Urapidil impurity compound, preparation method and application thereof
A technology of uradil and uradil hydrochloride, applied in organic chemistry, measuring devices, instruments, etc., can solve problems such as inability to carry out effective quality control, and achieve the effects of improved quality standards, high purity, and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] This example prepares a kind of urapidil impurity compound, the preparation method of this urapidil impurity compound is as follows:
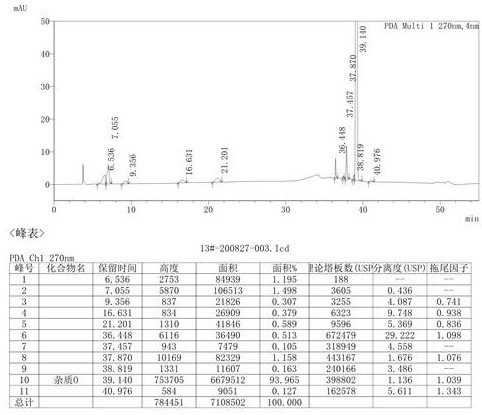
[0052] Add 10 g of urapidil, 20 mL of 95% ethanol, and 20 mL of water into the pressure reaction vessel, remove the air in the reaction vessel with carbon dioxide gas, pass the carbon dioxide gas to the set pressure of 60 psi, and keep the temperature at 60°C for 12 hours. After the reaction was completed, the reaction solution was concentrated to dryness under reduced pressure. Purification by column chromatography yielded 2.1 g of the impurity compound of Urapidil (yield 18.9%, purity 94.0%).
[0053] Product characterization of urapidil impurity compound:
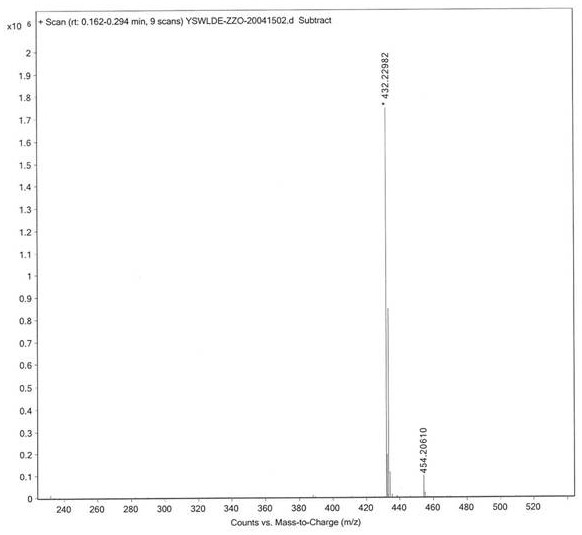
[0054] (1) Perform mass spectrometry analysis (ESI-MS) on the urapidil impurity compound prepared in Example 1: m / z=432.2[M+H]+, the mass spectrum of urapidil impurity compound is as follows figure 1 shown.
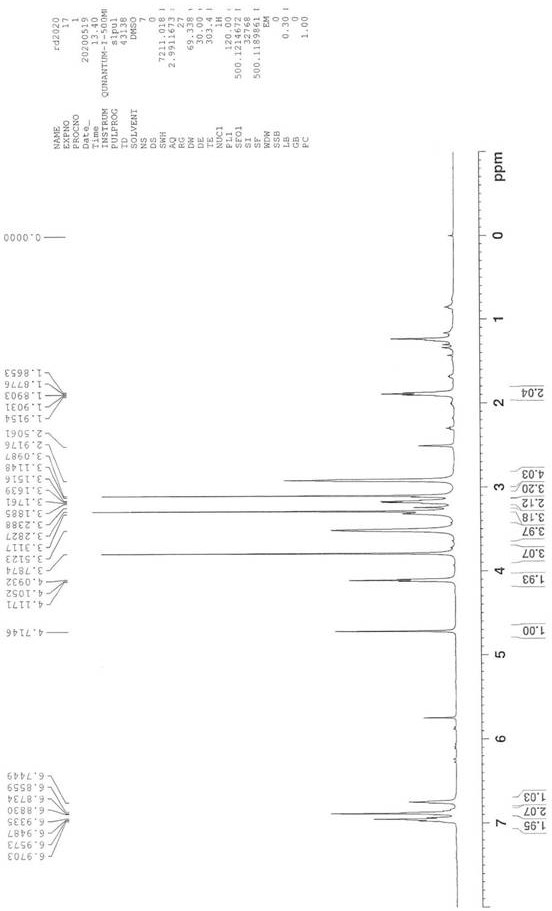
[0055] (2) NMR analysis of the urapidil impurity compound prepared in ...
Embodiment 2
[0058] The preparation method of urapidil impurity compound is as follows:
[0059] Add 10 g of urapidil, 20 mL of methanol, and 20 mL of water into the pressure reaction vessel, remove the air in the reaction vessel with carbon dioxide gas, pass the carbon dioxide gas to the set pressure of 40 psi, and keep the temperature at 50°C for 16 hours. After the reaction was completed, the reaction solution was concentrated to dryness under reduced pressure. Purified by column chromatography, the product of the impurity compound of urapidil was 1.8 g (yield 16.2%, purity 93.3%).
Embodiment 3
[0061] The preparation method of urapidil impurity compound is as follows:
[0062] Add 10 g of urapidil, 10 mL of acetone, and 20 mL of water into the pressure reaction vessel, remove the air in the reaction vessel with carbon dioxide gas, pass the carbon dioxide gas to the set pressure of 50 psi, and keep the temperature at 50°C for 16 hours. After the reaction was completed, the reaction solution was concentrated to dryness under reduced pressure. Purified by column chromatography to obtain 1.7 g of the impurity compound of urapidil (yield 15.3%, purity 92.1%).
[0063] The present invention characterizes the urapidil impurity compound prepared in Examples 2 and 3, and the result is consistent with the impurity characterization conclusion of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com