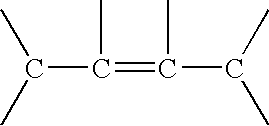
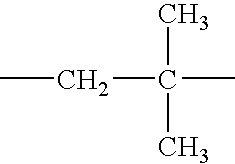
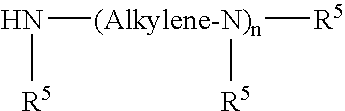Quaternary Ammonium Salt of a Polyalkene-Substituted Amine Compound
a technology of amine compound and quaternary ammonium salt, which is applied in the field of quaternary ammonium salt detergent, can solve the problems of poor fuel economy, high emission, and components that can degrade during engine operation and form deposits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
[0138]The invention will be further illustrated by the following examples, which sets forth particularly advantageous embodiments. While the examples are provided to illustrate the present invention, they are not intended to limit it.
example a
Preparation of Polyisobutene-dimethylamine (PIB DMA) via Chlorine
[0139]An apparatus suitable to handle chlorine and hydrogen chloride gas (glass reactor, glass stirrer, PTFE joints, glass thermowell for thermocouple) is connected to sodium hydroxide scrubbers. The glass vessel is charged with 1000 g (˜1 mole, 1 equiv.) of low vinylidene 1000 Mn polyisobutylene (PIB) and is heated to 110-120° C. and 1 mole (70 g, 1 equiv) chlorine bubbled into the reactor over 7 hours. The reaction is sparged with nitrogen at 110-120° C. overnight to remove HCl.
[0140]The resultant PIB chloride is transferred to an autoclave and the autoclave sealed. For every mole (˜1030 g) PIB chloride 1 mole of gaseous dimethylamine (45 g) is added and the reaction is heated to 160-170° C. for 8 hours, or until no further reduction in pressure is seen. The reaction is cooled to room temperature and the pressure is released. Enough Solvesso™ 150 solvent is added to make a 70% w / w actives solution and the reaction is...
example b
Preparation of Polyisobutene-dimethylamine (PIB DMA) via Hydroformylation
[0141]High vinylidene polyisobutylene (PIB) (500 g, Mn 950, 0.53 moles), 300 g dodecane and 2.8 g cobalt octacarbonyl is heated in a 2.5 litre autoclave at 280 bar 1:1 CO:H2 for 5 hours at 185° C., while stirring. The mixture is cooled to room temperature and the catalyst is removed by washing with 400 ml 10% aqueous acetic acid. The product is neutralized by washing, and the dodecane is stripped off to yield the PIB aldehyde and possibly also PIB alcohol.
[0142]A reactor equipped with stirrer, condenser and Dean and Stark trap is charged with 1 mole (˜980 g) of the above hydroformylated PIB plus 0.5 moles of 40% aqueous dimethylamine solution (112.5 g) and 500 ml cyclohexane. The reaction is heated to reflux until no more water is removed, and the cyclohexane is removed by distillation under vacuum. 1 kg of the azomethine product from above is reacted with 100 g Raney nickel and 25000 kPa hydrogen in an autocla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| specific gravity | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com