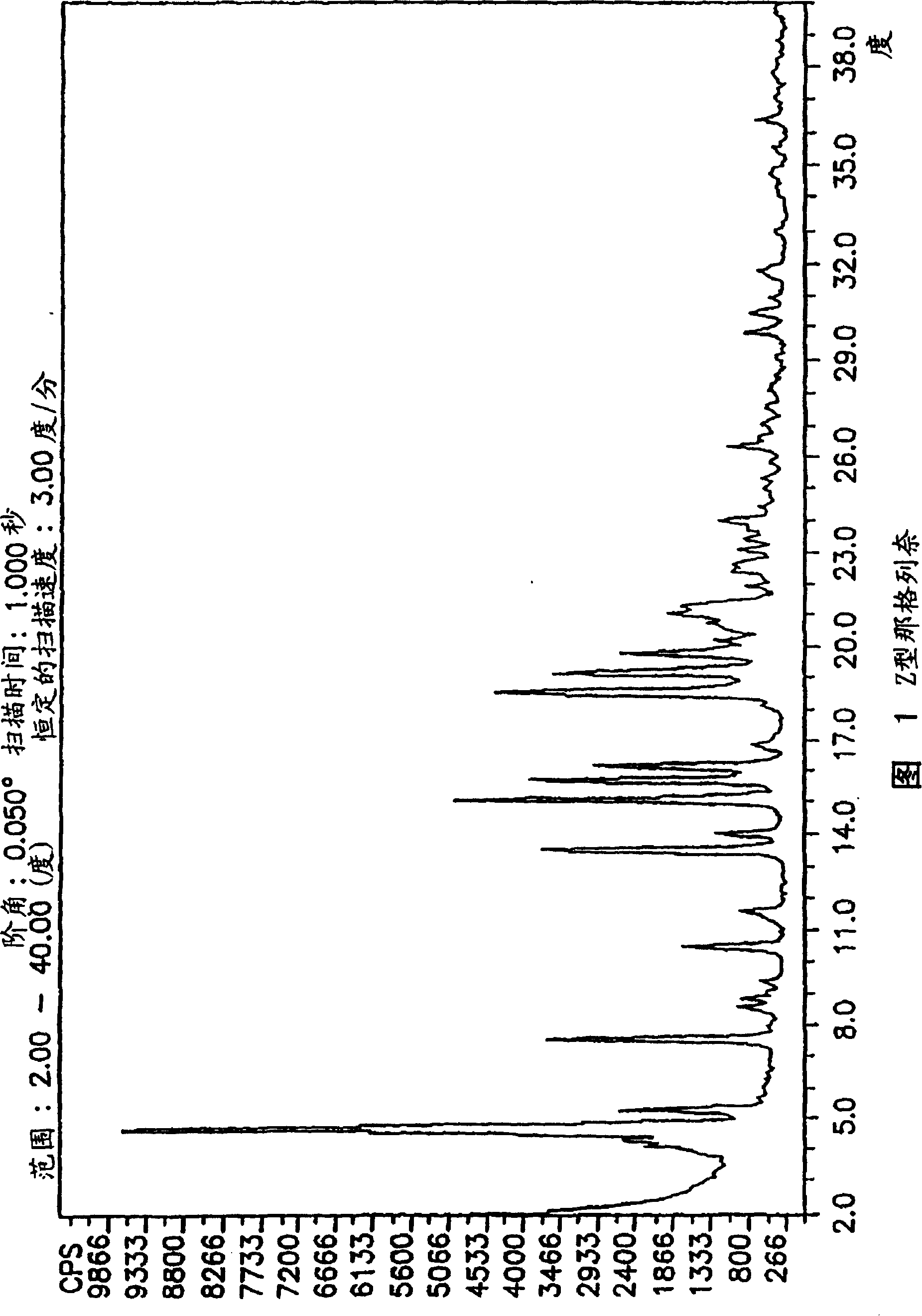
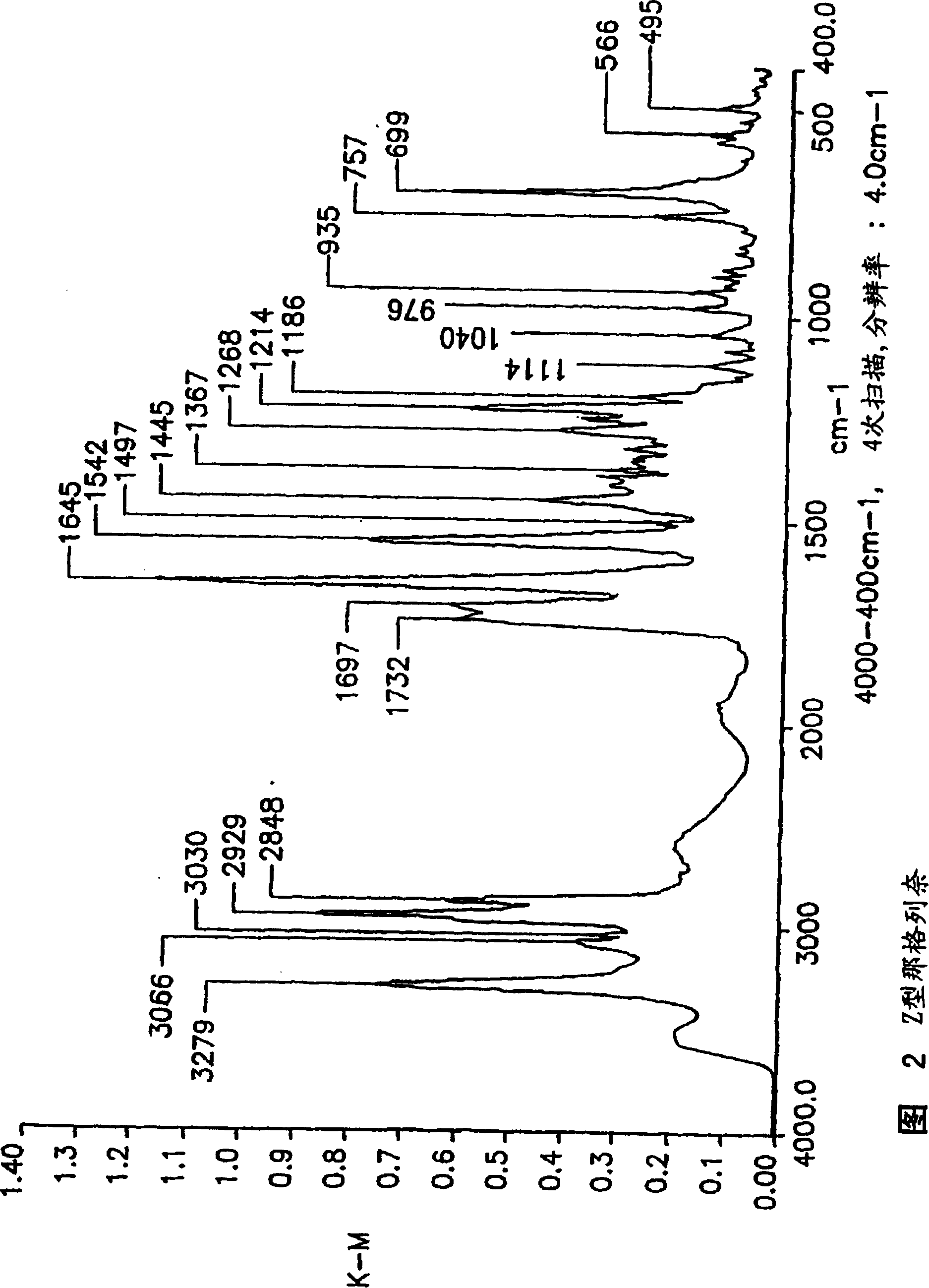
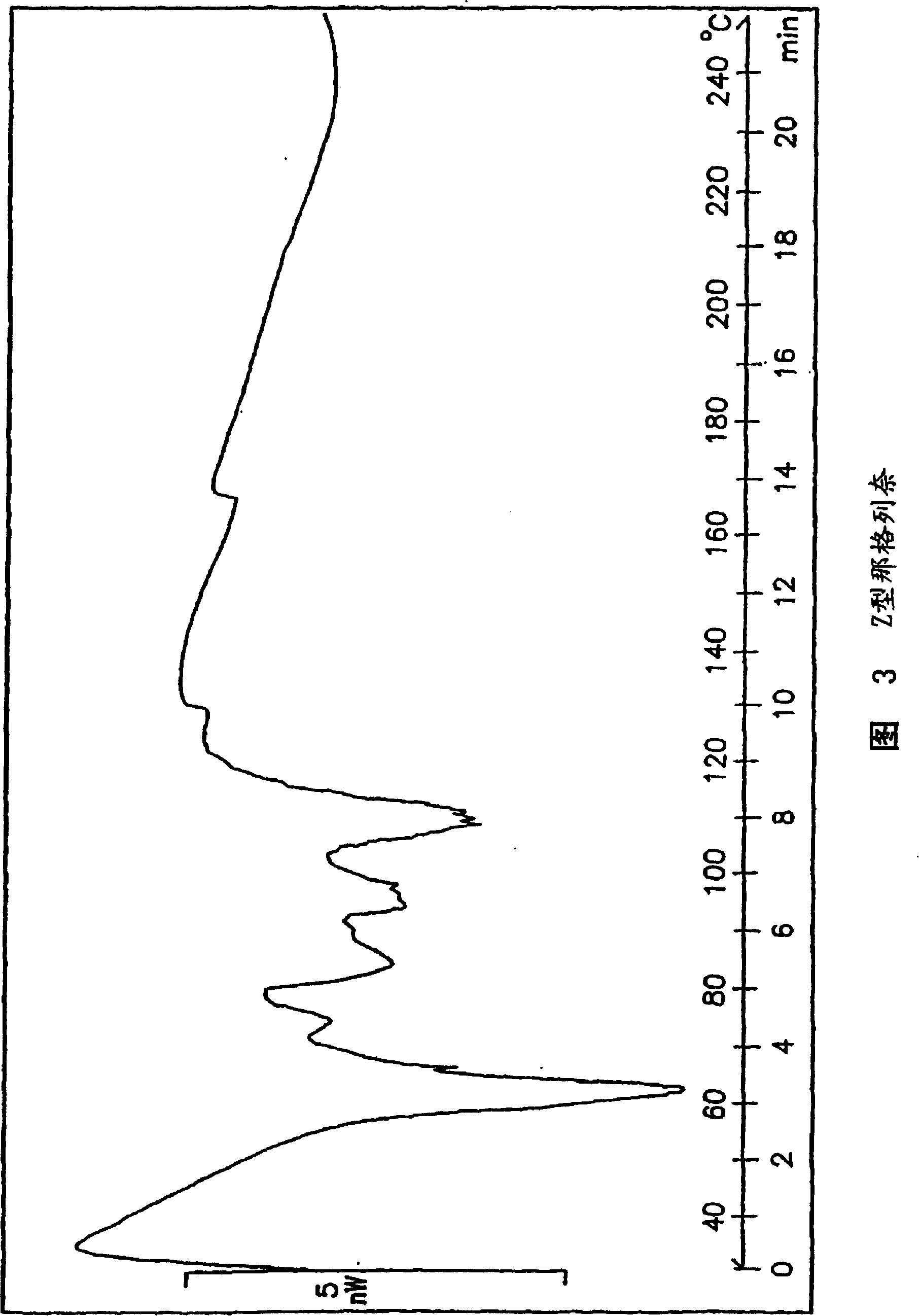
Process for preparing nateglinide and intermediates thereof
A technology for nateglinide and cis-isomers, applied in the field of preparation of nateglinide and its intermediates, capable of solving problems such as contamination, unwanted cis-isomers, and adverse cross-reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Preparation of IPCHAC by chlorination in the presence of amide
[0103] Add N,N-dimethylformamide (0.1ml) and pure trans-4-isopropylcyclohexanecarboxylic acid (9.92g) to thionyl chloride (8.32g) at room temperature. The isomer content is less than 0.1%]. The mixture was stirred at room temperature for 2 hours, and the volatiles were removed under reduced pressure in a 40°C bath to obtain 10.39 g of the desired product trans-4-isopropylcyclohexane acid chloride with a purity of 97% and a colorless liquid. No cis isomer was detected by GC. Yield: 96%.
Embodiment 2
[0105] Preparation of IPCHAC by chlorination in ethyl acetate in the presence of amide
[0106] To a mixture of trans-4-isopropylcyclohexanecarboxylic acid (20.0g) in ethyl acetate (8ml) was added N,N-dimethylformamide (0.1g), and the mixture was heated to 40°C, Thionyl chloride (16.1 g) was gradually added within 1 hour. The mixture was stirred at 40°C for 1 hour, and volatiles were removed under reduced pressure in a 40°C bath to obtain 22.43 g of the desired product trans-4-isopropylcyclohexane acid chloride with a purity of 98% and a colorless liquid. No cis isomer was detected by GC. The yield was 99%.
Embodiment 3
[0108] Preparation of IPCHAC by chlorination in the presence of amide
[0109] To pure trans-4-isopropylcyclohexanecarboxylic acid (250 g) placed in a 2 liter reactor was added N,N-dimethylformamide (0.63 g). The mixture was cooled to 18°C, and thionyl chloride (189.8 g) was gradually added within 15 minutes. The mixture was stirred for 1 hour, maintaining the temperature at about 15°C to obtain a clear solution. A second portion of trans-4-isopropylcyclohexanecarboxylic acid (250g) was added to the reactor, followed by N,N-dimethylformamide (0.63g). Another portion of thionyl chloride (189.8g) was added dropwise within 15 minutes, and the mixture was stirred for 1 hour, keeping the temperature at about 15°C to obtain a clear solution of the crude product of the desired product, which was directly used to prepare nateglinide . No cis isomer was detected by GC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com