Hydrobromic acid vortioxetine gastric-soluble tablet and preparation method thereof
A technology of vortioxetine hydrobromide and gastric dissolution, which is applied in the field of medicine and achieves good application prospects and market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
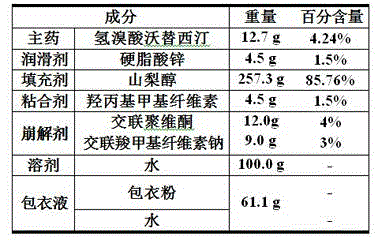
Embodiment 1
[0025] 1. Each component and its weight percentage are as follows:
[0026]
[0027] 2. Preparation method :
[0028] Take by weighing 12.7 g vortioxetine hydrobromide, 234.8 g mannitol, 12.0 g hydroxypropyl cellulose, 24.0 g microcrystalline cellulose and 9.0 g sodium starch glycolate respectively, after mixing and stirring evenly, add 100 g water wherein to continue Mix and stir evenly to obtain wet granules, pass through a 10-mesh sieve, dry in an oven at 65 °C for 1-2 hours, and pass through a 10-mesh sieve to obtain granules;
[0029] The granules obtained and the 7.5g magnesium stearate taken by weighing are added in the V-shaped mixer, and after mixing for 20-30 min, the mixture is obtained;
[0030] Add the mixture into the hopper of the tablet press, adjust the tablet weight to 0.15 g / tablet, and then perform tablet compression to obtain plain tablets;
[0031] Add the plain tablet into the coating pan, measure 61.1 g of the coating liquid, and spray coating ...
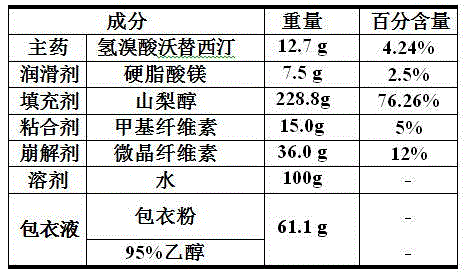
Embodiment 2
[0034] 1. Components and their weight percentages are shown in the following table:
[0035]
[0036] 2. Preparation method:
[0037] Weigh 12.7 g vortioxetine hydrobromide, 257.3 g mannitol, 4.5 g hydroxypropyl methylcellulose, 12.0 g crospovidone and 9.0 g croscarmellose sodium, mix and stir evenly Finally, add 27.8 g of water to it and continue mixing and stirring to obtain wet granules, pass through a 10-mesh sieve, dry in an oven at 65 °C for 1-2 h, and pass through a 10-mesh sieve to obtain granules;
[0038] The obtained granules and 4.5 g of zinc stearate weighed are added to a V-type mixer, and after mixing for 20 to 30 min, the mixture is obtained;
[0039] Add the mixture into the hopper of the tablet press, adjust the tablet weight to 0.15 g / tablet, and then perform tablet compression to obtain plain tablets;
[0040] Add the plain tablet into the coating pan, measure 61.1 g of the coating liquid, and spray coating at a speed of 3 g / kg per minute, the weig...
Embodiment 3
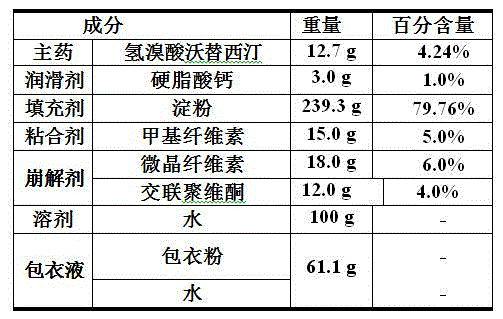
[0043] 1. Components and their weight percentages are shown in the following table:
[0044]
[0045] 2. Preparation method:
[0046] Weigh 12.7 g vortioxetine hydrobromide, 239.3 g starch, 15.0 g methylcellulose, 18.0 g microcrystalline cellulose and 12.0 g crospovidone, mix and stir evenly, add 100.0 g water to it Continue to mix and stir evenly to obtain wet granules, pass through a 10-mesh sieve, dry in an oven at 65 °C for 1-2 h, and pass through a 10-mesh sieve to obtain granules;
[0047] The obtained granules and 3.0 g of calcium stearate weighed are added to a V-shaped mixer, and after mixing for 20 to 30 min, the mixture is obtained;
[0048] Add the mixture into the hopper of the tablet press, adjust the tablet weight to 0.15 g / tablet, and then perform tablet compression to obtain plain tablets;
[0049] Add the plain tablet into the coating pan, measure 61.1 g of the coating liquid, and spray coating at a speed of 3 g / kg per minute, the weight of the prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com