Polymer-lipid delivery vehicles
a technology of polymer lipids and delivery vehicles, which is applied in the direction of drug compositions, capsule delivery, heavy metal active ingredients, etc., can solve the problems of significant solubility problems, high water insoluble, and delivery vehicle formulations of organic active agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
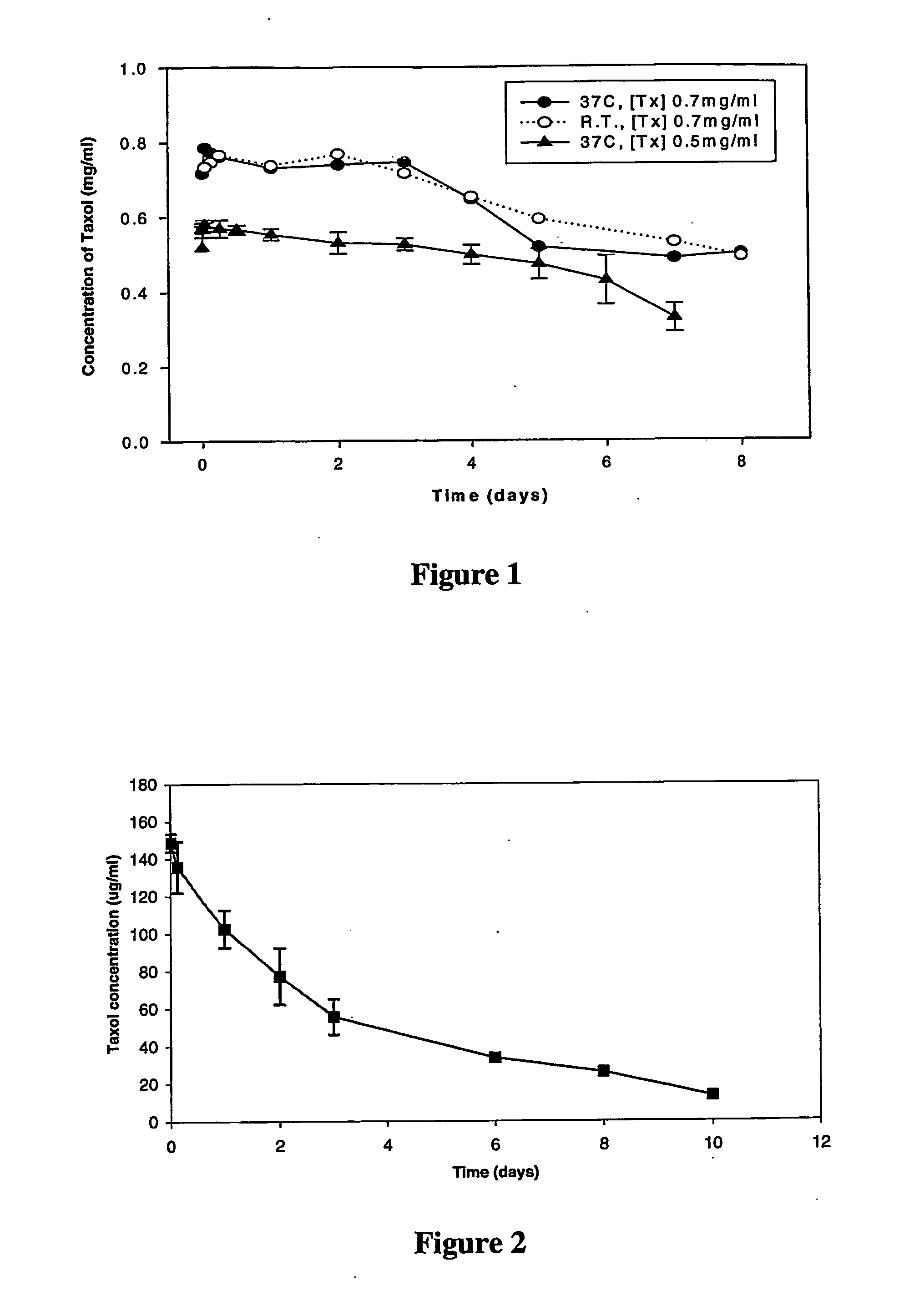
[0104] The Preparation of PCL Nanoparticles Stabilized by DPPC / DSPC / DSPE-PEG Mixtures
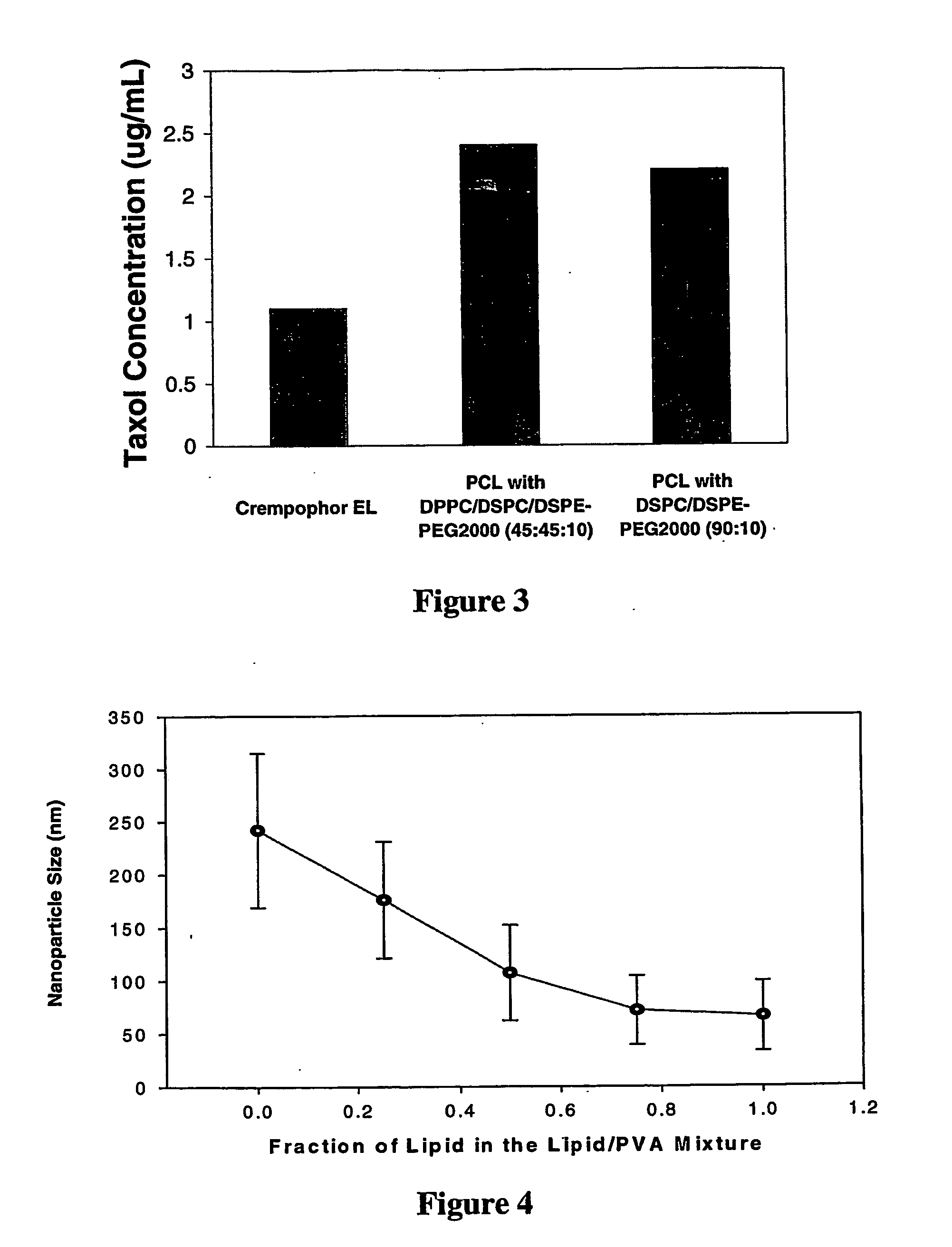
[0105] Nanoparticle systems containing DPPC / DSPC / DSPE-PEG2000 (45:45:10 mole ratio) and incorporating Taxol® were prepared using the method of the present invention. Poly(caprolactone) (PCL) was selected as the hydrophobic polymer making up the core of the particle. The nanoparticles were coated with a stabilizing lipid / PEG-lipid mixture during particle formation. In this method, the polymer and drug are dissolved in a solvent that is partially miscible with water and then mixed with an aqueous solution containing the lipid. The mixture is then homogenized, diluted with water while vortexing and dialyzed. In order to prepare lipid-coated nanoparticles by this method, it was necessary to dissolve the stabilizing lipid (lipid and PEG-lipid solution) in an ethanol / water mixture rather than water alone. The stability of the nanoparticles was assessed by measuring the size and polydispersity of the part...
example 2
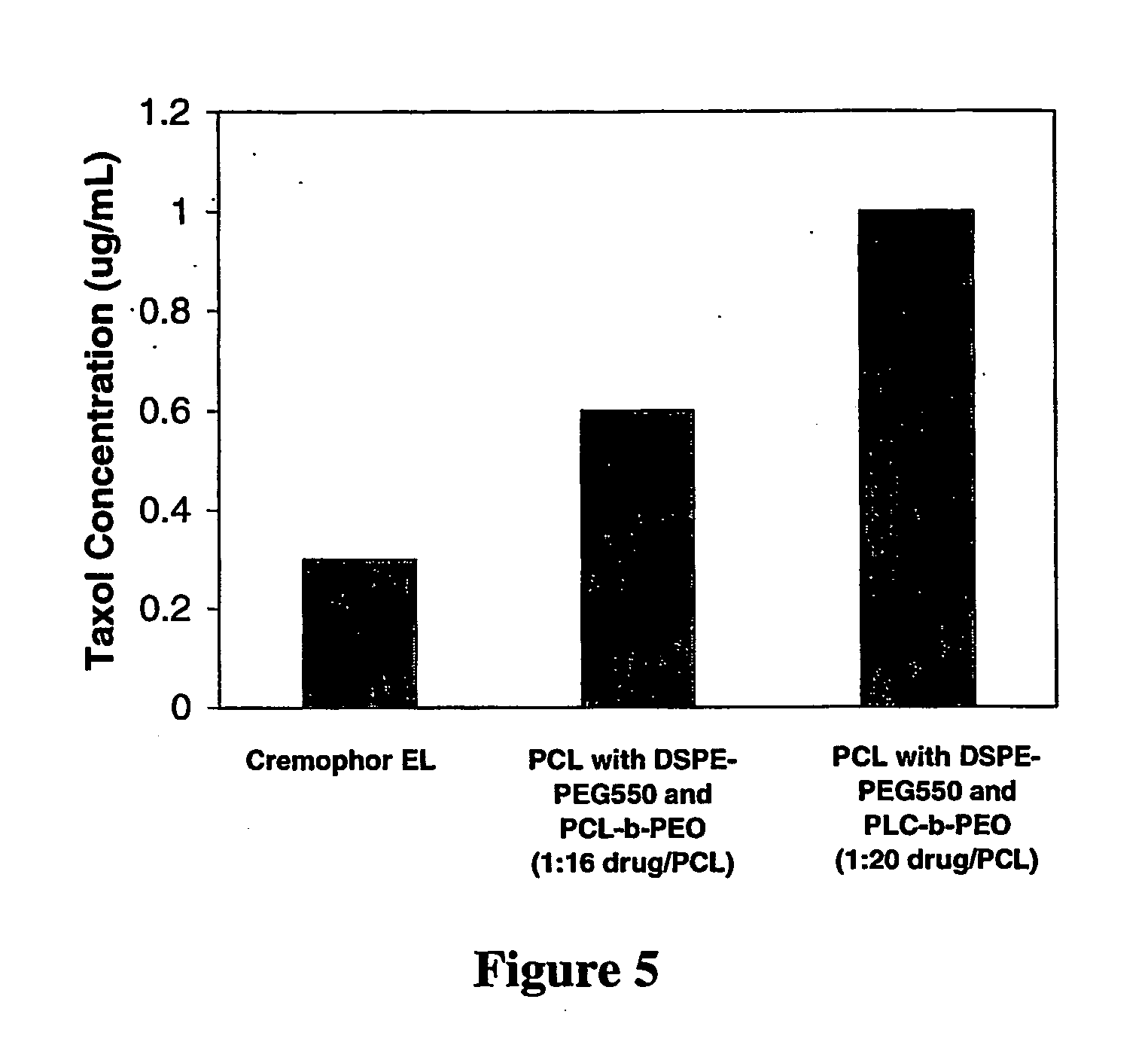
[0111] PCL Nanoparticles Cannot be Stabilized in the Absence of Stabilizing Lipid such as PEG-Lipid
[0112] In order to determine whether the presence of a stabilizing lipid such as DSPE-PEG was required for the preparation of lipid-coated PCL and PLA nanoparticles, nanoparticles were prepared in the absence of PEG-lipid, employing only DMPC or DPPC as the lipid coating.
[0113] The method was repeated in the same manner as in Example 1 to prepare PLA nanoparticles coated with DMPC and PCL particles coated with DPPC. PLA or PCL was dissolved with Taxol® at a 1:30 weight ratio in a 1 mL solution of 1:1 v / v benzyl alcohol:ethyl acetate. For the preparation of both nanoparticles, the drug and polymer precipitated out during the general procedure and therefore no size measurement could be obtained. These results thus indicate that stabilizing lipids, such as PEG-lipid conjugates are required in these systems to produce PCL or PLA nanoparticles that are stable in vitro. Examples of stabili...
example 3
[0114] The Preparation of PCL Nanoparticles Stabilized by PVA / DSPE-PEG Mixtures
[0115] The inventors next examined whether the in vitro stability of the PEG-lipid-coated nanoparticles could also be enhanced by the addition of a hydrophilic polymer surface stabilizer, poly(vinyl alcohol) (PVA). PVA contains a hydrophobic, hydrocarbon backbone that allows it to stably associate with the hydrophobic core of the particles, while the hydrophilic portion of the polymer extends into the aqueous medium. PVA of a molecular weight of 10,000 g / mole was employed in order to minimize the viscosity of the system during particle preparation, as higher molecular weight PVA may result in an increase of the solution viscosity leading to an increase in particle size.
[0116] Stock solutions of 100 mg / mL PCL in ethyl acetate, 10 mg / mL Taxol® in ethyl acetate and 5% w / w poly(vinyl alcohol) (PVA) in water were prepared. A DSPE-PEG750 stock solution at a concentration of 100 mg / mL was prepared in ethanol. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com