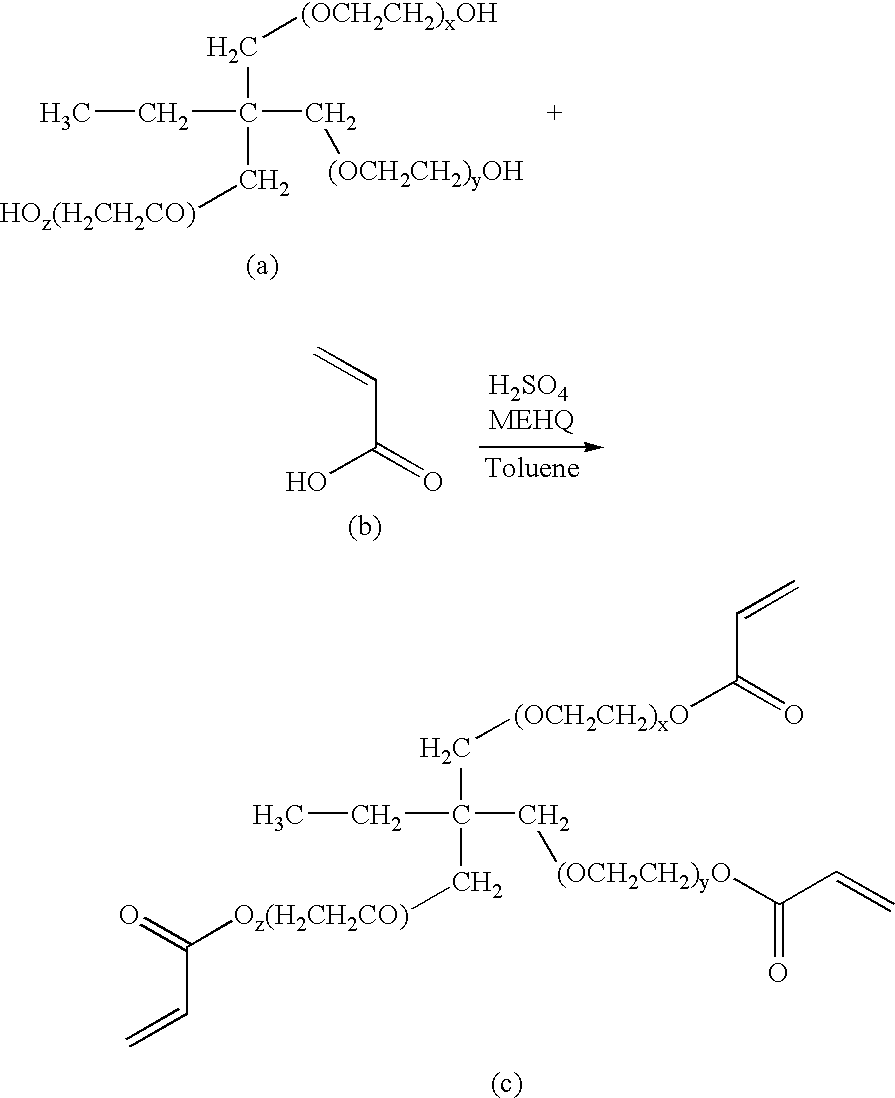
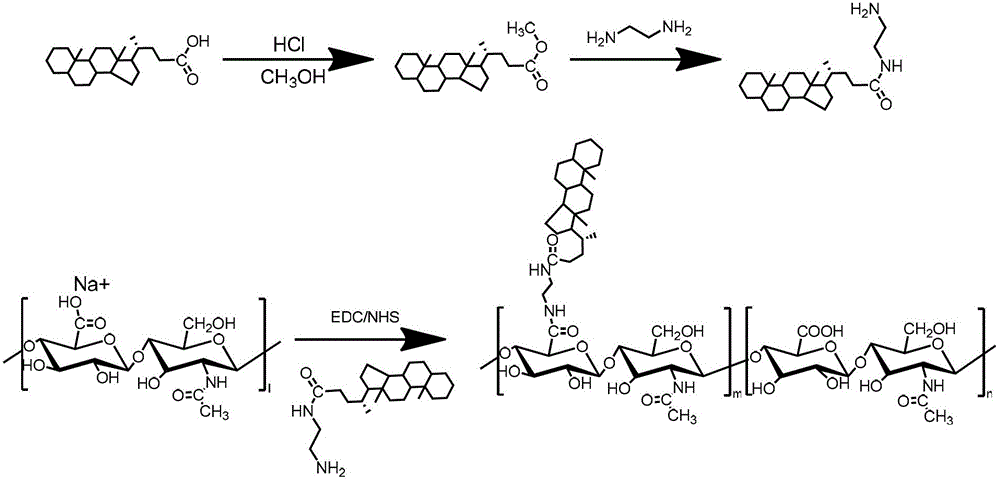
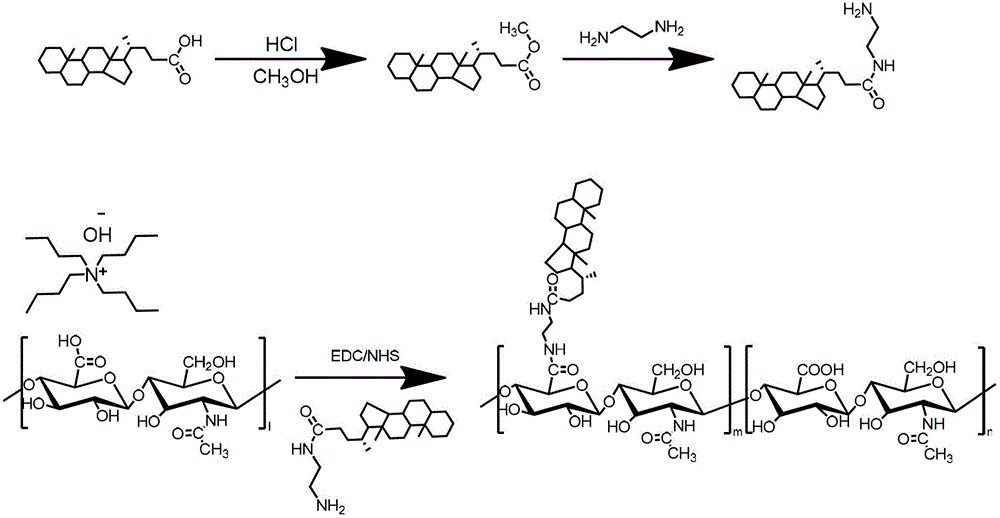
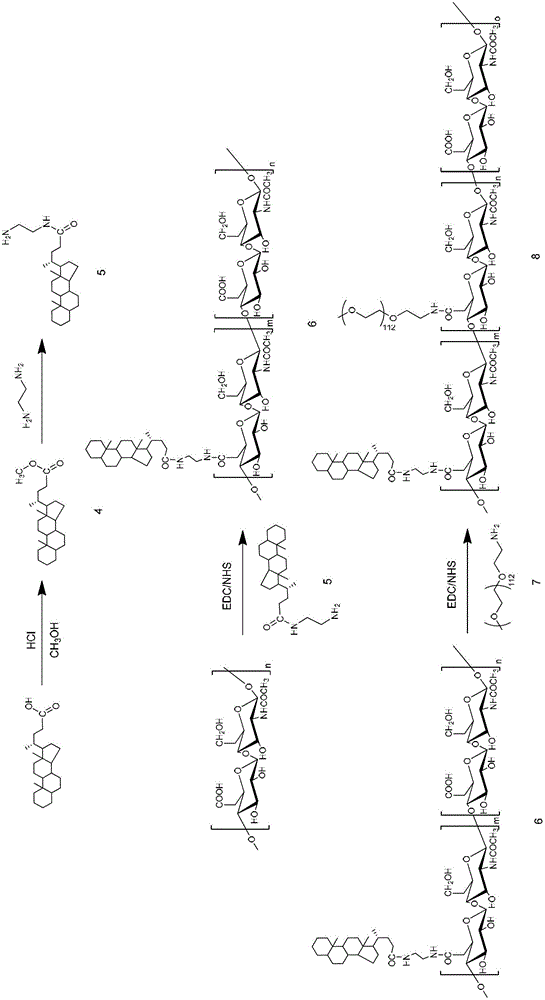
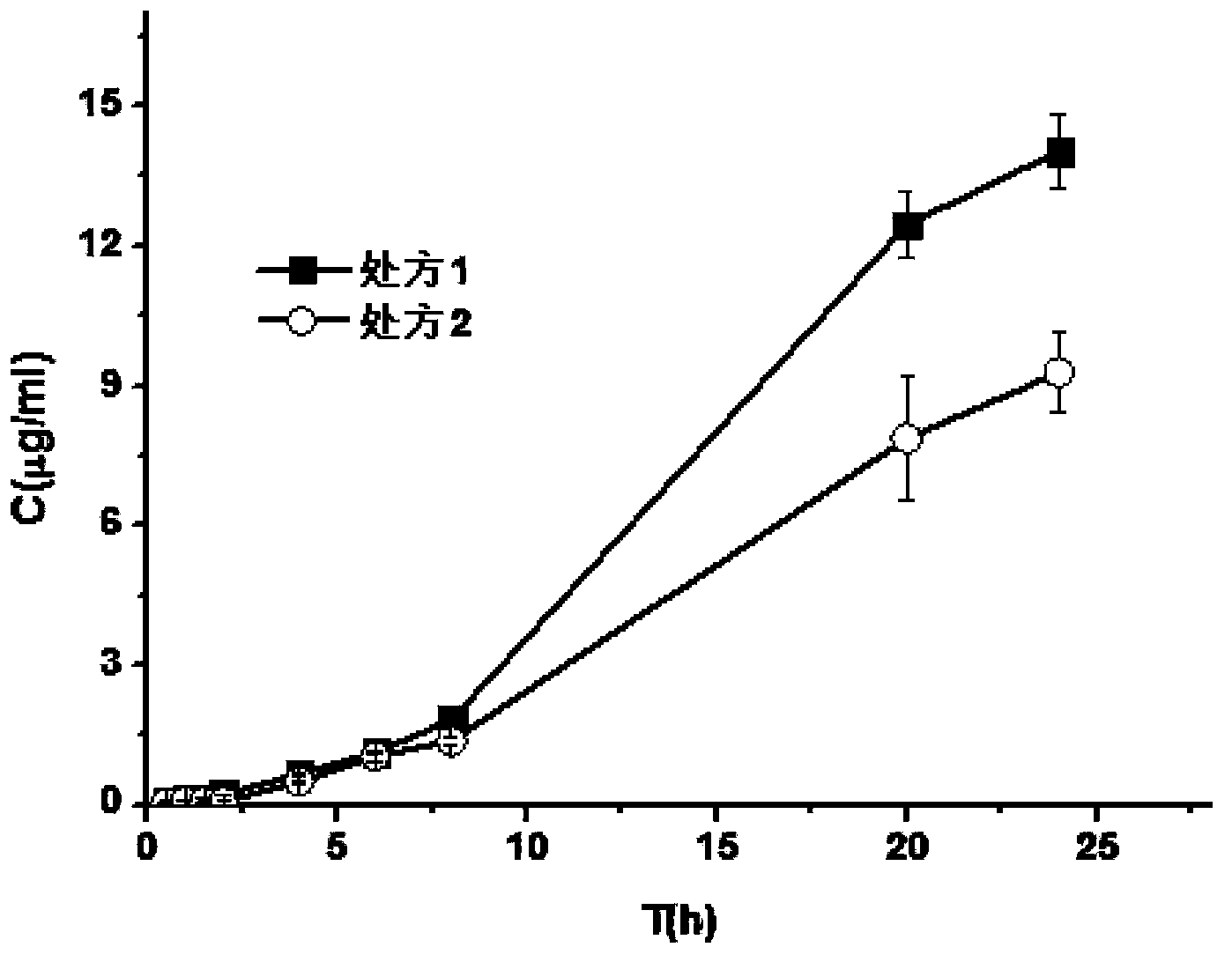
Patents
Literature
3019results about How to "Reduce adverse reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
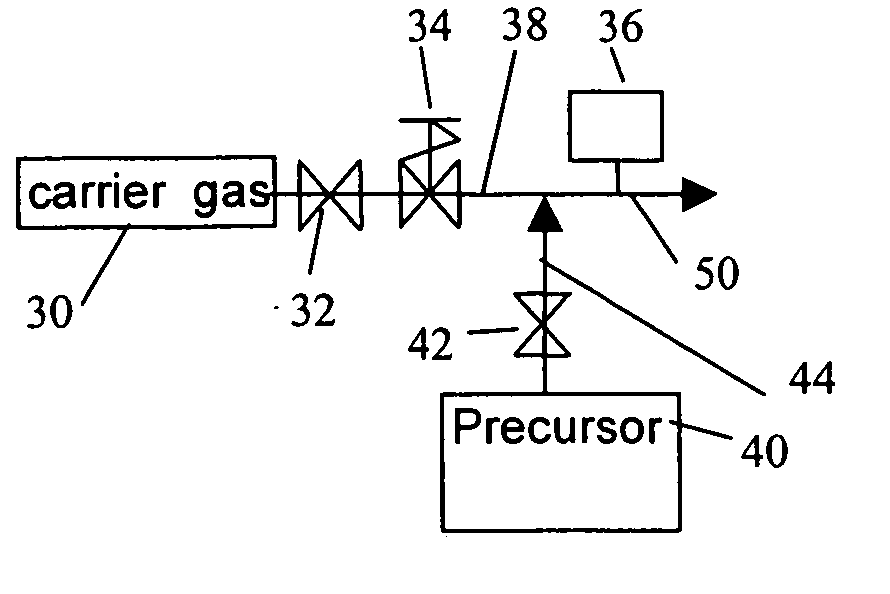
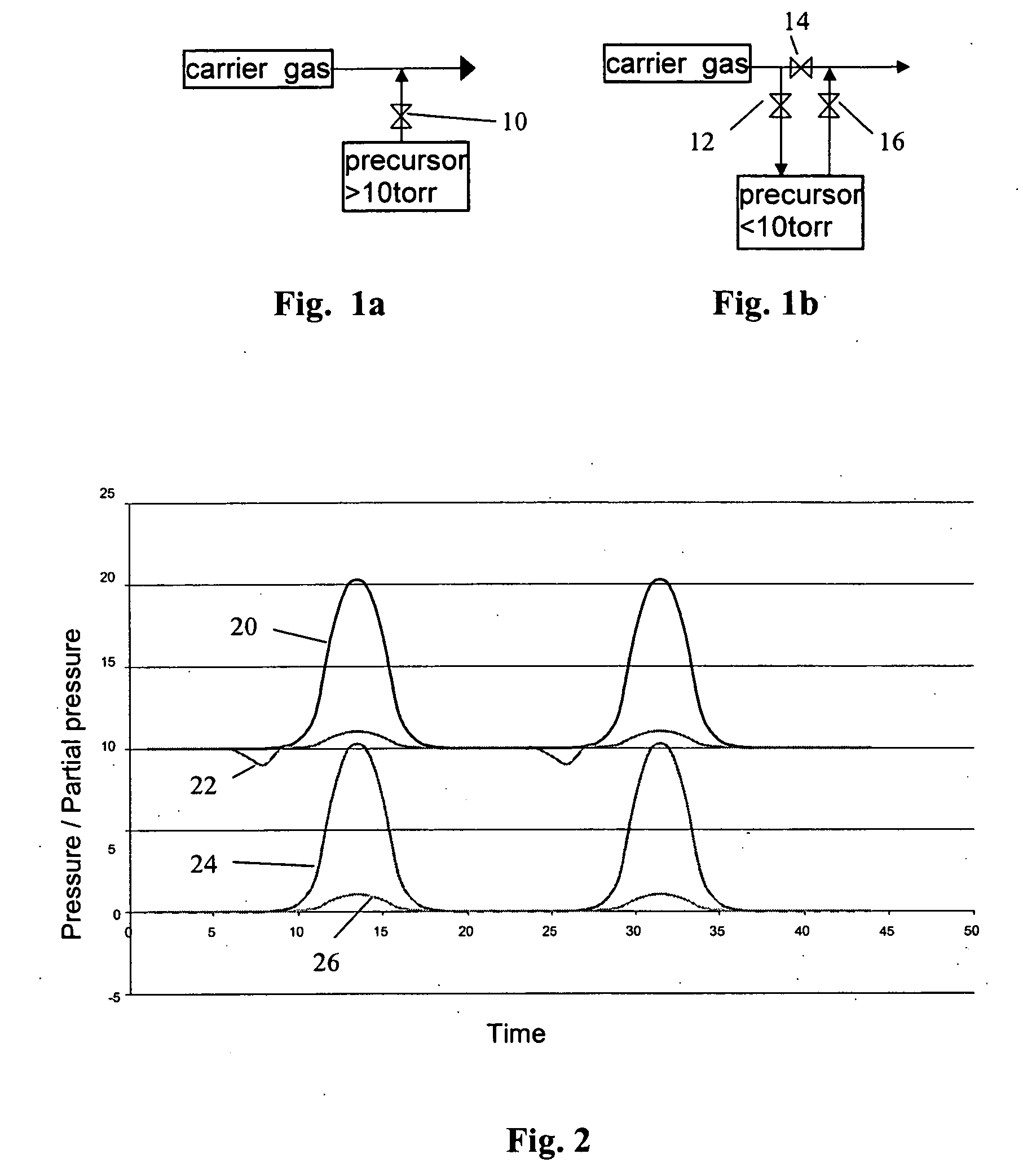
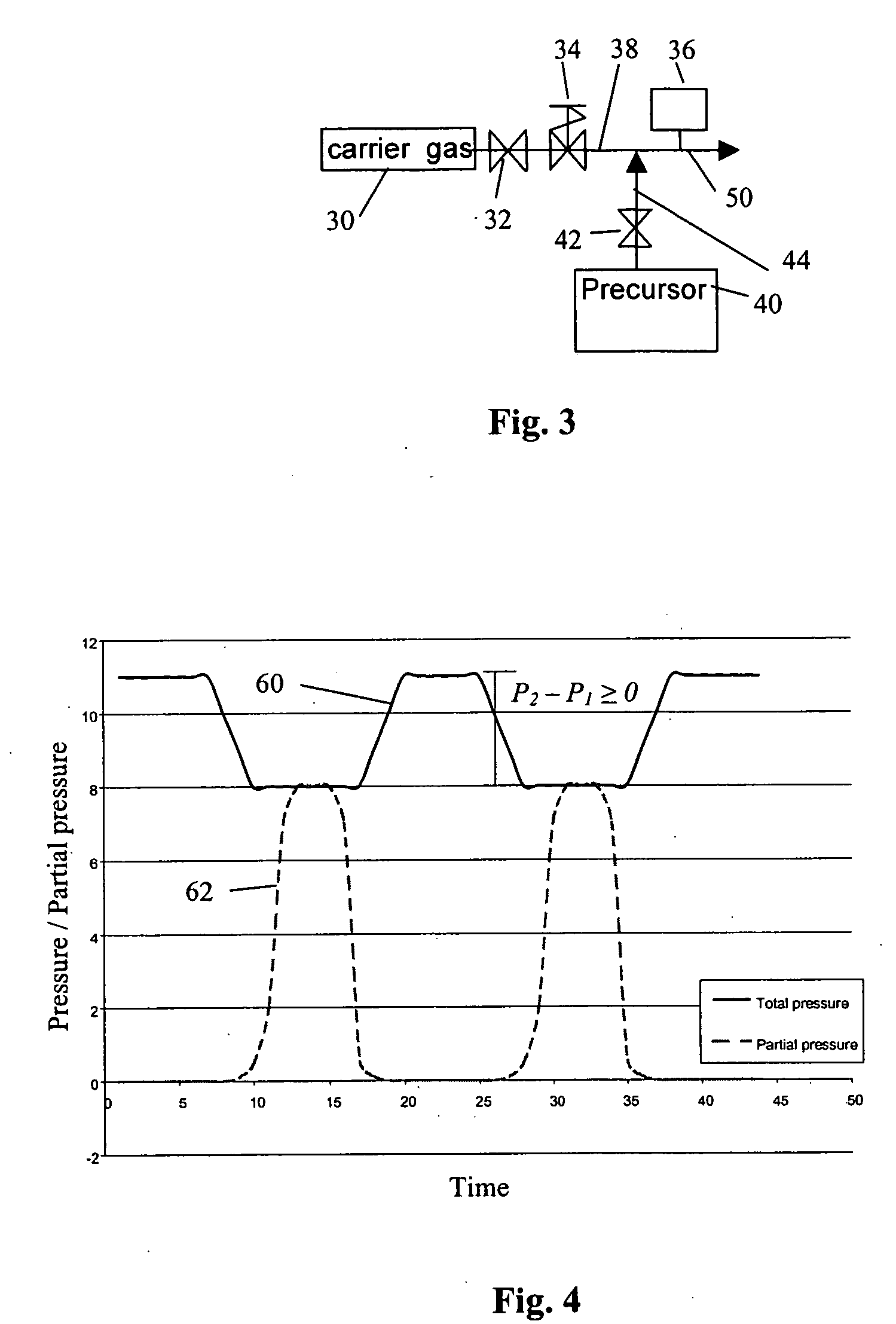
Method of pulsing vapor precursors in an ALD reactor
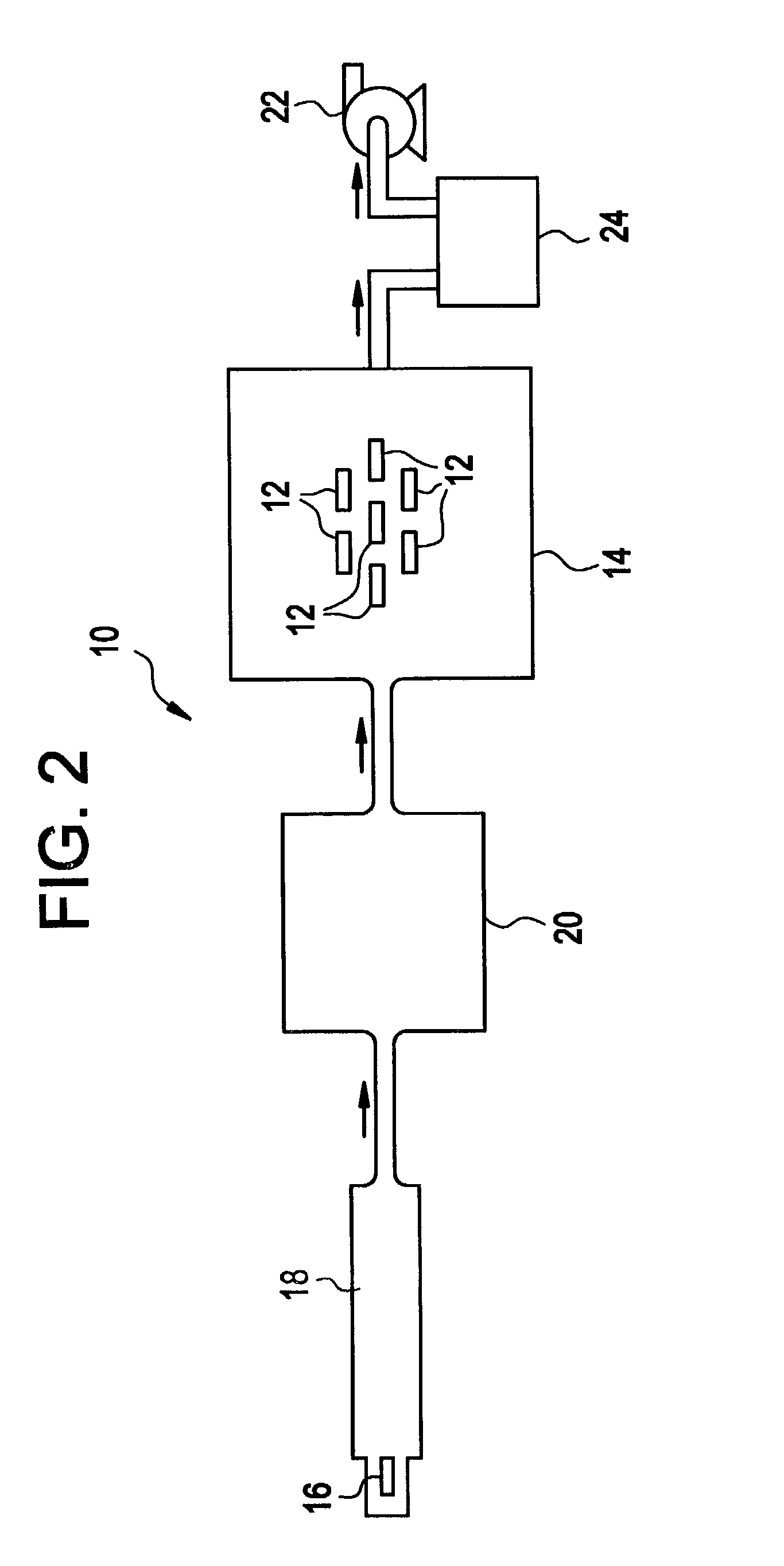
ActiveUS20060147626A1Faster film growthImproved pulse separationPolycrystalline material growthFrom chemically reactive gasesSource materialGas phase
A method of growing a thin film on a substrate by pulsing vapor-phase precursors material into a reaction chamber according to the ALD method. The method comprises vaporizing at least one precursor from a source material container maintained at a vaporising temperature, repeatedly feeding pulses of the vaporized precursor via a feed line into the reaction chamber at a first pressure, and subsequently purging the reaction chamber with pulses of inactive gas fed via the feed line at a second pressure. The second pressure is maintained at the same as or a higher level than the first pressure for separating successive pulses of said vaporized precursor from each other.
Owner:ASM IP HLDG BV
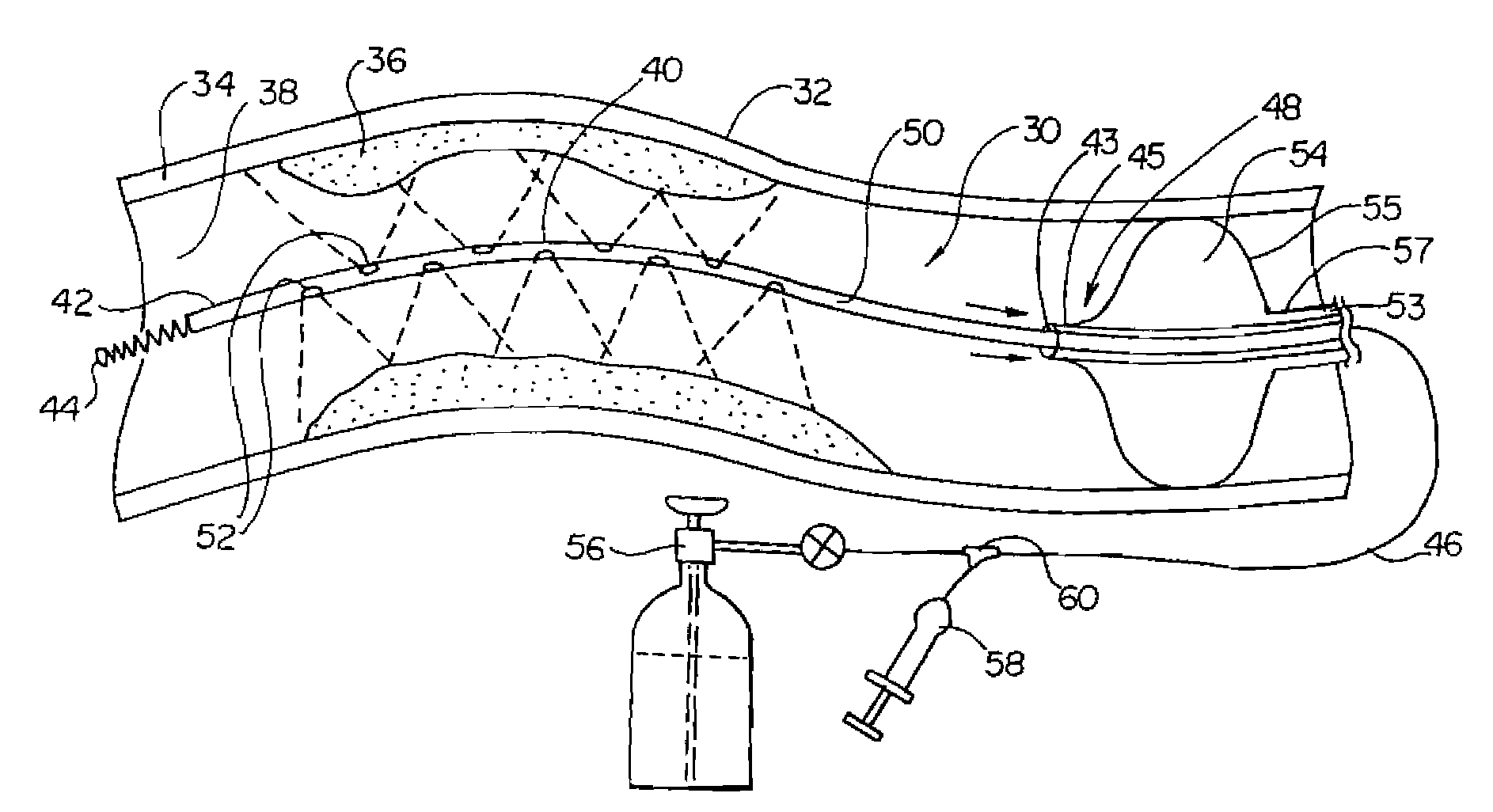
Cryotreatment device and method
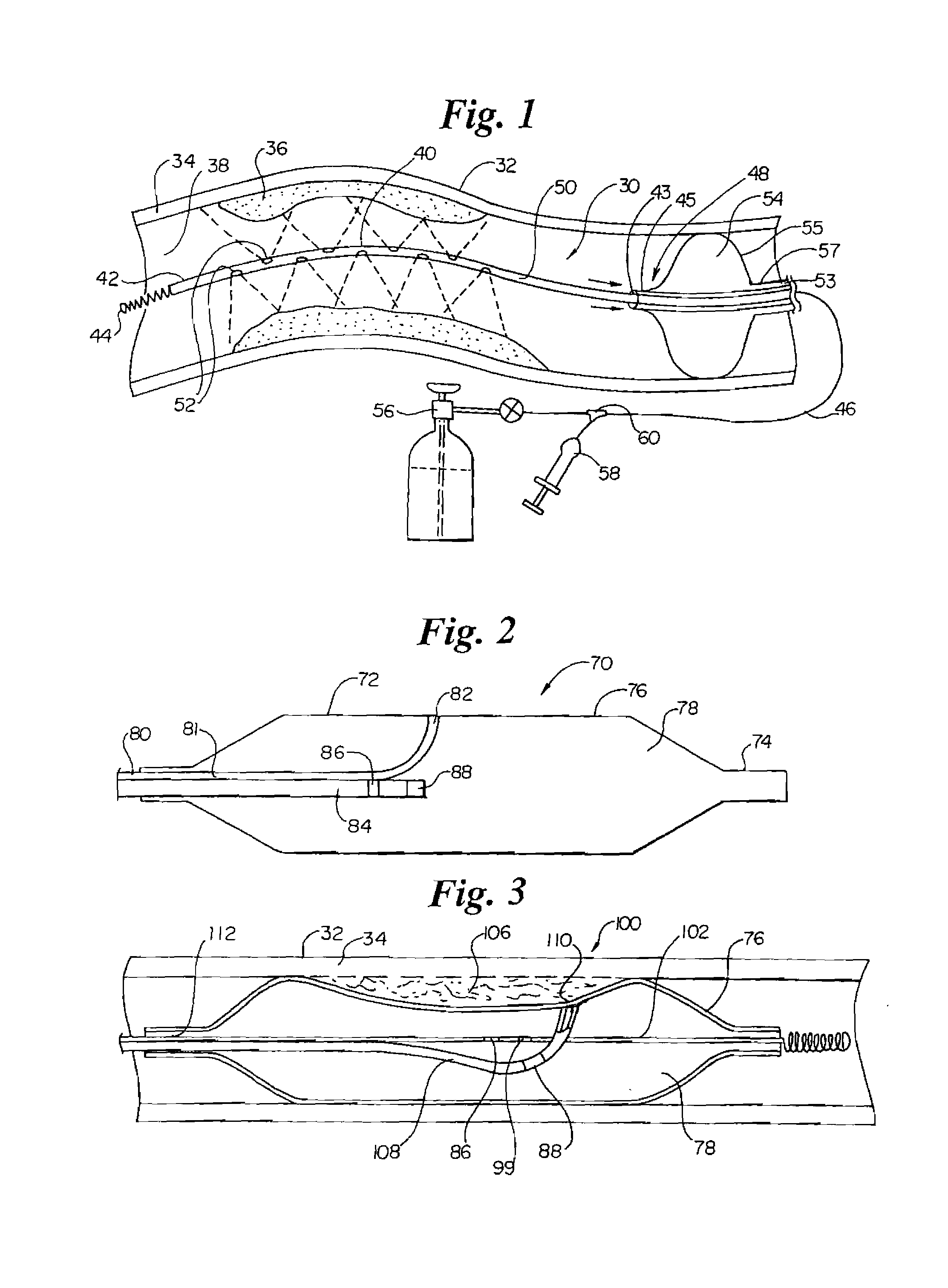
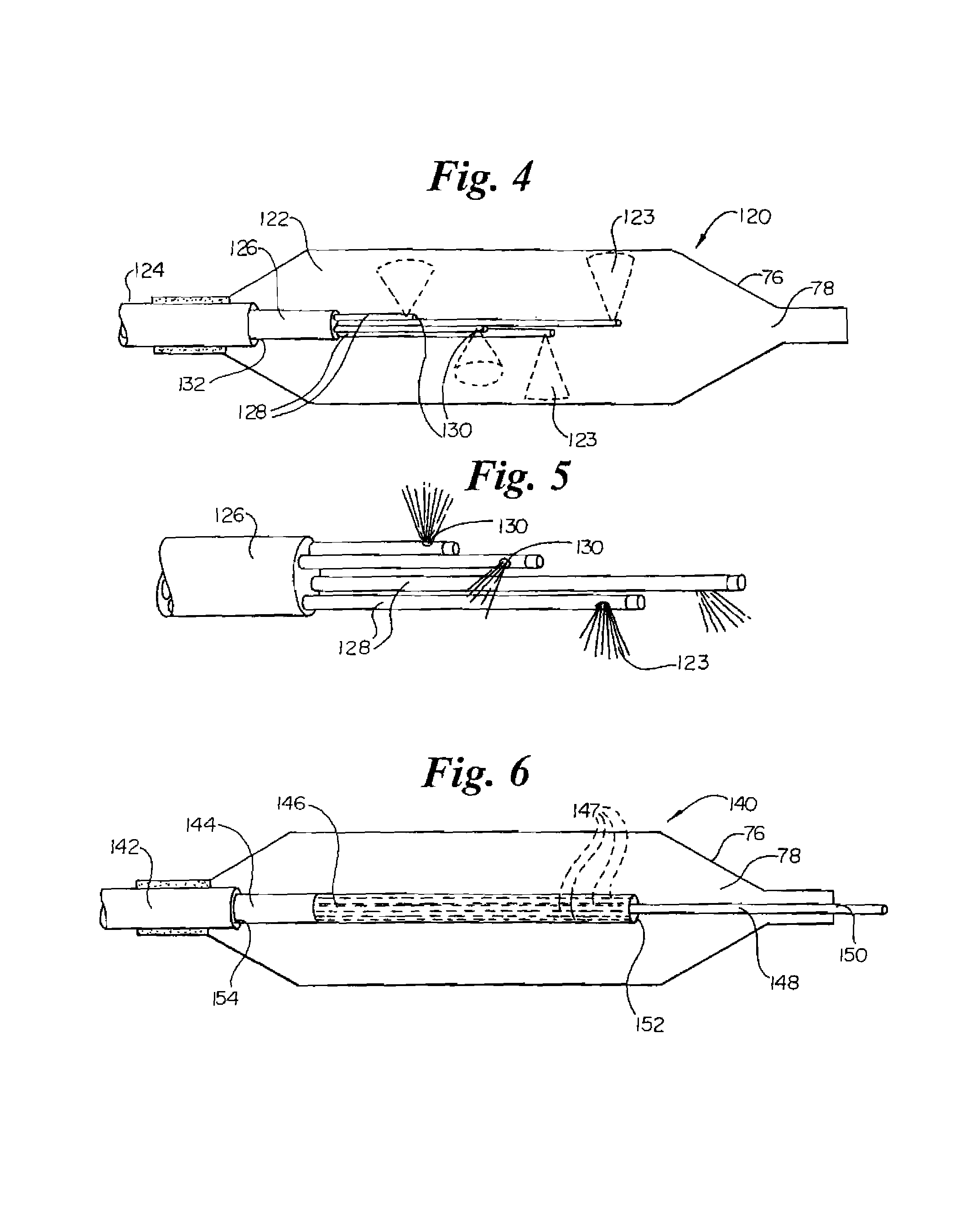
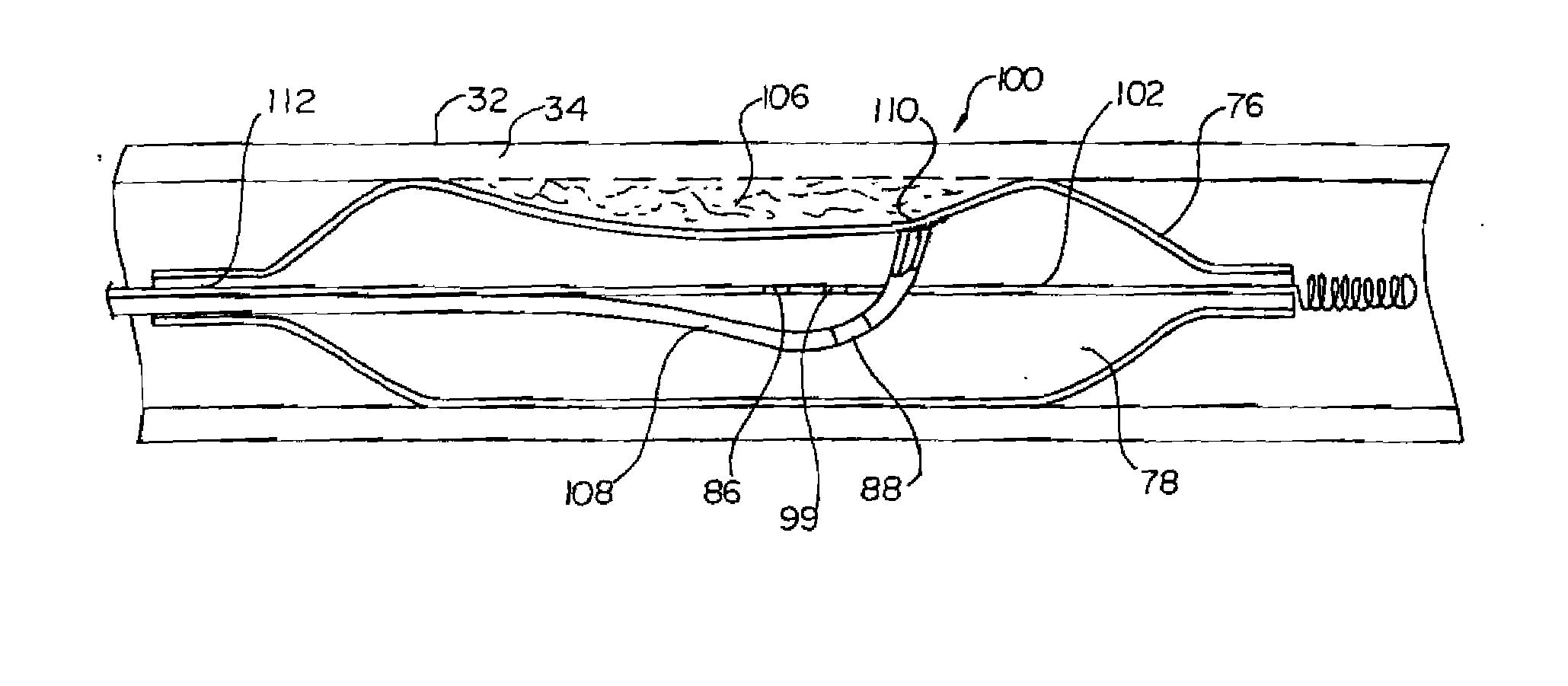
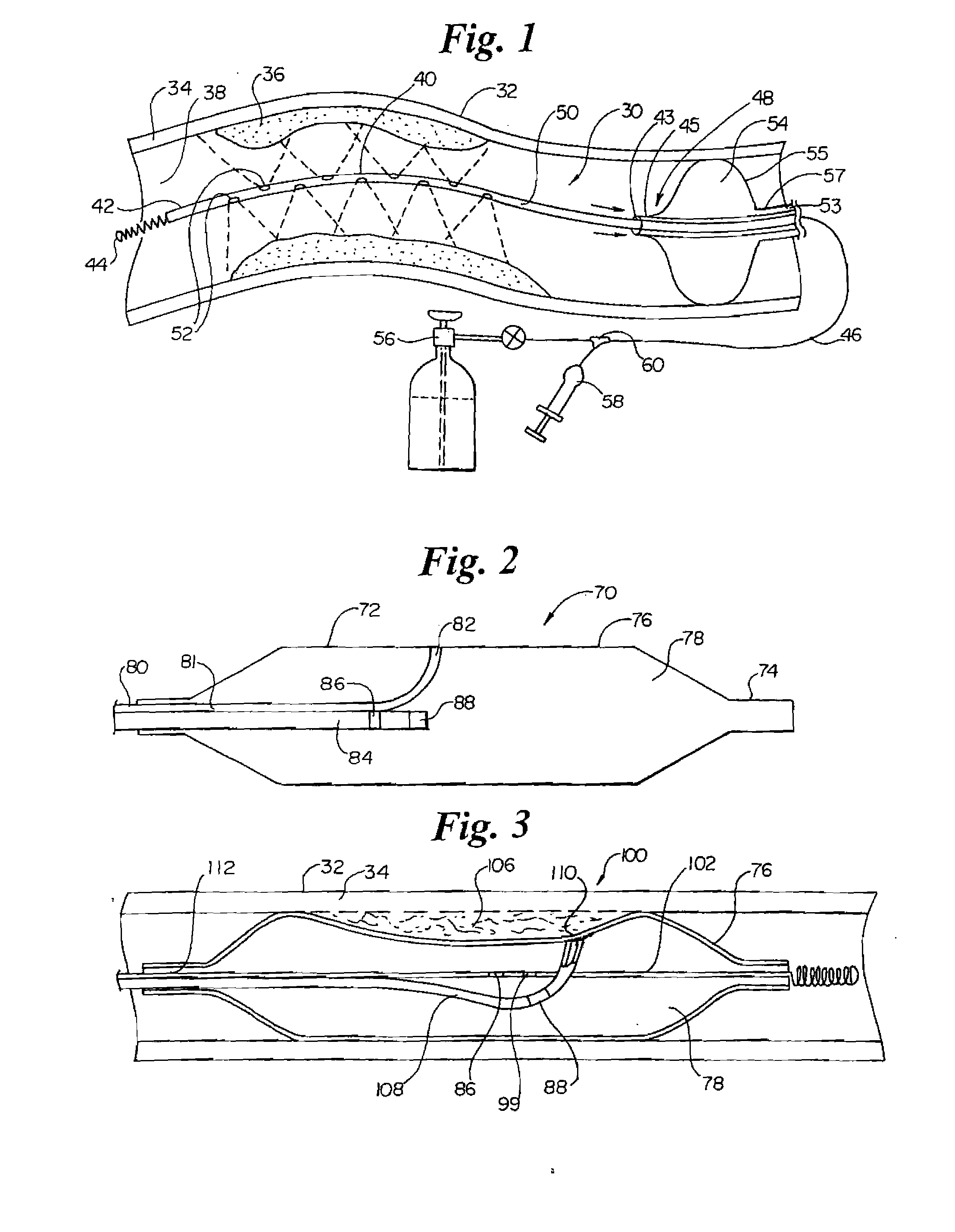
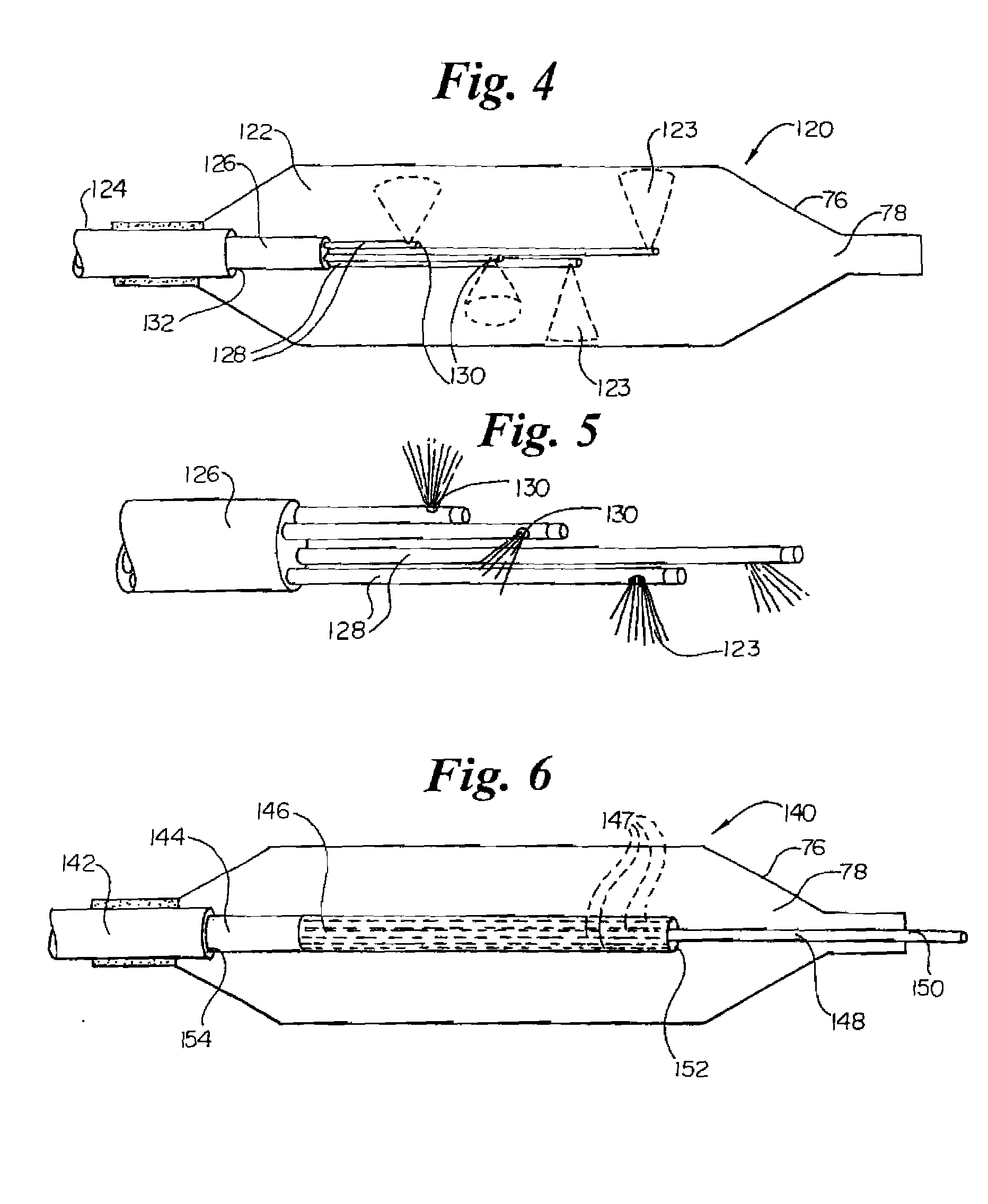
InactiveUS7220257B1Reduce adverse reactionsReduced responseStentsOther blood circulation devicesCoronary artery angioplastyPercent Diameter Stenosis
Devices and methods for cooling vessel walls to inhibit restenosis in conjunction with medical procedures such as coronary artery angioplasty. Stenosed vessel walls can be cooled prior to angioplasty, after angioplasty, or both. The invention is believed to inhibit restenosis through cooling to a temperature near freezing, preferably without causing substantial vessel wall cell death. One catheter device includes a distal tube region having coolant delivery holes radially and longitudinally distributed along the distal region. In some devices, holes spray coolant directly onto the vessel walls, with the coolant absorbed into the blood stream. In other embodiments, a balloon or envelope is interposed between the coolant and the vessel walls and the coolant returned out of the catheter through a coolant return lumen. Some direct spray devices include an occlusion device to restrict blood flow past the region being cooled. Pressure, temperature, and ultrasonic probes are included in some cooling catheters. Pressure control valves are included in some devices to regulate balloon interior pressure within acceptable limits. In applications using liquid carbon dioxide as coolant, the balloon interior pressure can be maintained above the triple point of carbon dioxide to inhibit dry ice formation. Some cooling catheters are coiled perfusion catheters supporting longer cooling periods by allowing perfusing blood flow simultaneously with vessel wall cooling. One coiled catheter is biased to assume a coiled shape when unconstrained and can be introduced into the body in a relatively straight shape, having a stiffening wire inserted through the coil strands.
Owner:BOSTON SCI SCIMED INC
Methods for conformal coating and sealing microchip reservoir devices
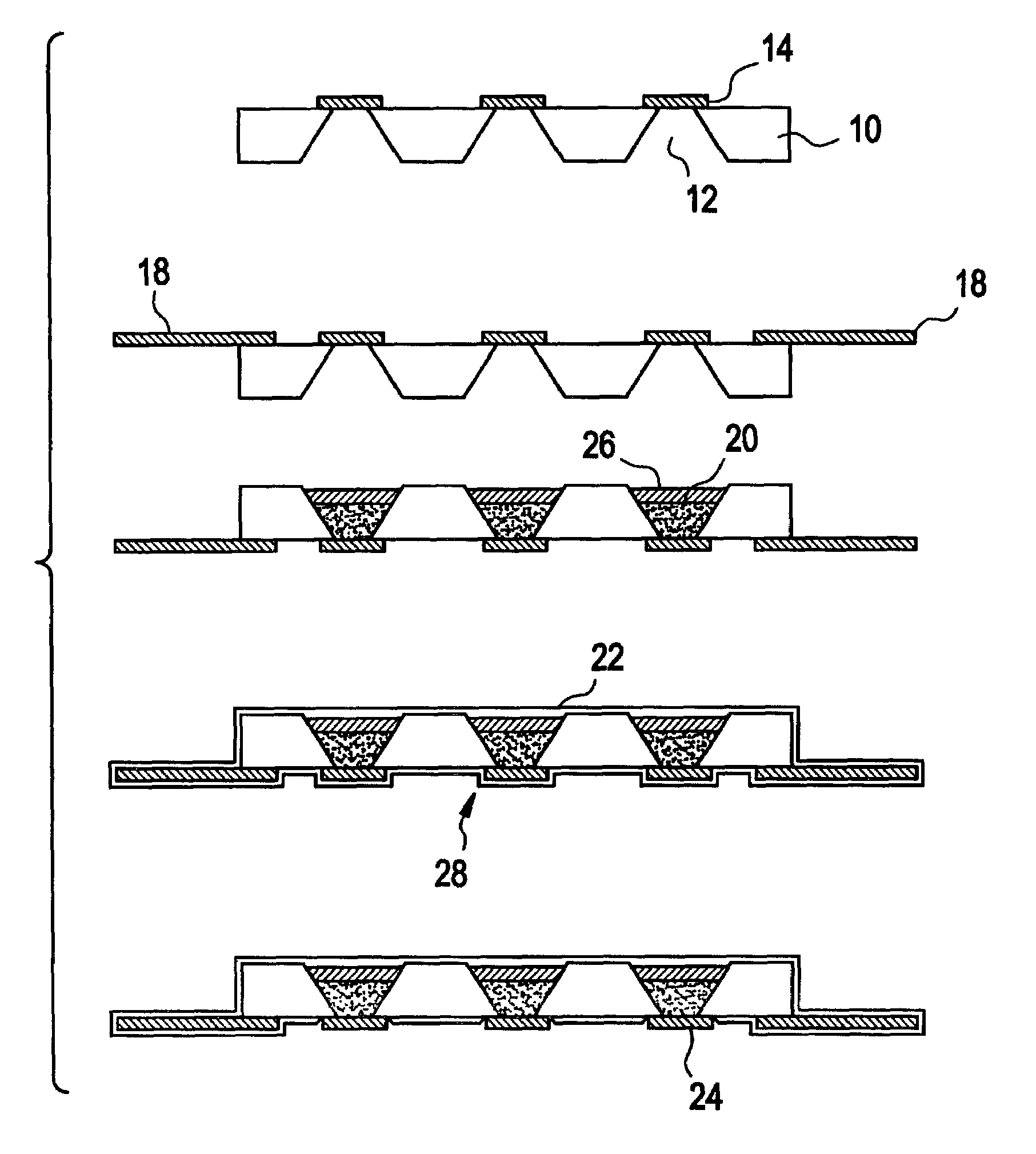
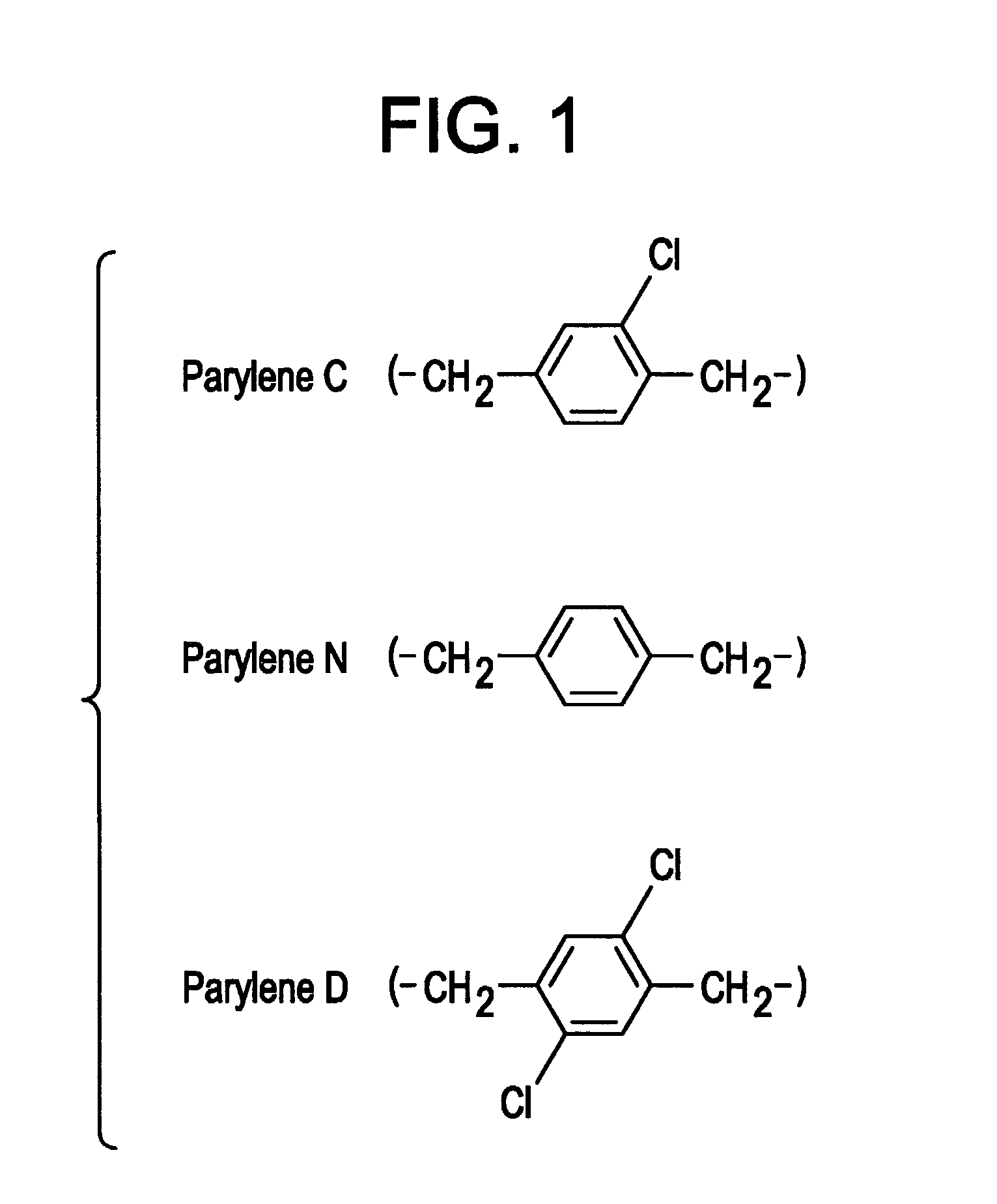
InactiveUS6973718B2Reduce adverse reactionsPrinted circuit assemblingLiquid surface applicatorsParyleneGas phase
Methods are provided for conformally coating microchip devices and for sealing reservoirs containing molecules or devices in a microchip device. One method comprises (i) providing a substrate having a plurality of reservoirs having reservoir openings in need of sealing; (ii) loading reservoir contents comprising molecules, a secondary device, or both, into the reservoirs; and (iii) applying a conformal coating barrier layer, such as a vapor depositable polymeric material, e.g., parylene, onto the reservoir contents over at least the reservoir openings to seal the reservoir openings. Another method comprises vapor depositing a conformal coating material onto a microchip device having at least two reservoirs and reservoir caps positioned over molecules or devices stored in the reservoirs, and providing that the conformal coating does not coat or is removed from the reservoir caps.
Owner:MICROCHIPS INC
Diet for dietotherapy and health preservation
ActiveCN102423073AReduce adverse reactionsReduce dosageConfectionerySweetmeatsPumpkin seedDry weight
The invention relates to a diet for dietotherapy and health preservation which is prepared by medicine-food materials, and belongs to the field of nutrition and health preservation; the basic formula comprises the following raw materials on a dry weight basis: 30-60 parts of black sesame, 4-6 parts of poria cocos, 2-8 parts of hawthorn, 2-8 parts of medlar, 2-5 parts of coix seeds, 1-5 parts of spine date seeds, 2-5 parts of lotus seeds, 2-5 parts of Chinese yam, 1-5 parts of lily, 5-10 parts of jujube, 1-5 parts of donkey-hide gelatin, 1-4 parts of roses, 2-8 parts of carrots, 2-5 parts of walnut kernels, 2-5 parts of pumpkin seeds, 2-5 parts of black soybeans, 2-5 parts of black fungus, 1-3 parts of honeysuckles, 1-3 parts of sea-tangle, and 1-3 parts of mulberry. The invention can alsobe combined with other materials to form a secondary formula by using the basic formula as a main component. The formula of the invention is not excessively cold or hot, has a moderate character and taste, is convenient for using, has a less using amount, can be eaten frequently for a long term, has a lot of efficacy and effect and definite effect, and the efficacy is confirmed to reach the expectation and to be satisfied through long-term eating by a lot of people.
Owner:李超建
Nano-bionic material for tissue repair and preparation method thereof
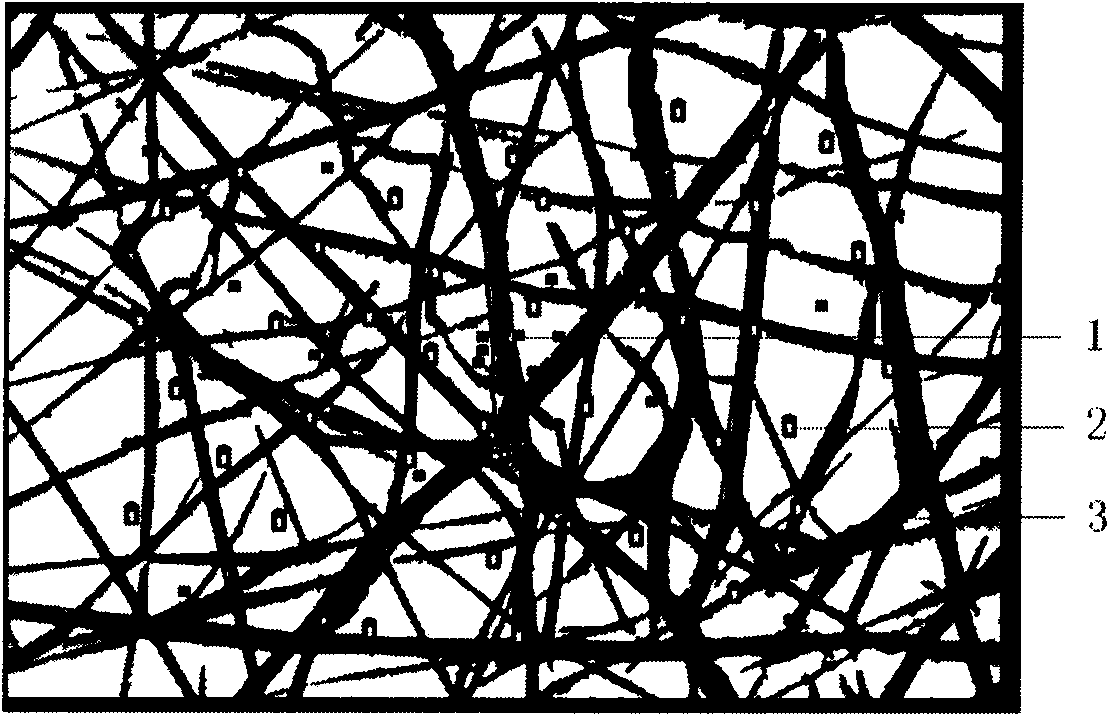
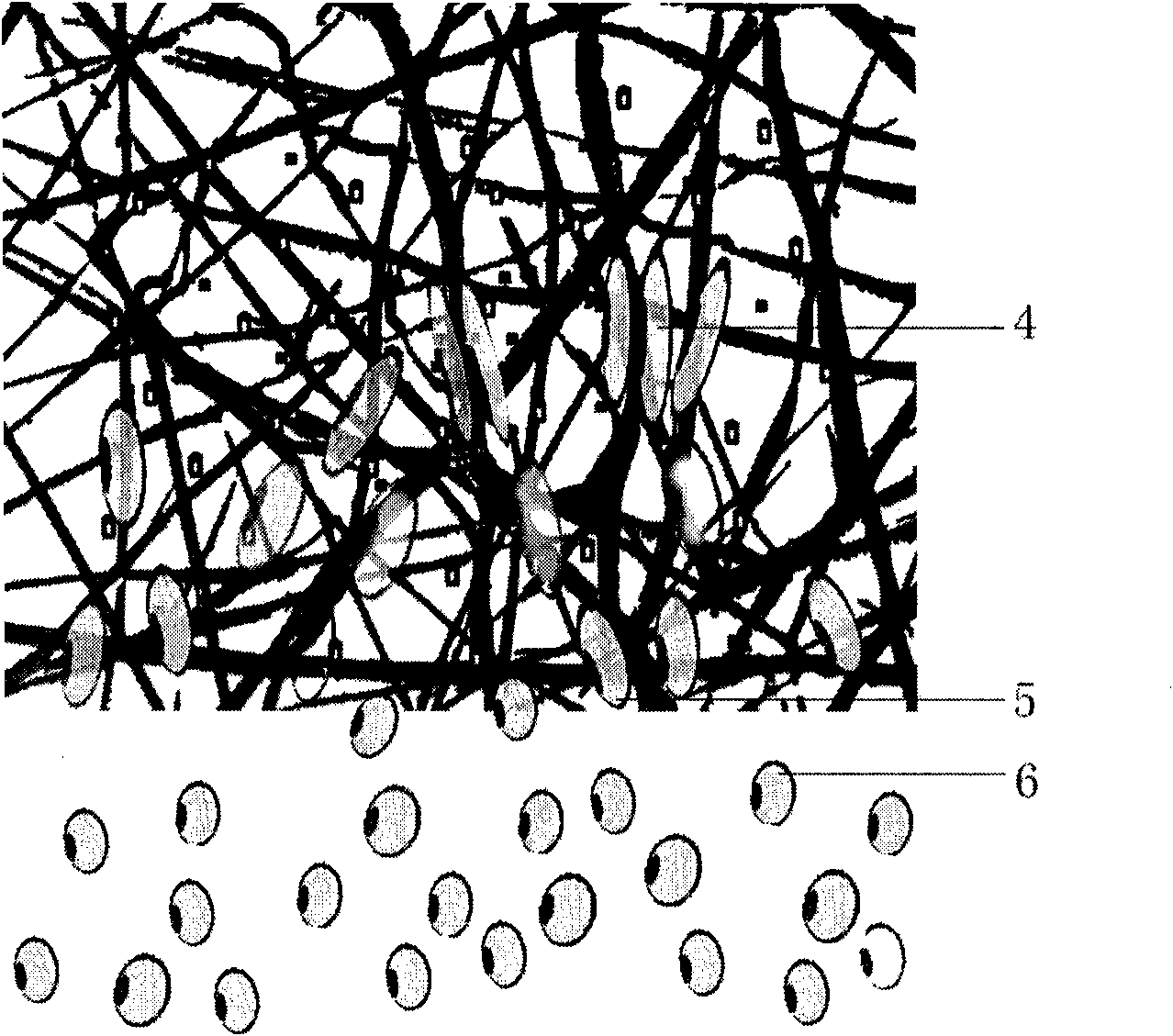
ActiveCN101829361AImprove regenerative abilityTo achieve the effect of graft repairProsthesisNano-scaffoldTissue repair
The invention provides a nano-bionic material for tissue repair and a preparation method thereof. The nano-bionic material comprises a nano-bionic bracket and hydrosol attached to the nano-bionic bracket, wherein one or more cell factors and / or cells are coated in the hydrosol. The preparation method of the nano-bionic material comprises the following steps of: preparing an electrospinning solution and a hydrosol solution containing the cell factors and / or the cells; preparing the nano-bionic bracket by using the electrospinning; printing the hydrosol solution containing the cell factors and / or the cells on the nano-bionic bracket by using a ink-jet printer; and the like, and repeating the electrospinning and the ink jet to obtain the nano-bionic material with different thicknesses. By combining with an electrospinning technology and a biological printing technology, the invention combines specific medicines and / or the cell factors and / or the cells in the nano-bracket and / or the surface of the nano-bracket, thereby greatly enhancing the effects of the nano-bionic material on tissue repair and tissue regeneration; in addition, the invention has broad application prospect.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
Cryotreatment device and method
InactiveUS20070250050A1Reduce adverse reactionsReduced responseStentsOther blood circulation devicesCoronary artery angioplastyPercent Diameter Stenosis
Devices and methods for cooling vessel walls to inhibit restenosis in conjunction with medical procedures such as coronary artery angioplasty. Stenosed vessel walls can be cooled prior to angioplasty, after angioplasty, or both. The invention is believed to inhibit restenosis through cooling to a temperature near freezing, preferably without causing substantial vessel wall cell death. One catheter device includes a distal tube region having coolant delivery holes radially and longitudinally distributed along the distal region. In some devices, holes spray coolant directly onto the vessel walls, with the coolant absorbed into the blood stream. In other embodiments, a balloon or envelope is interposed between the coolant and the vessel walls and the coolant returned out of the catheter through a coolant return lumen. Some direct spray devices include an occlusion device to restrict blood now past the region being cooled. Pressure, temperature, and ultrasonic probes are included in some cooling catheters. Pressure control valves are included in some devices to regulate balloon interior pressure within acceptable limits. In applications using liquid carbon dioxide as coolant, the balloon interior pressure can be maintained above the triple point of carbon dioxide to inhibit dry ice formation. Some cooling catheters are coiled perfusion catheters supporting longer cooling periods by allowing perfusing blood flow simultaneously with vessel wall cooling. One coiled catheter is biased to assume a coiled shape when unconstrained and can be introduced into the body in a relatively straight shape, having a stiffeninig wire inserted through the coil strands.
Owner:BOSTON SCI SCIMED INC
Crosslinkable macromers
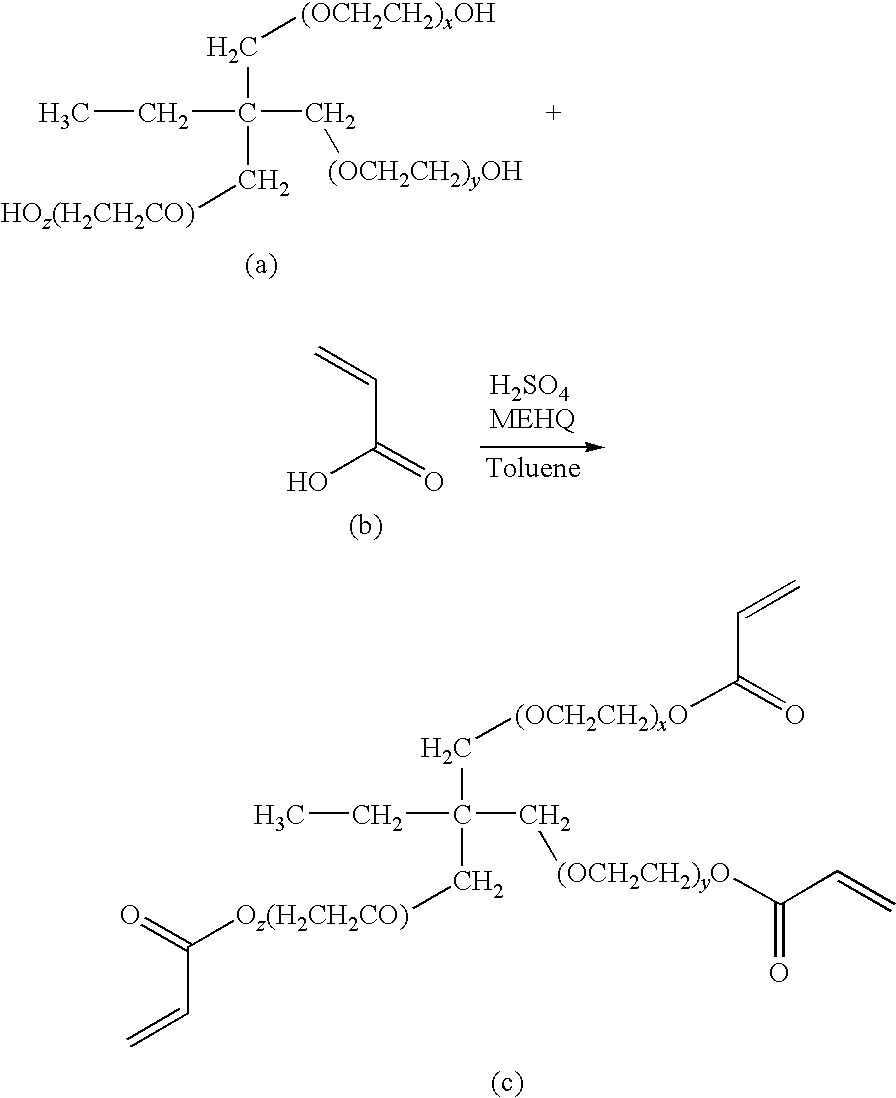
A crosslinkable macromer system and related methods of preparing the system and using the system in the form of a crosslinked matrix between a tissue site and an implant article such as a tissue implant or on the porous surface of a prosthetic device. The macromer system includes two or more polymer-pendent polymerizable groups and one or more multifunctional initiator groups. The polymerizable groups and the initiator group(s), when polymer-pendent, can be pendent on the same or different polymeric backbones. The macromer system provides advantages over the use of polymerizable macromers and separate, low molecular weight initiators, including advantages with respect to such properties as nontoxicity, efficiency, and solubility. A macromer system of the invention can be used as an interface between the tissue site and implant article in a manner sufficient to permit tissue growth through the crosslinked matrix and between the tissue site and implant. In a preferred embodiment, polymers with pendent polymerizable groups, for use in the macromer system, are prepared by reacting a polysaccharide polymer with a reactive moiety in an organic, polar solvent such as formamide.
Owner:SURMODICS INC
Risperidone slow-release microsphere, preparation method and application thereof
ActiveCN101653422AHigh drug loadingImprove complianceOrganic active ingredientsNervous disorderBlood concentrationMicrosphere
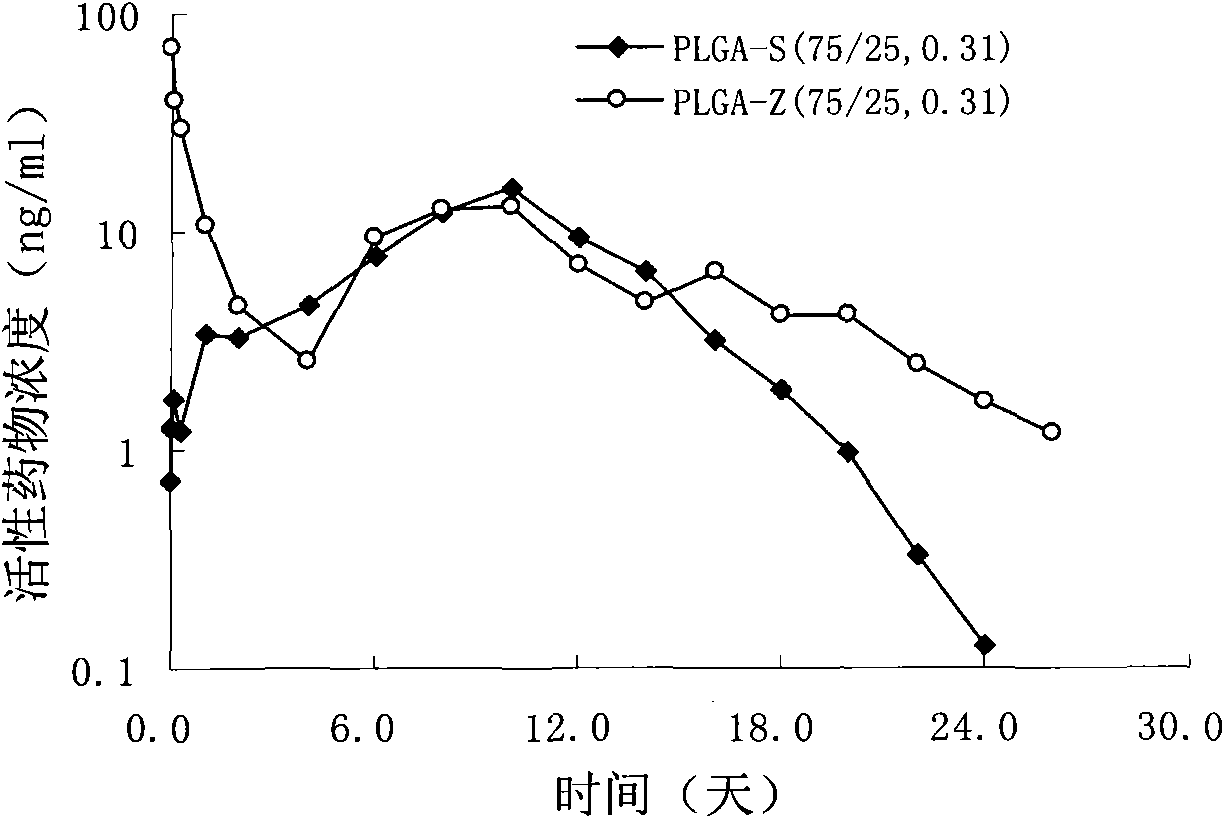
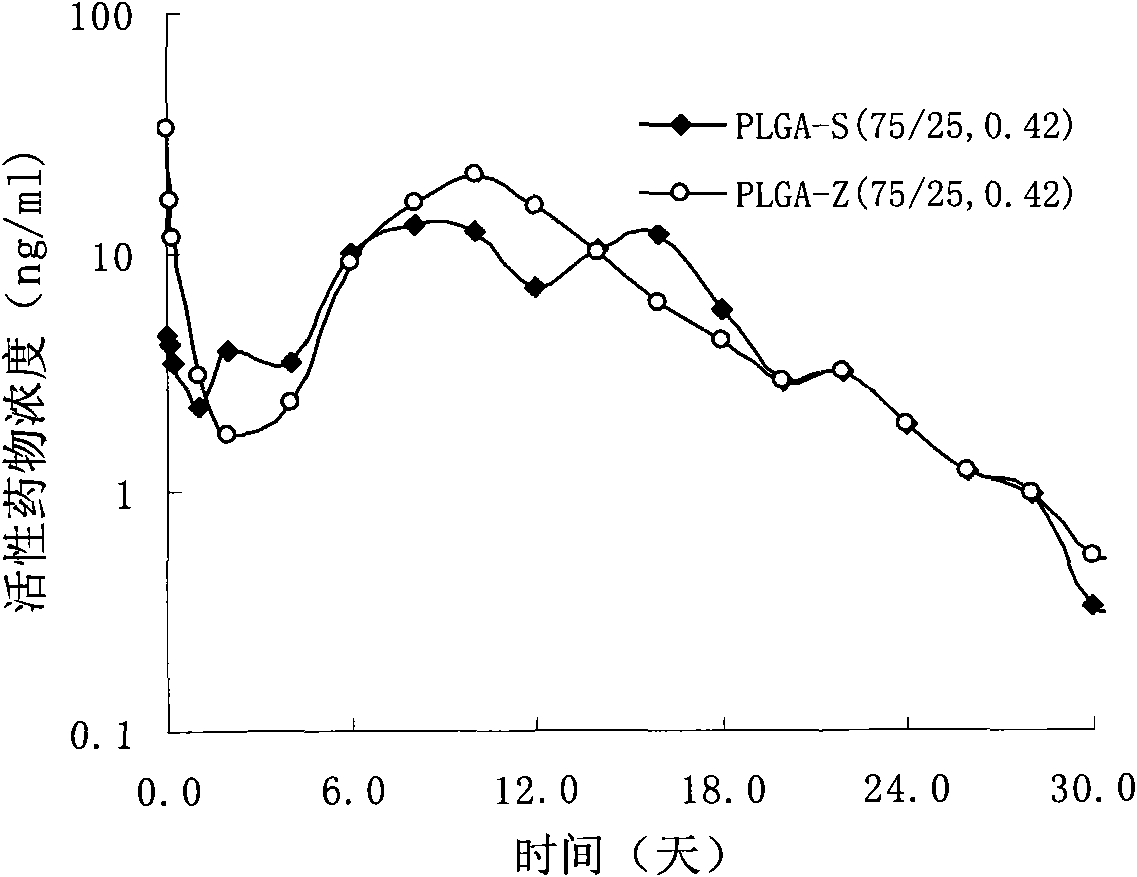
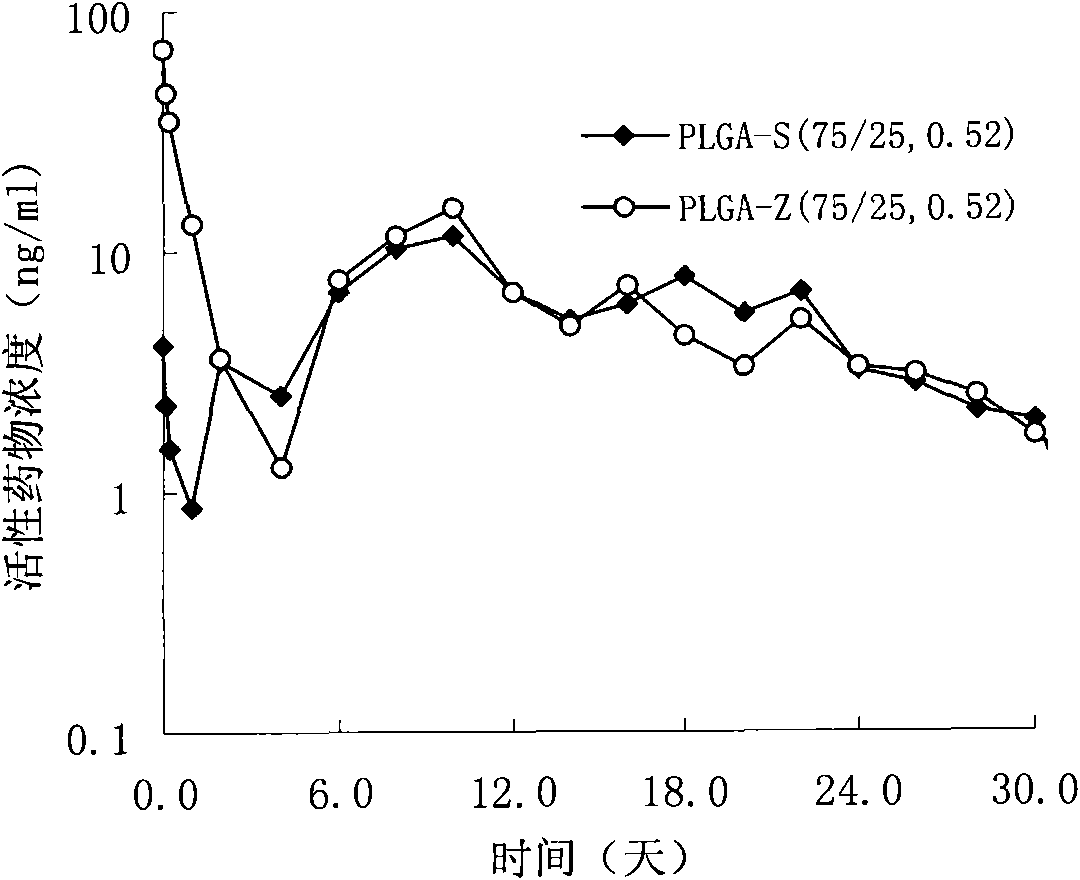
The invention provides a risperidone slow-release microsphere, a preparation method and an application thereof. The microsphere comprises risperidone or 9-hydroxy risperidone or the salt thereof and anon-end-capped lactide-glycollide copolymer. The risperidone slow-release microsphere provided by the invention has higher medicine-carrying quantity, no in-vivo sudden-release phenomenon, stable blood concentration and no medicine release lag period, reduces the administration frequency of a patient greatly, reduces the administration volume of each time, enhances the conformance of the patientand reduces the generation of adverse reactions.
Owner:SHANDONG LUYE PHARMA CO LTD +1
Compression Reactor And Process For Hydroprocessing
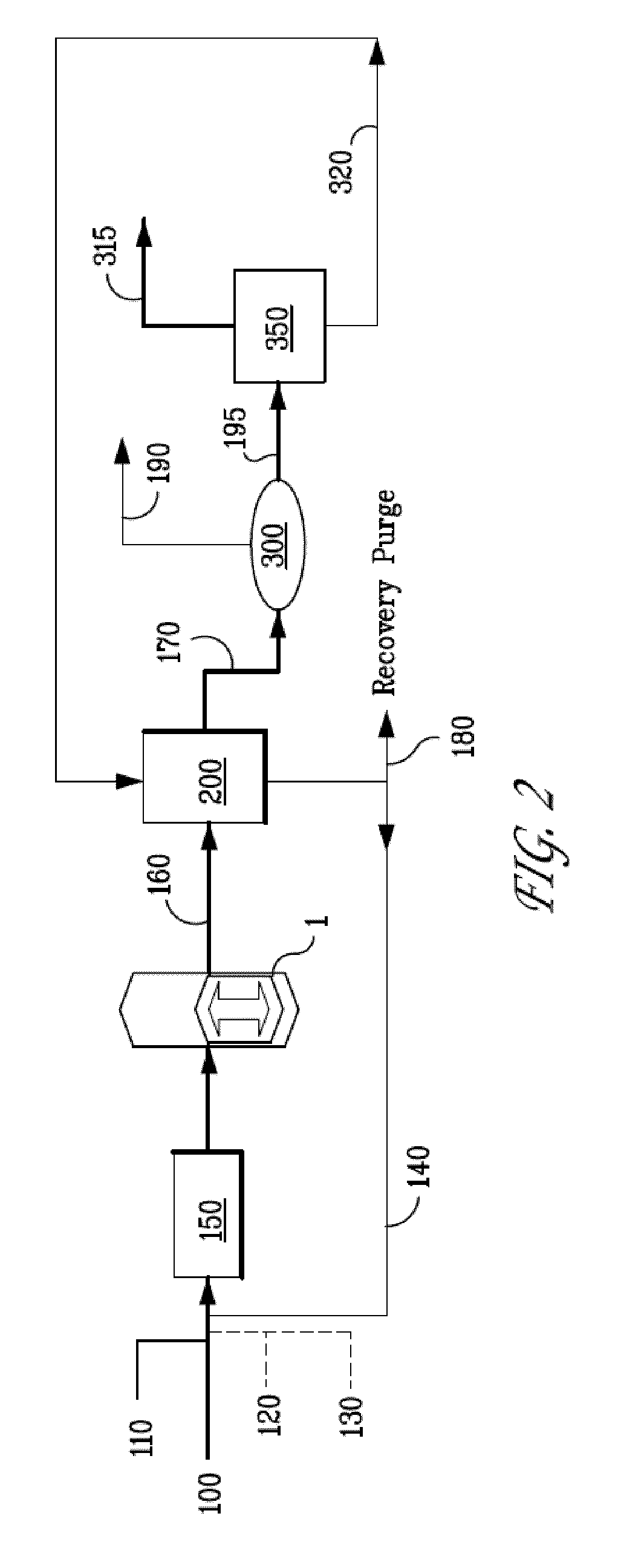
ActiveUS20110174682A1Reduce temperature and pressureReduce pressureThermal non-catalytic crackingUltra-high pressure processesLiquid hydrocarbonsResidence time
The present invention is directed to a process for hydroprocessing of a liquid hydrocarbon feedstock, comprising: (a) mixing liquid, partially vaporized and / or vaporized hydrocarbon feedstock with molecular hydrogen; (b) feeding said mixture into a compression reactor; (c) compressing said mixture to a pressure, a temperature and for a residence time sufficient to: i) thermally crack at least a portion of hydrocarbon molecules in said hydrocarbon feedstock, and ii) react hydrogen in the presence of a hydrogenation catalyst with unstable portions of the cracked molecules, forming a hydroprocessed product; and (d) expanding said mixture to reduce the pressure and temperature thereby reducing subsequent undesirable reactions.
Owner:EXXONMOBIL CHEM PAT INC
Crosslinkable macromers
InactiveUS6924370B2Improve performancePromote growthElectrotherapyDead plant preservationSolubilityFormamide
A crosslinkable macromer system and related methods of preparing the system and using the system in the form of a crosslinked matrix between a tissue site and an implant article such as a tissue implant or on the porous surface of a prosthetic device. The macromer system includes two or more polymer-pendent polymerizable groups and one or more initiator groups (e.g., polymer-pendent initiator groups). The polymerizable groups and the initiator group(s), when polymer-pendent, can be pendent on the same or different polymeric backbones. The macromer system provides advantages over the use of polymerizable macromers and separate, low molecular weight initiators, including advantages with respect to such properties as nontoxicity, efficiency, and solubility. A macromer system of the invention can be used as an interface between the tissue site and implant article in a manner sufficient to permit tissue growth through the crosslinked matrix and between the tissue site and implant. In a preferred embodiment, polymers with pendent polymerizable groups, for use in the macromer system, are prepared by reacting a polysaccharide polymer with a reactive moiety in an organic, polar solvent such as formamide.
Owner:SURMODICS INC
Hydrogenation purification method for siliceous distillate
ActiveCN101343565AIncrease capacityFast inactivationTreatment with hydrotreatment processesPurification methodsHydrogen
The invention relates to a silicon-containing distillate oil hydrofining method, which adopts the method that the silicon-containing distillate oil raw material and hydrogen pass through at least two hydrofining catalyst beds under the hydrofining condition, the silicon-containing distillate oil raw material first passes through a hydrogenising catalyst bed with the silicon catching function, and then passes through a conventional hydrofining catalyst bed; wherein the hydrogenising catalyst with the silicon catching function has a greater pore volume and a specific surface area and a relatively lower metal content. Compared with the prior art, the method has the advantages that the process is simple, the good hydrodesulfurization and hydrodedenitrification performance is kept on the premise of enhancing the silicon-containing ability, and the running period of the device is effectively prolonged. The method can be applied to various silicon-containing distillate oil hydrofining processes.
Owner:CHINA PETROLEUM & CHEM CORP +1
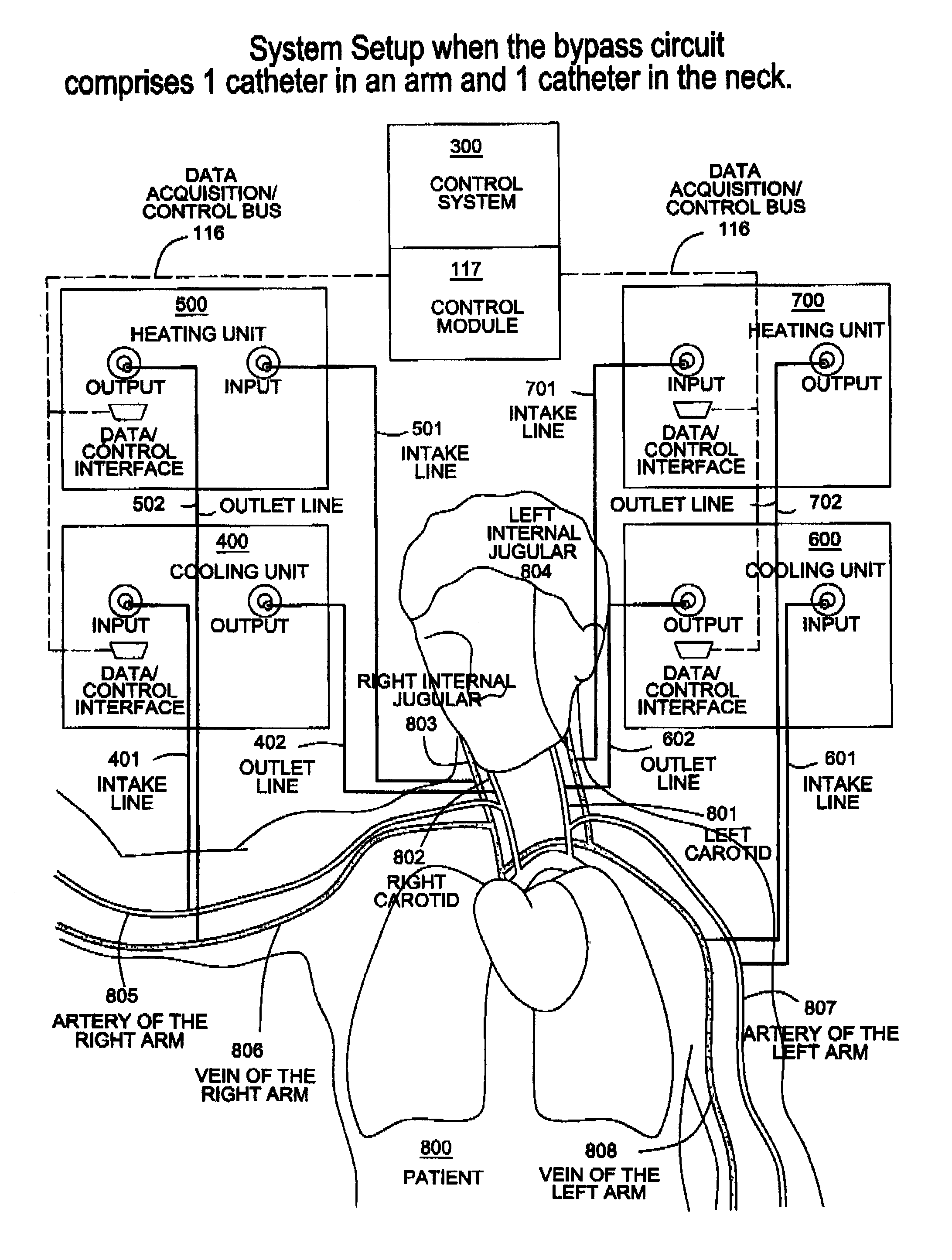
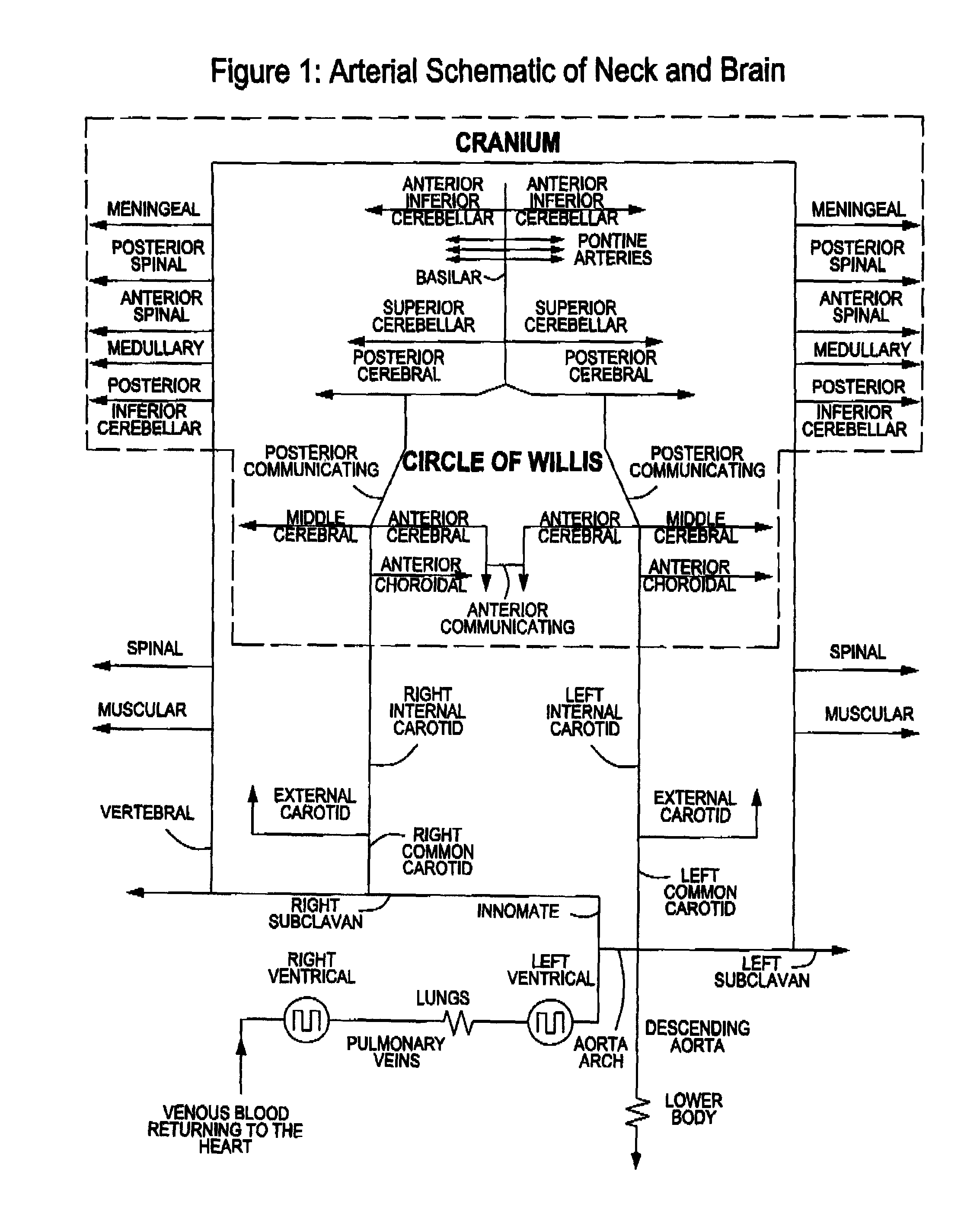
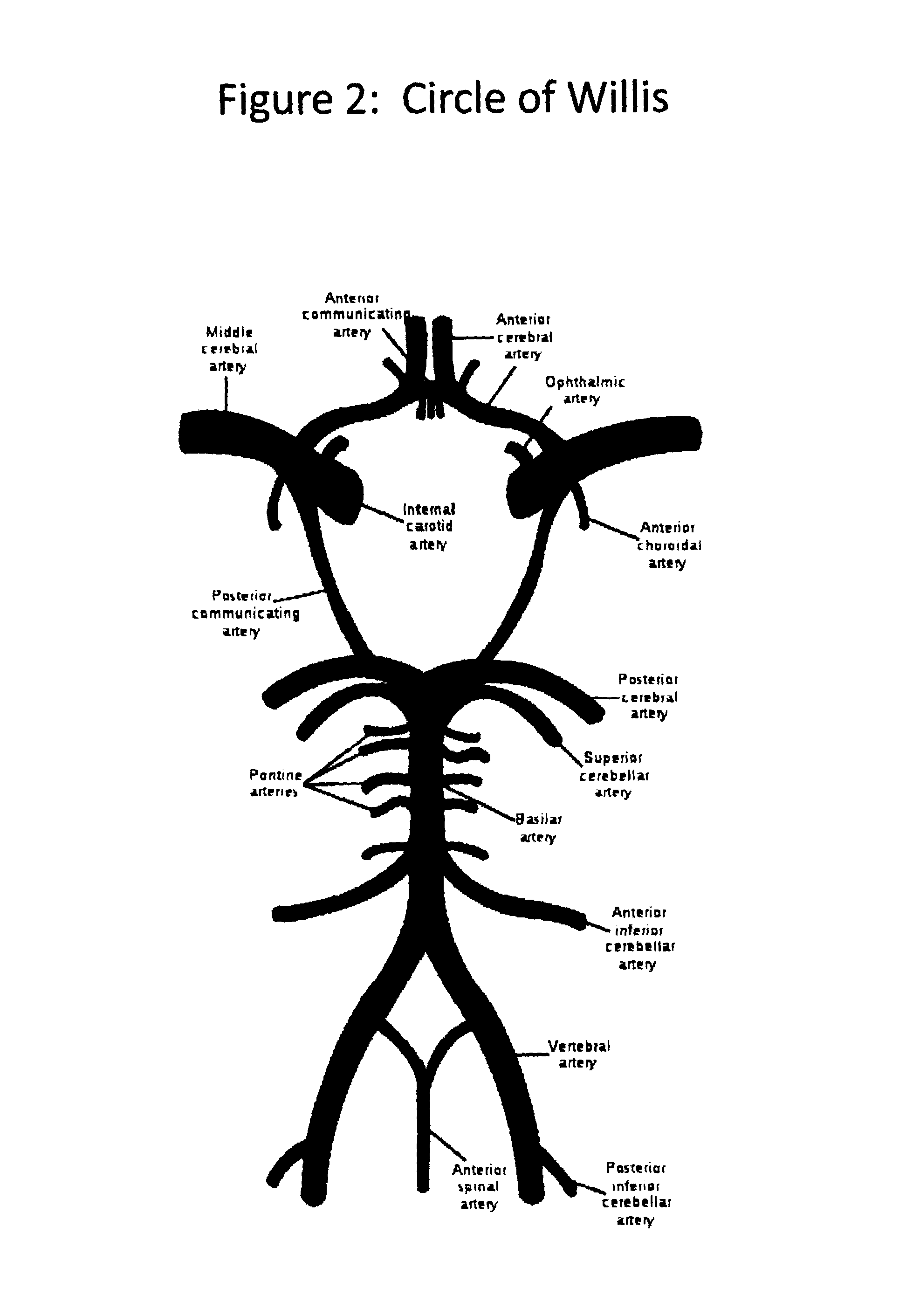
Apparatus, system and methods for extracorporeal blood processing for selectively cooling the brain relative to the body during hyperthermic treatment or to induce hypothermia of the brain
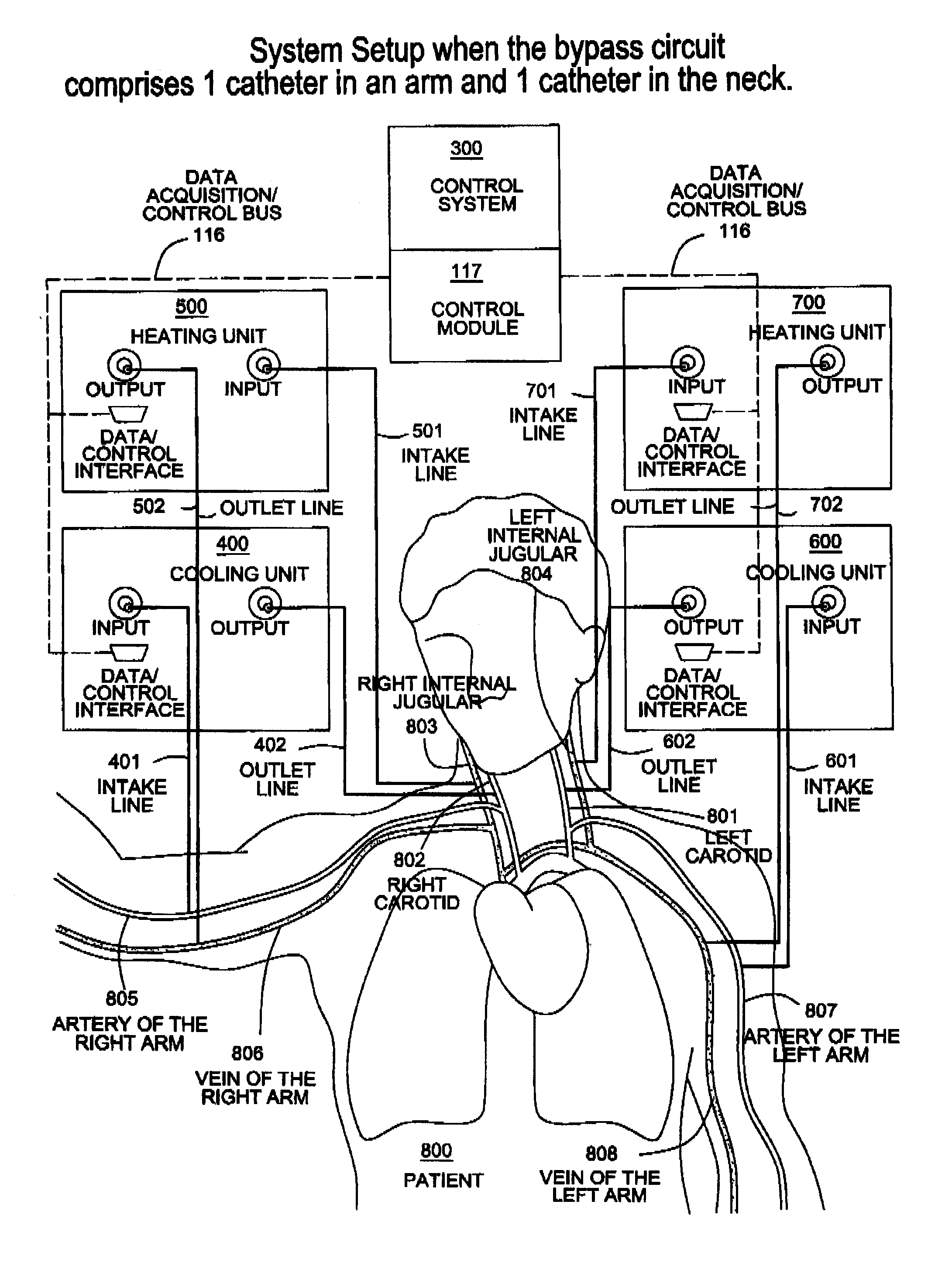
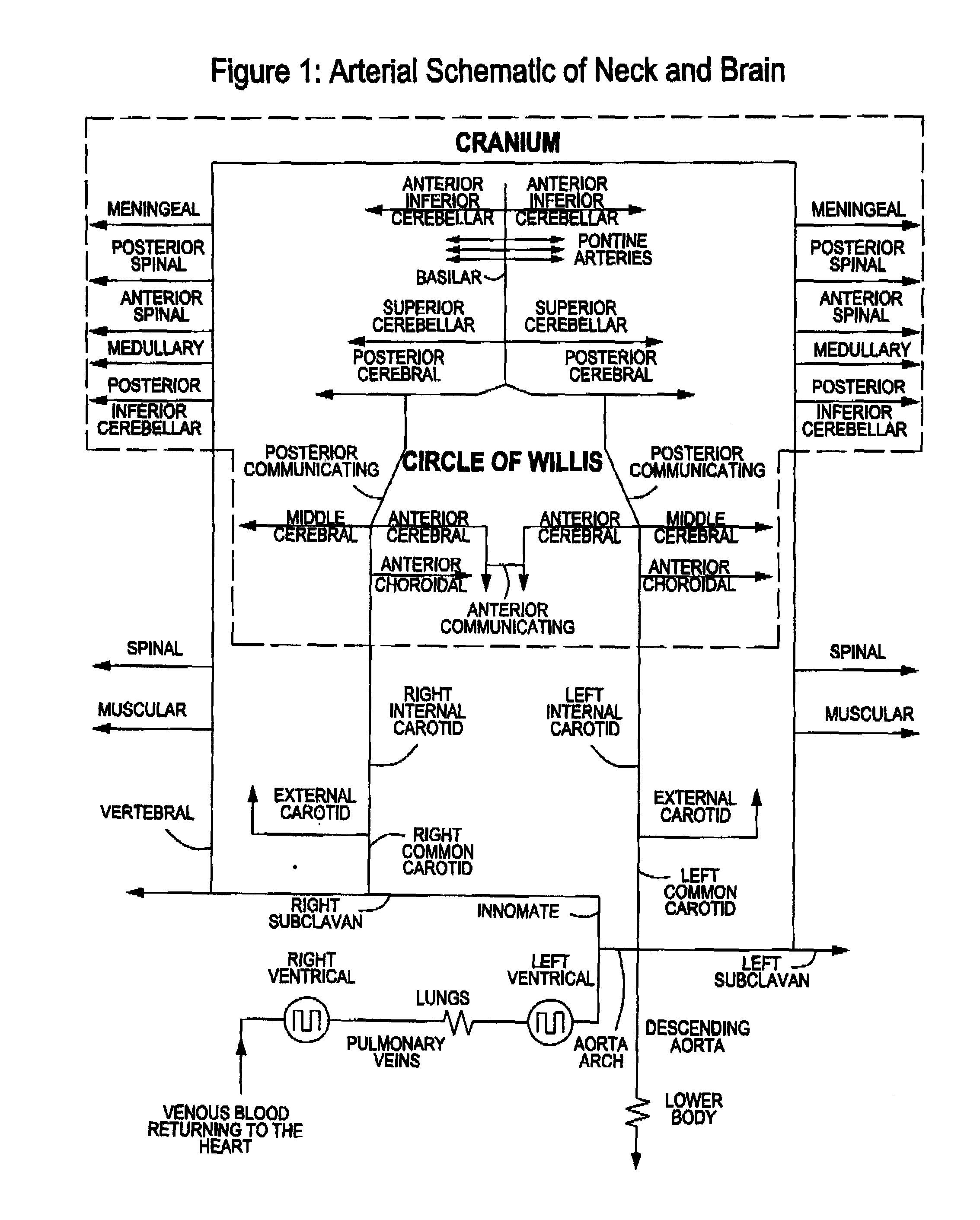
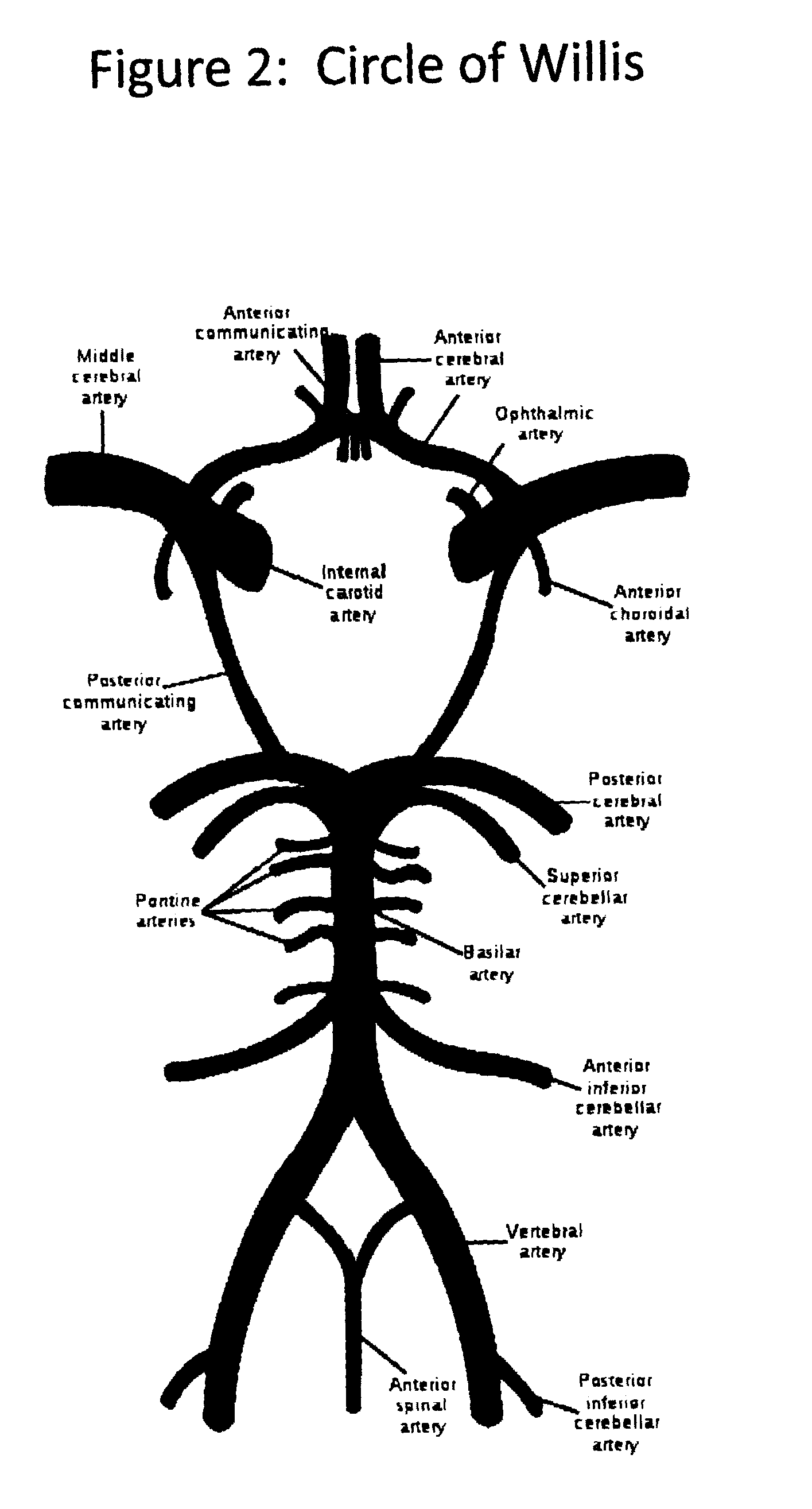
InactiveUS20120029408A1Reduce adverse reactionsReduce riskOther blood circulation devicesMedical devicesTherapeutic treatmentBlood processing
A system, apparatus and methods are provided for extra-corporeal blood treatment, and in particular for establishing and maintaining a neck down differential body temperature, while maintaining near normal brain temperatures, to protect the brain from extended or extreme hypothermia or hyperthermia. A blood treatment apparatus and system is provided for differential control of brain temperature and body temperature below the neck. For example, a first bypass circuit with heat exchanger for brain blood circulation maintains a near normal blood temperature, while a second bypass circuit for below the neck blood circulation provides for thermal treatment to induce a temperature differential, e.g. hyperthermia or hypothermia, relative to brain circulation. Such systems and apparatus have application, for example, for diagnostic and therapeutic treatments using hyperthermia, particularly for treatments of extended duration or at elevated temperatures above 42° C., for example, hyperthermia for treatment of cancer, infectious bacterial or viral diseases.
Owner:BEAUDIN STEVE ANDRE
Method for preparing ginseng polysaccharide
The invention refers to a preparation method of ginseng polysaccharide. The method includes the following step: extract it from the ginseng or the marc of the ginseng decocted, filtrate, concentrate and deposit the extracting solution, get the precipitate after centrifugence and dry it with low temperature to get the crude product of ginseng polysaccharide. We get the polysaccharide product after the ginseng polysaccharide goes through the boiling, depositing, filtration, trash extraction, frost thawing, getting rid of the protein and getting the supernatant and the supernatant passes through the concentration with low pressure and drying with low temperature. We can make it into all kinds of forms of prepared drugs after the ginseng polysaccharide passes through the experiments in pharmaceutics. The invention effectively increases the quality of the ginseng polysaccharide injection, fully exerts the immunoloregulation function of the ginseng polysaccharide, increases the anti tumor effect of chemotherapy medicament and is used to reduce the side effect caused by the knubbly radiotherapy and chemotherapy as well as adjuvant for curing the tumor.
Owner:JILIN SIHUAN PHARM CO LTD
Tandospirone citrate, preparation method thereof, formulations and quality control method
ActiveCN101362751ARaise quality standardsImprove clinical anxiolytic effectNervous disorderOrganic chemistryCITRATE ESTERQuality control
The invention provides citrate tandospirone which is characterized by comprising a compound I with the weight percent content not more than 0.5 percent and a compound II with the weight percent content not more than 0.5 percent. The structure formulas of the compound I and the compound II are as the right formulas. The invention also provides a preparation method and a quality control method of the citrate tandospirone. The results of a pharmacodynamic test and clinical observation show that after the quality standard of the citrate tandospirone is improved, the effect of clinical antianxiety is obviously improved and untoward effect is obviously reduced.
Owner:SICHUAN CREDIT PHARMA
Slow-releasing preparation containing metformin hydrochloride and glipizide and its preparation method
InactiveCN101057849AEvenly distributedReduce local irritationOrganic active ingredientsMetabolism disorderMedicineMetformin Hydrochloride
The invention discloses a diabecron and glipizide -containing slow-release agent and the method for preparing the same. The glipizide micro-pill takes blank micro-pill as carrier, and combines glipizide and other medical findings with it. The diabecron-containing slow-release micro-pill comprises diabetosan pill, slow-release coating membrane material or other medical findings. The method for preparing diabecron-containing slow-release micro-pill takes extrusion rolling method or blank micro-pill loading method. The product is characterized by safety, high efficient, low toxicity and convenient usage. It can be used to treat non-insulin-dependent diabetes mellitus.
Owner:QIQIHAR MEDICAL UNIVERSITY
Crosslinkable macromers
InactiveUS20060240072A1Increased toxicityImprove bindingBone implantAbsorbent padsSolubilityFormamide
A crosslinkable macromer system and related methods of preparing the system and using the system in the form of a crosslinked matrix between a tissue site and an implant article such as a tissue implant or on the porous surface of a prosthetic device. The macromer system includes two or more polymer-pendent polymerizable groups and one or more multifunctional initiator groups. The polymerizable groups and the initiator group(s), when polymer-pendent, can be pendent on the same or different polymeric backbones. The macromer system provides advantages over the use of polymerizable macromers and separate, low molecular weight initiators, including advantages with respect to such properties as nontoxicity, efficiency, and solubility. A macromer system of the invention can be used as an interface between the tissue site and implant article in a manner sufficient to permit tissue growth through the crosslinked matrix and between the tissue site and implant. In a preferred embodiment, polymers with pendent polymerizable groups, for use in the macromer system, are prepared by reacting a polysaccharide polymer with a reactive moiety in an organic, polar solvent such as formamide.
Owner:SURMODICS INC
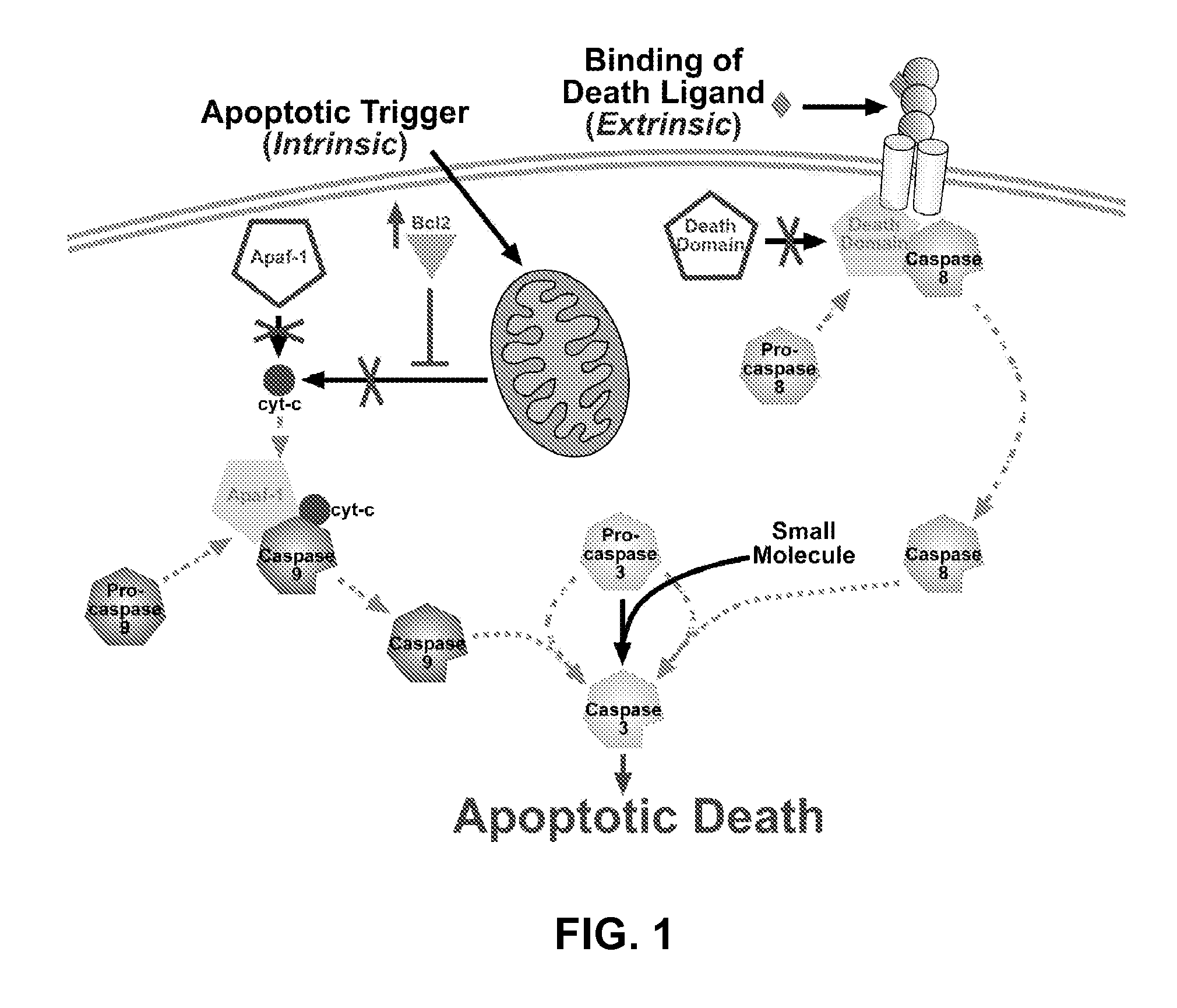
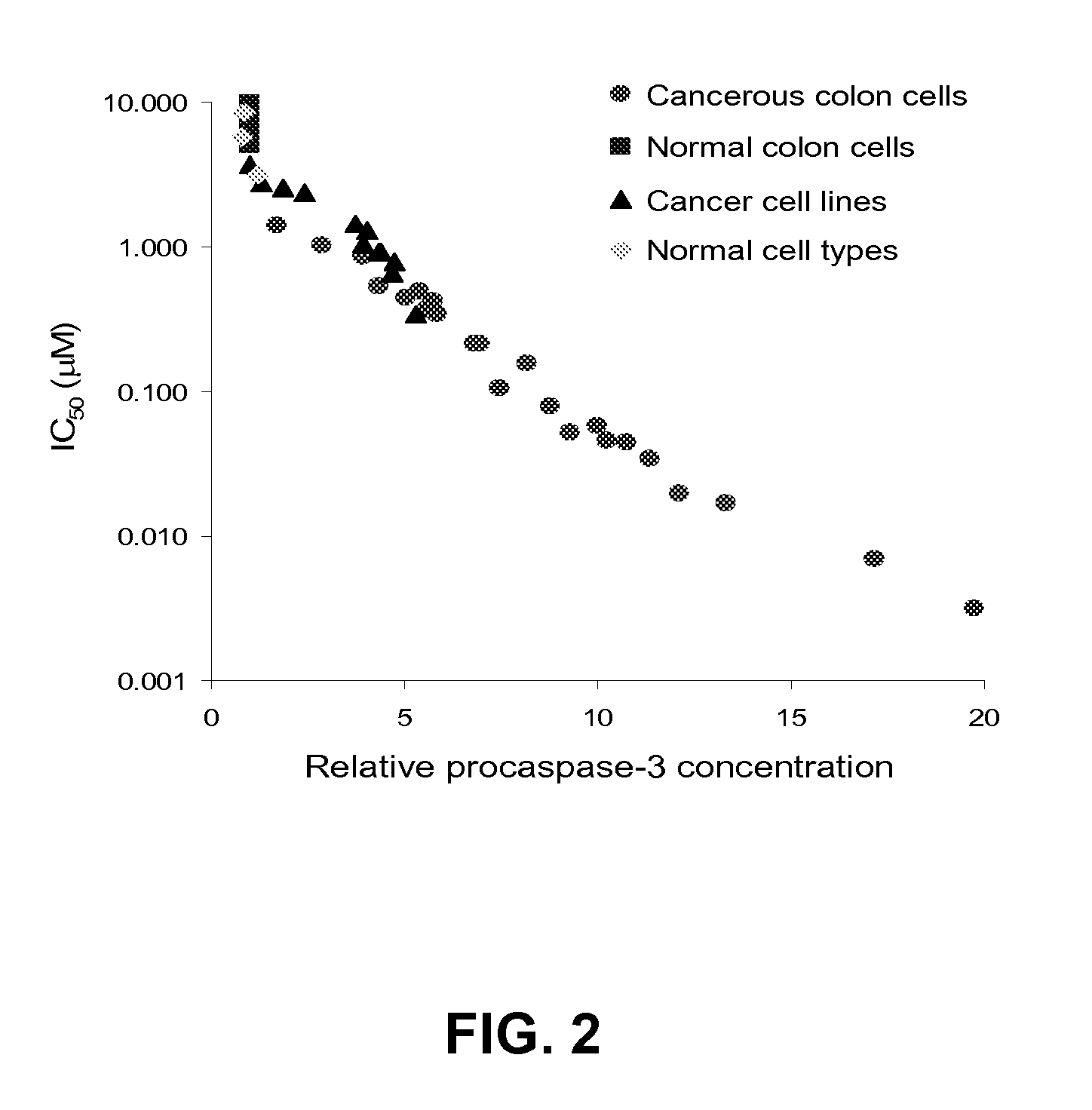
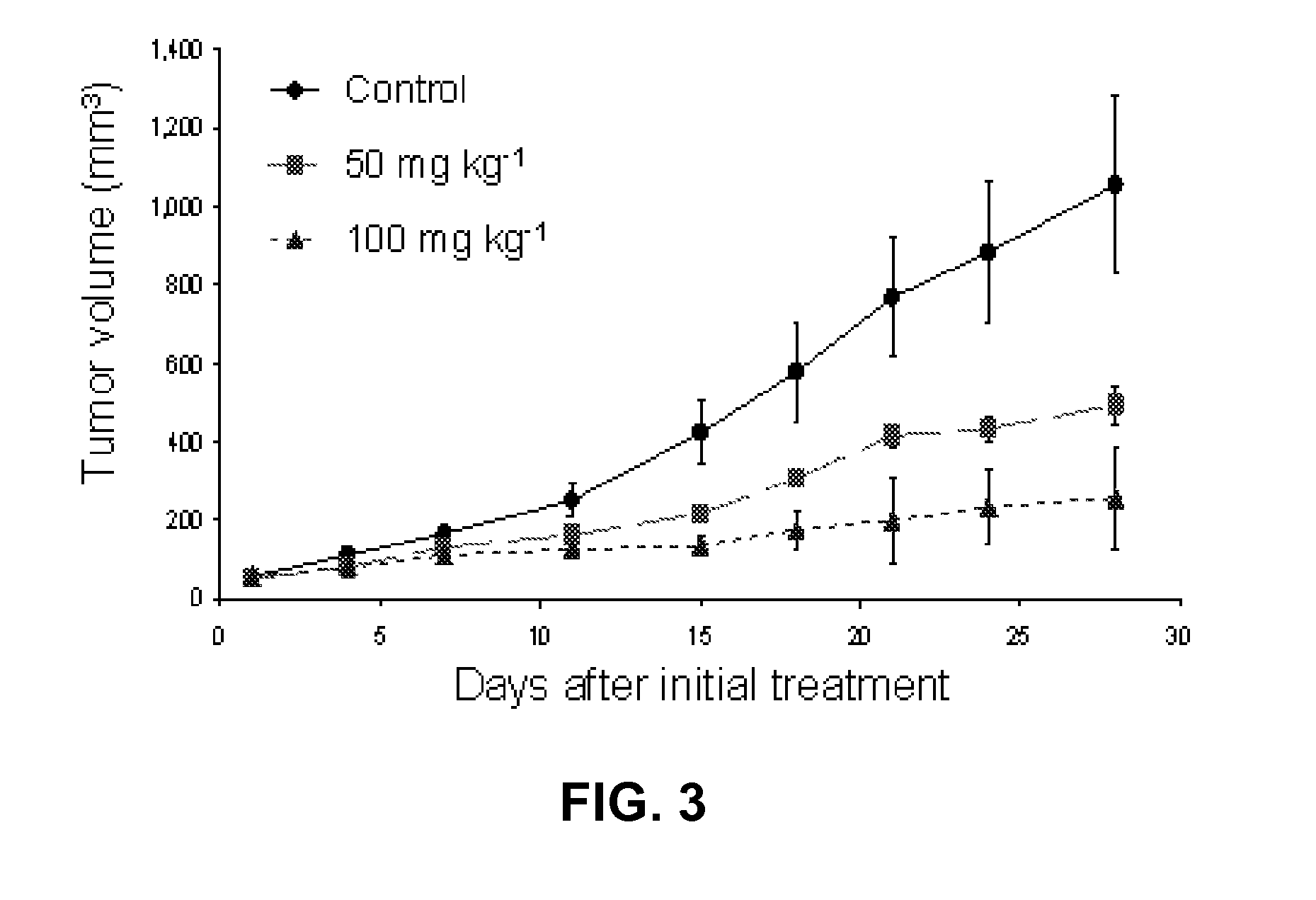
Design, synthesis and evaluation of procaspase activating compounds as personalized Anti-cancer drugs
ActiveUS20120040995A1Reduce adverse reactionsEffective compoundOrganic active ingredientsOrganic chemistryAnti cancer drugsNeurotoxic effect
Compositions and methods are disclosed in embodiments relating to induction of cell death such as in cancer cells. Compounds and related methods for synthesis and use thereof, including the use of compounds in therapy for the treatment of cancer and selective induction of apoptosis in cells are disclosed. Compounds are disclosed that have lower neurotoxicity effects than other compounds.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ILLINOIS
Panthenol and natural organic extracts for reducing skin irritation
InactiveUS20050136085A1Reducing irritation response and contact sensitizationReduce irritation responseCosmetic preparationsBiocideHair ColorantsActive agent
Panthenol combined with natural organic extracts are well suited for reducing skin irritation that is normally elicited by exposure to irritant active agents or compounds, particularly found in hair permanent and relaxing compositions, hair colorants and depilatories.
Owner:BELLAMY DAVID
A kind of gefitinib dispersible tablet and its preparation method and application
ActiveCN102266300AImprove bioavailabilityPromote dissolutionOrganic active ingredientsPill deliveryMedicineActive agent
The invention discloses a gefitinib dispersible tablet, a preparation method and an application thereof. The gefitinib dispersible tablet of the invention comprises the following components by weight: 10-65% of gefitinib, 1-30% of fillers, 10-50% of disintegrants, 1-60% of acidifiers, 0.1-20% of adhesives, and 0.1-30% of lubricants and glidants. According to the invention, gefitinib is wrapped bythe acidifier or gefitinib and the acidifier are wrapped with each other so as to reach the embedding effect. Compared with commercially available common tablets, the gefitinib dispersible tablet of the invention does not contain surfactants, has good dissolvability, dispersibility and disintegrability, and can be disintegrated completely within one minute. The gefitinib dispersible tablet prepared by the method of the invention has a high dissolution rate, good bioavailability, rapid distribution in vivo, and stable quality, and the preparation method is simple and practical, and is applicable to industrial production.
Owner:GUANGDONG PHARMA UNIV
Metformin hydrochloride enteric-coated tablets quality control method
ActiveCN101339178AFacilitated releaseGuaranteed to dissolveComponent separationColor/spectral properties measurementsPhosphateMetformin Hydrochloride
The invention discloses a quality control method of metformin hydrochloride enteric coated tablet, comprising the aspects of character, identification, examination and content measurement; wherein, release examination comprises the release quantity examination of acid in hydrochloric acid solution of 0.1 mol / l and the release quantity examination in phosphate buffer with the pH value of 6.8; the examination of relevant substances comprises the following steps: dicyandiamide is taken as reference, sulfonic group cation exchange bonded silica is taken as filler, ammonium dihydrogen phosphate solution of 1.7 percent with the pH value of 3 is mobile phase and the high performance liquid chromatography is used for examining the relevant substances. The invention controls the release quantity of the metformin hydrochloride enteric coated tablet in gastric juice strictly, reduces the adverse reaction of patients effectively, improves the release quantity of the metformin hydrochloride enteric coated tablet in the buffer solution (simulated intestinal juice) and ensures the dissolution of the enteric coated tablet in the intestinal juice effectively; the invention also adds the examination of dicyandiamide impurity under the examination item and enhances the safety of the medicine.
Owner:贵州天安药业股份有限公司
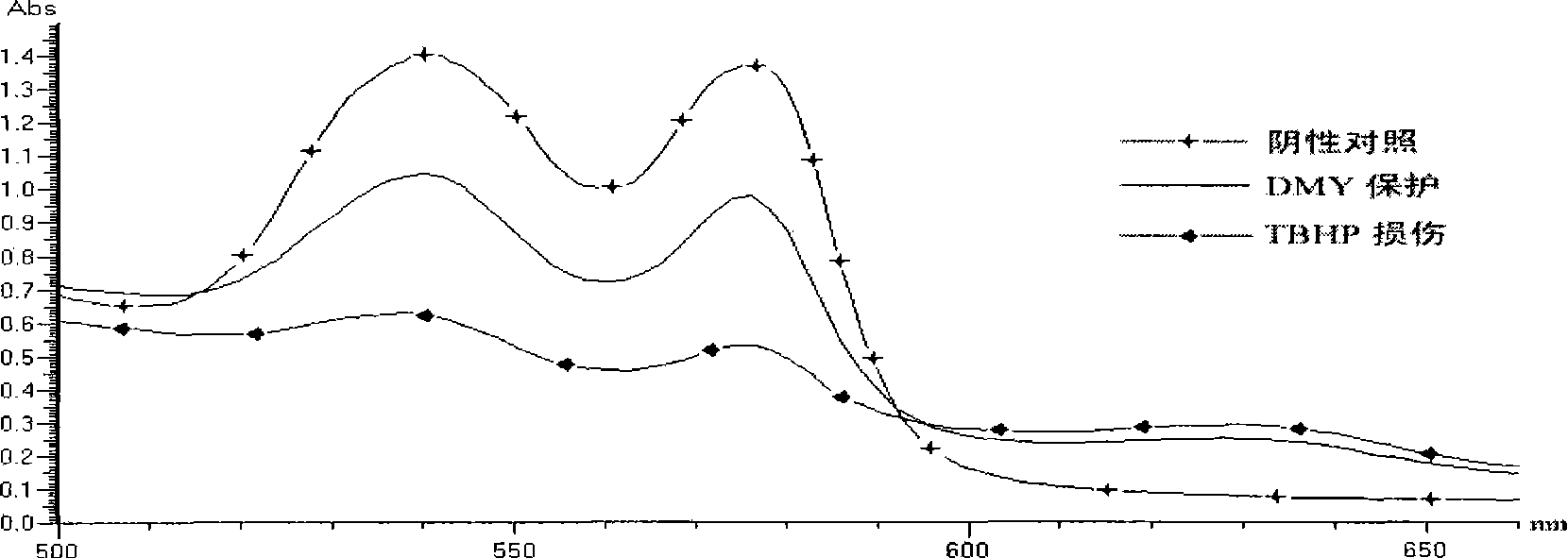
Application of dihydromyricetin in preparing medicament for preventing and treating adverse reaction of tumor chemoradiotherapy
InactiveCN101485655ADamage suppressionInhibition of poisoningOrganic active ingredientsAntinoxious agentsOncologyTumor cell apoptosis
The invention discloses application of dihydromyricetin in preparing a medicine for preventing and treating untoward reactions in radiotherapy and chemotherapy of tumor. In particular, the application is to prepare medicines for preventing and treating untoward reactions in radiotherapy and chemotherapy of tumor, preventing and treating arrest of bone marrow and baldness, resisting mutation, preventing and treating the generation of secondary tumors, preventing and treating tumor transfer, and preventing and treating breast cancer, cervical carcinoma, intestinal cancer and the like. The invention creatively finds that the dihydromyricetin has the effects of removing free radicals, inhibiting reaction chains of the free radicals, resisting the mutation, inhibiting the expression of the tumor gene, causing the death of tumor cells, preventing and treating the tumor generation, preventing and treating the secondary tumors and the transfer of the secondary tumors and preventing and treating infection. Therefore, as the medicine for preventing and treating untoward reactions in radiotherapy and chemotherapy of tumor, the dihydromyricetin can inhibit and alleviate chemical damage, prevent and treat damage of a mutagen and further prevent gene mutation or the secondary tumors; and by combination with the radiotherapy and chemotherapy, the effects of preventing untoward reactions in radiotherapy and chemotherapy of tumor and preventing generation of tumors are achieved.
Owner:SOUTH CHINA UNIV OF TECH
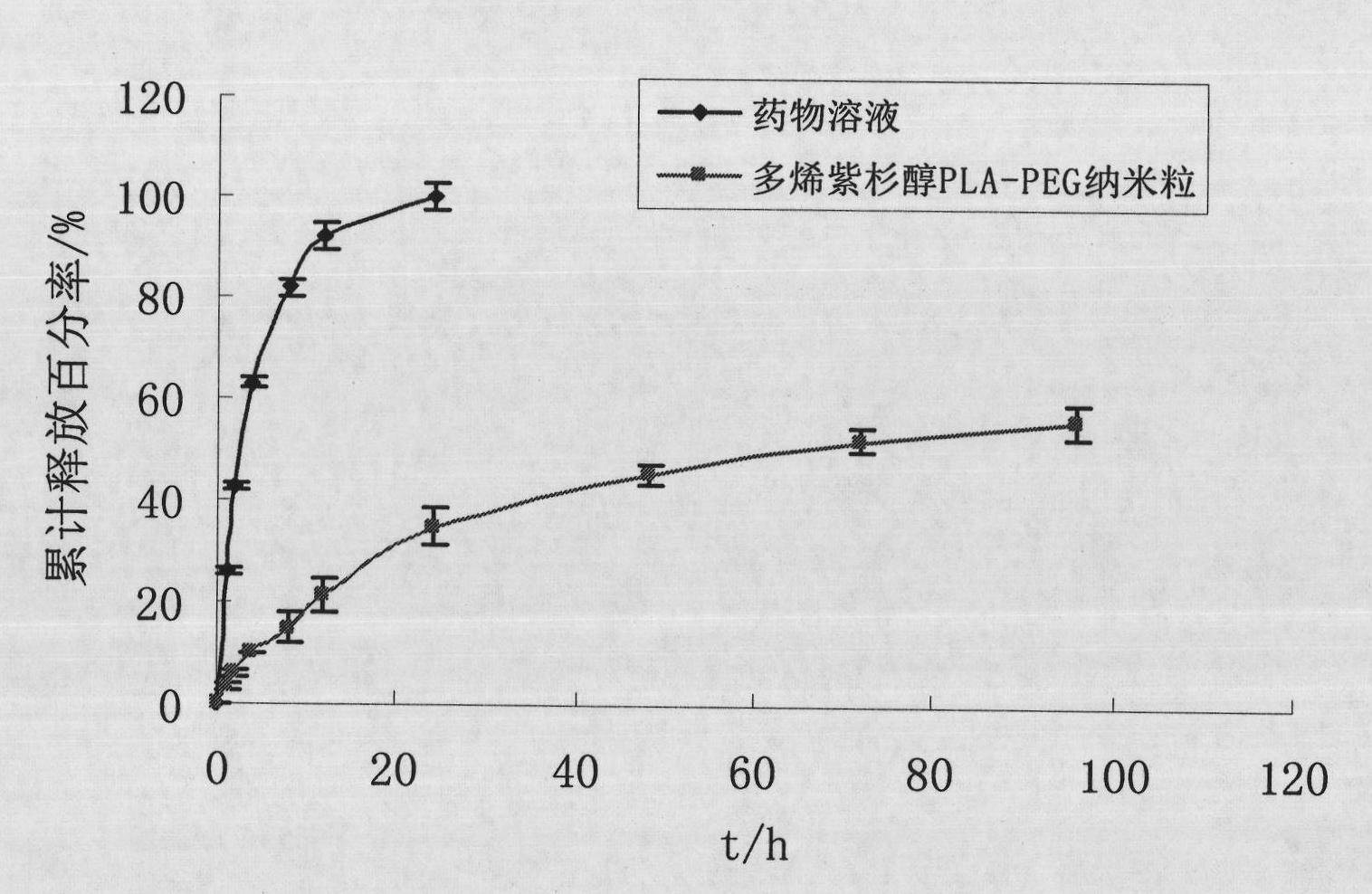
Preparation method of polyene-containing taxol nanoparticle mixed micelle preparation and freeze-drying agent
InactiveCN101804021AImprove solubilityHigh metabolic stabilityOrganic active ingredientsPowder deliveryMixed micelleFreeze-drying
The invention discloses a preparation method of a polyene-containing taxol nanoparticle mixed micelle preparation and a freeze-drying agent, which prepares docetaxel PLA-PEG nanoparticles or micelle or nanoparticle mixed micelle through a modified solvent evaporation method, takes PLA-PEG copolymer as a carrier, and wraps docetaxel in a PLA hydrophobic core. When in use, the docetaxel PLA-PEG containing long cycle freeze-dried preparation only needs to be added with water and is dissolved, and uniform nanoparticle suspension, micellar solution or mixed micellar nanoparticle suspension can be prepared. The preparation method does not need tween-80 and ethanol solubilization, only takes the biodegradable PLA-PEG as the carrier, and does not contain any surfactant; and compared with the docetaxel injection on sale, the preparation can reduce the toxicity and the adverse reactions of the medicine, and improve the clinical application safety of the medicine.
Owner:SHANDONG UNIV
Quick-disintegration tablets of calcium atovastatine, and its prepn. method
ActiveCN1911209AIncreased or improved disintegrationEnhanced or improved dissolutionMetabolism disorderPill deliveryLubricantChemistry
Owner:CSPC OUYI PHARM CO LTD
Adjuvant compositions and methods for delivering vaccines
InactiveUS20080292663A1Strong immune responseReduce adverse reactionsInorganic non-active ingredientsPharmaceutical delivery mechanismAntigenAdjuvant
An adjuvant composition is provided that is comprised of a calcium compound, lecithin, and an acrylic polymer. Pharmaceutical compositions are provided which include an antigen and the adjuvant. Methods are provided for stimulating an immune response in a human or animal subject by administering a composition comprising an antigen and the adjuvant composition to human or animal subjects.
Owner:ADVANCED BIOADJUVANTS
Apparatus, system and methods for extracorporeal blood processing for selectively cooling the brain relative to the body during hyperthermic treatment or to induce hypothermia of the brain
InactiveUS8834404B2Reduce adverse reactionsReduce riskOther blood circulation devicesMedical devicesTherapeutic treatmentBlood processing
Owner:BEAUDIN STEVE ANDRE
Preparation of hyaluronic-acid-based double-targeting nano-composite medicament and application of double-targeting nano-composite medicament
InactiveCN103143027AHigh mechanical strengthAccelerate the degradation rateOrganic active ingredientsPharmaceutical non-active ingredientsTumor targetDispersity
The invention relates to a hyaluronic-acid-based double-targeting nano-composite medicament and a preparation method thereof. Hydrophobic group ursodeoxycholic acid is included in a hyaluronic acid nano-polymer structure and can form an amphipathic polymer and automatically generate micelles in an aqueous solution, and polyethylene glycol can be introduced into the micelles to improve the dispersity and stability of a composite. An anti-tumor medicament can enter a nano-carrier through electrostatic adsorption or physical inclusion to generate a nano-medicament composite, wherein the nano-medicament composite is selectively concentrated in a tumor cell under an active targeting effect of hyaluronic acid and a surface CD44 receptor of a tumor cell, and promotes a tumor tissue to absorb the nano medicament-carrying composite by using a passive osmotic accumulation effect (EPR) at the same time. After an anti-tumor medicament is wrapped by a modified hyaluronic acid polymer, the anti-tumor medicament has the advantages of improving the bioavailability of the medicament, improving the targeting property, reducing the toxic and side effects, prolonging the half-life period of the medicament, being stably stored and the like, so that the tumor targeting therapy efficiency is improved in many ways.
Owner:XIAMEN UNIV
Tranexamic acid skin externally applied nano-preparation, as well as preparation method and use thereof
InactiveCN103565743AIncrease percutaneous permeabilityGood curative effectPeptide/protein ingredientsEmulsion deliveryUse medicationMedicine
The invention relates to a tranexamic acid skin externally applied nano-preparation, as well as a preparation method and a use thereof. The nano-preparation is prepared from tranexamic acid, medicated oil, an emulsifier, pharmaceutical additives, a thickener and pure water. The tranexamic acid skin externally applied nano-preparation provided by the invention has good physiological skin compatibility and can effectively promote the percutaneous absorption of tranexamic acid, improve the effect of preventing or treating pigmentation of the tranexamic acid and avoid toxicity and side effects, which are caused by systemic administration of the tranexamic acid.
Owner:CENT HOSPITAL XUHUI DISTRICT SHANGHAI CITY +1
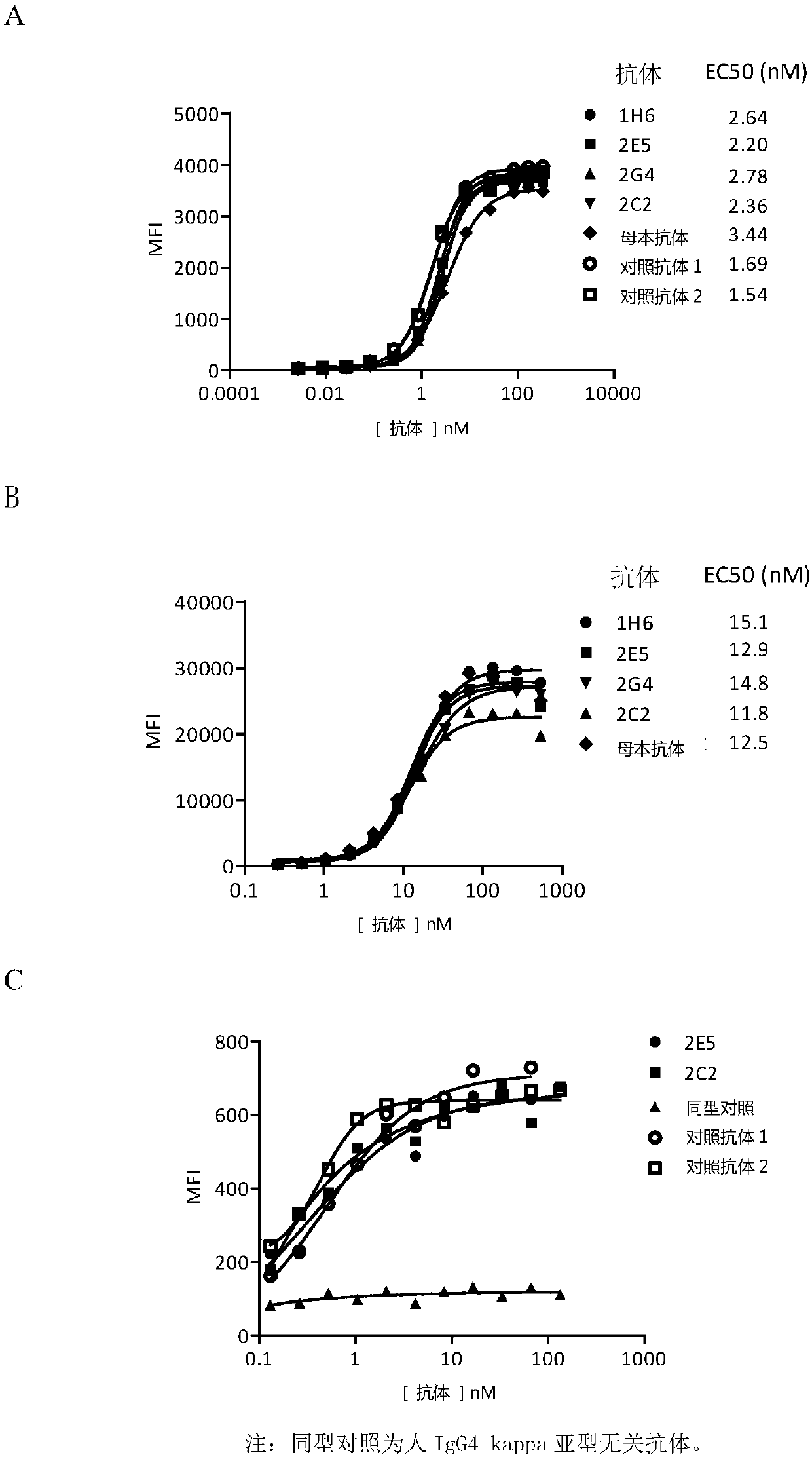
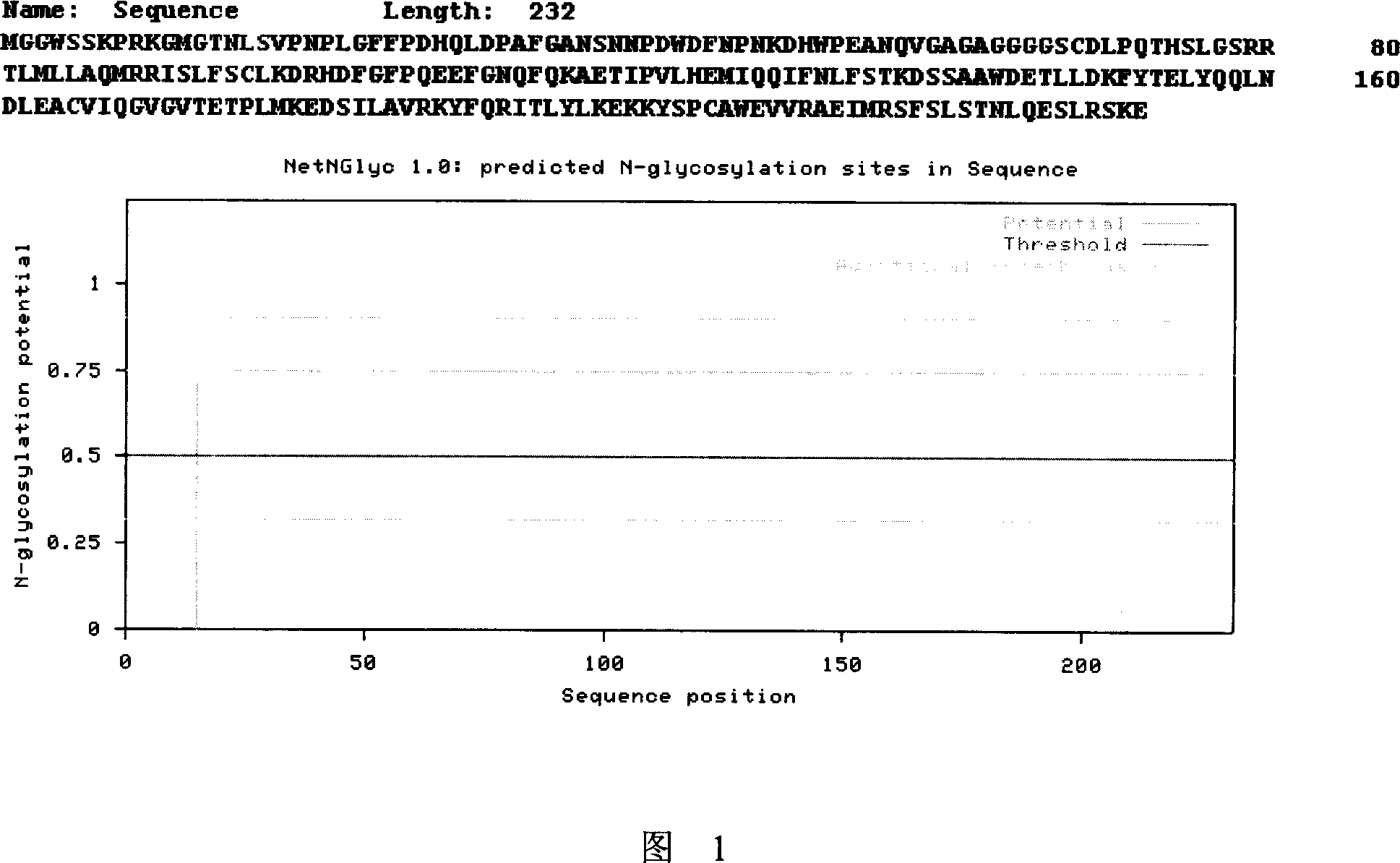
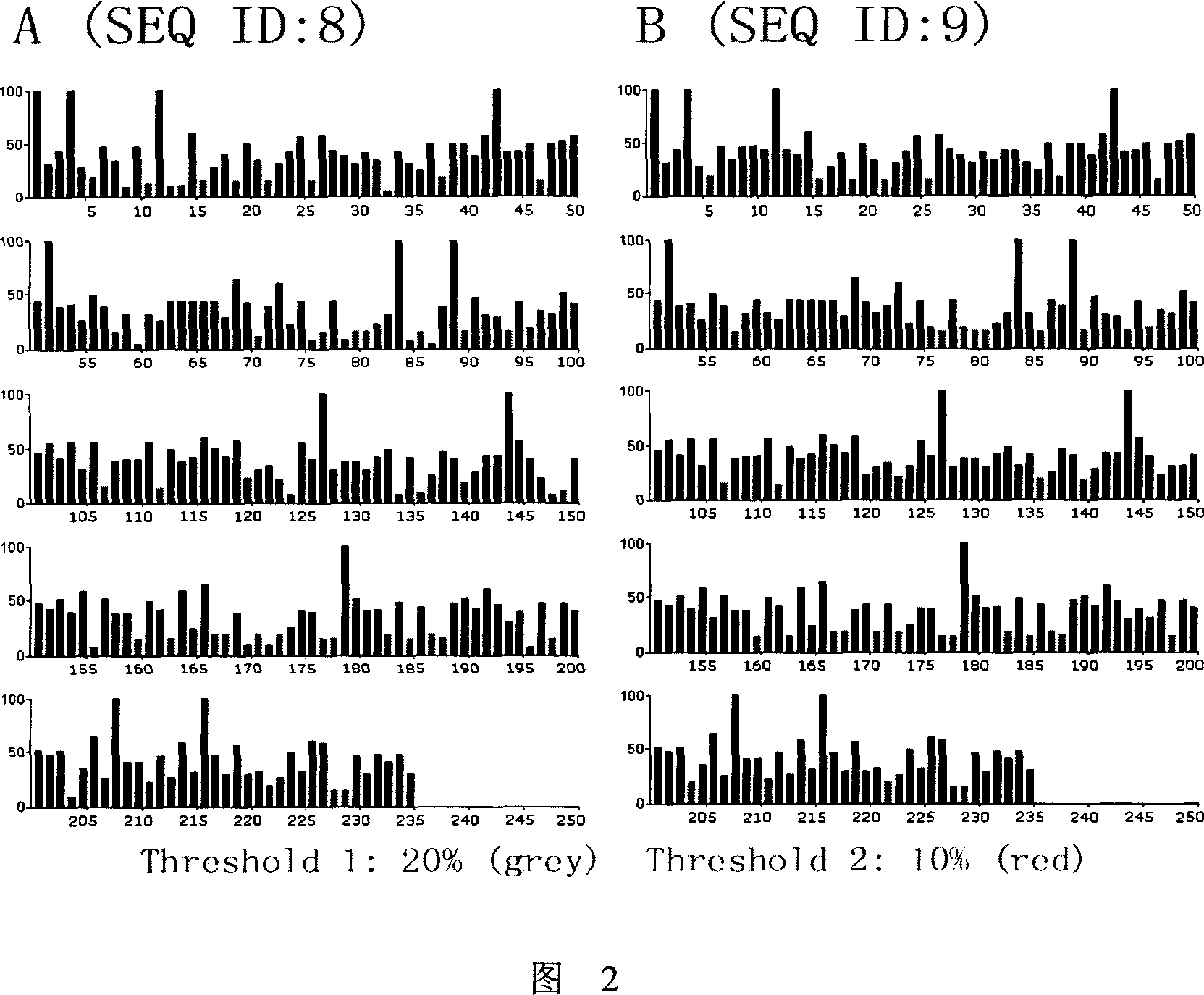
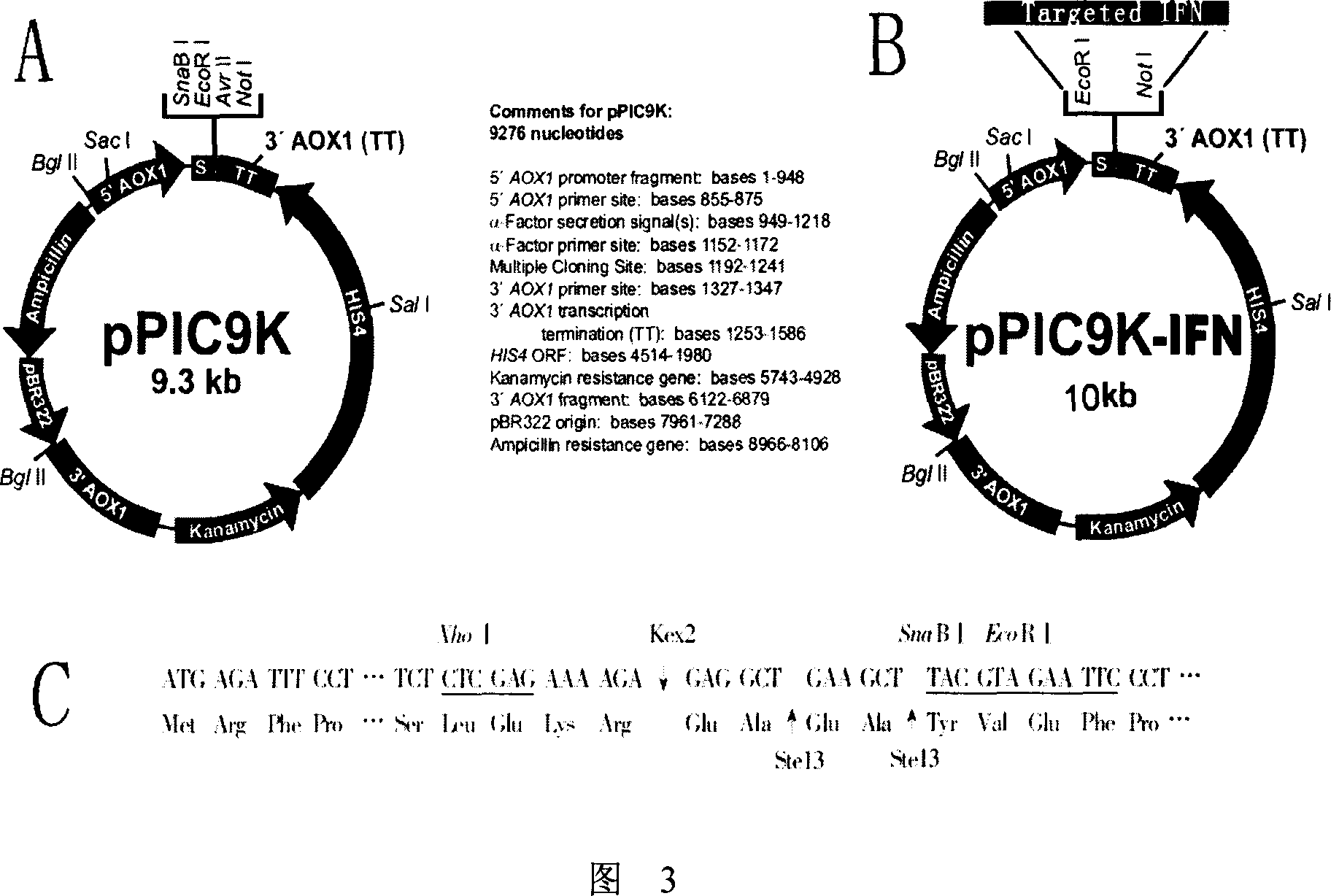
Novel monoclonal antibody against PD-1
ActiveCN107840887AHigh binding affinityPromote proliferationGenetically modified cellsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainNucleic acid sequencing
The invention provides a monoclonal antibody against PD-1, especially a human monoclonal antibody against PD-1. The antibody specifically binds to PD-1 in virtue of high affinity and comprises a heavychain and a light chain. The invention also provides a nucleic acid sequence coding the antibody, a cloning or expression vector, a host cell, a method for expressing or isolating the antibody, and an immunoconjugate and therapeutic composition containing the antibody. The invention further provides application of the anti-PD-1 antibody to treatment of various cancers.
Owner:CSTONE PHARM (SUZHOU) CO LTD +2
Special target medicine and its use
A specific target medicine, the nucleic acid sequence for coding it, the expression carrier containing said nucleic acid sequence, the host cell transformed from said carrier, and its application in preparing the medicines for treating hepatism, such as viral hepatitis, hepatocirrhosis and liver tumor, are disclosed.
Owner:SHANGHAI HEP PHARMA
Crosslinkable macromers
InactiveUS20050136091A1Increased toxicityImprove bindingPowder deliverySurgical adhesivesSolubilityFormamide
A crosslinkable macromer system and related methods of preparing the system and using the system in the form of a crosslinked matrix between a tissue site and an implant article such as a tissue implant or on the porous surface of a prosthetic device. The macromer system includes two or more polymer-pendent polymerizable groups and one or more initiator groups (e.g., polymer-pendent initiator groups). The polymerizable groups and the initiator group(s), when polymer-pendent, can be pendent on the same or different polymeric backbones. The macromer system provides advantages over the use of polymerizable macromers and separate, low molecular weight initiators, including advantages with respect to such properties as nontoxicity, efficiency, and solubility. A macromer system of the invention can be used as an interface between the tissue site and implant article in a manner sufficient to permit tissue growth through the crosslinked matrix and between the tissue site and implant. In a preferred embodiment, polymers with pendent polymerizable groups, for use in the macromer system, are prepared by reacting a polysaccharide polymer with a reactive moiety in an organic, polar solvent such as formamide.
Owner:SURMODICS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com