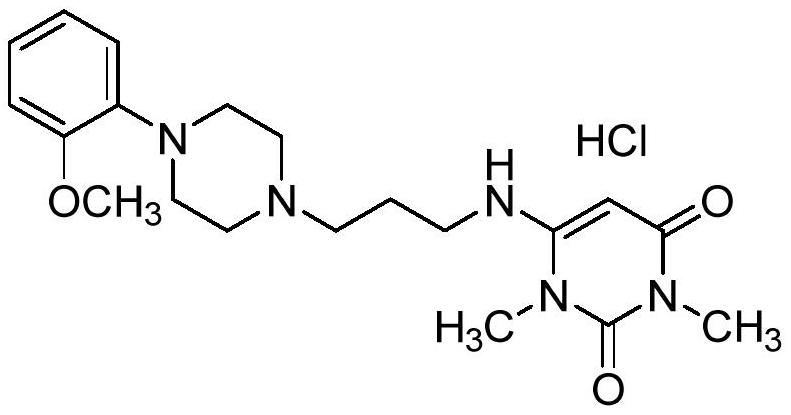
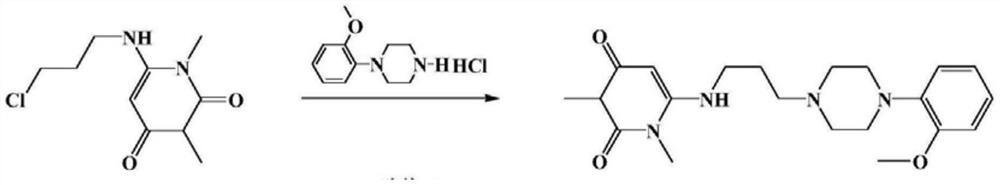
A kind of preparation method of urapidil hydrochloride
A technology of uradil hydrochloride and uradil, which is applied in organic chemistry and other fields, and can solve the problems of large pollution, low yield, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] The preparation of example 1 urapidil hydrochloride
[0033] A. Preparation of Urapidil
[0034] Add 3-[4-(2-methoxyphenyl)piperazin-1-yl]propylamine (50.00g, 0.20mol) and 6-chloro-1,3-dimethylurea to a 500ml clean three-necked reaction flask Pyrimidine purified water (35.01g, 0.20mol), sodium hydroxide (24.06g, 0.60mol) and purified water (500ml), mechanically stirred, heated to 60°C for 2 hours, cooled to room temperature, suction filtered, and the filter cake was ℃ blast drying for 6 hours to obtain 41.2 g of urapidil with a yield of 53.0% and a purity of 96.5%.
[0035] B. Preparation of Urapidil Hydrochloride
[0036] Add absolute ethanol (200.00g) and urapidil (20.00g, 0.05mol) to a 500ml clean three-necked reaction flask and heat up to 70-80°C, dropwise add a mixed solution of hydrochloric acid and ethanol solution (7.32g concentrated hydrochloric acid and 8.00g After the addition, keep stirring at 70-80°C for 1.0h, cool down to room temperature, filter with s...
example 2
[0037] The preparation of example 2 urapidil hydrochloride
[0038] A. Preparation of Urapidil
[0039] Add 3-[4-(2-methoxyphenyl)piperazin-1-yl]propylamine (50.00g, 0.20mol) and 6-chloro-1,3-dimethylurea to a 500ml clean three-necked reaction flask Pyrimidine purified water (35.01g, 0.20mol), potassium hydroxide (33.66g, 0.60mol) and purified water (500ml), stirred mechanically, heated to 60°C for 2 hours, cooled to room temperature, suction filtered, and the filter cake was ℃ blast drying for 6 hours to obtain 38.9 g of urapidil with a yield of 50.1% and a purity of 95.8%.
[0040] B. Preparation of Urapidil Hydrochloride
[0041] Add absolute ethanol (200.00g) and urapidil (20.00g, 0.05mol) to a 500ml clean three-necked reaction flask and heat up to 70-80°C, dropwise add a mixed solution of hydrochloric acid and ethanol solution (7.32g concentrated hydrochloric acid and 8.00g After the addition, keep stirring at 70-80°C for 1.0h, cool down to room temperature, filter wit...
example 3
[0043] Preparation of Urapidil Hydrochloride
[0044] A. Preparation of Urapidil
[0045] Add 3-[4-(2-methoxyphenyl)piperazin-1-yl]propylamine (50.00g, 0.20mol) and 6-chloro-1,3-dimethylurea to a 500ml clean three-necked reaction flask Pyrimidine purified water (35.01g, 0.20mol), anhydrous sodium carbonate (63.59g, 0.60mol) and purified water (500ml), stirred mechanically, heated to 60°C for 2 hours, cooled to room temperature, suction filtered, and the filter cake was 66.23 g of urapidil was obtained by blast drying at 50°C for 6 hours, with a yield of 85.2% and a purity of 97.9%.
[0046] B. Preparation of Urapidil Hydrochloride
[0047] Add dehydrated ethanol (400.00g) and urapidil (40.00g, 0.10mol) to a 500ml clean three-necked reaction flask and heat up to 70-80°C, add dropwise a mixed solution of hydrochloric acid and ethanol solution (14.64g concentrated hydrochloric acid and 16.00g After the addition, keep stirring at 70-80°C for 1.0h, cool down to room temperature,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com