Glipizide compound as well as pharmaceutical composition containing glipizide compound and preparation method of glipizide compound
A technology of glipizide and compounds, applied in the field of glipizide compounds and pharmaceutical compositions containing the compounds and their preparation, can solve the problems of poor compliance, low solubility of glipizide, difficulty in swallowing, etc., and achieve dissolution Consistent speed, excellent dissolution in vitro, and improved water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] [embodiment 1] preparation of glipizide compound
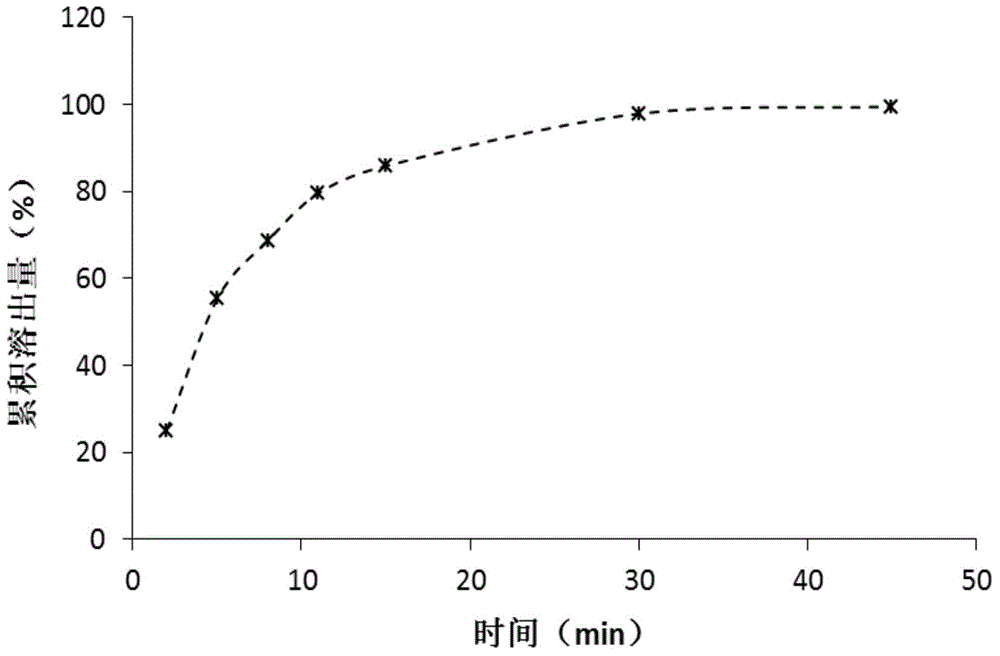
[0044]Get glipizide crude drug 500g, add the mixed solution of DMF / isopropanol, wherein the volume ratio of DMF and isopropanol is 2:1, the consumption ratio of glipizide crude drug and mixed solution is 1g: 10ml, Heat to 40°C, after the glipizide raw material is dissolved, add activated carbon for decolorization, the amount of added activated carbon is 0.2% g / ml of the total volume of the liquid, stir and adsorb for 25 minutes, filter for decarbonization and sterilization; the filtrate is transferred to the reaction In the kettle, slowly add acetone at a stirring rate of 25r / min, the volume ratio of acetone to the mixed solution is 3:1, after the addition is complete, cool down to 0°C, filter, wash with acetone for 3 times, and dry under reduced pressure for 5h. A white crystalline powder was obtained.
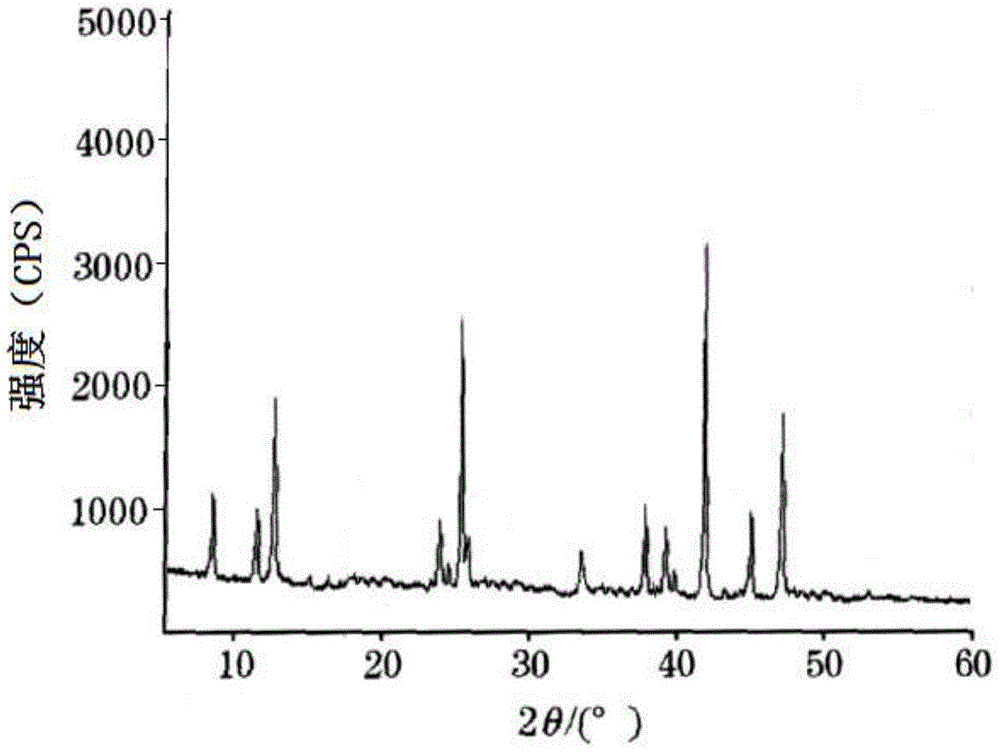
[0045] Gained white crystalline powder uses Cu-Kα ray to measure the X-ray powder diffraction pattern that obtains ...
Embodiment 2
[0046] [embodiment 2] preparation of glipizide compound
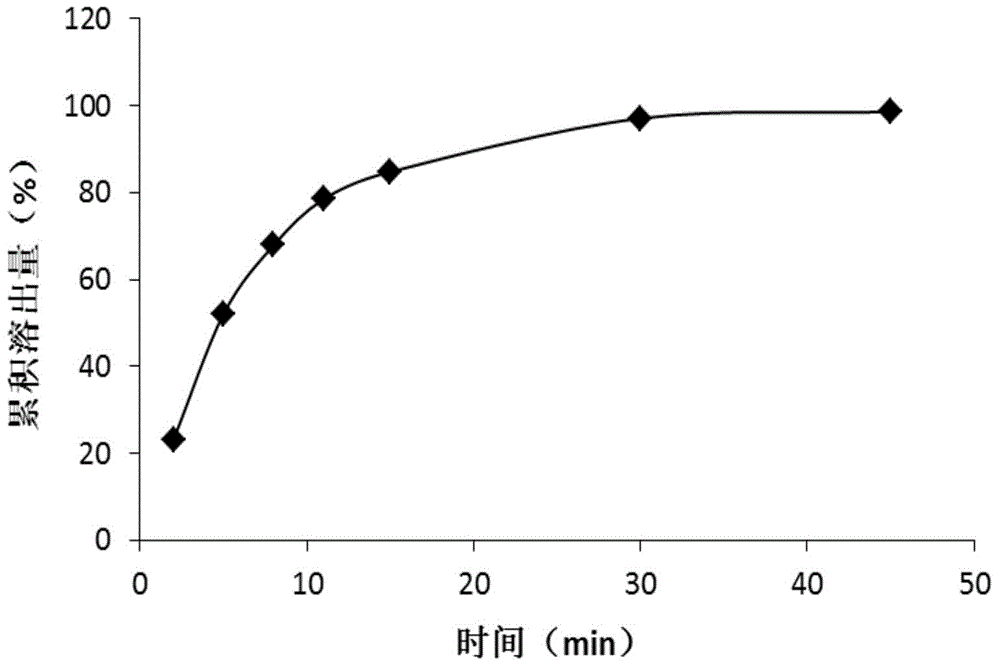
[0047] Get glipizide bulk drug 500g, add the mixed solution of DMF / isopropanol, wherein the volume ratio of DMF and isopropanol is 4:1, the consumption ratio of glipizide bulk drug and mixed solution is 1g: 15ml, Heat to 45°C, after the glipizide raw material is dissolved, add activated carbon for decolorization, the amount of added activated carbon is 0.2% g / ml of the total volume of the liquid, stir and adsorb for 25 minutes, filter for decarbonization and sterilization; the filtrate is transferred to the reaction In the kettle, slowly add acetone under the condition of a stirring rate of 30r / min, the volume ratio of acetone to the mixed solution is 6:1, after the addition is completed, cool down to 5°C, filter, wash with acetone for 3 times, and dry under reduced pressure for 5h. A white crystalline powder was obtained.
[0048] Gained white crystalline powder uses Cu-Kα ray to measure the X-ray powder diffraction p...
Embodiment 3
[0049] [embodiment 3] preparation of glipizide compound
[0050] Get glipizide bulk drug 500g, add the mixed solution of DMF / isopropanol, wherein the volume ratio of DMF and isopropanol is 3:1, the consumption ratio of glipizide bulk drug and mixed solution is 1g: 12ml, Heat to 42°C, after the glipizide raw material is dissolved, add activated carbon for decolorization, the amount of added activated carbon is 0.2% g / ml of the total volume of the liquid, stir and adsorb for 25 minutes, filter for decarbonization and sterilization; the filtrate is transferred to the reaction In the kettle, slowly add acetone at a stirring rate of 28r / min, the volume ratio of acetone to the mixed solution is 5:1, after the addition is complete, cool down to 3°C, filter, wash with acetone for 3 times, and dry under reduced pressure for 5h. A white crystalline powder was obtained.
[0051] Gained white crystalline powder uses Cu-Kα ray to measure the X-ray powder diffraction pattern that obtains a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com