Preparation method of urapidil
A technology of uradil and dimethyluracil, which is applied in the field of preparation of uradil, can solve the problems of expensive foreign products, no scale-up production, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
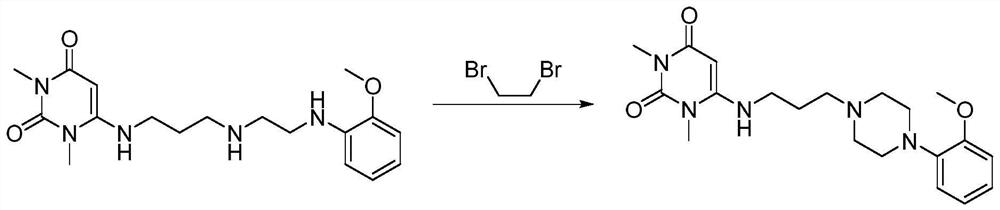
[0044] The present embodiment provides a kind of preparation method of urapidil, specifically comprises the following steps:
[0045] Step 1, the synthesis of 6-(3-hydroxypropylamino)-1,3-dimethyluracil
[0046] In a 500ml three-necked flask, add 3-amino-1-propanol (36.3g, 483.9mmol), add ammonium chloride (8.6g, 161.3mmol) and 1,3-dimethyl-6-amino Uracil (25.0g, 161.3mmol), heated to 100°C and stirred for 30min, then heated to 150°C, stirred for 2.5h to dissolve the system, and continued to maintain the temperature for 3h, TLC showed that the reaction was complete. Cool to 80°C and add 75ml of ethanol, the system dissolves, then slowly cool down to 10°C, a large amount of white solids precipitate out. Filtration, the filter cake was washed twice with acetone (2*100ml), and the product was dried at room temperature to obtain 6-(3-hydroxypropylamino)-1,3-dimethyluracil (24.1g white solid, yield 70.2% ), HPLC purity 97.1%.
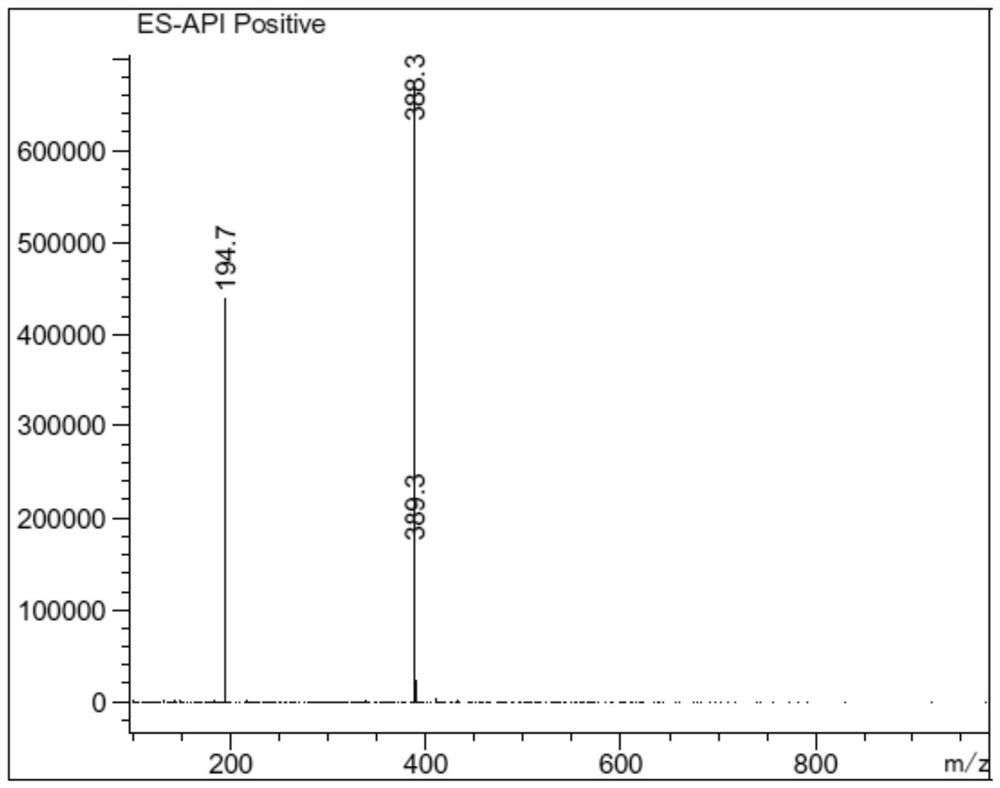
[0047] LC-MS: m / z=214.1 [M+H] +
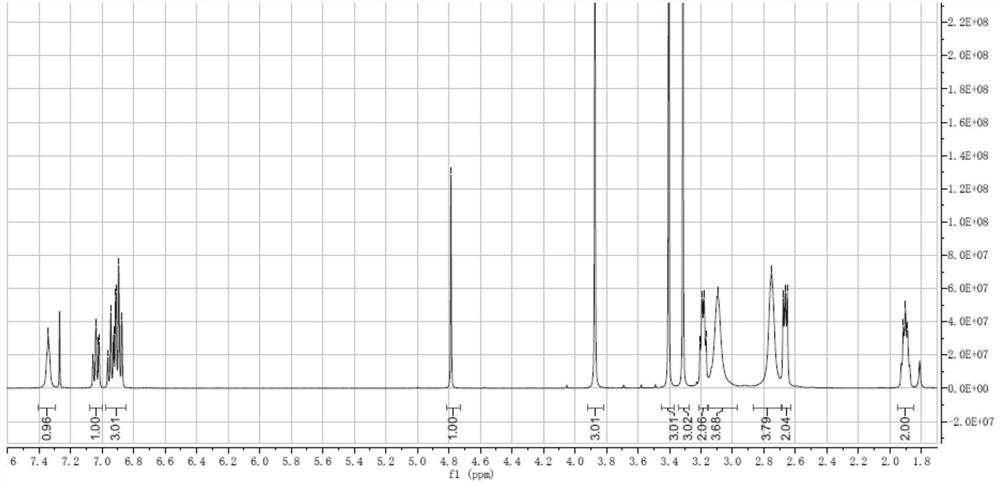
[0048] 1H NMR (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com