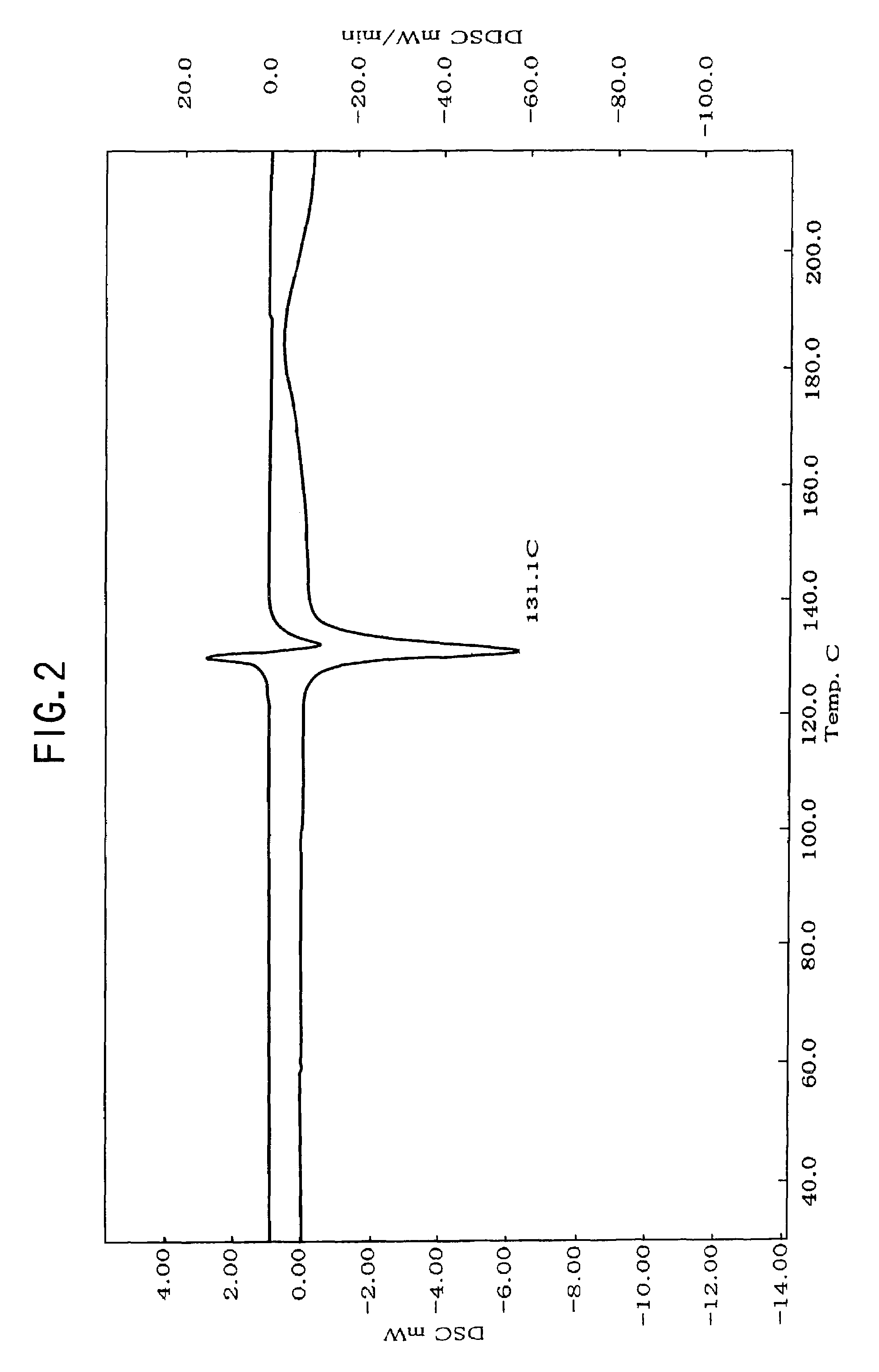
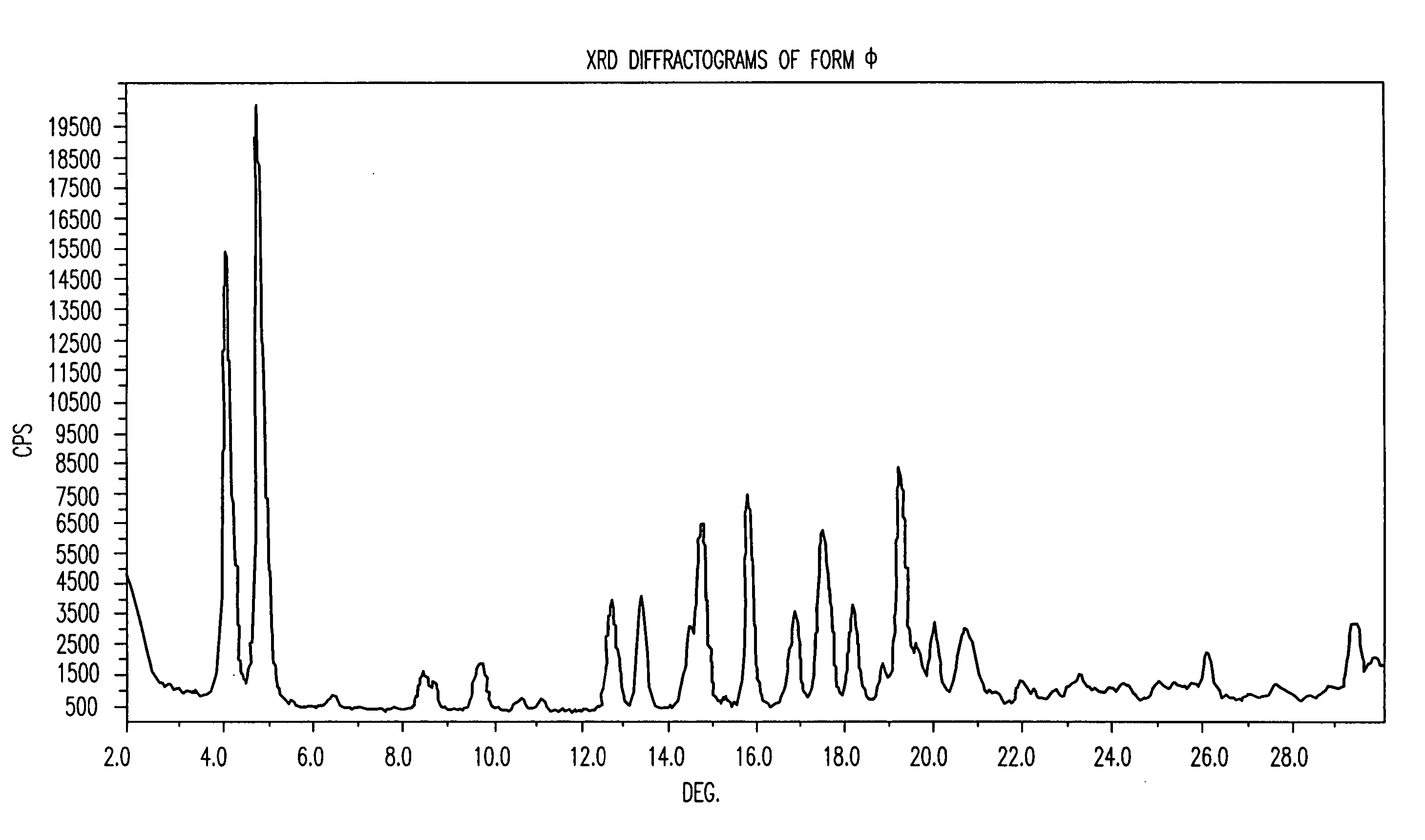
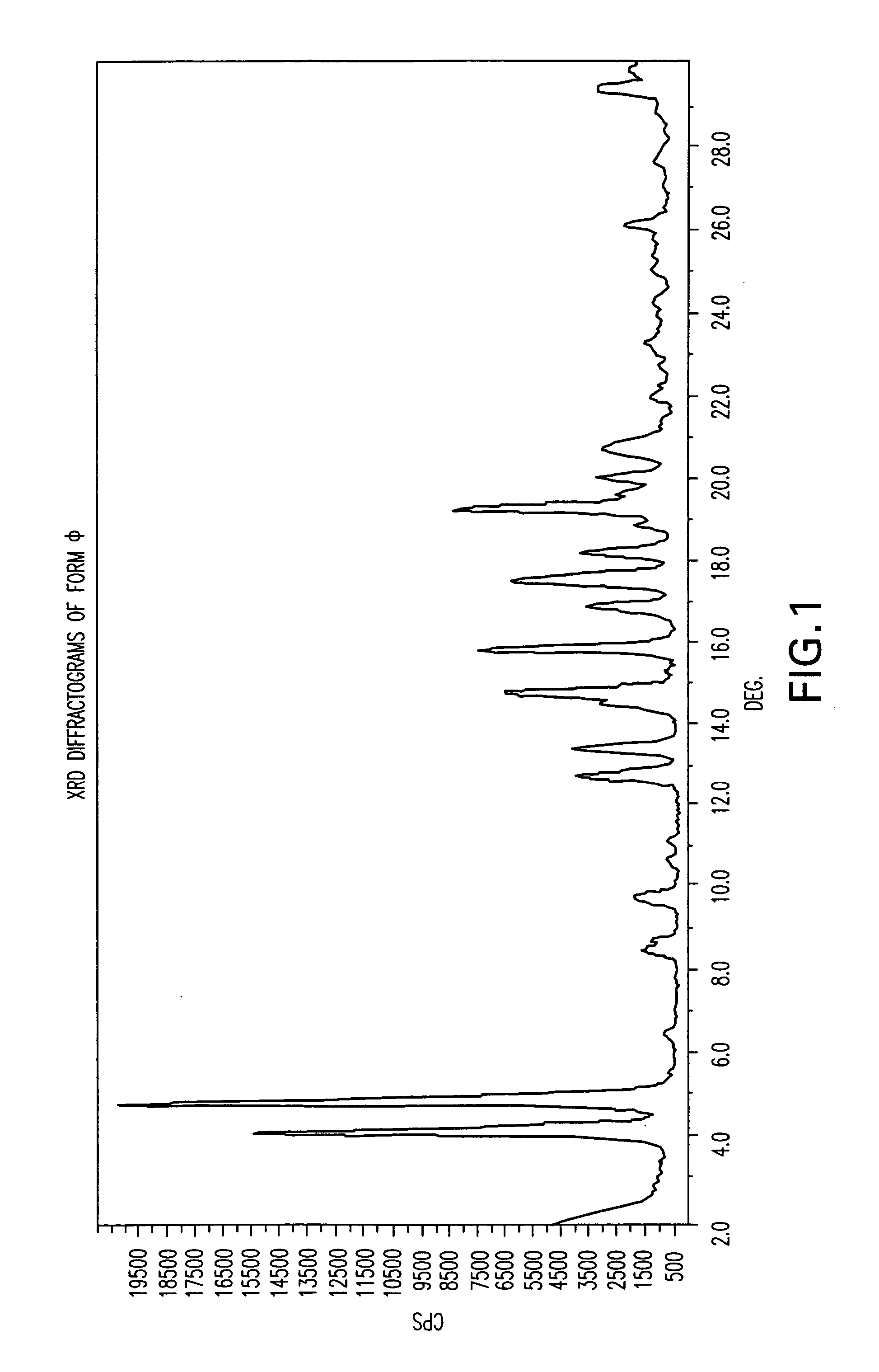
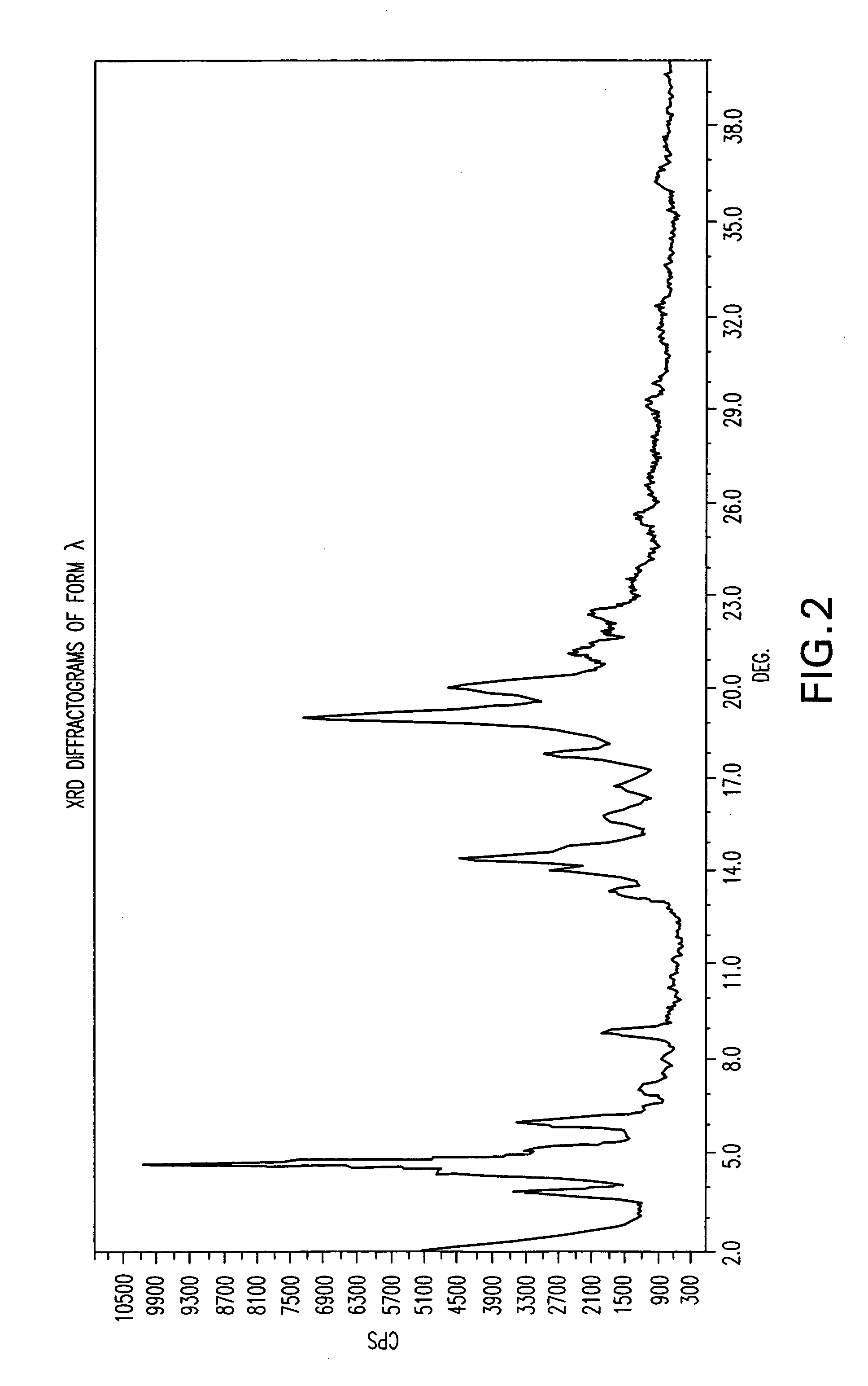
Patents
Literature
35 results about "Nateglinide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
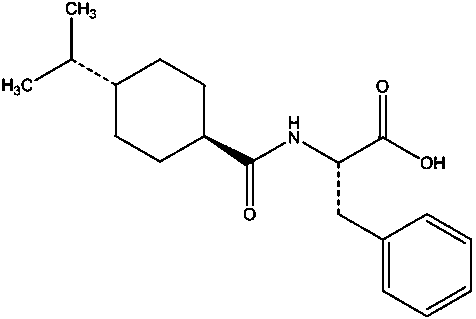
Nateglinide is used alone or with other medications to control high blood sugar along with a proper diet and exercise program. It is used in people with type 2 diabetes.
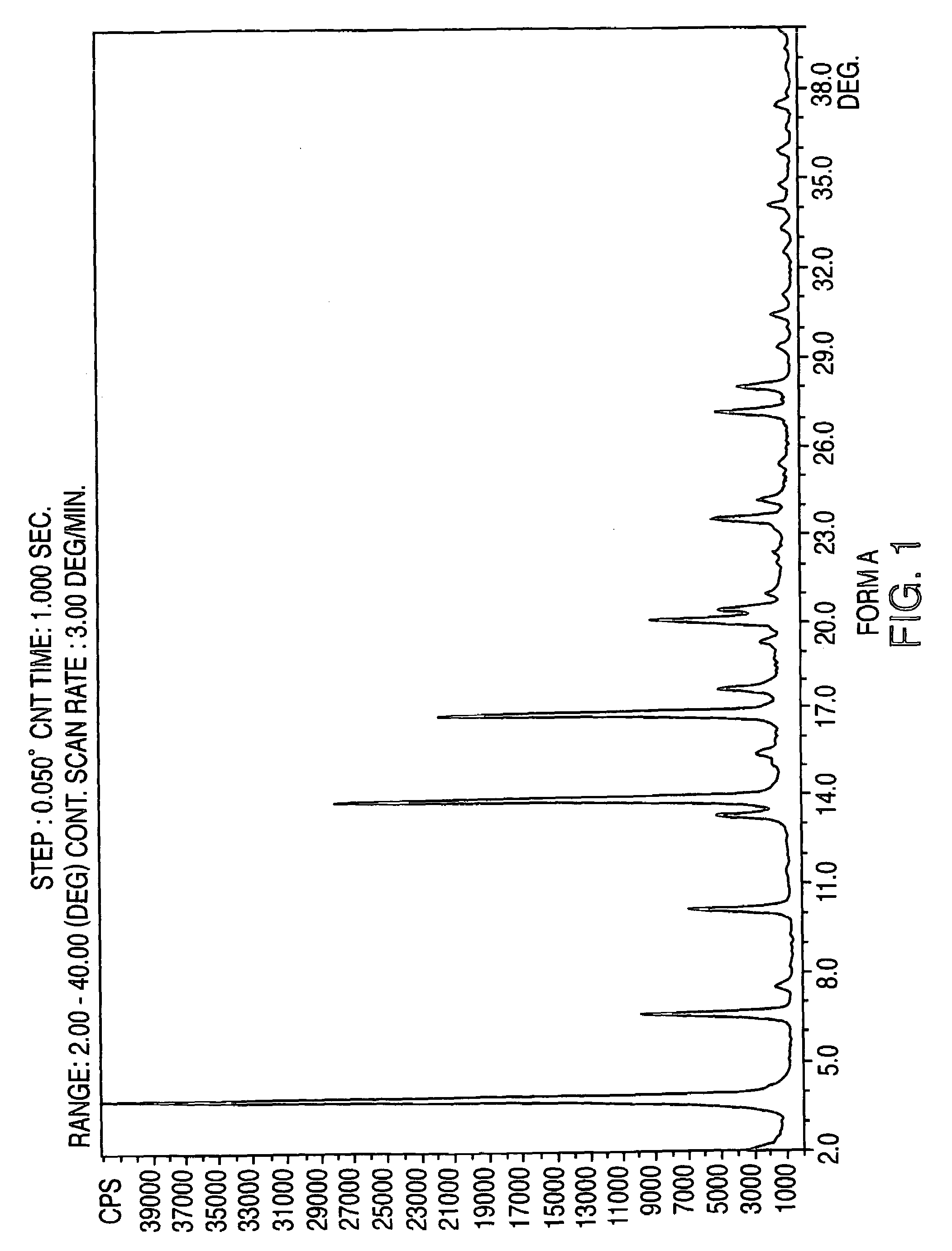
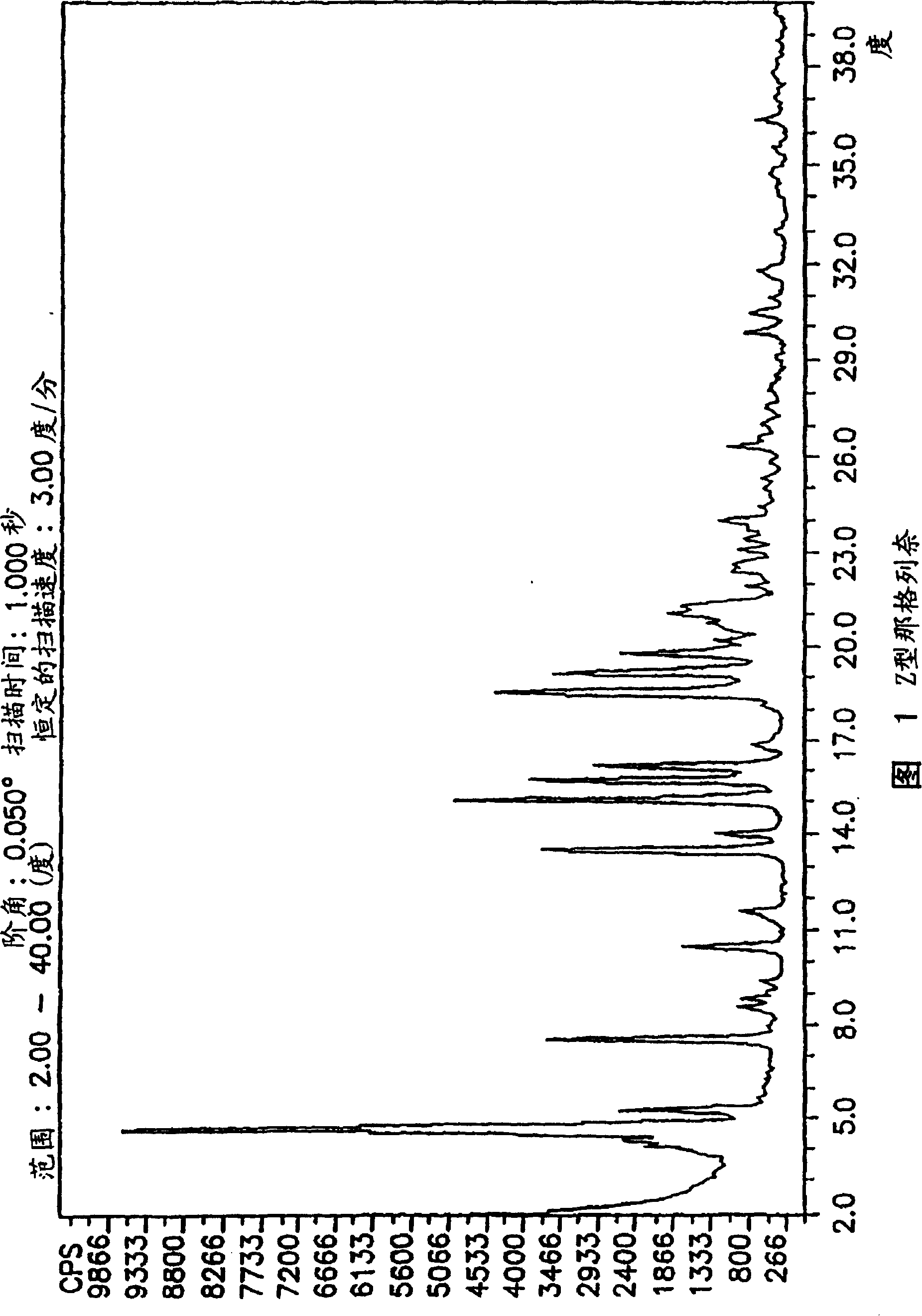
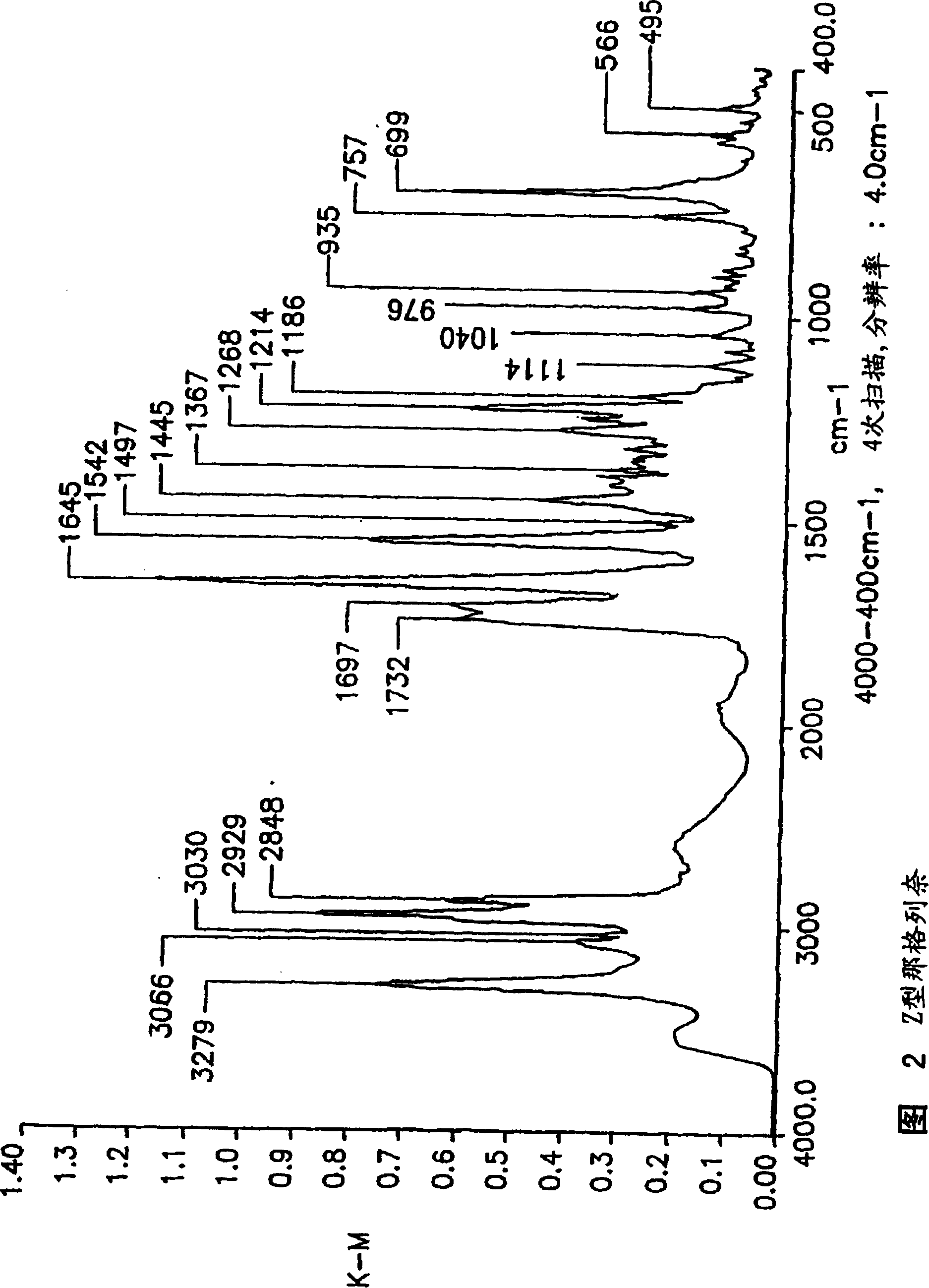
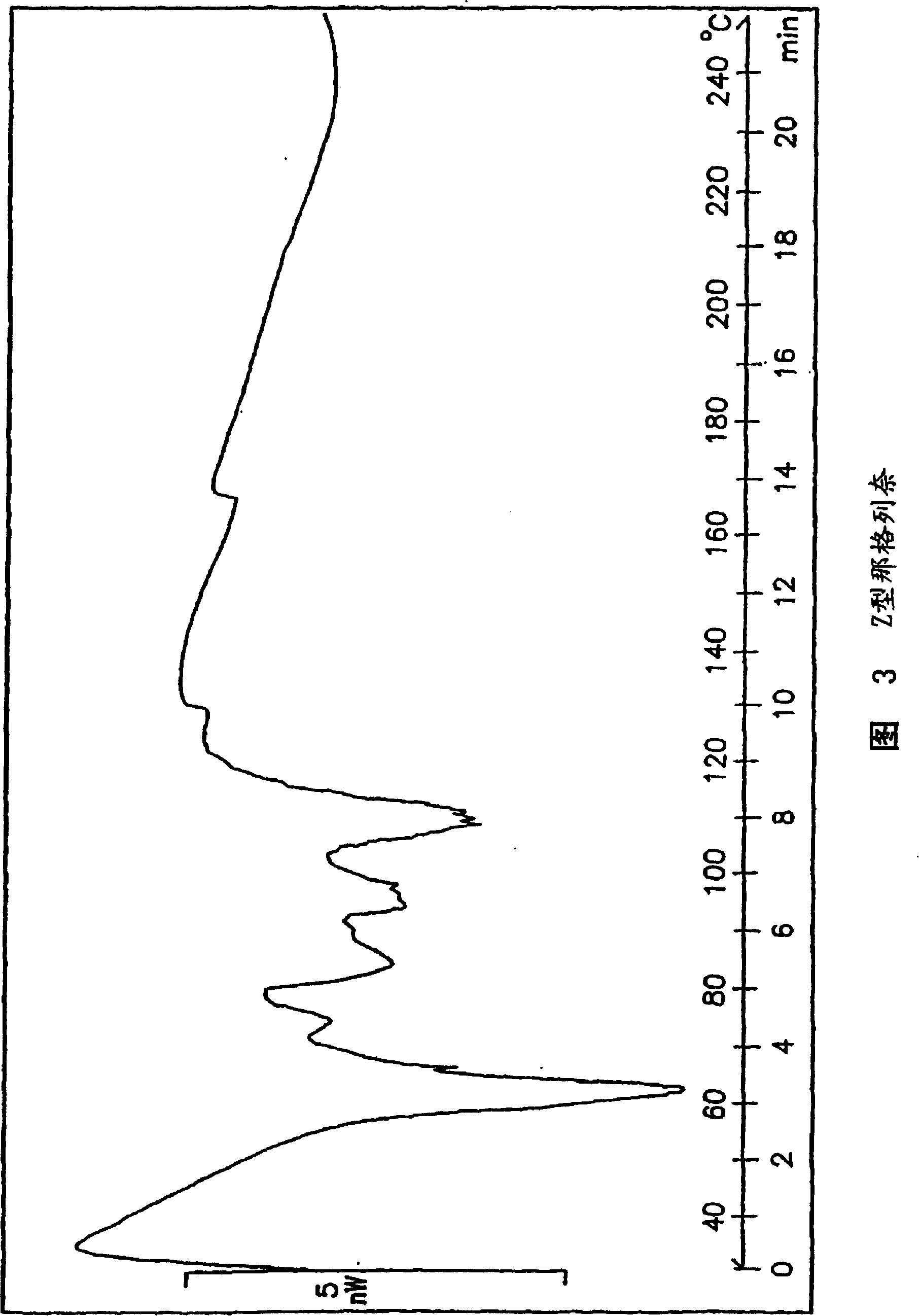
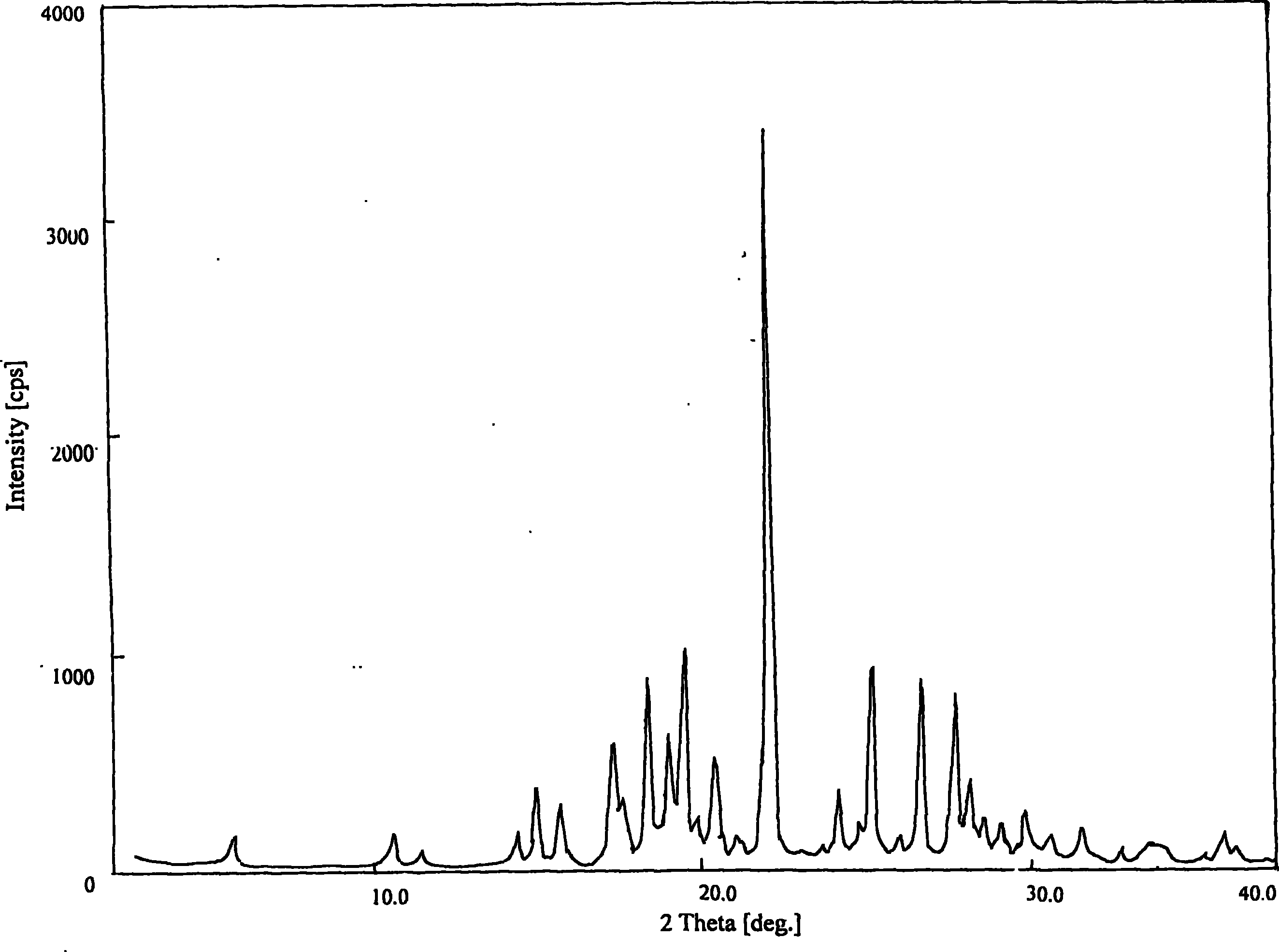
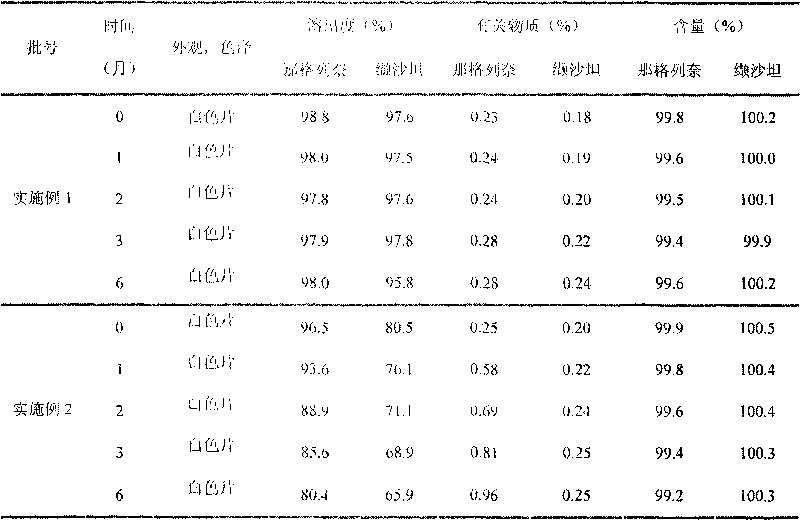
Methods for producing nateglinide B-type crystals
Owner:AJINOMOTO CO INC
Process for preparing nateglinide and intermediates thereof
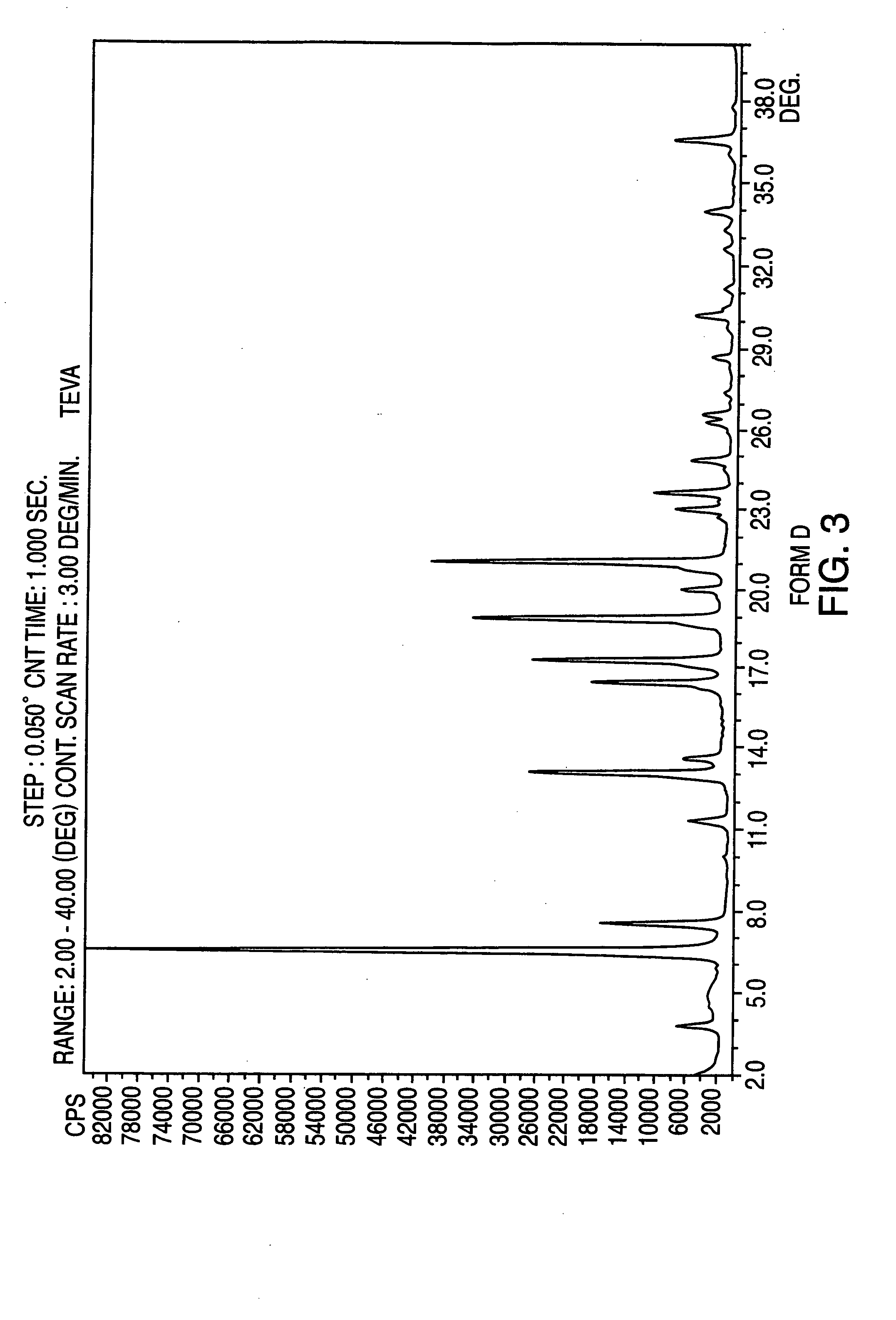
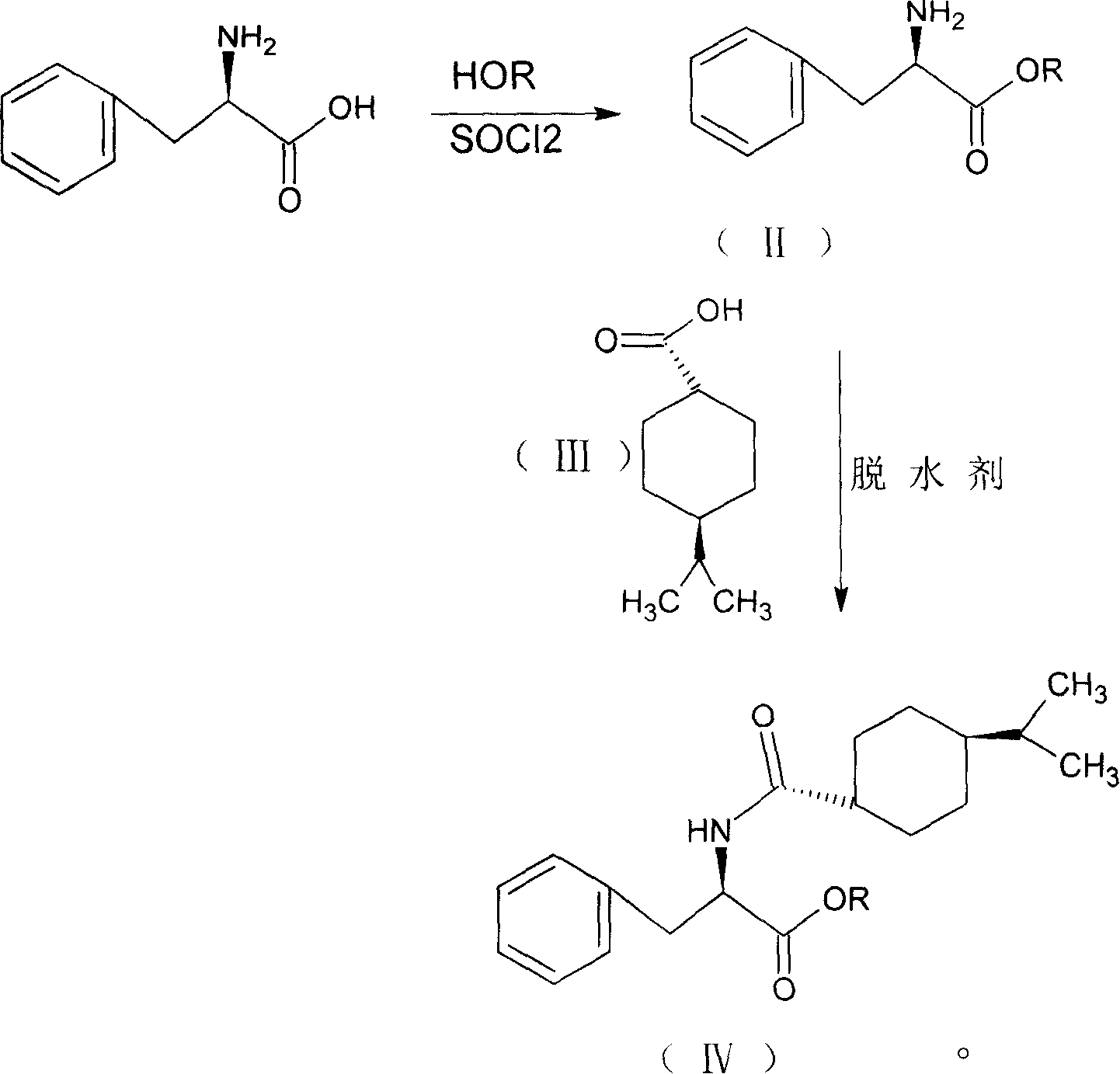
Provided is a process for preparation of an intermediate in the synthesis of nateglinide. Trans-4-isopropylcyclohexane acid chloride is formed by reacting 4-isopropylcyclohexanecarboxyl acid with thionyl chloride in the presence of an effective amount of an organic amide.Also provided are processes for preparation of nateglinide by acylation of a suitable salt of D-phenylalanine with trans-4-isopropylcyclohexane acid chloride in both a single and a two phase system, and in water free of a co-solvent.
Owner:TEVA PHARM USA INC
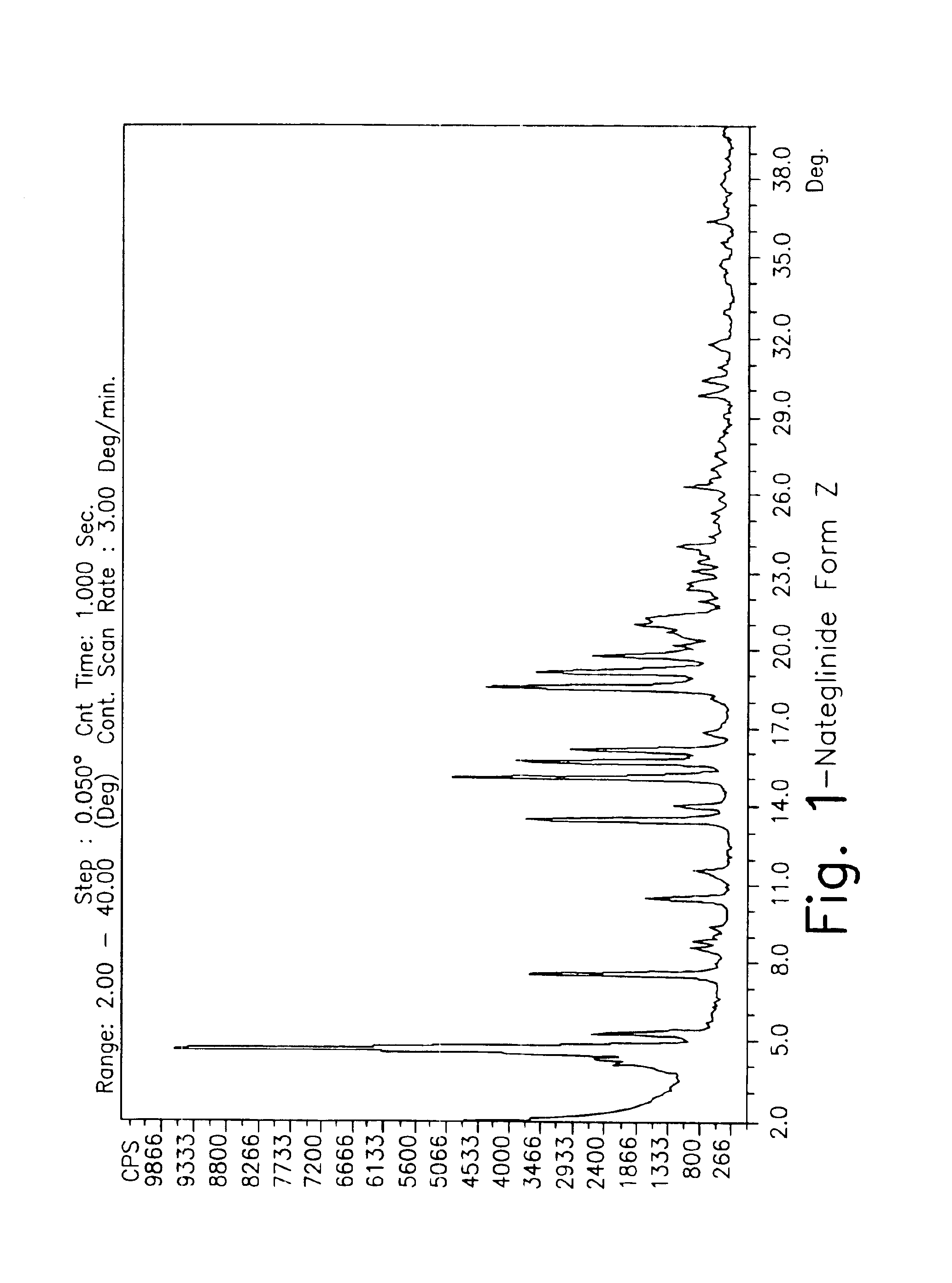
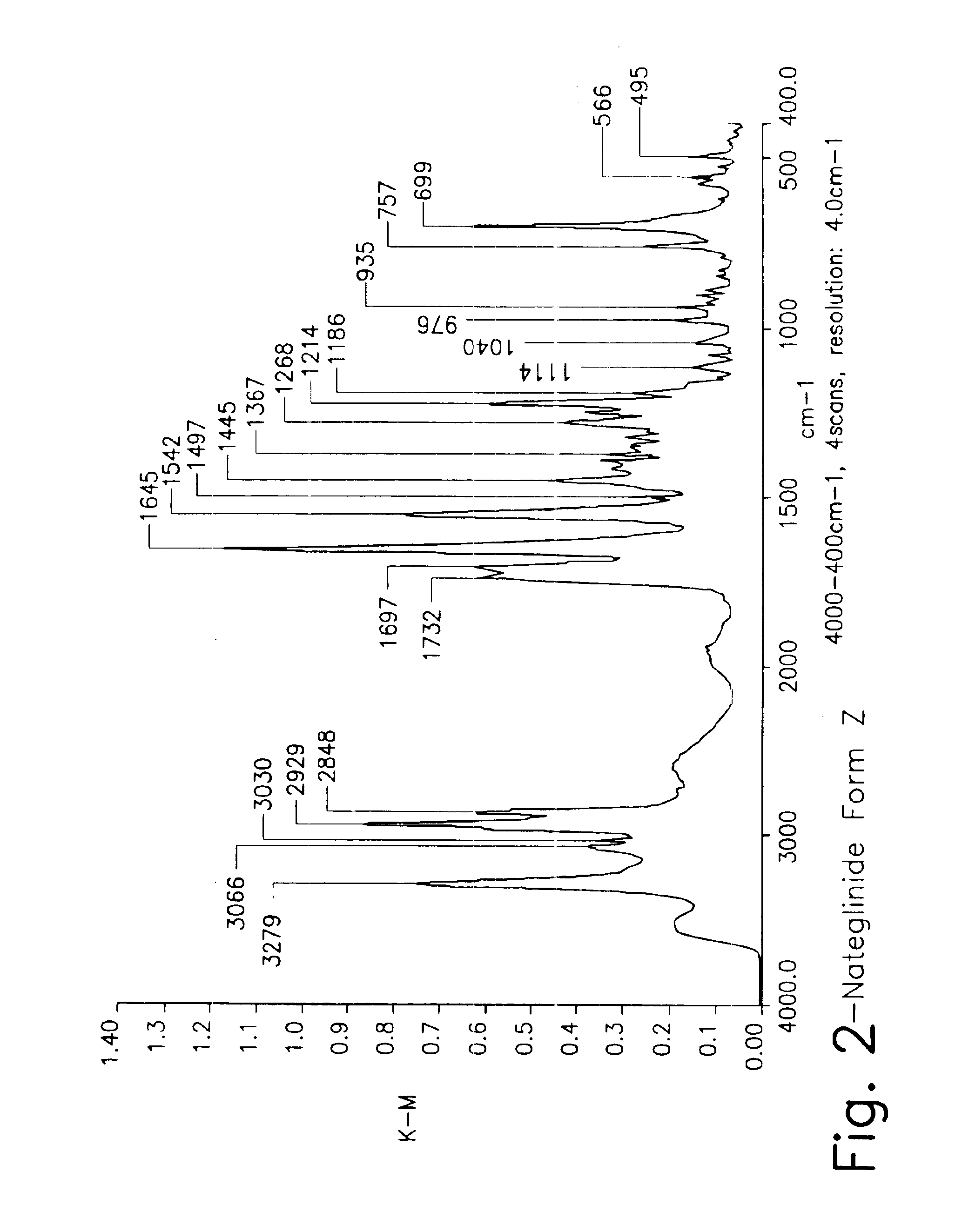
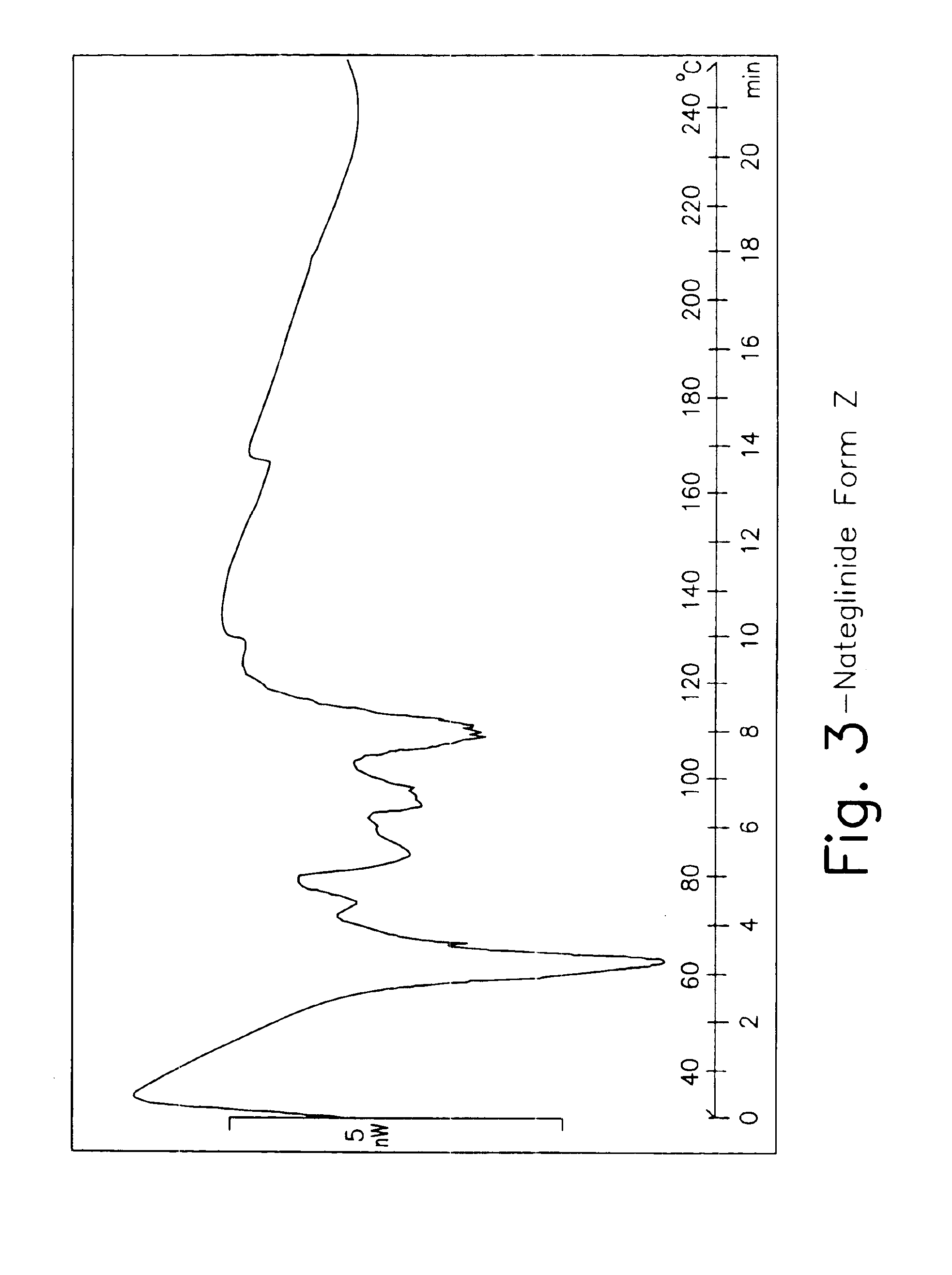
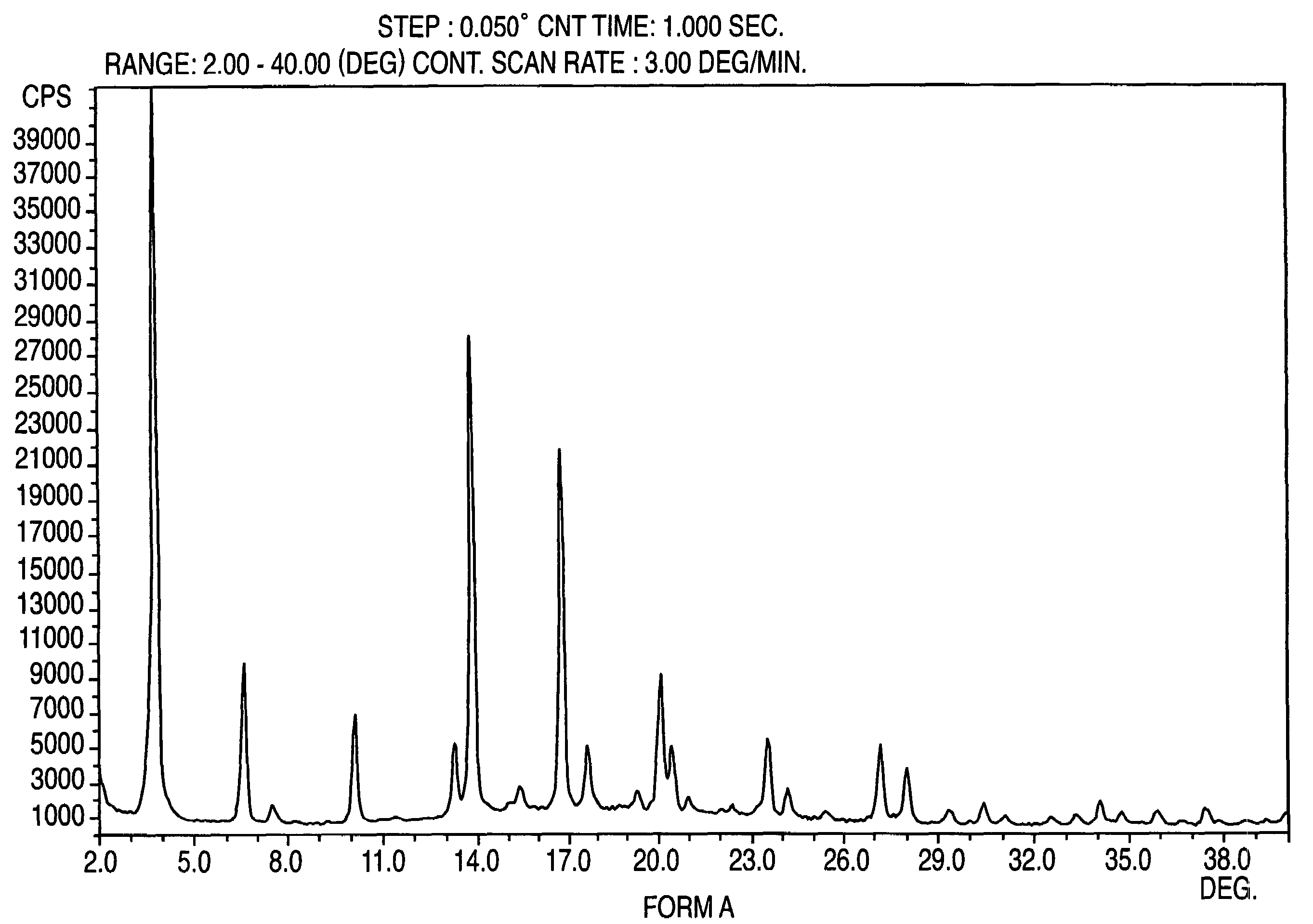
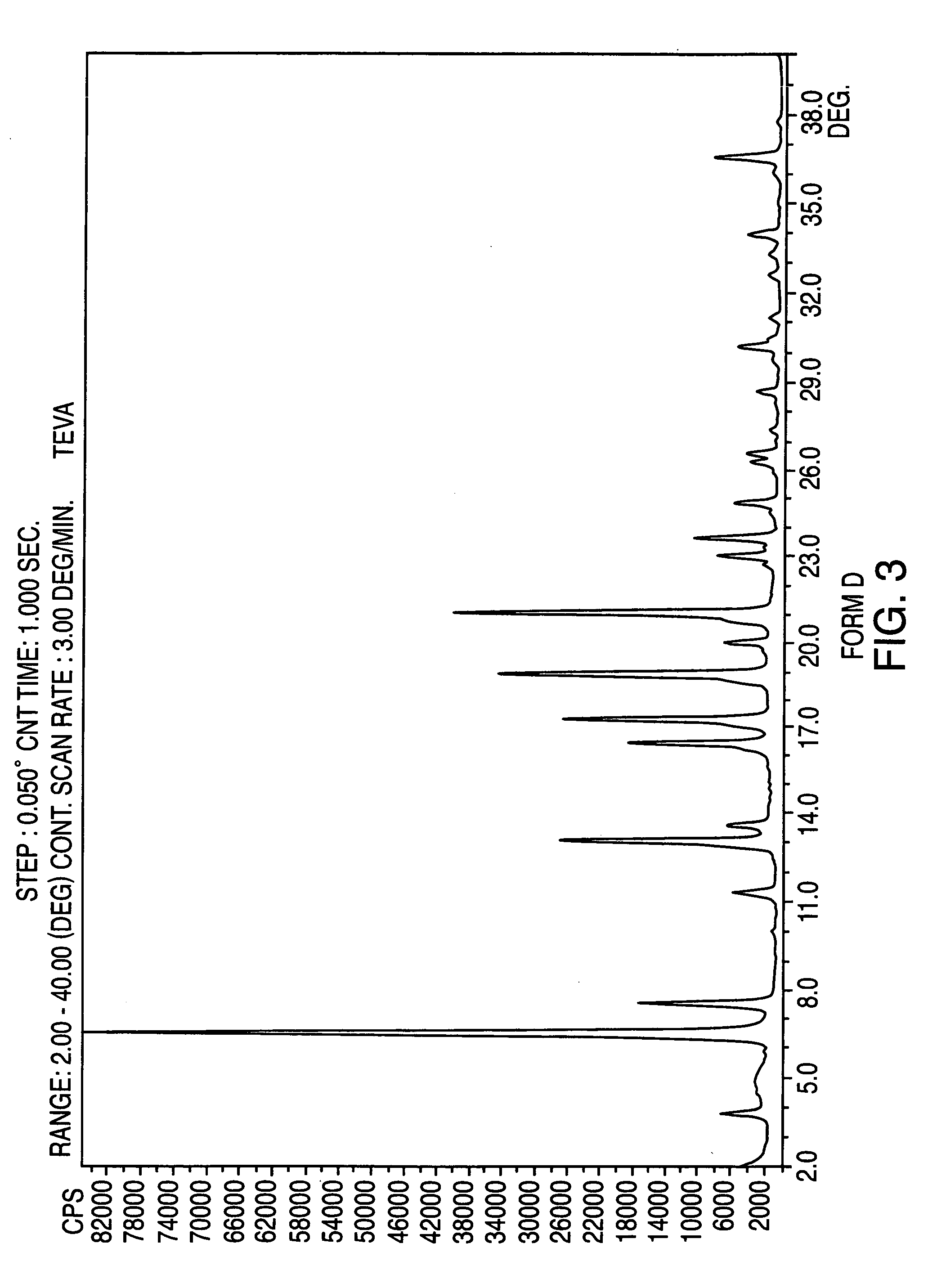
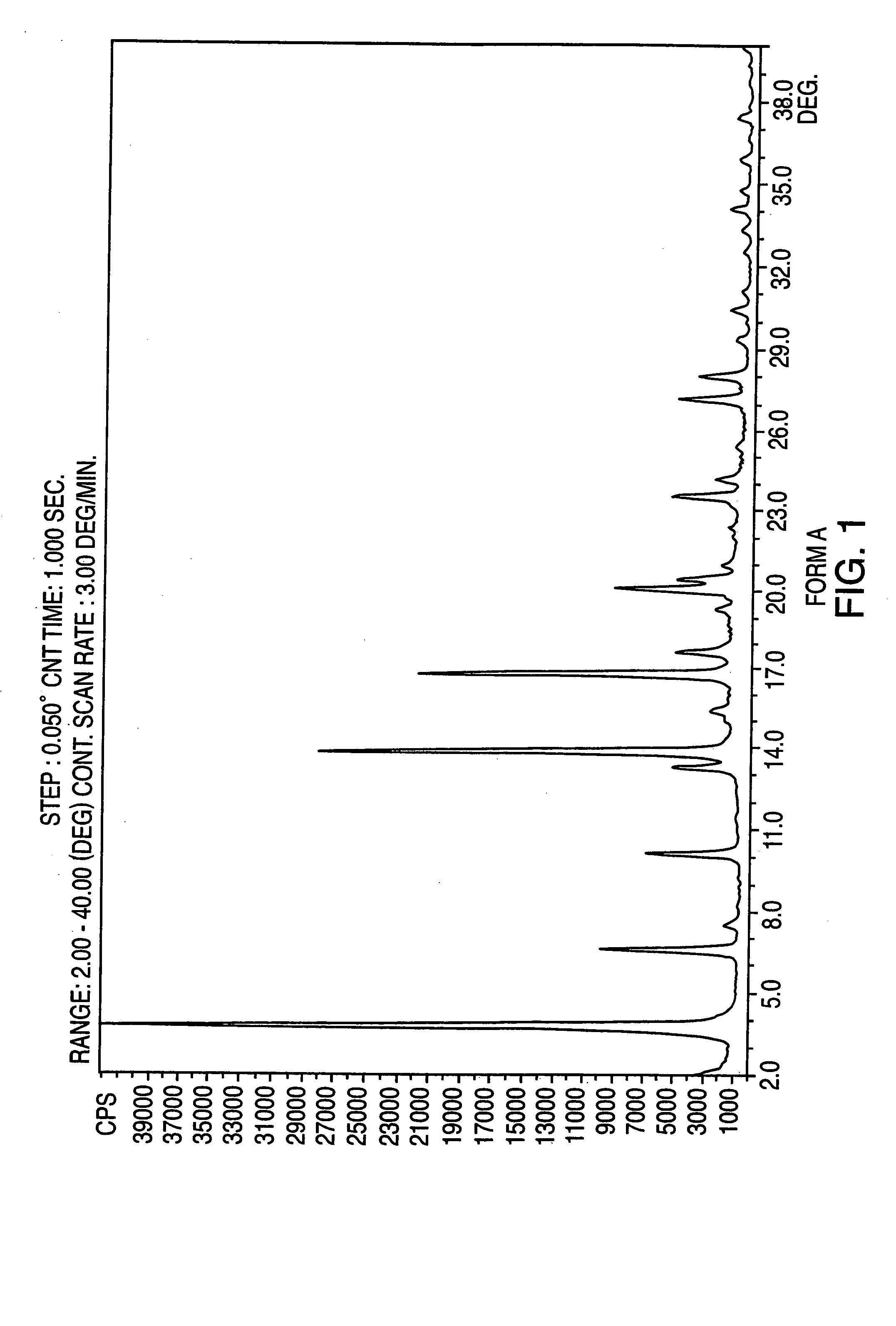
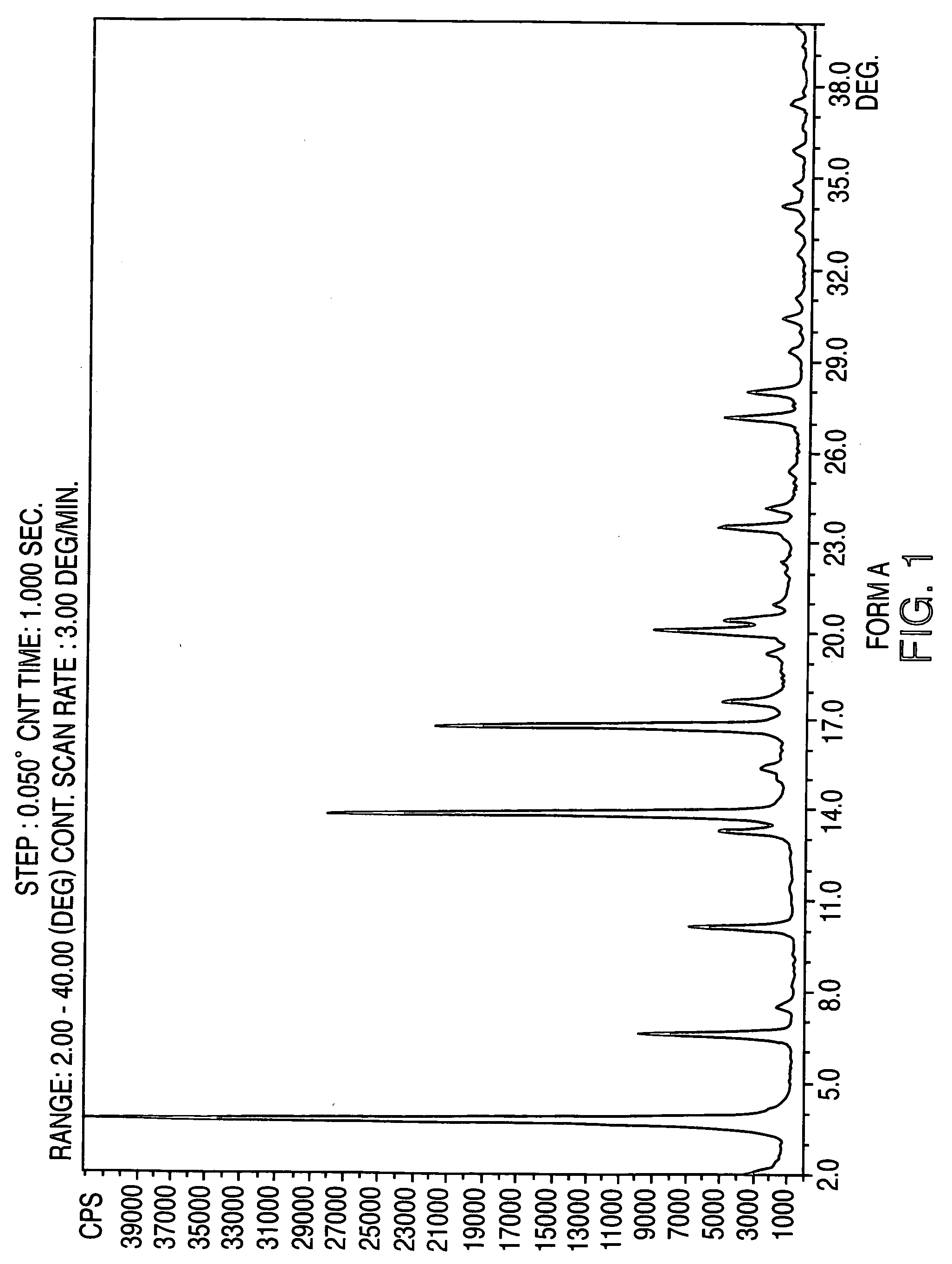
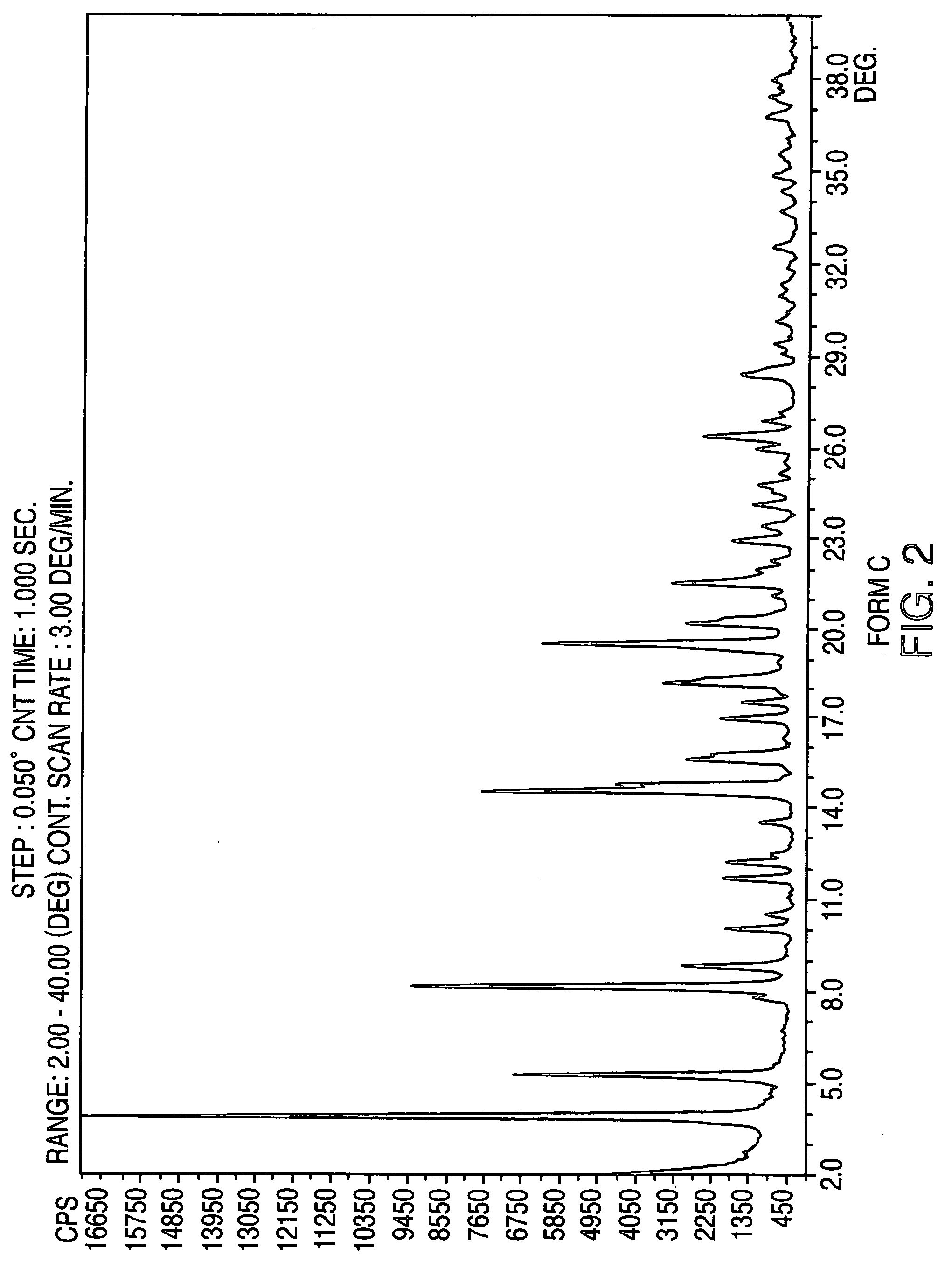
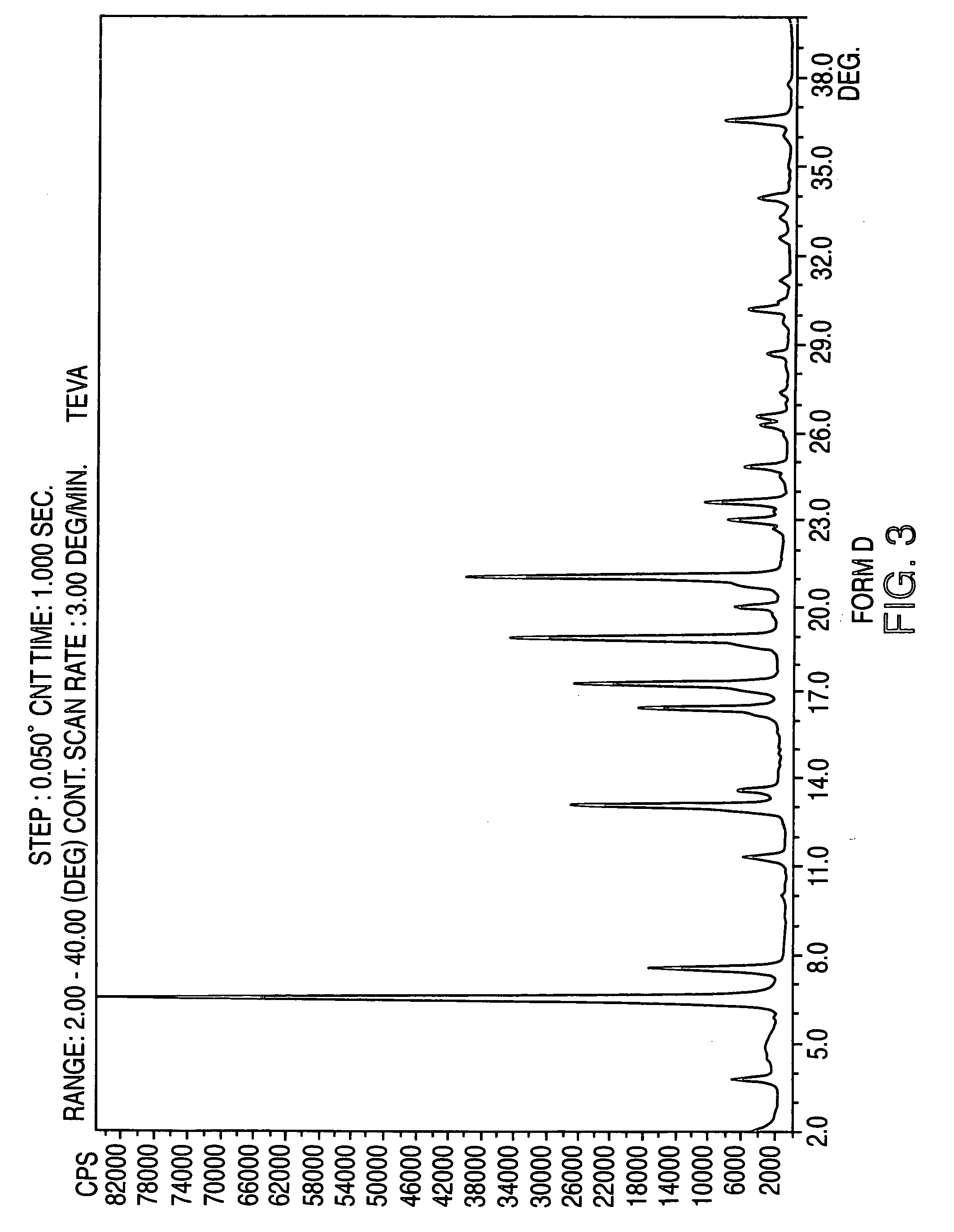
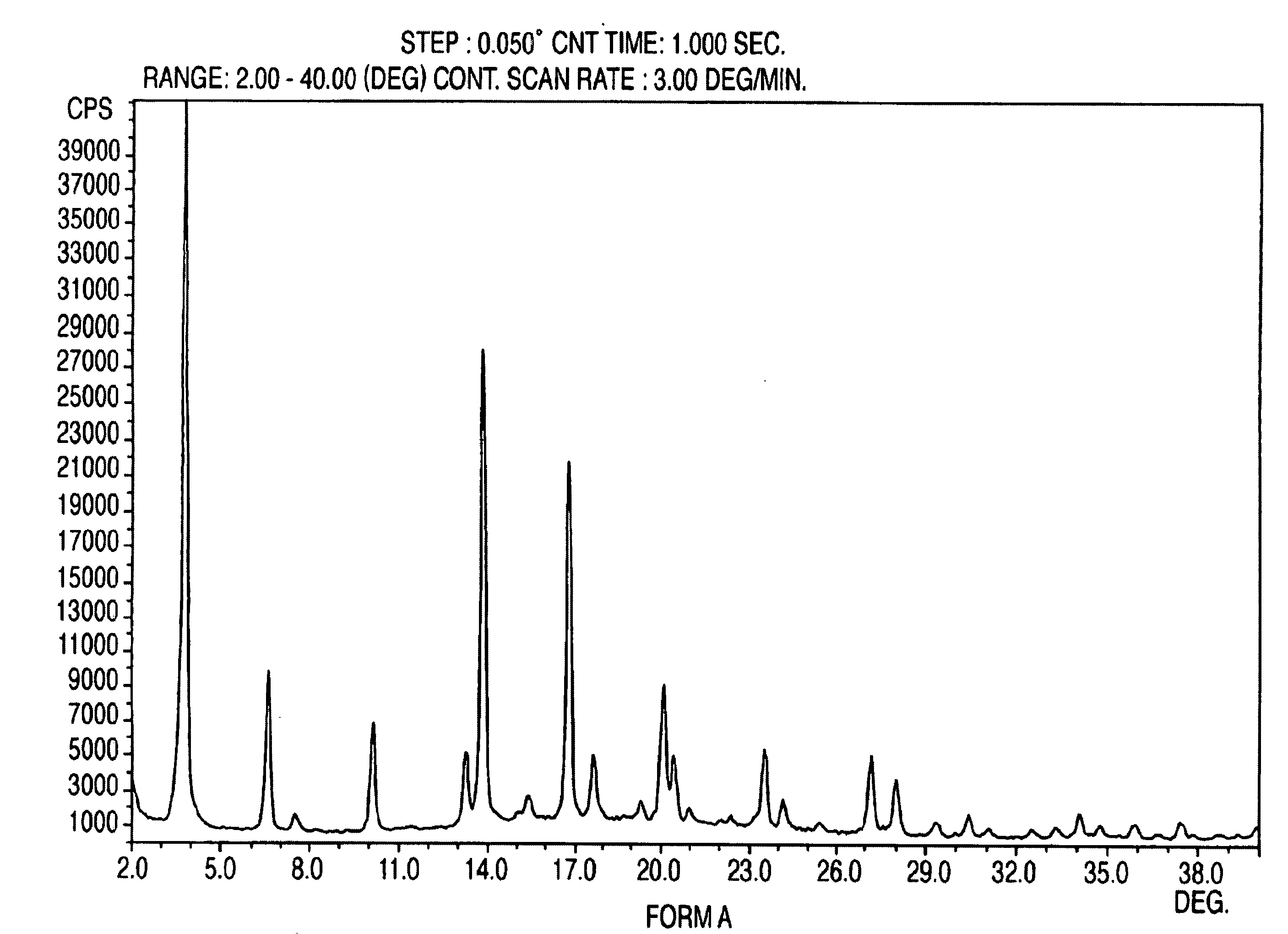
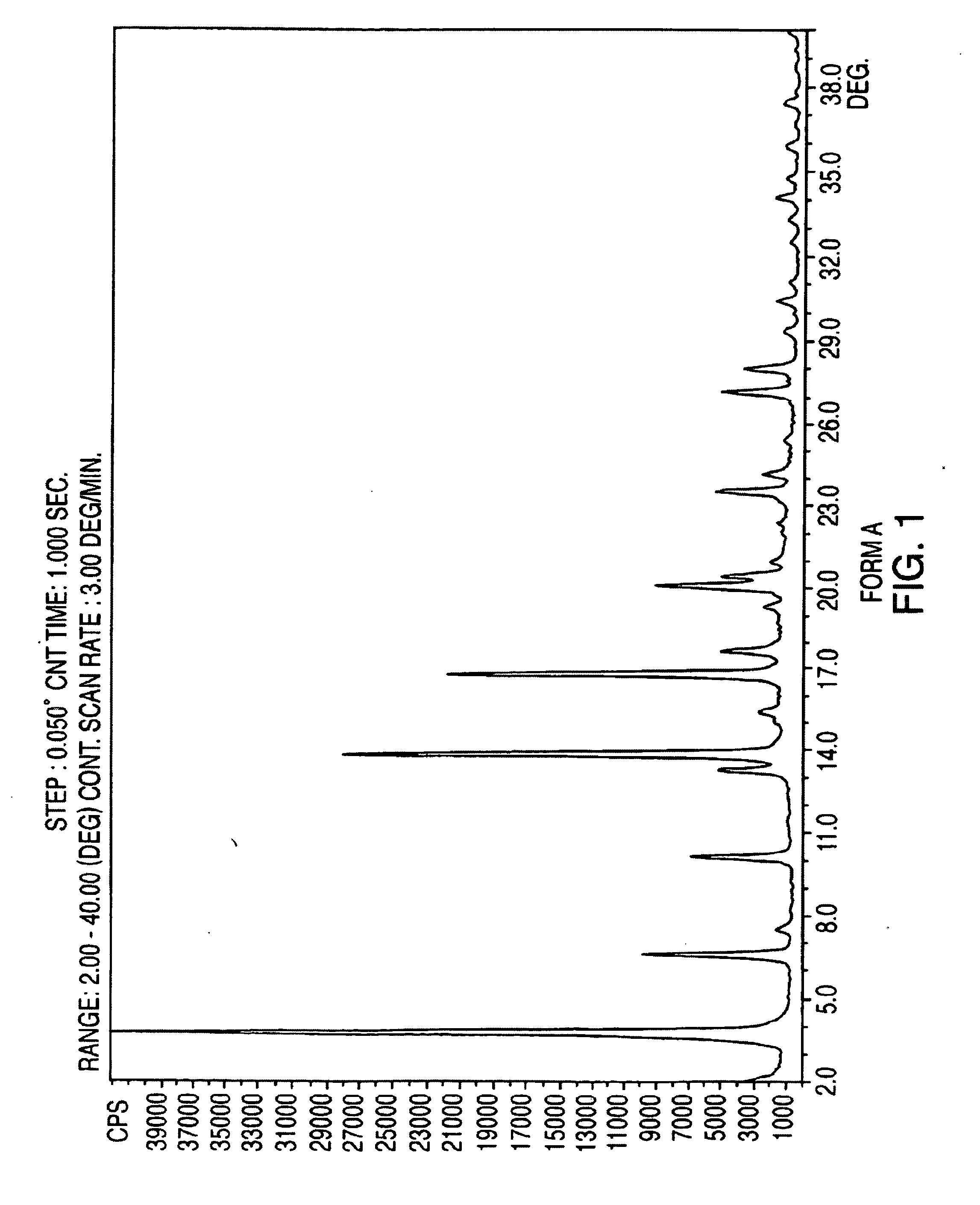
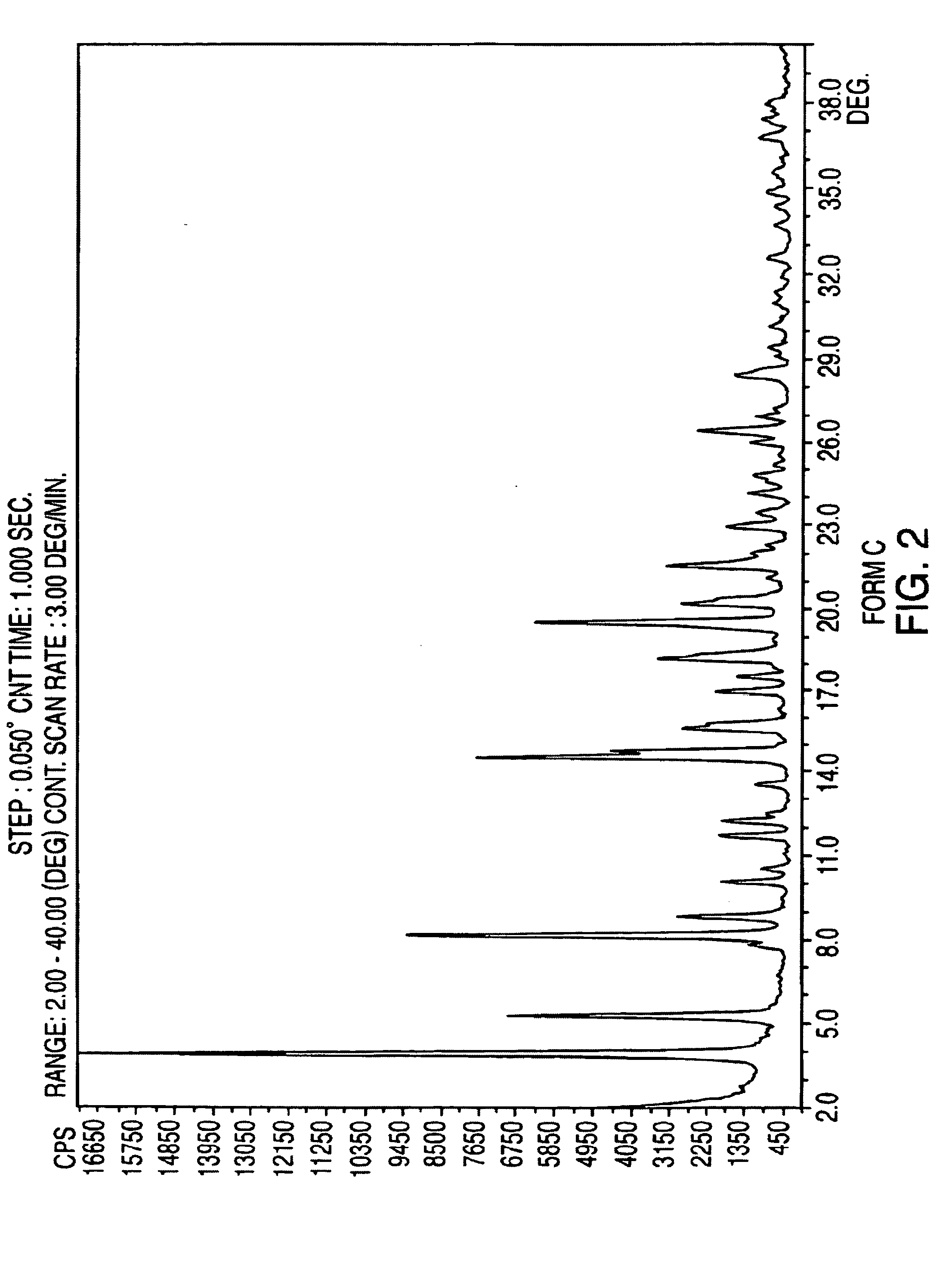
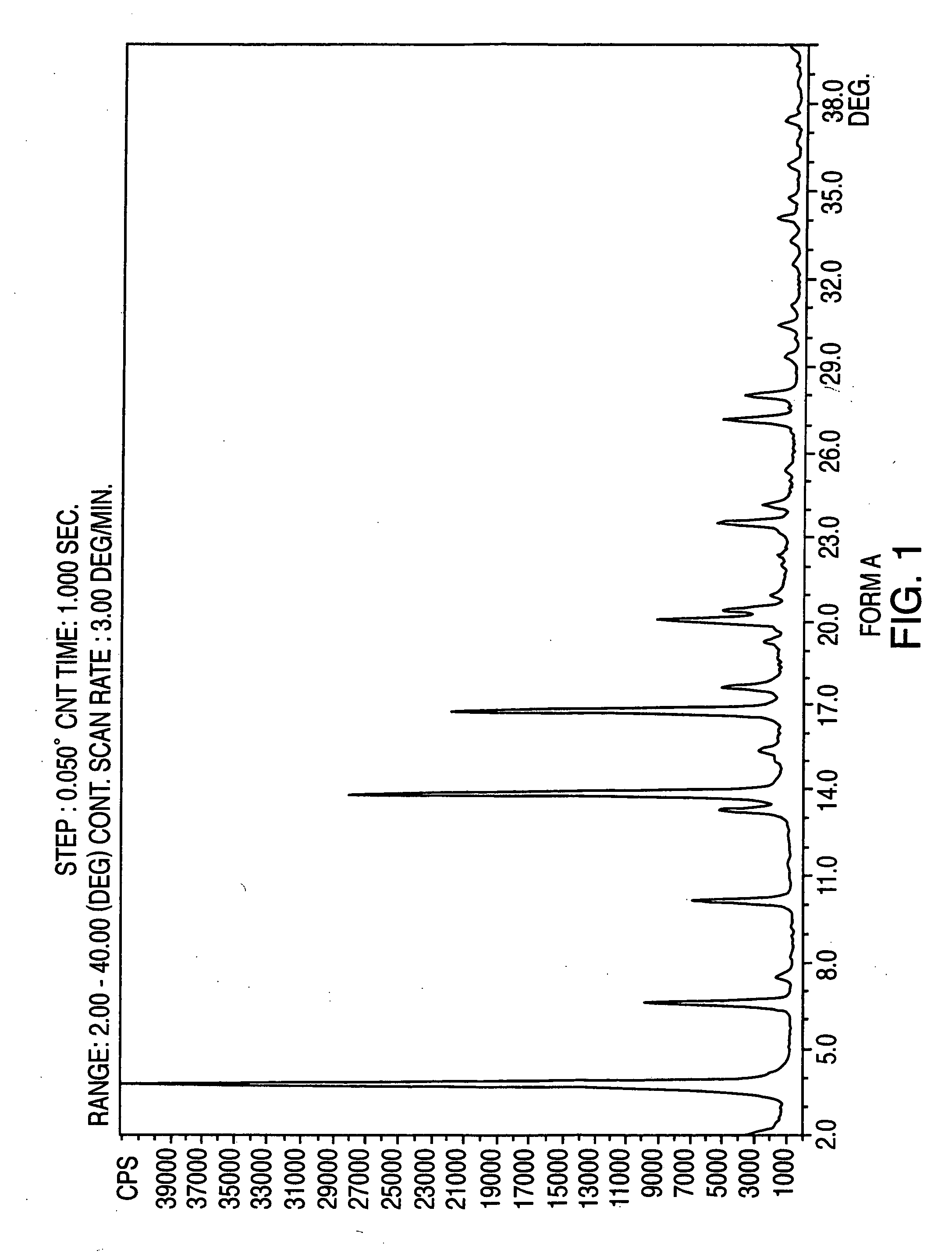
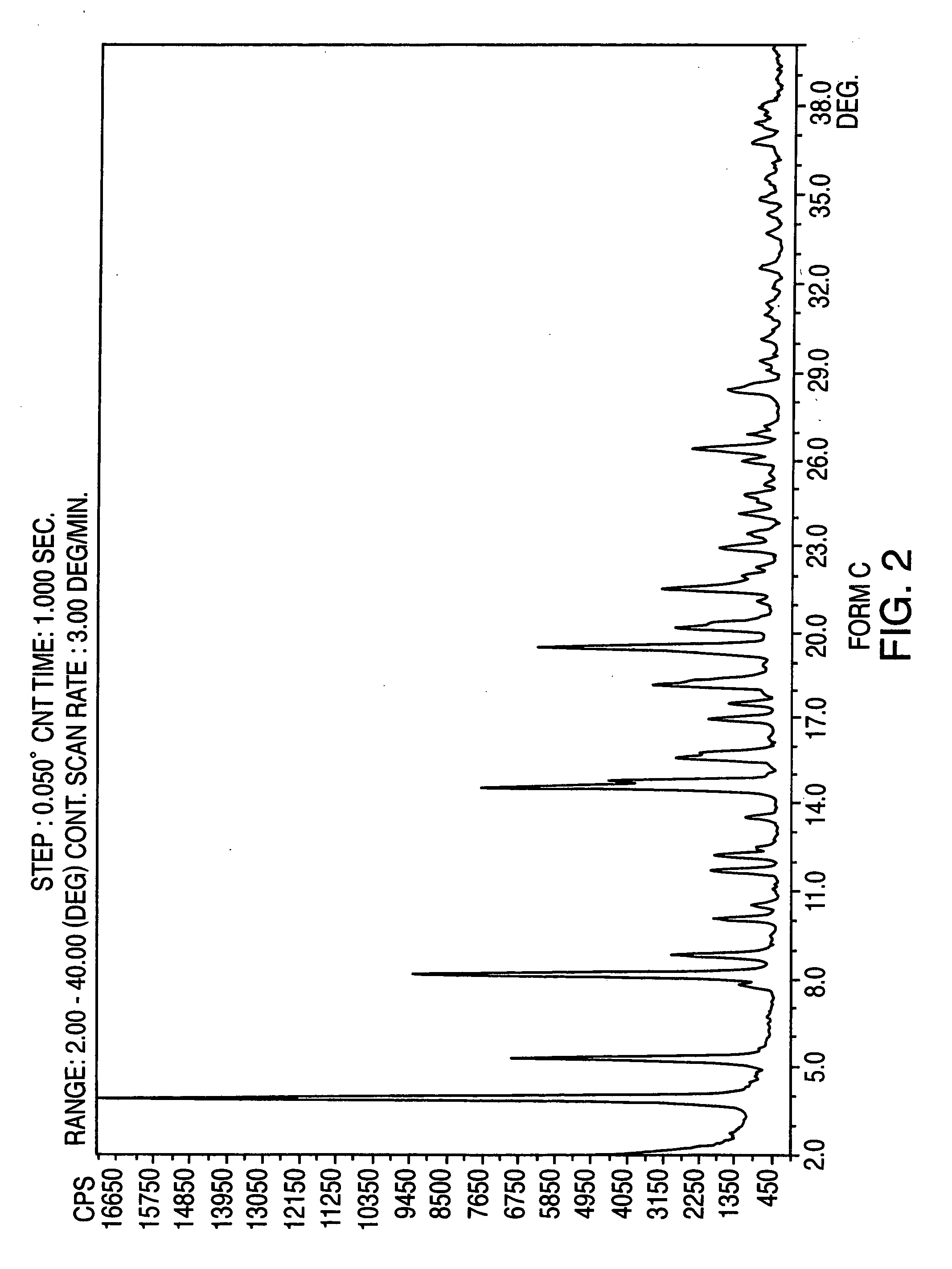
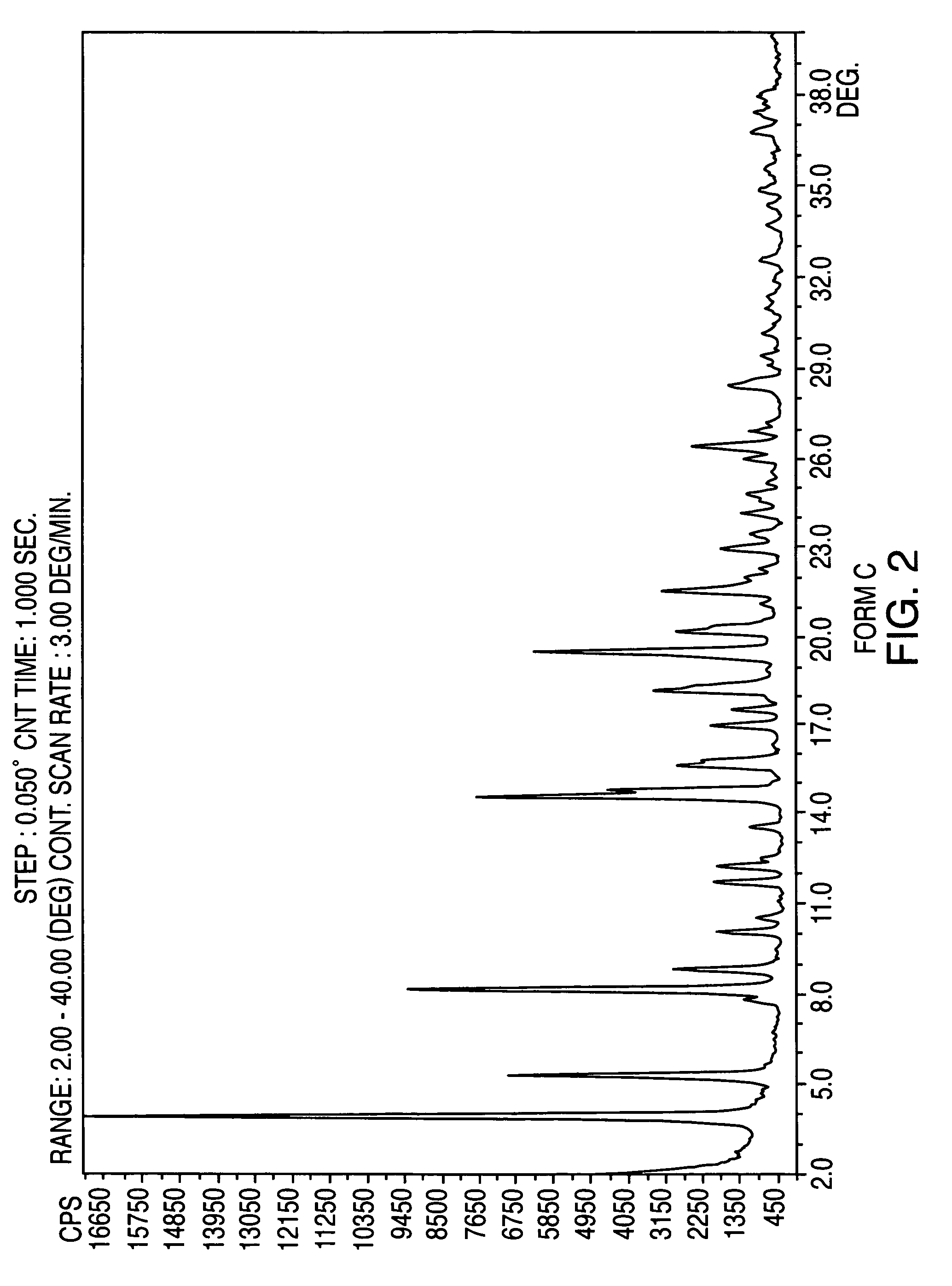
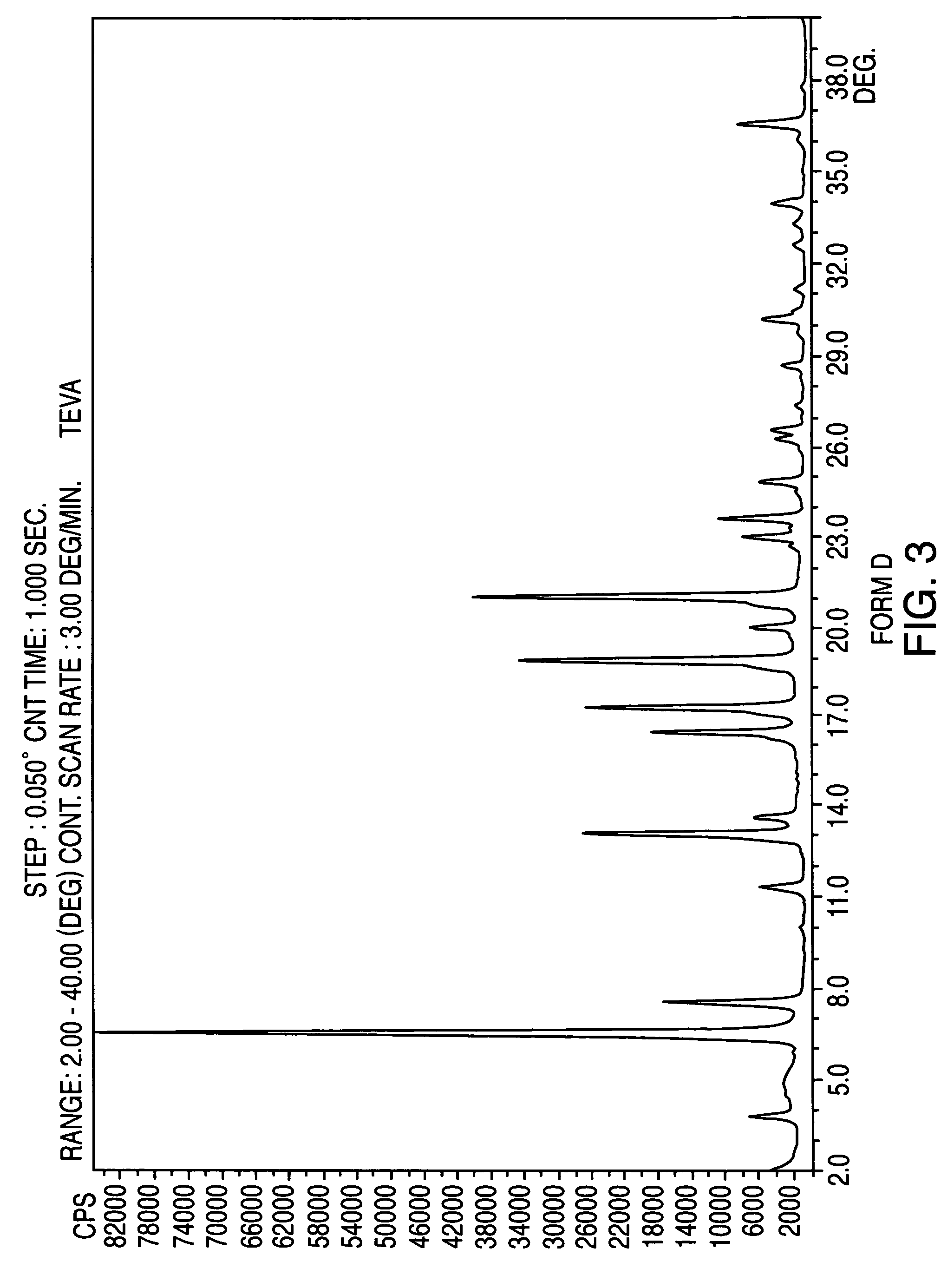
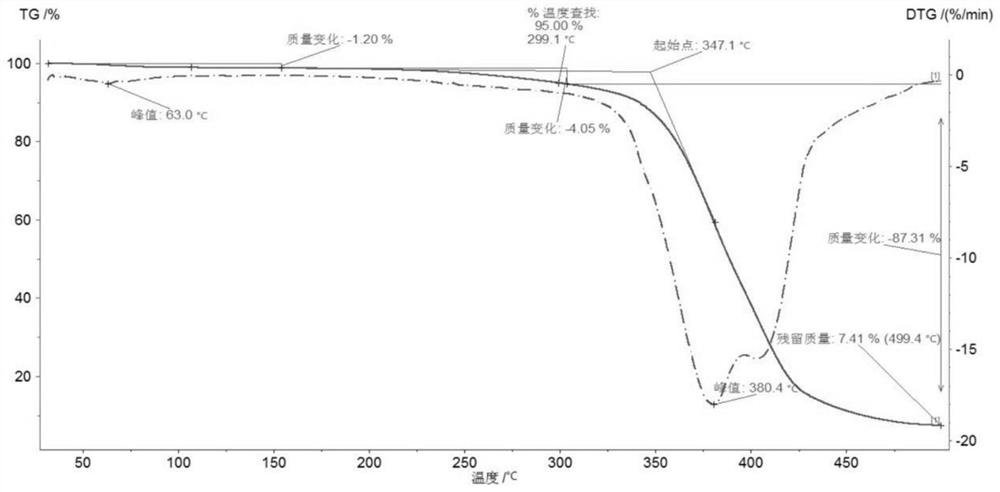
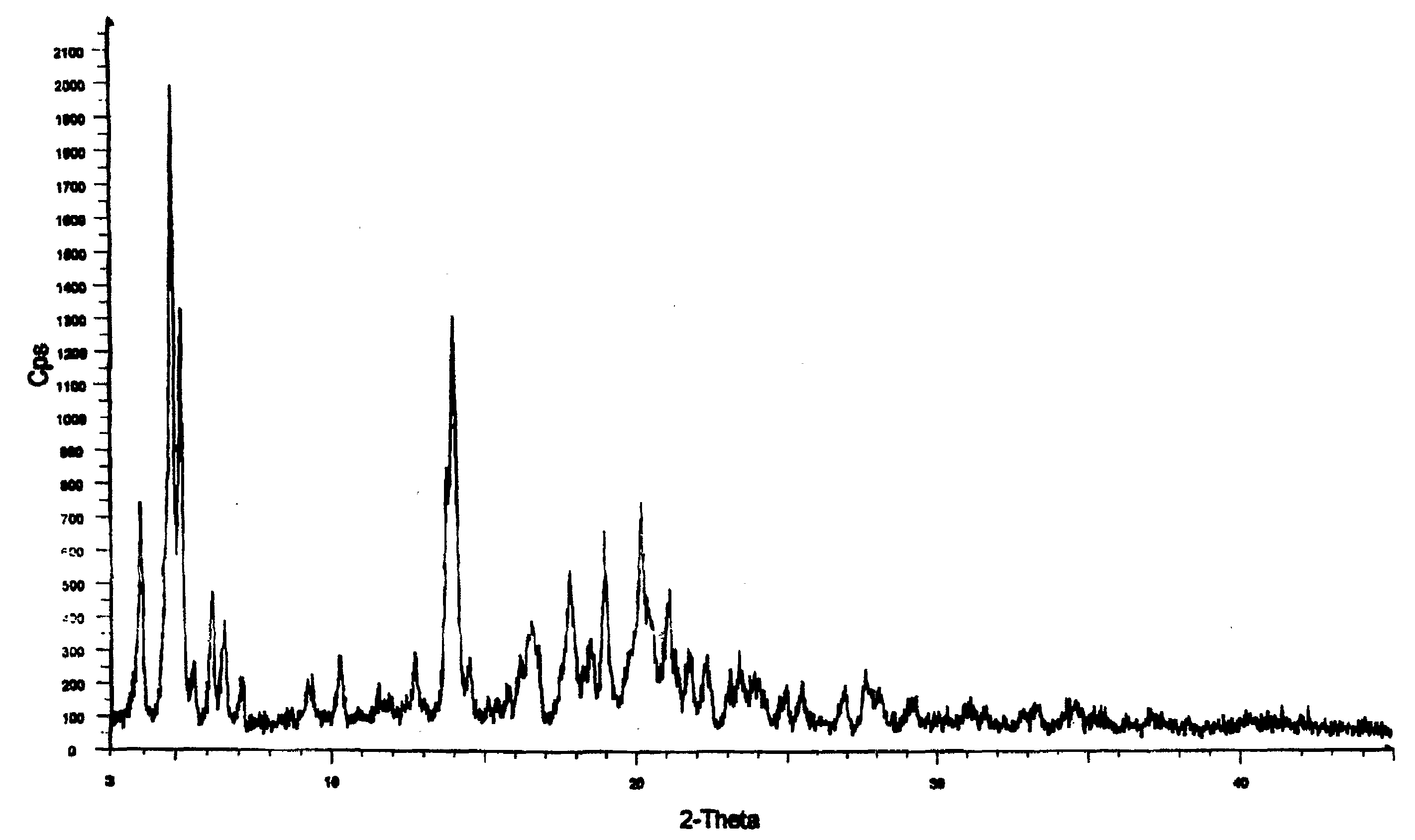
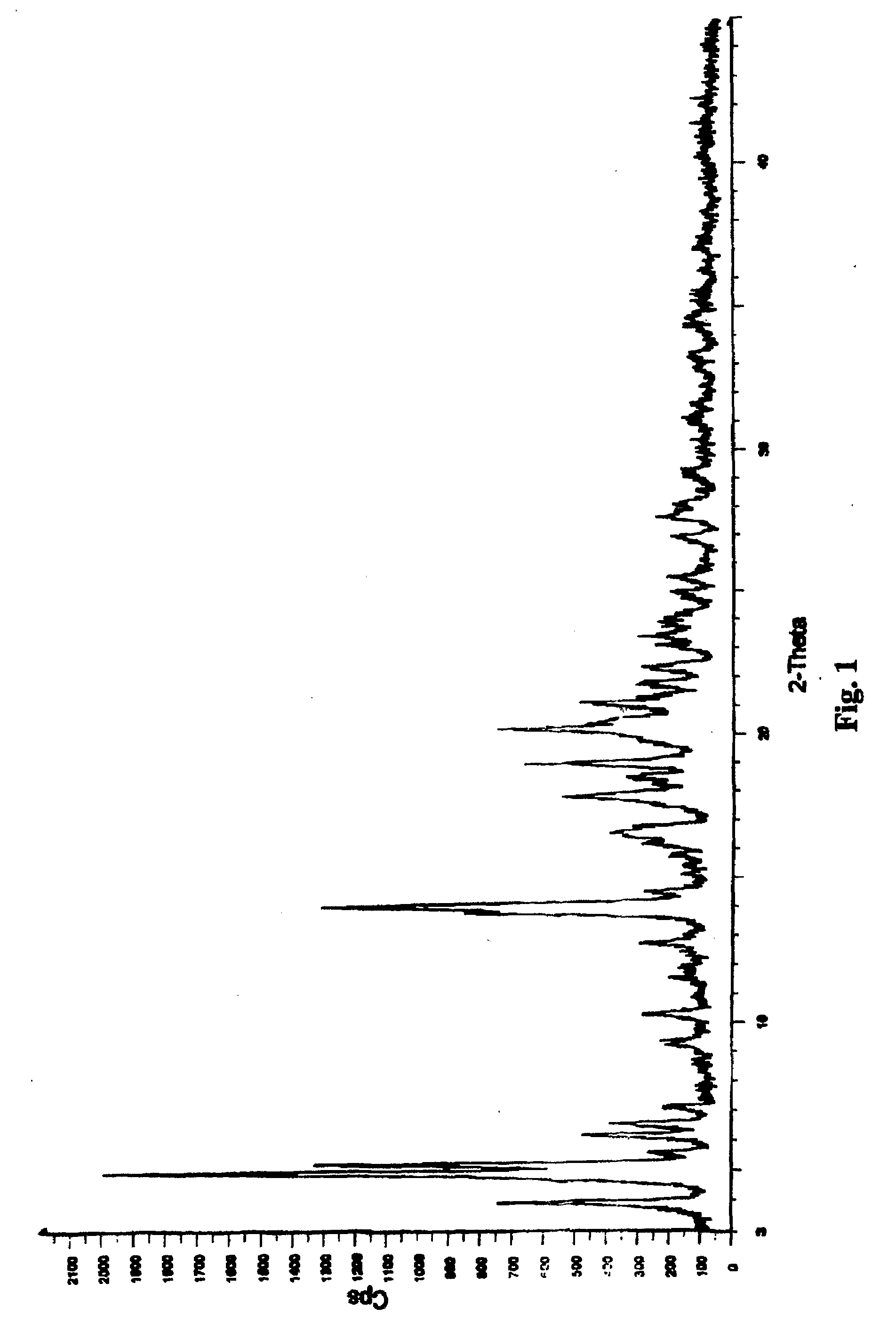
Crystalline form of nateglinide
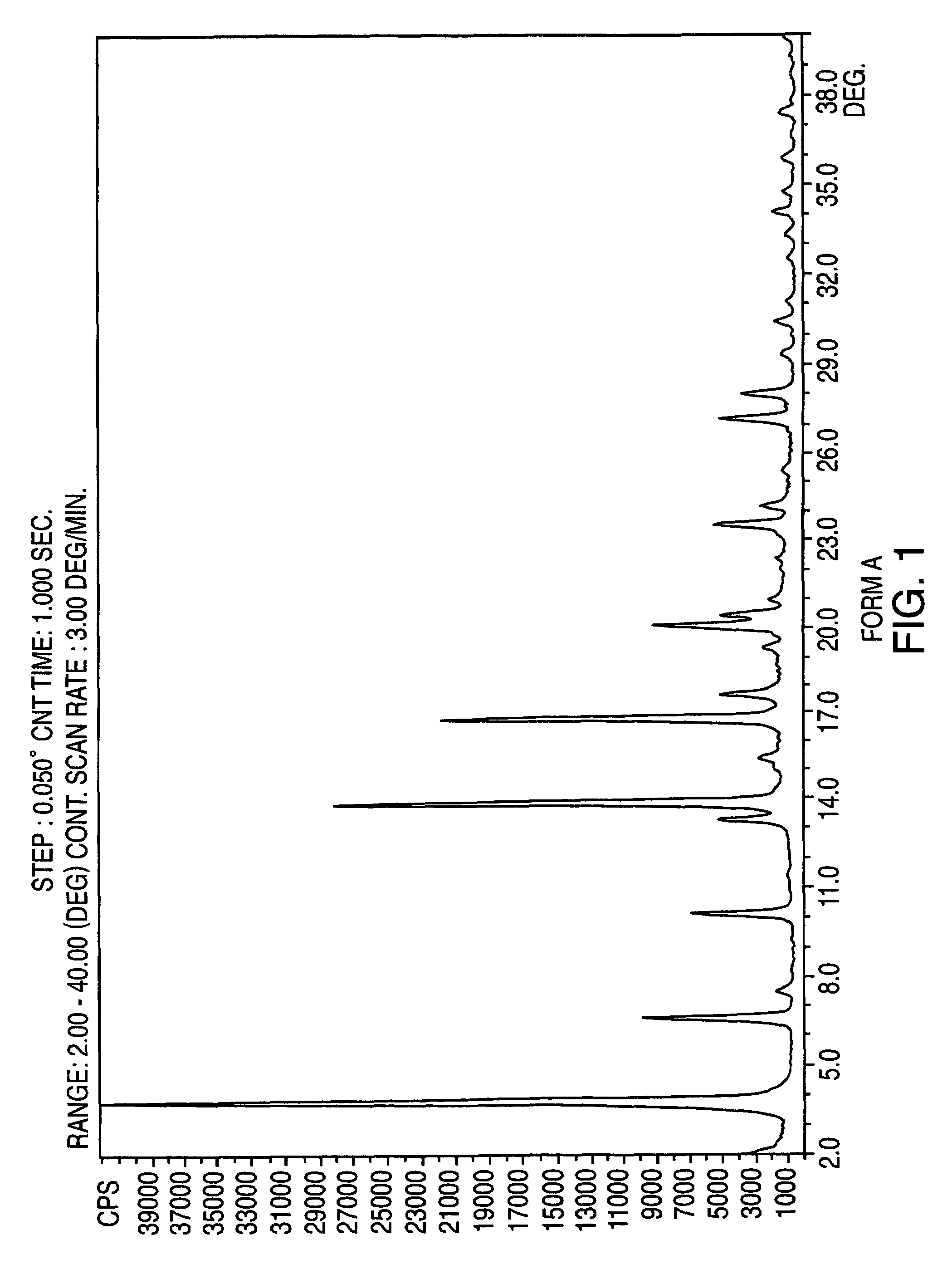
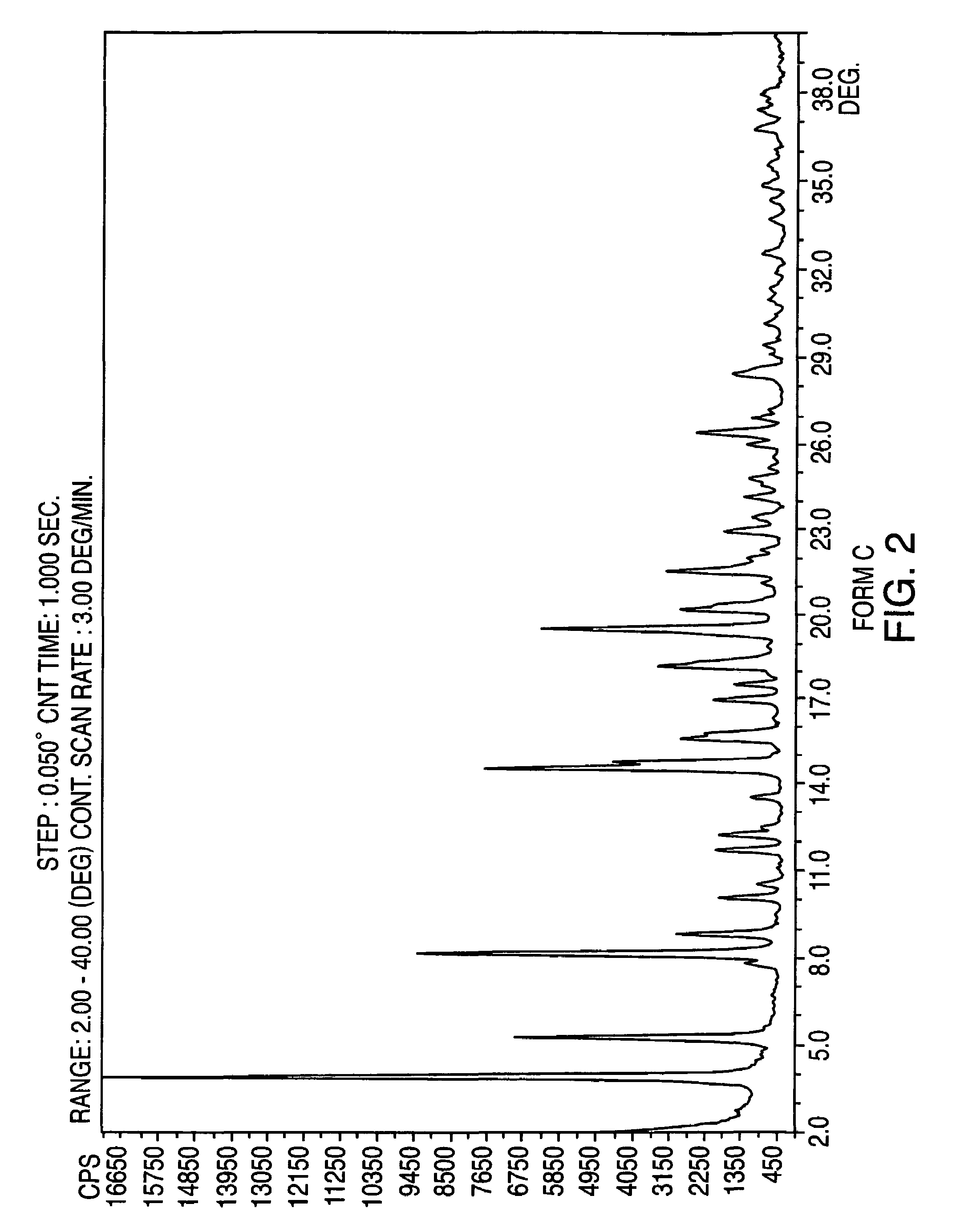
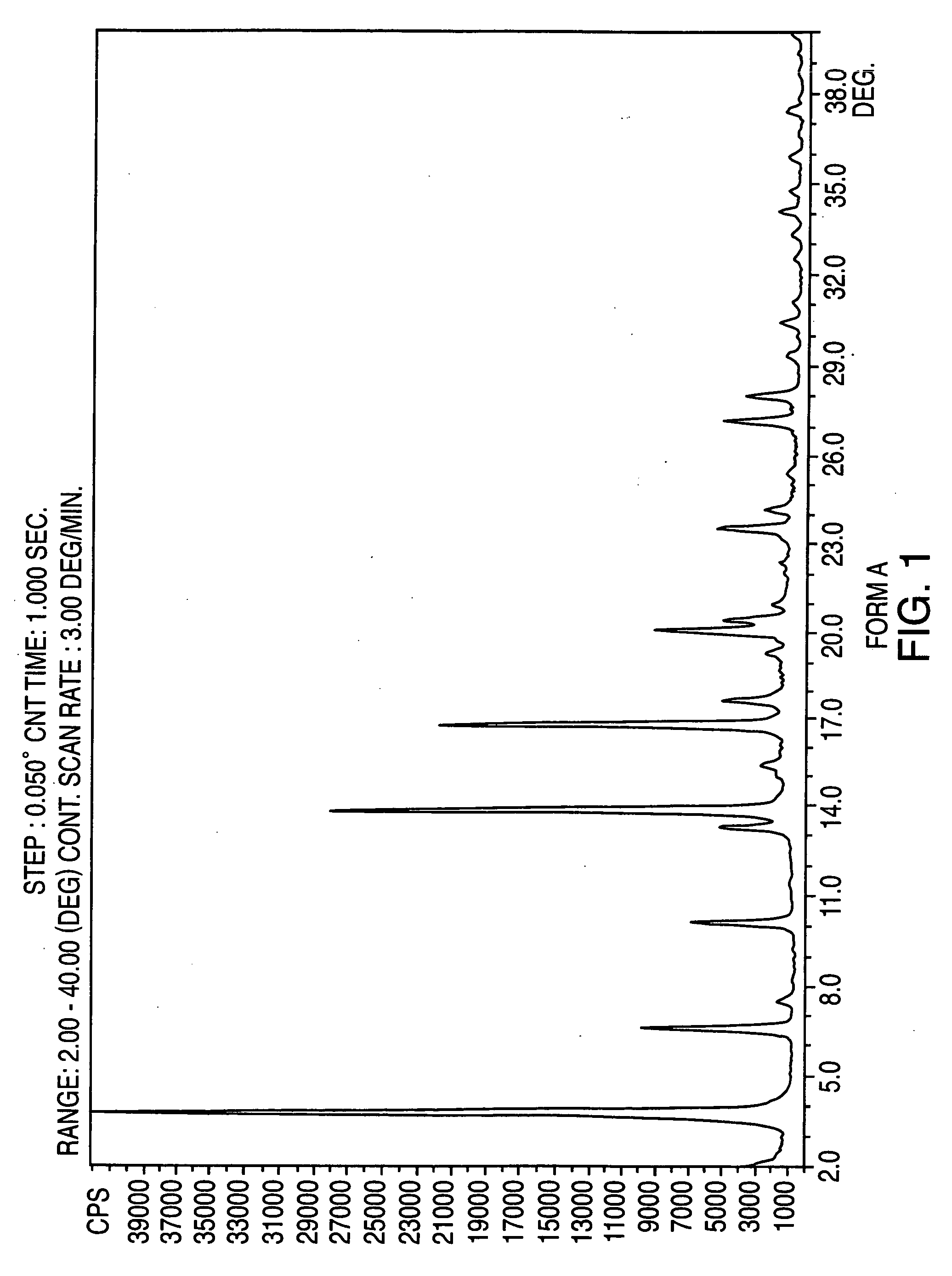
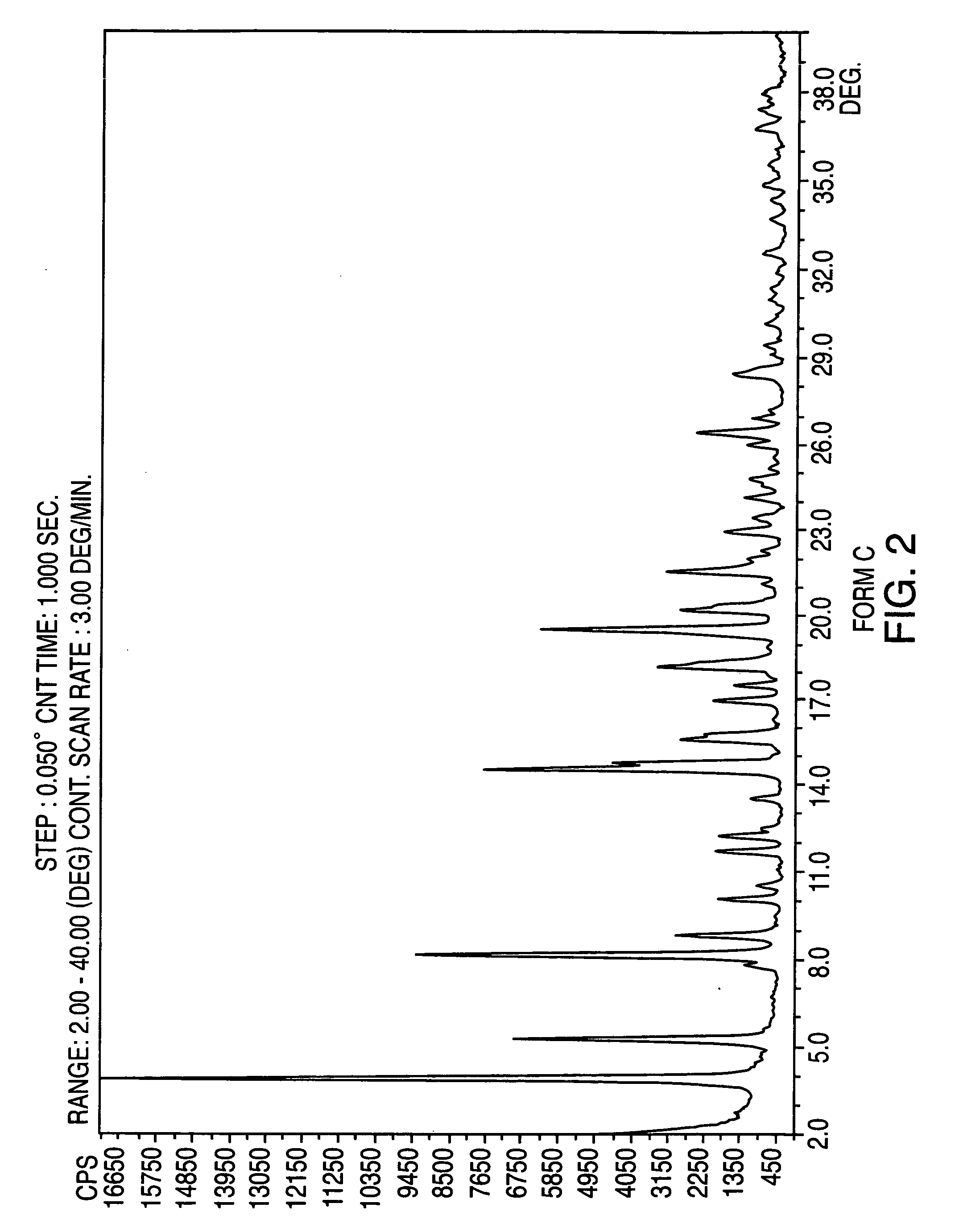
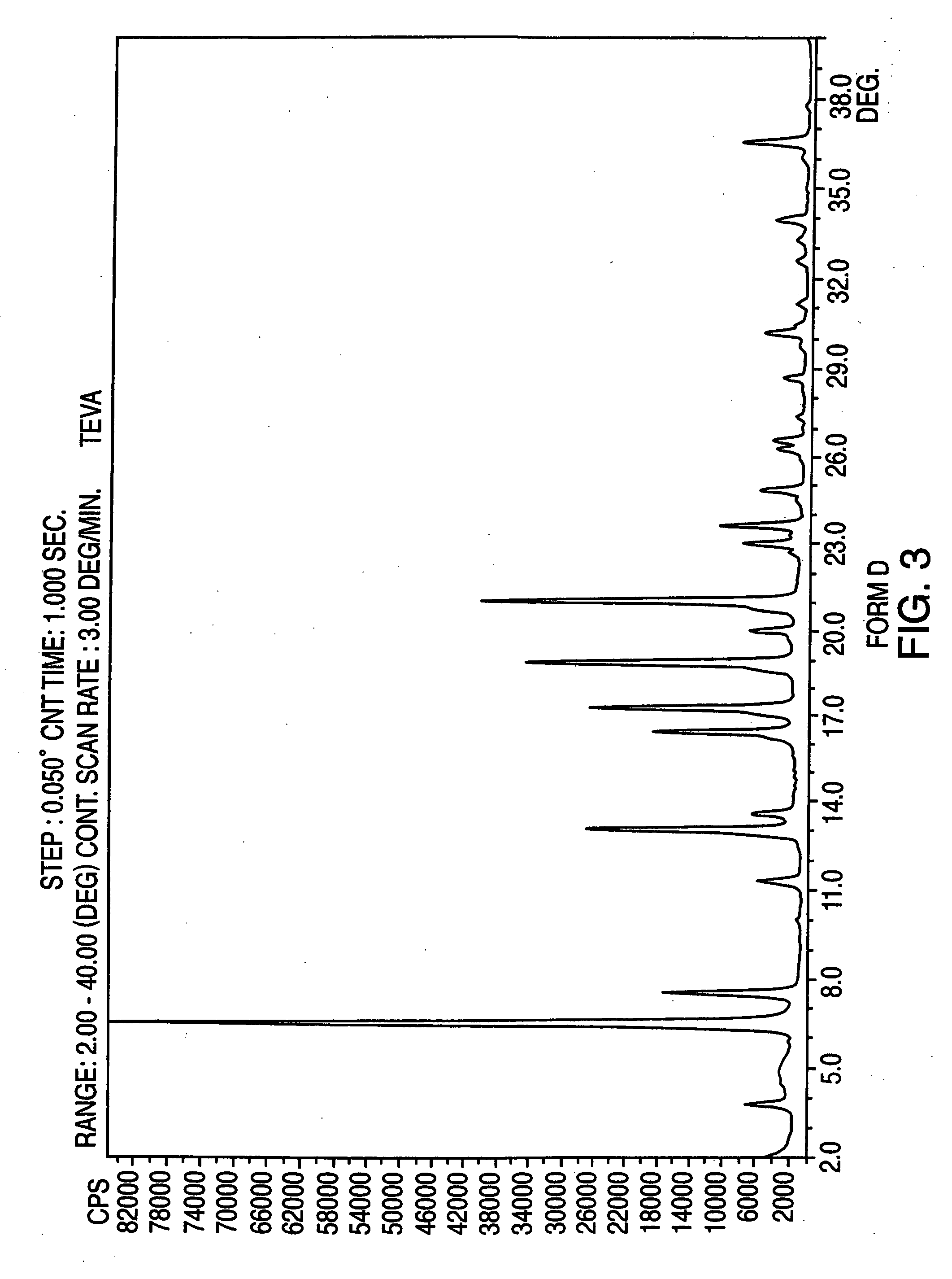
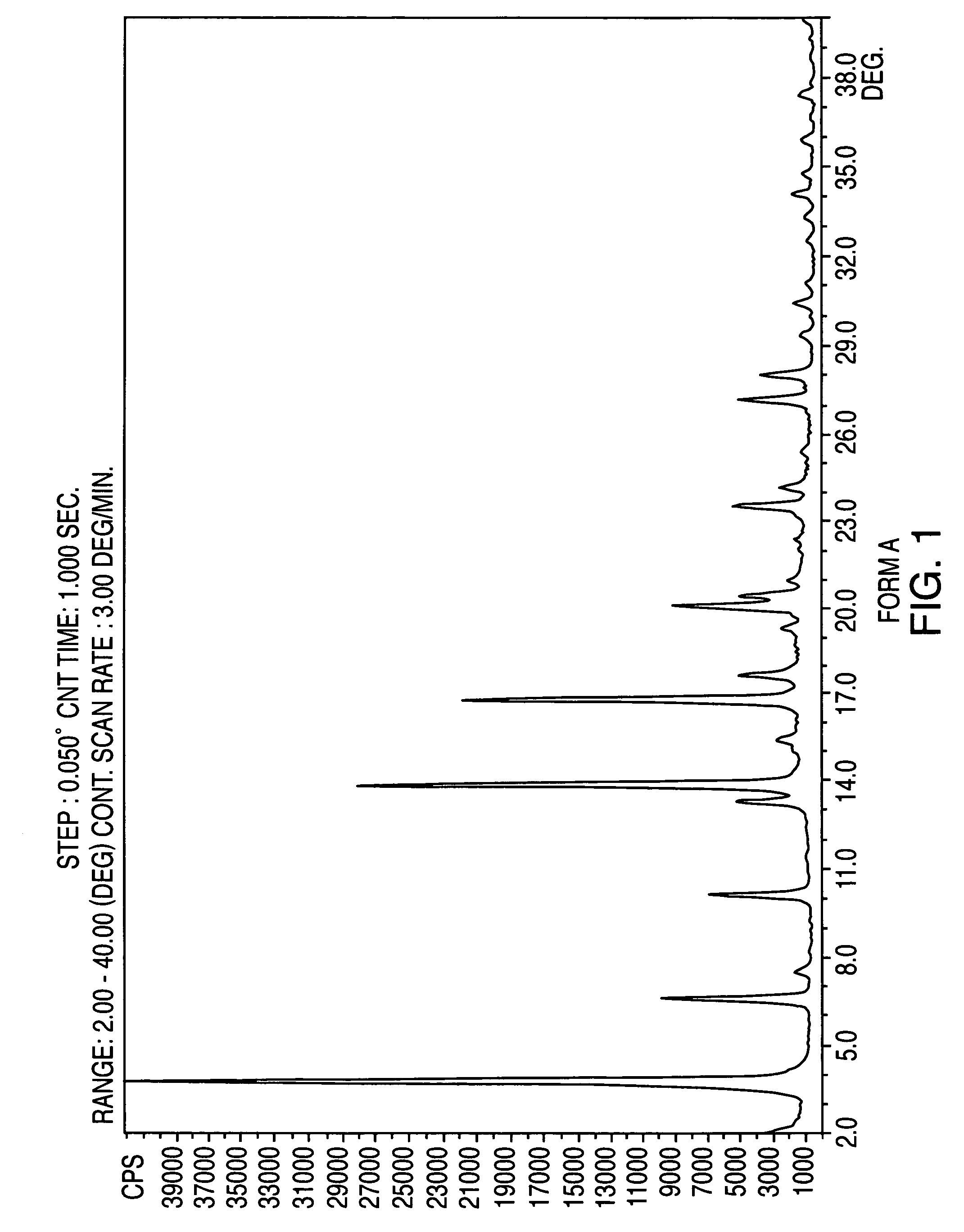
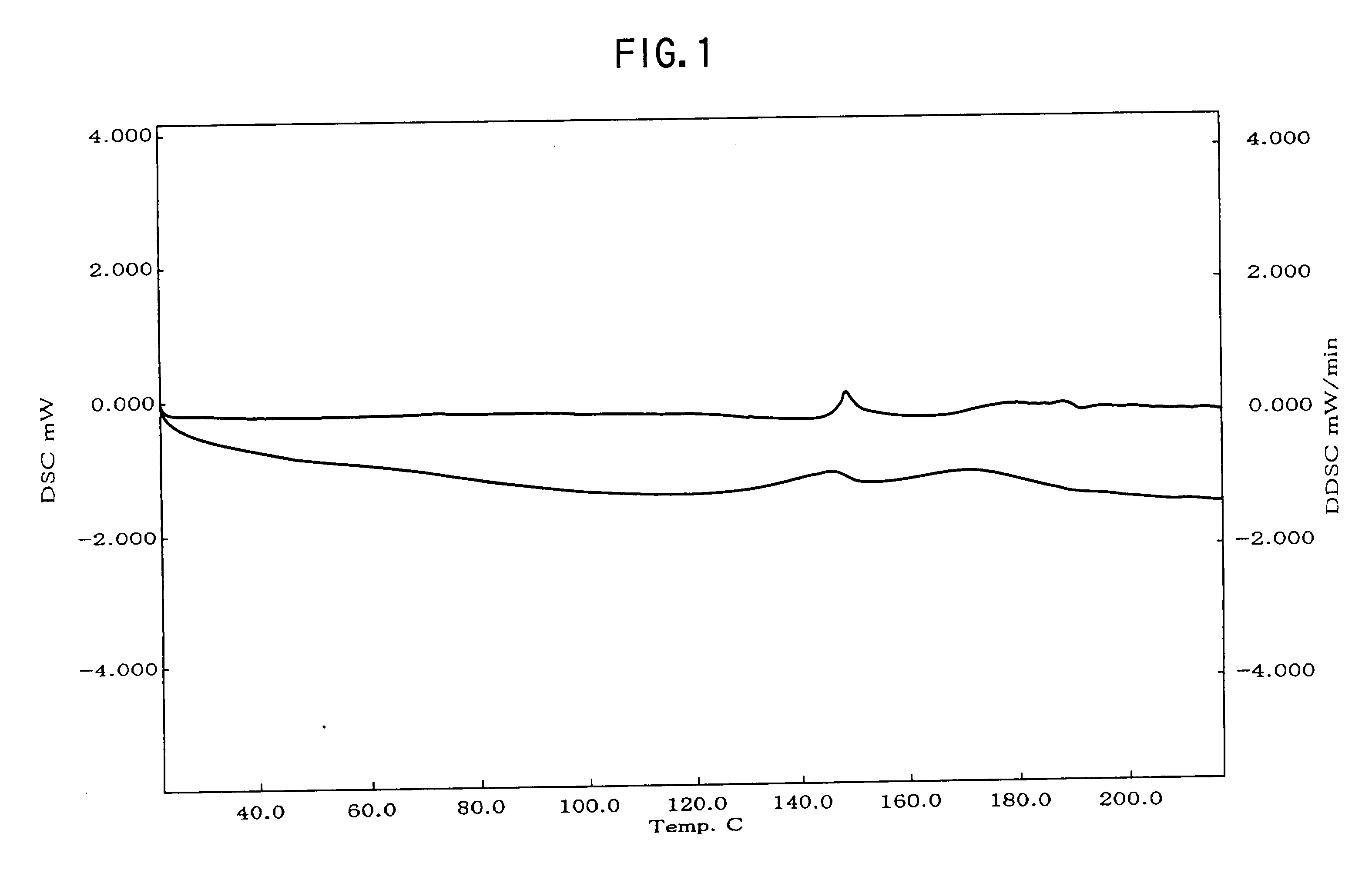
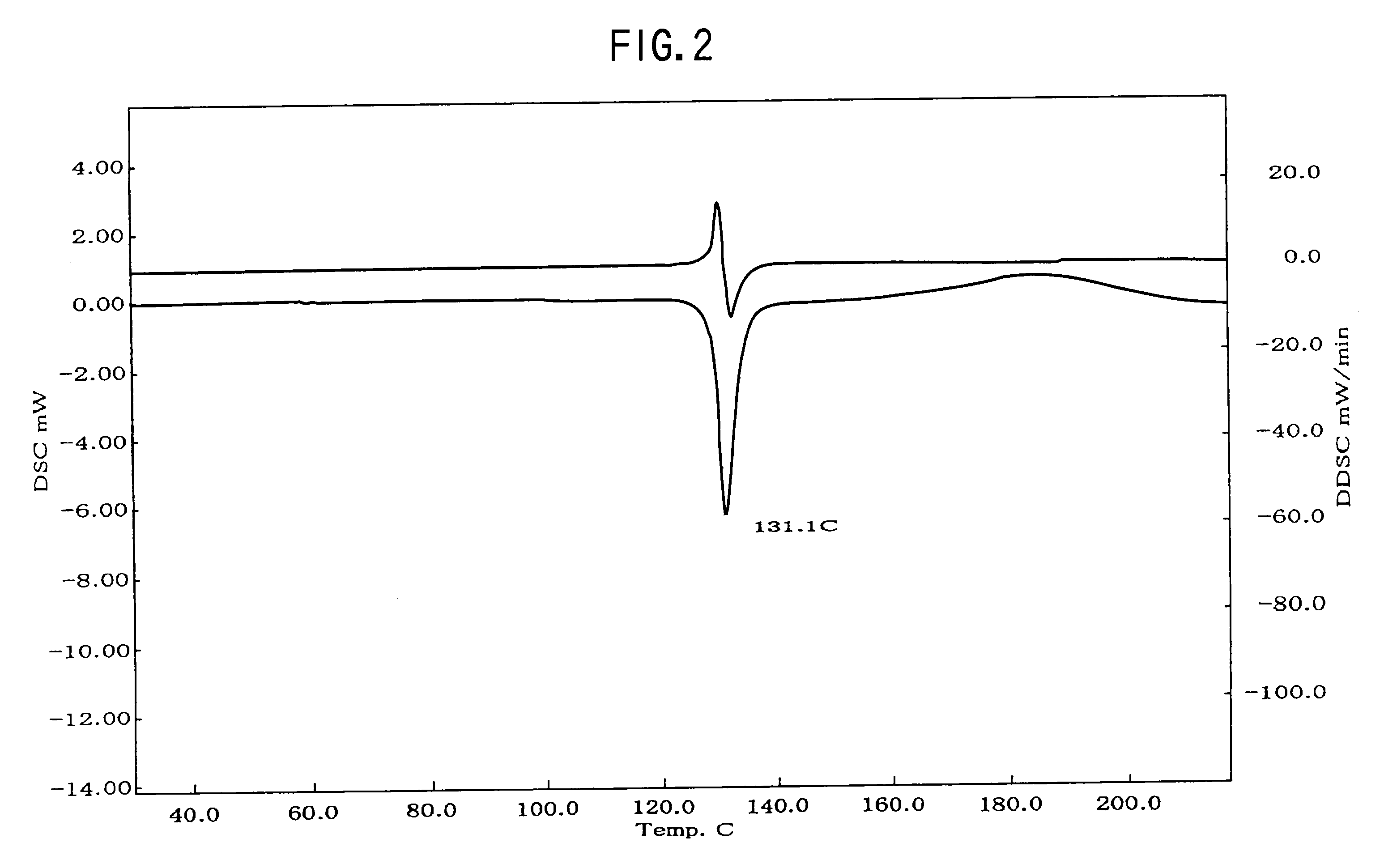
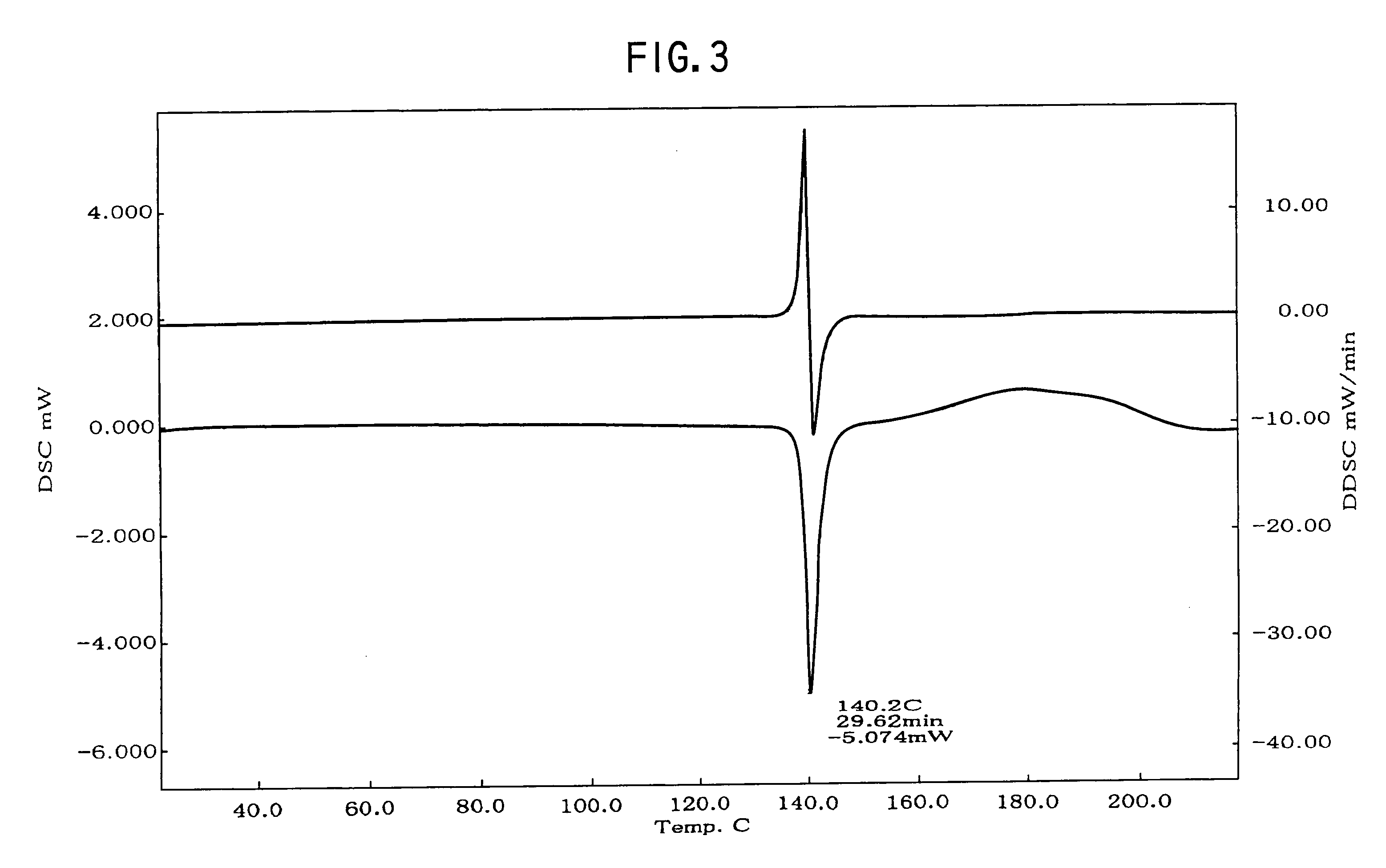
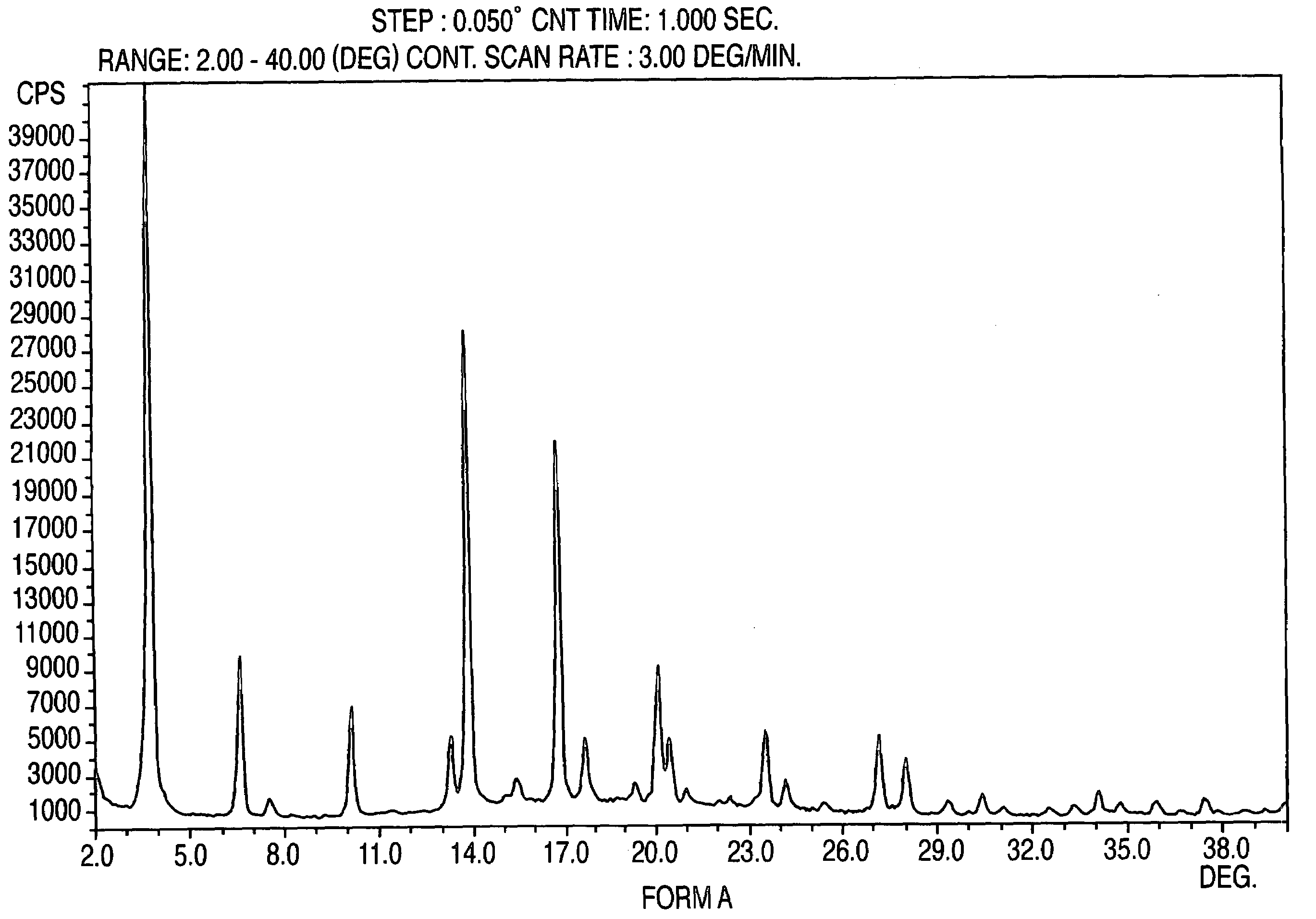
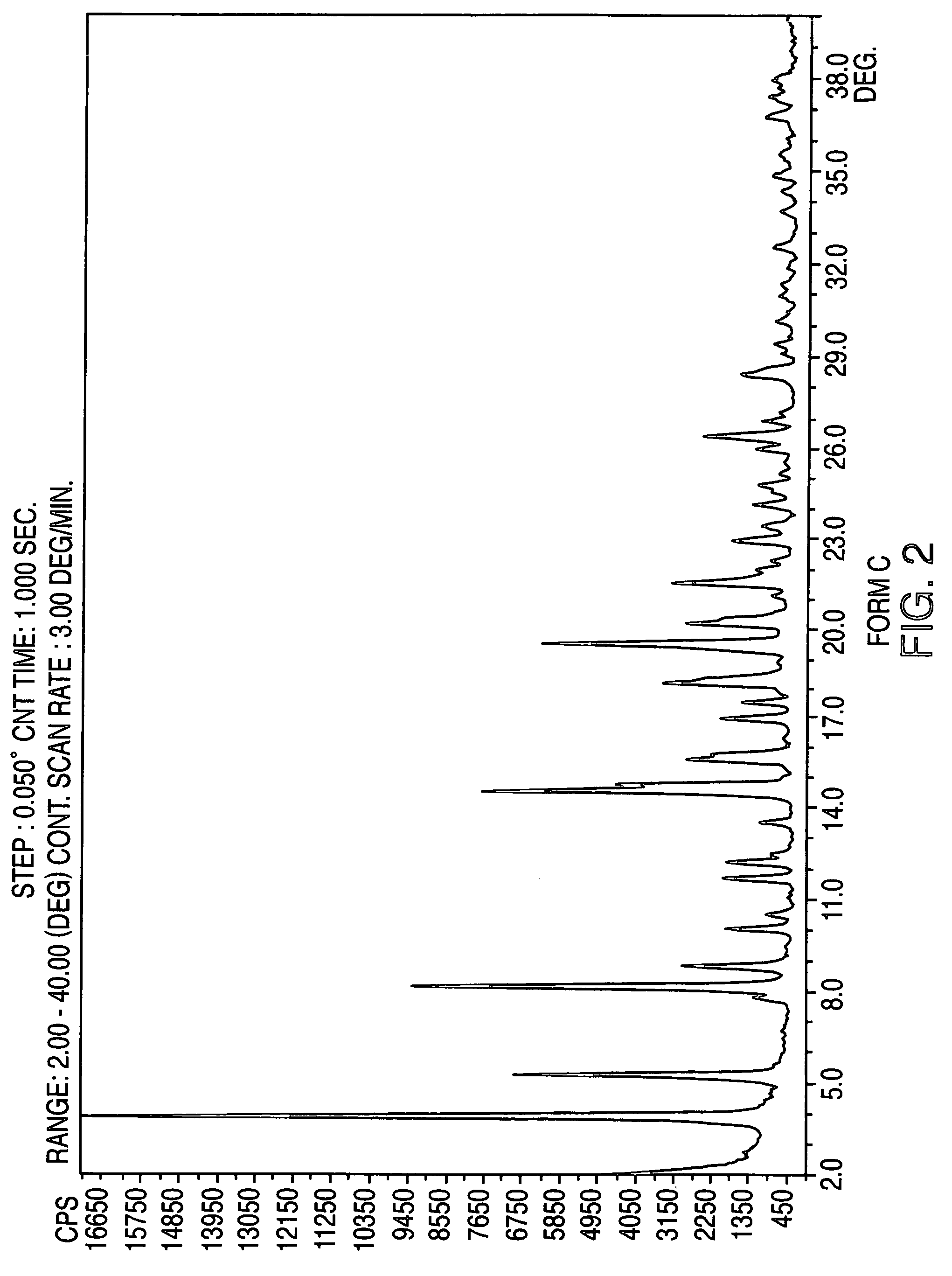
Provided are crystalline forms of nateglinide, labeled Forms A, C, D, F, G, I, J, K, L, M, N, O, P, Q, T, U, V, Y, α, β, γ, δ, ε, σ, θ and Ω, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:TEVA PHARM USA INC
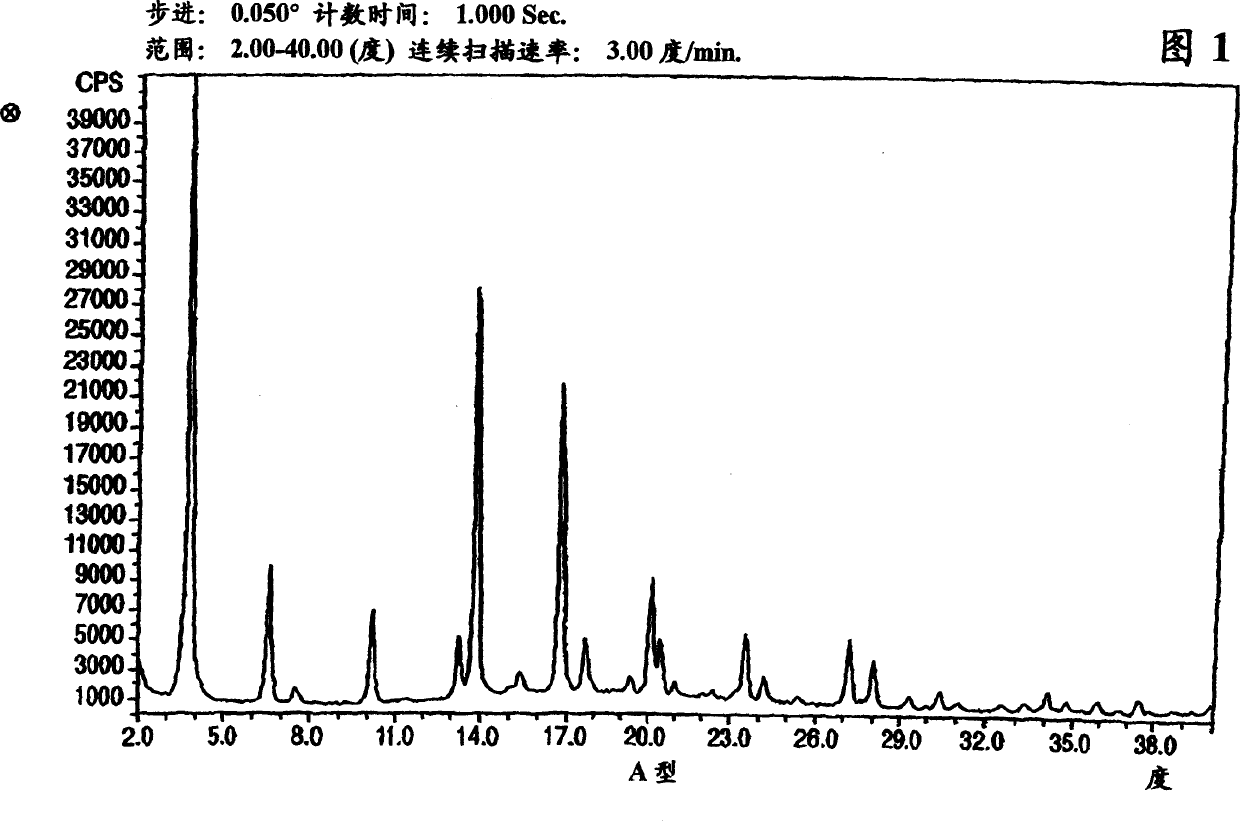
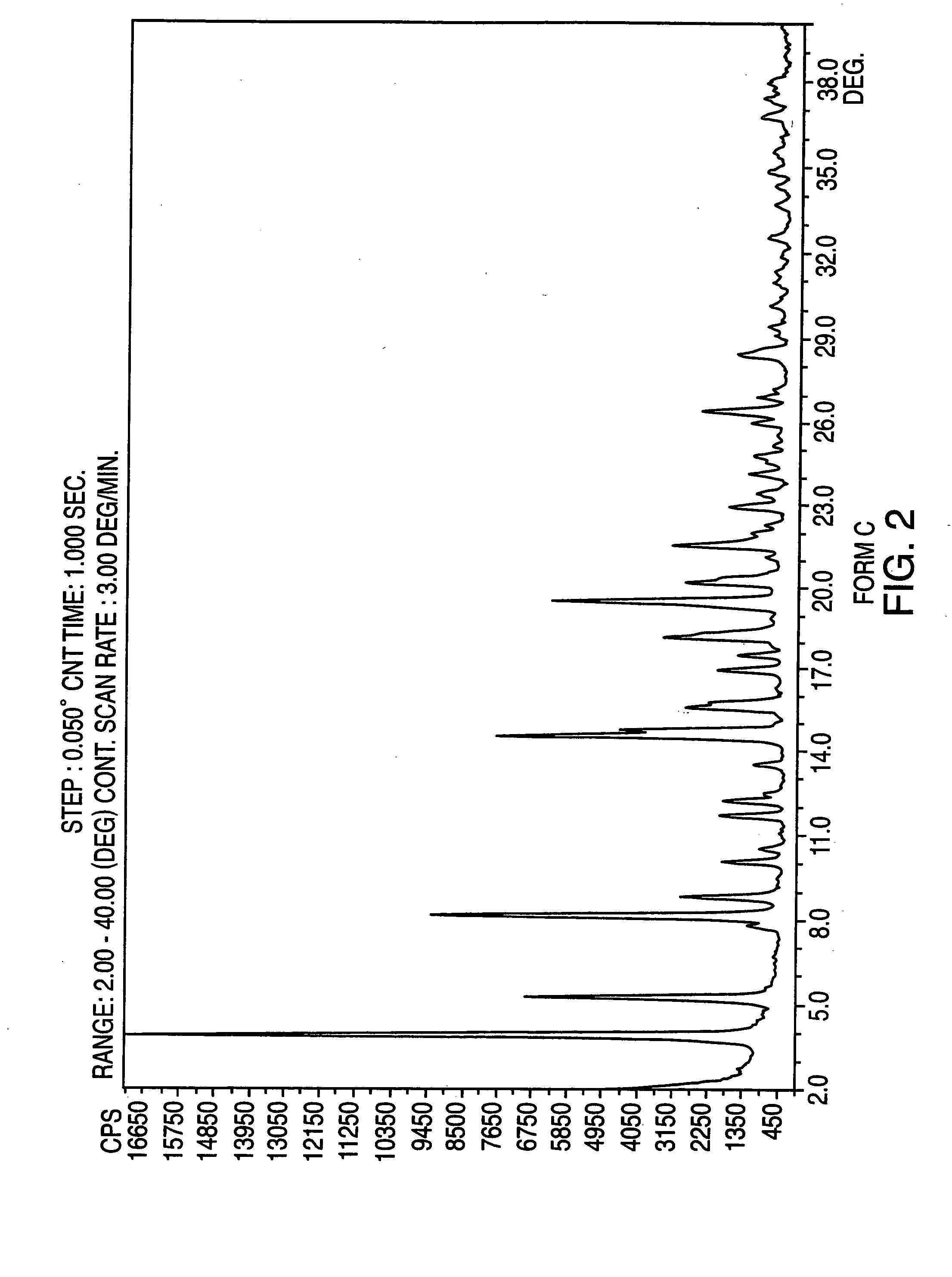
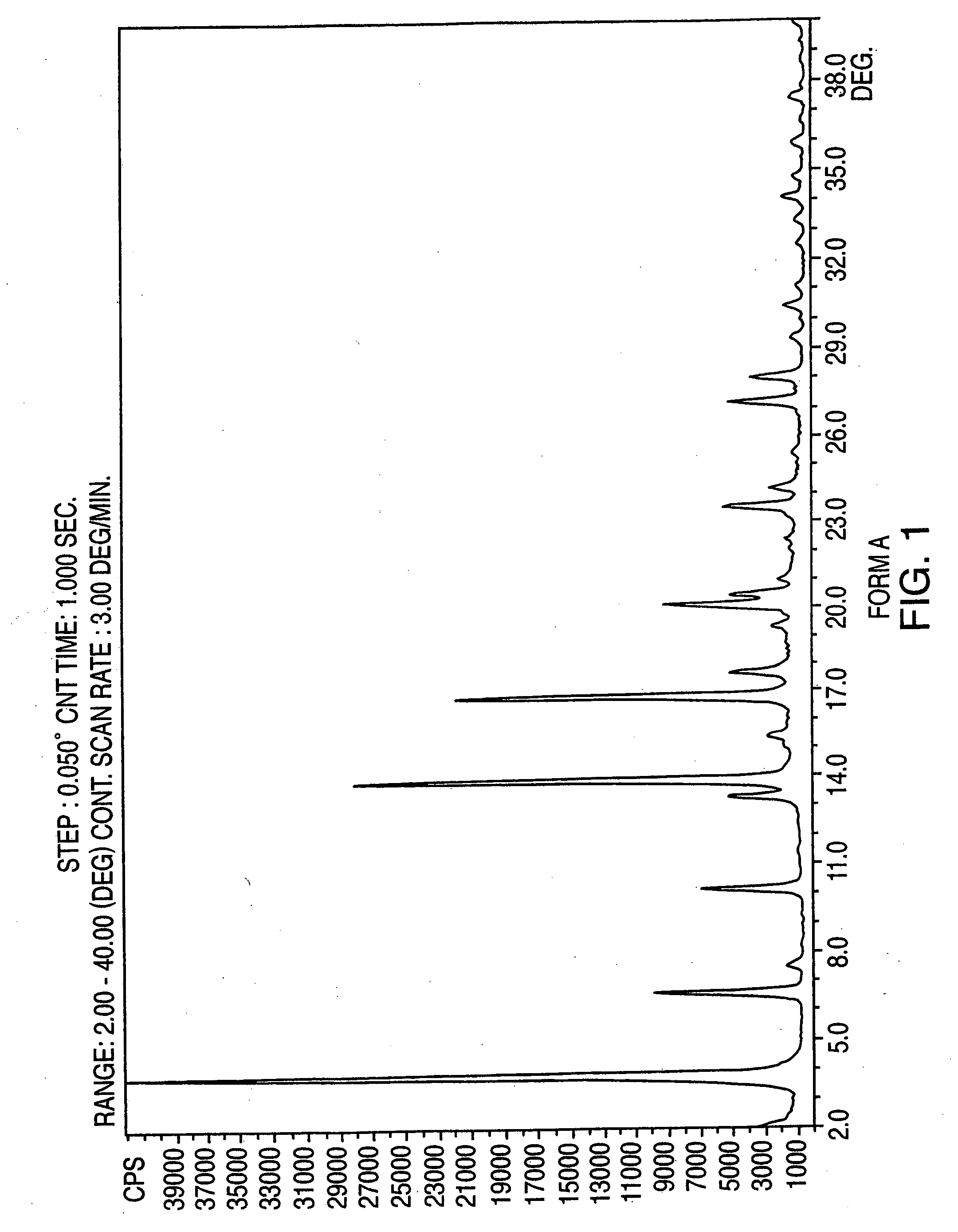
Crystalline form of nateglinide
InactiveUS20050014836A1BiocidePeptide/protein ingredientsPharmaceutical formulationMedicinal chemistry
Provided are crystalline forms of nateglinide, labeled Forms A, C, D, F, G, I, J, K, L, M, N, O, P, Q, T, U, V, Y, α, β, γ, δ, ε, σ, θ and Ω, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:TEVA PHARM USA INC
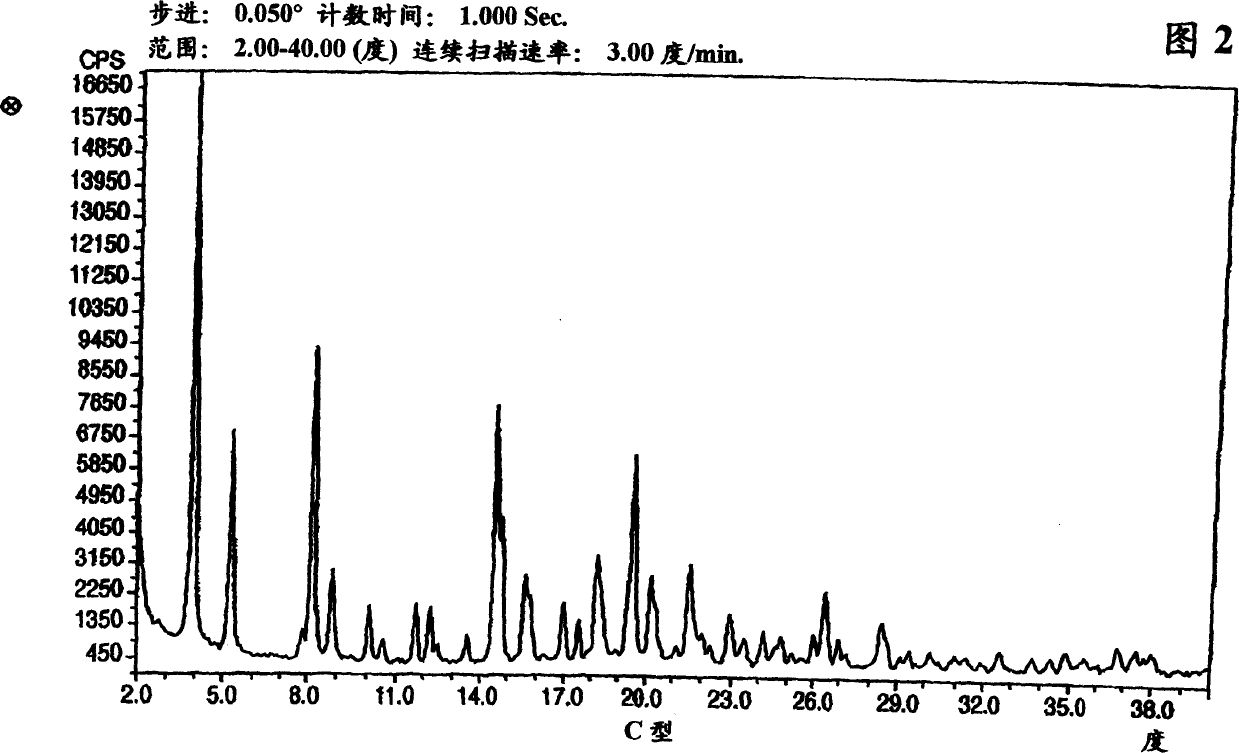
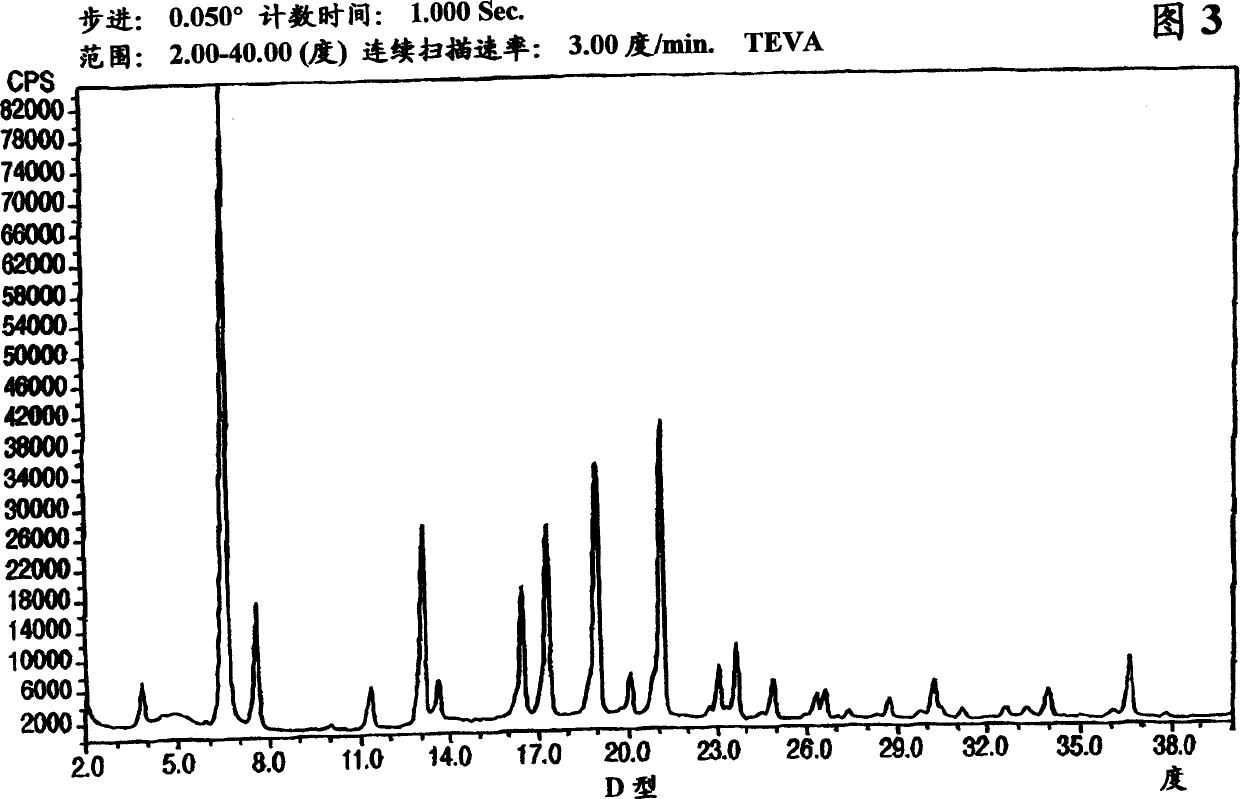
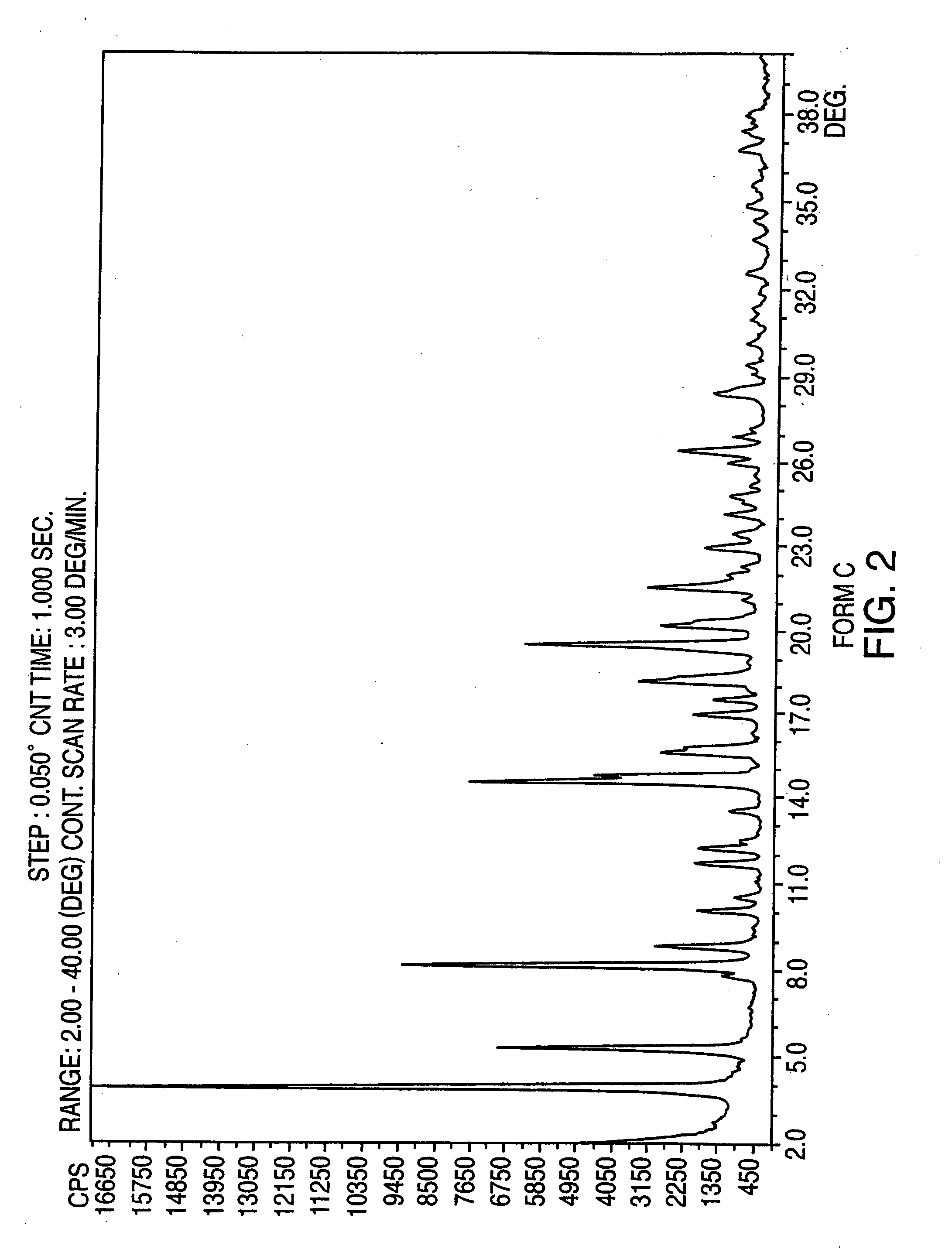
Polymorphic forms of nateglinide
InactiveCN1723190AMetabolism disorderCarboxylic acid amide separation/purificationGreek letter epsilonPharmaceutical formulation
Provided are crystalline forms of nateglinide, labeled forms A, C, D, F, G, I, J, K, L, M, N, O, P, Q, T, U, V, Y, alpha, beta, gamma, delta, epsilon, sigma, theta and omega, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:TEVA PHARMA IND LTD
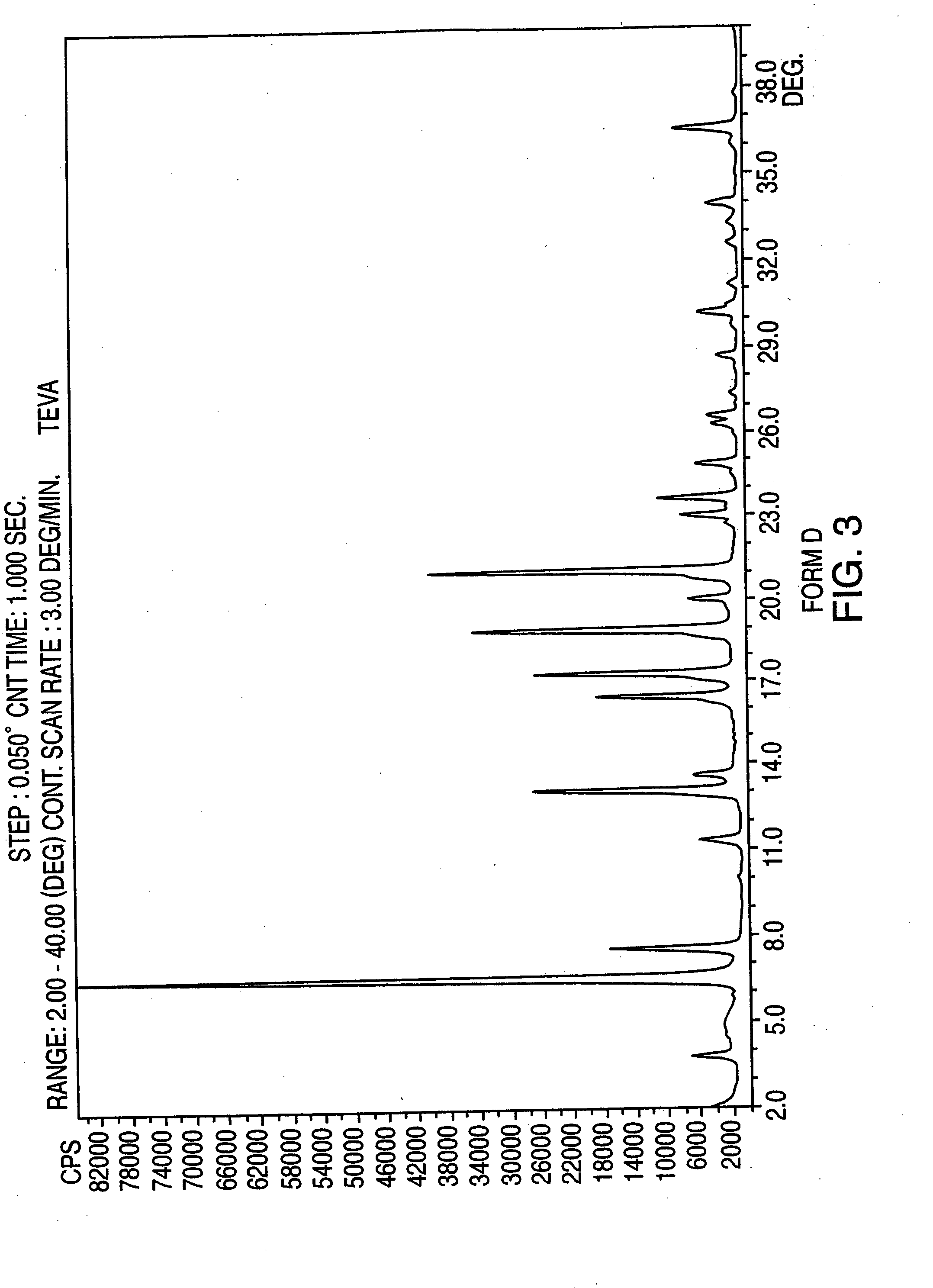
Polymorphic forms of nateglinide
InactiveUS20050090552A1BiocidePeptide/protein ingredientsPharmaceutical formulationMedicinal chemistry
Provides are crystalline forms of nateglinide, labeled Forms A, C, D, F, G, I, J, K, L, M, N, 0, P, Q, T, U, V, Y, α, β, γ, δ, ε, σ, θ and Ω, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:TEVA PHARMA IND LTD +1
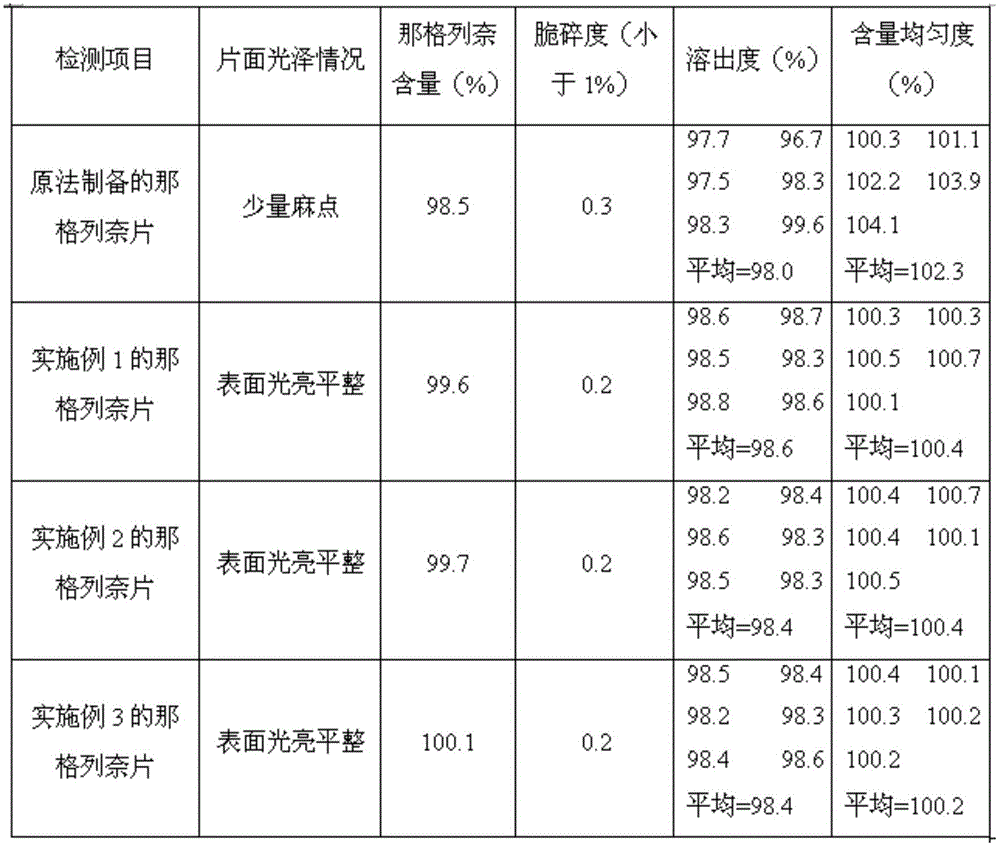
Nateglinide tablet and preparation method thereof
InactiveCN101612133ARapid disintegration of the drugQuality improvementOrganic active ingredientsMetabolism disorderCarboxymethyl starchAdditive ingredient
Owner:JIANGSU WANBANG BIOPHARMLS +1
Methods for producing nateglinide crystals
InactiveUS7208622B2Organic compound preparationCarboxylic acid amide separation/purificationD-PhenylalanineKetone solvents
There is provided methods for producing nateglinide crystals, which comprises the steps of adding an acid(s) to a reaction mixture containing nateglinide to make it acidic, the reaction mixture being obtained by reacting trans-4-isopropylcyclohexylcarbonyl chloride with D-phenylalanine in a mixed solvent of ketone solvent and water in the presence of an alkali; and then adjusting the temperature of the mixture to 58° C. to 72° C. and the concentration of ketone solvent to more than 8 wt % and less than 22 wt % to conduct precipitation of nateglinide crystals. This producing method is the industrially beneficial methods for crystallization of nateglinide.
Owner:AJINOMOTO CO INC
Antidiabetic preparation for oral administration
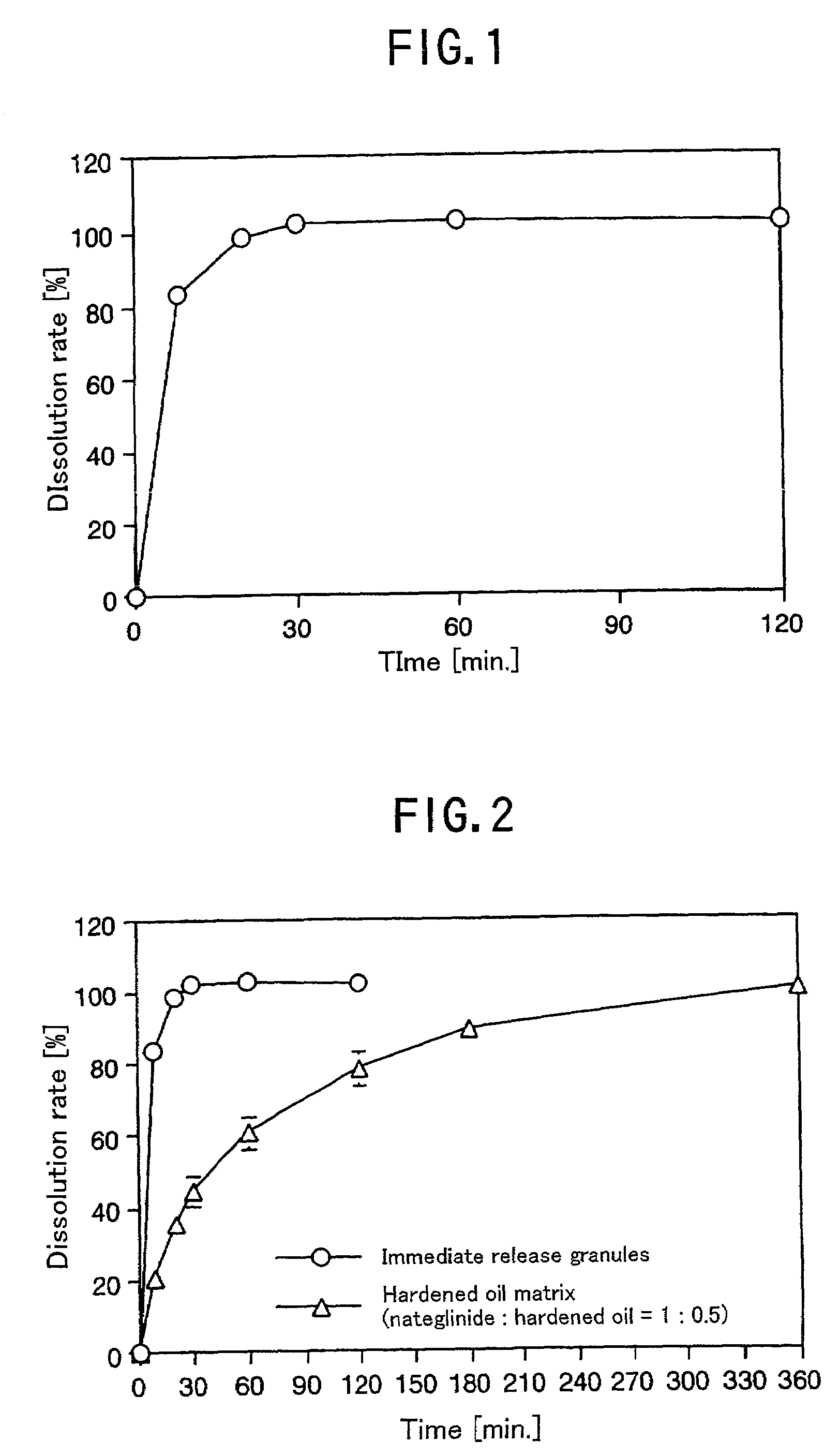
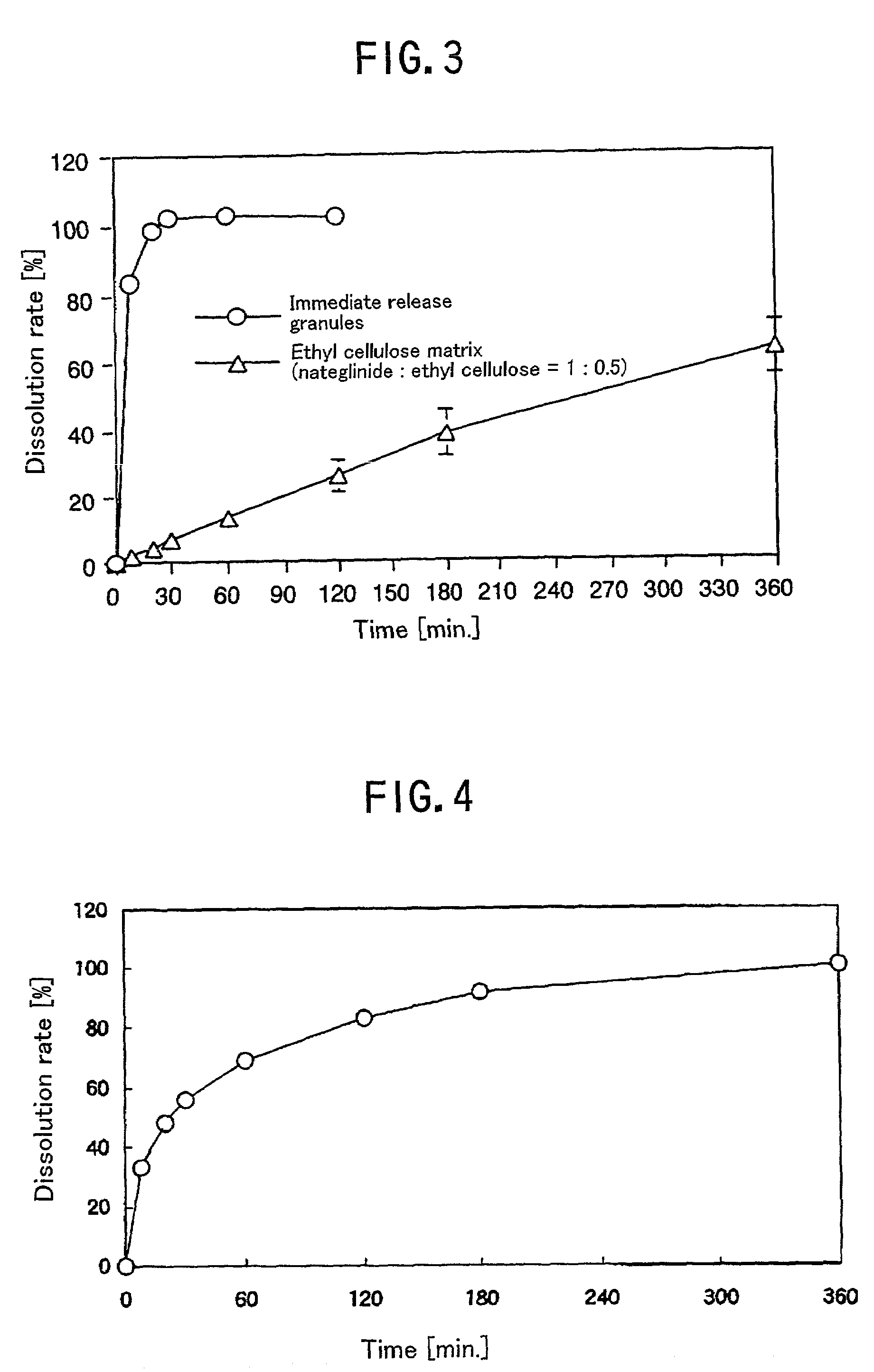
There is provided a single preparation which directly decreases both of the post prandial blood glucose level and the fasting blood glucose level close to normal levels, by release-sustaining a drug capable of decreasing the post prandial blood glucose level of diabetic patients close to the normal level, or mixing a controlled release drug capable of decreasing the post prandial blood glucose level close to the normal level with an immediate release drug. It is particularly preferable that the drug capable of decreasing the post prandial blood glucose level close to the normal level is nateglinide.
Owner:AJINOMOTO CO INC
Nateglinide-containing preparation
The present invention discloses, as a immediate-release preparation useful as an antidiabetic, a nateglinide-containing preparation comprising nateglinide as an active ingredient wherein the nateglinide is amorphous.
Owner:AJINOMOTO CO INC
Polymorphic forms of nateglinide
InactiveUS20060004102A1Lower blood sugar levelsBiocidePeptide/protein ingredientsMedicinal chemistryCrystallization
Owner:TEVA PHARM USA INC
Polymorphic forms of nateglinide
InactiveUS20050014949A1Organic active ingredientsOrganic compound preparationPharmaceutical formulationMedicinal chemistry
Provides are crystalline forms of nateglinide, labeled Forms A, C, D, F, G, I, J, K, L, M, N, O, P, Q, T, U, V, Y, α, β, γ, δ, ε, σ, θ and Ω, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:TEVA PHARM USA INC
Polymorphic forms of nateglinide
InactiveUS20080319075A1BiocidePeptide/protein ingredientsPharmaceutical formulationMedicinal chemistry
Provides are crystalline forms of nateglinide, labeled Forms A, C, D, F, G, I, J, K, L, M, N, O, P, Q, T, U, V, Y, α, β, γ, δ, ε, σ, θ and Ω, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:YAHALOMI RONIT +5
Polymorphic forms of nateglinide
InactiveUS20050075400A1Organic active ingredientsBiocidePharmaceutical formulationMedicinal chemistry
Provides are crystalline forms of nateglinide, labeled Forms A, C, D, F, G, I, J, K, L, M, N, O, P, Q, T, U, V, Y, α, β, γ, δ, ε, σ, θ and Ω, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:TEVA PHARMA IND LTD +1
Synthesis of pharmaceutical
InactiveCN101190887ALow costOrganic compound preparationCarboxylic acid amides preparationD-PhenylalanineSynthesis methods
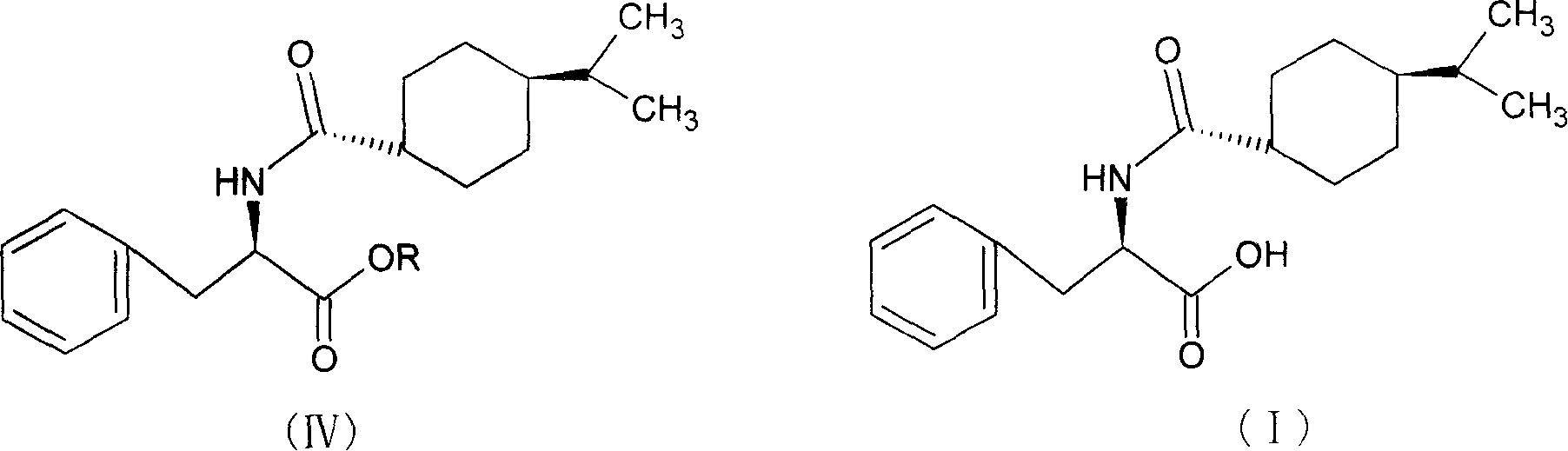
The invention relates to a synthesis method of a nateglinide, in particular to the synthesis of the nateglinide by a one-pot method. Original material D-phenylalanine is used as raw material in the method, D-phenylalanine esterified substance (II) is obtained through esterification, a compound (IV) is obtained through acylation of isopropylcyclohexanecarboxylic acid (III), and then the compound (IV) is ester hydrolyzed so as to obtain the nateglinide. The technique of the method has simple technique process and easy and convenient post-treatment; meanwhile, the method has the advantages of strong operability, being easy to be industrialized and cost saving.
Owner:TIANJIN PHARMA GROUP CORP
Polymorphic forms of nateglinide
InactiveUS7420084B2Organic compound preparationOrganic chemistry methodsPharmaceutical formulationMedicinal chemistry
Provides are crystalline forms of nateglinide, labeled Forms A, C, D, F, G, I, J, K, L, M, N, 0, P, Q, T, U, V, Y, α, β, γ, δ, ε, σ, θ and Ω, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:TEVA PHARM USA INC
Solid compound of dapagliflozin as well as preparation method and application of solid compound
The invention discloses a dapagliflozin solid-form compound as well as a preparation method and application thereof. The compound is in a solid form formed by dapagliflozin, nateglinide, candesartan cilexetil, anagliptin or enalapril. The invention also comprises a preparation method and application of the dapagliflozin solid compound. According to the dapagliflozin solid compound, the two components are combined in a hydrogen bond or other non-covalent bond mode on the basis that original molecular covalent bonds are not damaged, a new eutectic or amorphous substance is formed, the two active components exist in a specific stoichiometric ratio, no organic solvent is contained, and the preparation method is simple. And good biocompatibility is achieved. Different from a mixture obtained by simply and physically mixing the two active ingredients, the compound provided by the invention can effectively improve various physicochemical properties of the medicine, improve the stability, solubility and bioavailability of the medicine and effectively improve the hygroscopicity of the medicine.
Owner:HINYE PHARM CO LTD
Polymorphic forms of nateglinide
InactiveUS20070004804A1Organic active ingredientsBiocidePharmaceutical formulationMedicinal chemistry
Provides are crystalline forms of nateglinide, labeled Forms A, C, D, F, G, I, J, K, L, M, N, 0, P, Q, T, U, V, Y, α, β, γ, δ, ε, δ, σ, 2 and Ω, processes for their preparation and processes for preparation of other crystalline forms of nateglinide. Also provided are their pharmaceutical formulations and methods of administration.
Owner:TEVA PHARM USA INC
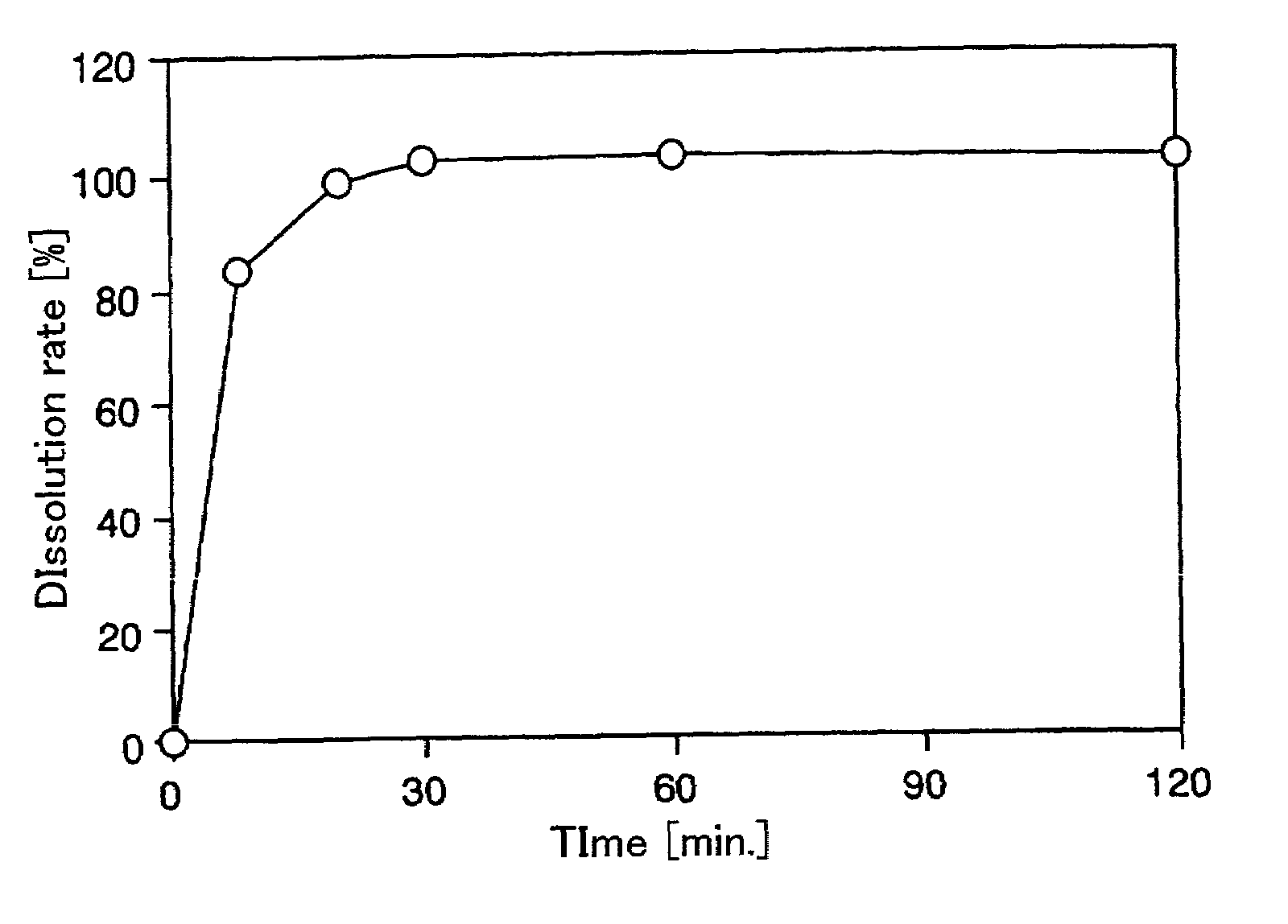
Nateglinide-containing preparation
InactiveUS20090203791A1High dissolution rateBiocideOrganic active ingredientsDiabetes mellitusImmediate release
The present invention discloses, as a immediate-release preparation useful as an antidiabetic, a nateglinide-containing preparation comprising nateglinide as an active ingredient wherein the nateglinide is amorphous.
Owner:AJINOMOTO CO INC
Polymorphic forms of nateglinide
InactiveUS7358390B2Organic active ingredientsOrganic compound preparationPharmaceutical formulationMedicinal chemistry
Owner:TEVA PHARM USA INC
Green food for treating diabetes and preparation process thereof
The invention discloses a green food for treating diabetes and a preparation process thereof, and belongs to the technical field of foods. The preparation process comprises the following steps: dryingand grinding oat, radix puerariae, Chinese yams, coix seeds, konjak, Chinese wolfberry fruits and other components respectively to obtain oat powder, radix puerariae powder, Chinese yam powder, coixseed powder, konjak powder and Chinese wolfberry fruit powder; carrying out biological extraction on oat beta glucose and a polygonatum sibiricum extract, and drying at low temperature to obtain oat beta glucose particles and polygonatum kingianum extract particles; and mixing oat powder, radix puerariae powder, Chinese yam powder, coix seed powder, konjaku flour, Chinese wolfberry fruit powder, oat beta glucose particles and polygonatum kingianum extract particles to obtain the green food. The total content of the oat powder and the cooked Chinese yams exceeds 50%; it is ensured that the content of metformin and nateglinide satisfies the function of treating and inhibiting diabetes mellitus; the fineness of the oat powder and the cooked Chinese yam is 320-350 meshes; and the cell wall breaking rate of the oat powder and the cooked Chinese yam is greater than 90%, so that respective nutrient substances are effectively amplified, and the absorption rate of people reaches 90% or above.
Owner:苏州隆欣达生物科技有限公司
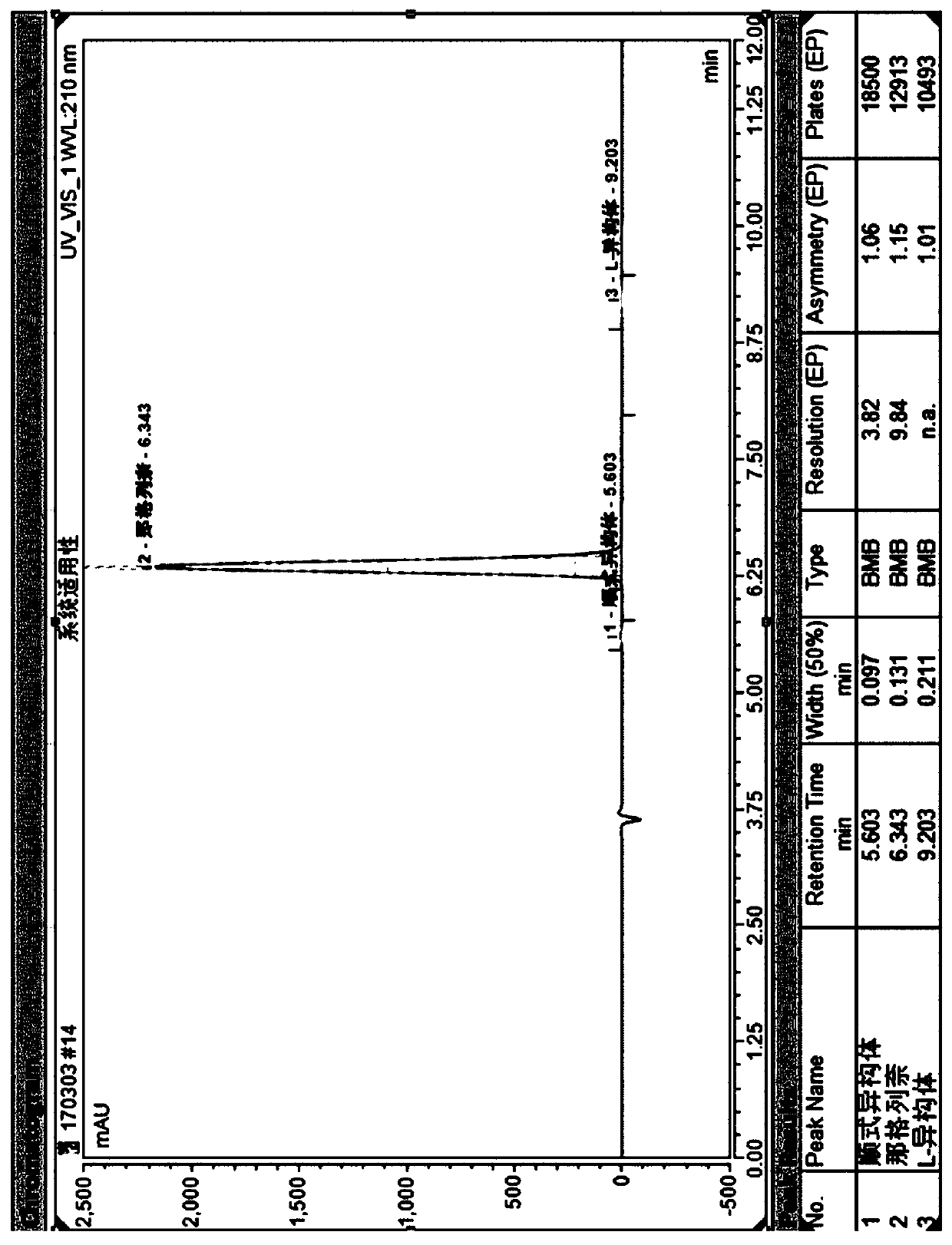
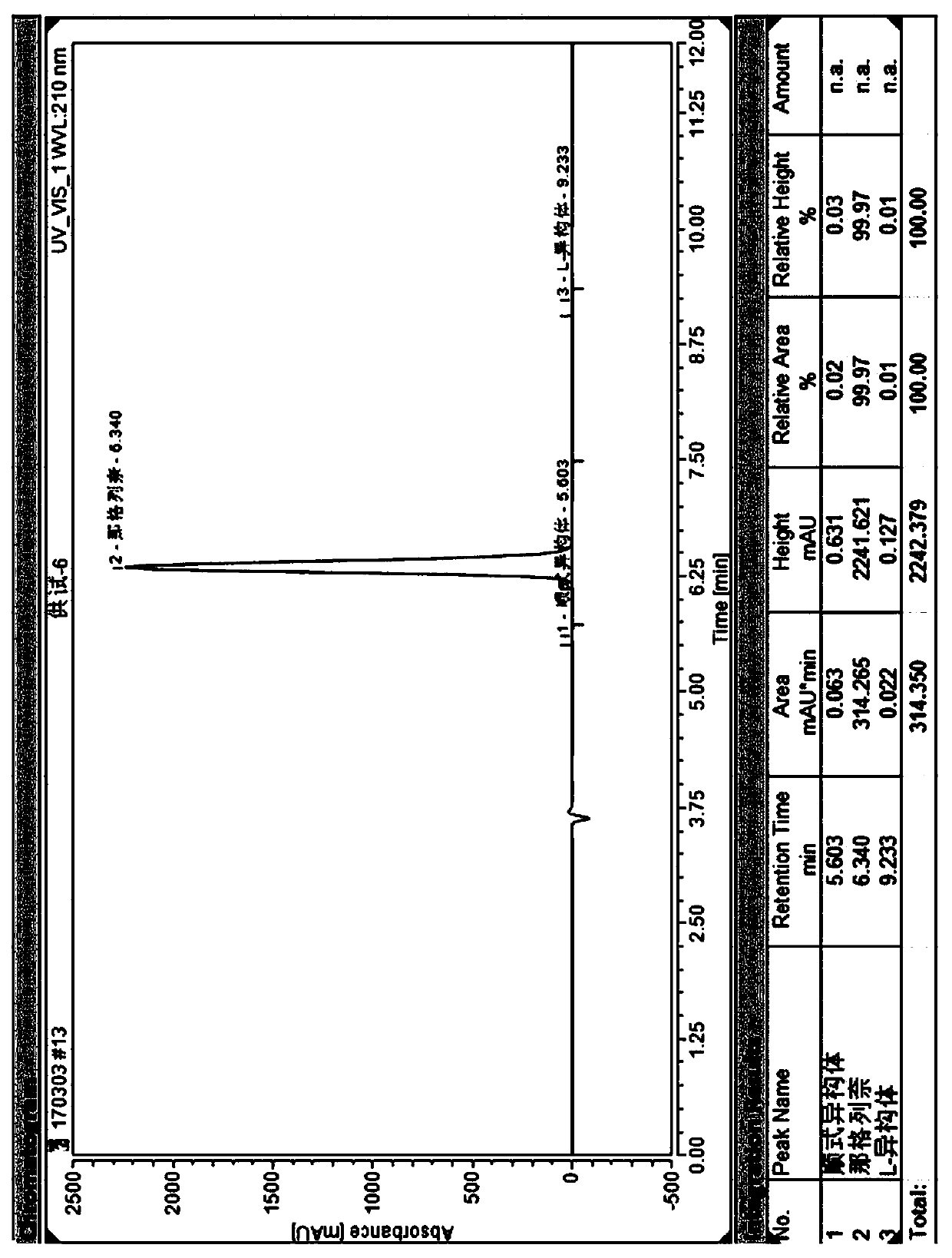
Method for separating nateglinide and its stereoisomers by high performance liquid chromatography
Owner:JIANGSU DEYUAN PHARMA
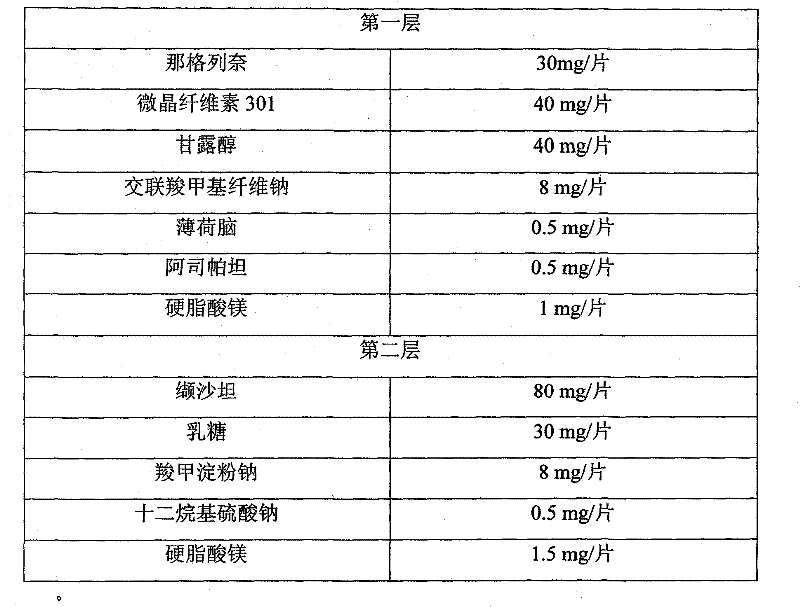
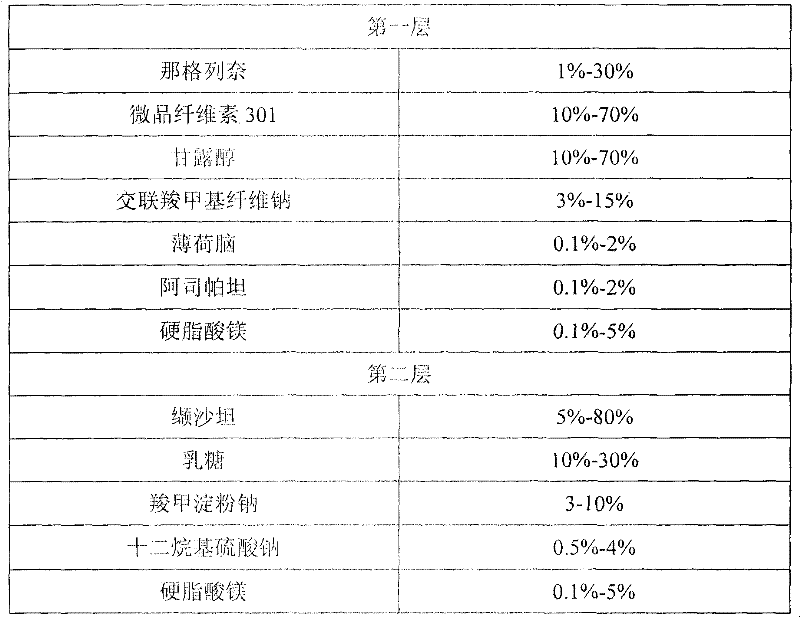
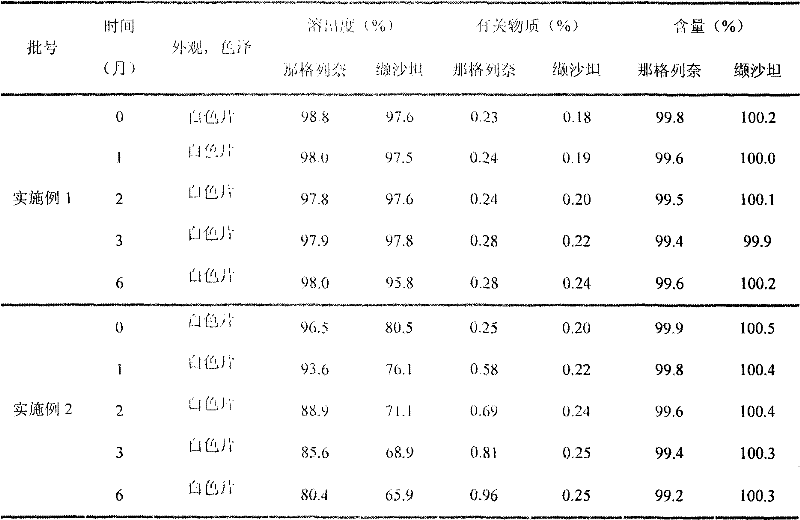
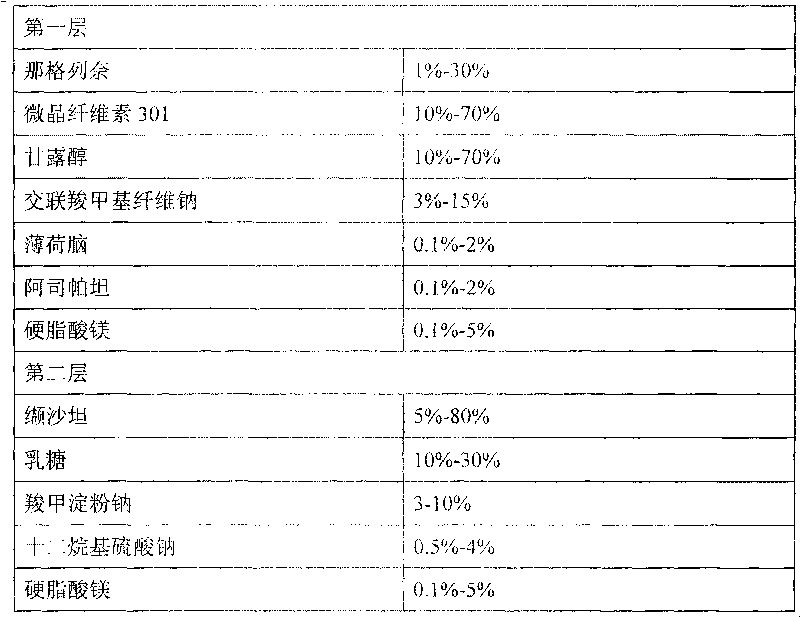
Compound nateglinide valsatan medicinal composition
The invention relates to a compound nateglinide valsatan medicinal composition, which is a dual-layer tablet and comprises a first layer of nateglinide and a second layer of valsatan, wherein the first layer is delivered from a self-soluble tablet to be immediately released; and the second layer is released from a self-disintegration or corroded tablet matrix.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Process for preparing nateglinide and intermediates thereof
InactiveCN100384813COrganic compound preparationOrganic chemistry methodsIsopropylcyclohexaneAcyl group
Provided is a method for preparing an intermediate in the synthesis of nateglinide. Trans-4-isopropylcyclohexane acid chloride is formed by reacting 4-isopropylcyclohexanecarboxylic acid with thionyl chloride in the presence of an effective amount of an organic amide. Also provided is the acylation of a suitable salt of D-phenylalanine with trans-4-isopropylcyclohexane acid chloride in single-phase and two-phase systems and in water free of co-solvents to prepare Nagar Lennet's method.
Owner:TEVA PHARMA IND LTD
New crystal type of netoglitazone and preparation method thereof
InactiveCN101037420BImprove stabilitySimple processOrganic active ingredientsOrganic chemistryDiabetes mellitusMedicine
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE
Process for the preparation of nateglinide
InactiveUS9150499B2High yieldHigh purityMetabolism disorderOrganic compound preparationD-PhenylalanineEnantiomer
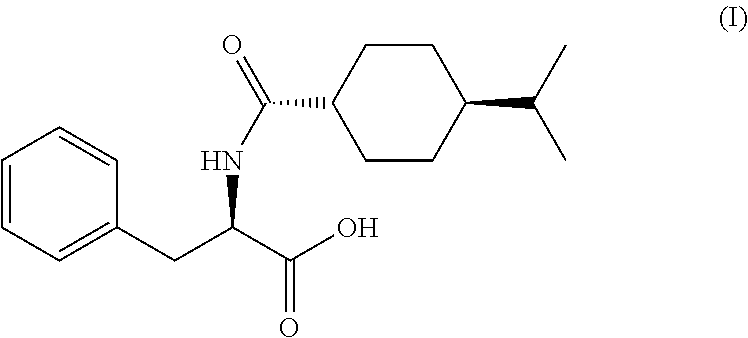
The present invention relates to a process for the preparation of substantially pure nateglinide of formula (I), substantially free from the cis-isomer and L-enantiomer and preparation of enantiomerically pure nateglinide form B, directly from the hydrolysis of a (−)-N-(trans-4-isopropylcyclohexyl-1-carbonyl)-D-phenylalanine alkyl ester in a ketonic solvent or water or mixture thereof.
Owner:CIPLA LTD
Nateglinide tablet and method for preparing nateglinide tablet through direct compression method
InactiveCN105534931ALess prone to numbnessNot prone to splintersOrganic active ingredientsMetabolism disorderMedicineCompression method
The invention discloses a nateglinide tablet and a method for preparing the nateglinide tablet through a direct compression method, wherein each one thousand of nateglinide tablets are prepared from 30g of nateglinide, 45g of diluent, 35g of disintegrating agent, 1g of lubricating agent and 3g of flow aid, the preparation method for preparing the nateglinide tablet through direct compression by using a compressible auxiliary material comprises a process of weighing and matching, screening, mixing, tabletting, inner packing and packing under the D level condition, the nateglinide tablet and the method, provided by the invention solve the problems of cracking tablet, rough tablet, sticking and the like of a nateglinide tablet prepared through a traditional wet method.
Owner:ANHUI GLOBAL PHARM CO LTD
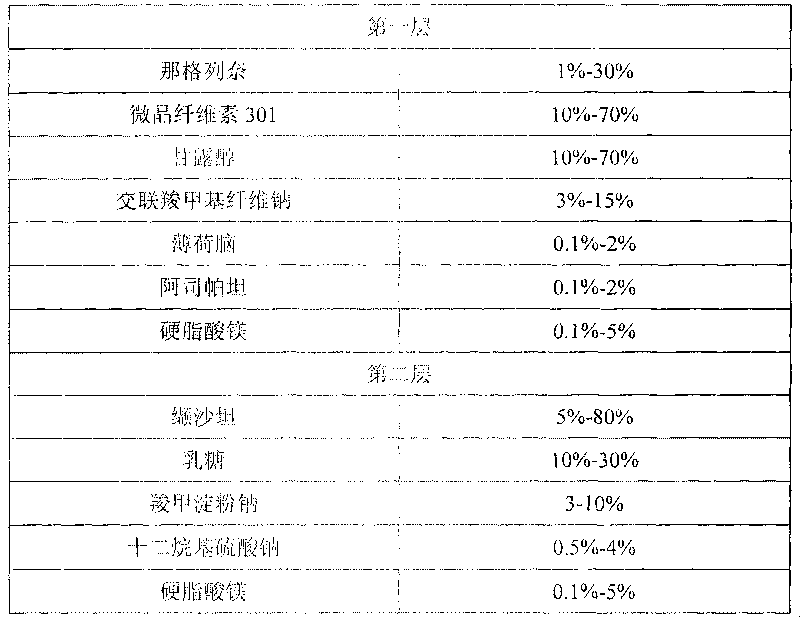
Compound nateglinide valsatan medicinal composition
The invention relates to a compound nateglinide valsatan medicinal composition, which is a dual-layer tablet and comprises a first layer of nateglinide and a second layer of valsatan, wherein the first layer is delivered from a self-soluble tablet to be immediately released; and the second layer is released from a self-disintegration or corroded tablet matrix.
Owner:TIANJIN HANKANG PHARMA BIOTECH
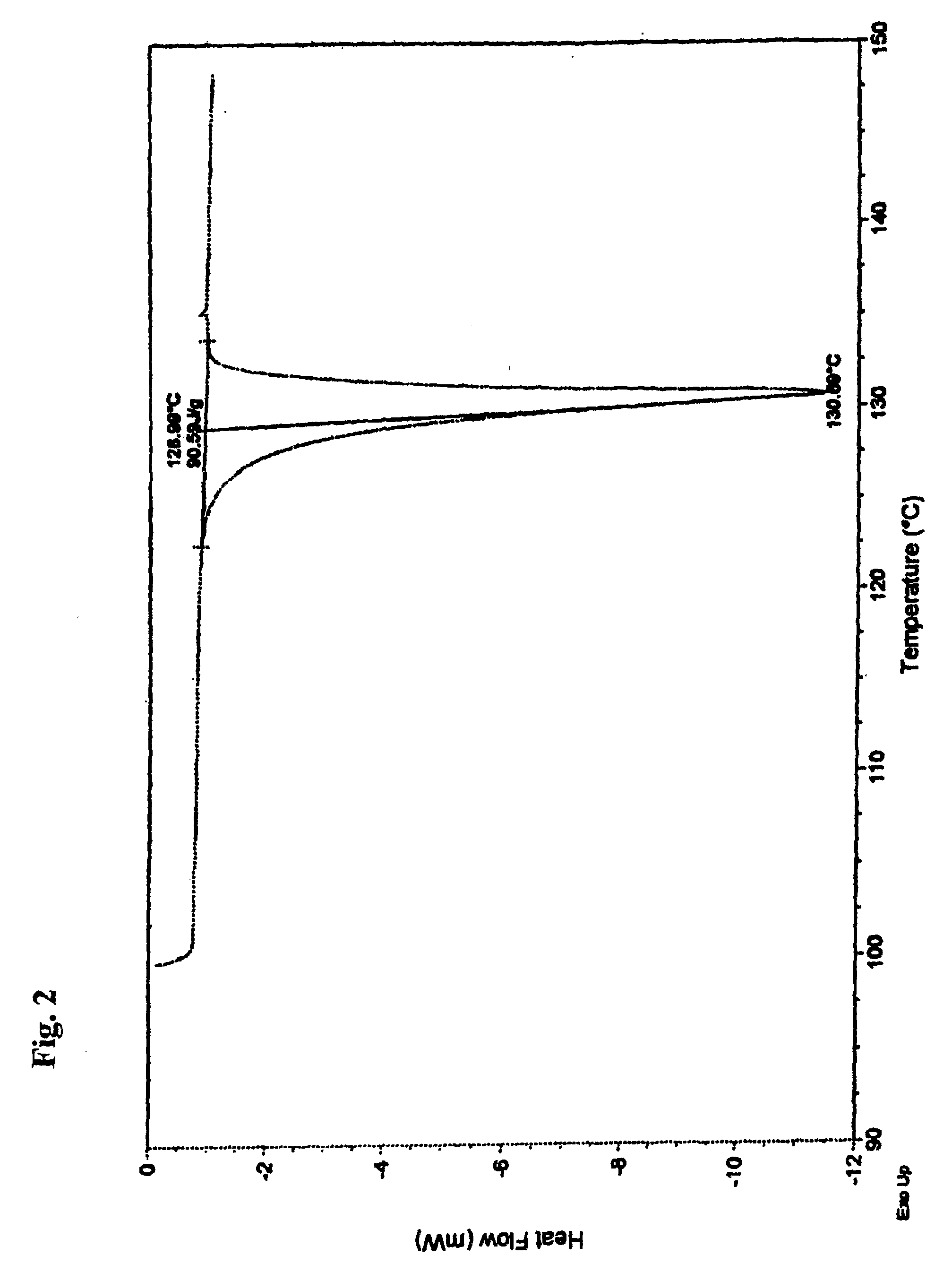
Stable Nateglinide Form B Compositions
A process for preparing nateglinide Form B comprises dissolving nateglinide in a solvent and adding the solution, at temperatures of 40-45° C., to a hydrocarbon liquid that is at temperatures of 40-45° C. Then, water is added and the mixture is allowed to cool, producing crystals of nateglinide Form B.
Owner:DR REDDYS LAB LTD +1
Additive for improving plasticity of plastic
The invention discloses an additive for improving plastic plasticity, which comprises the following components according to the ratio of parts by weight: 20-40 parts of potassium sulfate solution, and 12-20 parts of modified glycerin epoxy resin , 0.3-0.7 parts of sodium carbonate, 0.01-0.03 parts of ammonium chloride, 0.01-0.02 parts of methyl formate, 0.1-0.4 parts of regulator, 0.02-0.03 parts of nateglinide and 1-3 parts of potassium dihydrogen phosphate. The additive for improving the plasticity of the present invention can effectively improve the plasticity of the plastic, increase its transverse and longitudinal tensile stress, so that the plastic has a better plasticizing effect; further, the raw materials of the present invention are easy to obtain, and the preparation method is simple and easy. operate.
Owner:南宁市钜丰塑业有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com