Tandospirone citrate, preparation method thereof, formulations and quality control method
A tandospirone citrate, quality technology, applied in the field of high-purity tandospirone citrate, can solve the problem of unfavorable large-scale production, no tandospirone synthesis process impurity components, structure and properties, high production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Preparation of cis-exo-bicyclo[2.2.1]heptane-2.3-dicarboximide of the present invention
[0056]
[0057] The transformation product is obtained by transformation of norbornene diacid anhydride at 190-210 ° C, and the transformation product is hydrogenated under the condition of palladium carbon as a catalyst to obtain a hydride, and the hydride is ammoniated with ammonia water to obtain cis-exo-bicyclic [2.2.1 ] Heptane-2.3-dicarboximide.
Embodiment 2
[0058] Example 2 Another preparation scheme of cis-exo-bicyclo[2.2.1]heptane-2.3-dicarboximide of the present invention:
[0059]
[0060] Add 97g of maleimide to the reaction flask, add 1000ml of ethyl acetate, stir to dissolve, add 93g of cyclopentadiene, stir for 24 hours, evaporate 75% of the solvent under reduced pressure, filter, wash and filter with an appropriate amount of ether Cake to obtain 163 g of the specific configuration, with a yield of about 86%. Dissolve 163g of the specific configuration in 2500ml of THF, add 163g of cyclohexene and 5g of 5% palladium carbon, feed hydrogen at a pressure greater than 0.01MPa, and reflux the reactant for 9h. After cooling, evaporate the solvent under reduced pressure. The residue was recrystallized from toluene to obtain 161 g of the product, with a yield of about 98%. The purity of the cis-outer-bicyclo[2.2.1]heptane-2.3-dicarboximide obtained by this method meets the high-purity cis-outer-bicyclo[2.2.1]heptane described...
Embodiment 3
[0061] Example 3 The quality control method of cis-exo-bicyclo[2.2.1]heptane-2.3-dicarboximide of the present invention
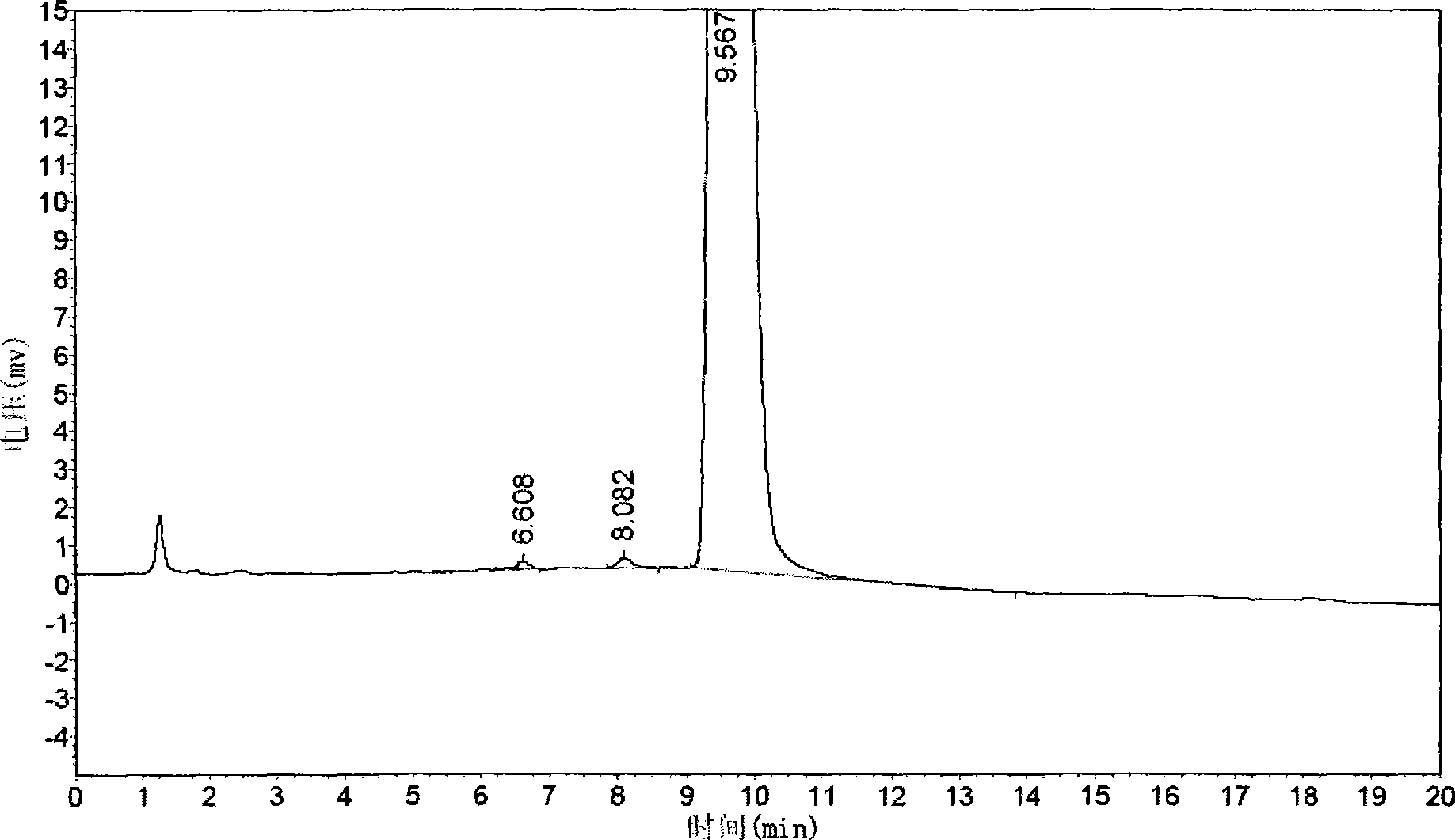
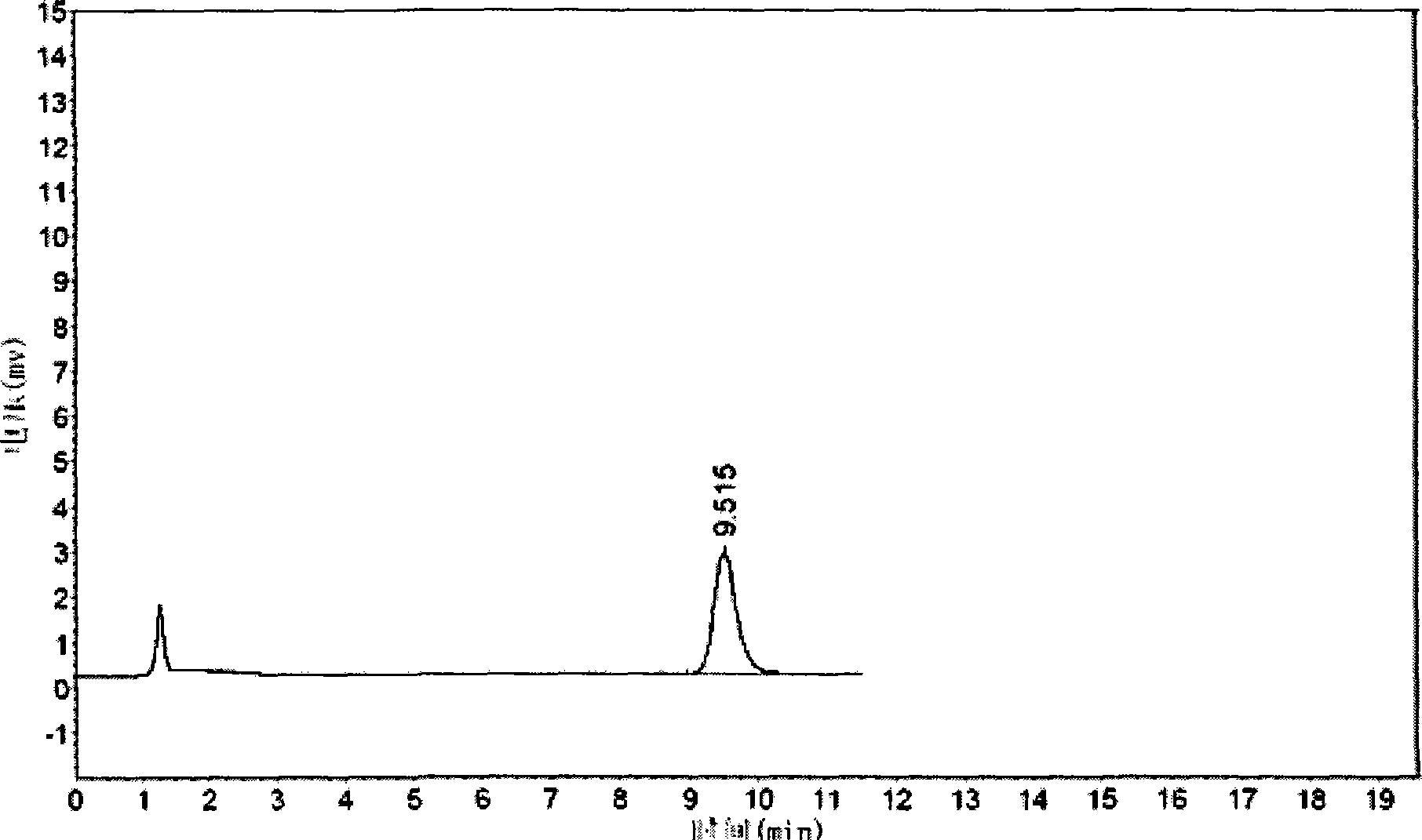
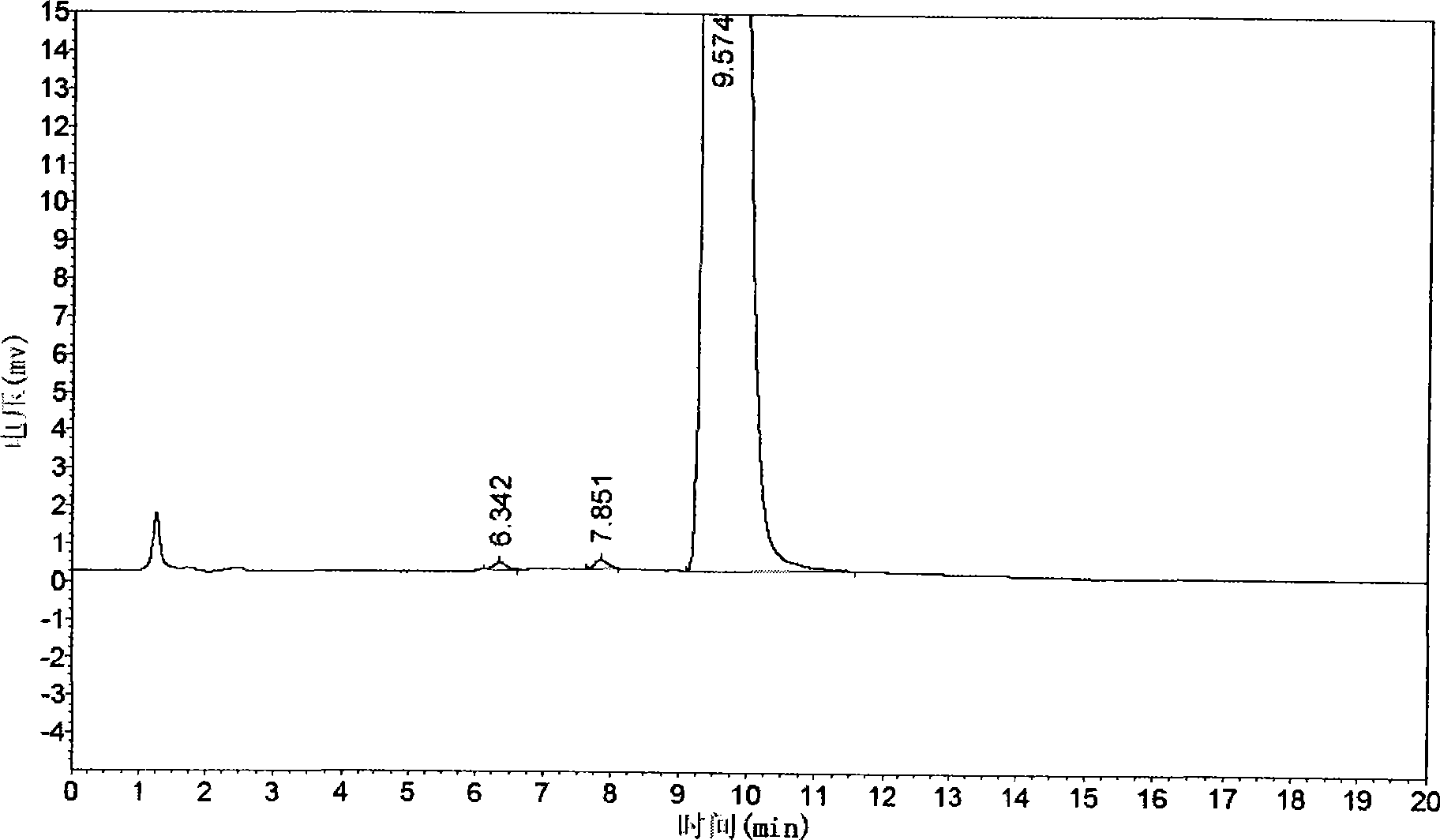
[0062] The quality of cis-exo-bicyclo[2.2.1]heptane-2.3-dicarboximide prepared in Examples 1 and 2 was measured.
[0063] The purity of cis-exo-bicyclo[2.2.1]heptane-2.3-dicarboximide was determined according to the "Operating Procedures for High Performance Liquid Chromatography".
[0064] Chromatographic conditions and system suitability test use octadecylsilane bonded silica gel as filler; 0.01mol / L potassium dihydrogen phosphate solution (adjust pH to 7.5 with 10% sodium hydroxide solution)-acetonitrile (80:20) is Mobile phase; detection wavelength is 243nm. The number of theoretical plates calculated based on the peak of cis-exo-bicyclo[2.2.1]heptane-2.3-dicarboximide should not be less than 1500.
[0065] Determination method Take about 0.1g of cis-exo-bicyclo[2.2.1]heptane-2.3-dicarboximide, put it in a 10ml measuring bottle, add mobile phase to di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com