Insulin modification method
A technology for modifying and modifying insulin, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., which can solve the problems of low quality of life and low patient compliance of patients, and achieve the goal of promoting blood circulation in the body. Absorption, good drug dispersion, strong targeting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
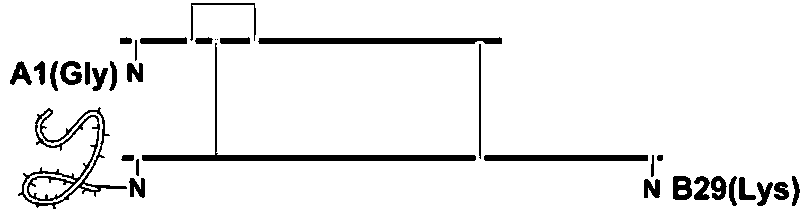
[0030] Dissolve 1 g of porcine insulin in 10 ml of DMSO, and add 1.0 ml of triethylamine. Add 84 mg of di-tert-butyl dicarbonate and stir at room temperature for 30 min. Then, a mixed solvent of 14 ml of acetic acid and 100 ml of water was added to stop the reaction. Freeze-drying, dissolving in a mixed solution of water and acetonitrile with a molar ratio of 2:3, and separating through a C4 chromatographic column to obtain product A.
[0031] Weigh 1.0250g of HPMA monomer, 0.234g of RAFT reagent 2-cyano-2-propylbenzodisulfide, 0.0769g of AIBN solvent with a mass concentration of 1%, dissolve in 2.5513g of DMAc, shake well and transfer into poplar In the test tube, put it in a 70°C oil bath for 24 hours after refrigerating and pumping for 4 times, and then pass air to cool down to stop the reaction and mark it as B.
[0032] Weigh 0.62 g of product B and 50 mg of product A, add 2 ml of triethylamine, and then add 10 ml of DMSO at 25°C for 10 h, then dialyze to remove small m...
Embodiment 2
[0035] Dissolve 1 g of porcine insulin in 10 ml of DMF, and add 1.0 ml of triethylamine. Add 60 mg of di-tert-butyl dicarbonate and stir at room temperature for 60 min. Then, a mixed solvent of 15 ml of acetic acid and 100 ml of water was added to stop the reaction. Freeze-drying, dissolving in a mixed solution of water and acetonitrile with a molar ratio of 2:3, and separating through a C4 chromatographic column to obtain product A.
[0036] Weigh 1.52g of methyl acrylate, 0.356g of RAFT-NHS, and 0.06g of ammonium persulfate, dissolve in 3.5g of acetonitrile, shake well and transfer to a Young’s test tube, freeze and drain 3 times, then place in a 50°C oil bath After 18 hours of reaction, the reaction was stopped by air cooling and marked as B.
[0037] Weigh 0.75g of product B and 50mg of product A respectively, add 1ml of triethylamine, and then add 10ml of DMSO at room temperature 25°C for 10h, then dialyze to remove small molecules and freeze-dry to obtain 0.8gC, a coup...
Embodiment 3
[0040]Dissolve 1 g of porcine insulin in 10 ml of water, and add 1.0 ml of triethylamine. Add 65 mg of di-tert-butyl dicarbonate and stir at room temperature for 50 min. Then, a mixed solvent of 25 ml of acetic acid and 100 ml of water was added to stop the reaction. Freeze-drying, dissolving in a mixed solution of water and acetonitrile with a molar ratio of 2:3, and separating through a C4 chromatographic column to obtain product A.
[0041] Weigh - 1.25g of methyl methacrylate monomer, 0.234g of RAFT reagent S-ethyl-S'-(2-methyl-2-propionyl) trithiocarbonate, 0.085g mass fraction is 1 % of azobisisopropylimidazoline solvent, dissolved in 3.5g of ethanol, shake well and transfer to Young's test tube, freeze and drain for 5 times, put it in a 65°C oil bath for 10 hours, and then cool down with air to stop the reaction Marked as B.
[0042] Weighed 0.9g of product B and 50mg of product A, added 3ml of triethylamine, and then added 10ml of DMSO at room temperature 25°C for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com