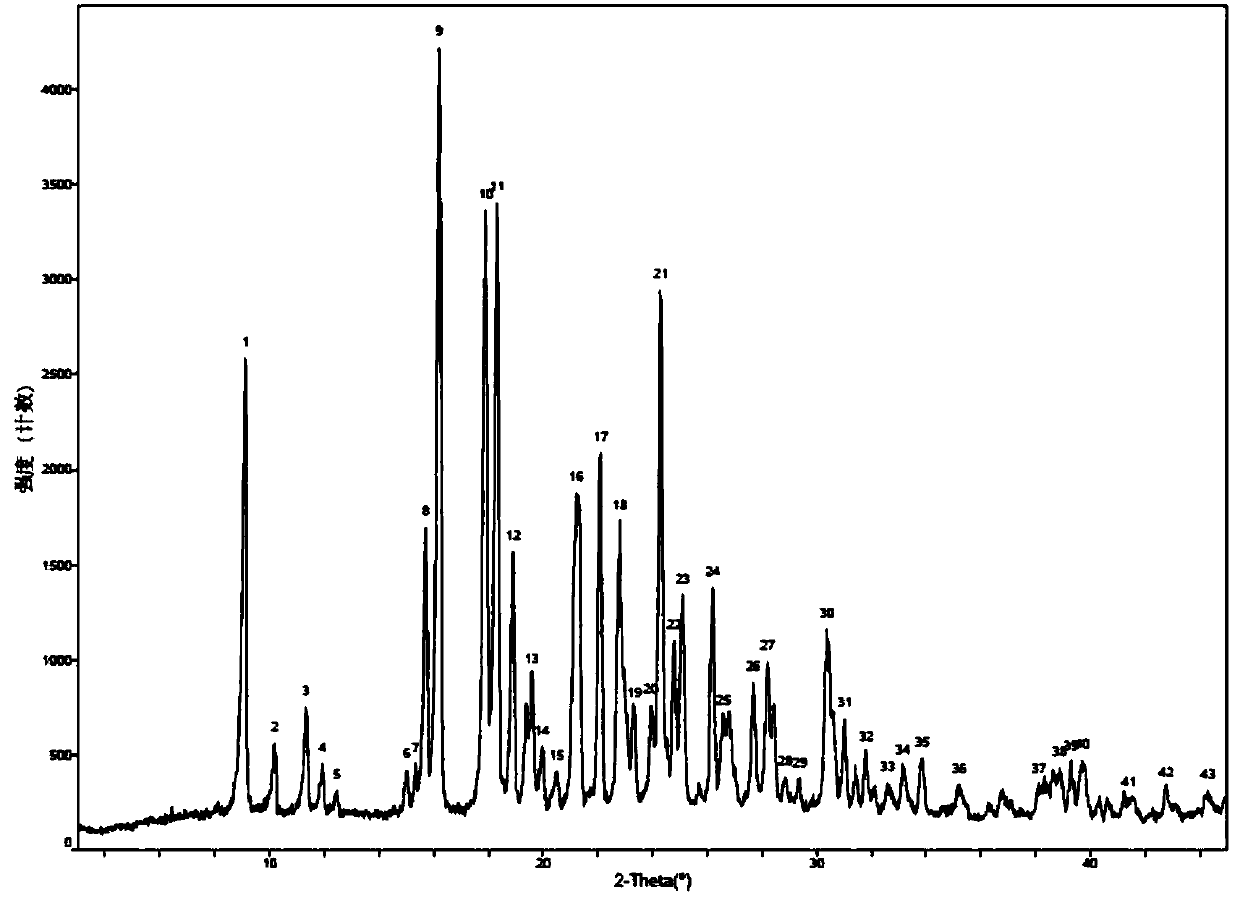
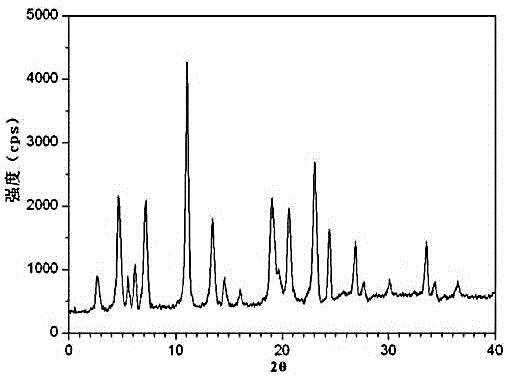
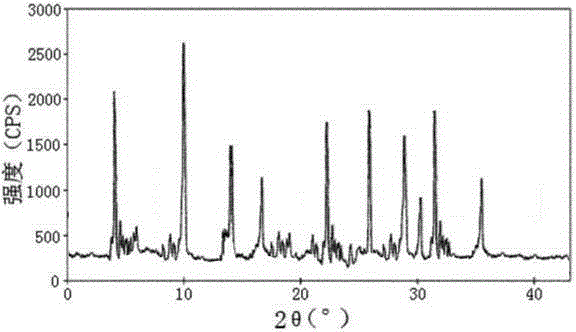
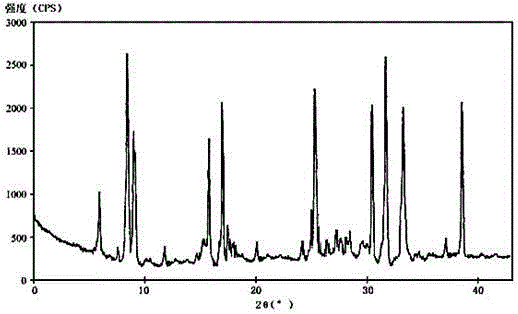
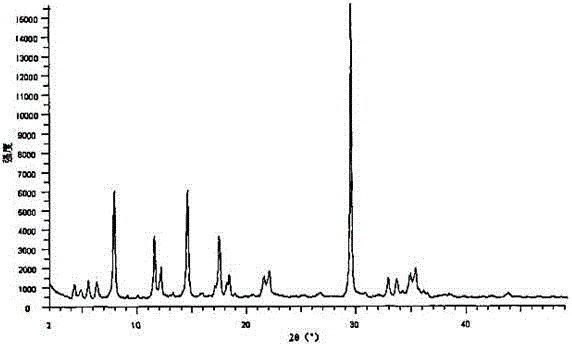
The invention relates to an urapidil hydrochloride crystal form and a preparation method thereof, specifically to an urapidil hydrochloride crystal form, wherein the X-ray powder diffraction pattern measured by using Cu-Kalpha shows that the crystal form has characteristic diffraction peaks at the following 2[theta] angles: 9.115+ / -0.2 DEG, 15.708+ / -0.2 DEG, 16.203+ / -0.2 DEG, 17.899+ / -0.2 DEG, 18.331+ / -0.2 DEG, 18.904+ / -0.2 DEG, 22.100+ / -0.2 DEG, and 24.294+ / -0.2 DEG.
Owner:LUOXIN PHARM SHANGHAI CO LTD +1
Preparation method of urapidil
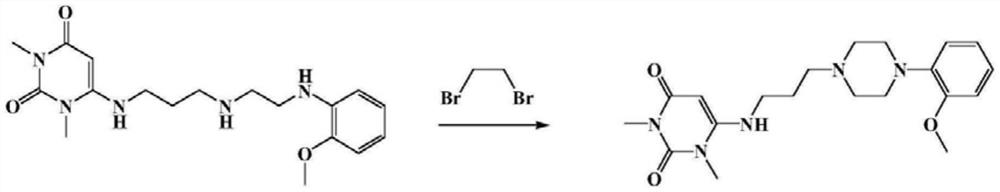
The invention provides a preparation method of urapidil. The preparation method comprises the following steps: mixing 1, 3-dimethyl-6-aminouracil with 3-amino-1-propanol, and carrying out a reaction so as to prepare 6-(3-hydroxypropylamino)-1, 3-dimethyluracil; mixing 6-(3-hydroxypropylamino)-1, 3-dimethyluracil and thionyl chloride to react so as to prepare 6-(3-chloropropylamino)-1, 3-dimethyluracil; and enabling 6-(3-chloropropyl amino)-1, 3-dimethyluracil to react with 1-(2-methoxyphenyl) piperazine hydrochloride so as to obtain the urapidil. The preparation method has the advantages of simple operation, cheap and easily available reagents, few side reactions, high yield, good purity of the obtained product, and facilitation of industrial production.
Owner:苏州中科新药篮生物医药科技有限公司
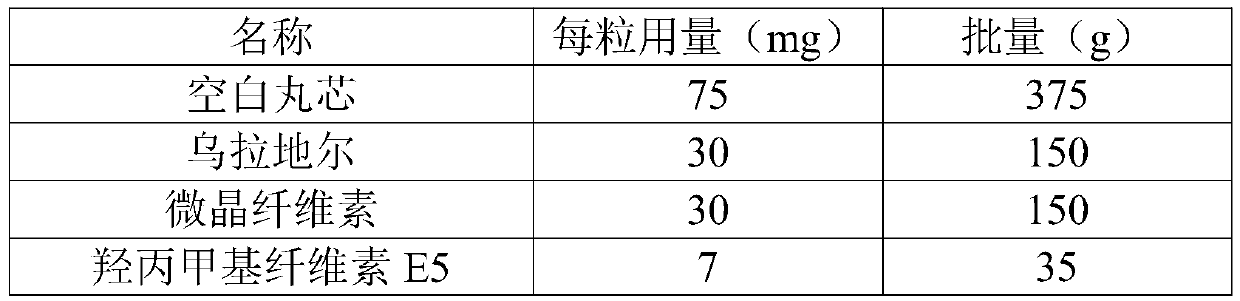
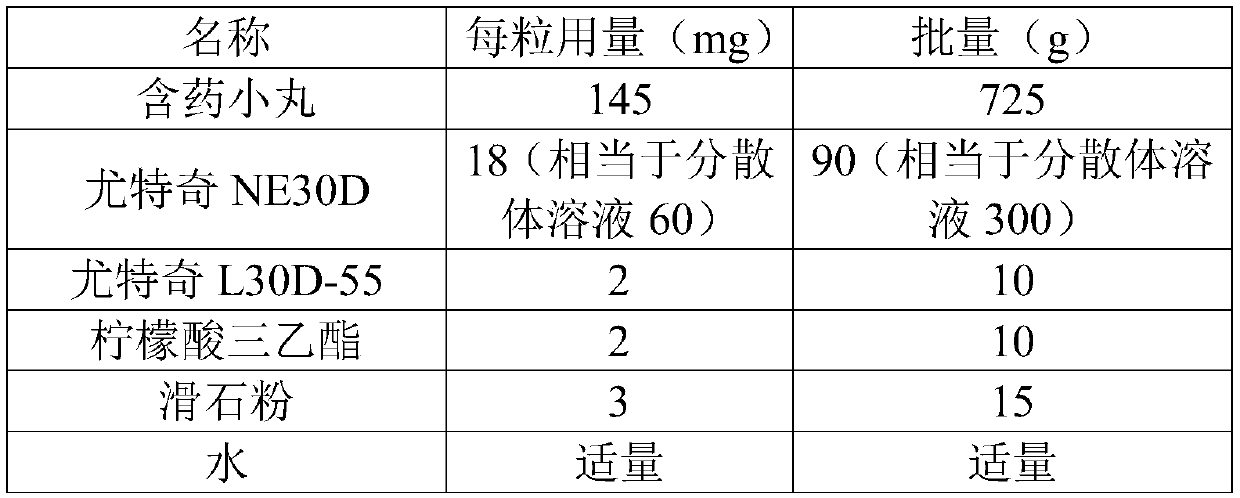
Urapidil sustained release preparation and preparation method thereof
InactiveCN111568871ASustained releaseSmooth releaseOrganic active ingredientsPharmaceutical non-active ingredientsAnti-Adhesion AgentPharmaceutical Substances
The invention belongs to the field of medicine, and particularly relates to a urapidil sustained release preparation and a preparation method thereof. The urapidil sustained release preparation consists of a medicine-containing small pill, an isolation coating layer and a sustained release coating layer, wherein the medicine-containing small pill comprises a blank pellet, a main medicine layer anda solvent; the main medicine layer comprises 20 to 40 parts of urapidil, 7 to 15 parts of a bonding agent, 30 to 40 parts of a diluent and 3 to 10 parts of an anti-sticking agent through being metered in parts by mass; the isolation coating layer comprises an isolation coating material, talcum powder and a solvent; and the sustained release coating layer comprises 70 to 85 parts of a sustained release coating material, 0 to 8 parts of triethyl citrate, 6 to 15 parts of talcum powder and a solvent through being metered in parts by mass. The invention develops a novel urapidil sustained releasepreparation recipe, and has the advantages of high product stability and no burst release phenomenon; meanwhile, the continuous uniform and sustained medicine release of the medicine can be ensured;and in addition, the urapidil sustained release preparation has the characteristics of simple preparation process and high efficiency.
Owner:北京华氏开元医药科技有限公司
Medicine urapidil composition capsule for treating hypertensive crisis of old people
InactiveCN105125521AImprove stabilityLow content of impurity IOrganic active ingredientsOrganic chemistryNicotinamideUrapidil
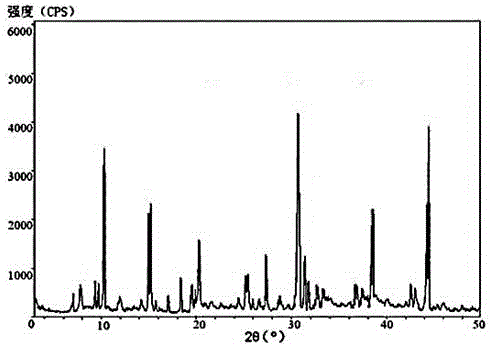
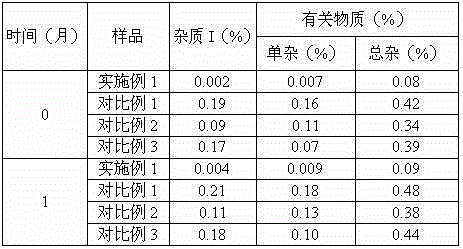
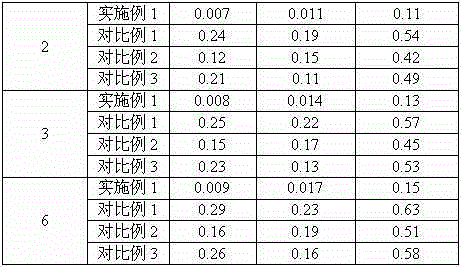
The invention related to a medicine urapidil composition capsule for treating hypertensive crisis of old people and belongs to the technical field of medicine. The composition is prepared from urapidil, microcrystalline cellulose, nicotinamide, povidone K30, 95% ethyl alcohol and superfine dilica powder. The urapidil is a novel crystal form compound, an X-ray powder diffraction pattern measured through a Cu-K alpha ray is shown in the graph 1, the urapidil is different from urapidil reported in the prior art, it is found through tests that compared with the prior art, the novel crystal form compound is better in stability and extremely low in content of impurities I, and the total impurity content is also controlled within a low range and has small change along with the prolonging of storage time. The solubility in water and fluidity of the urapidil crystal form compound are obviously improved compared with those in the prior art, the capsule prepared through the novel crystal form compound is high in dissolution rate, good in stability, high in bioavailability and very suitable for clinical application.
Owner:杨献美
Hypotensive drug urapidil hydrochloride composition water injection
InactiveCN105106112AImprove stabilityImprove solubilityOrganic active ingredientsOrganic chemistryChlorideNuclear chemistry
The invention discloses a hypotensive drug urapidil hydrochloride composition water injection, and belongs to the technical field of medicines. The composition consists of urapidil hydrochloride and calcium chloride; urapidil hydrochloride is crystal; an X-ray powder diffraction diagram obtained by measurement by using Cu-Ka rays is represented in figure 1. The new crystal form of the urapidil hydrochloride provided by the invention is different from a crystal form structure in the prior art; a test shows that compared with the prior art, the hypotensive drug urapidil hydrochloride composition water injection has the advantages that the compound with the new crystal form is high in stability, the content of an impurity I is extremely low, and the total content of impurities is also controlled within a lower range; furthermore, with the prolonging of the storage time, the change of the content is smaller. Meanwhile, the dissolving property and the flowability of the urapidil hydrochloride crystal compound in water are obviously improved when compared with the prior art; the water injection prepared from the compound of the new crystal form is simple in constituent, high in stability and very suitable for clinical application.
Owner:杨献美
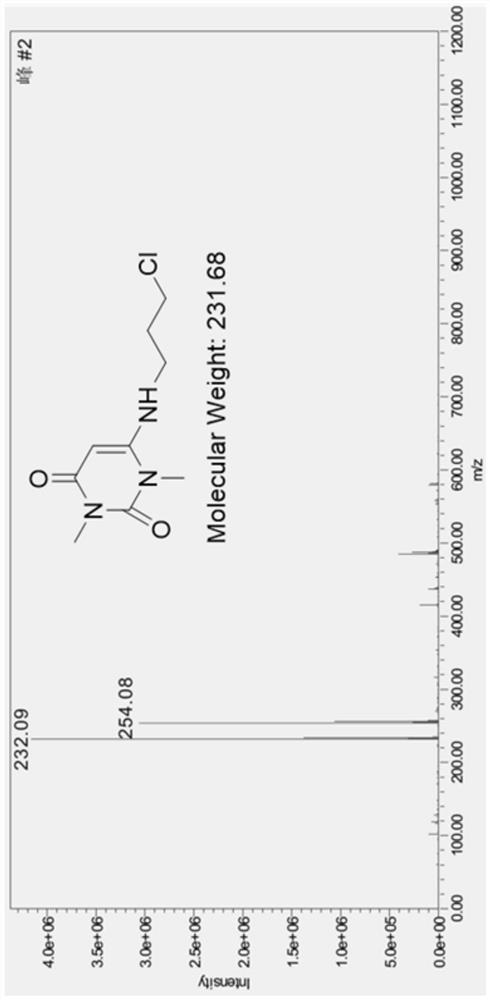
Urapidil oxidation product and preparation method thereof
ActiveCN104529912ARaise quality standardsAvoid product qualityOrganic chemistryImpurityQuality standard
The invention relates to an urapidil oxidation product and a reparation method thereof. The product is 6-[[3-[4-(2-methoxyphenyl)-1-piperazinyl]propyl]nitroxyl]-1,3-dimethyl-2,4(1H,3H)-pyrimidinedione. According to the invention, the compound is obtained, and is subjected to structure confirmation, such that the structure of the compound is confirmed. The compound is adopted as an impurity reference substance in urapidil related substance inspection, such that impurity change situations of urapidil can be directly effectively monitored. The implementation of the invention assists in improving urapidil quality standard, such that urapidil product quality can be better controlled.
Owner:REYOUNG PHARMA
Medicine for treating cardiovascular and cerebrovascular disease
Disclosed is a medicament for treating cardiovascular and cerebrovascular diseases which comprises the components of (by weight ratio) sodium ascorbate 2-3, chlorhydric pyridoxine 0.1-0.15, thiamine 0.1-0.15, methyldopa 0.05-0.1, Urapidil 0.05-0.1, puerarin 0.1-0.15, xinkeshu tablet 0.05-0.1, xuekang capsule 0.05-0.1, ginkgo leaf tablet 0.1-0.15, fresh vegetable and fruit maceration extract 20-40, and distilled water 77.3-56.
Owner:邱季端 +1
Urapidil large volume injection, its preparation method and application
ActiveCN100353944COrganic active ingredientsPharmaceutical delivery mechanismMANNITOL/SORBITOLCurative effect
Owner:杨立新
Urapidil hydrochloride injection and preparation method thereof
PendingCN114344255AImproving Sterility Assurance LevelsReduce generationOrganic active ingredientsInorganic non-active ingredientsSolventBuffering agent
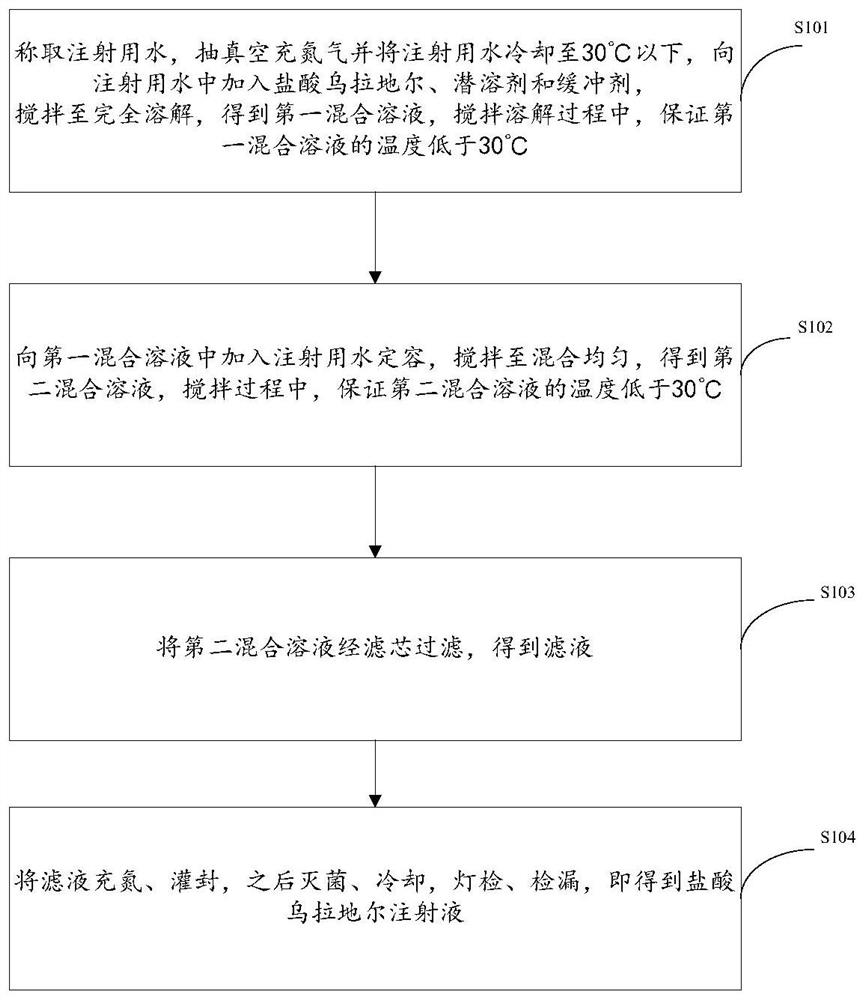
The invention relates to the technical field of medicines, in particular to urapidil hydrochloride injection and a preparation method thereof. The preparation method provided by the invention comprises the following steps: weighing water for injection, cooling to 30 DEG C or below, vacuumizing and filling nitrogen, adding urapidil hydrochloride, a latent solvent and a buffer agent into the water for injection, stirring until the urapidil hydrochloride, the latent solvent and the buffer agent are completely dissolved to obtain a first mixed solution, and ensuring that the temperature of the first mixed solution is lower than 30 DEG C in the stirring and dissolving process; adding water for injection of which the temperature is lower than 30 DEG C into the first mixed solution to make the volume constant, stirring until the materials are uniformly mixed to obtain a second mixed solution, and ensuring that the temperature of the second mixed solution is lower than 30 DEG C in the stirring process; filtering the second mixed solution through a filter element to obtain filtrate; and filling nitrogen into the filtrate, encapsulating, sterilizing, cooling, carrying out lamp inspection, and carrying out leak detection to obtain the urapidil hydrochloride injection.
Owner:WUHAN DOCAN PHARMA
A kind of preparation method of urapidil hydrochloride
Owner:HEBEI YIPIN PHARMA +1
Hypotensive drug urapidil hydrochloride composition freeze-dried powder injection
InactiveCN105193746AImprove stabilityImprove solubilityPowder deliveryOrganic active ingredientsMedicineBiology
The invention discloses a hypotensive drug urapidil hydrochloride composition freeze-dried powder injection and belongs to the technical field of medicine. The composition comprises urapidil hydrochloride and an excipient. The excipient is trehalose, and the urapidil hydrochloride is a novel crystal form compound. The X-ray powder diffraction pattern obtained through Cu-K alpha ray measurement is shown in the picture 1 and is different from urapidil hydrochloride reported in the prior art. Tests prove that compared with the prior art, the solubility and mobility in water of the novel crystal form compound are obviously improved, and the freeze-dried powder injection prepared through the novel crystal form compound is good in stability, good in stability after being combined with a solvent, extremely low in insoluble particle content, and quite suitable for clinic application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
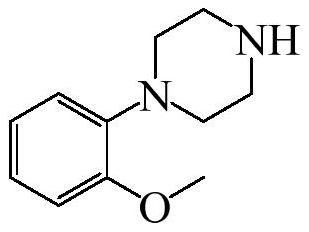
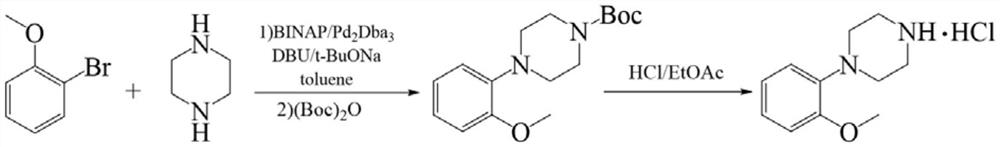
Preparation method of urapidil intermediate 1-(2-methoxyphenyl) piperazine and salt thereof
PendingCN114685399AHigh purityAvoid introducingOrganic chemistry methodsOrganic synthesisMethanesulfonyl chloride
The invention belongs to the field of organic synthesis, and particularly relates to a preparation method of urapidil intermediate 1-(2-methoxyphenyl) piperazine and salt thereof. According to the invention, o-methoxyphenol is used as a starting material to prepare the urapidil intermediate 1-(2-methoxyphenyl) piperazine by using a brand-new synthesis route, and methylsulfonyl chloride is adopted to activate hydroxyl on o-methoxyphenol, so that the condensation reaction condition of o-methoxyphenol and imino on piperazine is mild; and the intermediate product does not need to be separated in the intermediate process of the sulfonylation reaction and the condensation reaction, and the operation is simple and convenient. The condensation reaction adopts a mode of adding o-methoxyphenyl methanesulfonate into piperazine, so that two imino groups on piperazine are prevented from being subjected to condensation reaction at the same time, the selectivity of a target product is ensured, excessive impurities are prevented from being introduced into a system, and the purity of the product is improved. In terms of o-methoxyphenol, the total yield of the urapidil intermediate 1-(2-methoxyphenyl) piperazine prepared by the preparation method disclosed by the invention can reach 88% or above. Meanwhile, the preparation method is obvious in cost advantage and suitable for industrial production.
Owner:河北广祥制药有限公司
Urapidil hydrochloride freeze-dried powder for injection and preparing method thereof
ActiveCN100506211CEliminate first pass effectEasy to storePowder deliveryOrganic active ingredientsSolubilityHeating time
A freeze-dried powder of urapidil hydrochloride for injection and a preparation method thereof. The freeze-dried powder is composed of urapidil hydrochloride and a certain proportion of auxiliary materials. Its preparation is realized according to the following steps: dissolve the main and auxiliary materials in water, decolorize with activated carbon, freeze to -60~-30°C after subpackaging, 3~8 hours, vacuumize, slowly heat to 0~20°C, heating time 10 ~20 hours, keep the temperature and heat for 2~6 hours, then raise the temperature to 20~50℃, heat up for 2~6 hours, then keep warm for 0.5~4 hours, press the plug, roll the cap, and pack it into the finished product warehouse after passing the full inspection. The method adopts a gradient cooling process, and the finished product has plump appearance, good solubility, easy storage and transportation, and good stability.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD +1
Urapidil composition granule for treating pregnancy-induced hypertension
InactiveCN105055335AImprove stabilityLow content of impurity IOrganic active ingredientsOrganic chemistryCelluloseSucrose
The invention discloses a urapidil composition granule for treating pregnancy-induced hypertension, belonging to the technical field of medicines. The composition is prepared from urapidil, sucrose, hydroxypropyl methylcellulose, poloxamer and absolute ethyl alcohol. Urapidil is a compound in a new crystal form and is different from urapidil reported in the prior art. An X-ray powder diffraction pattern obtained through measurement by using a Cu-Kalpha ray is shown in a drawing 1 in the specification. Through tests, compared with the prior art, the compound in the new crystal form has good stability and lower impurity I content. The total impurity content is also controlled in a lower range and is slightly changed with increase of the storage time. The solubility and flowability of the urapidil crystalline compound provided by the invention in water are obviously improved compared with the solubility and flowability of the prior art. The granule prepared by utilizing the compound in the new crystal form has good stability and high bioavailability and is very suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Preparation method of 6-(3-chloropropyl)amino-1,3-dimethyluracil
InactiveCN113402469AReduce typesAvoid damageOrganic chemistryHypertension medicationsPharmaceutical Substances
The invention belongs to the technical field of drug synthesis processes, and provides a preparation method of 6-(3-chloropropyl)amino-1,3-dimethyluracil. 6-(3-hydroxypropyl)amino-1,3-dimethyluracil is used as a raw material, and 6-(3-chloropropyl)amino-1,3-dimethyluracil is obtained by chlorination with thionyl chloride. According to the reaction, thionyl chloride is directly used as a reaction reagent and solvent, the yield can reach 85% or above, the highest yield can reach 90% or above, and the highest purity can reach 98% or above. Compared with the prior art, the method has the advantages that the yield and the purity are obviously improved, the post-treatment is convenient, the post-treatment solvent can be recycled, and more importantly, the use of 1,2-dichloroethane which is carcinogenic and harmful to human and environment is avoided. When the method is used for synthesizing an antihypertensive drug urapidil, not only are the types of solvents reduced, but also the analysis of a 1,2-dichloroethane residual solvent in the drug quality analysis is avoided, and the method is a green and safe preparation method which is very suitable for industrial production.
Owner:沈阳信康药物研究有限公司
Urapidil impurity compound, preparation method and application thereof
PendingCN112094239AHigh purityAvoid product qualityOrganic chemistryComponent separationOrganic solventChemical compound
The invention belongs to the technical field of medicine impurity preparation, and discloses an urapidil impurity compound, a preparation method and application thereof. The preparation method of theurapidil impurity compound comprises the following steps that 1), urapidil is dissolved in a mixed solvent of an organic solvent and water, and after heating dissolution is completed, carbon dioxide is introduced for a pressurization reaction; and 2) after the reaction is finished, the reaction solution isconcentrated and dried under reduced pressure, and purifying is carried out by column chromatography to obtain the urapidil impurity compound. The impurity compound meets the requirements of an impurity reference substance in quality control, can be used for quality control in a urapidil or urapidil hydrochloride synthesis process, can be used as the impurity reference substance for accurate quantitative detection of urapidil or urapidil hydrochloride, and is beneficial to improvement ofquality control of corresponding bulk drugs.
Owner:燃点(南京)生物医药科技有限公司
Medicine urapidil composition for treating senile hypertensive crisis
InactiveCN105078897AImprove stabilityImprove solubilityOrganic active ingredientsPowder deliveryChemical compoundPharmaceutical drug
The invention discloses a medicine urapidil composition for treating senile hypertensive crisis, and belongs to the technical field of medicines. The composition comprises urapidil and anhydrous sodium carbonate. The urapidil is crystal, and an X-ray powder diffraction pattern shown in Figure 1 is obtained through Cu-Kalpha radioactive measurement. The new crystalline form of urapidil provided by the invention is different from a crystal structure in the prior art. Experiments show that the composition with the new crystalline form is high in stability and very low in content of an impurity I compared with those in the prior art; the content of all impurities is controlled at a lower range, and the content of the impurities is reduced along with the extension of storage time; the solubility and the flowability of the composition in water are obviously improved compared with those in the prior art; a powder-injection prepared from the composition is high in stability, high in stability after being mixed with a solvent, very low in content of insoluble particles, and very suitable for clinical application.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Urapidil composition freeze-dried powder injection for treating hypertension
InactiveCN105055346AImprove stabilityImprove solubilityOrganic active ingredientsPowder deliveryPharmaceutical drugExcipient
The invention discloses a urapidil composition freeze-dried powder injection for treating hypertension, belonging to the technical field of medicines. The composition comprises urapidil and an excipient. Urapidil is a compound in a new crystal form and is different from urapidil reported in the prior art. An X-ray powder diffraction pattern obtained through measurement by using a Cu-Kalpha ray is shown in a drawing 1 in the specification. Through tests, compared with the prior art, the compound in the new crystal form has good stability and lower impurity I content. The total impurity content is also controlled in a lower range and is slightly changed with increase of the storage time. The powder injection has the beneficial effects that the solubility and flowability of the powder injection in water are obviously improved compared with the solubility and flowability of the prior art; and the freeze-dried powder injection prepared by utilizing the compound in the new crystal form has good stability after undergoing compatibility with solvents, has low content of insoluble particles and is very suitable to apply clinically.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
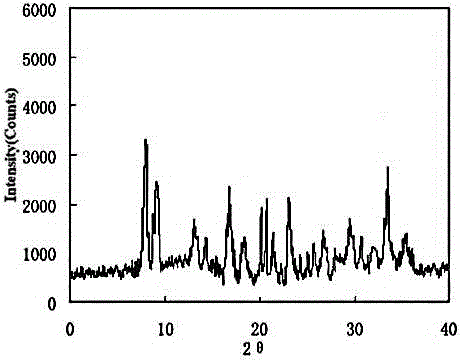
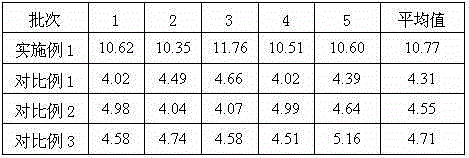
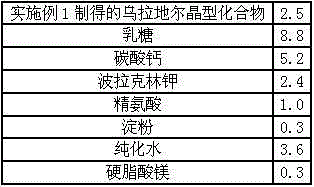
Medicine urapidil composition tablet for treating hypertension
InactiveCN105125513AImprove stabilityLow content of impurity IOrganic active ingredientsOrganic chemistryArginineMagnesium stearate
The invention belongs to the technical field of medicine and relates to a medicine urapidil composition tablet for treating hypertension. The composition is prepared from urapidil, lactose, calcium carbonate, polacrilin potassium, arginine, starch, purified water and magnesium stearate. The urapidil is a novel crystal form compound, an X-ray powder diffraction pattern measured through a Cu-K alpha ray is shown in the graph 1, the urapidil is different from urapidil reported in the prior art, it is found through tests that compared with the prior art, the novel crystal form compound is better in stability and extremely low in content of impurities I, and the total impurity content is also controlled within a low range and has small change along with the prolonging of storage time. The solubility in water and fluidity of the urapidil crystal form compound are obviously improved compared with those in the prior art, and the tablet prepared through the novel crystal form compound is high in dissolution rate, good in stability, high in bioavailability and very suitable for clinical application.
Owner:杨献美
Method for preparing hydrochloride urapidil
The invention relates to a preparation method of the urapidil hydrochloride. The method comprises the following procedures, the urapidil is dissolved in the alcohols or / and the ketones solvent, the organic solution of the hydrogen chloride is slowly added after the heating dissolution for controlling pH at the range of 2.0 to 4.0, then the mixture is crystallized by decreasing the temperature into the range of 20 to 70 DEG C, and the urapidil hydrochloride is filtrated and dried by the decompression drying. The content is not lower than 99.5 percent, the clarity and the pH meet the purity requirement of the raw material used in the injection. The invention has the advantages of simple method, stable quality, easy operation and being suitable for the industrial production.
Owner:YAOPHARMA CO LTD
Anti-hypertension urapidil medicine compound
InactiveCN105111152AImprove stabilityLow content of impurity IOrganic chemistryPharmaceutical drugCombinatorial chemistry
The invention discloses an anti-hypertension urapidil medicine compound, and belongs to the technical field of medicine. X-ray powder diffraction patterns of the anti-hypertension urapidil medicine compound measured by Cu-Ka rays are shown as a figure 1. Compared with the prior art, the anti-hypertension urapidil medicine compound has the advantages that the anti-hypertension urapidil medicine compound which is a urapidil crystal-form compound is good in stability and rarely contains impurities I, and the total impurity content of the anti-hypertension urapidil medicine compound is within a narrow range under the control and rarely changes along with extension of the storage time; the solubility and the flowability of the urapidil crystal-form compound in water are obviously improved, accordingly, the anti-hypertension urapidil medicine compound brings convenience for preparing preparations, and the efficacy of the anti-hypertension urapidil medicine compound can be greatly improved.
Owner:QINGDAO HUAZHICAO PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com