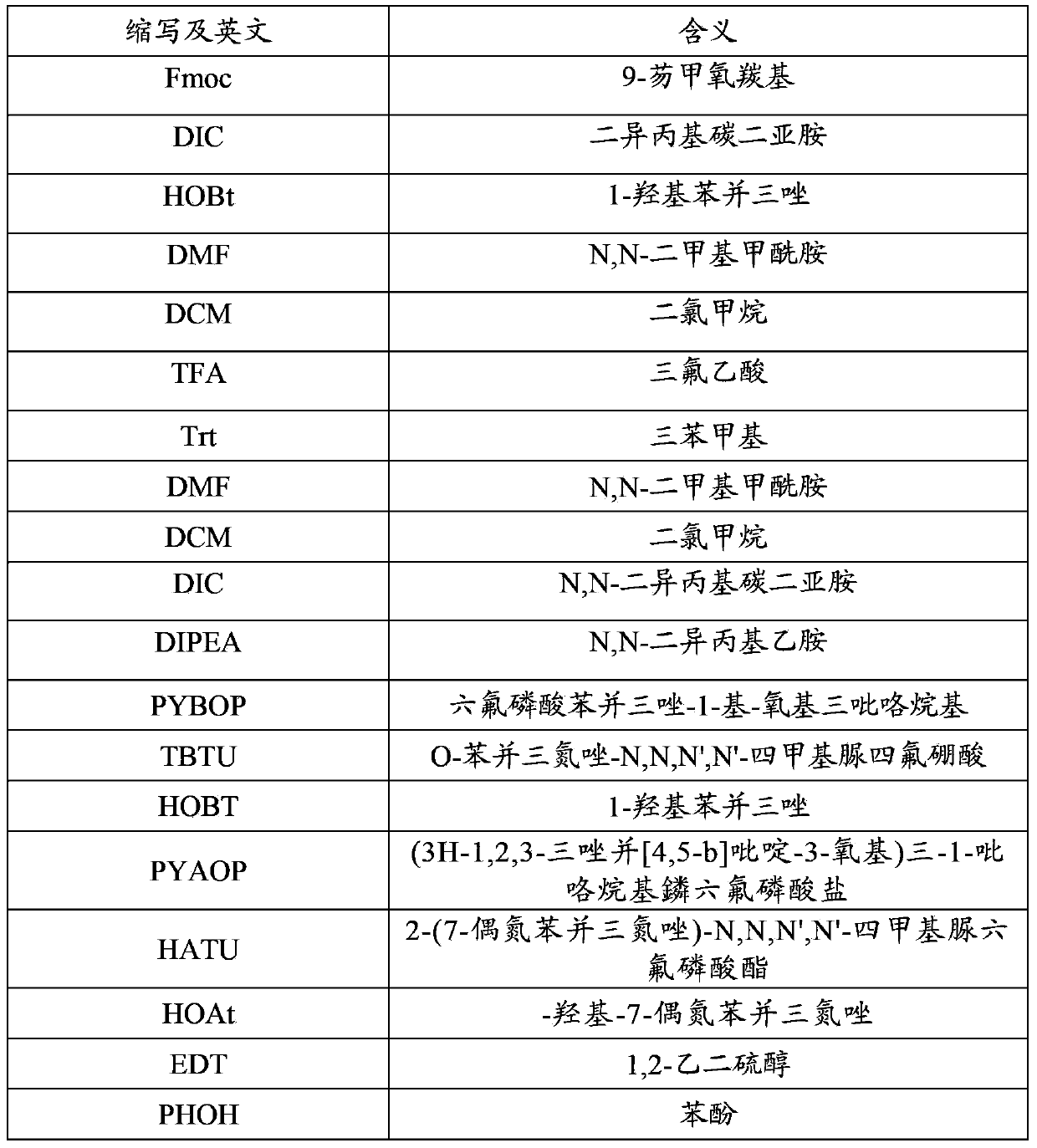
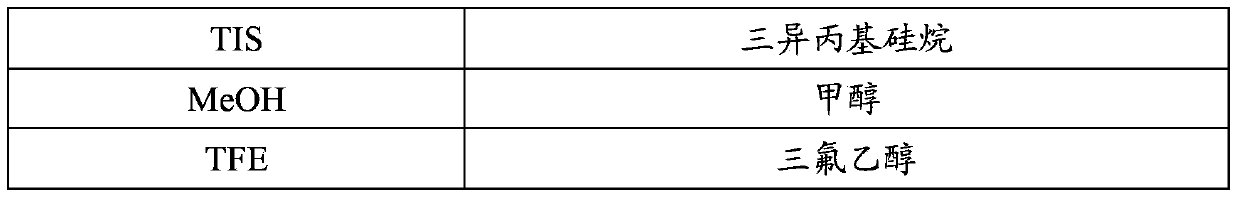
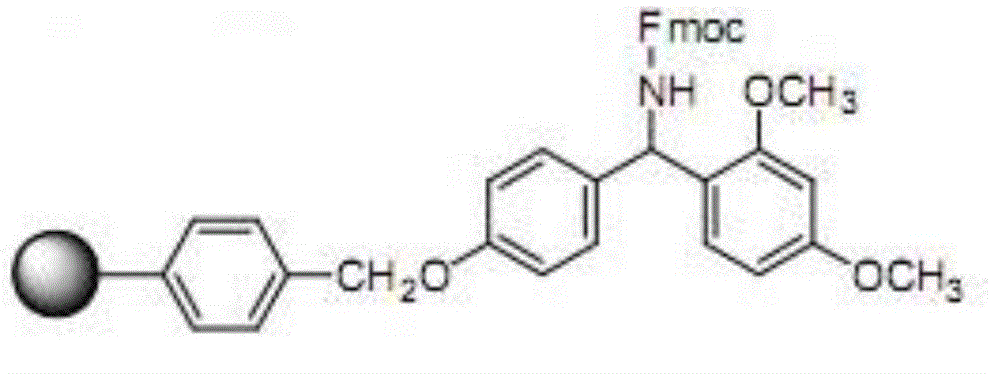
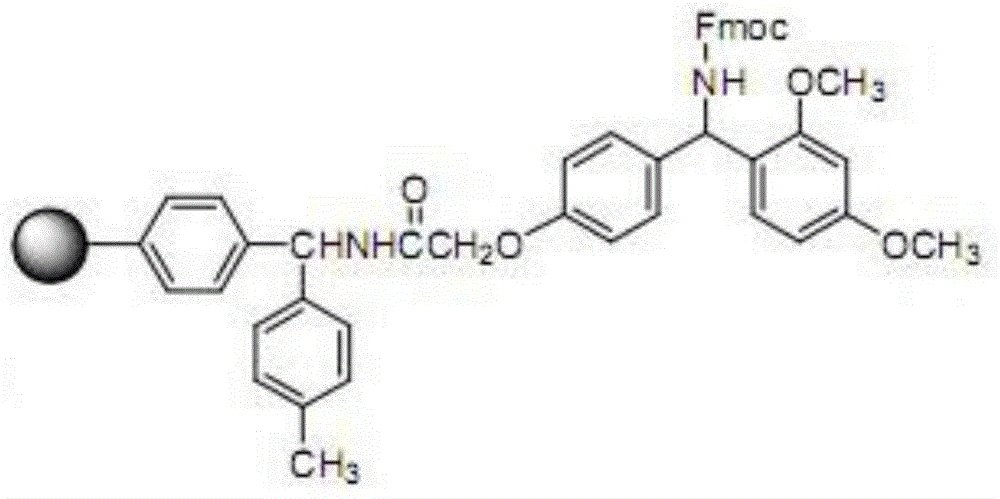
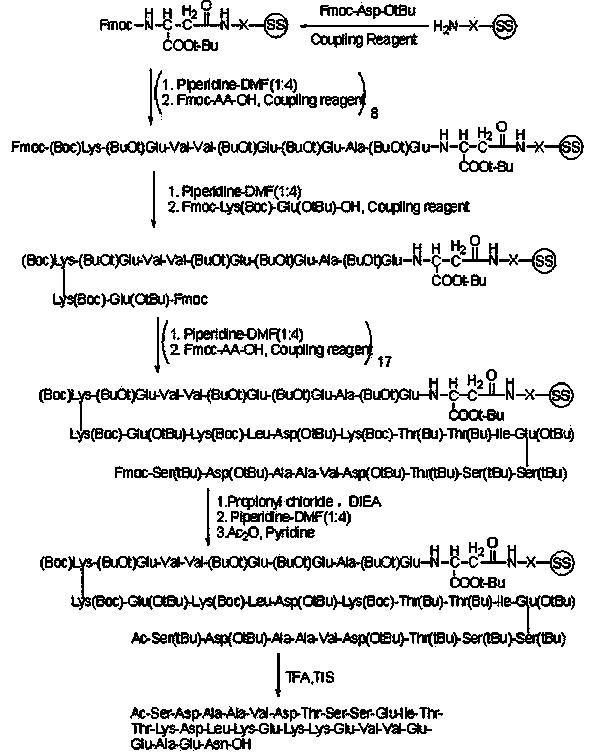
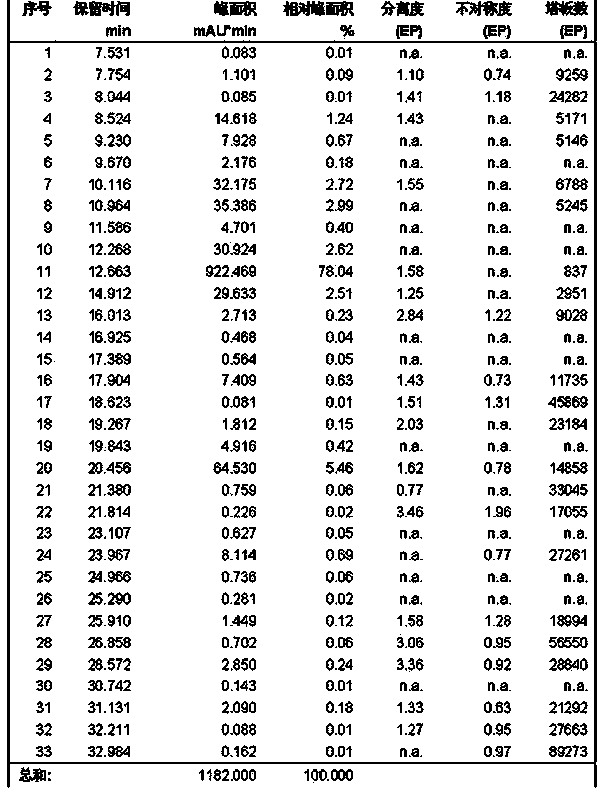
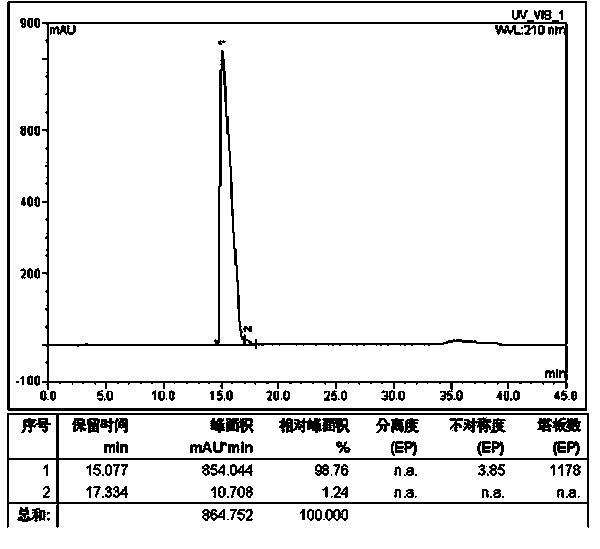
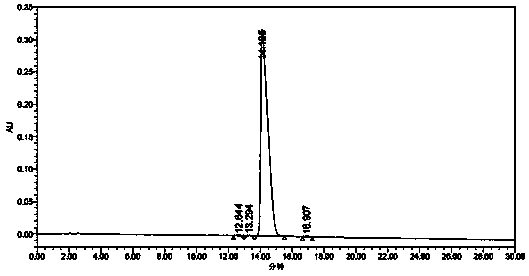
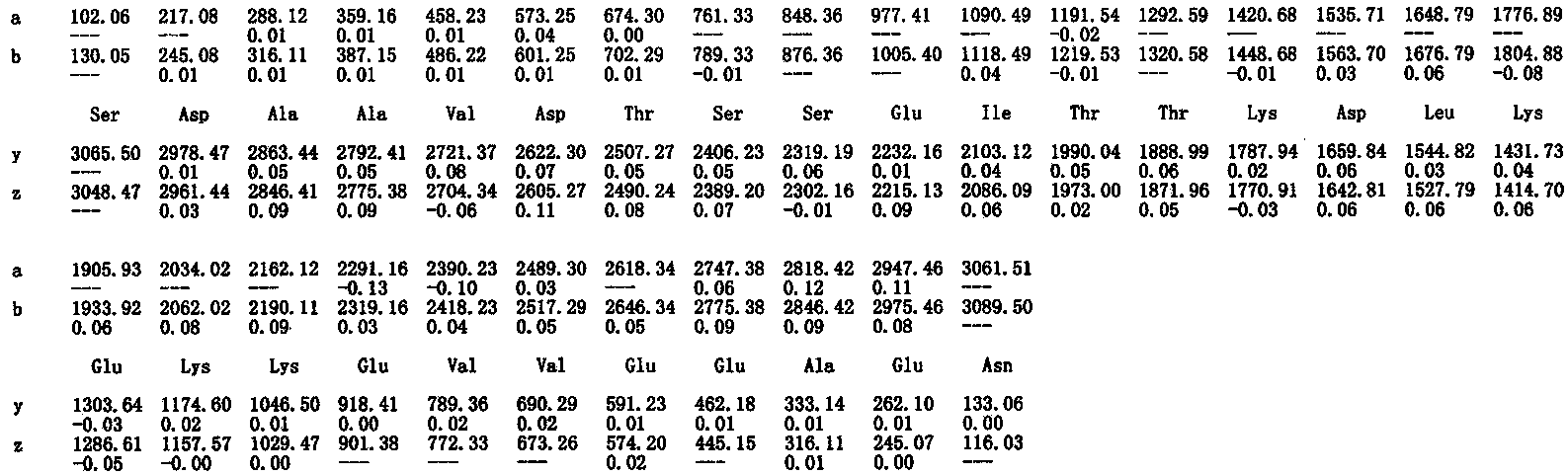
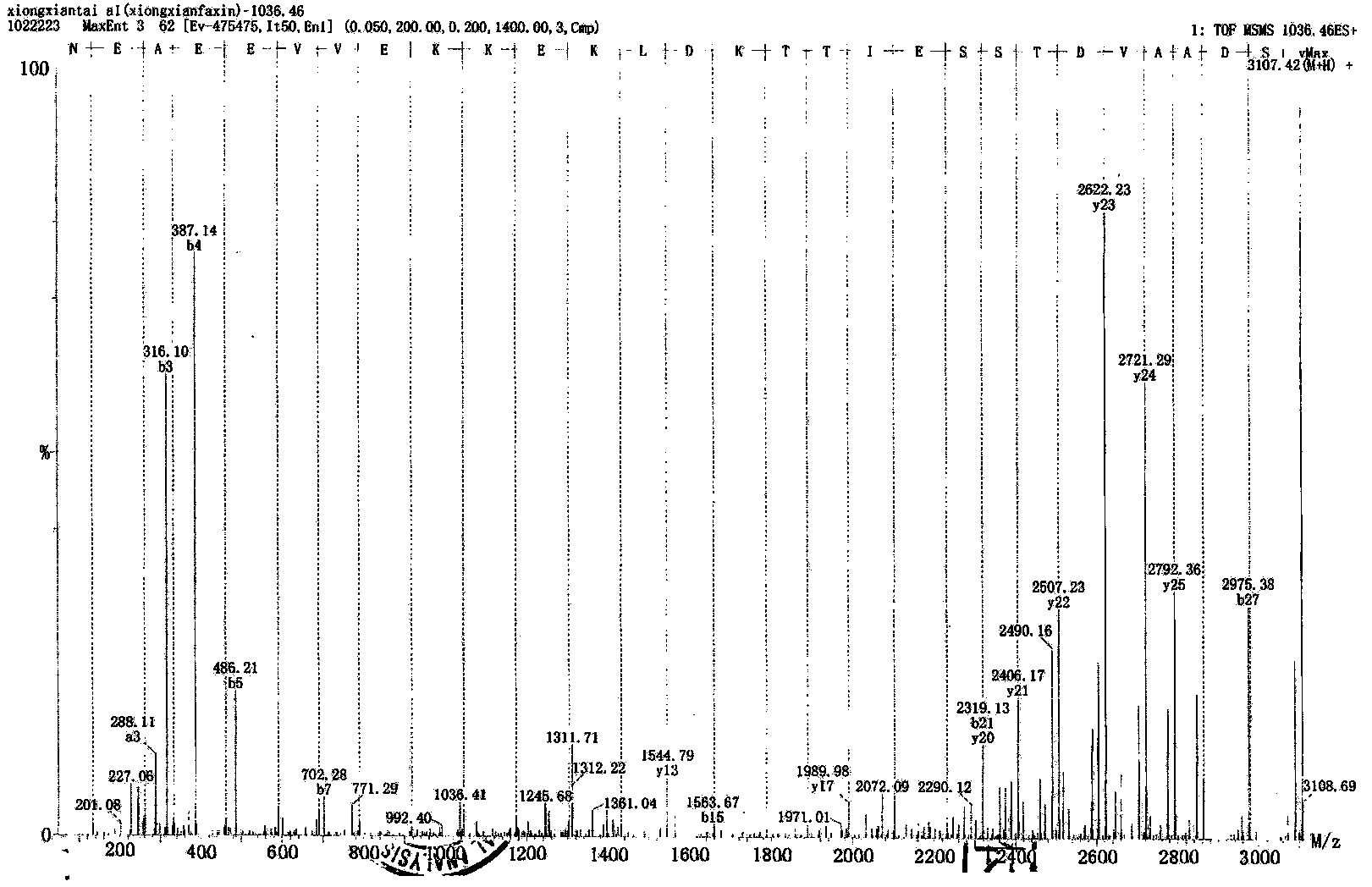
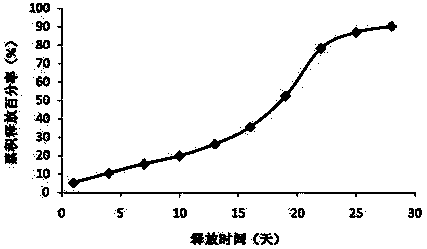
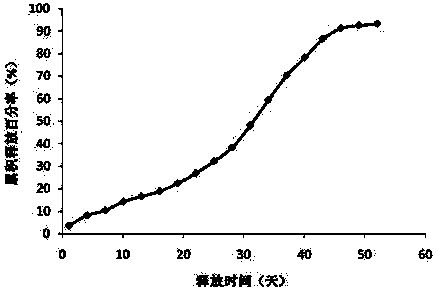
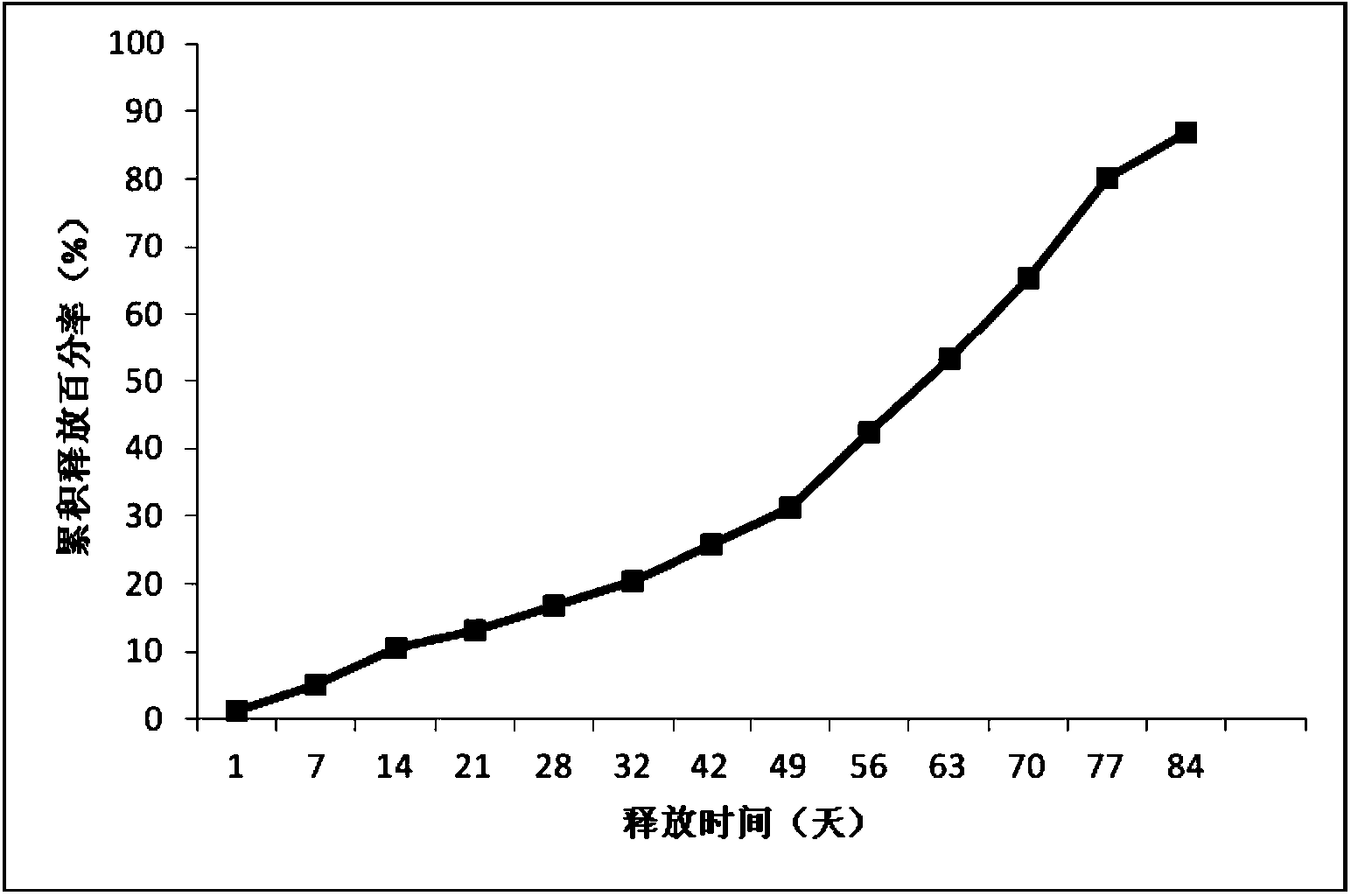
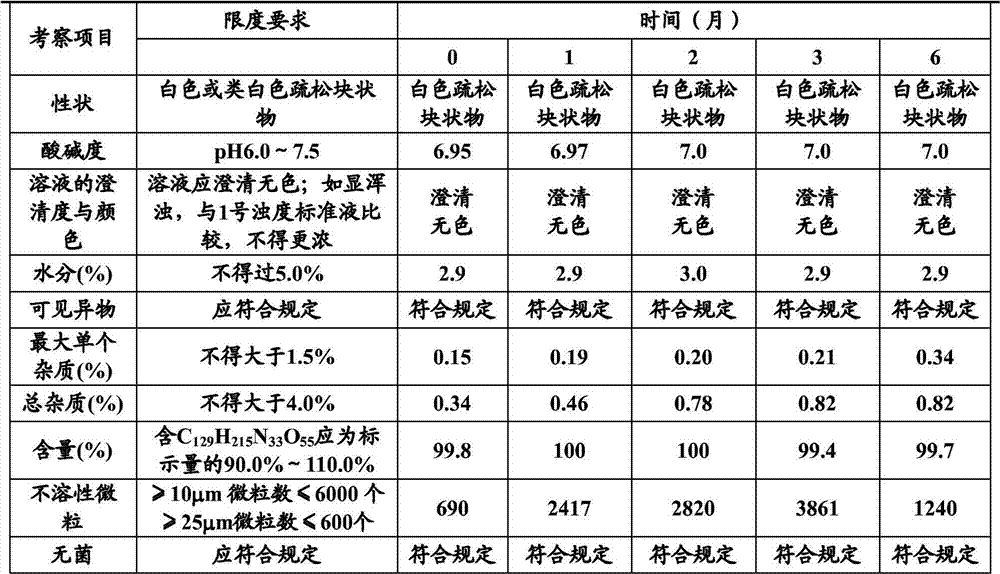
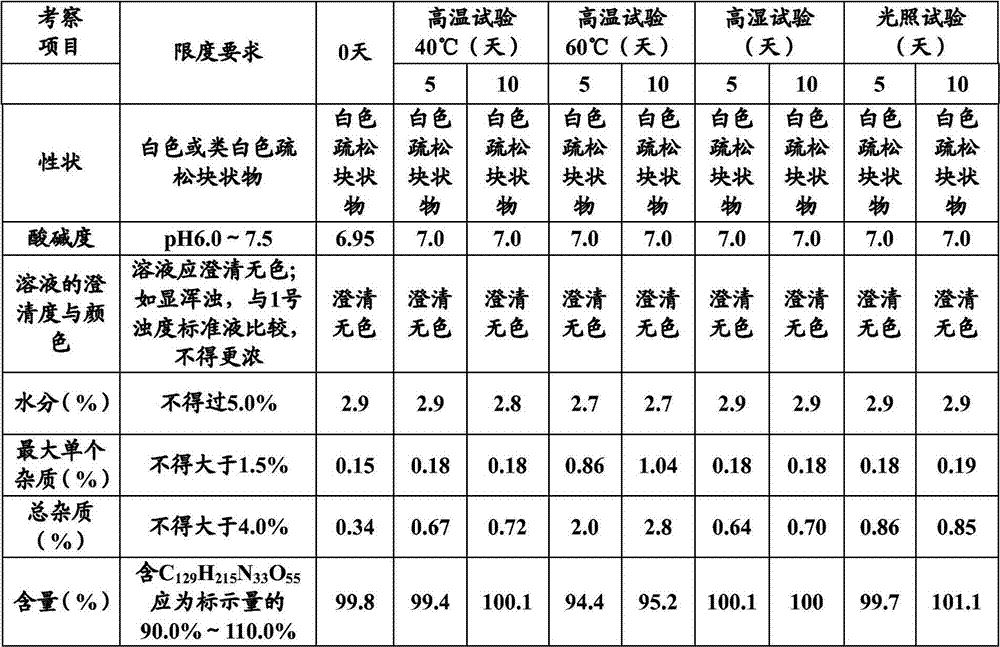
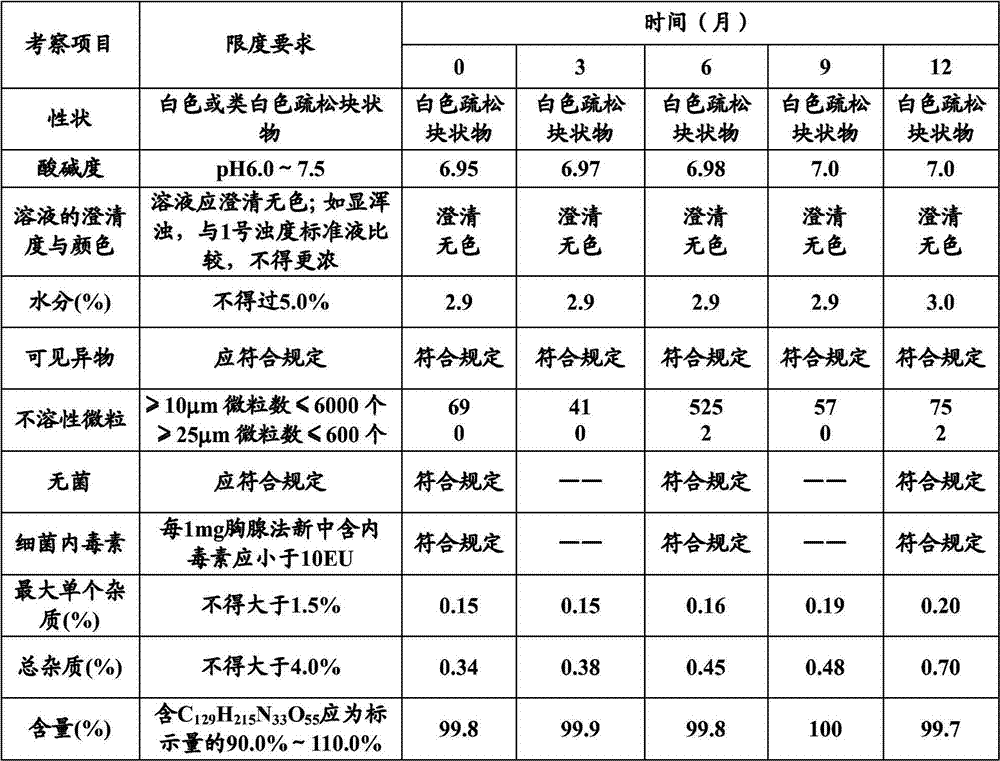
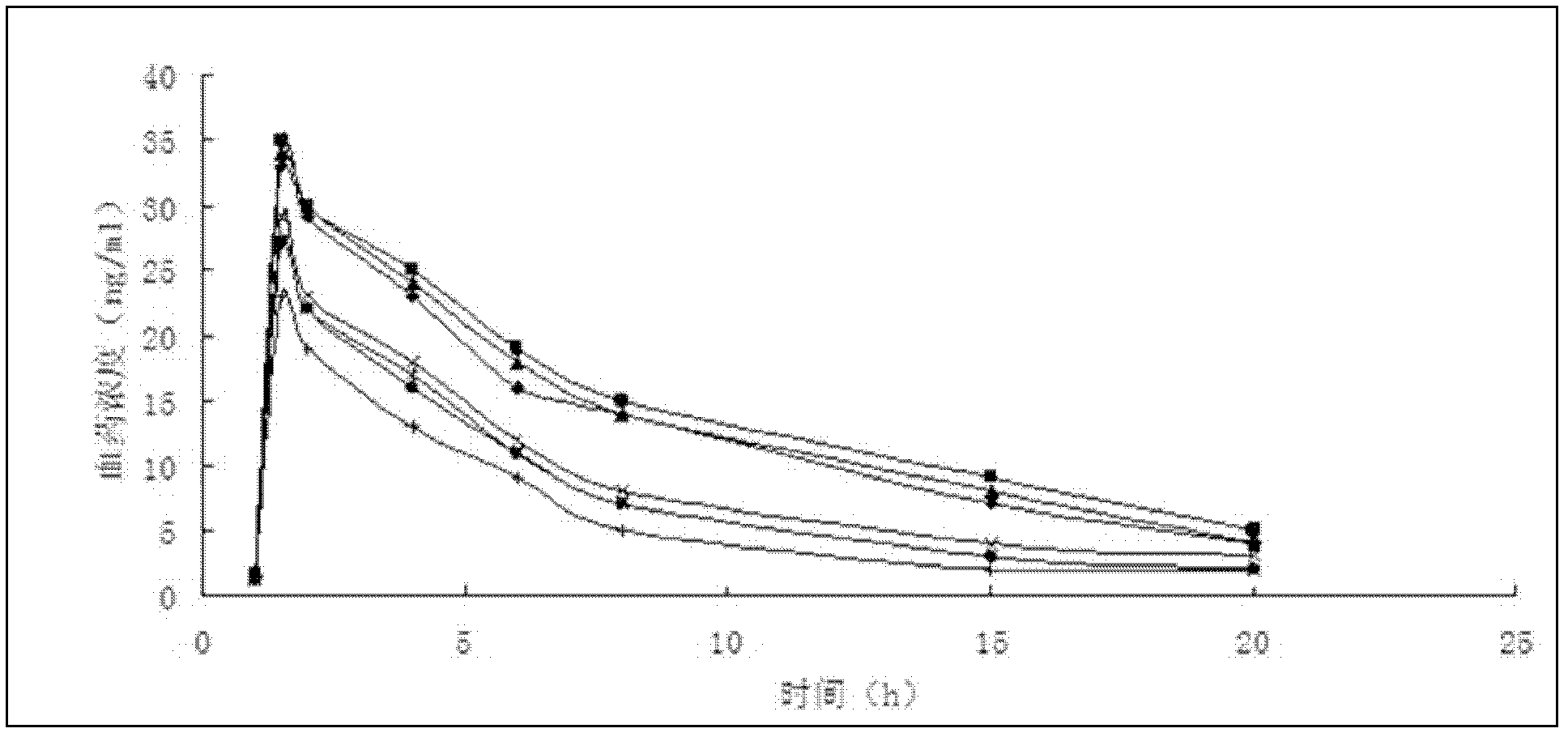
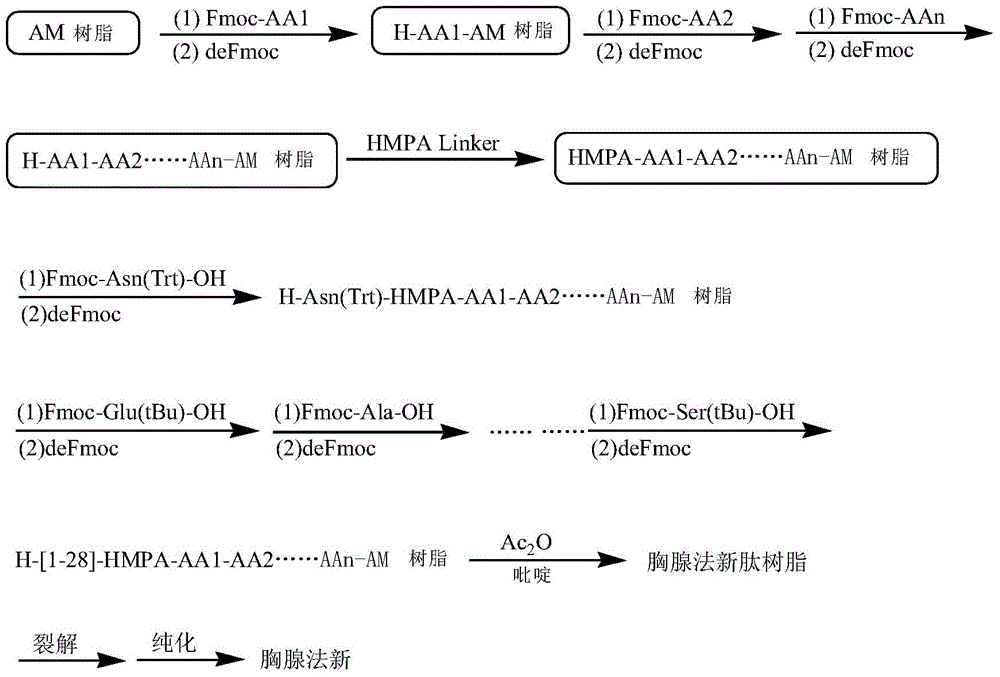
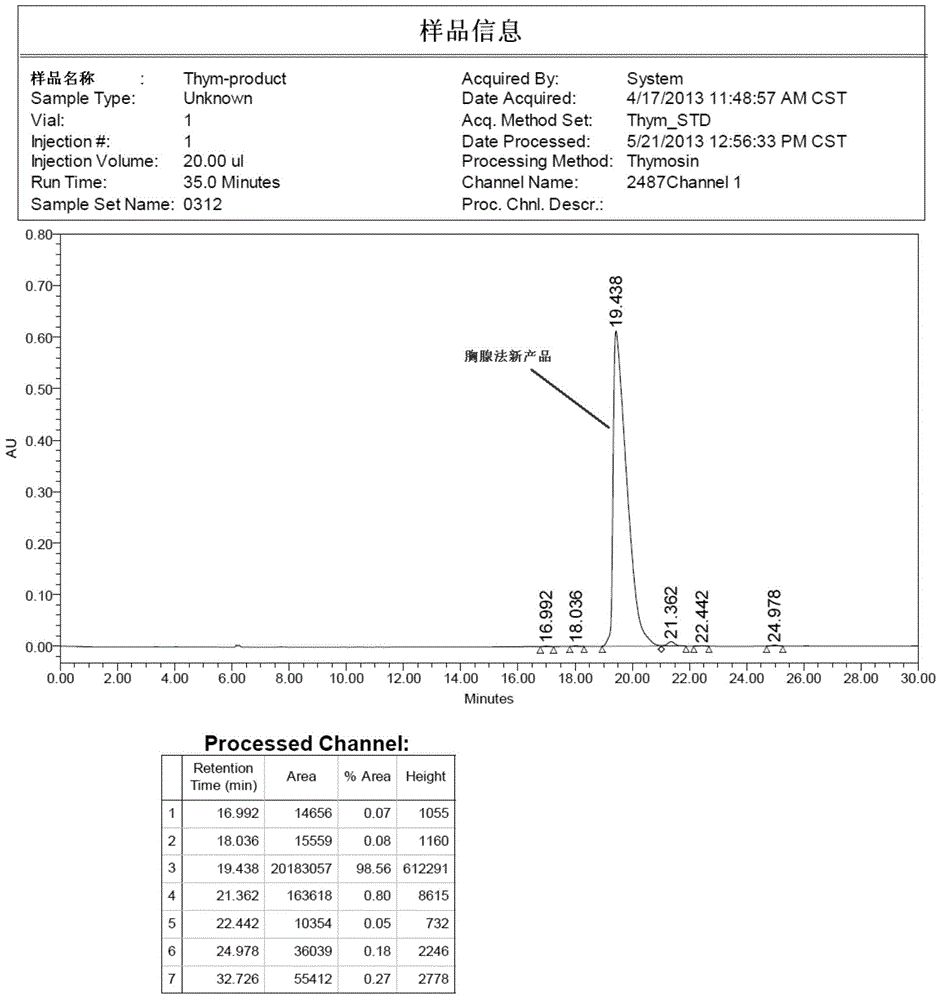
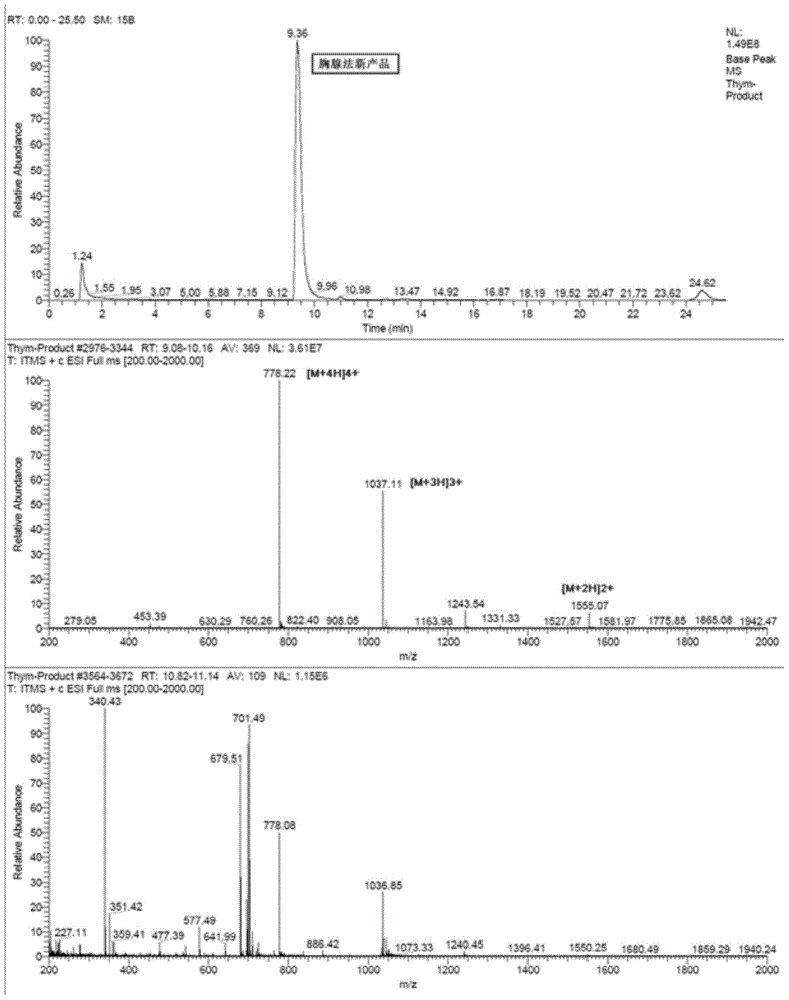
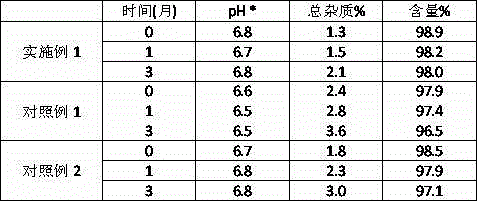
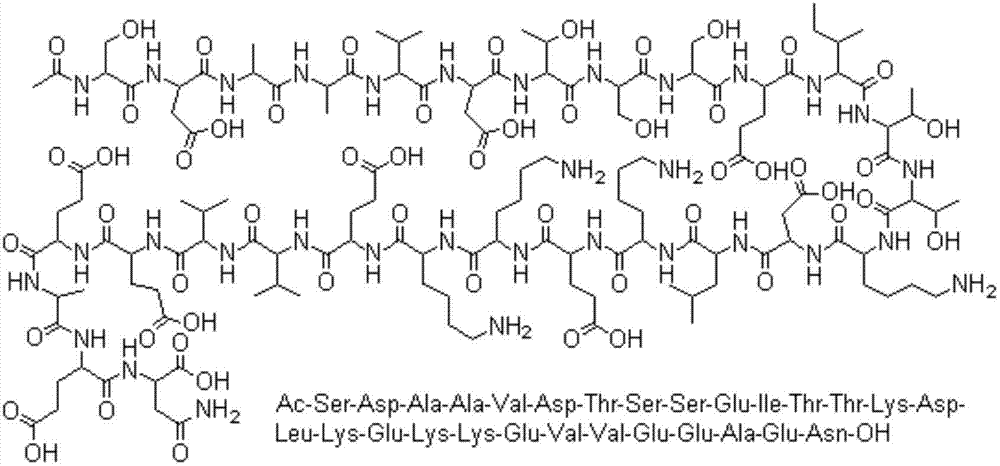
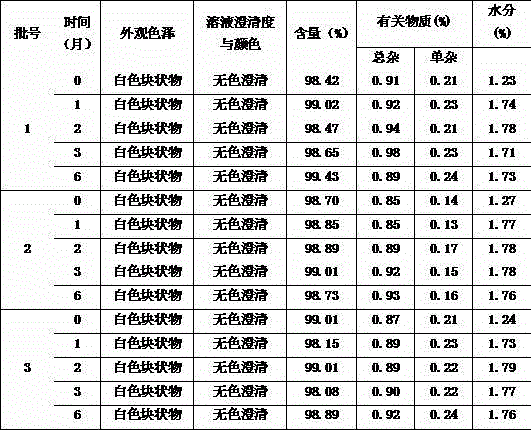
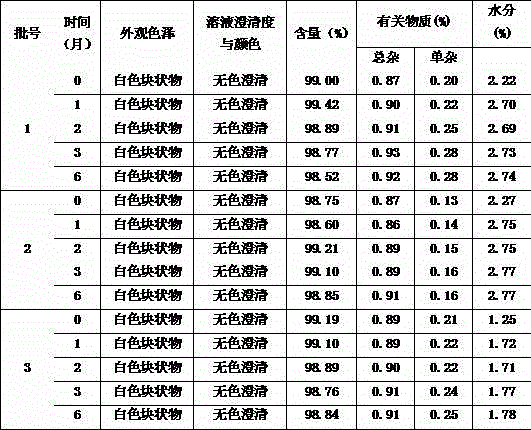
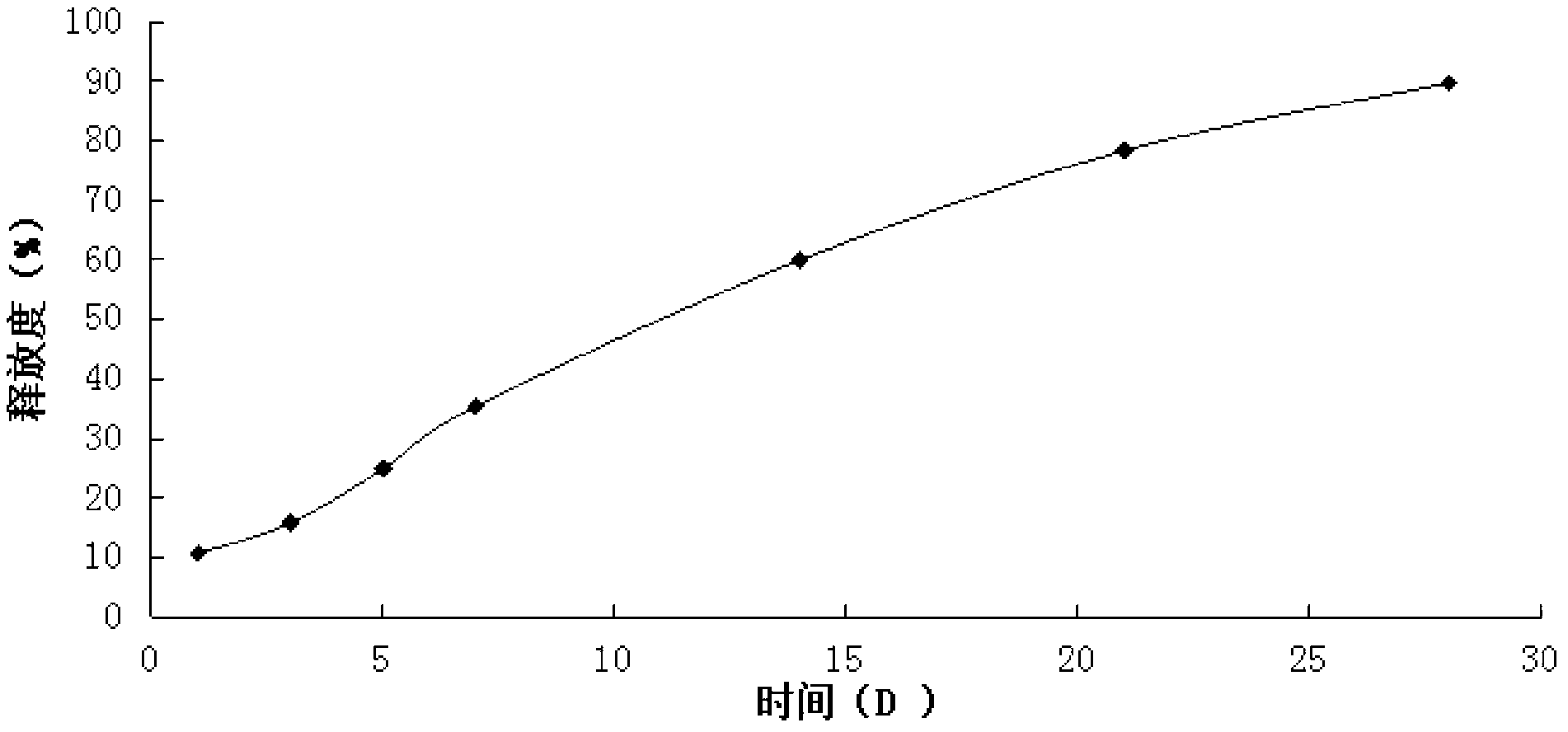
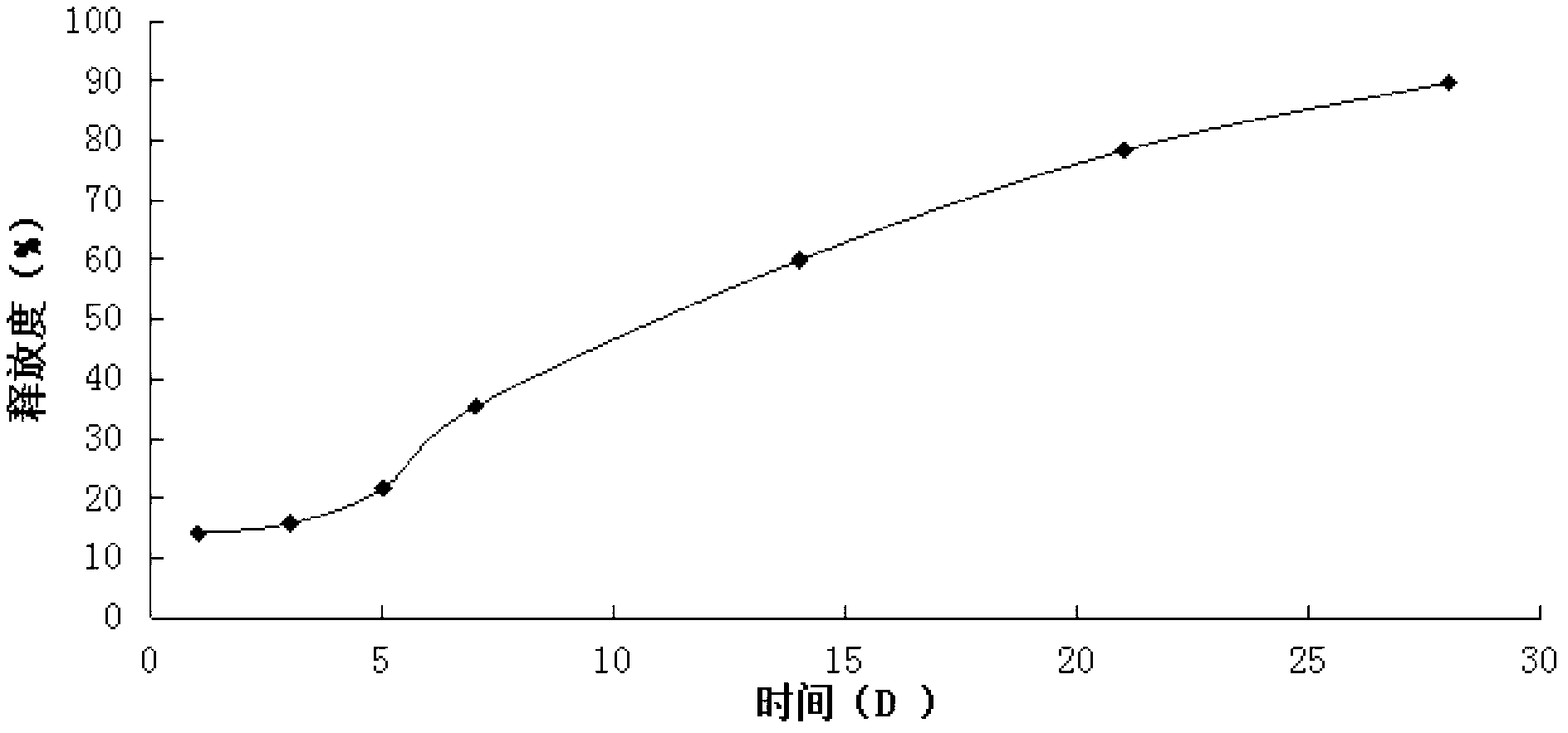
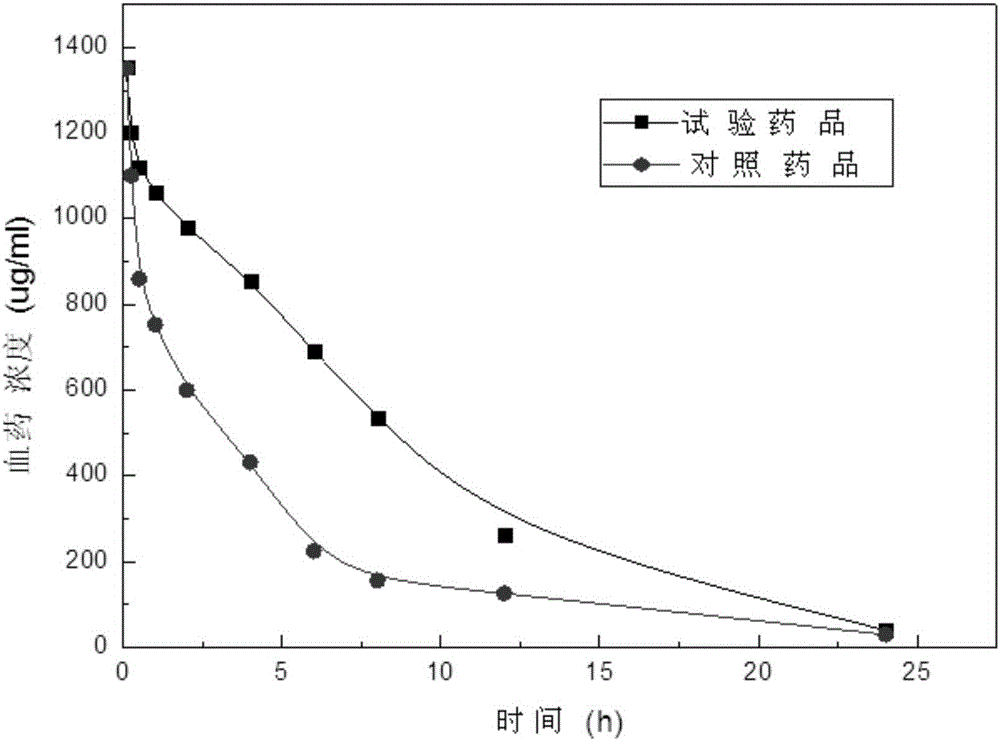
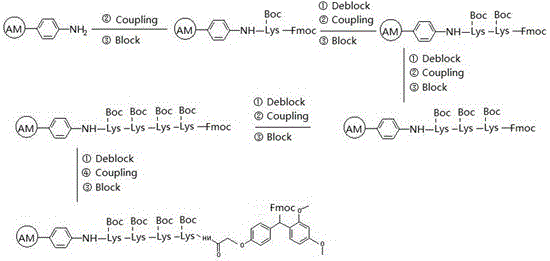
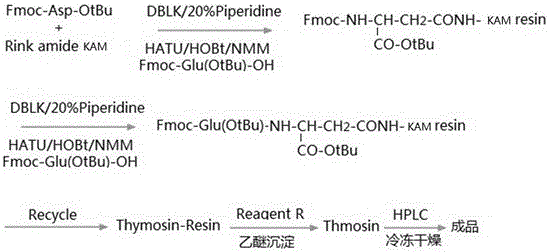
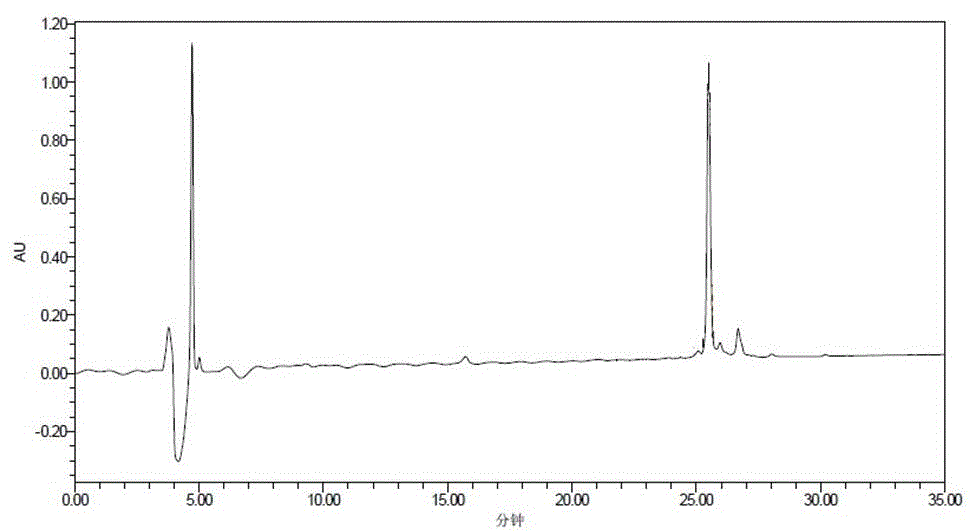
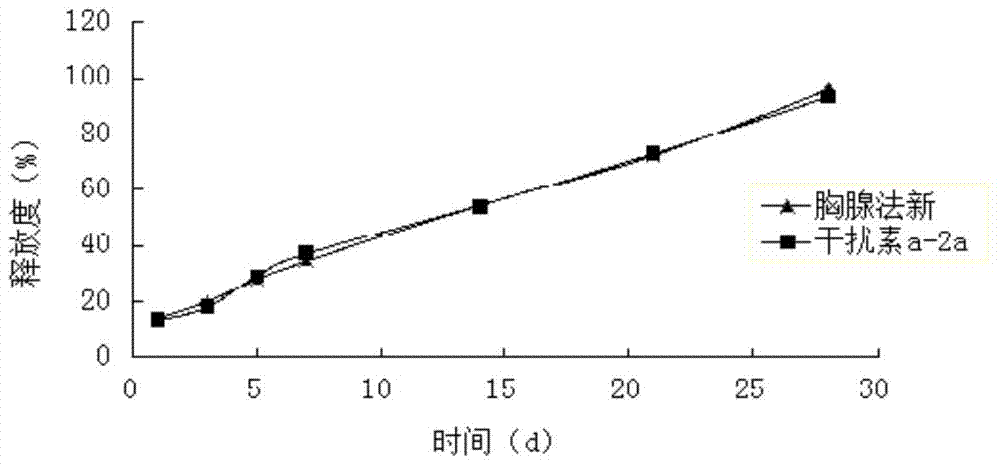
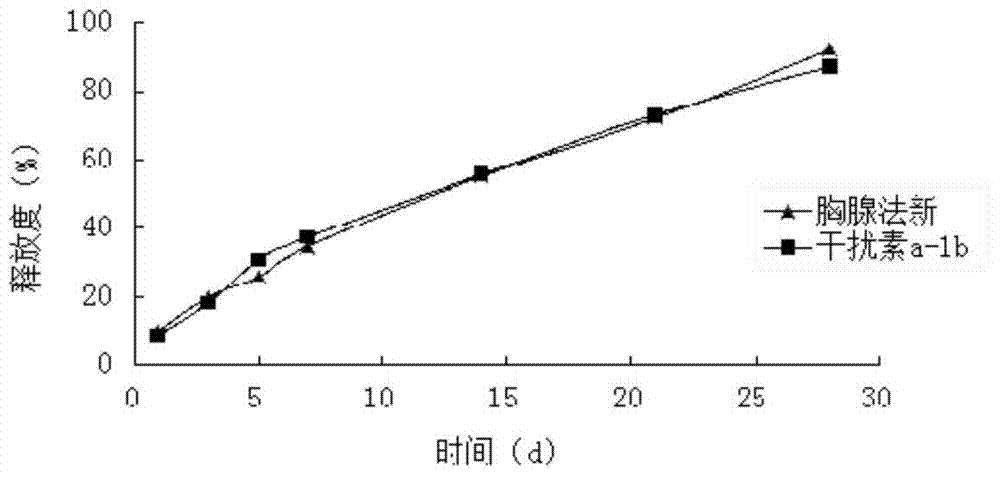
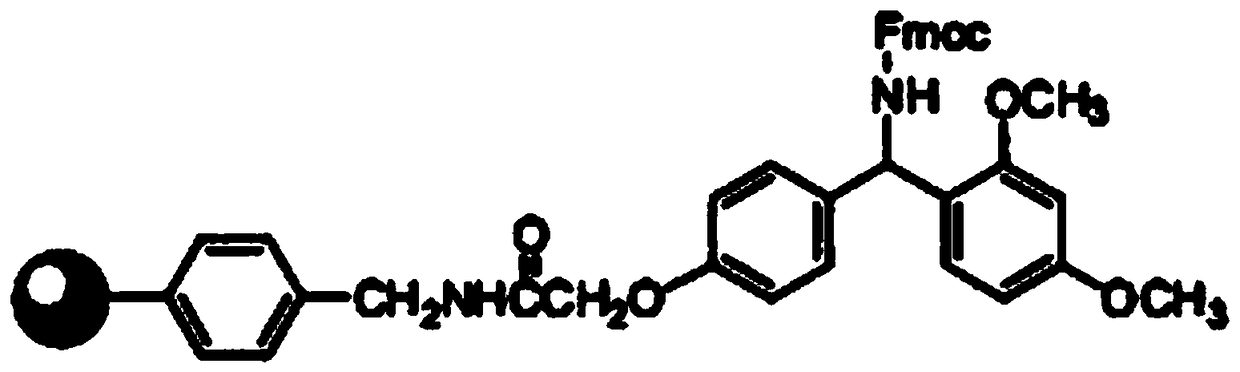
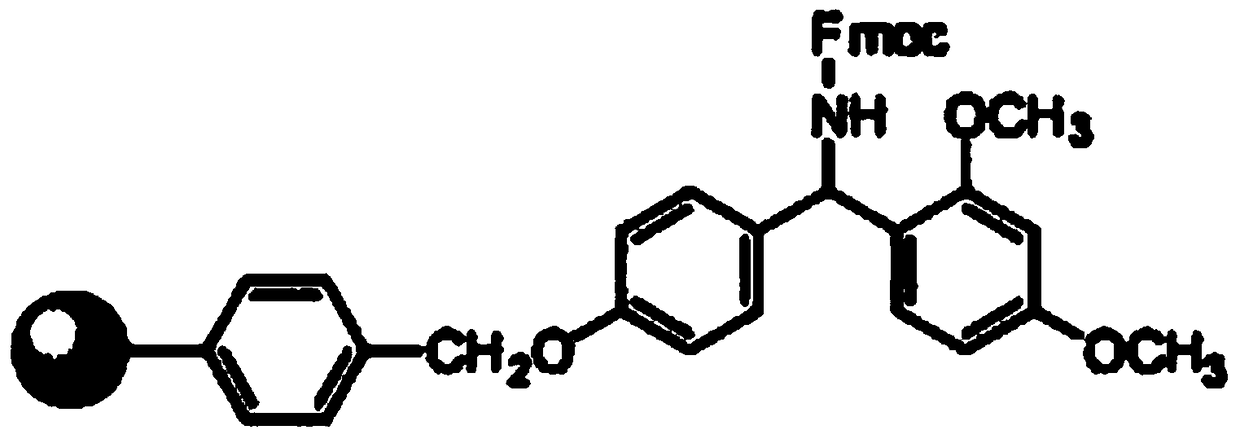
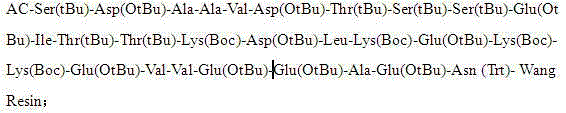Patents
Literature
60 results about "Thymalfasin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
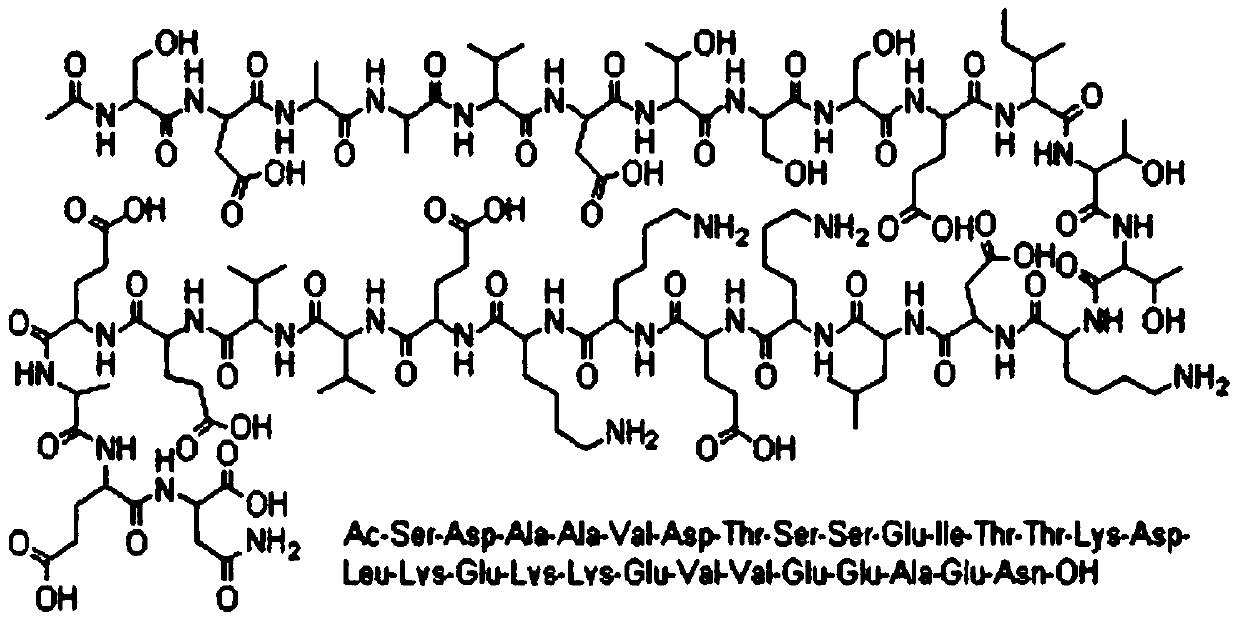
A synthetic analogue of thymosin-alpha-1, a 28-amino acid protein derived from the precursor protein prothymosin-alpha. Exhibiting a variety of immunoregulating properties, thymosin-alpha-1 induces differentiation of murine T-cell precursors and human thymocytes and the terminal differentiation of functionally immature cord blood lymphocytes and induces production of IL-2, high affinity IL-2 receptors, and B-cell growth factors by peripheral blood mononuclear cells. T-helper and cytotoxic/suppressor T-cell populations are targets of thymosin activity. Thymosin-alpha-1 has been shown to increase the efficiency of antigen presentation by macrophages and to be an endogenous modulator of alpha-thrombin activity. (NCI04)
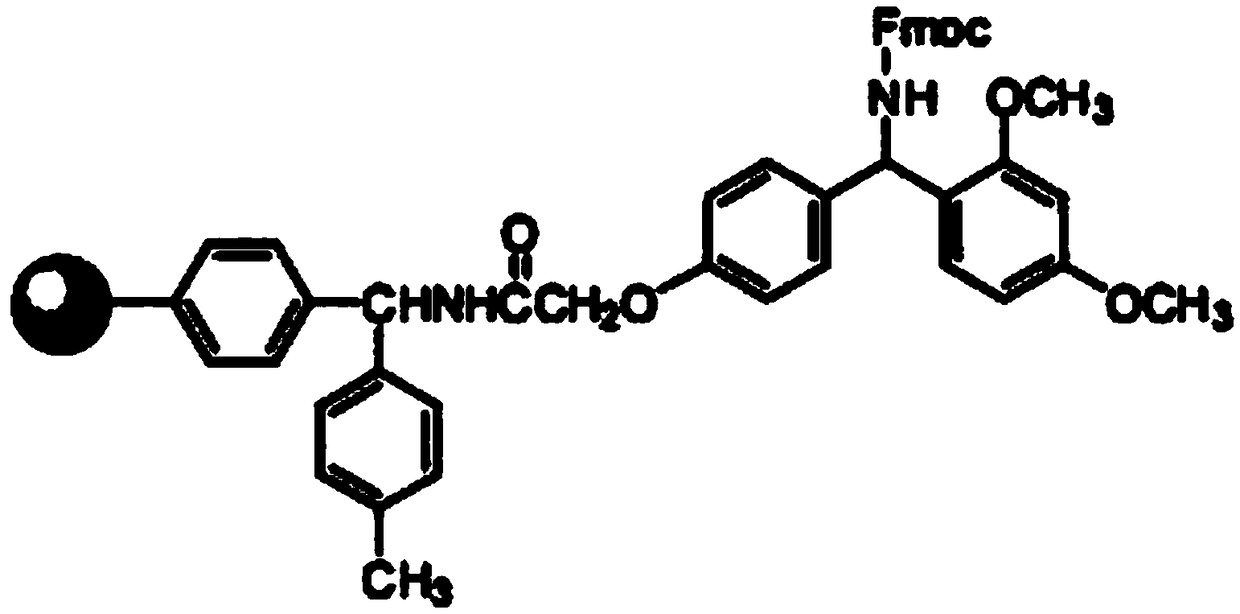
Method for synthesizing thymalfasin
ActiveCN103497245AEase of mass productionEasy to purifyThymosin peptidesPeptide preparation methodsThymalfasinCombinatorial chemistry
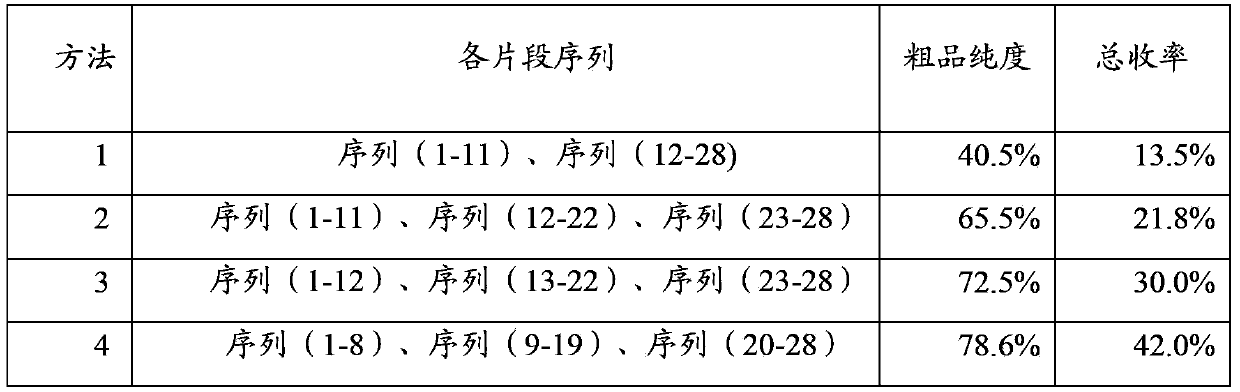
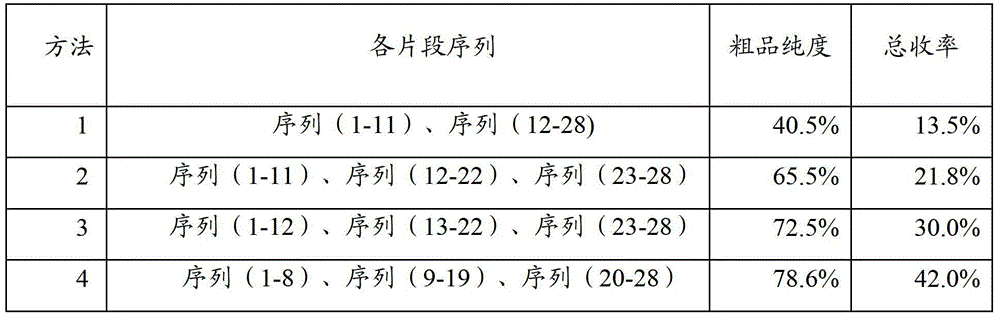
The invention relates to the field of medicine synthesis, and discloses a method for synthesizing thymalfasin. According to the method of the invention, based on the amino acid sequence from the C terminal to the N terminal of the thymalfasin peptide chain, fragments of 1-8, 9-19 and 20-28 are synthesized, and the three polypeptide fragments are coupled to obtain thymalfasin. According to the invention, a plurality of fragments are synthesized simultaneously; the synthetic period is reduced by 2 / 3; intermediates are easy to purify; the cost is low; the purity of the finished products is high; by-products are few; the product yield is high; and the method facilitates large-scale production of thymalfasin.
Owner:HYBIO PHARMA
Method for preparing thymalfasin through dipeptide fragment liquid-solid bonding
InactiveCN104987382ASolve the problem of low coupling efficiencyAvoid formingThymosin peptidesPeptide preparation methodsDipeptideFluid phase
The invention belongs to the field of polypeptide synthesis, and relates to a method for preparing thymalfasin through dipeptide fragment liquid-solid bonding. By means of a liquid phase mode, a dipeptide fragment of continuous amino acid is synthesized, the dipeptide fragment is used for batch charging solid phase synthesis, and the problems that loci are difficult to couple, and a deletion peptide is prone to generation are solved. Meanwhile, purity and yield of crude peptides are increased. The technology is used for preparing the thymalfasin so that the purity of the crude peptides can be over 75%. Compared with the prior art, a synthetic route is simple, the problems that the difficult loci are not easy to couple and the deletion peptide is prone to generation are mainly solved, synthesis cost and purification cost are reduced, and industrial large-scale production is facilitated.
Owner:JINAN KANGHE MEDICAL TECH
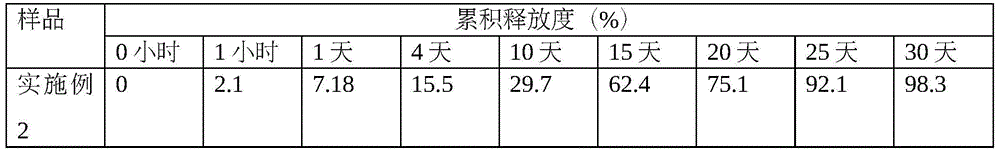
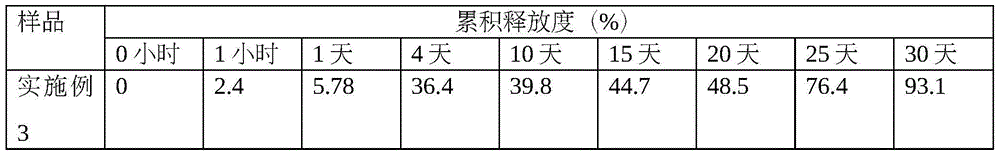
Thymalfasin sustained-release microsphere preparation and preparation method thereof
InactiveCN102106828AReduce dosing frequencyPeptide/protein ingredientsPharmaceutical non-active ingredientsAcetic acidLactide
The invention provides a thymalfasin sustained-release microsphere preparation and a preparation method thereof. The thymalfasin sustained-release microsphere preparation comprises the following components in percentage by weight: 0.1 to 10 percent of thymalfasin, and 85 to 99 percent of poly(glycolide-co-lactide), wherein the poly(glycolide-co-lactide) is copolymer of glycolide and lactide, the ratio of the glycolide to the lactide is (25:75)-(75:25), the molecular weight of the poly(glycolide-co-lactide) is between 5,000 and 35,000 dalton, and the intrinsic viscosity is between 0.1 and 0.5dL / g. The pH value of the external water phase of the thymalfasin sustained-release microsphere preparation is between 3.0 and 5.0, and can be regulated by adopting acetic acid, hydrochloric acid, sulfuric acid and the like. The inventor discovers that by controlling the pH value of the external water phase, the stability of the thymalfasin can be improved and the entrapment rate in a preparation process also can be improved, so that the drug-loading rate of sustained-release microspheres can be improved.
Owner:HYBIO PHARMA
Method for synthesizing thymalfasin
InactiveCN104098688AShort synthesis cycleHigh yieldThymosin peptidesPeptide preparation methodsSide chainCombinatorial chemistry
The invention relates to the field of pharmaceutical synthesis, in particular to a method for synthesizing thymalfasin, and aims to solve the technical problems of difficulty in separation and purification, low total yield and high production cost of a conventional method. According to the scheme, the method for synthesizing thymalfasin comprises the steps as follows: a, a polypeptide fragment 1 and a polypeptide fragment 2 provided with protecting groups on side chains are synthesized; b, a C terminal of the polypeptide fragment 1 and an N terminal of the polypeptide fragment 2 are coupled, and the protecting group at the N terminal is removed to obtain polypeptide resin I; c, according to an amino acid sequence of thymalfasin, amino acids from the eleventh to the first are sequentially coupled one by one according to the order from the C terminal to the N terminal, then the protecting group at the N terminal is removed, and acetylation is performed to obtain thymalfasin resin; and d, the thymalfasin resin is subjected to acidolysis to remove the C terminal resin and all protecting groups to obtain a coarse thymalfasin product, and thymalfasin is obtained after purification. With the adoption of the method, the product yield can be greatly improved, and the synthesis cycle is shortened.
Owner:CHENGDU SHENGNUO BIOPHARM
Method used for synthesizing thymalfasin
ActiveCN103980357AHigh yieldEasy to purifyThymosin peptidesPeptide preparation methodsCouplingFreeze-drying
The invention discloses a method used for solid-phase synthesis of thymalfasin, and relates to a novel technology used for preparing thymalfasin via Fmoc strategy solid-phase method. The method comprises following steps: (1) synthesis of Fmoc-Asp(RinkAmideMBHA resin)-OtBu is realized using Fmoc-Asp-OtBu and RinkAmideMBHA with an appropriate substitution degree; (2) Fmoc-Asp(RinkAmideMBHA resin)-OtBu is subjected to stepwise coupling or fragment coupling so as to obtain thymosin resin, wherein coupling of Glu18 and Lys19 is realized via fragment one-step peptide connection, and the rest amino acids are subjected to stepwise coupling, a mixed deprotection agent is used for deprotection in deprotection processes before coupling of Asp6, Thr12, Leu16, Lys17, Glu18-Lys19, and an special acylation reagent is used for terminal blocking of thymosin resin before Asp2 coupling; and (3) thymosin resin is subjected to cracking so as to obtain a crude peptide, and thymalfasin is obtained via RP-HPLC purification, salt transport, and freeze-drying. The invention provides the solid-phase synthesis method of thymalfasin with high product purity and convenient for purification.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Method for preparing high-purity thymalfasin
ActiveCN103880945AExtended reaction timeShort reaction timeThymosin peptidesPeptide preparation methodsWang resinThymalfasin
The invention discloses a method for preparing high-purity thymalfasin. The preparation method comprises: firstly preparing a first resin polypeptide fragment, a second resin polypeptide fragment, a third resin polypeptide fragment, a fourth resin polypeptide fragment and a fifth resin polypeptide fragment respectively; cleaving the second resin polypeptide fragment, the third resin polypeptide fragment, the fourth resin polypeptide fragment and the fifth resin polypeptide fragment to obtain crude peptide fragments; purifying the obtained second, third, fourth and fifth crude polypeptide fragments respectively; linking each purified polypeptide fragment to the first resin polypeptide fragment; carrying out an acetylation reaction on resin polypeptide fragments to obtain thymalfasin wang resin; cleaving to obtain the thymalfasin crude product; then purifying the obtained thymalfasin crude product twice; and collecting the mobile phase containing thymalfasin in a collection and purification process, evaporating to dryness under reduced pressure and centrifuging to obtain thymalfasin, and vacuum drying to obtain thymalfasin products. The thymalfasin is prepared by the method disclosed by the invention, the reaction time is effectively shortened, the reaction yield and the quality of the final product are improved; and the purity of thymalfasin prepared is more than 99%.
Owner:郑州大明药物科技有限公司
Thymalfasin sustained-release microspheres and preparation method thereof
InactiveCN103432570AReduce usageEasy to solvePeptide/protein ingredientsDigestive systemMicrosphereFreeze-drying
The invention relates to the technical field of medicines and discloses thymalfasin sustained-release microspheres and a preparation method thereof. The method comprises the following steps: freeze-drying and sieving an aqueous solution of thymalfasin; collecting the thymalfasin powder; grinding and sieving polylactic acid or a polylactide-co-glycolide acid (PLGA); mixing the obtained polymer powder and the thymalfasin powder to obtain a mixture of raw and accessory materials; melting and extruding the mixture of raw and accessory materials to obtain a hot-melting extrudate; cooling to cure the hot-melting extrudate; cutting, grinding and sieving to obtain the thymalfasin sustained-release microspheres. According to the invention, the thymalfasin sustained-release microspheres are prepared by a hot-melting extrusion technology without needing an organic solvent, the problem of residual organic solvent is completely avoided, a few impurities are contained, and the drug release is steady; an advantage of continuously releasing the thymalfasin dose is realized, thus the administration time is reduced, the administration pain of a patient is eased, and the thymalfasin sustained-release microspheres are suitable to be popularized and applied to treatment.
Owner:HYBIO PHARMA
Stable thymalfasin preparation and preparation method thereof
InactiveCN102813632APromote circulationAvoid damagePowder deliveryPeptide/protein ingredientsMANNITOL/SORBITOLPhosphate
Owner:苏州科耐尔医药科技有限公司
Thymalfasin liposome preparation for injecting
InactiveCN102579347ARound shape and not aggregatedImprove stabilityPeptide/protein ingredientsDigestive systemSolubilitySucrose
The invention discloses a thymalfasin liposome preparation for injecting and a preparation method thereof. The thymalfasin liposome preparation for injecting is prepared from thymalfasin, cholesterol, egg yolk lecithin, phosphatidyl inositol, sucrose ester and a pharmaceutically-acceptable carrier in a specific weight ratio, wherein the carrier is preferably mannitol and fucose. A liposome injection disclosed by the invention has high preparation stability, a liposome is prevented from cracking due to fusion, ice crystals and the like in a cooling process, and the liposome still keeps high packing rate and high stability after long-time storage. Due to the adoption of the thymalfasin liposome preparation, the solubility of thymalfasin is increased, the quality of a preparation product is improved, toxic and side effects are reduced, the retaining time of a medicament in body circulation is prolonged, the bioavailability of the medicament is increased, and a curative effect is increased remarkably; and moreover, the preparation method is simple, and is suitable for industrial production.
Owner:HAINAN LINGKANG PHARMA CO LTD
Immunomodulator polypeptide slow-release microsphere preparation and preparation method thereof
InactiveCN103961320AImprove adaptabilityEasy to acceptPeptide/protein ingredientsPharmaceutical non-active ingredientsSide effectMicrosphere
The invention belongs to the field of medical preparations, and relates to an immunomodulator polypeptide slow-release microsphere preparation and a preparation method thereof. Particularly, the immunomodulator polypeptide comprises thymopentin, thymalfasin and thymosin beta4. The slow-release microsphere comprises 0.1-30% (w / w) of immunomodulator polypeptide, 60-80% of biodegradable high polymer material with biocompatibility, of which the molecular weight is 5,000-200,000 Dalton, and 0.1-20% of other pharmaceutical acceptable auxiliary materials on the basis of the total weight of the microsphere. According to the slow-release microsphere disclosed by the invention, the mean grain size is 5-60mu m; the encapsulation efficiency is greater than 90%; the slow release period of the slow-release microsphere can be up to a few days and months, the medication times is obviously reduced, the bioavailability is improved, the toxic and side effects of the medicine are reduced, and clinical treatment is facilitated. The product is good in production process repeatability and good in feasibility.
Owner:SHENZHEN JYMED TECH
Solid-phase resin and its preparation method and use
The invention discloses solid-phase resin and its preparation method and use. The solid-phase resin has a structure shown in the formula I of HMPA-AAn-AM resin I. In the formula I, AA represents the same or different side chain-protection amino acids such as Arg, Lys, Asn, Gln, Asp, Glu, Pro and Gly, and n represents an integer of 0-8. The solid-phase resin can be used for thymalfasin solid-phase synthesis.
Owner:HAINAN SHUANGCHENG PHARMA
Lyophilized composition for injection containing thymalfasin and preparation method thereof
InactiveCN103142514AImprove stabilityImprove quality reliabilityPowder deliveryPeptide/protein ingredientsThymus GlandsEngineering
The invention belongs to the technical field of medicines, and in particular relates to a lyophilized composition for injection containing thymalfasin and a preparation method thereof. By changing the preparation process of the lyophilized composition for injection containing thymalfasin, the lyophilized composition for injection containing thymalfasin higher in stability and quality can be obtained. Meanwhile, the condition that concentration of a pH adjustor is locally overhigh with a short time in the process of dissolving the liquid of auxiliary materials and thymalfasin is avoided. The lyophilized composition for injection containing thymalfasin provided by the invention has the advantages of high reliability in quality, easiness in industrialized production, energy saving and the like.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Thymalfasin compound and novel preparation method thereof
InactiveCN102617727ASolve processing problemsSolve puzzlesHormone peptidesPeptide/protein ingredientsSide effectUltrafiltration
The invention discloses a thymalfasin compound prepared by the following processing steps: dissolving a thymalfasin crude product in an alcohol solvent or trifluoroacetic acid, adding a buffer solution at the pH of 2.0-4.0, adding activated carbon to perform adsorption, filtering, collecting filtrate, and carrying out decompression concentration; utilizing a preparation type neutral alumina chromatographic column to perform separating purification to the concentrated solution, collecting eluent, sequentially using a plate frame and a filter membrane to perform filtration, utilizing an ultrafiltration membrane to perform ultrafiltration concentration of the filtrate, and obtaining secondary concentrated solution; slowing adding ethyl ether into the obtained concentrated solution, mixing, performing gradient cooling, precipitating thymalfasin, performing centrifuged washing to the precipitated thymalfasin, and obtaining the purified thymalfasin after drying through a solid drier. The thymalfasin obtained by the method is high in purity, toxic and side effects of prepared medicines for curing chronic hepatitis B and illness causing damage of various immunity functions in the using process are reduced, product quality of a preparation is improved, and the thymalfasin is suitable for industrial mass production.
Owner:灵康药业集团股份有限公司
Thymalfasin-containing freeze-dried preparation
ActiveCN102949356AImprove stabilityGood antibacterial effectPowder deliveryPeptide/protein ingredientsFreeze-dryingThymalfasin
The invention relates to a thymalfasin-containing freeze-dried preparation. The freeze-dried preparation is prepared from thymalfasin serving as an active ingredient and pharmaceutically-acceptable auxiliary materials. Tert-butyl alcohol is added into a prescription, and the pH value of a solution is adjusted by adopting an acetate buffer solution before freeze drying, so that the freeze drying period is shortened, degradation of main medicaments in a preparation process is suppressed, the stability of main medicaments is enhanced, and the quality of a product is ensured.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Thymalfasin sustained release micro-sphere preparation and preparing method thereof
InactiveCN104984327AReduce dosing frequencyProlong the time of action in the bodyPeptide/protein ingredientsDigestive systemSide effectHalf-life
The invention provides a thymalfasin sustained release micro-sphere preparation and a preparing method thereof. The thymalfasin sustained release micro-sphere preparation comprises, by micro-sphere weight, 0.1%-30% of thymalfasin, 50%-99.9% of a biodegradable high polymer material which ranges from 5000 Dalton to 200000 Dalton in molecular weight and has the biocompatibility and 0%-20% of other acceptable auxiliary materials on pharmacy. The invention further provides the preparing method for preparing the thymalfasin sustained release micro-sphere preparation, namely a high-pressure static microcapsule molding method. By means of thymalfasin sustained release micro-spheres prepared with the method, effective embedding and sustained release of the thymalfasin are achieved, the sustained release effect can reach 40 days, the toxic and side effect of the thymalfasin can be effectively reduced, the bioavailability is improved, and the metabolic half-life is prolonged; and meanwhile, the number of drug administration times is decreased, and economic and mental burdens of a patient are relieved.
Owner:山东博创生物科技有限公司
Solution for preparing thymalfasin preparation and method for preparing thymalfasin preparation by virtue of solution
ActiveCN104587452AImprove stabilityStay porousPeptide/protein ingredientsDigestive systemFreeze-dryingInjections water
The invention provides a solution for preparing a thymalfasin preparation and a method for preparing the thymalfasin preparation by virtue of the solution. The pH value of the solution is 4-5, and each 1000ml of the solution contains 1-3g of a thymalfasin preparation, 35-55g of a stabilizer, 50-70ml of a cosolvent and the balance of a pH regulator and injection water. The thymalfasin preparation prepared by the solution disclosed by the invention is better in stability and shorter in freeze-drying cycle; and meanwhile, a freeze-dried product is porous.
Owner:SHANGHAI SOHO YIMING PHARMA
Thymalfasin in-situ gel preparation and preparation method thereof
ActiveCN103156806AReduce releaseRelieve painPeptide/protein ingredientsAerosol deliveryGel preparationBlood concentration
The invention relates to the field of pharmaceutic preparations, and in particular relates to a thymalfasin in-situ gel preparation and a preparation method thereof. The thymalfasin in-situ gel preparation is composed of thymalfasin, an in-situ gel material and an organic solvent, wherein the mass-volume ratio of the thymalfasin, the in-situ gel material and the organic solvent based on g / g / mL is (2-4.5):(15-35):(100-150). The thymalfasin in-situ gel preparation provided by the invention has sustained release function, can be used for preventing the peak valley phenomenon of blood concentration, reducing the admission time as the medicament action time is long, greatly easing the pain of patients and improving the compliance of the patients and has no irritation for the skin and connective tissues. The method for preparing the thymalfasin in-situ gel preparation is simple, high in medicament loading capacity and low in cost, and is conductive to large-scale production.
Owner:HYBIO PHARMA
Thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system and preparation method
ActiveCN103126999AAvoid degradationAvoid degradation reactionsPeptide/protein ingredientsAntiviralsOral medicationMicrosphere
The invention relates to a novel thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system and a preparation method. Chitosan serves as a membrane material, beta-cyclodextrin serves as a clathration material, and a composite microsphere is prepared in a reversed phase suspension-chemical crosslinking method. According to the novel thymalfasin chitosan / beta-cyclodextrin composite microsphere drug delivery system, the composite microsphere carries thymalfasin. According to the novel thymalfasin drug delivery system, the obtained composite microsphere performs good protection and controlled-release functions on the thymalfasin, provides feasibility for oral administration medicines and has good economic benefits and application prospect.
Thymalfasin crystal and pharmaceutical composition thereof
ActiveCN105753962ANot easy to produceImprove solubilityPowder deliveryThymosin peptidesSolubilityFreeze-drying
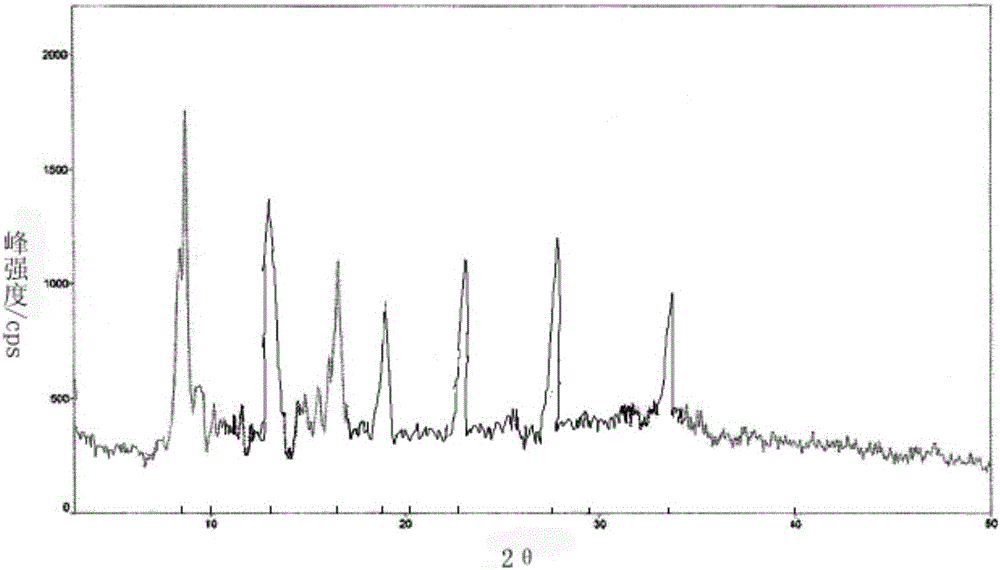
The invention belongs to the technical field of medicine and particularly relates to a thymalfasin crystal and a pharmaceutical composition thereof.An X-ray powder diffraction pattern obtained by Cu-K alpha ray measurement of the thymalfasin crystal is as shown in the figure 1.The pharmaceutical composition of the thymalfasin crystal is freeze-dried powder injection which is prepared from, by weight, 1-2 parts of the thymalfasin crystal, 45-70 parts of mannitol, 0.1-0.5 part of monosodium phosphate, 2.6-3.5 parts of sodium hydrogen phosphate and 100-200ml of tert-butyl alcohol.The thymalfasin crystal is high in stability and solubility and less prone to deterioration in long-time storage and has a gradual blood concentration curve, and bioavailability is remarkably improved.
Owner:广东泽盛药业有限公司
Synthesis process of thymalfasin
InactiveCN106543279AElimination of beta sheetsEasy to operateHormone peptidesPeptide preparation methodsAcetic anhydrideSide chain
The invention provides a synthesis process of thymalfasin and belongs to the field of solid phase polypeptide synthesis. The synthesis process of the thymalfasin is characterized in that firstly, AM resin is transformed, two to six side chain protection hydrophilic amino acid residues are coupled to the AM resin, and Lys, Arg, Glu, and Asp amino acid serve as the hydrophilic amino acid; and then, Rink amide Linker is coupled to the resin, and Fmoc-AAn...AA2-AA1-AM resin is prepared. The linear distance between synthetic polypeptide and the resin is prolonged, and hydrophilia of the resin is increased; the transformed resin is used for the synthesis of the thymalfasin, thymalfasin solid phase synthesis adopts the linear continuous synthesis from C terminal to N terminal; the best synthesis efficiency is obtained by controlling the amino acid excess multiple and the coupling time; after peptide chain synthesis is finished, Fmoc protection is removed; acetic anhydride acetylizes polypeptide N-terminal; decomposition is conducted through a cracking reagent; and the thymalfasin is obtained after diethyl ether precipitating. According to the synthesis process of the thymalfasin, the prepared resin performs efficiently when the thymalfasin synthesis is carried out, and the beta sheet of the thymalfasin is eliminated well in the process of the solid phase synthesis.
Owner:岳阳新华达制药有限公司
Purification method of thymalfasin
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Compound in-situ gel long-acting injection for treating chronic hepatitis and preparation method thereof
ActiveCN103494769AImprove product qualityNo obvious side effectsPeptide/protein ingredientsAerosol deliverySide effectMagnesium salt
The invention relates to the technical field of medicine, and discloses a compound in-situ gel long-acting injection for treating chronic hepatitis and a preparation method thereof. The compound in-situ gel long-acting injection for treating chronic hepatitis, disclosed by the invention, is prepared from 5-24 parts of zinc salt interferon a or magnesium salt interferon a, 150-800 parts of thymalfasin, 1800-12000 parts of in-situ gel materials, and 8000-100000 parts of solvent. The compound in-situ gel long-acting injection disclosed by the invention is prepared from the thymalfasin, the interferons and proper in-situ gel materials. Thus, the administration frequency is reduced; the medicine effect can be stably and lastingly released for a long period of time; the compound in-situ gel long-acting injection is free of obvious toxic and side effects, and high in medicine concentration and encapsulation efficiency; the product quality is superior to the specified standard; the compound in-situ gel long-acting injection is suitable for popularization and application in treatment of the chronic hepatitis.
Owner:HYBIO PHARMA
Synthesis method of thymalfasin
InactiveCN108314725AShort synthesis cycleHigh yieldHormone peptidesPeptide preparation methodsSide chainSynthesis methods
The invention discloses a synthesis method of thymalfasin. The synthesis method comprises the following steps: a, synthesizing a polypeptide segment 1 and a polypeptide segment 2, which have protecting groups on side chains; b, coupling a C end of the polypeptide segment 1 and an N end of the polypeptide segment 2; then removing the protecting group of the N end to obtain polypeptide resin I; c, sequentially coupling the eleventh to the first amino acids one by one according to the sequence from the C end to the N end and removing the protecting group of the N end; then carrying out acetylation to obtain thymalfasin resin; d, removing the resin of the C end and all the protecting groups from the thymalfasin resin to obtain a thymalfasin crude product; purifying to obtain the thymalfasin. By adopting the method disclosed by the invention, the yield of the product can be greatly improved and the synthesis period is shortened.
Owner:CHENGDU SHENGNUO BIOPHARM
Method for synthesizing thymalfasin
ActiveCN103497245BEase of mass productionEasy to purifyHormone peptidesPeptide preparation methodsThymalfasinCombinatorial chemistry
The invention relates to the field of medicine synthesis, and discloses a method for synthesizing thymalfasin. According to the method of the invention, based on the amino acid sequence from the C terminal to the N terminal of the thymalfasin peptide chain, fragments of 1-8, 9-19 and 20-28 are synthesized, and the three polypeptide fragments are coupled to obtain thymalfasin. According to the invention, a plurality of fragments are synthesized simultaneously; the synthetic period is reduced by 2 / 3; intermediates are easy to purify; the cost is low; the purity of the finished products is high; by-products are few; the product yield is high; and the method facilitates large-scale production of thymalfasin.
Owner:HYBIO PHARMA
Thymalfasin-containing drug composition and preparation thereof
ActiveCN103800293AImprove solubilityImprove stabilityPowder deliveryPeptide/protein ingredientsSolubilityPharmaceutical drug
The invention belongs to the technical field of medicine, and provides a thymalfasin-containing drug composition and a preparation thereof. According to the present invention, the lyophilized powder provided for injection and prepared from the composition has characteristics of good solubility, good stability, good reconstituting property, easy use and easy storage and transportation; and the preparation method has characteristics of simpleness, easy industrialization production, and low production cost.
Owner:长春海悦药业股份有限公司
Sustained-release microsphere preparation of goserelin composition
InactiveCN104436169AHigh acceptanceReduce dosing frequencyPeptide/protein ingredientsGranular deliverySide effectMedicine
The invention belongs to the field of a pharmaceutical preparation, and relates to a sustained-release microsphere preparation of a goserelin composition and a preparation method of the sustained-release microsphere preparation. Specifically, the goserelin composition includes goserelin and a polypeptide composition capable of enhancing immunity, wherein the polypeptide capable of enhancing immunity comprises thymalfasin, thymopentin and thymosin beta4. The sustained-release microsphere consists of 0.1-40% (w / w) of goserelin and polypeptide capable of enhancing immunity in terms of the total weight of the microsphere, 60-99.9% of a biodegradable and biocompatible high polymer material which is 5,000-200,000Dalton in molecular weight in terms of the weight of the microsphere, and 0-10% of other pharmaceutically acceptable accessories in terms of the weight of the microsphere. The sustained-release microsphere disclosed by the invention is 5-20microns in average grain size and encapsulation efficiency is more than 80%. The sustained-release duration of the sustained-release microsphere can last for several days or several months, so that administration frequency is obviously reduced, bioavailability is improved, the toxic and side effects of medicine are reduced, and the sustained-release microsphere is conducive to clinic treatment. The production process of the finished product is good in reproducibility and good in feasibility.
Owner:SHENZHEN JYMED TECH
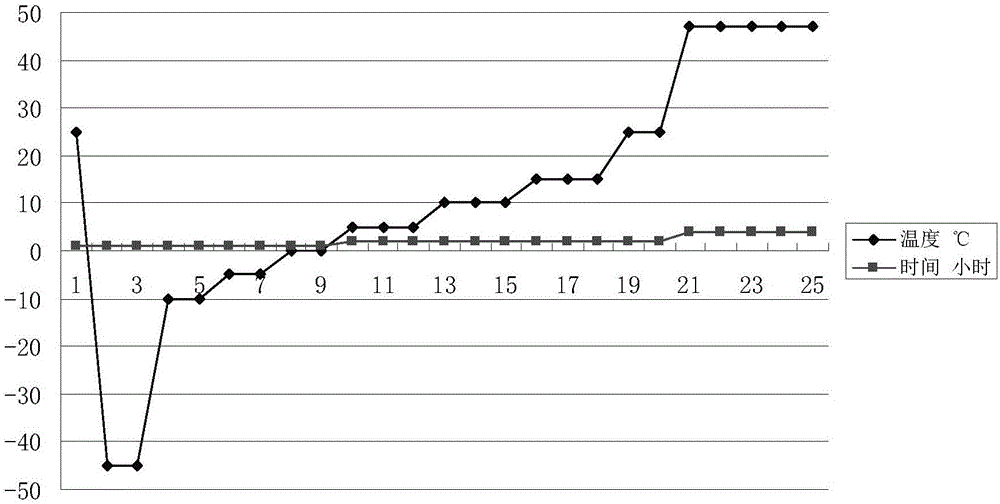
Freeze-drying process of thymalfasin for injection
InactiveCN106581644AGood resolubilityEasy to usePowder deliveryPeptide/protein ingredientsFreeze-dryingThymalfasin
The invention discloses a freeze-drying process of thymalfasin for injection. The freeze-drying process is characterized by including the first step of pre-freezing, the second step of primary sublimation drying and the third step of secondary sublimation drying. In the second step of primary sublimation drying, seven-stage step temperature rise is adopted, wherein a, a coolant is heated to -10 DEG C+ / -1 DEG C, and heat preservation is conducted for 1-1.5 h; when the vacuum degree reaches 15 Pa+ / -3 Pa, automatic pulsed gas infiltration is conducted, and when the vacuum degree reaches 25 Pa+ / -5 Pa, gas infiltration is stopped; b, the coolant is heated to -5 DEG C+ / -1 DEG C, heat preservation is conducted for 1-1.5 h, and gas infiltration is conducted till the vacuum degree reaches 25 Pa+ / -5 Pa; c, the coolant is heated to 0 DEG C+ / -1 DEG C, heat preservation is conducted, and gas infiltration is conducted till the vacuum degree reaches 25 Pa+ / -5 Pa; d, the coolant is heated to 5 DEG C+ / -1 DEG C, heat preservation is conducted, and gas infiltration is conducted till the vacuum degree reaches 25 Pa+ / -5 Pa; e, the coolant is heated to 10 DEG C+ / -1 DEG C, heat preservation is conducted, and gas infiltration is conducted till the vacuum degree reaches 25 Pa+ / -5 Pa; f, the coolant is heated to 15 DEG C+ / -1 DEG C, heat preservation is conducted, and gas infiltration is conducted till the vacuum degree reaches 25 Pa+ / -5 Pa; g, the coolant is heated to 25 DEG C+ / -1 DEG C, heat preservation is conducted, and gas infiltration is conducted till the vacuum degree reaches 33 Pa+ / -3 Pa. By means of the freeze-drying process, it can be ensured that the moisture content and impurity limit of the finished product meet the requirements of National Drug Standards, and the time of the whole freeze-drying process can be shortened.
Owner:苏州天马医药集团天吉生物制药有限公司
Thymalfasin peptide resin pyrolysis method
InactiveCN109762057AReduce dosageReduce the risk of explosionHormone peptidesPeptide preparation methodsSodium bicarbonateVacuum pressure
The invention discloses a thymalfasin peptide resin pyrolysis method, and belongs to the field of polypeptide solid-phase synthesis. The method comprises the steps of taking thymalfasin peptide resin,adding a lysate which is 6-12 times the volume of the resin, conducting pyrolysis at 10-30 DEG C for 1-3 h, and filtering out resin to obtain a filtrate; adding the filtrate into ether which accountsfor 6 times or more of the volume of the filtrate; adding a 0.5M-1.5M sodium bicaronate or ammonium hydroxide solution which is 0.5-1 time the volume of the solution into the solution; adding an alkaline solution until the solution is clarified; conducting standing and layering, taking an upper-layer organic phase, adding a moderate amount of water for washing, merging the upper-layer organic phase with a lower-layer aqueous phase, and conducting vacuum pressure reduction to remove residual ether; adding acid to adjust the pH value to 2-8, and conducting filtering and purification. By means of the method, there is no need to use ether to wash crude peptide, the use amount of the ether is greatly reduced, the centrifuging operation is omitted at the same time, and the fire blast risk of the ether is lowered; the crude peptide drying step is omitted, the production labor hour is shortened, the risk of thymalfasin degradation is also lowered, and the purity of crude products is improved.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
A new method for preparation of thymus by liquid-solid combination of dipeptide fragments
InactiveCN104987382BSolve the problem of low coupling efficiencyAvoid formingThymosin peptidesPeptide preparation methodsCrystallographyDipeptide
The invention belongs to the field of polypeptide synthesis, and relates to a method for preparing thymalfasin through dipeptide fragment liquid-solid bonding. By means of a liquid phase mode, a dipeptide fragment of continuous amino acid is synthesized, the dipeptide fragment is used for batch charging solid phase synthesis, and the problems that loci are difficult to couple, and a deletion peptide is prone to generation are solved. Meanwhile, purity and yield of crude peptides are increased. The technology is used for preparing the thymalfasin so that the purity of the crude peptides can be over 75%. Compared with the prior art, a synthetic route is simple, the problems that the difficult loci are not easy to couple and the deletion peptide is prone to generation are mainly solved, synthesis cost and purification cost are reduced, and industrial large-scale production is facilitated.
Owner:JINAN KANGHE MEDICAL TECH
Method for preparing Thymalfasin
PendingCN113321723AAvoid it happening againReduce usageThymosin peptidesPeptide preparation methodsSide chainCombinatorial chemistry
The invention discloses a method for preparing Thymalfasin. The method comprises the following steps: (1) preparing a Fmoc-Asp-resin complex; (2) preparing a protected Thymalfasin-resin complex; (3) removing a lateral-chain protective group by employing a cutting reagent, splitting Thymalfasin from resin, and carrying out ethyl ether precipitation, so as to obtain a Thymalfasin crude product; and (4) purifying the crude product by employing high-performance liquid chromatography, thereby obtaining refined Thymalfasin. The method for preparing the Thymalfasin, provided by the invention, is simple, convenient and efficient, and the problem that Thymalfasin preparation processes are tedious and are low in efficiency and high in cost at present is solved.
Owner:JIANGSU GENSCRIPT BIOTECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com