Preparation method of urapidil intermediate 1-(2-methoxyphenyl) piperazine and salt thereof
A technology of methoxyphenyl and urapidil, which is applied in the field of preparation of urapidil intermediate 1-piperazine and its salt, can solve the problems of low yield, heavy metal residue, high cost of raw materials, etc., and improve product purity , easy operation, guaranteed selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
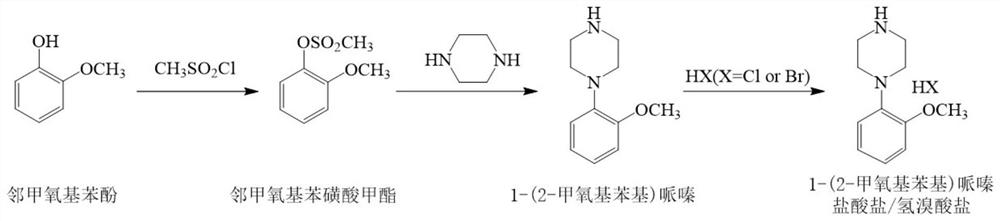
[0047] The invention provides a preparation method of urapidil intermediate 1-(2-methoxyphenyl)piperazine, comprising the following steps:
[0048] Step 1, in the organic solvent, under the action of an acid binding agent, slowly adding methylsulfonyl chloride to o-methoxyphenol to carry out sulfonylation reaction to generate o-methoxyphenyl methanesulfonate;
[0049] Step 2. In the organic solvent, under the action of an acid binding agent, slowly add the o-methoxyphenyl methanesulfonate to piperazine to carry out a condensation reaction to generate a urapidil intermediate 1-(2-methoxyl group) phenyl) piperazine crude.
[0050] The organic solvent in the preparation method is preferably at least one of dichloromethane, dichloroethane or chloroform.
[0051] The acid binding agent is preferably at least one of pyridine, triethylamine or N-methylmorpholine.
[0052] The synthetic route used in the present invention is different from the prior art, and o-methoxy phenol and met...
Embodiment 1
[0060] The present embodiment provides a preparation method of urapidil intermediate 1-(2-methoxyphenyl)piperazine, which specifically includes:
[0061] Take a 2000mL four-necked flask, add 560mL of chloroform, 80g of o-methoxyphenol and 66.27g of pyridine, control the temperature to -10°C, add 84.90g of methylsulfonyl chloride dropwise for 20min, and obtain o-methoxybenzene after the reaction is incubated for 30min. Methanesulfonate reaction solution;
[0062] Take another 2000mL four-necked flask, add 208mL of chloroform, 69.39g of piperazine, and 136.45g of pyridine, turn on stirring, control the temperature to be 25°C, and add the above-mentioned o-methoxyphenylmethanesulfonate reaction solution dropwise for 60min, and keep the reaction 60min;
[0063] After the reaction, 307 mL of deionized water was added, fully washed and left to stand for liquid separation. The organic phase obtained by separation was washed with 115 mL of 0.1 mol / L hydrochloric acid and 115 mL of de...
Embodiment 2
[0065] The present embodiment provides a preparation method of urapidil intermediate 1-(2-methoxyphenyl)piperazine, which specifically includes:
[0066] Take a 2000mL four-necked flask, add 560mL of dichloromethane, 80g of o-methoxyphenol and 78.26g of triethylamine, control the temperature to 20°C, drop 77.52g of methylsulfonyl chloride over 20min, and obtain o-methylsulfonyl chloride after 150min of insulation reaction. Oxyphenylmethanesulfonate reaction solution;
[0067] Take another 2000mL four-neck flask, add 122mL of dichloromethane, 69.39g of piperazine, and 157.78g of triethylamine, turn on stirring, control the temperature to be -5°C, and add the above-mentioned o-methoxyphenyl methanesulfonate dropwise for 90min The reaction solution was incubated for 30 min;
[0068] After the reaction, 409 mL of deionized water was added, and after sufficient washing, the solution was allowed to stand for liquid separation. The organic phase obtained by separation was washed wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com