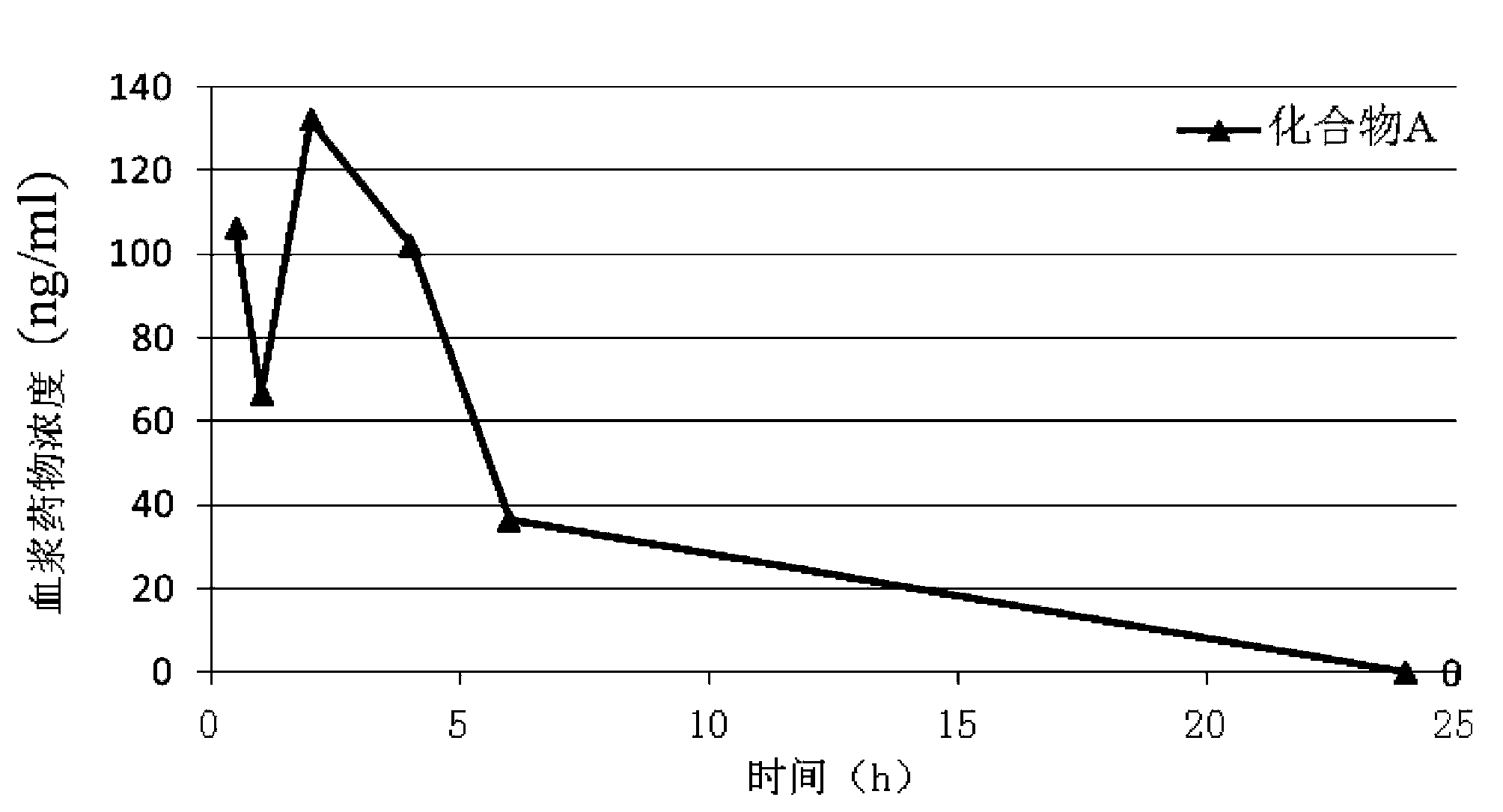
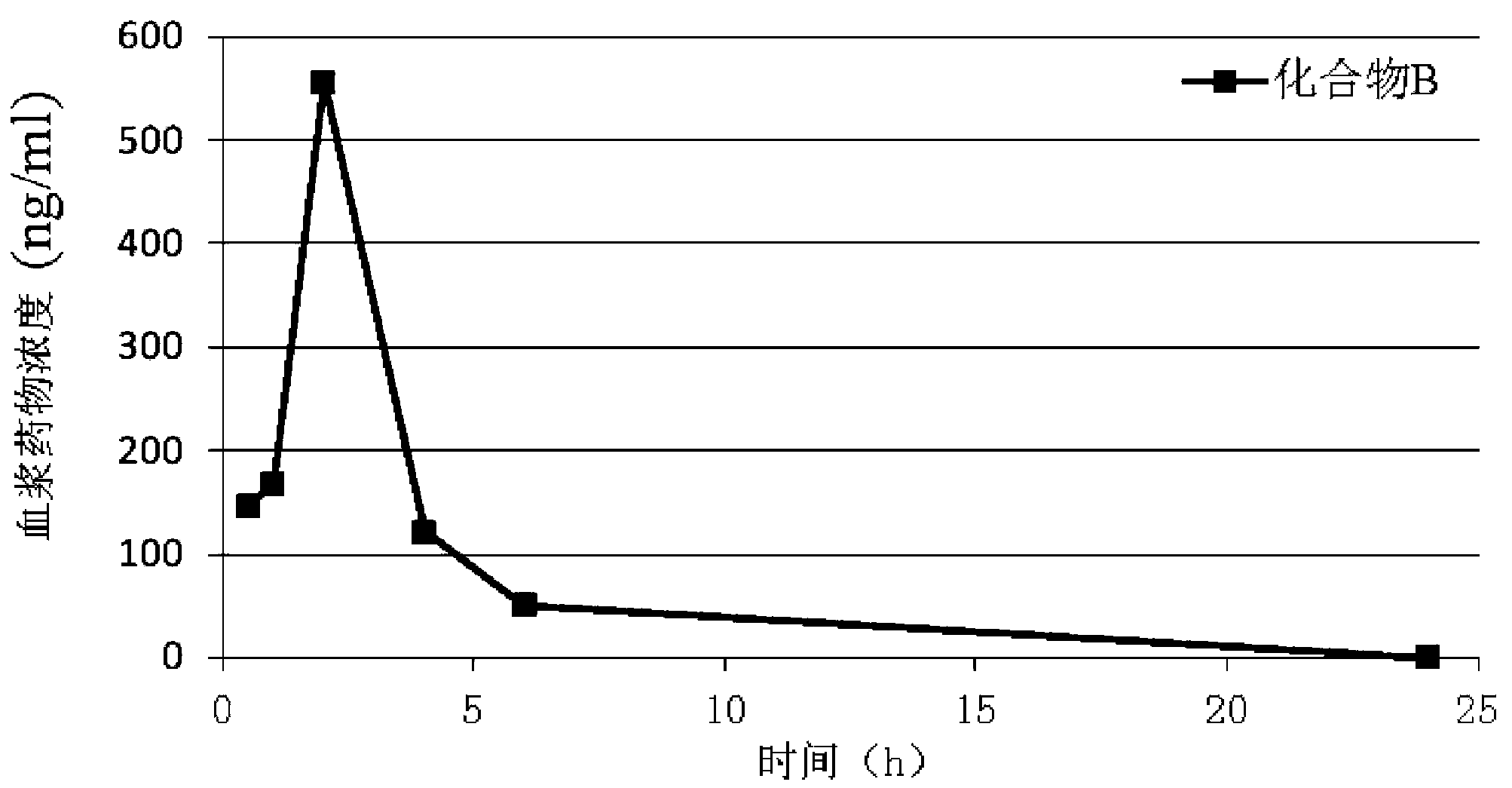
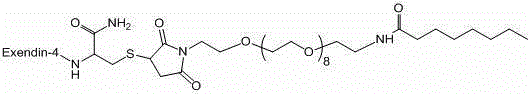
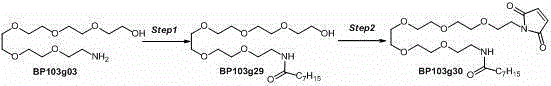
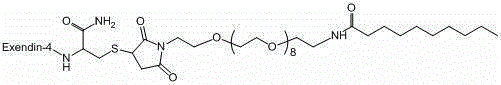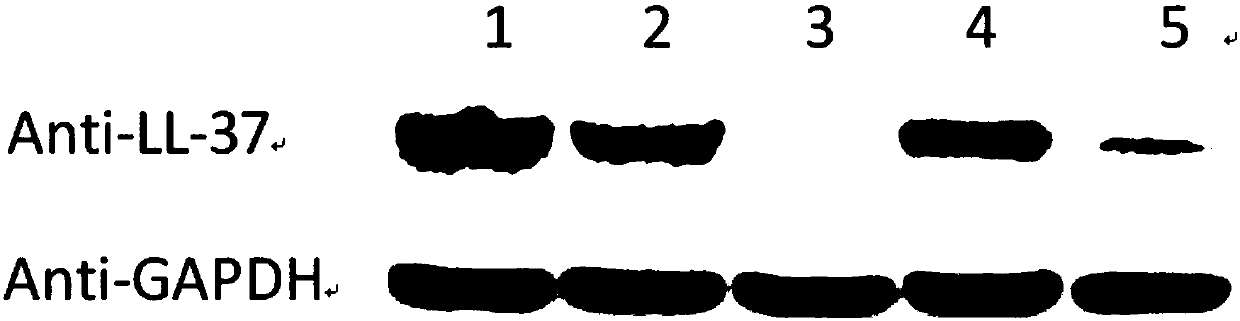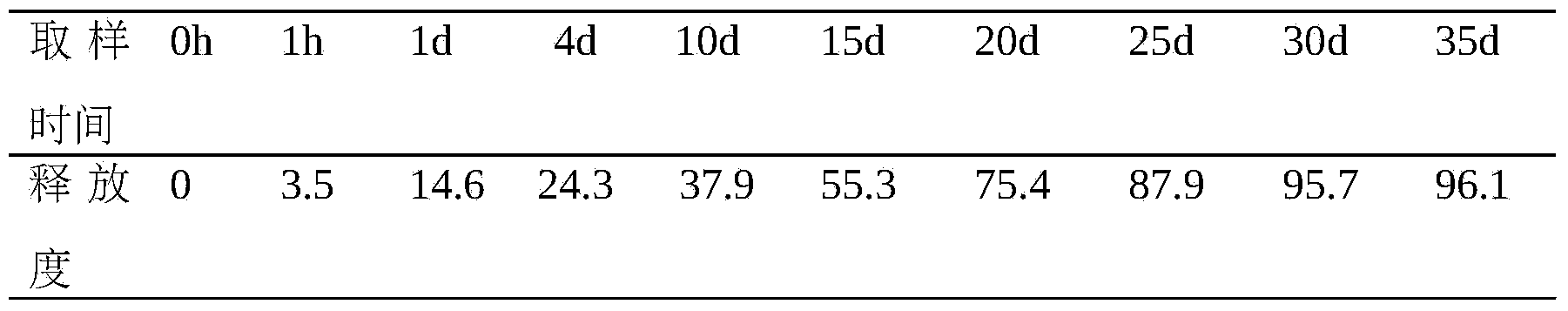Patents
Literature
36results about How to "Prolong the time of action in the body" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Suspension preparation of temperature change painless nano sulfadiazine metallic compound hyaluronic acid
ActiveCN104013574AStrong penetrating powerImprove bioavailabilityOrganic active ingredientsSolution deliveryCelluloseChlorhexidine
The invention discloses a suspension preparation of temperature change painless nano sulfadiazine metallic compound hyaluronic acid. The formula comprises a sulfadiazine metallic compound, a hyaluronic acid substance, an analgetic, a dispersing agent aid, a suspension aid and water, wherein the sulfadiazine metallic compound comprises sulfadiazine silver and sulfadiazine zinc; the hyaluronic acid substance is sourced from biological fermentation and animal tissue extraction and the like and can be hyaluronic acid or hyaluronate or crosslinked hyaluronic acid or crosslinked hyaluronate; the analgetic comprises ropivacaine, bupivacaine, levobupivacaine, lidocaine and other local anesthetics or combination of the local anesthetics; the dispersing agent aid comprises chlorhexidine, Tween series, oleic acid or sodium oleate; the suspension aid can comprise glycerinum, celluloses, poloxamer series and hyaluronic acid substances. The suspension preparation is mainly used for burn, scald or the surface of a wound caused by explosion or anabrosis, has the main clinical characteristics that pain is rapidly relieved, the surface of the wound is subjected to rapid film formation and rapid healing of the wound is promoted, and the conventional main administration modes refer to spraying, smearing and coating.
Owner:LIPONT PHARMA
FAK and EGFR targeted RNA interference plasmid-lipidosome antineoplastic complex
InactiveCN101280317APlay a protective effectTreatment advantageGenetic material ingredientsPharmaceutical non-active ingredientsAbnormal tissue growthLife quality
The invention discloses the tumor gene therapy field, which particularly relates to a RNA interference plasmid-liposome resistant tumor complex of targeting FAK and EGFR. A FAK and EGFR double-gene RNA interference vector prepared by the invention can effectively inhibit the growth of the lung cancer cell of human and can induce the apoptosis; the tumor inhibiting experiment in the vivo also indicates that the FAK and EGFR double-gene RNA interference vector-liposome resistant tumor complex can obviously inhibit the growth of a plurality of the tumor, and the lifetime of the tumor bearing mice is prolonged. In the invention, the FAK and EGFR double-gene RNA interference vector is wrapped through the cationic liposome, and the chemotherapy and the radiotherapy are taken as the resistant tumor medicine of the active component together, compared with each individual operation, the resistant tumor effect is obviously optimized, and both used amounts can be reduced. By adopting the invention, the effect is obvious, the dose can be greatly reduced, the life quality of the patient is improved, and thereby the invention has a good market developing prospect.
Owner:SICHUAN UNIV
RNA interference expression plasmid- cationic liposome-heparin antineoplastic complexes targeting human FAK and PLK1 gene
InactiveCN101358200AReduce chargeReduce the charge fromGenetic material ingredientsPharmaceutical non-active ingredientsLife qualityTreatment field
Belonging to the field of tumor gene therapy, the present invention provides an anti-tumor RNA interference plasmid-lipidosome-heparin complex targeting the human FAK and FLK1 genes. The RNA interference expression vector can express a RNA interference sequence aimed at a target site shown by SEQ ID NO:1 on the FAK gene and a RNA interference sequence aimed at a target site shown by SEQ ID NO:2 on the FLK1 gene in the body. The FAK and PLK1 dual-gene RNA interference plasmid prepared by the present invention can effectively inhibit the growth of lung cancer cells and induce the lung cancer cells to be apoptosed. A tumor inhibition experiment conducted in the body also indicates that the FAK and PLK1 dual-gene RNA interference plasmid-lipidosome-heparin complex can notably inhibit the growth of manifold tumors and prolong the lifetime of tumor-bearing mice. The product of the anti-tumor RNA interference plasmid-lipidosome-heparin complex has obvious efficacy and can reduce dosage and improve the life quality of patients, thus having a good application prospect.
Owner:SICHUAN UNIV
Preparation method and application of targeted multi-function double drug-loading liposome
ActiveCN104490786AAvoid scavengingProlong the time of action in the bodyPharmaceutical non-active ingredientsEmulsion deliveryHalf-lifeFilm material
The invention belongs to the preparation and application technical field of the liposome and specifically discloses a preparation method and application of targeted multi-function double drug-loading liposome. The raw material for the targeted multi-function double drug-loading liposome comprises the basic film material phospholipid, polyethylene glycol modified phospholipid capable of prolonging the half-life period of the liposome medicine blood, phospholipid capable of having fluorescence imaging, phospholipid having targeted function, lipid-soluble drug and water-soluble drug treating the tumor and contrast agent capable of strengthening nuclear magnetism imaging signal. The medicine can solve the clinic use defect of the medicine with poor water solubility through the package of the phospholipid, the method of administration of the conventional medicine is changed by combining with the water-soluble drug, the targeting action is achieved for the tumor tissue for effectively restraining the tumor growth, reducing the production of the side reaction; the medicine action effect and the tumor change condition can be observed in real time through MRI and great clinical application value is achieved.
Owner:INNOVATION ACAD FOR PRECISION MEASUREMENT SCI & TECH CAS
Indolone compounds or derivatives thereof and applications thereof
ActiveCN104003925AIncrease blood concentrationProlonged anti-cancer half-lifeOrganic active ingredientsOrganic chemistryAngiogenesis growth factorVascular Endothelial Growth Factor Receptor
The invention relates to indolone compounds shown as a general formula (a) or derivatives thereof and applications thereof. According to the invention, indolone compounds are substituted with deuterium to obtain deuterium-enriched indolone compounds; the indolone compounds can simultaneously act on the following three key receptor families involved in angiogenesis process: vascular endothelial growth factor receptor (VEGFR), fibroblast growth factor receptor (FGFR) and platelet-derived growth factor receptor (PDGFR), so as to inhibit angiogenesis and achieve the effect of treating cancers. The deuterated indolone compounds or the derivatives thereof have the advantage of high efficiency when used asdrugs. The general formula (a) is as shown in the specification.
Owner:SICHUAN UNIV
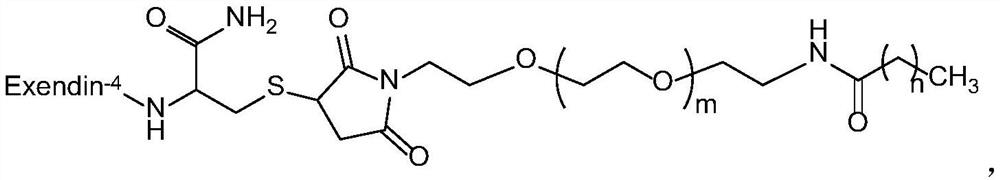
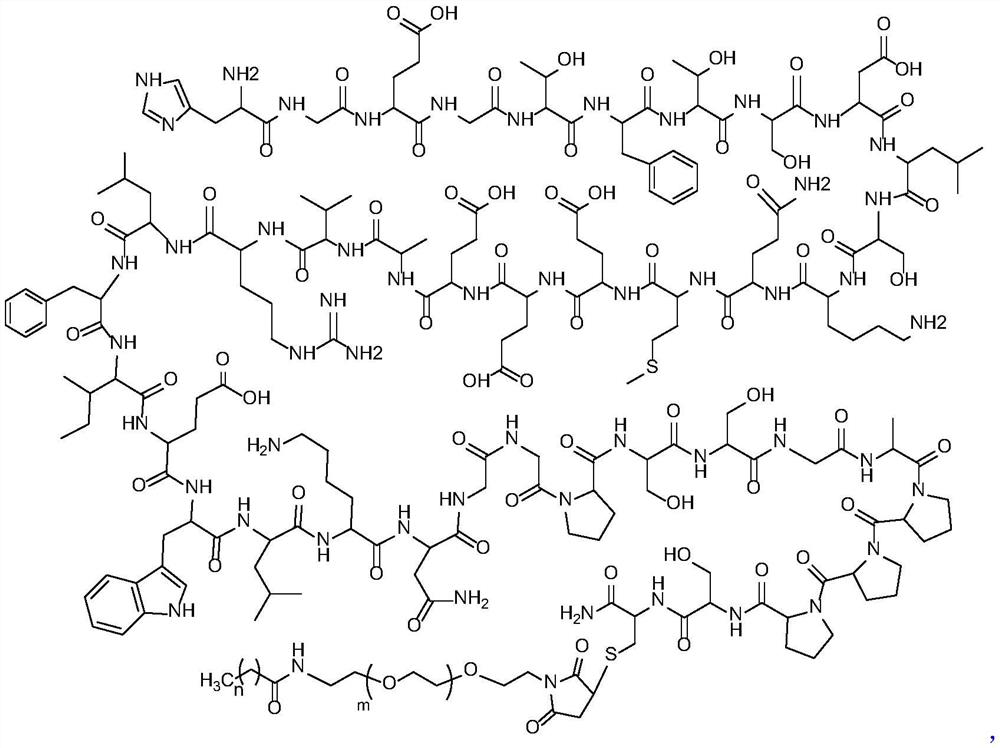
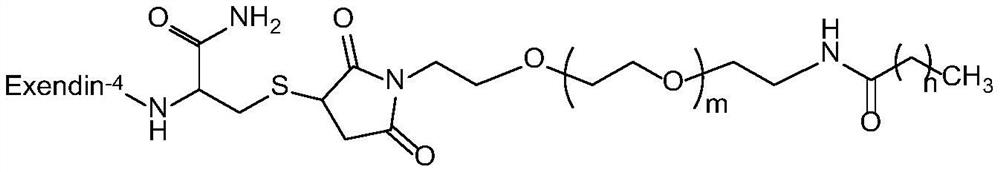
Exenatide modified compound and uses thereof
ActiveCN106554404AHigh activityProlonged hypoglycemiaPeptide/protein ingredientsMetabolism disorderDrugExenatide
The invention discloses an exenatide modified compound, a use of the exenatide modified compound in preparation of a medicine which serves as a GLP-1 receptor stimulant, a use of the exenatide modified compound in preparation of medicines used for preventing and / or curing diseases and / or symptoms relevant to low GLP-1 receptor activity, a use of the exenatide modified compound in preparation of medicines used for diseases and / or symptoms relevant to carbohydrate metabolism, a use of the exenatide modified compound in preparation of medicines used for diabetes, a use of the exenatide modified compound in preparation of medicines used for fatty liver, and a use of the exenatide modified compound in preparation of medicines used for weight losing.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Thymalfasin sustained release micro-sphere preparation and preparing method thereof
InactiveCN104984327AReduce dosing frequencyProlong the time of action in the bodyPeptide/protein ingredientsDigestive systemSide effectHalf-life
The invention provides a thymalfasin sustained release micro-sphere preparation and a preparing method thereof. The thymalfasin sustained release micro-sphere preparation comprises, by micro-sphere weight, 0.1%-30% of thymalfasin, 50%-99.9% of a biodegradable high polymer material which ranges from 5000 Dalton to 200000 Dalton in molecular weight and has the biocompatibility and 0%-20% of other acceptable auxiliary materials on pharmacy. The invention further provides the preparing method for preparing the thymalfasin sustained release micro-sphere preparation, namely a high-pressure static microcapsule molding method. By means of thymalfasin sustained release micro-spheres prepared with the method, effective embedding and sustained release of the thymalfasin are achieved, the sustained release effect can reach 40 days, the toxic and side effect of the thymalfasin can be effectively reduced, the bioavailability is improved, and the metabolic half-life is prolonged; and meanwhile, the number of drug administration times is decreased, and economic and mental burdens of a patient are relieved.
Owner:山东博创生物科技有限公司
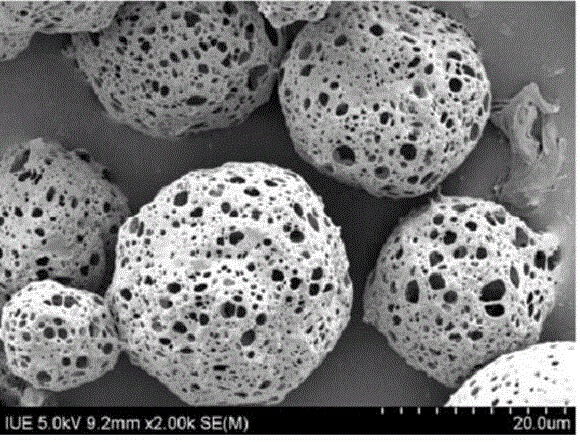
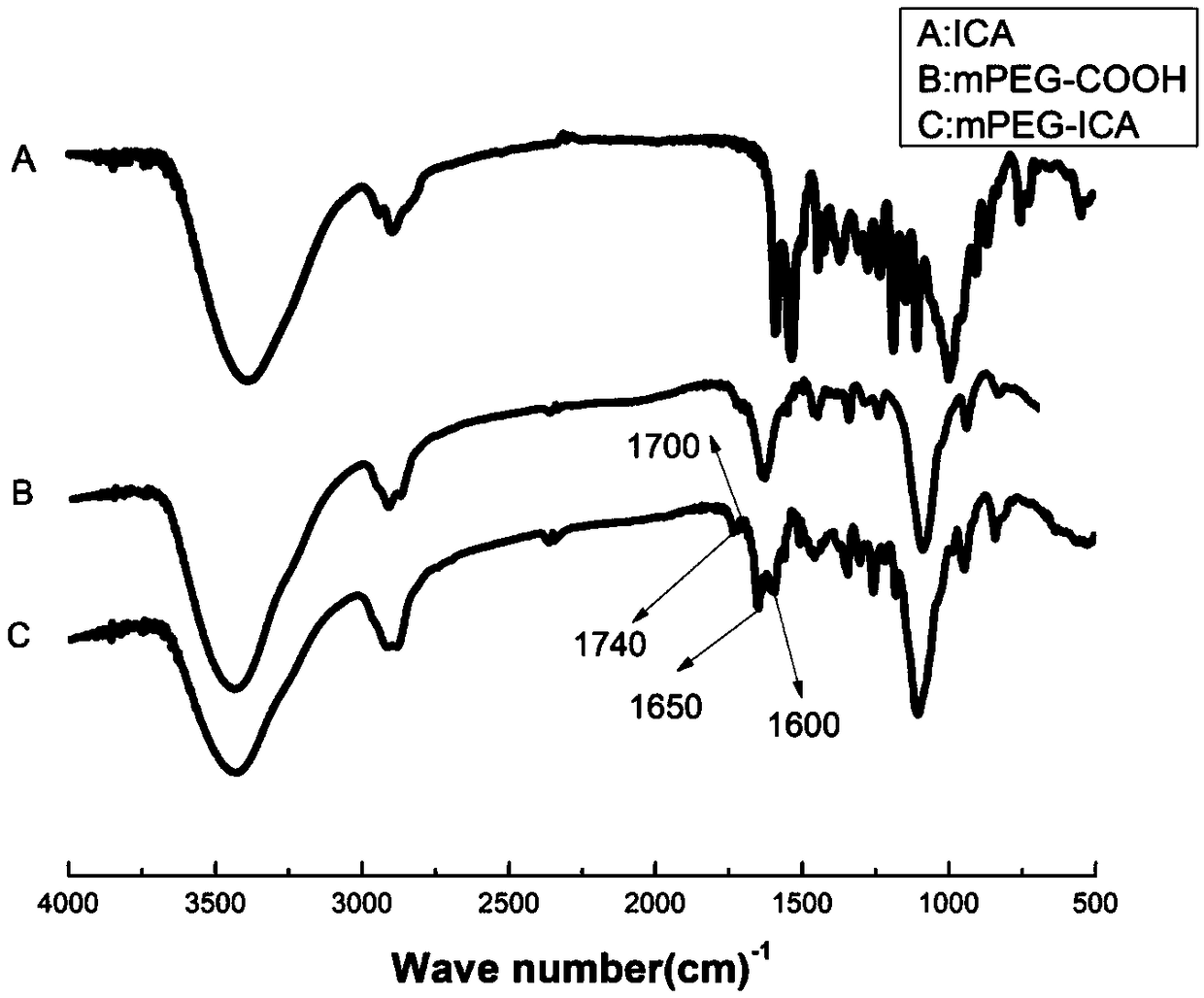
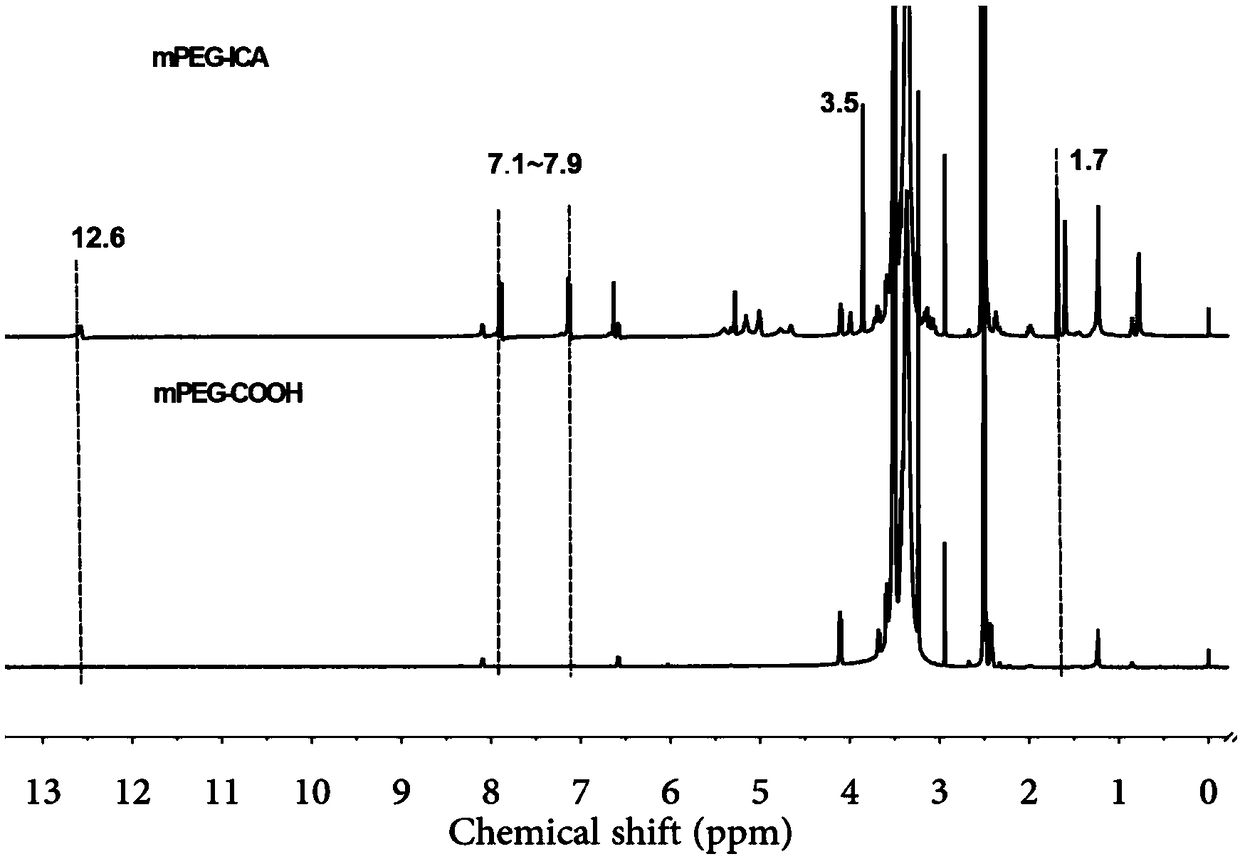
Carboxylated mPEG (polyethylene glycol methyl ether)-ICA (icariin) nanoparticle, preparation method and application
PendingCN109464674AGood water solubilityProlong the time of action in the bodyPowder deliveryOrganic active ingredientsSolubilityMicrosphere
The invention discloses a carboxylated mPEG (polyethylene glycol methyl ether)-ICA (icariin) nanoparticle, a preparation method and an application. The nanoparticle comprises ICA as a hydrophobic endand carboxylated mPEG as a hydrophilic end. In an aqueous solution, the mPEG part is linked to form a hydrophilic shell, ICA is used as a hydrophobic core, and a nano-scale mPEG-ICA polymer microsphere of a spherical core-shell structure is formed spontaneously. According to the carboxylated mPEG-ICA nanoparticle, the water solubility of ICA can be increased, the acting time of medicines in vivo can be prolonged, and the slow release effect can be enhanced.
Owner:HUBEI UNIVERSITY OF MEDICINE
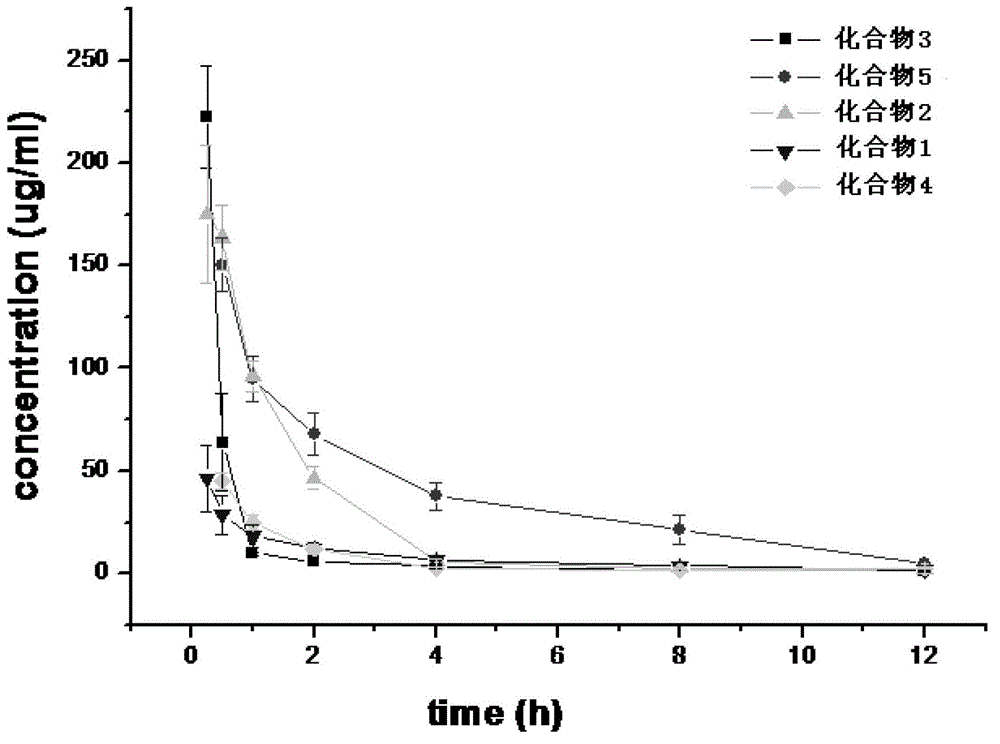
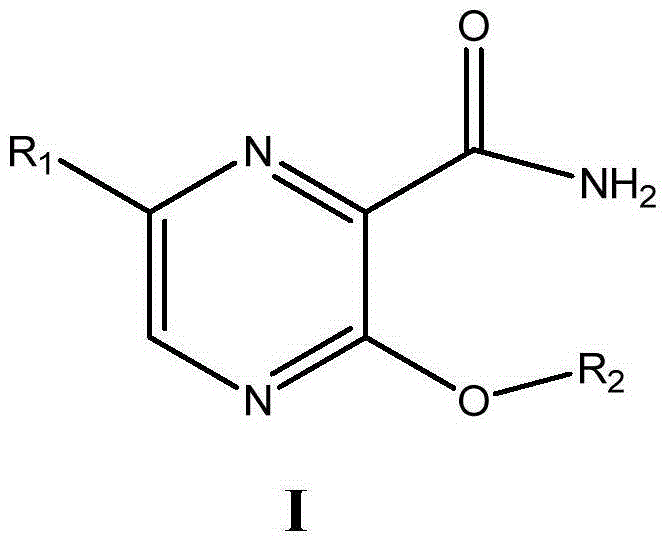
Preparation method and purpose of 3-alkoxy-substituent-2-pyrazinyl formamide compounds
ActiveCN102850282AImprove bioavailabilityProlong the time of action in the bodyOrganic chemistryAntiviralsAntiviral drugAlkoxy group
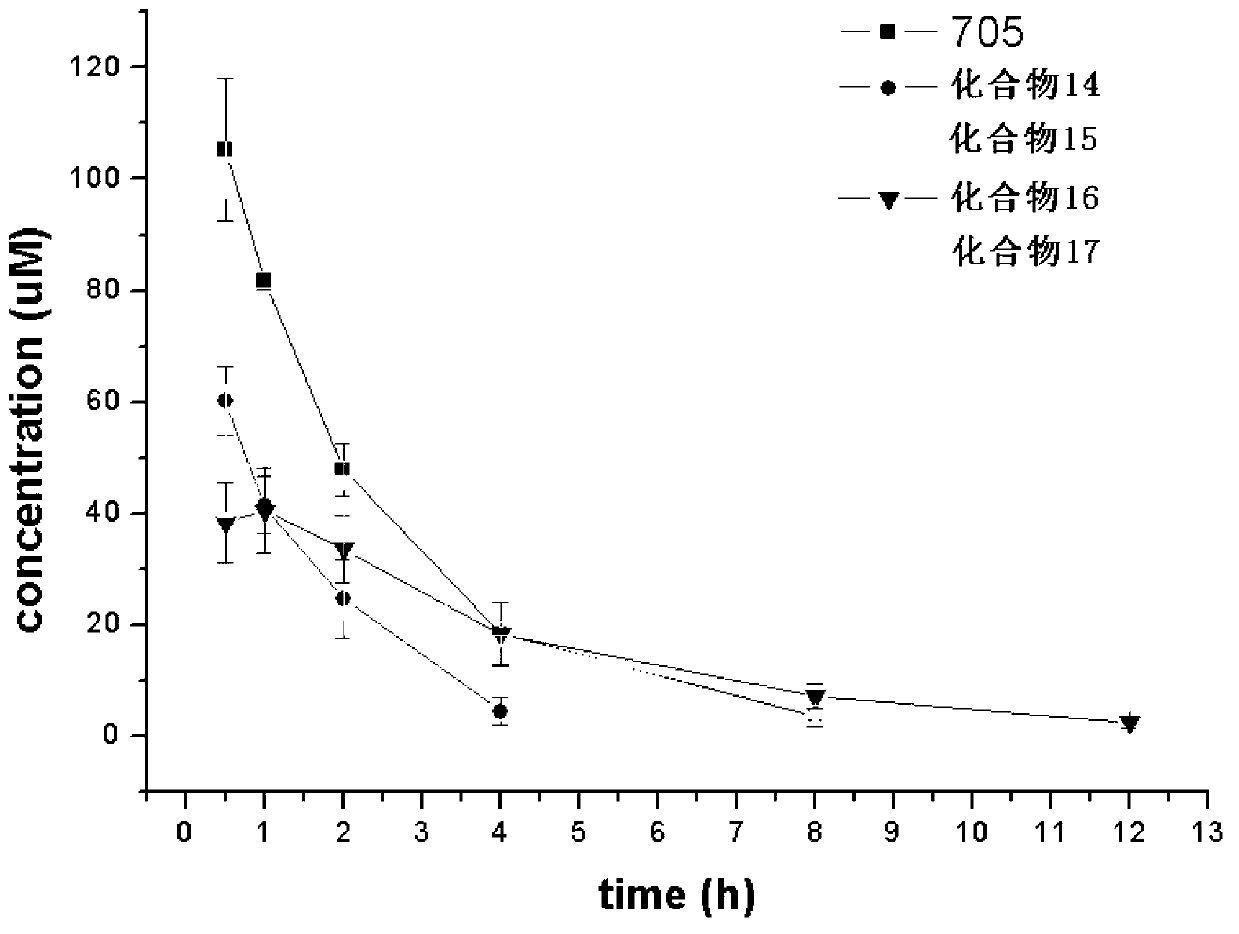
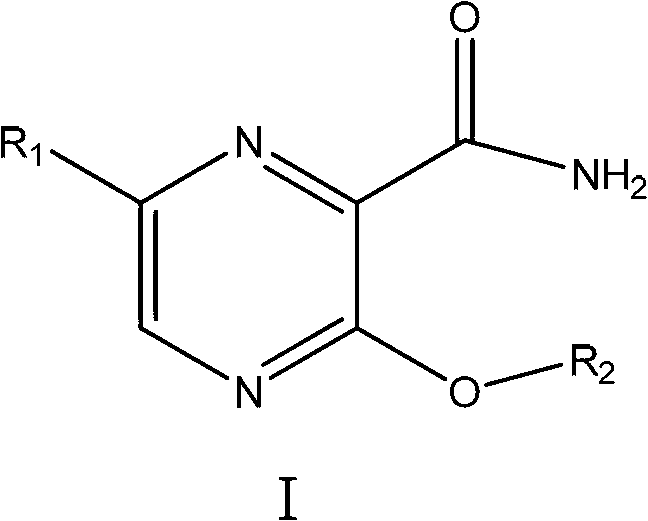
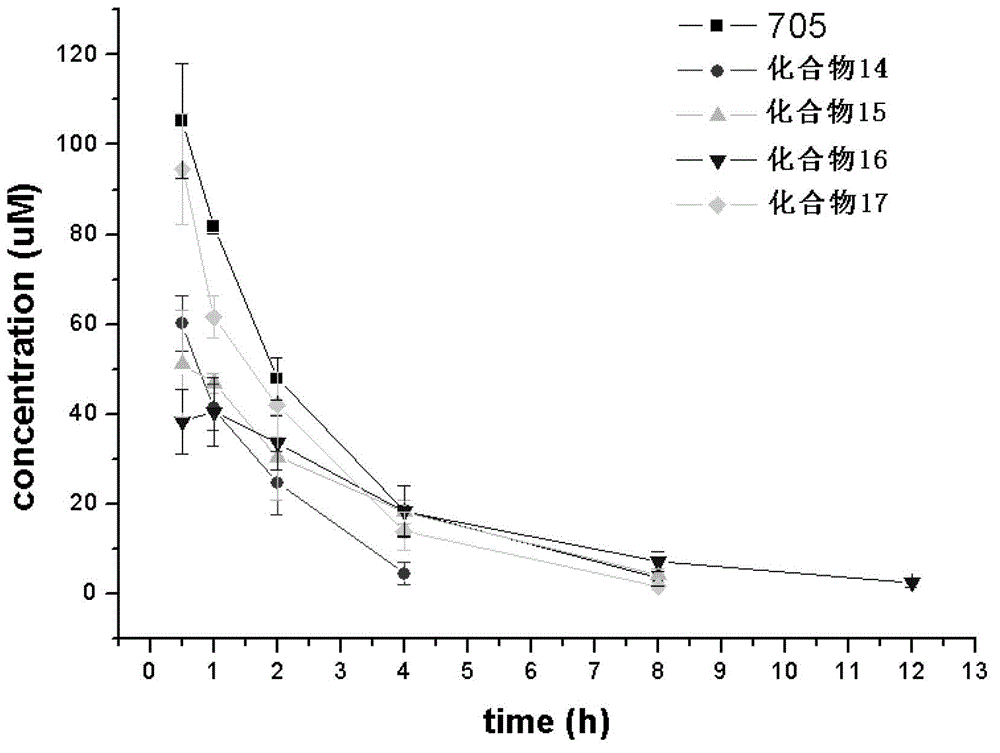
The invention related to 3-alkoxy-substituent-2-pyrazinyl formamide compounds with a structure represented by the general formula I, and an application thereof in preparing antiviral medicines. The compounds provided by the invention perform antiviral effects in vivo through a process of being metabolized into T1105 or T705 by esterase or P450 enzyme. Compared with prototype medicines T1105 or T705, the compounds with the general formula I have substantial advantages of high bioavailability and long in-vivo action time.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and preparation method thereof
ActiveCN103735528ARelease constant speedSmall toxicityOrganic active ingredientsPharmaceutical delivery mechanismSide effectMotility
The invention provides a trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet and a preparation method thereof. The osmotic pump controlled release tablet is formed by a drug-containing layer, a booster layer and a coating film, wherein the drug-containing layer comprises the following components in percentage by weight: 10-50% of trimetazidine hydrochloride, 30-80% of suspension agent and the balance of other auxiliary materials; the booster layer comprises the following components in percentage by weight: 20-90% of swelling agent, 5-70% of osmotic active substance and 0.5-5% of lubricating agent; the semipermeable coating film comprises 10-20g of semipermeable high polymer material and 1-5g of water-soluble pore-forming agent every 100 tablets. The trimetazidine hydrochloride bi-layer osmotic pump controlled release tablet can realizes constant release of drug in the body of a patient without being affected by pH value of a medium environment, enzyme, gastrointestinal motility and food, and is capable of maintaining the stability of plasma concentration of drug, reducing toxic and side effects of drug, decreasing dosing frequency and improving compliance of the patient.
Owner:SHENYANG PHARMA UNIVERSITY
Sphaelactone dimethylamine lipidosome atomizing inhalant and application thereof
ActiveCN105726520AGood treatment effectProlong the time of action in the bodyOrganic active ingredientsPharmaceutical non-active ingredientsPhospholipidTherapeutic effect
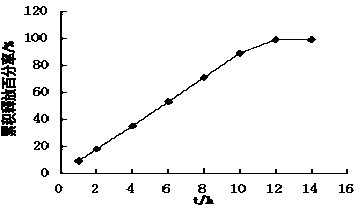
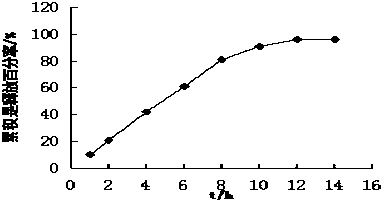
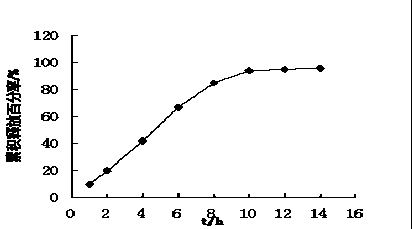
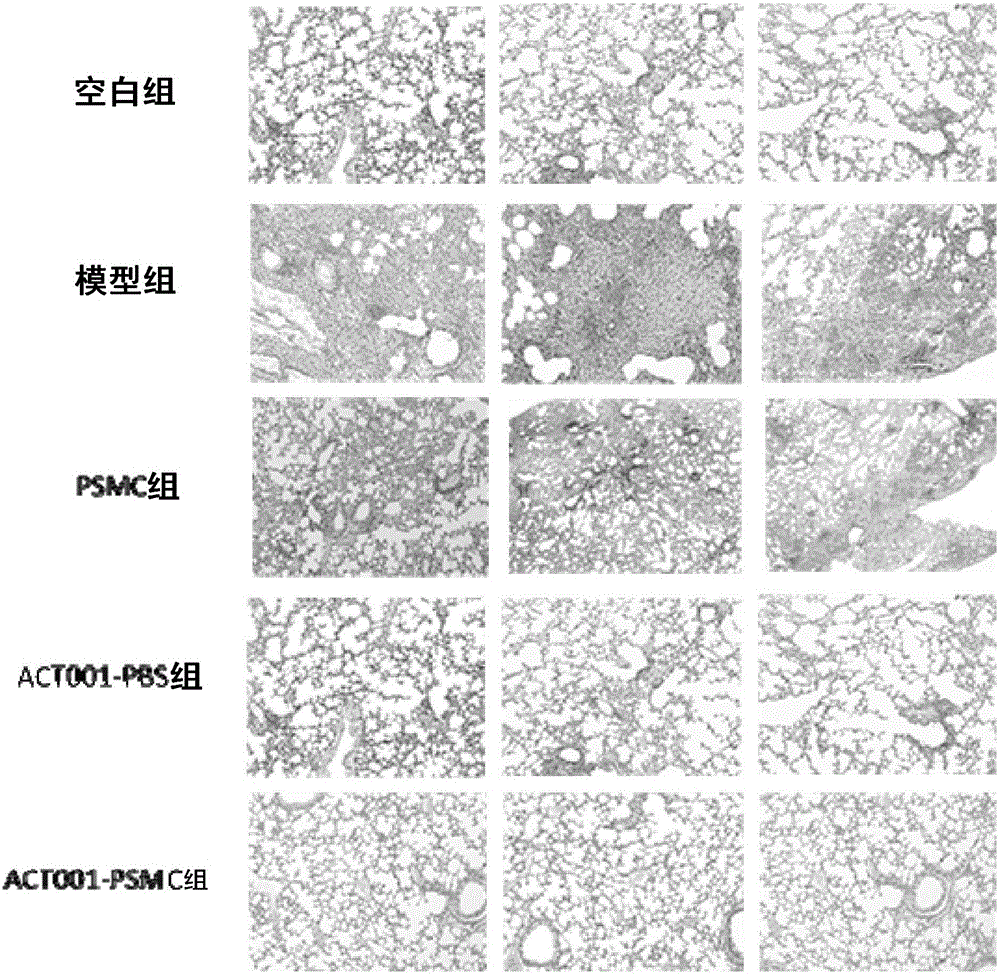
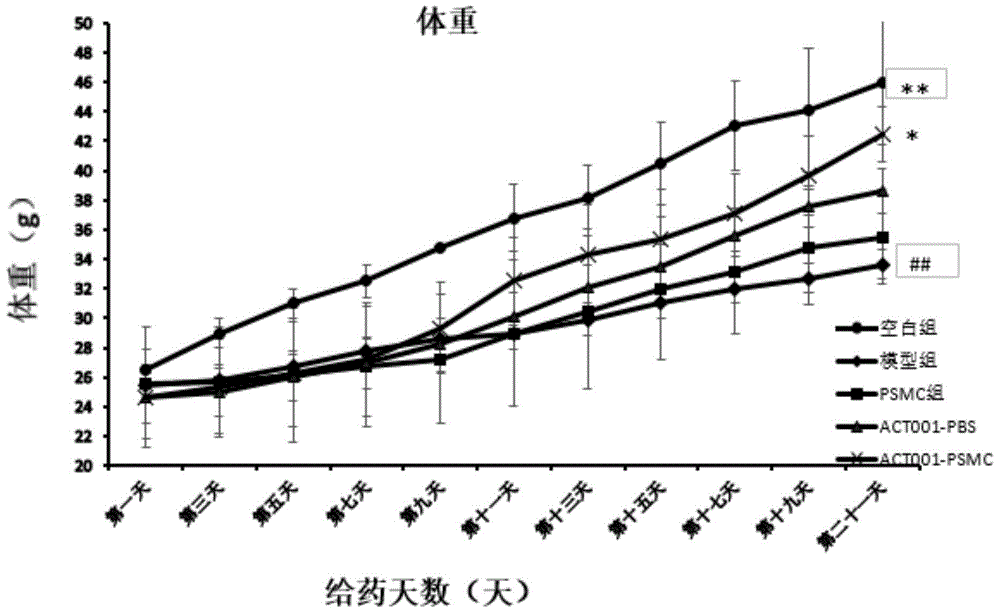
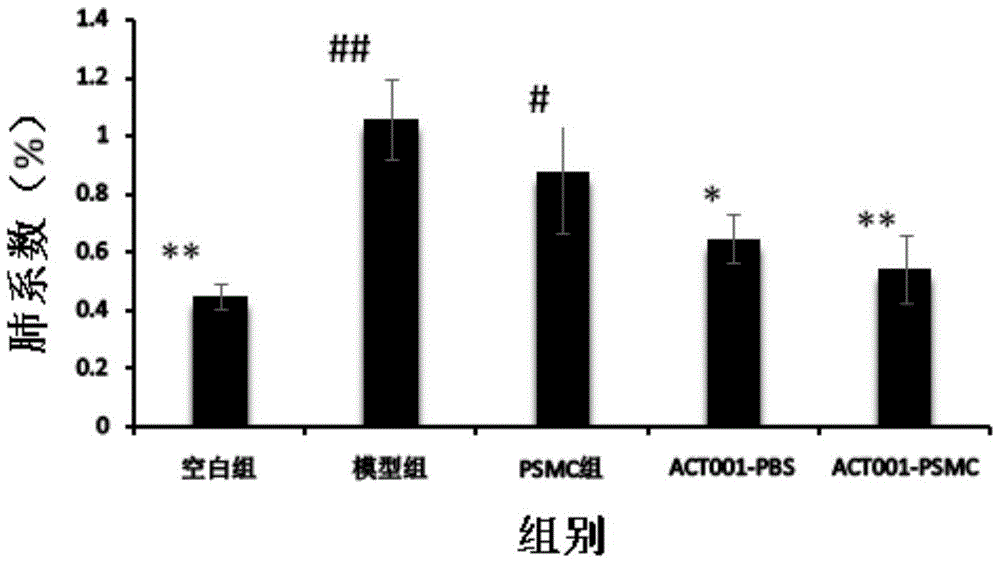
The invention innovatively provides sphaelactone dimethylamine lipidosome.The lipidosome uses a similar surface active substance as the carrier and includes medicine effective components, namely, sphaelactone dimethylamine and derivatives of sphaelactone dimethylamine.The application of the sphaelactone dimethylamine lipidosome in medicine for treating the pulmonary fibrosis is achieved.Compared with the prior art, the sphaelactone dimethylamine lipidosome atomizing inhalant with the PSMC as the carrier and the application of the inhalant in medicine for treating the pulmonary fibrosis have the advantages that through the phospholipid bilayer structure, the same as that of cytomembrane, of PSMC and the lubricating and protecting effects of phospholipids, the combining rate of medicine and alveolar cells is increased, the pharmacokinetics characteristics are changed, the medicine in-vivo acting time is prolonged, and the treatment effect of sphaelactone dimethylamine on the pulmonary fibrosis is remarkably improved.
Owner:NANKAI UNIV
Anti-microbial fusion proteins and application thereof
InactiveCN107603997AHigh molecular weightProlong the time of action in the bodyAntibacterial agentsPeptide/protein ingredientsBiotechnologyAntimicrobial peptides
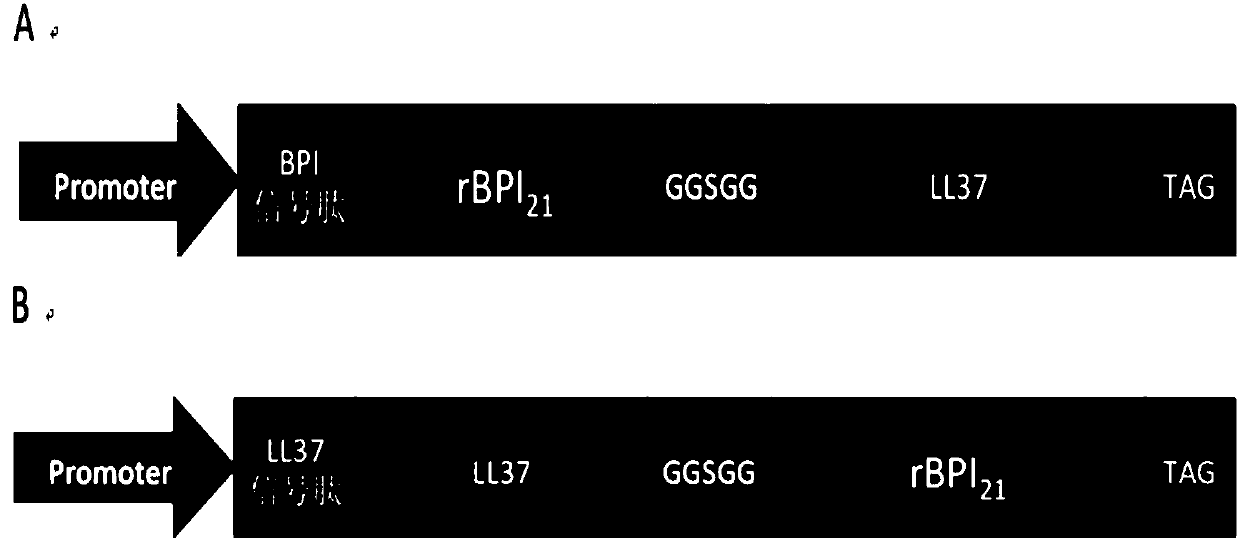
The invention belongs to the field of biotechnological genetic engineering, and mainly relates to construction of an expression vector of anti-microbial fusion proteins and application thereof. The construction aims at the problems of narrow antimicrobial spectrum and bad antimicrobial effects of antimicrobial peptides in the prior art, the anti-microbial fusion proteins utilize codon preference,a fusion protein gene is designed and synthesized, a recombined vector is constructed, and after the vector is introduced into host cells, and the fusion proteins containing an antimicrobial protein segment rBPI21 and an antimicrobial peptide LL-37 are obtained. The fusion proteins have large molecular weight, can prolong internal action time of fusion proteins, have wider antimicrobial spectrum and largely enhanced sterilization effects, can be widely applied to the anti-microbial, anti-endotoxin and other technology fields, and provide a new selection for biological sterilization medicaments.
Owner:重庆赛托斯创生物科技发展有限公司
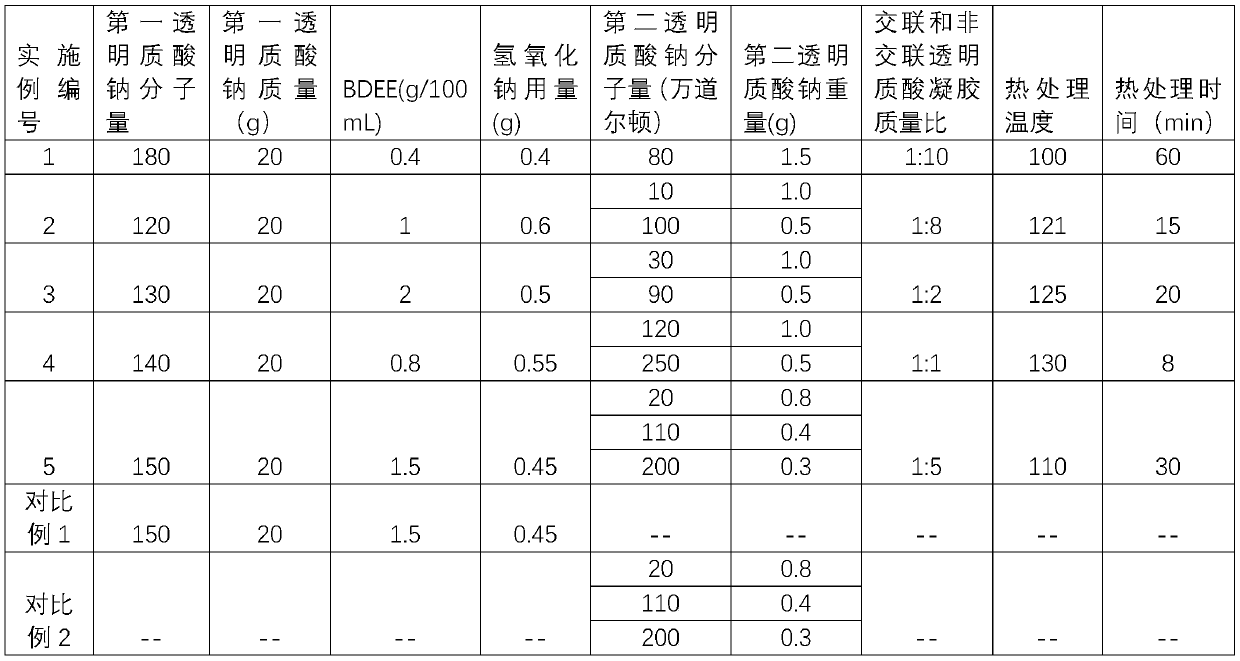
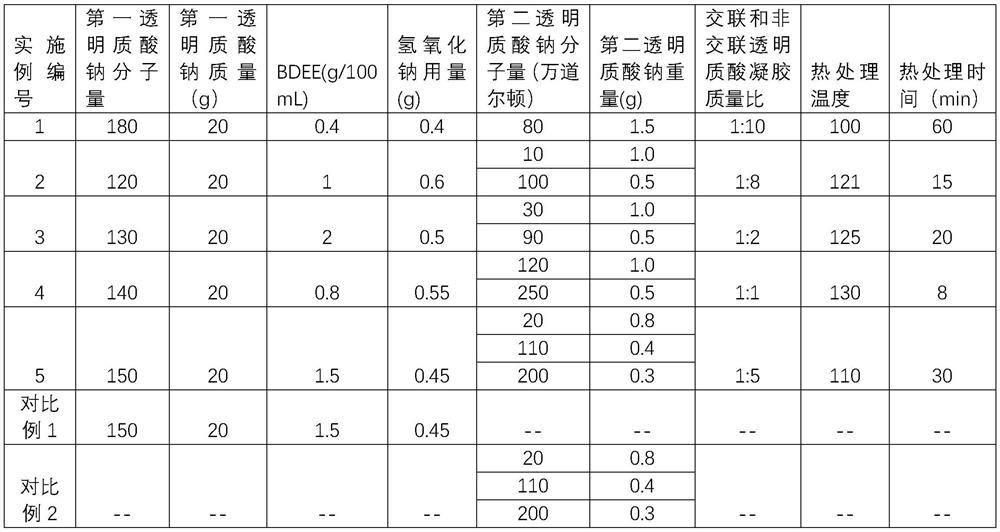
Composition containing hyalurates and preparation method thereof
ActiveCN110982126AIncreased resistance to hyaluronidaseProlong the time of action in the bodyTemperature treatmentBiology
A composition containing hyalurates is provided. The composition comprises a double-gel system consisting of crosslinked hyalurate gel and non-crosslinked hyalurate gel. The pH of the double-gel system is 6.0-8.0, the average particle size is 100-300 [mu]m, the kinematic viscosity is 10-80 mm<2> / s, and the extruding force is 5-30 N. The invention also provides a preparation method of the composition containing hyalurates. The method includes preparing the crosslinked hyalurate gel by utilizing a first hyaluronic acid or hyalurate; preparing the non-crosslinked hyalurate gel by utilizing a second hyaluronic acid or hyalurate; mixing the crosslinked hyalurate gel and the non-crosslinked hyalurate gel; and performing high-temperature treatment to obtain the double-gel system. The composition,compared with traditional hyaluronic acid water-gloss products, has enhanced resistance to hyaluronidase, longer action time in vivo, low gel viscosity, a high slip degree and enhanced fluidity, is easier to extrude and operate during clinical use, and can reduce the occurrence of side reactions such as skin hills and redness.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD +1
Thymalfasin slow release microsphere preparation and preparation method thereof
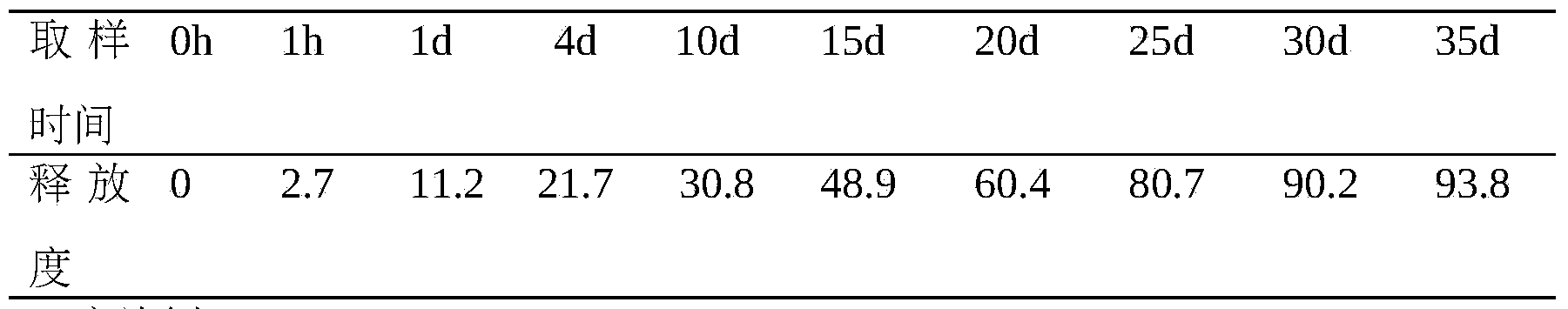
InactiveCN105935353AReduce dosing frequencyProlong the time of action in the bodyPeptide/protein ingredientsDigestive systemSide effectMicrosphere
The invention provides a thymalfasin slow release microsphere preparation and a preparation method thereof. The thymalfasin slow release microsphere preparation is a preparation prepared from thymalfasin accounting for 0.1-30% of the weight of microspheres, a biodegradable and biocompatible polymer material accounting for 50-99.9% of the weight of the microspheres and having a molecular weight of 5000-200000dalton, and other pharmaceutically acceptable auxiliary materials accounting for 0-20% of the weight of the microspheres. The invention also provides the preparation method of the thymalfasin slow release microsphere preparation. The method is a high-voltage electrostatic microcapsule molding method. The thymalfasin slow release microsphere preparation realizes effective embedding and slow releasing of thymalfasin, can continuously release for 40d, and also has the advantages of effectively reduction of the toxic and side effects of thymalfasin, improvement of the bioavailability, prolongation of the metabolism half life, administration frequency reduction, and reduction of the economy and spirit burden of patients.
Owner:山东博创生物科技有限公司
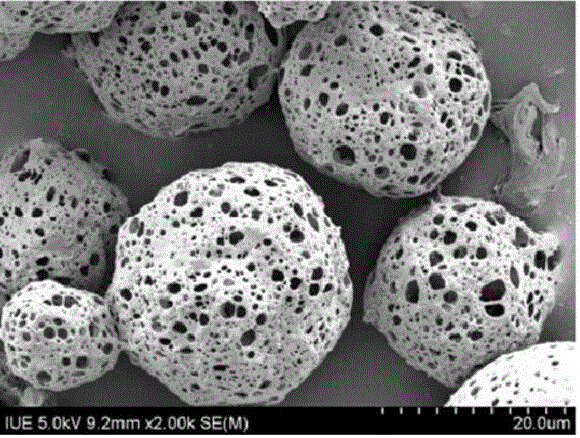
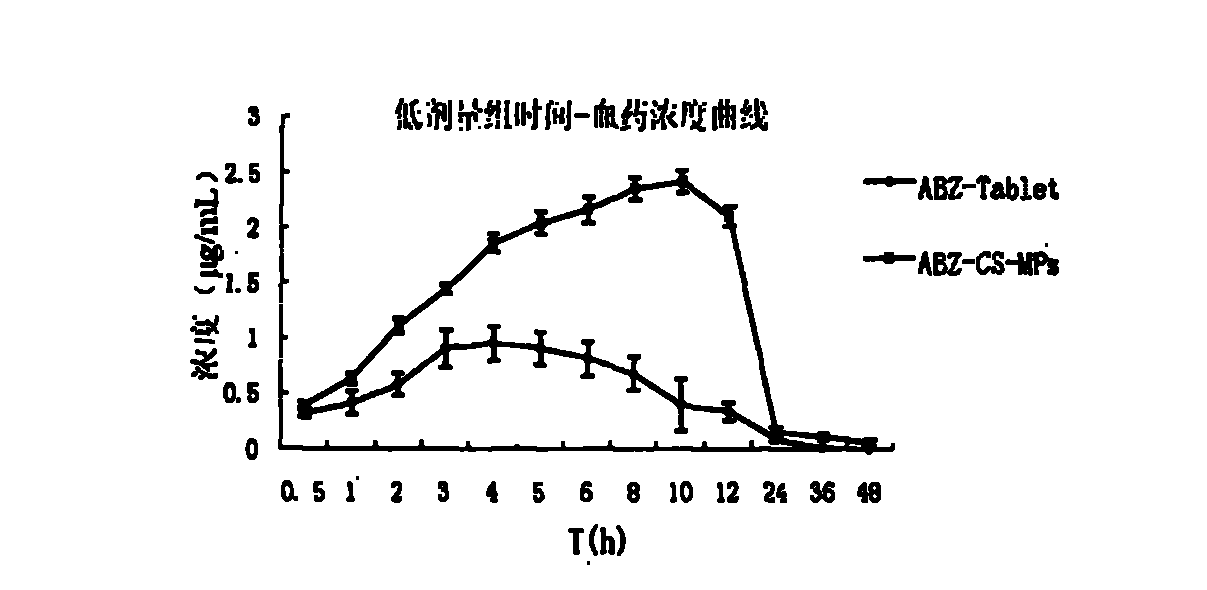
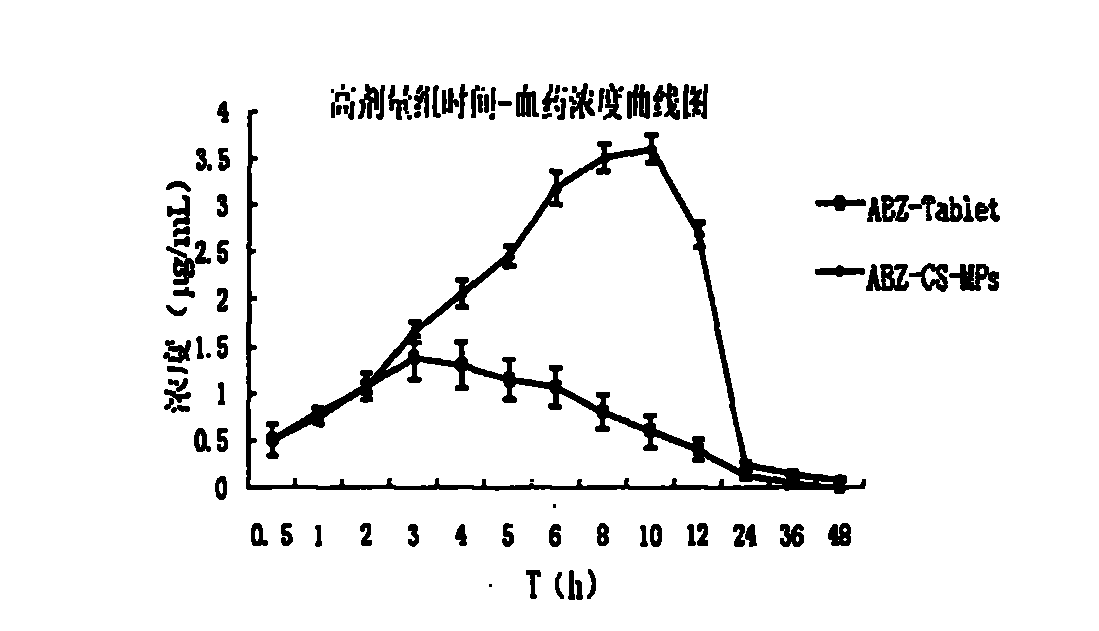
Albendazole chitosan microsphere composition and preparation method thereof
InactiveCN102525947AImprove oral bioavailabilityImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsRelease timeOil phase
The invention relates to an albendazole chitosan microsphere composition and a preparation method thereof. The albendazole chitosan microsphere composition consists of albendazole and chitosan. The preparation method comprises the following steps of: dissolving albendazole and chitosan in a dilute acetic acid solution to form an aqueous phase, and dissolving a surfactant in oil to form an oil phase, wherein the volume ratio of the aqueous phase to the oil phase is (1:2)-(1:8); injecting the aqueous phase into the oil phase and stirring to obtain an emulsion; adding a crosslinking curing agent into the emulsion while stirring; after the crosslinking curing of microspheres is finished, performing centrifugal separation; washing with petroleum ether; and drying to obtain the albendazole chitosan microspheres. The albendazole chitosan microspheres provided by the invention have the characteristics of narrow particle size distribution, high encapsulation efficiency and simple preparation method; and compared with albendazole tablets, the albendazole chitosan microsphere composition has the advantages that the bioavailability is obviously improved to reach about 500% of the bioavailability of the albendazole tablets, the action time of the medicine can be prolonged, and the in-vivo medicine release time is increased to 48 hours.
Owner:石河子大学医学院第一附属医院
Bacillus adhaerens and its use in preparing health food having thrombolysis property
InactiveCN1177039CHigh activityStrong thrombolytic effectBacteriaBacteria material medical ingredientsAcid-fastSolubility
Bacilliis natto NLSSc with the preservation number CGMCC No. 0724 is Gram staining positive, non-acid fast, straight or near straight, (0.6-0.8)x(1.5-2.0) micron sized and aerobic; has mesial and un-swelled spore with the spore number inside one sporange cell not more than one, meso diamino heptane diacid and glycin contained in the cell wall, no characteristic saccharum and peripheral flagellum; and can be grown well in five kinds of natural organic culture medium and can form relatively thick mycoderm and butter fat colored colony with smooth edge in soybean cake powder culture medium. Bacilliis natto can be cultured in culture medium with nitrogen source to obtain thrombus dissolving matter natto kinase and the cultured matter may be extracted to obtain powdered matter as the additive for thrombolytic health food.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Epothilone B polymer nanoparticle preparation for treating tumors and preparation method thereof
InactiveCN105963276AAvoid hydrolysisAvoid phagocytosisOrganic active ingredientsPharmaceutical non-active ingredientsDrug releaseIn vivo
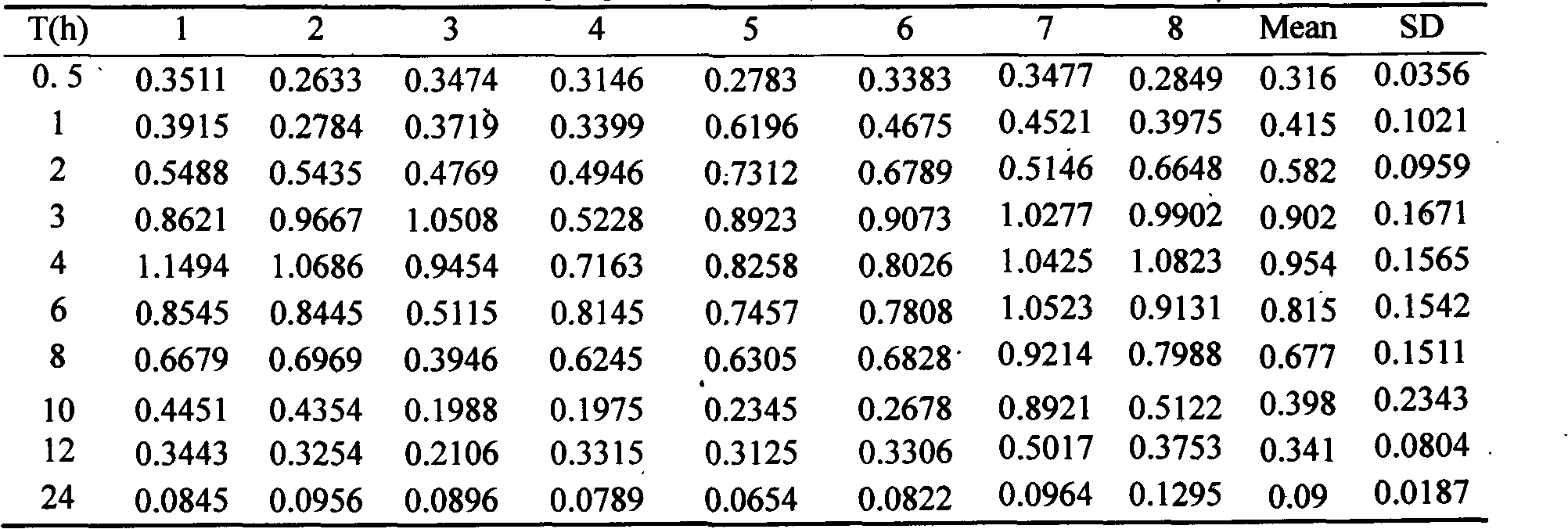
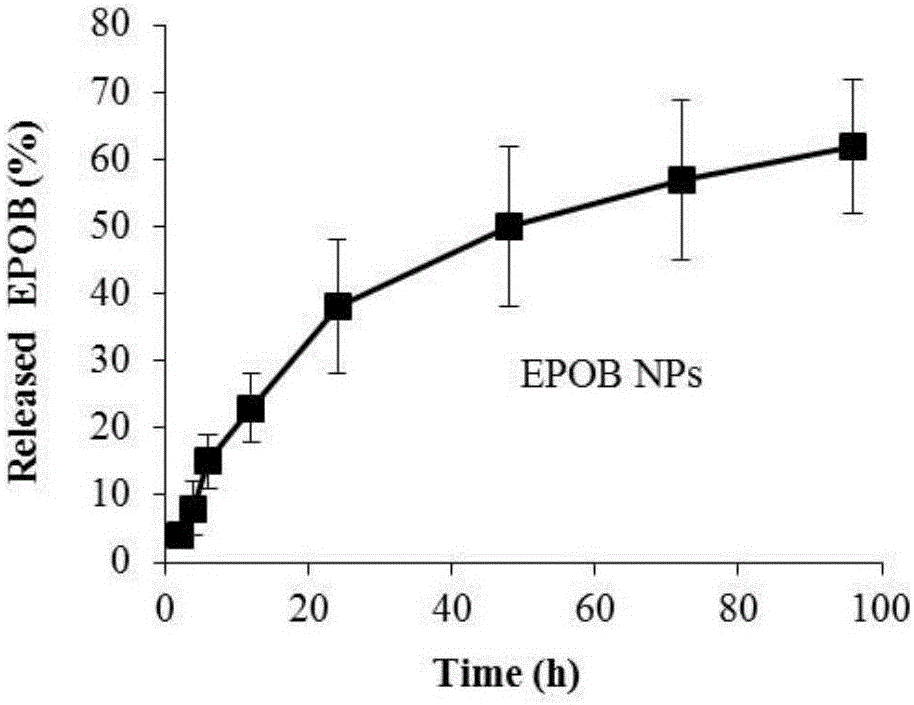
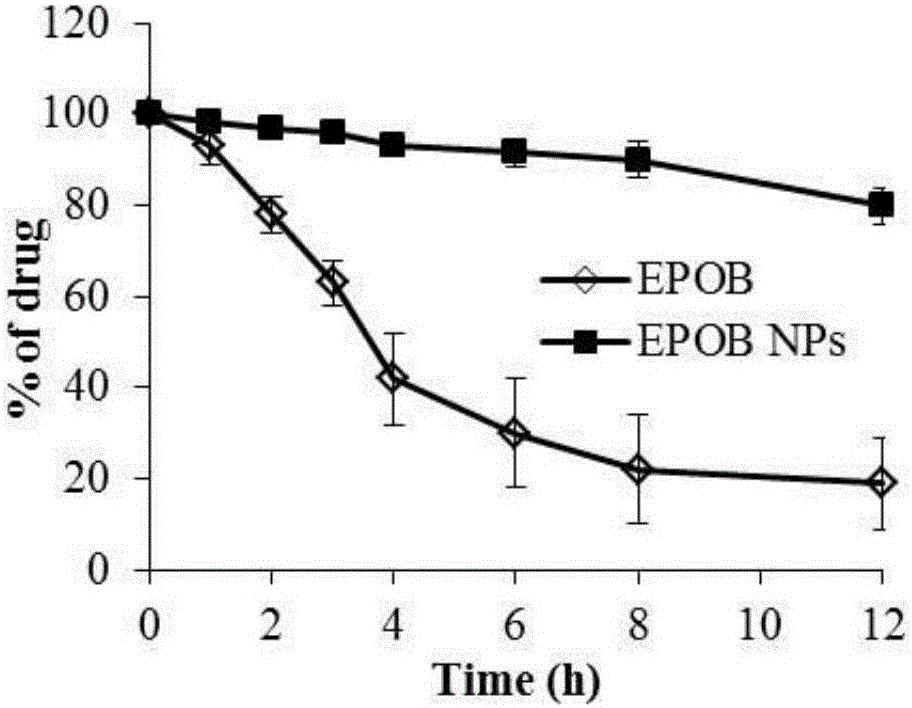
The invention discloses an epothilone B polymer nanoparticle preparation for treating tumors and a preparation method thereof. The polymer nanoparticle preparation is composed of epothilone B, a polymer carrier material and a lyoprotectant; the nanoparticle preparation has a particle size of 10-200 nm. The preparation method of the nanoparticle preparation comprises the following steps: mixing an organic solvent solution of the polymer carrier material and epothilone B with an aqueous phase containing an emulsifier according to a ratio, homogenizing, removing an organic solvent, concentrating, filtering and disinfecting, and lyophilizing to obtain the preparation. By changing the prescription process parameters of the polymer carrier material such as composition, concentration and organic solvent removal temperature, drug loading capacity, drug release speed and other features are adjusted; the preparation prepared herein has good slow release effect and can effectively prevent epothilone B from being hydrolyzed and deactivated by esterase in body, significantly prolonging in-vivo circulation time, improving antitumor activity and lowering medicine toxicity.
Owner:JIANGNAN UNIV
Medicinal composition containing lentinan, and preparation method and use thereof
InactiveCN106692266AConvenient clinical administrationImprove developmentOrganic active ingredientsAntiviralsBiotechnologyDisease
The invention discloses a medicinal composition containing lentinan. The medicinal composition comprises, by weight, 0.5-5% of lentinan, 1-10% of yeast glucan, 2-10% of levamisole hydrochloride, 5-30% of licorice root extract, and the balance of anhydrous glucose. The invention also discloses a preparation method of the medicinal composition, and a use of the composition in enhancement of the immunity of animals. The medicinal composition which is processed through an ultrafine crushing technology has a high medicine absorption rate, substantially promotes the growth of animal immune organs and enhances the immunity of animal bodies; the medicinal composition has a substantial curative effect on viral disease induced immunosuppression and poor immunity, and substantially reduces the mortality of pathogenetic animals; the medicinal composition substantially increases the antibody tilter of vaccine immunized serum of the animals in order to substantially enhance the vaccine immunization effect; and the composition has the advantages of simple production steps, convenience in clinic use, and convenience in colony administration.
Owner:胡大坤
Pharmaceutical composition sustained-release implant containing caspofungin acetate and preparation thereof
InactiveCN104324014AProlong the time of action in the bodyEliminate the peak and valley phenomenon of drug concentration in the bodyAntimycoticsPharmaceutical delivery mechanismEchinocandinSide effect
The invention relates to a pharmaceutical composition sustained-release implant containing caspofungin acetate and a preparation method thereof. The pharmaceutical composition sustained-release implant is characterized in that the active ingredient of the medicine is echinocandins antifungal drug caspofungin acetate. In addition, the sustained-release implant also comprises polyene and triazole antifungal drugs with a synergistic effect with the caspofungin. The active ingredient of the medicine is coated in a high polymer material carrier with biodegradability and biocompatibility according to a certain ratio. The active ingredient is prepared into sustained-release microspheres to be directly pressed into tablets of a certain shape, and the tablets are used for implant, so that a long-acting sustained-release effect is achieved. The sustained-release period of the sustained-release implant lasts for several days or several months, the drug administration frequency is obviously reduced, the bioavailability is improved, the toxic and side effects are reduced, and thus the pharmaceutical composition sustained-release implant containing caspofungin acetate is beneficial for clinical treatment.
Owner:SHENZHEN JYMED TECH
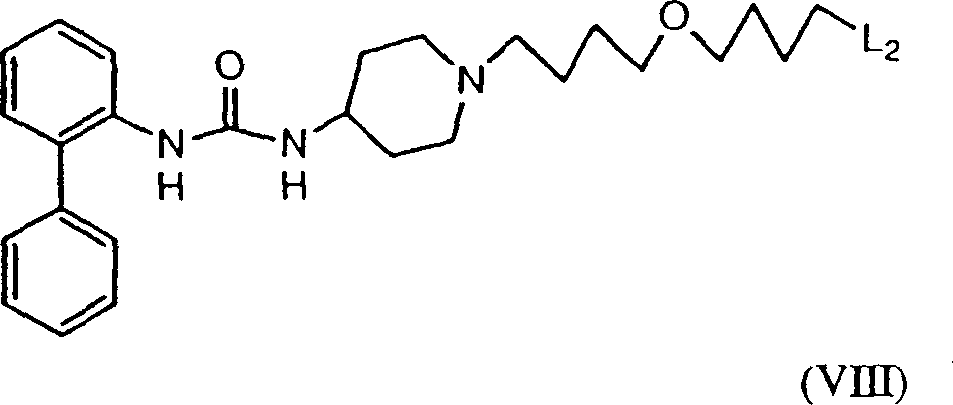
Urea compounds having muscarinic receptor antagonist activity
InactiveCN1407970ALong metabolic half-lifeProlong the time of action in the bodySenses disorderNervous disorderCarbamateAgonist
Owner:THERAVANCE BIOPHARMA R&D IP LLC
FAK and EGFR targeted RNA interference plasmid-lipidosome antineoplastic complex
InactiveCN101280317BPlay a protective effectTreatment advantageGenetic material ingredientsPharmaceutical non-active ingredientsAbnormal tissue growthLife quality
Owner:SICHUAN UNIV
A composition containing hyaluronate and its preparation method
ActiveCN110982126BIncreased resistance to hyaluronidaseImprove liquidityAnti-hyaluronidaseBiochemistry
The present invention provides a hyaluronate-containing composition comprising a two-gel system composed of a cross-linked hyaluronate gel and a non-cross-linked hyaluronate gel, the pH of the two-gel system 6.0-8.0, the average particle size is 100-300μm, and the kinematic viscosity is 10-80mm 2 / s, the pushing force is 5‑30N. The present invention also provides a method for preparing a hyaluronate-containing composition, comprising: using the first hyaluronic acid or hyaluronate to prepare a cross-linked hyaluronate gel; using the second hyaluronic acid or hyaluronate Hyaluronate to prepare non-cross-linked hyaluronate gel; mixing the cross-linked hyaluronate gel and the non-cross-linked hyaluronate gel, followed by high temperature treatment to obtain a double gel system. Compared with traditional hyaluronic acid water-light products, the composition prepared by the present invention has enhanced anti-hyaluronidase ability, longer in vivo action time, low gel viscosity, high slip, enhanced fluidity, and easier clinical use. Pushing operation can reduce side effects such as skin bumps and redness.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD +1
Modified exenatide and its use
ActiveCN106554404BActivity has no effectDoes not affect receptor agonistic activityPeptide/protein ingredientsMetabolism disorderFatty liverDiabetes mellitus
The invention discloses an exenatide modified compound, a use of the exenatide modified compound in preparation of a medicine which serves as a GLP-1 receptor stimulant, a use of the exenatide modified compound in preparation of medicines used for preventing and / or curing diseases and / or symptoms relevant to low GLP-1 receptor activity, a use of the exenatide modified compound in preparation of medicines used for diseases and / or symptoms relevant to carbohydrate metabolism, a use of the exenatide modified compound in preparation of medicines used for diabetes, a use of the exenatide modified compound in preparation of medicines used for fatty liver, and a use of the exenatide modified compound in preparation of medicines used for weight losing.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Method for preparing Gd3<+> magnetic resonance imaging contrast agent with graphene oxide serving as carrier
InactiveCN103330950BGood water dispersion stabilityHigh longitudinal relaxation efficiencyNMR/MRI constrast preparationsDispersion stabilityChloroacetic acids
The invention discloses a method for preparing a Gd3<+> magnetic resonance imaging contrast agent with graphene oxide serving as a carrier, and belongs to the technical field of preparation of medical materials. The method comprises the following steps of: 1) dispersing graphene oxide into distilled water to obtain a graphene oxide dispersing solution; 2) adding an alkaline substance and chloroacetic acid or sodium monochloroacetate to the graphene oxide dispersing solution, ultrasonically dispersing, centrifugally washing and processing, and dialyzing to obtain carboxylation graphene oxide; and 3) dispersing the carboxylation graphene oxide into the distilled water to obtain a carboxylation graphene oxide dispersing solution, adding an aqueous solution of gadolinium salt to the carboxylation graphene oxide dispersing solution, adding a DTPA (Diethylene Triamine Pentacetic Acid) aqueous solution after the stirring reaction is done, uniformly mixing to obtain a mixing solution, and dialyzing to obtain carboxylation graphene oxide with Gd3<+> loaded on the surface, thus obtaining the Gd3<+> magnetic resonance imaging contrast agent. The method is simple and feasible, and the Gd3<+> is firmly loaded; and the preparation magnetic resonance imaging contrast agent is high in water-borne dispersion stability and has high longitudinal relaxation rate.
Owner:XI AN JIAOTONG UNIV
Suspension preparation of hyaluronic acid with temperature-denaturable painless nanometer sulfadiazine metal compound
ActiveCN104013574BStrong penetrating powerImprove bioavailabilityOrganic active ingredientsSolution deliveryCelluloseChlorhexidine
The invention discloses a suspension preparation of temperature change painless nano sulfadiazine metallic compound hyaluronic acid. The formula comprises a sulfadiazine metallic compound, a hyaluronic acid substance, an analgetic, a dispersing agent aid, a suspension aid and water, wherein the sulfadiazine metallic compound comprises sulfadiazine silver and sulfadiazine zinc; the hyaluronic acid substance is sourced from biological fermentation and animal tissue extraction and the like and can be hyaluronic acid or hyaluronate or crosslinked hyaluronic acid or crosslinked hyaluronate; the analgetic comprises ropivacaine, bupivacaine, levobupivacaine, lidocaine and other local anesthetics or combination of the local anesthetics; the dispersing agent aid comprises chlorhexidine, Tween series, oleic acid or sodium oleate; the suspension aid can comprise glycerinum, celluloses, poloxamer series and hyaluronic acid substances. The suspension preparation is mainly used for burn, scald or the surface of a wound caused by explosion or anabrosis, has the main clinical characteristics that pain is rapidly relieved, the surface of the wound is subjected to rapid film formation and rapid healing of the wound is promoted, and the conventional main administration modes refer to spraying, smearing and coating.
Owner:LIPONT PHARMA
3-Substituted oxy-2-pyrazine carboxamide compounds and their use
ActiveCN102850282BImprove bioavailabilityProlong the time of action in the bodyOrganic chemistryAntiviralsAntiviral drugPyrazine
Disclosed are a 3-alkoxy-substituted-2-pyrazinyl formamide compound having a structure represented by formula I and use thereof in preparing anti-viral medications. The compound disclosed exerts anti-viral effects in vivo by means of metabolism into T1105 or T705 through esterate or P450 enzyme.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
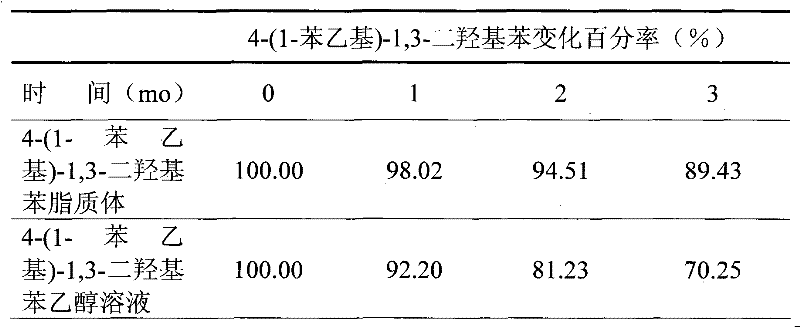
4-(1-phenethyl)-1,3-dihydroxy benzene liposome and preparation method thereof
ActiveCN101474154BImprove stabilityAvoid destructionCosmetic preparationsHydroxy compound active ingredientsBenzeneDermatomal
The invention relates to the technical field of medicine and cosmetics, in particular to lipidosome preparation formulation of 4-(1- phenethyl)-1 and 3-dihydroxy benzene and a preparation method thereof. The invention prepares the 4-(1- phenethyl)-1, 3-dihydroxy benzene into lipidosome, overcomes the defects of the insufficient application of existing 4-(1- phenethyl)-1 and 3-dihydroxy benzene inpercutaneous administration preparation and cosmetics, improves the stability and percutaneous performance of the 4-(1- phenethyl)-1and 3-dihydroxy benzene and reduces the acrimony of the 4-(1- phenethyl)-1 and 3-dihydroxy benzene for the skin, thus better exerting the healing effect.
Owner:苏州工业园区黎姿化妆品有限公司
Insulin modification method
ActiveCN109999181AGood drug dispersibilityPromote absorptionPeptide/protein ingredientsMetabolism disorderFreeze-dryingSolvent
The invention discloses an insulin modification method. Chemical coupling is adopted to combine a water-soluble polymer and the insulin through covalent bonding, wherein firstly the insulin is dissolved in a solvent, triethylamine and di-tert-butyl dicarbonate are added and stirred for a reaction, and a product A is obtained through freeze-drying and separation; then, a water-soluble polymer monomer is dissolved in a solvent, an RAFT reagent and an initiator are added for a reaction to obtain a product B; the product A and the product B are weighed, a solvent triethylamine is added, a productC is obtained through dialysis and freeze-drying, the product C is dissolved in water, trifluoroacetic acid is added for a reaction, and a coupled product D of the polymer and the insulin is obtainedthrough dialysis and freeze-drying. The chemically modified insulin is protected from degradation and inactivation by enzymes and the acid environment in the gastrointestinal environment through the water-soluble polymer, so that the degradation of the digestive enzymes of the gastrointestinal tract is effectively inhibited, and the product property is stable.
Owner:BEIJING UNIV OF CHEM TECH
Compound hedgehog hydnum dry-mixed suspension and its preparing method
InactiveCN1813817ALess irritatingEnhance pharmacological effectsHeavy metal active ingredientsPowder deliverySucrose sulfateMethyl cellulose
The present invention discloses a compound hericium dry suspension preparation. It is made up by using 600 g of hericium sporophore, 100 g of sucralfate, 50 g of magnesium trisilicate, 40 g of bismuth subnitrate, 5-15 g of polyvinylpyrrole, 50-160 g of lactose, 120-200 g of hydroxypropyl methyl cellulose, 2-6 g of Tween-80, 10-50 g of aromadenadrin powder and 3-15 g of sodium glutamate through the processes of pulverizing, granulating and filling. It has good therapeutic effect for curing ulcer of digestive tract.
Owner:倪友洪
A kind of modification method of insulin
ActiveCN109999181BGood drug dispersibilityPromote absorptionPeptide/protein ingredientsMetabolism disorderFreeze-dryingPancreatic hormone
The invention discloses an insulin modification method. Chemical coupling is adopted to combine a water-soluble polymer and the insulin through covalent bonding, wherein firstly the insulin is dissolved in a solvent, triethylamine and di-tert-butyl dicarbonate are added and stirred for a reaction, and a product A is obtained through freeze-drying and separation; then, a water-soluble polymer monomer is dissolved in a solvent, an RAFT reagent and an initiator are added for a reaction to obtain a product B; the product A and the product B are weighed, a solvent triethylamine is added, a productC is obtained through dialysis and freeze-drying, the product C is dissolved in water, trifluoroacetic acid is added for a reaction, and a coupled product D of the polymer and the insulin is obtainedthrough dialysis and freeze-drying. The chemically modified insulin is protected from degradation and inactivation by enzymes and the acid environment in the gastrointestinal environment through the water-soluble polymer, so that the degradation of the digestive enzymes of the gastrointestinal tract is effectively inhibited, and the product property is stable.
Owner:BEIJING UNIV OF CHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com