Glipizide tablets and preparation method thereof
A technology of glipizide tablets and parts by weight, applied in the field of medicine, can solve problems such as failure to provide, high production cost, complicated process parameters, etc., and achieve the effects of easy implementation, stable release and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
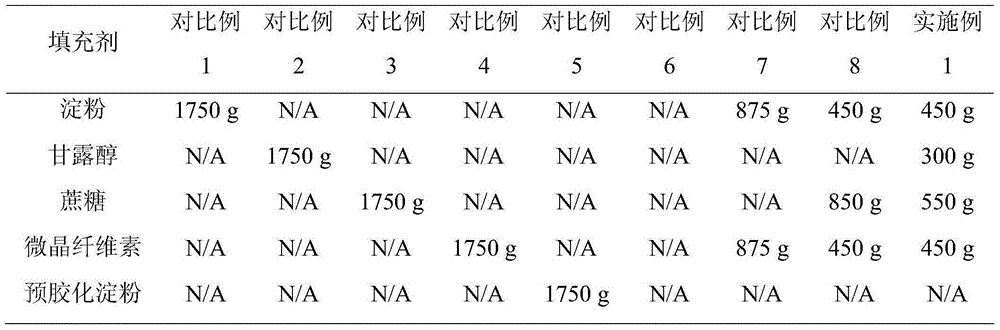
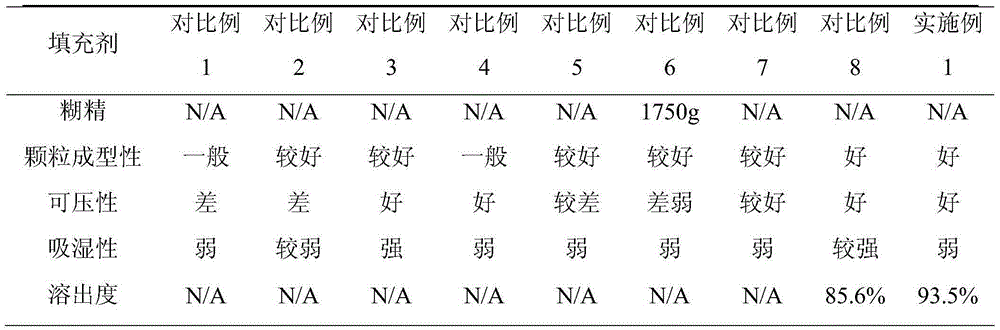
[0030] The glipizide is crushed through a 100-mesh sieve, and the filler is crushed through a 100-mesh sieve. Weigh 50 g of glipizide and 1750 g of filler, add them into a wet granulator, and mix well. Add 400 g of absolute ethanol and 400 g of purified water to prepare a soft material, and granulate with a 20-mesh sieve. The wet granules are dried with a fluidized granulation dryer, and the granules are sized with a 20-mesh screen of a crushing and sizing machine. Add 1800 g of the granules, 100 g of the disintegrant low-substituted hydroxypropyl cellulose and 10 g of the lubricant magnesium stearate into the mixer, and mix for 10 minutes. The mixed powder is compressed into tablets with a rotary tablet machine.
Embodiment 2-3
[0039] The glipizide is crushed through a 100-mesh sieve, and the filler is crushed through a 100-mesh sieve. Weigh 50 g of glipizide, 450 g of starch, 300 g of mannitol, 550 g of sucrose, and 450 g of microcrystalline cellulose, add them into a wet granulator, and mix well. Add 400 g of absolute ethanol and 400 g of purified water to prepare a soft material, and granulate with a 20-mesh sieve. The wet granules are dried with a fluidized granulation dryer, and the granules are sized with a 20-mesh screen of a crushing and sizing machine. Add 1800 g of granules, 10 g of disintegrant and lubricant magnesium stearate into the mixer, and mix for 10 minutes. The mixed powder is compressed into tablets with a rotary tablet machine.
Embodiment 4-5
[0047] The glipizide is crushed through a 100-mesh sieve, and the filler is crushed through a 100-mesh sieve. Weigh 50 g of glipizide, 450 g of starch, 300 g of mannitol, 550 g of sucrose, and 450 g of microcrystalline cellulose, add them into a wet granulator, and mix well. Add binder to prepare soft material, granulate with 20 mesh sieve. The wet granules are dried with a fluidized granulation dryer, and the granules are sized with a 20-mesh screen of a crushing and sizing machine. Add 1800 g of the granules, 100 g of the disintegrant low-substituted hydroxypropyl cellulose and 10 g of the lubricant magnesium stearate into the mixer, and mix for 10 minutes. The mixed powder is compressed into tablets with a rotary tablet machine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com