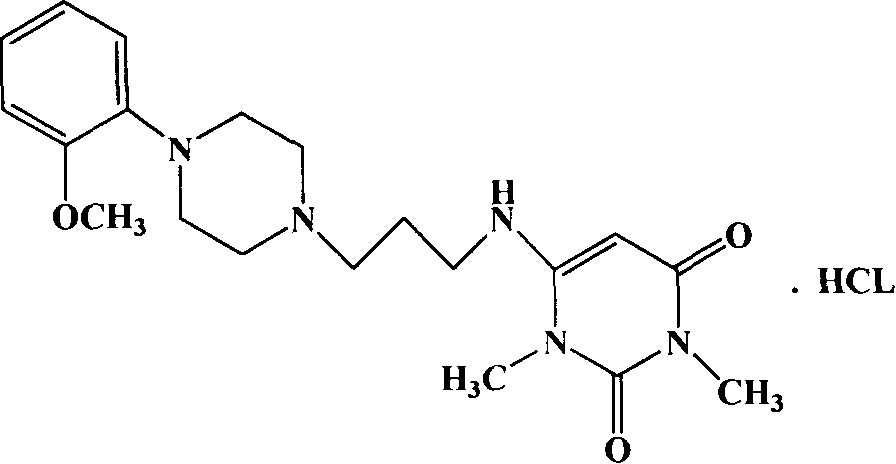
Method for preparing hydrochloride urapidil
A technology of uradil hydrochloride and uradil, applied in the direction of organic chemistry, can solve the problems of unqualified product content, pH, clarity, difficult pH control, etc., and achieve the effect of simple method, easy operation and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
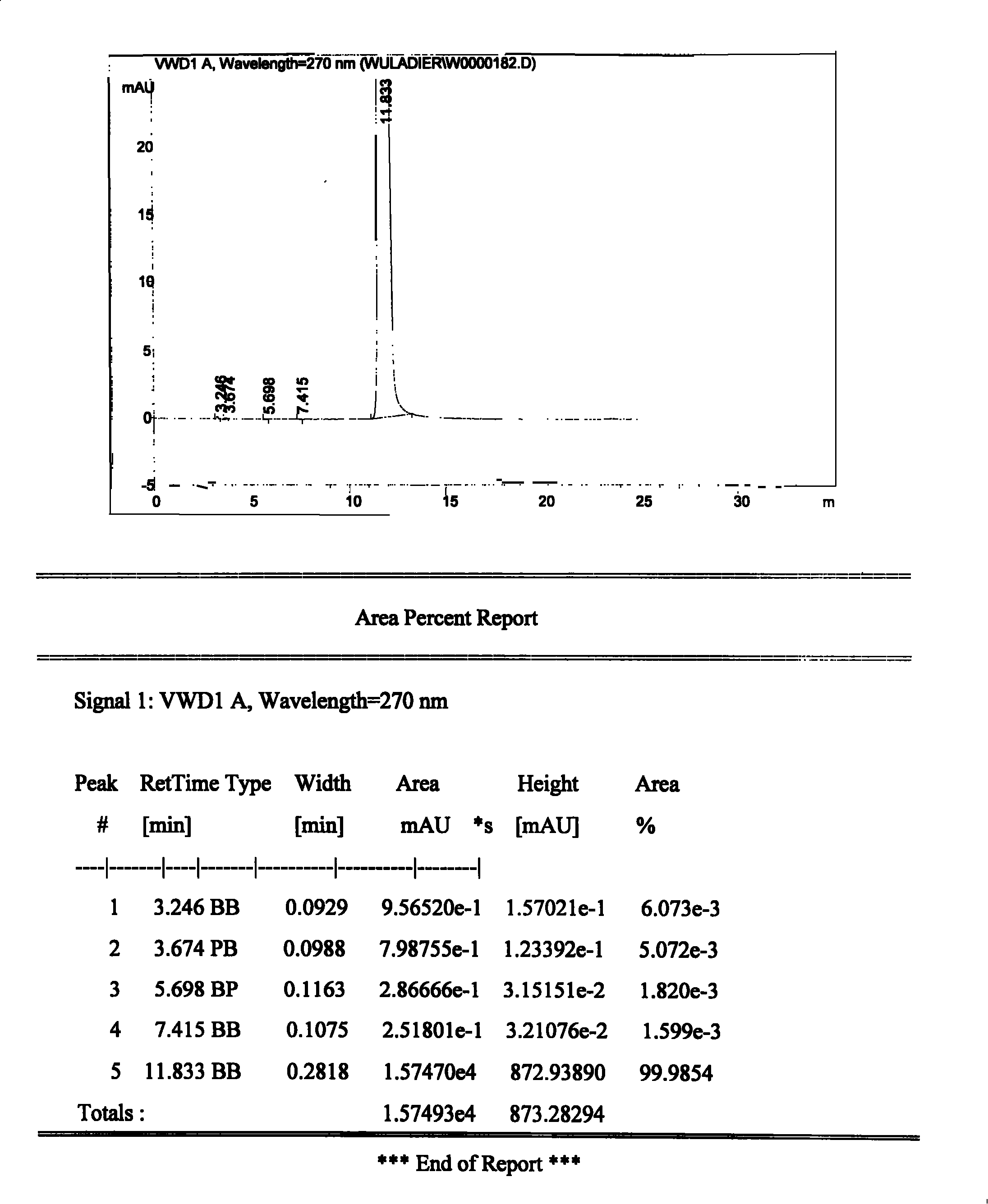
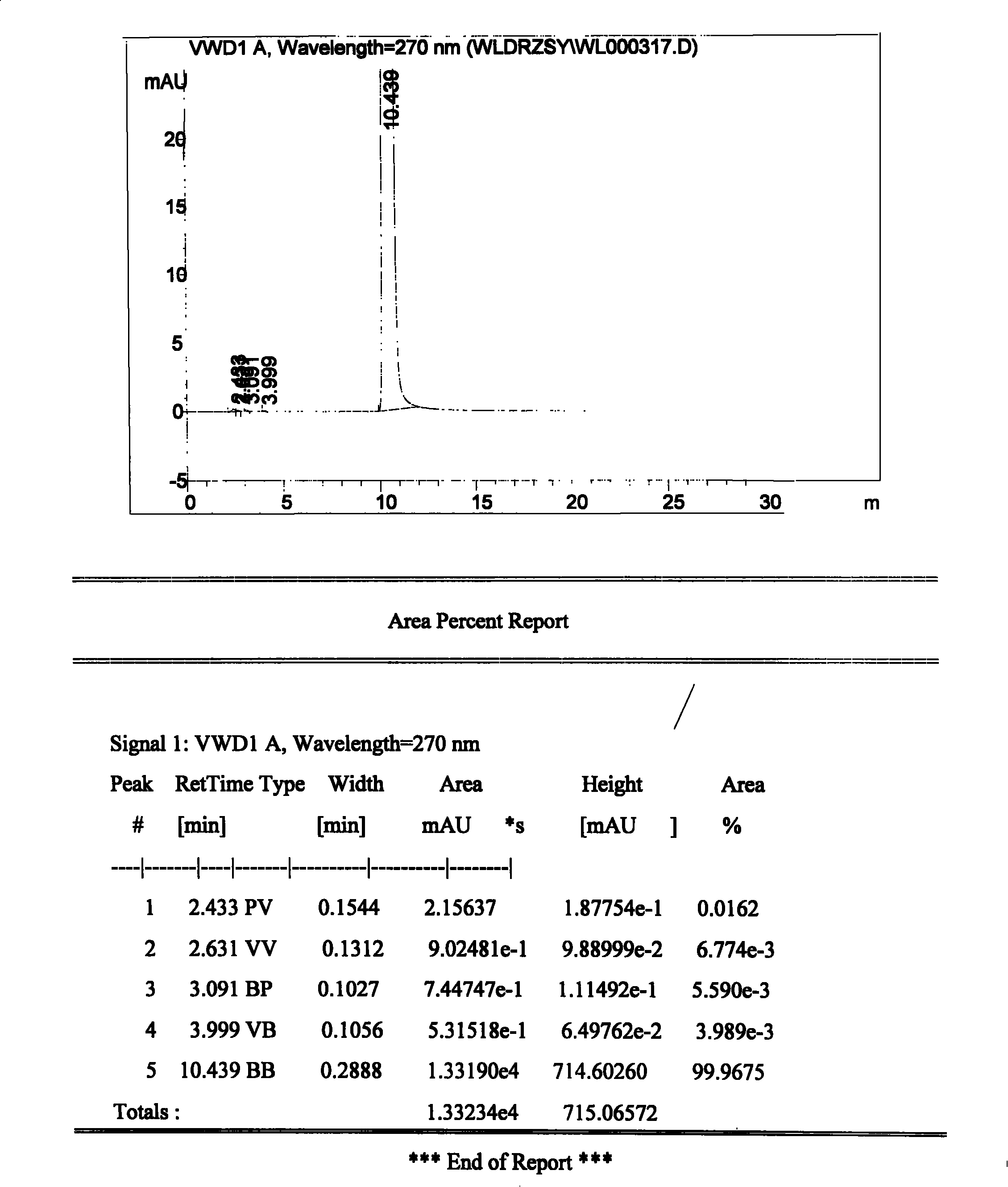
[0020] In a 250mL three-necked flask equipped with a reflux condenser, add 160ml of absolute ethanol and 8.1g of urapidil refined product, heat to 90°C while stirring, and dropwise add hydrogen chloride ethanol solution (obtained by passing hydrogen chloride gas into ethanol solvent) Use precision pH test paper to control the pH of the solution at about 3-4, continue to stir and keep warm for 30 minutes, the system is still a clear and transparent solution, then slowly cool down to about 40°C, crystallize at constant temperature for 1 hour, heat filter, and use the filter cake Wash once with a small amount of absolute ethanol, and filter with suction. The filter cake was dried under reduced pressure and <60°C for 2 hours to obtain 6.6 g of white crystalline urapidil hydrochloride, with a yield of 75.4%, a total yield of 61.1% (theoretical value 10.9 g), and a content of 100.5 g in dry terms. %, related substances are 0.02%, pH=4.76, clarity <No. 2 standard turbidity liquid.
Embodiment 2
[0022] In a 250mL three-necked flask equipped with a reflux condenser, add 160ml of absolute ethanol and 8.1g of urapidil refined product, heat to 80°C under stirring, and dropwise add hydrogen chloride ethanol solution (obtained by passing hydrogen chloride gas into ethanol solvent) Use precision pH test paper to control the pH of the solution at 2-3, continue to stir and keep warm for 30 minutes, the system is still a clear and transparent solution, then slowly cool down to about 70°C, add an appropriate amount of urapidil hydrochloride seed crystals, and crystallize at constant temperature After 1 hour, hot filter, the filter cake was washed once with a small amount of absolute ethanol, and suction filtered. The filter cake was dried under reduced pressure at <60°C for 2 hours to obtain 4.1 g of white crystalline urapidil hydrochloride, with a yield of 37.6%, a dry product content of 101.0%, related substances 0.05%, pH=4.39, Clarity <No. 2 standard turbidity liquid.
Embodiment 3
[0024] In a 250mL three-necked flask equipped with a reflux condenser, add 160ml of absolute ethanol and 8.1g of urapidil refined product, heat to 60°C under stirring, and dropwise add hydrogen chloride ethanol solution (obtained by passing hydrogen chloride gas into ethanol solvent) Use precision pH test paper to control the pH of the solution at about 3-4, continue to stir and keep warm for 30 minutes, the system is still a clear and transparent solution, then slowly cool down to about 40°C, add a small amount of urapidil hydrochloride seed crystals, and analyze at constant temperature Crystallize for 1 hour, heat filter, wash the filter cake once with a small amount of absolute ethanol, and filter with suction. The filter cake was dried under reduced pressure at <60°C for 2 hours to obtain 7.7 g of white crystalline urapidil hydrochloride, with a yield of 70.6%, a dry product content of 99.9%, related substances of 0.02%, pH=5.12, Clarity <No. 2 standard turbidity liquid. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com