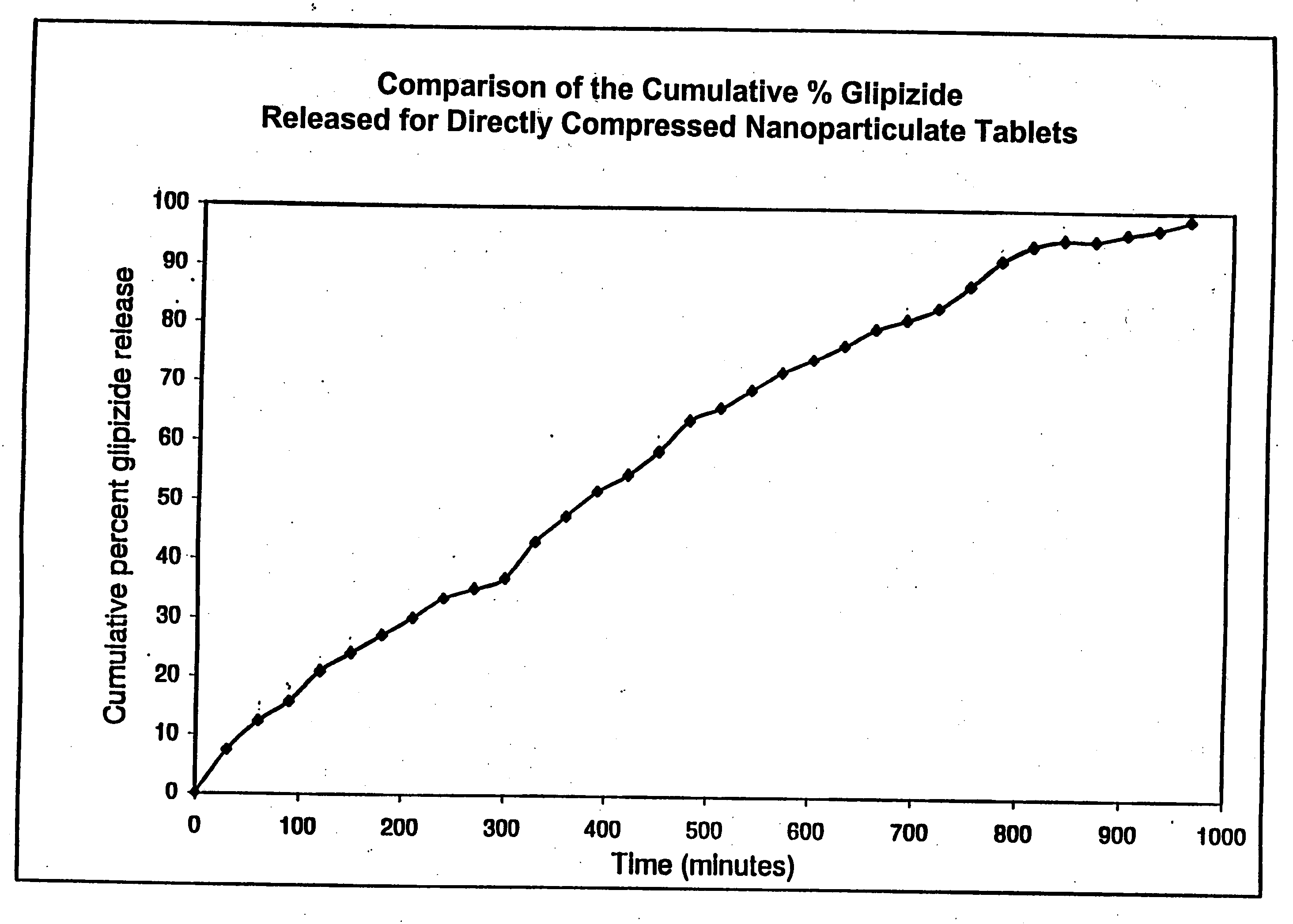
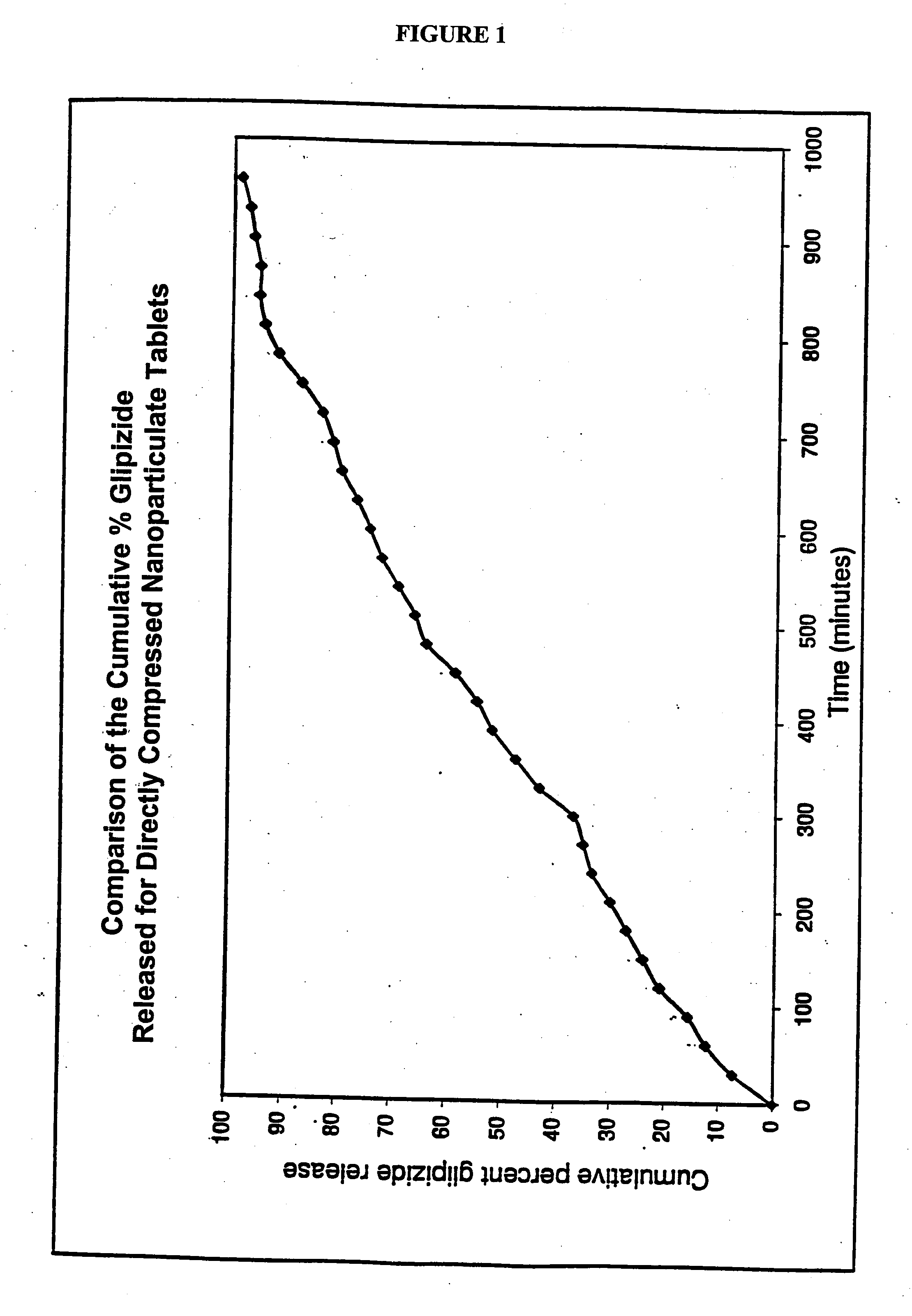
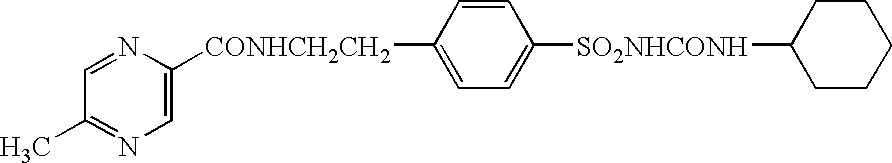
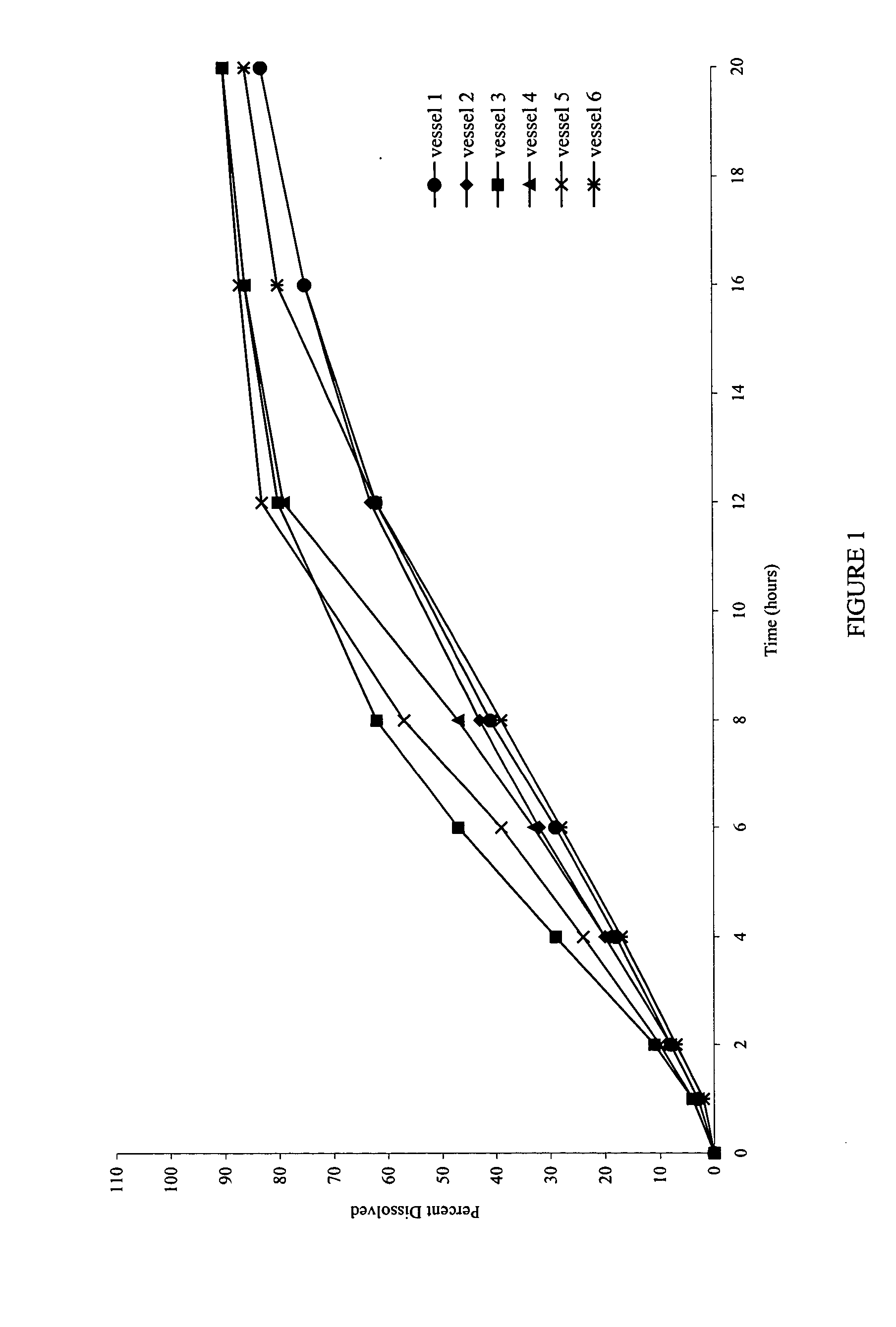
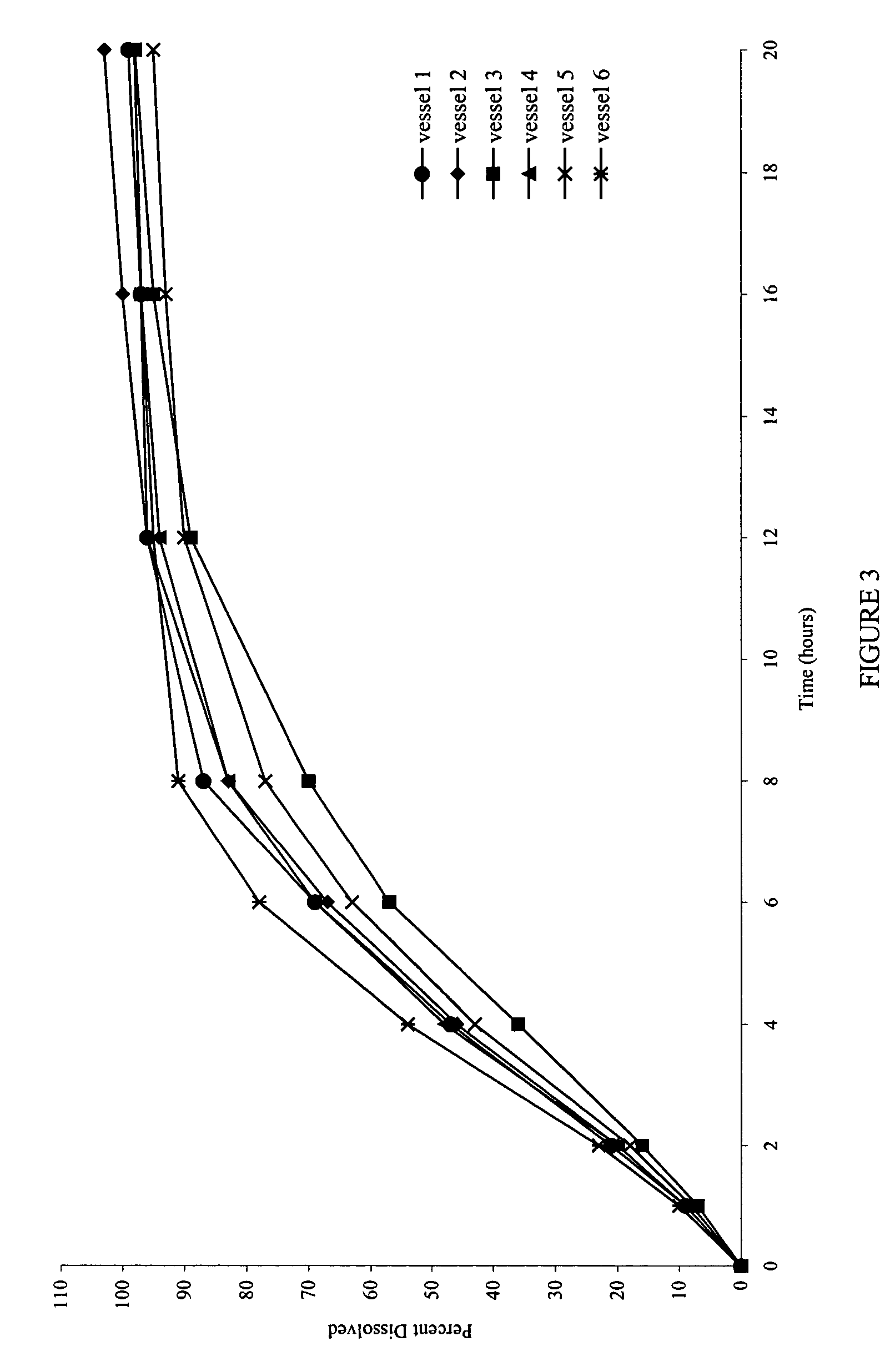
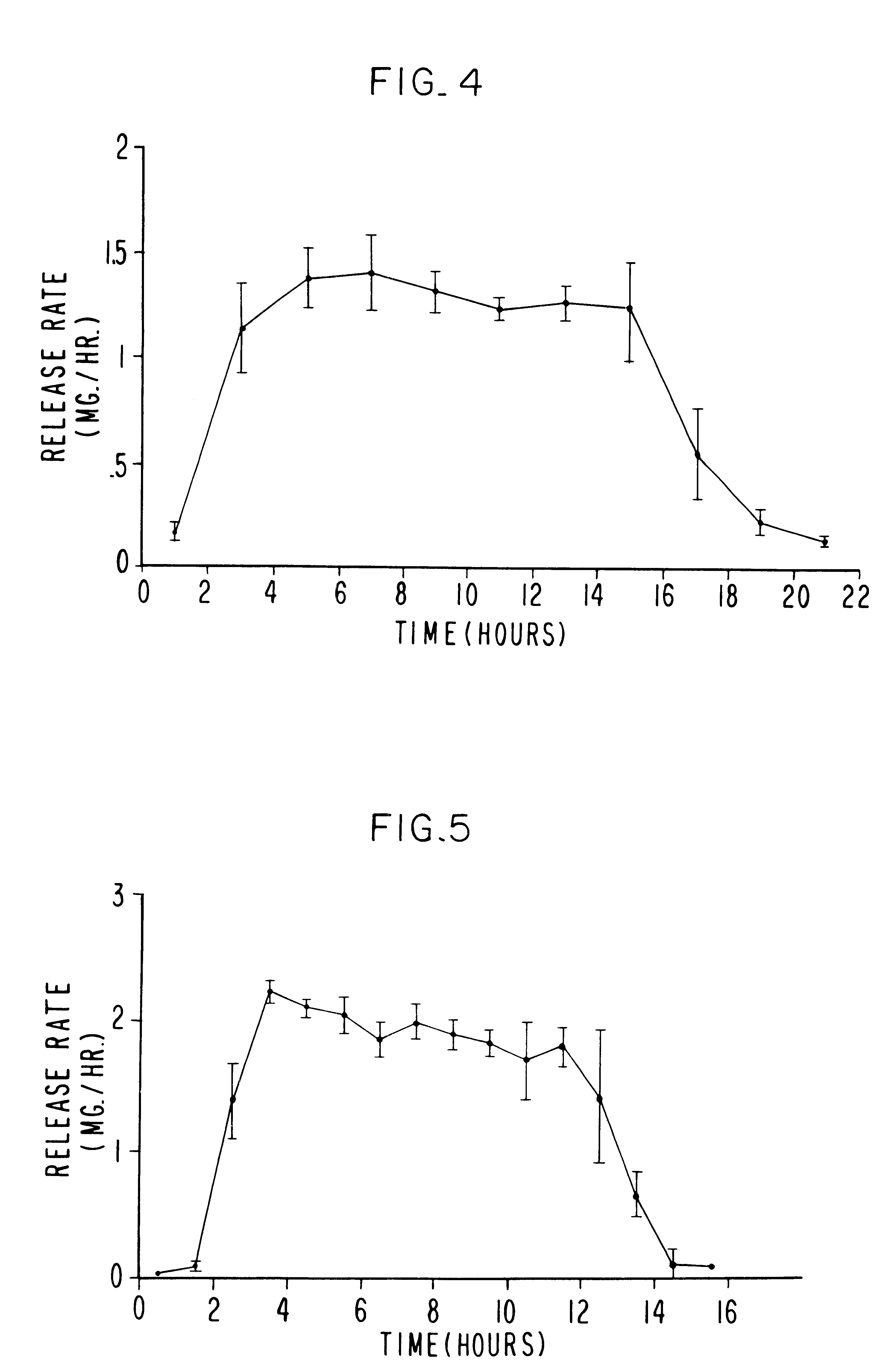
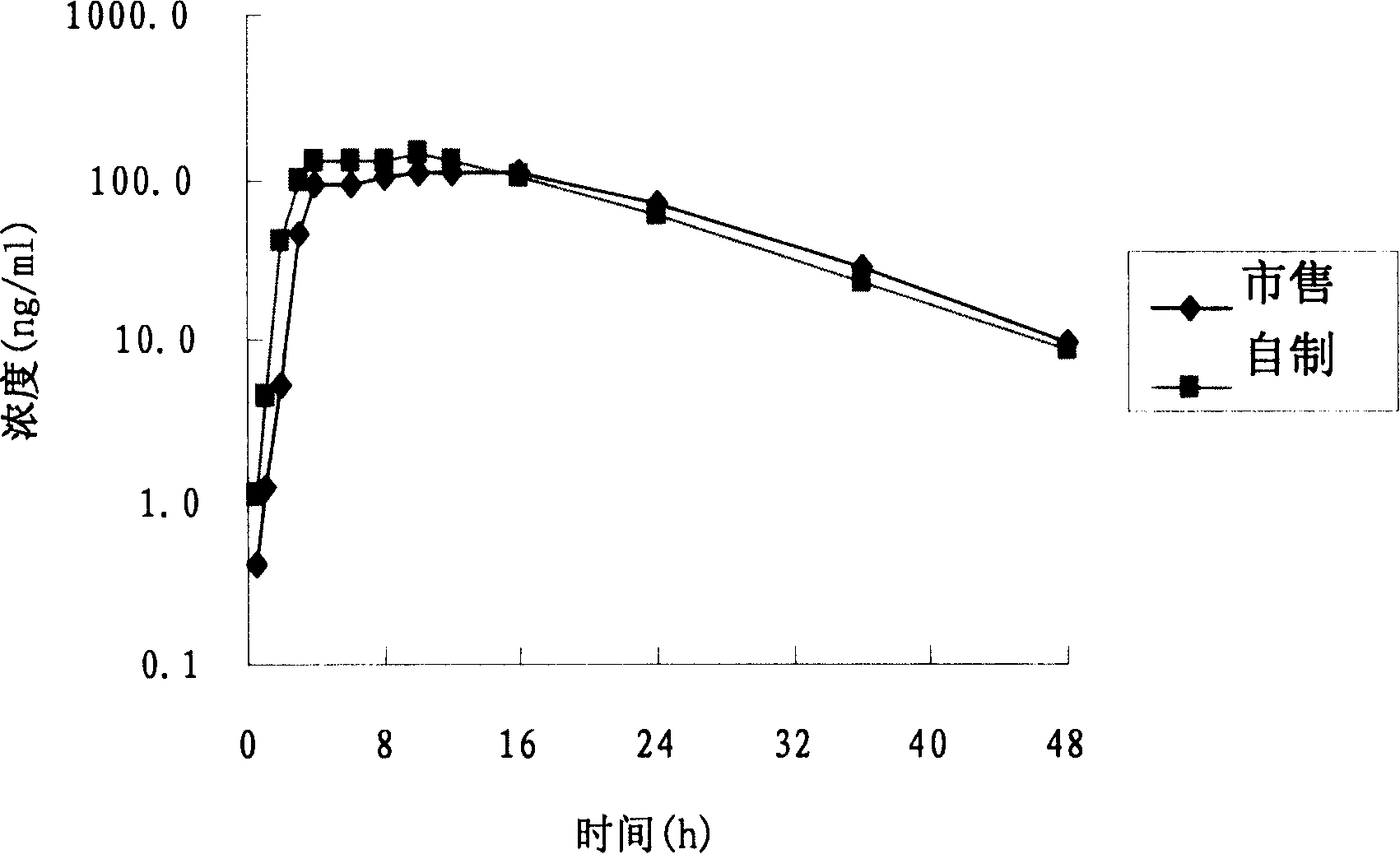
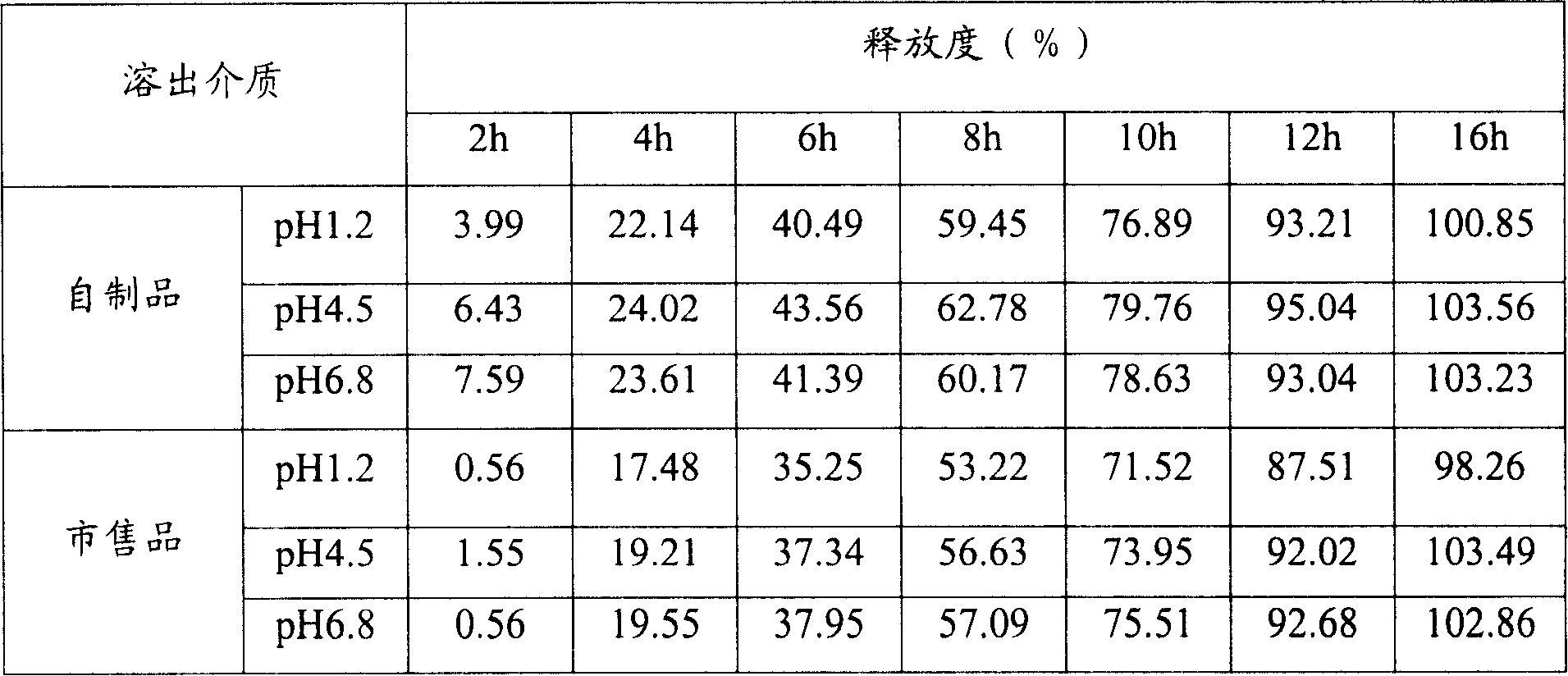
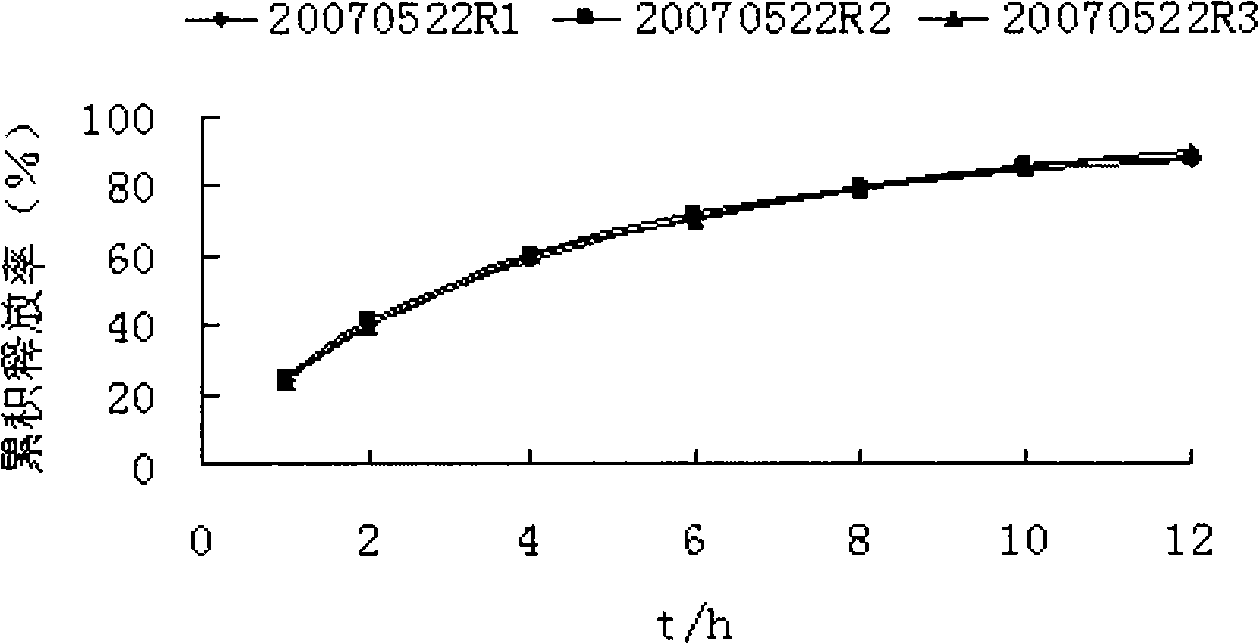
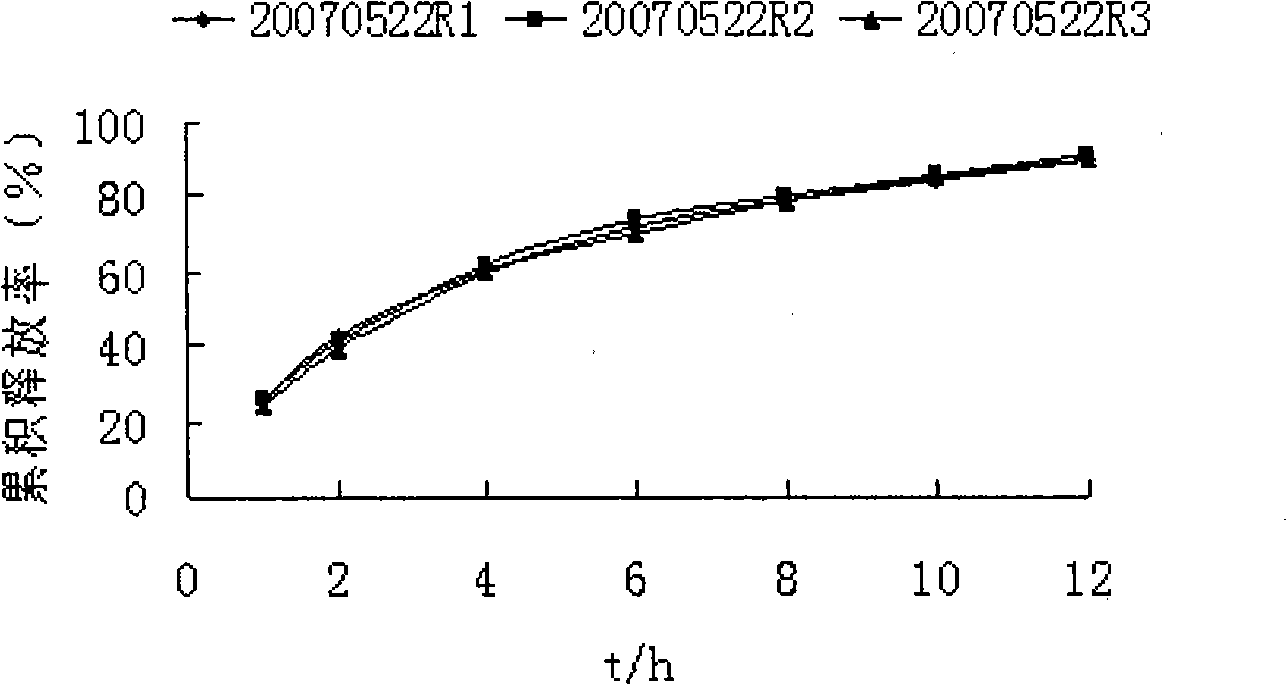
Patents
Literature
119 results about "Glipizide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Glipizide is used with a proper diet and exercise program to control high blood sugar in people with type 2 diabetes. It may also be used with other diabetes medications.
Osmotic delivery of therapeutic compounds by solubility enhancement
ActiveUS20050053653A1Low water solubilityImprove solubilityMetabolism disorderPill deliveryChemical compoundAqueous solubility
The present invention is directed to the oral osmotic delivery of therapeutic compounds that have limited solubility in an aqueous environment due to inherent hydrophobicity or to saturation limitations in the core of the osmotic system. The present invention is suitable for the osmotic delivery of glipizide and other hydrophobic drugs, but runs the spectrum to other therapeutic agents with higher aqueous solubilities, yet having a solubility limitation in an osmotic dosage unit due to high drug load.
Owner:SUPERNUS PHARM INC
Osmotic delivery of therapeutic compounds by solubility enhancement
ActiveUS7611728B2Low water solubilityImprove solubilityMetabolism disorderPill deliveryChemical compoundAqueous solubility
The present invention is directed to the oral osmotic delivery of therapeutic compounds that have limited solubility in an aqueous environment due to inherent hydrophobicity or to saturation limitations in the core of the osmotic system. The present invention is suitable for the osmotic delivery of glipizide and other hydrophobic drugs, but runs the spectrum to other therapeutic agents with higher aqueous solubilities, yet having a solubility limitation in an osmotic dosage unit due to high drug load.
Owner:SUPERNUS PHARM INC
Method for lowering blood glucose
InactiveUS6361795B1Promote insulin secretionRaise insulin levelsMetabolism disorderSulfonylurea active ingredientsGlucose polymersD-Glucose
Owner:ALZA CORP
Slow-releasing preparation containing metformin hydrochloride and glipizide and its preparation method
InactiveCN101057849AEvenly distributedReduce local irritationOrganic active ingredientsMetabolism disorderMedicineMetformin Hydrochloride
The invention discloses a diabecron and glipizide -containing slow-release agent and the method for preparing the same. The glipizide micro-pill takes blank micro-pill as carrier, and combines glipizide and other medical findings with it. The diabecron-containing slow-release micro-pill comprises diabetosan pill, slow-release coating membrane material or other medical findings. The method for preparing diabecron-containing slow-release micro-pill takes extrusion rolling method or blank micro-pill loading method. The product is characterized by safety, high efficient, low toxicity and convenient usage. It can be used to treat non-insulin-dependent diabetes mellitus.
Owner:QIQIHAR MEDICAL UNIVERSITY
Antidiabetic formulation and method
InactiveUS20070141154A1Addressing Insufficient ControlBiocideMetabolism disorderPharmaceutical formulationMetformin
An antidiabetic pharmaceutical formulation is provided, especially adapted for treating Type II diabetes, which includes a combination of metformin and glipizide in a manner to control moisture in the formulation so that the glipizide does not hydrolyze, yet the metformin is compressible, if necessary. A method for treating diabetes is also provided employing the above formulation.
Owner:BRISTOL MYERS SQUIBB CO
Novel synthesis route of glipizide
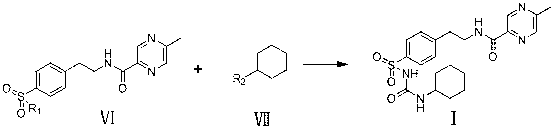
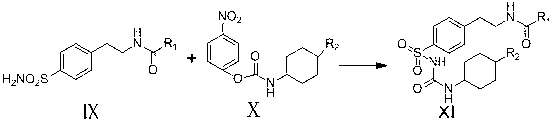
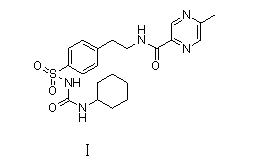
The invention relates to a novel synthesis route of glipizide, which is characterized by comprising the following steps of protecting 4-(2-amino ethyl)benzenesulfonic acid ammonia (II) by Boc anhydride to obtain a compound (III); reacting the compound (III) with cyclohexyl isocyanate to obtain a compound (IV); carrying out deprotection on the compound (IV) to obtain a compound (V); and reacting the compound (V) with 2-methyl-5-pyrazine carboxylic acid to obtain the glipizide (I) with the single impurity which is less than or equal to 0.5% and the high purity which is more than or equal to 99%. The process is simple, the yield is high, the purity is high and the single impurity is low; and the process is environment-friendly and industrial production is easy to realize.
Owner:WUHAN WUYAO PHARMA
Method for preparing high stripping-degree hautriwaic glipizide capsule
The preparation method of metformin hydrochloride glipizide capsule includes the following steps: using 250-500 weight portions of metformin hydrochloride, sieving it by using sieve with 100 meshes, using 2.5 weight portions of glipizide, sieving it by using sieve with 200 meshes or pulverizing it to make its fineness greater than 200 meshes, then uniformly mixing the above-mentioned materials, using dilute ethyl alcohol solution of polyvidone as adhesive, making soft material, granulating by using sieve with 24 meshes, drying at 50-60deg.C, finishing granules by using sieve with 20 meshes, adding 1% of magnesium stearate and capsulizing so as to obtain the invented finished product.
Owner:周卓和
Preparation method of glipizide osmotic pump controlled release tablet
ActiveCN102133205AFast dissolutionEasy to acceptMetabolism disorderSulfonylurea active ingredientsSolubilityTreatment effect
The invention relates to a preparation method of a glipizide osmotic pump controlled release tablet. A glipizide tablet core is prepared by the following steps of semipermeable membrane wrapping, laser boring and damp-proof layer wrapping. The preparation method is characterized in that the preparation of the glipizide tablet core comprises preparation of solid dispersoid and preparation of the tablet core. In the preparation method, a solid dispersion technology is utilized, so that the dissolving speed of medicines is improved; the solubility of the glipizide is improved through the fluxing auxiliary materials; and a proper amount of penetration promoter is added to adjust the osmotic pressure so as to achieve constant-speed or approximately-constant-speed release. The invention has the advantages that the process is simple, the cost is low, the release tablet is easy to accept by patients and the treating effect is good.
Owner:SHANDONG XINHUA PHARMA CO LTD
Oral disintegration tablet for dropping blood sugar and preparation method
InactiveCN1726916ARaise quality standardsStrong specificityOrganic active ingredientsMetabolism disorderSulfonylurea Antidiabetic AgentAdhesive
Owner:江西省药物研究所
Glipizide controlled release tablets and preparation method thereof
ActiveCN101172101AShort time lagImprove thermal stabilityOrganic active ingredientsMetabolism disorderEthylene HomopolymersControlled Release Tablet
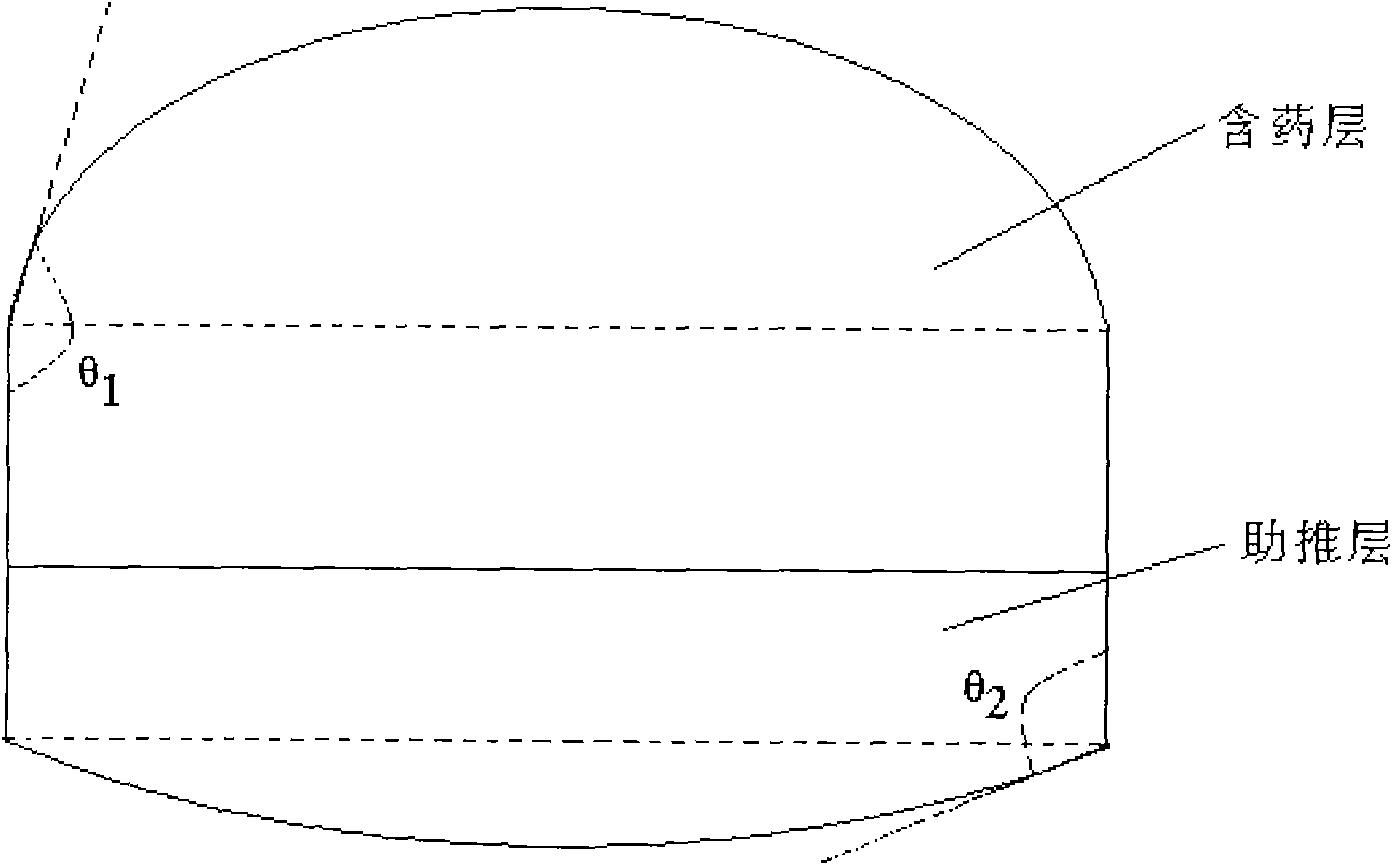
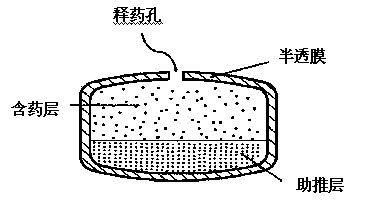
The invention provides a glipizide loaded controlled release preparation, which comprises a pastille layer and a boosting layer with the weight ratio of 1:0.5 to 3, the pastille layer comprises glipizide and carrier, the carrier is ethane ketopyrrolidine homopolymer and / or ethane ketopyrrolidine copolymer occupying 40 to 99 percent of the weight of the pastille layer; the boosting layer at least comprises infiltration promoting polymer occupying 10 to 80 percent of the weight of the boosting layer, 10 to 80 percent of insoluble polymer and residual osmotic pressure accelerant; the glipizide of the invention can be controlled to release a drug so as to lead the preparation to attain the purpose that the drug is administrated one time a day, and the glipizide can be released in 24 hours.
Owner:OCEAN STAR INT
Metformin hydrochloride and Glipizide sustained-release pellet and method of preparing the same
ActiveCN101278919AAvoid Difficulty ScreeningAvoid screening timeOrganic active ingredientsMetabolism disorderSustained release pelletsBlood concentration
The invention discloses a metformin hydrochloride and glipizide sustained-release pellet and a preparation method thereof. In the preparation method, a sustained release coated pellet of the metformin hydrochloride and a sustained release pellet of the glipizide are prepared respectively; and the two pellets are filled in capsules in a proportion of 250g-500g of the metformin hydrochloride and 2.5g-10g of the glipizide in every 1000 capsules; wherein, the coated pellet of the metformin hydrochloride is prepared by pill pericardium sustained release coating membrane; the sustained release pellet of the glipizide is prepared directly by extrusion-spheronization method. In the invention, the two pellets are filled into one capsule, thereby being convenient for quality control; octodecyl alcohol is taken as sustained release material for the sustained-release pellet of the glipizide, which is convenient for the forming of the pellet so as to reduce bursting release effectively. The metformin hydrochloride and the glipizide slowly release a drug within 12 hours, which reduces the frequency of taking medicine, stabilizes blood concentration better and reduces untoward effect, thereby having good marketing prospect.
Owner:国药控股星鲨制药(厦门)有限公司
Glipizide sustained-release granular formulation and preparation method thereof
The invention discloses a glipizide sustained-release granule and a preparation method thereof. The glipizide sustained-release granule is mainly prepared from glipizide bulk drugs, sustained-release materials and other appropriate auxiliary materials. The glipizide sustained-release granule can deaccelerate the release rate of main drugs, reduce the frequency of administration and improve the patient compliance. The glipizide sustained-release granule provided by the invention has the advantages of high quality controllability and stability of the preparation process.
Owner:山东淄博新达制药有限公司
Glipizide tablets for treating diabetes and preparation method thereof
InactiveCN105943514AStable industrialized mass productionSimple structureMetabolism disorderRotary stirring mixersWestern medicineMedicine
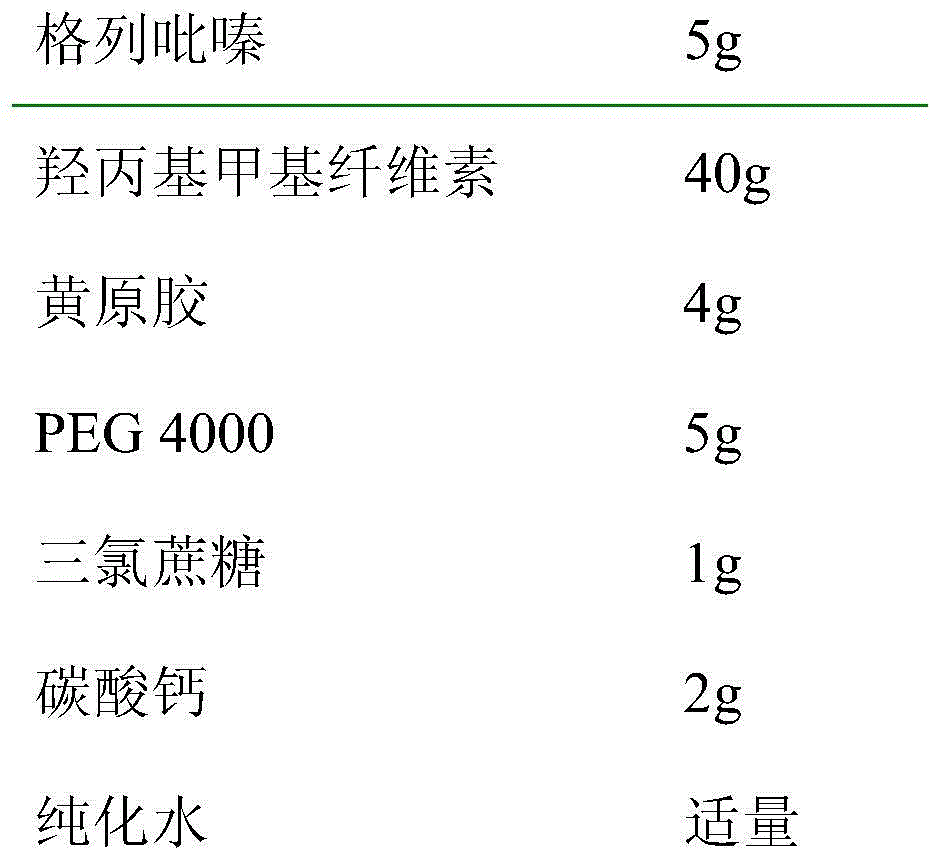
The invention relates to glipizide tablets for treating diabetes, belonging to the technical field of Western medicine preparations. The glipizide tablets consist of the following components: 5-15 parts of glipizide, 20-40 parts of hydroxypropyl methyl cellulose, 30-50 parts of calcium carbonate and the like; and the components are mixed by a specific particle stirrer. The tablets provided by the invention are stably and slowly released.
Owner:NANJING HUAKUANG INFORMATION CONSULTING CENT
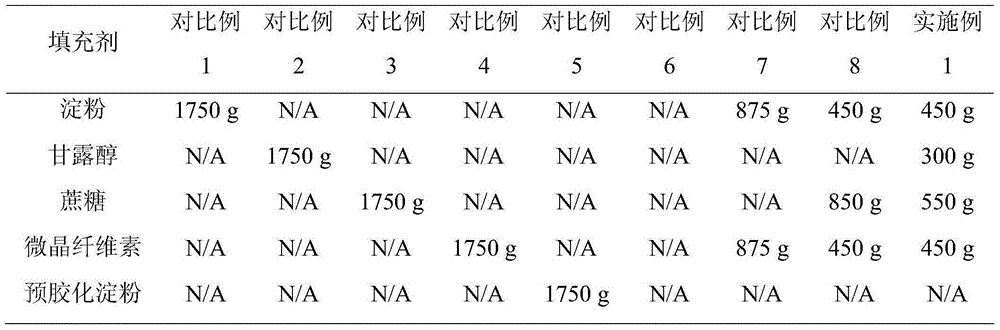
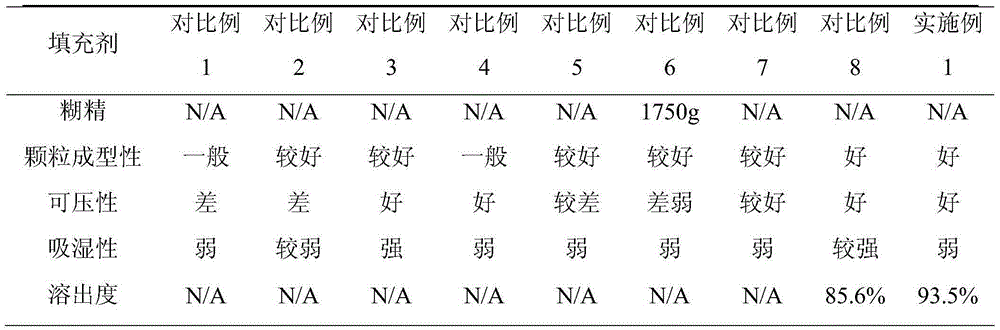
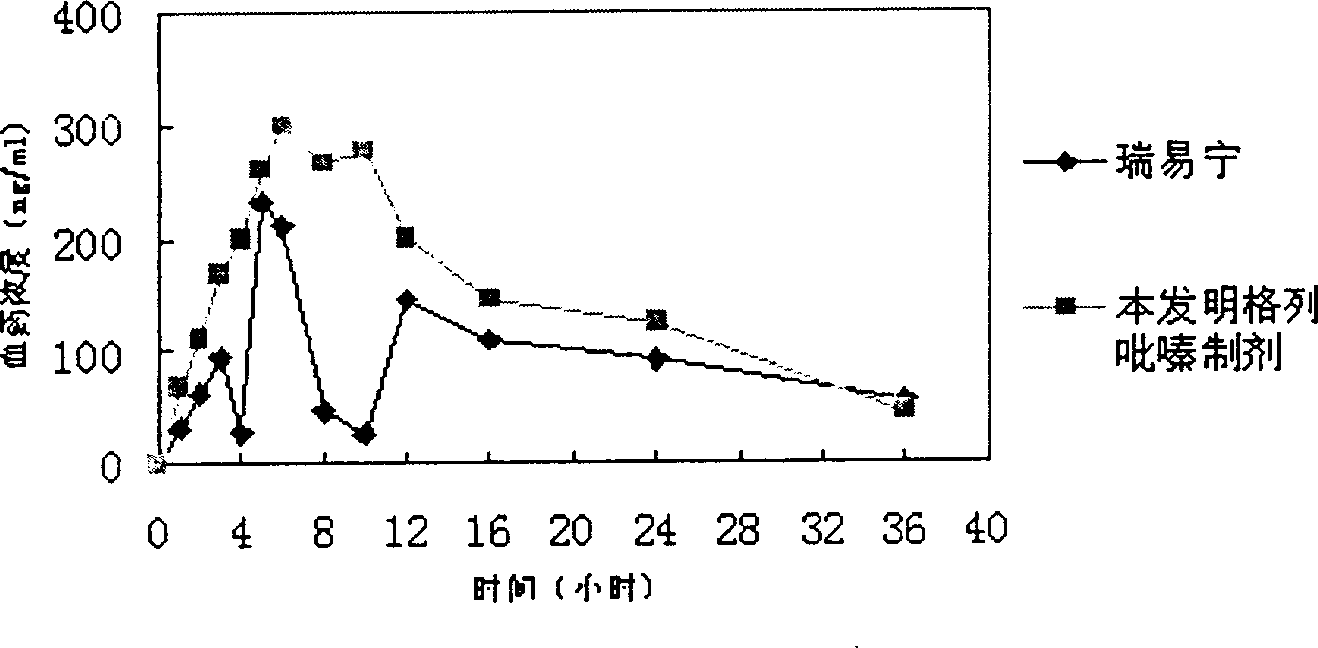
Glipizide tablets and preparation method thereof
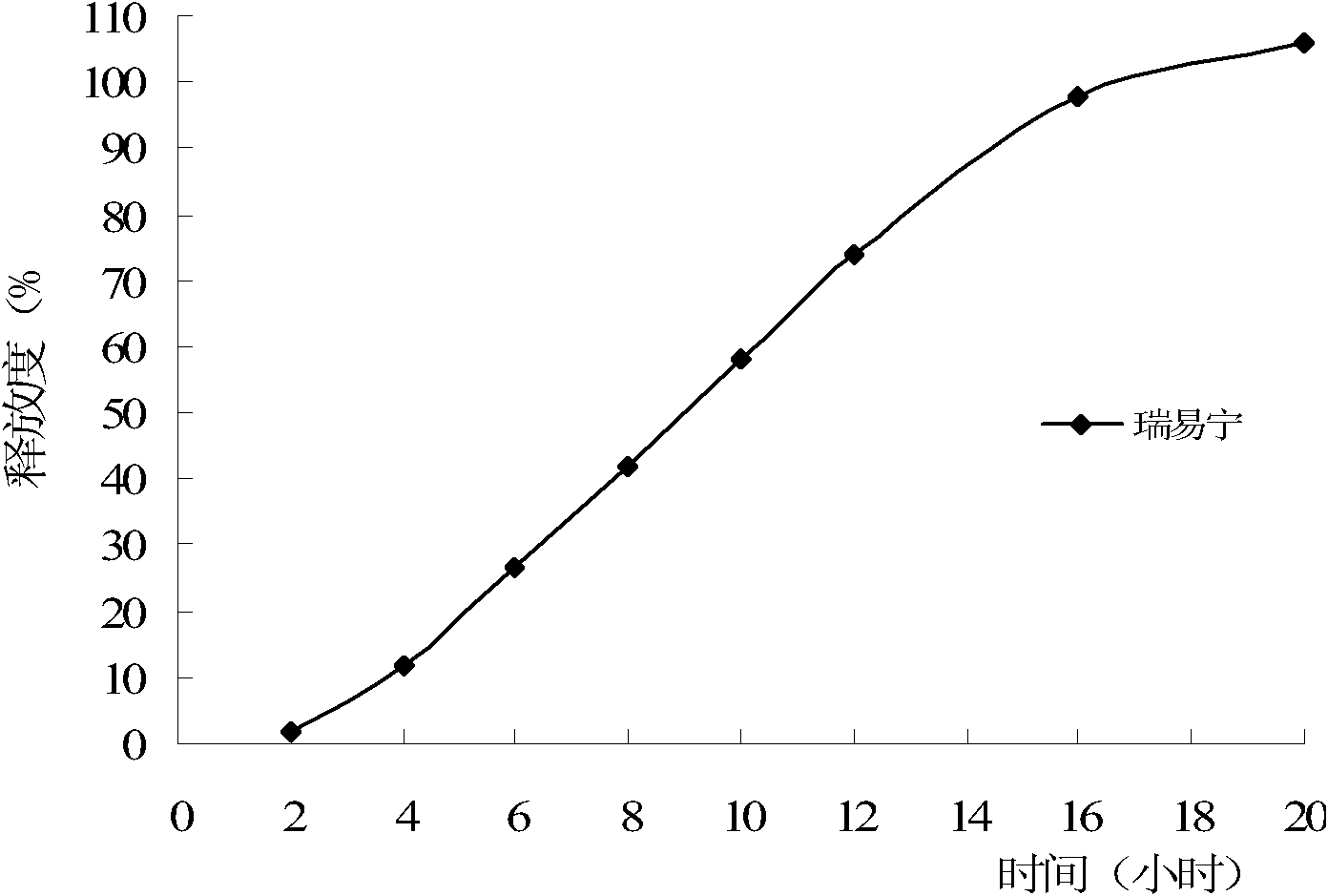
ActiveCN104997747ASmooth releaseHigh dissolution rateMetabolism disorderSulfonylurea active ingredientsSucroseMedicine
The invention belongs to the field of medicines and particularly relates to glipizide tablets and a preparation method thereof. The glipizide tablets comprise raw materials in parts by weight as follows: 4-6 parts of glipizide, 87-263 parts of a filler, 5-15 parts of a disintegrating agent and 0.5-1.5 parts of a lubricant, wherein the filler preferably comprises components in parts by weight as follows: 24-72 parts of starch, 15-45 parts of mannitol, 26-78 parts of sugar and 22-68 parts of microcrystalline cellulose. The glipizide tablets have stable drug release property and high dissolution rate. The invention further provides the preparation method of the glipizide tablets. The preparation method has a simple and reasonable process and is easy to implement.
Owner:REYOUNG PHARMA
Glipizide osmotic pump type controlled release tablet
ActiveCN102151252ARelease rate does not decreaseRelease less residueMetabolism disorderSulfonylurea active ingredientsCelluloseMedicine
The invention provides a glipizide osmotic pump type controlled release tablet. The controlled release tablet adopts ethylene cellulose and polyvidone as semi-permeable membrane materials and is unsymmetrical in appearance, not only can overcome the defect that a semi-permeable membrane is aged and reduce the drug release residue, but also can simplify the identification of laser drilling and is stable to place when being conveyed on a conveyor belt; and a hole is easier to be drilled in the middle to facilitate the stable release of the drugs. The invention also provides a method for improving the aging resistance of the glipizide osmotic pump type controlled release tablet, which is characterized in that the ethylene cellulose-the polyvidone is adopted as the semi-permeable membrane material. In addition, the invention further provides an application of an ethylene cellulose-the polyvidone combination for preparing the aging resistance of the glipizide osmotic pump type controlled release tablet.
Owner:北京天衡药物研究院南阳天衡制药厂
Antidiabetic formulation and method
InactiveUS7183321B2Addressing Insufficient ControlBiocideMetabolism disorderPharmaceutical formulationMetformin
An antidiabetic pharmaceutical formulation is provided, especially adapted for treating Type II diabetes, which includes a combination of metformin and glipizide in a manner to control moisture in the formulation so that the glipizide does not hydrolyze, yet the metformin is compressible, if necessary. A method for treating diabetes is also provided employing the above formulation.
Owner:BRISTOL MYERS SQUIBB CO
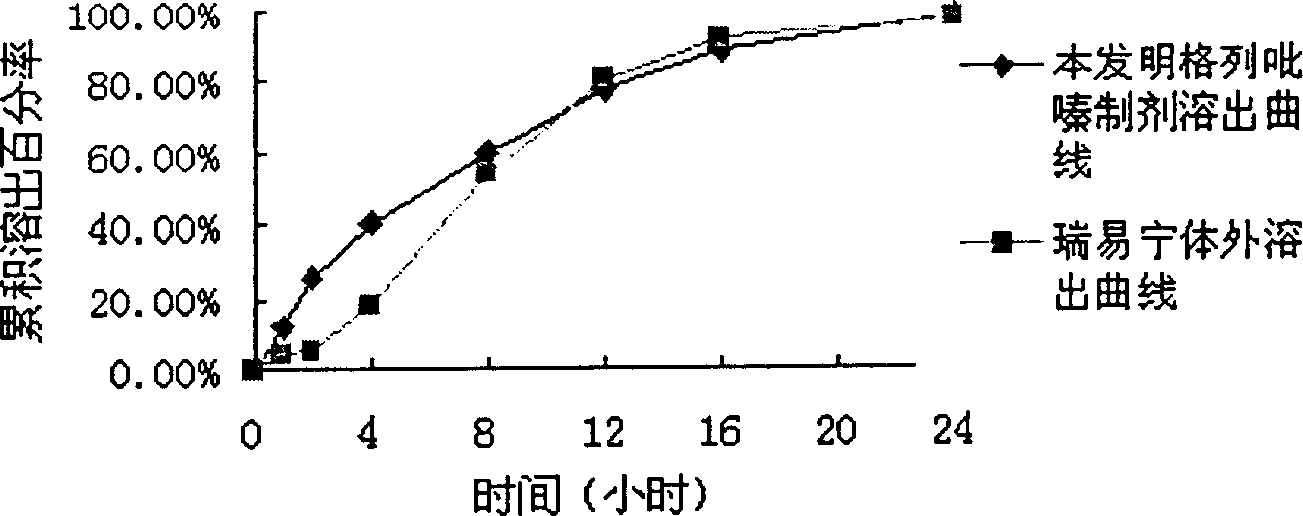
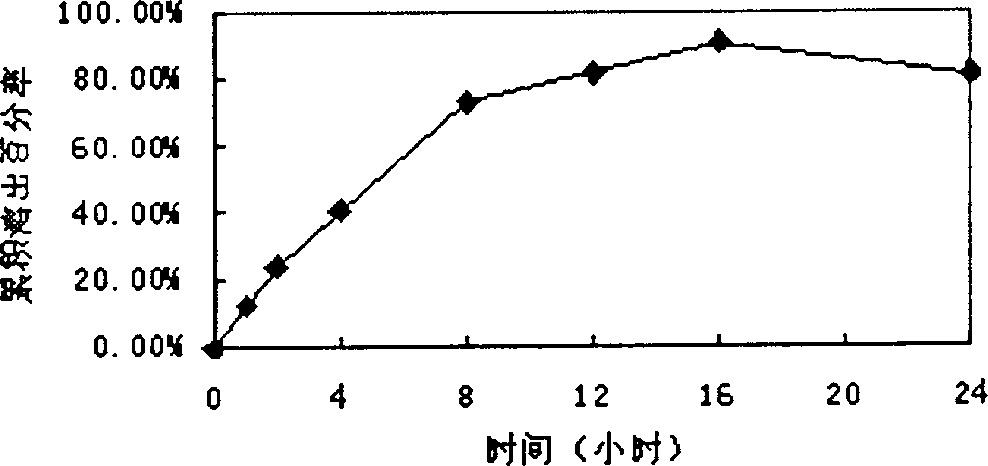
Glipizide controlled release composition as well as preparation method thereof
ActiveCN103565774AWell mixedUniform particle size distributionCosmetic preparationsMetabolism disorderControlled releaseOrganosolv
The invention relates to a glipizide controlled release composition which consists of a double-layer tablet core and a semipermeable membrane controlled release coating membrane. The double-layer tablet core consists of a medicine containing layer and a boosting layer, wherein the medicine containing layer and the boosting layer of the composition are pelletized by a water suspension. The invention further relates to a preparation method of the glipizide controlled release composition. The preparation method comprises the following steps: pelletizing the medicine containing layer; pelletizing the boosting layer; preparing the double-layer tablet core; coating; and punching, wherein the medicine containing layer and the boosting layer are pelletized by the water suspension. The glipizide controlled release composition prepared by the preparation method provided by the invention is uniform in particle distribution, good in tabletting and forming effect, uniform in content, low in residual rate of organic solvents and good in dissolving out effect. The preparation method is simple and feasible and is favorable for industrial production.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
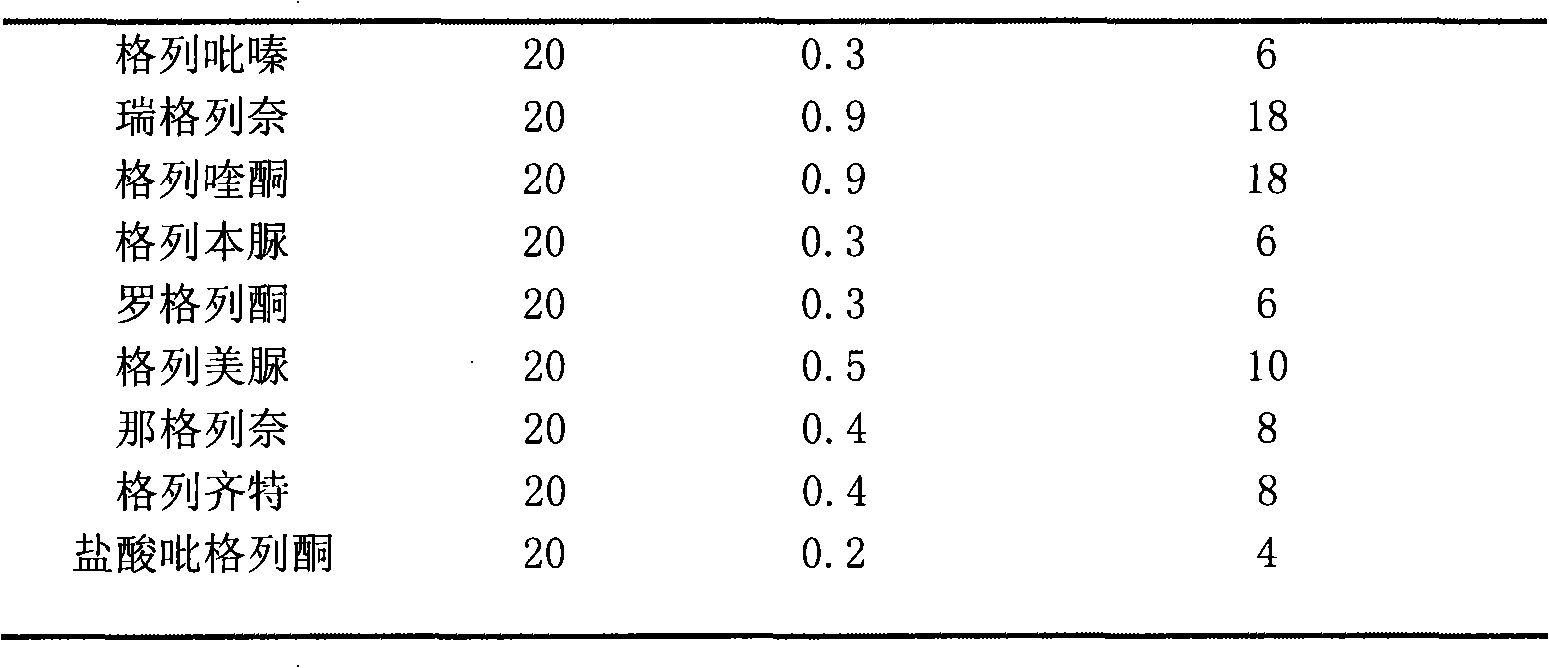
Qualitative analysis detection method for low polarity sugar-reducing chemical medicament in traditional Chinese medicine
InactiveCN101285803AImprove identification sensitivityHigh sensitivityComponent separationTesting medicinal preparationsRetention timeUltraviolet
The invention discloses a qualitative analysis detection method for illegally mixed high-polar chemical anti-diabetic components in anti-diabetic traditional Chinese medicine products. The method comprises the following steps that: 1) high efficient liquid phase chromatography conditions: an ammonium acetate-triethylamine-acetonitrile moving phase system and a C18 chromatographic column with certain specification are used, the wavelength is detected by ultraviolet, and the flow rate is 1.0ml / min; 2) analysis result: glibenclamide, glipizide, gliclazide, glimepiride, gliquidone, repaglinide, nateglinide, rosiglitazone and pioglitazone hydrochloride can realize the complete separation; 3) result judgment: when retention time of a chromatographic peak in an anti-diabetic traditional Chinese medicine product is consistent with that of anti-diabetic medicine in the step 2) and the apparent absorption is shown out, which indicate that the anti-diabetic medicine is contained in the sample to be tested. The method has the advantages of quickness, simplicity, convenience, high sensitivity, strong specialization, broad coverage and so on.
Owner:北京市东城区药品检验所
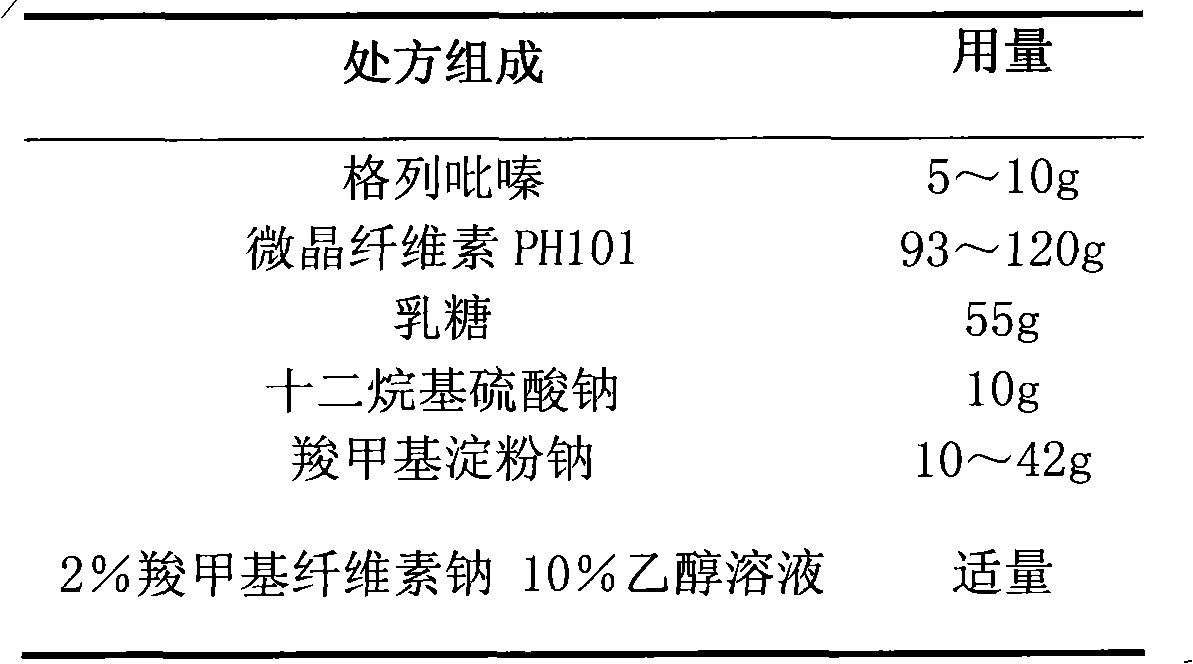
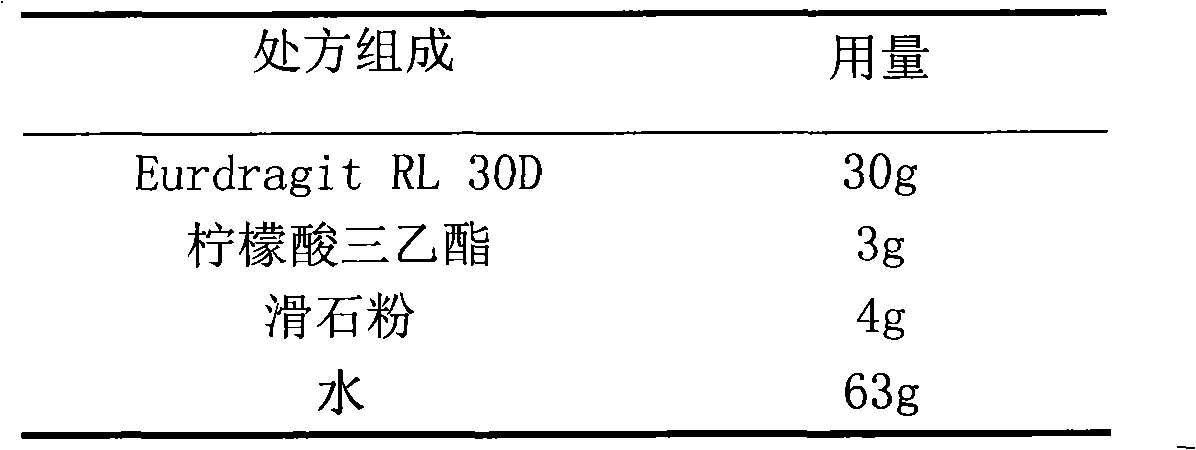
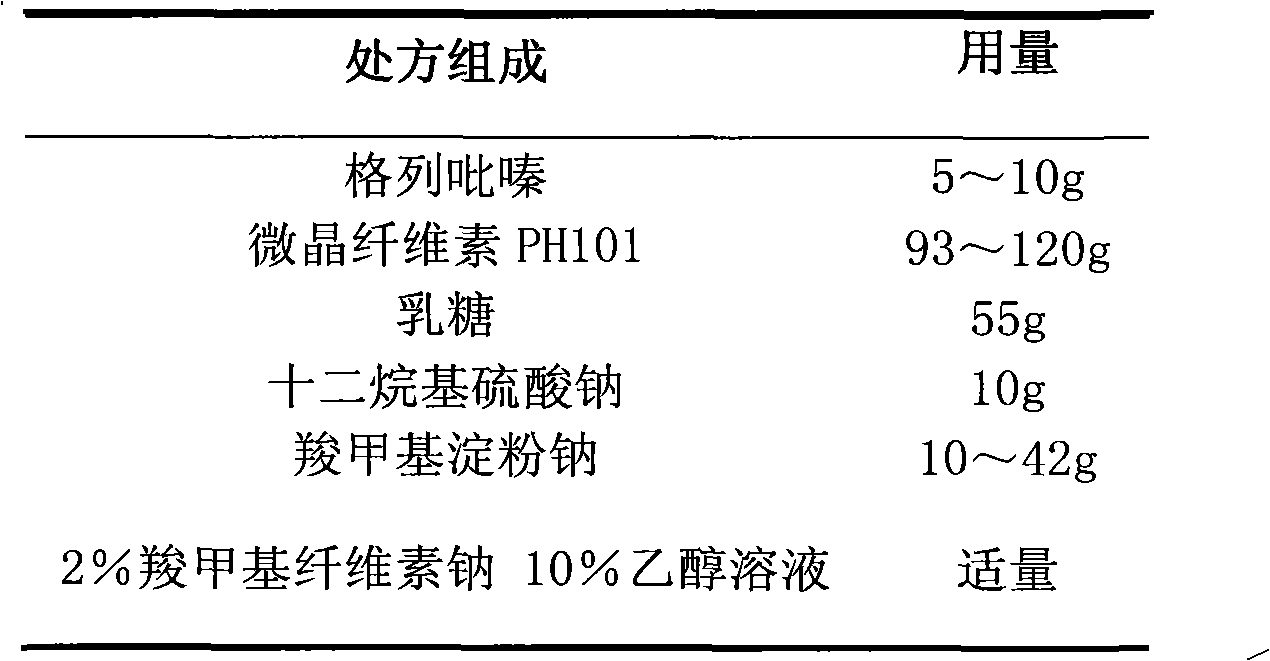
Glipizide film-controlled slow-release pellet capsule
ActiveCN103211787AAccelerated agingReduce permeabilityMetabolism disorderSulfonylurea active ingredientsSustained release pelletsMedicine
The invention relates to a glipizide film-controlled slow-release pellet capsule. A slow-release film of the glipizide film-controlled slow-release pellet utilizes Eurdragit RL 30D as a film-formation material. A pellet core of the glipizide film-controlled slow-release pellet contains sodium carboxymethyl starch having high expansibility, and also contains pharmaceutically-acceptable common excipients for the slow-release pellet, wherein preferably, the excipients comprise microcrystalline cellulose, lactose and sodium dodecyl sulfate, and the pellet core comprises 5 to 20wt% of sodium carboxymethyl starch. The slow-release film comprises Eurdragit RL 30D, triethyl citrate as a plasticizer and talcum powder as an antiplastering aid, wherein preferably, a ratio of Eurdragit RL 30D to triethyl citrate to talcum powder is 30: 3: 4 and a film weight increasing ratio is in a range of 17 to 36%. The glipizide film-controlled slow-release pellet comprises the pellet core containing sodium carboxymethyl starch having high water expansibility and thus after absorbing water, the glipizide film-controlled slow-release pellet obviously expands so that the slow-release film is stretched; the thickness of the slow-release film is reduced; the water-permeable micropore size is increased; and permeability is improved and the permeability reduction caused by film aging is counteracted. Therefore, in middle and later stages, the glipizide film-controlled slow-release pellet release rate is basically constant; in the last stage, residues are less; and stable release performances can be kept in the period of validity.
Owner:内蒙古天衡医院管理有限公司
Glipizide osmotic pump controlled release tablet
ActiveCN104414992ASimple methodImprove thermal stabilityMetabolism disorderSulfonylurea active ingredientsAdhesiveSuspending Agents
Disclosed is a glipizide osmotic pump controlled release tablet. The glipizide osmotic pump controlled release tablet comprises, from inside to outside, a double-layer tablet core containing a drug layer and a promoting layer and an insoluble semi-permeable membrane; the drug layer comprises drug release holes and an optional moisture-proof film coating; the drug layer, based on the total weight, contains 1-30 wt.% of glipizide, 30-95 wt.% of a suspending agent, 2-30 wt.% of a retardant, 0-10 wt.% of an adhesive, 0-2 wt.% of a colorant and 0-2 wt.% of a lubricant; the promoting layer, based on the total weight, comprises 30-80 wt.% of an expanding agent, 1-30 wt.% of a retardant, 0-2 wt.% of a colorant, 5-40 wt.% of an osmotic pressure active substance and 0-1 wt.% of a lubricant; and the weight gain of the semi-permeable membrane is 5-20% of the weight of the tablet core. The invention also discloses a preparation method of the glipizide osmotic pump controlled release tablets. The method is convenient for production, and the product is easy to store, and can better achieve the purpose of controlled release.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Glibenclamide, gliclazide and glipizide triple test card and test method thereof
InactiveCN102012427AEasy to manufactureLow detection costPreparing sample for investigationWestern medicineGlass fiber
The invention relates to a glibenclamide, gliclazide and glipizide triple test card and a test method thereof, belonging to the technical field of testing of western medicine components which are illegally added into traditional Chinese medicines. A test bar is arranged in a shell of the triple test card and comprises a sample pad, a plurality of sections of colloidal gold membranes, a nitrocellulose membrane and a water-absorbing membrane which are sequentially stuck on a supporting backboard, wherein the plurality of sections of the colloidal gold membranes are glass fiber membranes containing colloidal gold markers of an anti-glibenclamide antibody, an anti-gliclazide antibody and an anti-glipizide antibody sequentially, three test strips which respectively contain a glibenclamide protein conjugate, a gliclazide protein conjugate and a glipizide protein conjugate are arranged on the nitrocellulose membrane, and the triple test card additionally comprises a quality control strip containing an anti-rabbit antibody or an anti-mouse antibody. The triple test card has the advantages of being capable of simultaneously detecting glibenclamide, gliclazide and glipizide which are illegally added in a glucose-lowering traditional Chinese medicine. The test card is easy for preparation, convenient and fast for use and accurate in result, and can be used for saving the test cost.
Owner:长沙安迪生物科技有限公司
Preparation method of glipizide crystals
ActiveCN106187921ALarge granularityComplete crystal formOrganic chemistry methodsLiquid ratioGranularity
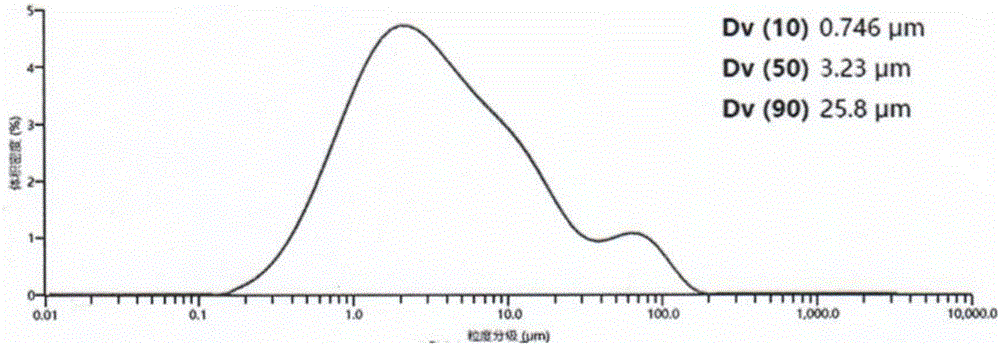
The invention belongs to the technical field of bulk pharmaceutical chemical preparation and particularly relates to a preparation method of glipizide crystals. According to the technical scheme, the preparation method comprises the first step that glipizide is added into an isopropanol mixed solvent, the solid-to-liquid ratio of the solution is 0.05 g / ml to 0.1 g / ml, and stirring is conducted continuously for 30-60 minutes at the temperature of 40-45 DEG C for dissolution; the second step that dilute alkaline liquor is added, the pH of the solution is regulated to 8-9, and filtering and decoloring are conducted; the filtrate is transferred into a crystallizer, a hydrochloric acid solution is added, the pH of the solution is regulated to 6-7, seed crystals are added and cultured for 1-2 h, then the temperature is dropped to 5-10 DEG C, and the crystals are cultured for 1-3 h; filtering is conducted, a cleaning solvent is used for washing filter cakes, products are dried, and the high-granularity glipizide products are obtained. According to the preparation method of the high-granularity glipizide crystals, the obtained products do not aggregate, the main granularity is 10 microns or above, and granularity distribution is uniform.
Owner:迪嘉药业集团股份有限公司
Glipizide oral cavity disintegrating lyophilized tablets and method for preparing the same
InactiveCN101134021ALittle side effectsFast absorptionOrganic active ingredientsMetabolism disorderFreeze-dryingOrally disintegrating tablet
The present invention relates to an oral disintegrant freeze-dried tablet which can be quickly disintegrated in the mouth cavity and absorbed for reducing blood sugar effectively. It is made up by using glipizide as main medicine, adding filling agent, adhesive and correctives and making them undergo the process of freeze-drying treatment.
Owner:HAILING PHARMA PLANT HAINAN PROV +1
Glipizide oral instant film and preparation method thereof
ActiveCN104666280AEasy to takeEasy to carryMetabolism disorderSulfonylurea active ingredientsBULK ACTIVE INGREDIENTPharmaceutical formulation
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a prescription of a glipizide oral instant film and a preparation method thereof. The oral instant film comprises medical active ingredients and pharmaceutically adaptable auxiliary materials. The film comprises the following components in percentage by weight: 1-30% of glipizide, 40-80% of a macromolecular film-forming material, 0-30% of a plasticizer, 0-20% of a corrigent and 0-20% of other auxiliary materials. The film prepared by the glipizide film can be taken without water. The film placed in the oval cavity can be quickly dispersed and dissolved (equivalent to an oral solution) and is swallowed into stomach with saliva. After the film is orally taken, no disintegration process is available, so that the film has the advantages of being quick to absorb, quick to response and high in bioavailability. The product is accurate in dosage, and dust explosion in the production process is nearly avoided, so that the problem on labor protection and environmental pollution can be solved.
Owner:HEFEI HUAFANG PHARMA SCI & TECH
Sustained release preparation of glipizide/metformin hydrochloride and its preparation method
InactiveCN1602873APromote secretionImprove utilizationOrganic active ingredientsMetabolism disorderIrritationMetformin Hydrochloride
The invention relates to a compound sustained-release preparation composed of glipizide and metformin hydrochloride, and its preparing method. The weight ratio of glipizide to metformin hydrochloride is 1 : 100-200, the best ratio is 1:100 and it adds a proper amount of medicinal auxiliary to the preparation. The compound sustained-release tablet can not only make advantages complementation on glipizide and metformin hydrochloride, promote and increase secretion of beta-cell insulin, and raise the utilization of glucose in tissues, such as muscle, etc, but also reduce the stimulus of metformin hydrochloride to stomach intestine, and largely strengthen curative effect and drug application safety.
Owner:海南国栋药物研究所有限公司
Glipizide enteric sustained-release preparation composition and method for preparing the same
InactiveCN101502517AReduce the frequency of takingSlow release rateOrganic active ingredientsMetabolism disorderPatient complianceIrritation
The invention discloses a glipizide enteric sustained-release preparation combination and a preparation method thereof. The glipizide enteric sustained-release preparation combination is mainly prepared from glipizide bulk drugs, sustained-release materials, enteric materials and other appropriate auxiliary materials. The glipizide enteric sustained-release preparation provided by the invention can not only prevent glipizide from disintegrating in the stomach and causing irritation to gastric mucosa and avoid the adverse reactions caused by the administration, such as nausea, abdominal pain, diarrhea and the like, but also deaccelerate the release rate of drugs, reduce the frequency of administration and improve the patient compliance. The invention provides a novel form of drug featuring higher safety and better patient compliance and having the advantages of high quality controllability and stability of the preparation process.
Owner:山东淄博新达制药有限公司
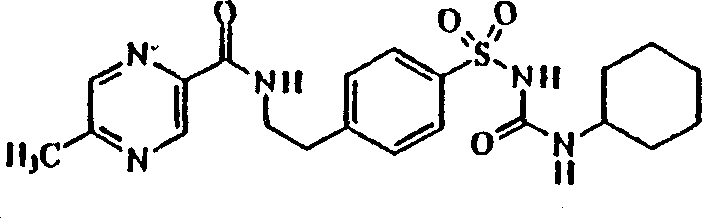
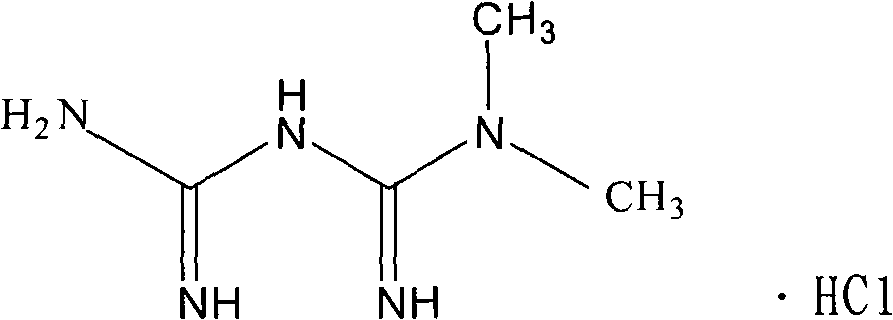
Glipizide compound as well as pharmaceutical composition containing glipizide compound and preparation method of glipizide compound
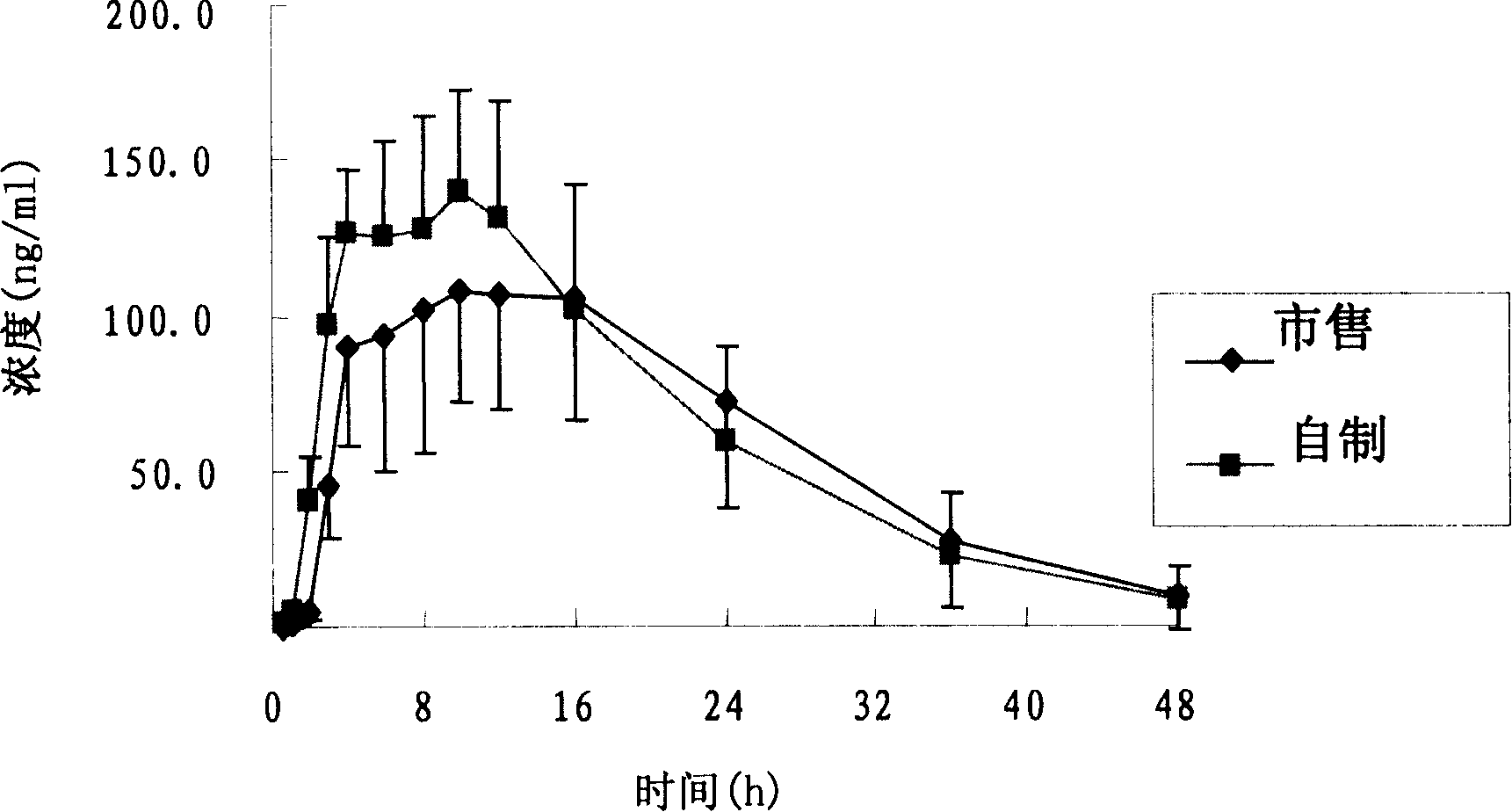
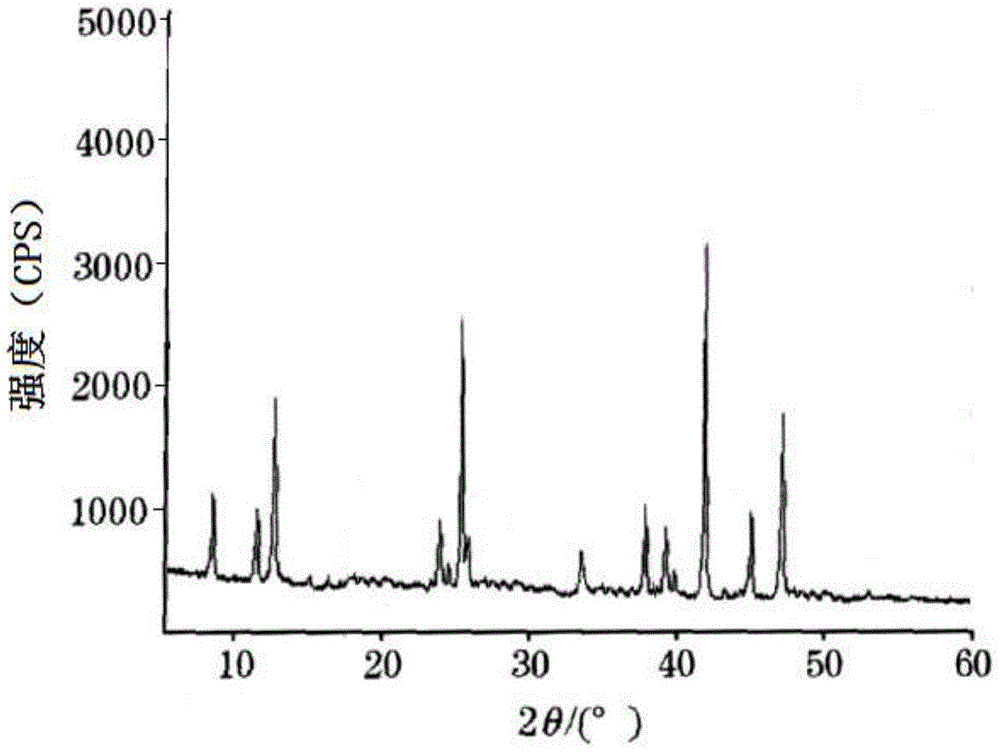
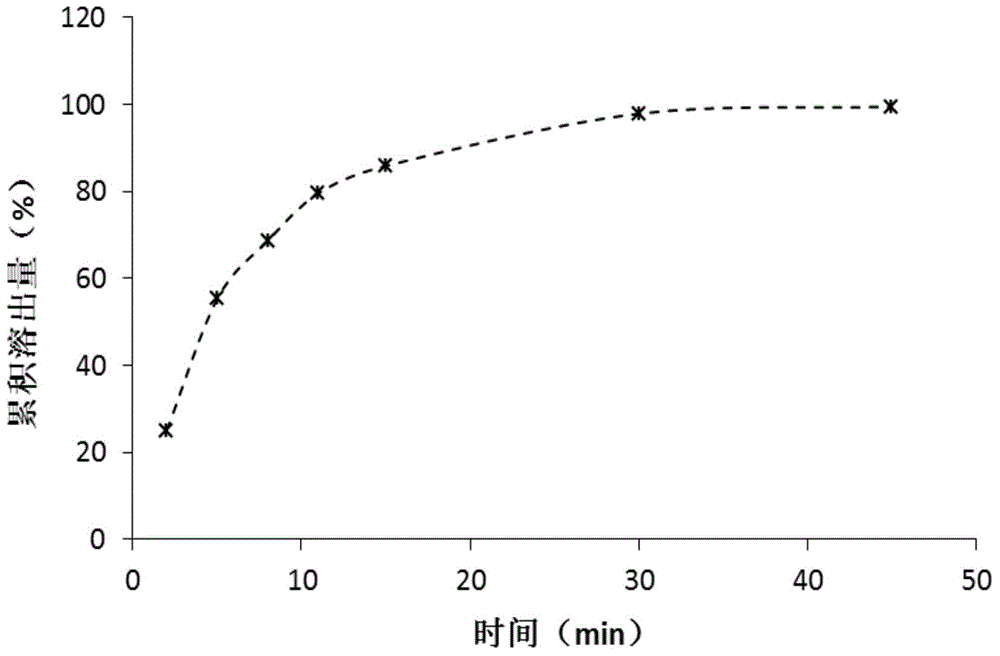
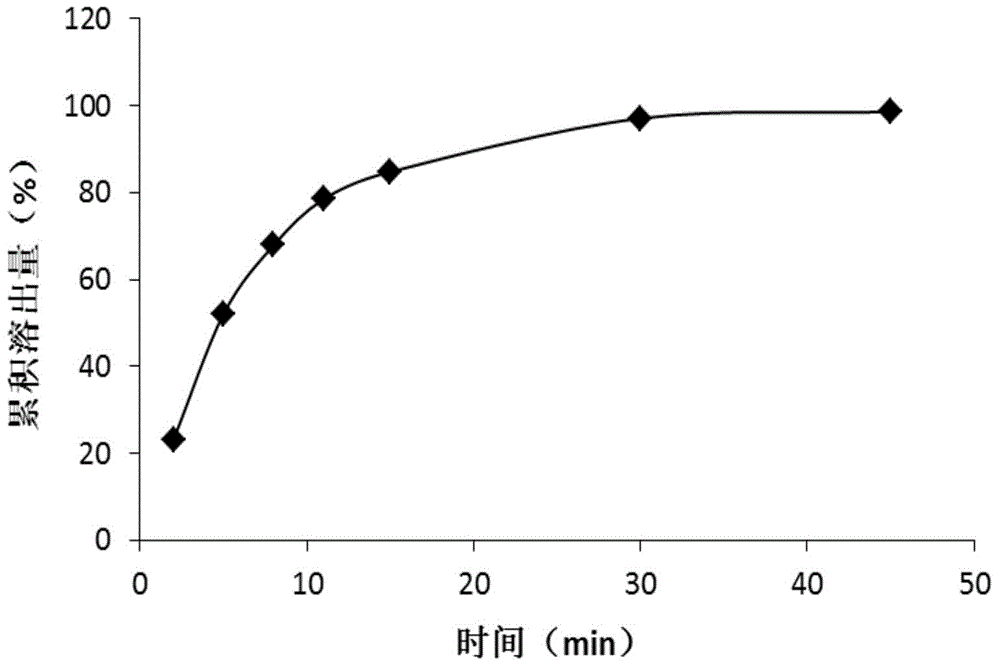
ActiveCN104086490AGood water solubilityImplement synchronous releaseOrganic chemistryMetabolism disorderSolubilityClinical efficacy
The invention belongs to the technical field of medicines and particularly relates to a glipizide compound as well as a pharmaceutical composition containing the glipizide compound and a preparation method of the glipizide compound. The glipizide compound has a structural formula shown in the specification, and the X-ray powder diffraction pattern, measured by virtue of Cu-Kalpha ray of the glipizide compound is shown in Figure 1. The glipizide compound provided by the invention is a novel crystalline form compound of glipizide, thus the water solubility of the compound can be significantly increased; and the purpose of synchronization release of a pharmaceutical composition comprising the compound and metformin hydrochloride can be achieved, in-vitro dissolution is excellent, the clinical curative effect of the pharmaceutical composition is equivalent to that of the commercially available bulk drug and the pharmaceutical composition has better security.
Owner:HUNAN WARRANT PHARMA
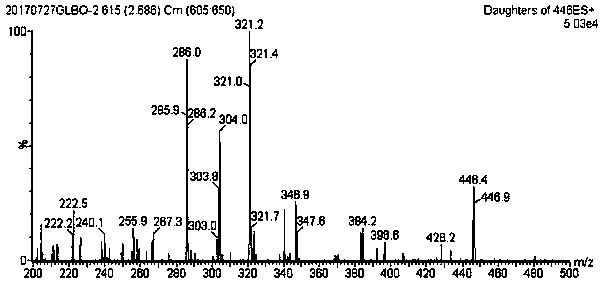
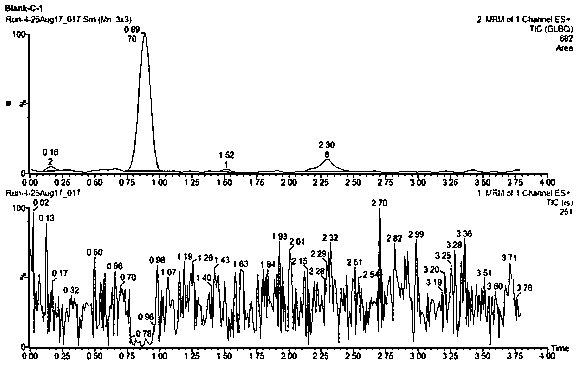
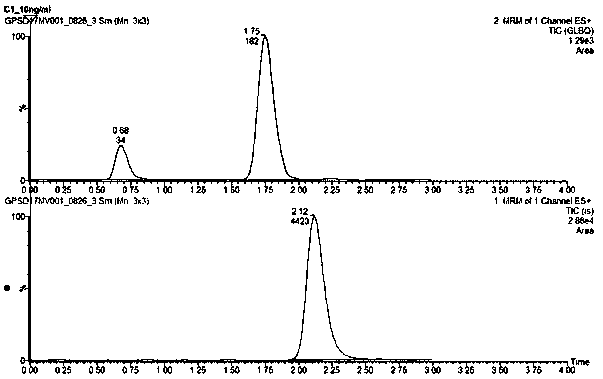
Method for using liquid chromatogram-tandem mass spectrometry to measure concentration of glipizide in plasma
InactiveCN108469479AMeet the requirements of quantitative testingEasy to operateComponent separationPlasma samplesAlcohol
The invention belongs to the field of medicine detection, and relates to a method for using liquid chromatogram-tandem mass spectrometry to measure concentration of glipizide in plasma, in particularto a method for measuring the concentration of glipizide in plasma of a beagle. The method comprises the following steps of 1, preparation of a plasma sample; 2, sedimentation of protein; 3, measuringthrough the liquid chromatogram-tandem mass spectrometry. An interior label working solution gliclazide is added in the sample, and methyl alcohol is adopted for settling, volution and centrifuging.Then the liquid chromatogram-tandem mass spectrometry is adopted for measuring the concentration of glipizide in the plasma. The method adopts methyl alcohol for settling the protein, has the advantages that liquid chromatogram-tandem mass spectrometry is conducted, the operation is simple, the detection limit is low, and the repeatability is high, and is applicable to pharmacokinetic studies, toxicokinetic studies, bioequivalence studies and the like.
Owner:沈阳信达泰康医药科技有限公司
Slow controlled released combsn. preparation of semisolid framework of containing glipizide
InactiveCN1695622ASolve the problem that the material utilization is lower than that of ordinary tabletsImprove stabilityOrganic active ingredientsMetabolism disorderControlled releaseSemi solid
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com