Patents
Literature
193results about "Micromachined delivery" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
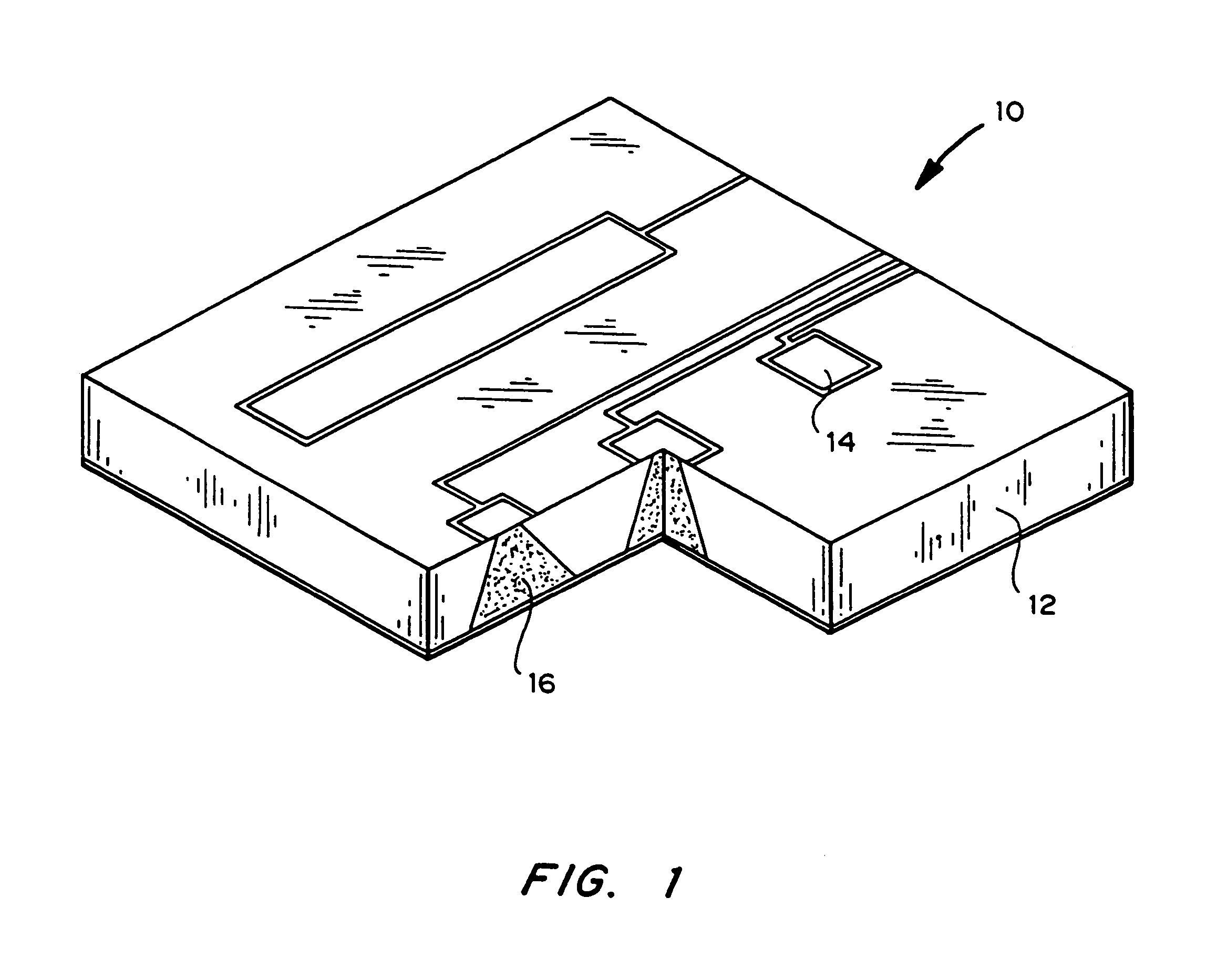
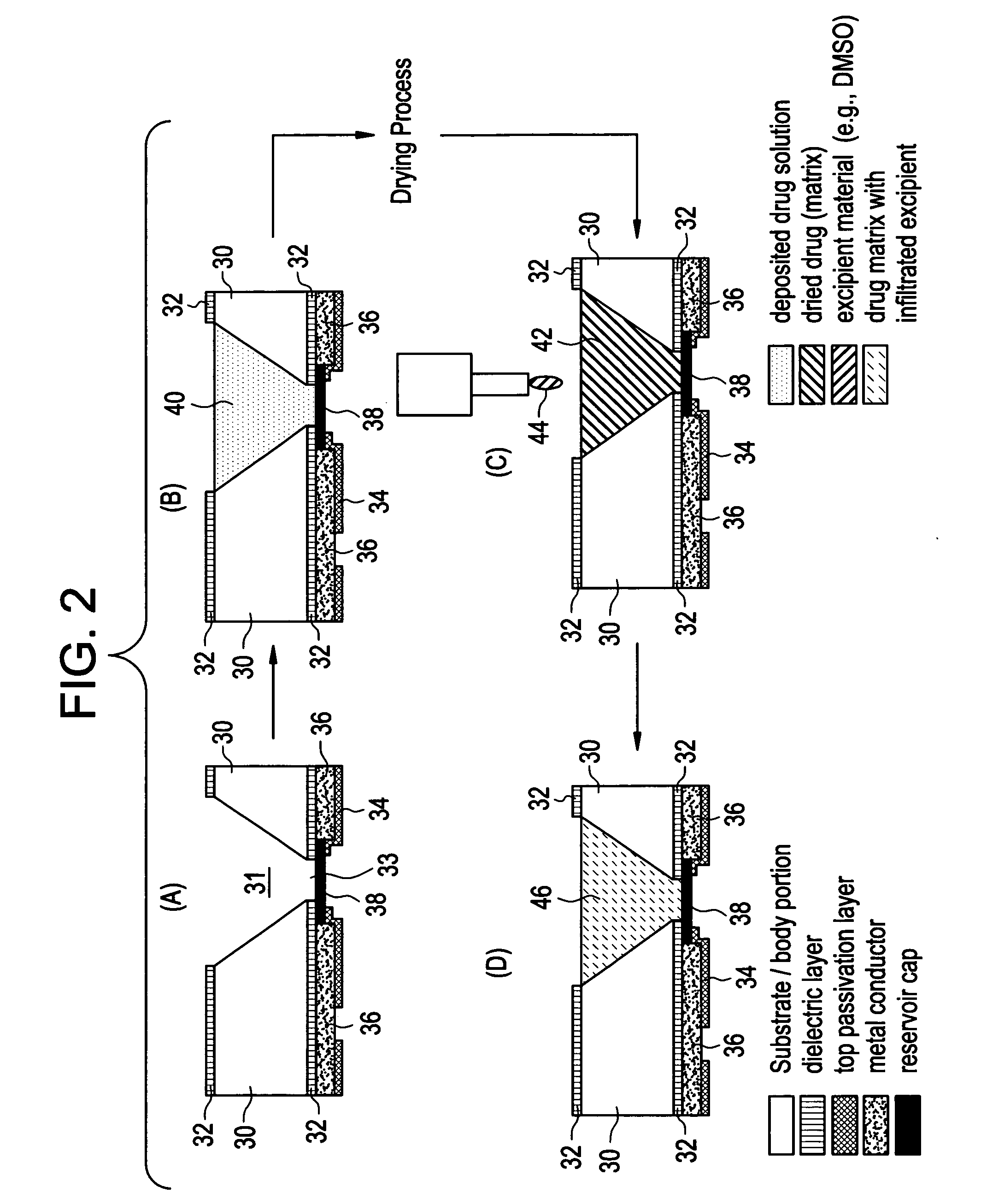
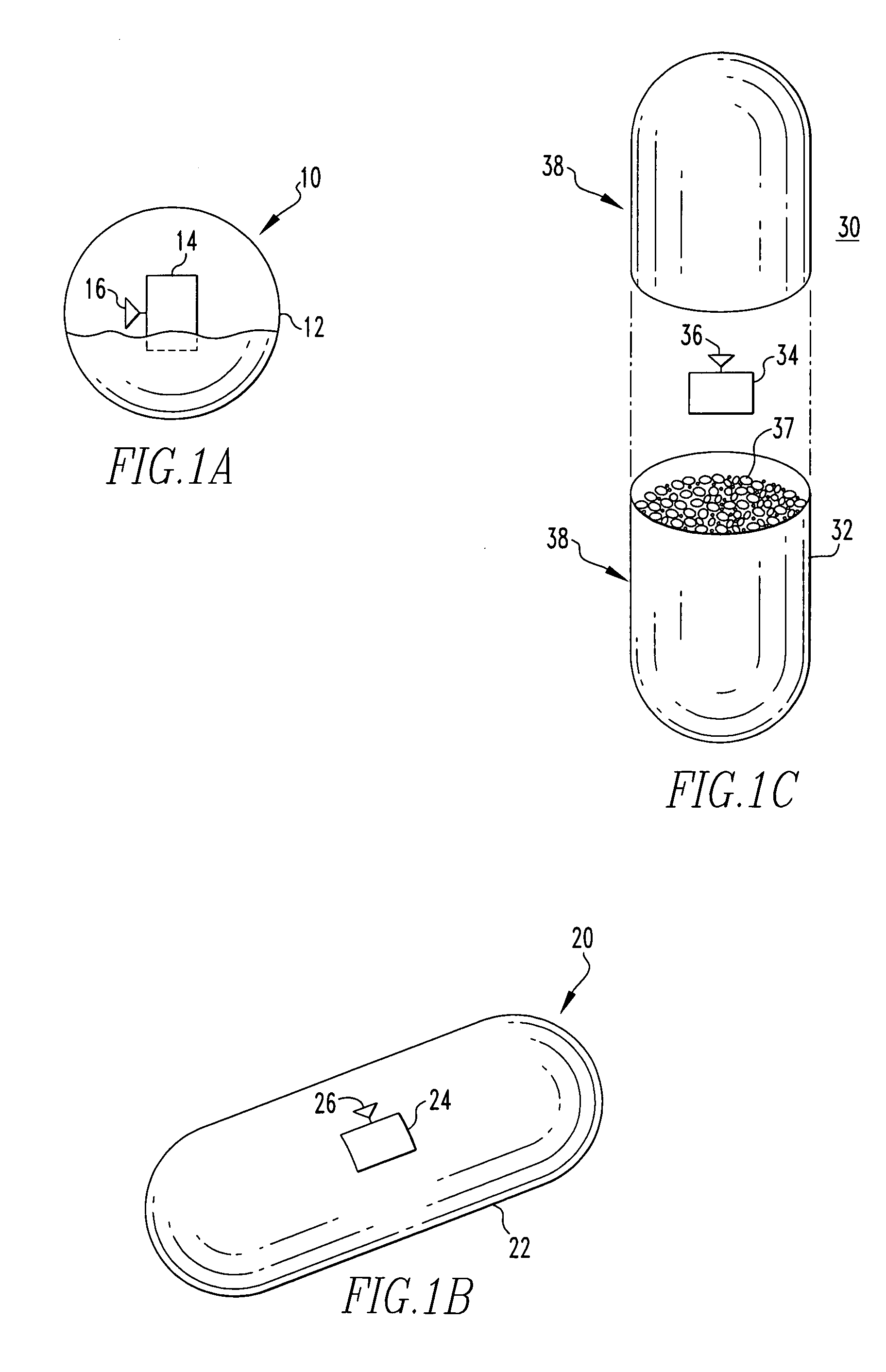
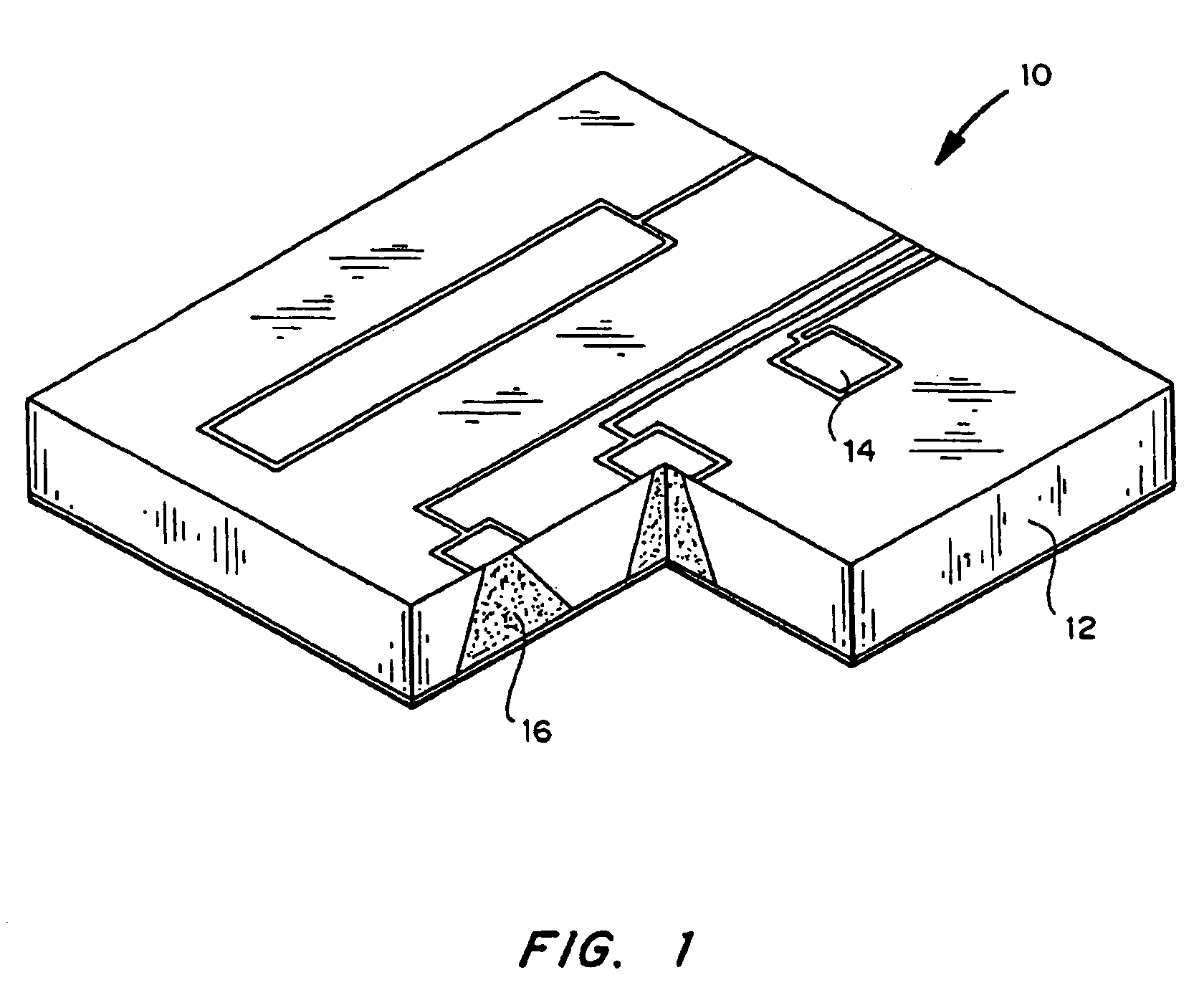
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
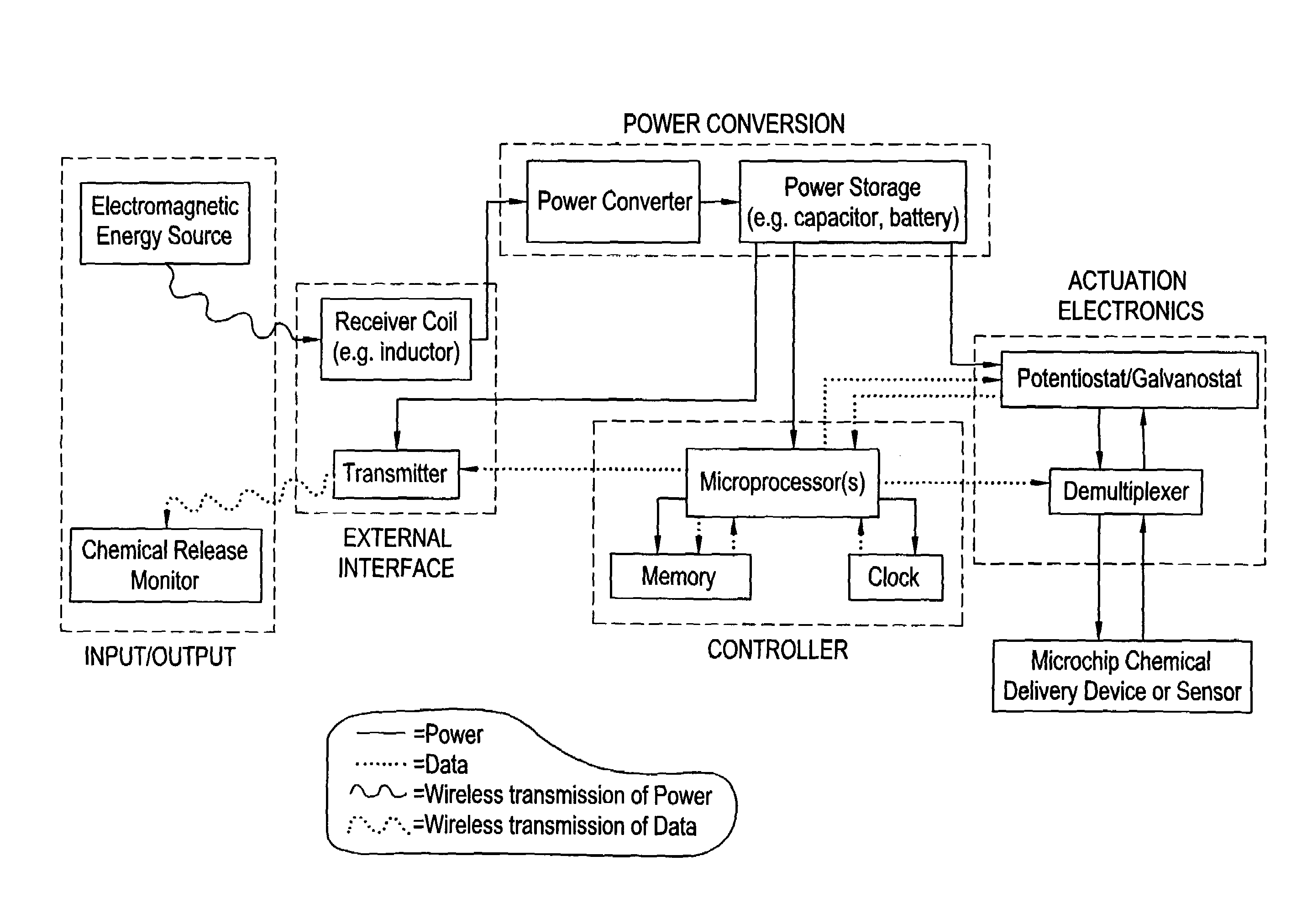
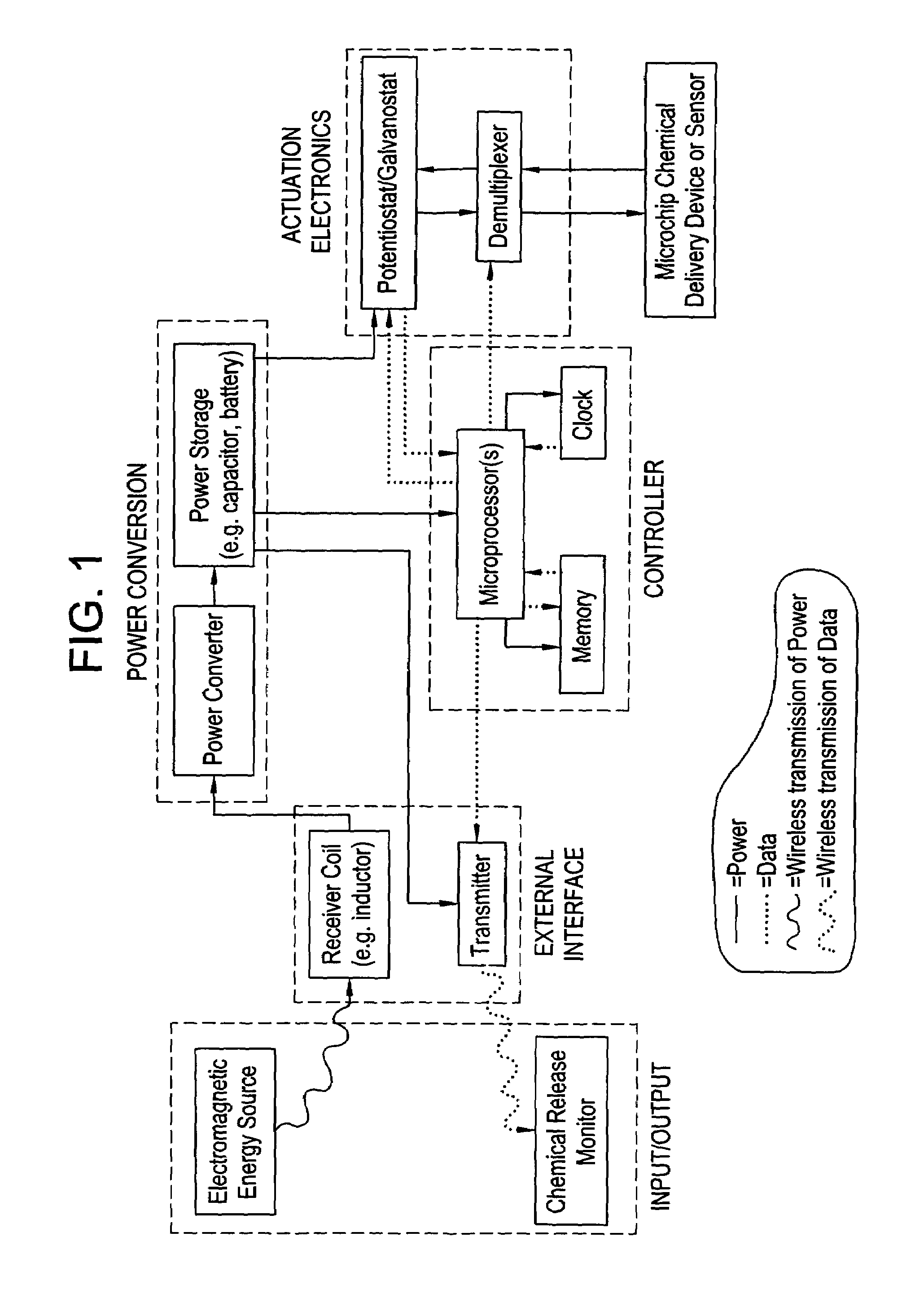
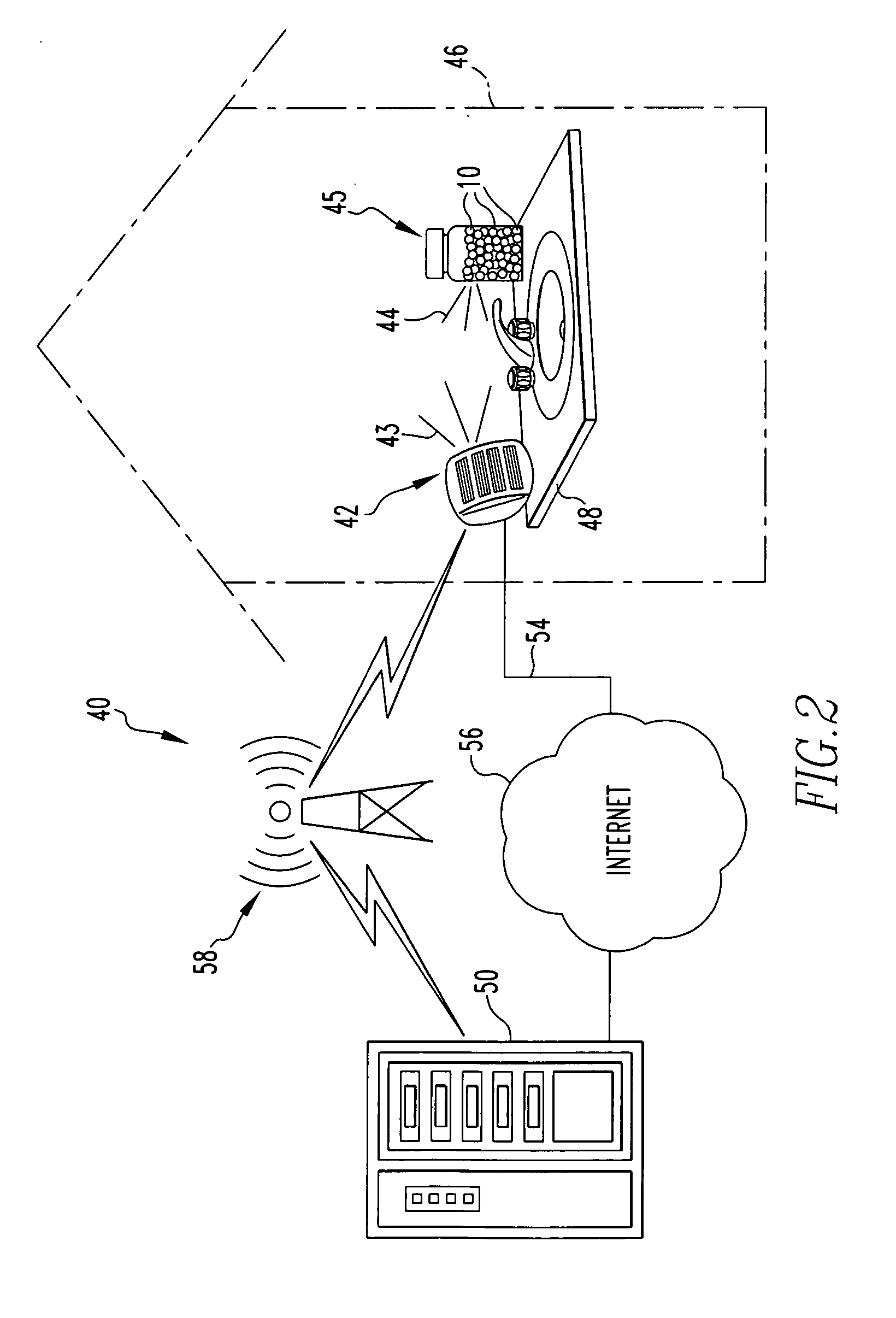
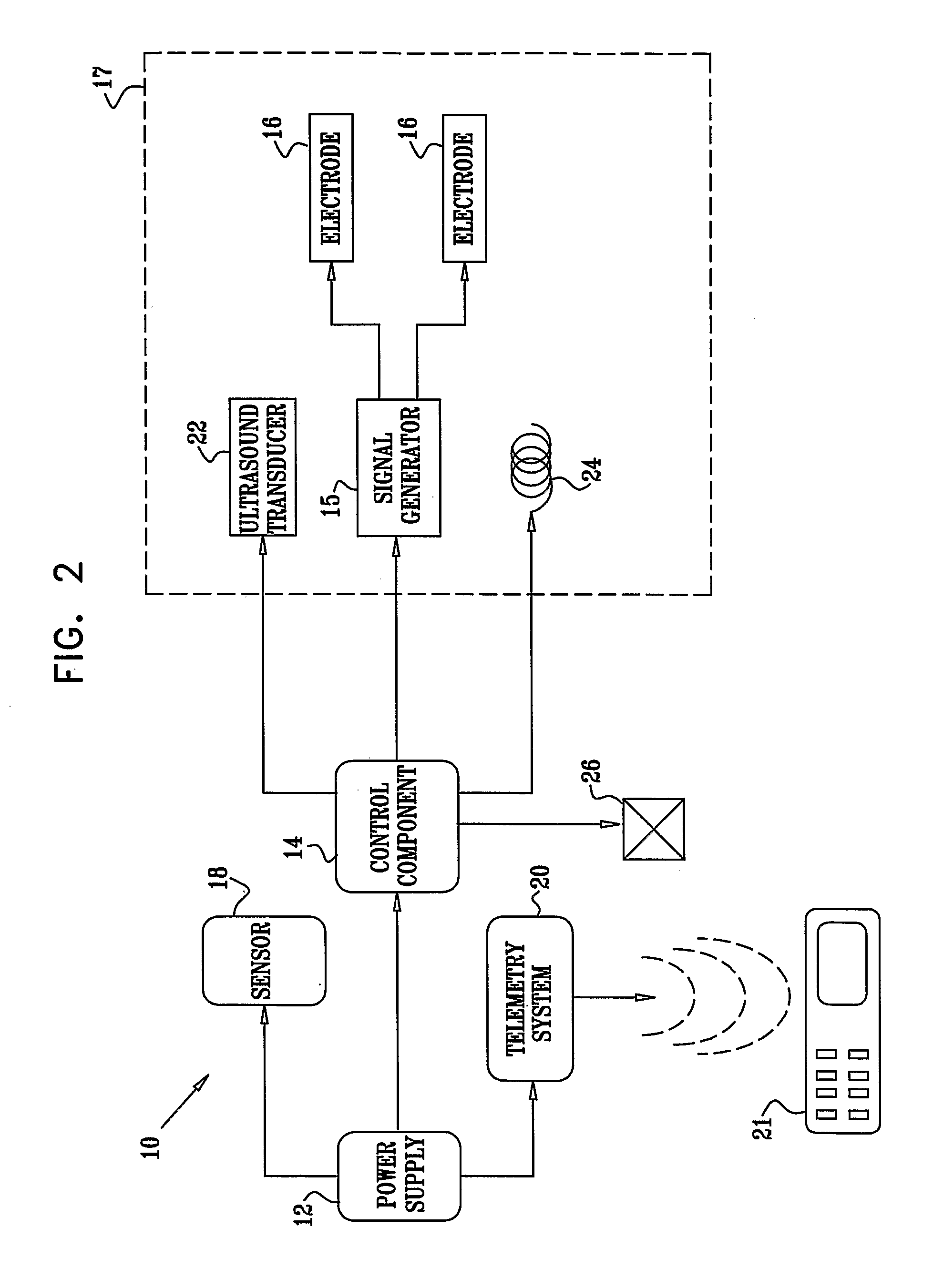
Microchip reservoir devices using wireless transmission of power and data
InactiveUS7226442B2Easy to disassembleElectroencephalographyElectrotherapyWireless transmissionElectric power
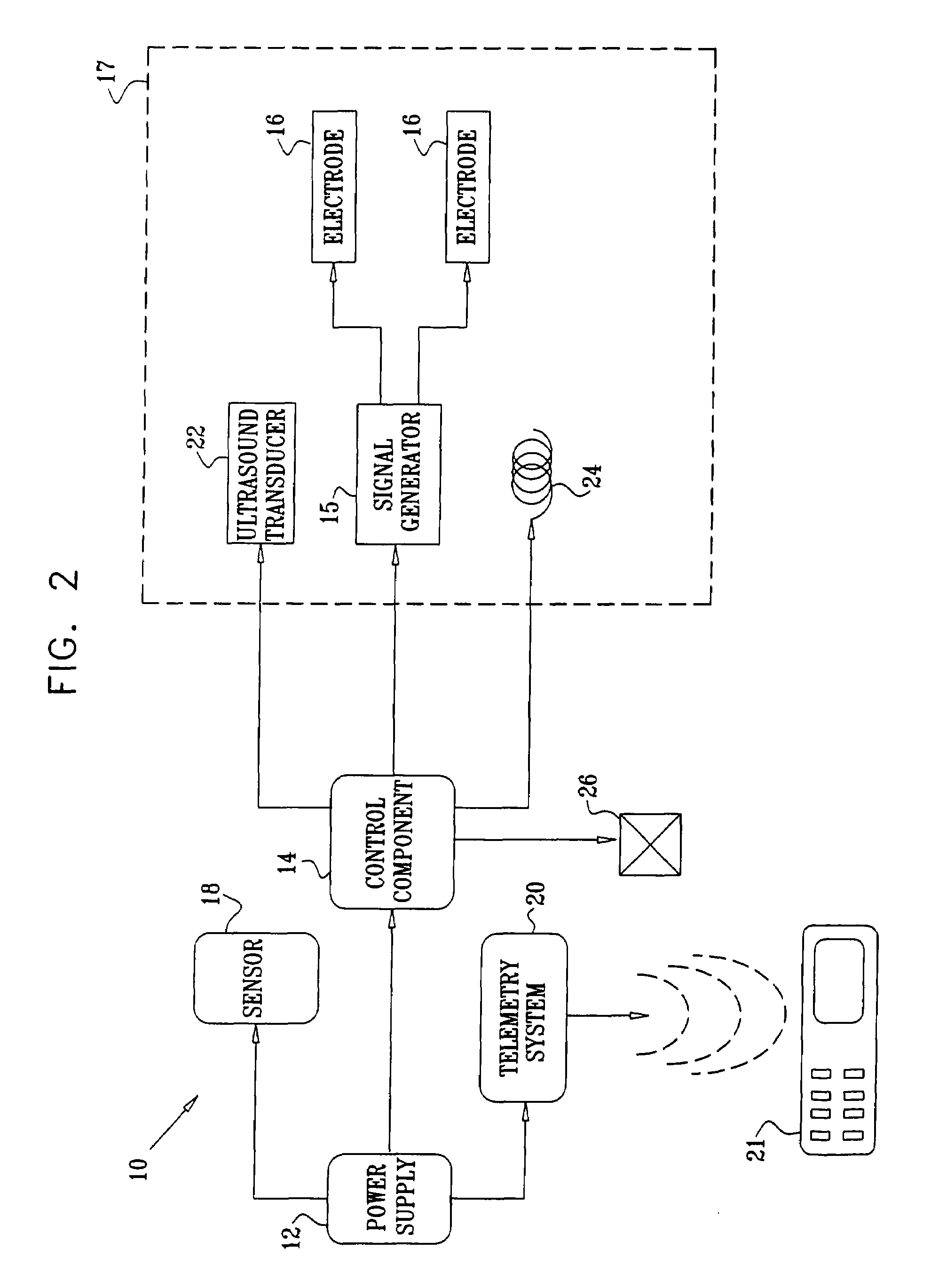
Devices, systems, and methods are provided for wirelessly powering and / or communicating with microchip devices used for the controlled exposure and release of reservoir contents, such as drugs, reagents, and sensors. In one embodiment, the system includes (1) a microchip device comprising a substrate having a plurality of reservoirs containing reservoir contents for release or exposure; and (2) a rechargeable or on-demand power source comprising a local component which can wirelessly receive power from a remote transmitter wherein the received power can be used, directly or following transduction, to activate said release or exposure of the reservoir contents. In another embodiment, the system comprises (1) a microchip device comprising a substrate a plurality of reservoirs containing reservoir contents for release or exposure; and (2) a telemetry system for the wireless transfer of data between the microchip device and a remote controller.
Owner:DARE MB INC
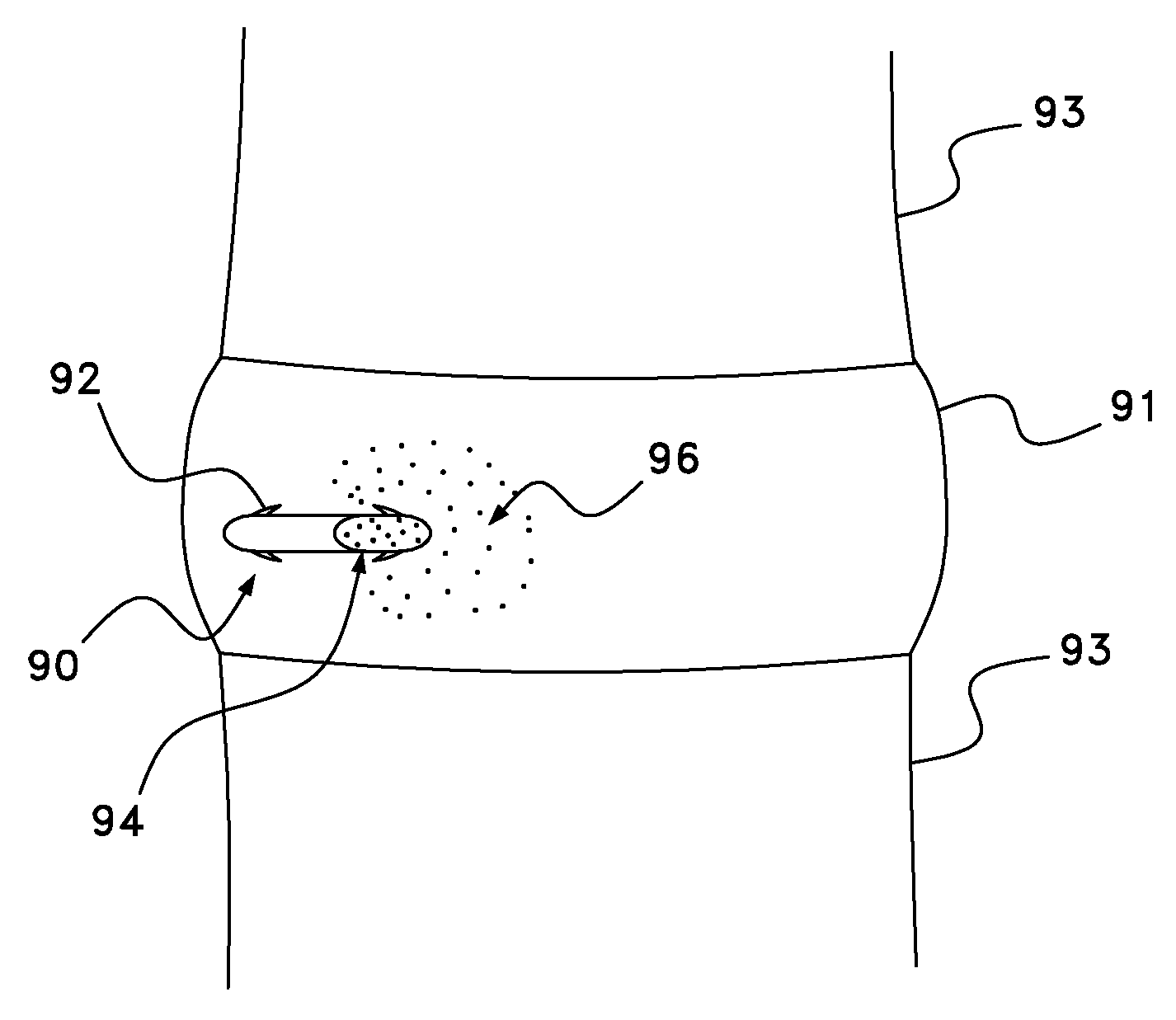
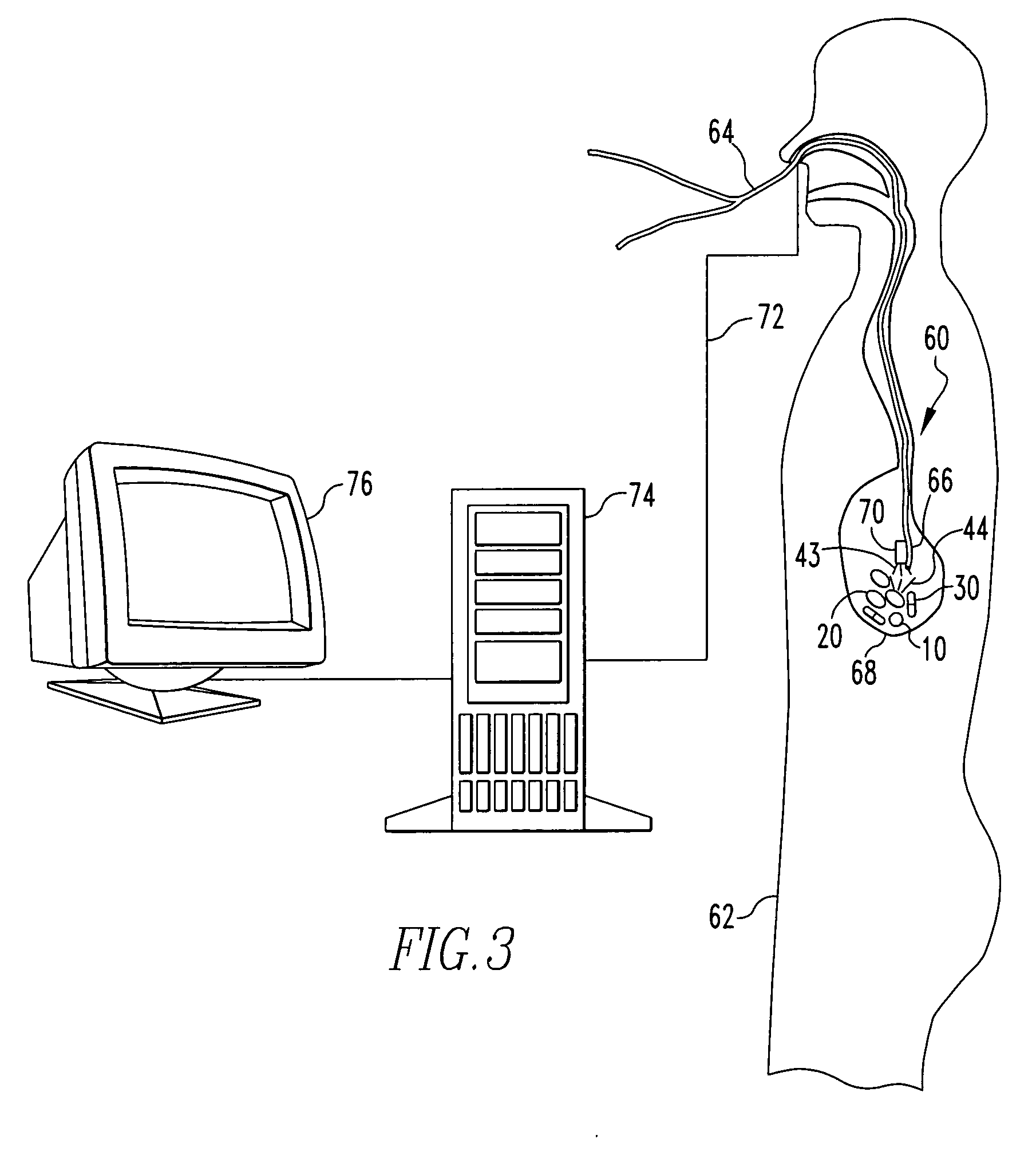
Active drug delivery in the gastrointestinal tract
InactiveUS20050058701A1Easy accessPromote absorptionInternal electrodesBody temperature measurementMedicineDrug administration
Apparatus for drug administration is provided, including an ingestible capsule, which includes a drug, stored by the capsule, and an environmentally-sensitive mechanism, adapted to change a state thereof responsively to a disposition of the capsule within a gastrointestinal (GI) tract of a subject. The capsule further includes first and second electrodes, and a control component, adapted to facilitate passage of the drug, in response to a change of state of the environmentally-sensitive mechanism, through an epithelial layer of the GI tract by driving the first and second electrodes to apply a series of pulses at a current of less than about 5 mA, at a frequency of between about 12 Hz and about 24 Hz, and with a pulse duration of between about 0.5 milliseconds and about 3 milliseconds.
Owner:E PILL PHARMA
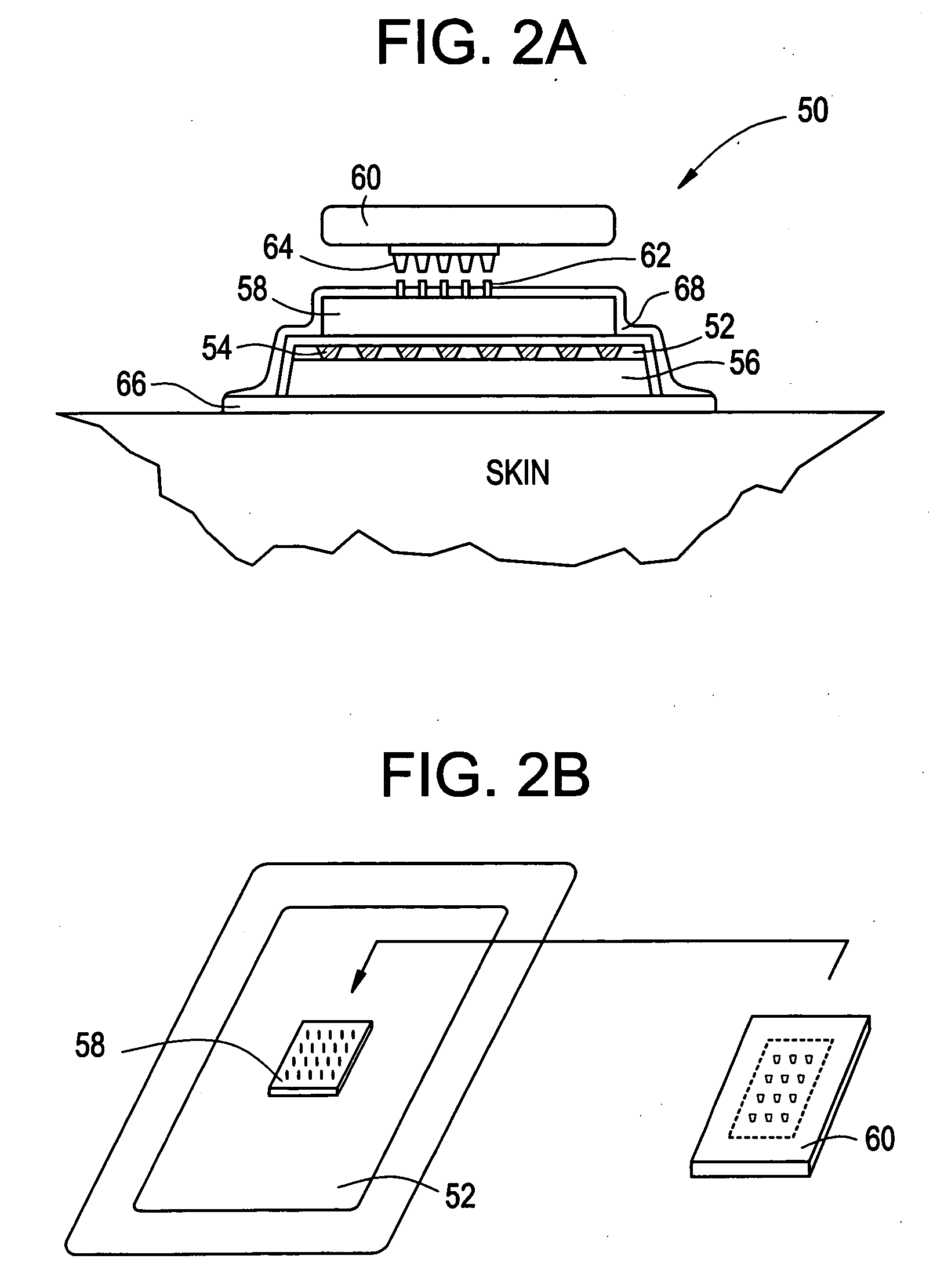
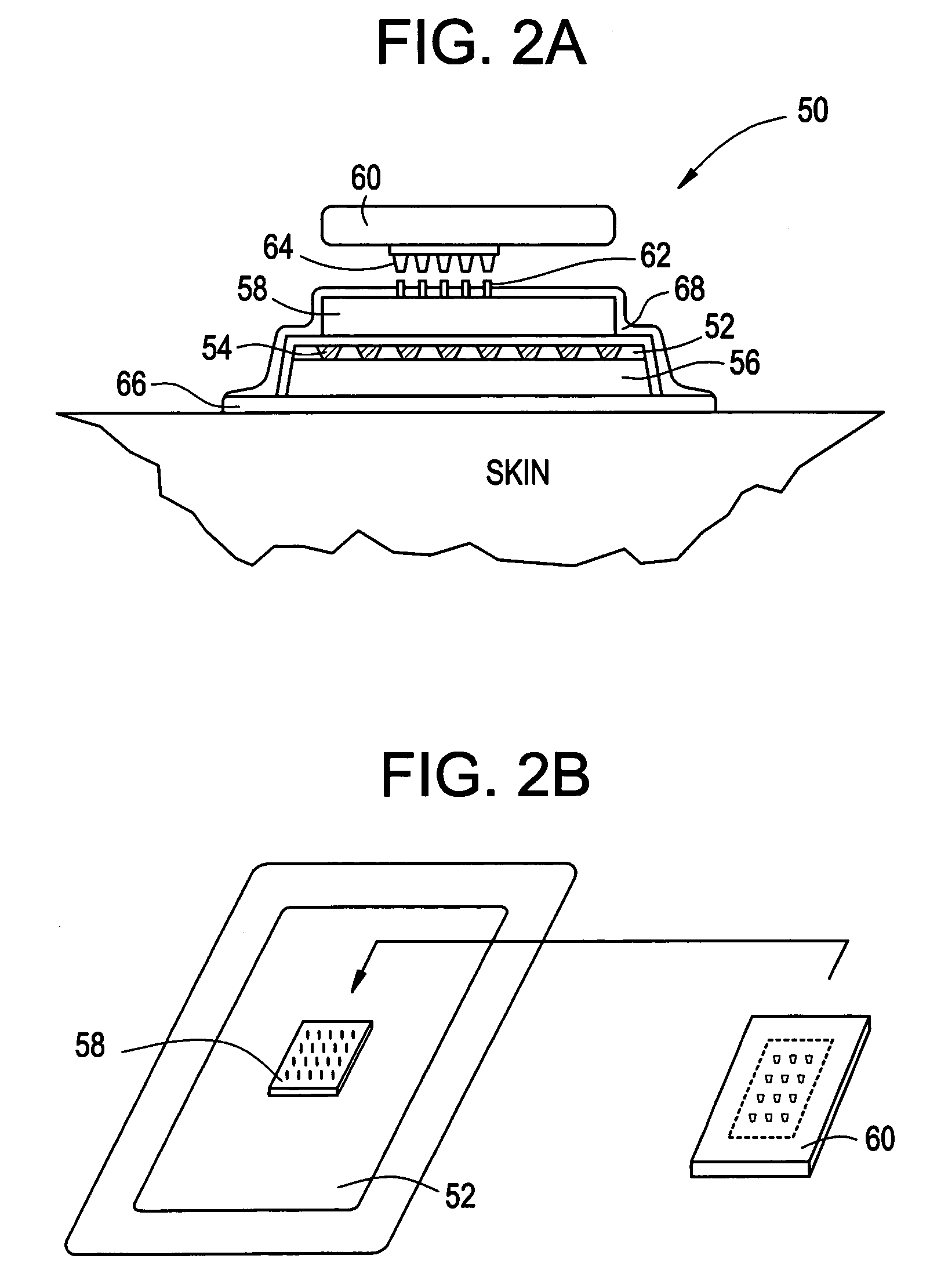
Multi-reservoir device for transdermal drug delivery and sensing
Devices and methods are provided for transdermal administration of a pharmaceutical agent to a patient in need thereof. The device includes a substrate, a plurality of discrete reservoirs in the substrate, one or more pharmaceutical agents stored in the reservoirs, discrete reservoir caps that prevent the pharmaceutical agent from passing out from the reservoirs until desired, control means for actuating release of the one or more pharmaceutical agents from one or more of the reservoirs by disintegrating or permeabilizing the reservoir caps, means for securing the device to the skin of the patient, and means for transporting the pharmaceutical agent to the skin following release from the one or more of the reservoirs. In another embodiment, the device is adapted for diagnostic sensing instead of or in addition to drug delivery.
Owner:MICROCHIPS BIOTECH INC
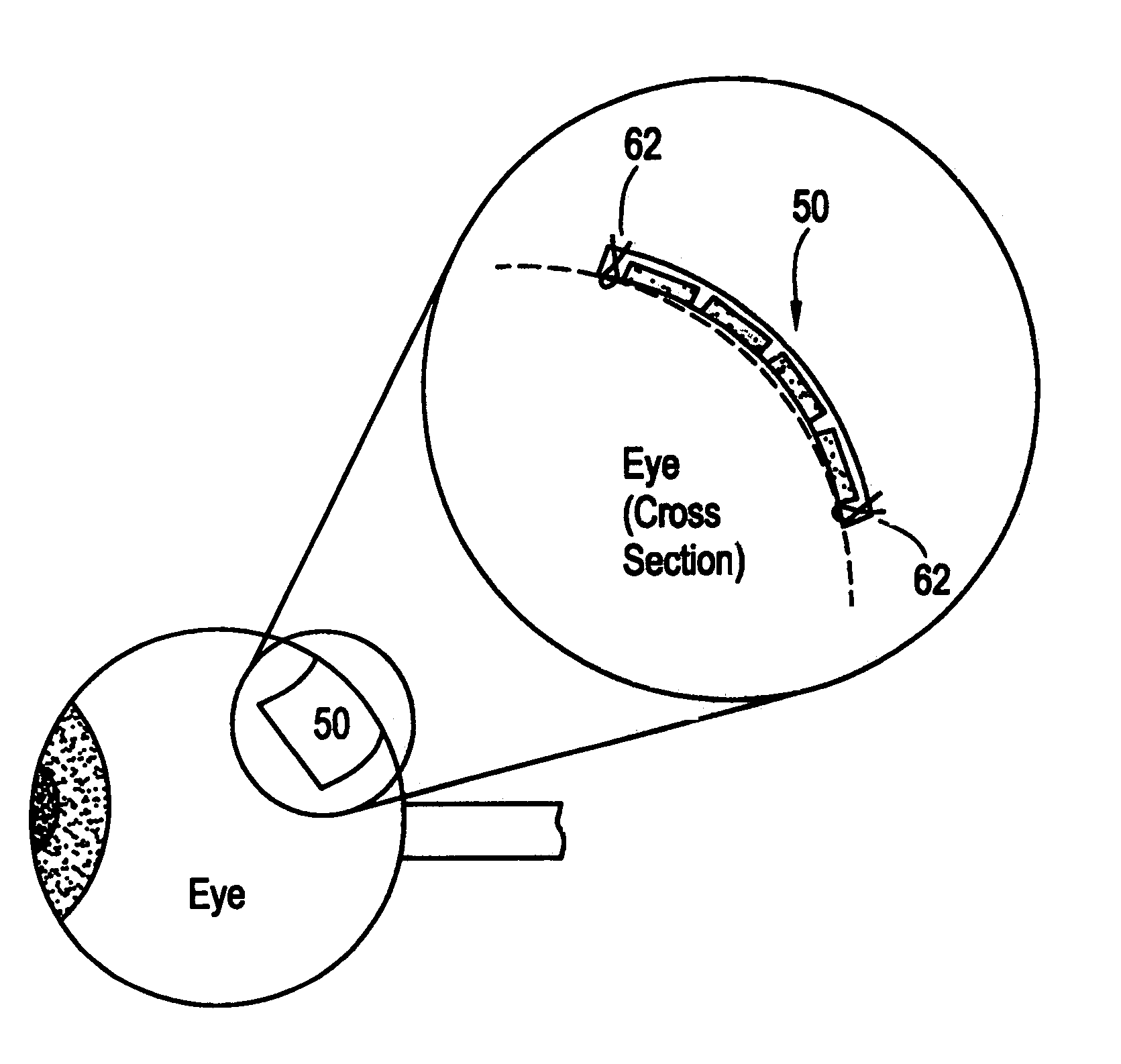
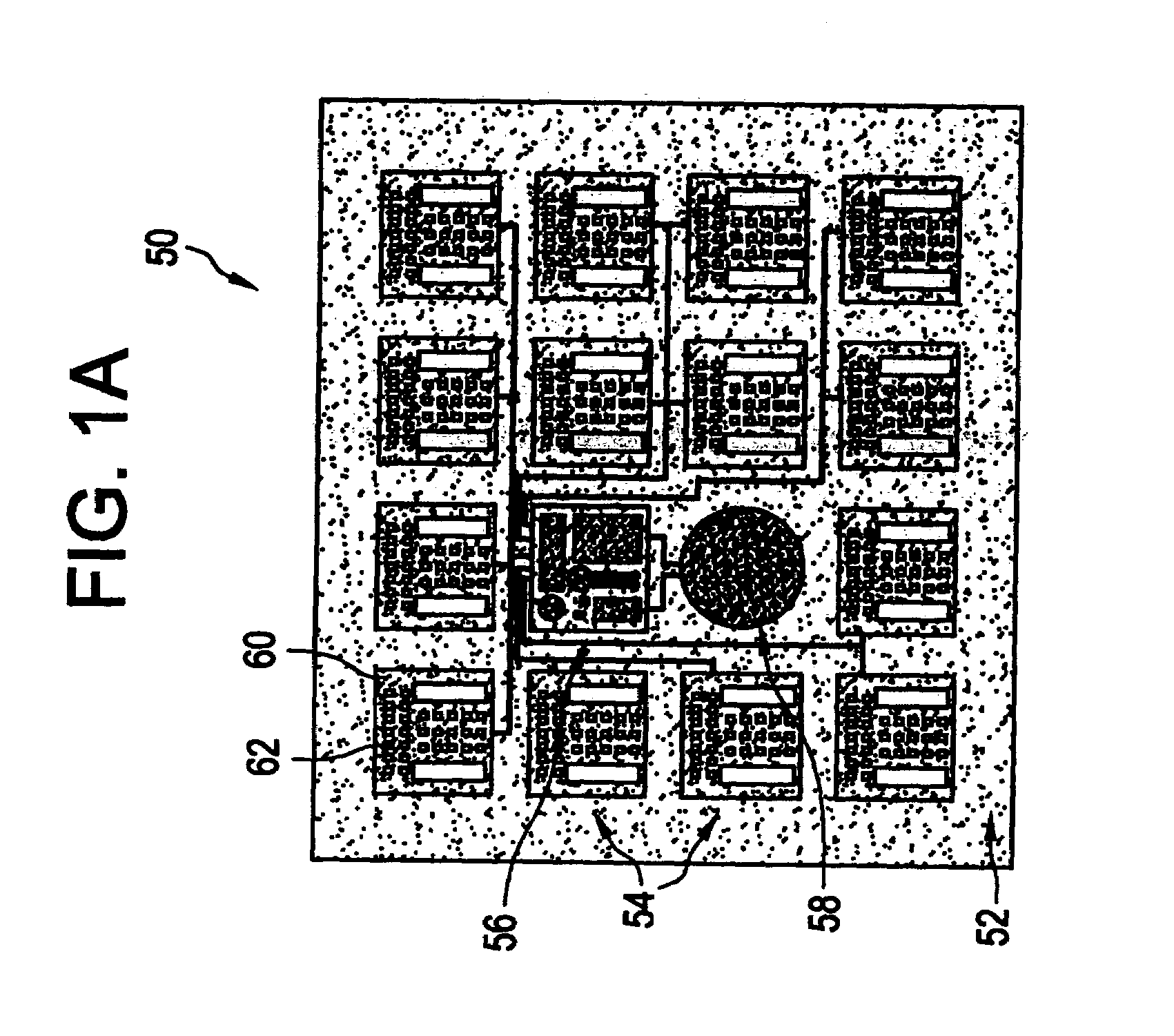
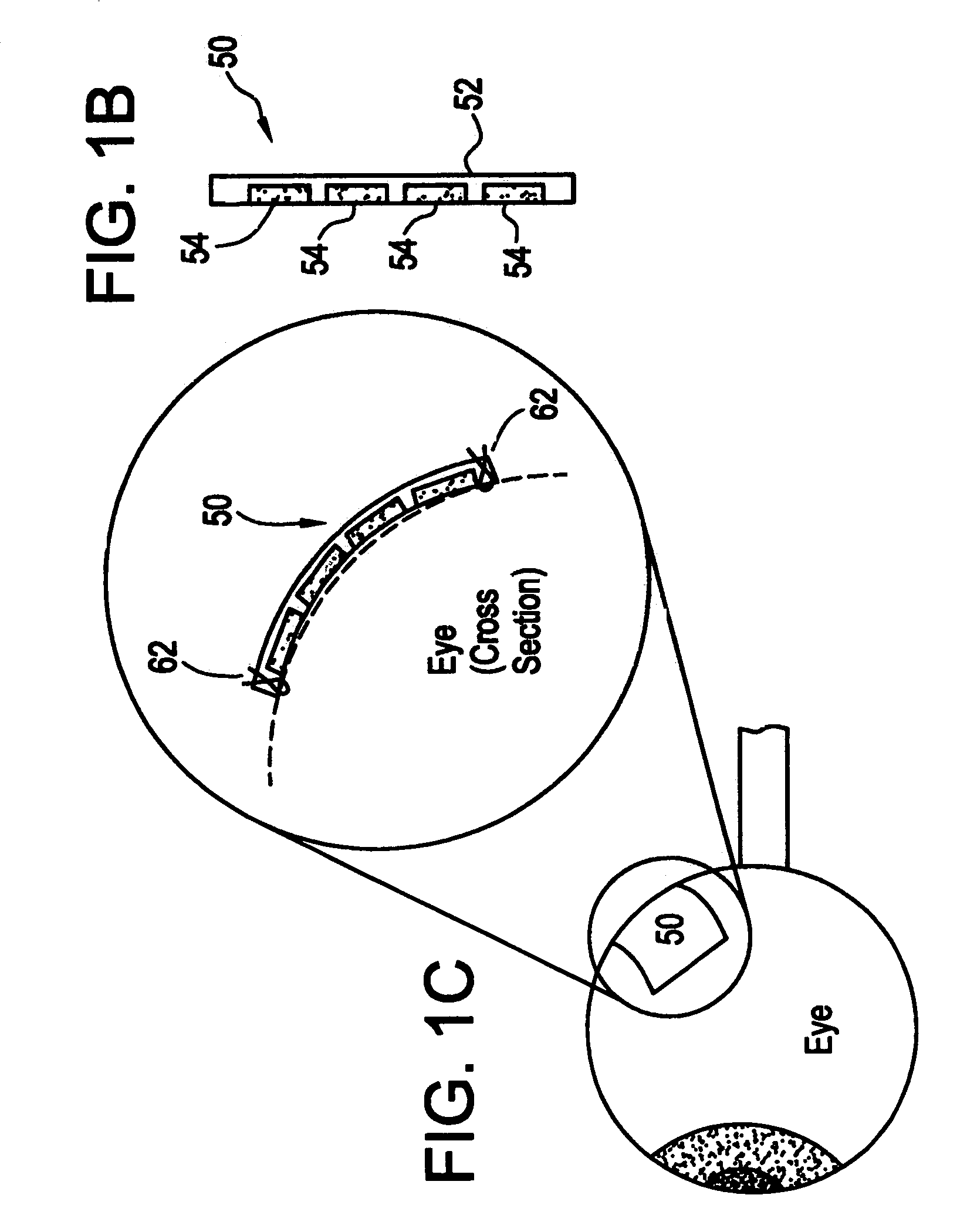
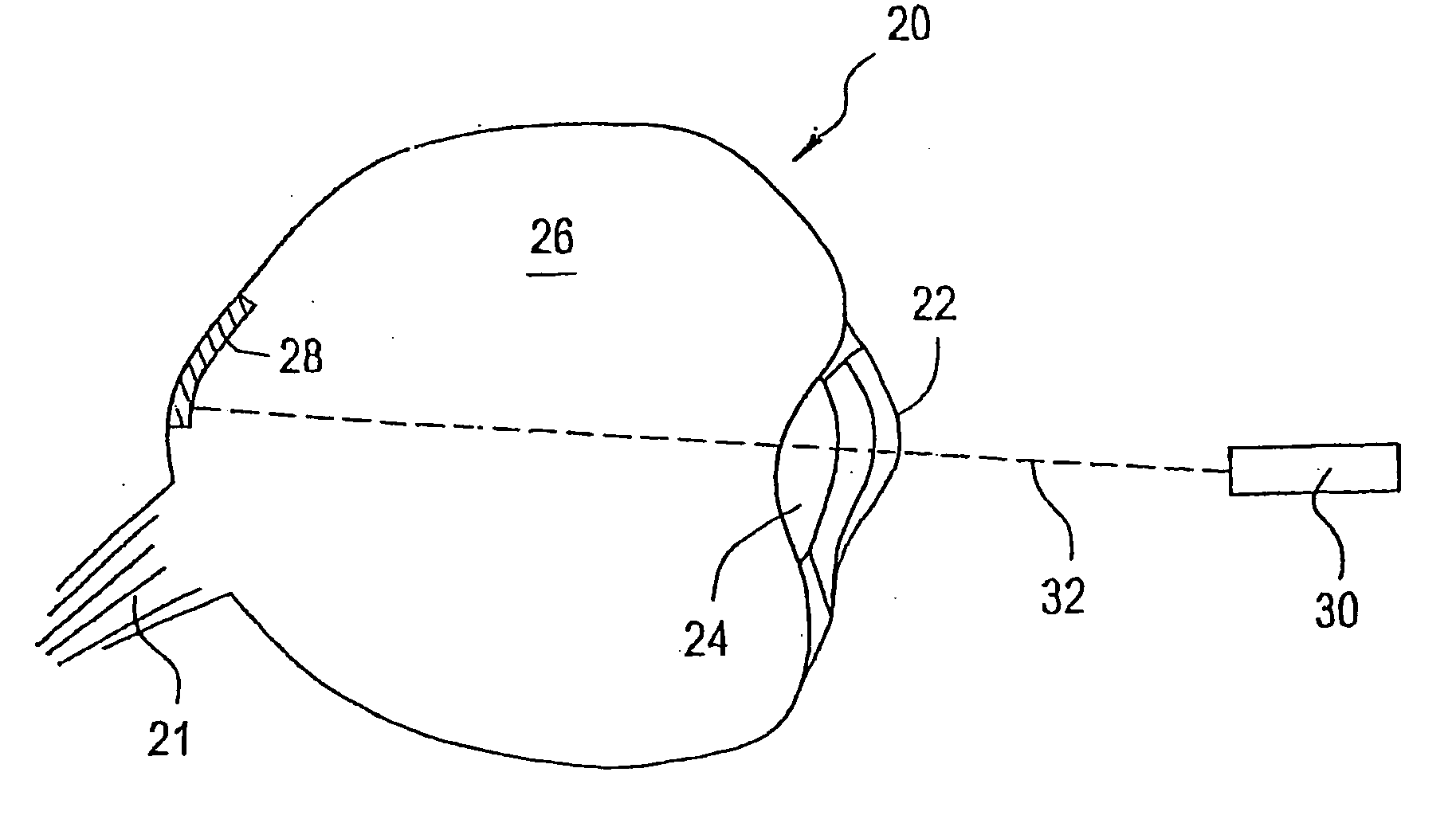
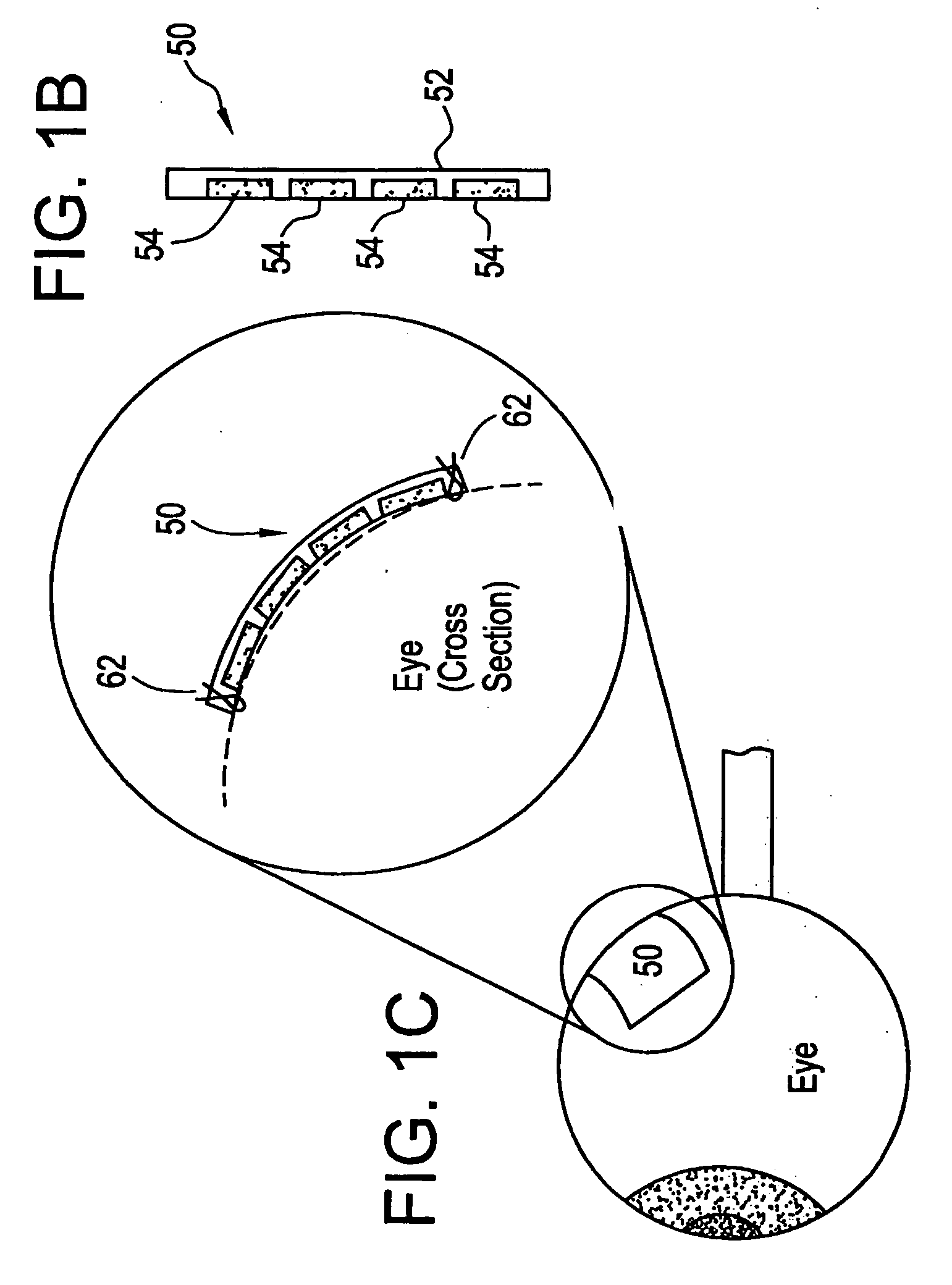
Flexible microchip devices for ophthalmic and other applications
InactiveUS6976982B2Small sizeVariable shapeEye surgerySolid-state devicesControlled releaseBiomedical engineering
Microchip device arrays that can conform to a curved surface are provided for the controlled release or exposure of reservoir contents. The arrays comprise two or more microchip device elements, each of which includes a plurality of reservoirs that contain molecules for controlled release or components for selective exposure, and a means for flexibly connecting the device elements. The reservoirs can contain one or more drugs and / or one or more secondary devices, such as a sensor or a component thereof. Preferably, the microchip devices contain and controllably release therapeutic, prophylactic, and diagnostic molecules to and into the eye of a patient in need thereof.
Owner:MICROCHIPS BIOTECH INC
Hermetically sealed microchip reservoir devices
InactiveUS20050077584A1Fixed microstructural devicesVolume/mass flow measurementGlucose sensorsHermetic seal
Devices are provided for the controlled exposure or release of contents stored in hermetically sealed reservoirs. The devices comprise a primary substrate having a front side and a back side, and including one or more hermetic sealing materials; a plurality of reservoirs in the primary substrate positioned between the front side and the back side; reservoir contents, which comprise chemical molecules (such as drugs) or a secondary device (such as a glucose sensor), located inside the reservoirs; a hermetic sealing substrate having a surface composed of one or more hermetic sealing materials; and a hermetic seal formed between and joining the primary substrate and the hermetic sealing substrate, wherein the hermetic seal independently seals the reservoirs.
Owner:MICROCHIPS BIOTECH INC
Drug depot implant designs and methods of implantation
ActiveUS20070243225A1Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
Multi-reservoir device for transdermal drug delivery and sensing
Devices and methods are provided for transdermal administration of a pharmaceutical agent to a patient in need thereof. The device includes a substrate, a plurality of discrete reservoirs in the substrate, one or more pharmaceutical agents stored in the reservoirs, discrete reservoir caps that prevent the pharmaceutical agent from passing out from the reservoirs until desired, control means for actuating release of the one or more pharmaceutical agents from one or more of the reservoirs by disintegrating or permeabilizing the reservoir caps, means for securing the device to the skin of the patient, and means for transporting the pharmaceutical agent to the skin following release from the one or more of the reservoirs. In another embodiment, the device is adapted for diagnostic sensing instead of or in addition to drug delivery.
Owner:MICROCHIPS BIOTECH INC
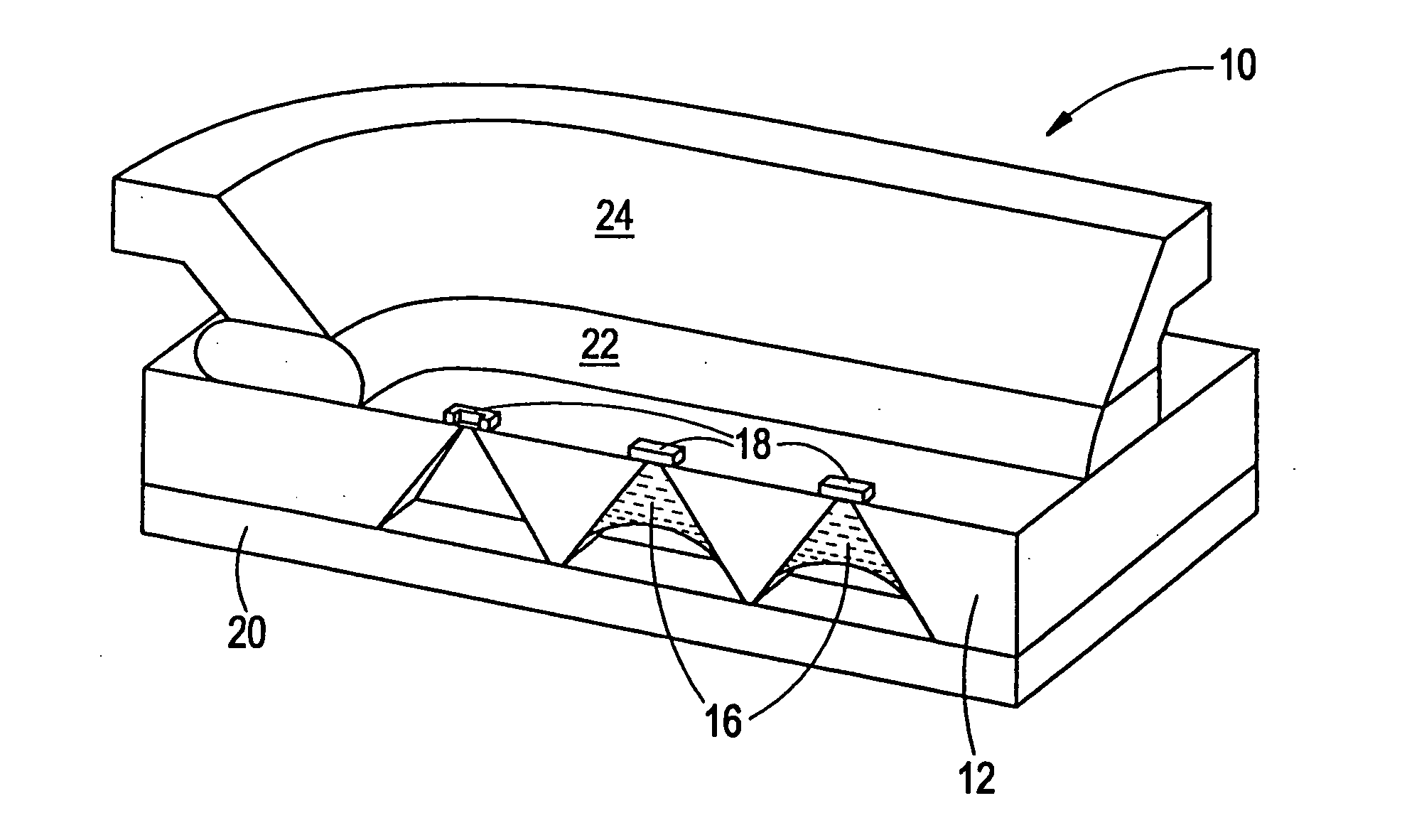
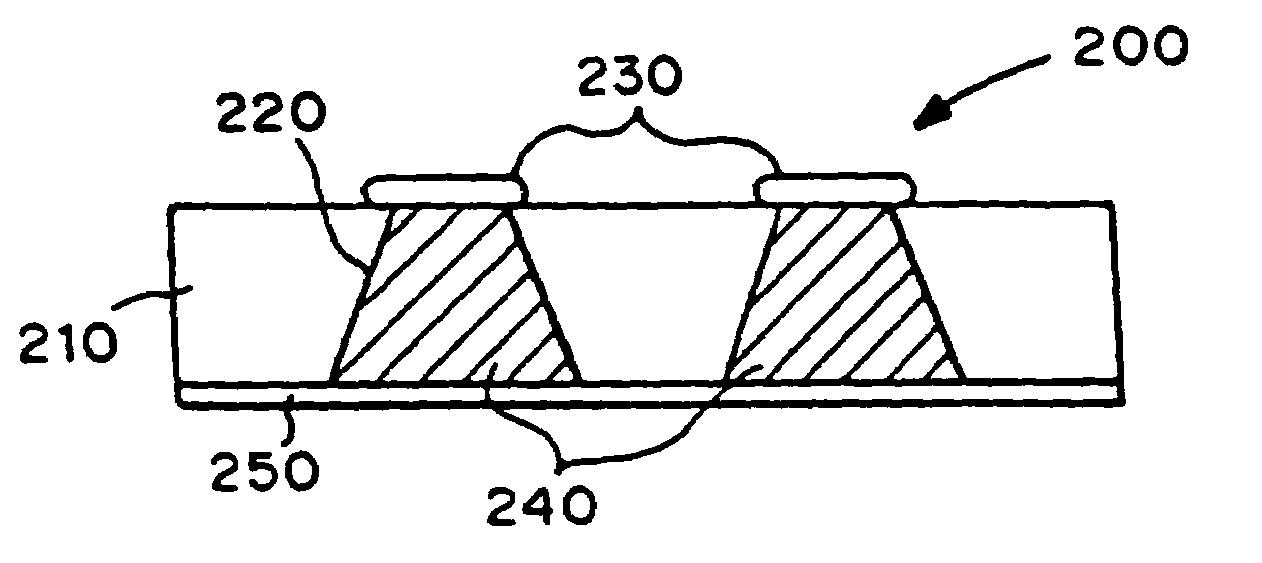
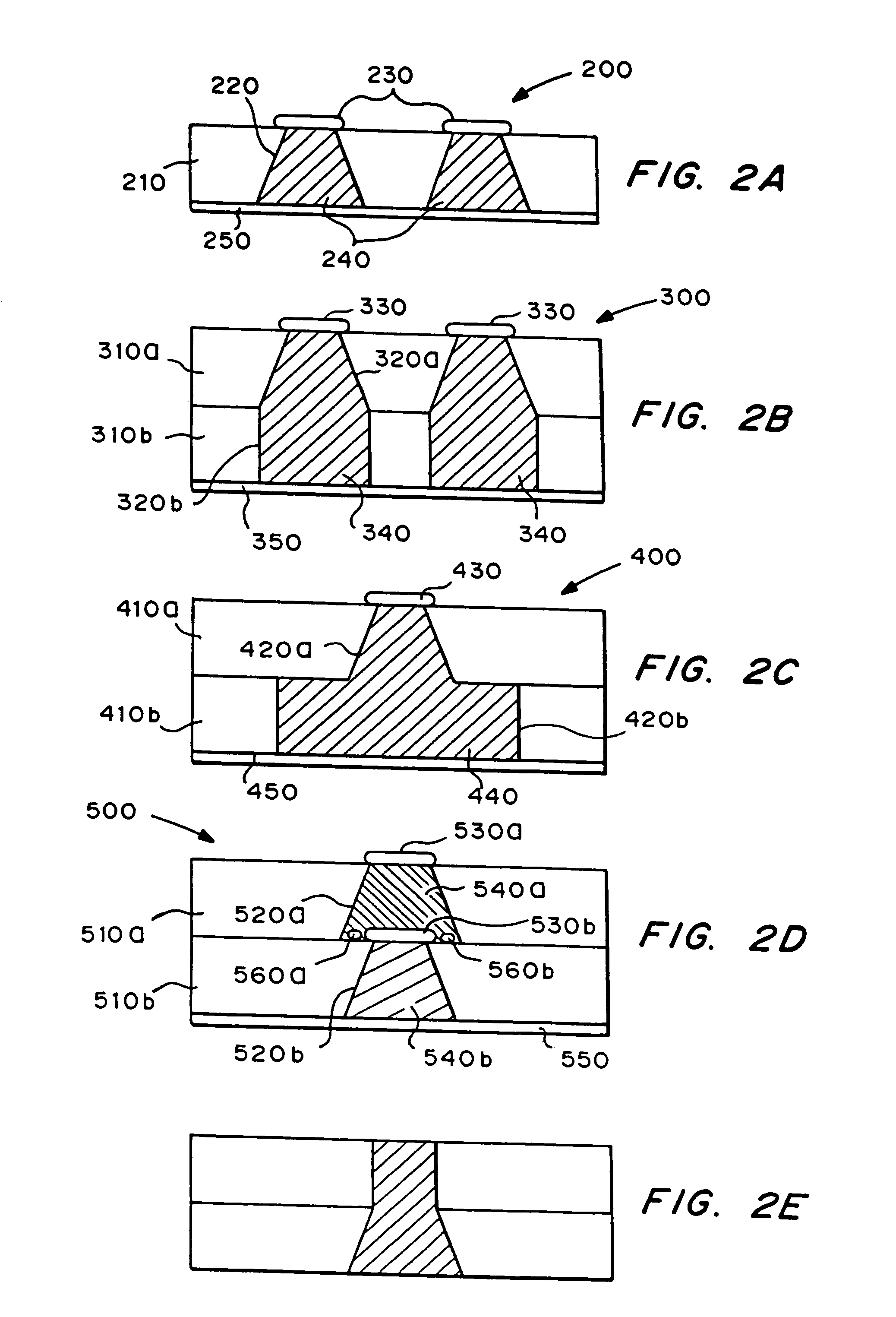
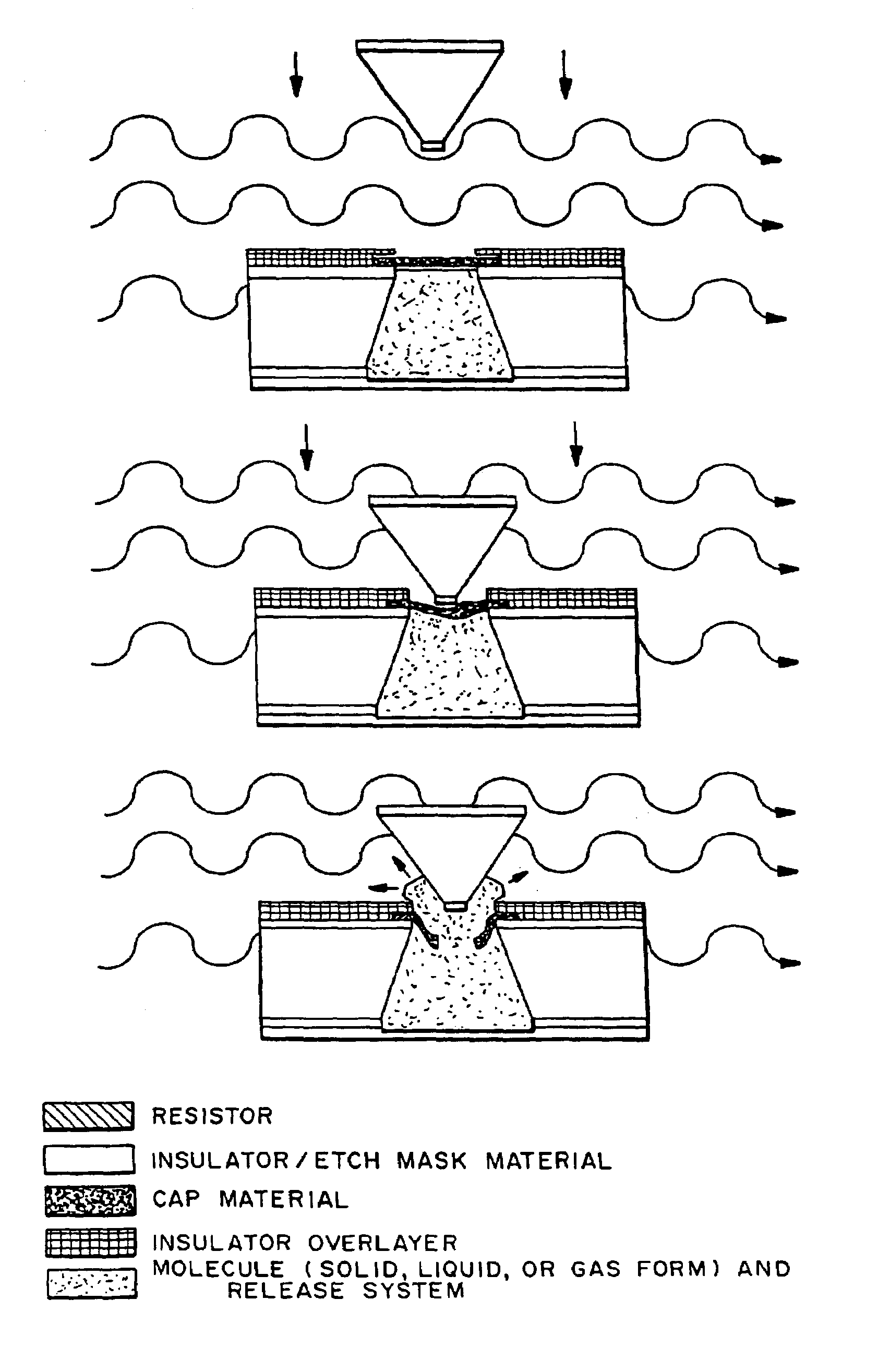
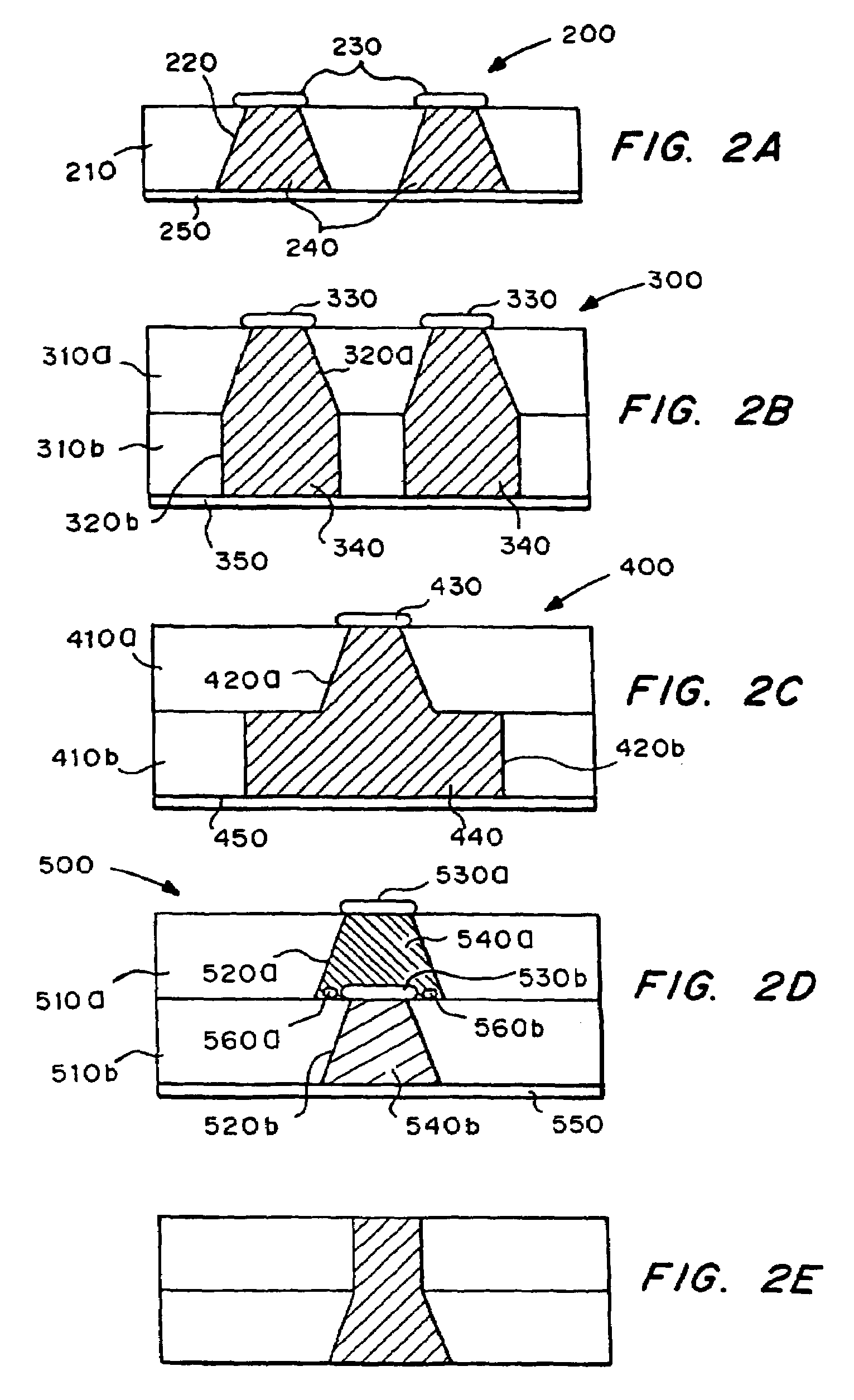
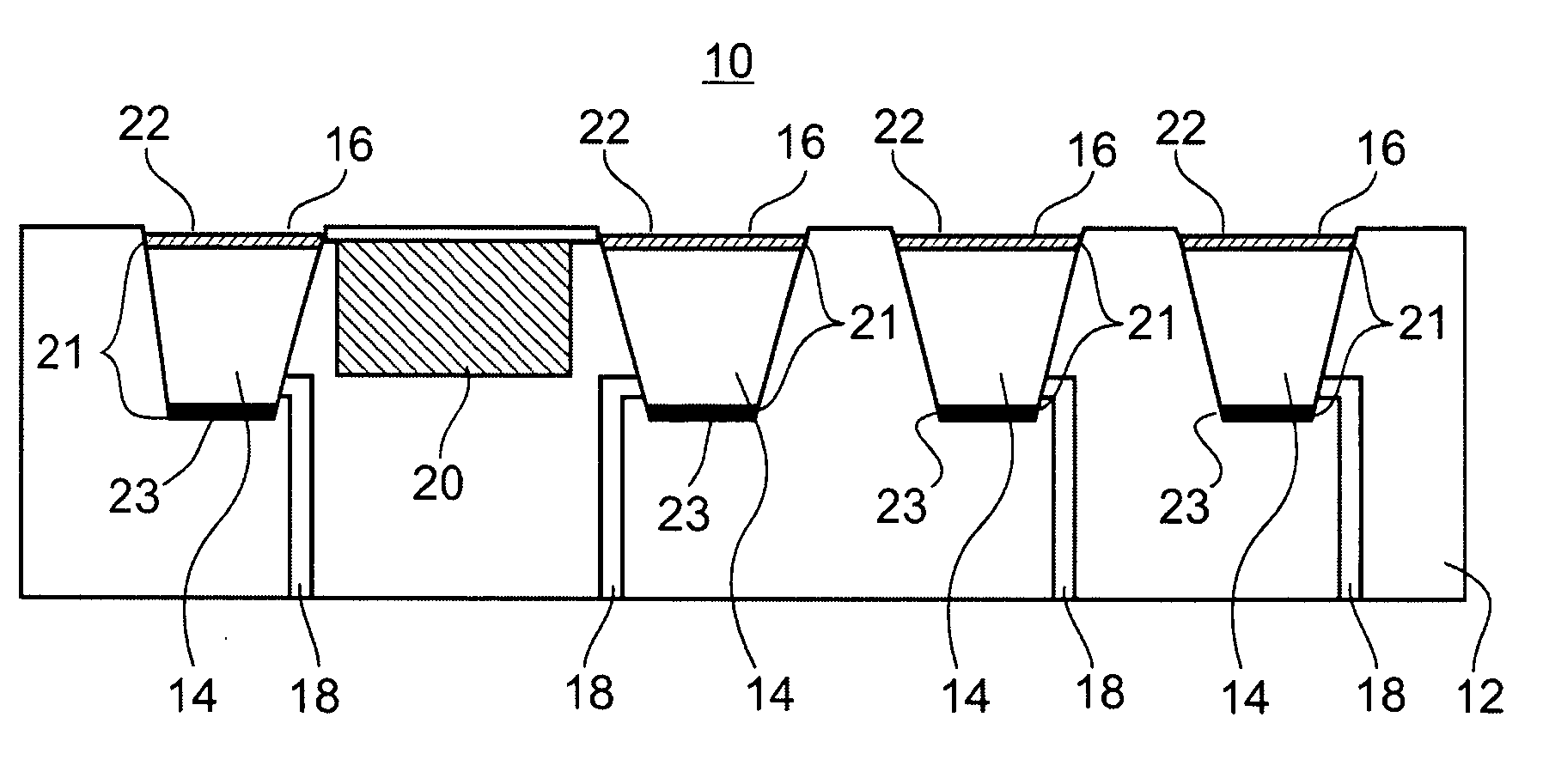
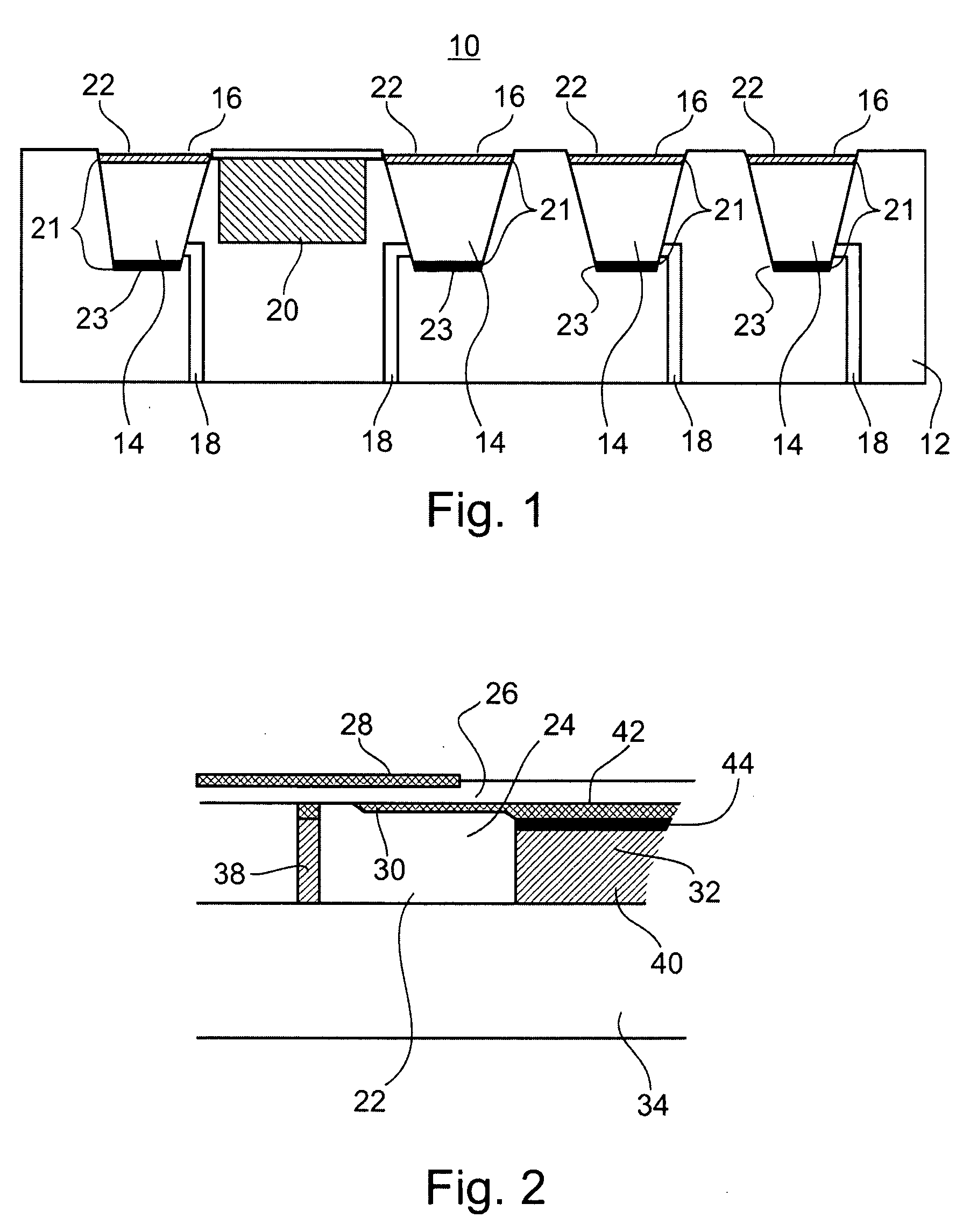
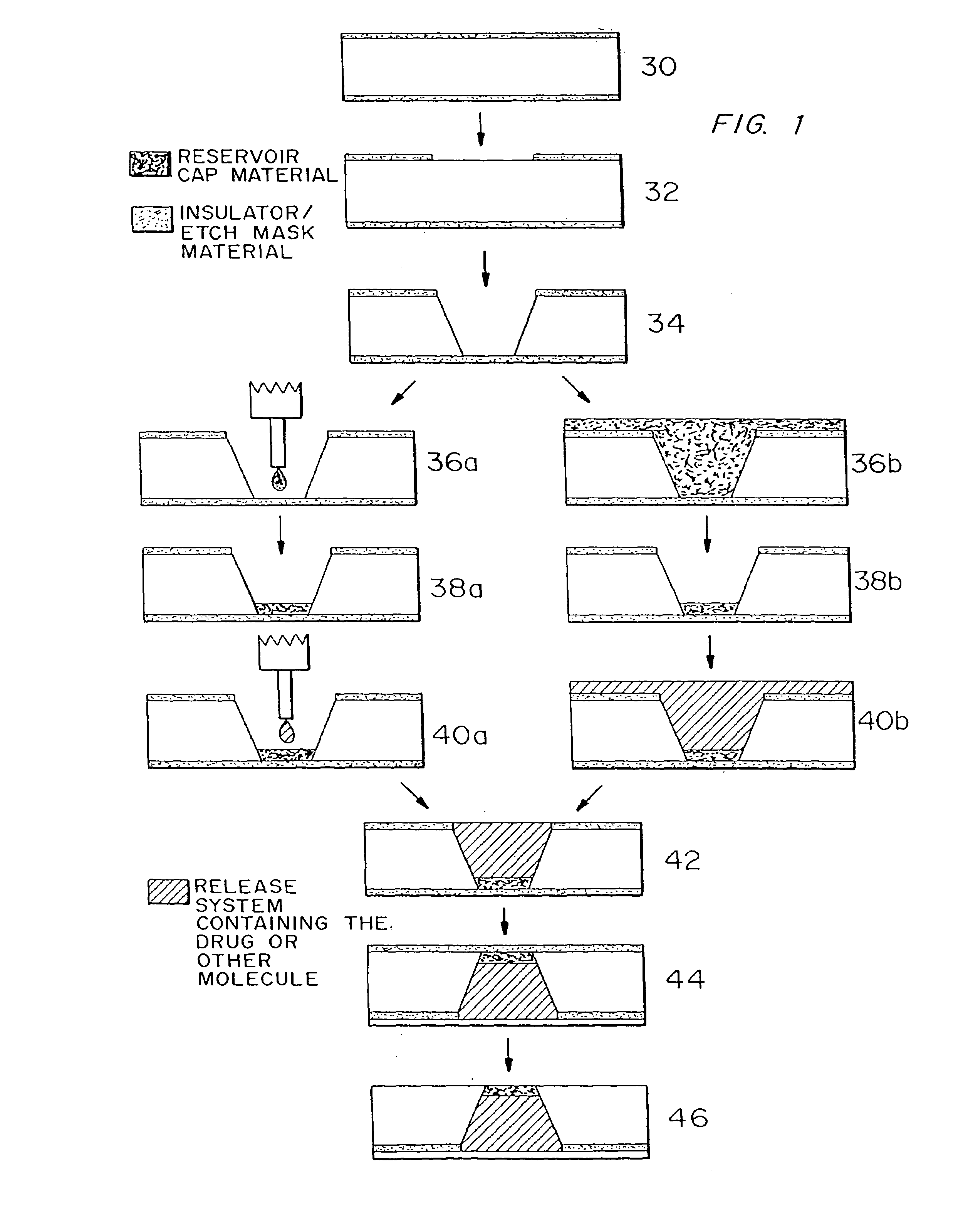
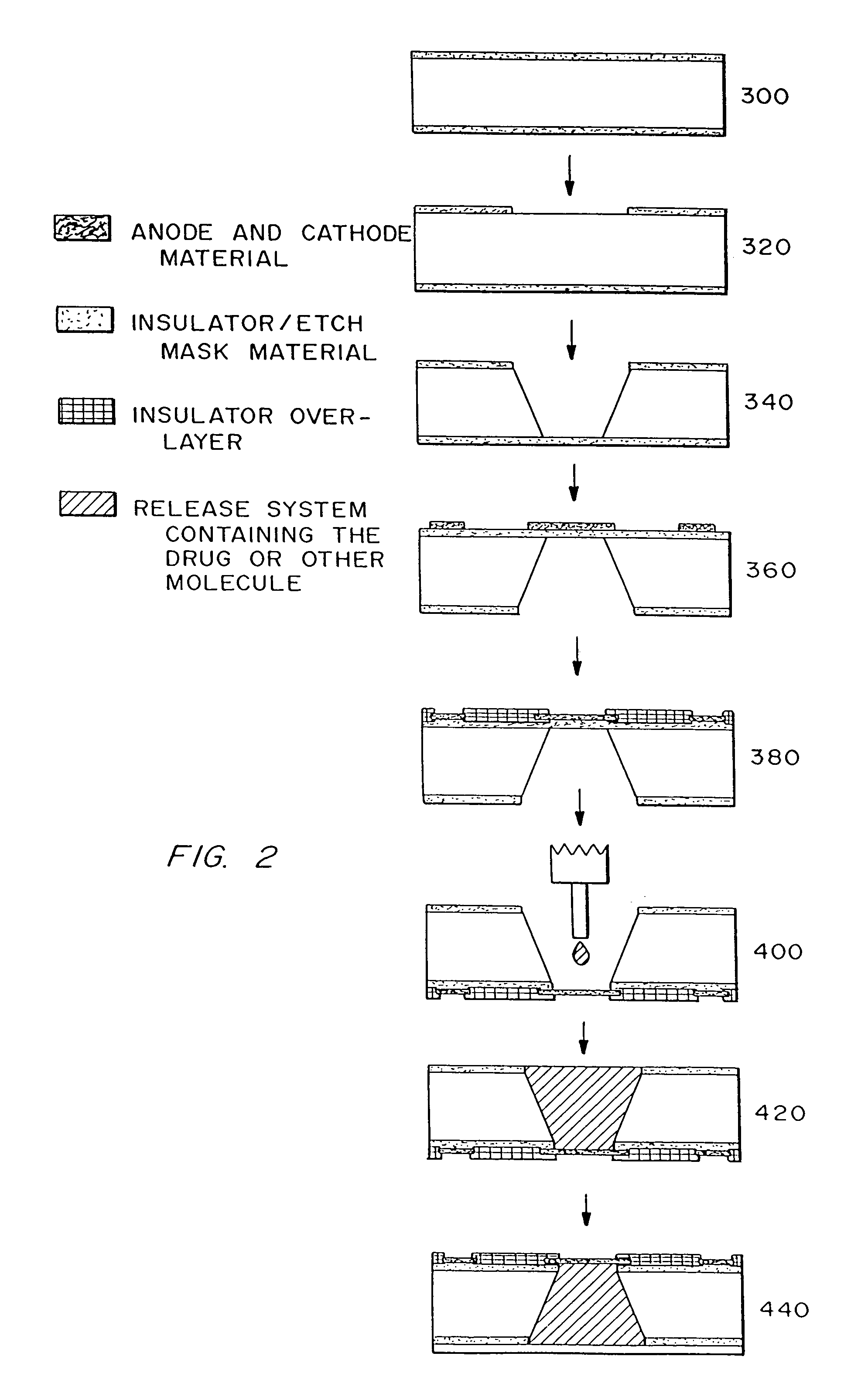
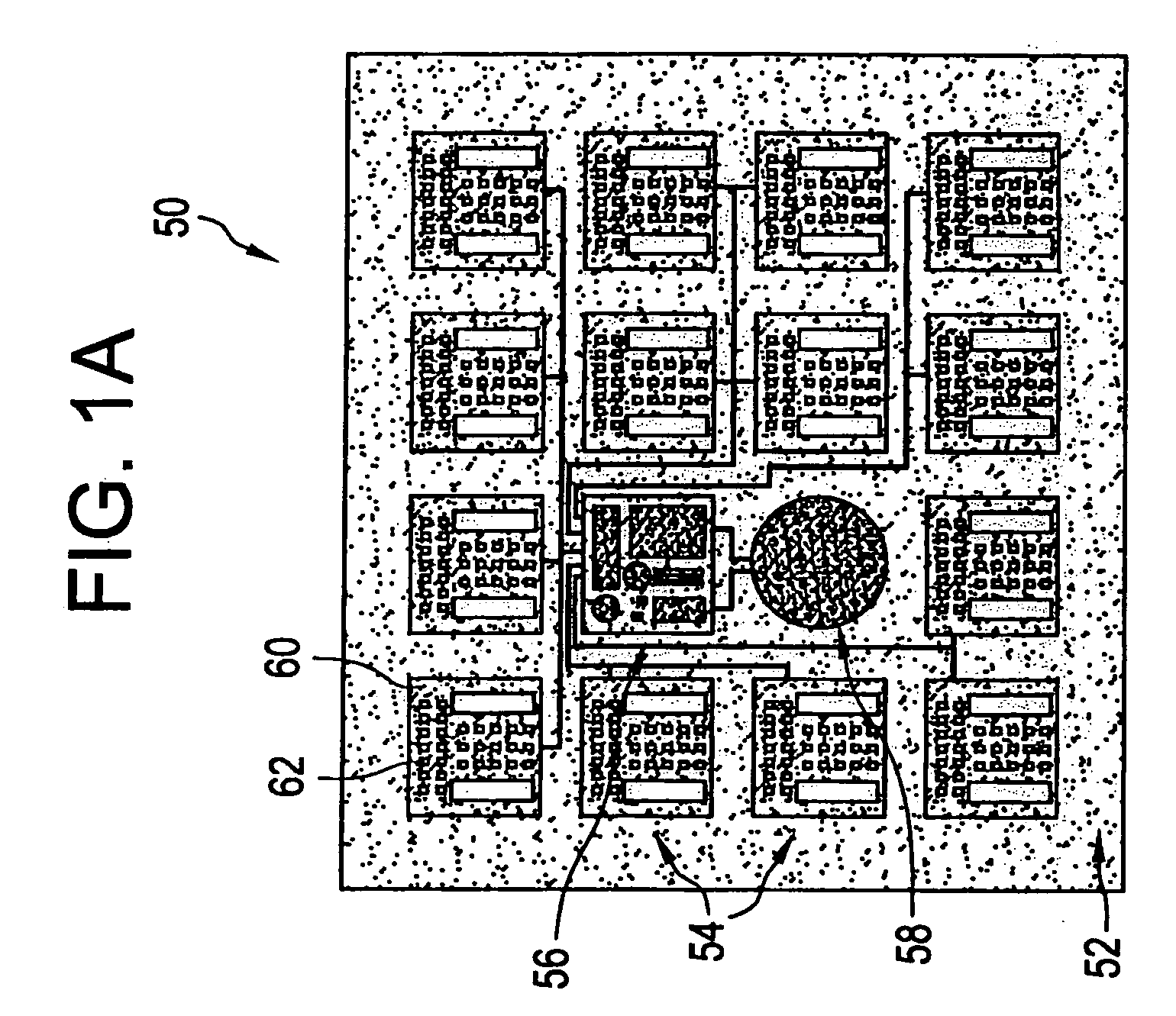
Microchip devices with improved reservoir opening
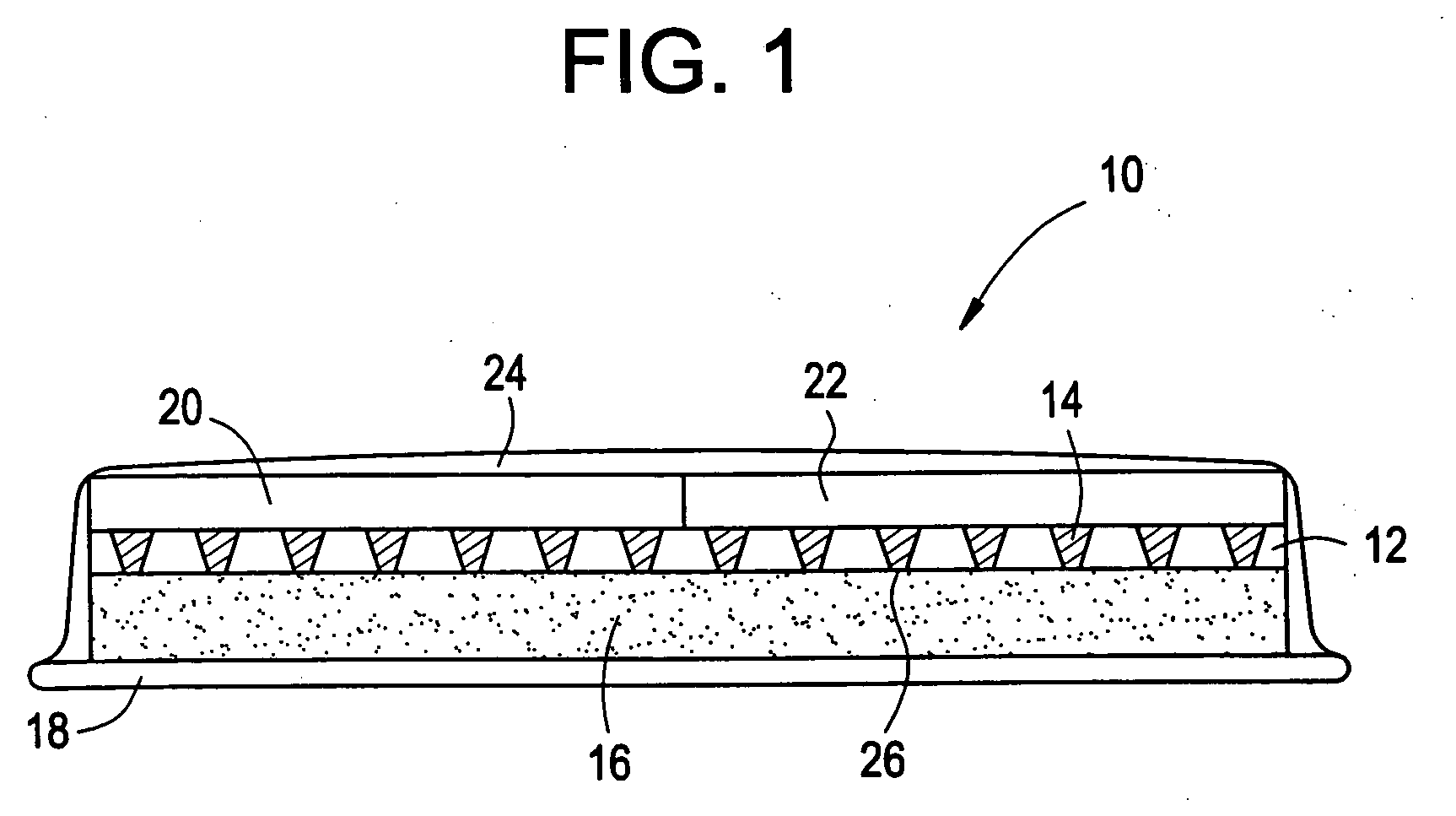
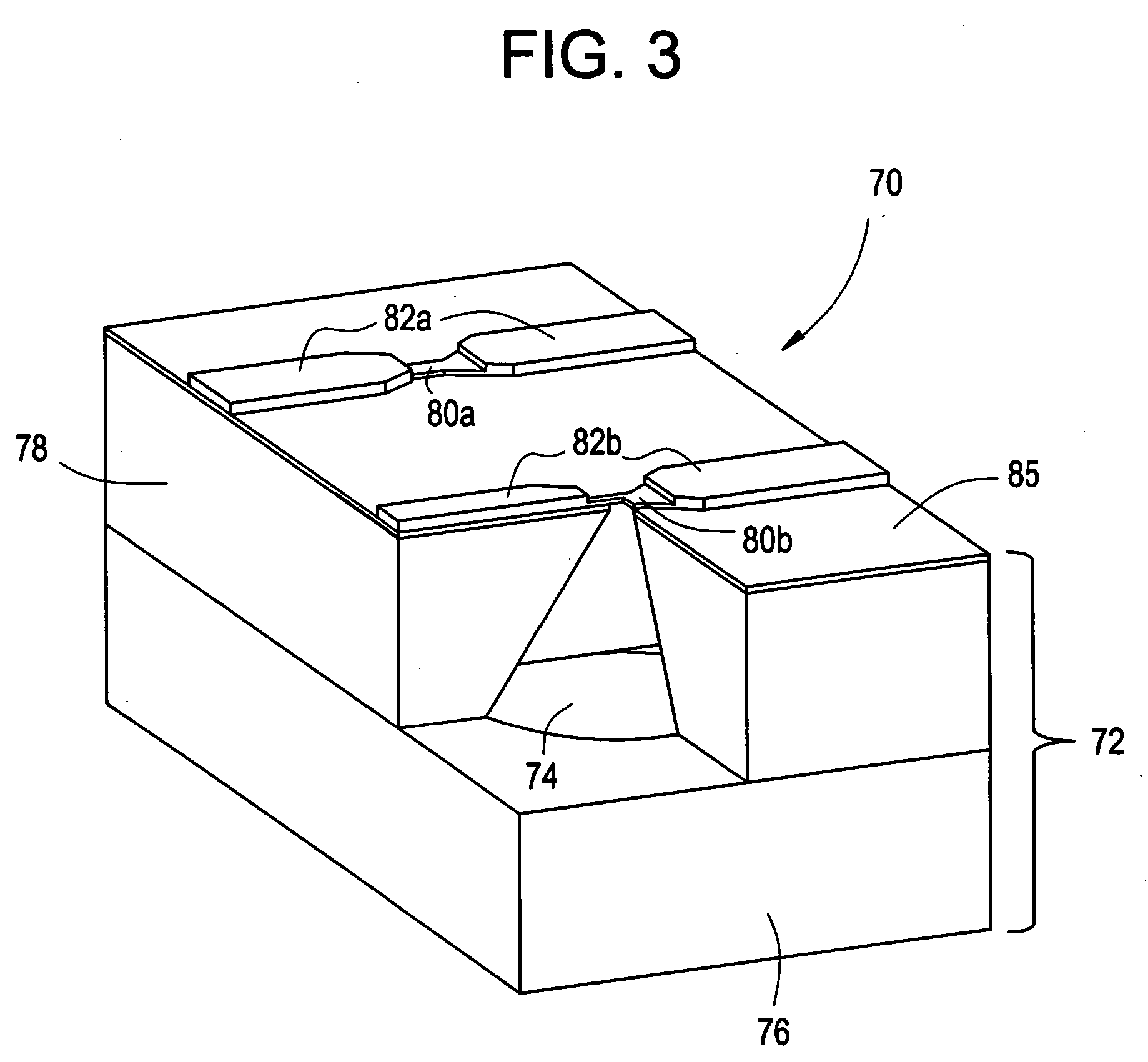
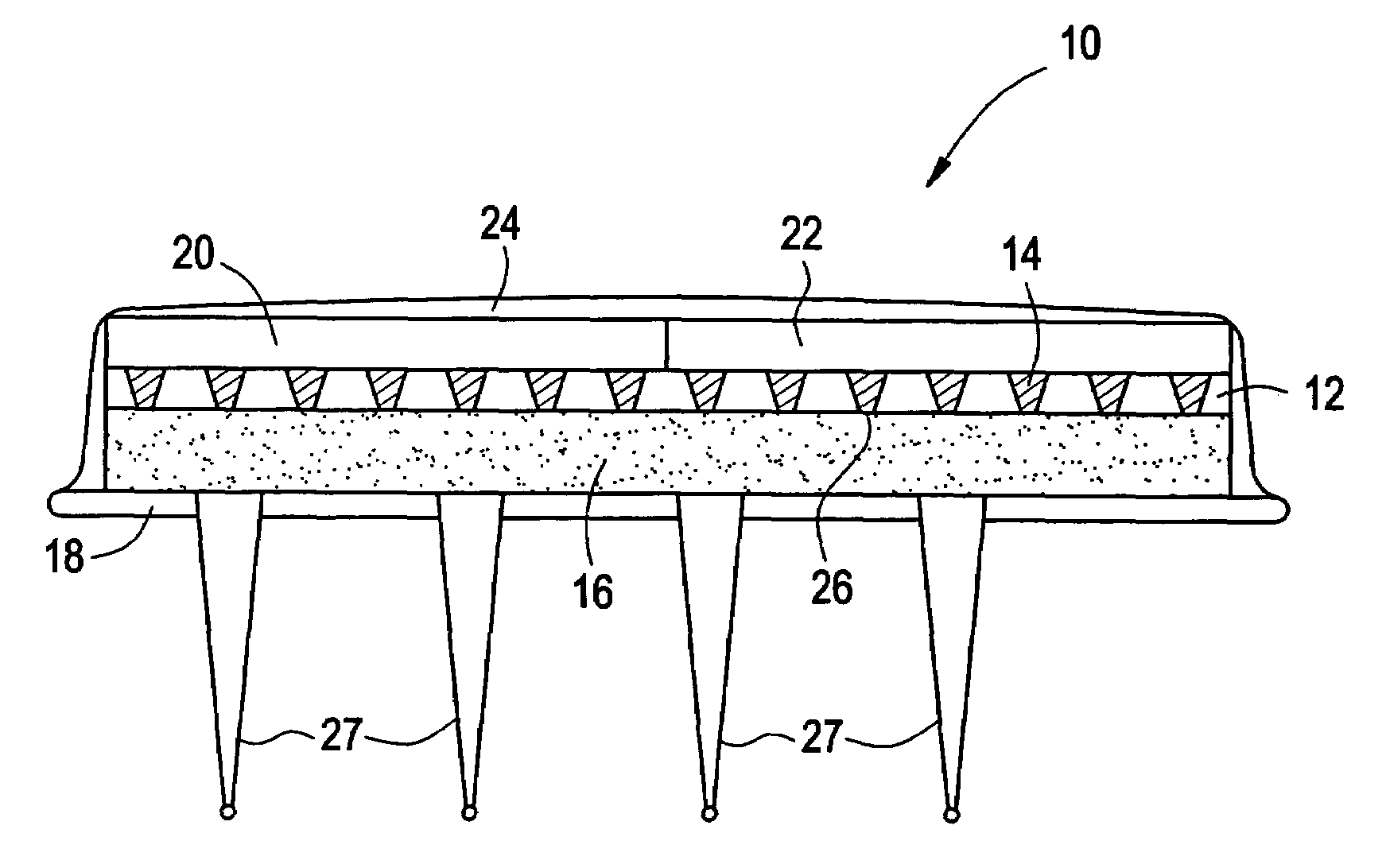
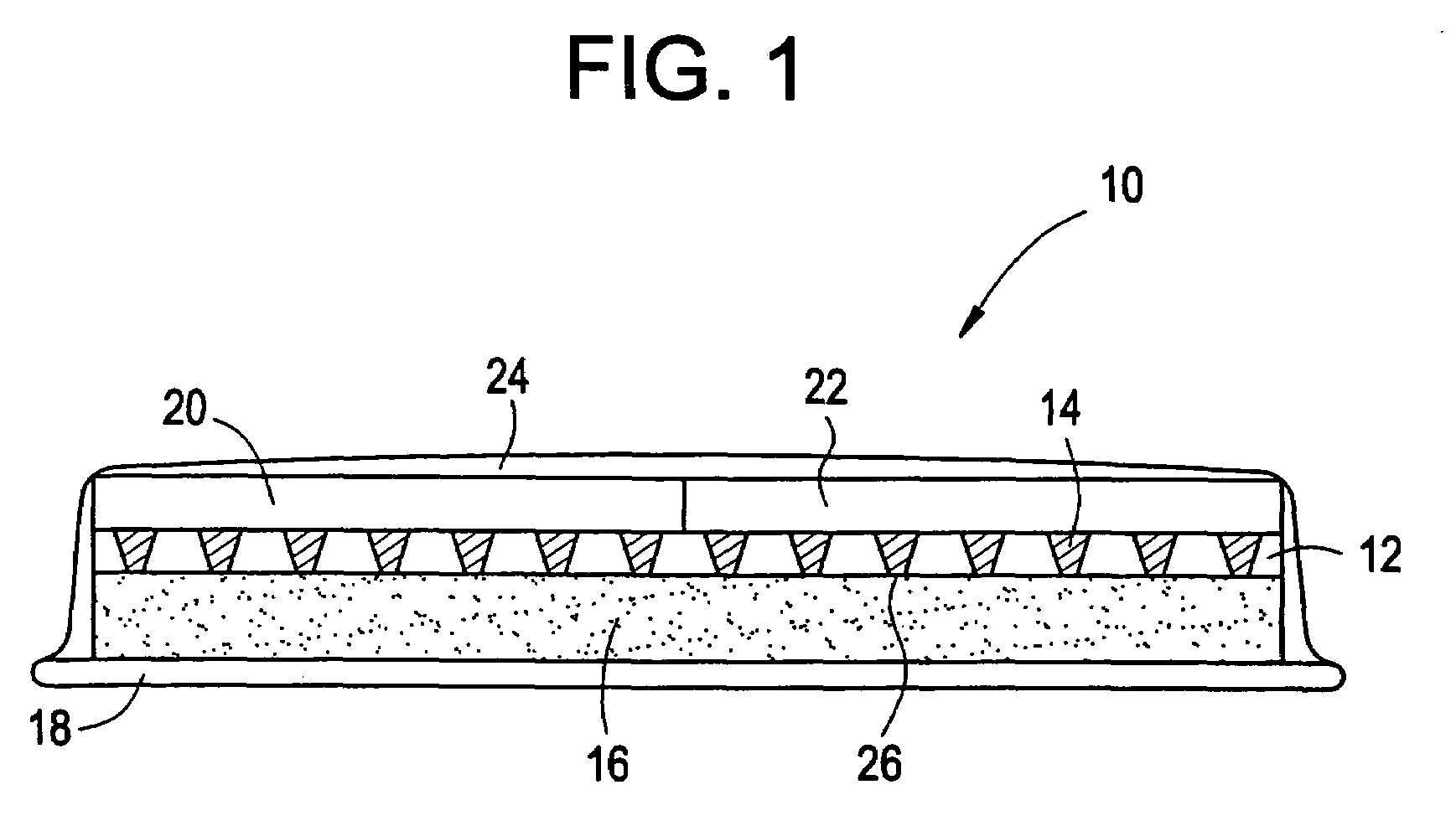
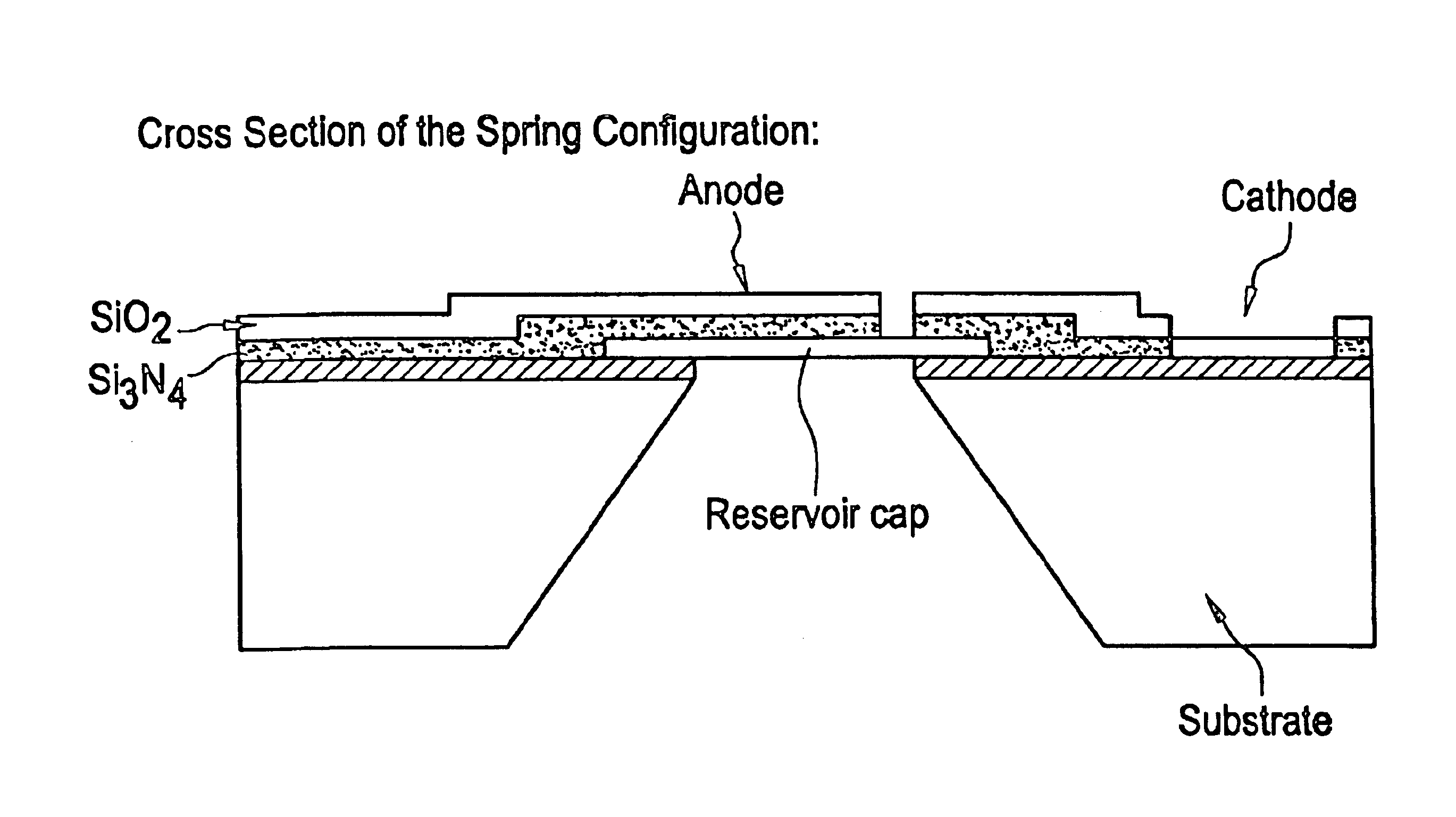
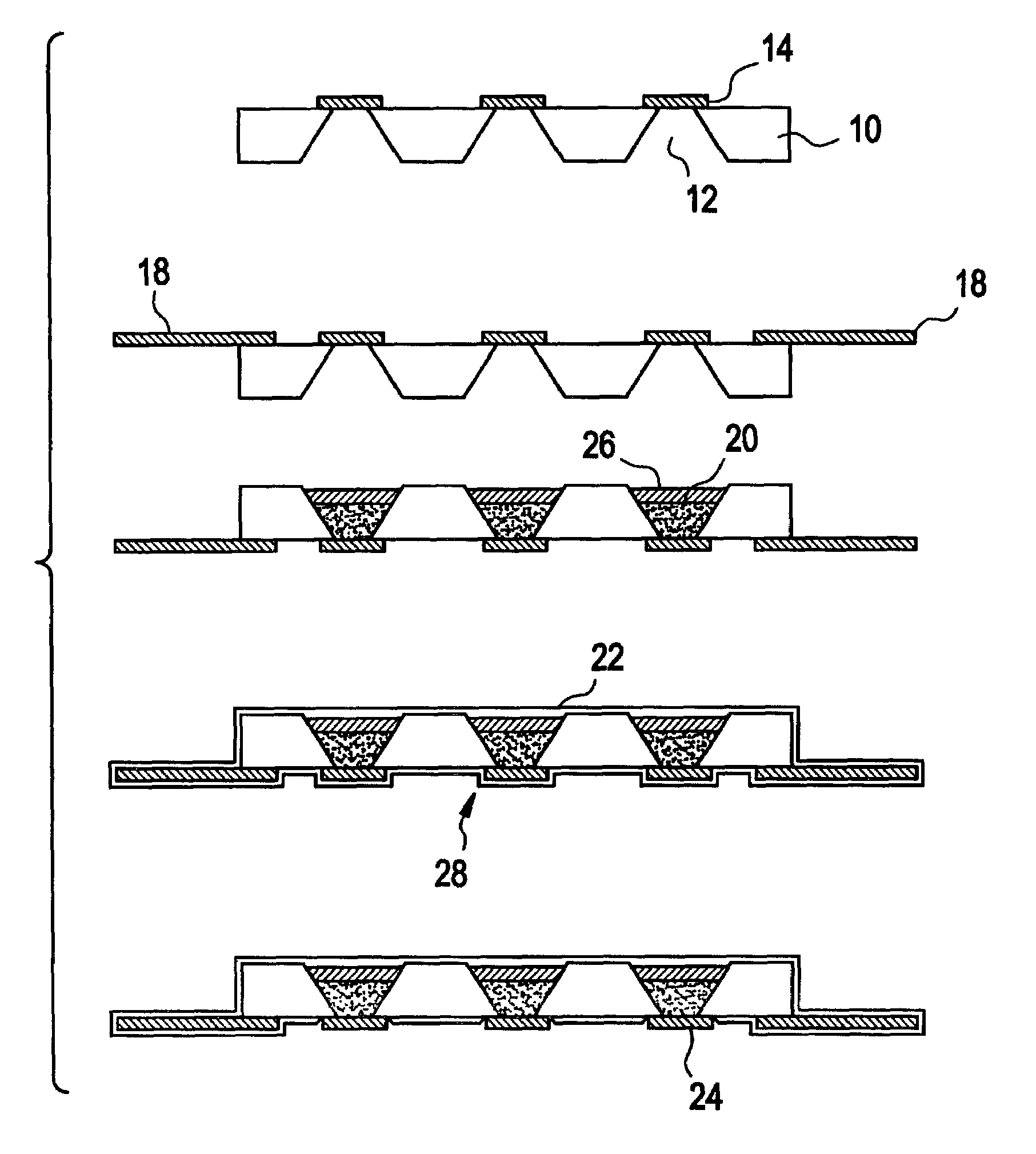
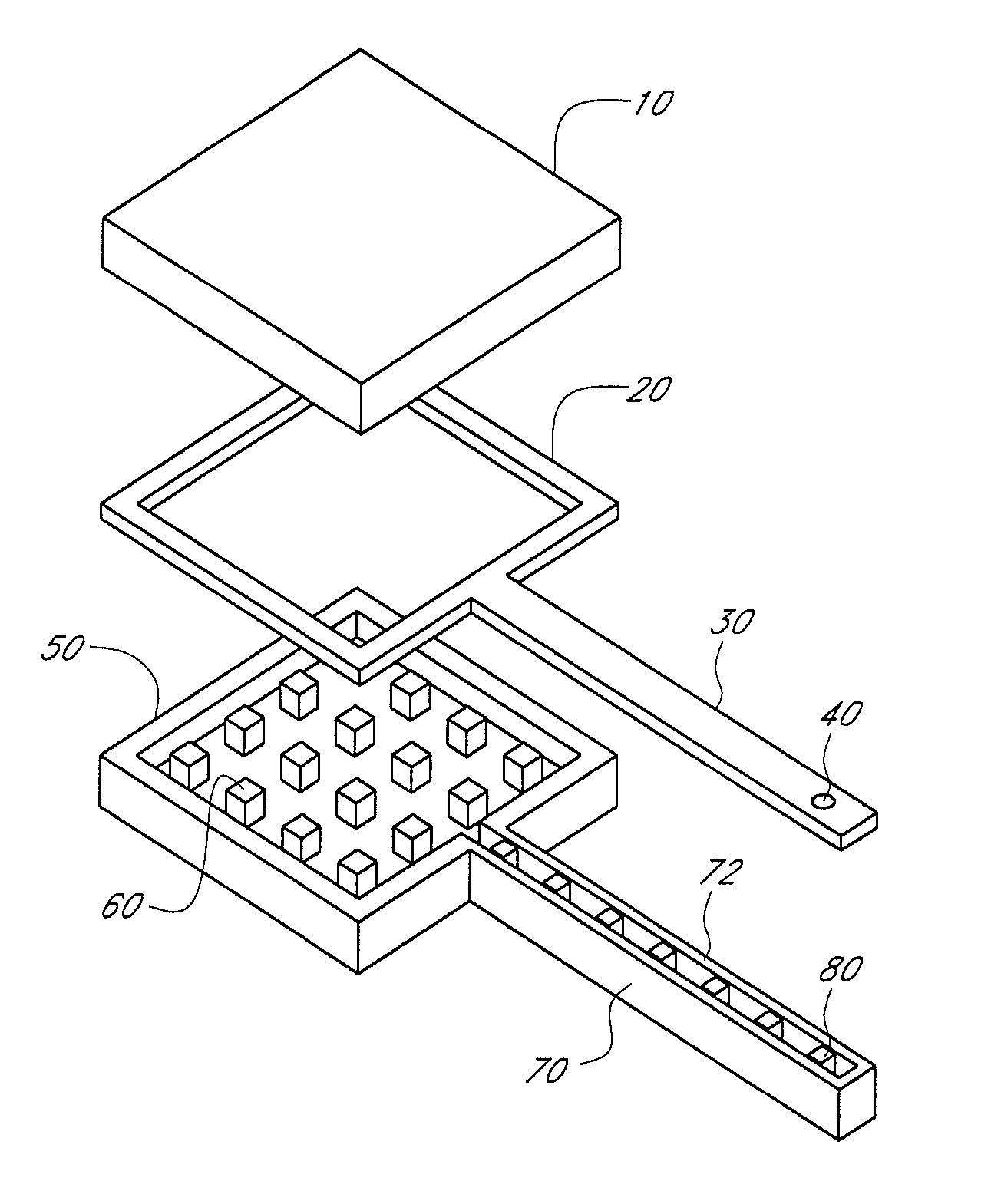
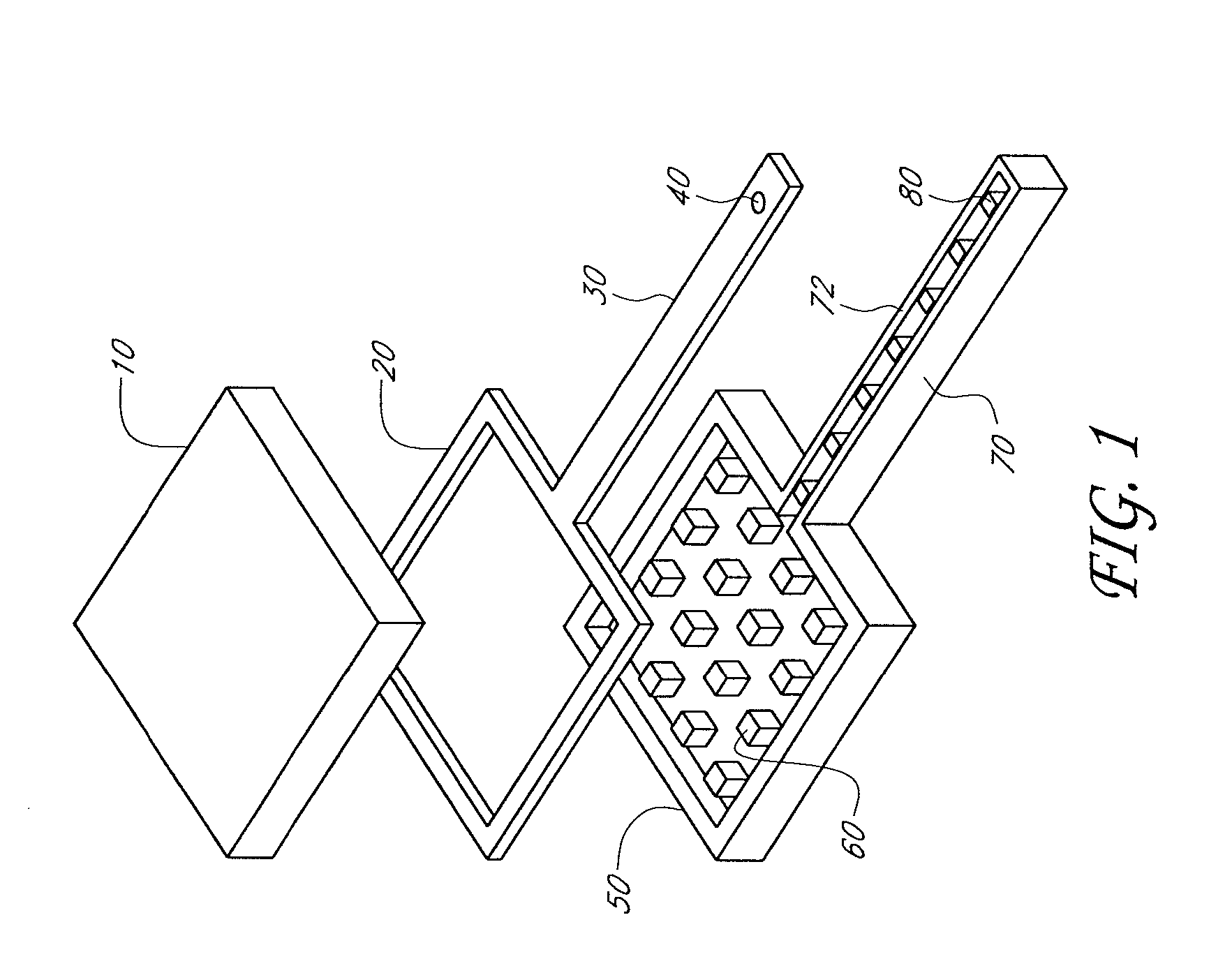
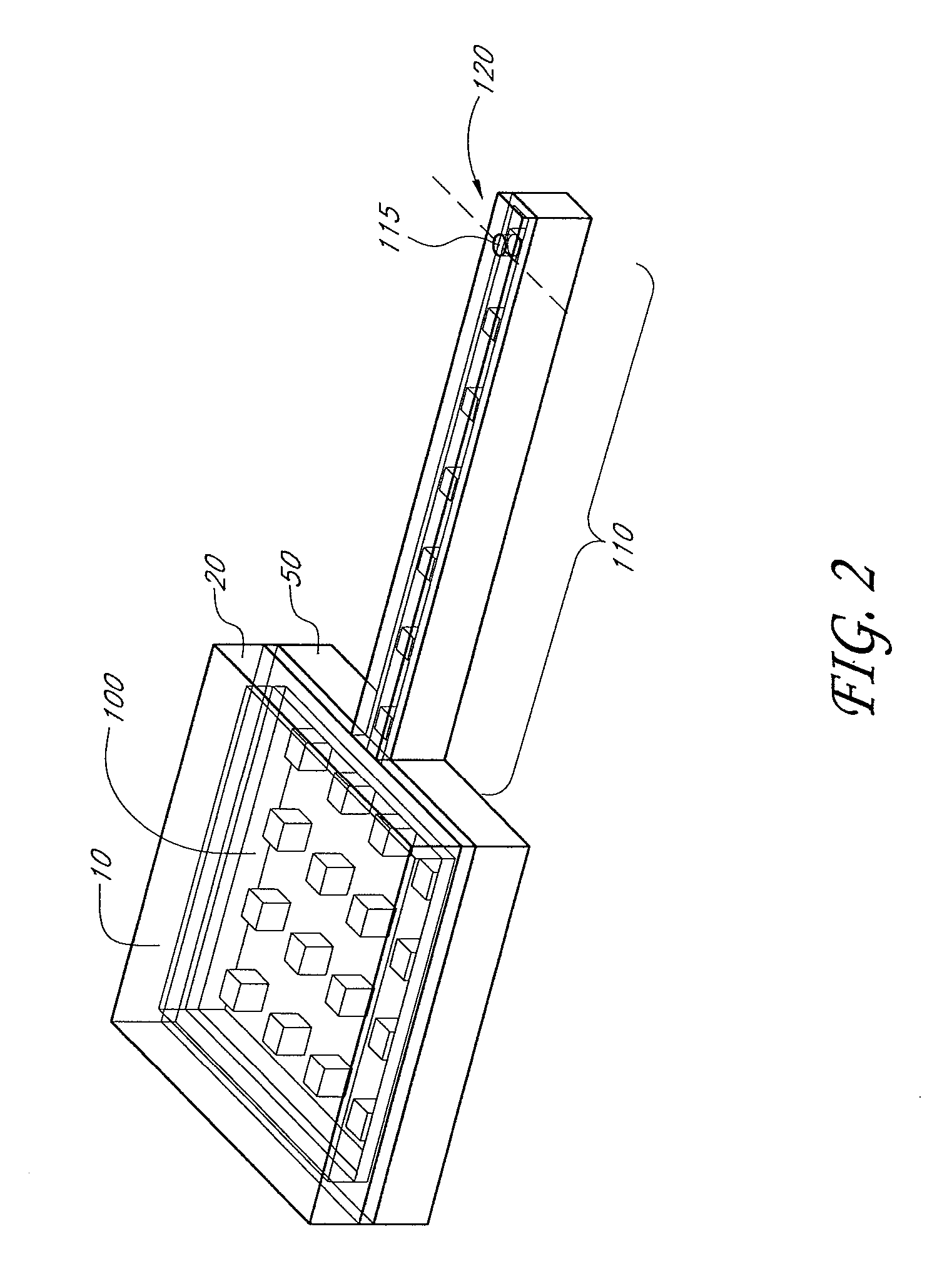
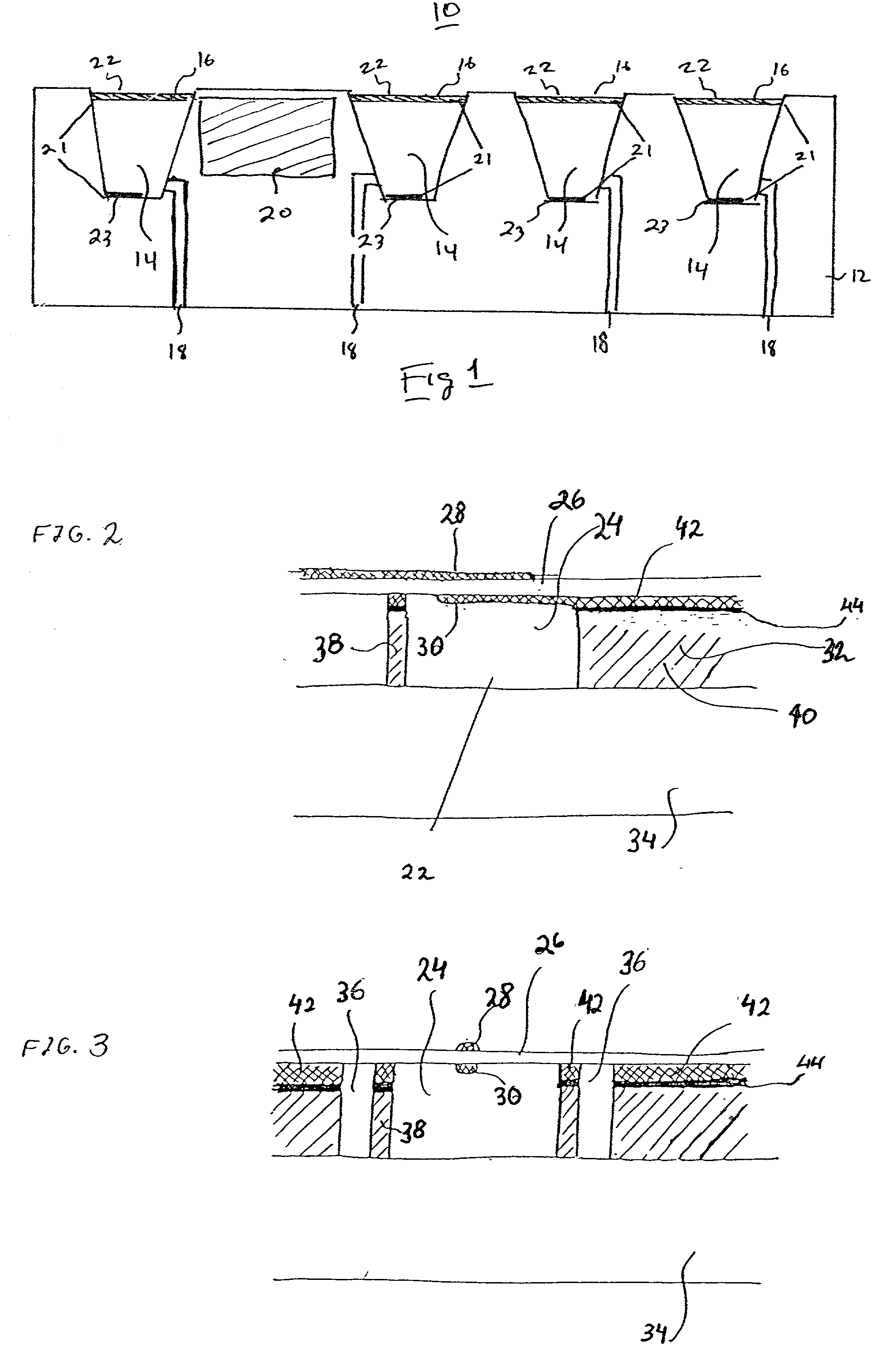
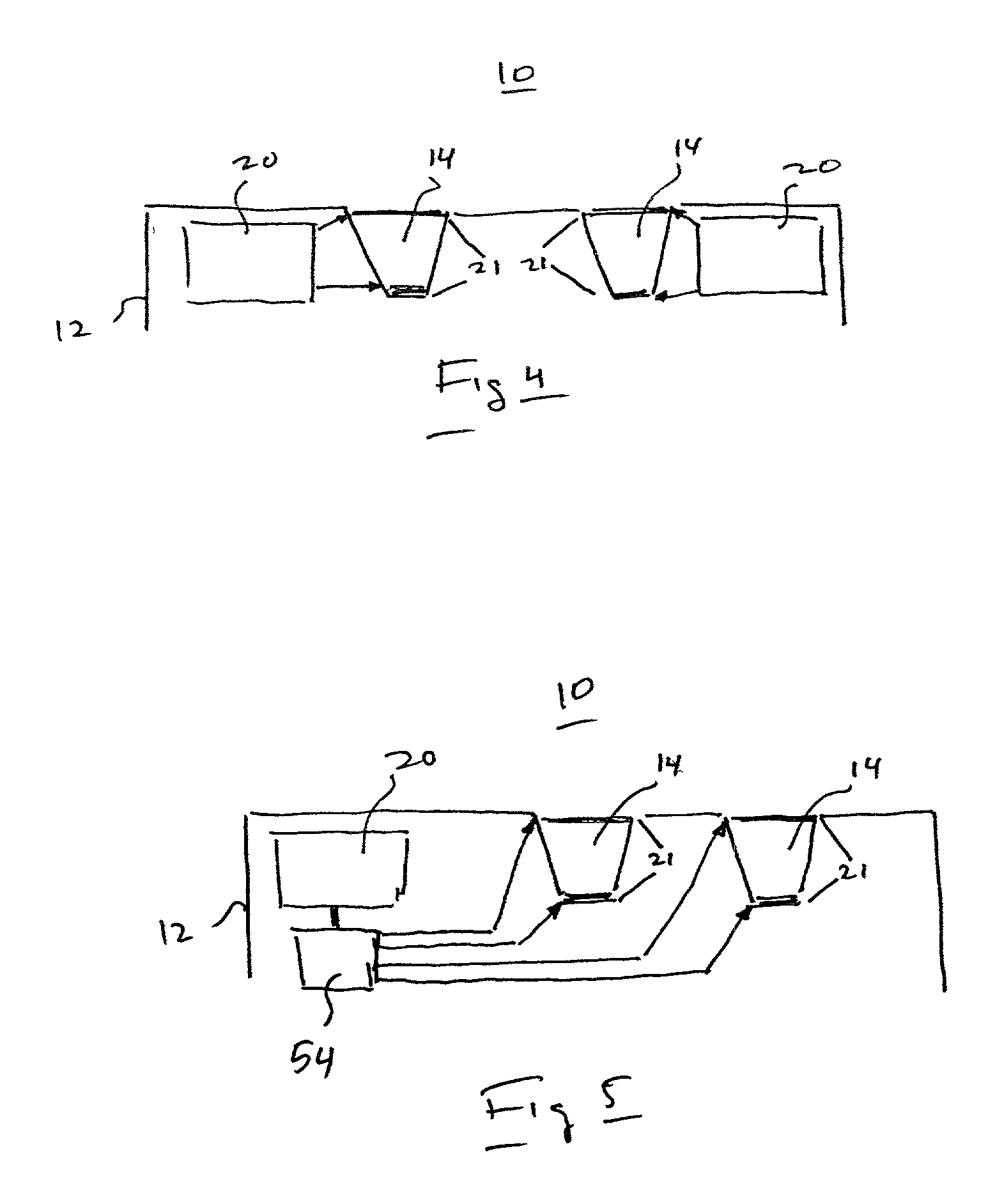
InactiveUS6875208B2Improve uniformityImprove reliabilityMedical devicesMicromachined deliveryControlled releaseCurrent distribution
Microchip devices and methods of manufacture thereof are provided to increase the uniformity and reliability of active exposure and release of microchip reservoir contents. In one embodiment, the microchip device for the controlled release or exposure of molecules or secondary devices comprises: (1) a substrate having a plurality of reservoirs; (2) reservoir contents comprising molecules, a secondary device, or both, located in the reservoirs; (3) reservoir caps positioned on the reservoirs over the reservoir contents; (4) electrical activation means for disintegrating the reservoir cap to initiate exposure or release of the reservoir contents in selected reservoirs; and (5) a current distribution means, a stress induction means, or both, operably engaged with or integrated into the reservoir cap, to enhance reservoir cap disintegration.
Owner:MASSACHUSETTS INST OF TECH
Local Delivery of Drugs or Substances Using Electronic Permeability Increase
InactiveUS20080188837A1Easy accessPromote absorptionMedical devicesMicromachined deliverySubstance useTarget tissue
An apparatus is provided for use in conjunction with a drug delivered to a gastrointestinal (GI) tract of a subject. The apparatus includes an ingestible capsule, which includes one or more electrodes, and a control component, adapted to drive the electrodes to apply an electrical current that induces local delivery of the drug in target tissue of the GI tract. Additional embodiments are also described.
Owner:E PILL PHARMA
Methods for conformal coating and sealing microchip reservoir devices
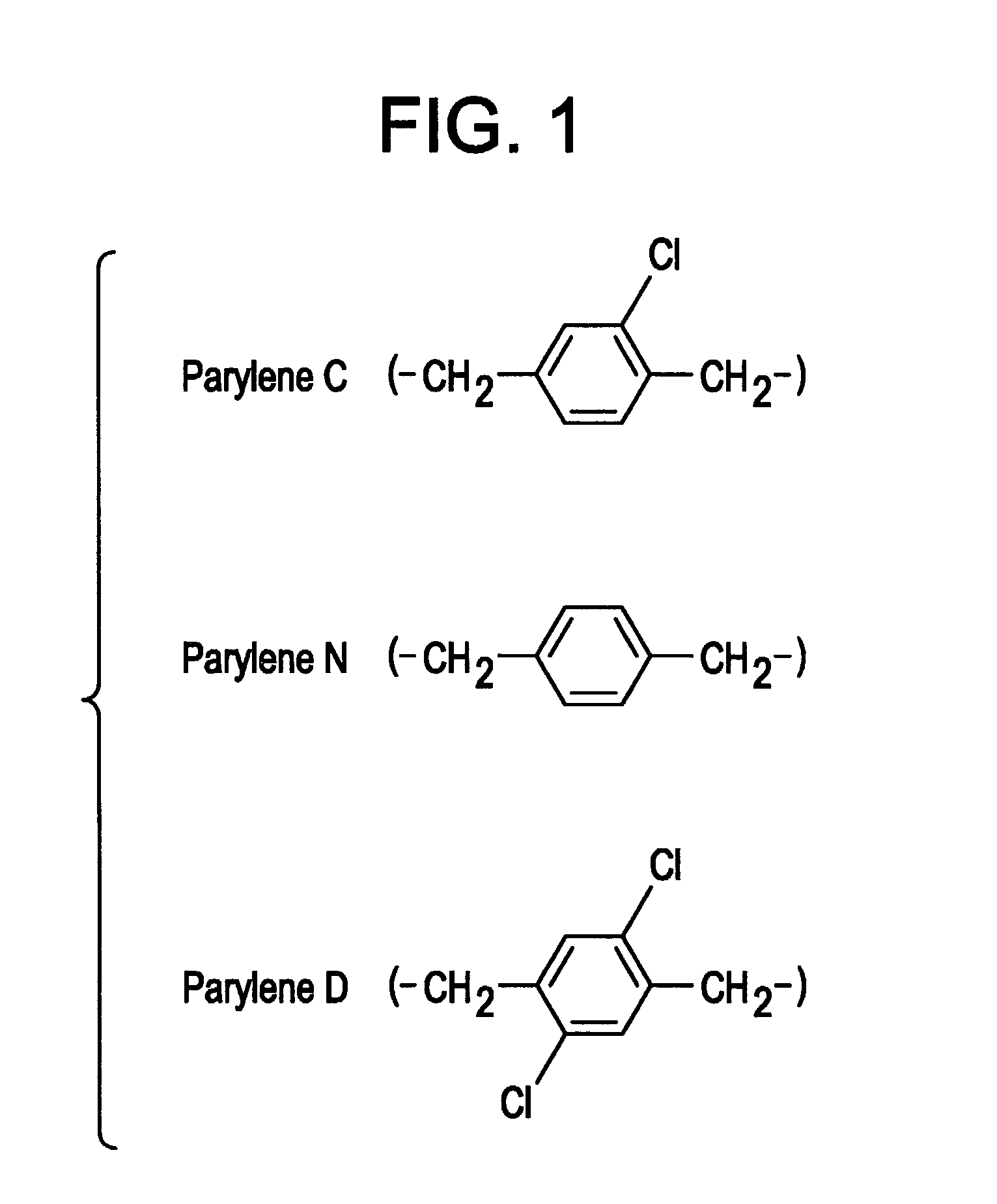
InactiveUS6973718B2Reduce adverse reactionsPrinted circuit assemblingLiquid surface applicatorsParyleneGas phase
Methods are provided for conformally coating microchip devices and for sealing reservoirs containing molecules or devices in a microchip device. One method comprises (i) providing a substrate having a plurality of reservoirs having reservoir openings in need of sealing; (ii) loading reservoir contents comprising molecules, a secondary device, or both, into the reservoirs; and (iii) applying a conformal coating barrier layer, such as a vapor depositable polymeric material, e.g., parylene, onto the reservoir contents over at least the reservoir openings to seal the reservoir openings. Another method comprises vapor depositing a conformal coating material onto a microchip device having at least two reservoirs and reservoir caps positioned over molecules or devices stored in the reservoirs, and providing that the conformal coating does not coat or is removed from the reservoir caps.
Owner:MICROCHIPS INC
Drug depot implant designs and methods of implantation
ActiveUS20070243228A1Uniform drug distributionMinimal disruptionBiocidePeptide/protein ingredientsSkeletal injurySacroiliac joint
The present invention relates to novel drug depot implant designs for optimal delivery of therapeutic agents to subjects. The invention provides a method for alleviating pain associated with neuromuscular or skeletal injury or inflammation by targeted delivery of one or more therapeutic agents to inhibit the inflammatory response which ultimately causes acute or chronic pain. Controlled and directed delivery can be provided by drug depot implants, comprising therapeutic agents, specifically designed to deliver the therapeutic agent to the desired location by facilitating their implantation, minimizing their migration from the desired tissue location, and without disrupting normal joint and soft tissue movement.
Owner:WARSAW ORTHOPEDIC INC
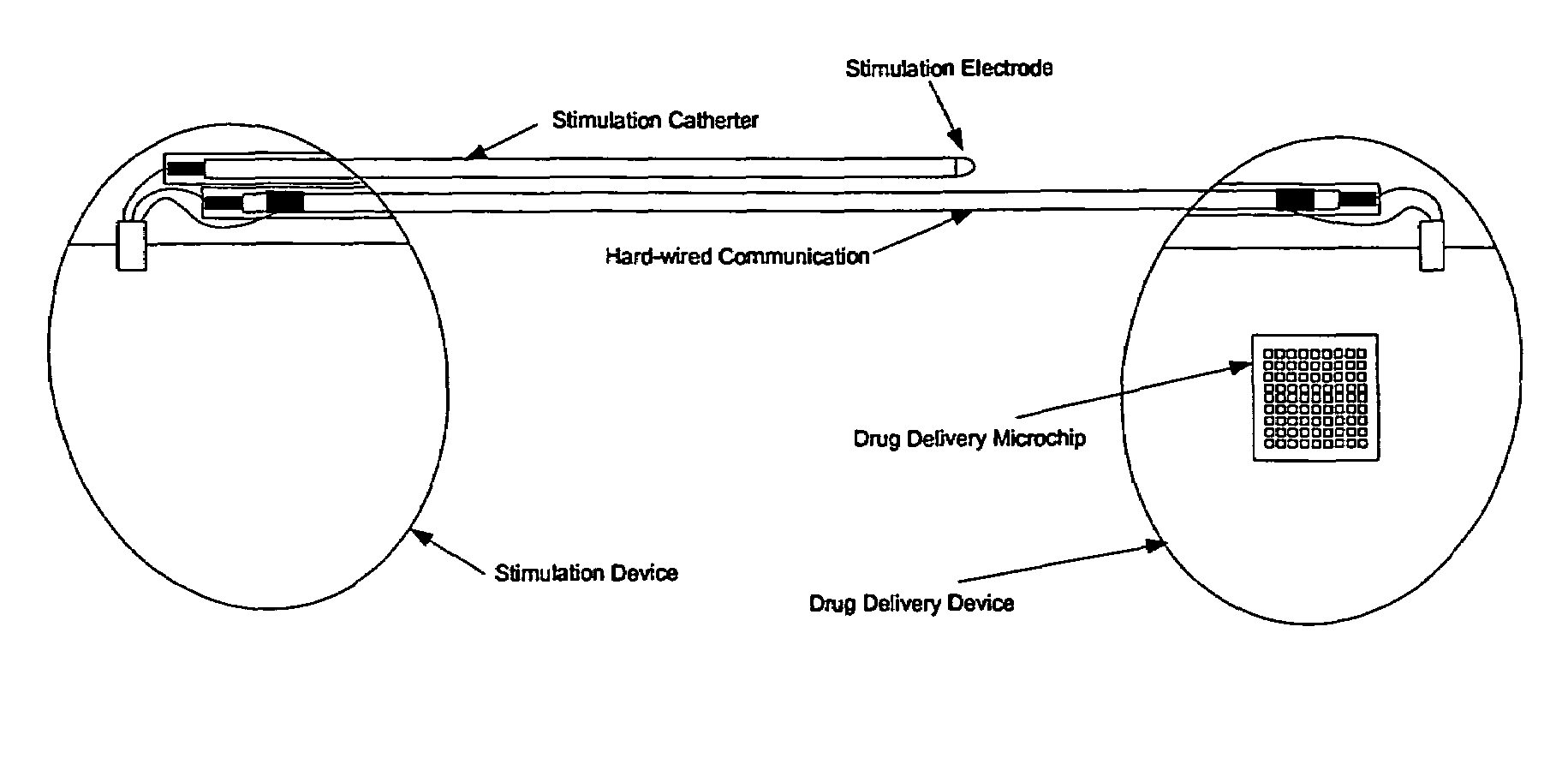
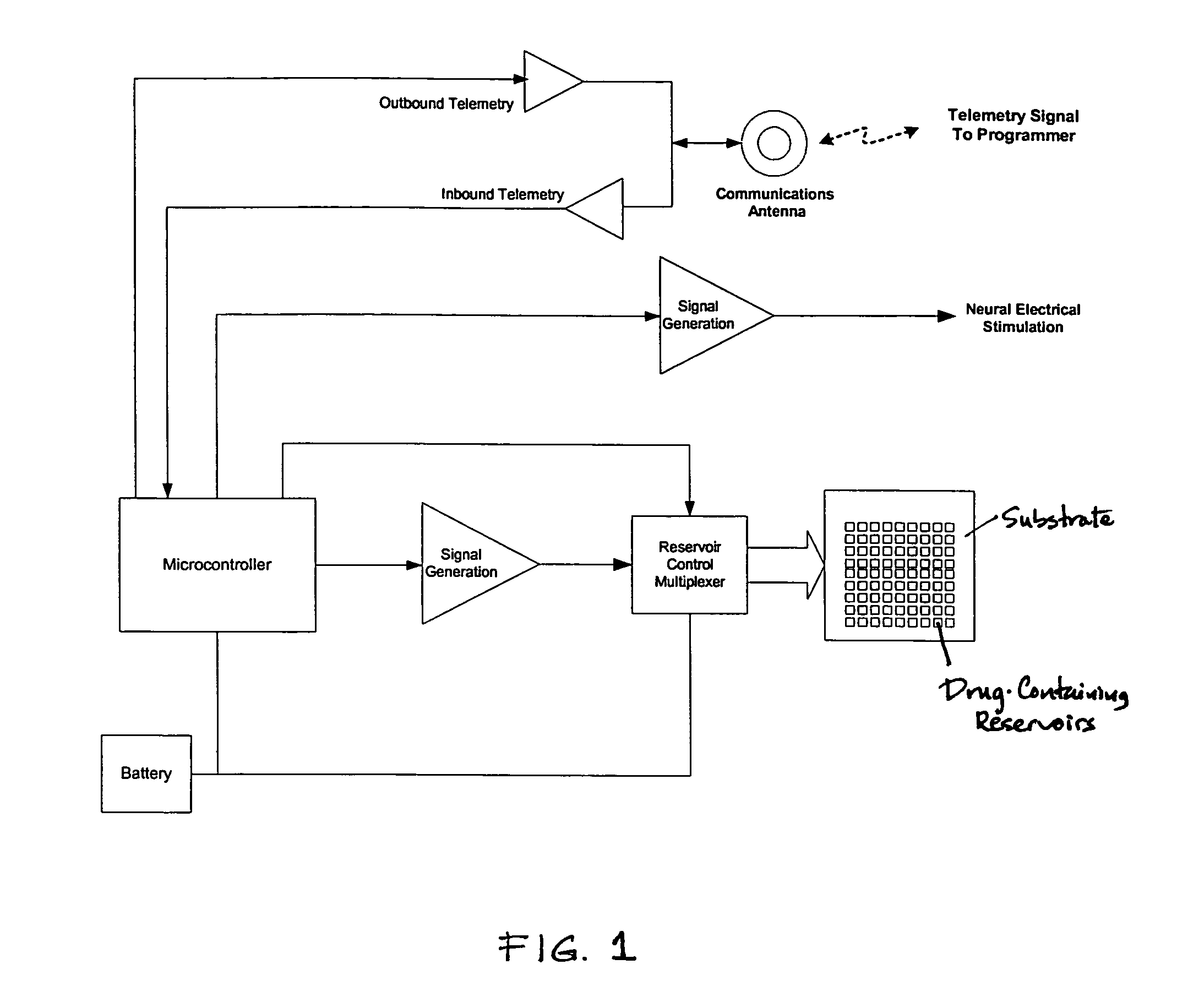
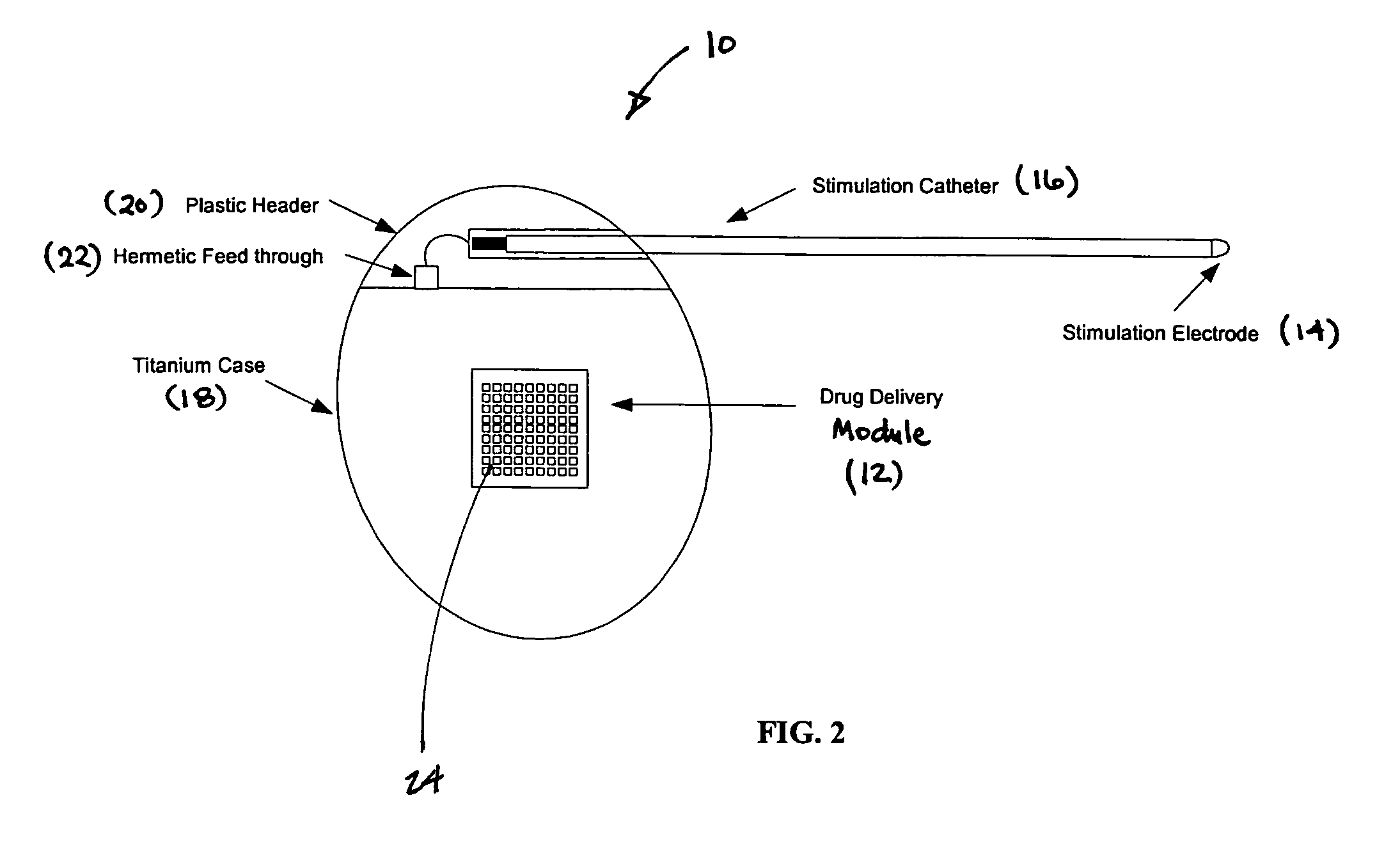
Medical device for neural stimulation and controlled drug delivery
InactiveUS7599737B2Reduce stimulation thresholdReduce stimulationElectrotherapyMicromachined deliveryMicrocontrollerElectrical stimulations
Medical devices and methods are provided for electrical stimulation of neural tissue and controlled drug delivery to a patient. The device includes an implantable drug delivery module which comprises a plurality of reservoirs, a release system comprising at least one drug contained in each of the reservoirs, and control means for selectively releasing a pharmaceutically effective amount of drug from each reservoir; a neural electrical stimulator which comprises a signal generator connected to at least one stimulation electrode for operable engagement with a neural tissue of the patient; and at least one microcontroller for controlling operational interaction of the drug delivery module and the neural electrical stimulator. The microcontroller may control the signal generator and the control means of the drug delivery module. The device may further include a sensor operable to deliver a signal to the microcontroller, for example to indicate when to deliver electrical stimulation, drug, or both.
Owner:DARE MB INC
Stent for controlled release of drug
Devices for the controlled release of one or more drugs into a patient are provided. The devices include an implantable stent, at least two reservoirs in the stent, and a release system contained in each of the at least two reservoirs, wherein the release system may have one or more drugs for release at a controlled rate. Reservoir caps optionally are provided to control the time at which release of the one or more drugs is initiated.
Owner:BOSTON SCI SCIMED INC
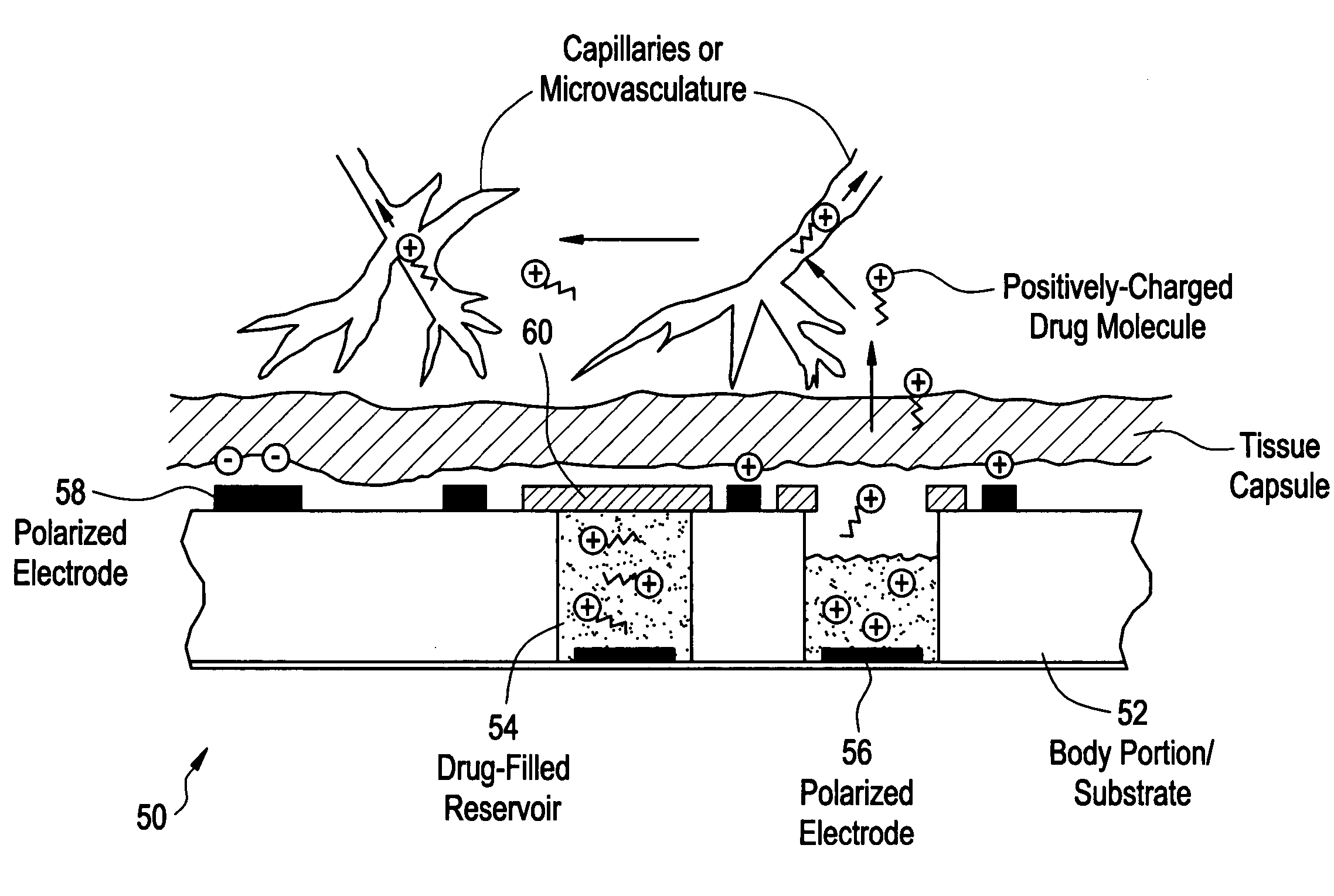
Devices and methods for measuring and enhancing drug or analyte transport to/from medical implant
InactiveUS20050267440A1Easy to transportEfficient propulsionElectrocardiographyAntipyreticControlled drugsAnalyte
Methods and devices are provided for enhancing mass transport through any fibrous tissue capsule that may form around an implanted medical device following implantation. Methods and devices are also provided to enhance vascularization around the implanted device, which also will aid in mass transport to / from the device. The device preferably comprises multiple reservoirs containing (i) a drug formulation for short- or long-term, controlled drug delivery, (ii) sensors for sensing an analyte in the patient, or (iii) a combination thereof.
Owner:MICROCHIPS INC
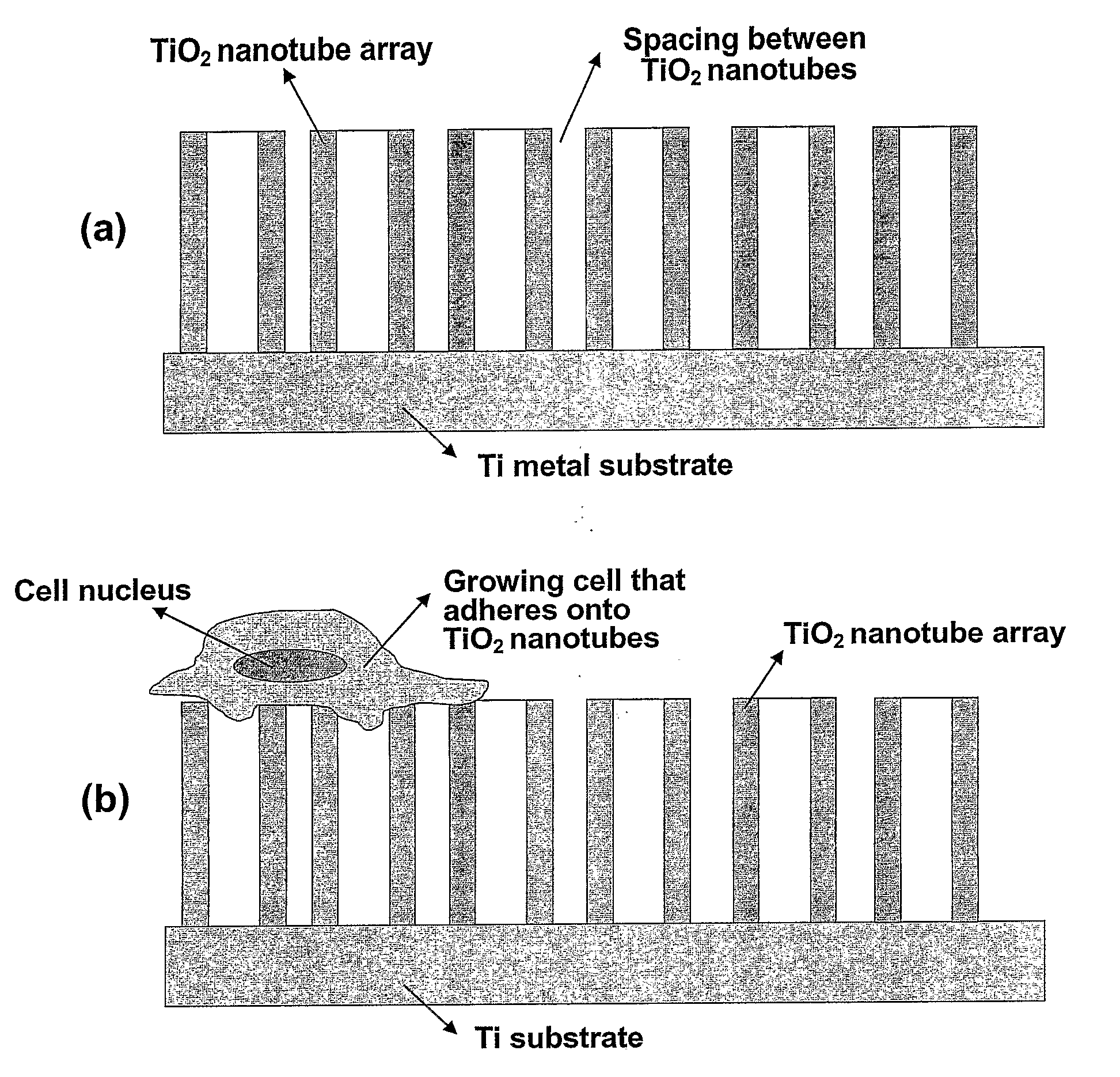
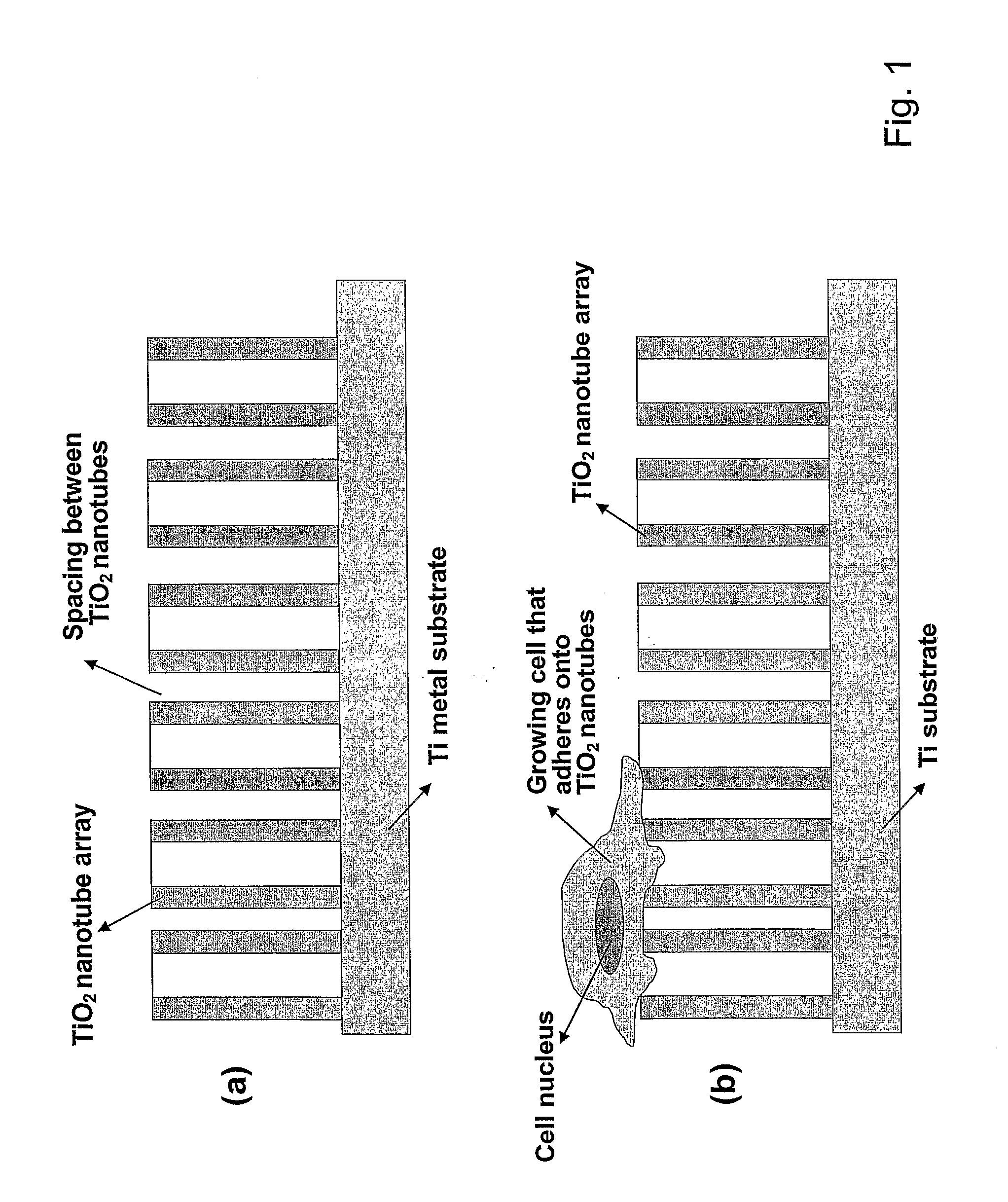
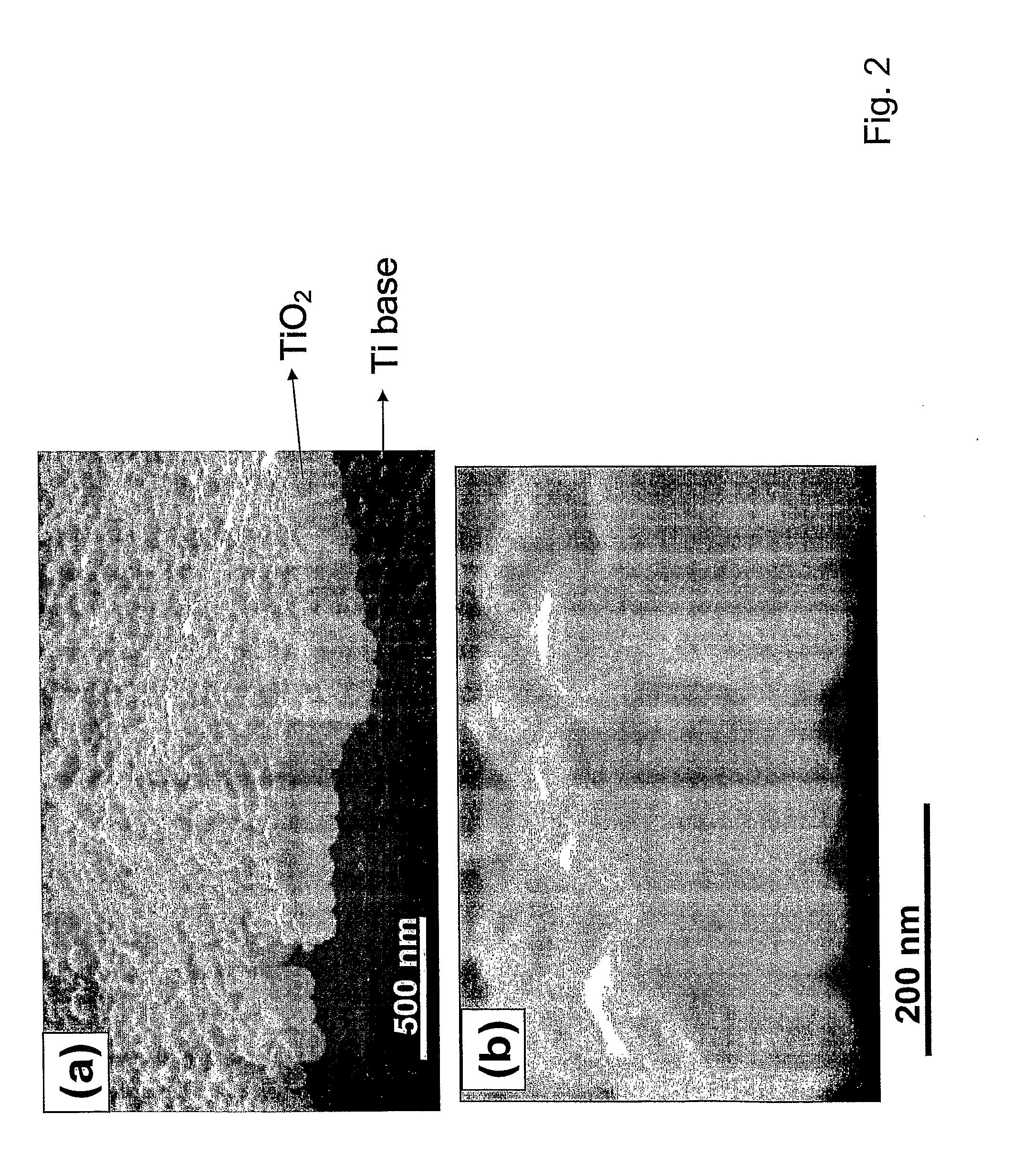
Compositions comprising nanostructures for cell, tissue and artificial organ growth, and methods for making and using same
ActiveUS20090220561A1Improve bone formationIncreased durabilityBioreactor/fermenter combinationsElectrolysis componentsIn vivoNanostructure
The invention provides articles of manufacture comprising biocompatible nanostructures comprising nanotubes and nanopores for, e.g., organ, tissue and / or cell growth, e.g., for bone, kidney or liver growth, and uses thereof, e.g., for in vitro testing, in vivo implants, including their use in making and using artificial organs, and related therapeutics. The invention provides lock-in nanostructures comprising a plurality of nanopores or nanotubes, wherein the nanopore or nanotube entrance has a smaller diameter or size than the rest (the interior) of the nanopore or nanotube. The invention also provides dual structured biomaterial comprising micro- or macro-pores and nanopores. The invention provides biomaterials having a surface comprising a plurality of enlarged diameter nanopores and / or nanotubes.
Owner:RGT UNIV OF CALIFORNIA
Radio frequency identification pharmaceutical tracking system and method
InactiveUS20060210626A1Effective treatmentQuickly and efficiently be identifiedDrug and medicationsSignalling system detailsOrogastric tubeRadio frequency
An ingestible dosage form includes a pill containing a pharmaceutical content and an RF tag associated with the pill. The RF tag is configured to output a wireless response signal in response to a wireless excitation signal received by the RF tag from an RF reader. The ingestible dosage form includes memory for storing pill identifying information. The wireless response signal includes the pill identifying information which may be received and processed by the RF reader. A system for monitoring patient medicine intake compliance includes a monitoring device placed within communicative proximity of ingestible dosage forms to transmit the wireless excitation signal and receive the wireless response signal including pill identifying information from the ingestible dosage forms. A device for determining an identity of a pill and quantity thereof ingested by an individual includes a nasogastric / orogastric tube having an RF tag reader attached thereto for detecting RF tagged pills.
Owner:SPAEDER JEFFREY A
Implantable drug delivery device
Implantable devices for controlled delivery of a drug are provided which include a substrate; at least two reservoirs in the substrate, each reservoir having an opening; at least one therapeutic agent in each of the reservoirs; a reservoir cap sealing each opening; a mechanical rupturing mechanism which moves into contact with and ruptures the reservoir cap to permit release the therapeutic agent from the reservoir through the opening; and a mixing chamber adjacent the reservoirs, wherein upon release of the therapeutic agent from at least one of the reservoirs, the therapeutic agent is combined with a carrier fluid in the mixing chamber and then transported to a delivery site in a human or animal.
Owner:BOSTON SCI SCIMED INC
Low temperature methods for hermetically sealing reservoir devices
Methods are provided for hermetically sealing an opening in a reservoir of a containment device. The method comprises applying a polymeric material to an opening in a reservoir of a containment device, the reservoir comprising reservoir contents (such as a drug or a sensor) to be hermetically isolated within the reservoir, the applied polymeric material closing off the opening and forming a temporary seal; and adhering a hermetic sealing material onto the polymeric material to hermetically seal the opening. The reservoir can be a micro-reservoir. The containment device can comprises an array of two or more of reservoirs, and the method comprises hermetically sealing each of the two or more reservoirs.
Owner:MICROCHIPS BIOTECH INC
Prolonged Transit Time of Permeability-Enhancing Drug Eluting Pill
InactiveUS20080275430A1Promote absorptionShorten speedMedical devicesMicromachined deliveryPower flowMedicine
Apparatus is provided for drug administration. The apparatus includes an ingestible capsule, which includes a drug, stored by the capsule. The apparatus also includes an environmentally-sensitive mechanism, adapted to change a state thereof responsively to a disposition of the capsule within a gastrointestinal (GI) tract of a subject; one or more drug-passage facilitation electrodes; and a control component, adapted to facilitate passage of the drug, in response to a change of state of the environmentally-sensitive mechanism, by driving the drug-passage facilitation electrodes to apply an electrical current. The apparatus further includes a velocity-reduction element adapted to reduce a velocity of the capsule through the GI tract for at least a portion of the time that the control component is facilitating the passage of the drug. Additional embodiments are also described.
Owner:E PILL PHARMA
MEMS device and method for delivery of therapeutic agents
An implantable device for delivering a therapeutic agent to a patient is provided. The device includes a reservoir configured to contain a liquid comprising the therapeutic agent. The device further includes a cannula in fluid communication with the reservoir. The cannula has an outlet configured to be in fluid communication with the patient. The device further includes a first electrode and a second electrode, at least one of the first electrode and the second electrode is planar. The device further includes a material in electrical communication with the first and second electrodes. A voltage applied between the first electrode and the second electrode produces gas from the material, the gas forcing the liquid to flow from the reservoir to the outlet.
Owner:UNIV OF SOUTHERN CALIFORNIA
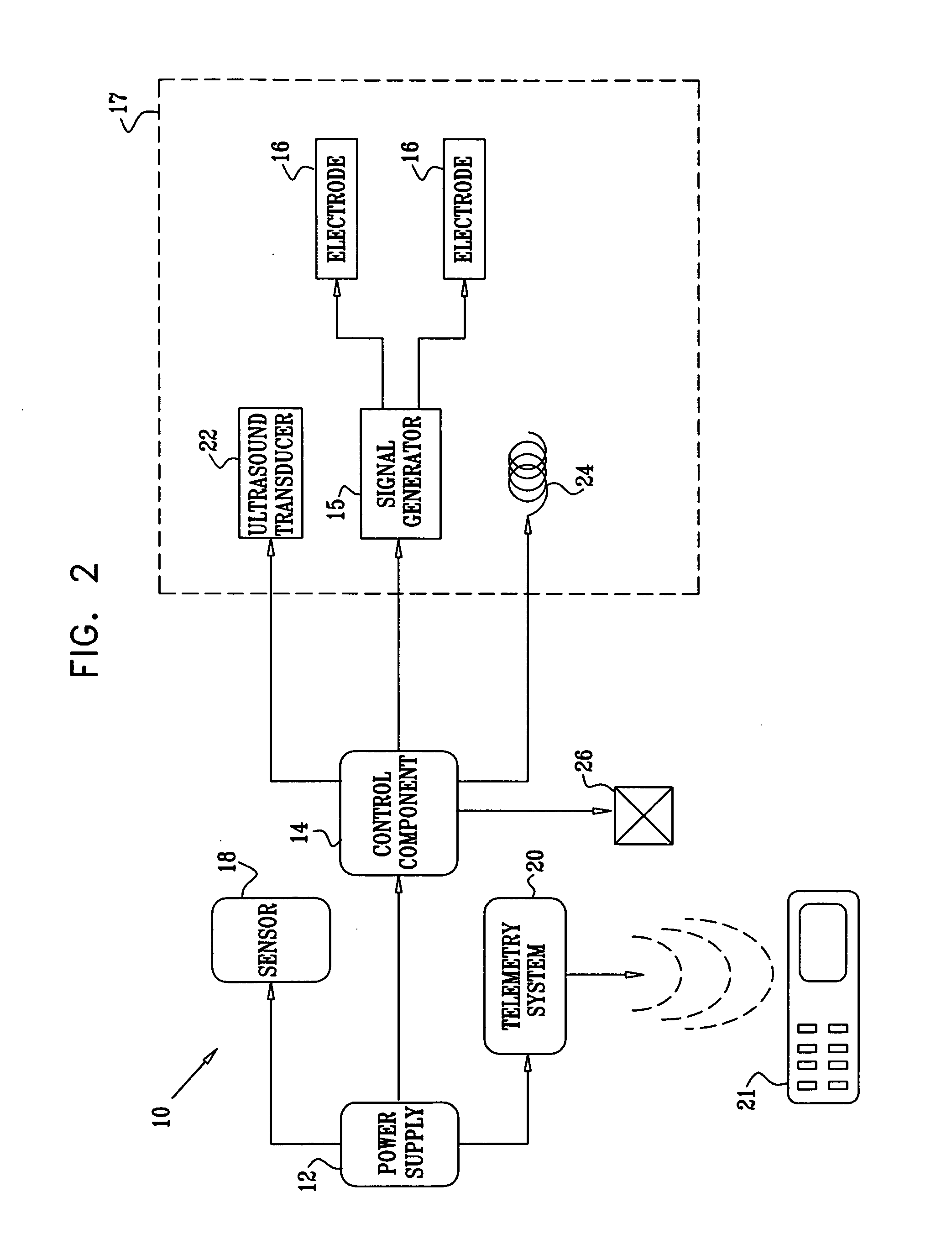
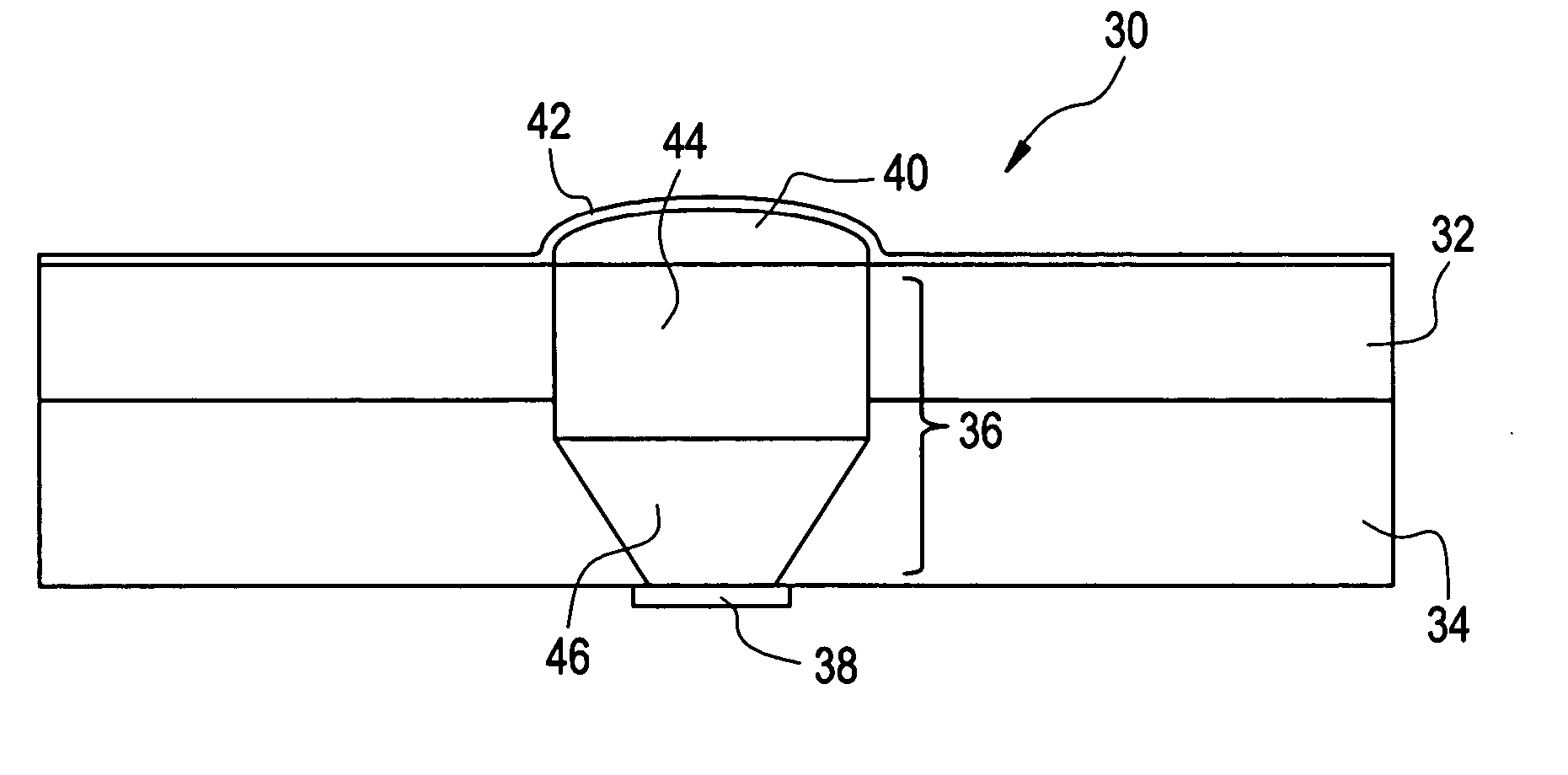
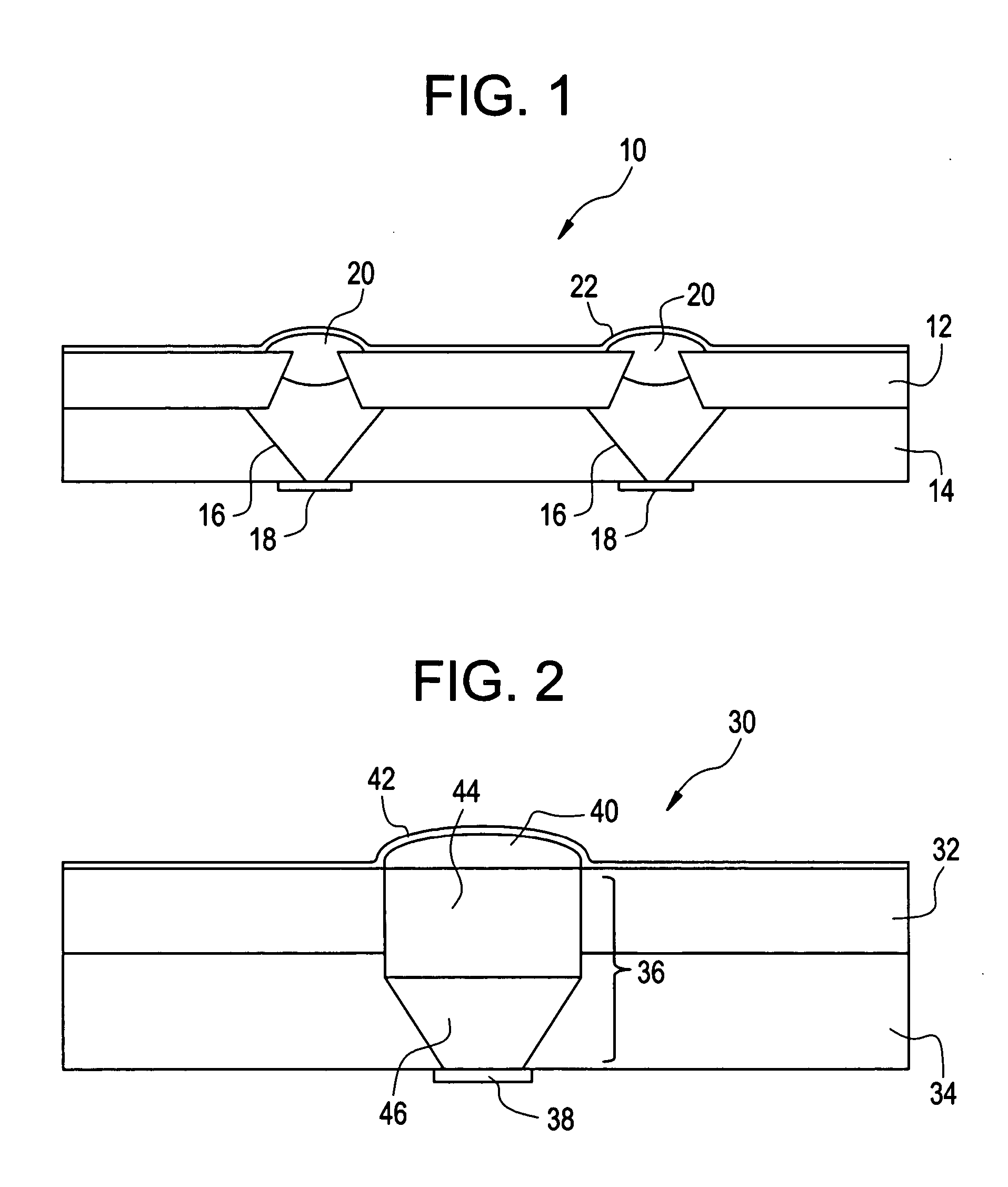
Active Drug Delivery in the Gastrointestinal Tract
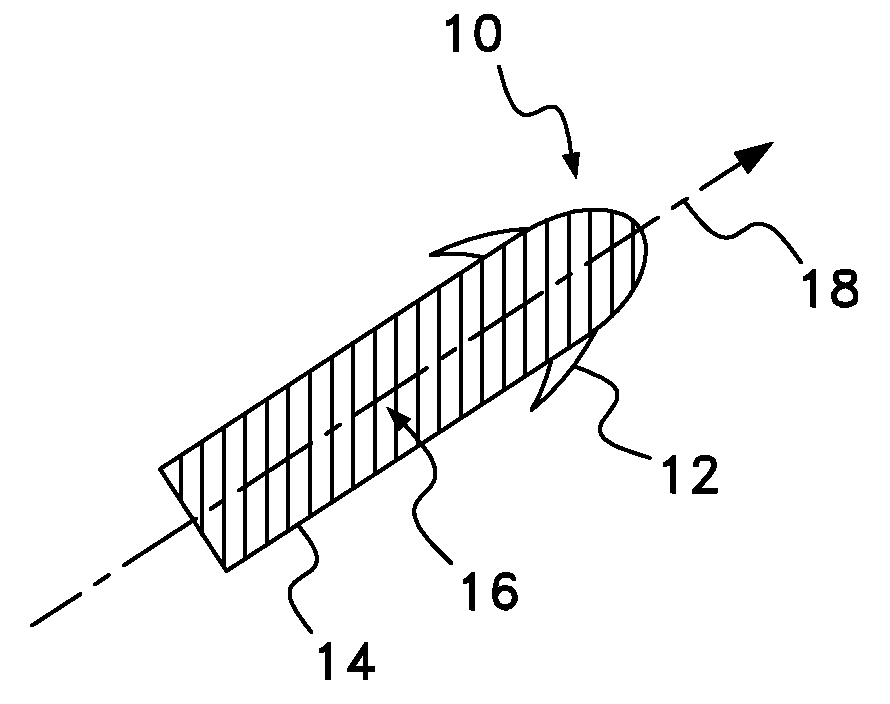
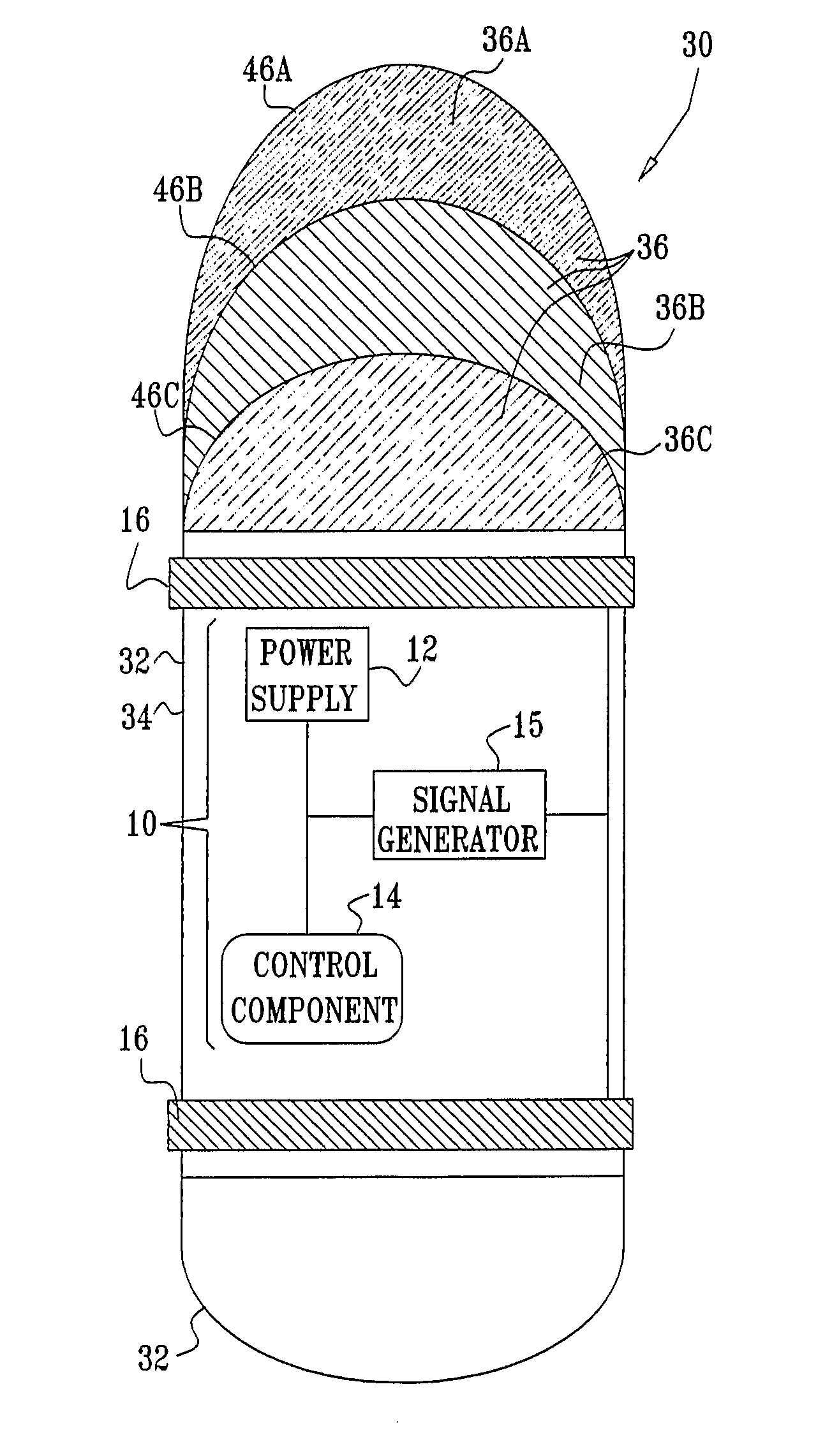
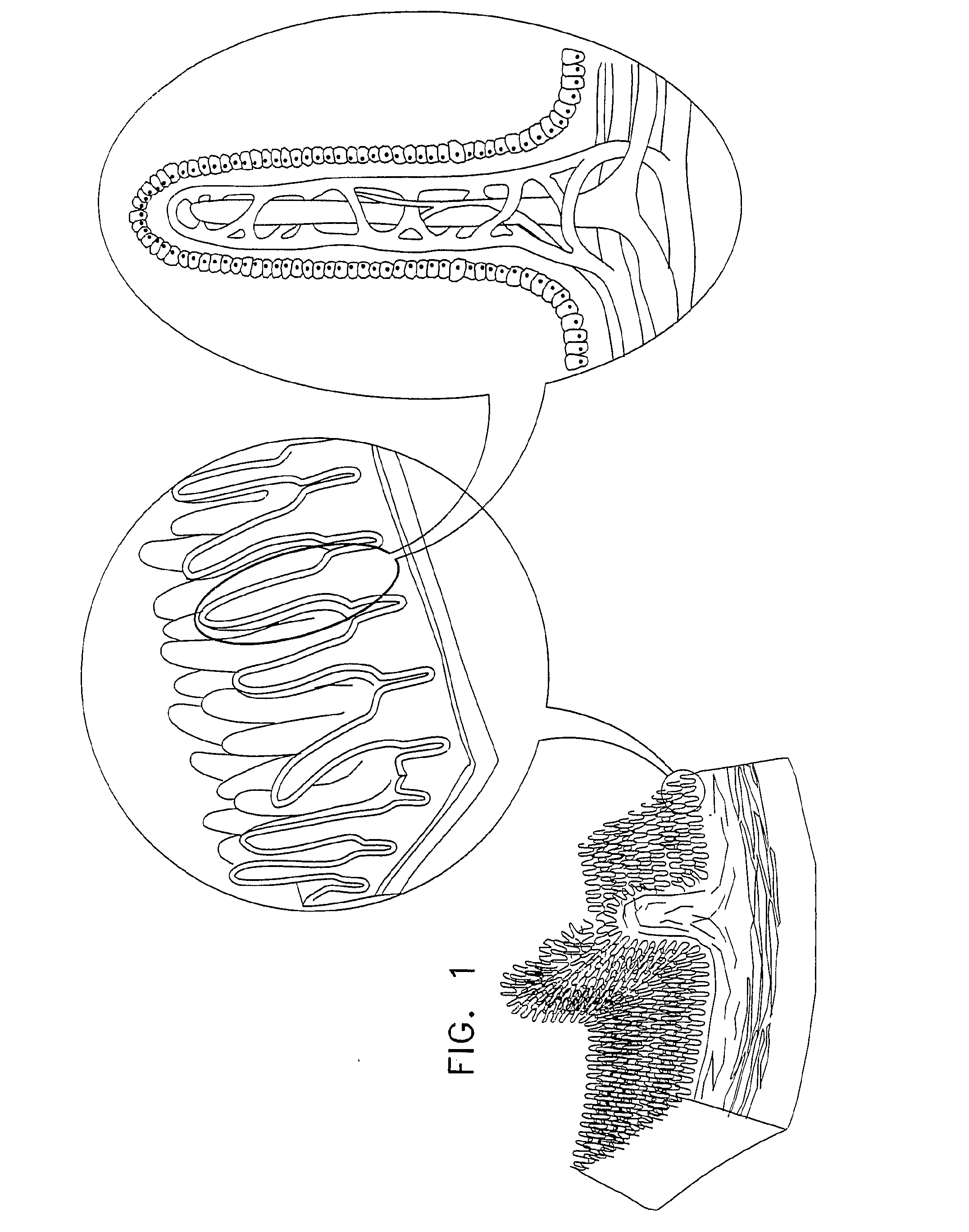
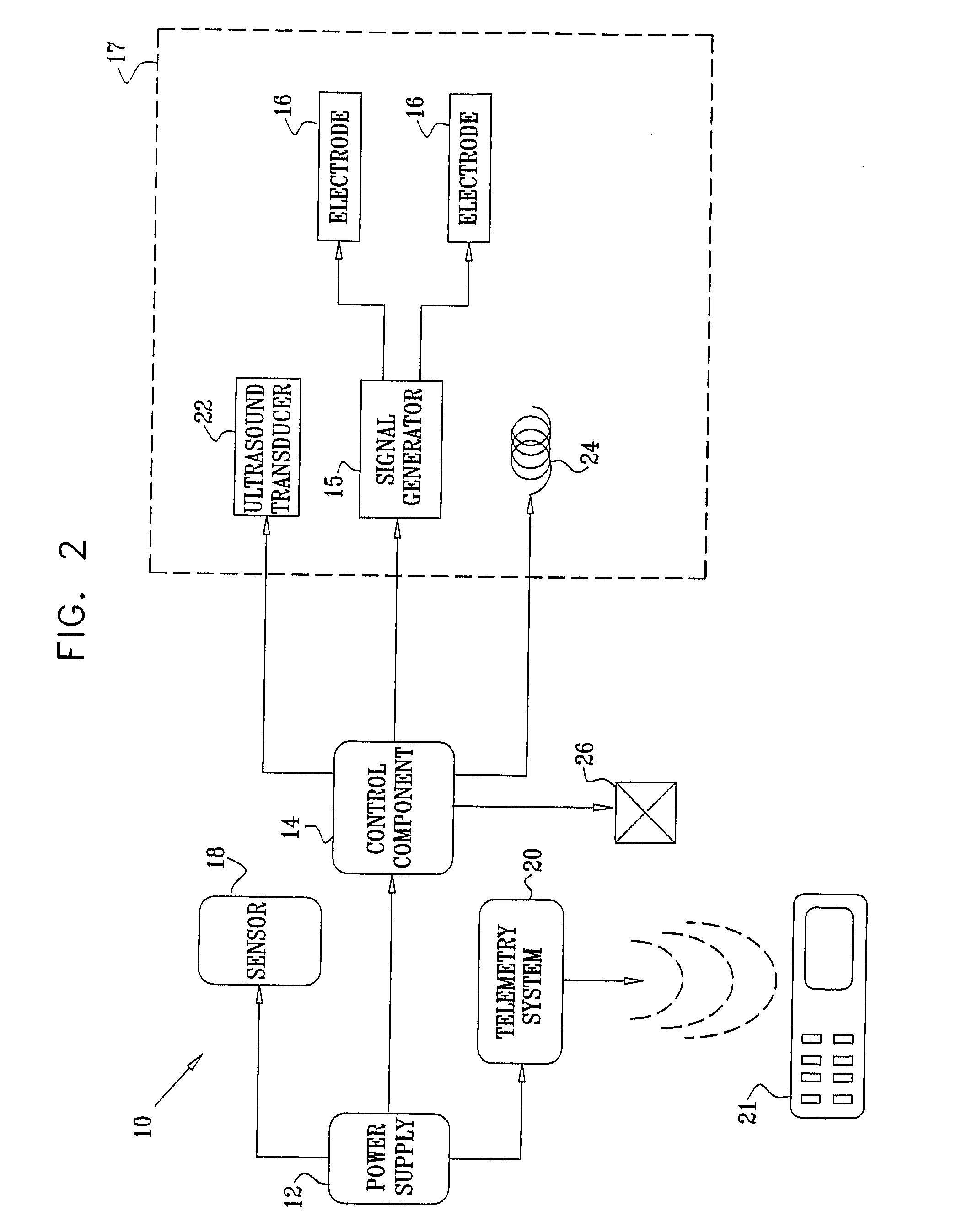
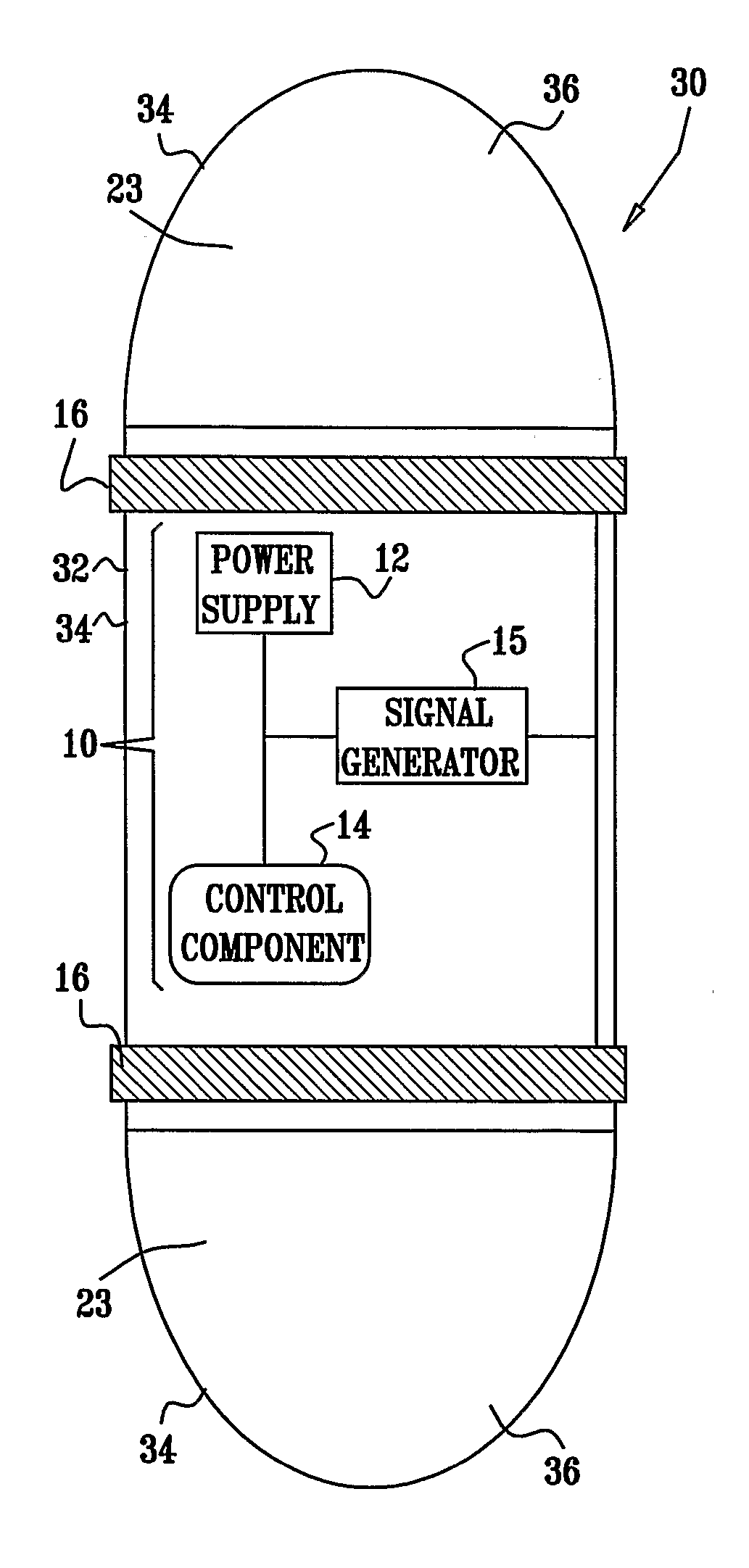
InactiveUS20080063703A1Promote absorptionEasy accessInternal electrodesMedical devicesMedicineDrug administration
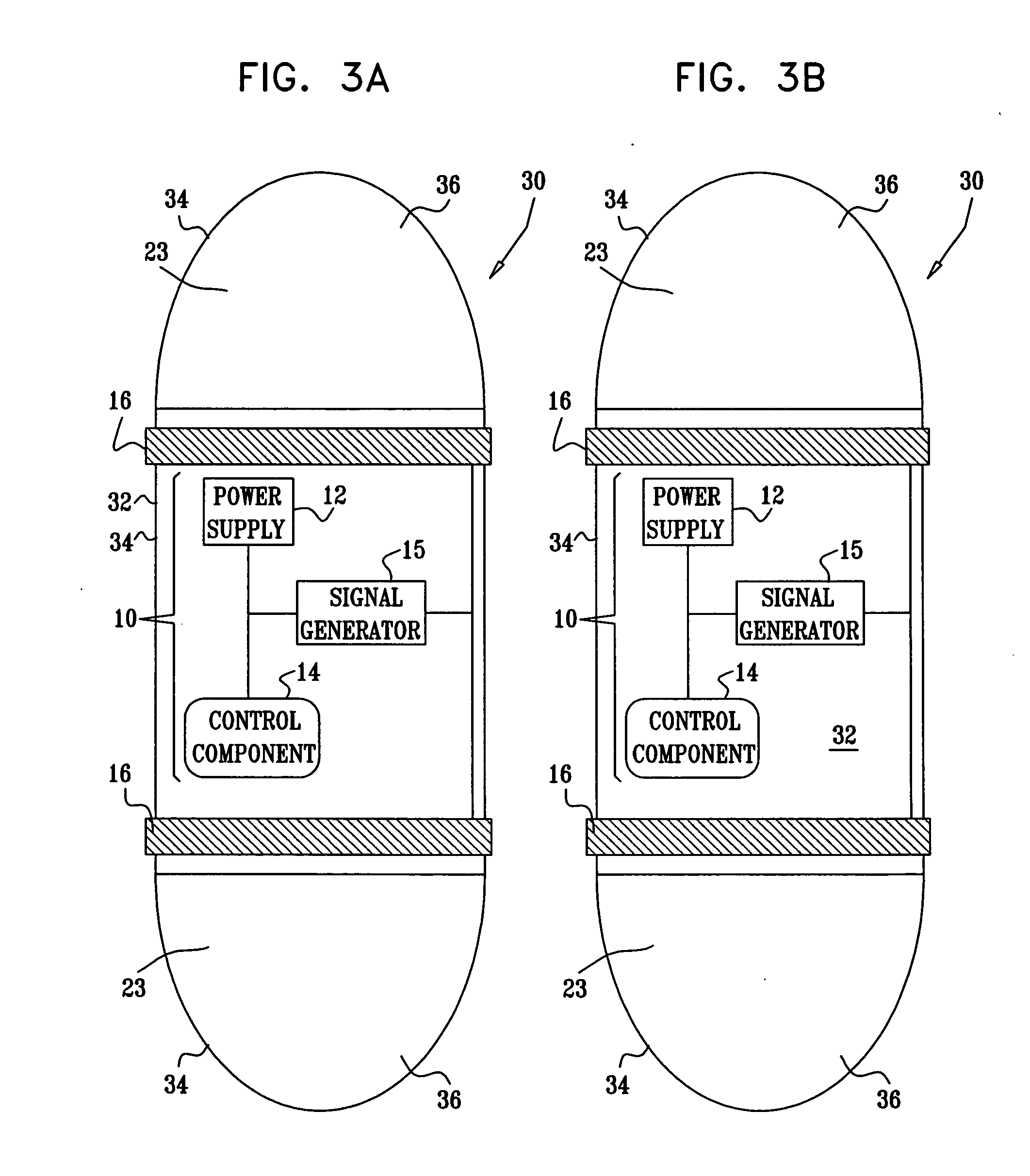
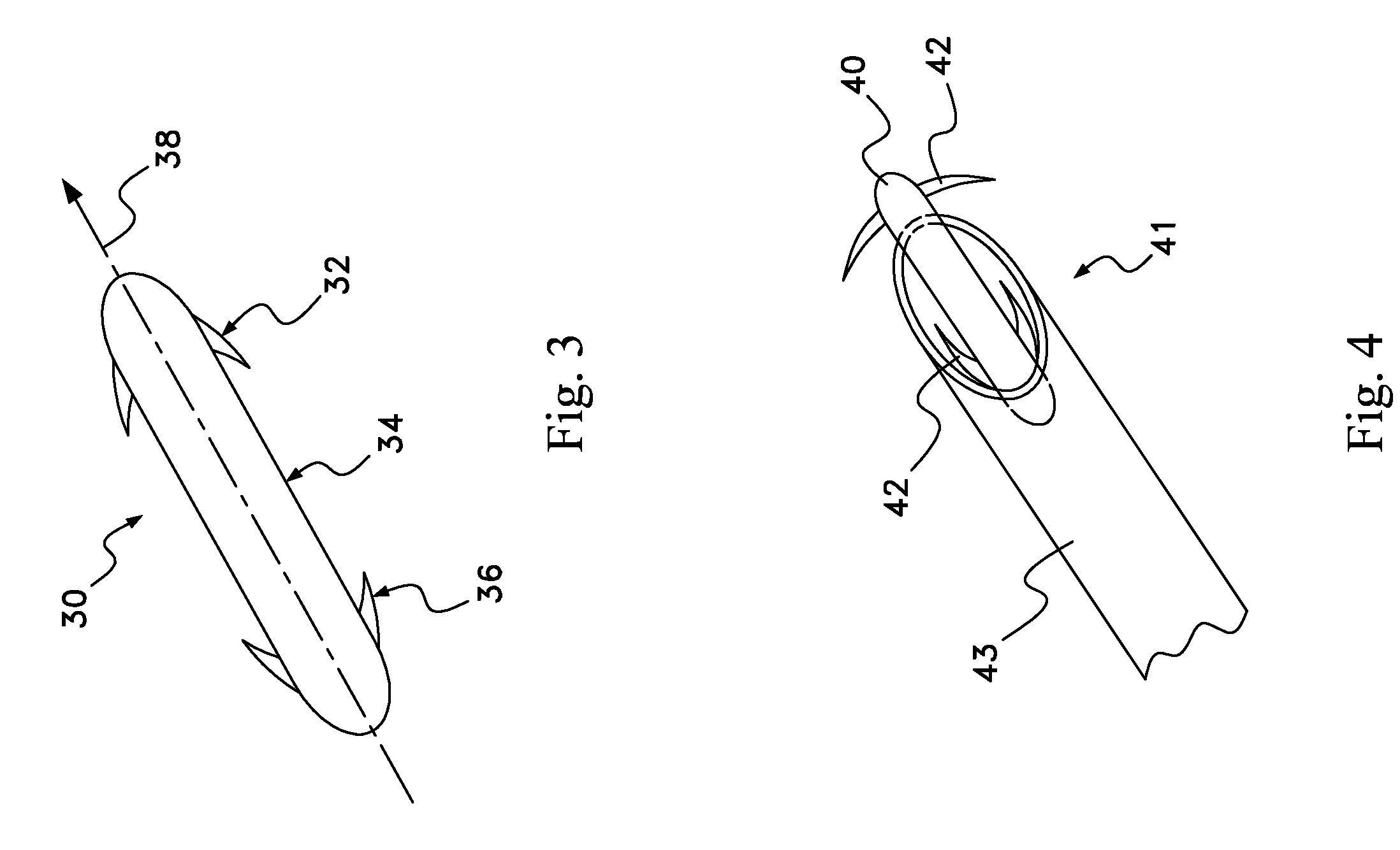
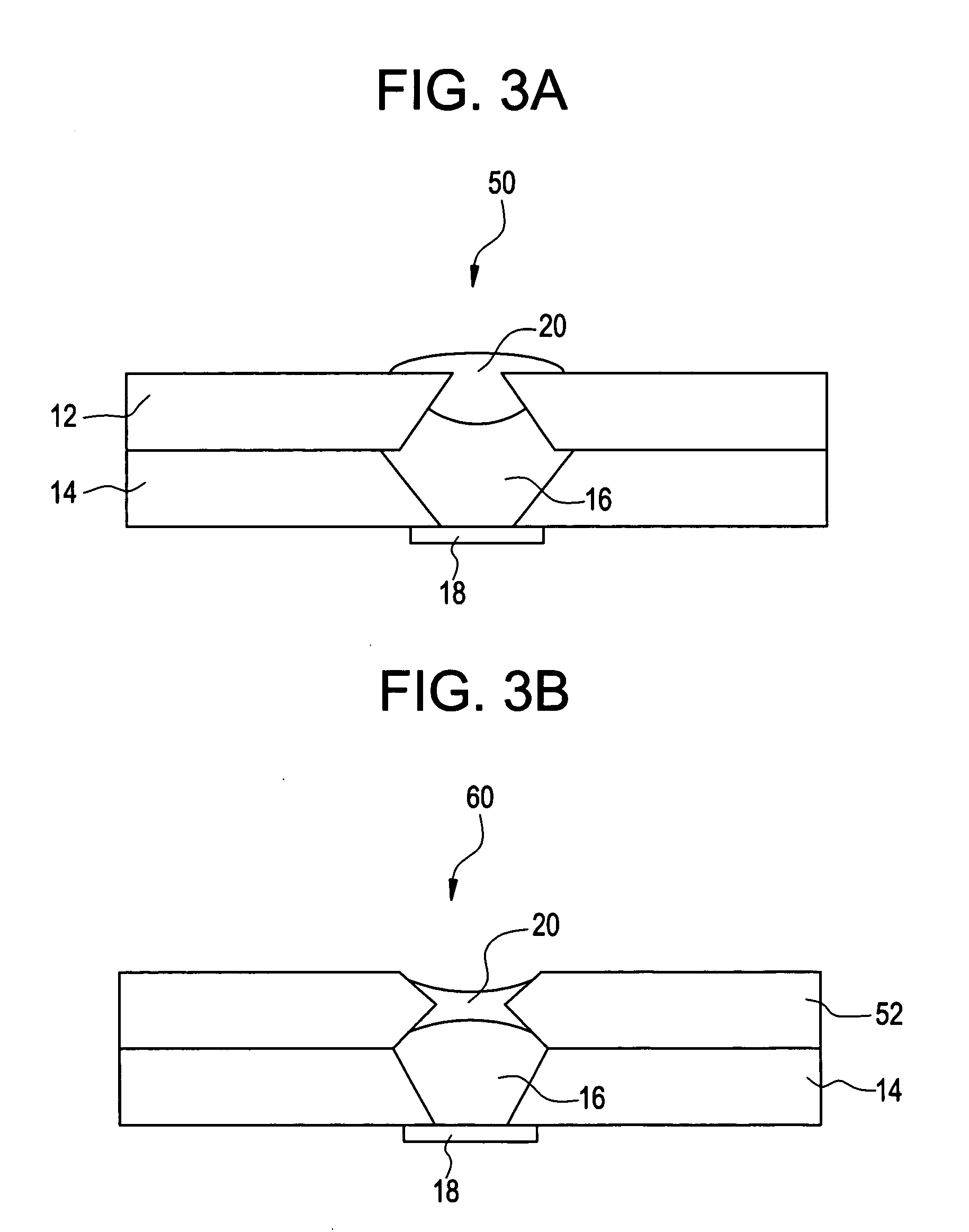
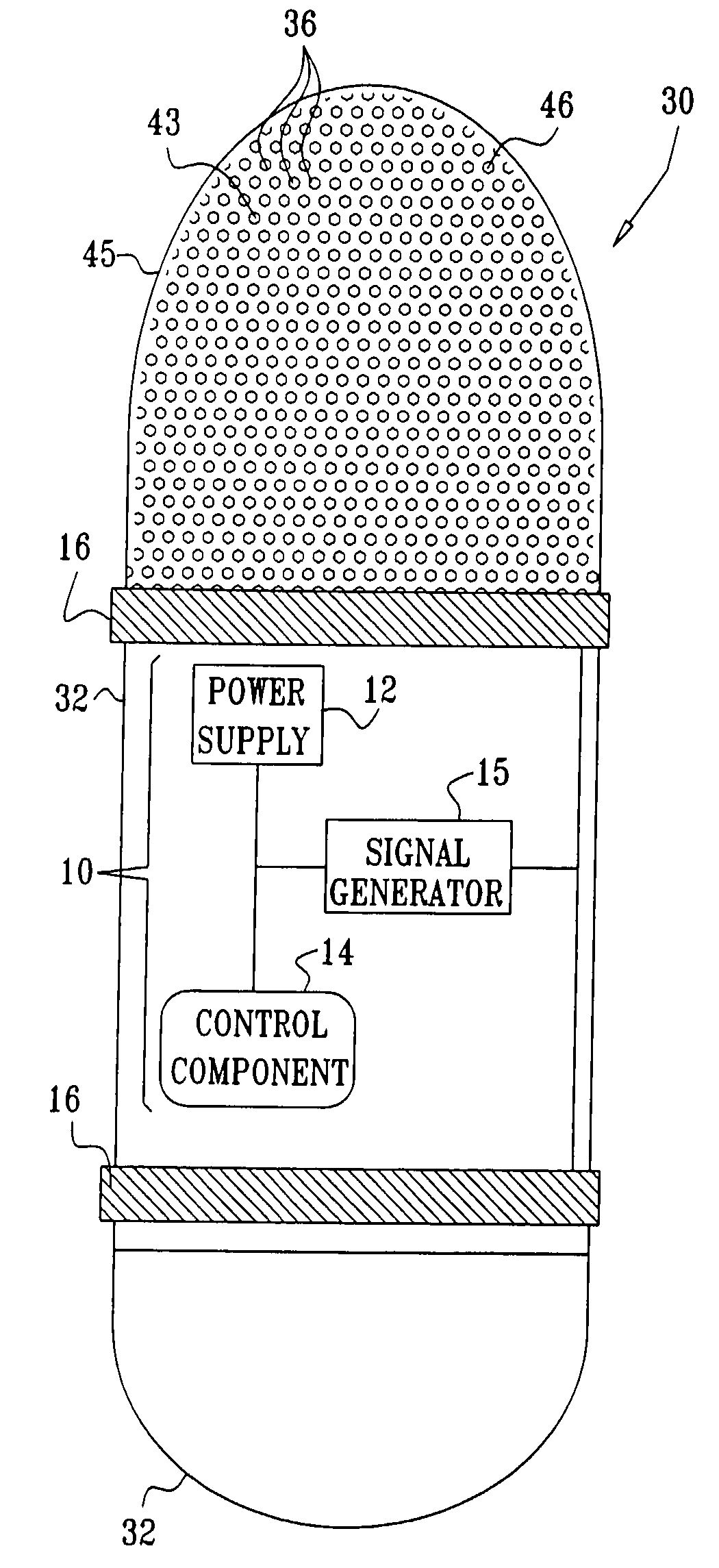
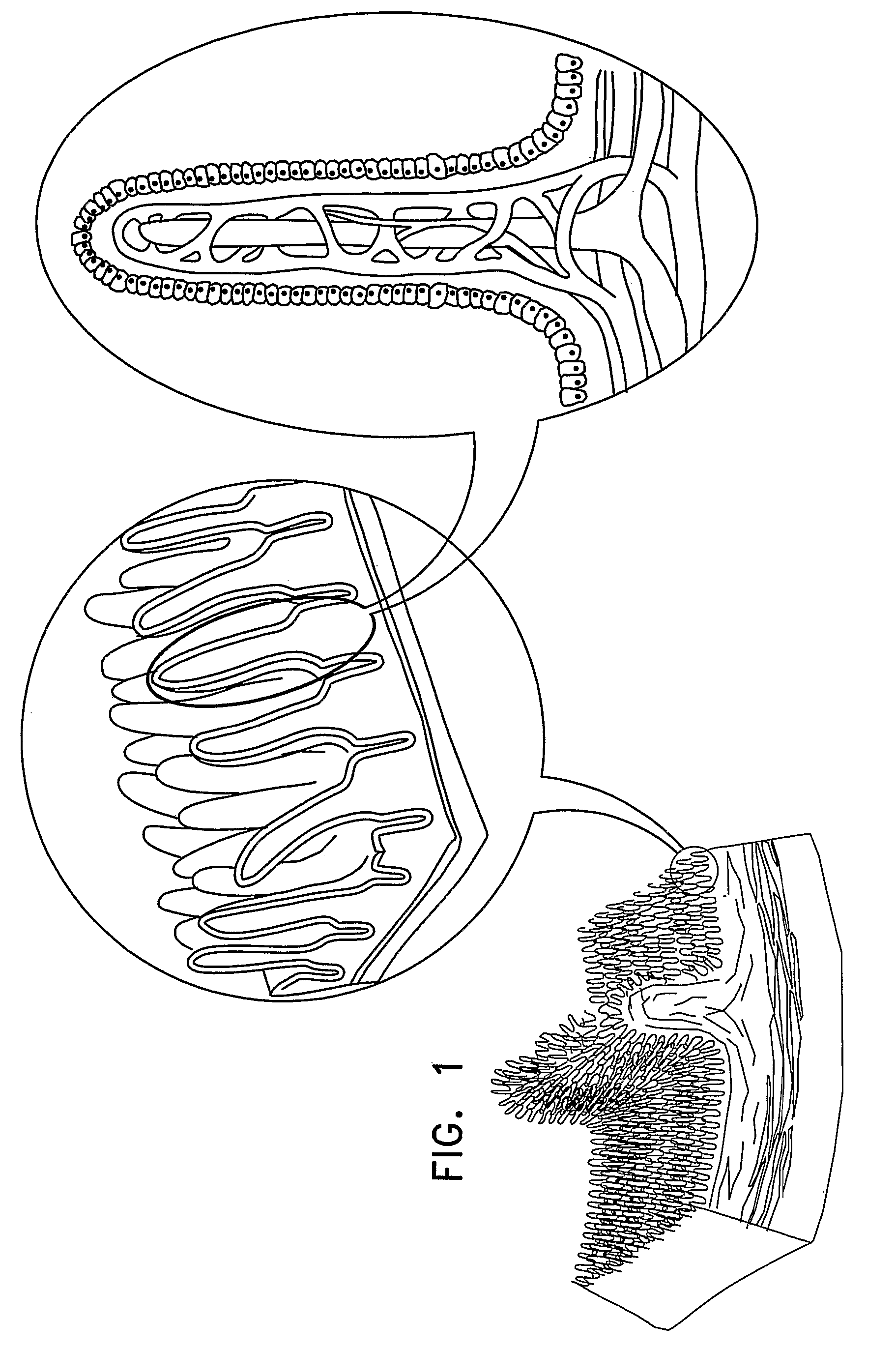
Apparatus (30) for drug administration is provided, including an ingestible capsule (32), which includes a drug (36), stored by the capsule (32), and an environmentally-sensitive mechanism (18), adapted to change a state thereof responsively to a disposition of the capsule (32) within a gastrointestinal (GI) tract (50) of a subject. The capsule (32) further includes first and second electrodes (16), and a control component (14), adapted to facilitate passage of the drug (36), in response to a change of state of the environmentally-sensitive mechanism (18), through an epithelial layer of the GI tract (50) by driving the first and second electrodes (16) to apply a series of pulses at a current of less than about 10 mA, at a frequency of between about 12 Hz and about 24 Hz, and with a pulse duration of between about 0.5 milliseconds and about 3 milliseconds. Other embodiments are also described.
Owner:E PILL PHARMA
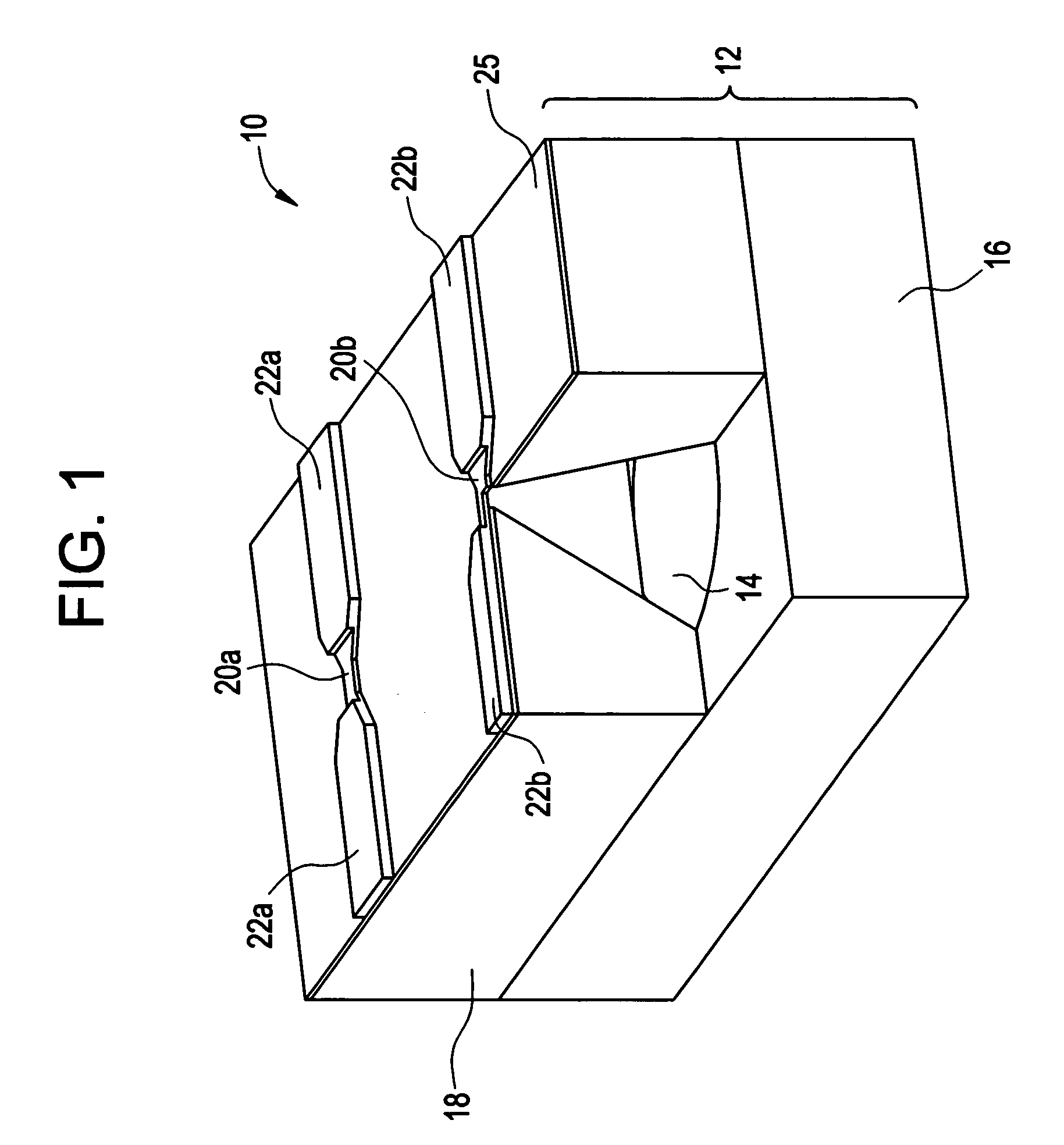
Devices for intrabody delivery of molecules and systems and methods utilizing same
InactiveUS20040032187A1Piezoelectric/electrostriction/magnetostriction machinesMicromachined deliveryControl releaseTransducer
A device for controlled release of molecules is provided. The device including: (a) a device body having at least one reservoir therein for containing the molecules, the at least one reservoir being formed with a barrier impermeable to the molecules thereby preventing release thereof from the at least one reservoir; and (b) at least one acoustic transducer being attached to, or forming a part of, the device body, the at least one acoustic transducer being for converting an acoustic signal received thereby into an electrical signal, the electrical signal leading to barrier permeabilization and therefore release of the molecules from the at least one reservoir.
Owner:REMON MEDICAL TECH
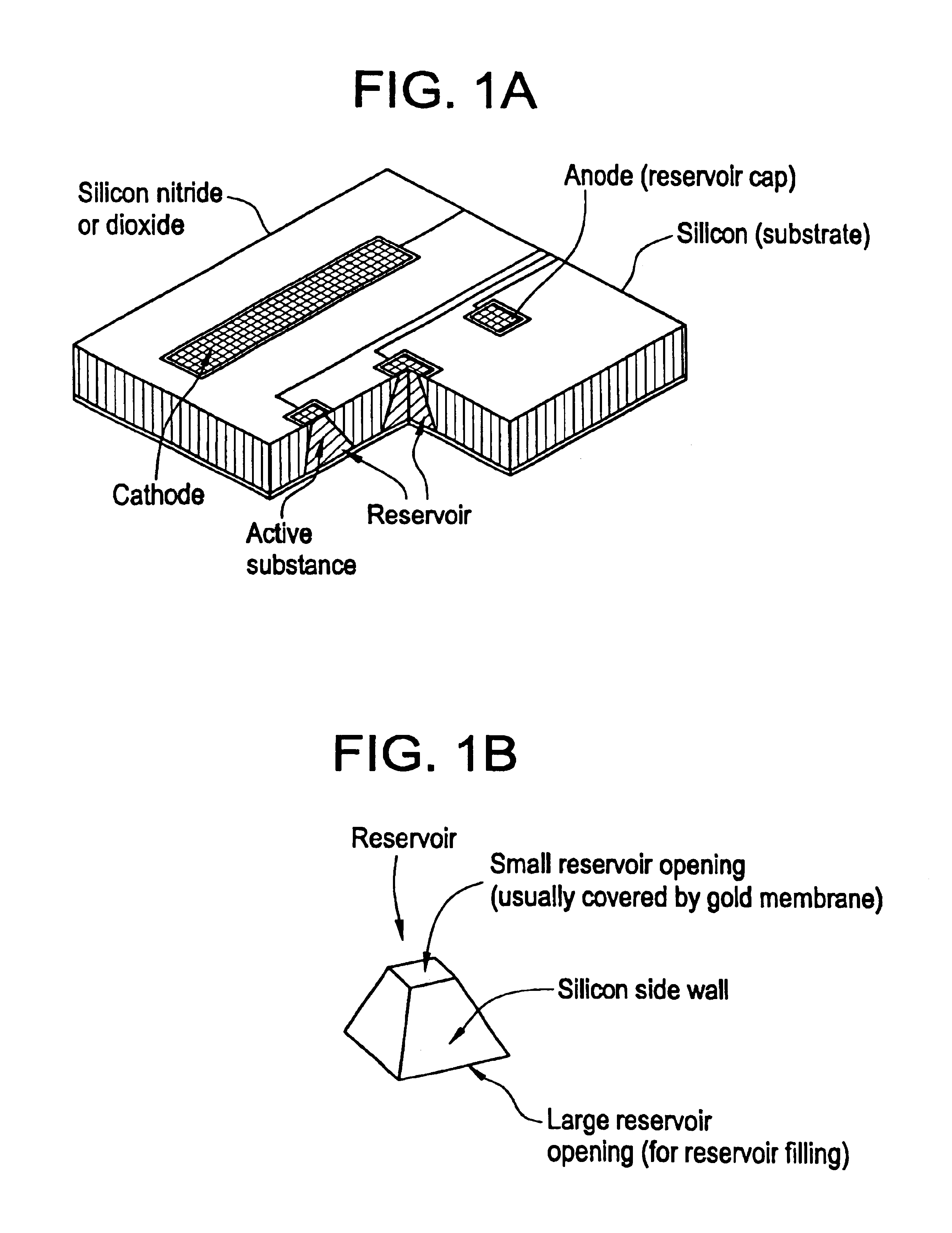
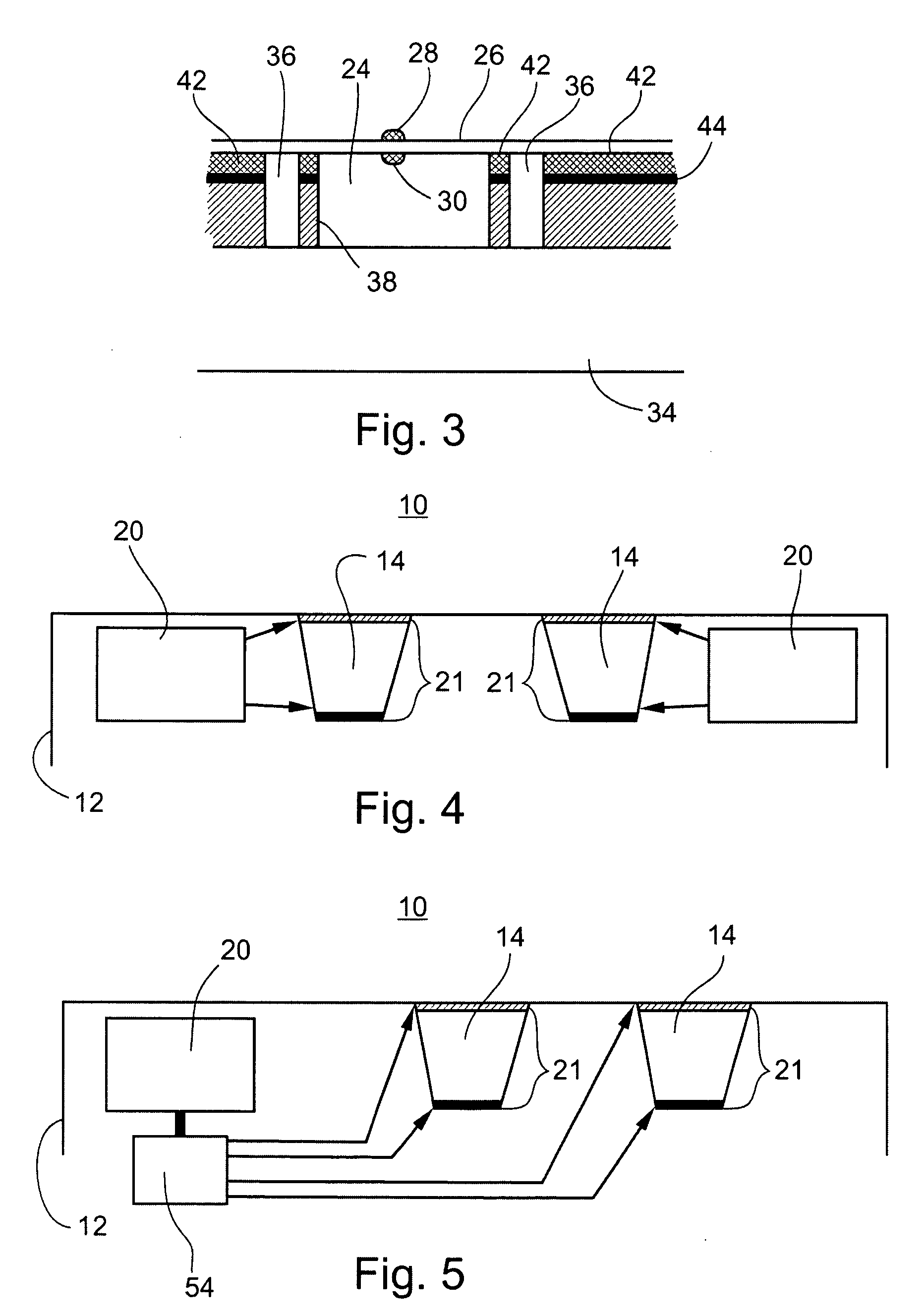
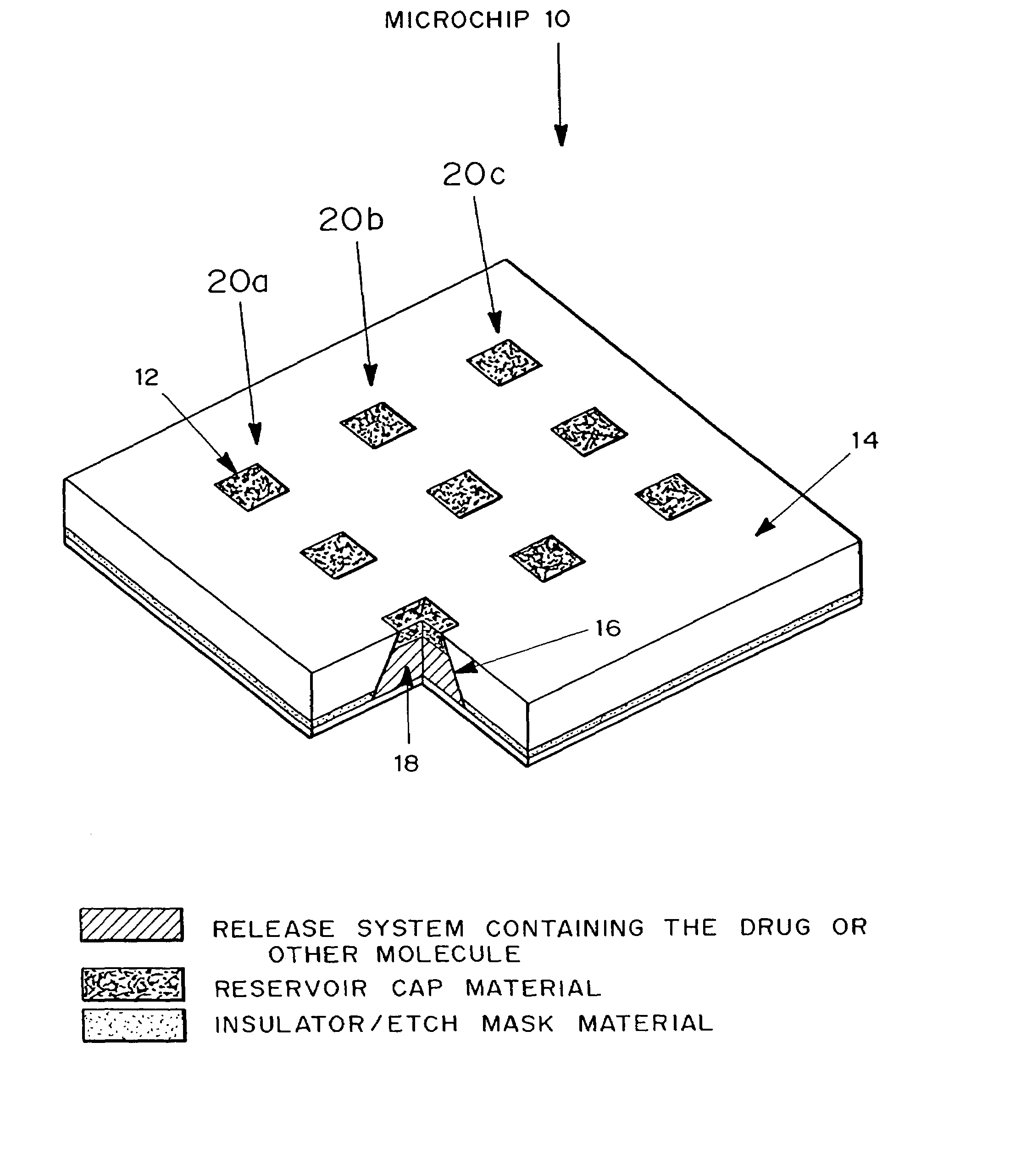
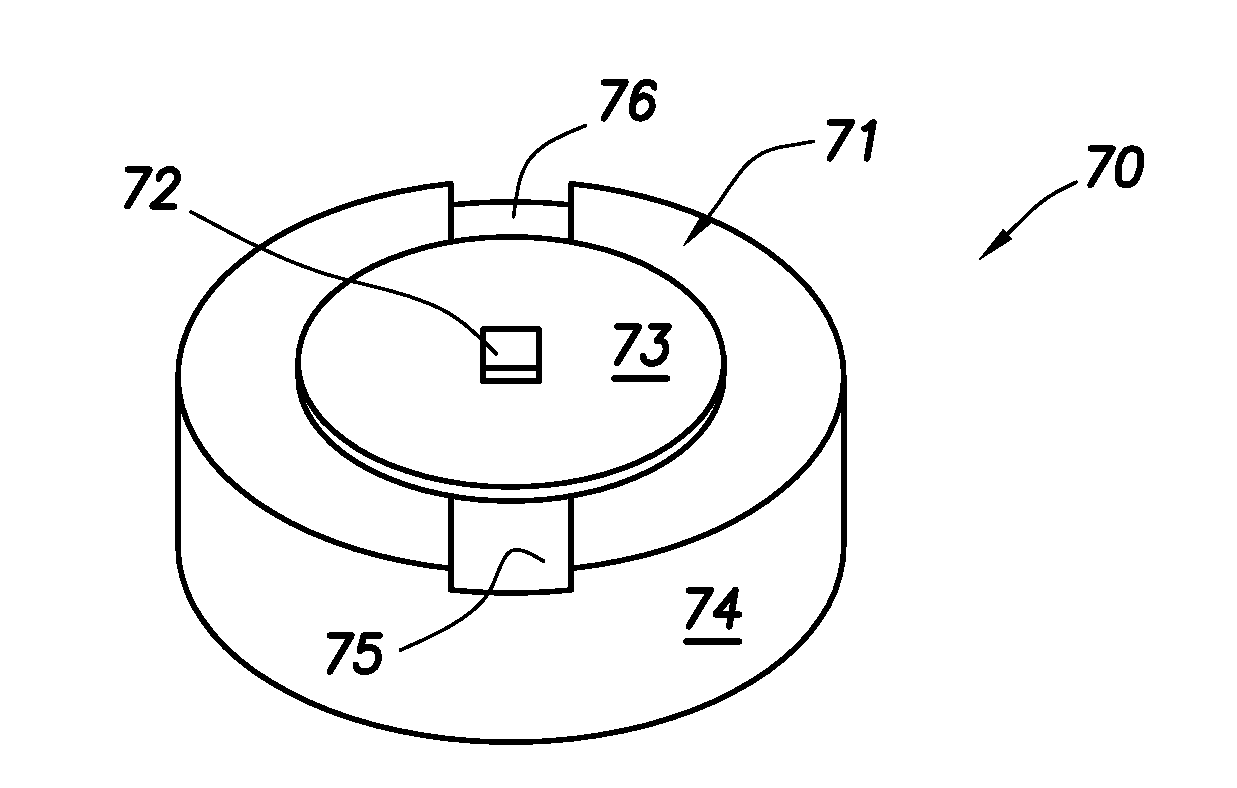
Microchip drug delivery devices
Devices are provided for the controlled release of drug or other molecules. The devices include (1) a substrate, which optionally includes two or more substrate portions bonded together, (2) at least two reservoirs in the substrate, (3) a release system disposed in the reservoirs that includes the molecules for release and optionally a matrix material, and (4) active or passive means for controlling release of the molecules from the reservoirs. In one embodiment, a reservoir cap is positioned on, or within a portion of, the reservoir and over the molecules, so that the molecules are controllably released from the device by diffusion through or upon disintegration of the reservoir cap.
Owner:MASSACHUSETTS INST OF TECH
Implantable, tissue conforming drug delivery device
A drug delivery device for implantation into a patient is provided that includes at least one device element which comprises a substrate having a plurality of discrete reservoirs disposed therein, said reservoirs containing drug molecules and a degradable matrix material which controls in vivo release of the drug molecules from the reservoirs, wherein the drug delivery device is adapted to conform to a curved bone tissue surface. In one embodiment, the substrate is flexible and conforms to a bone tissue surface. In another embodiment, the device has two or more device elements attached to or integral with a flexible supporting layer.
Owner:MICROCHIPS BIOTECH INC
Highly reliable ingestible event markers and methods for using the same
ActiveUS20110054265A1Improve reliabilityTransmission systemsCell seperators/membranes/diaphragms/spacersDiagnostic agentActive agent
Ingestible event markers having high reliability are provided. Aspects of the ingestible event markers include a support, a control circuit, a first electrochemical material, a second electrochemical material and a membrane. In addition, the ingestible event markers may include one or more components that impart high reliability to the ingestible event marker. Further, the ingestible event markers may include an active agent. In some aspects, the active agent, such as a pharmaceutically active agent or a diagnostic agent may be associated with the membrane.
Owner:OTSUKA PHARM CO LTD
Method and device for the controlled delivery of parathyroid hormone
InactiveUS7497855B2Efficiently providePeptide/protein ingredientsMicromachined deliveryOsteopoikilosisMedical device
Method and devices are provided for extended and controlled delivery of parathyroid hormone to a patient. The method includes implanting a medical device into the patient, the medical device comprising a substrate, a plurality of reservoirs in the substrate, a release system contained in each of the reservoirs, wherein the release system comprises parathyroid hormone; and controllably releasing a pharmaceutically effective amount of the parathyroid hormone from the reservoirs. The parathyroid hormone can be released intermittently, such as once daily over an extended period (e.g., two months, ten months, or more.). The device can further include reservoirs containing a bone resorption inhibitor or other drug for release. The devices are useful in delivering PTH for the treatment and prevention of bone loss, such as associated with osteoporosis.
Owner:MICROCHIPS BIOTECH INC
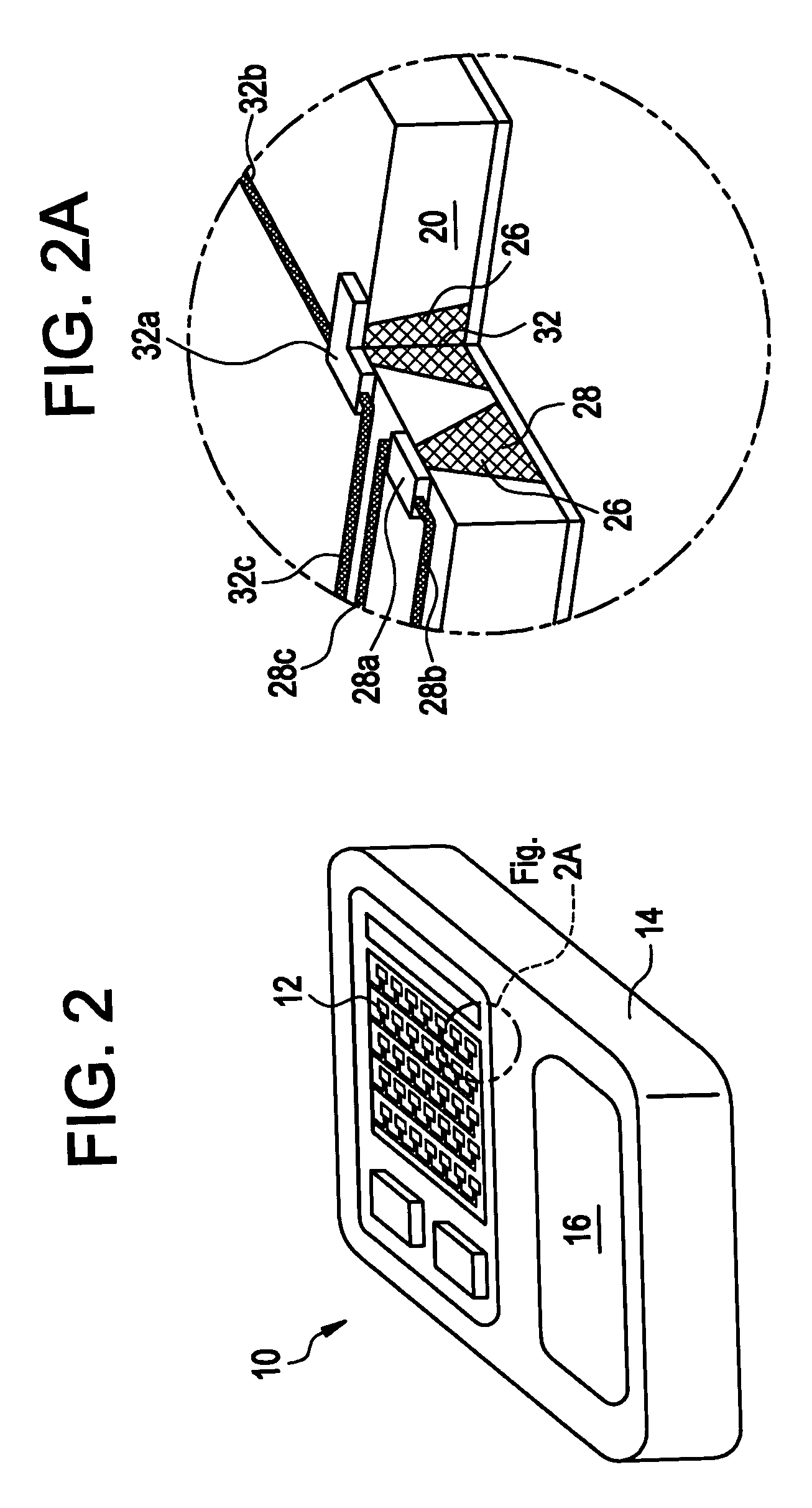
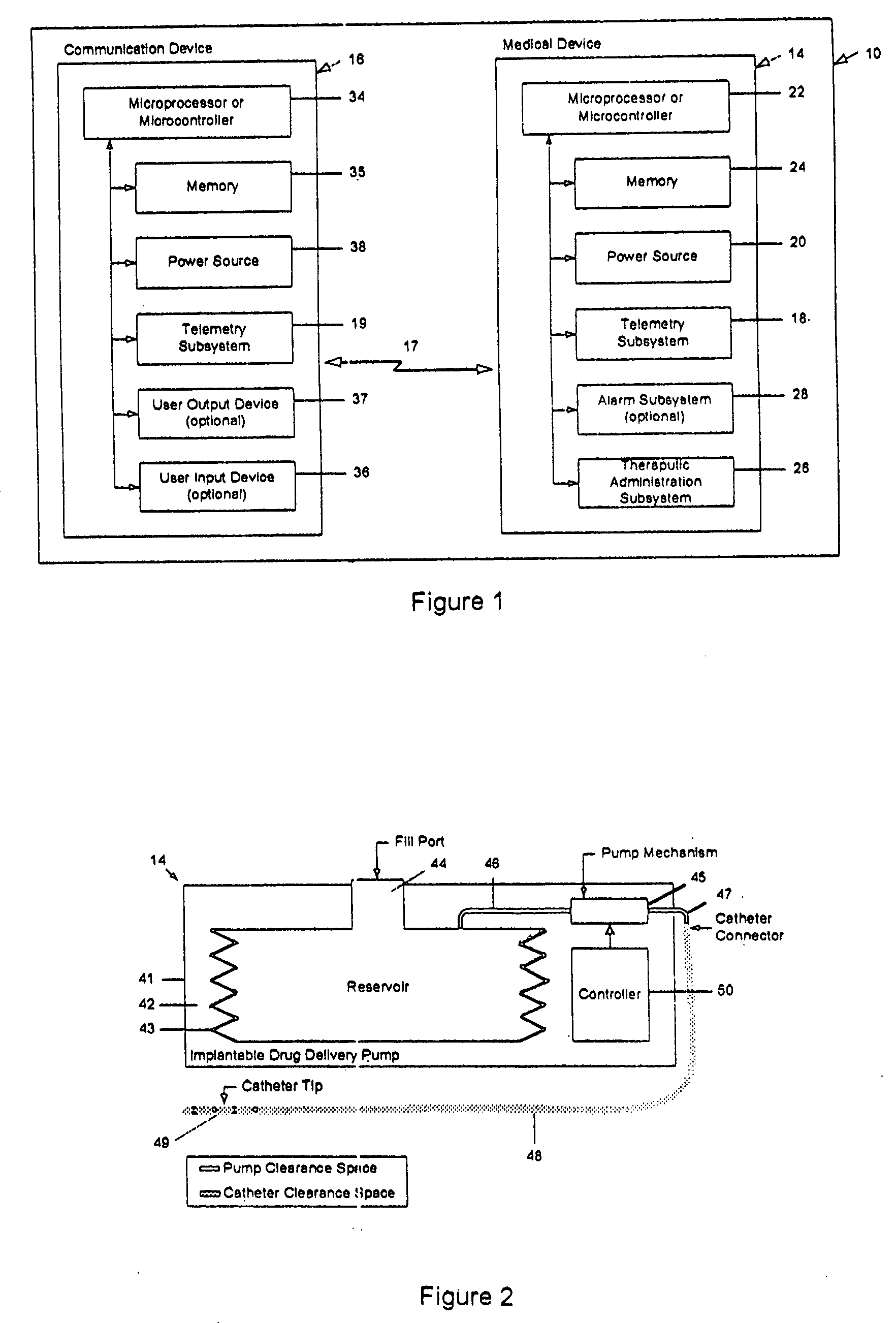
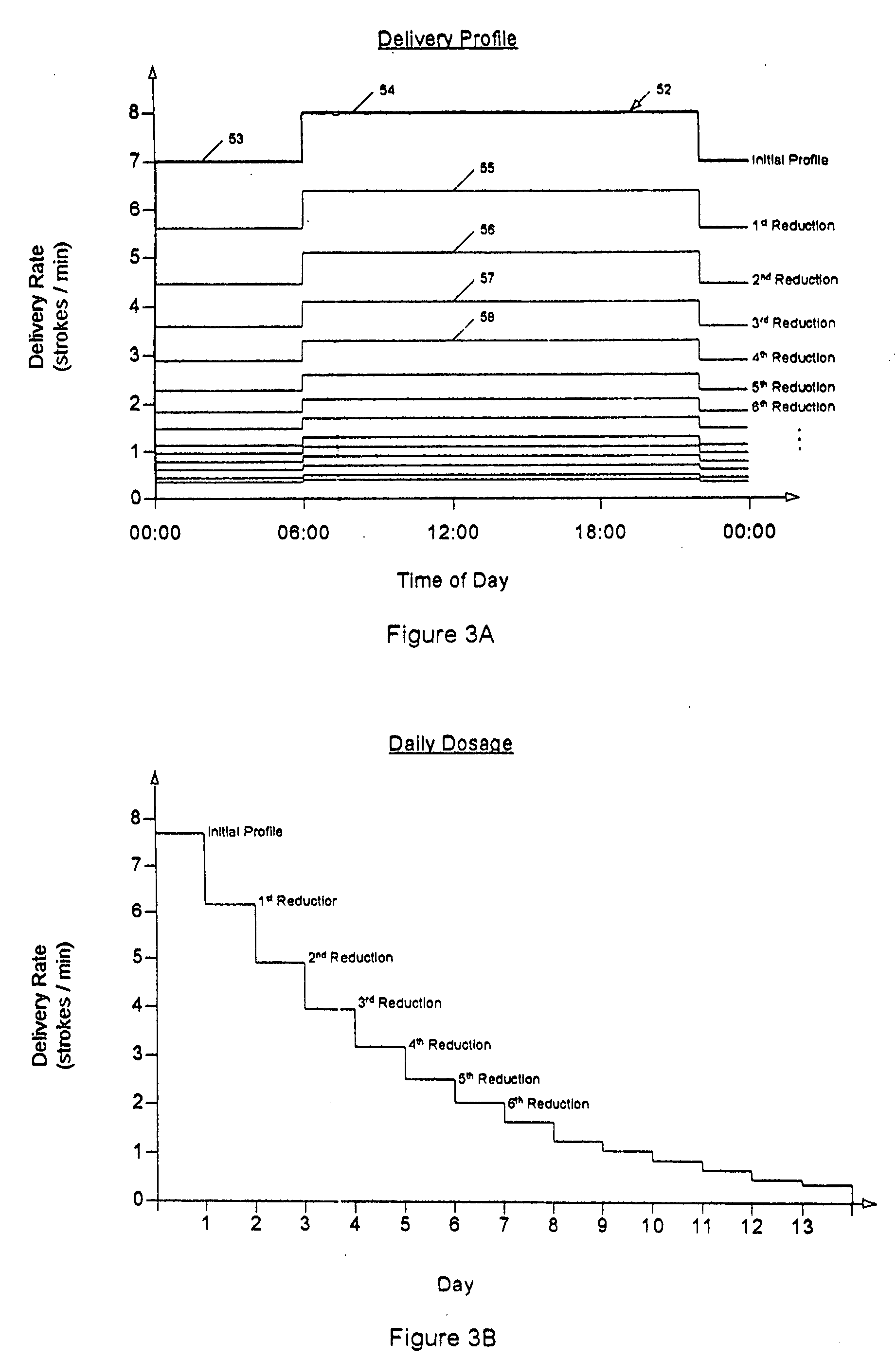
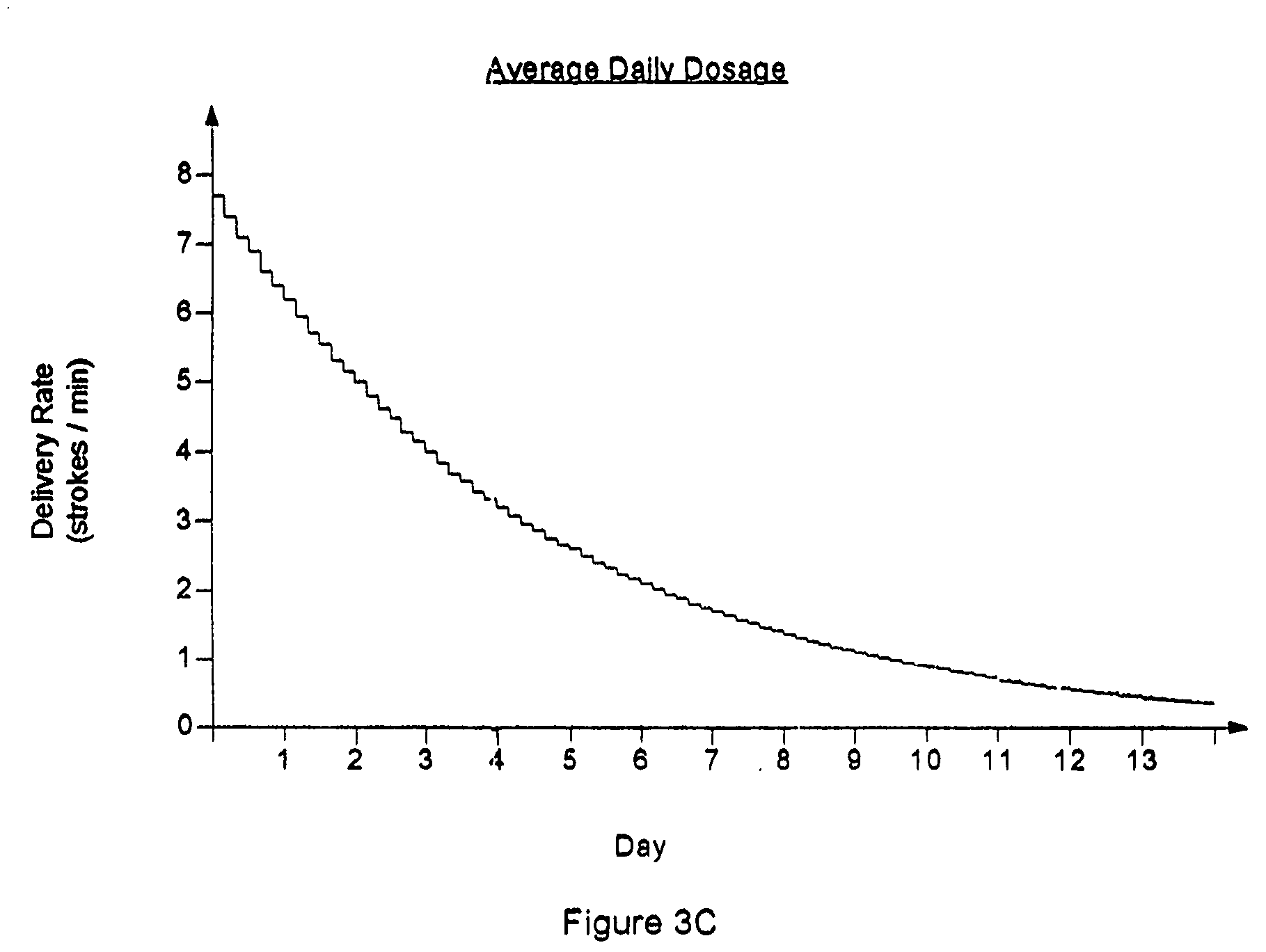
Drug delivery apparatus and method for automatically reducing drug dosage
InactiveUS20060047270A1Reduce probabilityMedical devicesPressure infusionMicrocontrollerDrug reservoir
A drug delivery device which includes a fluid drug reservoir, a catheter, a controllable fluid transfer device, e.g., a pump mechanism or valve, and a drug delivery control means. The drug delivery control means comprises a controller, e.g., a microprocessor or microcontroller which is operable to automatically reduce the rate of drug delivery over a certain reduction interval (e.g., multiple days) from an initial dosage value to a final dosage value.
Owner:INFUSION SYST
Nanochanneled Device and Related Methods
ActiveUS20100152699A1Amenability to selectHigh mechanical strengthServomotor componentsDecorative surface effectsBiomedical engineeringCorrelation method
Owner:THE OHIO STATE UNIV RES FOUND +1
Devices for intrabody delivery of molecules and systems and methods utilizing same
A device for controlled release of molecules is provided. the device including: (a) a device body having at least one reservoir therein for containing the molecules, the at least one reservoir being formed with a barrier impermeable to the molecules thereby preventing release thereof from the at least one reservoir; and (b) at least one acoustic transducer being attached to, or forming a part of, the device body, the at least one acoustic transducer being for converting an acoustic signal received thereby into an electrical signal, the electrical signal leading to barrier permeabilization and therefore release of the molecules from the at least one reservoir.
Owner:REMON MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com