Patents
Literature
33 results about "Soluble Tablet" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
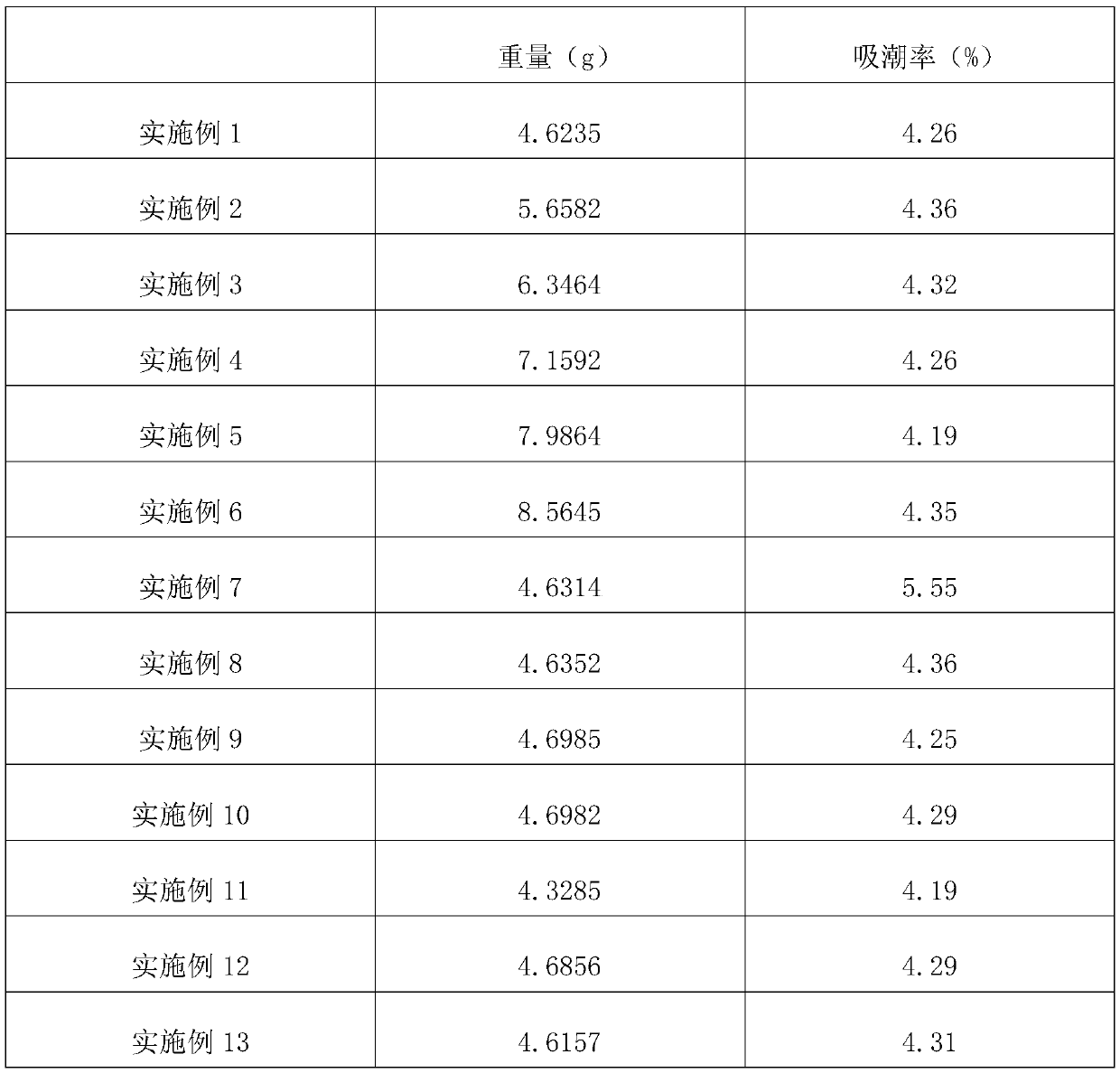
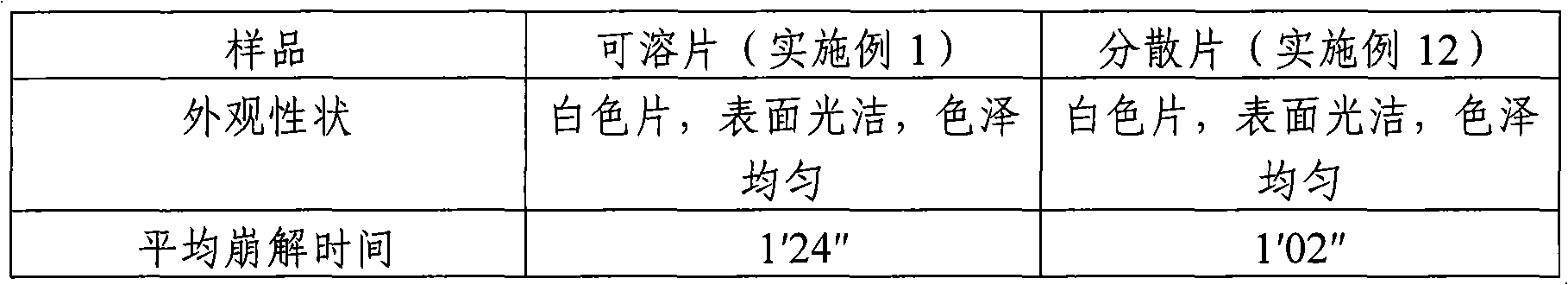
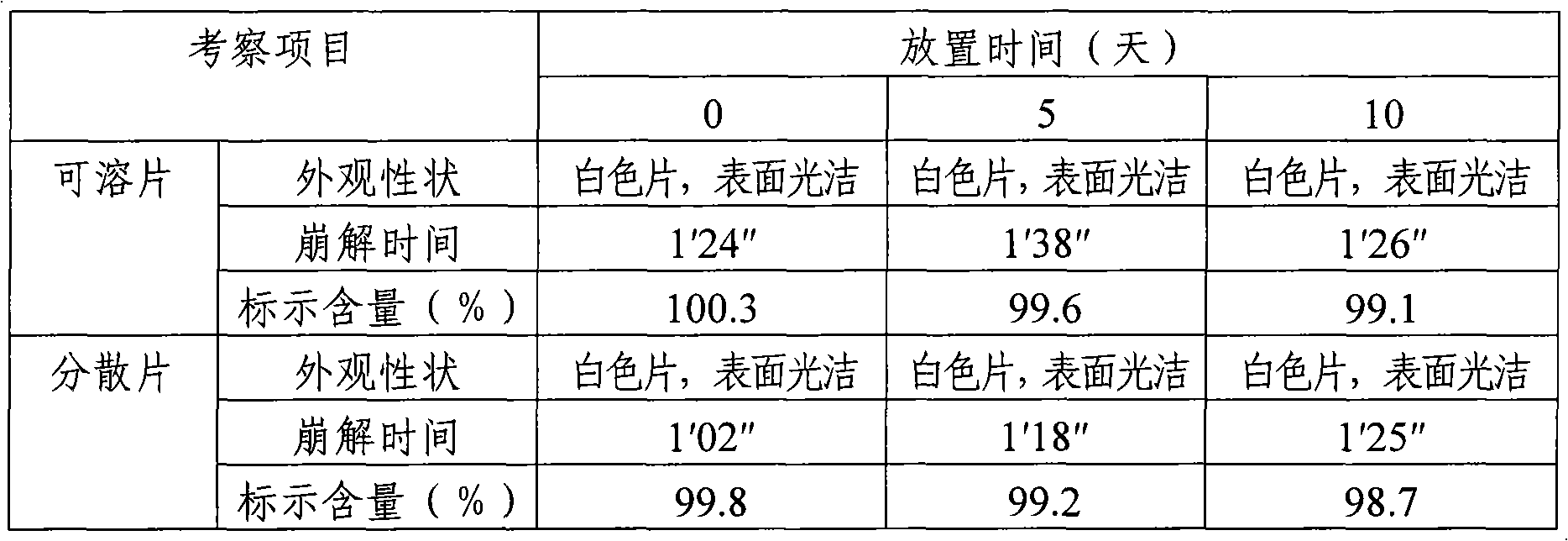
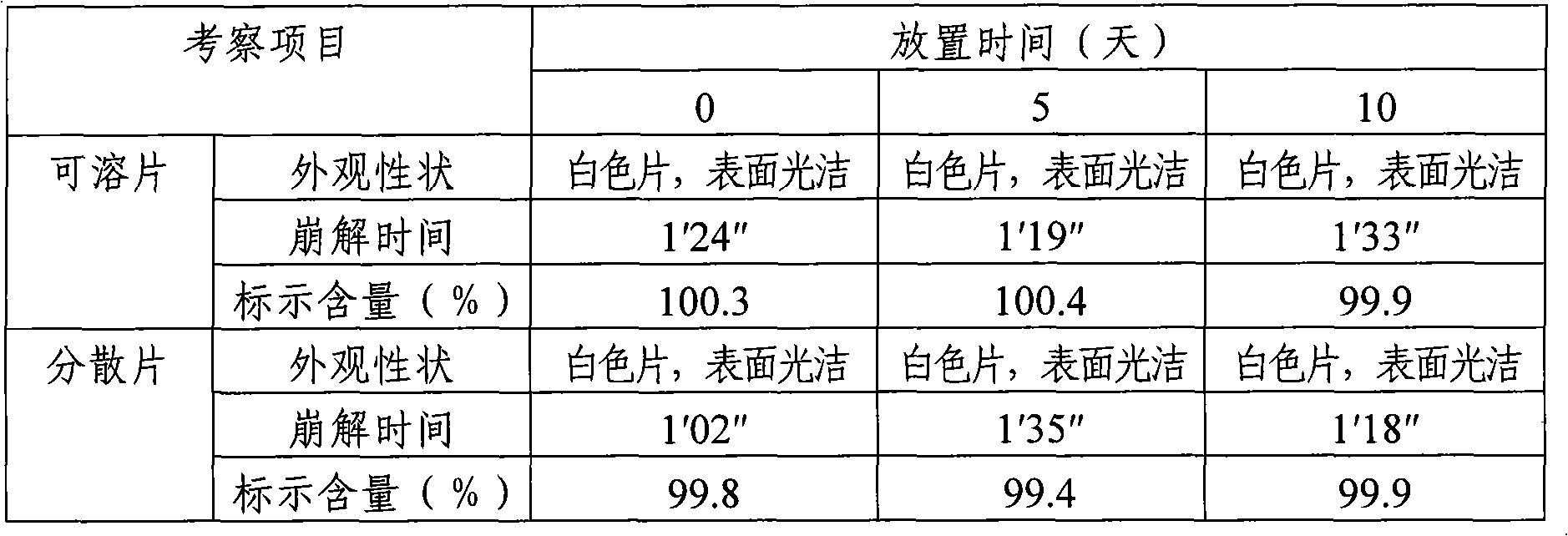
Paracetamol Soluble Tablets are used for headache, migraine, tension headaches, toothache, neuralgia, rheumatic and muscle pain, period pain, sore throat and for relieving the fever, aches and pains of cold and flu.
Process for the preparation of a granulate suitable to the preparation of rapidly disintegrable mouth-soluble tablets and compositions obtained thereby
InactiveUS6149938AGood water solubilityPill deliveryPharmaceutical non-active ingredientsParticle compositionSoluble Tablet
A process for making a granulate composition suitable to the preparation of an oral solid form that can disintegrate rapidly inside the buccal cavity is provided as well as the granulate compositions and obtained.
Owner:ALPEX PHARMA SA
Pitavastatin soluble tablet composition and preparation method thereof
InactiveCN1698609AQuality improvementHigh dissolution rateMetabolism disorderPill deliveryPitavastatinDissolution
The invention relates to a pitavastatin soluble tablet composition and preparation process wherein the composition is prepared from soluble medicinal auxiliary materials by a predetermined proportion, the composition becomes solution state in oral liquid, thus facilitating the dissolution, absorption and application of pitavastatin calcium.
Owner:南京馗珂生物医药技术有限公司
Pesticide formulation containing pyrithiobac-sodium
InactiveCN102037970ASpray evenlyIncrease production capacityBiocideAnimal repellantsAdhesivePesticides/herbicides
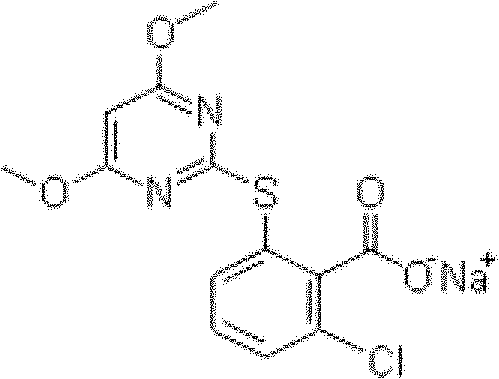
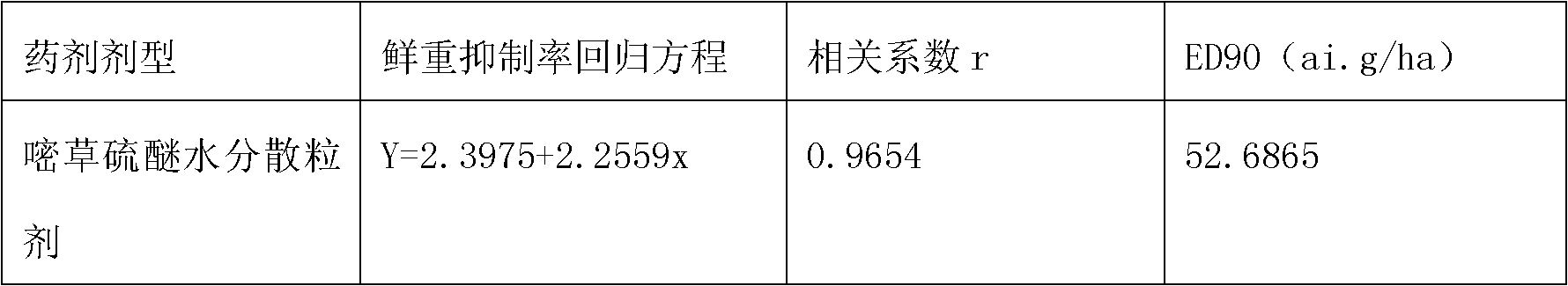
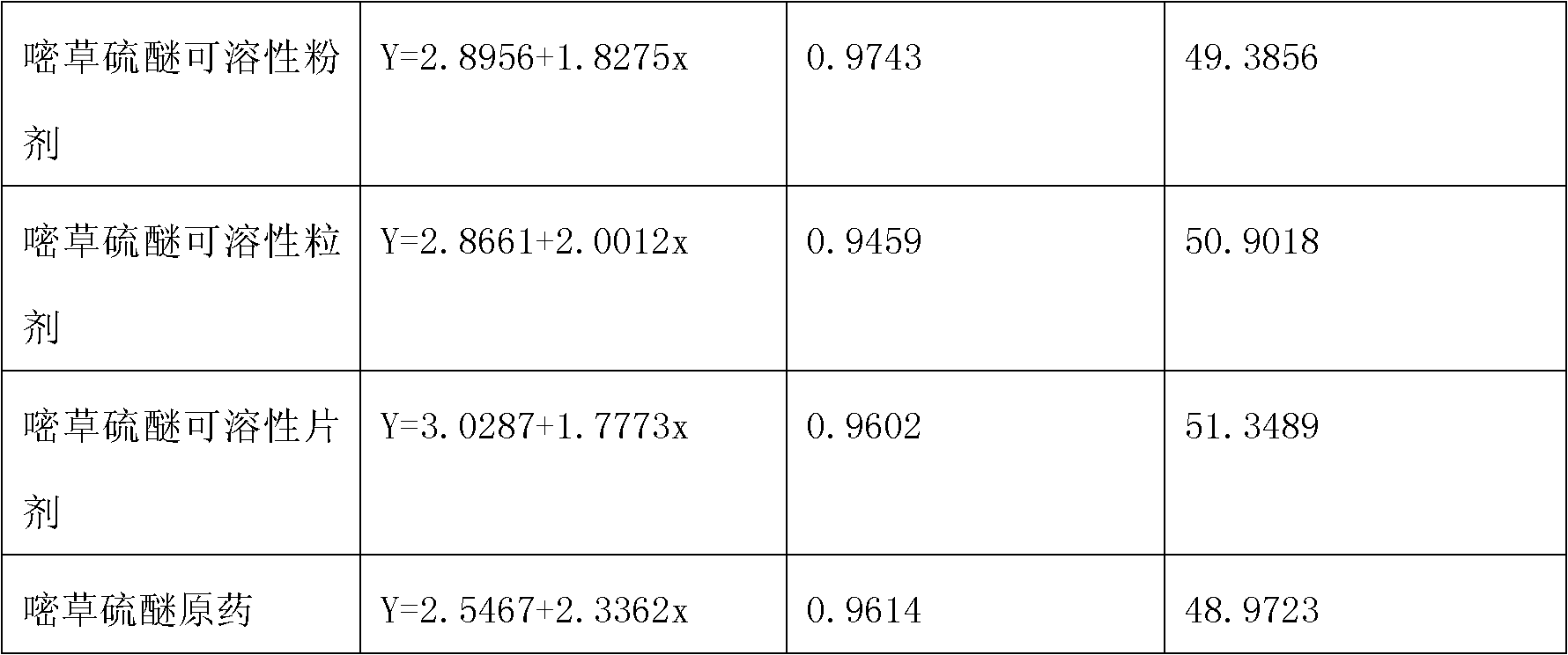
The invention belongs to the field of weed killer, in particular to a pyrithiobac-sodium containing soluble powder, soluble granules and soluble tablets. The invention aims at providing a pyrithiobac-sodium containing pesticide new formulation as well as improving the utilization rate of active ingredients and enhancing environmental compatibility by improving the formulation. The technical scheme thereof includes that a pyrithiobac-sodium containing soluble powder, soluble granules or soluble tablets comprises the components such as pyrithiobac-sodium crude drug, wetting agent, dispersing agent and a carrier, wherein the soluble tablets also comprise adhesive, lubricating agent and disintegrating agent. The invention has the beneficial effects: firstly, product is completely dissolved in water when in use, thus spraying is more uniform; secondly, the pyrithiobac-sodium containing soluble powder, soluble granules and soluble tablets are solid, thus being convenient for production and transportation and reducing production cost.
Owner:QINGDAO HANSEN BIOLOGIC SCI
Imatinib mesylate gastric-soluble pellet tablet and preparation method thereof
ActiveCN105663064AEasy to takeEasy to carryOrganic active ingredientsPill deliveryAdhesiveFluidized bed
The invention discloses an imatinib mesylate pellet tablet and a preparation method thereof. The preparation method comprises the following steps: firstly, preparing a main drug, namely imatinib mesylate, into a gastric-soluble coating pellet by a fluidized bed coating process, and then mixing the coating pellet with a filling agent, a disintegrating agent, an adhesive, a plasticizing agent, a lubricating agent and the like to prepare the gastric-soluble tablet by a compressing dry granulation technology. The tablet can be directly swallowed and can be quickly disintegrated into pellets in water so as to be convenient for patients with dysphagia and especially children to take. The accurate dose of the tablet can be ensured, and the tablet can be taken safely and conveniently.
Owner:HENAN LIFE PHARMA CO LTD
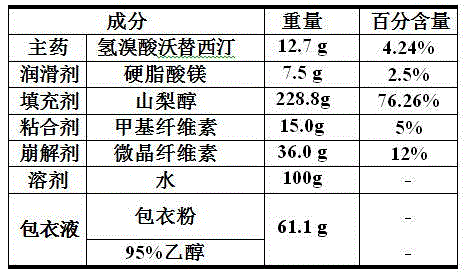
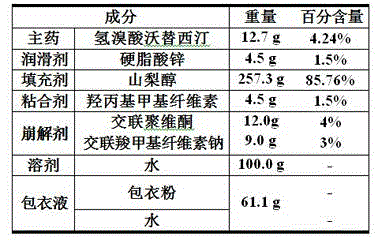
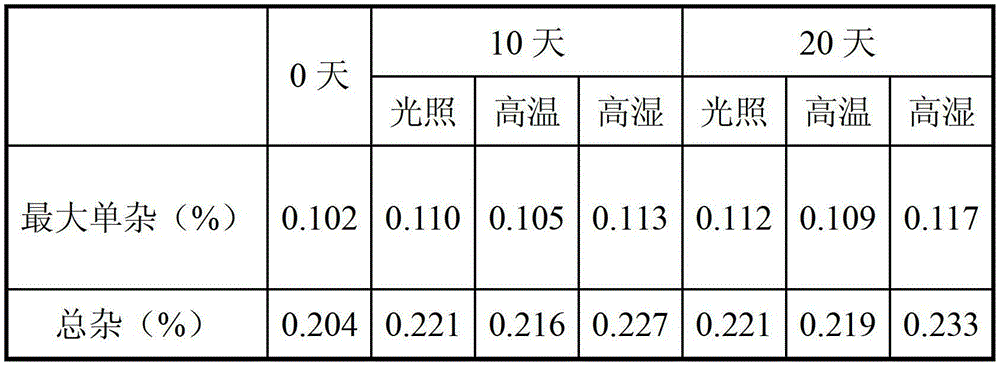
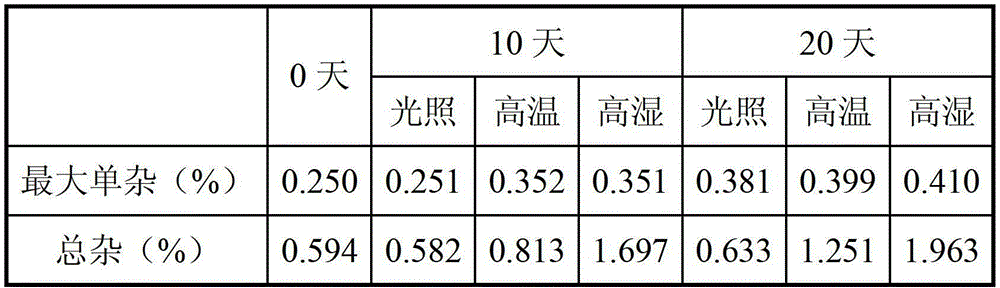
Hydrobromic acid vortioxetine gastric-soluble tablet and preparation method thereof
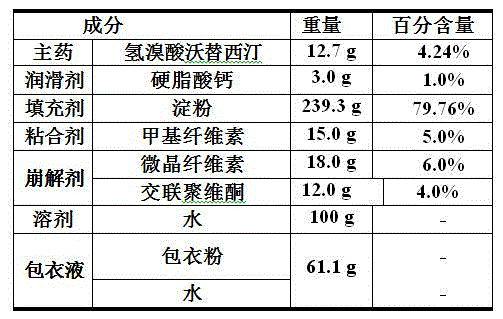
The invention relates to the technical field of medicines and in particular relates to a hydrobromic acid vortioxetine gastric-soluble tablet and a preparation method thereof. The hydrobromic acid vortioxetine gastric-soluble tablet comprises the following components in percentage by weight: 4.24% of hydrobromic acid vortioxetine, 1.0-5.0% of a lubricating agent, 81-91% of a filling agent, 2-5% of a binding agent and 2-15% of a disintegrating agent. Dissolution rate of the hydrobromic acid vortioxetine gastric-soluble tablet is similar to the foreign original standard, so that the hydrobromic acid vortioxetine gastric-soluble tablet has good application prospect and market prospect.
Owner:郑州大明药物科技有限公司
Dextral ibuprofen amino acid salt tablets and preparation method thereof
InactiveCN101978955ATake a small doseGood curative effectOrganic active ingredientsPeptide/protein ingredientsSugar Coated TabletFiller Excipient
The invention discloses dextral ibuprofen amino acid salt tablets and a preparation method thereof. The tablets are prepared from the following raw materials in part by weight: 1 part of dextral ibuprofen amino acid salt, 0.2 to 20 parts of diluent (or filler), 0.01 to 1 part of adhesive, 0.01 to 1 part of disintegrating agent, 0.01 to 1 part of flavoring agent and 0.001 to 0.1 part of lubricating agent. Wet granulation, dry granulation and tabletting or direct powder tabletting and tablet coating are adopted in the preparation process of the tablets. Based on the dextral ibuprofen, the specification of the tablets can be 30 to 300 milligrams, preferably 37.5 milligrams or integral multiple of 37.5 milligrams. The dextral ibuprofen amino acid salt serving as an effective ingredient of the tablets has the advantages of quick response, accurate dose, low side effect and small individual difference. The tablets can be prepared into common tablets, chewable tablets, effervescent tablets, soluble tablets, oral fast dissolving tablets, sugar coated tablets, film coated tablets and the like.
Owner:AEROSPACE CENT HOSPITAL
Olanzapine gastric soluble tablets and preparation method thereof
ActiveCN103142525ASimple processShorten the production cycleOrganic active ingredientsNervous disorderMoisture absorptionMannitol
The invention relates to olanzapine gastric soluble tablets and a preparation method thereof. Particularly, the olanzapine gastric soluble tablets comprise the following components in percentage by weight: 2 to 15 percent of olanzapine, 40 to 82 percent of mannitol, 5 to 30 percent of microcrystalline cellulose, 0.5 to 2 percent of silicon dioxide, and 0.5 to 3 percent of magnesium stearate. The olanzapine gastric soluble tablets have the overall effects of quick dissolubility and stable preparation quality, and do not have the remarkable moisture absorption phenomenon when being placed in the air for a long time. The preparation method is simple in process steps, low in cost and energy consumption and short in production cycle, and thus is suitable for wide popularization and application.
Owner:JIANGSU HANSOH PHARMA CO LTD
Ribavirin dissolvable tablet for infant
InactiveCN101190205AEasy to calculateEasy to calculate dosageOrganic active ingredientsAntiviralsDrugs solutionWater soluble
The invention relates to a ribavirin soluble tablet which is a specification special for children, the components of which include effective dosage of ribavirin and the water-soluble medicinal excipient which can rapidly disintegrate in water and release medicine. When the tablet is taken by patients, the dosage of administration can be determined according to the weights of patients, therefore, the intake dosage of medicine can be more accurate and the safety can be improved. The invention is a beverage with sweet and sour taste when being dissolved, so the invention is easy to be accepted by children in term of the way of administration. Meanwhile, the solution of the medicine is beneficial for full absorption and is high in bioavailability.
Owner:何德义
Soluble tablet containing clopidogrel
InactiveCN101601660AEasy to storeEasy to carryOrganic active ingredientsPharmaceutical non-active ingredientsBULK ACTIVE INGREDIENTClopidogrel
The invention discloses a clopidogrel soluble tablet and a preparation method thereof. The soluble tablet contains an active ingredient of clopidogrel or pharmaceutically acceptable salts and pharmaceutical excipients, wherein the pharmaceutical excipients comprise soluble excipients. The ratio of the clopidogrel to the soluble excipients is 1:1-10 in percentage by weight. The soluble tablet can also contain a disintegrant and a lubricant. The preparation method for the clopidogrel soluble tablet can be a direct tabletting method or a wet granulation tabletting method. Compared with a common tablet, the clopidogrel soluble tablet has the advantages of fast disintegration and formation of solution state in water within a short period of time, is easy in storage and use and particularly convenient for a patient with swallowing difficulty, and significantly improves the compliance of the patient.
Owner:SHENZHEN NEPTUNUS PHARM CO LTD
Alkali food and preparation process thereof
InactiveCN101720936AImprove immunityNutritional supplementsFood preparationSodium bicarbonateAdditive ingredient
The invention relates to alkali food for improving acid constitution and a preparation process thereof. The alkali food comprises the following main components in percentage by weight: 50 to 95 percent of algae powder and 5 to 50 percent of sodium bicarbonate. By the conventional pharmaceutical process, the alkali food can be made into capsules, tablets, pills, particles, powder, intestine soluble capsules and intestine soluble tablets. The alkali food has the function of improving the acid constitution of a human body.
Owner:李大鹏
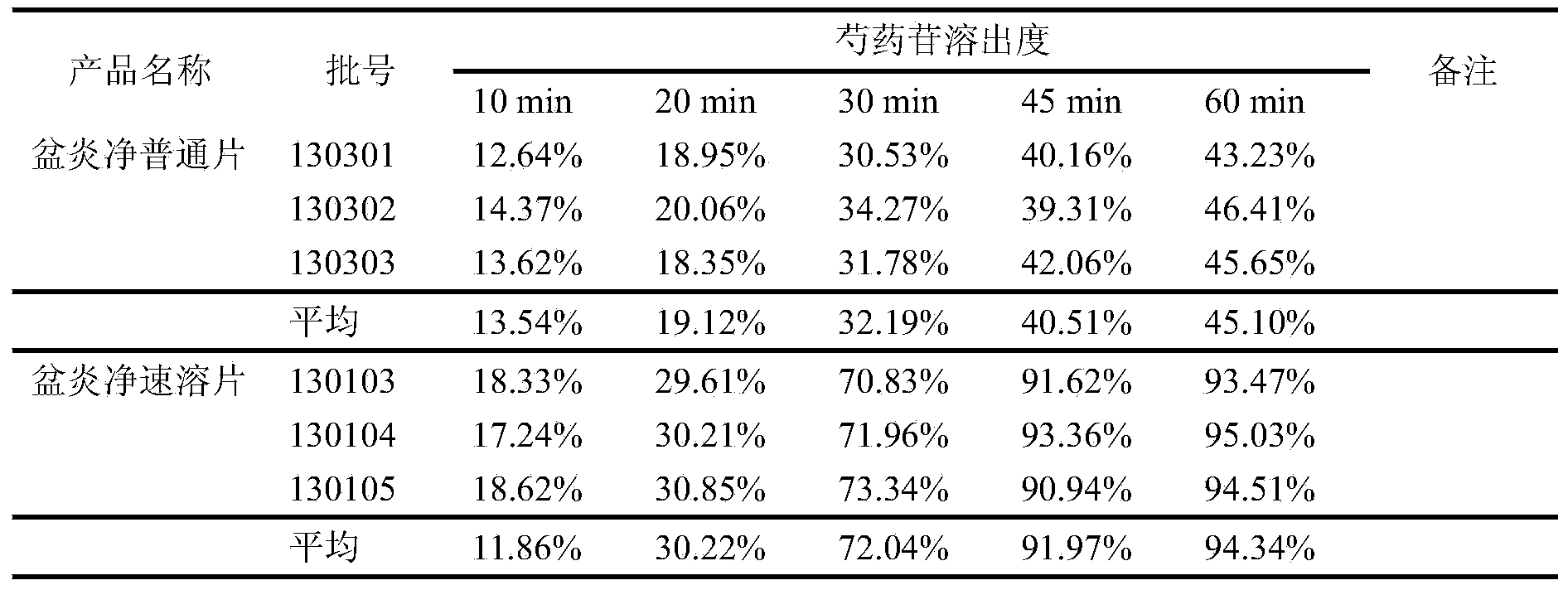
Pelvic inflammation treating tablet and preparation method thereof
InactiveCN104013684AImprove tablet qualityHigh dissolution ratePill deliveryPharmaceutical non-active ingredientsCarboxymethyl starchClinical efficacy
The invention provides a pelvic inflammation treating tablet and a preparation method thereof, and belongs to the technical field of medicines. The pelvic inflammation treating tablet comprises the following auxiliary materials by weight percent according to the total weight of the tablet: 10-35% of lactose, 1-3% of sodium carboxymethyl starch, 2-5% of microcrystalline cellulose, 0.5-2% of superfine silica powder and 6-15% of medicinal ethanol. Undissolved tablets of traditional Chinese medicine tablets are changed into soluble tablets and instant tablets, so that the problems that all extract tablets of the traditional Chinese medicine are slow to disintegrate and slow to take effect are fundamentally solved, the bioavailability of the traditional Chinese medicine product is improved, and the clinical curative effect of the product is enhanced. The novel medicinal auxiliary materials are refined, and the components are scientific, so that the disintegration time limit of the product is accelerated by 3-4 times on the original basis, the dissolution rate of effective components in the product is increased, and the inherent quality of the traditional Chinese medicine tablet is greatly improved.
Owner:HUNAN PALLADIUM PHARMA
Paediatrics amoxicillin Na dissolve sheet
InactiveCN101199518AImprove complianceQuick effectAntibacterial agentsInorganic non-active ingredientsDrugs solutionAmoxicillin Sodium
The invention relates to an amoxicillin sodium soluble tablet with special specification for children, comprising effective amount of amoxicillin sodium and water-soluble medicinal excipient which disintegrates fast to release efficacy in water. When the patient takes the tablet, the patient can determine the taken number of the tablets according to the body weight of the patient. Thus, personalized administration can be realized; the medicine intake can be more accurate; and the safety is higher. After the medicine is dissolved in the water, the water can become a sweet and sour beverage, so the taken method is more acceptable by children with good compliance. At the same time, the medicine solution is easy to be fully absorbed by the body , and the bioavailability is higher.
Owner:何德义
Paediatrics aminophylline insant sheet
InactiveCN101199531AEasy to calculateEasy to calculate dosagePill deliveryPharmaceutical non-active ingredientsDrugs solutionTheophylline
The invention relates to an aminophylline soluble tablet with special specification for children, comprising effective amount of aminophylline and water-soluble medicinal excipient which disintegrates fast in the water to release the efficacy. When the patient takes the tablets, the patient can determine the number of the tablets according to the weight of the patient, so the personalized administration can be realized, the drug intake is more accurate, and the safety is higher. After the drug is dissolved in the water, the water becomes a sweet and sour beverage, so the taken method is more acceptable by the children. At the same time, the drug solution is easy to be fully absorbed with high bioavailability.
Owner:何德义
Astragalus mongholicus tablet, and its preparation and active constituent content measuring method
The present invention provides a medicine for curing the diseases of cardio palmus, short breath, collapse, spontaneous perspiration, edema, chronic nephritis, proctoptosis and carbuncles. Said medicine is made up by using astragalus root extract as main component, can be made into various tablet form including general tablet (containing sugar-coated tablet), disintegrant tablet, chewing tablet, enteric soluble tablet and dispersing tablet, etc.
Owner:SICHUAN GUOKANG PHARMA
Herbicidals of imidazoleethyniacin
A novel herbicide in the form of emulsified oil, microemulsion, concentrated soluble tablet, slow-releasing agent, soluble powder, particles, wettable powder, suspension or powder contains imazethapyr and / or emulsifier, solvent, assistant and filler.
Owner:王现全 +1
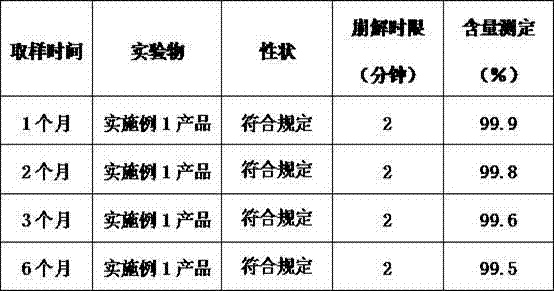
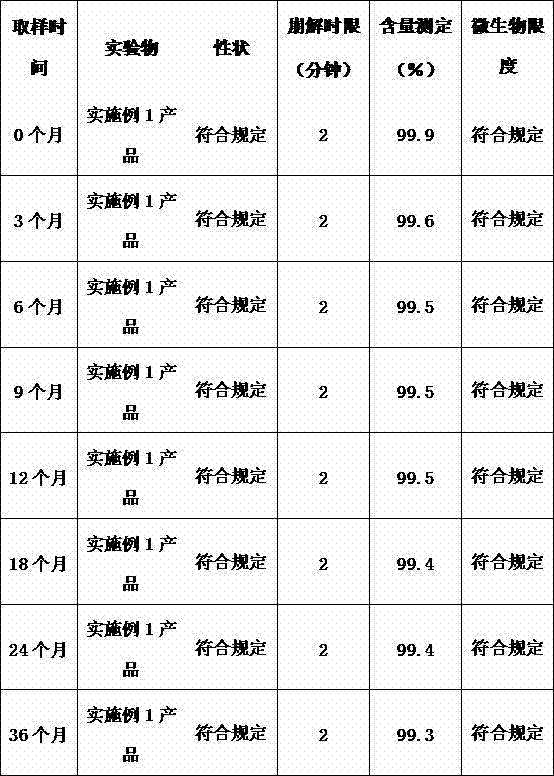
Vitamin C soluble tablet and preparation method thereof
ActiveCN103877050AMeet needsIncrease usageOrganic active ingredientsMetabolism disorderVitamin CCalcium EDTA
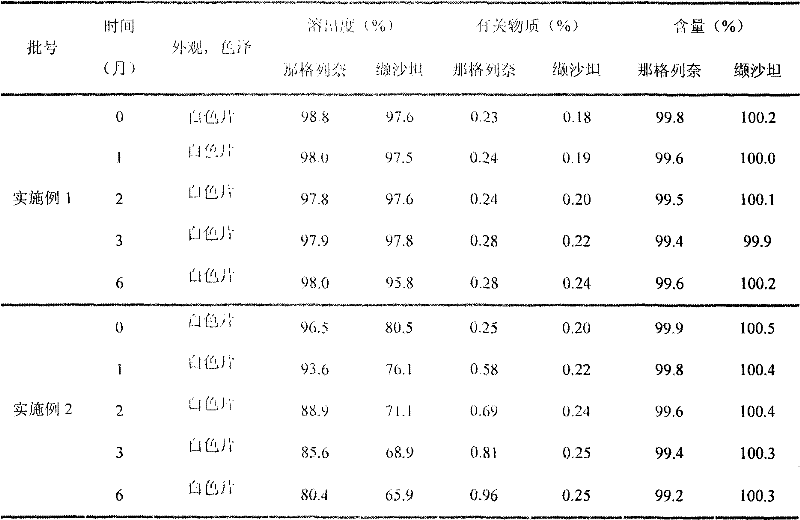
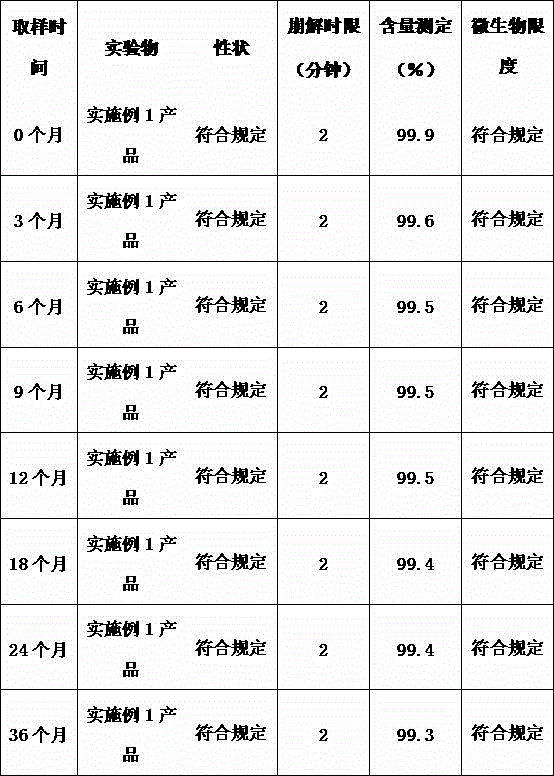
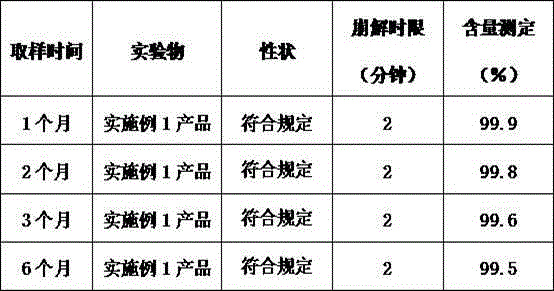
The invention relates to a vitamin C soluble tablet. The medicinal composition is vitamin C or calcium salt dihydrate thereof. The invention also provides a preparation method of the vitamin C soluble tablet. A reasonable auxiliary material formula is selected, so that the vitamin C soluble tablet can be quickly disintegrated within 2 minutes in water and becomes a settled solution form, so that the drug is quickly obtained. By adopting the preparation method disclosed by the invention, vitamin C can be well protected, and oxidation is reduced. Meanwhile, the preparation time is also shortened when the tablet with excellent performance is obtained, and the cost is reduced. The preparation method disclosed by the invention is simple and convenient in process, easy to carry out, and applicable to massive industrial production.
Owner:北京朗迪制药有限公司
Colon target-positioning preparation for treating colonitis and preparing method
InactiveCN101069695ATo achieve the purpose of colon positioningGood curative effectDigestive systemPharmaceutical non-active ingredientsUpper gastrointestinalMedicine
The present invention provides a kind of colon target orientation preparation for curing colitis and its preparation method. Said preparation is formed from 250-500g of montmorillonite, 3-15g of binding agent and 0.20-3.0g of lubricating agent. Said colon target orientation preparation can be made into oral enteric soluble tablet or oral enteric soluble capsule. Besides, said invention also provides the concrete steps of its preparation method.
Owner:刘庆芸
Intraorally disintegrating valdecoxib compositions
Orally disintegrating valdecoxib fast-dissolving tablets and methods of preparing such dosage forms are provided. The compositions are useful for treating or preventing cyclooxygenase-2 mediated conditions and disorders.
Owner:PHARMACIA CORP
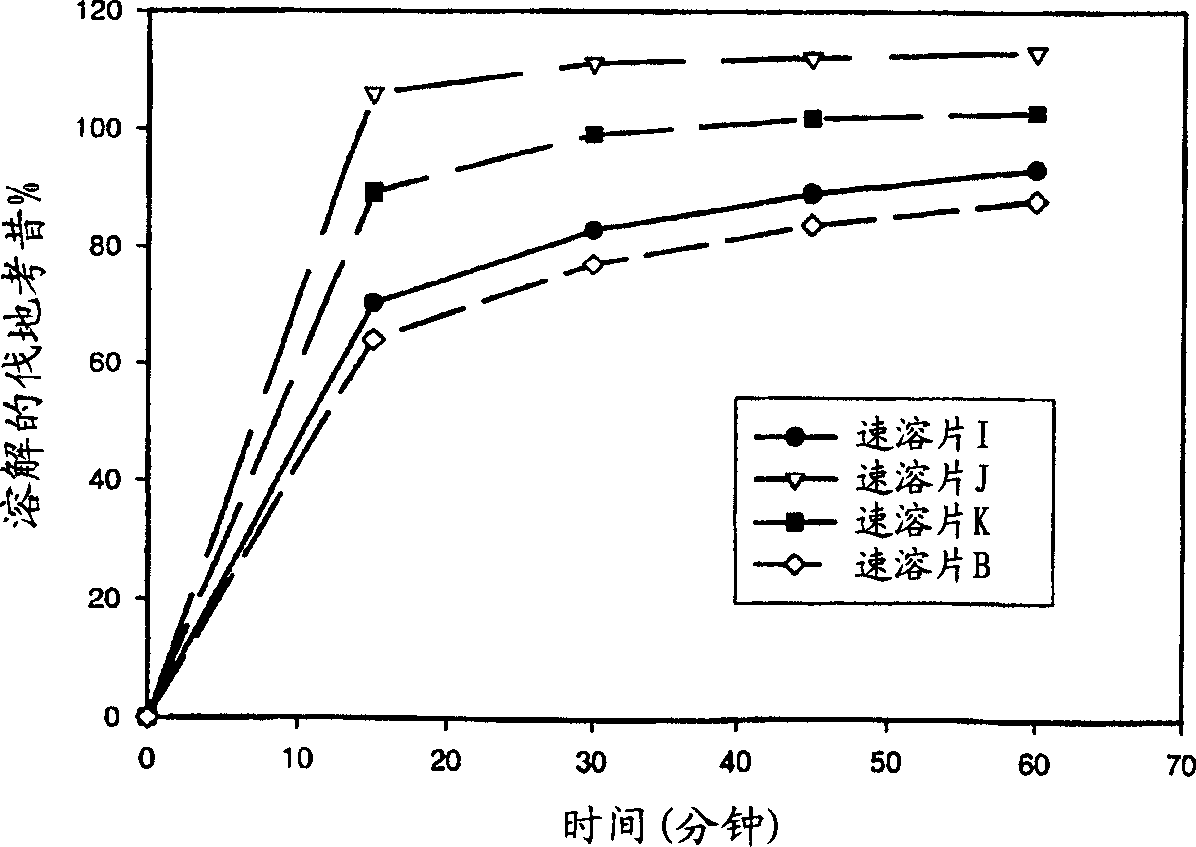
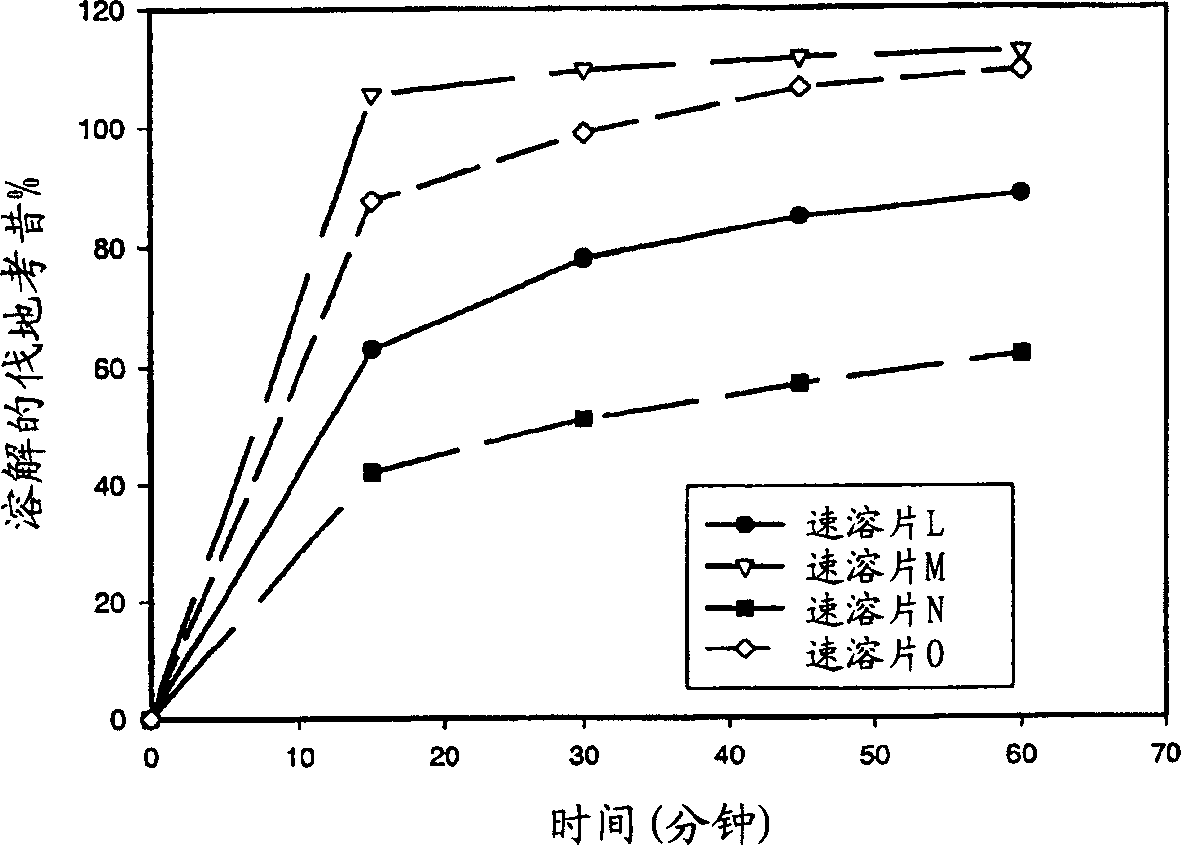
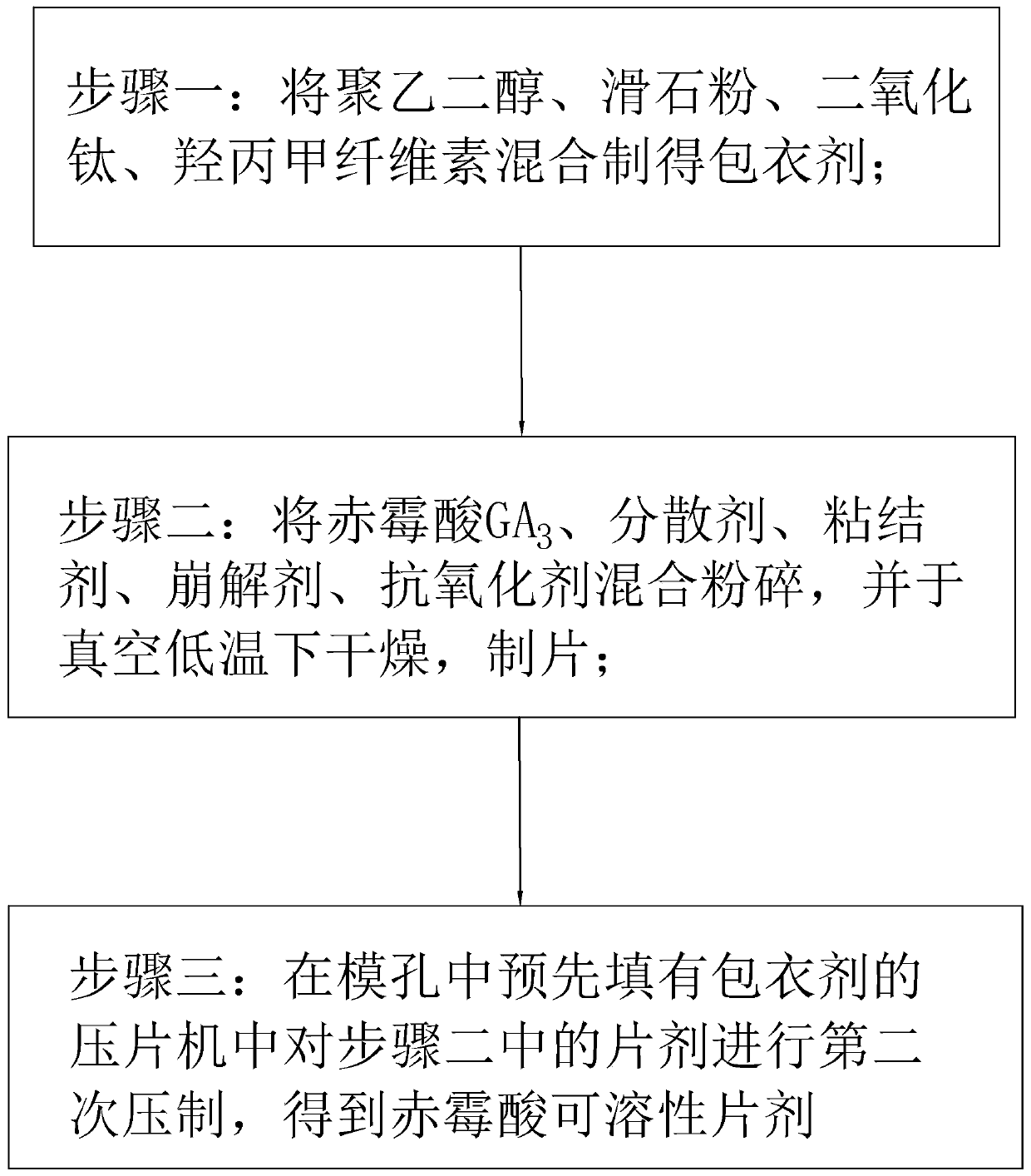
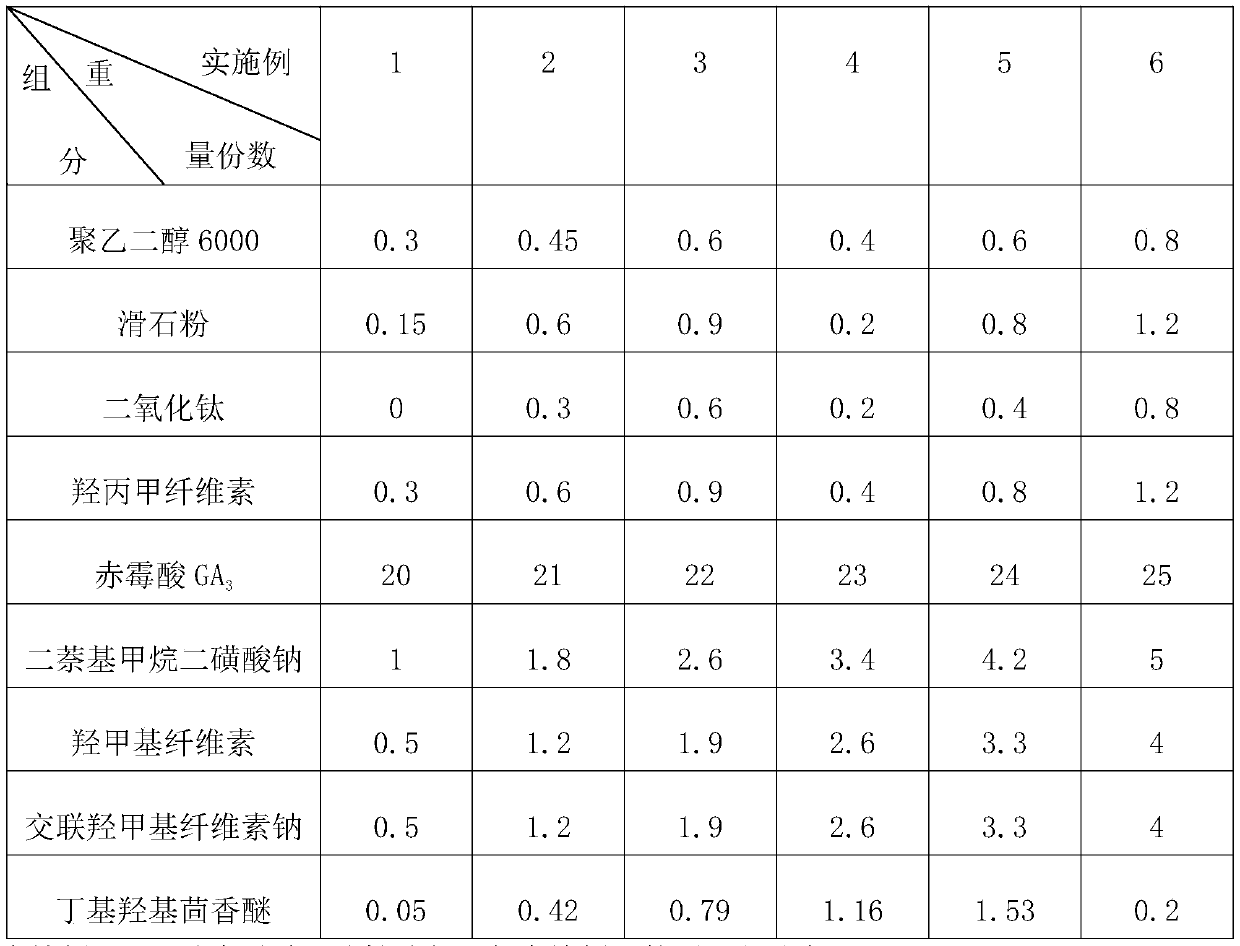
Gibberellic-acid soluble tablet and preparing method thereof
The invention discloses a gibberellic-acid soluble tablet and a preparing method thereof, and relates to the technical field of pesticide preparing. The gibberellic-acid soluble tablet and the preparing method thereof are characterized in that the gibberellic-acid soluble tablet is prepared from, by weight, 20-25 parts of gibberellic acid GA3, 1-5 parts of a dispersing agent, 0.5-4 parts of a binding agent, 0.5-4 parts of a disintegrating agent, 0.05-0.2 part of an antioxidant and 3-5 parts of a coating agent, wherein the coating agent is prepared from, by percentage content, 10%-20% of polyethylene glycol, 5%-30% of talcum powder, 0%-20% of titanium dioxide and 10%-30% of hydroxypropyl methylcellulose. The tablet is packaged with a layer of coating, the humidity resistance of the gibberellic-acid soluble tablet is greatly improved, and the shelf life is prolonged.
Owner:PILARQUIM SHANGHAI
Hydrochloric acid boningmycin solid preparation and preparation method thereof
ActiveCN102462668AImprove complianceImprove stabilitySaccharide peptide ingredientsAntiviralsFiller ExcipientAdhesive
The invention provides a hydrochloric acid boningmycin solid preparation, which is a soluble tablet or a dispersible tablet. The compositions and weight percentages of the hydrochloric acid boningmycin solid preparation are as follows: 0.5 to 50wt% of hydrochloric acid boningmycin, 15 to 99.4wt% of fillers achieving a filling effect, 0 to 20wt% of disintegrants achieving a disintegration effect, 0 to 10 wt% of adhesives achieving an adhesion effect and 0.1 to 5wt% of lubricants achieving a lubrication effect. The invention also provides a preparation method of the soluble tablet and the dispersible tablet. By adopting the hydrochloric acid boningmycin tablet provided by the invention, on the one hand, the deficiency that preparations such as ointment, gel, and the like have poor stabilityafter being placed for a long time under a room temperature condition is overcome, and the storage and the carrying are facilitated; and on the other hand, before being used, the tablet is added to aspecial solvent so as to be dissolved or dispersed to form a water solution or an injectable suspension with certain viscosity, and thereby the tablet also has the advantage of a semi-solid or liquidexternal preparation on the aspects of the use and the pharmaceutical effect.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
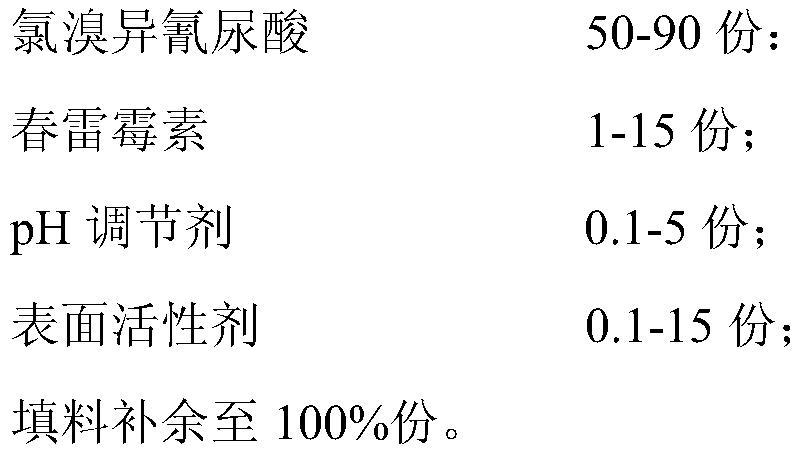
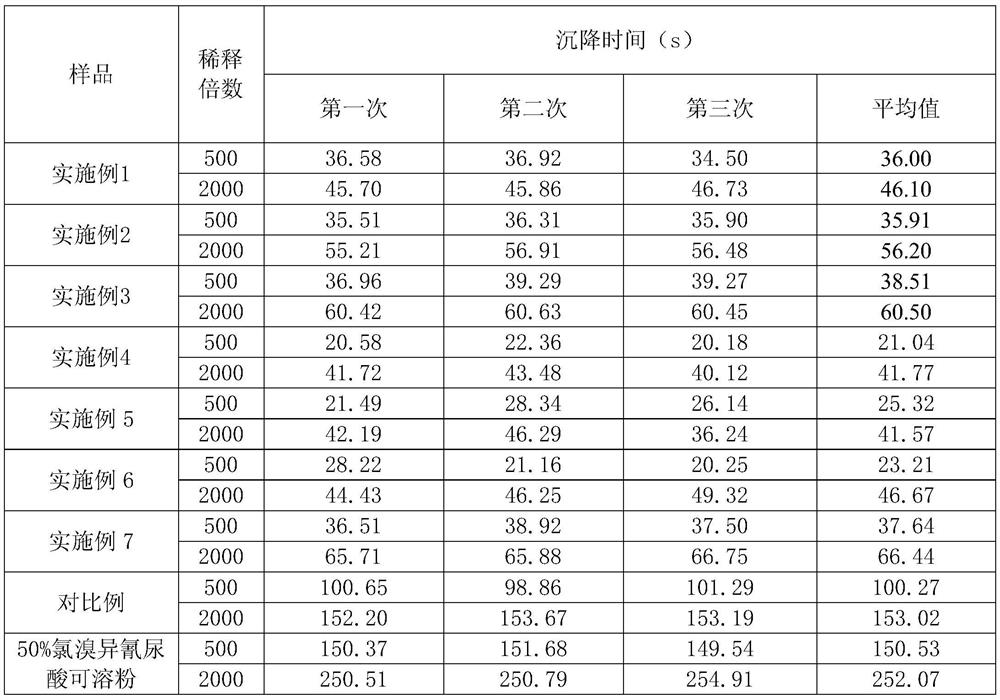
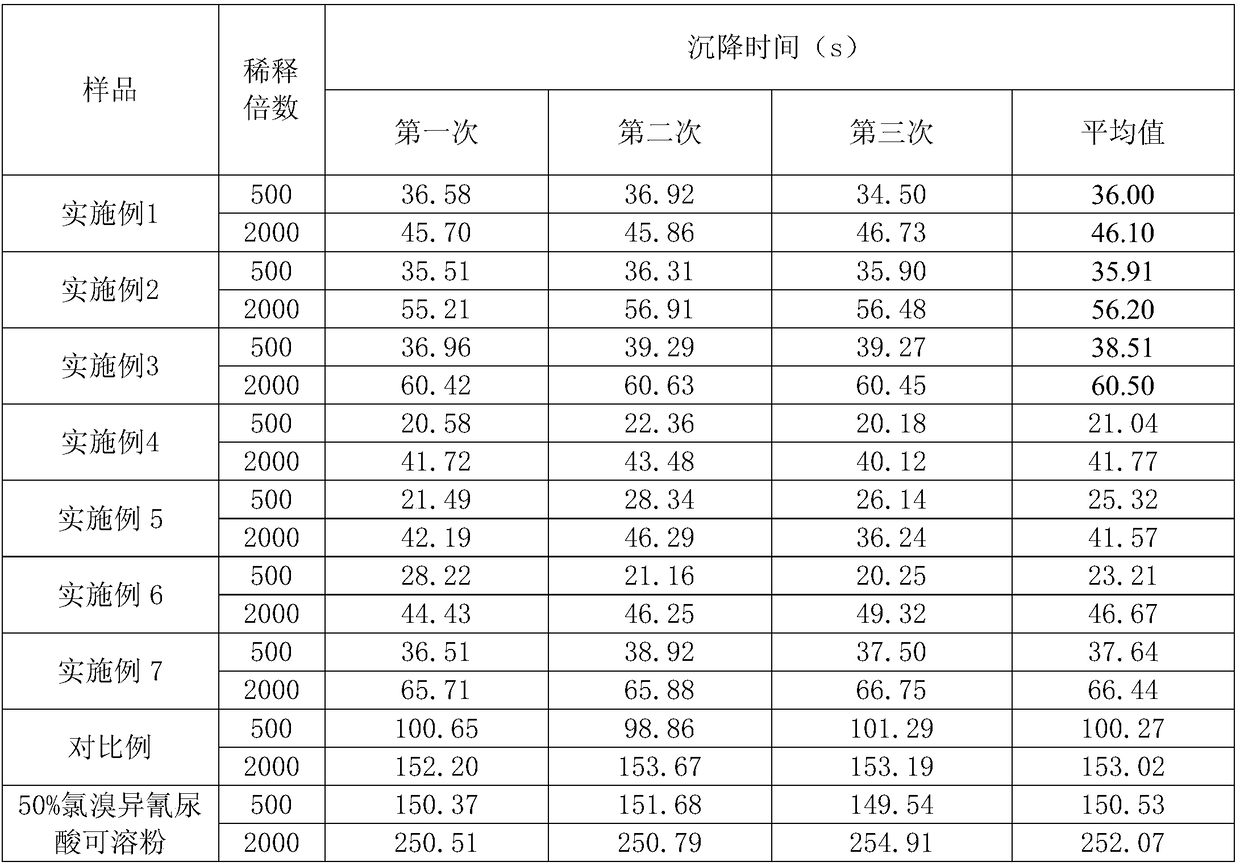
A kind of soluble tablet containing chlorobromoisocyanuric acid and kasugamycin and preparation method thereof
ActiveCN108294011BReduce harmReduce pollutionBiocideDisinfectantsEnvironmental engineeringChloroform
The invention discloses a soluble tablet containing chlorobromoisocyanuric acid and kasugamycin and a preparation method thereof. The soluble tablet uses chlorobromoisocyanuric acid and kasugamycin as active ingredients, and the soluble tablet has completely water-soluble , No insoluble impurities, rapid dissolution characteristics. The soluble tablet is granulated by dry method and produced by a tablet press machine, with low production energy consumption, no dust drift during production and use, and no pollution to human body and environment. The dissolvable tablet conforms to the national requirements for the development of pesticide products in the direction of green environmental protection, can better utilize the advantages of chlorobromoisocyanuric acid and kasugamycin products, and prolong the product life cycle.
Owner:江苏邦盛生物科技有限责任公司
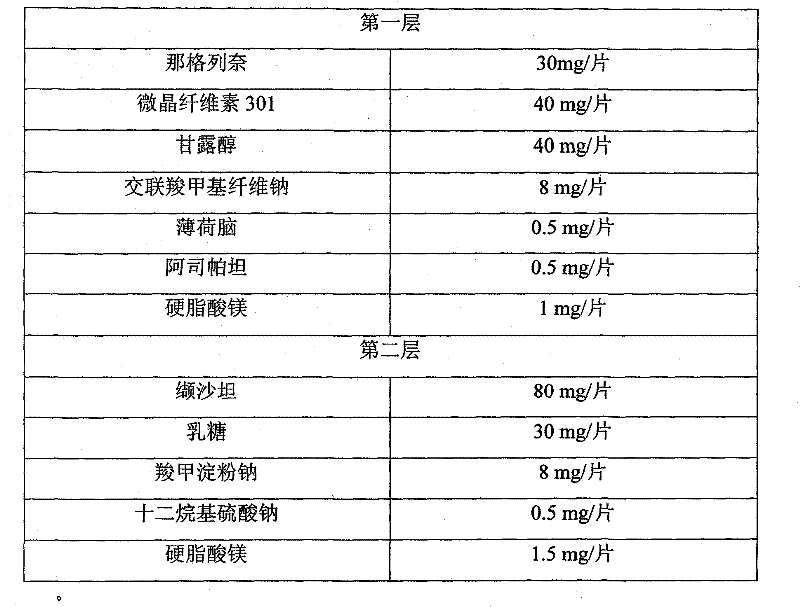
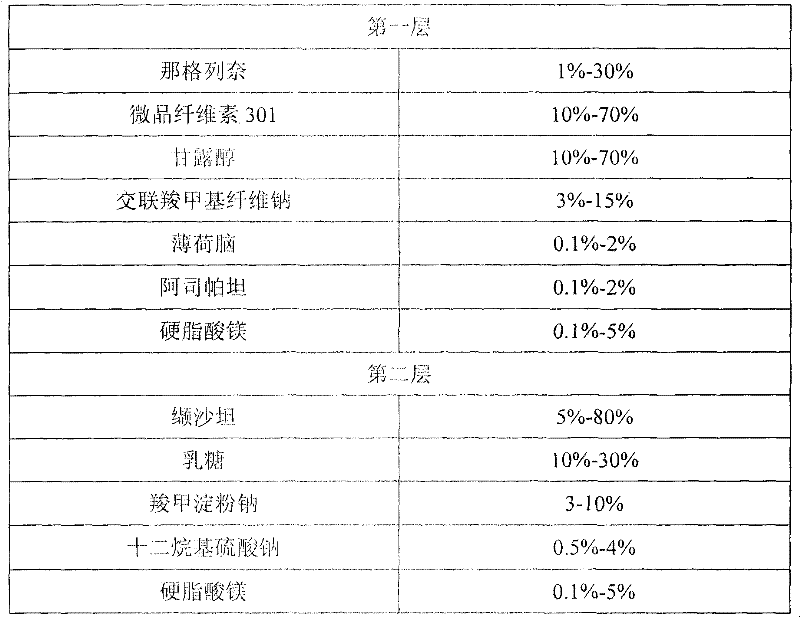
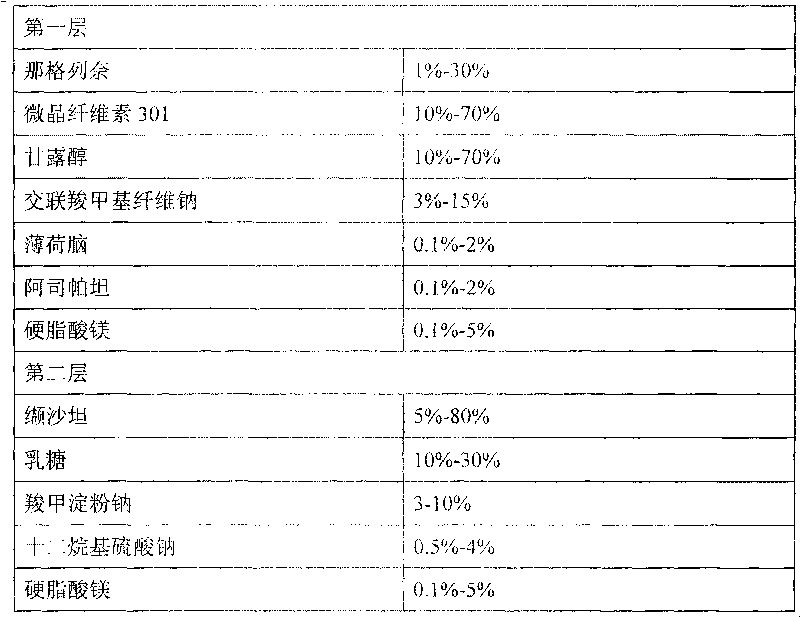
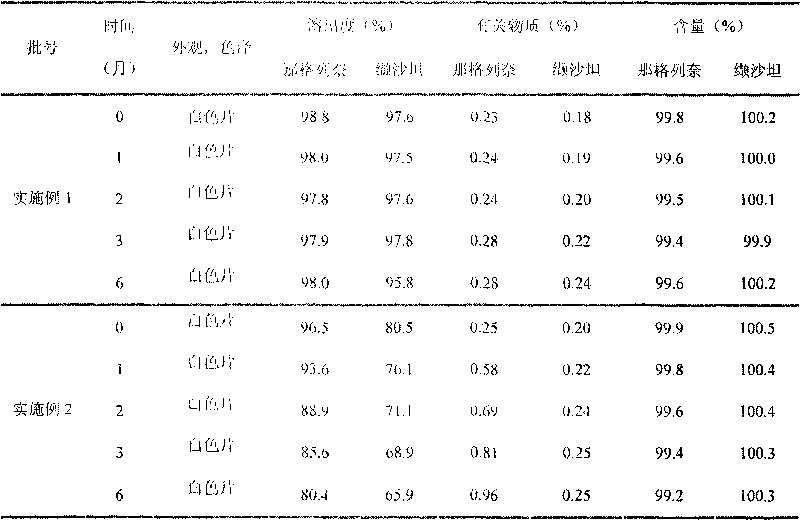
Compound nateglinide valsatan medicinal composition
The invention relates to a compound nateglinide valsatan medicinal composition, which is a dual-layer tablet and comprises a first layer of nateglinide and a second layer of valsatan, wherein the first layer is delivered from a self-soluble tablet to be immediately released; and the second layer is released from a self-disintegration or corroded tablet matrix.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Vitamin C soluble tablet and preparation method thereof
ActiveCN103877050BMeet needsIncrease usageOrganic active ingredientsMetabolism disorderVitamin CCalcium EDTA
The invention relates to a vitamin C soluble tablet. The medicinal composition is vitamin C or calcium salt dihydrate thereof. The invention also provides a preparation method of the vitamin C soluble tablet. A reasonable auxiliary material formula is selected, so that the vitamin C soluble tablet can be quickly disintegrated within 2 minutes in water and becomes a settled solution form, so that the drug is quickly obtained. By adopting the preparation method disclosed by the invention, vitamin C can be well protected, and oxidation is reduced. Meanwhile, the preparation time is also shortened when the tablet with excellent performance is obtained, and the cost is reduced. The preparation method disclosed by the invention is simple and convenient in process, easy to carry out, and applicable to massive industrial production.
Owner:北京朗迪制药有限公司
Soluble tablet containing chloroisobromine cyanuric acid and kasugamycin and preparation method thereof
The invention discloses a soluble tablet containing chloroisobromine cyanuric acid and kasugamycin and a preparation method thereof. With chloroisobromine cyanuric acid and kasugamycin as active ingredients, the soluble tablet has the advantages of complete water solubility without insoluble impurities and rapid dissolution. Dry granulation is adopted for the soluble tablet, a tableting machine isused for production, the production energy consumption is low, no dust flies in the production and use process, and the soluble tablet causes no pollution to the human body and the environment. The soluble tablet meets the requirements of China for the development of pesticide products towards the environmental protection direction, the advantages of chloroisobromine cyanuric acid and kasugamycincan be better exerted, and the life cycle of the products is prolonged.
Owner:江苏邦盛生物科技有限责任公司
A kind of imatinib mesylate stomach-dissolving pellets and preparation method thereof
ActiveCN105663064BEasy to takeEasy to carryOrganic active ingredientsPill deliveryFluidized bedMedicine
The invention discloses an imatinib mesylate pellet tablet and a preparation method thereof. The preparation method comprises the following steps: firstly, preparing a main drug, namely imatinib mesylate, into a gastric-soluble coating pellet by a fluidized bed coating process, and then mixing the coating pellet with a filling agent, a disintegrating agent, an adhesive, a plasticizing agent, a lubricating agent and the like to prepare the gastric-soluble tablet by a compressing dry granulation technology. The tablet can be directly swallowed and can be quickly disintegrated into pellets in water so as to be convenient for patients with dysphagia and especially children to take. The accurate dose of the tablet can be ensured, and the tablet can be taken safely and conveniently.
Owner:HENAN LIFE PHARMA CO LTD
A kind of thymosin enteric-coated tablet and preparation method thereof
ActiveCN111297820BSolve the fluctuation of treatment effectGood treatment effectOrganic active ingredientsPeptide/protein ingredientsImmunomodulationsEnteric coating
The invention relates to a thymosin enteric-coated tablet and a preparation method thereof. The thymosin enteric-coated tablet comprises thymosin, elinic acid, a pharmaceutically acceptable carrier, an enteric coating and an optional absorption promoter. The addition of Potentinic acid in the thymosin enteric-coated tablet of the present invention can not only effectively solve the release fluctuation of thymosin in the intestinal tract due to pH changes, but also has a considerable promotion effect on the activity of thymosin, further enhancing the strength of the thymosin enteric-coated tablet. immunomodulatory effect.
Owner:H H T WHITE SWAN PHARMACY GRP
Hydrochloric acid boningmycin solid preparation and preparation method thereof
ActiveCN102462668BImprove complianceImprove stabilityAntiviralsSaccharide peptide ingredientsFiller ExcipientAdhesive
The invention provides a hydrochloric acid boningmycin solid preparation, which is a soluble tablet or a dispersible tablet. The compositions and weight percentages of the hydrochloric acid boningmycin solid preparation are as follows: 0.5 to 50wt% of hydrochloric acid boningmycin, 15 to 99.4wt% of fillers achieving a filling effect, 0 to 20wt% of disintegrants achieving a disintegration effect, 0 to 10 wt% of adhesives achieving an adhesion effect and 0.1 to 5wt% of lubricants achieving a lubrication effect. The invention also provides a preparation method of the soluble tablet and the dispersible tablet. By adopting the hydrochloric acid boningmycin tablet provided by the invention, on the one hand, the deficiency that preparations such as ointment, gel, and the like have poor stabilityafter being placed for a long time under a room temperature condition is overcome, and the storage and the carrying are facilitated; and on the other hand, before being used, the tablet is added to aspecial solvent so as to be dissolved or dispersed to form a water solution or an injectable suspension with certain viscosity, and thereby the tablet also has the advantage of a semi-solid or liquidexternal preparation on the aspects of the use and the pharmaceutical effect.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
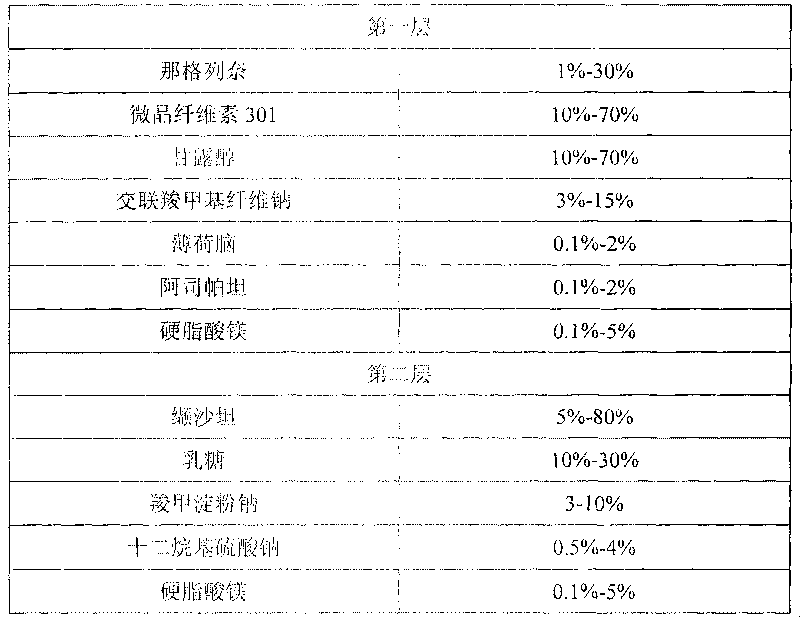
Compound nateglinide valsatan medicinal composition
The invention relates to a compound nateglinide valsatan medicinal composition, which is a dual-layer tablet and comprises a first layer of nateglinide and a second layer of valsatan, wherein the first layer is delivered from a self-soluble tablet to be immediately released; and the second layer is released from a self-disintegration or corroded tablet matrix.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Pitavastatin soluble tablet composition and preparation method thereof
Owner:南京馗珂生物医药技术有限公司
Child soluble amantadine hydrochloride soluble tablet
InactiveCN101204385BEasy to calculateEasy to operateAntiviralsPill deliveryDrugs solutionWater soluble
The invention relates to an amantadine hydrochloride soluble tablet exclusively used for children, which is made up of an effective dose of hydrochloric acid admantadine and a water soluble medical excipient that can rapidly disintegrate and release drugs. Patients determine the dose of the tablet according to weight, thus realizing personalized medicine administration and ensuring the medicine intake dose more appropriate with higher security. Once in solution, the medicine instantly becomes a sweet and sour beverage, which is more easily to be accepted by children; meanwhile the medicine solution is easily to be absorbed fully and the bioavailability is high.
Owner:何德义
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com