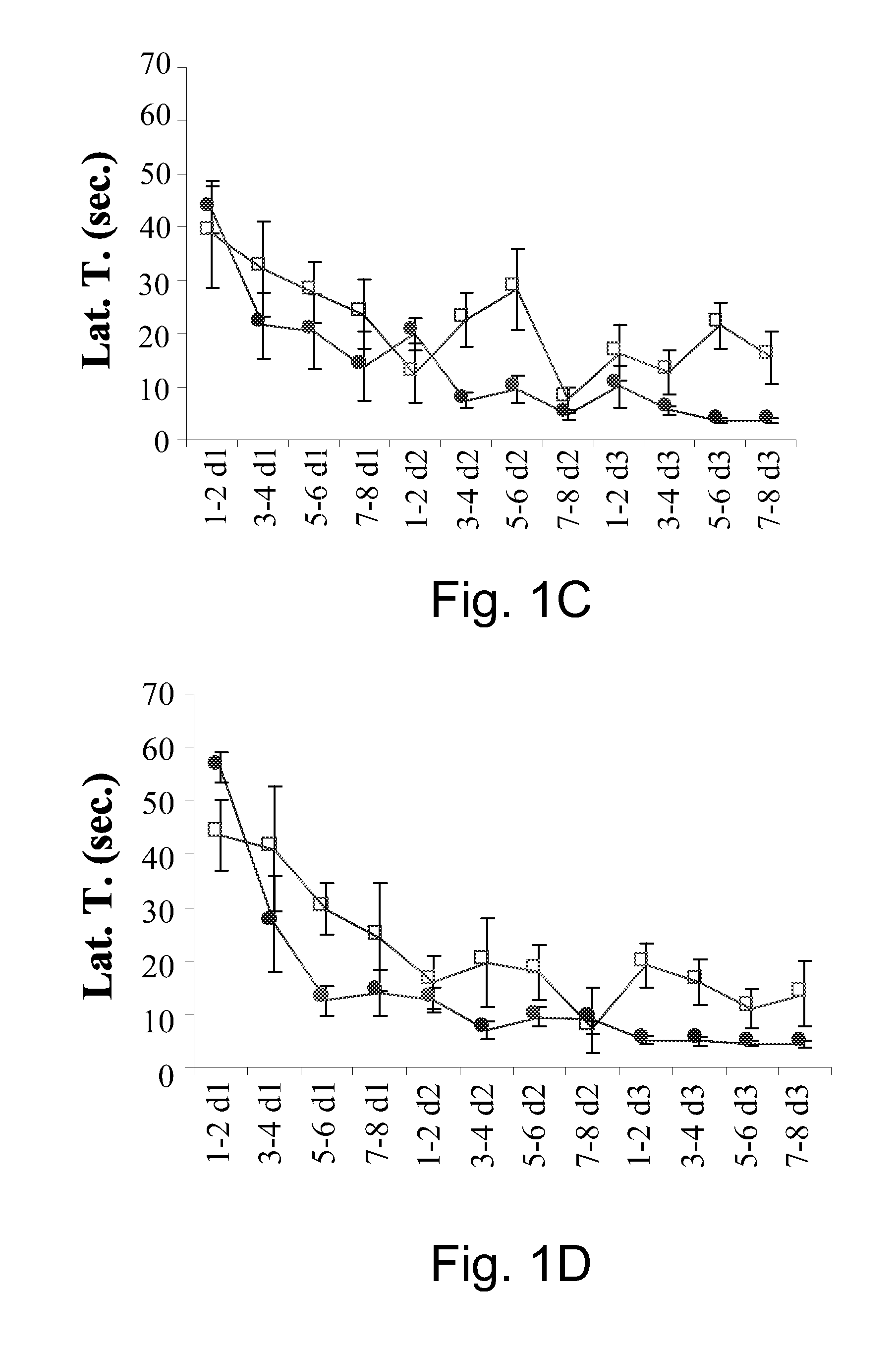
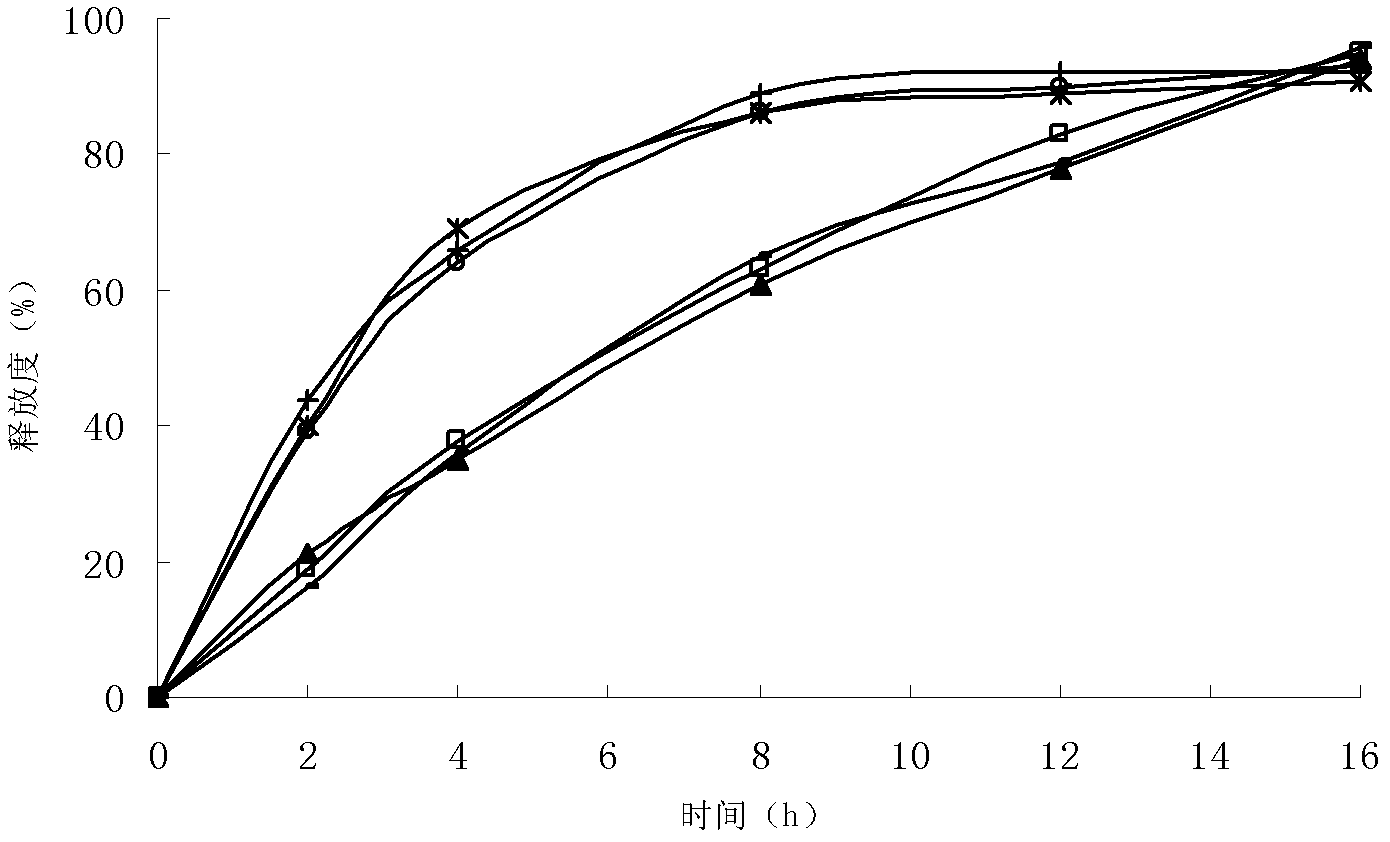
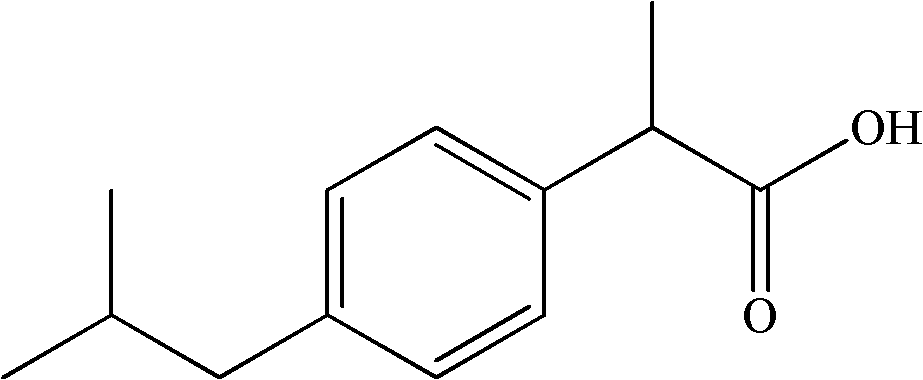
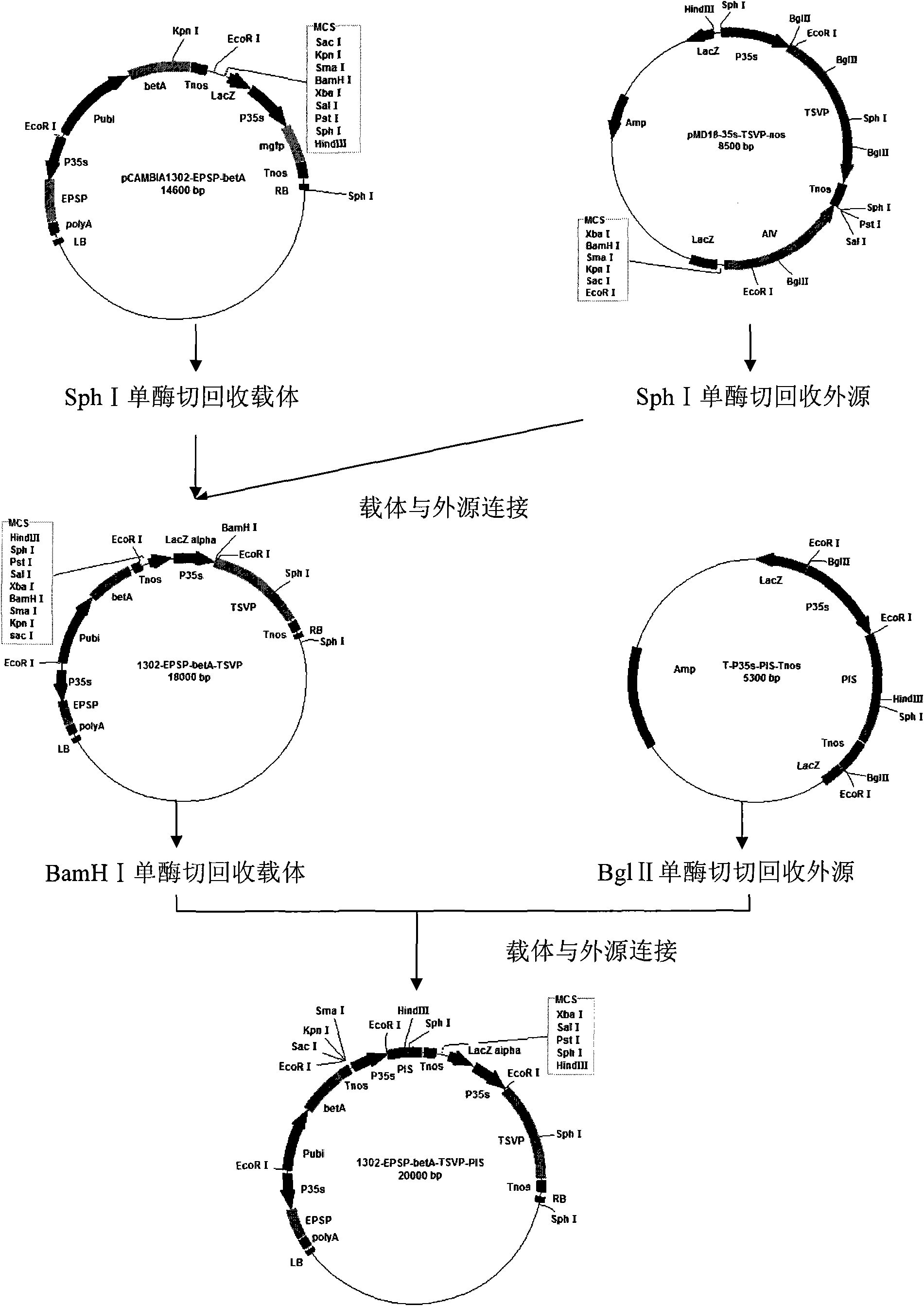
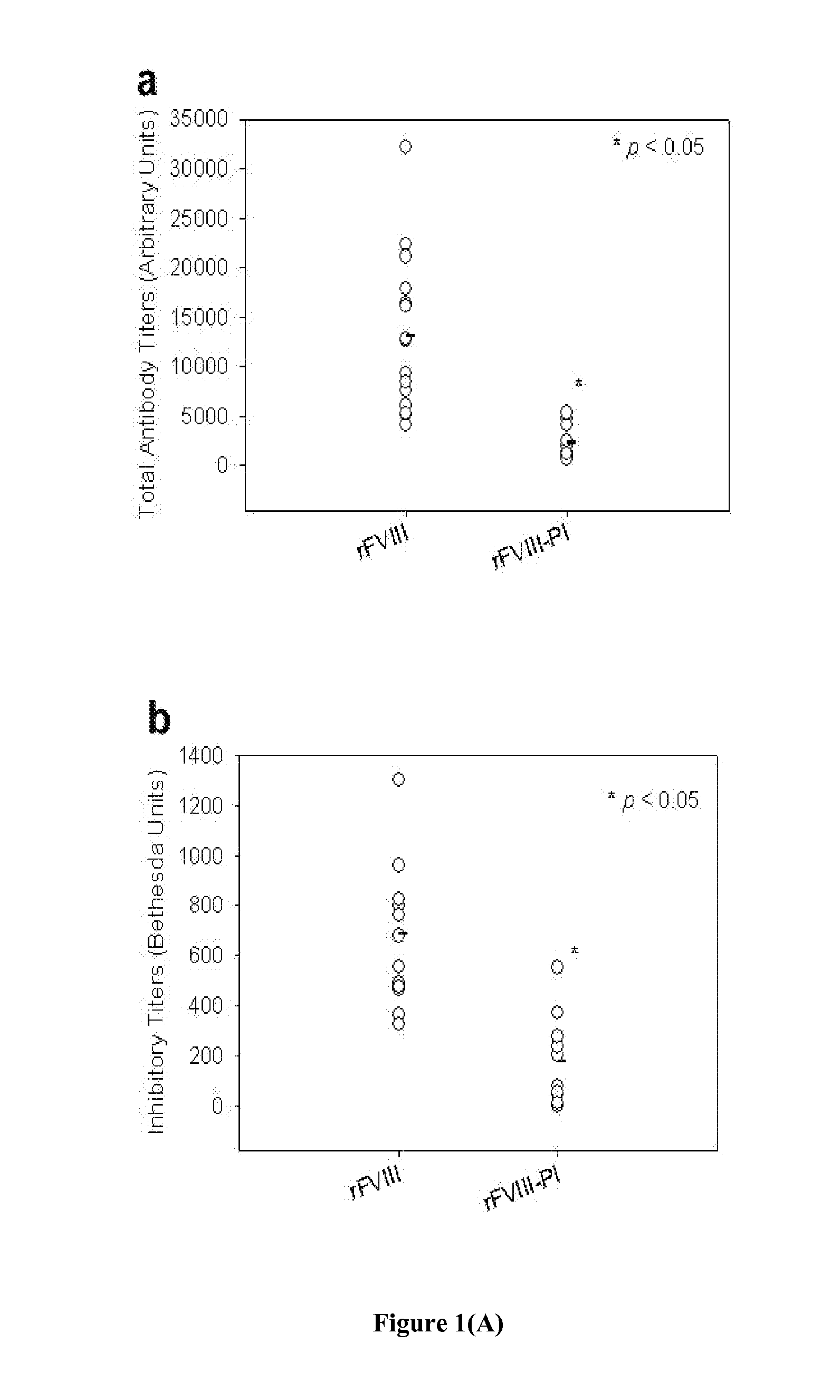
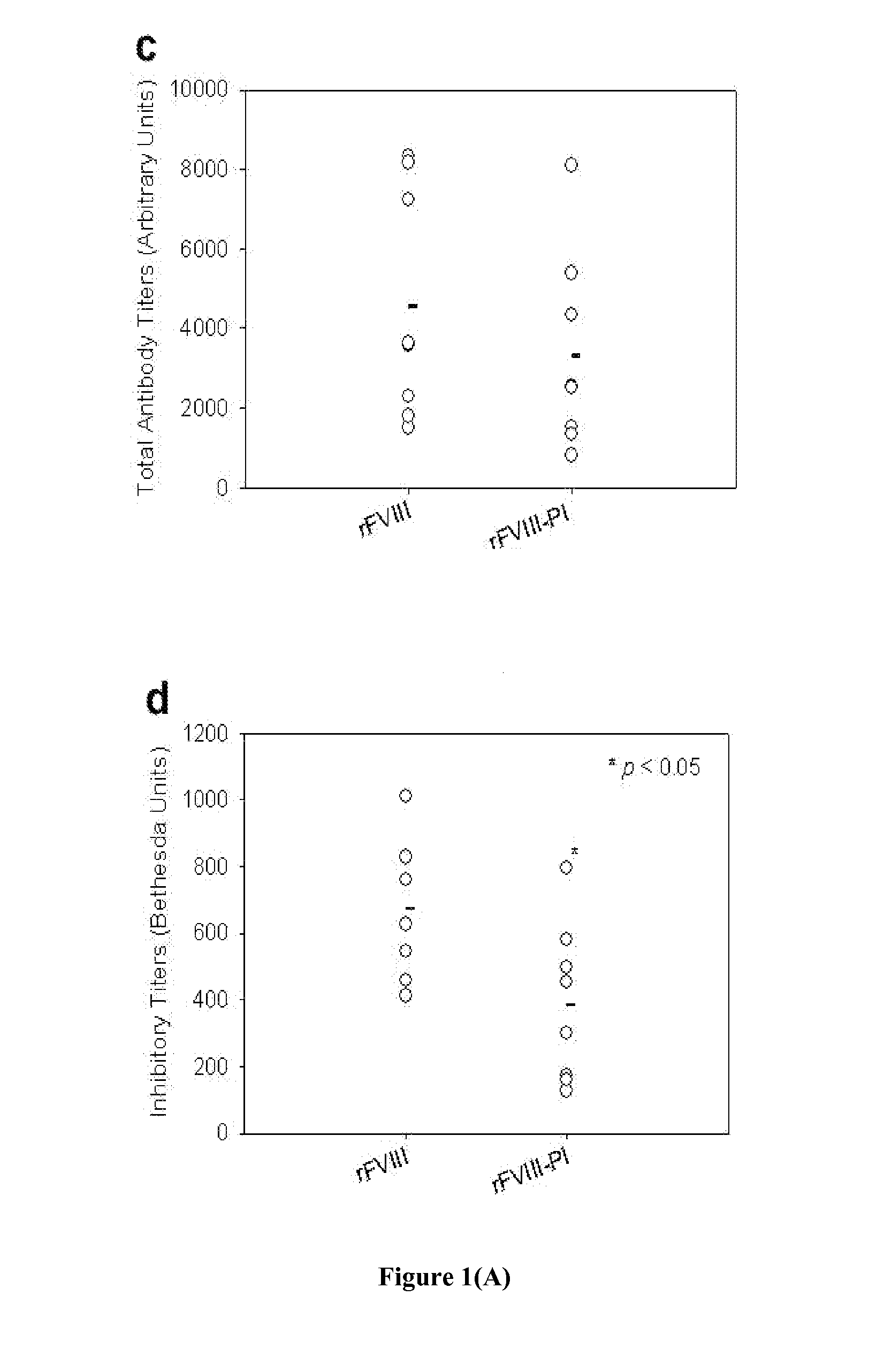
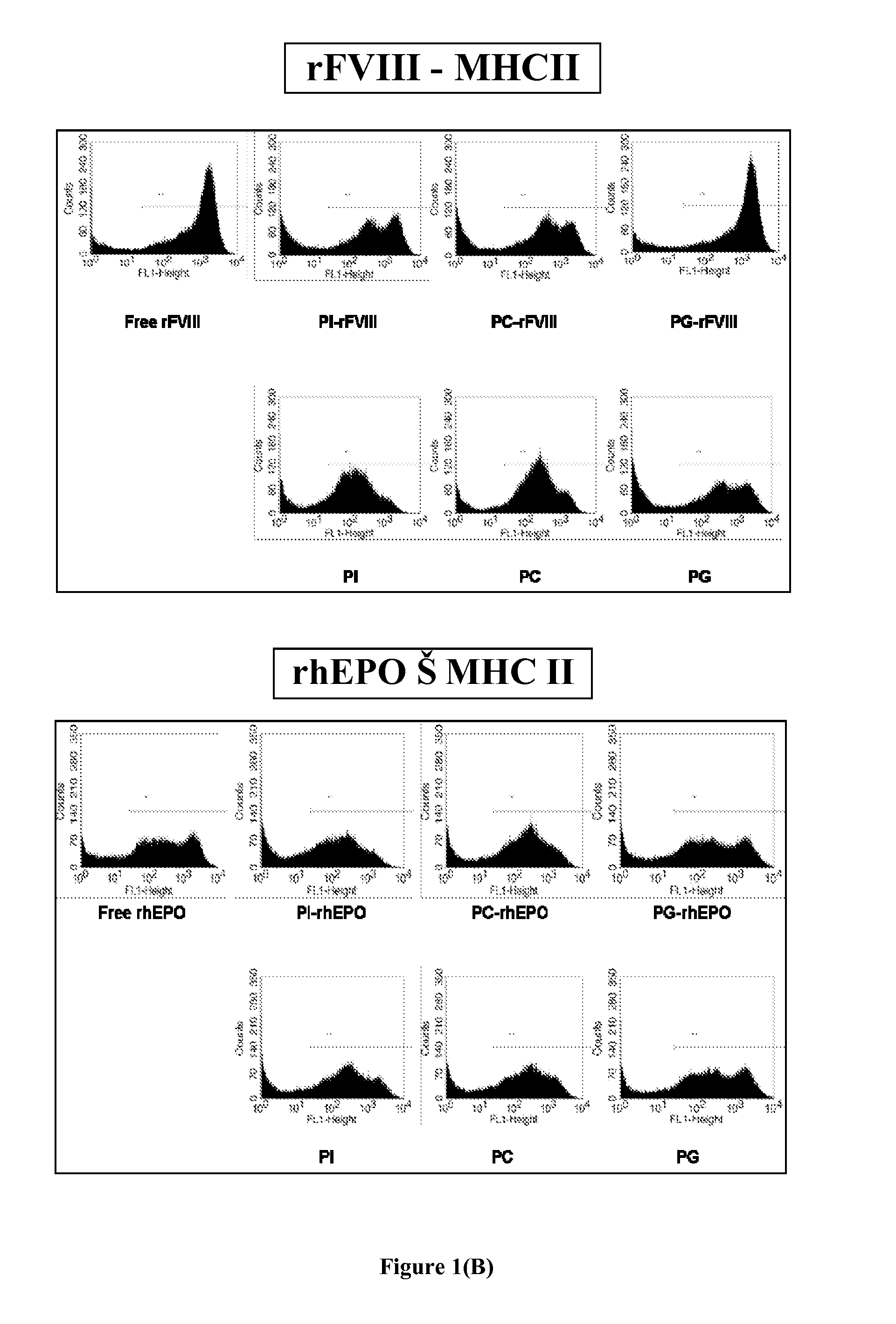
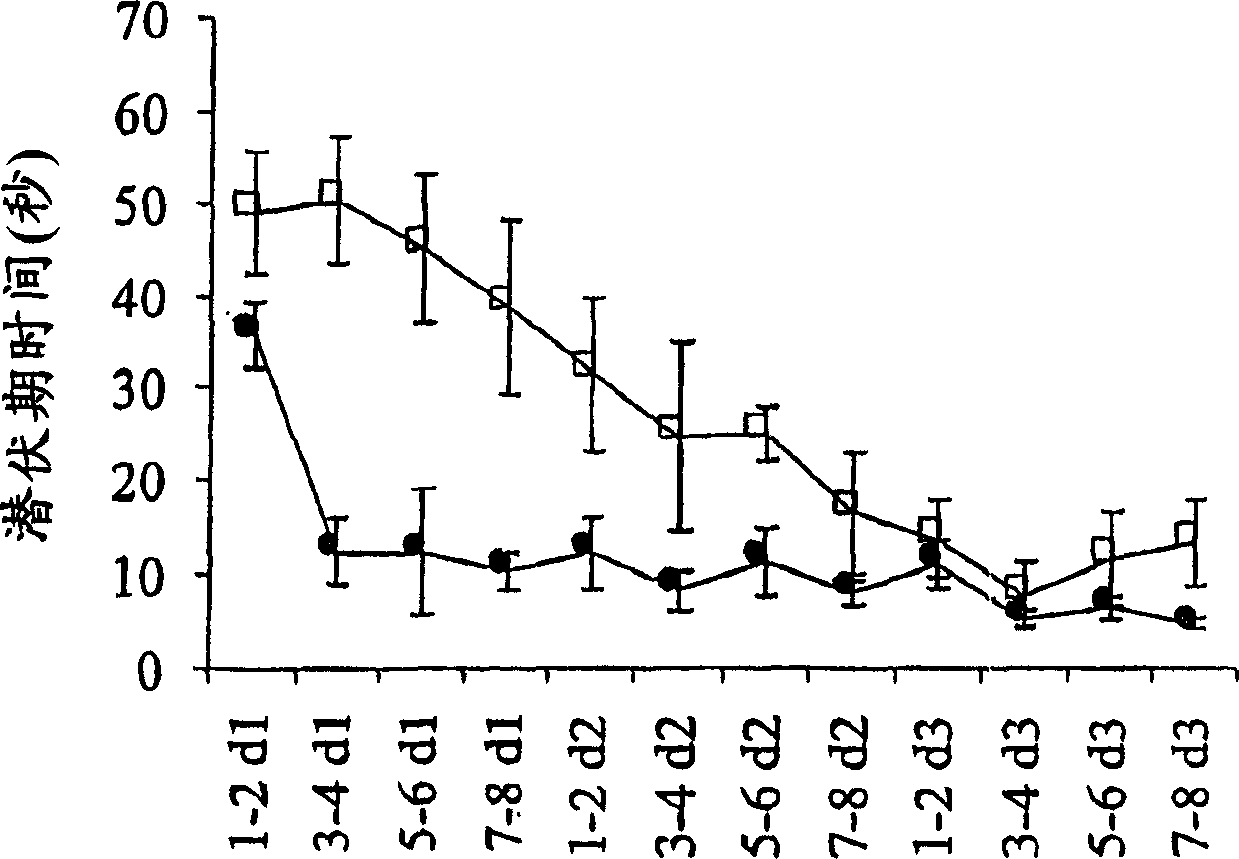
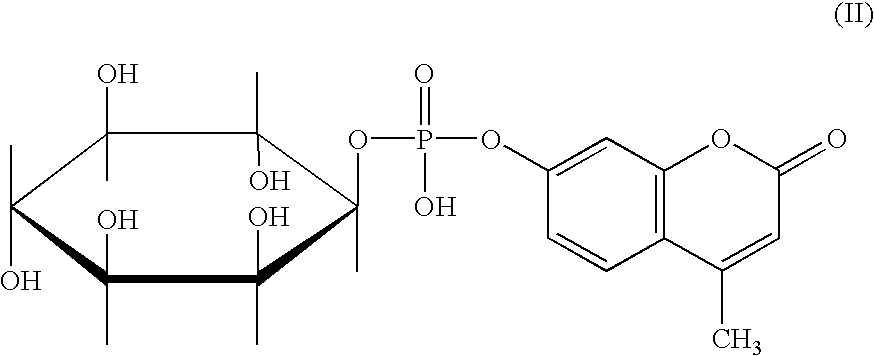
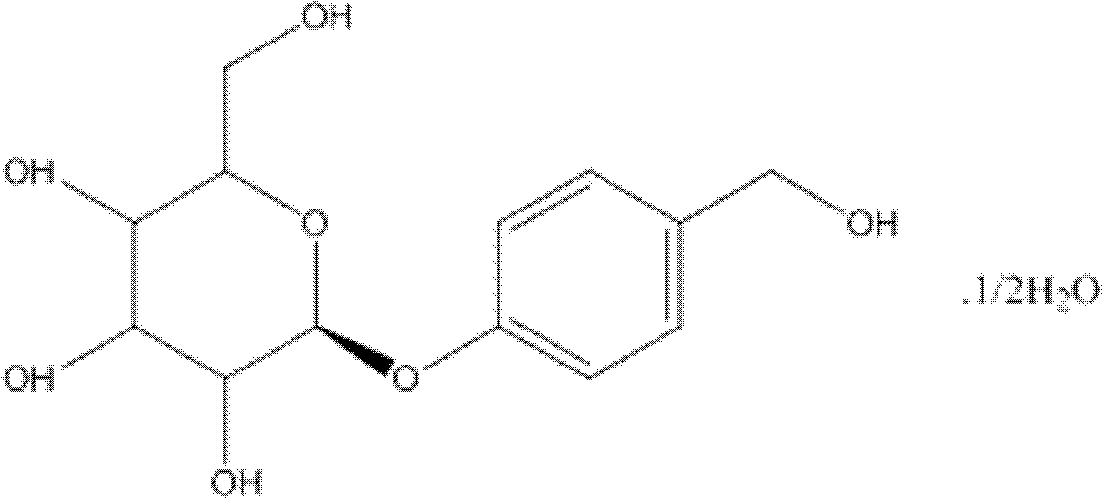
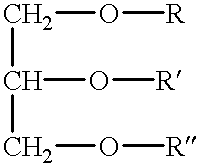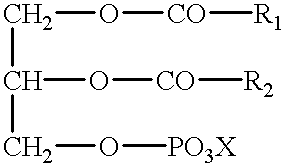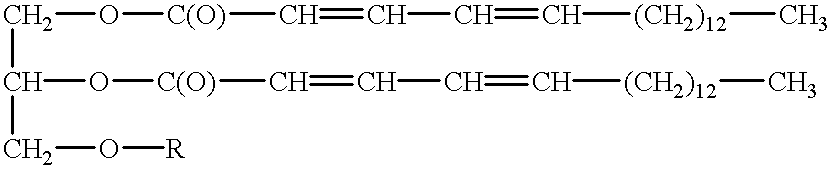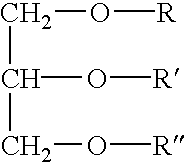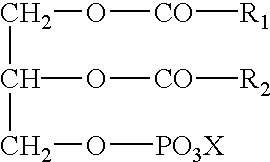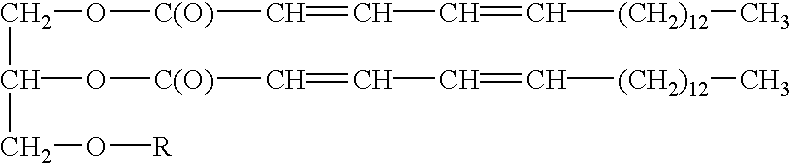Patents
Literature
83 results about "Phosphatidyl inositol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polymerizable fatty acids, phospholipids and polymerized liposomes therefrom
InactiveUS6187335B1Improve stabilityImprove abilitiesFatty acid chemical modificationOrganic chemistryIntestinal structureLipid formation
The invention relates to an oral drug delivery system which delivers biologically active substances to the mucosal tissue of the intestine utilizing novel polymerized liposomes. Novel polymerizable fatty acids having a polymerizable group, a surfactant group, and a functional group, and optionally coupled to ligands which target mucosal tissue in the intestine are disclosed. Novel negatively charged polymerizable lipids which have phosphatidyl inositol (PI), phosphatidyl glycerol (PG) or phosphatidyl serine (PS) groups on a polymerizable backbone are also described.
Owner:DOR BIOPHARMA
Polymerizable fatty acids, phospholipids and polymerized liposomes therefrom
InactiveUS6500453B2Improve stabilityImprove abilitiesBiocideFatty acid chemical modificationIntestinal structureLipid formation
The invention relates to an oral drug delivery system which delivers biologically active substances to the mucosal tissue of the intestine utilizing novel polymerized liposomes. Novel polymerizable fatty acids having a polymerizable group, a surfactant group, and a functional group, and optionally coupled to ligands which target mucosal tissue in the intestine are disclosed. Novel negatively charged polymerizable lipids which have phosphatidyl inositol (PI), phosphatidyl glycerol (PG) or phosphatidyl serine (PS) groups on a polymerizable backbone are also described.
Owner:ORASOMAL TECH
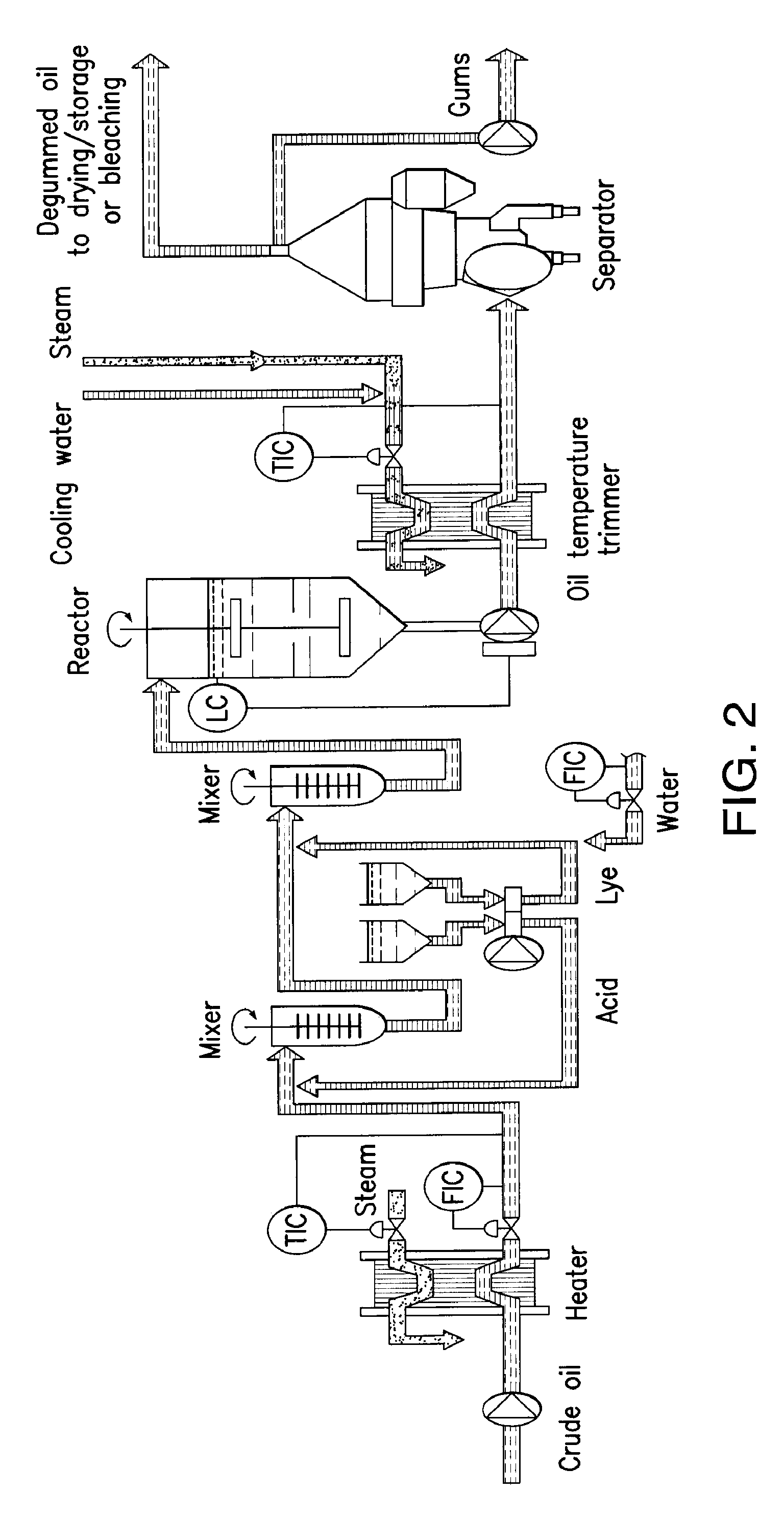
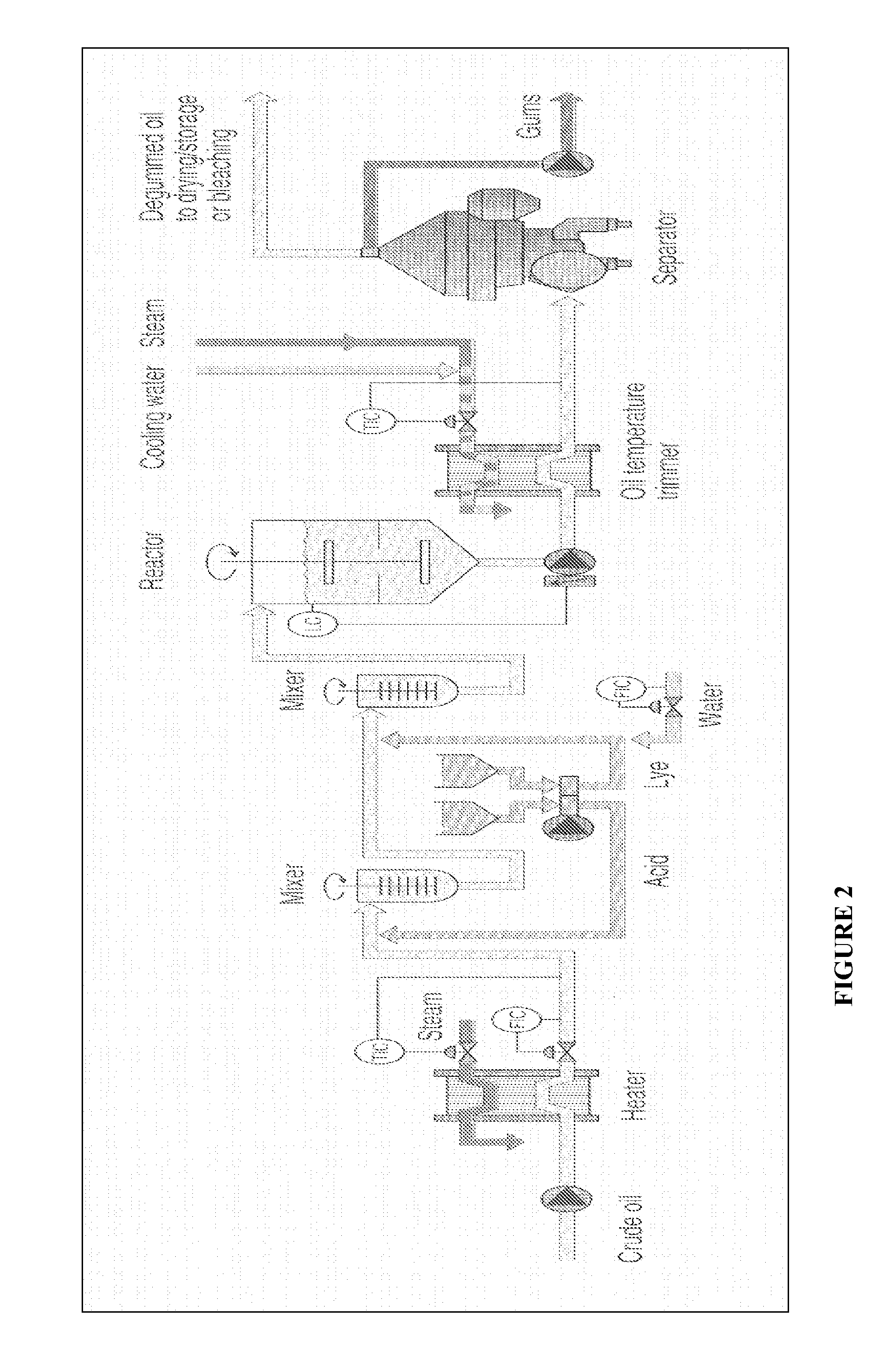
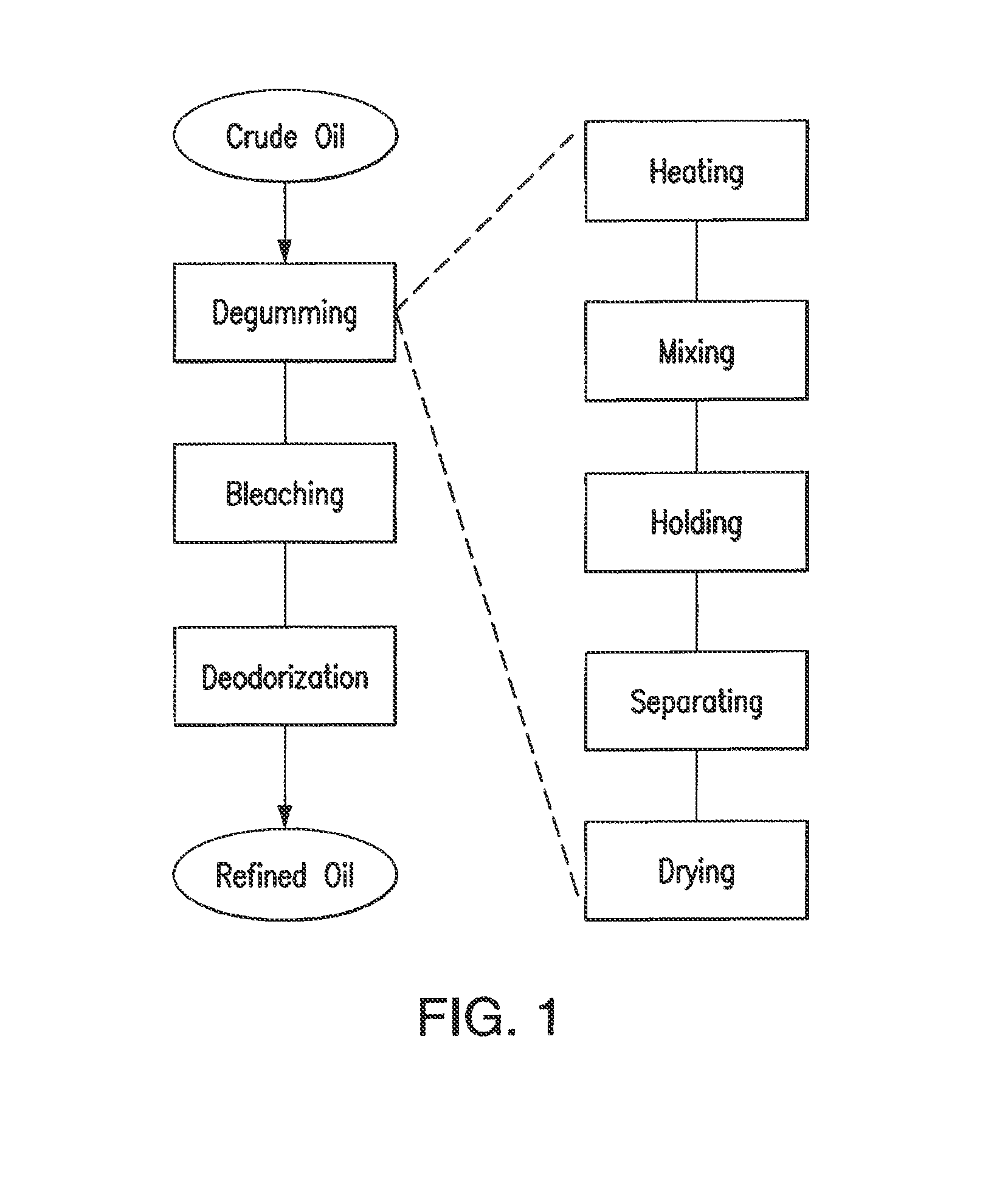
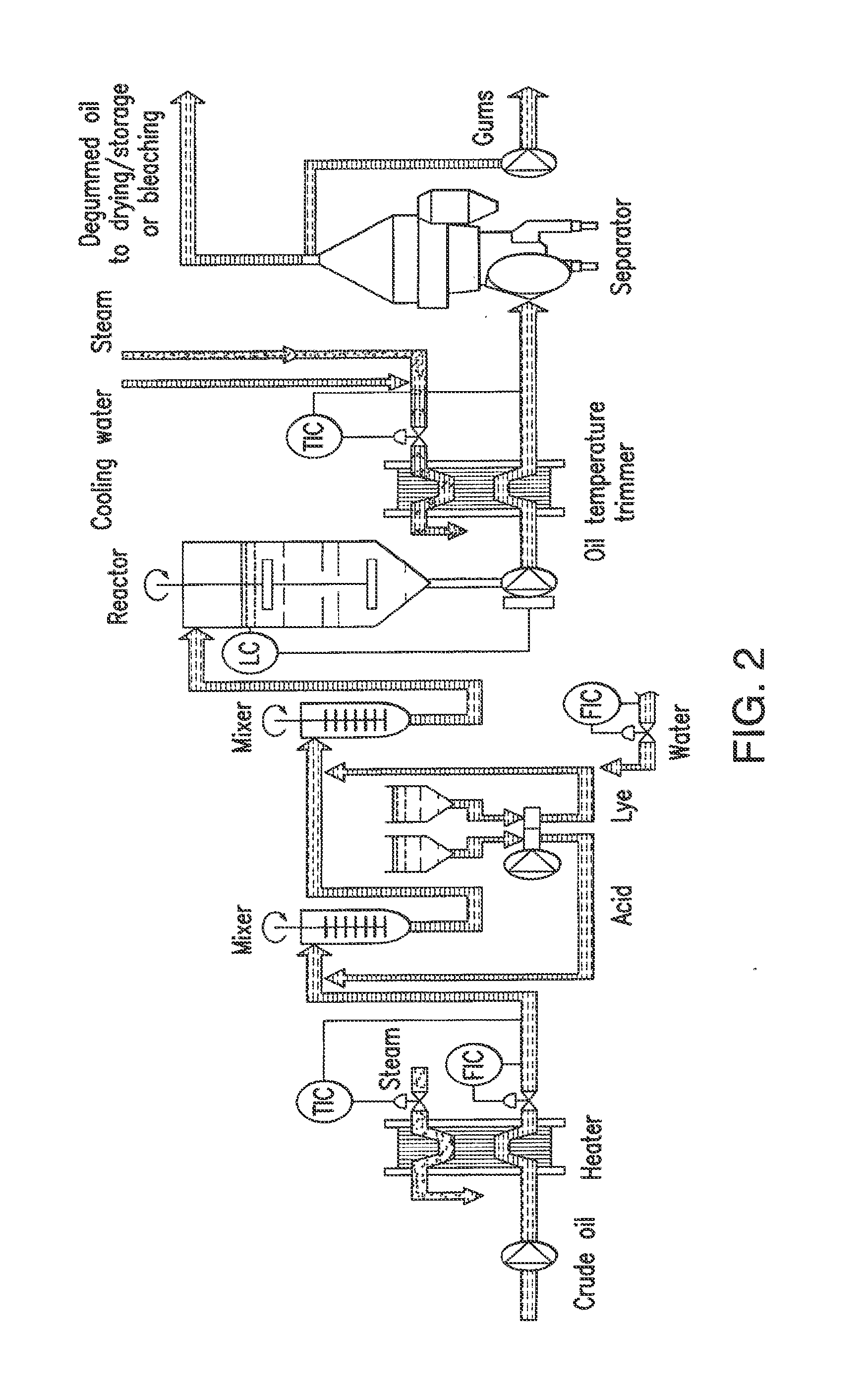
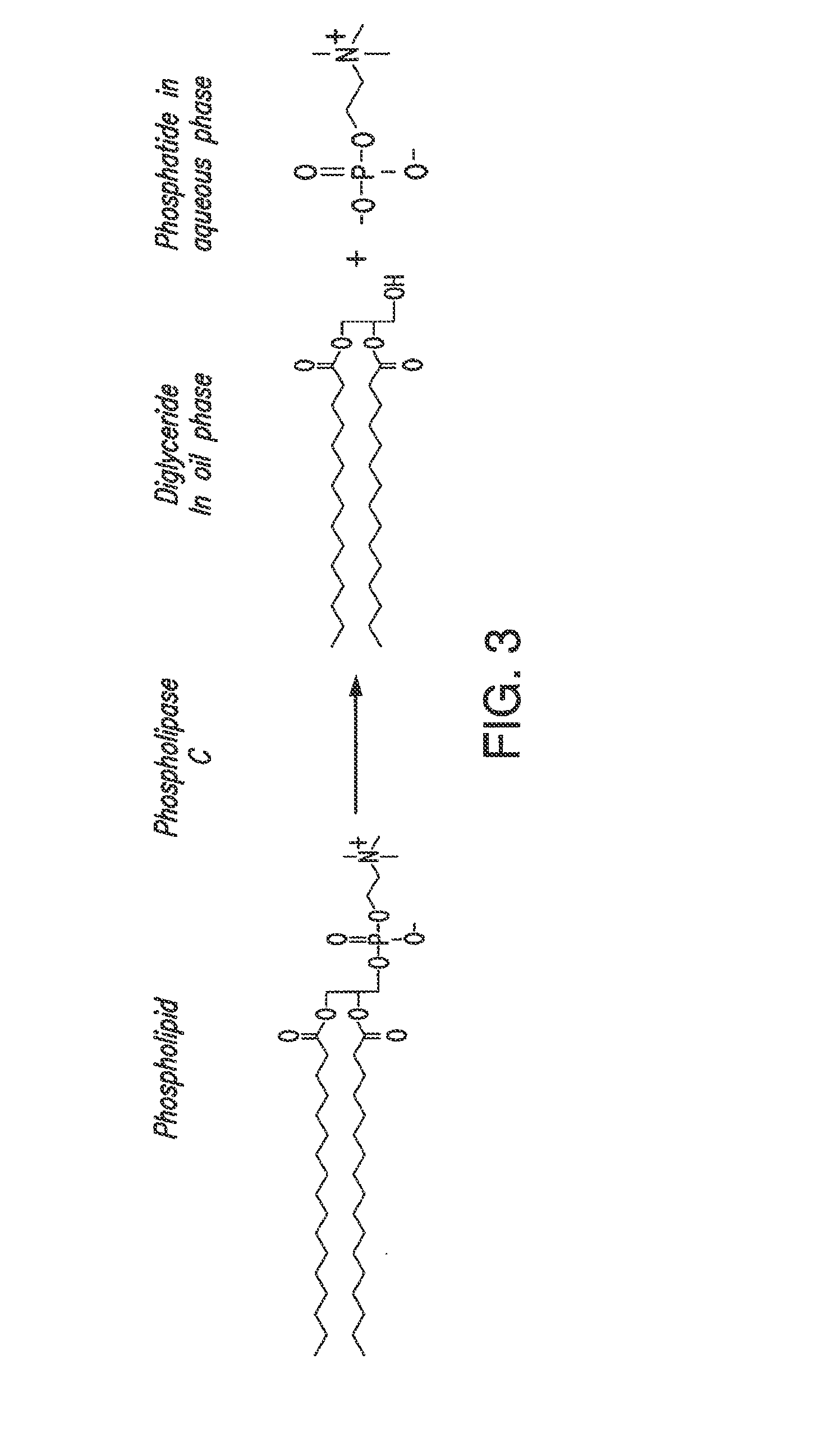
Oil degumming methods
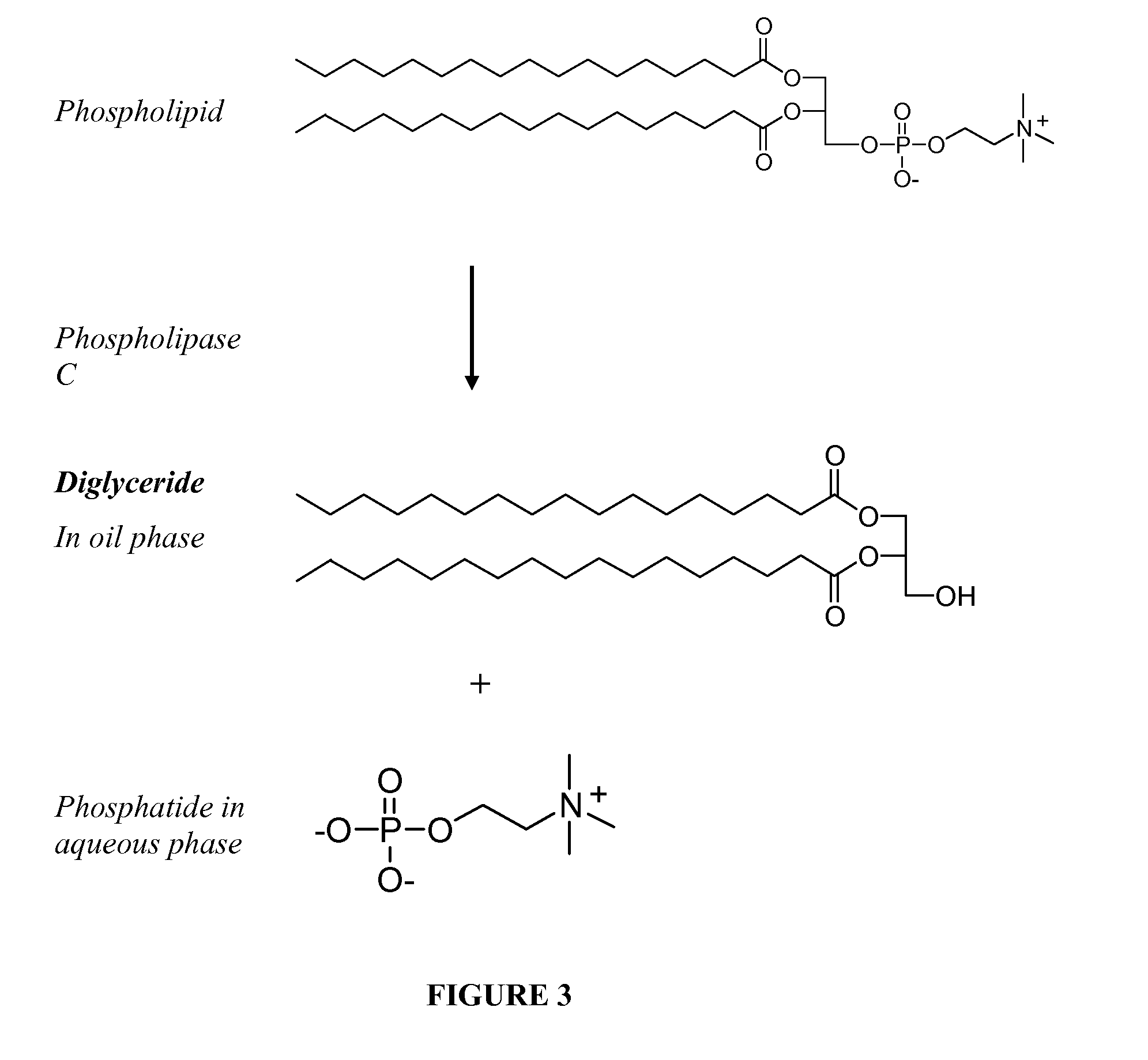
ActiveUS20130011887A1Improve reaction speedImprove heat resistanceFatty acids production/refiningOther chemical processesVegetable oilPhospholipase
In alternative embodiments, the invention provides phosphatidylinositol-specific phospholipase C (PI-PLC) enzymes, nucleic acids encoding them, antibodies that bind specifically to them, and methods for making and using them. Industrial methods and products comprising use of these phospholipases are also provided. In certain embodiments, provided herein are methods for hydration of non hydratable phospholipids (NHPs) within a lipid matrix. The methods enable migration of NHPs to an oil-water interface thereby allowing the NHPs to be reacted and / or removed from the lipids. In certain embodiments, provided is a method for removing NHPs, hydratable phospholipids, and lecithins from vegetable oils to produce a degummed oil or fat product that can be used for food production and / or non-food applications. In certain embodiments, provided herein are methods for hydration of NHPs followed by enzymatic treatment and removal of various phospholipids and lecithins. The methods provided herein can be practiced on either crude or water-degummed oils.
Owner:DSM IP ASSETS BV +1
Phospholipases, nucleic acids encoding them and methods for making and using them
ActiveUS20120210467A1Improve heat resistanceImprove thermal stabilityAntibacterial agentsImmobilised enzymesVegetable oilAntiendomysial antibodies
In alternative embodiments, the invention provides phosphatidylinositol-specific phospholipase C (PI-PLC) enzymes, nucleic acids encoding them, antibodies that bind specifically to them, and methods for making and using them. Industrial methods and products comprising use of these phospholipases are also provided. In certain embodiments, provided herein are methods for hydration of non hydratable phospholipids (NHPs) within a lipid matrix. The methods enable migration of NHPs to an oil-water interface thereby allowing the NHPs to be reacted and / or removed from the lipids. In certain embodiments, provided is a method for removing NHPs, hydratable phospholipids, and lecithins from vegetable oils to produce a degummed oil or fat product that can be used for food production and / or non-food applications. In certain embodiments, provided herein are methods for hydration of NHPs followed by enzymatic treatment and removal of various phospholipids and lecithins. The methods provided herein can be practiced on either crude or water-degummed oils.
Owner:DSM IP ASSETS BV
Compositions and methods for treating hypophosphatasia
ActiveUS20090238814A1Extend your lifeWeight increaseHydrolasesPeptide/protein ingredientsMembrane bound enzymeBone tissue
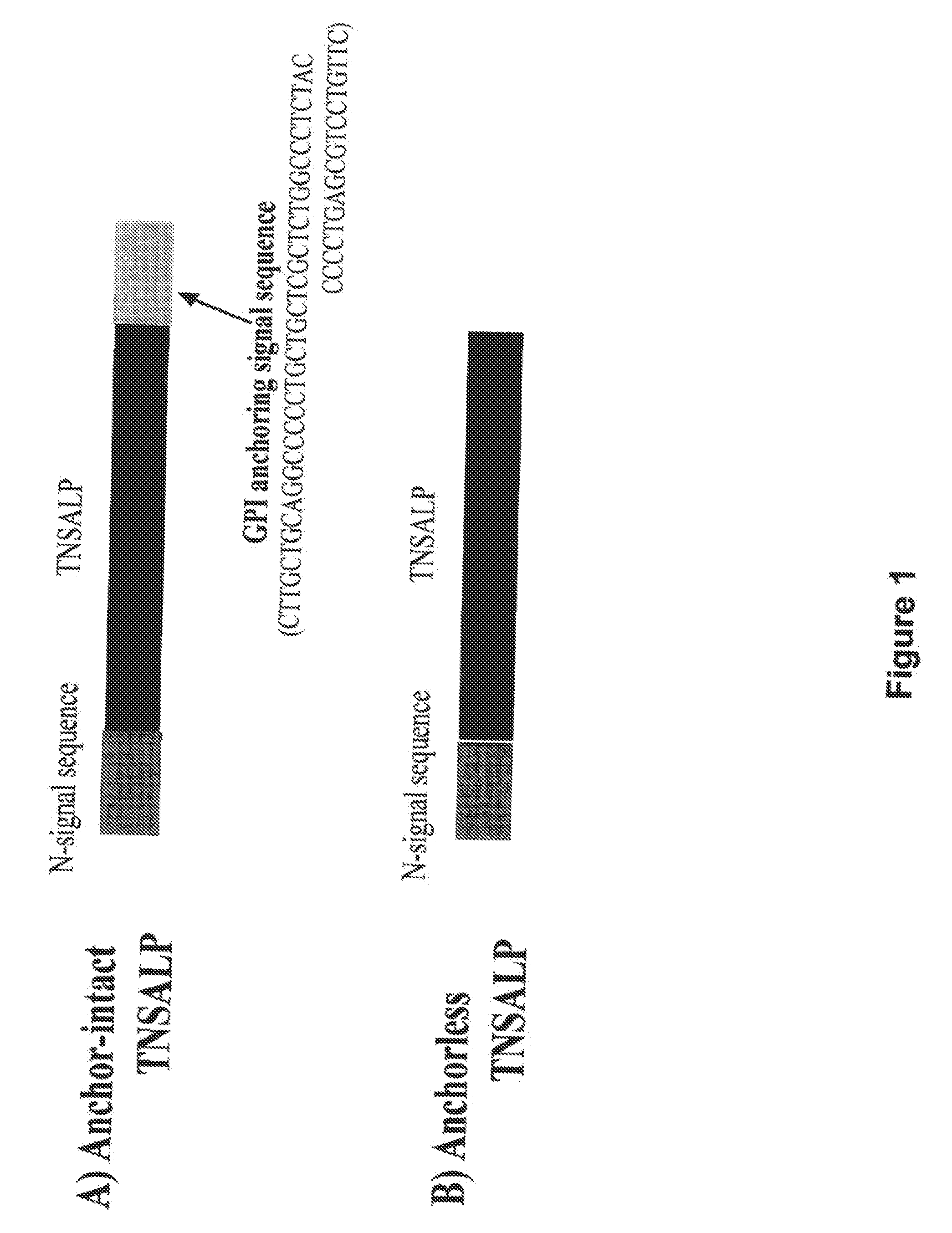
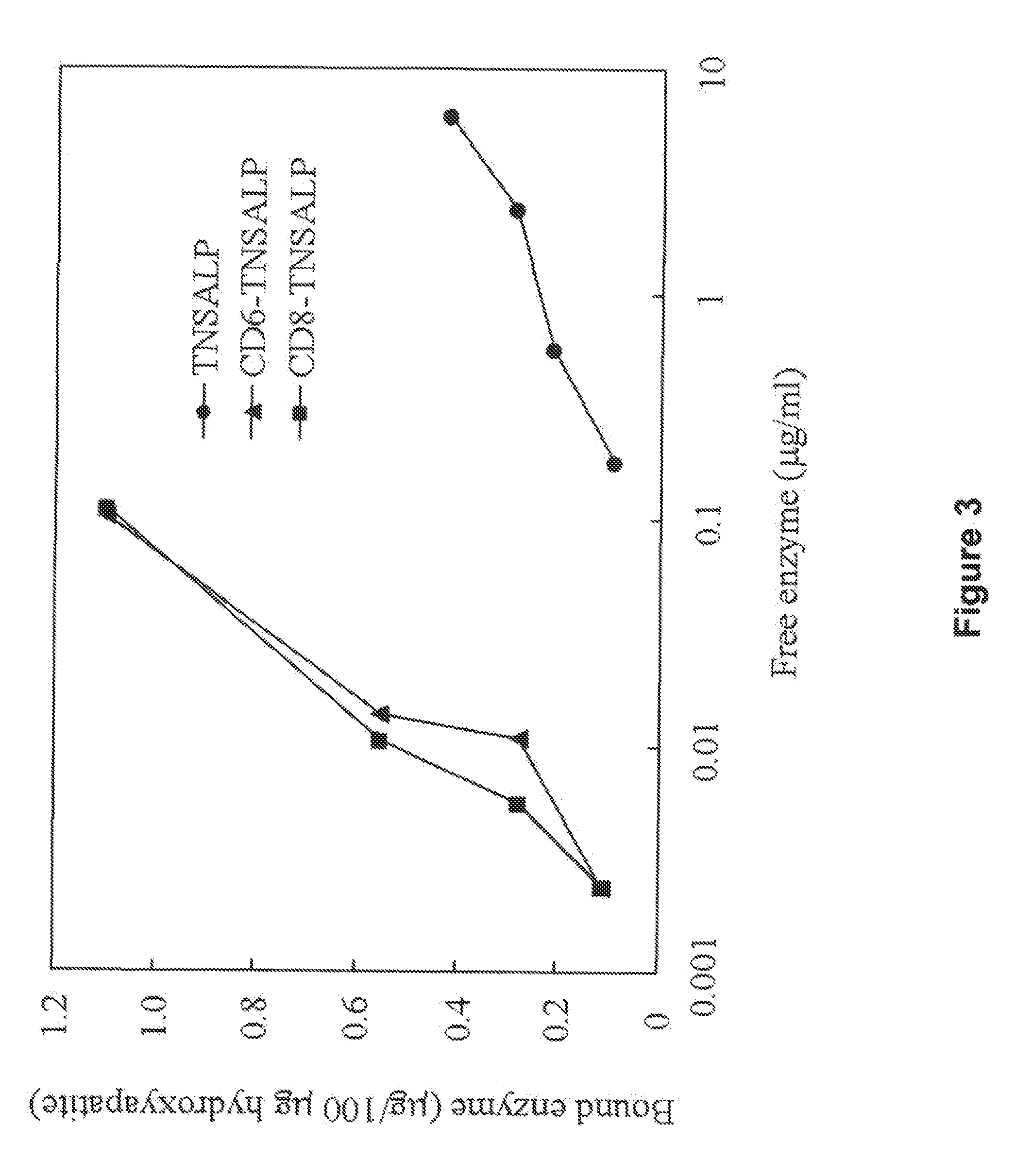
The present invention provides compositions and methods for use in enzyme replacement therapy. The inventors disclose a method of producing membrane bound enzymes in an active soluble form by eliminating the glycosylphosphatidylinositol (GPI) membrane anchor. In particular the inventors disclose a soluble active form of the membrane bound enzyme TNSALP which they produced by deleting the GPI anchor single peptide sequence. They have further shown that this composition is useful for treatment of hypophosphatasia. The inventors also disclose oligo acid amino acid variants thereof which specifically target bone tissue.
Owner:SAINT LOUIS UNIVERSITY +2
Polar lipid mixtures, their preparation and uses
InactiveUS20110294757A1Milk preparationOrganic active ingredientsPhosphatidyl inositolPhosphatidylethanolamine
Disclosed herein are polar lipid mixtures, comprising glycerophospholipids such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS) and phosphatidyl-inositol (PI), and sphingolipids such as sphyngomyelin (SM). Most importantly, the ratio of phospholipids in said mixture is comparable to that of HMF, and is represented by SM>PC>PE>PS>PI or SM=PC>PE>PS>PI. Processes for the preparation of said mixtures and uses thereof are also described herein.
Owner:ENZYMOTEC
Lipids containing omega-3 and omega-6 fatty acids
InactiveUS20080085319A1Enhance memoryReduce stressBiocideEdible oils/fats ingredientsLipid formationAcyl group
A lipid preparation including a glycerophospholipid or salt, conjugate and derivatives thereof, particularly phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidyl-inositol (PI), phosphatidylglycerol (PG) and phosphatidic acid (PA), and poly-unsaturated fatty acid (PUFA) acyl groups, particularly long-chain poly-unsaturated fatty acid (LC-PUFA) acyl groups such as omega-3 and / or omega-6 acyl groups, wherein said PUFA is covalently bound to said glycerophospholipid. The preparation possesses an improved bioactivity, and is useful in the treatment of various cognitive and mental conditions and disorders and for maintenance of normal functions of brain-related systems and processes.
Owner:ENZYMOTEC
Pidotimod liposome solid preparation
InactiveCN102579350AImprove bioavailabilityQuality improvementOrganic active ingredientsAntipyreticSide effectRetention time
The invention discloses a pidotimod liposome solid preparation and a preparation method thereof. The pidotimod liposome with excellent quality is prepared by selecting pidotimod, phosphatidylinositol, cholesterol, stigmasterol and cremophor HEL 40 in specific weight ratios and then the pidotimod liposome is prepared into the solid preparation by a common preparation method. The liposome solid preparation and preparation method provided by the invention have the following beneficial effects: the liposome solid preparation has high encapsulation efficiency and uniform grain size; the retention time of medicaments in blood circulation is long; and the preparation method is simple and practical, is beneficial to improvement of the product quality and reduction of the toxic or side effect and is suitable for industrial large-scale production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for enriching phosphatidyl inositol from antarctic krill
InactiveCN102746941AHigh enrichment efficiencyFatty-oils/fats productionPhosphatidyl inositolAntarctic krill
The invention discloses a method for enriching phosphatidyl inositol from antarctic krill. The method includes the steps: (1) adding 1 kilogram of frozen antarctic krill to 1-1.3 liters of ethanol solution with the volume fraction of 90-100%, stirring to extract for 4-6 times under natural conditions, keeping extraction each time for 1-3h, and filtering and combining extracting solutions so as to obtain filtrate; (2) concentrating the obtained filtrate under the pressure of negative 0.07-negative 0.09Mpa at the temperature of 60-65 DEG C until 8-10% of the volume of the filtrate remains so as to obtain concentrated extracting solution; (3) adding isometric normal hexane into the obtained concentrated extracting solution, uniformly mixing and statically layering so as to obtain transparent reddish bottom solution; and (4) removing the normal hexane from the obtained bottom solution by vaporizing under the pressure of negative 0.07-negative 0.09Mpa at the temperature of 50-55 DEG C so as to obtain oily solution, namely, a phosphatidyl-inositol-enriched product, wherein the product comprises 2.70-2.76% of phospholipid, and the phosphatidyl inositol accounts for 50.5-51.92% of the phospholipid. Re-extraction, separation, purification and the like can be further performed on the basis so as to obtain the phosphatidyl inositol.
Owner:SHANDONG NORMAL UNIV
Method for heightening salt tolerance and drought tolerance of cotton by polymeric stress-resistant gene
InactiveCN101624604AImprove salt and drought toleranceVector-based foreign material introductionPlant genotype modificationEscherichia coliAgricultural science
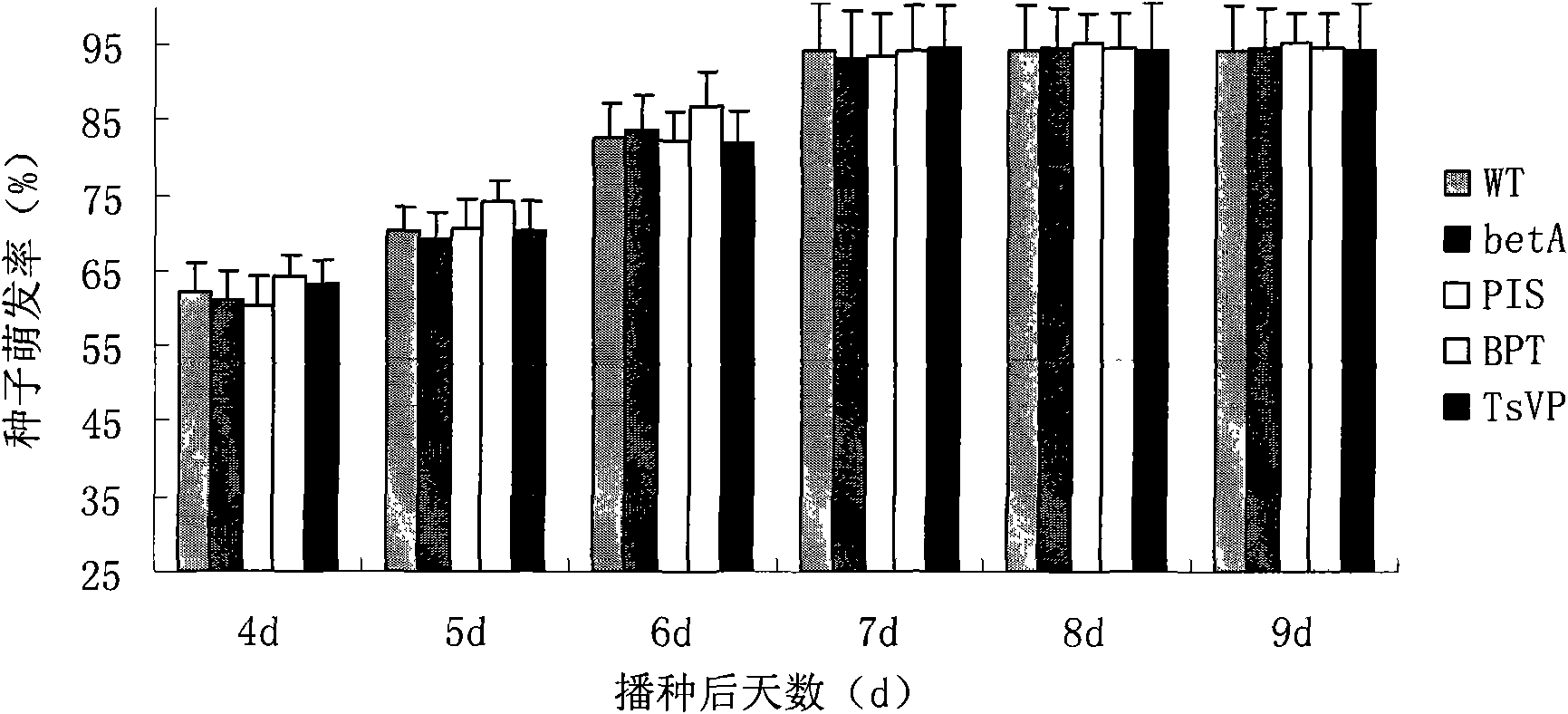
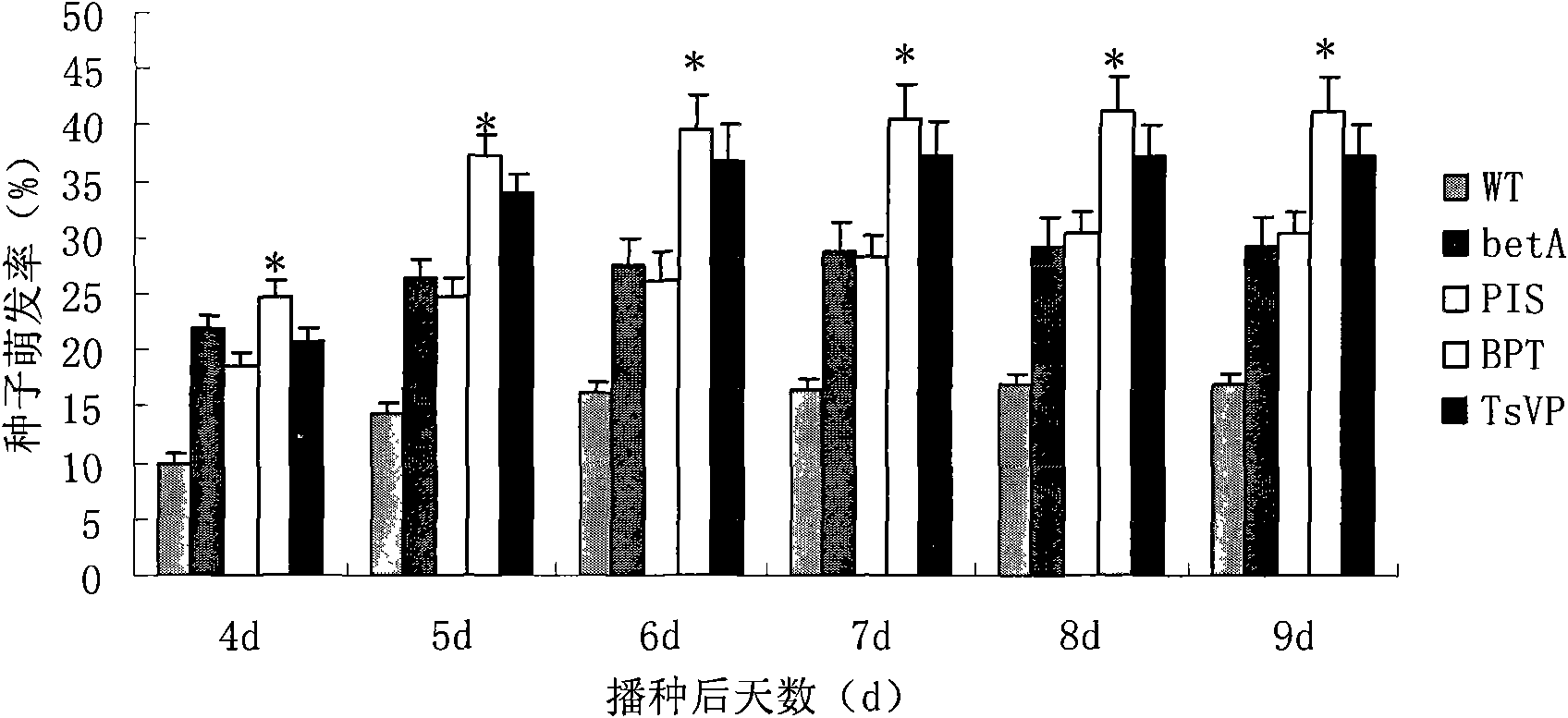
The invention discloses a method for heightening the salt tolerance and the drought tolerance of cotton by polymeric stress-resistant gene, comprising the following steps of: recombining phosphatidyl inositol synthetase gene PIS from a signal transduction pathway of a plant, tonoplast pyrophosphatase gene Ppase isolated from an ion region, and choline dehydrogenase gene betA which is from colon bacillus and gives glycine betaine synthesis capability into a plant expression carrier; transplanting the recombinant into cotton cells to be effectively expressed; regenerating transgene plantlet; selecting transgene homozygote with the obviously heightened salt tolerance and drought tolerance from the transgene plantlet and filial generation thereof; and breeding out new cotton germplasm with good salt tolerance and salt tolerance, so as to create new material for breeding the cotton with good salt tolerance and salt tolerance for breeding the conventional variety or hybrid variety of the cotton.
Owner:SHANDONG UNIV
Lipids containing omega-3 and omega-6 fatty acids
InactiveUS20080085320A1Enhance memoryReduce stressOrganic active ingredientsBiocideLipid formationAcyl group
Owner:ENZYMOTEC
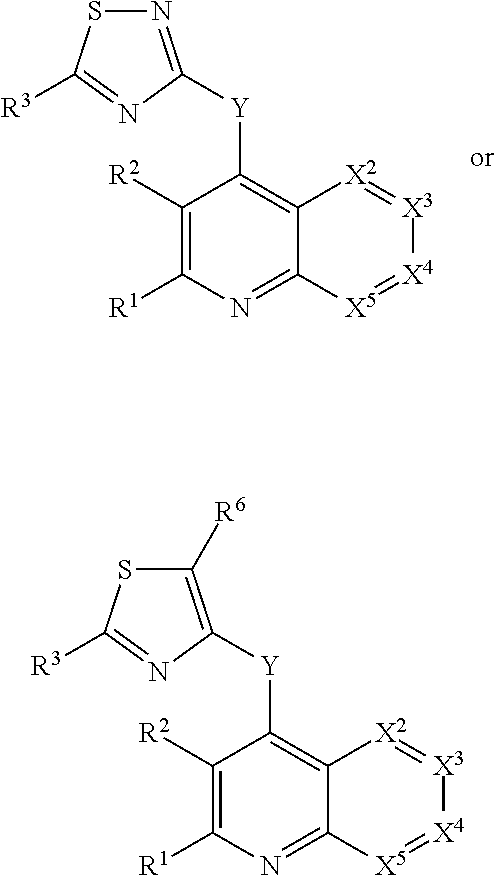
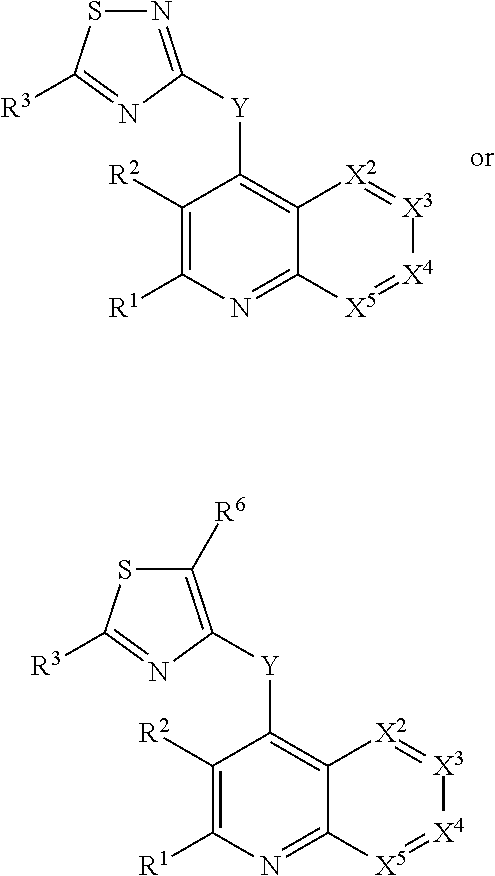
Condensed heteroaryl derivatives
The present invention provides a pharmaceutical composition which is useful as a phosphatidylinositol 3 kinase (PI3K) inhibitor and an antitumor agent, and it provides a novel bicyclic or tricyclic fused heteroaryl derivative or a salt thereof which possesses an excellent PI3K inhibiting activity and cancer cell growth inhibiting activity.
Owner:ASTELLAS PHARMA INC +2
Oil degumming methods
ActiveUS20140371476A1Improve heat resistanceImprove thermal stabilityOrganic chemistryPhosphorus-oxygen lyasesPhospholipaseVegetable oil
In alternative embodiments, the invention provides phosphatidylinositol-specific phospholipase C (PI-PLC) enzymes, nucleic acids encoding them, antibodies that bind specifically to them, and methods for making and using them. Industrial methods and products comprising use of these phospholipases are also provided. In certain embodiments, provided herein are methods for hydration of non hydratable phospholipids (NHPs) within a lipid matrix. The methods enable migration of NHPs to an oil-water interface thereby allowing the NHPs to be reacted and / or removed from the lipids. In certain embodiments, provided is a method for removing NHPs, hydratable phospholipids, and lecithins from vegetable oils to produce a degummed oil or fat product that can be used for food production and / or non-food applications. In certain embodiments, provided herein are methods for hydration of NHPs followed by enzymatic treatment and removal of various phospholipids and lecithins. The methods provided herein can be practiced on either crude or water-degummed oils.
Owner:BUNGE GLOBAL INNOVATION LLC
Preparationj process of high pureness phosphatidyl inositol
The invention relates to a high-purity PI preparing method. The solubility of PE is ov higher in basic alcohol than alcohol, using the basic alcohol to extract can effectively eliminate PC and PE from soybean phospholipids; dissolving the obtained PI crude product in a nonpolar solvent, adding in basic substance-containing hydrous polar solvent for liquid-liquid extraction, extracting PI into the hydrous polar solvent, and then adding in metallic salt for purifying, able to obtain high-purity PI.
Owner:SHANDONG NORMAL UNIV
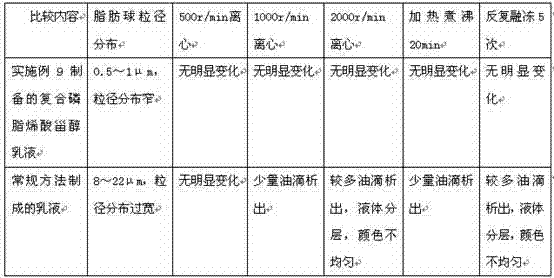
Water-based emulsion containing lecithin and DHA and preparation method and application of water-based emulsion
InactiveCN104490773AImprove bioavailabilityCosmetic preparationsOrganic active ingredientsGlycerolConjugated linoleic acid
The invention provides an emulsion composition containing lecithin, DHA, other fat-soluble substances and purified water. The content of the purified water in the emulsion composition is more than 70% by weight, the weight ratio of the lecithin to the DHA is 1: (0.005-1), the particle size of fat balls in the emulsion is less than 5 mu m, and the other fat-soluble substances comprise one or more of the following components: arachidonic acid, linolenic acid, alpha-linolenic acid, gamma-linolenic acid, linoleic acid, conjugated linoleic acid, conjugated linoleic acid glyceride, phosphatidylserine, polyene phosphatidyl choline, phosphatidyl ethanolamine, phosphatidyl inositol, phosphatidyl glycerol, phytosterol, plant sterol ester, plant stanol ester and medium and long-chain fatty acid. The invention further provides a preparation method of the emulsion composition and application of the emulsion composition in the functional aspects of supplementing phospholipids and unsaturated fatty acids by oral administration, protecting heart and brain blood vessels, reducing blood lipids, reducing blood pressure, treating fatty liver and the like.
Owner:FUZHOU QIANZHENG PHARMA
Lipidic Compositions for Induction of Immune Tolerance
InactiveUS20120164189A1Improve the level ofDecrease in ILSSPowder deliverySnake antigen ingredientsL serineTolerability
This invention provides a method for inducing immune tolerance toward an antigen comprising the antigen in lipidic particles or lipidic compositions. The lipidic particles are made up of phosphatidylserine and phosphatidylcholine, or phosphatidylinositol and phosphatidylcholine. The lipidic compositions comprise the antigen and O-phospho-L-serine. Administration of these composition results in inducing immune tolerance to the antigen.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Glycerophospholipids containing omega-3 and omega-6 fatty acids
InactiveCN1897955AGuaranteed functionImprove and treat ADHDOrganic active ingredientsSenses disorderGlycerolOmega-6 fatty acid
Disclosed is a lipid preparation comprising a glycerophospholipid or salt, conjugate and derivatives thereof, particularly phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidyl-inositol (PI), phosphatidylglycerol (PG) and phosphatidic acid (PA), and poly-unsaturated fatty acid (PUFA) acyl groups, particularly long-chain poly-unsaturated fatty acid (LC-PUFA) acyl groups such as omega-3 and / or omega-6 acyl groups, wherein said PUFA is covalently bound to said glycerophospholipid. The disclosed preparations possess an improved bioactivity, and are useful in the treatment of various cognitive and mental conditions and disorders and for maintenance of normal functions of brain-related systems and processes, for example ADHD.
Owner:ENZYMOTEC
Potentially fluorogenic compounds
InactiveUS6416970B1Avoid disadvantagesMicrobiological testing/measurementGroup 5/15 element organic compoundsPhosphateStaphylococcus species
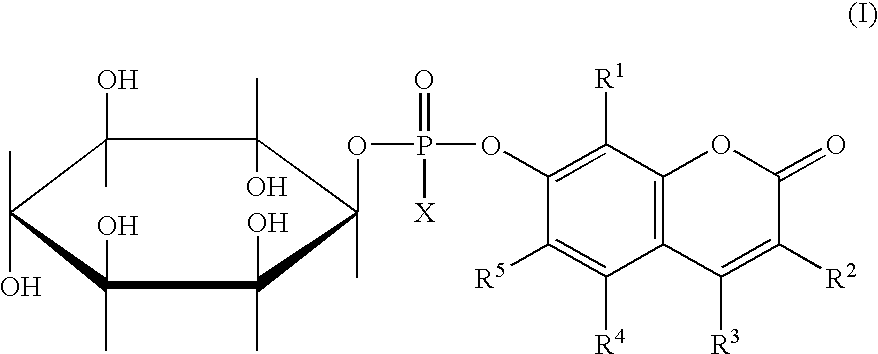
The invention provides compounds of formula (I) in which R1, R2, R3, R4 and R5 are independently selected from hydrogen and chromogenic substituents, and X is selected from the group consisting of hydroxyl; OR6 wherein R6 is selected from the group consisting of C1-C4 alkyl; and O-Me+ wherein Me+ is a cation derived from an organic or inorganic base. A preferred species of formula (I) is 4-Methylumbelliferyl myo-inositol-1-phosphate and salts thereof with an organic or inorganic base. The invention also provides fluorogenic methods for detecting various pathogenic bacteria, e.g., Listeria, Staphylococcus and Clostridium species, using substrates containing at least one formula (I) compound. A kit for detecting a phosphatidyl-inositol-specific phospholipase C enzyme as an indication of bacterial activity is disclosed.
Owner:BIOSYNTH
Method for preparing compound of vegetative phosphatidylinositol in high purity
InactiveCN1727351ASimple production processThe production process is effectivePhosphorous compound active ingredientsEmulsion deliveryHuman cancerCancer cell
A process for preparing the high-purity (more than 96%) vegetative phosphatidylinositol (PI) from soybean includes such steps as extracting in solvent, separating, and purifying by macroreticular resin chromatography. Said PI has high activity of selectively killing more kinds of human cancer cells and preventing and treating senile dementia.
Owner:商宗一
Thymalfasin liposome preparation for injecting
InactiveCN102579347ARound shape and not aggregatedImprove stabilityPeptide/protein ingredientsDigestive systemSolubilitySucrose
The invention discloses a thymalfasin liposome preparation for injecting and a preparation method thereof. The thymalfasin liposome preparation for injecting is prepared from thymalfasin, cholesterol, egg yolk lecithin, phosphatidyl inositol, sucrose ester and a pharmaceutically-acceptable carrier in a specific weight ratio, wherein the carrier is preferably mannitol and fucose. A liposome injection disclosed by the invention has high preparation stability, a liposome is prevented from cracking due to fusion, ice crystals and the like in a cooling process, and the liposome still keeps high packing rate and high stability after long-time storage. Due to the adoption of the thymalfasin liposome preparation, the solubility of thymalfasin is increased, the quality of a preparation product is improved, toxic and side effects are reduced, the retaining time of a medicament in body circulation is prolonged, the bioavailability of the medicament is increased, and a curative effect is increased remarkably; and moreover, the preparation method is simple, and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for enriching phosphatidyl inositol from antarctic krill
InactiveCN102746941BHigh enrichment efficiencyFatty-oils/fats productionPhosphatidyl inositolConcentrated extract
The invention discloses a method for enriching phosphatidyl inositol from antarctic krill. The method includes the steps: (1) adding 1 kilogram of frozen antarctic krill to 1-1.3 liters of ethanol solution with the volume fraction of 90-100%, stirring to extract for 4-6 times under natural conditions, keeping extraction each time for 1-3h, and filtering and combining extracting solutions so as to obtain filtrate; (2) concentrating the obtained filtrate under the pressure of negative 0.07-negative 0.09Mpa at the temperature of 60-65 DEG C until 8-10% of the volume of the filtrate remains so as to obtain concentrated extracting solution; (3) adding isometric normal hexane into the obtained concentrated extracting solution, uniformly mixing and statically layering so as to obtain transparent reddish bottom solution; and (4) removing the normal hexane from the obtained bottom solution by vaporizing under the pressure of negative 0.07-negative 0.09Mpa at the temperature of 50-55 DEG C so as to obtain oily solution, namely, a phosphatidyl-inositol-enriched product, wherein the product comprises 2.70-2.76% of phospholipid, and the phosphatidyl inositol accounts for 50.5-51.92% of the phospholipid. Re-extraction, separation, purification and the like can be further performed on the basis so as to obtain the phosphatidyl inositol.
Owner:SHANDONG NORMAL UNIV
Gastrodin multiphase liposome injection
InactiveCN102626390AHigh encapsulation efficiencySmall toxicityOrganic active ingredientsSenses disorderCholesterolSoybean Phospholipids
The invention discloses a gastrodin multiphase liposome injection and a preparation method thereof. Gastrodin, soybean phosphatidylinositol, cholesterol, Twain 80, polyoxyethylene 40, hydrogenated castor oil, trehalose and EDTA-2Na which have specific weight ratios are prepared into the gastrodin multiphase liposome injection. The gastrodin multiphase liposome injection provided by the invention has excellent preparation stability and meets the demand of intravenous injection; after the gastrodin multiphase liposome injection is stored for a long time, the excellent encapsulation of the liposome is kept; the solubility of gastrodin is increased; the quality of preparation product is increased; the toxic side effect is reduced; the retention time of medicine in systemic circulation is increased; the bioavailability of the medicine is increased; the curative effect is obviously increased; and the preparation method is simple and is suitable for industrial production in bulk.
Owner:灵康药业集团股份有限公司
Method for detecting impurities in choline alfoscerate
The invention discloses a method for detecting impurities in choline alfoscerate. The method comprises the following steps: with a mixed solution of 0.0005-0.002mol / L of ammonium formate solution and acetonitrile as a moving phase A and with a mixed solution of 0.03-0.05mol / L of ammonium formate solution and acetonitrile as a moving phase B, performing primary liquid chromatography detection on a choline alfoscerate test sample solution by a binary gradient elution process to obtain the contents of the impurities glycerol, choline chloride and glycerol phosphatidyl inositol; with acetonitrile and water as a moving phase, performing secondary liquid chromatography detection on the choline alfoscerate test sample to obtain the content of the impurity, namely glycerin phosphatidyl ethanolamine. According to the method provided by the invention, different chromatographic conditions can be set up for detecting different impurities so as to obtain the contents of all the known impurities, so that one-sidedness of judging the quality of the choline alfoscerate by only detecting some impurities and an aim of monitoring the quality of the choline alfoscerate comprehensively and effectively is achieved.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Method for detecting PTEN (Phosphatase and tensin homolog) gene and PI3K/AKT protein and application of method in cancer treatment
InactiveCN103361430AThe test result is accurateReliable test resultsMicrobiological testing/measurementBiological testingA-DNAOncology
Owner:BEIJING QINLAN BIOTECH
Injection containing nervonic acid and phospholipid compound as well as preparation method and application of injection
ActiveCN104523715ASmall particle sizeNarrow particle size distributionOrganic active ingredientsNervous disorderDiseaseNervonic acid
The invention provides a fat milk injection containing phospholipid, nervonic acid and water for injection. In the injection, the content of the water for injection is larger than 70% in weight ratio; the particle size of fat globules in the injection is smaller than 1 micrometer; the phospholipid comprises lecithin, phosphatidyl ethanolamine and phosphatidyl inositol, wherein the phospholipid and the nervonic acid are combined by a weight ratio of 1:(0.005-1). The invention also provides a preparation method of the fat milk injection and application of the fat milk injection in injection and replenishment of the nervonic acid, the phospholipid, unsaturated fatty acid and medicines for preventing and treating diseases such as cranial nerve degenerative changes, cerebral injuries and disordered brain function.
Owner:哈尔滨医大药业股份有限公司
Traditional Chinese medicine preparation for treating cirrhosis and preparation method thereof
PendingCN105853947ATo achieve the goal of treating both symptoms and root causesNo side effectsDigestive systemAlgae medical ingredientsSide effectBULK ACTIVE INGREDIENT
The invention discloses a traditional Chinese medicine preparation for treating cirrhosis and a preparation method thereof. The traditional Chinese medicine preparation is prepared from, by weight, 3-5 parts of hirudo, 1-3 parts of Rhizoma curcumae, 10-20 parts of Pu-er raw tea, 5-10 parts of Radix morindae officinalis, 0.2-0.8 part of pig lung extract, 5-20 parts of kelp, 5-15 parts of root of Litsea glutinosa and 10-25 parts of fried hedgehog hide, wherein active ingredients of pig lung extract are one or multiple of phosphatidylcholine, dipalmitoyl phosphatidylcholine, phosphatidyl ethanolamine, phosphatidylserine, phosphatidyl-inositol and sphingomyelin. The preparation method includes: subjecting raw materials to smashing, water extracting or supercritical CO2 extracting and mixing. A principle of regulating kidney, gallbladder and spleen at the same time is adopted, the traditional Chinese medicine preparation has efficacy of clearing heat for detoxification, soothing liver for choleresis, promoting diuresis to treat stranguria, calming liver to regulate qi, activating blood to remove stasis, strengthening heart to lower blood pressure, dispelling wind to stop pain and regulating immunity, is safe, reliable, free of toxic and side effect, short in treatment course, quick in action, low in cost, capable of treating both symptoms and root causes and worthy of wide popularization and application clinically.
Owner:THE SECOND HOSPITAL OF DALIAN MEDICAL UNIV
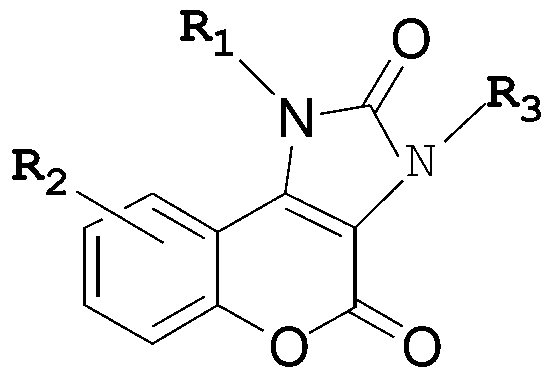
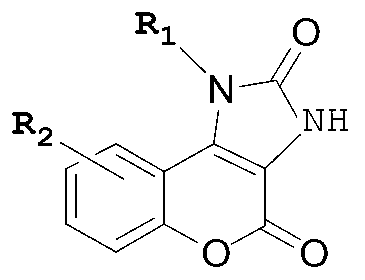
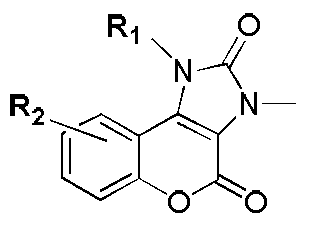
Five-membered urea ring-coumarin derivative or pharmaceutical salt and application thereof
The invention relates to a small molecule medicament based on PI3K (Phosphatidyl Inositol 3-kinase) / mTOR (Mammalian Target of Rapamycin) double targets, belongs to the technical field of chemical medicines, and in particular relates to a five-membered urea ring-coumarin derivative or a pharmaceutical salt and an application thereof. A compound claimed by the invention has a structure shown as formula I described in the specification, and pharmacological experiments show that the compound has excellent inhibitory activity in a plurality of tumor cell strains.
Owner:SICHUAN UNIV
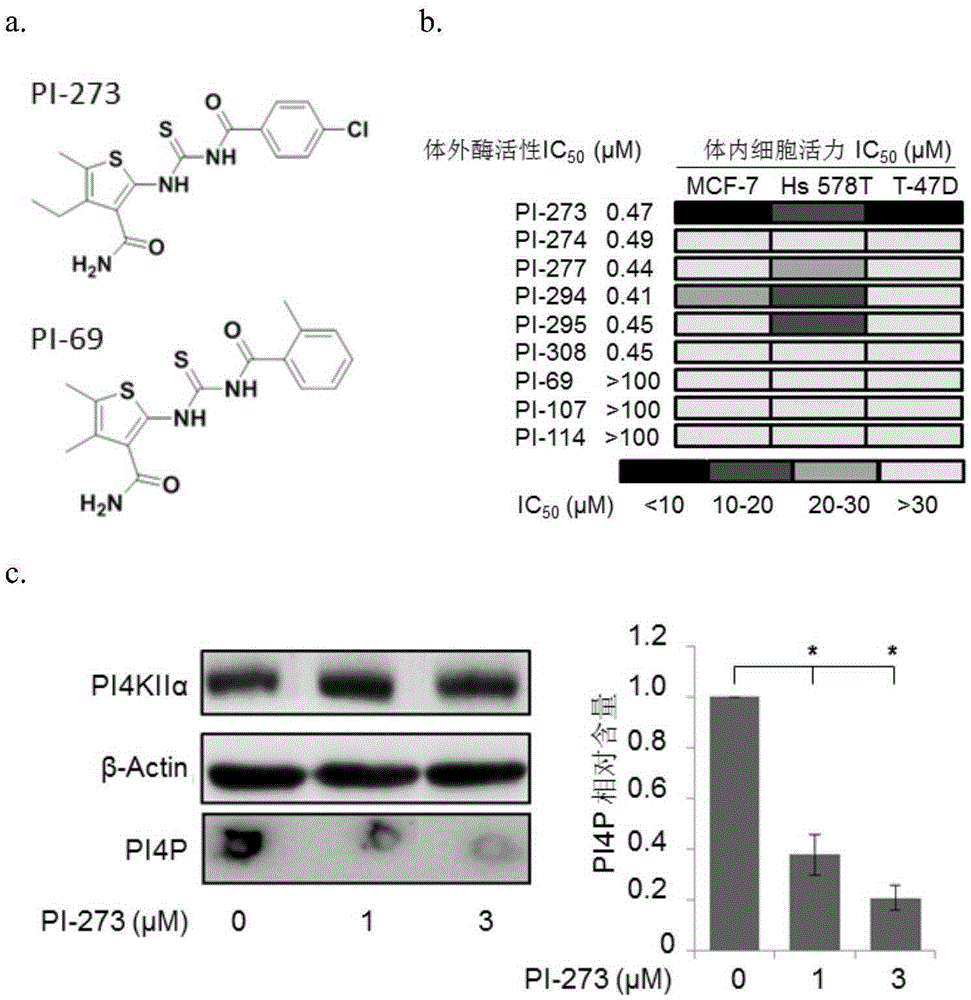
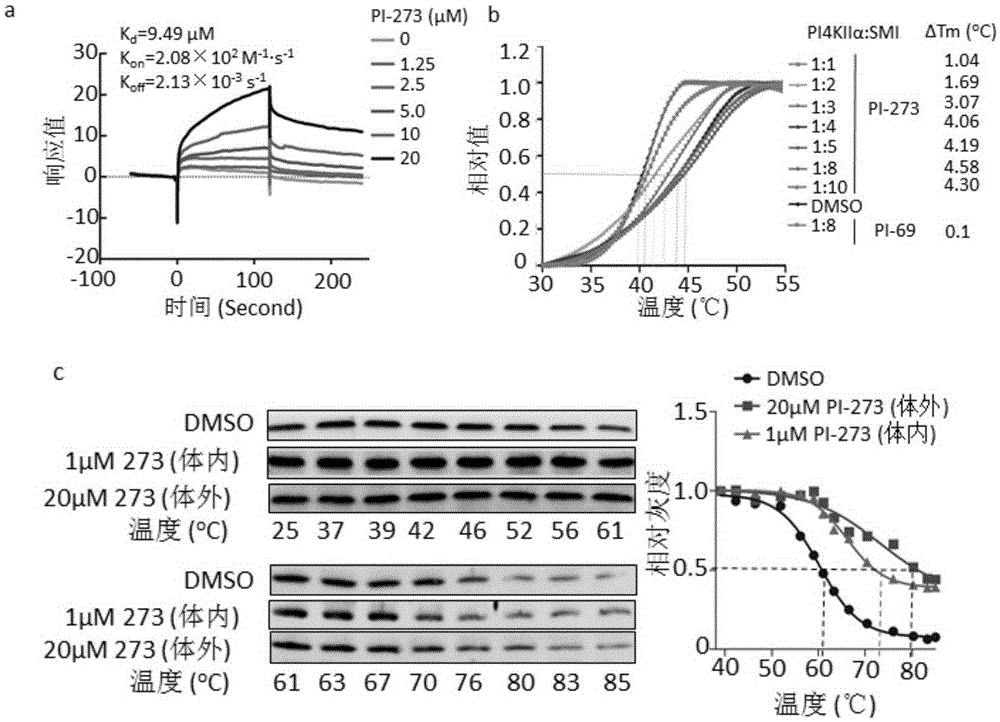
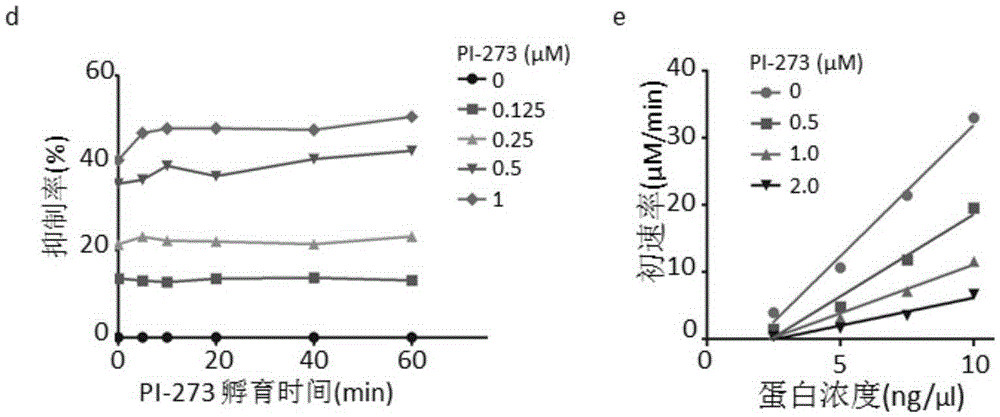
Application of phosphatidyl inositol 4-position kinase type II alpha subtype specific inhibitor PI-273
The invention discloses application of phosphatidyl inositol 4-position kinase type II alpha (PI4KIIalpha) subtype specific inhibitor PI-273 and provides application of the PI-273 in PI4KIIalpha enzyme inhibitor as well as application of the PI-273 in preparation of a product for inhibiting the enzyme activity of the PI4KIIalpha. Experiments in the invention show that subtype specific small-molecule inhibitor of the PI4KIIalpha is screened according to a crystal structure of a human PI4KIIalpha for the first time; the PI-273 can be used for significantly inhibiting the growth of tumor tissues induced by MCF-7; the PI4KIIalpha subtype specific inhibitor PI-273 provides a new strategy for treating breast cancer.
Owner:INSITUTE OF BIOPHYSICS CHINESE ACADEMY OF SCIENCES +1
Olmesartan ester liposome solid preparation
InactiveCN103040777AImprove product qualityUniform particle sizeOrganic active ingredientsPill deliverySide effectCholesterol
The invention discloses an olmesartan ester liposome solid preparation and a preparation method thereof. Active ingredient-olmesartan ester, particularly-combined phosphatidyl inositol, di-stearoyl phosphatidyl glycerol, cholesterol succinate and tween 80 are prepared into liposome, the stability, the dissolution rate and the bioavailability of the preparation is greatly increased, the action is stable and lasting, and the curative effect is significant. The product quality of the preparation is increased, and the toxic and side effects are reduced.
Owner:海南路易丹尼生物科技有限公司
Heterocyclic compounds and their uses
Compounds having the structure which are selective inhibitors of P13K (phosphatidylinositol 3-kinase-delta), for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions.
Owner:AMGEN INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com